Abstract
Background:
Monocyte activation may contribute to neuronal injury in aviremic HIV-infected adults; data are lacking in children. We examined the relation between monocyte activation markers and early and long-term neurodevelopmental outcomes in early-treated HIV-infected children.
Setting:
Prospective study of infant and child neurodevelopmental outcomes nested within a randomized clinical trial () and extended cohort study in Kenya.
Methods:
HIV-infected infants (N=67) initiated ART at age <5 months. Plasma soluble (s) CD163 (sCD163), sCD14 and neopterin were measured pre-ART (entry) and 6 months later. Milestone attainment was ascertained monthly during 24 months and neuropsychological tests (NPTs) were performed at 5.8–8.2 years post-initiation of ART (N=27). The relationship between neurodevelopment and sCD163, sCD14 and neopterin at entry and 6 months post-ART was assessed using Cox proportional hazards models and linear regression.
Results:
Infants with high entry sCD163 had unexpected earlier attainment of supported sitting (5 vs 6 mo.; P=0.006) and supported walking (10 vs 12 mo.; P =0.02) with trends in adjusted analysis. Infants with high 6-month post-ART sCD163 attained speech later (17 vs 15 mo.; P=0.006; aHR, 0.47; P=0.02), threw toys later (18 vs 17 mo.; P =0.01; aHR, 0.53; P =0.04), and at median 6.8 years post-ART, had worse NPT scores (adj. mean z-score differences, cognition, −0.42; P =0.07; short-term memory, −0.52; P =0.08; nonverbal test performance, −0.39, P =0.05).
Conclusion:
Prior to ART, monocyte activation may reflect transient neuroprotective mechanisms in infants. Following ART and viral suppression, monocyte activation may predict worse short- and long-term neurodevelopment outcomes.
Keywords: monocyte, neurocognitive, antiretroviral, perinatal HIV, CD163, neurodevelopment
Background
In 2018, there were an estimated 1.6 million HIV-infected children aged <15 years, most of whom were in sub-Saharan Africa [1]. HIV infected children have higher risk of neurodevelopmental delay [2] and neurocognitive deficits [3, 4]. Neurocognitive deficits may not be reversible by antiretroviral therapy (ART) [5, 6], and may persist even in infants who begin treatment as early as 5 months of age [7].
The precise mechanisms of HIV-related neuropathogenesis are not well defined. However, HIV-infected CD4+ T lymphocytes or monocytes likely cross the blood-brain-barrier (BBB) into the central nervous system (CNS) [8]. HIV replication triggers local inflammation and neuronal damage. HIV-mediated local inflammation increases the permeability of the blood brain barrier (BBB) [9], and promotes recruitment of additional target cells from the periphery [8]. Monocytes and their cellular derivatives are important for CNS HIV entry and replication and subsequent neuronal injury [8]. HIV can be detected in perivascular macrophages and microglia in post-mortem brain tissue [10, 11]. Brain viral isolates replicate well in cultured macrophages [8], and HIV-infected monocyte-derived macrophages produce neurotoxic factors in vitro [12, 13]. Adults with high CSF levels of monocyte and macrophage activation markers such as neopterin, sCD14, and sCD163 have higher concentrations of CSF HIV RNA [14], neurofilament light chain, a soluble marker of neuronal injury [15–17], and lower neuropsychological test (NPT) performance [18].
Monocyte expansion and activation in the periphery may contribute to the CNS viral reservoir and exacerbate local CNS inflammation. Adults with HIV-associated dementia had higher circulating concentrations of mature CD14+CD16+ monocytes than those with no dementia, and these cells were neurotoxic in vitro [19]. Separately, CD14+CD16+ cells accumulated in brain perivascular spaces, and co-located with p24 antigen in adults with HIV encephalopathy [20]. Adults with higher HIV DNA concentrations in peripheral blood CD14+ cells have worse cognitive outcomes, including dementia, and higher levels of markers for neuronal injury and CSF inflammation [21–23]. Data for these processes in HIV-infected children are more limited and contradictory; one study noted higher peripheral monocyte concentration and percentages in children (mean age 2.7 years) with HIV encephalopathy [24]. In contrast, treated and untreated children (median age 6 years) with higher percentage of activated (CD14+/CD16+/HLA-DR+) monocytes and non-classical (CD14low/CD16+) monocytes had higher NPT performance [25].
