Abstract
Usnic acid, as active dibenzofuran derivative, isolated and characterized from some lichen species. The aim of manuscript was to evaluate antioxidant, anticholinergic and antidiabetic potentials of usnic acid as an important natural product. Antioxidant profile of usnic acid determined by eight distinguishes bioanalytical antioxidant methods including 1,1-diphenyl-2-picrylhydrazyl (DPPH·), 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS•+), superoxide anion radical (O2•−) and N,N-dimethyl-p-phenylenediamine (DMPD•+) scavenging activities, cupric ion (Cu2+), ferric ion (Fe3+) and Fe3+-TPTZ reducing abilities and ferrous ion (Fe3+) chelating activity. Usnic acid was found as potent DPPH· (IC50: 49.50 μg/mL), DMPD•+ (IC50: 33.00 μg/mL), O2•− (IC50: 18.68 μg/mL), and ABTS•+ (IC50: 10.41 μg/mL) scavenging effects. Also, the inhibition effects of usnic acid were tested against some metabolic enzymes including acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) linked to neurodegenerative diseases. Both enzymes play important roles in cholinergic transmission by hydrolyzing the neurotransmitter acetylcholine in cholinergic synapses, central nervous system, neuromuscular junctions and autonomic ganglia. Their inhibitors were used for clinical treatment of some neurodegenerative conditions including myasthenia gravis, Alzheimer's disease, apathy, glaucoma, postural tachycardia syndrome and dementia. Furthermore, usnic acid showed the potent inhibition profiles against AChE (IC50: 1.273 nM) and BChE (IC50: 0.239 nM) enzymes. The results clearly showed that usnic acid is an important natural product with antioxidant and anticholinergic potentials.
Keywords: Usnic acid, Antioxidant activity, Acetylcholinesterase, Butyrylcholinesterase, Enzyme inhibition
1. Introduction
Usnic acid was firstly isolated from lichens including Usnea in 1844 [1]. It is the most widespread and well-studied secondary lichen metabolite and has a large spectrum of biological activities such as antibacterial and cytotoxic, antiproliferative, antiviral, antimicrobial, antiprotozoal, antimycotic, antiparasitic, anti-pyretic, anesthetic, anti-inflammatory effects [2] and antitumor effects in different cell types [3]. Also, it was proven that usnic acid wound-healing effect [[4], [5], [6], [7]].
Reactive oxygen species (ROS) occur in living organisms during normal cellular metabolism and can be harmful decisive biomolecules including lipids, carbohydrates, nucleic acids, and proteins [[8], [9], [10]]. Also, ROS, which have been implicated in many diseases, are produced in the all-living organisms as primary immune defense [11,12]. Recently, oxidative stress and ROS had been accepted as an important environmental risk for different chronic disorders such as cancer, immunodeficiency syndrome, age-related pathologies, cardiovascular diseases, arteriosclerosis, diabetes, and obesity [13,14]. Antioxidant defense system includes antioxidant components and antioxidant enzymes. They can repair or remove the damaged biomolecules in living organisms [[15], [16], [17]]. Plants include a lot biological active phytochemicals and metabolites such as phenols, polyphenols that possess structural features, which had antioxidant activities [[18], [19], [20], [21]]. Hence, they have been intensely researched for their possible effectiveness and health promoting benefits including antioxidant activity. For this reason, many works had been performed on antioxidants and crude extracts from plants [22]. Also, there is an increased demand in safer and natural antioxidants for food and pharmaceutical applications related to human health. This growing trend canalized consumer demand to antioxidants from natural source like medicinal plants [23]. Phenolic compounds are plant secondary metabolites and reduce oxidative damage and prevent human health against degenerative disorders including cataract, cardiovascular diseases, cancer, hypercholesterolemia, rheumatoid arthritis, diabetes, and arteriosclerosis [24].
Alzheimer’s disease (AD) generally effects memory and behavior of elderly people in worldwide. This neurological disease clinically includes the growing degeneration of brain tissue, which influenced by acetylcholine (ACh) deficiency [25]. AChE converts ACh to choline (Ch) and acetate [26]. It was reported that decreasing of ACh levels in hippocampus and cortex had great biochemical changes in patients with AD [[27], [28], [29], [30]]. Natural substances as AChE inhibitors (AChEIs) had a common usage in clinical trials medicine, especially for the AD treatment. Phenolic compounds had been also identified as AChEIs and providing pioneer compounds for AD treatment [[31], [32], [33], [34], [35]].
The aim of this study was to determine the antioxidant activity of usnic acid using by Fe3+, Cu2+ and Fe3+-TPTZ reducing antioxidant power, ABTS•+, DPPH, O2•− and DMPD•+ scavenging activities and compare to standard antioxidant molecules of α-tocopherol and trolox. Another goal of this study was to demonstrate the inhibition effect of usnic acid against both cholinergic enzymes (BChE and AChE), which linked to some neurodegenerative diseases.
2. Material and methods
2.1. Chemicals
Usnic acid, neocuproine, DMPD, ABTS, BHA, DPPH·, BHT, α-tocopherol, trolox and other chemicals were purchased from Sigma-Aldrich (Germany).
2.2. Reducing ability assays
Fe3+-reducing effect of usnic acid was done accordance with the Oyaizu’s method [36] with minor revision as described previously [37]. Briefly, certain concentrations of usnic acid (10−30 μg/mL) were transferred to same volume of buffer solution (pH 6.6, 1.25 mL 0.2 M) and K3Fe(CN)6 solution (1.25 mL, 1 %). The mixture was incubated at 50 °C for 20 min. and acidified with TCA (10 %, 1.25 mL). Finally, 0.5 mL of FeCl3 solution (0.1 %) was transferred and the absorbance of mixture was spectrophotometrically recorded at 700 nm [38].
Cu2+ reducing effects of usnic acid was measured according to the Apak’s method [39] as described in details [40]. To this end, same volumes (0.25 mL) of CuCl2 solution (10 mM), 250 μL of neocuproine solution (7.5 mM) and 0.25 mL of acetate buffer (1.0 M) were added to usnic acid solution (contains 10−30 μg/mL) in a glass test tube. Total volume of mixture was set to 2 mL with deionized water. Then, glass tubes were closed and detained at r.t. Finally, their absorbances were spectrophotometrically measured at 450 nm [41].
The FRAP reducing power is relied on Fe3+-TPTZ reduction of in acidic medium. Reduced form of TPTZ-Fe2+ is spectrophotometrically measured at 593 nm. FRAP reagent includes 2.25 mL solution of TPTZ (10 mM) with 2.25 mL of FeCl3 (20 mM) in 2.5 mL of buffer (pH 3.6, 0.3 M). Then, 0.2 mL of sample was transferred to 1.8 mL of FRAP reagent. Finally their absorbances were spectrophotometrically determined at 593 nm. Then, usnic acid (10−30 μg/mL) was dissolved in 5 mL of buffer solution and left at 37 °C for half hour. Lastly, the samples’ absorbances were recorded at the indicated wavelength [42].
