Highlights
-
•
We used fMRI to study young children with prenatal alcohol exposure (PAE).
-
•
We measured the functional connectome and its stability within and across participants.
-
•
The PAE group had similar graph theory metrics to controls.
-
•
The PAE group, but not controls, had increasing intra-participant stability with age.
-
•
Controls, but not the PAE group, had increasing inter-participant stability with age.
Keywords: Prenatal alcohol exposure, Young children, Brain development, Passive viewing fMRI, Functional connectome, Inter individual stability, Intra individual stability
Abstract
Prenatal alcohol exposure (PAE) can lead to altered brain function and structure, as well as lifelong cognitive, behavioral, and mental health difficulties. Previous research has shown reduced brain network efficiency in older children and adolescents with PAE, but no imaging studies have examined brain differences in young children with PAE, at an age when cognitive and behavioral problems often first become apparent. The present study aimed to investigate the brain's functional connectome in young children with PAE using passive viewing fMRI. We analyzed 34 datasets from 26 children with PAE aged 2–7 years and 215 datasets from 87 unexposed typically-developing children in the same age range. The whole brain functional connectome was constructed using functional connectivity analysis across 90 regions for each dataset. We examined intra- and inter-participant stability of the functional connectome, graph theoretical measurements, and their correlations with age. Children with PAE had similar inter- and intra-participant stability to controls. However, children with PAE, but not controls, showed increasing intra-participant stability with age, suggesting a lack of variability of intrinsic brain activity over time. Inter-participant stability increased with age in controls but not in children with PAE, indicating more variability of brain function across the PAE population. Global graph metrics were similar between children with PAE and controls, in line with previous studies in older children. This study characterizes the functional connectome in young children with PAE for the first time, suggesting that the increased brain variability seen in older children develops early in childhood, when participants with PAE fail to show the expected age-related increases in inter-individual stability.
1. Introduction
Children with prenatal alcohol exposure (PAE) can have difficulties with language, attention, learning, and behavior compared to unexposed children (Jacobson and Jacobson, 2002; Riley et al., 2011; Riley and McGee, 2005). Structural magnetic resonance imaging (MRI) studies have shown brain alterations underlying these difficulties in children with PAE, including decreased total brain volume (Donald et al., 2015; Guerri et al., 2009; Lebel et al., 2011; Moore et al., 2014; Nguyen et al., 2017), increased (Sowell et al., 2008b; Yang et al., 2012) and decreased (Robertson et al., 2016; Zhou et al., 2018; Zhou 2011) cortical thickness, and altered white matter microstructure (lower fractional anisotropy and/or higher mean diffusivity) (Fan et al., 2015; Lebel et al., 2008; Paolozza et al., 2017; Sowell et al., 2008a; Treit et al., 2013; Wozniak et al., 2009, 2006; Wozniak and Muetzel, 2011). Functional MRI studies show that PAE is associated with altered functional connectivity at rest in infants (Donald et al., 2016), children and youth (Fan et al., 2017; Little et al., 2018; Long et al., 2018; Wozniak et al., 2011), and adults (Santhanam et al., 2011), as well as both increased and decreased functional connectivity within brain networks during working memory tasks (Infante et al., 2017; Roussotte et al., 2012). However, no MRI studies have examined brain functional connectivity in young children with PAE (i.e., 2–6 years). Cognitive and behavioral problems in individuals with PAE can persist throughout life, but often first become apparent in early childhood (Chen et al., 2006; Emond et al., 2007). Identifying emerging differences in brain function during this critical stage is important to understanding the origins of later abnormalities.
Recently, the use of graph-theory based analysis to study brain development has attracted attention. This approach constructs a connectivity matrix representing brain regions and their structural or functional connections with each other, and then quantifies the topological features of this connectivity matrix, or graph (Achard et al., 2006; Bassett and Bullmore, 2006; Bullmore and Sporns, 2009). An increasing number of studies have investigated the development of the brain's functional connectome from infancy to adulthood (Cao et al., 2017; Huang et al., 2015; Supekar et al., 2009; Zhang et al., 2018; Zhao et al., 2019; Zuo et al., 2017). While functional brain abnormalities are evident in school-age children and youth with PAE, very little previous research has investigated how PAE alters the brain's functional connectome. One previous study showed reduced global efficiency and higher path length in youth aged 10–17 years with PAE compared to unexposed controls (Wozniak et al., 2013). A second study in a different sample (aged 7–17 years) found no differences in global brain metrics, but noted substantially more atypical values of global efficiency, clustering coefficient and characteristic path length in the PAE group than in controls (Wozniak et al., 2017). Functional connectome analysis has demonstrated whole brain network alterations in other neurodevelopmental disorders (Di Martino et al., 2014; Uddin, 2010; Uddin and Karlsgodt, 2018), and shows potential in clinical diagnosis and intervention (Castellanos et al., 2013). A better understanding of the functional connectome in young children with PAE would provide a more complete picture of the effects of PAE at the whole-brain level and potentially help inform future research on interventions.
In the present study, we aimed to investigate the functional connectome of young children with PAE using passive viewing fMRI. Unlike resting state fMRI that requires participants to lie still for several minutes with minimalm stimulus, passive viewing fMRI allows participant to watch videos during their scan. Because it provides a more natural condition, passive viewing can decrease head motion during data collection (Vanderwal et al., 2015), and it is increasingly used in children (Vanderwal et al., 2018, 2015; Xu et al., 2018). Several previous studies have successfully examined brain activity, variability, and/or network development using naturalistic-viewing fMRI (Emerson et al., 2015; Vanderwal et al., 2018, 2017, 2015), including in young children (Long et al., 2017; Rohr et al., 2018). Passive viewing fMRI has also been applied to young children and older adults with major depressive disorder and adults with autism spectrum disorder (ASD) (Bolton et al., 2018; Gaffrey et al., 2011; Guo et al., 2015). Here, we examined functional connectome measures including network efficiency and centrality, as well as inter- and intra-individual stability. We expected that young children with PAE would show decreased network efficiency and increased network path length compared to exposed controls, as seen in older childhood (Wozniak et al., 2013). Previous studies have not examined inter- or intra-participant stability in children with PAE, but based on higher rates of atypical metrics seen in older children with PAE (Wozniak et al., 2013) and higher variability in behavior (Ali et al., 2018), we expected to see lower inter- and intra-participant stability in the PAE group.
2. Materials and methods
2.1. Participants
This study recruited 52 children with PAE (26 males/26 females, aged 2.78 to 7.22 years) from Alberta, Canada. Participants with PAE were recruited via advertisements (print and online) in newsletters and parent groups (n = 18), early intervention services and diagnostic clinics (n = 25), and through Child and Family Services (n = 9). PAE was confirmed via child welfare files (containing information from birth families), social workers, police records, medical files, and semi-structured interviews with caregivers and birth families, where possible. No participants had been diagnosed with fetal alcohol spectrum disorder (FASD), the developmental disorder associated with PAE, because they are too young to be assessed in most diagnostic clinics in Alberta, which typically see children only after they are 7-years old. 153 unexposed typically-developing children (82 males/71 females; aged 1.97 to 8.04 years) were included from an ongoing study of typical brain development (Reynolds et al., 2019). These typically-developing controls were originally recruited from the Calgary community via advertisements and from the Alberta Pregnancy Outcomes and Nutrition (APrON) study, an ongoing research study that recruited women in pregnancy and is focused on child outcomes related to maternal nutrition and mental health in pregnancy (Kaplan et al., 2014). For recruitment details for the APrON study, please refer to (Kaplan et al., 2014). The absence of prenatal alcohol exposure in controls was verified by maternal report at multiple stages of pregnancy (Kaplan et al., 2014).