CD163 is a hemoglobin scavenger receptor expressed only by monocytes and macrophages, and it is shed in response to innate immune activation [26, 27]. The CD14brightCD16+ monocyte subpopulation comprises a more mature phenotype and expresses particularly high levels of CD163 [28]. Similarly, CD14 is a receptor for lipopolysaccharide (LPS), and it is also shed during innate immune activation [29]. Adults with high plasma soluble CD14 (sCD14) and soluble CD163 (sCD163) concentrations have worse neurocognitive outcomes [18, 30–33], cerebral atrophy and neuronal injury [34], and lower synaptophysin and microtubule associated protein 2 (suggesting synaptodendritic damage), and microglial activation [35]. Individuals with high plasma sCD14 and high sCD163 also have worse NPT performance, despite viral suppression on ART [33, 36]. Neopterin is produced by activated macrophages and is a CSF marker for CNS macrophage activation [37]. Adults with elevated CSF neopterin have higher CSF HIV RNA [14] and poorer NPT performance [37]. Adults with higher CSF neopterin and sCD14 concentrations have higher plasma neopterin and sCD14, respectively [16, 18, 37].
In this study of HIV-infected Kenyan children who began ART before 5 months of age, we evaluated the relationship between measures of monocyte and macrophage activation in plasma, before ART initiation and after 6 months of ART, and infant neurodevelopmental and school-age neurocognition. We hypothesized that infants with higher plasma concentration of markers of monocyte and macrophage activation would attain neurodevelopmental milestones later and would have worse neurocognitive function at school-age, despite low plasma virus level.
Methods
Study population.
This study included children enrolled as infants in a randomized trial involving early ART initiation and treatment for 24 months in both arms, then randomization to continued versus interrupted treatment () [38]. Briefly, HIV infected infants were enrolled following routine HIV screening (2007–2009). Entry criteria were confirmation of HIV infection, age <5 months and no previous ART except for prophylaxis for prevention of mother-to-child transmission (PMTCT). Infants started ART within 2 weeks after study entry. Infants received ritonavir-boosted lopinavir (if previously exposed to nevirapine) or nevirapine combined with lamivudine and zidovudine; infants enrolling later received abacavir instead of zidovudine. Each study visit included ascertainment of growth parameters. Blood samples were collected at entry, then every 3 months after ART initiation. Infants remained in monthly follow-up for the randomized trial for up to 42 months and then were invited to participate in extended quarterly follow-up for 60 additional months. Of 140 infants in the original trial, 41 were excluded from this analysis because they enrolled at an older age, limiting milestone ascertainment. Thirty-two infants had no available specimen for biomarker assays at entry and 6 months (29 were lost, died or withdrew prior to 6 months and 3 remained on study but had no specimen at 6 months) (See Figure, Supplemental Digital Content 1). This report includes data from 67 infants with biomarker data for at least one visit during the first 6 months of ART (entry or 6 months or both). Caregivers of study participants provided written informed consent and the University of Washington and the University of Nairobi/Kenyatta National Hospital Institutional Review Boards each provided ethical approval for this study.
Laboratory.
CD4+ T cell count and percentage and plasma HIV RNA were ascertained as described [38]. Plasma concentrations of sCD14, sCD163 and neopterin were assessed using commercially available kits (sCD14 and sCD163, R&D Systems, Inc. Minneapolis, MN; neopterin, GenWay Biotech Inc. San Diego, CA).
Neurodevelopmental milestone attainment.
Neurodevelopmental milestone attainment was assessed as described [39] at entry and monthly visits by caregiver self-report and clinician observation for 24 months. Milestones were selected and adapted based on items in the Denver Developmental Screening Test [40]. Briefly, these included sitting with support (defined as sitting with a straight back on the caregiver’s lap or on the floor between the caregiver’s legs), sitting unsupported (defined as sitting on a flat surface or on the caregiver’s lap without needing support), walking with support (defined as walking well with the help of someone holding one or both hands or using furniture or a wall), walking unsupported (walking at least a few steps unsupported) monosyllabic speech (defined as saying one-syllable words or sounds, referring to a specific person or object), throwing toys (defined as throwing a toy such as a ball while playing) and naming objects defined as pointing to and naming common household items). For missing milestone data, the age at attainment was calculated by subtracting the date of birth from the date of the visit at which the milestone was first observed by study clinicians.
