Human colonic mucosa is covered with a mucus layer several hundred microns in thickness, which serves as a barrier physically separating the underlying epithelium from bacteria, and hindering the movement of molecules derived from food and microbiota.1, 2, 3 Despite its importance, a thick, continuous mucus layer that mimics in vivo functions has not been achieved in in vitro colonic epithelial models constructed from primary intestinal epithelial cells. The organoid “mini-gut” model possesses goblet cells, but mucus is secreted and accumulated within the organoids’ inaccessible lumen making the mucus properties difficult to quantify.4, 5 Prior monolayer models exposed the tissue luminal surface by culturing primary cells on scaffolds, but the mucus layer was very thin and diffuse and unable to act as a physiologic barrier.6, 7 In this study, we report strategies to generate a thick mucus layer and demonstrate its function act as a physiological barrier to bacteria and toxins.
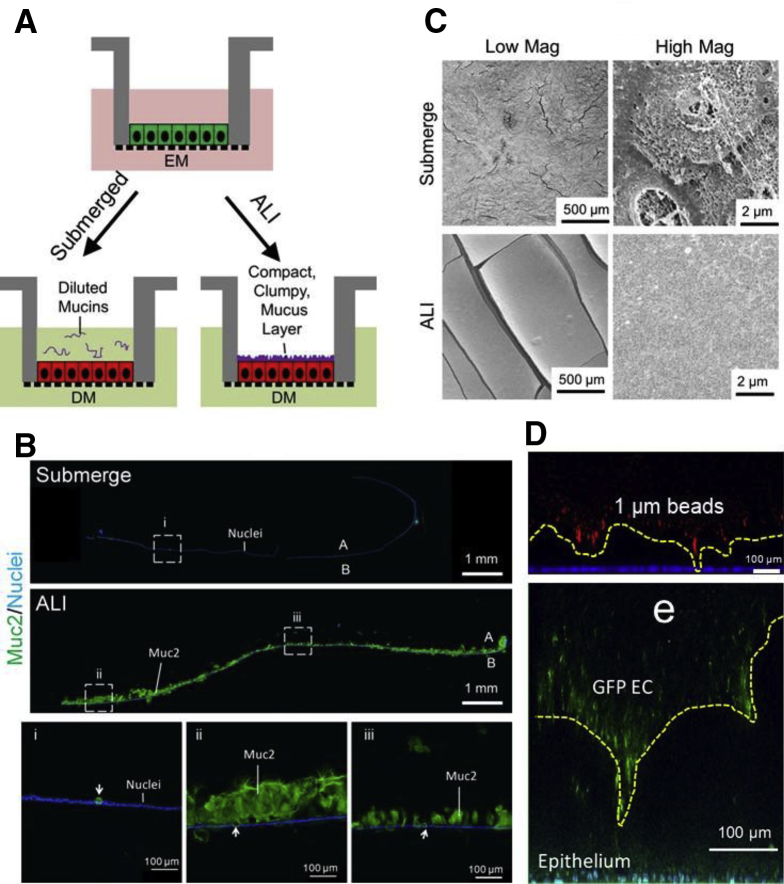
An air-liquid interface (ALI) similar to that used for respiratory cell culture8 was tested based on the hypothesis that overlying aqueous medium with convective mixing might dilute the mucus as it formed. Removal of the fluid might then enable accumulation of a thick, condensed mucus. Human colonic epithelial monolayers were differentiated for 5 days as either a submerged or as an ALI culture (Supplementary Figure 1A). A contiguous mucus layer with irregular thickness (71–381 μm) was observed in the ALI culture by immunofluorescence (Supplementary Figure 1B, D) but was absent from the submerged culture. The mucus layer appeared to fully cover the epithelium when viewed by scanning electron microscopy (Supplementary Figure 1C). Additionally, the mucus layer served as a barrier to 1-μm red fluorescent beads and green fluorescent protein–expressing Escherichia coli separating them from the epithelium (Supplemental Figure 1D).
Supplemental Figure 1.