The study was originally planned with a longitudinal design, and all participants’ families were invited to return for subsequent scans approximately every six months before school age, and annually thereafter. After removing scans with severe head motion (see details in Data preprocessing), unfinished scans (any fMRI scans with < 250 vol), and scans where participants were asleep, a total of 249 fMRI datasets (215 datasets from 87 control participants, 34 datasets from 26 participants with PAE, less head motion, non-sleep and full 250 vol) were included in the present study (Table 1). Because data collection from the children with PAE began after the control data collection, fewer participants with PAE had multiple scans.
Table 1.
Demographics of the available datasets included in the current study, including the total number of participants, age, family income brackets (unit: Canadian dollar, 8 brackets from “under $25,000″ to “over $175,000″), full scale intelligence quotient (FSIQ), head motion (average relative frame-wise displacement), and the number of participants who had multiple scans. Age, family income, and FSIQ were significantly different between the two groups (age: PAE > Control, t = 3.27, p = 0.002; family income: PAE < Control, t = −6.06, p = 2.49E-08; FSIQ: PAE < Control, t = −5.28, p = 1.05E-06).
| Demographics of the datasets included in the current study | |||
|---|---|---|---|
| Controls | PAE | All Participants | |
| Number of participants | 87 (45 female) | 26 (15 female) | 113 (60 female) |
| Age* | 4.86 ± 1.21 | 5.57 ± 0.95 | 4.96 ± 1.20 |
| Age range | 3.35 - 6.97 | 1.97 - 8.00 | 1.97 - 8.00 |
| Family income (median bracket)* | $125,000–149,999/year | $75,000–99,999/year | $125,000–149,999/year |
| FSIQ* | 109 ± 12 | 93 ± 12 | 104 ± 14 |
| Head Motion (mm) | 0.21 ± 0.18 | 0.24 ± 0.29 | 0.22 ± 0.20 |
| One Scan | 38 (44%) | 19 (73%) | 57 (50%) |
| Two Scans | 12 (14%) | 6 (23%) | 18 (16%) |
| Three Scans | 14 (16%) | 1 (4%) | 15 (13%) |
| Four Scans | 11 (13%) | / | 11 (10%) |
| Five Scans | 7 (8%) | / | 7 (6%) |
| Six Scans | 3 (3%) | / | 3 (3%) |
| Seven Scans | 2 (2%) | / | 2 (2%) |
| Total Scans | 215 | 34 | 249 |
Parents or legal guardians of all participants provided written informed consent, and all participants provided verbal assent. The study was approved by the conjoint health research ethics board at the University of Calgary.
2.2. MRI parameters
Imaging was conducted on a GE 3T MR750w (General Electric, Waukesha, WI) scanner equipped with a 32-channel head-coil at the Alberta Children’s Hospital in Calgary, Canada. Children were not sedated, and were watching a movie of their choice for the entire duration of the MRI scan. Headphones were equipped to receive movie audio and to communicate with scanning staff. T1-weighted data acquisition used an FSPGR BRAVO sequence with flip angle = 12º, 210 slices, TR = 8.23 ms, TE = 3.76 ms, voxel size = 0.9 × 0.9 × 0.9 mm, matrix size = 512 × 512, inversion time = 540 ms. Data acquisition for fMRI used a gradient- echo-planar imaging (EPI) sequence with TR = 2 s, TE = 30 ms, flip angle = 60º, 36 slices, voxel size = 3.59 × 3.59 × 3.6 mm, matrix size = 64 × 64, 250 vol, total scan time 8:10 min. The entire protocol including other modalities lasted 45–60 min, depending on whether sequences needed to be repeated. In some cases, children requested that the scan be stopped, or they were moving too much to collect high quality data, and the scan was stopped early.
2.3. Data preprocessing
Data preprocessing was completed in AFNI and FSL (Cox, 1996; Jenkinson et al., 2012). Each participant's T1-weighted image was skull stripped and segmented into gray matter, white matter, and cerebrospinal fluid (CSF) images and co-registered to their individual fMRI space. Total brain volume was calculated as the sum of gray and white matter volumes and used as a covariate due to its potential effect on the graph measures (Yan et al., 2011). The first 10 volumes of the passive viewing fMRI data were removed to allow for environmental adaptation and signal stabilization; 240 volumes remained. Correction of slice timing and head motion were performed. The averaged relative frame-wise displacement (FD) was calculated for further analysis (Jenkinson et al., 2002). A 36-parameter matrix was created from the averaged signals from the individual whole brain mask, CSF mask, white matter mask, the six head motion parameters, and their temporal derivatives and quadratic term signals (Satterthwaite et al., 2013). A spike matrix was created from volumes that had high relative FD (> 0.3 mm) (Power et al., 2014). Then the combination of the 36-parameter matrix and the spike matrix were regressed out of the fMRI signals. Datasets with spike volumes longer than 4 min (i.e., less than 4 min of low-motion data) were excluded from the study. Finally, the residual fMRI signals were band-pass filtered (0.009 to 0.08 Hz), underwent linear-trend removal, spatially transformed to MNI standard space using a pediatric template aged from 4.5 to 8.5 years (Fonov et al., 2011), and spatially smoothed with a 4 mm full width at half maximum kernel. Slice timing, head motion correction, T1-weighted image segmentation, head motion outlier detection, co-registration, and spatial normalization and smoothing were done in FSL (Jenkinson et al., 2012). Regression of the nuisance signals, band-pass filtering and linear trend removal were done using AFNI version AFNI_17.3.03 (Cox, 1996).