Neuropsychological assessments.
All children in follow-up through age 6.1–8.5 years underwent the same NPT testing battery. The battery included the Kaufman Assessment Battery for Children 2nd edition (KABC) (global cognitive ability, visual-spatial processing, short-term memory, learning, delayed memory, and nonverbal test performance), the Behavior Rating Inventory of Executive Functioning (BRIEF) (executive function), the Visual Test of Variables of Attention (TOVA) (attention), and Bruininck’s-Oseretsky Test of Motor Proficiency (BOT) 2nd edition Brief Form (motor) (See Table, Supplemental Digital Content 2). Raw scores for each domain or scale were scaled and standardized as percentiles using US norms.
Assessments were conducted by study staff with either bachelor’s degree or graduate level training in psychology. Test administration instructions were translated to Kiswahili and back-translated to ensure accuracy. Tests were administered in the child’s preferred language (Kiswahili or English). All testers were fluent in both languages. Testers received one-on-one training from a doctoral level neuropsychologist. Testers did not begin to test for the study until their performance of a full battery tests was deemed proficient by a trainer. Testers participated in periodic internal group and external review of video recorded assessments for quality assurance.
Statistical Methods.
Cut-off points for dichotomous variables.
Dichotomous variables for biomarkers were pre-specified based on overall median levels (for the entry and 6 month timepoints) for sCD163 and sCD14 (sCD163, >1,100 ng/ml; sCD14, >3,700 ng/ml). Two cut-offs were required for neopterin to allow sufficient numbers within groups (median neopterin at entry, >35 nmol/L and median neopterin at follow-up, >20 nmol/L). Lower HIV RNA was defined as plasma HIV RNA <1000 copies/mL.
Cross sectional analysis of cofactors for biomarker levels at entry.
Two-sample t-tests and χ2 tests, as appropriate, were performed to evaluate the relationship between entry plasma HIV RNA levels, CD4 T cell percentage (CD4%), WHO disease stage, receipt of PMTCT, age at initiation of ART, and growth parameters (z-scores for weight-for-age (WAZ), height-for-age (HAZ), and weight-for-height (WHZ)) and dichotomous variables for each biomarker. Similar analyses were performed for biomarker concentrations as continuous variables.
Prospective analysis of biomarker levels and neurodevelopmental outcomes.
The primary analysis was designed to determine the association of activation markers following initiation of ART with subsequent neurodevelopmental outcomes. In addition to biomarkers following initiation of ART, biomarkers at entry (pre-ART) were assessed for associations with neurodevelopmental outcomes. Kaplan-Meier survival methods were used to calculate median age at milestone attainment for groups of infants defined by low vs high biomarker concentrations (as defined above) overall. Models stratified by low vs high plasma HIV RNA at 6 months post-initiation of ART were also evaluated, based on our hypothesis that the relation between monocyte activation and neurodevelopmental outcomes might differ by virological status. The relationship between age at milestone attainment and biomarker levels as dichotomous variables was also examined using Cox proportional hazards models, adjusted for 6-month plasma HIV RNA level, CD4%, and WAZ (each as continuous variables). Models including HIV RNA levels were evaluated first and then models including CD4% were evaluated secondarily. Models including both HIV RNA level and CD4% together were not evaluated due to colinearity. Finally, the relationships between biomarkers as continuous variables and age at milestone attainment were evaluated using Cox proportional hazards models.
Univariable and multivariable linear regression models were used to evaluate the relationship between high vs low sCD163 levels at 6 months and neurocognitive domain specific Z-scores at school-age. Separate models adjusted for entry or 6-month HIV RNA level, CD4% and WAZ, respectively were evaluated, as described above. P-values ≤0.05 were considered significant. To account for multiple comparisons, we used the Benjamini-Hochberg procedure and a false discovery rate of 0.25 as described [41, 42].