2.3. Fe2+ chelating activity
The ferrous ions (Fe2+) chelating ability of usnic acid was done according to the previous method [43] with some modification [44]. Fe2+-binding ability of usnic acid was spectrophotometrically recorded at 522 nm [45]. Briefly, to an aliquot of 100 μL FeCl2 (0.6 mM) was transferred to 0.4 mL of usnic acid (10−30 mg/mL). The disrupting complex formation as (%) was obtained by following formula: Chelated Fe2+ (%) = (1-As/Ac) x 100. Where Ac and As are the absorbances of control and sample [46].
2.4. Radicals scavenging activities
The DPPH radical scavenging effects of usnic acid were performed as the first radical scavenging method [47] as described in previous study [48,49]. DPPH• is used for estimation of radical scavenging ability of pure substance or plant extracts. For this aim, an aliquot (0.5 mL, 0.1 mM) of DPPH• in ethanol was added to sample solution (1.5 mL) in ethyl alcohol (10−30 μg/mL) and incubated for half hour in dark. The absorbance samples were measured at 517 nm. Analyses were achieved in triplicate.
At the same manner, ABTS•+ scavenging activity of usnic acid is determined based on previous method [50]. The 2.0 mM ABTS solution in deionized water and 2.3 mM oxidizing agent of K2S2O8 resulted the ABTS cation radical (ABTS•+). The inquired absorbance (0.750 ± 0.025) was adjusted with buffer solution (0.1 mM; pH 7.4). Finally, 3.0 mL of certain concentrations of usnic acid (10−30 μg/mL) were interacted to 1.0 mL of ABTS radicals and the remaining absorbance was spectrophotometrically recorded at 734 nm.
DMPD•+ scavenging effects of usnic acid were realized using the previous method [50]. For this purpose, a portion of DMPD•+ solution (1 mL, 0.1 M) was transferred to 100 mL of acetate buffer (pH 5.3, 0.1 M,) containing certain concentrations of usnic acid (10−30 μg/mL). Then, 0.2 mL of FeCl3 (50 mM) was transferred to this solution and their absorbance was spectrophotometrically measured at 505 nm.
Superoxide anion radical (O2•−) was formed in quadruple system of methionine, riboflavin and illuminate system according to the method of Beauchamp and Fridovich [51] with minor modification [52]. The resulting radical product (O2•−) was assayed by the NBT reduction, which spectrophotometrically recorded at 560 nm.
All radical scavenging capacities (RSC) of usnic acid were calculated as millimolar in the reaction mixture. All radicals scavenging effects (RSC) were calculated as following: RSC (%) = (1 - λs / λc) x 100. In here λc and λs describe the absorbances of control and samples. Half maximal scavenging of chelating concentration (IC50) was estimated by plotting percentages against sample of usnic acid concentrations (μg/mL) [53].
2.5. Enzymes inhibition studies
2.5.1. AChE and BChE inhibition studies
Cholinergic enzymes inhibitory activities of usnic acid were done accordance with Ellman’s method [54] as given in a previous study [55]. Electric eel serum AChE and equine serum BChE were used for this purpose. Briefly, certain usnic concentration (10−30 μg/mL) in buffer (Tris/HCl, 1.0 M, 100 μL, pH 8.0) was transferred to the enzymes solution (50 μL, 5.32 × 10−3 EU). The mixtures were left for 10 min at 20 °C. Then, 50 μL of DTNB (5,5′-dithio-bis(2-nitro-benzoic)acid) (0.5 mM) and acetylthiocholine iodide (AChI) / butrylcholine iodide (BChI) were transferred to mixtures. Finally, the reaction medium was started and absorbances of incubated mixture were spectrophotometrically recorded at 412 nm [56].
2.6. Statistical analysis
The result values are average of triplicate analysis. The experimental data were calculated as mean ± standard deviation. Variance ANOVA including one-way analysis was realized. Significant differences between means were recorded by Duncan’s Multiple Range tests. p<0.05 was regarded as significant and p<0.01 as very significant.
3. Results
3.1. Antioxidant results
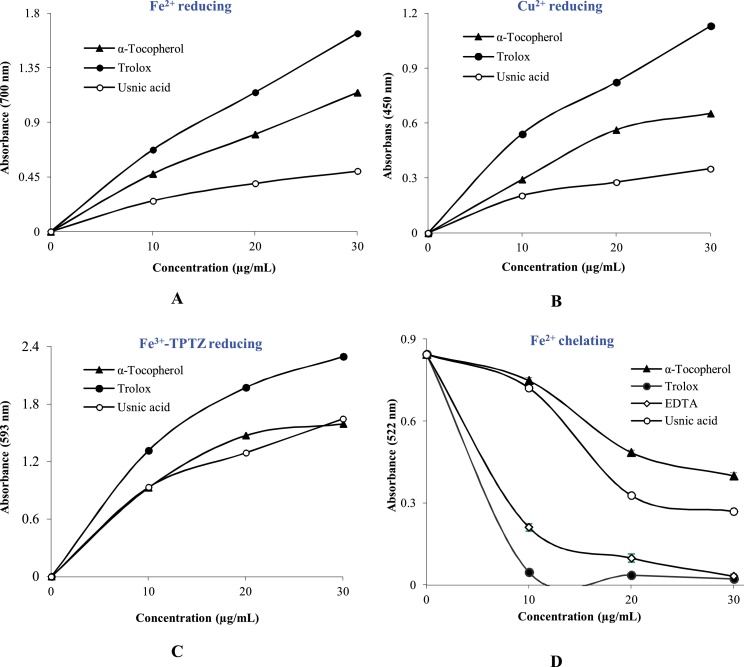
Fe[(CN-)6]3 reduction methods can easily measured the reducing power of usnic acid. Ferric ions (Fe3+) addition to usnic acid leads to occurring of Fe4[Fe(CN−)6]3 complex, which had a maximum absorbance at 700 nm [57]. In this context, usnic acid had effective reducing effects by using Fe[(CN-)6]3 reduction, Cu2+ and Fe3+-TPTZ reducing methods. Fe3+ reductive abilities of usnic acid were performed according to the Oyaizu method [36]. As summarized in Table 1 and Fig. 1A, usnic acid (r2: 0.9470) showed potent Fe3+ reducing profile (p<0.01). The Fe3+ reducing ability of usnic acid and standards decreased in following orders: Trolox (2.177 ± 0.07, r2:0.9736) > α-tocopherol (1.644 ± 0.17, r2:0.9118) > Usnic acid (0.278, r2: 0.9567).
Table 1.