2.4. Functional connectome constructions
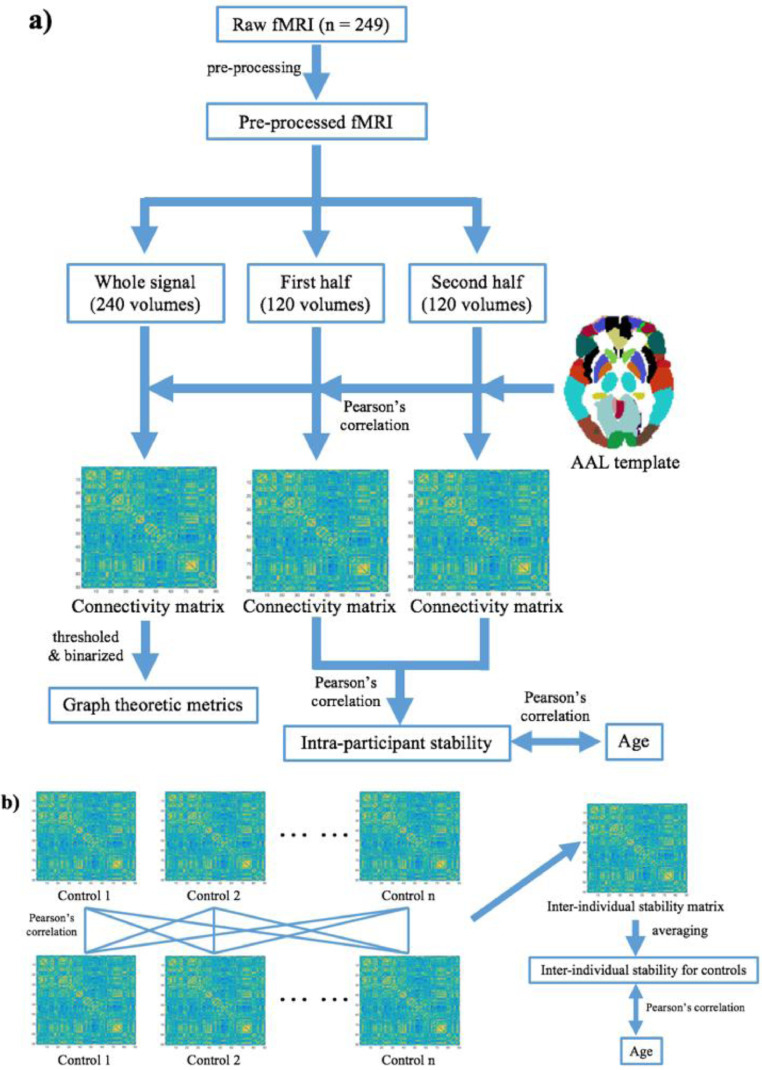
We used an adapted version of the stability analysis performed by Vanderwal and colleagues (Vanderwal et al., 2017) to investigate intra-individual stability of the functional connectome. This analysis involved splitting the fMRI signal into two halves for each participant and comparing the similarity between halves. To complete this, each participant's fMRI time series was split into two equal datasets with 120 time points each. Each half of the dataset, as well as the entire dataset (all time points), were separately run through the following functional connectome analysis in AFNI (Cox, 1996). The Automated Anatomical Labeling (AAL) template was used to subdivide each participant's brain into 90 regions excluding the cerebellum (Bullmore and Sporns, 2009; Kaiser, 2017; Rorden and Brett, 2000; Tzourio-Mazoyer et al., 2002). The average time series was calculated within each AAL region, and then Pearson's correlation coefficients were calculated between the averaged time series of each pair of AAL regions and transformed to Fisher's z scores to create a 90 × 90 connectivity matrix for each dataset (Fig. 1a). Thus, three connectivity matrices were produced for each participant: one on the first half of their data, one on the second half, and one for the entire dataset.
Fig. 1.
Schematic showing the functional connectome analysis pipeline. a) 90×90 connectivity matrices based on the AAL template were calculated for each participant based on the entire fMRI dataset, then the first half and the second half of each dataset separately. Intra-participant stability was calculated as the correlation between connectivity matrices from the first and the second half of the data. Correlations between intra-participant stability and age were examined for the PAE and control groups. c) To evaluate inter-individual stability, cross-correlation analysis was performed between entire dataset connectivity matrices for each participant within the same group, e.g., control group in the figure, and averaged to create one value per participant. These were examined for correlations with age in each group.
2.5. Analysis of the functional connectome
First, we examined intra-participant stability during movie watching by correlating connectivity matrices from the first and the second halves of the dataset for each participant (Fig. 1a). Correlation coefficients were transformed to Fisher's z scores and a permutation comparison test was performed between the PAE and control groups. Age, sex, head motion (i.e., averaged relative FD), and total brain volume were included as covariates. Linear correlations between age and the intra-participant stability metric were calculated for each group with sex, head motion, and total brain volume as covariates.
Second, inter-individual stability was examined within each group (Fig. 1b). Pearson's correlation coefficients were calculated between each subject's whole connectivity matrix and that of every other participant in the same group (i.e., PAE or controls). Then a 215 × 215 correlation matrix for the unexposed control group and a 34 × 34 correlation matrix for the PAE group were generated, and the average value of each row was calculated to generate an inter-individual stability value for each person in each dataset. A permutation comparison test was performed between the inter-individual stability of two groups with age, sex, head motion, and total brain volume as covariates. The relationship between inter-individual stability and age was examined with sex, head motion, and total brain volume as covariates.
2.6. Graph-theory based metrics calculation
As in previous studies, the graph theoretical measurements were calculated for the thresholded and binarized individual connectivity matrix of the whole time series (Wozniak et al., 2017, 2013). The threshold of the matrix was set at r = 0.17 as p < 0.01. The graph theoretic metrics (global and nodal level of clustering coefficient, shortest path length, small-worldness, local efficiency, global efficiency, betweenness centrality and network degree centrality) were calculated for each binary matrix using the GRETNA toolbox (Wang et al., 2015). Clustering coefficient measures the connection density among all neighbors of each node; shortest path length measures the fastest pathway to transfer information between any pair of nodes; sigma characterizes the ratio between clustering coefficient and shortest path length compared with the random networks; local efficiency measures the efficiency of transferring information from each node and its neighbors; global efficiency measures the efficiency of transferring information from each node to all other nodes in the whole graph, with higher efficiency indicating that fewer pathways are required to reach to other nodes in the network; betweenness centrality measures the intensity of the shortest pathway that passes each node; degree centrality measures the number of the connections of each node (Bassett and Bullmore, 2006; Buckner et al., 2009; Bullmore and Sporns, 2009; Humphries et al., 2006; Power et al., 2013; Rubinov and Sporns, 2010; Wang et al., 2010). Permutation tests between the two groups for each metric were performed controlling for age, sex, head motion, and total brain volume. The threshold for nodal metrics was set at p < 0.05. For completeness, we report results both uncorrected and corrected using false discovery rate (FDR) for multiple comparisons. We also analyzed metrics for linear correlations with age, and for group-age interactions. All permutation tests were performed in the GRETNA toolbox (Wang et al., 2015). The nodal level results were displayed by BrainNet Viewer toolbox (Xia et al., 2013).
3. Results
Children with PAE had significantly smaller total brain volume than controls: mean(PAE) = 1.08 × 106 mm3, mean(Controls) = 1.14 × 106 mm3; t = 2.70, p = 0.007.
Intra-participant stability between the first half and the second half of the data was high in both groups: z(PAE) = 0.82 ± 0.11; z(Controls) = 0.80 ± 0.11, and no significant difference was found between groups (p = 0.37). Intra-participant stability increased significantly with age only in the PAE group: r(PAE) = 0.36, p = 0.05; r(Controls) = 0.08, p = 0.27, and PAE > Controls at p = 0.12 (Fig. 2a).