Results
At entry, the median age of the infants was 3.8 months (interquartile range [IQR], 3.1 to 4.0) and 46.3% were male (Table 1). The median birth weight was 3.0 kg (2.7, 3.4). Most infants (87.8%) were ever breastfed. Nearly all infants (97.1%) were cared for by their biological mothers. Infants were generally undernourished with a median WAZ of −2.29 (IQR, −3.59, −1.03). Most were immunosuppressed, with a median CD4% of 19% (IQR, 14, 24). More than half (55.2%) received antiretrovirals for PMTCT, administered to infants directly, to their mothers, or both.
Table 1.
Entry characteristics of HIV-infected infants initiating ART.
| Characteristic | N | Median (IQR) or N (%) | |
|---|---|---|---|
| Infant | |||
| Age (months) | 67 | 3.8 | (3.1, 4.0) |
| Male | 67 | 31 | (46.3) |
| Clinical and growth | |||
| WHO Stage 3 or 4 diagnosis | 67 | 30 | (44.8) |
| CD4 percentage (%) | 67 | 19 | (14, 25) |
| Plasma HIV RNA log10 copies/ml | 63 | 6.6 | (6.0, 7.0) |
| Previously hospitalized | 67 | 38 | (56.7) |
| Received antiretrovirals for PMTCT | 58 | 32 | (55.2) |
| Growth | |||
| WAZ | 67 | −2.29 | (−3.59, −1.03) |
| HAZ | 67 | −2.01 | (−3.05, −0.94) |
| WHZ | 67 | −0.58 | (−1.71, 0.75) |
| Primary caregiver characteristics | |||
| Age (years) | 65 | 25 | (22, 30) |
| Married | 67 | 53 | (79.1) |
| Education (years) | 59 | 8 | (8, 11) |
| Maternal CD4 count (cells/μL) | 64 | 344 | (190, 481) |
Note. IQR, interquartile range, WAZ, weight-for-age Z-score; HAZ, height-for-age Z-score, WHZ, weight-for-height Z-score; PMTCT, prevention of mother-to-child transmission of HIV.
Infants with high entry sCD14 concentration had higher entry plasma HIV RNA level (means, 6.95 vs 6.33 log10 copies/ml; P=0.004) and those with high entry neopterin had lower entry CD4% (means 17% vs 22%; P=0.03) than those with low entry sCD14 and low entry neopterin, respectively (See Table, Supplemental Digital Content 3). In Kaplan-Meier analyses, infants with high entry plasma sCD163 concentration had earlier age at attainment of sitting supported and walking supported (median differences in age, sitting supported, 1 month; P=0.006, and walking supported, 2 months; P=0.02) (Table 2). Trends for these association persisted in multivariable models adjusted for plasma HIV RNA level and WAZ at entry (See Table, Supplemental Digital Content 4).
Table 2.
Kaplan-Meier analyses comparing age at attainment of milestones between infants with low vs high sCD163, sCD14 and neopterin at entry and 6 months following ART initiation.
| Milestone | sCD163 (ng/ml) | sCD14 (ng/ml) | Neopterin (nM) | |||
|---|---|---|---|---|---|---|
| ≤1100 at entry N=16 [7] |
>1100 at entry N=26 [2] |
≤3700 at entry N=27 [6] |
>3700 at entry N=15 [3] |
≤35 at entry N=21 [4] |
>35 at entry N=21 [5] |
|
| Sitting supported | 6 (6, 8) | 5 (4,6)*** | 5 (4, 6) | 5 (5, 6) | 4 (5, 6) | 5 (5, 6) |
| Sitting unsupported | 7 (7, 9) | 6 (6, 7) [4] | 7 (6, 7) [8] | 6 (6, 8) | 6 (6, 7) | 7 (6, 8) [7] |
| Walking supported | 12 (12, 15) [8] | 10 (9, 12) [5]* | 10 (9, 12) [8] | 10 (9, 12) [5] | 10 (9, 12) | 11 (10, 13) [9] |
| Walking unsupported | 14 (13, 17) [8] | 15 (14, 17) [6] | 14 (13, 17) [9] | 15 (14, 17) [5] | 14 (13, 16) [5] | 17 (14, 17) [9] |
| ≤1100 at 6 mo N=29 [0] |
>1100 at 6 mo N=22 [1] |
≤3700 at 6 mo N=24 [1] |
>3700 at 6 mo N=27 [0] |
≤20 at 6 mo N=25 [0] |
>20 at 6 mo N=25 [0] |
|
| Walking unsupported | 15 (13, 16) | 17 (13, 19) [2] | 15 (14, 17) | 15 (13, 19) [1] | 15 (13, 20) | 15 (13, 17) [1] |
| Monosyllabic speech | 15 (13, 16) | 17 (15, 21) [3]*** | 15 (13, 19) [2] | 15 (14, 17) [1] | 15 (13, 17) | 15 (14, 17) [2] |
| Throwing toys | 17 (15, 18) | 18 (17, 19)** | 16 (15, 18) | 18 (17, 19) | 17 (16, 19) | 18 (16, 19) |
| Naming objects | 21 (19, 24) | 23 (20, 24) [4] | 22 (19, 23) [2] | 23 (20, 24) [2] | 22 (19, 24) | 22 (20, 24) [3] |
Note, Shown are median age (mos), (interquartile range). N, number at risk [number censored, except where noted].
