Abstract
Background & Aims
Intestinal Ca2+ absorption early in life is vital to achieving optimal bone mineralization. The molecular details of intestinal Ca2+ absorption have been defined in adults after peak bone mass is obtained, but they are largely unexplored during development. We sought to delineate the molecular details of transcellular Ca2+ absorption during this critical period.
Methods
Expression of small intestinal and renal calcium transport genes was assessed by using quantitative polymerase chain reaction. Net calcium flux across small intestinal segments was measured in Ussing chambers, including after pharmacologic inhibition or genetic manipulation of TRPV6 or Cav1.3 calcium channels. Femurs were analyzed by using micro–computed tomography and histology.
Results
Net TRPV6-mediated Ca2+ flux across the duodenum was absent in pre-weaned (P14) mice but present after weaning. In contrast, we found significant transcellular Ca2+ absorption in the jejunum at 2 weeks but not 2 months of age. Net jejunal Ca2+ absorption observed at P14 was not present in either Trpv6 mutant (D541A) mice or Cav1.3 knockout mice. We observed significant nifedipine-sensitive transcellular absorption across the ileum at P14 but not 2 months. Cav1.3 knockout pups exhibited delayed bone mineral accrual, compensatory nifedipine-insensitive Ca2+ absorption in the ileum, and increased expression of renal Ca2+ reabsorption mediators at P14. Moreover, weaning pups at 2 weeks reduced jejunal and ileal Cav1.3 expression.
Conclusions
We have detailed novel pathways contributing to transcellular Ca2+ transport across the distal small intestine of mice during development, highlighting the complexity of the multiple mechanisms involved in achieving a positive Ca2+ balance early in life.
Keywords: Calcium Channel, Pediatric, Bone, Development
Abbreviations used in this paper: Cav1.3, voltage-dependent L-type calcium channel, alpha 1D subunit; HA, hemagglutinin; JCa2+, Ca2+ flux; KO, knockout; μCT, micro–computed tomography; PCR, polymerase chain reaction; PMCA1, plasma membrane Ca2+-ATPase 1; TAL, thick ascending limb; Trpv6, transient receptor potential vanilloid 6; WT, wild-type
Graphical abstract
See editorial on page 647.
Summary.
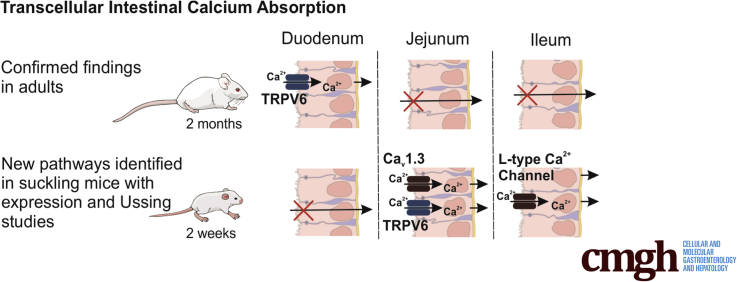
Maintaining a positive calcium balance is vital for bone mineralization during postnatal development. We delineate transcellular calcium absorption pathways in the jejunum and ileum, which are present only early in life and contribute to a positive calcium balance.
The greatest net positive calcium (Ca2+) balance occurs in infancy.1 This process is vital to mineralizing bone throughout development.2 An estimated 60% of osteoporosis risk can be attributed to a failure to reach optimal peak bone mass density by early adulthood.3 In women, the incidence of fractures due to osteoporosis is greater than breast cancer and cardiovascular disease combined and represents a major health care burden.4, 5 Infancy and childhood are thus critical periods for long-term skeletal health.
Bone Ca2+ deposition rate is greatest in infancy and is a direct function of intestinal absorption.1, 6 Unfortunately, studies to date have not fully examined how intestinal absorption is maximized in infants to meet increased demand. Intestinal Ca2+ absorption can occur via passive paracellular or active transcellular pathways.7 The current hypothesized model of transcellular absorption in both humans and rodents consists of apical entry into the enterocyte through the Ca2+-selective channel, transient receptor potential vanilloid 6 (TRPV6), intracellular binding to calbindin-D9k, and basolateral extrusion via the plasma membrane Ca2+-ATPase 1 (PMCA1) or sodium-calcium exchanger.8, 9, 10 Interestingly, Trpv6 knockout (KO) or mutant mice (Trpv6 mt) do not display a severe Ca2+ wasting phenotype, strongly inferring another apical Ca2+ entry channel.10, 11, 12 Voltage-dependent L-type calcium channel, alpha 1D subunit (Cav1.3) has been proposed as a complementary channel to TRPV6.13, 14 Consistent with this, one group has reported that global Cacna1d knockout (Cav1.3 KO) mice have lower weight and decreased bone mineral density at 20 weeks in males; however, others report normal growth.15, 16 Regardless, the role of Cav1.3 in intestinal Ca2+ absorption has not been directly assessed.
The molecular components of the proposed transcellular absorption pathway are expressed in the duodenum and large intestine of adult animals, whereas paracellular absorption or secretion predominates in the jejunum and ileum.17, 18, 19 In contrast, existing evidence suggests that alternative Ca2+ absorption mechanisms are present during development compared with older animals.18, 20, 21 However, the exact molecular details conferring increased intestinal Ca2+ absorption and their contribution to bone mineralization early in life have yet to be determined.
We therefore sought to delineate the molecular details of transcellular Ca2+ absorption from the small intestine and how they contribute to bone mineralization during early postnatal development. We report net transcellular Ca2+ flux before weaning across jejunum and ileum but not duodenum at 2 weeks, with the opposite pattern present at 2 months. Furthermore, we find that TRPV6 and Cav1.3 are necessary for this absorption across jejunum and that Cav1.3 may mediate absorption across ileum, although compensation is present in Cacna1d KO pups. Furthermore, Cacna1d KO pups exhibit delayed bone mineralization and renal compensation to increase Ca2+ reabsorption. Together, this work defines the molecular details in mice of how the small intestine facilitates increased demand of Ca2+ early in life to meet requirements of growth.
Results
Expression of Transcellular Ca2+ Absorption Mediators Is Absent From the Duodenum of Young Mice
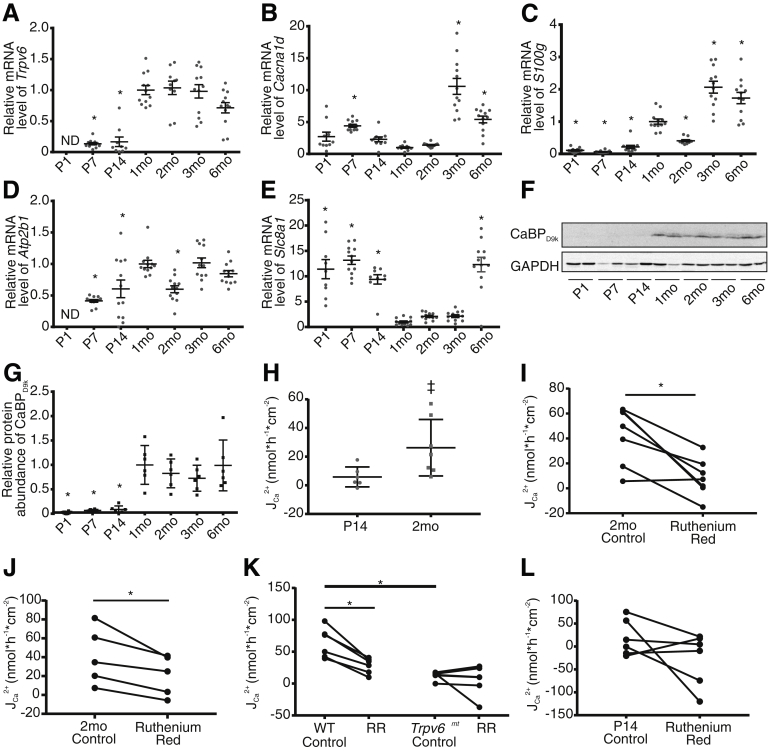
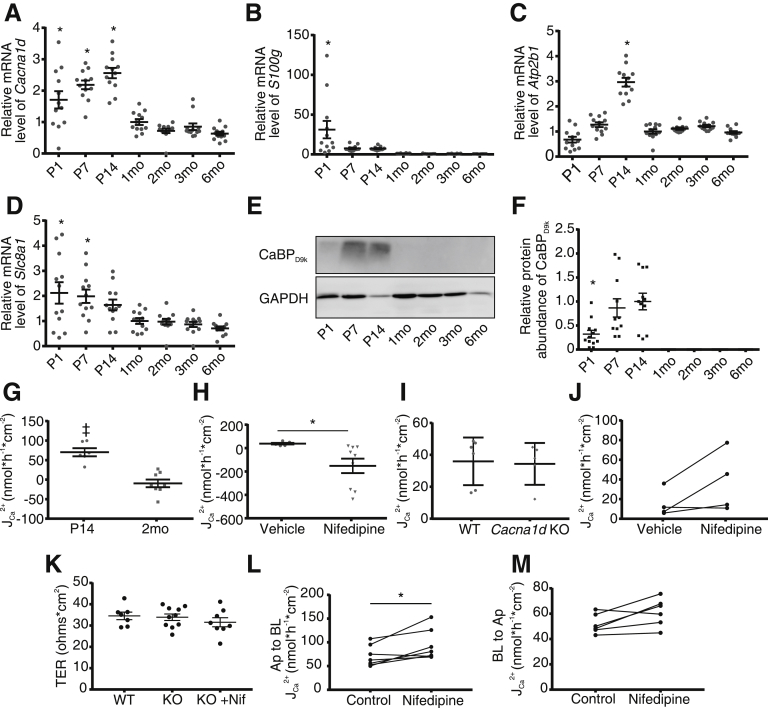
To assess how transcellular Ca2+ absorption changes with age, we first examined the expression of known mediators in the duodenum before weaning at postnatal day 1 (P1), P7, and P14 and after weaning at 1–6 months in wild-type (WT) mice. Trpv6 was undetectable at P1 and increased 6-fold from P14 to 1 month (Figure 1A). Cacna1d, encoding the L-type Ca2+ channel Cav1.3, was greatest at P7 and 3 months (Figure 1B). Expression of S100g, encoding calbindin-D9k, was very low at P1, P7, and P14 but increased with age (Figure 1C). Atb2b1, encoding the basolateral PMCA1, followed a similar pattern (Figure 1D). Slc8a1, encoding sodium-calcium exchanger 1, showed bimodal pattern with greater expression before weaning and at 6 months (Figure 1E). Calbindin-D9k protein was detectable by immunoblot only at and after 1 month (Figure 1F and G). Together, these results suggest that the transcellular Ca2+ absorption pathway is poorly expressed or not present before weaning in the duodenum.
Figure 1.