Fig. 2.
a) The PAE group (blue), but not typical controls (black), had significant age-related increases of intra-participant stability during movie viewing fMRI. b) Controls (black) had significant age-related increases in inter-participant stability, but the PAE group (blue) did not. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Inter-individual stability was not significantly different between groups (p = 0.12): PAE = 0.40 ± 0.06; Controls = 0.39 ± 0.05. Inter-individual stability increased significantly with age in controls, but in not the PAE group: r(PAE) = 0.14, p < 0.44; r(Controls) = 0.16, p < 0.02; PAE < Controls at p = 0.91 (Fig. 2b).
No significant differences were detected in the global graph theoretical measurements between two groups (Table 2). Nodal level analysis detected distributed differences between groups, though none of the results survived FDR correction at p<0.05 (Fig. 1S, Table 1 and 2 in Supplementary materials). Because of the significant age difference between the groups, the graph theory analysis was repeated on the full PAE group compared to a subset of control participants who were closely age- and sex-matched (n = 34; age: 5.57 ± 0.95); group differences remained non-significant (all uncorrected p-values >0.07).
Table 2.
Group comparisons for whole brain graph theoretical measurements.
| Metrics | PAE | Controls | P value |
|---|---|---|---|
| Clustering coefficient | 0.59 ± 0.05 | 0.59 ± 0.06 | 0.27 |
| Shortest path length | 1.58 ± 0.04 | 1.58 ± 0.04 | 0.26 |
| Small-worldness | 0.87 ± 0.03 | 0.87 ± 0.02 | 0.26 |
| Local efficiency | 0.79 ± 0.03 | 0.79 ± 0.03 | 0.27 |
| Global efficiency | 0.64 ± 0.02 | 0.63 ± 0.02 | 0.25 |
| Betweenness centrality | 35.67 ± 1.93 | 35.84 ± 1.88 | 0.48 |
| Degree centrality | 27.26 ± 2.92 | 27.35 ± 3.32 | 0.17 |
4. Discussion
In this study of young children with PAE, we used graph theory methods to investigate functional network features. Our data showed similar network metrics as well as intra-participant and inter-participant stability of the functional connectome in children with PAE compared to controls. Children with PAE, but not controls, showed increasing intra-participant stability with age, but lacked the age-related increases in inter-participant stability seen in controls. Our results provide important context for previous findings of atypical connectome metrics across older children with PAE group (Wozniak et al., 2017), suggesting that increased variability originates in early childhood.
4.1. Stability of functional connectome during passive viewing
Intra-participant stability of the functional connectome was high (r = ~ 0.67) in both PAE and control groups during passive viewing fMRI. This level of stability is similar to a previous study in older typically-developing children (Vanderwal et al., 2017), which found correlations of functional connectivity matrices between the first and the second half of the data were approximately 0.60 when watching video clips. The high intra-participant stability in the PAE group was unexpected because children with PAE often have behavioral challenges, atypical neurocognitive responses to visual (Green et al., 2013; Paolozza et al., 2014), auditory (Stephen et al., 2012), and emotional stimuli (Petrenko et al., 2017), as well as altered functional connectivity among brain regions (Donald et al., 2016; Fan et al., 2017; Little et al., 2018; Long et al., 2018). It may be that movie viewing, as opposed to behavioral tasks, moderates the intra-individual variability in children with PAE and leads to higher stability. More challenging behavioral tasks may elicit more variable responses in children with PAE, while passive viewing is not sufficiently challenging to elicit high variability. This high stability of brain function suggests that naturalistic viewing fMRI could be a reliable paradigm for resting neuroimaging studies in young children, even those with PAE.
Previous resting-state fMRI studies in PAE instructed participants to close their eyes, or acquired data during natural sleep in infants, and found significant between-group differences in functional connectivity compared to unexposed controls (Donald et al., 2016; Little et al., 2018; Long et al., 2018; Wozniak et al., 2013; Wozniak, 2011). It is possible that the passive viewing status here, with its relatively high intra-individual stability, may be less taxing on the brain and thus may not show differences evident in more difficult cognitive tasks. Alternatively, there may be fewer differences in the brain's functional connectivity at the younger ages studied here.
Intra-participant connectome stability increased significantly with age in children with PAE, suggesting ongoing adjustment of functional networks during childhood. The unexposed controls, however, presented a flat trend of intra-participant stability. Increasing intra-individual stability with age in children with PAE may reflect decreased variability in cognitive processing during passive viewing. Electroencephalography (EEG) studies have shown that signal variability increases with age from childhood to adulthood in typically-developing individuals, and that higher variability (i.e., decreased stability) is associated with better task performance (Garrett et al., 2013; McIntosh et al., 2010). This suggests that variability in neuronal responses is essential to appropriate cognitive processing and the relative lack of variability may underlie impaired cognitive processing in children with PAE. Dramatic changes in cognition and behavior occur across childhood (Hutchison and Morton, 2015; Macdonald et al., 2006; Marusak et al., 2017; Qin et al., 2015), skills that may be impaired in children with PAE (Jacobson and Jacobson, 2002; Riley et al., 2011; Riley and McGee, 2005). Thus, network reorganization related to increasing intra-participant stability may reflect abnormal brain development underlying cognitive and behavioral difficulties in this population.
4.2. Inter-participant stability of functional connectome
Using cross-correlations between connectivity matrices (Li et al., 2017; Vanderwal et al., 2017), we found similar inter-individual stability in children with PAE and unexposed controls, but inter-individual stability in the PAE group did not show the age-related increases observed in controls. This may suggest that children with PAE have highly variable development rates, with some children showing faster brain development than others, perhaps associated with the extent of PAE. More similar development rates in controls would lead to increasing inter-individual stability, while more variable rates of brain development in children with PAE would keep inter-individual stability low relative to controls. Higher inter-individual variability in the human brain has been detected in other neurodevelopmental disorders, including adults with ASD (Humphreys et al., 2008) and young adults with attention deficit hyperactivity disorder (Depue et al., 2010). One study in older children with PAE showed more atypical functional connectome metrics in PAE compared to controls (Wozniak et al., 2017). For comparison purposes, we applied a similar analysis testing how many participants with PAE were >1 standard deviation from the mean of the whole sample. We found that 15% of the children with PAE in our study had atypical global functional connectome metrics (local and global efficiency, clustering coefficient and shortest path length), considerably lower than the 50% observed in older children and adolescents with PAE (Wozniak et al., 2017). Our current results showed greater inter-individual variability within the PAE group than in controls, and suggest divergent trajectories in the functional connectome, where stability across individuals increases with age in controls, but these increases are absent in individuals with PAE. This difference in development may be associated with the higher frequency of atypical functional connectomes in older children and adolescents with PAE. The lack of increasing inter-participant stability in the PAE group may reflect disordered functional network development that becomes more apparent with age as functional demand increases. Indeed, older children with PAE had altered functional brain network connectivity (Fan et al., 2017; Little et al., 2018; Long et al., 2018; Santhanam et al., 2011; Wozniak et al., 2011). The altered development of inter- and intra-individual stability may contribute to diverse cognitive impairments often observed in children with PAE (Kodituwakku, 2009). Though the relationship between network measures and cognitive function was not examined in the current study, previous studies have shown high correlations between network measures (e.g., shortest path length and network efficiency) and cognitive scores in older children with PAE (Wozniak et al., 2017, 2013).
