Highlights
-
•
Fractionating insight into objects aids its neuroanatomical exploration in dementia.
-
•
Distinctive neural correlates seem to underpin different insight objects in FTD.
-
•
Altered insight into disease/health condition mostly involves right frontal areas.
-
•
Altered insight into social cognition implicates frontal, temporal and limbic areas.
-
•
Frontal, medial temporal and parietal areas underpin insight into memory problems.
Keywords: Insight, Neural correlates, Frontotemporal dementia
Abbreviations: ACC, Anterior Cingulate Cortex; AD, Alzheimer's disease; ADL, Activities of Daily Living; ALS, Amyotrophic Lateral Sclerosis; AMG, Amygdala; AQ-D_iADL, Awareness of Deficit - Dementia scale, instrumental activities of daily living domain; BA, Brodmann Area; bvFTD, Frontotemporal dementia behavioural variant; CT, computed tomography; CBD, Corticobasal Degeneration; CBS, Corticobasal Syndrome; CERAD, Consortium to establish a registry in Alzheimer's disease; DLPFC, Dorsolateral Prefrontal Cortex; DS, Discrepancy Score; DTI, Diffusion Tensor Imaging; EEG, Electroencephalography; ERP, Evoked Related Potentials; FC, Frontal Cortex; FDG-PET/PET, Fluorodeoxyglucose (18F) positron emission tomography; FDR, False Discovery Rate; FIS, Fronto-insular Stroke; fMRI, Functional Magnetic Resonance; FOK, Feeling of Knowing; FP, Frontopolar; FrSBe, Frontal System Behaviour Scale; FSL, FMRIB's Software Library,Oxford University's Centre for Functional Magnetic Resonance Imaging of the Brain; FTD, Frontotemporal Dementia; FTLD, Frontotemporal Lobar Degeneration; FWE, Family Wise Error; GM, Grey Matter; HBD, Heart Beat Detection; HC, Cognitively Healthy Controls; HEP, Heart Evoked Potentials; HP, Hippocampus; iADL, Instrumental Activities of Daily Living; ICC, Inferior Cingulate Cortex; IFG, Inferior Frontal Gyrus; ITG, Inferior Temporal Gyrus; Ke, Cluster extent; LA, Logopenic Aphasia; mm2, square milimetre; MC, Multiple Comparisons; MARS, Memory Awareness Rating Scale; MCC, Mid Cingulate Cortex; MEG, Magnetoencephalography; MFG, Middle Frontal Gyrus; MND, Motor Neuron Disease; MPFC, Medial Prefrontal Cortex; MRI, Magnetic Resonance Imaging; MRS, Magnetic Resonance Spectroscopy; MTG, Middle Temporal Gyrus; nfPAA/PNFA/PPA, Progressive non-fluent Primary Aphasia; OFC, Orbitofrontal Cortex; PCC, Posterior Cingulate Cortex; PET, Positron Emission Tomography; PHP, Parahippocampus; PiD, Pick's Disease; PSP, Progressive Supranuclear Palsy; R2, R-squared; RFT, Random Field Theory; RS, Resting State; SD, Semantic Dementia; SFG, Superior Frontal Gyrus; SPECT, Single-Photon Emission Computed Tomography; SPM, Statistical Parametric Mapping, University College London's Wellcome Trust Centre for Neuroimaging ("The FIL"); STG, Superior Temporal Gyrus; TFCE, Threshold-free Cluster Enhancement; VBM, Voxel-based Morphometry; X2, Chi squared
Abstract
Altered insight into disease or specific symptoms is a prominent clinical feature of frontotemporal dementia (FTD). Understanding the neural bases of insight is crucial to help improve FTD diagnosis, classification and management. A systematic review to explore the neural correlates of altered insight in FTD and associated syndromes was conducted. Insight was fractionated to examine whether altered insight into different neuropsychological/behavioural objects is underpinned by different or compatible neural correlates. 6 databases (Medline, Embase, PsycINFO, Web of Science, BIOSIS and ProQuest Dissertations & Theses Global) were interrogated between 1980 and August 2019. 15 relevant papers were found out of 660 titles screened. The studies included suggest that different objects of altered insight are associated with distinctive brain areas in FTD. For example, disease unawareness appears to predominantly correlate with right frontal involvement. In contrast, altered insight into social cognition potentially involves, in addition to frontal areas, the temporal gyrus, insula, parahippocampus and amygdala. Impaired insight into memory problems appears to be related to the frontal lobes, postcentral gyrus, parietal cortex and posterior cingulate. These results reflect to a certain extent those observed in other neurodegenerative conditions like Alzheimer's disease (AD) and also other brain disorders. Nevertheless, they should be cautiously interpreted due to variability in the methodological aspects used to reach those conclusions. Future work should triangulate different insight assessment approaches and brain imaging techniques to increase the understanding of this highly relevant clinical phenomenon in dementia.
1. Introduction
Colloquially, ‘insight’ is understood as the capacity to have a deep understanding of a specific situation, or, as the word itself may suggest, an ‘internal sight’ (Oxford English Dictionary, 2017). In a clinical context, ‘insight’ refers to a conscious knowledge of health conditions and the capability to identify or judge the presence or severity of disease or symptoms (Zanetti et al., 1999). Likewise, the term ‘altered insight’ embraces an inaccurate self-perception of a particular health status and/or its associated symptoms and potential consequences (Markova et al., 2005; McGlynn and Schacter, 1989; Mullen et al., 1996).
Altered insight cuts across different brain disorders including multiple forms of dementia (Wilson et al., 2016), traumatic brain injury (TBI) and schizophrenia (SCZ) (David et al., 2012), among others. Reduced insight is commonly observed in Alzheimer's disease (AD) and is especially prominent in frontotemporal dementia (FTD) (Wilson et al., 2016). Severely impaired insight has been reported in 75% of cases with FTD (Wedderburn et al., 2008), and is one of the main clinical features of behavioural variant FTD (bvFTD) (Wedderburn et al., 2008; Neary et al., 1998; Rascovsky et al., 2011). Although altered insight is a critical symptom in FTD, its neural foundations have not yet been fully explored.
Exploring the neural correlates of altered insight in FTD and associated syndromes has significant clinical relevance for several reasons. Diagnosing FTD is frequently clinically challenging and often delayed (van Vliet et al., 2013). Identifying potential neuroimaging biomarkers specific to the patterns of reduced insight, a highly frequent symptom observed in FTD variants (Wilson et al., 2016; Wedderburn et al., 2008; Neary et al., 1998; Rascovsky et al., 2011), is of great clinical interest. The timely detection of cognitive decline and dementia may be interfered by altered insight (Iliffe et al., 2005; Koch et al., 2010) probably due to its role in the recognition and reporting of neuropsychological and behavioural symptoms (Markova et al., 2005; McGlynn and Schacter, 1989; Mullen et al., 1996). Greater understanding of the neural underpinnings of insight will aid disease prognostication, and may provide surrogate markers of disease progression for use in clinical trials.
Altered insight in dementia is associated with difficulties in performing activities of daily living (ADL), impaired decision making, dysthymia, apathy, psychosis, reduced adherence to treatment, increase in risky behaviours and caregiver burden (Zamboni and Wilcock, 2011; Aalten et al., 2005; Aalten et al., 2006). As a consequence, altered insight can impair quality of life and limit independence. Affected individuals may continue to perform everyday tasks such as cooking or driving when they are unsafe to do so, due to their reduced insight into cognitive or motor symptoms. Moreover, altered insight can lead to delayed presentation to clinical services, refusal of appropriate clinical investigations, and delayed diagnosis.
There is a theoretical importance to the study of the brain areas implicated in altered insight in dementia. The notion of ‘self’ encompasses a system of personal representations (or mental constructs), traits and attitudes that shape behaviour and social interactions (Orfei et al., 2008) and provide a sense of personal identity (Mograbi et al., 2009). These will clearly be influenced by the degree of insight one has into personal abilities and disabilities. Congruently, exploring insight can offer a window onto neural mechanisms underlying the self itself (Mograbi et al., 2009) and other self-referential processes such as self-evaluation, self-awareness and consciousness, and can therefore potentially make a significant contribution to our understanding of human cognitive neuroscience (Zamboni and Wilcock, 2011).
Research into the neural correlates of insight has been hampered by the high complexity of the term and a certain lack of consistency in its definition (Markova and Berrios, 2011). Patients’ reduced conscious knowledge of their own disease status or specific symptoms has been differently conceptualized as anosognosia, unawareness, lack of insight, denial, or impaired metacognition, among other terms (David et al., 2012; Markova and Berrios, 2011; Gilleen et al., 2010). Anosognosia is often used to refer to reduced insight into specific symptoms such as amnesia (Vogel et al., 2005; Shibata et al., 2008) or a broader disease unawareness in dementia (Prigatano, 2009). This scenario differs from its original connotation referring to the clinical consequences of stroke (McGlynn and Schacter, 1989). The concepts of unawareness and lack of insight have had similar definitions in the literature (McGlynn and Schacter, 1989; Mullen et al., 1996). The term metacognition has been recently used in dementia when investigating self-perception and self-monitoring (DeLozier and Davalos, 2016; Eslinger et al., 2005).
A potential resolution to the problem of conceptualizing insight is to define it at the object level (Markova et al., 2005). Insight only emerges when it is related to something (an object) either pathological or non-pathological, and it cannot be expressed without having something to have insight into (Markova et al., 2005; Markova and Berrios, 2011; Markova and Berrios, 2001). From this approach, object is understood as a “particular mental or physical state (e.g., mental illness, neuropsychological deficit) in relation to which insight is being assessed” (26 p.245). Thus, the relational nature of the term is highlighted, and insight can be fractionated into different clinical phenomena that are targeted at their respective objects (Markova and Berrios, 2011). For instance, insight into having a specific disease (e.g. dementia) would differ from insight into a specific function such as memory, language or walking ability (Markova and Berrios, 2011; Gilleen et al., 2010). This approach may facilitate the search for the neural correlates of altered insight in dementia (Markova and Berrios, 2011; Gilleen et al., 2010).
Altered insight is not an ‘all-or-nothing’ phenomenon (Aalten et al., 2005). One may over or underestimate one's abilities, to a greater or lesser degree. Previous work in neuropsychiatric disorders has shown insight to be more severely reduced in patients with AD compared to people with TBI and SCZ (Gilleen et al., 2010). In dementia, individuals with FTD tends to show less insight into their symptoms than people with AD (Salmon et al., 2008). Insight is also more severely affected in bvFTD than in the language variants of FTD (Banks and Weintraub, 2009). Additionally, some patients may exhibit impaired insight into some objects but not others. For example, impaired insight into cognitive functions or into behavioural disorders can be presented independently in patients with AD (Starkstein et al., 1996).
Emerging evidence supports the hypothesis that insight is object specific. Research suggests that several types of self-referential mechanisms can be differentiated across particular brain areas (Mograbi et al., 2009). For instance, a global conception of the self has been correlated with midline cortical anterior and posterior structures including the prefrontal, parietal, cingulate and retrosplenial cortices (Mograbi et al., 2009). On the other hand, encoding of self-relatedness for external and internal inputs may be mostly situated at ventral midline cortical areas, whereas revaluation of self-related stimuli seems to principally face dorsal midline cortical networks. For its part, temporal functions might be sustained by interactions between posterior midline cortical areas and the hippocampal complex (Mograbi et al., 2009).
Alternatively, disorders presenting with variable types of altered insight can illustrate the potential neuroanatomical specialization of this symptom. The right hemisphere is particularly implicated in the altered insight seen post stroke (Bisiach et al., 1986), whereas in Anton's syndrome, where an individual loses vision but continues to believe they can see, a bilateral deterioration of the visual cortex in the occipital lobes occurs (Prigatano, 2009). On the other hand, the striking neurological sign known as ‘alien limb’, which consists of sense of estrangement towards a limb that moves involuntarily, has been associated to callosal, parietal and frontal brain anomalies not only mainly in corticobasal syndrome (CBS), but also in Creutzfeldt-Jakob disease (CJD) (Graff-Radford et al., 2013).