P=0.02;
P=0.01;
P=0.006 (all P-values noted with asterisks retained significance using Benjamini Hochberg).
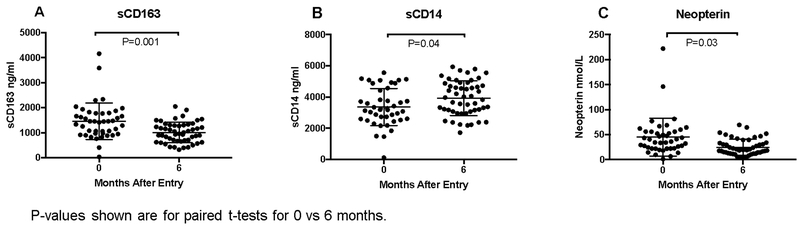
Twenty-six infants had biomarker data at entry and 6 months after starting ART. Monocyte activation levels changed significantly from entry to 6 months after ART initiation. Soluble CD163 and neopterin decreased (mean differences, −530 ng/ml, P=0.001 and −11.26 ng/ml, P=0.03, respectively) whereas sCD14 increased (mean difference, 707 ng/ml, P=0.04; Figure 1).
Figure 1.
Concentration of plasma (A) sCD163, (B) sCD14 and (C) neopterin at entry and 6 months in HIV-infected infants initiating ART. Bars indicate means and standard deviations.
We hypothesized that infants with high monocyte activation in spite of lower plasma virus would have poor neurodevelopmental outcomes. Of 67 infants with biomarker data, 51 had biomarker data at 6 months post initiation of ART. Overall, infants with high sCD163 at 6 months after initiating ART were older at achievement of milestones compared to those with low sCD163 (median differences in age, speech, 2 months; P=0.006 and throwing toys, 1 month; P=0.01) (Table 2). In stratified analyses, these differences were significant only for the subset of infants with 6-month HIV RNA <1000 copies/ml (median differences in age, speech, 2 months; P=0.003; throwing toys, 2 months; P=0.02) (See Table, Supplemental Digital Content 5). The subset lacking 6-month HIV RNA<1000 copies/ml had similar ages at attainment of milestones (P=0.3 and P=0.5, respectively). In Cox proportional hazards models adjusted for 6-month plasma virus concentration and WAZ, infants with high 6-month sCD163 attained speech later (adjusted hazard ratio (aHR), 0.47; P=0.02) and threw toys later (aHR, 0.53; P=0.04) (See Table, Supplemental Digital Content 4). Infants with high vs low sCD14 and neopterin had similar age at milestone attainment (Table 2). In analyses of soluble biomarker levels as continuous variables, there were no associations between biomarker levels and age at milestone attainment.