It may be that brain networks of young children with PAE have difficulty reorganizing themselves during development due to impaired underlying structure. Widespread structural brain alterations have been reported in children with PAE (Donald et al., 2015; Lebel et al., 2011; Nguyen et al., 2017). Furthermore, altered development of white matter has been observed in older children with PAE (Gautam et al., 2015; Treit et al., 2013), who have differing trajectories of gray matter volume changes which suggest reduced brain plasticity (Lebel et al., 2012). Structural and functional connectivity both develop quickly in early childhood, though at different rates and with varying relationships to each other (Cao et al., 2017; Gilmore et al., 2018; Supekar et al., 2010). The current findings of abnormal development of functional networks in young children with PAE may coincide with altered structural development during this time period.
4.3. Graph theoretical measurements in preschool children with PAE
We found no significant differences in global functional connectome metrics in children with PAE. One of the two previous studies on the functional connectome in older children with PAE found significantly decreased global efficiency and increased shortest path length in children and youth with PAE (Wozniak et al., 2013), while the other study found no differences in mean global metrics (Wozniak et al., 2017). Our results in young children with PAE are in line with the latter study. Previous studies have demonstrated altered structural connectivty in children aged 5–13 years (Lebel et al., 2008) and infants with PAE (Donald et al., 2015), while other studies have shown abnormal functional connectivity in corticostriatal networks during a working-memory task (Roussotte et al., 2012) and natural sleep (Donald et al., 2016). No previous studies have investigated node-level changes in the functional connectome in PAE. We found distributed changes in frontal, motor and occipital cortex, yet none of them survived multiple comparison corrections, suggesting little-to-no difference in functional connectome nodes in youth children with PAE.
4.4. Limitations
There were several limitations in our study. This study used passive viewing fMRI, which can increase compliance relative to fixation on a cross or an eyes closed condition (Harned II and Strain, 2001; Vanderwal et al., 2015), especially in difficult-to-scan populations such as young children and children with PAE. However, children in our study were watching different movies and the details were not recorded for each scan. Thus, there may be increased variability between participants compared to passive viewing fMRI studies that use the same clips for all participants (Rohr et al., 2018; Vanderwal et al., 2018). The sample size of children with PAE was relatively small with few longitudinal scans, especially compared to the control group, which may reduce the reliability of the findings for the PAE group. The relationship between age and inter-individual stability in control group was significant, but relatively small (r = 0.16); future studies may help clarify whether this represents a meaningful change. The age range of participants is a strength for the age-related changes analysis, but developmental differences may also obscure group differences that would be apparent in a narrower age group. Future studies will help clarify functional brain differences in young children with PAE.
5. Conclusions
Here, we used passive-viewing fMRI to describe functional connectome development in young children with PAE. Children with PAE and unexposed controls both showed similar graph theory metrics, as well as intra- and inter-participant stability of their functional connectomes. Children with PAE showed age-related increases in intra-individual stability of functional connectomes, and no age-related changes in inter-individual stability. This suggests early origins of the higher inter-individual variability that exists in older children with PAE and these differences may underlie the altered brain function observed in PAE by resting-state and task-based fMRI studies.
Conflict of Competing Interest
CL's spouse is an employee of General Electric Healthcare
Acknowledgements
This work was supported by grants from the Alberta Children’s Hospital Research Institute (ACHRI) and the Kids Brain Health Network. Salary support was provided by the University of Calgary I3T program (XL), CIHR (CL), as well as the Hotchkiss Brain Institute and ACHRI (PK).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2019.102082.
Appendix. Supplementary materials
References
- Achard S., Salvador R., Brandon W., Suckling J., Bullmore E.T. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J. Neurosci. 2006;26:63–72. doi: 10.1523/JNEUROSCI.3874-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S., Kerns K.A., Mulligan B.P., Olson H.C., Astley S.J. An investigation of intra-individual variability in children with fetal alcohol spectrum disorder (FASD) Child Neuropsychol. 2018;24:617–637. doi: 10.1080/09297049.2017.1302579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett D.S., Bullmore E. Small-world brain networks. Neuroscience. 2006;12:512–523. doi: 10.1177/1073858406293182. [DOI] [PubMed] [Google Scholar]
- Bolton T.A.W., Jochaut D., Giraud A.-.L., Van De Ville D. Brain dynamics in ASD during movie-watching show idiosyncratic functional integration and segregation. Hum. Brain Mapp. 2018;39:2391–2404. doi: 10.1002/hbm.24009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L., Sepulcre J., Talukdar T., Krienen F.M., Liu H., Hedden T., Andrews-Hanna J.R., Sperling R.A., Johnson K.A. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J. Neurosci. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Cao M., Huang H., He Y. Developmental connectomics from infancy through early childhood. Trends Neurosci. 2017;40:494–506. doi: 10.1016/j.tins.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos F.X., Di Martino A., Craddock R.C., Mehta C.M., Milham M.P. Clinical applications of the functional connectome. Neuroimage. 2013;80:527–540. doi: 10.1016/j.neuroimage.2013.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., John P., Duggan A.K., Hardy J.B., Eaton W.W. Mild cognitive impairment in early life and mental health problems in adulthood. Am. J. Public Health. 2006;96:1772–1778. doi: 10.2105/AJPH.2004.057075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Depue B.E., Burgess G.C., Willcutt E.G., Bidwell L.C., Ruzic L., Banich M.T. Symptom-correlated brain regions in young adults with combined-type ADHD: their organization, variability, and relation to behavioral performance. Psychiatry Res. Neuroimaging. 2010;182:96–102. doi: 10.1016/j.pscychresns.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A., Fair D.A., Kelly C., Satterthwaite T.D., Castellanos F.X., Thomason M.E., Craddock R.C., Luna B., Leventhal B.L., Zuo X.N., Milham M.P. Unraveling the miswired connectome: a developmental perspective. Neuron. 2014;83:1335–1353. doi: 10.1016/j.neuron.2014.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald K.A., Eastman E., Howells F.M., Adnams C., Riley E.P., Woods R.P., Narr K.L., Stein D.J. Neuroimaging effects of prenatal alcohol exposure on the developing human brain: a magnetic resonance imaging review. Acta Neuropsychiatr. 2015;27:251–269. doi: 10.1017/neu.2015.12. [DOI] [PubMed] [Google Scholar]