Transcellular JCa2+flux across the duodenum is not detectable at P14 but mediated by TRPV6 in 2-month-old mice. Relative expression of (A) Trpv6, (B) Cacna1d, (C) S100g, (D) Atb2b1, and (E) Slc8a1 in the duodenum across ages (n = 12/group). Expression is normalized to Gapdh and relative to 1 month. (F) Representative immunoblot from 3 replicates and (G) semi-quantification of calbinidin-D9k. Protein abundance is normalized to GAPDH and presented relative to the 1-month group (n = 6/group). Groups compared by 1-way analysis of variance with Dunnett multiple comparisons test. *P < .05 compared with 1-month group. (H) Net JCa2+ across ex vivo sections of mouse duodenum is not different from 0 in P14 mice (n = 6, P = .095) but significantly greater than 0, consistent with absorption, in 2-month-old mice (n = 7, ‡P = .013) (two-tailed, one-sample t test). Net JCa2+ is significantly reduced in 2-month-old mice after addition of 100 μmol/L ruthenium red apically under (I) apical hyperosmolar (n = 6; two-tailed, paired t test; *P = .006) and (J) iso-osmolar conditions (n = 5; two-tailed, paired t test; *P = .02). (K) Net JCa2+ is significantly lower in 2-month-old Trpv6mt mice compared with WT littermates (n = 6/group; two-tailed, unpaired t test; *P < .001). One hundred μmol/L apical ruthenium red significantly decreases net JCa2+ in WT (*P = .002) but not Trpv6mt mice (P = .474) (two-tailed, paired t test). Both paired experiments were performed under apical hyperosmolar conditions. (L) One hundred μmol/L ruthenium red did not decrease net JCa2+ in P14 mice (n = 6; two-tailed, paired t test; P = .2). Data are presented as mean ± standard error of the mean. CaBPD9k, calbindin-D9k; ND, not detected; RR, ruthenium red; Trpv6mt, Trpv6 mutant.
Net Ca2+ Absorption Occurs Across the Duodenum at 2 Months and Is Mediated by Transient Receptor Potential Vanilloid 6
To functionally validate the expression pattern changes observed with age, we sought to examine Ca2+ flux (JCa2+) across the duodenum of mice at P14 and 2 months. Net JCa2+ from P14 mice was not different from 0, whereas net JCa2+ from 2-month-old mice demonstrated absorption (Figure 1H). Net JCa2+ was significantly decreased in the presence of 100 μmol/L apical ruthenium red,22 implicating TRPV6 activity (Figure 1I). This experiment was performed with hyperosmolar apical buffer to stimulate Trpv6 activity13, 23; however, this decrease was also noted with ruthenium red under iso-osmolar conditions (Figure 1J). Similarly, net JCa2+ was significantly greater in WT compared with Trpv6 mutant (Trpv6 mt) littermates expressing a pore mutation (D541A) rendering the channel non-permeable to Ca2+.24 Furthermore, ruthenium red significantly decreased net JCa2+ in WT but not Trpv6 mt mice (Figure 1K). Importantly, the drug had no effect on net JCa2+ in P14 mice (Figure 1L). These results demonstrate TRPV6-mediated, transcellular Ca2+ absorption across duodenum, which develops by 2 months of age.
Pre-Weaned Mice Express Transcellular Ca2+ Absorption Mediators in the Jejunum
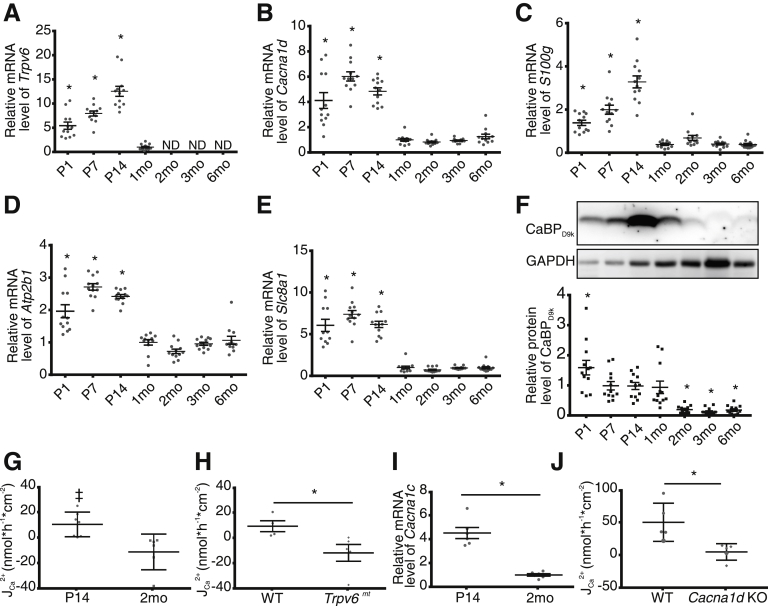
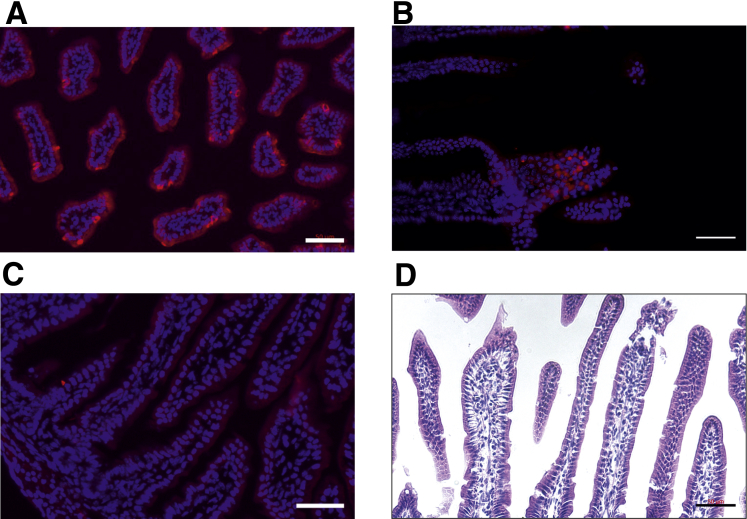
We next examined the expression of the transcellular pathway in the jejunum. To our surprise, we identified Trpv6 expression from P1 to P14 (Figure 2A). Minimal expression was detected at 1 month, but not at any older age (Figure 2A). Cacna1d expression was significantly higher at P1 to P14 relative to 1 month of age (Figure 2B). Similarly, expression of S100g, Atp2b1, and Slc8a1 was significantly greater from P1 to P14 (Figure 2C–E). Calbindin-D9k protein was detected from P1 to 1 month but was nearly undetectable from 2 to 6 months (Figure 2F). Cav1.3 has been identified apically in the jejunum of rats.14 To determine whether we could detect Cav1.3 protein in the jejunum of P14 pups, we fixed tissue from mice expressing hemagglutinin (HA)-tagged Cacna1d.25 We observed HA immunoreactivity in the jejunum of HA-Cacna1d mice but not WT mice (Figure 3). Together, these results suggest the presence of a transcellular Ca2+ absorption pathway in the jejunum in the first 2 weeks of life in mice, with apical entry mediated by TRPV6, Cav1.3, or both channels.
Figure 2.
Net Ca2+absorption across the jejunum of P14 mice is mediated by TRPV6 and Cav1.3 and is not present at 2 months. Relative expression of (A) Trpv6, (B) Cacna1d, (C) S100g, (D) Atp2b1, and (E) Slc8a1 by age (n = 12/group). Expression is normalized to Gapdh and relative to 1 month. (F) Representative calbindin-D9k (CaBPD9k) immunoblot of 12 replicates and quantification by age (n = 12/group). Groups compared by 1-way analysis of variance with Dunnett multiple comparisons test. *P < .05 compared with 1-month group. (G) Net JCa2+ across ex vivo sections of mouse jejunum are greater than 0, indicating absorption at P14 (n = 7; ‡P = .03) but not 2-month-old mice (n = 6; P = .11; two-tailed, one-sample t test). (H) Net JCa2+ is significantly reduced across the jejunum of P14 Trpv6mt mice compared with WT littermates (n = 4 WT and 5 mt; two-tailed unpaired t test; *P = .04). (I) Greater expression of Cacna1c, encoding Cav1.2, at P14 (n = 6/group; two-tailed unpaired t test; *P < .0001) normalized to Gapdh. (J) Significantly reduced net JCa2+ across the jejunum of P14 Cacna1d KO mice compared with WT mice (n = 5/group; Mann-Whitney test; *P = .008). Data are presented as mean ± standard error of the mean. CaBPD9k, calbindin-D9k; ND, not detected; Trpv6mt, Trpv6 mutant.
Figure 3.
Cav1.3 expression in jejunum of P14 mice. (A and B) Immunoreactivity (red) of HA reveals apical localization of HA-tagged Cav1.3 on sections (7 μm) of jejunum from P14 mice expressing HA-tagged Cacna1d. (C) Section of jejunum from WT mice (control) shows no HA immunoreactivity. Cell nuclei were stained with bisbenzimide (Hoechst 33342) (blue). (D) Hematoxylin-eosin staining of sections of jejunum of Cav1.3-HA mice. Scale bars: 50 μm.
TRPV6 and Cav1.3 Are Required for Net Transcellular Ca2+ Absorption Across the Jejunum at P14
Because of the expression patterns observed, we next sought to measure transcellular JCa2+ across the jejunum. We found net apical to basolateral JCa2+ in P14 but not 2-month-old mice (Figure 2G). To specifically implicate TRPV6 in this process, we repeated the studies using Trpv6 mt P14 mice. We observed significantly lower net JCa2+ across jejunum of Trpv6 mt mice compared with WT littermates (Figure 2H). Together, these studies infer a role for TRPV6 in Ca2+ absorption across the jejunum of pre-weaned mice.
Next, we aimed to determine the potential role of the L-type Ca2+ channel Cav1.3 in net Ca2+ absorption across jejunum of P14 mice. We first examined the expression of other, potentially confounding L-type Ca2+ channels, specifically Cacna1s, Cacna1c, and Cacna1f. Cacna1s and Cacna1f were not detected at P14 or 2 months. However, Cacna1c, encoding Cav1.2, was detected at both ages, with 5-fold greater expression at P14 (Figure 2I). Importantly, Cav1.2 is more sensitive than Cav1.3 to nifedipine, so this drug could not be used to specifically implicate Cav1.3.26 To specifically implicate Cav1.3 in net JCa2+ observed at P14, we repeated the Ca2+ flux studies in Cacna1d KO pups.16 Net JCa2+ was abolished in Cacna1d KO compared with WT animals, indicating that Cav1.3 is required for Ca2+ absorption from the jejunum of P14 mice (Figure 2J).
The Ileum of Younger Animals Expresses Transcellular Ca2+ Absorption Mediators
The presence of a transcellular Ca2+ absorption pathway in the ileum of mice before weaning was also examined. Trpv6 expression was not detected at any age. Cacna1d expression was greater from P1 to P14 compared with 1- to 6-month-old mice (Figure 4A). A similar pattern was observed for S100g, Atp2b1, and Slc8a1 (Figure 4B–D). Calbindin-D9k protein expression was detected on immunoblot (Figure 4E), with semi-quantification (Figure 4F) at P1–P14 but not at 1 month of age or older. Together, these data infer transcellular Ca2+ absorption occurs across the ileum before weaning but not after.