Air-liquid interface (ALI) culture generates a compact, clumped-appearing mucus layer. (A) Schematic of culture formats. (B) Immunofluorescence staining of sectioned monolayers. Green: Muc2; blue: Hoechst 33342. White arrows indicate goblet cells. (C) Scanning electron microscopy images. Top panel: submerged culture. Bottom panel: ALI culture. (D) The mucus layer was overlaid with 1-μm red fluorescent beads (top, red) or GFP-expressing EC (bottom, green). The nuclei of intestinal cells were stained with Hoechst 33342 (blue/aqua). The yellow dashed line shows the boundary between the mucus and microbeads or EC. A, apical; B, basal.
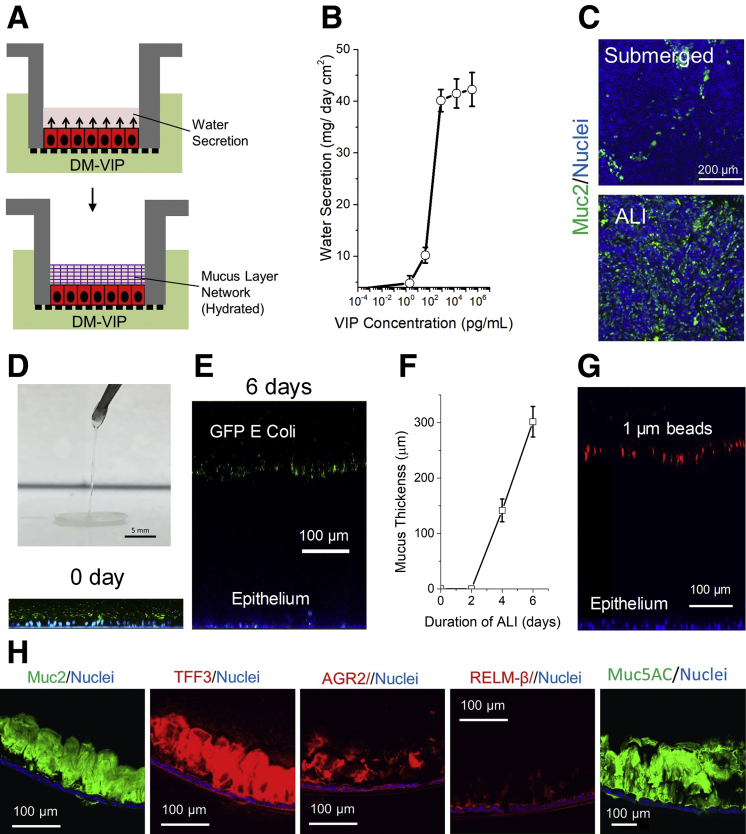
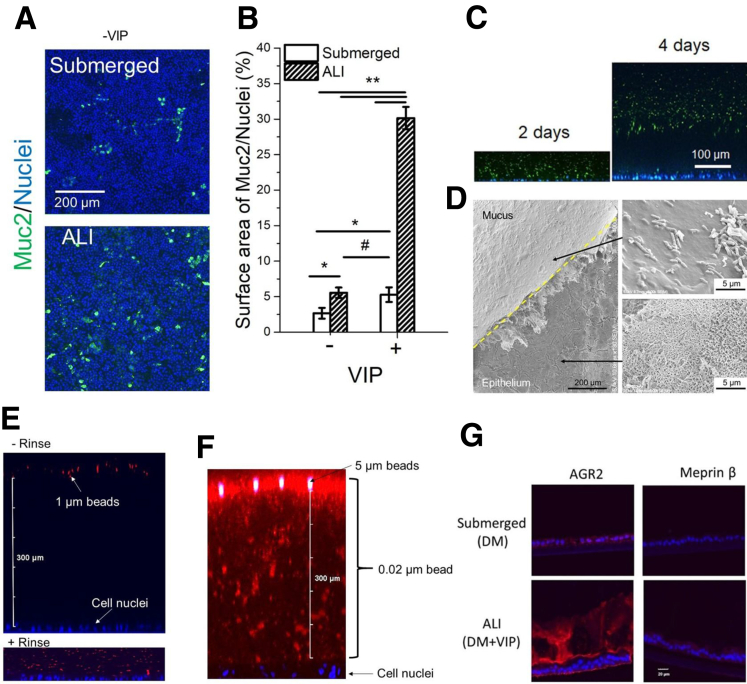
The ALI culture was modified by adding an endogenous intestinal hormone, vasoactive intestinal peptide (VIP),9, 10 to mediate the luminal water homeostasis with a goal of supporting hydrated mucus layer of uniform thickness (Figure 1A). VIP stimulated the apical water secretion in a dose-dependent manner with an effective dose 50 of 210 pg/mL (Figure 1B). When the monolayers were differentiated under ALI culture with VIP, a layer of liquid (42 mg/cm2) accumulated on the apical side within 24 hours and was maintained during 5 days of differentiation. The ALI culture with VIP significantly increased the number of goblet cells (Figure 1C; Supplementary Figure 2A, B). The apical mucus volume increased in quantity over time and by day 5 a slippery hydrogel could be lifted off the epithelium (Figure 1D). The thickness of the bacteria-separating mucus layer was readily adjusted by the duration of ALI culture (Figure 1E, F; Supplemental Figure 2C). The thickness of the mucus was even across the surface (302 ± 28 μm), likely due to its ability to flow and redistribute as a result of its water content