Summary
Digital health promises a paradigm shift for medicine where biomarkers in individuals are continuously monitored to improve diagnosis and treatment of disease. To that end, a technology for minimally invasive quantification of endogenous analytes in bodily fluids will be required. Here, we describe a strategy for designing and fabricating hydrogel microfilaments that can penetrate the skin while allowing for optical fluorescence sensing. The polyacrylamide formulation was selected to provide high elastic modulus in the dehydrated state and optical transparency in the hydrated state. The microfilaments can be covalently tethered to a fluorescent aptamer to enable functional sensing. The microfilament array can penetrate the skin with low pain and without breaking, contact the dermal interstitial fluid, and be easily removed from the skin. In the future, hydrogel microfilaments could be integrated with a wearable fluorometer to serve as a platform for continuous, minimally invasive monitoring of intradermal biomarkers.
Subject Areas: Bioelectronics, Polymers, Biomedical Materials
Graphical Abstract

Highlights
-
•
Polyacrylamide hydrogel microfilaments were fabricated via replica molding
-
•
Microfilaments are stiff, when dehydrated, and optically transparent, when hydrated
-
•
Fluorescent aptamer can be tethered to hydrogel matrix for in situ analyte sensing
-
•
Microfilaments penetrate skin with low pain and contact interstitial fluid
Bioelectronics; Polymers; Biomedical Materials
Introduction
The emerging paradigm of digital health will require minimally invasive techniques for continuous sensing of biomarkers in internal bodily fluids such as interstitial fluid and blood. A commonly used technology is continuous glucose monitors (CGMs), which monitor glucose levels in the interstitial fluid with high accuracy (Slattery and Choudhary, 2017, Forlenza et al., 2017, Welsh et al., 2019a, Welsh et al., 2019b). However, insertion is painful and the device can cause discomfort during use (Slattery and Choudhary, 2017, Forlenza et al., 2017, Ramchandani et al., 2011), such that up to 41% of diabetic patients have discontinued the use of CGMs (Slattery and Choudhary, 2017). In alternative approaches, noninvasive monitoring of analytes in extracorporeal fluids such as sweat (Alizadeh et al., 2018, Currano et al., 2018, Nyein et al., 2018, Parlak et al., 2018) must account for physiological variables such as differing sweat secretion rates and compositions among individuals and daily conditions (Nyein et al., 2018, Alizadeh et al., 2018).
This tradeoff in comfort against accuracy has motivated the development of microneedles that can painlessly interface with the interstitial fluid to facilitate accurate and continuous monitoring. Although most applications of microneedles have focused on delivery of drugs or vaccines (Yan et al., 2010, Chen et al., 2009, Sullivan et al., 2010, Yu et al., 2015, Ito et al., 2012, Mcvey et al., 2014), other works have focused on sampling of blood (Li et al., 2009, Li et al., 2013, Tsuchiya et al., 2007) and interstitial fluid (Mukerjee et al., 2004, Yang et al., 2013, Caffarel-Salvador et al., 2015) for analyte sensing. Such approaches include hollow microneedle arrays for fluid collection with off-site analysis (Blicharz et al., 2018, Li et al., 2013), solid arrays for skin pre-treatment before fluid collection (Wang et al., 2005), and integrated sampling and sensing approaches (Windmiller et al., 2011, Zahn et al., 2005, Invernale et al., 2014, Jina et al., 2014). Although the fluid extraction process in traditional microneedles is minimally invasive, current challenges include reliability, contamination, and variable yield (often less than 10 μL for interstitial fluid) (Kiang et al., 2017, Lei and Prow, 2019). Further, integrated sampling and sensing approaches are susceptible to delayed sensing as the fluid is transported to an external electrochemical sensor. Thus, an in situ approach—where the sensing takes place within the interstitial fluid, without the need to transport the fluid elsewhere—could be more suitable for continuous sensing.
Hydrogels have great potential for in situ biosensing because of their biocompatibility, hydrophilicity, and tunable structure (Le Goff et al., 2015). Moreover, fluorescent nanoparticles or sensors can be incorporated into the hydrogel matrix to enable in situ sensing of multiple analytes (Park and Park, 2018, Tan et al., 2016). To date, many applications of hydrogels require injection (Kanick et al., 2019, Chien et al., 2017), which is invasive and makes the hydrogel difficult to remove. In addition, fluorescent sensors can diffuse out of the hydrogel, resulting in a loss of signal and limiting the operation lifetime (Li et al., 2019). Here, we describe a strategy for fabricating hydrogel structures toward in situ sensing in the body without invasive injection or removal of the hydrogel. The proposed structures are sufficiently stiff and sharp to penetrate the skin to directly access interstitial fluid and can be covalently conjugated to fluorescent sensors for sustained sensing.
Results and Discussion
Design and Fabrication of Array of Stiff and Sharp Hydrogel Microfilaments
Our design consists of an array of hydrogel “microfilaments” that are chemically tethered to fluorescent sensors, which rapidly respond to analyte levels. In contrast to traditional hollow silicon-based microneedles, the hydrogel microfilaments are non-hollow and porous. Whereas silicon-based microneedles require a complex scheme for fabrication and extraction of fluid for sensing, the non-hollow structure of the microfilaments allows for easy fabrication (via molding, for example) and the porosity ensures that analytes can diffuse into the filaments and interact with conjugated sensor molecules. Further, the microfilaments are designed to be mechanically stiff in the dehydrated state and soft in the hydrated state (i.e. upon contact with internal bodily fluids). We chose polyacrylamide as the hydrogel material, as acrylamide-based copolymers are biocompatible and used in Food and Drug Administration (FDA)-approved medical devices for prolonged wear (such as soft contact lenses (Nicolson and Vogt, 2001) and urinary incontinence treatment devices (Toozs-Hobson et al., 2012)). However, the hydrogel, which is soft in the hydrated state, must overcome the skin's compliant surface before penetrating the stratum corneum (Park and Prausnitz, 2010) and contacting dermal interstitial fluid.
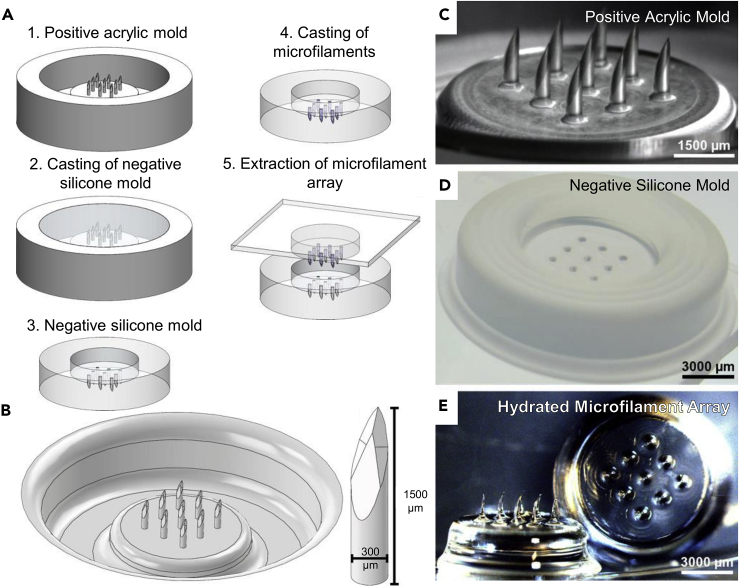
As such, we fabricated stiff and sharp hydrogel structures by using desiccated high-percentage polyacrylamide, which provides high mechanical strength and stiffness, and fashioning beveled tips in order to minimize the insertion force. Geometrically, the hydrated microfilaments are 1,500 μm tall and 300 μm in diameter (i.e. exhibiting an aspect ratio of 5) and have a 74° diamond-shaped bevel. (The dimensions of the desiccated, de-swelled microfilaments were approximately 1,100 μm in height and 200 μm in diameter.) The designed depth ensured contact with dermal interstitial fluid, the total volume ensured a sufficiently large fluorescence signal from the conjugated fluorescent sensor, and the bevel ensured piercing of the stratum corneum. We placed the polyacrylamide microfilaments into an array with a diameter of 7 mm, containing 9 microfilaments that are spaced 1,500 μm apart tip-to-tip and placed in a 3 × 3 configuration. The hydrogel microfilament array was fabricated via molding (Figure 1A). Positive microfilament array molds were designed using SolidWorks CAD software (Figure 1B) and produced by CNC milling of machinable acrylic plastic (Figure 1C). Compared with fabrication techniques used to fabricate silicon-based microneedles (Barrett et al., 2015), CNC milling of acrylic molds (Guckenberger et al., 2015) is simple to perform, cost-effective, and allows for high-aspect geometry. Acrylic plastic was chosen as the mold due to its ease of machinability for fine features, particularly a diamond-shaped bevel at the end of the hydrogel microfilament for ease of insertion into the skin. We chose a 5-bevel design, as they have been shown to be less painful when compared with traditional 3-bevel designs (Hirsch et al., 2012). A silicone negative mold (Figure 1D) is then replicated from the positive mold.