Determination of absorbance of 20 μg/mL for usnic acid and standards for Fe3+ reducing, Fe3+-TPTZ reducing and Cu2+ reducing activities of usnic acid and standard compounds such as α-tocopherol and trolox.
| Antioxidants | Fe3+ reducing | Fe3+-TPTZ reducing | Cu2+ reducing |
|---|---|---|---|
| α-Tocopherol | 0.802 | 1.473 | 0.564 |
| Trolox | 1.148 | 1.973 | 0.825 |
| Usnic acid | 0.278 | 1.293 | 0.277 |
Fig. 1.
Antioxidant activity of usnic acid. A. Fe3+-Fe2+ reducing ability assay, B. Cupric ions (Cu2+) reducing ability by Cuprac assay, C. Fe3+-TPTZ+ complex reducing ability assay, D. Ferrous ions (Fe2+) chelating ability assay.
Cu2+ reducing abilities of usnic acid was given in Table 1. A good correlation was found between the Cu2+ reducing effect and usnic acid (r2:0.9254) and sample concentrations depending on concentration (10−30 μg/mL). However, at 30 μg/mL concentration, the marked absorbance of reducing power was displayed by usnic acid (0.277; r2: 0.9254). Cu2+ ions reducing ability of usnic acid and standards were as follows (Fig. 1B): Trolox (0.825, r2: 0.9811) > α-Tocopherol (0.564, r2: 0.9277) > Usnic acid (0.277, r2: 0.9254). On the other hand, FRAP effects of usnic acid declined in the following arrangement (Table 1 and Fig. 1C): Trolox (2.432 ± 0.015, r2: 0.9611) > α-Tocopherol (2.327 ± 0.001, r2: 0.9998) > Usnic acid (1.293, r2: 0.9355). Increased reducing ability of a pure compound indicated its higher antioxidant activity.
Also, usnic acid had strong Fe2+ chelating effect. The difference between the control values and certain usnic acid concentrations (10−30 μg /mL) were statistically found significant (p < 0.01). Usnic acid exhibited IC50 value of 18.68 μg/mL (Fig. 1D and Table 2). When the Fe2+ chelating effect of usnic acid was compared to α-tocopherol, trolox and EDTA, usnic acid exhibited demonstrated potent Fe2+ chelating ability. IC50 values of the Fe2+ chelating capacity of the same standard metal chelators including of α-tocopherol, trolox and EDTA were found to be 27.72, 8.05, and 6.24 nM, respectively. Based on these results, usnic acid was statistically higher than α-tocopherol (p > 0.05) but lower than that of trolox and trolox and EDTA, which a strong metal chelator (p < 0.05).
Table 2.
Determination of half maximal concentrations (IC50, μg/mL) of usnic acid and standards for DPPH• scavenging, ABTS•+ scavenging, DMPD•+ scavenging and superoxide radicals O2•− scavenging activities of usnic acid and standard compounds such as α-tocopherol and trolox.
| Antioxidants | DPPH∙ Scavenging |
ABTS∙+ Scavenging | DMPD∙+ Scavenging |
O2·− Scavenging |
Metal Chelating |
|---|---|---|---|---|---|
| α-Tocopherol | 9.76 | 5.02 | -* | 21.01 | 27.72 |
| Trolox | 8.01 | 4.30 | 22.35 | 30.13 | 8.05 |
| EDTA | – | – | – | – | 6.24 |
| Usnic acid | 49.50 | 10.41 | 33.00 | 20.38 | 18.68 |
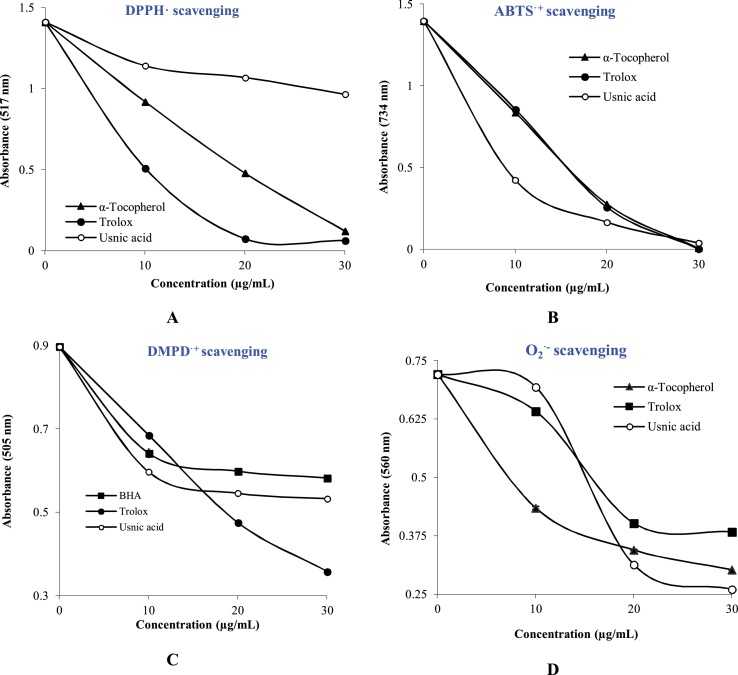
As seen in Table 2 and Fig. 2A, a significant scavenging ability (p<0.01) in the DPPH· concentration and radical scavenging ability of usnic acid was observed. When an antioxidant component or plant extracts reacts with DPPH radicals, they can donate hydrogen and consequently reduced DPPH·. The color changes were spectrophotometrically recorded at 517 nm. IC50 values of DPPH· scavenging were fond as 49.50 μg/mL (r2: 0.9128) for usnic acid, 9.76 μg/mL (r2: 0.9951) for α-tocopherol and 8.01 μg/mL (r2: 0.8355) for trolox. The tested usnic acid in this study exhibited effective radical scavenging activity against ABTS radicals (p > 0.001). As seen in Table 2, Usnic acid had effective ABTS radicals scavenging in a dose-dependently (10−30 μg/mL, p < 0.001). IC50 values for Usnic acid in this assay were calculated as 10.41 μg/mL (r2: 0.8267). Also, IC50 values were found as for 5.02 μg/mL (r2: 0.9779) for α-tocopherol and 4.30 μg/mL (r2: 0.9756) for trolox (Fig. 2B). As shown in Table 2, usnic acid was an effective DMPD•+ scavenger in a dose-dependently (10–30 μg/mL). IC50 values of usnic acid were calculated as 33.00 μg/mL (r2: 0.7417). IC50 values were determined as for 22.35 μg/mL (r2: 0.9842) for trolox (Fig. 2C). Also usnic acid had marked O2•+ scavenging activity with IC50 value of 20.38 μg/mL (Table 2 and Fig. 2D). Lastly, IC50 values of usnic acid were calculated to be 18.68 nM for Fe2+ chelating effects (Fig. 1D). Also, same value for α-tocopherol, trolox and EDTA as a powerful metal chelator were found as 27.72, 8.05 and 6.24 nM, respectively.