The Dissociable Interactions and Conscious Experience (DICE) model was initially developed to account for the differentiation between explicit and implicit memory processes (Schacter, 1989). Later, this approach was used to describe the neural processes underpinning altered insight (McGlynn and Schacter, 1989). It is theorized that the brain networks supporting altered insight into specific objects differ from those that configure broader overarching awareness of health status (McGlynn and Schacter, 1989). The model includes an operational centre in charge of mediating insight named the Conscious Awareness System (CAS). The CAS is strategically set up throughout posterior callosal, cingulate and parietal brain areas, and receives inputs from objects such as perception, memory, and language (McGlynn and Schacter, 1989; Agnew and Morris, 1998). Thus, damage to partial areas of the CAS or one of its connections may result in the expression of isolated altered insight into a specific object (McGlynn and Schacter, 1989; Agnew and Morris, 1998). Frontal lobes and executive functioning are also postulated to play a crucial role in this model. In DICE, disruptions involving the entire CAS or executive functions can create overarching altered insight into health status (McGlynn and Schacter, 1989; Agnew and Morris, 1998).
Studies conducted in AD patients suggest that distinctive neural substrates underlie insight into different objects. For instance, overarching insight into having dementia has been linked with AD pathology in the right prosubiculum of the hippocampus on histopathological analysis (Marshall et al., 2004), whereas insight into broad cognitive and functional impairment correlated with low metabolic rate in right lateral and dorsolateral frontal cortex (Harwood et al., 2005). Altered insight into memory impairment in AD appears to be linked to reduced blood flow in the left orbitofrontal cortex (Shibata et al., 2008) or hypometabolism in medial and lateral frontal lobes, left inferior parietal areas and the cingulate on functional brain imaging studies (Hanyu et al., 2008), whereas this symptom appears to be related to dysfunctions in inferior frontal areas in both MCI and AD (Vogel et al., 2005).
Variable associations between different types of insight and brain involvement have been also reported in patients with stroke, vascular dementia (VD), TBI and SCZ. Correlations between impaired insight into hemiplegia and damage to frontal, parietal, insula and basal ganglia areas have been found in many studies conducted with patients with strokes (Pia et al., 2004). Disease unawareness in patients with VD has been associated with frontal lobes and basal ganglia dysfunction (Starkstein et al., 1996), while impaired insight into ADL ability was linked to damage to the insula and both right- and left-sided stroke disease (Tezuka et al., 2013). TBI studies highlight prefrontal and anterior temporal lobe areas as being crucial for insight into disease (Prigatano, 2005). In contrast, failure at judging personal traits and skills have been associated with decreased mid-line blood flow in the prefrontal and retrosplenial cortices in TBI (Schmitz et al., 2006). Clinical insight into SCZ, meaning insight into having a SCZ diagnosis, its symptoms and consequences, has been linked with activity in regions adjacent to frontal, temporal and parietal lobes, along with thalamus, basal ganglia, and cerebellum (Xavier and Vorderstrasse, 2016). Cognitive insight in SCZ, referring to the ability to evaluate personal experiences, has correlated instead with the prefrontal cortex and hippocampal functioning (Xavier and Vorderstrasse, 2016). Altogether, these results imply that different objects of insight may have distinct neuroanatomical substrates in AD and other brain disorders. It is therefore plausible that this may also occur in FTD.
The overarching aim of this systematic review is to examine the neural bases of altered insight in FTD and its associated syndromes. We explore what the neural correlates of altered insight are in FTD and whether they differ across different objects of altered insight, either broad objects (i.e. altered insight into presence of disease/diagnosis or health condition) or more specific objects (i.e. altered insight into particular neuropsychological, neuropsychiatric or behavioural objects). Consequently, evidence for and against the concept of altered insight into different objects being subserved by different or compatible brain regions is reviewed. We hypothesized that altered insight into different objects is underpinned by distinctive brain areas. We predict that by utilising a more precise definition of subtypes of insight it will be possible to better understand the neural correlates of altered insight. Detailed dissection of the neural correlates of different types of insight used in different studies may yield important information as to whether altered insight in FTD occurs at a broad or more specific object level. Exploring the neuroanatomical foundations of altered insight can contribute to the understanding of a clinical phenomenon critical for the early diagnosis and effective management of FTD and other forms of dementia.
2. Methods
2.1. Procedure and definitions
The present systematic review was carried out in accordance with the procedures proposed by the Preferred Reported Items for Systematic Reviews and Meta-analyses (PRISMA) protocol guidelines (www.prisma-statement.org) (Liberati et al., 2009; Moher et al., 2009) and was correspondingly registered on the International prospective register of systematic reviews PROSPERO database (CRD42019078504) (Booth et al., 2012; Booth et al., 2011). The focus of this work was formulated considering the PICOS/PECOS approach (Liberati et al., 2009; Moher et al., 2009). This led to the design of a search strategy intended to be as inclusive as possible to properly address the main purpose of this systematic review.
Altered insight was defined as a relational clinical phenomenon characterised by reduced self-knowledge or awareness of an object, either broad objects (i.e. disease diagnosis or health condition) or more specific objects (i.e. particular cognitive, behavioural, functional or neuropsychiatric symptoms) (Markova et al., 2005; Markova and Berrios, 2011, 2000). Concerning broad objects of insight, it should be noted that two connotations were considered in this systematic review: altered insight into the presence of a disease and diagnosis, or overall insight into health condition (either referring to self-perception of being in good or poor health and ability, or where authors combined insight scores into multiple objects, either cognitive or behavioural). FTD was defined as a neurodegenerative disease with diverse presentations, including behavioural (bvFTD), language (progressive non-fluent aphasia -PNFA-, semantic dementia -SD-, logopenic aphasia -LA-) and associated motor variants (motor neuron disease -MND-/amyotrophic lateral sclerosis -ALS-/FTD) (Rascovsky et al., 2011; Cairns et al., 2007; Mackenzie et al., 2009, 2010; Kovacs, 2016; Piguet et al., 2011; Gorno-Tempini et al., 2011; Strong et al., 2017, 2009). Although LA can be explained by AD pathology in roughly 56% of the cases (Giannini et al., 2017) and therefore classified as such (Rohrer et al., 2012), it has been stated that frontotemporal lobar degeneration can account for 38% of the cases of LA (Giannini et al., 2017), which the authors considered as sufficient justification to include it in the current review.
Diverse approaches were considered for the assessment of altered insight. In terms of the brain imaging methods used to explore the neural correlates of impaired insight in FTD, both structural and functional techniques were considered, including magnetic resonance imaging (MRI), functional MRI, positron emission tomography (PET), single-photon emission computed tomography (SPECT) and other methods used in cognitive neuroscience including EEG (electroencephalography) (Table 1).
Table 1.
Generic model of the systematic review search strategy.
| Keywords* | Inputs⁎⁎ |
|---|---|
| FTD, bvFTD, PNFA, SD, LA, MND, ALS, FTLD PiD, CBD, PSP. | Frontotemporal dementia and its associated syndromes |
| insight, lack of insight, awareness, unawareness, self-appraisal, anosognosia, metacognition. | Altered insight |
| neural correlates, anatomical or neuroanatomical bases, histopathological techniques, MRI, CT, DTI, fMRI, PET, SPECT, MRS, EEG, MEG, RS. | Neural correlates and brain imaging methods |
Search strategy performed onto five online databases including Medline, Embase, PsycINFO, Web of Science, BIOSIS and ProQuest Dissertations &Theses Global.
After every key word the command ‘OR’ was added.
Inputs were overlaid using the command ‘AND’.
2.2. Data sources and search strategy
A thorough search was performed across 6 online databases including Medline, Embase, PsycINFO, Web of Science, BIOSIS and ProQuest Dissertations & Theses Global in August 2019. References cited by the group of selected papers and pertinent reviews were manually examined to seek additional relevant papers. A generic model of the search strategy and key words used in this systematic review can be seen in Table 1. This generic search strategy was modified to fit with the specific headings and/or sub-headings proposed by each of the databases. Examples of the particular search strategies run onto each database can be found in the supplementary section of this article (Supplementary Material 1). Papers found across the databases included in this systematic review were merged into a unified files that were then imported into EndNote X9 (Thomson Reuters, 2018), where the final number of publications was deduplicated.
2.3. Study selection
Two independent reviewers (CMN and AT) screened titles and abstracts and where necessary full-texts. Agreement for relevant papers in the final selection was reached through the aid of the online tool Covidence (www.covidence.org) (Mavergames, 2013). Any disagreement was solved by discussion until consensus was obtained.
Inclusion criteria:
-
i
Studies of human beings published in English language between 1980 and August 2019.
-
ii
Studies seeking to correlate insight into any object (broad objects defined as insight into presence of disease/diagnosis status or health condition, or specific objects focused on particular neuropsychological, functional, behavioural or neuropsychiatric domains) with the outcomes of either structural or functional brain imaging.
-
iii
Studies with samples of patients with any form of FTD and/or associated syndromes.
Exclusion criteria:
-
i
Investigations conducted on animals.
-
ii
Articles published in languages different from English.
-
iii
Studies which did not include brain imaging.
-
iv
Investigations conducted solely with cognitively healthy normal controls.
2.4. Data extraction and analysis
Making use of a pre-defined structured data extraction form, two independent reviewers (CMN and AT) extracted the following details from each paper included in the final selection: participant groups (including diagnoses), insight assessment methodology, object of insight measured, brain imaging modality and analysis methods, and study findings. A quality assessment based on a modified version of the Newcastle-Ottawa Scale (Wells et al., 2014; Sharmin et al., 2017) for cross-sectional studies (Supplementary Material 2) was performed by two independent reviewers (CMN and AT) over the final selection of articles. Complementary, an examination utilising Crombie's items along with a global qualitative critical appraisal was conducted across those papers (Zeng et al., 2015). The results from the final selection of papers were organised according to broad or specific objects of insight and their respective neural correlates.
3. Results
3.1. Study selection
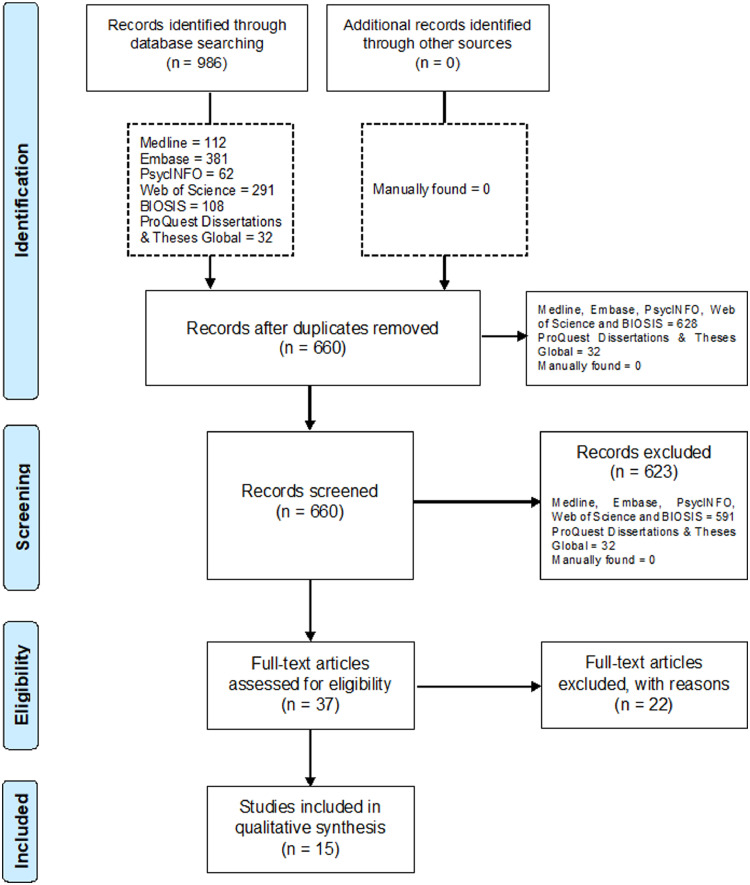
A total of 660 records were identified and screened across the databases explored. 623 results were excluded following title or abstract screening where applied, which corresponded to postgraduate dissertation projects and theses (32), conference abstracts (126), other reviews (127), clinical cases (29), book sections (9) and other publications exploring FTD on issues not pertinent for this review, neuropsychiatric conditions other than FTD, diverse behavioural studies in dementia with no neuroimaging method utilised or general irrelevant topics (300) (Fig. 1). Among the 37 papers shortlisted for full reading, 22 were considered not pertinent since they did not make use of neuroimaging or focused on neurogenerative diseases other than FTD. 15 studies met the selection criteria of this systematic review (Amanzio et al., 2016; Bastin et al., 2012; Garcia-Cordero et al., 2016; Hornberger et al., 2014; Ichikawa et al., 2013; Levy et al., 2018; Massimo et al., 2013; McMurtray et al., 2006; Mendez and Shapira, 2005; Miller et al., 1997; Rosen et al., 2010; Ruby et al., 2007; Shany-Ur et al., 2014; Sollberger et al., 2014; Zamboni et al., 2010) (Tables 2 and 3).