Figure 1.
Fabrication of Hydrogel Microfilament Arrays
(A) Schematic diagram of the fabrication process for hydrogel microfilament arrays.
(B) CAD design for a microfilament array in the hydrated state.
(C) Positive acrylic mold fabricated via CNC milling.
(D) Negative silicone mold, using a fast-curing platinum-cured silicone.
(E) Fabricated microfilament array in the hydrated state.
The sensor-laden polyacrylamide microfilament array was formed by casting a mixture of methacryl-functionalized fluorescent sensor and polyacrylamide precursor solution in the negative silicone mold followed by exposure to collimated ultraviolet (UV) light. The polyacrylamide precursor solution was composed of 30% w/v acrylamide, 2% w/v cross-linking agent (N,N'-methylenebisacrylamide), 0.5% v/v photoinitiator (2-hydroxy-2-methylpropiophenone, or Darocur 1173), and 100 nM fluorescent sensor. The microfilament array was extracted immediately after photopolymerization and subsequently rinsed under vacuum in two fresh baths of phosphate-buffered saline (PBS) to remove unpolymerized polyacrylamide precursor residues. The hydrogel was then desiccated to dry and harden the microfilaments, yielding the final sensor-conjugated microfilament array (Figure 1E).
Optimization of Polymer Formulation for Optical, Swelling, and Mechanical Properties
In addition to biocompatibility (Hadjesfandiari and Parambath, 2018) and mechanical strength, polyacrylamide, as used in the microfilaments, offers favorable diffusive and optical properties for real-time sensing in situ. It has high porosity (Holmes and Stellwagen, 1991) to enable diffusion of analytes from the dermal interstitial fluid to the aptamer sensor and is optically transparent to enable transmission of light. The optical and mechanical properties can be easily tuned by controlling the monomer formulation, specifically the acrylamide percentage (%AAm) as well as the ratio of acrylamide to N,N'-methylenebisacrylamide cross-linker (AAm/MBAm) (Bansil and Gupta, 1980). Figure 2A summarizes the optical and mechanical properties that were considered for optimization of the polymer formulation. To determine the hydrogel composition that would yield desirable optical properties, the absorbance of different hydrogel compositions was measured using the microplate reader. Measurement of absorbance at the peak excitation and emission wavelengths for 6-carboxyfluorescein (FAM) and 6-carboxytetramethylrhodamine (TAMRA) fluorophores was performed on a range of polyacrylamide compositions that polymerize without leaving opaque residue (Figure 2B). Excessively high acrylamide concentration or insufficient cross-linker leaves acrylamide unpolymerized, leading to opaque and brittle hydrogel; the limit was found to be around 32% w/v acrylamide. These absorbance measurements were then used to calculate the percent transmittance at the specified wavelengths (Figure S1). Both %AAm and AAm/MBAm significantly affected absorbance (p < 0.0001). For a set %AAm, absorbance decreased with increasing AAm/MBAm values; for example, absorbance decreased (p < 0.05) for all wavelengths assessed when AAm/MBAm values increased from 10 to 20 (at a fixed %AAm). To simplify measurements of the other parameters in the subsequent experiments, only formulations with AAm/MBAm ratios of 15, 17.5, and 20 and %AAm of 20, 25, and 30 were selected. The absorbance values at 470 nm, 520 nm, 550 nm, and 580 nm for these polyacrylamide compositions are summarized in Table S1.
Figure 2.
Optical and Mechanical Properties of Polyacrylamide Hydrogel
(A) Design criteria selected for optimization of polyacrylamide formulation.
(B) Absorbance values of hydrated polyacrylamide disks with different formulations. Top left: λ = 470 nm, the excitation wavelength for FAM (n = 4). Top right: λ = 520 nm, the emission wavelength for FAM (n = 4). Bottom left: λ = 550 nm, the excitation wavelength for TAMRA (n = 4). Bottom right: λ = 580 nm, the emission wavelength for TAMRA (n = 4). See also Figure S1 and Table S1.
(C) The shrinkage percentage of polyacrylamide hydrogel for different polymer formulations (n = 6). See also Figure S2
(D) The Young's modulus of dehydrated polyacrylamide hydrogel for different polymer formulations (n = 4). Data analyzed using a two-way ANOVA with Tukey post-hoc test. “ns” indicates not significant, “*” indicates significant at p < 0.05, “**” indicates significant at p < 0.01, and “****” indicates significant at p < 0.0001. Line indicates comparison between groups. Data are represented as mean ± standard deviation.
Microfilament integrity during insertion is facilitated by having sharp tips to minimize the insertion force and overcome the skin's compliant surface (Park and Prausnitz, 2010). Hydrogels typically have high water content. As such, when left to desiccate, significant deformation in shape of the hydrogels is observed. Since morphology retention is necessary to preserve the beveled tip for easy insertion, it is important to understand the extent of shrinking and the effect on microfilament morphology following desiccation. Figure 2C shows the shrinkage percentage of different polyacrylamide compositions, as determined by the change in radius before and after hydrogel desiccation. Polyacrylamide formed from precursor solutions containing higher %AAm exhibit significantly (p < 0.0001) decreased shrinkage percentage for all AAm/MBAm ratios tested, likely due to an increase in dry weight that inhibits collapse of the matrix upon dehydration. A scanning electron microscopic (SEM) image of a polyacrylamide microfilament array taken two days after fabrication (Figure S2) demonstrated that challenging features for microfabrication—such as high aspect ratio, diamond-shaped bevels, and sharp tips—are preserved after shrinkage as brought on by desiccation.