Figure 4.
P14 but not 2-month-old mice display net apical to basolateral calcium flux across the ileum, mediated by L-type Ca2+channel. Expression of (A) Cacna1d, (B) S100g, (C) Atp2b1, and (D) Slc8a1 by age (n = 12/group). Expression is normalized to Gapdh and relative to 1 month. (E) Representative immunoblot of 12 repeats and (F) semi-quantification of calbindin-D9k (CaBPD9k) demonstrates expression in ileum only in younger mice. Results are normalized to GAPDH and displayed relative to P14 age (n = 12/group). Groups are compared by 1-way analysis of variance with Dunnett multiple comparisons test; *P < .05. (G) Net JCa2+ across ex vivo sections of mouse ileum are greater than 0 at P14 (n = 6; ‡P = .001) but not 2 months (n = 6; P = .359) (two-tailed, one-sample t tests). (H) Ten μmol/L apical nifedipine decreases net JCa2+ in P14 mice compared with vehicle (n = 5 vehicle and 9 nifedipine; Mann-Whitney test; *P = .001). (I) No difference in net JCa2+ across ileum between WT and Cacna1d KO mice at P14 (n = 5 WT and 6 KO; Mann-Whitney test; P = .54). (J) Ten μmol/L apical nifedipine does not decrease net JCa2+ in Cacna1d KO mice at P14 mice (n = 4; Wilcoxon matched-pairs signed rank test; P = .25). (K) Transepithelial resistance (TER) across the ileum of P14 WT, Cacna1d KO, or Cacna1d KO with nifedipine (1-way analysis of variance; P = .5) (n = 7–10/group). Unidirectional apical to basolateral (L) and basolateral to apical (M) JCa2+ across ileum of P14 Cacna1d KO mice before (control) and after apical addition of 10 μmol/L nifedipine (paired t test; *P < .05) (n = 6). Data are presented as mean ± standard error of the mean.
Net Transcellular Ca2+ Absorption Occurs Across the Ileum at 2 Weeks but not 2 Months
To determine whether transcellular Ca2+ absorption occurs across the ileum of P14 mice, we measured Ca2+ flux across this segment ex vivo in Ussing chambers. In FVB/N WT mice, net JCa2+ was significantly greater than 0 in P14 but not 2-month-old mice (Figure 4G). Because Trpv6 was not detectable at any age in ileum, we sought to implicate Cav1.3 in mediating the net absorption. To do so, we repeated the flux studies in P14 mice in the presence of vehicle or 10 μmol/L nifedipine and observed a significant inhibition of net JCa2+ (Figure 4H). To more specifically implicate Cav1.3, we repeated the experiments in WT and Cacna1d KO mice at P14. However, we did not observe lower net JCa2+ in the KO mice (Figure 4I). To determine whether another L-type Ca2+ channel was compensating for the loss of Cacna1d, we repeated net JCa2+ studies with 10 μmol/L nifedipine. Again, no difference was observed between groups (Figure 4J). This made us examine the results more closely. The transepithelial resistance was not different across the ileum of WT and Cacna1d KO with or without nifedipine (Figure 4K). Unidirectional apical to basolateral 45Ca2+ flux was slightly increased after addition of nifedipine; however, basolateral to apical 45Ca2+ flux also increased slightly after nifedipine treatment (Figure 4L and M), likely because of increased tissue permeability over time in this ex vivo experiment. Regardless, together, these results suggest net transcellular Ca2+ absorption across the ileum of mice at P14 is mediated by an L-type Ca2+ channel with compensation by a non–L-type Ca2+ channel after genetic deletion of Cav1.3.
Delayed Bone Mineralization in Cacna1d KO Pups
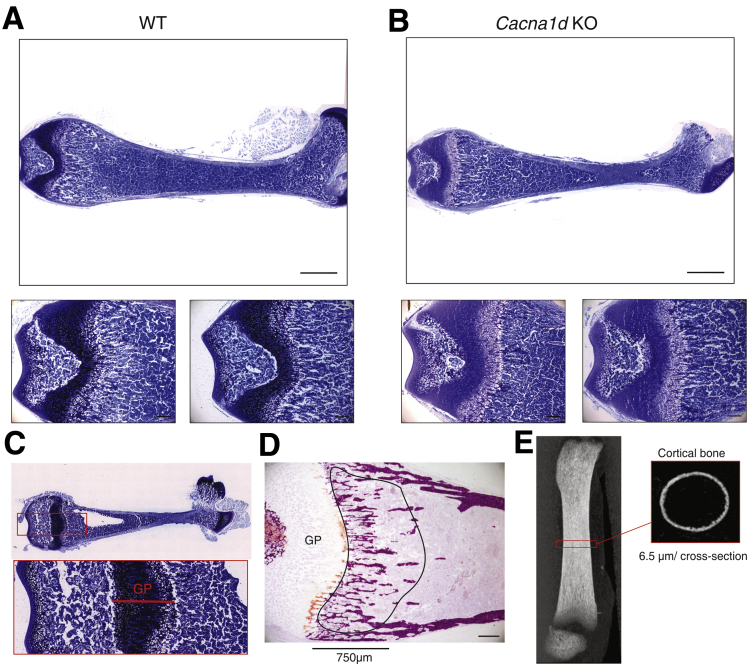
We next queried whether the loss of net transcellular Ca2+ absorption from the jejunum of Cacna1d KO and Trpv6 mt pups altered bone mineralization at P14. Femur growth plate thickness measured on toluidine blue–stained sections was greater in Cacna1d KO (Figure 5A–C, Table 1) but not Trpv6 mt (Table 2) compared with WT pups. These results suggest delayed bone mineralization in the Cacna1d KO mice. No other differences were observed in trabecular bone between WT and Cacna1d KO (Table 1) or WT and Trpv6 mt (Table 2) pups as determined by alizarin red staining (Figure 5D). Similarly, no differences were observed for cortical bone parameters as assessed by micro–computed tomography (μCT) (Figure 5E) for either WT vs Cacna1d KO pups (Table 1) or WT vs Trpv6 mt pups (Table 2). Together, these data suggest that Cav1.3 contributes to maintaining a positive Ca2+ balance during postnatal growth, whereas TRPV6 is not critical at this age.
Figure 5.
Bone phenotype of Cacna1d KO pups. Representative toluidine blue–stained sections from fixed non-decalcified femurs of (A) WT and (B) Cacna1d KO mice at P14 (P13-P15). The growth plate thickness was measured in middle of the section as indicated below. Scale bar = 1 mm (upper panels) and 0.2 mm (lower panels). (C) Representative toluidine blue–stained sections obtained from non-decalcified femur (top) and enlarged region covering the growth plate (GP) used to determine thickness of growth plate shown in Tables 1 and 2. (D) Representative alizarin red stain used to visualize calcified bone (red) and to calculate trabecular parameters shown in Tables 1 and 2. Region of interest (ROI) starting at growth plate (GP) and covering primary spongiosa over 750 μm is indicated. (E) Lateral scout view of femur indicating midshaft section used to analyze cortical bone.
Table 1.
Trabecular and Cortical Bone Parameters of P14 WT and Cacna1d KO Mice
| Male |
Female |
|||
|---|---|---|---|---|
| WT | Cacna1d KO | WT | Cacna1d KO | |
| Trabecular bone | ||||
| N | 6 | 5 | 5 | 6 |
| BV/TV (%) | 20.3 ± 0.92 | 19.2 ± 2.68 | 20.7 ± 2.1 | 17.2 ± 1.03 |
| Trabecular number (1/mm) | 0.012 ± 0.001 | 0.011 ± 0.001 | 0.013 ± 0.001 | 0.011 ± 0.001 |
| Trabecular width (μm) | 16.5 ± 0.37 | 16.6 ± 0.88 | 16.1 ± 0.35 | 15.6 ± 0.42 |
| Trabecular separation (μm) | 65.4 ± 3.06 | 75.0 ± 9.8 | 64.3 ± 6.63 | 76.4 ± 5.95 |
| Growth plate thickness (μm) | 343.4 ± 14.6 | 426.2 ± 30.9a | 361.1 ± 25.5 | 484.5 ± 17.2b |
| Cortical bone | ||||
| N | 6 | 7 | 4 | 9 |
| Bone volume (mm3) | 0.056 ± 0.003 | 0.059 ± 0.005 | 0.061 ± 0.004 | 0.067 ± 0.003 |
| Endocortical volume (mm3) | 0.26 ± 0.01 | 0.25 ± 0.01 | 0.26 ± 0.01 | 0.27 ± 0.01 |
| Cross-sectional thickness (mm) | 0.043 ± 0.002 | 0.045 ± 0.003 | 0.047 ± 0.002 | 0.050 ± 0.002 |
| Perimeter (mm) | 3.87 ± 0.09 | 3.76 ± 0.09 | 3.74 ± 0.07 | 3.87 ± 0.03 |
| Tissue mineral density (g/cm3) | 0.98 ± 0.01 | 0.98 ± 0.01 | 0.99 ± 0.01 | 1.01 ± 0.01 |
NOTE. Trabecular bone parameters were calculated from bone sections stained with alizarin red and cortical bone parameters as measured by μCT. Data are presented as mean ± standard error of the mean (unpaired, two-tailed t test KO vs WT for each sex).
BV/TV, bone volume/tissue volume.
P < .05.
P < .01.
Table 2.
Trabecular and Cortical Bone Parameters of P14 WT and Trpv6Mt Mice
| N | WT | Trpv6mt | |
|---|---|---|---|
| Trabecular bone | |||
| BV/TV (%) | 3 | 21.6 ± 1.25 | 21.4 ± 1.6 |
| Trabecular number (1/mm) | 3 | 0.013 ± 0.0009 | 0.013 ± 0.0002 |
| Trabecular width (μm) | 3 | 17.3 ± 0.44 | 19.0 ± 1.12 |
| Trabecular separation (μm) | 3 | 63.3 ± 6.0 | 60.2 ± 2.1 |
| Growth plate thickness (μm) | 3 | 652.5 ± 18.0 | 630.2 ± 19.6 |
| Cortical bone | |||
| Femur length (mm) | 5 | 8.26 ± 0.25 | 8.07 ± 0.14 |
| Bone volume (mm3) | 5 | 0.074 ± 0.004 | 0.078 ± 0.004 |
| Endocortical volume (mm3) | 5 | 0.18 ± 0.007 | 0.19 ± 0.006 |
| Cross-sectional thickness (mm) | 5 | 0.062 ± 0.002 | 0.068 ± 0.004 |
| Perimeter (mm) | 5 | 3.37 ± 0.09 | 3.42 ± 0.05 |
| Tissue mineral density (g/cm3) | 5 | 1.04 ± 0.01 | 1.05 ± 0.01 |
NOTE. Trabecular bone parameters calculated from bone sections stained with alizarin red and cortical bone parameters as measured by μCT. Data are presented as mean ± standard error of the mean (unpaired Student t test).