Fig. 1.
PRISMA Flow Diagram.
Table 2.
Final selection of relevant papers: participants, insight assessment methodology, insight object(s) assessed, brain imaging methods, and study quality.
| Paper | Participants | Insight assessment methodology | Insight object(s) assessed | Brain imaging methods | Study Quality* |
|---|---|---|---|---|---|
| Mendez and Shapira (2005) | 29 FTD | Insight question from CERAD plus 3 extra insight questions 4-point Likert scale ranging from total unawareness (0) to normal awareness (3) |
Presence of disease Behavioural change | Visual rating of PET and SPECT images | Fair |
| Miller et al. (1997) | 30 FTD 30 AD |
Clinical judgment Presence or absence of patients’ insight into illness | Presence of disease | Visual inspection of SPECT images | Fair |
| McMurtray et al. (2006) | 74 bvFTD | Clinical judgment using Frontotemporal Dementia Inventory (degree of characterization of the object in reference ranging from 1 -not characteristic at all- to 5 -extremely characteristic-) | Behavioural change | Visual rating of SPECT images | Good |
| Levy et al. (2018) | 26 bvFTD 29 CBS 12 PPA | Clinical judgment on the NRS item ‘Inaccurate insight and self-appraisal’ ranging from 1 (not present) to 7 (extremely severe) Participant - informant DS on the FrSBe. | Presence of disease and health status Executive dysfunction |
MRI with automated parcellation of cerebral cortex into 68 regions of interest using FreeSurfer | Good |
| Ichikawa et al. (2013) | 8 ALS 8 FTLD 11 HC |
Clinical judgement Combination of DS between patients’ & clinicians’ judgement on an Anosognosia scale (scores ranging from 0 to 32) |
Overall motor, cognitive and emotional functioning. | Longitudinal changes of areas of bilateral anterior and inferior horns on CT images using Synapse | Good |
| Hornberger et al. (2014) | 24 bvFTD 18 SD 13 PNFA 15 AD 11 LA |
Patient - informant DS on the Insight Questionnaire | Specific scores on: Diagnosis and treatment Social behaviour Emotion Language Motivation/ organization Plus analysis of an overall insight score across all objects |
VBM analysis of MRI using FSL | Good |
| Massimo et al. (2013) | 49 bvFTD 73 AD | DS between retrospective self-appraisal and standardized scores on language and episodic memory tasks | Multi-domain self appraisal (average performance on language and episodic memory tasks) | VBM analysis of MRI using SPM5 | Good |
| Rosen et al. (2010) | 2 ALS 2 MCI 9 AD 10 FTD 5 SD 5 PPA 4 CBD 2 HC |
DS between retrospective self-rating of performance and standardize scores on attention, episodic memory, language & executive function tasks | Overall cognitive performance (average performance on working memory, attention, episodic memory and executive function tasks) | VBM analysis of MRI using SPM5 | Fair |
| Shany-Ur et al. (2014) | 35 AD 21 bvFTD 7 right-temporal variant FTD 8 svPPA 7 nfPPA 46 HC |
Participant-informant DS using the Patient Competency Rating Scale (PCRS) | ADL competency Cognitive ability Interpersonal ability Emotional ability Composite score of all the above objects |
VBM analysis of MRI using SPM5 | Good |
| Sollberger at al. (2014) | 28 bvFTD 16 svPPA 4 nfPPA 23 AD 12 CBS 19 HC |
Participant-informant DS using the Interpersonal Reactivity Index (IRI) | Empathy | VBM analysis of MRI using SPM5 | Good |
| Ruby et al. (2007) | 16 bvFTD 16 HC | Participant-informant DS using questionnaires on behaviour prediction and personality assessment | Behavioural and personality changes | VBM analysis of PET images using SPM2 | Good |
| Bastin et al. (2012) | 8 bvFTD 26 HC |
Participant - informant DS on the MARS Participants’ performance prediction on a FOK task |
Memory (autonoetic consciousness) | FDG-PET analysis using SPM8 | Good |
| Zamboni et al. (2010) | 27 bvFTD 12 aphasic variants of FTD 31 CBS 14 HC |
Participant-informant DS on the FrSBe; clinical judgement | Executive function | VBM analysis of MRI using SPM5 | Good |
| Amanzio et al. (2016) | 23 bvFTD 30 HC | Participant - informant DS on the AQ-D_iADL | iADL | VBM analysis of MRI using SPM8 | Good |
| Garcia-Cordero et al., 2016 | 18 bvFTD 21 AD 18 FIS 42 HC |
Participants’ ratings on their confidence to count their own heart beats (HBD) over accuracy, learning and feedback stages | Interoceptive awareness | VBM and lesion mapping analyses of MRI using SPM12, MRI resting state analysis using SPM8 and HEP/ERP analysis of EEG. | Good |
Judgements made according to the quality assessment conducted with an adapted version of the Newcastle-Ottawa Scale (see Table 7).
Table 3.
Final selection of relevant papers: strength of correlation between altered insight assessment and neuroimaging metrics, and main findings.
| Paper | Strength of correlation between altered insight assessment and neuroimaging metrics | Study findings | |||
|---|---|---|---|---|---|
| Statistical analyses; Coefficient used | Threshold | Threshold cluster extent where applicable | p value used for interpretation | ||
| Mendez and Shapira (2005) | Factorial analysis of variance (ANOVA), F-test | Not specified | Not applicable (visual inspection) | p < 0.001 for main effect; p < 0.01 for right-left hemispheres and frontal-temporal lobes effects; not significant interaction (however, right frontal predominance) | Loss of insight in FTD was associated with hypoperfusion/hypometabolism in the right hemisphere, especially in the frontal lobes. |
| Miller et al. (1997) | X2 | Not specified | Not applicable (visual inspection) | p = 0.04 | Patients with FTD showed an early loss of personal awareness related to uneven frontotemporal dysfunctions, either bilateral or unilateral |
| McMurtray et al. (2006) | Ordinal regression; F and Bonferroni tests | p < 0.005 | Not applicable (visual inspection) | p < 0.001 | Loss of insight into behavioural change was associated with right frontal hypoperfusion |
| Levy et al. (2018) | Regression; R2 | Not specified | Not specified | R2 = =0.45 for self-regulation mask | - Outcomes from measures of clinical judgement were more robust than patient-informant DS when correlating with brain structures. - Altered global insight correlated with left OFC and right rostral ACC in the whole cohort of patients (bvFTD, CBS and PPA). - Episodic memory functioning did not predict altered insight. |
| Ichikawa et al. (2013) | Two-tailed Fisher correlation | p < 0.05 | N/A (areas of horns measured in mm2) | p = 0.0016 for anterior horn; p < 0.0001 for inferior horn | - Significant positive correlations between anosognosia scores and increase of anterior and inferior horns sizes (indexes of frontotemporal atrophy) in ALS, but especially ALS with FTLD. - Longitudinal increases of horn sizes were significantly more rapid in non-demented ALS patients compared with controls. - The anosognosia score was predominantly correlated with longitudinal enlargement of the inferior horn size (index of medial temporal lobes atrophy) in non-demented ALS patients. |
| Hornberger et al. (2014) | Voxel-wise general linear model; Covariate only model; T-contrast | Significant clusters formed by TCE method (5000 permutations); p < 0.001, FDR corrected for each voxel | 20 voxels | p < 0.001 | - Scores on insight into Diagnosis and treatment and Language domains did not differ among groups and were not covaried with GM volumes. -Whole group (bvFTD, SD, PNFA, AD and LA) significant correlations were found between GM volumes and insight into social interactions, emotion processing, and motivation/organization, but not on dementia subgroup analysis. -Social interactions insight correlated with left OFC, left PHPC, right MTG, bilateral insula, and right AMG atrophy. - Emotion processing insight correlated with bilateral FP cortices, right DLPC, supplementary motor area, bilateral ACC, and left AMG atrophy. - Motivation insight covaried with bilateral OFC, left ACC, right FP cortical atrophy. - Overall insight covaried with bilateral OFC and right FP cortical atrophy. |
| Massimo et al. (2013) | Regression | p < 0.05 FDR corrected for both voxel and cluster level analyses | 15 voxels | p < 0.05 | Impaired capacity to self-appraise cognitive performances correlated with GM density across ventral and rostral medial prefrontal regions in AD and bvFTD and especially with the subgenual cingulate (BA 25) in bvFTD. |
| Rosen et al. (2010) | Covariates only model | p < 0.05 corrected for MC using FWE correction | 25 voxels | p < 0.05 | Altered self-appraisal correlated with tissue content mainly in the right ventromedial prefrontal cortex in the whole cohort (behavioural and language variants of FTD, CBD, ALS, MCI, AD and HC). |
| Shany-Ur et al. (2014) | General linear models; T-test | p < 0.05 FWE corrected | Study specific T-threshold at p < 0.05 after 1000 permutations | p < 0.05 | - Whole group analysis (behavioural and language variants of FTD, AD and HC). Participants were split into under- and over-estimators. - Overestimating ADL competency correlated with atrophy of widespread right frontal regions, anterior insula, putamen, thalamus, medial & lateral temporal lobes & pons. - Overestimating cognitive functioning was associated with atrophy of right middle frontal & middle temporal gyri. - Overestimating emotional control correlated with atrophy in bilateral OFC, insula and right SFG. - Overestimating interpersonal abilities was linked with atrophy of putamen and fusiform gyrus. - Overestimating overall functioning was associated with atrophy of right orbital inferior frontal gyrus, middle frontal gyrus, caudate head, and putamen, left superior frontal gyrus, and the pons. |
| Sollberger at al. (2014) | Multiple regression design (covariates only); T-test | p < 0.001 voxel-wise | p < 0.001 corrected for MC at p < 0.05 based on cluster extent and a custom-fit error distribution determined by 1000 permutations | p < 0.001 uncorrected | - Overestimation of empathic concern correlated with GM volumes in right-hemispheric anterior inferolateral temporal regions in the whole cohort of participants behavioural and language variants of FTD, AD, AD, CBS y HC). - bvFTD and nfPPA mostly overestimated their empathic concern compared to HC. |
| Ruby et al. (2007) | Behavioural-metabolic correlation analyses; Z-scores | p < 0.05 corrected at cluster level | 315 voxels | p < 0.05 | - Decreased metabolic activity in the left temporal pole correlated with reduced insight into behavioural change in bvFTD group - Reduced insight into personality changes did not exhibit significant neural correlates |
| Bastin et al. (2012) | Factorial analysis | p<0.05 FWE corrected for MC at the voxel level | 20 voxels | p < 0.05 | bvFTD patients with reduced autonoetic consciousness exhibited hypometabolism across the anterior medial prefrontal cortex, the left dorsolateral prefrontal cortex (near the superior frontal sulcus), parietal regions, and the posterior cingulate cortex. |
| Zamboni et al. (2010) | Full factorial model; One tailed T-test | p < 0.001 uncorrected | 1568 voxels | p < 0.001 uncorrected; p < 0.05 FWE corrected and FDR | - Combined cohort (behavioural and language variants of FTD, CBS and HC) showed correlations between reduced insight into behavioural change and GM loss in a region extending from the right superior temporal sulcus to the right ITG (posterior region of the right superior temporal sulcus, adjacent to the temporoparietal junction). - bvFTD patients underestimated their current behavioural disturbances and overestimated their premorbid ones. |
| Amanzio et al. (2016) | Explorative univariate linear regression | p < 0.005 corrected for MC; statistical inferences made according to RFT | 150 voxels; small clusters filtered with a p FWE corrected>0.05 (Ke> FWE corrected) | p < 0.001 | bvFTD patients exhibited significant correlations between decreased awareness of performance on iADL and regional GM volume changes in the MPFC (predominantly MCC, dorsal anterior insula and cuneous) and areas of the anterior and posterior cerebellum. |
| García-Cordero et al. (2016) | Multiple regressions; T-tests and Spearman correlations | Structural and functional analysis: p > 0.001 uncorrected; lesion analysis: p < 0.05 | 50 voxels in structural analysis and 10 voxels in functional analysis | p < 0.05 or p < 0.001 |
Correlations between interoceptive awareness & structural MRI: - Combined bvFTD, AD & HC groups: IFG, STG, temporal pole, ACC, AMG, HPC and PHPC. - bvFTD group alone: temporal and parietal cortices plus the MCC, PHPC and AMG. Correlations between interoceptive awareness & resting state MRI: - Combined bvFTD, AD & HC group: IFG, HPC and PHPC. - bvFTD group alone: inferior, middle and superior frontal gyri including the prefrontal cortex. |
3.2. Characteristics of the selected studies
Most of the studies (13 papers) focused on exploring the neural substrates of altered insight into presence of disease/diagnosis (2 papers) or health condition status (7 papers) or reduction of insight into social cognition (4 papers). Other publications examined the neural bases of impaired insight into other specific objects such as memory, executive functions, motivation, ADL or interoception (Table 4).