- Donald K.A., Ipser J.C., Howells F.M., Roos A., Fouche J.P., Riley E.P., Koen N., Woods R.P., Biswal B., Zar H.J., Narr K.L., Stein D.J. Interhemispheric functional brain connectivity in neonates with prenatal alcohol exposure: preliminary findings. Alcohol. Clin. Exp. Res. 2016;40:113–121. doi: 10.1111/acer.12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson R.W., Short S.J., Lin W., Gilmore J.H., Gao W. Network-level connectivity dynamics of movie watching in 6-year-old children. Front. Hum. Neurosci. 2015;9:631. doi: 10.3389/fnhum.2015.00631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emond A., Ormel J., Veenstra R., Oldehinkel A.J. Preschool behavioral and social-cognitive problems as predictors of (Pre) adolescent disruptive behavior. Child Psychiatry Hum. Dev. 2007;38:221–236. doi: 10.1007/s10578-007-0058-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J., Meintjes E.M., Molteno C.D., Spottiswoode B.S., Dodge N.C., Alhamud A.A., Stanton M.E., Peterson B.S., Jacobson J.L., Jacobson S.W. White matter integrity of the cerebellar peduncles as a mediator of effects of prenatal alcohol exposure on eyeblink conditioning. Hum. Brain Mapp. 2015;36:2470–2482. doi: 10.1002/hbm.22785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J., Taylor P.A., Jacobson S.W., Molteno C.D., Gohel S., Biswal B.B., Jacobson J.L., Meintjes E.M. Localized reductions in resting-state functional connectivity in children with prenatal alcohol exposure. Hum. Brain Mapp. 2017;38:5217–5233. doi: 10.1002/hbm.23726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonov V., Evans A.C., Botteron K., Almli C.R., McKinstry R.C., Collins D.L., Group B.D.C. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage. 2011;54:313–327. doi: 10.1016/j.neuroimage.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffrey M.S., Luby J.L., Belden A.C., Hirshberg J.S., Volsch J., Barch D.M. Association between depression severity and amygdala reactivity during sad face viewing in depressed preschoolers: an fMRI study. J. Affect. Disord. 2011;129:364–370. doi: 10.1016/j.jad.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett D.D., Samanez-Larkin G.R., Macdonald S.W.S.S., Lindenberger U., McIntosh A.R., Grady C.L. Moment-to-moment brain signal variability: a next frontier in human brain mapping. Neurosci. Biobehav. Rev. 2013;37:610–624. doi: 10.1016/j.neubiorev.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam P., Lebel C., Narr K.L., Mattson S.N., May P.A., Adnams C.M., Riley E.P., Jones K.L., Kan E.C., Sowell E.R. Volume changes and brain-behavior relationships in white matter and subcortical gray matter in children with prenatal alcohol exposure. Hum. Brain Mapp. 2015;36:2318–2329. doi: 10.1002/hbm.22772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore J.H., Knickmeyer R.C., Gao W. Imaging structural and functional brain development in early childhood. Nat. Rev. Neurosci. 2018;19:123–137. doi: 10.1038/nrn.2018.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green C.R., Lebel C., Rasmussen C., Beaulieu C., Reynolds J.N. Diffusion tensor imaging correlates of saccadic reaction time in children with fetal alcohol spectrum disorder. Alcohol. Clin. Exp. Res. 2013;37:1499–1507. doi: 10.1111/acer.12132. [DOI] [PubMed] [Google Scholar]
- Guerri C., Bazinet A., Riley E.P. Foetal alcohol spectrum disorders and alterations in brain and behaviour. Alcohol Alcohol. 2009;44:108–114. doi: 10.1093/alcalc/agn105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C.C., Nguyen V.T., Hyett M.P., Parker G.B., Breakspear M.J. Out-of-sync: disrupted neural activity in emotional circuitry during film viewing in melancholic depression. Sci. Rep. 2015;5:11605. doi: 10.1038/srep11605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harned II R.K., Strain J.D. MRI-compatible audio/visual system: impact on pediatric sedation. Pediatr. Radiol. 2001;31:247–250. doi: 10.1007/s002470100426. [DOI] [PubMed] [Google Scholar]
- Huang H., Shu N., Mishra V., Jeon T., Chalak L., Wang Z.J., Rollins N., Gong G., Cheng H., Peng Y., Dong Q., He Y. Development of human brain structural networks through infancy and childhood. Cereb. Cortex. 2015;25:1389–1404. doi: 10.1093/cercor/bht335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys K., Hasson U., Avidan G., Minshew N., Behrmann M. Cortical patterns of category-selective activation for faces, places and objects in adults with autism. Autism Res. 2008;1:52–63. doi: 10.1002/aur.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries M.D., Gurney K., Prescott T.J. The brainstem reticular formation is a small-world, not scale-free, network. Proc. Biol. Sci. 2006;273:503–511. doi: 10.1098/rspb.2005.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison R.M., Morton J.B. Tracking the brain's functional coupling dynamics over development. J. Neurosci. 2015;35:6849–6859. doi: 10.1523/JNEUROSCI.4638-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante M.A., Moore E.M., Bischoff-Grethe A., Tapert S.F., Mattson S.N., Riley E.P. Altered functional connectivity during spatial working memory in children with heavy prenatal alcohol exposure. Alcohol. 2017;64:11–21. doi: 10.1016/j.alcohol.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson J.L., Jacobson S.W. Effects of prenatal alcohol exposure on child development. Alcohol Res. Health. 2002;26:282–286. [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. FSL. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Kaiser M. Mechanisms of connectome development. Trends Cogn. Sci. 2017;21:703–717. doi: 10.1016/j.tics.2017.05.010. [DOI] [PubMed] [Google Scholar]
- Kaplan B.J., Giesbrecht G.F., Leung B.M.Y., Field C.J., Dewey D., Bell R.C., Manca D.P., O’Beirne M., Johnston D.W., Pop V.J., Singhal N., Gagnon L., Bernier F.P., Eliasziw M., McCargar L.J., Kooistra L., Farmer A., Cantell M., Goonewardene L., Casey L.M., Letourneau N., Martin J.W., Team A.P.N.S. The alberta pregnancy outcomes and nutrition (APrON) cohort study: rationale and methods. Matern. Child Nutr. 2014;10:44–60. doi: 10.1111/j.1740-8709.2012.00433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodituwakku P. Neurocognitive profile in children with fetal alcohol spectrum disorders. Dev. Disabil. Res. 2009;15:218–224. doi: 10.1002/ddrr.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Mattson S.N., Riley E.P., Jones K.L., Adnams C.M., May P.A., Bookheimer S.Y., O’Connor M.J., Narr K.L., Kan E., Abaryan Z., Sowell E.R. A longitudinal study of the long-term consequences of drinking during pregnancy: heavy in utero alcohol exposure disrupts the normal processes of brain development. J. Neurosci. 2012;32:15243–15251. doi: 10.1523/JNEUROSCI.1161-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Rasmussen C., Wyper K., Walker L., Andrew G., Yager J., Beaulieu C. Brain diffusion abnormalities in children with fetal alcohol spectrum disorder. Alcohol. Clin. Exp. Res. 2008;32:1732–1740. doi: 10.1111/j.1530-0277.2008.00750.x. [DOI] [PubMed] [Google Scholar]