BV/TV, bone volume/tissue volume.
Renal and Intestinal Compensation for Loss of Cav1.3
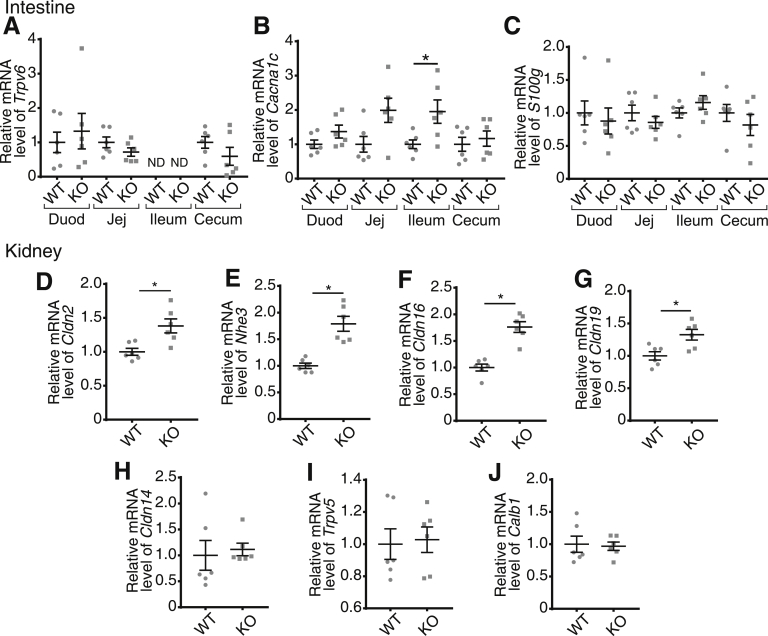
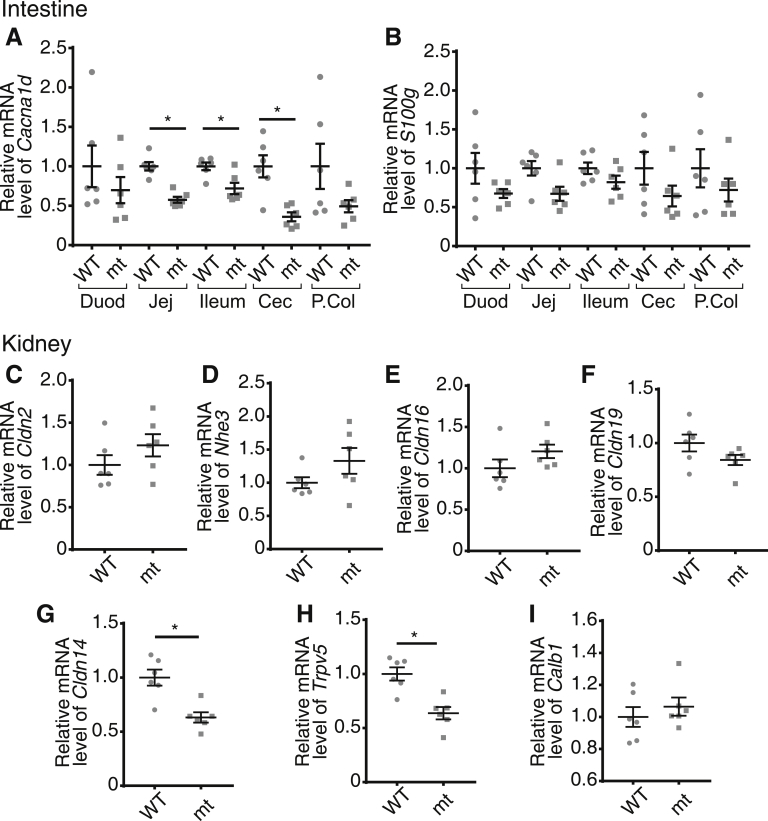
To understand the lack of a severe bone phenotype in Cacna1d KO and Trpv6 mt pups, we examined the expression of genes that might compensate for the loss of jejunal Ca2+ absorption in the intestine and kidney. We observed no difference in Trpv6 or S100g expression along the length of the intestine in Cacna1d KO pups (Figure 6A and C). However, we did find a 2-fold increase in Cacna1c, encoding Cav1.2, expression in the ileum but not in other segments (Figure 6B). It is unlikely that Cav1.2 contributes to compensatory increased net JCa2+ in this segment because we observed nifedipine-insensitive flux in the Cacna1d KO pups (Figure 4J). In contrast, we observed significant up-regulation of mediators of renal Ca2+ reabsorption in the proximal tubule and thick ascending limb (TAL), the segments responsible for a combined 90% of renal Ca2+ reabsorption.7 Specifically, the Cldn2 and Nhe3 genes that encode a calcium permeable pore and generate the driving force for Ca2+ reabsorption from the proximal tubule, respectively, were increased in Cacna1d KO pups (Figure 6D and E).27, 28, 29 Furthermore, we observed increased expression of Cldn16 and Cldn19, genes that encode the Ca2+ permeable pore in the TAL (Figure 6F and G).30 No differences were observed in expression of Cldn14, which blocks Ca2+ reabsorption in the TAL,31 or Trpv5 and Calb1, which mediate transcellular Ca2+ reabsorption in the distal nephron (Figure 6H–J).8 Interestingly, Trpv6 mt pups had significantly decreased expression of Cacna1d in the jejunum, ileum, and cecum (Figure 7A), although no differences were observed in S100g expression (Figure 7B). Contrary to findings in Cacna1d KO pups, renal expression of Cldn2, Nhe3, Cldn16, and Cldn19 was not different in Trpv6 mt compared with WT mice (Figure 7C–F). We did identify a significant decrease in both Cldn14 and Trpv5 expression (Figure 7G–I). Together, these results suggest compensatory increases in renal Ca2+ reabsorption in Cacna1d KO pups.
Figure 6.
Renal compensation in Cacna1d KO mice at P14. Quantitative real-time PCR results of (A) Trpv6, (B) Cacna1c encoding Cav1.2, and (C) S100g along the intestine. Renal expression of (D) Cldn2, (E) Nhe3, (F) Cldn16, (G) Cldn19, (H) Cldn14, (I) Trpv5, and (J) Calb1 encoding calbindin-D28k reveals compensatory increases in Cacna1d KO pups. Small intestine and kidney results are normalized to Gapdh; cecum results are normalized to β-actin. All expression results are displayed relative to WT group for each tissue. *P < .05 vs WT by Mann-Whitney test. (n = 6/group). Data are presented as mean ± standard error of the mean. Duod, duodenum; Jej, jejunum.
Figure 7.
Compensatory expression changes in Trpv6mtpups. Quantitative real-time PCR results of (A) Cacna1d and (B) S100g encoding calbindin-D9k along the intestine from Trpv6mt pups relative to WT expression in each tissue. Quantitative real-time PCR expression of mediators of renal Ca2+ reabsorption, (C) Cldn2, (D) Nhe3, (E) Cldn16, (F) Cldn19, (G) Cldn14, (H) Trpv5, and (I) Calb1 encoding calbindin-D28k in Trpv6mt pups relative to WT. Small intestine and kidney results are normalized to Gapdh; cecum and proximal colon (P.Col) results are normalized to β-actin. All expression results are relative to WT group for each tissue. *P < .05 vs WT by Mann-Whitney test. (n = 6/group). Data presented as mean ± standard error of the mean. Duod, duodenum; Jej, jejunum; mt, mutant.
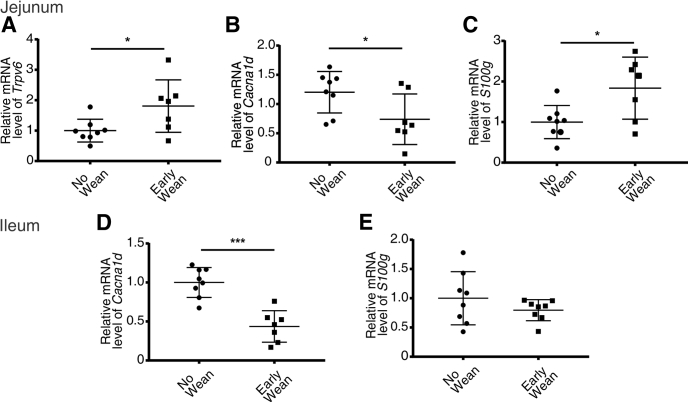
Early Weaning Alters Expression of Trpv6, Cacna1d, and S100g in the Jejunum and Ileum
To determine whether weaning from breast milk to a regular chow diet results in the changes in expression observed, we weaned pups at P12, roughly 7 days before they are typically weaned, collected tissue 48 hours later, and compared gene expression to littermates that were not weaned early. We observed a 2-fold increase in expression of Trpv6 and a 1.8-fold increase in S100g in the jejunum of pups weaned early. Furthermore, jejunal Cacna1d was decreased by 38% in pups weaned early (Figure 8A–C). In the ileum, Cacna1d expression was also decreased by 66% with early weaning, whereas no difference was observed in S100g (Figure 8D and E). Together, these results suggest that expression of these pathways is regulated by a bioactive compound in breast milk and/or dietary calcium changes.
Figure 8.
Early weaning to rodent chow alters Trpv6, Cacna1d, and S100g expression in jejunum and ileum at P14. Quantitative real-time PCR results of (A) Trpv6, (B) Cacna1d, and (C) S100g in jejunum and (D) Cacna1d and (E) S100g in ileum. Tissue was taken from mice at P14 after either early weaning to rodent chow at P12 or not. Results are normalized to β-actin. *P < .05, ***P < .0001 vs P14 mice not weaned by Mann-Whitney or unpaired t test. (n = 7–8/group). Data are presented as mean ± standard error of the mean.
Discussion
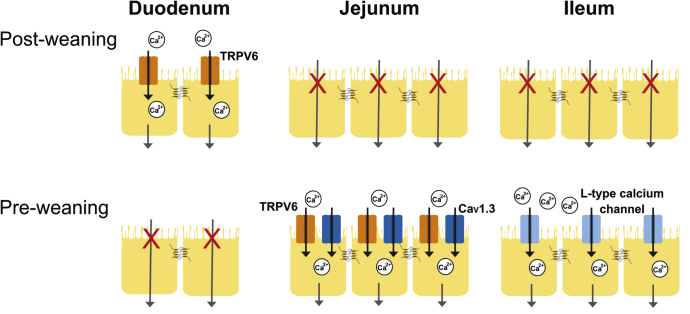
The lifetime osteoporosis risk is independently related to bone mineral content accrued early in life.32 Infancy and adolescence represent the 2 periods of greatest Ca2+ accretion into bone. When normalized to body weight, the rate of calcium deposition into bone is greatest in the first year of life.1, 6 A positive calcium balance is necessary for this deposition rate and is a linear function of intestinal absorption. Thus, intestinal absorptive capacity is greatest during infancy.1 We identify active Ca2+ absorption across distal small intestinal segments in mice from 1 day to 6 months of age. Our results failed to identify transcellular Ca2+ absorption in the duodenum until after weaning, whereas significant net absorption from the jejunum and ileum occurs only in early postnatal development. Furthermore, TRPV6 and Cav1.3 mediate this novel absorption pathway identified in the jejunum, whereas absorption across the ileum is mediated by an L-type Ca2+ channel, likely Cav1.3 (Figure 9).