unlike that in the ALI culture without VIP. The mucus effectively separated 1-μm red fluorescent beads from the underlying epithelium (Figure 2G, Supplemental Figure 2E). Scanning electron microscopy images additionally demonstrated a confluent layer of uninterrupted mucus upon which microbes could be visualized (Supplemental Figure 2D). The mucus layer contained other expected constituents including Muc5AC, TFF3, and AGR2 (Figure 1H; Supplementary Figure 2G). As the single mucus layer acted as barrier to microbes, its properties resembled that of the compact or microbe-impermeant layer observed in vivo. However, vigorous washing removed the mucus layer entirely, a property most closely associated with the diffuse mucus layer bordering the luminal contents in vivo (Supplementary Figure 2E). Overlaying the mucus layer with a mixture of 0.02- and 5-μm beads suggested the presence of a single layer (Supplementary Figure 2F).
Figure 1.
VIP-assisted ALI culture generates physiologic mucus layer above intestinal epithelial cells. (A) Culture schematic. (B) Dose-dependent water secretion of VIP at 24 hours. (C) Immunofluorescence of monolayers differentiated under submerged and ALI conditions in the presence of VIP. Green: anti-Muc2; blue: Hoechst 33342. (D) Removal of hydrated mucus with forceps. (E) Side-view confocal micrograph showing tissues with mucus separating bacteria from epithelial cells at 0 and 6 days, respectively. Green: green fluorescent protein (GFP)–expressing Escherichia coli; blue: Hoechst 33342. (F) Plot of mucus thickness vs duration of ALI. (G) Representative side-view confocal micrograph showing 1 μm red fluorescent beads separated from epithelial cells by mucus. (H) Immunofluorescence staining of paraffin-embedded, sectioned monolayers. DM, differentiation medium.
Supplemental Figure 2.