Twenty-seven of 67 children (40.2%) were followed for 5.8–8.2 years and underwent a detailed NPT between the ages of 6.1–8.5 years (Supplemental Digital Content 1). Children who had NPTs had similar ages at attainment of neurodevelopmental milestones compared to children who did not have an assessment (all p-values >0.2) (data not shown). For this subset, median age at initiation of ART was 3.9 (IQR, 3.4, 4.3) months and median entry CD4% was 18% (IQR, 14, 23). At the time of NPT assessment, median age was 6.8 years (IQR, 6.3, 7.4) and median CD4% and plasma virus level were 36% and 2.18 1og10 copies/ml. All children were diagnosed as having WHO Stage 1 disease and their median WAZ was −0.79. Nine of 27 children had elevated sCD163 at 6 months post initiation of ART (during infancy). Compared to children with lower sCD163 at 6 months, these 9 children had worse global cognitive ability (unadj. mean z-score differences, −0.45; P=0.05), short-term memory (−0.63; P=0.06), and nonverbal test performance (−0.41; P=0.04) (Table 3 and Figure 2). Results were similar in analyses adjusted for 6-month plasma virus level and WAZ (adj. mean differences, global cognitive ability, −0.42; P=0.07; short-term memory, −0.52; P=0.08; nonverbal test performance, −0.39, P=0.05). Results for separate models adjusted for CD4% and WAZ were also similar (adj. mean differences, global cognitive ability, −0.47, P=0.04; short-term memory, −0.63, P=0.06; learning, −0.73, P=0.02; nonverbal test performance, −0.37, P=0.07; delayed recall, −0.77, P=0.03).
Table 3.
Linear regression analyses of low vs high sCD163 at 6 months following ART initiation (infancy) and school-age neurocognitive and motor scores.
| 6 mo sCD163 >1100 ng/ml vs ≤1100 ng/ml | ||||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusteda | |||||
| Domain | N | Mean difference (95% CI) | P | N | Mean difference (95% CI) | P |
| Global cognitive ability | 27 | −0.45 (−0.90, −0.00) | 0.05b | 26 | −0.42 (−0.86, 0.027) | 0.07b |
| Visual-spatial processing | 27 | −0.20 (−0.65, 0.26) | 0.4 | 26 | −0.17 (−0.63, 0.29) | 0.5 |
| Short-term memory | 27 | −0.63 (−1.31, 0.04) | 0.06b | 26 | −0.52 (−1.12, 0.08) | 0.08b |
| Learning | 27 | −0.56 (−1.22, 0.11) | 0.1b | 26 | −0.57 (−1.26, 0.12) | 0.1b |
| Delayed memory | 21 | −0.46 (−1.29, 0.38) | 0.3 | 21 | −0.43 (−1.29, 0.43) | 0.3 |
| Nonverbal test performance | 27 | −0.41 (−0.80 to −0.02) | 0.04b | 26 | −0.39 (−0.78, −0.00) | 0.05b |
| Executive function | 27 | −0.24 (−1.00 to 0.51) | 0.5 | 26 | −0.19 (−1.02, 0.62) | 0.6 |
| Attention | 24 | −0.28 (−0.89, 0.34) | 0.4 | 23 | −0.31 (−0.89, 0.26) | 0.3 |
| Motor | 26 | 0.40 (−0.38, 1.18) | 0.3 | 25 | 0.42 (-0.37 to 1.20) | 0.3 |
Note, CI, confidence interval.
Adjusted for 6-mo. weight-for age Z-score and plasma HIV RNA level.
Retained significance using Benjamini Hochberg.
Figure 2.
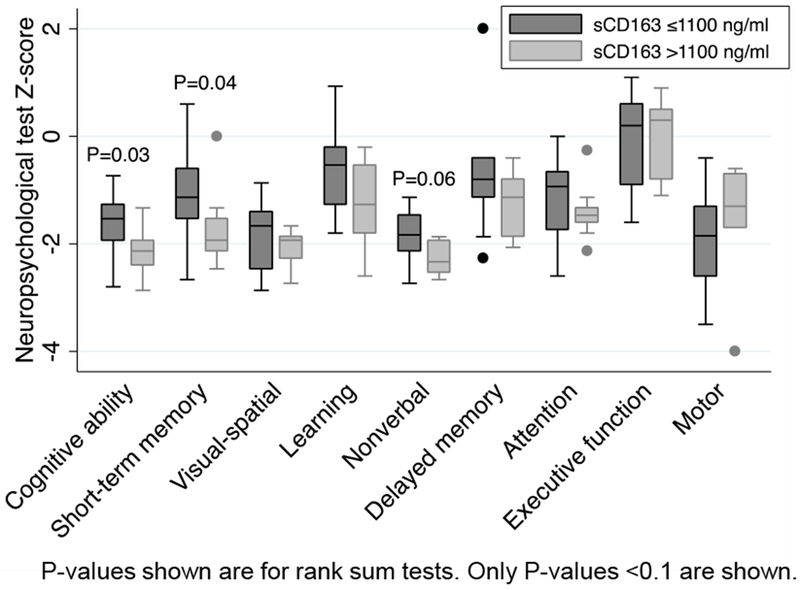
Box plots comparing neurocognitive ability in HIV-infected children by high vs low sCD163 at 6 months post start of ART. Dark gray, low sCD163; light gray, high sCD163.