Figure 9.
Summary of apical entry mechanisms contributing transcellular Ca2+absorption across the small intestine before and after weaning. Significant net transcellular Ca2+ absorption across the duodenum is mediated by apical TRPV6 and is present only after weaning. In the jejunum, significant net transcellular Ca2+ absorption is present only before weaning and is mediated by apical TRPV6 and Cav1.3. Similarly, significant net transcellular Ca2+ absorption occurs only before weaning across the ileum and is mediated by an L-type calcium channel.
Previous studies have examined expression of the transcellular Ca2+ absorption pathway in the duodenum at various ages.21, 33 TRPV6 and calbindin-D9k were first identified at 1 week in mice, which is consistent with our findings.21, 34 We observed Trpv6 at P7 and P14; however, the expression was far below that of older mice. Similarly, calbindin-D9k was nearly undetectable before 1 month. Calbindin-D9k expression is induced by transcellular Ca2+ absorption and maintains a low free cytosolic Ca2+ concentration.35 The dramatic shift in expression of the apical entry channel, ie, Trpv6, and intracellular buffer, ie, calbindin-D9K, indicates that this pathway is not present in the duodenum of mice before 1 month. Indeed, we observed absorption at 2 months but not P14. This is consistent with previous in situ ligated loop studies in rats demonstrating duodenal absorption occurs only via an unsaturable, paracellular process up to P14, with increasing prevalence of a saturable, transcellular process thereafter.18, 36 We extended this observation and reveal that TRPV6 is essential to net transcellular duodenal Ca2+ absorption at 2 months through pharmacologic inhibition and a TRPV6 pore mutant. This is consistent with previous findings that lumen to serum 45Ca2+ flux after oral gavage of 3-month-old Trpv6 KO mice is reduced by 40%–50% compared with WT.12 Importantly, transcellular Ca2+ absorption across the duodenum does not contribute to net absorption before weaning. Therefore, there must be other mechanisms mediating Ca2+ absorption at a young age.
Previous studies failed to identify transcellular Ca2+ absorption in the jejunum and ileum while noting significant paracellular secretion in 9- to 15-week-old mice.17, 19 A study using in situ ligated loops in 16-day-old rats measured absorption along the length of the small intestine.37 However, this technique does not fully capture serosal to lumen recycling and thus cannot definitively demonstrate transcellular absorption. We observed transcellular absorption across the jejunum and ileum before weaning but not thereafter. In addition, our gene expression profiling supported our functional observations. Importantly, we detected calbindin-D9k protein in mice up to P14. Previous work detected calbindin-D9k in rat ileum at 2 months but at a level less than-one fifth of duodenum. However, other work has failed to find expression in mice at 1 month of age.18, 20 These findings illustrate that active Ca2+ uptake from distal small bowel is an alternative pathway to meet the high requirements of infancy.
We further reveal the molecular identity of this developmental Ca2+ absorption pathway in the jejunum. Net transcellular JCa2+ was absent from the jejunum of both Trpv6 mt and Cacna1d KO mice at P14, clearly implicating both channels. Prior work using perfused jejunal loops of adult rats found decreased unidirectional lumen to serosal flux on apical addition of nifedipine and therefore suggested that Cav1.3 contributes to intestinal Ca2+ absorption at later ages.38 However, their study has been contradicted by further work in rodents.19 Collectively, these results illustrate the potential role of Cav1.3, but not in early postnatal development. Our study clearly illustrates the importance of Cav1.3 in the jejunum before weaning. It is unclear whether TRPV6 and Cav1.3 directly or indirectly interact to mediate Ca2+ absorption in this segment; however, both appear to be necessary.
The ileum is the longest intestinal segment with the longest sojourn time and thus could contribute significantly to a positive Ca2+ balance early in life.39 Some authors have speculated the existence of transcellular Ca2+ absorption across the ileum of mice and humans.13, 40, 41 However, before the current work, no functional measurements were performed before weaning. Morgan et al14 observed JCa2+ that was inhibited by L-type Ca2+ channel blockers in the jejunum of older rats. Combined with protein detected in the jejunum and ileum, the authors suggested that Cav1.3 mediates Ca2+ absorption. However, direct functional measurements on the ileum did not support this. Ussing chambers studies on 9- to 15-week-old mice did not find net JCa2+.17 Further work using in situ ligated loops in 1-month-old rats found only passive diffusion without a transcellular component.18 However, we found significant net transcellular Ca2+ absorption across the ileum of mice at P14 that was not present at 2 months. Furthermore, this net flux was inhibited by nifedipine at a concentration that blocks both Cav1.2 and Cav1.3.38 To specifically implicate Cav1.3, we repeated the JCa2+ flux studies across ileum of Cacna1d KO pups. However, these animals did not have decreased net absorption. Interestingly, the net flux observed in the Cacna1d KO ileum was no longer inhibited by nifedipine, suggesting that Cav1.3 mediates flux in WT animals, but that a non–L-type Ca2+ channel compensates when Cav1.3 is knocked out. The identification of this third transcellular Ca2+ absorption pathway is the focus of further studies.
To determine whether the absence of net transcellular Ca2+ absorption across the jejunum of P14 mice negatively impacted the ability to maintain a positive Ca2+ balance resulting in poorly mineralized bone, we examined the bone phenotype of Cacna1d KO and Trpv6 mt pups. We observed a significantly thicker growth plate in the Cacna1d KO pups relative to WT, suggesting delayed mineralization of bone in these animals. These findings are consistent with increased growth plate thickness and morphologic changes in bones of rabbits treated with nifedipine.42 Although Platzer et al16 reported normal growth, Li et al15 found decreased bone mineral content and cross-sectional area of cortical bone of male Cacna1d KO mice at 18 weeks of age, which is consistent with our observation. Li et al attributed the observed bone phenotype to the loss of Cav1.3 in osteoblasts. Because of our findings of a key role of Cav1.3 in jejunal Ca2+ absorption and bone mineralization at P14, it is possible that later bone mineral content differences are the result of reduced intestinal Ca2+ absorption early in life. It is not possible to delineate the effect of bone versus gut with the available global Cacna1d KO model.
In a different mutant Trpv6 mouse strain, a 9.3% reduction in femoral bone mineral density was observed in Trpv6 KO mice at 3 months of age on a 1% Ca2+ diet but not a diet without Ca2+.12 We did not find altered bone parameters in Trpv6 mt pups, suggesting adequate intestinal Ca2+ absorption or renal compensation to mineralize bone. Absorption across the ileum at P14, where Trpv6 is not expressed, likely compensates for loss of jejunal absorption.39 Interestingly, infants born with TRPV6 mutations have skeletal abnormalities detectable in utero.43, 44 This human phenotype is likely the result of decreased placental Ca2+ transfer, as has been observed in mice.45, 46 Because bone mineralization normalizes by 2 years in these infants, humans also appear to compensate for the loss of TRPV6 in early development. We should also acknowledge that our work and most previous molecular studies on intestinal calcium absorption have been performed on rodents, and confirmation of these molecular pathways in humans should be done.
The Cacna1d KO pups do not have a severe bone phenotype because of 2 compensatory mechanisms. First, transcellular Ca2+ absorption across the ileum of Cacna1d KO mice is replaced by nifedipine-insensitive flux, which is consistent with the up-regulation of a yet unidentified calcium absorption pathway. Second, Cacna1d KO mice also display renal compensation in the proximal tubule and TAL of the nephron to maintain Ca2+ balance.30, 31 These results suggest that increased renal Ca2+ reabsorption is necessary to maintain appropriate Ca2+ balance, which is consistent with suboptimal Ca2+ absorption across the intestine in the absence of Cav1.3.
To elucidate whether weaning itself caused the changes in Ca2+ absorption observed, we weaned mice early to a regular rodent chow diet. Unexpectedly, we observed an increase in Trpv6 and S100g expression in the jejunum, but not the ileum, in the pups weaned early. Trpv6 and S100g expression is up-regulated by hormones found in breast milk including epidermal growth factor and prolactin, which may stimulate expression in suckling pups.47, 48 However, a change from a high to low calcium diet with early weaning may also lead to activation of vitamin D via parathyroid hormone, which, in turn, increases Trpv6 and S100g expression, thus explaining the increased expression observed in the jejunum.49 There is a paucity of data regarding the free Ca2+ concentration available in each segment of the intestine from breast milk or chow diet, which is then available for absorption. It is likely that the postprandial lumen Ca2+ concentrations are in the millimolar range. Cacna1d expression was decreased with early weaning similar to our observations with age, suggesting its regulation by a bioactive compound in breast milk such as prolactin.50 Further studies are required to delineate the mechanisms mediating the intestinal Ca2+ absorption changes observed at weaning.
In conclusion, we identified pathways mediating active transcellular Ca2+ absorption in the jejunum and ileum early in life. TRPV6 and Cav1.3 mediate this absorption in the jejunum. Pharmacologic blockade of L-type Ca2+ channels prevents net absorption in the ileum where TRPV6 is absent. The loss of Cav1.3 induces a compensatory increase in Ca2+ absorption from the ileum and renal Ca2+ reabsorption in pre-weaned mice despite delayed bone mineralization. Furthermore, we have demonstrated that a change in diet from breast milk to solid food causes shifts in expression of these pathways in the jejunum and ileum. We have therefore identified molecular details of how active Ca2+ uptake from the intestine contributes to the increased demand early in life.
Methods
Mice
FVB/N (Taconic Biosciences, Rensselaer, NY) and Trpv6 mt mice24 were maintained on a 12-hour light/dark cycle with drinking water and chow ad libitum (Lab Diet Irradiated Rodent Diet 5053, 4% fat, 0.81% calcium). Experiments were approved by the University of Alberta animal ethics committee, Health Sciences Section (AUP00000213). Experiments on the Cacna1d and HA-tagged Cacna1d KO mice16 were conducted in agreement with the European Communities Council Directive (2010/63/EU) in accordance with the German law on the use of laboratory animals and approved by the regional board for scientific animal experiments of Saarland. Trpv6 mt mice were genotyped by polymerase chain reaction (PCR).24, 51 For the early weaning experiments, half of the mice in a litter (FVB/N mice) were weaned at P12 to the standard rodent chow diet, and the littermates remained with the dam. After 48 hours, tissue was collected from all pups. Experiments involving mice included both female and male mice in approximately equal numbers except for the bone phenotype analysis of P14 Trpv6 mt mice.