Fig. 2.
Radical scavenging activity of usnic acid. A. DPPH· scavenging assay, B. ABTS•+ scavenging assay, C. DMPD•+ scavenging assay. D. O2•− scavenging assay.
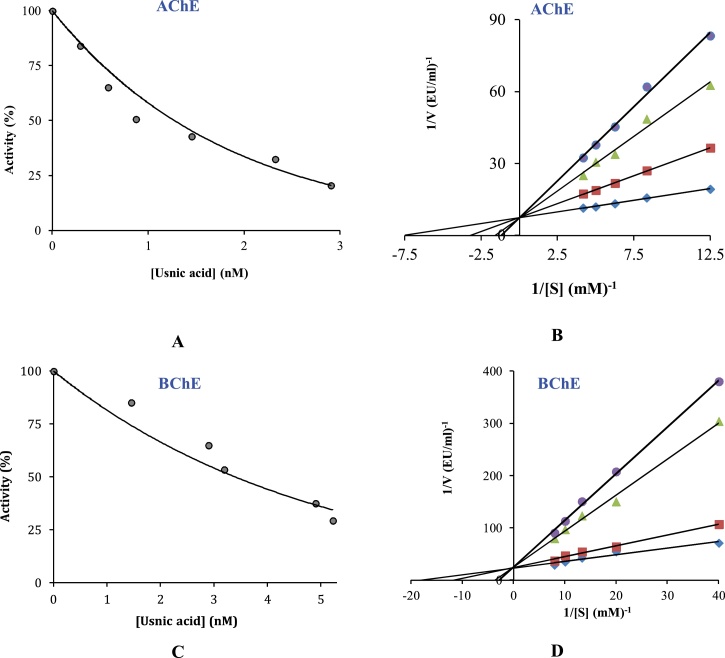
Usnic acid had IC50 values of 1.273 nM for AChE and 3.397 nM for BChE. Also, tacrine as clinical used inhibitors had Ki values of 124.58 μM (r2: 0.9744) and 97.70 μM (r2: 0.9978) against AChE, and BChE, respectively. Usnic acid demonstrated effective inhibition profiles on AChE and BChE (Table 3).
Table 3.
The enzyme inhibition results (IC50 and Ki values) of usnic acid against acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) enzymes. Tacrine was used as positive control for acetylcholinesterase and butyrylcholinesterase enzymes.
| Inhibitors | AChE |
BChE |
||
|---|---|---|---|---|
| IC50 (nM) | Ki (nM) | IC50 (nM) | Ki (nM) | |
| Usnic acid | 1.273 | 0.239 | 3.397 | 1.425 |
| Tacrine | 1.795 | 0.160 | 3.666 | 1.984 |
4. Discussion
Lichens are an interesting source of potential biological active substances. They are symbiosis between algae and fungi or cyanobacteria produce a number of bioactive compounds. Lichen-specific compounds are a unique class of secondary metabolites [[58], [59], [60]]. Recently, there has been an increased interest in lichens with biological and pharmacological active molecules. Usnic acid is a best-known and commercially valued lichen metabolite. It is a pure substance used in creams, toothpaste, deodorants, mouthwash, and sunscreen products. Also, its ecological and biological effects including anti-growth, anti-insect and anti-herbivore properties had been well documented [61]. Reducing potential of usnic acid was determined by three different and distinct reducing systems including CUPRAC, FRAP and Fe3+ reducing abilities. An antioxidant molecule or plant extracts can be reductants and inactivate oxidant agents and ROS. An increase in absorbance indicates an increased reducing ability because to an increased of complex formation (Fig. 1A). The results demonstrated that both usnic acid could donate electron and neutralize free radicals and ROS. Fe[(CN-)6]3 reduction methods can easily measured reducing power of usnic acid. Ferric ions (Fe3+) addition to usnic acid leads to occurring of Fe4[Fe(CN−)6]3 complex, which had a maximum absorbance at 700 nm [62]. Cuprac assay is rapid, stable, cheap, selective, and suitable method [63]. The FRAP method is performed at acidic medium to maintain iron solubility [64].
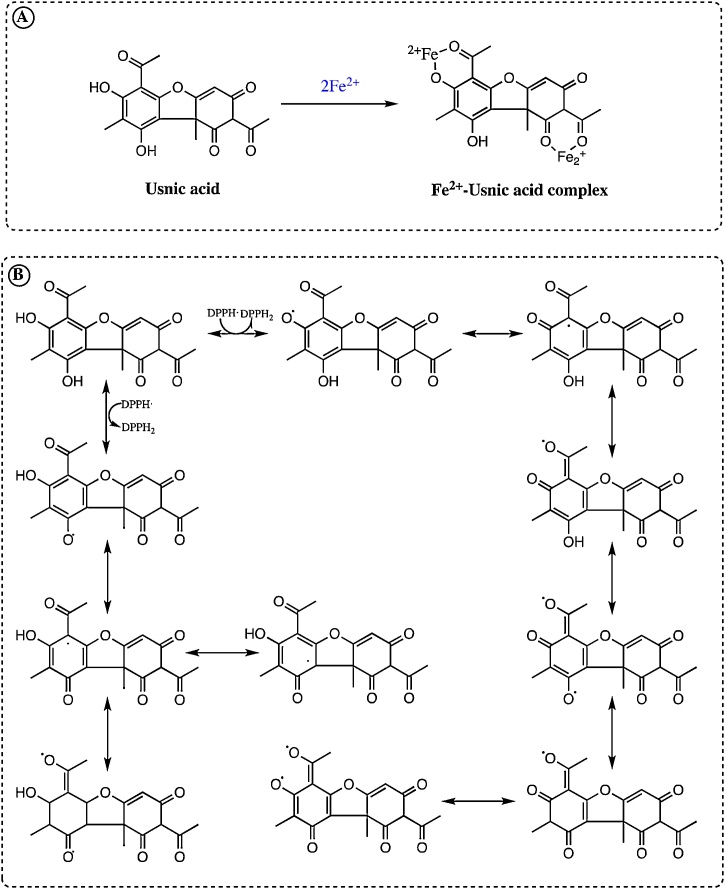
The possible Fe2+ chelating mechanism of usnic acid was given in Fig. 3A. In the current study, EDTA was used as an additional standard metal chelator. Usnic acid interfered with the formation of the ferrozine-Fe2+ complex. Usnic acid had Fe2+ chelating effect and was able to capture Fe2+ ions before ferrozine as a metal biding agent. The structure of usnic acid and its binding sites for metal chelation was shown in Fig. 4A. It may chelate the Fe2+ with its hydroxyl and carboxyl groups bounded phenolic ring. It was well known that the compounds with structures containing functional groups of −CO, −COH and −COOH could easily coordinate to metal ions including Fe2+. Also, it was demonstrated that phenolic molecules such as kaempferol, which is a natural flavanol found chelated Cu2+ and Fe2+ ions through the above-indicated functional groups [65]. Also, The compounds had two or more of the following functional groups: −OH, −COOH, –SH, –NR2, −CO, –H2PO3, –S– and –O– in a function-structure configuration, can easily show metal chelating ability [66].