- Lebel C., Roussotte F., Sowell E.R. Imaging the impact of prenatal alcohol exposure on the structure of the developing human brain. Neuropsychol. Rev. 2011;21:102–118. doi: 10.1007/s11065-011-9163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Yin S., Zhu X., Ren W., Yu J., Wang P., Zheng Z., Niu Y.N., Huang X., Li J. Linking inter-individual variability in functional brain connectivity to cognitive ability in elderly individuals. Front. Aging Neurosci. 2017;9:1–13. doi: 10.3389/fnagi.2017.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little G., Reynolds J., Beaulieu C. Altered functional connectivity observed at rest in children and adolescents prenatally exposed to alcohol. BRAIN Connect. 2018;8:503–515. doi: 10.1089/brain.2017.0572. [DOI] [PubMed] [Google Scholar]
- Long X., Benischek A., Dewey D., Lebel C. Age-related functional brain changes in young children. Neuroimage. 2017;155:322–330. doi: 10.1016/j.neuroimage.2017.04.059. [DOI] [PubMed] [Google Scholar]
- Long X., Little G., Beaulieu C., Lebel C. Sensorimotor network alterations in children and youth with prenatal alcohol exposure. Hum. Brain Mapp. 2018;39:2258–2268. doi: 10.1002/hbm.24004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald S.W.S., Nyberg L., Backman L. Intra-individual variability in behavior : links to brain structure, neurotransmission and neuronal activity. TRENDS Neurosci. 2006;29:474–480. doi: 10.1016/j.tins.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Marusak H.A., Calhoun V.D., Brown S., Crespo L.M., Sala-Hamrick K., Gotlib I.H., Thomason M.E. Dynamic functional connectivity of neurocognitive networks in children. Hum. Brain Mapp. 2017;38:97–108. doi: 10.1002/hbm.23346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh A.R., Kovacevic N., Lippe S., Garrett D., Grady C., Jirsa V. The development of a noisy brain. Arch. Ital. Biol. 2010;148:323–337. [PubMed] [Google Scholar]
- Moore E.M., Migliorini R., Infante M.A., Riley E.P. Fetal alcohol spectrum disorders: recent neuroimaging findings. Curr. Dev. Disord. Rep. 2014;1:161–172. doi: 10.1007/s40474-014-0020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen V.T., Chong S., Tieng Q.M., Mardon K., Galloway G.J., Kurniawan N.D. Radiological studies of fetal alcohol spectrum disorders in humans and animal models: an updated comprehensive review. Magn. Reson. Imaging. 2017;43:10–26. doi: 10.1016/j.mri.2017.06.012. [DOI] [PubMed] [Google Scholar]
- Paolozza A., Rasmussen C., Pei J., Hanlon-Dearman A., Nikkel S.M., Andrew G., McFarlane A., Samdup D., Reynolds J.N. Deficits in response inhibition correlate with oculomotor control in children with fetal alcohol spectrum disorder and prenatal alcohol exposure. Behav. Brain Res. 2014;259:97–105. doi: 10.1016/j.bbr.2013.10.040. [DOI] [PubMed] [Google Scholar]
- Paolozza A., Treit S., Beaulieu C., Reynolds J.N. Diffusion tensor imaging of white matter and correlates to eye movement control and psychometric testing in children with prenatal alcohol exposure. Hum. Brain Mapp. 2017;38:444–456. doi: 10.1002/hbm.23371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrenko C.L.M., Pandolfino M.E., Quamma J., Carmichael Olson H. Emotional understanding in school-aged children with fetal alcohol spectrum disorders: a promising target for intervention. J. Popul. Ther. Clin. Pharmacol. 2017;24:e21–e31. doi: 10.22374/1710-6222.24.2.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Mitra A., Laumann T.O., Snyder A.Z., Schlaggar B.L., Petersen S.E. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Schlaggar B.L., Lessov-Schlaggar C.N., Petersen S.E. Evidence for hubs in human functional brain networks. Neuron. 2013;79:798–813. doi: 10.1016/j.neuron.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J., Chen S.-.G., Hu D., Zeng L.-.L., Fan Y.-.M., Chen X.-.P., Shen H. Predicting individual brain maturity using dynamic functional connectivity. Front. Hum. Neurosci. 2015;9:418. doi: 10.3389/fnhum.2015.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J.E., Grohs M.N., Dewey D., Lebel C. Global and regional white matter development in early childhood. bioRxiv. 2019;196 doi: 10.1016/j.neuroimage.2019.04.004. [DOI] [PubMed] [Google Scholar]
- Riley E.P., Infante M.A., Warren K.R., Court A., Diego S., Warren K.R. Fetal alcohol spectrum disorders: an cverview. Neuropsychol. Rev. 2011;21:73–80. doi: 10.1007/s11065-011-9166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley E.P., McGee C.L. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp. Biol. Med. (Maywood). 2005;230:357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- Robertson F.C., Narr K.L., Molteno C.D., Jacobson J.L., Jacobson S.W., Meintjes E.M. Prenatal alcohol exposure is associated with regionally thinner cortex during the preadolescent period. Cereb. Cortex. 2016;26:3083–3095. doi: 10.1093/cercor/bhv131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr C.S., Arora A., Cho I.Y.K., Katlariwala P., Dimond D., Dewey D., Bray S. Functional network integration and attention skills in young children. Dev. Cogn. Neurosci. 2018;30:200–211. doi: 10.1016/j.dcn.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C., Brett M. Stereotaxic display of brain lesions. Behav. Neurol. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Roussotte F.F., Rudie J.D., Smith L., O'Connor M.J., Bookheimer S.Y., Narr K.L., Sowell E.R. Frontostriatal connectivity in children during working memory and the effects of prenatal methamphetamine, alcohol, and polydrug exposure. Dev. Neurosci. 2012;34:43–57. doi: 10.1159/000336242. [DOI] [PubMed] [Google Scholar]
- Rubinov M., Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Santhanam P., Coles C.D., Li Z., Li L., Lynch M.E., Hu X. Default mode network dysfunction in adults with prenatal alcohol exposure. Psychiatry Res. - Neuroimaging. 2011;194:354–362. doi: 10.1016/j.pscychresns.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T.D., Elliott M.A., Gerraty R.T., Ruparel K., Loughead J., Calkins M.E., Eickhoff S.B., Hakonarson H., Gur R.C., Gur R.E., Wolf D.H. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell E.R., Johnson A., Kan E., Lu L.H., Van Horn J.D., Toga A.W., O'Connor M.J., Bookheimer S.Y. Mapping white matter integrity and neurobehavioral correlates in children with fetal alcohol spectrum disorders. J. Neurosci. 2008;28:1313–1319. doi: 10.1523/JNEUROSCI.5067-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell E.R., Mattson S.N., Kan E., Thompson P.M., Riley E.P., Toga A.W. Abnormal cortical thickness and brain-behavior correlation patterns in individuals with heavy prenatal alcohol exposure. Cereb. Cortex. 