Table 4.
Number of papers published (and its respective percentage) according to the objects of altered insight explored in frontotemporal dementia and associated syndromes*.
| Objects of insight | Number of papers | Percentage |
|---|---|---|
| Disease/diagnosis | 2 | 13.33% |
| Health condition | 7 | 46.67% |
| Social cognition | 4 | 26.67% |
| Memory | 1 | 6.67% |
| Motivation/Apathy | 1 | 6.67% |
| Activities of daily living | 2 | 13.33% |
| Executive functions | 1 | 6.67% |
| Interoception | 1 | 6.67% |
The commonest method used to assess insight was participant-informant discrepancy score (7 studies), followed by retrospective self-assessment versus actual performance discrepancy score and clinical judgement (4 papers respectively) (Table 5).
Table 5.
Methodology used for insight assessment and brain imaging.
| Brain imaging technique | Method of insight assessment | Total | ||
|---|---|---|---|---|
| Participant-informant discrepancy score | Self-ratings versus performances | Clinical judgment | ||
| Structural* | 6 | 2 | 1 | 9 |
| Functional⁎⁎ | 1 | 1 | 3 | 5 |
| Combined⁎⁎⁎ | 0 | 1 | 0 | 1 |
| Total | 7 | 4 | 4 | 15 |
No study used DTI, MEG, MRS or histological observations to explore any modality of lack of insight in FTD.
8 studies (88.89%) used MRI [Amanzio et al. (2016), Hornberger et al. (2014), Levy et al. (2018), Massimo et al. (2013), Rosen et al. (2010), Shany-Ur et al. (2014), Sollberger et al. (2014) and Zamboni et al. (2010)] and 1 (11.11%) used CT [Ichikawa et al. (2013)).
All studies used either PET or SPECT [Bastin et al. (2012), McMurtray et al. (2006), Mendez and Shapira (2005), Miller et al. (1997) and Ruby et al. (2007)].
1 study used structural MRI, EEG and resting state functional MRI [Garcia-Cordero et al. (2016)].
The predominant brain imaging method used was structural brain imaging (9 studies, 8 with MRI and 1 with CT). In contrast, less preferred techniques were functional neuroimaging (5 studies with PET or SPECT) and combined brain imaging methods (1 study with MRI, EEG and resting state). No studies using DTI, MEG, MRS or histological observations were identified (Table 5).
In terms of the cohorts studied, most of the works included bvFTD patients (14 papers), language variants of FTD (13 papers; 6 PNFA, 5 SD and 2 LA) and healthy controls (9 papers). Less frequent diagnostic samples included across the publications were patients with ALS, CBS and AD (Table 6).
Table 6.
Frontotemporal dementia-related diagnostic cohorts included across the studies reporting neural correlates of altered insight into diverse objects.
| Diagnoses | Numbers of studies including the diagnosis in reference | Percentage |
|---|---|---|
| bvFTD | 14 | 93.33% |
| nfPPA | 6 | 40.00% |
| SD | 5 | 33.33% |
| LA | 2 | 13.33% |
| ALS | 2 | 13.33% |
| CBS | 4 | 26.67% |
| AD | 5 | 33.33% |
| Others* | 2 | 13.33% |
| Controls | 9 | 60.00% |
Others included patients with MCI and frontal strokes.
3.3. Quality assessment of the papers selected for the present systematic review
The adapted version of the Newcastle-Ottawa Scale for cross-sectional studies utilised here (Supplementary Material 2) evaluated the accuracy of participant selection, variables used to classify comparability of groups, potentially confounding variables accounted for, and the validity of outcomes measures. Using this scale, the quality of the articles finally selected for this systematic were rated as fair (4–6 stars) or good (7–9 stars) (Table 7). According to Crombie's items (Zeng et al., 2015), they included study designs coherent with their objectives, along with valid measures for the variables under study and appropriate statistical analyses (Supplementary Material 3, Table 8). In addition, a qualitative examination suggested that they all included clear aims, collected data from reliable sources and used appropriate diagnostic inclusion and exclusion criteria to select participants (Supplementary Material 3, Table 9). Only a minority of studies reported dropouts during the data collection process (Supplementary Material 3, Table 9).
Table 7.
Quality assessment for the papers included in the present systematic review according to an adapted version of the Newcastle-Ottawa Scale for cross-sectional studies.
| Paper | Selection (out of 4 stars) | Comparability (out of 2 stars) | Outcome (out of 3 stars) | Total (out of 9 stars) |
|---|---|---|---|---|
| Amanzio et al. (2016) | ★★★ | ★★ | ★★ | ★★★★★★★ (7) |
| Bastin et al. (2012) | ★★ | ★★ | ★★ | ★★★★★★ (6) |
| Garcia-Cordero et al., 2016 | ★★ | ★★ | ★★ | ★★★★★★ (6) |
| Hornberger et al. (2014) | ★★★ | ★ | ★★ | ★★★★★★ (6) |
| Ichikawa et al. (2013) | ★★★★ | ★★ | ★★ | ★★★★★★★★ (8) |
| Levy et al. (2018) | ★★★ | ★ | ★★ | ★★★★★★ (6) |
| Massimo et al., 2013 | ★★★ | ★ | ★★ | ★★★★★★ (6) |
| McMurtray et al. (2006) | ★★★ | ★ | ★ | ★★★★★ (5) |
| Mendez and Shapira (2005) | ★★★ | ★ | ★★★★ (4) | |
| Miller et al. (1997) | ★★★ | ★ | ★ | ★★★★★ (5) |
| Rosen et al. (2010) | ★★★ | ★★ | ★★★★★ (5) | |
| Ruby et al. (2007) | ★★★★ | ★★ | ★★ | ★★★★★★★★ (8) |
| Shany-Ur et al. (2014) | ★★★★ | ★★ | ★★ | ★★★★★★★★ (8) |
| Sollberger at al. (2014) | ★★★★ | ★★ | ★★ | ★★★★★★★★ (8) |
| Zamboni et al. (2010) | ★★★ | ★ | ★★ | ★★★★★★ (6) |
Thresholds for converting the Newcastle-Ottawa scales to Agency for Healthcare Research and Quality (AHRQ) standards (good, fair, and poor) as applied elsewhere (Sharmin et al., 2017):
- 3 or 4 stars in selection domain plus 1 or 2 stars in comparability domain plus 2 or 3 stars in outcome/exposure domain = good quality.
- 2 stars in selection domain plus 1 or 2 stars in comparability domain plus 2 or 3 stars in outcome/exposure domain = fair quality.
- 0 or 1 star in selection domain plus 0 stars in comparability domain plus 0 or 1 stars in outcome/exposure domain = poor quality.
3.4. Broad objects of altered insight
3.4.1. Altered insight into presence of disease/diagnosis
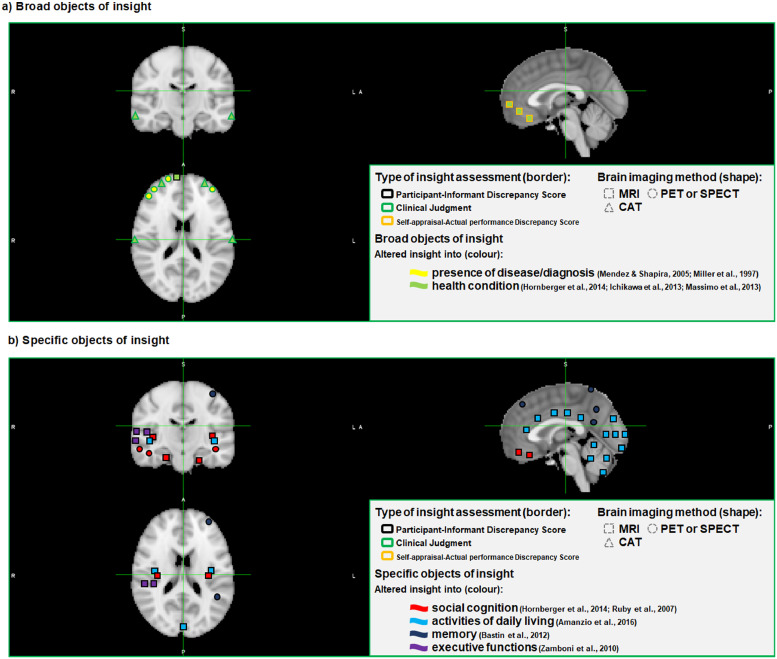
Utilising clinical judgment, Mendez and Shapira (2005) reported predominant right frontal dysfunctions (hypometabolism or hypoperfusion) in FTD patients showing impaired insight into disease status using either PET or SPECT, whereas Miller et al. (1997) observed links between this object and variable bilateral, left- or right-sided frontotemporal dysfunction in FTD (Tables 2 and 3 and Fig. 2a).
Fig. 2.
Brain areas involved in altered insight into different objects in patients with frontotemporal dementia and associated syndromes
MRI = magnetic resonance imaging; PET = positron emission tomography; SPECT = single-photon emission computed tomography; CT = computed axial tomography
This figure was created with the Montreal Neurological Institute (MNI) template. The coloured figures represent spatial approximations only (see Tables 2 and 3 for more accurate details).
Garcia-Cordero et al. (2016), Levy et al. (2018), McMurtray et al. (2006), Rosen et al. (2010), Shany-Ur et al. (2014) and Sollberger et al. (2014) were not illustrated in this figure.
3.4.2. Altered insight into health condition
Using clinical judgement and SPECT and in accordance with Mendez and Shapira's results (Mendez and Shapira, 2005), McMurtray et al. (2006) encountered associations between altered insight into health condition and right frontal hypoperfursion. In contrast, Levy et al.'s study (Levy et al., 2018) used clinical judgement and structural MRI brain scans, identifying correlations between this object and shrinkage of grey matter over the left orbitofrontal cortex and right regions of the anterior cingulate in FTD, CBS and PPA. After conducting a longitudinal study with CT brain scans, Ichikawa et al. (2013) found altered insight into health condition, measured through discrepancy scores between patients’ and clinical judgements, to correlate with sizes of the anterior and inferior horns (indexes of frontotemporal atrophy), in ALS and especially in the ALS-FTD patients.
In a slightly different vein, Hornberger et al.'s study (Hornberger et al., 2014) reported neural correlates for overall insight, which represented an average of components such as diagnosis/treatment, social cognition, language and motivation/organization. Using patient-informant discrepancy scores and structural brain imaging, correlations between altered overall insight and reduction of grey matter across bilateral ventromedial/orbitofrontal and left frontopolar areas were found in a mixed sample of behavioural and language variants of FTD and AD. Significant correlations did not emerge when each patient group was analysed separately (Hornberger et al., 2014) (Table 2 and Fig. 2a).
Massimo et al. (2013) and Rosen et al. (2010) examined the discrepancy between subjective ratings of task performance and objective standardised performance scores, and explored correlations with structural MRI findings. Poor self-appraisal accuracy into multi-domain cognitive decline (average performance on grammatical, comprehension and visual episodic memory tasks) correlated with grey matter density in the ventral and rostral medial prefrontal cortex areas in AD and bvFTD, whilst exclusively with the subgenual cingulate (BA 25) in bvFTD (Massimo et al., 2013). Additionally, altered self-appraisal into multi-domain cognitive impairment (average performance on working memory/attention, episodic memory and executive functions tasks) was correlated with grey matter content in the right ventromedial prefrontal cortex in patients with a wide range of diagnoses including FTD, AD, ALS, MCI and controls (Rosen et al., 2010). On the other hand, through a patient-informant discrepancy score-based assessment, Shany-Ur's MRI study (Shany-Ur et al., 2014) found that overestimation of overall cognitive functioning corresponded with atrophy of bilateral superior and middle frontal gyri, right inferior frontal and cingulate gyri, middle temporal gyrus, insula and caudate in FTD, AD and controls (Tables 2 and 3, Fig. 2a).