In addition to having a sharp, beveled tip, the microfilaments must have high mechanical strength to penetrate the skin and access dermal interstitial fluid. In its hydrated state, polyacrylamide is soft and unable to pierce the skin. Although there has been recent effort to develop hydrated hydrogels with good mechanical properties, such as high compressive strength or Young's modulus, by modifying polymer formulation or polymerization method (Zhao, 2017), we optimized the polyacrylamide formulation to yield desired mechanical properties in the dehydrated state. Because the material hardens when low in water content, desiccated polyacrylamide microfilaments are able to penetrate the stratum corneum. To quantify the strength of the desiccated microfilaments, the Young's modulus was determined experimentally at different polyacrylamide compositions. Figure 2D shows that all the compositions selected based on highest optical transmission exhibit comparable and extremely high Young's modulus of approximately 1 GPa. These values are approximately one order of magnitude less than hard plastic (Crompton, 2012) and indicate that desiccated polyacrylamide is much stiffer than PDMS (Johnston et al., 2014). Conversely, in the hydrated state, the hydrogel has a Young's modulus of 461 ± 76 KPa, matching more closely that of surrounding skin (∼100 kPa (Liang and Boppart, 2010)) and hence reducing the risk of immune response due to mechanical mismatch (Stieglitz and Schuettler, 2013). At fixed %AAm, Young's modulus increased with AAm/MBAm ratio, presumably due to decrease in cross-linked clusters that introduce heterogeneity and soften the matrix (Denisin and Pruitt, 2016). Overall, any of the tested formulations may be used to fabricate the microfilament sensing platform with sufficiently high mechanical strength to penetrate the stratum corneum of the human skin.
Ultimately, the choice of polymer composition for microfilament fabrication is a balance of the different properties assessed. Absorbance measurements at the peak excitation and emission wavelengths for the reporter fluorophores indicate that formulations with AAm/MBAm ratios of 15, 17.5, or 20 had the highest transmittance. Assessments of mechanical properties demonstrate that all polyacrylamide compositions tested yielded sufficient strength to penetrate the stratum corneum, whereas formulations with higher %AAm showed reduced shrinkage. Thus, a polyacrylamide composition of 30% AAm and AAm/MBAm ratio of 15 was selected due to the high optical transmission at the desired wavelengths, good mechanical strength, and minimal shrinkage percentage. Finally, we demonstrated porosity of this polymer composition, using fluorescence recovery after photo bleaching, to 2-NBDG, a commercially available fluorescent analog of glucose (which is a small molecule as phenylalanine, our test analyte). The diffusivity was measured as 5.7 ± 1.9 μm2/s. Table 1 summarizes the properties of this polyacrylamide composition.
Table 1.
Properties of Polyacrylamide of Composition %AAm = 30 and AAm/MBAm = 15
| Composition |
|||
|---|---|---|---|
| %AAm = 30 and AAm/MBAm = 15 | |||
| Optical | Absorbance, transmittance at 470 nm | 0.058 ± 0.009 | 87.614 ± 1.820% |
| Absorbance, transmittance at 520 nm | 0.054 ± 0.009 | 88.270 ± 1.735% | |
| Absorbance, transmittance at 550 nm | 0.054 ± 0.009 | 86.801 ± 1.090% | |
| Absorbance, transmittance at 580 nm | 0.054 ± 0.009 | 88.373 ± 1.798% | |
| Mechanical | Young's modulus | 0.793 ± 0.329 GPa | |
| Deformation | Shrinkage percentage | 26.115 ± 1.547% | |
Summary of properties for selected polyacrylamide formulation with 30% acrylamide (%AAm) and acrylamide to N,N'-methylenebisacrylamide cross-linker ratio (AAm/MBAm) of 15. Data are represented as mean ± standard deviation.
Assessment of Sensor Functionality in Hydrated Hydrogel
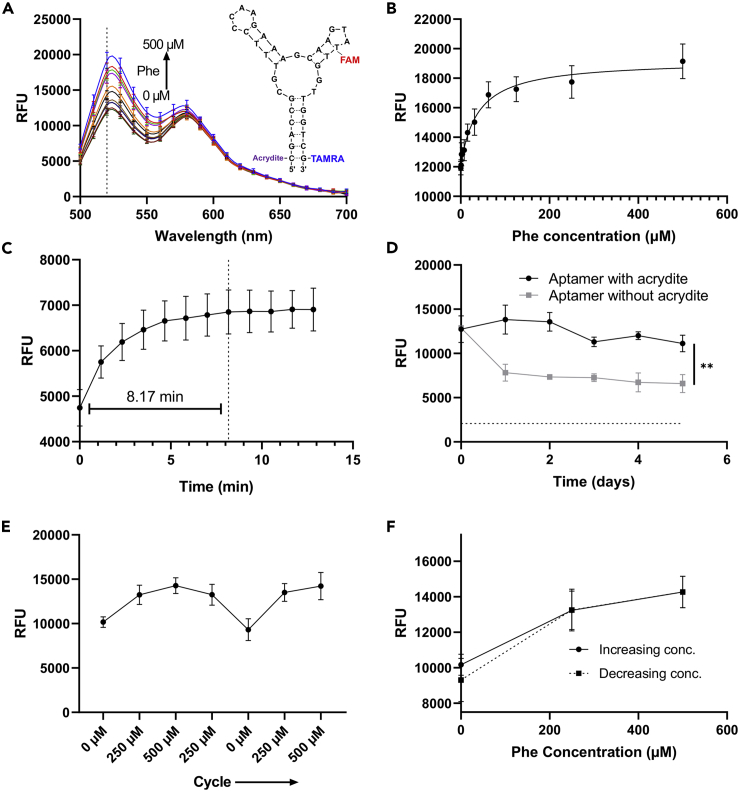
In our platform, the aptamer sensing moiety can be conjugated to microfilaments to enable passive in situ sensing, thereby bypassing delayed, complex, and potentially error-prone procedures that extract the interstitial fluid out of a microneedle onto an external electrochemical sensor. Here, we assessed whether a fluorescent aptamer sensor selected against phenylalanine can be covalently conjugated to the polyacrylamide matrix via co-polymerization and that the functionality is retained. The aptamer sensor (Figure 3A, inset) is composed of phenylalanine binding site, FAM and TAMRA fluorophore reporting unit, and an Acrydite handle to enable immobilization to the polyacrylamide matrix. As a future application, monitoring of phenylalanine is critical for patients with phenylketonuria, a genetic disease that leads to elevated phenylalanine levels, growth failure, and mental health disorders (Brumm et al., 2010, Williams et al., 2008). This sensor was selected as a model moiety, as aptamers are a flexible class of sensor that can be selected for a wide variety of target analytes (Ilgu and Nilsen-Hamilton, 2016) and easily functionalized to enable attachment. To demonstrate that the sensor functionality is maintained following conjugation to the hydrogel, hydrated hydrogel disks were exposed to solutions with different concentrations of phenylalanine. Figure 3A shows the fluorescence emission spectra of the hydrogel following excitation at 470 nm. The fluorescence peak intensity at 520 nm increased as the phenylalanine concentration increased from 0.0 to 500 μM (Figure 3B). Comparison to the emission spectra (Figure S3A) and fluorescence intensity at 520 nm (Figure S3B) for the aqueous sensor shows that the phenylalanine concentration dependence is maintained following chemical tethering to the polyacrylamide matrix. Disks were used instead of the microfilaments because the geometry was less fragile and could withstand the multiple read and wash cycles without breaking.
Figure 3.
Sensor Performance Following Tethering to Hydrated Hydrogel [30% AAm 15, AAm/MBAm]
(A) Fluorescence emission spectra of hydrated hydrogel exposed to different concentration of phenylalanine solution and excitation at λ = 470 nm (n = 8). Inset shows the schematic of phenylalanine-responsive fluorescent aptamer sensor.
(B) Fluorescence intensity concentration response for hydrated hydrogel with excitation λ = 470 nm and emission λ = 520 nm (n = 8). The curve was fit using a Padé approximant.
(C) Response time for hydrated hydrogel after addition of 500 μM phenylalanine solution (n = 8).