Isolation of Tissue
Murine tissue was taken as previously described,9 snap frozen in liquid nitrogen, and stored at –80°C until use. At each age, the length of the whole small intestine was measured. The duodenum was defined at the first ninth, the jejunum as the second two-ninths, and ileum as the remaining two-thirds of the length. For expression studies, tissue was taken from the middle of these defined sections. For experiments in Ussing chambers at P14, the duodenum was defined as the first 2 cm, jejunum from 5 to 7 cm, and ileum as 14–16 cm from the pyloric sphincter. For 2-month-old mice, the duodenum was taken as the first 4.6 cm, jejunum as 10–14.8 cm, and ileum as 22–26.8 cm.
Quantitative Real-time Polymerase Chain Reaction
Total RNA was isolated from tissue as described.52 Briefly, RNA was isolated by using the TRIzol method (Invitrogen, Carlsbad, CA; cat.# 15596026) and treated with DNAse (ThermoScientific, Vilnius, Lithuania; cat.# EN0521). RNA quality and quantity were measured with a Nanodrop 2000 (Thermo Fisher Scientific, Waltham, MA). Five micrograms of RNA was then reverse transcribed into cDNA (SuperScript II; Invitrogen; cat.# 18064014). Quantitative real-time PCR was performed in triplicate for each sample by using TaqMan Universal Master Mix II (Applied Biosystems, Foster City, CA; cat.# 4440042) and specific primers and probes on a ABI 7900HT Sequence Detection System (Applied Biosystems) as previously shown.52 Primer and probe sequences for murine Trpv6, S100g, Atp2b1, Slc8a1, Gapdh, 18s, and β-actin have been published elsewhere.9, 52 Sequences for murine Cacna1d primers and probe (forward: TCCTCTTCCTCTTCACCTACTG; reverse: AGTCAACCAGATAGCCAACAG; probe: CCCTTACCCGCCCTGTGATGT) were created by using IDT software (Integrated DNA Technologies, San Diego, CA), and specificity was assessed with NCBI Primer-BLAST. Samples were quantified by using the standard curve method where the standard curve was made of serial dilutions of cDNA from a positive control or the target tissue. A Ct value greater than 35 was considered negligible.
Immunoblotting
Tissue was lysed in RIPA buffer (50 mmol/L Tris, 150 mmol/L NaCl, 1 mmol/L EDTA, 1% Triton-X, 1% sodium dodecyl sulfate, 1% NP-40, pH 7.4) with phenylmethylsulfonyl fluoride to 1:1000 concentration (Thermo Scientific, Rockford, IL; cat.# 36978) and protease inhibitor set III to 1:100 concentration (Calbiochem, San Diego, CA; cat.# 535140) for 1 hour on ice and then centrifuged for 10 minutes at 14,000 centrifugal force at 4°C. The protein content of the supernatant was quantified against a standard curve of serial dilutions of bovine serum albumin (Sigma-Aldrich, St Louis, MO; cat.# A-9647) using a Nanodrop 2000 (Thermo Fisher Scientific, Waltham, MA).
For immunoblots, 150 μg protein from total lysate was run on a 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, electrotransferred to 0.45 μm polyvinylidene difluoride membrane (Merck Millipore, Burlington, MA; cat.# IVPH00010), and blocked overnight in tris-buffered saline tween with 5% milk. The blot was then incubated overnight at 4°C with either rabbit anti–calbindin-D9k (1:1000; Swant, Marly, Switzerland; cat.# CB9) or mouse anti-GAPDH (1:1000; Thermo Fisher Scientific, Rockford, IL; cat.# MA5-15738), followed by incubation for 1 hour at room temperature with horseradish peroxidase–conjugated donkey anti-rabbit or goat anti-mouse immunoglobulin G (1:5000; Santa Cruz Biotechnology Inc, Santa Cruz, CA; cat.# sc-2005, sc-2317) and visualized using Clarity Western ECL (Bio-Rad, Hercules, CA; cat.# 1705061) and a ChemiDoc Touch imaging system (Bio-Rad). Protein was semi-quantified with ImageJ (U. S. National Institutes of Health, Bethesda, MD), and each sample was normalized to GAPDH as a loading control and one age group as indicated to enable the combination of results from several blots.
Micro–Computed Tomography Analysis of Femora
Femurs from P14 Trpv6 mt and Cacna1d KO and WT mice were scanned by μCT at a resolution of 6.5 μm (Bruker μCT SkyScan 1172; Billerica, MA). The bones were wrapped into wet paper, placed in a plastic holder, and mounted vertically in the sample chamber for scanning. Voltage and current x-rays source were adjusted to 49 kV and 200 μA, respectively, and beam hardening was reduced by using a 0.5-mm Al filter; the exposure time was 5 seconds, and scanning angular rotation was set to 180° with an increment of 0.4° rotation step.53 NRecon (1.6.10.6) was used to reconstruct, and DataViewer (1.5.1.2) and CT Analyser (1.16.4.1+; all from Bruker) were used for bone analysis. A total of 50 cross sections (6.5 μm) exactly in the middle of femoral shaft were analyzed to access the cortical bone volume, endocortical volume, cross-sectional thickness, perimeter, and mineral density.
Non-Decalcified Bone Histology
Femurs were fixed in 4% PFA at 4°C and incubated in 30% (w/v) sucrose solution overnight. Samples were then embedded in an anterior-posterior orientation in tissue freezing medium (simulated colonic environment medium; CEM-001; Section-Lab Co Ltd, Hiroshima, Japan) according to Kawamoto and Shimizu54 and stored at –80°C until sectioning. Four 6-μm sections per bone were made in an anterior-posterior orientation at 2 different regions spaced at least 100 μm from each other. Two sections were stained with a modified toluidine blue staining to visualize cartilage. The thickness of the growth plate was determined from the middle of the section. The mean of either 2 per bone was taken as a single value. Two sections were stained with alizarin red to visualize calcified bone (red) and to calculate trabecular parameters as previously published.55
Immunohistochemistry
Sections of the jejunum were prepared and fixed with Zamboni’s fixative solution at 4°C for 12 hours and transferred to 30% (w/v) sucrose solution at 4°C for 12 hours. Samples were embedded in tissue freezing medium optical cutting temperature (Leica Microsystems GmbH, Wetzlar, Germany) and cut into 7-μm sagittal sections with a cryostat. Sections were incubated with primary anti-HA antibody overnight (1:1000, clone 3F10; Roche, Basel, Switzerland; cat.# 11867431001), followed by 1-hour incubation with a secondary antibody (donkey anti rat-Cy3; Jackson Immuno Research, Cambridgeshire, United Kingdom; cat.# 712-165-150), followed by incubation in the presence of 1 μg/mL bisbenzimide (Hoechst 33342; Sigma-Aldrich, Munich, Germany) for 5 minutes. Images were collected on an Axio Scan.Z1 microscope via the Plan-Apoichromat 20x/0.8 M27 objective, equipped with AxioVision 4.7 or Zen 2.3 software (all from Zeiss, Oberkochen, Germany).
Ussing Chamber Studies
Net 45Ca flux (JCa) was measured essentially as previously.17 Fresh intestinal tissue was excised from mice, linearized, mounted onto Ussing chamber sliders, and placed into the corresponding P2400/P2300 Ussing chambers connected to a VCC multichannel voltage/current clamp (Physiologic Instruments, San Diego, CA). Whole-thickness intestinal tissue was used for all experiments because our previous work found no difference between stripped tissue and full thickness for Ca2+ fluxes across all the intestinal segments.17 A maximum of 4 segments were mounted per tissue, and the mean of biological replicates was used for analysis. For experiments using P2407B sliders, the internal resistance offset of the voltage/current clamp was increased by the manufacturer to compensate for the artificially increased fluid resistance created by the small aperture of the sliders.
Tissue was bathed on both sides with 4 mL Kreb’s Ringer buffer (140 mmol/L Na, 5.2 mmol/L K, 120 mmol/L Cl, 1.2 mmol/L Mg, 1.2 mmol/L Ca, 2.8 mmol/L PO4, 2.5 mmol/L HCO3, pH = 7.4) at 37°C and bubbled with 5% CO2 (balance O2).56 The buffer contained 2 μmol/L indomethacin (from 10 mmol/L stock solubilized in 100% EtOH) (Sigma-Aldrich; cat.# I7378) bilaterally and 0.1 μmol/L tetrodotoxin (Alomone Labs, Jerusalem, Israel; cat.# T-550) basolaterally to inhibit prostaglandin synthesis and neuronal activity.57 The basolateral side contained 10 mmol/L dextrose, and the apical side contained 10 mmol/L mannitol to balance osmolarity.
After 15 minutes under open circuit conditions, 2-mV pulses were applied 3 times across the tissue for 20 seconds, and the resulting current was recorded to calculate the resistance of the tissue by using Ohm’s law. One side of each chamber was then spiked with 45Ca (5 μCi/mL) (PerkinElmer Health Sciences, Waltham, MA; cat.# NEZ013001MC) and the potential difference clamped to 0 mV across the tissue, and time was set to 0 minutes. Thereafter, samples of 50 μL were taken in quadruplicate from both sides at 15-minute intervals for 4 time points. For experiments with ruthenium red (Sigma-Aldrich, Oakville, Canada; cat.# R275-1), a 5 mmol/L stock was added apically to a final concentration 100 μmol/L at time 45 minutes, and after 20 minutes of incubation, samples were collected for 3 time points at 15-minute intervals. Where indicated, nifedipine (Sigma-Aldrich; cat.# N7634) was added to a final concentration 10 μmol/L apically.14 In P14 FVB/N mice (Figure 4H), experiments were performed under vehicle and nifedipine conditions on separate animals. For P14 Cacna1d KO mice (Figure 4J), experiments were performed on tissue from the same mouse as above for experiments with ruthenium red. After samples were collected, the tissue was clamped at 2 mV, as described above, to calculate the post-experiment resistance. Data were excluded if the transepithelial resistance changed by more than 40%.17 To further assess tissue viability, forskolin (LC Laboratories, Woburn, MA; cat.# F-9929) was added to a final concentration of 10 μmol/L bilaterally. The tissue was considered viable if an increase in short circuit current of greater than 50% was observed.17
Radioactivity of samples was measured with an LS6500 Multi-Purpose Scintillation Counter (Beckman Coulter, Brea, CA) as an average count per minute over 3 minutes. JCa2+ was calculated as the rate of appearance of 45Ca2+ in the “cold” chamber (ie, not spiked with 45Ca2+) in cpm/h divided by the specific activity of the hot chamber (ie, spiked with 45Ca2+) in cpm/mol and normalized to surface area of tissue exposed.58 Net JCa2+ was calculated as flux from apical to basolateral side minus flux from basolateral to apical side for tissues with a difference in resistance less than 25%.17 Because the potential difference across the tissue was clamped to 0 mV throughout the experiment and there were equimolar concentrations of Ca2+ in both hemichambers, there was no electrochemical gradient to drive net paracellular diffusion of Ca2+. Therefore, net JCa2+ represents transcellular flux, and a positive value indicates net absorption.17
Although transcellular Ca2+ absorption is known to occur across the duodenum of adult rodents, previous studies have not consistently found net absorption with protocols similar to those used in the current study.8, 17 It is known that TRPV6 is activated under hyperpolarizing conditions.13 Therefore, we sought to optimize experimental conditions for absorption in the duodenum by inducing a hyperpolarized state. Previously, it has been shown that apical hyperosmolar conditions induce hyperpolarization of the apical membrane of epithelial cells.23 We therefore increased the osmolarity of our apical buffer by 100 mOsm (Osmometer Model 3D3; Advanced Instruments, Inc, Pomona, CA) with the addition of 100 mmol/L mannitol. Net JCa2+ under apical hyperosmolar conditions across duodenum of 2-month-old FVB/N mice was 42.65 ± 8.7 nmol·h–1·cm–2 compared with 26.26 ± 7.5 nmol·h–1·cm–2 (n = 7/group; P = .18, two-tailed Student t test). Net JCa2+ across the duodenum of P14 FVB/N mice under apical hyperosmolar conditions was not different than under iso-osmolar conditions, 18.6 ± 16.0 nmol·h–1·cm–2 vs 5.86 ± 2.85 nmol·h–1·cm–2 (n = 6/group; P = .45, two-tailed Student t test), and not significantly different from 0 (P = .3, one sample t test). Data presented for the net JCa2+ across duodenum of all mice were obtained under apical hyperosmolar conditions except where indicated.