3.5. Specific objects of altered insight
3.5.1. Altered insight into social cognition
Findings on altered insight into social cognition can be divided into two aspects, those related to mentalizing and others linked with emotion processing. Mentalizing, also referred to as theory of mind, can be defined as inferring cognitive or emotional states in others (Amodio and Frith, 2006; Frith and Frith, 2012). Hornberger et al.'s study (Hornberger et al., 2014) found correlations between reduced awareness into mentalizing and atrophies across the insula bilaterally, the left orbitofrontal cortex, left parahippocampal zones, the right middle temporal gyrus and the right amygdala in a mixed group of FTD and AD patients. Using methods compatible with this work, Shany-Ur et al.'s study (Shany-Ur et al., 2014) and Sollberger et al. (2014) found similar results. Shany-Ur et al.'s study (Shany-Ur et al., 2014) reported correlations between overestimation of interpersonal abilities and atrophy of the right orbitofrontal cortex, fusiform gyrus, anterior insula and putamen in a mixed group of AD, FTD and controls. Sollberger et al.'s study (Sollberger et al., 2014) found significant correlations between insight into mentalizing and grey matter density in right-hemispheric anterior inferolateral temporal regions in a combined cohort of people with behavioural and language variant of FTD, CBS and AD and controls. In contrast, Ruby et al. (2007) observed relations between bvFTD patients’ insight into the capacity to interpret social situations and reduced metabolic activity in the left temporal pole using participant-informant discrepancy scores and PET (Table 2 and Fig. 2b).
Emotion processing can also be defined as the ability to recognise emotions expressed by others (Amodio and Frith, 2006; Frith and Frith, 2012). Hornberger et al.'s study (Hornberger et al., 2014) reported significant associations between altered insight into emotion processing ability and atrophy of bilateral frontopolar regions, right dorsolateral prefrontal cortex, the supplementary motor area, the anterior cingulate bilaterally, and the left amygdala in AD and FTD. On the other hand, Shany-Ur et al.'s study (Shany-Ur et al., 2014) found that overestimation of competency in social interactions was associated with atrophy of bilateral orbitofrontal and anterior insular areas, right superior frontal gyrus, anterior cingulate cortex and the caudate across a mixed sample of patients with FTD, AD and controls.
3.5.2. Altered insight into memory problems
Only one study reported neural correlates of metamemory, that is, self-awareness and self-monitoring of memory, in patients with FTD. Bastin et al. (2012) conducted a study aimed at measuring autonoetic consciousness with a feeling-of-knowing task and PET scans. Autonoetic consciousness, defined as the feeling of being able to recall the encoding context during memory retrieval, was markedly impaired in bvFTD patients. Those patients with reduced autonoetic consciousness showed reduced metabolism in the left anterior medial frontal cortex, the left middle frontal cortex near the superior frontal sulcus, the right postcentral gyrus, the left inferior parietal cortex, and the posterior cingulate cortex (Tables 2 and 3 and Fig. 2b). No structural studies exploring altered insight into memory problems in FTD and its associated syndromes were found.
3.5.3. Altered insight into dysexecutive behaviours and executive function problems
When exploring altered insight into dysexecutive disorders and decline in executive function with the use of MRI scans and patient-informant discrepancy scores, Zamboni et al. (2010) showed that grey matter loss in a region extending from the right superior temporal sulcus to the right inferior temporal gyrus (posterior region of the right superior temporal sulcus, adjacent to the temporoparietal junction) correlated with worsening of insight into behavioural disturbances in a cohort made up of patients with behavioural and language variants of FTD along with patients with CBS, where bvFTD patients tended to underestimate their present behavioural disturbances and overestimate their premorbid behavioural disturbances (Tables 2 and 3 and Fig. 2b).
3.5.4. Impaired insight into performances on activities of daily living
After conducting a structural study with patients with bvFTD and controls, utilising patient versus informant discrepancy scores and MRI, Amanzio et al. (2016) suggested that decreased density of grey matter in the cuneus, anterior and middle cingulate cortices and posterior cerebellum correlated with reduced insight into instrumental ADL. Complementary, Shany-Ur et al. (2014) found, with a similar methodological design, that overestimation of performance on instrumental ADL corresponded with atrophy in widespread right frontal regions (orbital inferior and superior frontal gyri, medial orbitofrontal cortex, dorsal middle and superior frontal gyri), medial and lateral temporal lobe regions, anterior insula, putamen, thalamus and the pons in FTD, AD and controls (Tables 2 and 3 and Fig. 2b).
3.5.5. Altered insight into motivation
Hornberger et al.''s MRI study (Hornberger et al., 2014) suggested that insight into motivation assessed by patient versus informant discrepancy score was associated to bilateral orbitofrontal, left anterior cingulate, and right frontopolar shrinkage in a mixed group of AD and FTD patients.
3.5.6. Altered insight into interoception
Garcia-Cordero et al. (2016) explored the neural correlates of interoceptive awareness within a cohort of patients with bvFTD, AD, frontal strokes and controls using EEG, structural MRI and resting state sequences. First, interoceptive accuracy was assessed by asking participants to tap a keyboard in time with their own heartbeat over 3 phases: no feedback, learning (with a stethoscope as means of feedback) and no feedback again. Then, interoceptive awareness was measured by asking participants to estimate how confident they thought they were at performing the task. Compared to controls, patients with AD and FTD showed significant deficits in their confidence in reporting biological changes. Interoceptive awareness was related to atrophy/vascular impairment/dysfunction across a broad frontotemporal, parietal and limbic-insular network.
4. Discussion
The present systematic review sheds certain lights on how different types of altered insight are mapped across the brain in FTD and associated symptoms; however, it also sparks several issues that require discussion.
4.1. Neural correlates of altered insight appear to vary according to the object of insight in reference in FTD and associated syndromes
The main goal of this systematic review was to investigate whether different objects of altered insight are linked to different or compatible brain areas in FTD. As hypothesized, overarching altered insight and altered insight into specific neuropsychological/behavioural domains seem to be underpinned by different brain areas in FTD and associated syndromes. This hypothesis is supported by the findings of this review.
Overarching altered insight (into the presence of disease/diagnosis or health condition) in FTD is predominately associated with right frontal hypometabolism/hypoperfusion (McMurtray et al., 2006; Mendez and Shapira, 2005) or shrinkages of frontal regions that contain left orbitofrontal cortex and right anterior cingulate (Levy et al., 2018). In contrast, different modalities of insight into specific neuropsychological/behavioural domains are apparently sustained by specific brain regions. For example, altered insight into social cognition correlates with grey matter density in right inferotemporal regions in FTD, AD, CBS and controls (Sollberger et al., 2014) or the orbitofrontal cortex and limbic subcortical regions when combining FTD and AD patients (Hornberger et al., 2014). Additionally, inaccuracy in estimating memory performances in bvFTD can be accounted for by dysfunctions of the frontal, parietal and limbic lobes (Bastin et al., 2012), whereas poor insight into performances in ADL in bvFTD and controls seems to be mediated by frontal medial regions, the insula and also posterior areas of the cerebellum (Amanzio et al., 2016).
4.2. Comparisons with neural foundations of altered insight in other brain disorders
The findings of the studies reviewed here on overarching altered insight resemble partly those seen in other neurodegenerative diseases. As noted in FTD (McMurtray et al., 2006; Mendez and Shapira, 2005), impaired disease/symptoms insight correlates with reduced blood flow in right frontal inferior and superior regions in AD (Starkstein et al., 1995). However, hypometabolism and atrophy in the left dorsal anterior cingulate has also been associated with reduced insight in AD (Guerrier et al., 2018). Conversely in other analyses, altered insight has not been shown to correlate with cortical thickness in a mixed group of MCI and AD (Senturk et al., 2017).
This scenario appears to be in accordance with observations made in other brain disorders. For instance, disease awareness appears to be associated to frontal lobes and basal ganglia in VD (Starkstein et al., 1996). In patients with TBI, overall insight into one's own personality traits has been paired with higher activity in the right anterior dorsal prefrontal cortex (Schmitz et al., 2006). Furthermore, patients with SCZ exhibit associations between disease unawareness and reductions of grey matter density in the right anterior cingulate, left posterior cingulate and bilateral temporal inferior regions (Ha et al., 2004), the prefrontal cortex (Shad et al., 2006) and total cranial volume (Flashman et al., 2000), together with hypoactivations of frontal, parietal, basal ganglia and limbic areas (Shad and Keshavan, 2015). However, results obtained from larger cohorts of SCZ patients have not found significant correlations between illness awareness and cortical thickness or subcortical involvement (Beland et al., 2019).
In healthy controls, activation in the right ventrolateral prefrontal cortex and the midbrain were respectively found when the assessment of insight was split into self-reflectiveness and self-certainty within an external memory paradigm (Buchy et al., 2014). Additionally, a cohort of healthy individuals and patients with subjective cognitive decline exhibited a significant correlation between altered insight into cognitive changes and grey matter volumes of the left posterior hippocampus and cerebellar areas (Sanchez-Benavides et al., 2018).
As observed in FTD, the brain areas of a particular insight object seem to be compatible with those that may be responsible for that object in control subjects and patients with different brain disorders. For example, social interoceptive accuracy in healthy controls has been accounted for by higher activations mainly in right frontal, cingulate and parahippocampal regions, whereas in SCZ patients this has been correlated with frontotemporal and occipital areas (Pinkham et al., 2018). While metamemory in healthy controls appears to be primarily underpinned by a wide network of medial temporal, frontotemporal and parietal areas (Chua et al., 2009), altered metamemory has been associated with reduced cortical thickness in right frontal and cingulate areas in a combined sample of controls and AD patients (Bertrand et al., 2018). In relation to AD patients only, insight into memory performance is linked with grey matter volumes in the left superior frontal gyrus (Fujimoto et al., 2017), while in amnestic MCI patients both the posterior cingulate cortex and the hippocampi are compromised (Vannini et al., 2017).
4.3. Study methodologies may shape their respective outcomes
The evidence analysed in this systematic review suggests that distinct insight objects are subserved by different neural correlates in FTD. However, this affirmation should be interpreted with caution due to the diversity of methodological approaches that led to such results. Considerable variability was seen in the conceptualization of insight, insight assessment methods, and brain imaging techniques used.
4.3.1. Conceptualization of insight
The articles selected for this systematic review used various labels to denote impaired self-knowledge of disease/symptoms. Whilst anosognosia (McMurtray et al., 2006; Mendez and Shapira, 2005) and reduced self-awareness mostly addressed disease unawareness, reduced insight itself, autonoetic consciousness or awareness embraced respectively the insight objects of social cognition (Hornberger et al., 2014), episodic memory (Bastin et al., 2012) and ADL (Amanzio et al., 2016). Other publications specified with insufficient precision the insight object under study. Anosognosia appeared to be occasionally extrapolated to a global condition when seeking the neural bases of altered insight into the presence of frontal behaviours (Zamboni et al., 2010). Congruently, unsuccessful post-test judgements on particular attentional, language and memory performances, once averaged, tended to be overgeneralized to mean lack of overall insight (Rosen et al., 2010) or impaired multi-domain self-appraisal (Massimo et al., 2013). Such a remarkable variety in the terms used to characterize altered insight in neuropsychiatric disorders has been also highlighted previously elsewhere (Zamboni and Wilcock, 2011; Gilleen et al., 2010; Markova and Berrios, 2000).
The wide array of terms used to label insight raises doubts as to whether researchers were investigating the same phenomena. This inconsistency can be attributed to the intrinsic complexity of the conceptualization of insight (Markova et al., 2005, 2014; Markova and Berrios, 2011). We propose that relating insight to a specified object will aid the search for its neural correlates in different neurodegenerative and neuropsychiatric conditions.
4.3.2. Insight assessment
A wide range of different insight assessment approaches have been reported, which coincides with the variability in the conceptualization of the term and may introduce further heterogeneity to the literature. This situation questions whether the resulting neural correlates of altered insight may be shaped by their definitions and insight assessment methods. Comparing the neural outcomes obtained from different types of insight evaluation procedures focused on a particular object over the same cohort may provide an answer (Table 2 and Fig. 2).
Levy et al. (2018) partially addressed such a problem contrasting results from participant-informant discrepancy scores and clinical judgment assessment methods in FTD and CBS. In comparison with participant-informant discrepancy scores, clinical judgement accounted for more variance of altered insight in orbitofrontal and anterior cingulate grey matter. However, these findings are debatable as the insight objects respectively contrasted differed, corresponding to altered insight into dysexecutive behaviours when participant-informant discrepancy score was used and disease unawareness when clinical judgement was used (Levy et al., 2018).