(A, B) Effect of culture method and VIP (0 and 330 ng/mL) on the number of goblet cells. (A) Immunofluorescence images of monolayers stained for Muc2 (green). (B) The percentage of the monolayer surface area positive for Muc 2 fluorescence. *P < .05; **P < .005; #not statistically significant. n = 3. (C, D) The hydrated mucus layer generated by VIP-assisted air-liquid interface (ALI) culture. (C) Side-view confocal micrograph showing tissues with bacteria-separating mucus accumulation at 2 and 4 days, respectively. Green: GFP-expressing EC; blue: Hoechst 33342. (D) Scanning electron microscopy of hydrated mucus layer (overlaid with GFP-expressing EC). The mucus layer was partially removed using tweezers to reveal the epithelium (lower arrow) below the mucus. The upper right panel shows bacteria (rod-shaped structures) on the surface of the mucus layer. (E–G) Characterizations of the hydrated mucus layer generated by VIP-assisted ALI culture. (E) Visualization of the mucus layer by overlaying 1-μm red fluorescent beads. Top: adding beads to the mucus layer without rinsing. Bottom: adding beads to the mucus layer after gently rinsing. (F) The mucus layer was overlaid with a mixture of 0.02-μm red fluorescent beads (#F8786; ThermoFisher) and 5_μm green fluorescent beads (#G0500; ThermoFisher) for 4 hours. (G) Immunofluorescence of sectioned monolayers. Red: AGR2 or Meprin β; blue: Hoechst 33342. There was minimal Meprin β expression, as was expected because the tissue is derived from the large and not small intestine and the β subunit is not predominant in the large intestine.8
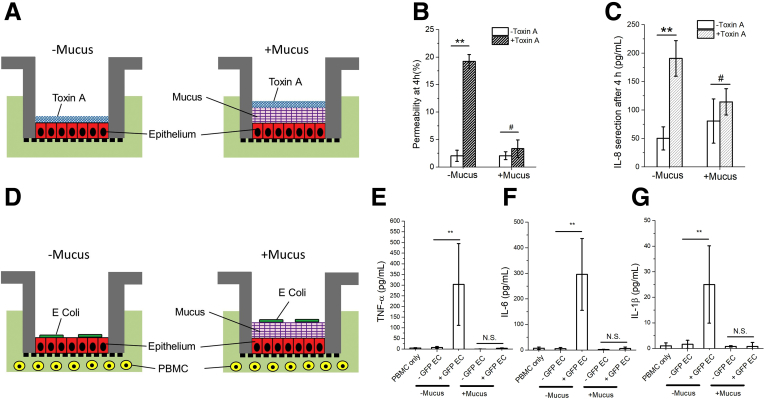
Figure 2.
Effect of Clostridium difficile toxin A and Escherichia coli (EC) on human colonic epithelium in the absence or presence of the VIP-enhanced mucus layer. (A) Cell-culture schematic for panels B and C. (B) Permeability and (C) IL-8 secretion of the epithelium after 4-hour exposure to toxin A. (D) Cell culture schematic for panels E, F, and G. (E–G) Production of inflammatory cytokines after a 24-hour co-culture of green fluorescent protein (GFP)–expressing EC, epithelium, and peripheral blood mononuclear cells (PBMC): (E) tumor necrosis factor alpha (TNF-α), (F) IL-6, and (G) IL-1β. Unpaired t test: **P < .005; #not statistically significant. n = 3 samples per condition. N.S., not significant.
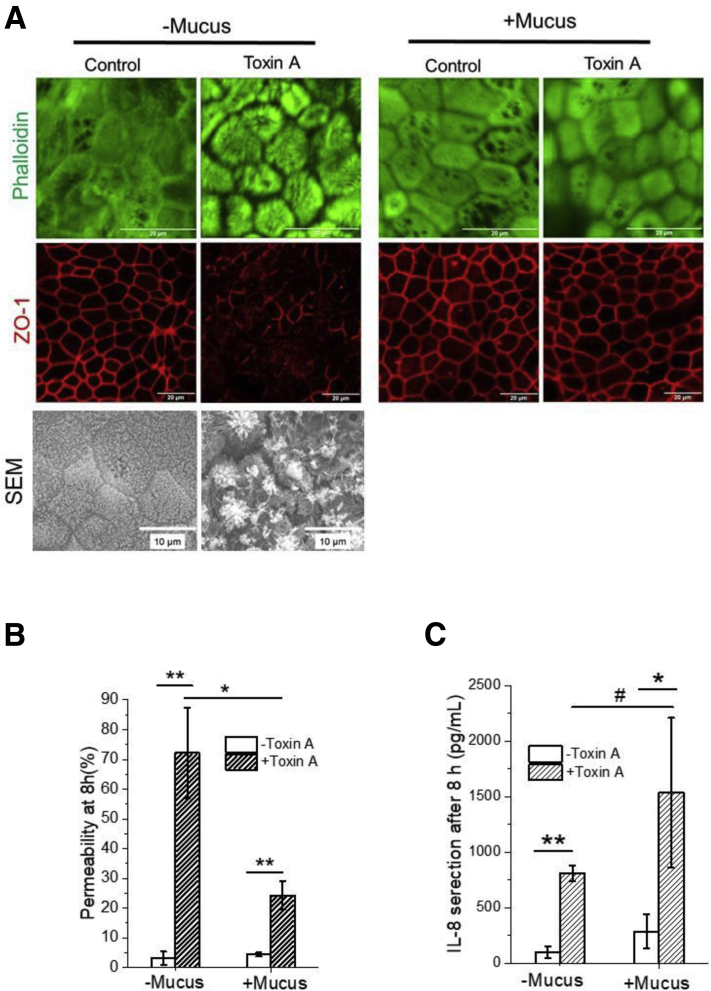