(D) Fluorescence intensity for hydrated hydrogel disks with excitation λ = 470 nm and emission λ = 520 nm after soaking in PBS for 5 days with PBS changed daily. Hydrogel disks were fabricated using aptamer with or without the 5′-acrydite modification. The dotted line indicates the average fluorescence for a hydrated hydrogel disk with no aptamer (n = 3). Data analyzed using two-tailed t test. “**” indicates significant at p < 0.01.
(E) Fluorescence intensity for hydrated hydrogel with excitation λ = 470 nm and emission λ = 520 nm after cycling through different concentrations of phenylalanine (n = 3).
(F) Hysteresis loop showing the fluorescence intensity for hydrated hydrogel with excitation λ = 470 nm and emission λ = 520 nm after increasing and decreasing phenylalanine concentration (n = 3).
Data are represented as mean ± standard deviation. See also Figures S3–S5.
Real-time in situ sensing requires the sensor to quickly respond to the changing environment. The response time for the aqueous sensor is less than 20 s (Figure S4), indicating rapid response to changes in phenylalanine concentration. The response time for the hydrogel-conjugated sensor is approximately 8 min for a disk approximately 6.4 mm in diameter and 1 mm in height (Figure 3C). As the dimensions for the microfilaments are up to an order of magnitude smaller than the disk, facilitating more rapid diffusion of the analyte through the hydrogel, we expect the response time for the microfilament array to be less than 8 min. Extended sensing requires that the aptamer be covalently attached to the polyacrylamide matrix such that the sensor does not diffuse out of the microfilaments, resulting in an erroneous decrease in fluorescence signal. To demonstrate this chemical tethering, sensor-laden polyacrylamide disks were fabricated, and the fluorescence intensity at 520 nm was tracked over 5 days where the disks were soaked in clean PBS buffer with the buffer replaced daily (Figure 3D). For hydrogel disks fabricated using aptamer without a 5′-acrydite modification, the fluorescence intensity decreased significantly within a day (p < 0.01) after soaking in buffer, likely due to diffusion of unconjugated sensor out of the gel. After this initial drop, the fluorescence intensity remains relatively constant and is greater than that for disks made without aptamer, indicating that some sensor may be physically encapsulated by the hydrogel matrix. For disks made with the 5′-acrydite modification, the fluorescence intensity remains relatively stable for the entire measurement period, indicative of successful tethering to the hydrogel.
Additionally, in situ sensing requires the sensor be reversible. To demonstrate reversible sensing, hydrogel disks were exposed to phenylalanine solutions with both increasing and decreasing concentration and the fluorescence intensity at 520 nm was recorded (Figure 3E). These data show that the phenylalanine aptamer sensor demonstrates reversible sensing, as an increase in phenylalanine concentration results in an increase in fluorescence intensity, whereas a decrease in concentration results in a subsequent decrease in intensity. The hysteresis loop for this reversible sensing (Figure 3F) shows that the sensor has minimal hysteresis. However, the aptamer sensor is susceptible to photobleaching (Figure S5), which may limit the duration of in situ sensing for a single microfilament array. (In the future, it may be possible to normalize for the effects of photobleaching, or monitor at 520 and 580 nm for ratiometric sensing, which is less sensitive to effects of photobleaching.)
Exploration of Design of Microfilament Array for Low Human Pain
Toward the goal of eliciting minimal pain on the user, we note that previous studies that studied microneedles and human pain sensation (Gill et al., 2008, Haq et al., 2009, Park et al., 2007) have demonstrated the predominant factors to be length of needle, and secondarily, number of needles, diameter, and sharpness. As such, previous studies (Gill et al., 2008, Haq et al., 2009) have investigated different geometric design parameters of the microneedle array (number of microneedles in array and needle height and diameter) on human pain assessments, but typically each parameter was investigated independently of the others, with compound effects unexplored. Here, we performed a human pain assessment using microneedles fabricated from polyether ether ketone (PEEK), which is FDA approved for experiments on human subjects and can be easily steam sterilized. (We measured the Young's modulus of PEEK to be 4.3 ± 0.2 GPa, within an order of magnitude, but even higher, of that of the dehydrated hydrogel; both the PEEK microneedles and hydrogel microfilaments are sufficiently stiff to avoid bending-induced fracture (Zahn et al., 2000).) We machined PEEK microneedles to identify geometric parameters that elicited low human pain and subsequently fabricated dehydrated polyacrylamide microfilaments with matching length, number of filaments in array, diameter, and bevel.
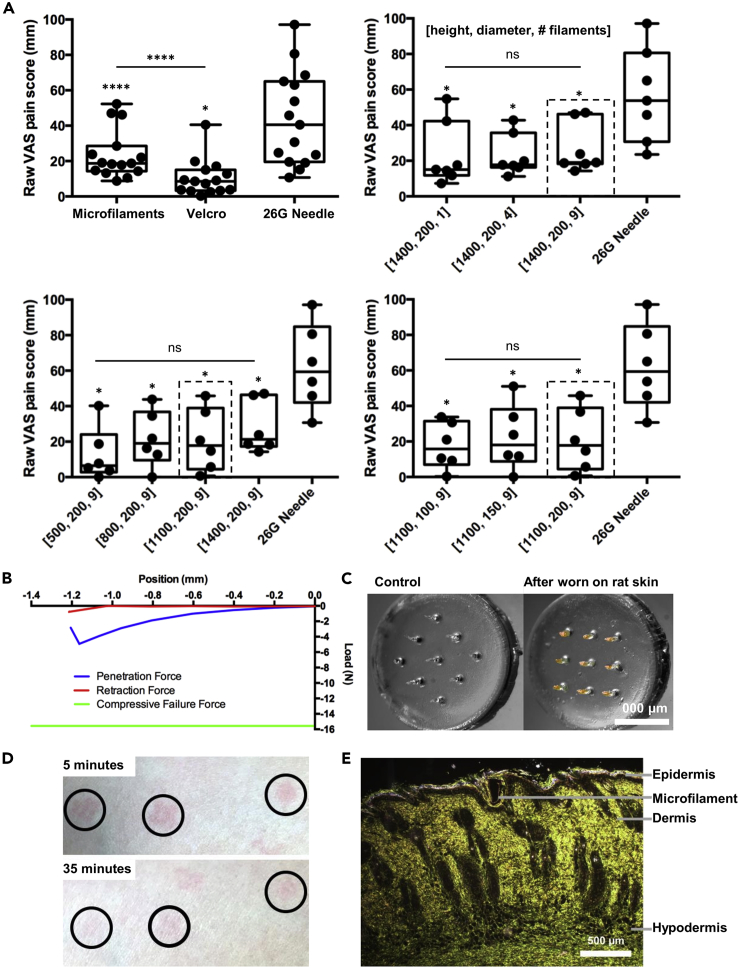
First, we recruited human subjects to assess pain levels induced by insertion of microneedles compared with pressing the hook surface of a hook-and-loop fastener (Velcro) against the skin. The raw visual analog scale (VAS) pain scores (Hawker et al., 2011) (Figure 4A, top left) and those normalized to a 26-guage hypodermic needle (Figure S6A) from 15 human subjects showed that the sensation experienced by the insertion of a 3 × 3 PEEK microneedle array with needles of 200 μm diameter and 1,400 μm height induced slightly more pain compared with a press of the Velcro patch against the skin but is still significantly less painful (p < 0.0001) compared with a standard 26-gauge hypodermic needle. (Qualitative feedback from human subjects also indicated there was minimal discomfort after insertion of device.)
Figure 4.