Fig. 3.
The effect of usnic acid on acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) enzymes. A and C: IC50 values of Usnic acid for AChE and BChE. B and D: Ki values of Usnic acid for AChE and BChE, which determined from Lineweaver-Burk graph.
Fig. 4.
A. Possible ferrous ions (Fe2+) chelating mechanism of usnic acid. B. Purposed radical scavenging mechanism between usnic acid and DPPH radicals.
DPPH·, O2•− DMPD·+, and ABTS·+ scavenging assays are the most putative and antioxidant methods to determine of the antioxidant ability of beverages, foods, and plants [67]. Usnic acid was computed from the bleaching property of the purple-colored ethanol solution of DPPH·. This radical scavenging ability is the most used and oldest method for determining of radical scavenging and antioxidant activities. In radical scavenging assay, the antioxidant compounds and plant extracts can reduce DPPH· to DPPH2 [68]. The structure of the usnic acid leads to interference in the DPPH·. After the interaction of usnic acid and radicals, DPPH· disappears after accepted an electron or hydrogen radical from usnic acid to become DPPH2. The featured reaction between DPPH· and usnic acid is shown in Fig. 3B. DPPH· scavenging mechanism of usnic acid has not been reported, so far. However, the best knowledge is that a phenolic group stabilizes radicals formed on phenolic carbon with their resonance structure. In usnic acid molecule, phenolic group has also two hydroxyl units. A withdrawing of hydrogen atoms from phenolic hydroxyl groups may occur easily. Usnic acid can be found in the triradical structures by removing three DPPH molecules using resonance structures. Different resonance structures for these triradical structures are shown in Fig. 3B.
ABTS radical scavenging can applicable for plant extracts including both hydrophilic and lipophilic compounds. ABTS·+ scavenging has a characteristic wavelength at 734 nm. ABTS·+ scavenging assay can be used in a large spectrum of pH range. This is allowed to study the effect of pH on antioxidant mechanisms for food extracts or components [69]. The last evaluated radical scavenging assay is DMPD•+ scavenging activity [70]. α-Tocopherol as hydrophobic antioxidants did not demonstrate scavenging activity in this assay [71]. There is a significant decrease (p<0.05) between DMPD•+ scavenging and usnic acid concentrations. The results shown that usnic acid can easily transfer hydrogen atoms to DMPD•+ and quenched this radical. Usnic acid is effectively blocked of O2•− generation (Table 2). Also, these results demonstrated that when compared to the standards, Usnic acid had remarkable O2•− scavenging activity.
Enzyme inhibitory activity of usnic acid was done using AChE and BChE enzymes. Considering the fact that usnic acid is found as effective indicated metabolic enzyme inhibition effects. Enzyme inhibitions are most studied therapeutic medium in cosmetic and pharmaceutical and food industries. Enzyme inhibitors are clinically used as drugs for managing of some health problems, including AD, obesity, and diabetes [72]. It was reported that reported there is side effects including gastrointestinal disturbances and hepatotoxicity of synthetic inhibitors. However, there are great interests for finding natural and novel inhibitors without side effects [73]. The inhibition data are summarized in Table 3. For evaluation of the effect of usnic acid on these enzymes, the following results had been described. AChE and BChE inhibition properties of usnic acid were determined according to the Ellman’s procedure [54]. AChE hydrolysis acetylcholine (ACh) to choline and acetate. The AChE inhibition increases the levels of ACh, thus AChE inhibition were considered as useful therapeutic approach to treat neurological disorders including AD [74]. As observed in antioxidant effects, the usnic acid had the effective AChE and BChE inhibition capacities. It was speculated that the major phenolics identified in usnic acid act as AChE inhibitors. It is known that phenolic compounds had cholinergic enzymes inhibitors [75].
5. Conclusions
For determination of bioactivity of usnic acid antioxidant activities including reducing ability, metal chelating and radical scavenging activity and cholinergic enzymes (AChE and BChE) inhibitory effects were evaluated and compared to the standards. The results demonstrated that usnic acid was found as effective antioxidant abilities in the indicated bioanalytical assays including Fe3+ and Cu2+ reducing, Fe2+ chelating, DPPH·, ABTS•+, DMPD•+ and O2•− radical scavenging abilities. In addition, this active lichen metabolite was found as effective antioxidant activity. Overall, this study suggested that usnic acid might be a promising potential source of benefit compound for some food and pharmaceutical applications.
Declaration of Competing Interest
The authors declare no conflict of interest.