To demonstrate the physiological barrier function of the mucus, intestinal epithelial monolayers were incubated with Clostridium difficile toxin A (Figure 2A) or E coli (Figure 2D) in the absence or presence of a mucus layer. In the absence of a mucus layer, toxin A disrupted apical F-actin structures and zonula occludens-1 tight junctions, diminished epithelial barrier function, and elicited epithelial-cell interleukin (IL)-8 secretion by 4 hours (Supplementary Figure 3A). In the presence of a mucus layer, the epithelial cells were protected from the destructive effects of toxin at 4 hours (Figure 2B, C). Although by 8 hours, the tissue demonstrated signs of toxin exposure (Supplementary Figure 3B, C). The in vitro mucus layer also served as a physical barrier to segregate E coli from the epithelium and underlying white blood cells for up to 24 hours with elimination of cytokine production (tumor necrosis factor alpha, IL-6, and IL-1β) in the presence of the mucus layer (Figure 2E–G). These results demonstrate that the mucus layer successfully slowed toxin movement, delaying intestinal damage, and acted as a physical barrier for bacteria, delaying or eliminating the immune response and emulating in vivo conditions.
Supplemental Figure 3.
Effect of Clostridium difficile toxin A on human colonic epithelium in the absence or presence of the VIP-enhanced mucus layer. (A) Confocal microscopic and Scanning electron microscopy images of F-actin (top panel) and zonula occludens-1 tight junction (middle panel). (B) Permeability and (C) IL-8 secretion of epithelium after 8-hour exposure to toxin A. Unpaired t test: *P < .05; **P < .005; #not statistically significant. n = 3. Scale bar = 20 μm.
Acknowledgements
The authors thank the Scott Magness Laboratory for kindly providing human colon specimens. The authors also thank Shee-Hwan Hwang and Mallory Maurer for technical assistance.
Footnotes
Conflicts of interest These authors disclose the following: Yuli Wang, Christopher E. Sims, and Nancy L. Allbritton have a financial interest in Altis Biosystems, Inc. The remaining author discloses no conflicts.
Funding Research reported in this publication was supported by the National Institutes of Health under Award R01DK109559 to Nancy L. Allbritton. This work was performed in part at the Chapel Hill Analytical and Nanofabrication Laboratory, a member of the North Carolina Research Triangle Nanotechnology Network, which is supported by the National Science Foundation (Grant ECCS-1542015) as part of the National Nanotechnology Coordinated Infrastructure.