A recent study investigated whether different insight assessment methods were linked with compatible neuroanatomical correlates in MCI and AD (Tondelli et al., 2018). Insight into disease status was measured by clinical judgement and patient-informant discrepancy scores, with compatible temporomedial neural correlates emerging from each measure. Moreover, discrepancy scores between self-appraisal and standardised scores on cognitive tasks yielded the same results (Tondelli et al., 2018). There is a need for comparison of different insight assessment methodologies in FTD cohorts, to establish whether experimental results will be influenced by the choice of assessment method.
4.3.3. Brain imaging
The influence of different brain imaging techniques on study results was not thoroughly investigated among the studies reviewed here. Only one study examined this issue to a certain extent. Garcia-Cordero et al. (2016) used structural and functional MRI to investigate the neural substrates of interoceptive awareness and both imaging techniques suggested similar findings (Garcia-Cordero et al., 2016).
4.4. Considerations relating to the critical appraisal of the papers included in the present systematic review
Although the studies reviewed in this manuscript presented well-defined methods and were of satisfactory quality, methodological issues remain. No study explicitly declared whether the researchers who conducted the evaluation of insight were blind to the diagnoses of the participants or imaging findings. Additionally, certain studies did not include cognitively healthy controls (Hornberger et al., 2014; Levy et al., 2018; McMurtray et al., 2006; Mendez and Shapira, 2005; Miller et al., 1997). Other studies excluded covariables like age, years of education, disease severity or total intracranial volume from the regression models used to link altered insight and brain involvement (Ichikawa et al., 2013; Levy et al., 2018; Massimo et al., 2013; Ruby et al., 2007), which could have influenced the reported results. In the same line, certain investigations applied weak statistical techniques to suggest neural substrates for altered insight in FTD (McMurtray et al., 2006; Mendez and Shapira, 2005; Miller et al., 1997). Other confounders are small groups sizes, and the use of mixed cohorts including participants with different cognitive diagnoses. For example, several studies included small FTD subgroups of roughly 10 participants (Bastin et al., 2012; Ichikawa et al., 2013; Rosen et al., 2010). Moreover, others combined FTD with AD (Massimo et al., 2013), FTD, AD and patients with strokes (Garcia-Cordero et al., 2016) or FTD, MCI, AD, ALS and CBS (Rosen et al., 2010). Aside from this, the heterogeneity of the pathology that causes FTD (Cairns et al., 2007; Mackenzie et al., 2009; Mackenzie et al., 2010; Kovacs, 2016) can result in syndromes characterised by different symptoms, either predominantly behavioural, language associated or motor (Rascovsky et al., 2011; Piguet et al., 2011; Gorno-Tempini et al., 2011; Strong et al., 2017, 2009), which at the same time can hinder the search of the neural basis of altered insight in FTD.
To the best of the authors’ knowledge this is the first systematic review on neural correlates of altered insight in FTD and associated syndromes conducted according to predefined structured procedures such as those proposed by the PRISMA statement (Liberati et al., 2009; Moher et al., 2009). Other reviews on the matter using alternative methodologies have already reported assessment methods for insight in dementia (Clare et al., 2005), neural bases of insight in AD and other dementias (Ecklund-Johnson and Torres, 2005); neuropsychological patterns of altered insight/unawareness (DeLozier and Davalos, 2016) and neuroanatomical correlates for lack of awareness (Zamboni and Wilcock, 2011) in AD and FTD.
4.5. Altered insight and models of awareness
The DICE model recognizes insight's neurocognitive multidimensionality; however it seems to oversimplify a highly complex phenomenon and explain insufficiently memory's crucial role in its formation (Agnew and Morris, 1998). The Cognitive Awareness Model (CAM) model vindicates memory as a critical centre for a ‘personal knowledge base’ (PKB). In CAM, a faulty functioning of a ‘memory comparator’ (MC) can trigger different forms of altered insight. Thus, inaccurate updates of the PKB generated by a MC can make a subject with altered insight assume their abilities are unchanged from their premorbid state (Agnew and Morris, 1998).
CAM was reformulated (CAM-R) to include other components that potentially support insight (Morris and Hannesdottir, 2004). A ‘personal data base’ (PDB), equivalent to the PKB, interacts with an ‘Autobiographical Conceptual Memory System’ (ACMS), ‘comparator mechanisms’ (CMs) and a ‘Metacognitive Awareness System’ (MAS) (Morris and Hannesdottir, 2004; Morris and Mograbi, 2013). In CAM-R, diverse types of impaired insight are a consequence of disruptions in CMs, which wrongly update the PDB. Complementary, the conscious recognition of both abilities and deficits is controlled by the MAS, which finally embodies insight mechanisms (Morris and Hannesdottir, 2004; Morris and Mograbi, 2013).
Other models like the Self-Memory System (SMS) (Conway, 2005) have also stressed the central role played by memory processes in the construction of self-images. In a similar line, it has been postulated that disruptions in the interplay between anterograde and retrograde memory may result in an incomplete upgrade of personal information (Mograbi et al., 2009). This reduced consolidation of self-knowledge (non-updated PDB) may generate then a “petrified self” made up of distorted previous representations (Mograbi et al., 2009).
Unlike DICE, the aforementioned models seem to fail at proposing specific neural correlates for different forms of altered insight. Recently, Berlingeri et al. (2015) experimentally tested the CAM-R with cognitive tasks and functional brain imaging in patients with dementia. Altered processing of new personal information (inability to update the PDB) and faulty consolidation (disintegrated PDB) correlated with dysfunctions in lateral temporal and insular cortices along with the hippocampal complex. Moreover, it was suggested that altered insight into memory was associated with the disrupted connectivity in the default mode network (DMN) (Berlingeri et al., 2015).
More research is needed to experimentally test models of insight in FTD and associated syndromes. Although the brain areas implicated in altered insight proposed by the cited models of awareness may be relatively equivalent with the findings summarized in this systematic review, the number of articles revised is insufficient to draw firmer conclusions on the neural circuits that underpin different types of insight. Further generalizations are subject to limitations and go beyond of the scope of this review.
4.6. Altered insight and neural networks
Several neural networks with structural and functional differentiations have attracted the attention of dementia researchers. Among them, the salience network (SN) processes external and internal significant socio-emotional information and includes the frontoinsula and the pregenual anterior cingulate (Ranasinghe et al., 2016). The semantic appraisal network (SAN), which is juxtaposed with the SN, is responsible for self-evaluation functions influenced by semantic frameworks and involves the temporal pole, ventral striatum, subgenual cingulate and amygdala (Ranasinghe et al., 2016). The DMN, distributed across temporoparietal, cingulate and hippocampal areas, corresponds to circuits that deactivate when performing certain cognitive functions, but activates in memory retrieval, mental state attribution and visual imagery (Zhou and Seeley, 2014).
Both SN and SAN are predominantly affected in FTD (Ranasinghe et al., 2016), which contrasts with the characteristic vulnerability of the DMN in AD (Zhou and Seeley, 2014). Aside from this, FTD appears to exhibit reductions of activity in frontotemporal areas of the DMN and enhanced posterior DMN connectivity, whereas AD tend to have enhanced SN connectivity (Zhou and Seeley, 2014). It has been proposed that the patterns of deterioration described across those neural networks can account for the alterations of self-projection (Irish et al., 2012), insight and other metacognitive phenomena (Zhou and Seeley, 2014) observed in FTD. Although it is possible to acknowledge certain overlap between the structures and functions of the mentioned networks and the findings analysed in this systematic review, more research is needed to clarify how insight can be shaped by those circuits.
4.7. Future directions
Larger group sizes with cognitively healthy control groups and triangulation of different neuropsychological methods for insight assessment and neuroimaging modalities are needed. Examining the findings obtained through different brain imaging techniques over the same insight object and cohort of participants may provide neuroanatomical validity to insight measures. Ideally, patients with clinical FTD should be subgrouped according to their exact clinical syndrome and likely underlying neuropathology, although this is challenging in a relatively rare disease. Counterintuitively, healthy controls from several studies reviewed here were inaccurate when judging their own abilities, although altered insight was significantly more severe in patients with bvFTD. It would be of great interest to elucidate whether different brain imaging techniques, for instance, voxel-based morphometry, cortical thickness, diffusion tensor imaging or resting state, yield compatible results in the examination of one particular object of insight measured through a unique or several different approaches over the same cohort. Longitudinal studies with repeated measures of insight and brain imaging over a number of time points will also help enhance our understanding of how different brain regions contribute to the deterioration in insight seen over the disease course in FTD.
Funding
Carlos Muñoz-Neira is sponsored by the Government of Chile through 'Becas Chile' and CONICYT - National Commission for Scientific and Technological Research [CONICYT - Comisión Nacional de Investigación Científica y Tecnológica] and University of Bristol (Grant Code G100030-150).
Declaration of Competing Interest
The authors of this paper declare no conflicts of interest.
Acknowledgements
The authors of this paper must thank the librarians Ms Catherine Borwick from Medical Libraries, University of Bristol, and Mr Bennet Jones from North Bristol NHS Trust Libraries for the help provided in the design of the search strategy and the conduction of the search itself of this systematic review.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2019.102066.