References
- 1.Campanella L., Delfini M., Ercole P., Iacoangeli A., Risuleo G. Molecular char- acterization and action of usnic acid: a drug that inhibits proliferation of mouse polyomavirus in vitro and whose main target is RNA transcription. Biochimie. 2002;84:329–334. doi: 10.1016/s0300-9084(02)01386-x. [DOI] [PubMed] [Google Scholar]
- 2.Piska K., Galanty A., Koczurkiewicz P., Zmudzki P., Potaczek J., Podolak I., Pękala E. Usnic acid reactive metabolites formation in human, rat, and mice T microsomes. Implication for hepatotoxicity. Food Chem. Toxicol. 2018;120:112–118. doi: 10.1016/j.fct.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Song Y., Yu Z., Song B., Guo S., Lei L., Ma X., Su Y. Usnic acid inhibits hypertrophic scarring in a rabbit ear model by T suppressing scar tissue angiogenesis. Biomed. Pharmacother. 2018;108:524–530. doi: 10.1016/j.biopha.2018.06.176. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Z., Zheng Y., Li Y., Bai H., Ma T., Song X., Zhao J., Gao L. The effects of sodium usnic acid by topical application on skin wound healing in rats. Biomed. Pharmacother. 2018;97:587–593. doi: 10.1016/j.biopha.2017.10.093. [DOI] [PubMed] [Google Scholar]
- 5.Araujo A.A.S., de Melo M.G.D., Rabelo T.K., Nunes P.S., Santos S.L., Serafini M.R., Santos M.R., Quintans-Júnior L.J., Gelain D.P. Review of the biological properties and toxicity of usnic acid. Nat. Prod. Res. 2015;6419:1–14. doi: 10.1080/14786419.2015.1007455. [DOI] [PubMed] [Google Scholar]
- 6.Zengin G., Aumeeruddy-Elalfi Z., Mollica A., Yilmaz M.A., Mahomoodally M.F. In vitro and in silico perspectives on biological and phytochemical profile of three halophyte species-A source of innovative phytopharmaceuticals from nature. Phytomedicine. 2018;38:35–44. doi: 10.1016/j.phymed.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Mahomoodally M.F., Vlaisavljevic S., Berezni S., Abdallah H.H., Zengine G., Mollica A.G.A.A., Lobine D., Aktumsek A. Lotus aegaeus (Gris.) Boiss and Iberis sempervirens L.: chemical fingerprints, antioxidant potential, and inhibition activities and docking on key enzymes linked to global health problems. Ind. Crops Prod. 2018;120:270–278. [Google Scholar]
- 8.Taslimi P., Gulçin İ. Antioxidant and anticholinergic properties of olivetol. J. Food Biochem. 2018;42(3) [Google Scholar]
- 9.Gülçin İ., Elias R., Gepdiremen A., Boyer L. Antioxidant activity of lignans from fringe tree (Chionanthus virginicus L.) Eur. Food Res. Technol. 2006;223:759–767. [Google Scholar]
- 10.Elmastas M., Gülçin İ., Işıldak Ö., Küfrevioğlu Ö.İ., İbaoğlu K., Aboul-Enein H.Y. Antioxidant capacity of bay (Laurus nobilis L.) leaves extracts. J. Iran. Chem. Soc. 2006;3(3):258–266. [Google Scholar]
- 11.Gülçin İ. Antioxidant properties of resveratrol: a structure-activity insight. Innov. Food Sci. Emerg. 2010;11:210–218. [Google Scholar]
- 12.Gülçin İ. Antioxidant activity of food constituents-An overview. Arch. Toxicol. 2012;86(3):345–391. doi: 10.1007/s00204-011-0774-2. [DOI] [PubMed] [Google Scholar]
- 13.Gülçin İ. Antioxidant activity of L-Adrenaline: an activity-structure insight. Chem. Biol. Interact. 2009;179(2–3):71–80. doi: 10.1016/j.cbi.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 14.Gülçin İ., Elias R., Gepdiremen A., Taoubi K., Köksal E. Antioxidant secoiridoids from fringe tree (Chionanthus virginicus L.) Wood Sci. Technol. 2009;43(3–4):195–212. [Google Scholar]
- 15.Bursal E., Gülçin İ. Polyphenol contents and in vitro antioxidant activities of lyophilized aqueous extract of kiwifruit (Actinidia deliciosa) Food Res. Int. 2011;44(5):1482–1489. [Google Scholar]
- 16.Halliwell B., Murcia M.A., Chirico S., Aruoma O.I. Free radicals and antioxidants in food and in vivo: what they do and how they work. Crit. Rev. Food Sci. Nutr. 1995;35:7–20. doi: 10.1080/10408399509527682. [DOI] [PubMed] [Google Scholar]
- 17.Gülçin İ., Elmastaş M., Aboul-Enein H.Y. Antioxidant activity of clove oil-A powerful antioxidant source. Arab. J. Chem. 2012;5(4):489–499. [Google Scholar]
- 18.Ko H.C., Lee J.Y., Jang M.G., Song H., Kim S.J. Seasonal variations in the phenolic compounds and antioxidant activity of Sasa quelpaertensis. Ind. Crops Prod. 2018;122:506–512. [Google Scholar]
- 19.Lu Y., Du Y., Qin X., Wu H., Huang Y., Cheng Y., Wei Y. Comprehensive evaluation of effective polyphenols in apple leaves and their combinatory antioxidant and neuroprotective activities. Ind. Crops Prod. 2019;129:242–252. [Google Scholar]
- 20.Köksal E., Gülçin İ. Antioxidant activity of cauliflower (Brassica oleracea L.) Turk. J. Agric. For. 2008;32(1):65–78. [Google Scholar]
- 21.Köksal E., Gülçin İ., Öztürk Sarıkaya S.B., Bursal E. On the in vitro antioxidant activity of silymarin. J. Enzyme Inhib. Med. Chem. 2009;24(2):395–405. doi: 10.1080/14756360802188081. [DOI] [PubMed] [Google Scholar]
- 22.Gülçin İ., Elias R., Gepdiremen A., Chea A., Topal F. Antioxidant activity of bisbenzylisoquinoline alkaloids from Stephania rotunda: Cepharanthine and fangchinoline. J. Enzyme Inhib. Med. Chem. 2010;25(1):44–53. doi: 10.3109/14756360902932792. [DOI] [PubMed] [Google Scholar]
- 23.Gülçin İ., Kaya R., Gören A.C., Akıncıoğlu H., Topal M., Bingöl Z., Çetin Çakmak K., Ozturk Sarikaya S.B., Durmaz L., Alwasel S. Anticholinergic, antidiabetic and antioxidant activities of Cinnamon (Cinnamomum verum) bark extracts: polyphenol contents analysis by LC-MS/MS. Int. J. Food Propert. 2019;22(1):1511–1526. [Google Scholar]
- 24.Gülçin İ., Huyut Z., Elmastaş M., Aboul-Enein H.Y. Radical scavenging and antioxidant activity of tannic acid. Arab. J. Chem. 2010;3:43–53. [Google Scholar]
- 25.Sehitoglu M.H., Han H., Kalin P., Gülçin İ., Ozkan A., Aboul-Enein H.Y. Pistachio (Pistacia vera L.) gum: a potent inhibitor of reactive oxygen species. J. Enzyme Inhib. Med. Chem. 2015;30(2):264–269. doi: 10.3109/14756366.2014.915395. [DOI] [PubMed] [Google Scholar]