Supplemental Information
Methods
Cell culture and generation of a mucus layer
The human-derived colonic epithelial stem cells were expanded in stem medium (SM) (formulation is listed in Supplementary Table 1) using a previously published protocol.1, 2, 3 Twelve-well Transwell inserts possessing a porous membrane (0.4-μm pore size) (#3460; Corning, Corning, NY) were coated with 1 vol% Matrigel in ice cold phosphate-buffered saline at 37°C overnight and rinsed with phosphate-buffered saline. Cells were plated as described previously in EM.3 The medium was switched to differentiation medium (DM) after 5 days.3 In the submerged culture, 1-mL DM was added in the apical reservoir and 2 mL was added in the basal reservoir. In the air-liquid interface culture, the medium in the apical reservoir was completely aspirated, 1-mL DM or DM vasoactive intestinal peptide (VIP) (DM containing 330-ng/mL VIP [#AS-22872; AnaSpec, Fremont, CA]) was added to the basal reservoir. DM or DM-VIP was changed daily. Cells were cultured for 10 days before assay.
Characterization of colonic epithelium and mucus layer
The cells and mucus layer were fixed with Carnoy’s solution (ethanol 6:acetic acid 3:chloroform 1, v/v/v) at 4°C for 2 hours, dehydrated in ethanol, paraffin-embedded, sectioned, and stained with anti-Muc2 antibody (#sc-15334, 200×; Santa Cruz Biotechnology, Dallas, TX) and Hoechst 33342 (#62249; ThermoFisher)2 or antibodies against TFF3 (#PA557279, 100×; ThermoFisher), AGR2 (#PA534517, 100×; ThermoFisher), RELM-β (#PA561896, 100×; ThermoFisher) and MUC5AC (#MA512178, 100×; ThermoFisher).4, 5, 6 For scanning electron microscopy imaging, the tissues were fixed with Carnoy’s solution or glyoxal at 4°C for 2 hours, dehydrated in a graded ethanol dried with a critical point dryer (Tousimis Semidri PVT-3, Rockville, MD), coated with 10-nm metal by a sputter coater (Cressington 108, Watford, England, UK), and inspected by scanning electron microscopy (FEI Quanta 200 ESEM; FEI, Hillsboro, OR).
To demonstrate the barrier capability, the cells were stained with Hoechst 33342 (2 μg/mL) from basal side and then green fluorescent protein–expressing Escherichia coli (EC) (0.5 mL) (#25922GFP; ATCC, Manassas, VA) at 200 million colony-forming units (CFU)/mL, or red fluorescent beads (1-μm diameter) (#F13083; ThermoFisher) at a density of 108 beads/mL was added to the apical reservoir. Twenty minutes after seeding, the Transwell insert were imaged by confocal microscopy.
Toxin A experiment
A mixture (20 μL) of natural Clostridium difficile toxin A protein (12 μg/mL) (#ab123999; Abcam, Cambridge, UK) and FITC-dextran (5 mg/mL) (#FD40S; Sigma-Aldrich, St Louis, MO) was added to the apical monolayer side. Hanks' balanced salt solution with calcium/magnesium, 10% fetal bovine serum (FBS), and 10-mM HEPES was added to the basal compartment. Samples (150 μL) were collected from the basal compartment at 2, 4, 8, and 24 hours. The fluorescence intensity of the collected samples was used to calculate the percent permeability. The cells were fixed in ethanol and stained with phalloidin (#R37110; ThermoFisher) and zonula occludens-1 antibody (#21773-1-AP; Proteintech Group, Rosemont, IL).
Co-culture with EC and peripheral blood mononuclear cells
Fresh human peripheral blood mononuclear cells (Physician's Plasma Alliance, Johnson City, TN) were suspended in RPMI containing 10% FBS and 100-μg/mL gentamicin at 2.86 million cells/mL. GFP-expressing EC (200 million CFU/mL) were washed and suspended in 0.2-mL RPMI with FBS and gentamicin (100 μg/mL).7 GFP-expressing EC (20 million CFU, 20 μL) was added to the apical epithelial side. Peripheral blood mononuclear cells (1.43 million, 500 μL) were added to the basal reservoir. After 24 hours, the basal reservoir media reservoir was collected, centrifuged (5000 rpm, 6 minutes), aliquoted, and stored at –20°C.
Quantification of cytokines
The concentrations of cytokines (interleukin [IL]-8, IL-6, IL-1β, and tumor necrosis factor alpha) were determined using enzyme-linked immunosorbent assay kits (ThermoFisher, Waltham, MA) (n = 3).