Exploration of Microfilament Array Design and Assessment of Skin Penetration and Healing
(A) Box plots of raw VAS pain scores after insertion of microfilament arrays with varying geometries. The dotted lines indicate the design that was identified as optimal for each group. Top left: 3 × 3 array of microfilaments with 200 μm diameter and 1,400 μm height microfilaments, application of a Velcro patch to the skin, or insertion of a 26-guage hypodermic needle (n = 15). Top right: microfilament array with 1, 4, or 9 microfilaments of 200 μm diameter and 1,400 μm height or insertion of a 26-guage hypodermic needle (n = 7). Bottom left: 3 × 3 microfilament array with 200 μm diameter and 500–1400 μm height microfilaments or insertion of a 26-guage hypodermic needle (n = 6). Bottom right: a 3 × 3 microfilament array with 100–200 μm diameter and 1,100 μm height microfilaments or insertion of a 26-guage hypodermic needle (n = 6). Data analyzed using a one-way ANOVA with Sidak post-hoc test. “ns” indicates not significant, “*” indicates significant at p < 0.05, “****” indicates significant at p < 0.0001. Symbol above group indicates comparison with 26G Needle. Line indicates comparison between groups. See also Figure S6.
(B) Representative force-displacement curve during insertion into and removal of a 3 × 3 microfilament array from human cadaver skin. The bottom line marks the compressive force required to fracture desiccated microfilaments during insertion into the skin.
(C) Image of microfilament array worn on rat skin for 1 h and a control array not worn. Arrays were subsequently treated with silver nitrate to indicate exposure to chloride ions. For visualization purposes, the red and yellow channels were boosted for the control and experimental arrays to highlight the silver deposits.
(D) Image of microfilament array penetration site on the skin surface of a human subject 5 or 35 min after device removal. The insertion sites have been circled.
(E) Bright-field micrograph of rat skin histological section 24 h after insertion of microfilament platform.
We tested insertion of arrays with 1, 4, or 9 microneedles arranged in a 1 × 1, 2 × 2, or 3 × 3 grid, respectively, keeping constant the diameter (200 μm), height (1400 μm), and bevel (74°). Raw (Figure 4A, top right) and normalized (Figure S6B) VAS pain scores indicated that the number of microneedles in an array did not significantly affect pain. Thus, we selected an array with 9 gel microfilaments in order to maximize the fluorescent signal. We also tested insertion of 3 × 3 arrays with needles of 200 μm diameter, while varying the height from 500 to 1,400 μm. The raw (Figure 4A, bottom left) and normalized (Figure S6C) VAS pain scores showed that, for the heights probed, microneedle height did not appreciably affect insertion pain, with no significant difference between 800 μm and 1,100 μm. Thus, to both maximize the fluorescent signal and minimize insertion pain, the design with a microfilament height of 1,100 μm was selected. Finally, insertion of 3 × 3 arrays with needle height of 1,100 μm and diameters ranging from 100 to 200 μm (Figures 4A, bottom right, S6D) showed an increase in the median pain score with increasing diameter, but the differences were not significant (as further confirmed by qualitative feedback from human subjects). Overall, we selected for the microfilament array to be a 3 × 3 array with microfilaments of 200 μm diameter and 1,100 μm height. Taking into account the shrinkage percentage of the chosen polyacrylamide formulation, this target geometry was achieved after desiccation of hydrated microfilaments of 300 μm diameter and 1,500 μm height.
Assessment of Skin Penetration and Healing
Finally, we performed a number of assessments to evaluate the ability of microfilaments to penetrate the skin and contact interstitial fluid. To assess the ability of the polyacrylamide microfilament sensor platform to penetrate the human skin without breaking and leaving polymer residues inside the skin, we measured the penetration and failure force. To measure the penetration force, we used human cadaver skin as a model (Wang et al., 2006, Park et al., 2005). Full-thickness human cadaver skin was mounted on the base platen of a material testing machine equipped with a 25-lb load cell. The microfilament array was affixed to the upper platen of the mechanical tester. The array was brought into contact with the tissue, and the force for insertion was recorded. The array was then displaced in the opposite direction, and the force for removal was recorded. Mechanical failure was considered due to axial loading. Comparison of the force required to penetrate the polyacrylamide microfilaments into human cadaver skin and the force required to fracture the microfilaments, Figure 4B, demonstrates that the microfilaments provide sufficient mechanical strength to penetrate the human skin without breaking and leaving residues inside the skin. The force required to penetrate a 3 × 3 array of microfilaments into human skin is around 5 N, whereas the compressive failure force is approximately 16 N (corresponding to a compressive strength on the order of hundreds of MPa).
To demonstrate the ability of polyacrylamide microfilaments to penetrate the stratum corneum and contact dermal interstitial fluid, we applied microfilaments on the skin of live, anesthetized hairless rats. We gently pressed the microfilament arrays into the skin, secured it in position with a skin adhesive, and after one hour, removed the microfilament array and treated it with silver nitrate to detect for the presence of chloride ions from dermal interstitial fluid. Figure 4C shows localization of silver deposits to the microfilament shafts, whereas the control array, not applied to rat skin, does not indicate the presence of chloride ions. The presence of silver deposits and their localization to the shafts of the microfilaments demonstrate that chloride ions were sampled not from the superficial layer of the skin, but rather from the interstitial fluid beneath the epidermis.
To assess the healing response of the human skin after insertion and removal of PEEK microfilament arrays, we captured images of the human skin surface after device removal. Figure 4D shows images of the microfilament array penetration sites on the skin of a human subject at 5 and 35 min after device removal. The faint redness on most of the penetration sites, indicating minimal inflammation, subsided within 2 h after removal of device. (As PEEK is biocompatible and the arrays were inserted for only ∼10 s, the observed inflammation was likely due to mechanical trauma and would likely be similar for a gel polyacrylamide array with the same length, number, diameter, and sharpness of filaments.) Finally, histological samples of polyacrylamide microfilament insertion sites on rat skin were obtained to examine the wound healing response in vivo. Figure 4E shows a histological image at a microfilament insertion site, with a microfilament still embedded within, further confirming that polyacrylamide microfilaments penetrated the epidermis to contact the dermal layer. With regard to the wound healing response in an in vivo model, over 24 h post application, the epidermis reformed and engulfed around the microfilament, indicative of fast healing of the puncture site. As expected from the small dimensions of microfilaments, the puncture site was observed to rapidly reseal.
Steps toward Translation
To translate the proposed system to the clinic, a number of challenges must be considered. For manufacturing, the demonstrated fabrication method can be scaled up by increasing the batch size (e.g. using larger molds with more arrays and curing in a UV oven) and automating the fabrication. Sterilization of the mold and polymer precursor solution can potentially be achieved by UV germicidal irradiation (Huebsch et al., 2005). Relating to the use of the platform, the patch is intended to be worn over the length of the desired measurement period, until the performance of the sensor has degraded due to photobleaching or until discomfort. To facilitate safe and reproducible insertion of the device, a simple applicator device could be designed in the future to apply the suitable force to penetrate the skin. Because the microfilaments are sufficiently stiff to avoid bending-induced fracture, we do not expect fragments of the microfilaments to be deposited into the skin; any small fragments lodged inside the skin, after removing the device, would reside primarily in the epidermis and be expected to be discarded via regular skin turnover within 2–4 weeks (S. Leo et al., 2014). During the manufacturing process, the microfilaments should be thoroughly washed after polymerization to remove unconjugated aptamers; aptamers covalently attached to the hydrogel are not expected to leach into the skin. Toward the goal of extended use, the effects of sweat and bathing on the performance of the system should be studied, as well as any potential immune response (although polyacrylamide (Hadjesfandiari and Parambath, 2018) and aptamers (Zhou and Rossi, 2017) have been demonstrated as non-immunogenic). Finally, the response time in vivo should be studied to take into account the time needed for interstitial fluid to diffuse through the microfilaments (which we currently estimate would be several minutes in vivo).