2008;18:136–144. doi: 10.1093/cercor/bhm039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen J.M., Kodituwakku P.W., Kodituwakku E.L., Romero L., Peters A.M., Sharadamma N.M., Caprihan A., Coffman B.A. Delays in auditory processing identified in preschool children with FASD. Alcohol. Clin. Exp. Res. 2012;36:1720–1727. doi: 10.1111/j.1530-0277.2012.01769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K., Musen M., Menon V. Development of large-scale functional brain networks in children. PLoS Biol. 2009;7 doi: 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K., Uddin L.Q., Prater K., Amin H., Greicius M.D., Menon V. Development of functional and structural connectivity within the default mode network in young children. Neuroimage. 2010;52:290–301. doi: 10.1016/j.neuroimage.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treit S., Lebel C., Baugh L., Rasmussen C., Andrew G., Beaulieu C. Longitudinal mri reveals altered trajectory of brain development during childhood and adolescence in fetal alcohol spectrum disorders. J. Neurosci. 2013;33:10098–10109. doi: 10.1523/JNEUROSCI.5004-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in spm using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uddin L.Q. Typical and atypical development of functional human brain networks: insights from resting-state fMRI. Front. Syst. Neurosci. 2010;4:1–12. doi: 10.3389/fnsys.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q., Karlsgodt K.H. Future directions for examination of brain networks in neurodevelopmental disorders. J. Clin. Child Adolesc. Psychol. 2018;47:483–497. doi: 10.1080/15374416.2018.1443461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwal T., Eilbott J., Castellanos F.X. Movies in the magnet: naturalistic paradigms in developmental functional neuroimaging. Dev. Cogn. Neurosci. 2018:1–15. doi: 10.1016/j.dcn.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwal T., Eilbott J., Finn E.S., Craddock R.C., Turnbull A., Castellanos F.X. Individual differences in functional connectivity during naturalistic viewing conditions. Neuroimage. 2017;157:521–530. doi: 10.1016/j.neuroimage.2017.06.027. [DOI] [PubMed] [Google Scholar]
- Vanderwal T., Kelly C., Eilbott J., Mayes L.C., Castellanos F.X. Inscapes: a movie paradigm to improve compliance in functional magnetic resonance imaging. Neuroimage. 2015;122:222–232. doi: 10.1016/j.neuroimage.2015.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Wang X., Xia M., Liao X., Evans A., He Y. GRETNA: a graph theoretical network analysis toolbox for imaging connectomics. Front. Hum. Neurosci. 2015;9:386. doi: 10.3389/fnhum.2015.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zuo X.-.N., He Y. Graph-based network analysis of resting-state functional MRI. Front. Syst. Neurosci. 2010;4:1–14. doi: 10.3389/fnsys.2010.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak J.R., Mueller B.A., Bell C.J., Muetzel R.L., Hoecker H.L., Boys C.J., Lim K.O. Global functional connectivity abnormalities in children with fetal alcohol spectrum disorders. Alcohol. Clin. Exp. Res. 2013;37:748–756. doi: 10.1111/acer.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak J.R., Mueller B.A., Chang P.-.N., Muetzel R.L., Caros L., Lim K.O. Diffusion tensor imaging in children with fetal alcohol spectrum disorders. Alcohol. Clin. Exp. Res. 2006;30:1799–1806. doi: 10.1111/j.1530-0277.2006.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak J.R., Mueller B.A., Mattson S.N., Coles C.D., Kable J.A., Jones K.L., Boys C.J., Lim K.O., Riley E.P., Sowell E.R. Functional connectivity abnormalities and associated cognitive deficits in fetal alcohol spectrum disorders (FASD) Brain Imaging Behav. 2017;11:1432–1445. doi: 10.1007/s11682-016-9624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak J.R., Mueller B.A., Muetzel R.L., Bell C.J., Hoecker H.L., Nelson M.L., Chang P.N., Lim K.O. Inter-Hemispheric functional connectivity disruption in children with prenatal alcohol exposure. Alcohol. Clin. Exp. Res. 2011;35:849–861. doi: 10.1111/j.1530-0277.2010.01415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak J.R., Muetzel R.L. What does diffusion tensor imaging reveal about the brain and cognition in fetal alcohol spectrum disorders. Neuropsychol. Rev. 2011;21:133–147. doi: 10.1007/s11065-011-9162-1. [DOI] [PubMed] [Google Scholar]
- Wozniak J.R., Muetzel R.L., Mueller B.A., McGee C.L., Freerks M.A., Ward E.E., Nelson M.L., Chang P.N., Lim K.O. Microstructural corpus callosum anomalies in children with prenatal alcohol exposure: an extension of previous diffusion tensor imaging findings. Alcohol. Clin. Exp. Res. 2009;33:1825–1835. doi: 10.1111/j.1530-0277.2009.01021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M., Wang J., He Y. BrainNet viewer: a network visualization tool for human brain connectomics. PLoS ONE. 2013;8:e68910. doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T., Falchier A., Sullivan E.L., Linn G., Ramirez J.S.B., Ross D., Feczko E., Opitz A., Bagley J., Sturgeon D., Earl E., Miranda-Domínguez O., Perrone A., Craddock R.C., Schroeder C.E., Colcombe S., Fair D.A., Milham M.P. Delineating the macroscale areal organization of the macaque cortex in vivo. Cell Rep. 2018;23:429–441. doi: 10.1016/j.celrep.2018.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C., Gong G., Wang J., Wang D., Liu D., Zhu C., Chen Z.J., Evans A., Zang Y., He Y. Sex- and brain size-related small-world structural cortical networks in young adults: a DTI tractography study. Cereb. Cortex. 2011;21:449–458. doi: 10.1093/cercor/bhq111. [DOI] [PubMed] [Google Scholar]
- Yang Y., Roussotte F., Kan E., Sulik K.K., Mattson S.N., Riley E.P., Jones K.L., Adnams C.M., May P.A., O'Connor M.J., Narr K.L., Sowell E.R. Abnormal cortical thickness alterations in fetal alcohol spectrum disorders and their relationships with facial dysmorphology. Cereb. Cortex. 2012;22:1170–1179. doi: 10.1093/cercor/bhr193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Shen D., Lin W. Resting-state functional MRI studies on infant brains: a decade of gap-filling efforts. Neuroimage. 2018:1–21. doi: 10.1016/j.neuroimage.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T., Xu Y., He Y. NeuroImage graph theoretical modeling of baby brain networks. Neuroimage. 2019;185:711–727. doi: 10.1016/j.neuroimage.2018.06.038. [DOI] [PubMed] [Google Scholar]
- Zhou D., Lebel C., Lepage C., Rasmussen C., Evans A., Wyper K., Pei J., Andrew G., Massey A., Massey D., Beaulieu C. Developmental cortical thinning in fetal alcohol spectrum disorders. Neuroimage. 2011;58:16–25. doi: 10.1016/j.neuroimage.2011.06.026. [DOI] [PubMed] [Google Scholar]
- Zhou D., Rasmussen C., Pei J., Andrew G., Reynolds J.N., Beaulieu C. Preserved cortical asymmetry despite thinner cortex in children and adolescents with prenatal alcohol exposure and associated conditions. Hum. Brain Mapp. 2018;39:72–88. doi: 10.1002/hbm.23818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X.N., He Y., Betzel R.F., Colcombe S., Sporns O., Milham M.P. Human connectomics across the life span. Trends Cogn. Sci. 2017;21:32–45. doi: 10.1016/j.tics.2016.10.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.