Quantification and Statistical Analysis
Statistical analyses were carried out by using GraphPad Prism 7.03 (GraphPad Software Inc, San Diego, CA). Groups were compared by unpaired t test, paired t test, one-way analysis of variance with Dunnett multiple comparisons test, or Mann-Whitney test as indicated in figure and table legends. All n indicated in figure legends represent samples from independent mice. The Brown-Forsythe test was used to assess equality of group variance. A non-parametric test was performed when variance was significantly different between groups. P <.05 was considered significant. All authors had access to the study data and had reviewed and approved the final manuscript. Figures were created using CorelDRAW 2017 and the Mind the Graph platform (www.mindthegraph.com).
Footnotes
Author contributions All authors contributed to experimental design, interpreting results, and manuscript revisions. MRB, JJL, KB, and AR conducted experiments and analyzed data; HD, PW, JE, VF, and RTA provided reagents; PW, JE, and VF bred and provided mice; MRB wrote the first draft; and all authors approved the final version of the manuscript.
Conflicts of interest The authors disclose no conflicts.
Funding This work was funded by grants from the Women and Children's Health Research Institute, which is supported by the Stollery Children’s Hospital Foundation, and the National Sciences and Engineering Research Council to RTA, who is the Canada Research Chair in Renal Epithelial Transport Physiology. MRB is supported by a Vanier Canada Graduate Scholarship and Alberta Innovates Clinician Fellowship. MRB’s research at the Universität des Saarlandes (UdS) was funded by an NSERC Michael Smith Foreign Study Supplement. H. Dimke is funded by the Danish Medical Research Council. Work at UdS was funded by Deutsche Forschungsgemeinschaft (DFG) by IRTG1830 (to JE, VF), Sonderforschungsbereich (SFB) 894 (to JE, PW), and SFB TRR152 (to VF).
References
- 1.Matkovic V. Calcium metabolism and calcium requirements during skeletal modeling and consolidation of bone mass. Am J Clin Nutr. 1991;54:245S–260S. doi: 10.1093/ajcn/54.1.245S. [DOI] [PubMed] [Google Scholar]
- 2.Bronner F. Recent developments in intestinal calcium absorption. Nutr Rev. 2009;67:109–113. doi: 10.1111/j.1753-4887.2008.00147.x. [DOI] [PubMed] [Google Scholar]
- 3.Bachrach L.K., Levine M.A., Cowell C.T., Shaw N.J. Clinical indications for the use of DXA in pediatrics. In: Sawyer A.J., editor. Bone densitometry in growing patients. Humana Press; New York, NY: 2007. pp. 59–72. [Google Scholar]
- 4.Cauley J., Wampler N., Barnhart J., Wu L., Allison M., Chen Z., Hendrix S., Robbins J., Jackson R. Incidence of fractures compared to cardiovascular disease and breast cancer: the Women’s Health Initiative Observational Study. Osteoporos Int. 2008;19:1717. doi: 10.1007/s00198-008-0634-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tarride J.E., Guo N., Hopkins R., Leslie W.D., Morin S., Adachi J.D., Papaioannou A., Bessette L., Brown J.P., Goeree R. The burden of illness of osteoporosis in Canadian men. J Bone Miner Res. 2012;27:1830–1838. doi: 10.1002/jbmr.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abrams S.A., Esteban N.V., Vieira N.E., Yergey A.L. Calcium absorption and endogenous fecal excretion in low birth weight infants. Pediatr Res. 1991;29:615–618. doi: 10.1203/00006450-199106010-00018. [DOI] [PubMed] [Google Scholar]
- 7.Alexander R.T., Rievaj J., Dimke H. Paracellular calcium transport across renal and intestinal epithelia. Biochem Cell Biol. 2014;92:467–480. doi: 10.1139/bcb-2014-0061. [DOI] [PubMed] [Google Scholar]
- 8.Hoenderop J.G.J., Nilius B., Bindels R.J.M. Calcium absorption across epithelia. Physiol Rev. 2005;85:373–422. doi: 10.1152/physrev.00003.2004. [DOI] [PubMed] [Google Scholar]
- 9.Alexander R.T., Beggs M.R., Zamani R., Marcussen N., Frische S., Dimke H. Ultrastructural and immunohistochemical localization of plasma membrane Ca2+-ATPase 4 in Ca2+-transporting epithelia. Am J Physiol Renal Physiol. 2015;309:F604–F616. doi: 10.1152/ajprenal.00651.2014. [DOI] [PubMed] [Google Scholar]
- 10.Woudenberg-Vrenken T.E., Lameris A.L., Weissgerber P., Olausson J., Flockerzi V., Bindels R.J., Freichel M., Hoenderop J.G. Functional TRPV6 channels are crucial for transepithelial Ca2+ absorption. Am J Physiol Gastrointest Liver Physiol. 2012;303:G879–G885. doi: 10.1152/ajpgi.00089.2012. [DOI] [PubMed] [Google Scholar]
- 11.Benn B.S., Ajibade D., Porta A., Dhawan P., Hediger M., Peng J.-B., Jiang Y., Oh G.T., Jeung E.-B., Lieben L. Active intestinal calcium transport in the absence of transient receptor potential vanilloid type 6 and calbindin-D9k. Endocrinology. 2008;149:3196–3205. doi: 10.1210/en.2007-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bianco S.D.C., Peng J.B., Takanaga H., Suzuki Y., Crescenzi A., Kos C.H., Zhuang L., Freeman M.R., Gouveia C.H.A., Wu J. Marked disturbance of calcium homeostasis in mice with targeted disruption of the Trpv6 calcium channel gene. J Bone Miner Res. 2007;22:274–285. doi: 10.1359/jbmr.061110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kellett G.L. Alternative perspective on intestinal calcium absorption: proposed complementary actions of Cav1.3 and TRPV6. Nutr Rev. 2011;69:347–370. doi: 10.1111/j.1753-4887.2011.00395.x. [DOI] [PubMed] [Google Scholar]
- 14.Morgan E.L., Mace O.J., Helliwell P.A., Affleck J., Kellett G.L. A role for Cav1.3 in rat intestinal calcium absorption. Biochem Biophys Res Commun. 2003;312:487–493. doi: 10.1016/j.bbrc.2003.10.138. [DOI] [PubMed] [Google Scholar]
- 15.Li J., Zhao L., Ferries I.K., Jiang L., Desta M.Z., Yu X., Yang Z., Duncan R.L., Turner C.H. Skeletal phenotype of mice with a null mutation in Cav 1.3 L-type calcium channel. J Musculoskelet Neuronal Interact. 2010;10:180–187. [PubMed] [Google Scholar]
- 16.Platzer J., Engel J., Schrott-Fischer A., Stephan K., Bova S., Chen H., Zheng H., Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102:89–97. doi: 10.1016/s0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 17.Rievaj J., Pan W., Cordat E., Alexander R.T. The Na+/H+ exchanger isoform 3 is required for active paracellular and transcellular Ca2+ transport across murine cecum. Am J Physiol Gastrointest Liver Physiol. 2013;305:G303–G313. doi: 10.1152/ajpgi.00490.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toverud S.U., Dostal L.A. Calcium absorption during development: experimental studies of the rat small intestine. J Pediatr Gastroenterol Nutr. 1986;5:688–695. [PubMed] [Google Scholar]
- 19.Reyes-Fernandez P.C., Fleet J.C. Luminal glucose does not enhance active intestinal calcium absorption in mice: evidence against a role for Ca(v)1.3 as a mediator of calcium uptake during absorption. Nutr Res. 2015;35:1009–1015. doi: 10.1016/j.nutres.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beggs M.R., Alexander R.T. Intestinal absorption and renal reabsorption of calcium throughout postnatal development. Exp Biol Med. 2017;242:840–849. doi: 10.1177/1535370217699536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song Y., Peng X., Porta A., Takanaga H., Peng J.-B., Hediger M.A., Fleet J.C., Christakos S. Calcium transporter 1 and epithelial calcium channel messenger ribonucleic acid are differentially regulated by 1, 25 dihydroxyvitamin D3 in the intestine and kidney of mice. Endocrinology. 2003;144:3885–3894. doi: 10.1210/en.2003-0314. [DOI] [PubMed] [Google Scholar]
- 22.Hoenderop J.G.J., Vennekens R., Müller D., Prenen J., Droogmans G., Bindels R.J.M., Nilius B. Function and expression of the epithelial Ca2+ channel family: comparison of mammalian ECaC1 and 2. J Physiol. 2001;537:747–761. doi: 10.1111/j.1469-7793.2001.00747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mukoh S., Kawasaki K., Yonemura D., Tanabe J. Hyperosmolarity-induced hyperpolarization of the membrane potential of the retinal pigment epithelium. Doc Ophthalmol. 1985;60:369–374. doi: 10.1007/BF00158926. [DOI] [PubMed] [Google Scholar]
- 24.Weissgerber P., Kriebs U., Tsvilovskyy V., Olausson J., Kretz O., Stoerger C., Vennekens R., Wissenbach U., Middendorff R., Flockerzi V. Male fertility depends on Ca2+ absorption by TRPV6 in epididymal epithelia. Science Signaling. 2011;4:ra27. doi: 10.1126/scisignal.2001791. [DOI] [PubMed] [Google Scholar]