Supplementary Table 1.
Formulation of Culture Media for Human Colonic Epithelial Cells
| SM | EM | DM | DM-VIP | |
|---|---|---|---|---|
| WRN-conditioned medium | 50 vol% | 50 vol% | ||
| Advanced DMEM/F12 | 50 vol% | 50 vol% | 100 vol% | 100 vol% |
| GlutaMax | 1× | 1× | 1× | 1× |
| HEPES | 10 mM | 10 mM | 10 mM | 10 mM |
| Murine EGF | 50 ng/mL | 50 ng/mL | 50 ng/mL | 50 ng/mL |
| N-acetyl cysteine | 1.25 mM | 1.25 mM | 1.25 mM | 1.25 mM |
| Primocin | 50 μg/mL | 50 μg/mL | 50 μg/mL | 50 μg/mL |
| B27 | 1× | 1× | ||
| Gastrin | 10 mM | 10 mM | ||
| A83-01 | 500 nM | 500 nM | 500 nM | |
| SB202190 | 3 μM | 3 μM | ||
| Y-27632 | 10 μMa | 10 μMa | ||
| Nicotinamide | 10 mM | |||
| PGE2 | 10 nM | |||
| VIP | 330 ng/mL |
Advanced DMEM/F-12, Dulbecco's Modified Eagle Medium/Ham's F-12; DM, differentiation medium; EGF, epidermal growth factor; EM, expansion medium; WRN, Wnt-3A, R-spondin 3, Noggin; PGE2, prostaglandin E2; SM, stem medium; VIP, vasoactive intestinal peptide.
Used in the first 48 hours after cell plating to prevent dissociation-induced cell apoptosis.
References
- 1.Johansson M.E.V. Proc Natl Acad Sci U S A. 2011;108:4659–4665. doi: 10.1073/pnas.1006451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansson G.C. Curr Opin Microbiol. 2012;15:57–62. doi: 10.1016/j.mib.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Werlang C. Nat Rev Mater. 2019;4:134–145. [Google Scholar]
- 4.Sato T. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 5.Dedhia P.H. Gastroenterology. 2016;150:1098–1112. doi: 10.1053/j.gastro.2015.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.In J. Cell Mol Gastroenterol Hepatol. 2016;2:48–62.e3. doi: 10.1016/j.jcmgh.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moon C. Mucosal Immunol. 2014;7:818–828. doi: 10.1038/mi.2013.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prunieras M. J Investig Dermatol. 1983;81:S28–S33. [Google Scholar]
- 9.Schwartz C.J. J Clin Invest. 1974;54:536–544. doi: 10.1172/JCI107790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu X. PLoS One. 2015;10 [Google Scholar]
References
- 1.Wang Y. Cell Mol Gastroenterol Hepatol. 2017;4:165–182. doi: 10.1016/j.jcmgh.2017.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y. Cell Mol Gastroenterol Hepatol. 2018;5:113–130. doi: 10.1016/j.jcmgh.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y. Anal Chem. 2018;90:11523–11530. doi: 10.1021/acs.analchem.8b02835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madsen J. J Histochem Cytochem. 2007;55:505–513. doi: 10.1369/jhc.6A7100.2007. [DOI] [PubMed] [Google Scholar]
- 5.Zhao F. Dev Biol. 2010;338:270–279. doi: 10.1016/j.ydbio.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnes S.L. J Immunol. 2007;179:7012–7020. doi: 10.4049/jimmunol.179.10.7012. [DOI] [PubMed] [Google Scholar]
- 7.Parlesak A. Scand J Immunol. 2004;60:477–485. doi: 10.1111/j.0300-9475.2004.01495.x. [DOI] [PubMed] [Google Scholar]
- 8.Lottaz D. Eur J Biochem. 1999;259:496–504. doi: 10.1046/j.1432-1327.1999.00071.x. [DOI] [PubMed] [Google Scholar]