Appendix. Supplementary materials
References
- Aalten P., Van Valen E., Clare L., Kenny G., Verhey F. Awareness in dementia: a review of clinical correlates. Aging Ment. Health. 2005;9(5):414–422. doi: 10.1080/13607860500143075. [DOI] [PubMed] [Google Scholar]
- Aalten P., van Valen E., de Vugt M.E., Lousberg R., Jolles J., Verhey F.R.J. Awareness and behavioral problems in dementia patients: a prospective study. Int. Psychogeriatr. 2006;18(1):3–17. doi: 10.1017/S1041610205002772. [DOI] [PubMed] [Google Scholar]
- Agnew S.K., Morris R.G. The heterogeneity of anosognosia for memory impairment in Alzheimer's disease: a review of the literature and a proposed model. Aging Ment. Health. 1998;2(1):7–19. [Google Scholar]
- Amanzio M., D'Agata F., Palermo S., Rubino E., Zucca M., Galati A. Neural correlates of reduced awareness in instrumental activities of daily living in frontotemporal dementia. Exp. Gerontol. 2016;83:158–164. doi: 10.1016/j.exger.2017.05.022. [DOI] [PubMed] [Google Scholar]
- Amodio D.M., Frith C.D. Meeting of minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci. 2006;7(4):268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Banks S.J., Weintraub S. Generalized and symptom-specific insight in behavioral variant frontotemporal dementia and primary progressive aphasia. J. Neuropsychol. Clin. Neurosci. 2009;21(3):299–306. doi: 10.1176/appi.neuropsych.21.3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastin C., Feyers D., Souchay C., Guillaume B., Pepin J.-.L., Lemaire C. Frontal and posterior cingulate metabolic impairment in the behavioral variant of frontotemporal dementia with impaired autonoetic consciousness. Hum. Brain Mapp. 2012;33(6):1268–1278. doi: 10.1002/hbm.21282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beland S., Makowski C., Konsztowicz S., Buchy L., Chakravarty M.M., Lepage M. Clarifying associations between cortical thickness, subcortical structures, and a comprehensive assessment of clinical insight in enduring schizophrenia. Schizophr. Res. 2019;204:245–252. doi: 10.1016/j.schres.2018.08.024. [DOI] [PubMed] [Google Scholar]
- Berlingeri M., Ravasio A., Cranna S., Basilico S., Sberna M., Bottini G. Unrealistic representations of "the self': a cognitive neuroscience assessment of anosognosia for memory deficit. Conscious Cognit. 2015;37:160–177. doi: 10.1016/j.concog.2015.08.010. [DOI] [PubMed] [Google Scholar]
- Bertrand E., Azar M., Rizvi B., Brickman A.M., Huey E.D., Habeck C. Cortical thickness and metacognition in cognitively diverse older adults. Neuropsychology. 2018;32(6):700–710. doi: 10.1037/neu0000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisiach E., Vallar G., Perani D., Papagno C., Berti A. Unawareness of disease following lesions of the right hemisphere: anosognosia for hemiplegia and anosognosia for hemianopia. Neuropsychologia. 1986;24(4):471–482. doi: 10.1016/0028-3932(86)90092-8. [DOI] [PubMed] [Google Scholar]
- Booth A., Clarke M., Dooley G., Ghersi D., Moher D., Petticrew M. The nuts and bolts of PROSPERO: an international prospective register of systematic reviews. Syst. Rev. 2012;1:2. doi: 10.1186/2046-4053-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth A., Clarke M., Ghersi D., Moher D., Petticrew M., Stewart L. An international registry of systematic-review protocols. Lancet. 2011;377(9760):108–109. doi: 10.1016/S0140-6736(10)60903-8. [DOI] [PubMed] [Google Scholar]
- Buchy L., Hawco C., Bodnar M., Izadi S., Dell'Elce J., Messina K. Functional magnetic resonance imaging study of external source memory and its relation to cognitive insight in non-clinical subjects. Psychiatry Clin. Neurosci. 2014;68(9):683–691. doi: 10.1111/pcn.12177. [DOI] [PubMed] [Google Scholar]
- Cairns N.J., Bigio E.H., Mackenzie I.R., Neumann M., Lee V.M., Hatanpaa K.J. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114(1):5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua E.F., Schacter D.L., Sperling R.A. Neural correlates of metamemory: a comparison of feeling-of-knowing and retrospective confidence judgments. J. Cognit. Neurosci. 2009;21(9):1751–1765. doi: 10.1162/jocn.2009.21123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clare L., Markova I., Verhey F., Kenny G. Awareness in dementia: a review of assessment methods and measures. Aging Ment. Health. 2005;9(5):394–413. doi: 10.1080/13607860500142903. [DOI] [PubMed] [Google Scholar]
- Conway M.A. Memory and the self. J. Mem. Lang. 2005;53(4):594–628. [Google Scholar]
- David A.S., Bedford N., Wiffen B., Gilleen J. Failures of metacognition and lack of insight in neuropsychiatric disorders. Philos. Trans. R. Soc. B. 2012;367(1594):1379–1390. doi: 10.1098/rstb.2012.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLozier S.J., Davalos D. A systematic review of metacognitive differences between Alzheimer's disease and frontotemporal dementia. Am. J. Alzheimers Dis. Dement. 2016;31(5):381–388. doi: 10.1177/1533317515618899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecklund-Johnson E., Torres I. Unawareness of deficits in alzheimer's disease and other dementias: operational definitions and empirical findings. Neuropsychol. Rev. 2005;15(3):147–166. doi: 10.1007/s11065-005-9026-7. [DOI] [PubMed] [Google Scholar]
- Eslinger P.J., Dennis K., Moore P., Antani S., Hauck R., Grossman M. Metacognitive deficits in frontotemporal dementia. J. Neurol. Neurosurg. Psychiatry. 2005;76(12):1630–1635. doi: 10.1136/jnnp.2004.053157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flashman L.A., McAllister T.W., Andreasen N.C., Saykin A.J. Smaller brain size associated with unawareness of illness in patients with schizophrenia. Am. J. Psychiatry. 2000;157(7):1167–1169. doi: 10.1176/appi.ajp.157.7.1167. [DOI] [PubMed] [Google Scholar]
- Frith C.D., Frith U. Mechanisms of social cognition. Annu. Rev. Psychol. 2012;63:287–313. doi: 10.1146/annurev-psych-120710-100449. [DOI] [PubMed] [Google Scholar]
- Fujimoto H., Matsuoka T., Kato Y., Shibata K., Nakamura K., Yamada K. Brain regions associated with anosognosia for memory disturbance in alzheimer's disease: a magnetic resonance imaging study. Neuropsychol. Dis. Treat. 2017;13:1753–1759. doi: 10.2147/NDT.S139177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cordero I., Sedeno L., de la Fuente L., Slachevsky A., Forno G., Klein F. Feeling, learning from and being aware of inner states: interoceptive dimensions in neurodegeneration and stroke. Philos. Trans. R. Soc. B-Biol. Sci. 2016;371(1708):10. doi: 10.1098/rstb.2016.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini L.A.A., Irwin D.J., McMillan C.T., Ash S., Rascovsky K., Wolk D.A. Clinical marker for Alzheimer disease pathology in logopenic primary progressive aphasia. Neurology. 2017;88(24):2276–2284. doi: 10.1212/WNL.0000000000004034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleen J., Greenwood K., David A.S. Lack of insight and awareness in schizophrenia and neuropsychiatric disorders. In: Miyoshi K, Morimura Y, Maeda K, editors. Neuropsychiatric Disorders. Springer Science & Business Media; 2010. pp. 33–50. [Google Scholar]
- Gorno-Tempini M.L., Hillis A.E., Weintraub S., Kertesz A., Mendez M., Cappa S.F. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff-Radford J., Rubin M.N., Jones D.T., Aksamit A.J., Ahlskog J.E., Knopman D.S. The alien limb phenomenon. J. Neurol. 2013;260(7):1880–1888. doi: 10.1007/s00415-013-6898-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrier L., Le Men J., Gane A., Planton M., Salabert A.S., Payoux P. Involvement of the cingulate cortex in anosognosia: a multimodal neuroimaging study in Alzheimer's disease patients. J. Alzheimers Dis. 2018;65(2):443–453. doi: 10.3233/JAD-180324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha T.H., Youn T., Ha K.S., Rho K.S., Lee J.M., Kim I.Y. Gray matter abnormalities in paranoid schizophrenia and their clinical correlations. Psychiatry Res. 2004;132(3):251–260. doi: 10.1016/j.pscychresns.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Hanyu H., Sato T., Akai T., Shimizu S., Hirao K., Kanetaka H. Neuroanatomical correlates of unawareness of memory deficits in early Alzheimer's disease. Dement. Geriatr. Cognit. 2008;25(4):347–353. doi: 10.1159/000119594. [DOI] [PubMed] [Google Scholar]
- Harwood D.G., Sultzer D.L., Feil D., Monserratt L., Freedman E., Mandelkern M.A. Frontal lobe hypometabolism and impaired insight in Alzheimer disease. Am. J. Geriatr. Psychiatry. 2005;13(11):934–941. doi: 10.1176/appi.ajgp.13.11.934. [DOI] [PubMed] [Google Scholar]
- Hornberger M., Yew B., Gilardoni S., Mioshi E., Gleichgerrcht E., Manes F. Ventromedial-frontopolar prefrontal cortex atrophy correlates with insight loss in frontotemporal dementia and Alzheimer's disease. Hum. Brain Mapp. 2014;35(2):616–626. doi: 10.1002/hbm.22200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa H., Ohno H., Murakami H., Ishigaki S., Ohnaka Y., Kawamura M. Self-rated anosognosia score may be a sensitive and predictive indicator for progressive brain atrophy in amyotrophic lateral sclerosis: an X-ray computed tomographic study. Eur. Neurol. 2013;69(3):158–165. doi: 10.1159/000345371. [DOI] [PubMed] [Google Scholar]
- Iliffe S., De Lepeleire J., Van Hout H., Kenny G., Lewis A., Vernooij-Dassen M. Understanding obstacles to the recognition of and response to dementia in different European countries: a modified focus group approach using multinational, multi-disciplinary expert groups. Aging Ment. Health. 2005;9(1):1–6. doi: 10.1080/13607860412331323791. [DOI] [PubMed] [Google Scholar]
- Irish M., Piguet O., Hodges J.R. Self-projection and the default network in frontotemporal dementia. Nat. Rev. Neurol. 2012;8(3):152–161. doi: 10.1038/nrneurol.2012.11. [DOI] [PubMed] [Google Scholar]
- Koch T., Iliffe S., project E.-.E. Rapid appraisal of barriers to the diagnosis and management of patients with dementia in primary care: a systematic review. BMC Fam. Pract. 2010;11:52. doi: 10.1186/1471-2296-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs G.G. Molecular pathological classification of neurodegenerative diseases: turning towards precision medicine. Int. J. Mol. Sci. 2016;17(2) doi: 10.3390/ijms17020189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S., Gansler D., Huey E., Wassermann E., Grafman J. Assessment of patient self-awareness and related neural correlates in frontotemporal dementia and corticobasal syndrome. Arch. Clin. Neuropsychol. 2018;33(5):519–529. doi: 10.1093/arclin/acx105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P. The Prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie I.R.A., Neumann M., Bigio E.H., Cairns N.J., Alafuzoff I., Kril J. Nomenclature for neuropathologic subtypes of frontotemporal lobar degeneration: consensus recommendations. Acta Neuropathol. 2009;117(1):15–18. doi: 10.1007/s00401-008-0460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie I.R.A., Neumann M., Bigio E.H., Cairns N.J., Alafuzoff I., Kril J. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol. 2010;119(1):1–4. doi: 10.1007/s00401-009-0612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markova I.S., Berrios G.E. Insight into memory deficits. In: Berrios GE, Hodges JR, editors. Memory Disorders in Psychiatric Practice. Cambridge University Press; Cambridge: 2000. pp. 204–233. [Google Scholar]
- Markova I.S., Berrios G.E. The 'object' of insight assessment: relationship to insight 'structure'. Psychopathology. 2001;34(5):245–252. doi: 10.1159/000049317. [DOI] [PubMed] [Google Scholar]
- Markova I.S., Berrios G.E. Awareness and insight in psychopathology: an essential distinction? Theor. Psychol. 2011;21(4):421–437. [Google Scholar]
- Markova I.S., Clare L., Wang M., Romero B., Kenny G. Awareness in dementia: conceptual issues. Aging Ment. Health. 2005;9(5):386–393. doi: 10.1080/13607860500142945. [DOI] [PubMed] [Google Scholar]
- Markova I.S., Clare L., Whitaker C.J., Roth I., Nelis S.M., Martyr A. Phenomena of awareness in dementia: heterogeneity and its implications. Conscious Cognit. 2014;25:17–26. doi: 10.1016/j.concog.2014.01.008. [DOI] [PubMed] [Google Scholar]
- Marshall G.A., Kaufer D.I., Lopez O.L., Rao G.R., Hamilton R.L., DeKosky S.T. Right prosubiculum amyloid plaque density correlates with anosognosia in Alzheimer's disease. J. Neurol. Neurosurg. Psychiatry. 2004;75(10):1396–1400. doi: 10.1136/jnnp.2003.030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimo L., Libon D.J., Chandrasekaran K., Dreyfuss M., McMillan C.T., Rascovsky K. Self-appraisal in behavioural variant frontotemporal degeneration. J. Neurol. Neurosurg. Psychiatry. 2013;84(2):148–153. doi: 10.1136/jnnp-2012-303153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavergames C.Covidence (Systematic Review Software). 2013.