- 25.Scharinger A., Eckrich S., Vandael D.H., Schönig K., Koschak A., Hecker D., Kaur G., Lee A., Sah A., Bartsch D. Cell-type-specific tuning of Cav1. 3 Ca2+-channels by a C-terminal automodulatory domain. Frontiers in Cellular Neuroscience. 2015;9:309. doi: 10.3389/fncel.2015.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu W., Lipscombe D. Neuronal CaV1. 3α1 L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J Neurosci. 2001;21:5944–5951. doi: 10.1523/JNEUROSCI.21-16-05944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu A.S., Cheng M.H., Angelow S., Gunzel D., Kanzawa S.A., Schneeberger E.E., Fromm M., Coalson R.D. Molecular basis for cation selectivity in claudin-2-based paracellular pores: identification of an electrostatic interaction site. J Gen Physiol. 2009;133:111–127. doi: 10.1085/jgp.200810154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muto S., Hata M., Taniguchi J., Tsuruoka S., Moriwaki K., Saitou M., Furuse K., Sasaki H., Fujimura A., Imai M., Kusano E., Tsukita S., Furuse M. Claudin-2 - deficient mice are defective in the leaky and cation-selective paracellular permeability properties of renal proximal tubules. Proc Natl Acad Sci U S A. 2010;107:8011–8016. doi: 10.1073/pnas.0912901107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan W., Borovac J., Spicer Z., Hoenderop J.G., Bindels R.J., Shull G.E., Doschak M.R., Cordat E., Alexander R.T. The epithelial sodium/proton exchanger, NHE3, is necessary for renal and intestinal calcium (re) absorption. Am J Physiol Renal Physiol. 2012;302:F943–F956. doi: 10.1152/ajprenal.00504.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hou J., Renigunta A., Gomes A.S., Hou M., Paul D.L., Waldegger S., Goodenough D.A. Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc Natl Acad Sci U S A. 2009;106:15350–15355. doi: 10.1073/pnas.0907724106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dimke H., Desai P., Borovac J., Lau A., Pan W., Alexander R.T. Activation of the Ca2+-sensing receptor increases renal claudin-14 expression and urinary Ca2+ excretion. Am J Physiol Renal Physiol. 2013;304:F761–F769. doi: 10.1152/ajprenal.00263.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heaney R.P., Abrams S., Dawson-Hughes B., Looker A., Marcus R., Matkovic V., Weaver C. Peak bone mass. Osteoporos Int. 2000;11:985–1009. doi: 10.1007/s001980070020. [DOI] [PubMed] [Google Scholar]
- 33.Lee G.-S., Jung E.-M., Choi K.-C., Oh G.T., Jeung E.-B. Compensatory induction of the TRPV6 channel in a calbindin-D9k knockout mouse: its regulation by 1,25-hydroxyvitamin D. J Cell Biochem. 2009;108:1175–1183. doi: 10.1002/jcb.22347. [DOI] [PubMed] [Google Scholar]
- 34.Lee G.-S., Lee K.-Y., Choi K.-C., Ryu Y.-H., Paik S.G., Oh G.T., Jeung E.-B. Phenotype of a calbindin-D9k gene knockout is compensated for by the induction of other calcium transporter genes in a mouse model. J Bone Miner Res. 2007;22:1968–1978. doi: 10.1359/jbmr.070801. [DOI] [PubMed] [Google Scholar]
- 35.Cui M., Li Q., Johnson R., Fleet J.C. Villin promoter-mediated transgenic expression of transient receptor potential cation channel, subfamily V, member 6 (TRPV6) increases intestinal calcium absorption in wild-type and vitamin D receptor knockout mice. J Bone Miner Res. 2012;27:2097–2107. doi: 10.1002/jbmr.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pansu D., Bellaton C., Bronner F. Developmental changes in the mechanisms of duodenal calcium transport in the rat. Am J Physiol. 1983;244:G20–G26. doi: 10.1152/ajpgi.1983.244.1.G20. [DOI] [PubMed] [Google Scholar]
- 37.Amnattanakul S., Charoenphandhu N., Limlomwongse L., Krishnamra N., Amnattanakul S., Charoenphandhu N., Limlomwongse L., Krishnamra N. Endogenous prolactin modulated the calcium absorption in the jejunum of suckling rats. J Physiol Pharmacol. 2005;83:595–604. doi: 10.1139/y05-045. [DOI] [PubMed] [Google Scholar]
- 38.Morgan E.L., Mace O.J., Affleck J., Kellett G.L. Apical GLUT2 and Cav1.3: regulation of rat intestinal glucose and calcium absorption. J Physiol. 2007;580:593–604. doi: 10.1113/jphysiol.2006.124768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duflos C., Bellaton C., Pansu D., Bronner F. Calcium solubility, intestinal sojourn time and paracellular permeability codetermine passive calcium absorption in rats. J Nutr. 1995;125:2348–2355. doi: 10.1093/jn/125.9.2348. [DOI] [PubMed] [Google Scholar]
- 40.Wasserman R.H. Vitamin D and the dual processes of intestinal calcium absorption. J Nutr. 2004;134:3137–3139. doi: 10.1093/jn/134.11.3137. [DOI] [PubMed] [Google Scholar]
- 41.Favus M.J. Factors that influence absorption and secretion of calcium in the small intestine and colon. Am J Physiol. 1985;248:G147–G157. doi: 10.1152/ajpgi.1985.248.2.G147. [DOI] [PubMed] [Google Scholar]
- 42.Duriez J., Flautre B., Blary M., Hardouin P. Effects of the calcium channel blocker nifedipine on epiphyseal growth plate and bone turnover: a study in rabbit. Calcif Tissue Int. 1993;52:120–124. doi: 10.1007/BF00308320. [DOI] [PubMed] [Google Scholar]
- 43.Burren C.P., Caswell R., Castle B., Welch C.R., Hilliard T.N., Smithson S.F., Ellard S. TRPV6 compound heterozygous variants result in impaired placental calcium transport and severe undermineralization and dysplasia of the fetal skeleton. Am J Med Genet A. 2018;176:1950–1955. doi: 10.1002/ajmg.a.40484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suzuki Y., Chitayat D., Sawada H., Deardorff M.A., McLaughlin H.M., Begtrup A., Millar K., Harrington J., Chong K., Roifman M., Grand K., Tominaga M., Takada F., Shuster S., Obara M., Mutoh H., Kushima R., Nishimura G. TRPV6 variants interfere with maternal-fetal calcium transport through the placenta and cause transient neonatal hyperparathyroidism. Am J Hum Genet. 2018;102:1104–1114. doi: 10.1016/j.ajhg.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suzuki Y., Kovacs C.S., Takanaga H., Peng J.B., Landowski C.P., Hediger M.A. Calcium channel TRPV6 is involved in murine maternal-fetal calcium transport. J Bone Miner Res. 2008;23:1249–1256. doi: 10.1359/JBMR.080314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fecher-Trost C., Lux F., Busch K.M., Raza A., Winter M., Hielscher F., Belkacemi T., van der Eerden B., Boehm U., Freichel M., Weissgerber P. Maternal transient receptor potential vanilloid 6 (Trpv6) is involved in offspring bone development. J Bone Miner Res. 2019;34:699–710. doi: 10.1002/jbmr.3646. [DOI] [PubMed] [Google Scholar]
- 47.Wang L., Zhu F., Yang H., Li J., Li Y., Ding X., Xiong X., Yin Y. Effects of dietary supplementation with epidermal growth factor on nutrient digestibility, intestinal development and expression of nutrient transporters in early-weaned piglets. J Anim Physiol Anim Nutr. 2019;103(2):618–625. doi: 10.1111/jpn.13059. [DOI] [PubMed] [Google Scholar]
- 48.Ajibade D.V., Dhawan P., Fechner A.J., Meyer M.B., Pike J.W., Christakos S. Evidence for a role of prolactin in calcium homeostasis: regulation of intestinal transient receptor potential vanilloid type 6, intestinal calcium absorption, and the 25-hydroxyvitamin D3 1α hydroxylase gene by prolactin. Endocrinology. 2010;151:2974–2984. doi: 10.1210/en.2010-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Christakos S. Mechanism of action of 1, 25-dihydroxyvitamin D3 on intestinal calcium absorption. Rev Endocr Metab Disord. 2012;13:39–44. doi: 10.1007/s11154-011-9197-x. [DOI] [PubMed] [Google Scholar]
- 50.Dorkkam N., Wongdee K., Suntornsaratoon P., Krishnamra N., Charoenphandhu N. Prolactin stimulates the L-type calcium channel-mediated transepithelial calcium transport in the duodenum of male rats. Biochem Biophys Res Commun. 2013;430:711–716. doi: 10.1016/j.bbrc.2012.11.085. [DOI] [PubMed] [Google Scholar]
- 51.Weissgerber P., Kriebs U., Tsvilovskyy V., Olausson J., Kretz O., Stoerger C., Mannebach S., Wissenbach U., Vennekens R., Middendorff R., Flockerzi V., Freichel M. Excision of Trpv6 gene leads to severe defects in epididymal Ca2+ absorption and male fertility much like single D541A pore mutation. J Biol Chem. 2012;287:17930–17941. doi: 10.1074/jbc.M111.328286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beggs M.R., Appel I., Svenningsen P., Skjødt K., Alexander R.T., Dimke H. Expression of transcellular and paracellular calcium and magnesium transport proteins in renal and intestinal epithelia during lactation. Am J Physiol Renal Physiol. 2017;313:F629–F640. doi: 10.1152/ajprenal.00680.2016. [DOI] [PubMed] [Google Scholar]
- 53.Bouxsein M.L., Boyd S.K., Christiansen B.A., Guldberg R.E., Jepsen K.J., Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 54.Kawamoto T., Shimizu M. A method for preparing 2- to 50-micron-thick fresh-frozen sections of large samples and undecalcified hard tissues. Histochem Cell Biol. 2000;113:331–339. doi: 10.1007/s004180000149. [DOI] [PubMed] [Google Scholar]
- 55.Dempster D.W., Compston J.E., Drezner M.K., Glorieux F.H., Kanis J.A., Malluche H., Meunier P.J., Ott S.M., Recker R.R., Parfitt A.M. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28:2–17. doi: 10.1002/jbmr.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grubb B.R. Bioelectric measurement of CFTR function in mice. Methods Mol Med. 2002;70:525–535. doi: 10.1385/1-59259-187-6:525. [DOI] [PubMed] [Google Scholar]
- 57.Clarke L.L. A guide to Ussing chamber studies of mouse intestine. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1151–G1166. doi: 10.1152/ajpgi.90649.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Charoenphandhu N., Tudpor K., Pulsook N., Krishnamra N. Chronic metabolic acidosis stimulated transcellular and solvent drag-induced calcium transport in the duodenum of female rats. Am J Physiol Gastrointest Liver Physiol. 2006;291:G446–G455. doi: 10.1152/ajpgi.00108.2006. [DOI] [PubMed] [Google Scholar]