- 26.Yiğit B., Yiğit M., Barut Celepci D., Gök Y., Aktaş A., Aygün M., Taslimi P., Gulçin İ. Novel benzylic substituted imidazolinium, tetrahydropyrimidinium and tetrahydrodiazepinium salts-potent carbonic anhydrase and acetylcholinesterase inhibitors. ChemistrySelect. 2018;3(27):7976–7982. [Google Scholar]
- 27.Bicer A., Taslimi P., Yakali G., Gülçin İ., Gültekin M.S., Turgut Cin G. Synthesis, characterization, crystal structure of novel bis-thiomethylcyclohexanone derivatives and their inhibitory properties against some metabolic enzymes. Bioorg. Chem. 2019;82:393–404. doi: 10.1016/j.bioorg.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Huseynova M., Taslimi P., Medjidov A., Farzaliyev V., Aliyeva M., Gondolova G., Şahin O., Yalçın B., Sujayev A., Orman E.B., Özkaya A.R., Gülçin İ. Synthesis, characterization, crystal structure, electrochemical studies and biological evaluation of metal complexes with thiosemicarbazone of glyoxylic acid. Polyhedron. 2018;155:25–33. [Google Scholar]
- 29.Wilkinson D. Pharmacotherapy of Alzheimer’s disease. Psychiatry. 2007;7:9–14. [Google Scholar]
- 30.Rezai M., Bayrak Ç., Taslimi P., Gulçin İ., Menzek A. The first synthesis, antioxidant and anticholinergic activities of 1-(4,5-dihydroxybenzyl)pyrrolidin-2-one derivative bromophenols including natural products. Turk. J. Chem. 2018;42:808–825. [Google Scholar]
- 31.Turkan F., Cetin A., Taslimi P., Karaman M., Gülçin İ. Synthesis, biological evaluation and molecular docking of novel pyrazole derivatives as potent carbonic anhydrase and acetylcholinesterase inhibitors. Bioorg. Chem. 2019;86:420–427. doi: 10.1016/j.bioorg.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Taslimi P., Turkan F., Cetin A., Burhan H., Karaman M., Bildirici İ., Gülçin İ., Şen F. Pyrazole[3,4-d]pyridazine derivatives: molecular docking and explore of acetylcholinesterase and carbonic anhydrase enzymes inhibitors as anticholinergics potentials. Bioorg. Chem. 2019;92 doi: 10.1016/j.bioorg.2019.103213. [DOI] [PubMed] [Google Scholar]
- 33.Çağlayan C., Demir Y., Küçükler S., Taslimi P., Kandemir F.M., Gulçin İ. The effects of hesperidin on sodium arsenite-induced different organ toxicity in rats on metabolic enzymes as antidiabetic and anticholinergics potentials: a biochemical approach. J. Food Biochem. 2019;43(2) doi: 10.1111/jfbc.12720. [DOI] [PubMed] [Google Scholar]
- 34.Eruygur N., Ataş M., Tekin M., Taslimi P., Koçyiğit U.M., Gulçin İ. In vitro antioxidant, antimicrobial, anticholinesterase and antidiabetic activities of Turkish endemic Achillea cucullata (Asteraceae) from ethanol extract. S. Afr. J. Bot. 2019;120:141–145. [Google Scholar]
- 35.Taslimi P., Aslan H.E., Demir Y., Öztaşkın N., Maraş A., Gulçin İ., Beydemir Ş., Göksu Ş. Diarilmethanon, bromophenols and diarilmetan compounds: discovery of potent aldose reductase, α-amylase and α-glycosidase inhibitors as new therapeutic approach in diabetes and functional hyperglycemia. Int. J. Biol. Macromol. 2018;119:857–863. doi: 10.1016/j.ijbiomac.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Jpn. J. Nutr. 1986;44:307–315. [Google Scholar]
- 37.Göçer H., Gülçin İ. Caffeic acid phenethyl ester (CAPE): correlation of structure and antioxidant properties. Int. J. Food Sci. Nutr. 2011;62(8):821–825. doi: 10.3109/09637486.2011.585963. [DOI] [PubMed] [Google Scholar]
- 38.Gülçin İ. Antioxidant activity of eugenol-a structure and activity relationship study. J. Med. Food. 2011;14(9):975–985. doi: 10.1089/jmf.2010.0197. [DOI] [PubMed] [Google Scholar]
- 39.Apak R., Guclu K., Ozyurek M., Karademir S.E., Ercag E. The cupric ion reducing antioxidant capacity and polyphenolic content of some herbal teas. Int. J. Food Sci. Nut. 2006;57:292–304. doi: 10.1080/09637480600798132. [DOI] [PubMed] [Google Scholar]
- 40.Köksal E., Gülçin İ. Antioxidant activity of cauliflower (Brassica oleracea L.) Turk. J. Agric. For. 2008;32:65–78. [Google Scholar]
- 41.Talaz O., Gülçin İ., Göksu S., Saracoglu N. Antioxidant activity of 5,10-dihydroindeno[1,2-b]indoles containing substituents on dihydroindeno part. Bioorg. Med. Chem. 2009;17(18):6583–6589. doi: 10.1016/j.bmc.2009.07.077. [DOI] [PubMed] [Google Scholar]
- 42.Çetinkaya Y., Göçer H., Menzek A., Gülçin İ. Synthesis and antioxidant properties of (3,4-dihydroxyphenyl)(2,3,4-trihydroxyphenyl)methanone and its derivatives. Arch. Pharm. 2012;345(4):323–334. doi: 10.1002/ardp.201100272. [DOI] [PubMed] [Google Scholar]
- 43.Dinis T.C.P., Madeira V.M.C., Almeida L.M. Action of phenolic derivates (acetoaminophen, salycilate, and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994;315:161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- 44.Huyut Z., Beydemir S., Gulçin İ. Antioxidant and antiradical properties of some flavonoids and phenolic compounds. Biochem. Res. Int. 2017 doi: 10.1155/2017/7616791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ozbey F., Taslimi P., Gulcin İ., Maraş A., Goksu S., Supuran C.T. Synthesis, acetylcholinesterase, butyrilcholinesterase, carbonic anhydrase inhibitory and metal chelating properties of some novel diaryl ether. J. Enzyme Inhib. Med. Chem. 2016;31(S2):79–85. doi: 10.1080/14756366.2016.1189422. [DOI] [PubMed] [Google Scholar]
- 46.Benzie I.F.F., Strain J.J. Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Method. Enzymol. 1999;299:15–27. doi: 10.1016/s0076-6879(99)99005-5. [DOI] [PubMed] [Google Scholar]
- 47.Blois M.S. Antioxidant determinations by the use of a stable free radical. Nature. 1958;26:1199–1200. [Google Scholar]
- 48.Gülçin İ. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid) Toxicology. 2006;217(2-3):213–220. doi: 10.1016/j.tox.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 49.Gülçin İ. Antioxidant and antiradical activities of L-Carnitine. Life Sci. 2006;78(8):803–811. doi: 10.1016/j.lfs.2005.05.103. [DOI] [PubMed] [Google Scholar]
- 50.Gülçin İ. Measurement of antioxidant ability of melatonin and serotonin by the DMPD and CUPRAC methods as trolox equivalent. J. Enzyme Inhib. Med. Chem. 2008;23(6):871–876. doi: 10.1080/14756360701626223. [DOI] [PubMed] [Google Scholar]