Beyond the continuous in situ monitoring in humans, a microfilament platform fabricated out of biocompatible hydrogel can be used for minimally invasive collection of interstitial fluid for downstream discrete analysis (similar to minimally invasive one-step blood-collection devices (Blicharz et al., 2018)), as the interstitial fluid sample resides within the microfilaments. Alternatively, the device could be adapted for monitoring chemical analytes in animal models to support basic science. As dermal interstitial fluid is rich with potential target analytes, the hydrogel microfilament platform could be adapted to monitor a wide variety of analytes, including electrolytes, saccharides, hormones, lactate, amino acids, proteins, enzymes, co-enzymes, triglycerides, oxygen, creatinine, urea, and reactive oxygen and nitrogen species (Ruckh and Clark, 2014, Paliwal et al., 2013).
Conclusion
In this work, we reported a microfilament platform fabricated from polyacrylamide hydrogel conjugated with phenylalanine aptamer sensor with fluorescence readout. To enable penetration of the stratum corneum, the microfilament platform was designed to be sharp and stiff. By optimizing the polymer formulation via %AAm and AAm/MBAm ratio, we were able to fabricate polyacrylamide hydrogel microfilament arrays with a sharp 5-bevel tip that was maintained after desiccation. Further, the desiccated microfilaments exhibited high mechanical strength with the compressive failure force exceeding both the force of insertion and removal. When rehydrated, the hydrogel demonstrated good transmittance at the desired wavelengths, and the functionality of a phenylalanine aptamer sensor attached via co-polymerization was preserved. The design of the microfilament array was optimized to minimize pain during insertion while maximizing the volume of the analyte-responsive microfilaments to thereby maximize fluorescence signal. The implemented microfilament design exhibited less insertion pain than a hypodermic needle and was more comparable to a piece of Velcro. Although the functionality of only one sensor was demonstrated, the microfilament platform can be adapted for alternative analytes by incorporating other sensors functionalized with acrylamide copolymers. This work points a path forward for using hydrogel microfilaments as skin patch continuous sensors without requiring surgery.
Limitations of the Study
The main limitation of this study is the lack of validation of a fully integrated system, which would include characterization of sensor performance from the microfilaments in an in vivo animal model. Considerable work has been done on portable fluorometer development (Alam et al., 2019, Kostov et al., 2014), such that in future studies, one of these designs could be adapted to interface with the platform presented. In addition, systematic studies of pore sizes for different polymer formulations, as well as tuning the formulation to accommodate sensing of different analytes (i.e. such that the Stokes radius of the analyte is less than 10% compared with the polyacrylamide pore radius (Deen et al., 1981)) could be performed. Finally, we have only demonstrated incorporation of a single sensor. However, aptamers can be selected for many different targets with chemistry similar to the utilized moiety. Thus, we anticipate alternative sensors could be incorporated without loss of functionality.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Tyler J. Poore and Willem J. Prins. This work was supported in part by grants from the National Institutes of Health (1R01DA045550) and the National Science Foundation (Graduate Research Fellowship under Grant No. DGE 1644869).
Author Contributions
N.T. and S.S. conceptualized the project. S.S. supervised the project. N.T. and D.C. designed experiments and analyzed data. N.T., D.C., F.M., V.G., and S.C. performed experiments. K.Y. designed and tested aptameric sensor candidates. K.Y. and M.S. provided materials. N.T. drafted a manuscript, and D.C. and S.S. wrote the manuscript.
Declaration of Interests
A patent based on these results has been filed by Columbia University.
Published: November 22, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.10.036.
Supplemental Information
References
- Alam M.W., Wahid K.A., Goel R.K., Lukong K.E. Development of a low-cost and portable smart fluorometer for detecting breast cancer cells. Biomed. Opt. Express. 2019;10:399–410. doi: 10.1364/BOE.10.000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizadeh A., Burns A., Lenigk R., Gettings R., Ashe J., Porter A., Mccaul M., Barrett R., Diamond D., White P. A wearable patch for continuous monitoring of sweat electrolytes during exertion. Lab Chip. 2018;18:2632–2641. doi: 10.1039/c8lc00510a. [DOI] [PubMed] [Google Scholar]
- Bansil R., Gupta M.K. Effect of varying crosslinking density on polyacrylamide gels. Ferroelectrics. 1980;30:63–71. [Google Scholar]
- Barrett C., Dawson K., O'mahony C., O'riordan A. Development of low cost rapid fabrication of sharp polymer microneedles for in vivo glucose biosensing applications. ECS J Solid State Sci Technol. 2015;4:S3053–S3058. [Google Scholar]
- Blicharz T.M., Gong P., Bunner B.M., Chu L.L., Leonard K.M., Wakefield J.A., Williams R.E., Dadgar M., Tagliabue C.A., El Khaja R. Microneedle-based device for the one-step painless collection of capillary blood samples. Nat. Biomed. Eng. 2018;2:151–157. doi: 10.1038/s41551-018-0194-1. [DOI] [PubMed] [Google Scholar]
- Brumm V.L., Bilder D., Waisbren S.E. Psychiatric symptoms and disorders in phenylketonuria. Mol. Genet. Metab. 2010;99(Suppl 1):S59–S63. doi: 10.1016/j.ymgme.2009.10.182. [DOI] [PubMed] [Google Scholar]
- Caffarel-Salvador E., Brady A.J., Eltayib E., Meng T., Alonso-Vicente A., Gonzalez-Vazquez P., Torrisi B.M., Vicente-Perez E.M., Mooney K., Jones D.S. Hydrogel-forming microneedle arrays allow detection of drugs and glucose in vivo: potential for use in diagnosis and therapeutic drug monitoring. PLoS One. 2015;10:e0145644. doi: 10.1371/journal.pone.0145644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.F., Prow T.W., Crichton M.L., Jenkins D.W.K., Roberts M.S., Frazer I.H., Fernando G.J.P., Kendall M.A.F. Dry-coated microprojection array patches for targeted delivery of immunotherapeutics to the skin. J. Control. Release. 2009;139:212–220. doi: 10.1016/j.jconrel.2009.06.029. [DOI] [PubMed] [Google Scholar]
- Chien J.S., Mohammed M., Eldik H., Ibrahim M.M., Martinez J., Nichols S.P., Wisniewski N., Klitzman B. Injectable phosphorescence-based oxygen biosensors identify post ischemic reactive hyperoxia. Sci. Rep. 2017;7:8255. doi: 10.1038/s41598-017-08490-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton T.R. Physical Testing of Plastics. Smithers Rapra Technology; 2012. 1-Mechanical properties of polymers; pp. 1–148. [Google Scholar]