- McGlynn S.M., Schacter D.L. Unawareness of deficits in neuropsychological syndromes. J. Clin. Exp. Neuropsychol. 1989;11(2):143–205. doi: 10.1080/01688638908400882. [DOI] [PubMed] [Google Scholar]
- McMurtray A.M., Chen A.K., Shapira J.S., Chow T.W., Mishkin F., Miller B.L. Variations in regional Spect hypoperfusion and clinical features in frontotemporal dementia. Neurology. 2006;66(4):517–522. doi: 10.1212/01.wnl.0000197983.39436.e7. [DOI] [PubMed] [Google Scholar]
- Mendez M.F., Shapira J.S. Loss of insight and functional neuroimaging in frontotemporal dementia. J. Neuropsychiatry Clin. Neurosci. 2005;17(3):413–416. doi: 10.1176/jnp.17.3.413. [DOI] [PubMed] [Google Scholar]
- Miller B.L., Ikonte C., Ponton M., Levy M., Boone K., Darby A. A study of the Lund-Manchester research criteria for frontotemporal dementia: clinical and single-photon emission CT correlations. Neurology. 1997;48(4):937–942. doi: 10.1212/wnl.48.4.937. [DOI] [PubMed] [Google Scholar]
- Mograbi D.C., Brown R.G., Morris R.G. Anosognosia in Alzheimer's disease–the petrified self. Conscious Cognit. 2009;18(4):989–1003. doi: 10.1016/j.concog.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the Prisma statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R.G., Hannesdottir K. Loss of ‘awareness’ in Alzheimer's disease. In: Morris RG, Becker JT, editors. The Cognitive Neuropsychology of Alzheimer's Disease. Oxford University Press; Oxford: 2004. pp. 275–296. [Google Scholar]
- Morris R.G., Mograbi D.C. Anosognosia, autobiographical memory and self knowledge in Alzheimer’s disease. Cortex. 2013;49(6):1553–1565. doi: 10.1016/j.cortex.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Mullen R., Howard R., David A., Levy R. Insight in Alzheimer's disease. Int. J. Geriatr. Psychol. 1996;11(7):645–651. [Google Scholar]
- Neary D., Snowden J.S., Gustafson L., Passant U., Stuss D., Black S. Frontotemporal lobar degeneration - A consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Orfei M.D., Robinson R.G., Bria P., Caltagirone C., Spalletta G. Unawareness of illness in neuropsychiatric disorders: phenomenological certainty versus etiopathogenic vagueness. Neuroscientist. 2008;14(2):203–222. doi: 10.1177/1073858407309995. [DOI] [PubMed] [Google Scholar]
- Oxford English Dictionary . Oxford University Press; 2017. Insight, n.1.http://www.oed.com/view/Entry/96831 Available from. [Google Scholar]
- Pia L., Neppi-Modona M., Ricci R., Berti A. The anatomy of anosognosia for hemiplegia: a meta-analysis. Cortex. 2004;40(2):367–377. doi: 10.1016/s0010-9452(08)70131-x. [DOI] [PubMed] [Google Scholar]
- Piguet O., Hornberger M., Mioshi E., Hodges J.R. Behavioural-variant frontotemporal dementia: diagnosis, clinical staging, and management. Lancet Neurol. 2011;10(2):162–172. doi: 10.1016/S1474-4422(10)70299-4. [DOI] [PubMed] [Google Scholar]
- Pinkham A.E., Klein H.S., Hardaway G.B., Kemp K.C., Harvey P.D. Neural correlates of social cognitive introspective accuracy in schizophrenia. Schizophr. Res. 2018;202:166–172. doi: 10.1016/j.schres.2018.07.001. [DOI] [PubMed] [Google Scholar]
- Prigatano G.P. Disturbances of self-awareness and rehabilitation of patients with traumatic brain injury: a 20-year perspective. J. Head Trauma Rehabil. 2005;20(1):19–29. doi: 10.1097/00001199-200501000-00004. [DOI] [PubMed] [Google Scholar]
- Prigatano G.P. Anosognosia: clinical and ethical considerations. Curr. Opin. Neurol. 2009;22(6):606–611. doi: 10.1097/WCO.0b013e328332a1e7. [DOI] [PubMed] [Google Scholar]
- Ranasinghe K.G., Rankin K.P., Pressman P.S., Perry D.C., Lobach I.V., Seeley W.W. Distinct subtypes of behavioral variant frontotemporal dementia based on patterns of network degeneration. JAMA Neurol. 2016;73(9):1078–1088. doi: 10.1001/jamaneurol.2016.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K., Hodges J.R., Knopman D., Mendez M.F., Kramer J.H., Neuhaus J. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(Pt 9):2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer J.D., Rossor M.N., Warren J.D. Alzheimer's pathology in primary progressive aphasia. Neurobiol. Aging. 2012;33(4):744–752. doi: 10.1016/j.neurobiolaging.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H.J., Alcantar O., Rothlind J., Sturm V., Kramer J.H., Weiner M. Neuroanatomical correlates of cognitive self-appraisal in neurodegenerative disease. Neuroimage. 2010;49(4):3358–3364. doi: 10.1016/j.neuroimage.2009.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby P., Schmidt C., Hogge M., D'Argembeau A., Collette F., Salmon E. Social mind representation: where does it fail in frontotemporal dementia? J. Cognit. Neurosci. 2007;19(4):671–683. doi: 10.1162/jocn.2007.19.4.671. [DOI] [PubMed] [Google Scholar]
- Salmon E., Perani D., Collette F., Feyers D., Kalbe E., Holthoff V. A comparison of unawareness in frontotemporal dementia and Alzheimer's disease. J. Neurol. Neurosurg. Psychiatry. 2008;79(2):176–179. doi: 10.1136/jnnp.2007.122853. [DOI] [PubMed] [Google Scholar]
- Sanchez-Benavides G., Grau-Rivera O., Cacciaglia R., Suarez-Calvet M., Falcon C., Minguillon C. Distinct cognitive and brain morphological features in healthy subjects unaware of informant-reported cognitive decline. J. Alzheimers Dis. 2018;65(1):181–191. doi: 10.3233/JAD-180378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter D.L. On the relation between memory and consciousness: dissociable interactions and conscious experience. In: Roediger HL, Craik FIM, editors. Varieties of Memory and Consciousness : Essays in Honour of Endel Tulving. L. Erlbaum Associates; Hillsdale, N.J.: 1989. pp. 355–389. [Google Scholar]
- Schmitz T.W., Rowley H.A., Kawahara T.N., Johnson S.C. Neural correlates of self-evaluative accuracy after traumatic brain injury. Neuropsychologia. 2006;44(5):762–773. doi: 10.1016/j.neuropsychologia.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Senturk G., Bilgic B., Arslan A.B., Bayram A., Hanagasi H., Gurvit H. Cognitive and anatomical correlates of anosognosia in amnestic mild cognitive impairment and early-stage Alzheimer's disease. Int. Psychogeriatr. 2017;29(2):293–302. doi: 10.1017/S1041610216001812. [DOI] [PubMed] [Google Scholar]
- Shad M.U., Keshavan M.S. Neurobiology of insight deficits in schizophrenia: an fMRI study. Schizophr. Res. 2015;165(2–3):220–226. doi: 10.1016/j.schres.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shad M.U., Muddasani S., Keshavan M.S. Prefrontal subregions and dimensions of insight in first-episode schizophrenia – a pilot study. Psychiatry Res. 2006;146(1):35–42. doi: 10.1016/j.pscychresns.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Shany-Ur T., Lin N., Rosen H.J., Sollberger M., Miller B.L., Rankin K.P. Self-awareness in neurodegenerative disease relies on neural structures mediating reward-driven attention. Brain. 2014;137(Pt 8):2368–2381. doi: 10.1093/brain/awu161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharmin S., Kypri K., Khanam M., Wadolowski M., Bruno R., Mattick R.P. Parental supply of alcohol in childhood and risky drinking in adolescence: systematic review and meta-analysis. Int. J. Environ. Res. Public Health. 2017;14(3) doi: 10.3390/ijerph14030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata K., Narumoto J., Kitabayashi Y., Ushijima Y., Fukui K. Correlation between anosognosia and regional cerebral blood flow in Alzheimer's disease. Neurosci. Lett. 2008;435(1):7–10. doi: 10.1016/j.neulet.2008.01.065. [DOI] [PubMed] [Google Scholar]
- Sollberger M., Rosen H.J., Shany-Ur T., Ullah J., Stanley C.M., Laluz V. Neural substrates of socioemotional self-awareness in neurodegenerative disease. Brain Behav. 2014;4(2):201–214. doi: 10.1002/brb3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkstein S.E., Sabe L., Chemerinski E., Jason L., Leiguarda R. Two domains of anosognosia in Alzheimer's disease. J. Neurol. Neurosurg. Psychiatry. 1996;61(5):485–490. doi: 10.1136/jnnp.61.5.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkstein S.E., Sabe L., Vazquez S., Teson A., Petracca G., Chemerinski E. Neuropsychological, psychiatric, and cerebral blood flow findings in vascular dementia and Alzheimer's disease. Stroke. 1996;27(3):408–414. doi: 10.1161/01.str.27.3.408. [DOI] [PubMed] [Google Scholar]
- Starkstein S.E., Vazquez S., Migliorelli R., Teson A., Sabe L., Leiguarda R. A single-photon emission computed tomographic study of anosognosia in Alzheimer's disease. Arch. Neurol. 1995;52(4):415–420. doi: 10.1001/archneur.1995.00540280105024. [DOI] [PubMed] [Google Scholar]
- Strong M.J., Abrahams S., Goldstein L.H., Woolley S., McLaughlin P., Snowden J. Amyotrophic lateral sclerosis – frontotemporal spectrum disorder (ALS-FTSD): revised diagnostic criteria. Amyotroph. Lateral Scler. Frontotemporal Degener. 2017:1–22. doi: 10.1080/21678421.2016.1267768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong M.J., Grace G.M., Freedman M., Lomen-Hoerth C., Woolley S., Goldstein L.H. Consensus criteria for the diagnosis of frontotemporal cognitive and behavioural syndromes in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 2009;10(3):131–146. doi: 10.1080/17482960802654364. [DOI] [PubMed] [Google Scholar]
- Tezuka K., Meguro K., Akanuma K., Tanaka N., Ishii H., Yamaguchi S. Overestimation of self-reported activities of daily living in vascular dementia patients with a right hemisphere lesion. J. Stroke Cerebrovasc. Dis. 2013;22(1):9–14. doi: 10.1016/j.jstrokecerebrovasdis.2011.05.019. [DOI] [PubMed] [Google Scholar]
- Thomson Reuters . Thomson Reuters; Philadelphia: 2018. EndNote X9. [Google Scholar]
- Tondelli M., Barbarulo A.M., Vinceti G., Vincenzi C., Chiari A., Nichelli P.F. Neural correlates of anosognosia in Alzheimer's disease and mild cognitive impairment: a multi-method assessment. Front. Behav. Neurosci. 2018;12:100. doi: 10.3389/fnbeh.2018.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vliet D., de Vugt, Bakker M.E., Pijnenburg C., Vernooij-Dassen MJFJ Y.A.L., Koopmans RTCM Time to diagnosis in young-onset dementia as compared with late-onset dementia. Psychol. Med. 2013;43(2):423–432. doi: 10.1017/S0033291712001122. [DOI] [PubMed] [Google Scholar]
- Vannini P., Hanseeuw B., Munro C.E., Amariglio R.E., Marshall G.A., Rentz D.M. Anosognosia for memory deficits in mild cognitive impairment: insight into the neural mechanism using functional and molecular imaging. Neuroimage Clin. 2017;15:408–414. doi: 10.1016/j.nicl.2017.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel A., Hasselbalch S.G., Gade A., Ziebell M., Waldemar G. Cognitive and functional neuroimaging correlates for anosognosia in Mild Cognitive Impairment and Alzheimer's disease. Int. J. Geriatr. Psychol. 2005;20(3):238–246. doi: 10.1002/gps.1272. [DOI] [PubMed] [Google Scholar]
- Wedderburn C., Wear H., Brown J., Mason S.J., Barker R.A., Hodges J. The utility of the Cambridge Behavioural Inventory in neurodegenerative disease. J. Neurol. Neurosurg. Psychiatry. 2008;79(5):500–503. doi: 10.1136/jnnp.2007.122028. [DOI] [PubMed] [Google Scholar]
- Wells G.A., Shea B., O'Connell D., Peterson J., Welch V., Losos M., et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses Department of Epidemiology and Commuunity Medicine, University of Ottawa, Room 3227A, 451 Smyth Road, Ottawa, Ontario K1J 8M5, Canada. 2014 [Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Wilson R.S., Sytsma J., Barnes L.L., Boyle P.A. Anosognosia in Dementia. Curr. Neurol. Neurosci. Rep. 2016;16(9):77. doi: 10.1007/s11910-016-0684-z. [DOI] [PubMed] [Google Scholar]
- Xavier R.M., Vorderstrasse A. Neurobiological basis of insight in schizophrenia: a systematic review. Nurs. Res. 2016;65(3):224–237. doi: 10.1097/NNR.0000000000000159. [DOI] [PubMed] [Google Scholar]
- Zamboni G., Grafman J., Krueger F., Knutson K.M., Huey E.D. Anosognosia for behavioral disturbances in frontotemporal dementia and corticobasal syndrome: a voxel-based morphometry study. Dement. Geriatr. Cognit. Disord. 2010;29(1):88–96. doi: 10.1159/000255141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamboni G., Wilcock G. Lack of awareness of symptoms in people with dementia: the structural and functional basis. Int. J. Geriatr. Psychol. 2011;26(8):783–792. doi: 10.1002/gps.2620. [DOI] [PubMed] [Google Scholar]
- Zanetti O., Vallotti B., Frisoni G.B., Geroldi C., Bianchetti A., Pasqualetti P. Insight in dementia: when does it occur? Evidence for a nonlinear relationship between insight and cognitive status. J. Gerontol. B-Psychol. 1999;54(2):P100–P1P6. doi: 10.1093/geronb/54b.2.p100. [DOI] [PubMed] [Google Scholar]
- Zeng X., Zhang Y., Kwong J.S., Zhang C., Li S., Sun F. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J. Evid. Based Med. 2015;8(1):2–10. doi: 10.1111/jebm.12141. [DOI] [PubMed] [Google Scholar]
- Zhou J., Seeley W.W. Network dysfunction in Alzheimer's disease and frontotemporal dementia: implications for psychiatry. Biol. Psychiatry. 2014;75(7):565–573. doi: 10.1016/j.biopsych.2014.01.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.