- 51.Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 52.Gülçin İ., Şat İ.G., Beydemir Ş., Küfrevioğlu Ö.İ. Evaluation of the in vitro antioxidant properties of extracts of broccoli (Brassica oleracea L.) Ital. J. Food Sci. 2004;16(1):17–30. [Google Scholar]
- 53.Balaydın H.T., Gülçin İ., Menzek A., Göksu S., Şahin E. Synthesis and antioxidant properties of diphenylmethane derivative bromophenols including a natural product. J. Enzyme Inhib. Med. Chem. 2010;25(5):2010685–2010695. doi: 10.3109/14756360903514164. [DOI] [PubMed] [Google Scholar]
- 54.Ellman G.L., Courtney K.D., Andres V., Featherston R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 55.Akincioglu A., Akıncıoğlu H., Gülçin I., Durdağı S., Supuran C.T., Göksu S. Discovery of potent carbonic anhydrase and acetylcholine esterase inhibitors: novel sulfamoylcarbamates and sulfamides derived from acetophenones. Bioorg. Med. Chem. 2015;23(13):3592–3602. doi: 10.1016/j.bmc.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 56.Oztaskin N., Çetinkaya Y., Taslimi P., Göksu S., Gülçin I. Antioxidant and acetylcholinesterase inhibition properties of novel bromophenol derivatives. Bioorg. Chem. 2015;60:49–57. doi: 10.1016/j.bioorg.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 57.Eruygur N., Ataş M., Tekin M., Taslimi P., Koçyiğit U.M., Gulçin İ. In vitro antioxidant, antimicrobial, anticholinesterase and antidiabetic activities of Turkish endemic Achillea cucullata (Asteraceae) from ethanol extract. South Afr. J. Bot. 2019;120:141–145. [Google Scholar]
- 58.Fernandez-Moriano C., Divakar P.K., Crespo A., Gomez-Serranillos M.P. Protective effects of lichen metabolites evernic and usnic acids against redox impairment-mediated cytotoxicity in central nervous system-like cells. Food Chem. Toxicol. 2017;105:262–277. doi: 10.1016/j.fct.2017.04.030. [DOI] [PubMed] [Google Scholar]
- 59.Gülçin İ., Oktay M., Küfrevioğlu Ö.İ., Aslan A. Determinations of antioxidant activity of lichen Cetraria islandica (L) Ach. J. Ethnopharmacol. 2002;79(3):325–329. doi: 10.1016/s0378-8741(01)00396-8. [DOI] [PubMed] [Google Scholar]
- 60.Galanty A., Koczurkiewicz P., Wnuk D., Paw M., Karnas E., Podolak I., Węgrzyn M., Borusiewicz M., Madeja Z., Czyz J., Michalik M. Usnic acid and atranorin exert selective cytostatic and anti-invasive effects on human prostate and melanoma cancer cells. Toxicol. In Vitro. 2017;40:161–169. doi: 10.1016/j.tiv.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 61.Ingolfsdottir K. Usnic acid. Phytochemistry. 2002;61:729–736. doi: 10.1016/s0031-9422(02)00383-7. [DOI] [PubMed] [Google Scholar]
- 62.Gocer H., Akıncıoğlu A., Öztaşkın N., Göksu S., Gülçin İ. Synthesis, antioxidant and antiacetylcholinesterase activities of sulfonamide derivatives of dopamine related compounds. Arch. Pharm. 2013;346(11):783–792. doi: 10.1002/ardp.201300228. [DOI] [PubMed] [Google Scholar]
- 63.Lu Y., Foo L.Y. Polyphenolics of Salvia-A review. Phytochemistry. 2002;59:117–140. doi: 10.1016/s0031-9422(01)00415-0. [DOI] [PubMed] [Google Scholar]
- 64.Oztaskin N., Taslimi P., Maraş A., Göksu S., Gülçin İ. Novel antioxidant bromophenols with acetylcholinesterase, butyrylcholinesterase and carbonic anhydrase inhibitory actions. Bioorg. Chem. 2017;74:104–114. doi: 10.1016/j.bioorg.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 65.Kazazic S.P., Butkovic V., Srazic D., Klasinc L. Gas-phase ligation of Fe+ and Cu+ ions with some flavonoids. J. Agric. Food Chem. 2006;54:8391–8396. doi: 10.1021/jf061401m. [DOI] [PubMed] [Google Scholar]
- 66.Fiorucci S.B., Golebiowski J., Cabrol-Bass D., Antonczak S. DFT study of quercetin activated forms involved in antiradical, antioxidant, and prooxidant biological processes. J. Agric. Food Chem. 2007;55:903–911. doi: 10.1021/jf061864s. [DOI] [PubMed] [Google Scholar]
- 67.Koksal E., Bursal E., Gulcin İ., Korkmaz M., Çağlayan C., Goren A.C., Alwasel S.H. Antioxidant activity and polyphenol content of Turkish thyme (Thymus vulgaris) monitored by LC-MS/MS. Int. J. Food Propert. 2017;20(3):514–525. [Google Scholar]
- 68.Aksu K., Özgeriş B., Taslimi P., Naderi A., Gülçin İ., Göksu S. Antioxidant activity, acetylcholinesterase and carbonic anhydrase inhibitory properties of novel ureas derived from phenethylamines. Arch. Pharm. 2016;349(12):944–954. doi: 10.1002/ardp.201600183. [DOI] [PubMed] [Google Scholar]
- 69.Oktay M., Gülçin İ., Küfrevioğlu Ö.İ. Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. Lebensm. Wissen. Technol. 2003;36(2):263–271. [Google Scholar]
- 70.Gülçin İ. Comparison of in vitro antioxidant and antiradical activities of L-Tyrosine and L-Dopa. Amino Acids. 2007;32:431–438. doi: 10.1007/s00726-006-0379-x. [DOI] [PubMed] [Google Scholar]
- 71.Topal F., Topal M., Gocer H., Kalın P., Koçyiğit U.M., Gülçin İ., Alwasel S.H. Antioxidant activity of taxifolin: an activity-structure relationship. J. Enzyme Inhib. Med. Chem. 2016;31(4):674–683. doi: 10.3109/14756366.2015.1057723. [DOI] [PubMed] [Google Scholar]
- 72.Okten S., Ekiz M., Koçyiğit U.M., Tutar A., Çelik İ., Akkurt M., Gökalp M., Taslimi P., Gulçin İ. Synthesis, characterization, crystal structures, theoretical calculations and biological evaluations of novel substituted tacrine derivatives as cholinesterase and carbonic anhydrase enzymes inhibitors. J. Mol. Struct. 2019;1175:906–915. [Google Scholar]
- 73.Gülçin İ., Tel A.Z., Gören A.C., Taslimi P., Alwasel S. Sage (Salvia pilifera): determination its polyphenol contents, anticholinergic, antidiabetic and antioxidant activities. J. Food Measure. 2019;13(3):2062–2074. [Google Scholar]
- 74.Xiao Z., Storms R., Tsang A. A quantitative starch-iodine method for measuring alpha-amylase and glucoamylase activities. Anal. Biochem. 2006;351:146–148. doi: 10.1016/j.ab.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 75.Oztürk Sarıkaya S.B., Topal F., Şentürk M., Gülçin İ., Supuran C.T. In vitro inhibition of α-carbonic anhydrase isozymes by some phenolic compounds. Bioorg. Med. Chem. Lett. 2011;21(14):4259–4262. doi: 10.1016/j.bmcl.2011.05.071. [DOI] [PubMed] [Google Scholar]