- Currano L.J., Sage F.C., Hagedon M., Hamilton L., Patrone J., Gerasopoulos K. Wearable sensor system for detection of lactate in sweat. Sci. Rep. 2018;8:15890. doi: 10.1038/s41598-018-33565-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen W.M., Bohrer M.P., Epstein N.B. Effects of molecular-size and configuration on diffusion in microporous membranes. Aiche J. 1981;27:952–959. [Google Scholar]
- Denisin A.K., Pruitt B.L. Tuning the range of polyacrylamide gel stiffness for mechanobiology applications. ACS Appl. Mater. Interfaces. 2016;8:21893–21902. doi: 10.1021/acsami.5b09344. [DOI] [PubMed] [Google Scholar]
- Forlenza G.P., Argento N.B., Laffel L.M. Practical considerations on the use of continuous glucose monitoring in pediatrics and older adults and nonadjunctive use. Diabetes Technol. Ther. 2017;19:S13–S20. doi: 10.1089/dia.2017.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill H.S., Denson D.D., Burris B.A., Prausnitz M.R. Effect of microneedle design on pain in human volunteers. Clin. J. Pain. 2008;24:585–594. doi: 10.1097/AJP.0b013e31816778f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guckenberger D.J., De Groot T.E., Wan A.M.D., Beebe D.J., Young E.W.K. Micromilling: a method for ultra-rapid prototyping of plastic microfluidic devices. Lab Chip. 2015;15:2364–2378. doi: 10.1039/c5lc00234f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjesfandiari N., Parambath A. 13-Stealth coatings for nanoparticles: polyethylene glycol alternatives. In: Parambath A., editor. Engineering of Biomaterials for Drug Delivery Systems. Woodhead Publishing; 2018. pp. 345–361. [Google Scholar]
- Haq M.I., Smith E., John D.N., Kalavala M., Edwards C., Anstey A., Morrissey A., Birchall J.C. Clinical administration of microneedles: skin puncture, pain and sensation. Biomed. Microdevices. 2009;11:35–47. doi: 10.1007/s10544-008-9208-1. [DOI] [PubMed] [Google Scholar]
- Hawker G.A., Mian S., Kendzerska T., French M. Measures of adult pain visual analog scale for pain (VAS pain), numeric rating scale for pain (NRS pain), McGill pain questionnaire (MPQ), short-form McGill pain questionnaire (SF-MPQ), chronic pain grade scale (CPGS), short form-36 bodily pain scale (SF-36 BPS), and measure of intermittent and constant osteoarthritis pain (ICOAP) Arthritis Care Res. 2011;63:S240–S252. doi: 10.1002/acr.20543. [DOI] [PubMed] [Google Scholar]
- Hirsch L., Gibney M., Berube J., Manocchio J. Impact of a modified needle tip geometry on penetration force as well as acceptability, preference, and perceived pain in subjects with diabetes. J. Diabetes Sci. Technol. 2012;6:328–335. doi: 10.1177/193229681200600216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D.L., Stellwagen N.C. Estimation of polyacrylamide-gel pore-size from ferguson plots of linear DNA fragments 2. Comparison of gels with different cross-linker concentrations, added agarose and added linear polyacrylamide. Electrophoresis. 1991;12:612–619. doi: 10.1002/elps.1150120903. [DOI] [PubMed] [Google Scholar]
- Huebsch N., Gilbert M., Healy K.E. Analysis of sterilization protocols for peptide-modified hydrogels. J. Biomed. Mater. Res. B Appl. Biomater. 2005;74b:440–447. doi: 10.1002/jbm.b.30155. [DOI] [PubMed] [Google Scholar]
- Ilgu M., Nilsen-Hamilton M. Aptamers in analytics. Analyst. 2016;141:1551–1568. doi: 10.1039/c5an01824b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Invernale M.A., Tang B.C., York R.L., Le L., Hou D.Y., Anderson D.G. Microneedle electrodes toward an amperometric glucose-sensing smart patch. Adv. Healthc. Mater. 2014;3:338–342. doi: 10.1002/adhm.201300142. [DOI] [PubMed] [Google Scholar]
- Ito Y., Hirono M., Fukushima K., Sugioka N., Takada K. Two-layered dissolving microneedles formulated with intermediate-acting insulin. Int. J. Pharmaceutics. 2012;436:387–393. doi: 10.1016/j.ijpharm.2012.06.047. [DOI] [PubMed] [Google Scholar]
- Jina A., Tierney M.J., Tamada J.A., Mcgill S., Desai S., Chua B., Chang A., Christiansen M. Design, development, and evaluation of a novel microneedle array-based continuous glucose monitor. J. Diabetes Sci. Technol. 2014;8:483–487. doi: 10.1177/1932296814526191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston I.D., Mccluskey D.K., Tan C.K.L., Tracey M.C. Mechanical characterization of bulk Sylgard 184 for microfluidics and microengineering. J. Micromech. Microeng. 2014;24:035017. [Google Scholar]
- Le Goff G.C., Srinivas R.L., Hill W.A., Doyle P.S. Hydrogel microparticles for biosensing. Eur. Polym. J. 2015;72:386–412. doi: 10.1016/j.eurpolymj.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanick S.C., Schneider P.A., Klitzman B., Wisniewski N.A., Rebrin K. Continuous monitoring of interstitial tissue oxygen using subcutaneous oxygen microsensors: in vivo characterization in healthy volunteers. Microvasc. Res. 2019;124:6–18. doi: 10.1016/j.mvr.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang T.K.L., Ranamukhaarachchi S.A., Ensom M.H.H. Revolutionizing therapeutic drug monitoring with the use of interstitial fluid and microneedles technology. Pharmaceutics. 2017;9:43. doi: 10.3390/pharmaceutics9040043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostov Y., Ge X.D., Rao G., Tolosa L. Portable system for the detection of micromolar concentrations of glucose. Meas. Sci. Technol. 2014;25:025701. doi: 10.1088/0957-0233/25/2/025701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei B.U.W., Prow T.W. A review of microsampling techniques and their social impact. Biomed. Microdevices. 2019;21:81. doi: 10.1007/s10544-019-0412-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo M.S., Lev-Tov H.A., Kamangar F., Maibach H., Sivamani R. Efficacy and Toxicity of Microneedle-Based Devices. In: Shah V., Maibach H., Jenner J., editors. Topical Drug Bioavailability, Bioequivalence, and Penetration. Springer-Verlag; New York: 2014. [Google Scholar]
- Li T., Barnett A., Rogers K.L., Gianchandani Y.B. A blood sampling microsystem for pharmacokinetic applications: design, fabrication, and initial results. Lab Chip. 2009;9:3495–3503. doi: 10.1039/b910508e. [DOI] [PubMed] [Google Scholar]
- Li C.G., Lee C.Y., Lee K., Jung H. An optimized hollow microneedle for minimally invasive blood extraction. Biomed. Microdevices. 2013;15:17–25. doi: 10.1007/s10544-012-9683-2. [DOI] [PubMed] [Google Scholar]
- Li Y., Young D.J., Loh X.J. Fluorescent gels: a review of synthesis, properties, applications and challenges. Mater. Chem. Front. 2019;3:1489–1502. [Google Scholar]
- Liang X., Boppart S.A. Biomechanical properties of in vivo human skin from dynamic optical coherence elastography. IEEE Trans. Biomed. Eng. 2010;57:953–959. doi: 10.1109/TBME.2009.2033464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcvey E., Sutter D., Rini C., Nosek L., Kapitza C., Rebrin K., Pettis R. Intradermal insulin infusion achieves faster insulin action than subcutaneous infusion for three day wear. Diabetes Technol. Ther. 2014;16:A27–A28. [Google Scholar]
- Mukerjee E., Collins S.D., Isseroff R.R., Smith R.L. Microneedle array for transdermal biological fluid extraction and in situ analysis. Sens. Actuators A Phys. 2004;114:267–275. [Google Scholar]
- Nicolson P.C., Vogt J. Soft contact lens polymers: an evolution. Biomaterials. 2001;22:3273–3283. doi: 10.1016/s0142-9612(01)00165-x. [DOI] [PubMed] [Google Scholar]
- Nyein H.Y.Y., Tai L.C., Ngo Q.P., Chao M., Zhang G.B., Gao W., Bariya M., Bullock J., Kim H., Fahad H.M., Javey A. A wearable microfluidic sensing patch for dynamic sweat secretion analysis. ACS Sens. 2018;3:944–952. doi: 10.1021/acssensors.7b00961. [DOI] [PubMed] [Google Scholar]
- Paliwal S., Hwang B.H., Tsai K.Y., Mitragotri S. Diagnostic opportunities based on skin biomarkers. Eur. J. Pharm. Sci. 2013;50:546–556. doi: 10.1016/j.ejps.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Park H.I., Park S.Y. Smart fluorescent hydrogel glucose biosensing microdroplets with dual-mode fluorescence quenching and size reduction. Acs Appl. Mater. Interfaces. 2018;10:30172–30179. doi: 10.1021/acsami.8b10768. [DOI] [PubMed] [Google Scholar]
- Park J.H., Prausnitz M.R. Analysis of the mechanical failure of polymer microneedles by axial force. J. Korean Phys. Soc. 2010;56:1223–1227. doi: 10.3938/jkps.56.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.H., Allen M.G., Prausnitz M.R. Biodegradable polymer microneedles: fabrication, mechanics and transdermal drug delivery. J. Controll. Release. 2005;104:51–66. doi: 10.1016/j.jconrel.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Park J.H., Yoon Y.K., Choi S.O., Prausnitz M.R., Allen M.G. Tapered conical polymer microneedles fabricated using an integrated lens technique for transdermal drug delivery. IEEE Trans. Biomed. Eng. 2007;54:903–913. doi: 10.1109/TBME.2006.889173. [DOI] [PubMed] [Google Scholar]
- Parlak O., Keene S.T., Marais A., Curto V.F., Salleo A. Molecularly selective nanoporous membrane-based wearable organic electrochemical device for noninvasive cortisol sensing. Sci. Adv. 2018;4:eaar2904. doi: 10.1126/sciadv.aar2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandani N., Arya S., Ten S., Bhandari S. Real-life utilization of real-time continuous glucose monitoring: the complete picture. J. Diabetes Sci. Technol. 2011;5:860–870. doi: 10.1177/193229681100500407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckh T.T., Clark H.A. Implantable nanosensors: toward continuous physiologic monitoring. Anal. Chem. 2014;86:1314–1323. doi: 10.1021/ac402688k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery D., Choudhary P. Clinical use of continuous glucose monitoring in adults with type 1 diabetes. Diabetes Technol. Ther. 2017;19:S55–S61. doi: 10.1089/dia.2017.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieglitz T., Schuettler M. 2-Material–tissue interfaces in implantable systems. In: Inmann A., Hodgins D., editors. Implantable Sensor Systems for Medical Applications. Woodhead Publishing; 2013. pp. 39–67. [Google Scholar]
- Sullivan S.P., Koutsonanos D.G., Martin M.D., Lee J.W., Zarnitsyn V., Choi S.O., Murthy N., Compans R.W., Skountzou I., Prausnitz M.R. Dissolving polymer microneedle patches for influenza vaccination. Nat. Med. 2010;16:915–920. doi: 10.1038/nm.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan B., Zhao H.M., Du L., Gan X.R., Quan X. A versatile fluorescent biosensor based on target-responsive graphene oxide hydrogel for antibiotic detection. Biosens. Bioelectron. 2016;83:267–273. doi: 10.1016/j.bios.2016.04.065. [DOI] [PubMed] [Google Scholar]
- Toozs-Hobson P., Al-Singary W., Fynes M., Tegerstedt G., Lose G. Two-year follow-up of an open-label multicenter study of polyacrylamide hydrogel (Bulkamid(R)) for female stress and stress-predominant mixed incontinence. Int. Urogynecol. J. 2012;23:1373–1378. doi: 10.1007/s00192-012-1761-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P.M., Cornwell M., Prausnitz M.R. Minimally invasive extraction of dermal interstitial fluid for glucose monitoring using microneedles. Diabetes Technol. Ther. 2005;7:131–141. doi: 10.1089/dia.2005.7.131. [DOI] [PubMed] [Google Scholar]
- Tsuchiya K., Isobata K., Sato M., Uetsuji Y., Nakamachi E., Kajiwara K., Kimura M. Design of painless microneedle for blood extraction system. Biomems Nanotechnology III. 2007;6799 [Google Scholar]
- Wang P.M., Cornwell M., Hill J., Prausnitz M.R. Precise microinjection into skin using hollow microneedles. J. Invest. Dermatol. 2006;126:1080–1087. doi: 10.1038/sj.jid.5700150. [DOI] [PubMed] [Google Scholar]
- Welsh J.B., Gao P., Derdzinski M., Puhr S., Johnson T.K., Walker T.C., Graham C. Accuracy, utilization, and effectiveness comparisons of different continuous glucose monitoring systems. Diabetes Technol. Ther. 2019;21:128–132. doi: 10.1089/dia.2018.0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh J.B., Zhang X., Puhr S.A., Johnson T.K., Walker T.C., Balo A.K., Price D. Performance of a factory-calibrated, real-time continuous glucose monitoring system in pediatric participants with type 1 diabetes. J. Diabetes Sci. Technol. 2019;13:254–258. doi: 10.1177/1932296818798816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R.A., Mamotte C.D., Burnett J.R. Phenylketonuria: an inborn error of phenylalanine metabolism. Clin. Biochem. Rev. 2008;29:31–41. [PMC free article] [PubMed] [Google Scholar]
- Windmiller J.R., Zhou N.D., Chuang M.C., Valdes-Ramirez G., Santhosh P., Miller P.R., Narayan R., Wang J. Microneedle array-based carbon paste amperometric sensors and biosensors. Analyst. 2011;136:1846–1851. doi: 10.1039/c1an00012h. [DOI] [PubMed] [Google Scholar]
- Yan G.A., Warner K.S., Zhang J., Sharma S., Gale B.K. Evaluation needle length and density of microneedle arrays in the pretreatment of skin for transdermal drug delivery. Int. J. Pharmaceutics. 2010;391:7–12. doi: 10.1016/j.ijpharm.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Yang S.Y., O'cearbhaill E.D., Sisk G.C., Park K.M., Cho W.K., Villiger M., Bouma B.E., Pomahac B., Karp J.M. A bio-inspired swellable microneedle adhesive for mechanical interlocking with tissue. Nat. Commun. 2013;4:1702. doi: 10.1038/ncomms2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J.C., Zhang Y.Q., Ye Y.Q., Disanto R., Sun W.J., Ranson D., Ligler F.S., Buse J.B., Gu Z. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc. Natl. Acad. Sci. U S A. 2015;112:8260–8265. doi: 10.1073/pnas.1505405112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn J.D., Talbot N.H., Liepmann D., Pisano A.P. Microfabricated polysilicon microneedles for minimally invasive biomedical devices. Biomed. Microdevices. 2000;2:295–303. [Google Scholar]
- Zahn J.D., Trebotich D., Liepmann D. Microdialysis microneedles for continuous medical monitoring. Biomed. Microdevices. 2005;7:59–69. doi: 10.1007/s10544-005-6173-9. [DOI] [PubMed] [Google Scholar]
- Zhao X.H. Designing toughness and strength for soft materials. Proc. Natl. Acad. Sci. U S A. 2017;114:8138–8140. doi: 10.1073/pnas.1710942114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J.H., Rossi J. Aptamers as targeted therapeutics: current potential and challenges. Nat. Rev. Drug Discov. 2017;16:181–202. doi: 10.1038/nrd.2016.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.