Discussion
We examined plasma markers of peripheral monocyte and macrophage activation in relation to infant and school-age neurodevelopmental outcomes in HIV-infected children who initiated ART in infancy. Infants with elevated sCD163 6-months after initiating ART had later age at attainment of milestones. In stratified analyses, these associations persisted in the subset of infants with lower virus levels. Consistent with these findings, ART-treated adults with elevated sCD163 had neurocognitive impairment and despite viral suppression [32, 33]. Among the subset of infants who remained in follow-up through school-age, those with elevated sCD163 at 6 months after ART initiation also had lower scores for neurocognitive ability and nonverbal skills, and trends for worse short-term memory and learning, suggesting long-lasting impacts of early monocyte activation in pediatric HIV.
The role of immune activation markers may be less clear in untreated infants. We observed that pre-treatment immune activation was associated with better neurocognitive outcomes. This may reflect the need for a balance between effective immune activation necessary to mount immune responses pre-ART and damaging activation that is residual following initiation of ART. Soluble CD163 may be shed by peripheral CD14+CD16+ monocytes [43] or monocyte subsets that have recently migrated from bone marrow [44] as part of response to reduce T lymphocyte activation in the periphery [43] or an anti-inflammatory response in the brain [44]. Thus elevated sCD163 in the periphery may reflect some protective mechanisms in addition to deleterious expansion of monocyte subsets which may cause brain injury, as has been demonstrated in untreated SIV-infected macaques [36, 44]. Intriguingly, in uninfected neonate and infant macaques peripheral monocyte turnover was significantly higher than in adult animals, and then lowered to adult levels at approximately 3–4 months of age [45]. Following SIV infection, monocyte turnover remained aberrantly high in both neonate and infant animals [45], suggesting HIV/SIV infection disrupts normal monocyte activity, which may otherwise be protective. In our study, age at HIV acquisition is unknown, since infants were <5 months of age at entry and HIV acquisition could have occurred late in utero, peripartum or during the postnatal period. Our findings of evidence of both protective and deleterious monocyte activation also mirror recent findings in HIV-infected adults during acute infection [46]. Adults with higher plasma sCD163 during early acute infection (Fiebig Stages I and II) had better CNS outcomes (NPT scores), whereas adults with higher plasma sCD163 later in acute infection (Fiebig Stage III) had worse CNS outcomes (lower markers of neuronal health [N-acetylaspartate] on brain imaging and lower NPT scores) [46].
Our data contrast with a prior study of late-diagnosed and treated school-aged children, which found that those with elevated subsets of activated monocytes following initiation of ART also had higher neurocognitive scores [25]. The authors of this prior study speculated that in long-term untreated HIV-infected children, immune activation may reflect protective mechanisms [25]. Altogether, these studies of adults, older children and infants suggest that timing of infection and ART with respect to individual immune maturation may both influence the balance between damaging and protective immune function.
We did not find associations between either plasma concentrations of neopterin or sCD14 pre- or following ART initiation and neurodevelopmental outcomes. Soluble CD14 was higher in adults with neurocognitive impairment, cerebral atrophy and neuronal injury among subsets with advanced or untreated HIV [30, 34], and adults with viral suppression in one study [33] but not others [18, 30, 34]. In a prior analysis of infants in our cohort, infants had robust immune recovery following ART [47]. Plasma neopterin may reflect macrophage activation in tissues other than the CNS, and thus may lack specificity for neurodevelopmental outcomes. Expression of neopterin and sCD163 are also regulated by distinct cytokine pathways [27, 28, 37], and thus may exhibit differential predictive value for neurocognitive impairment across cohorts or compartments.
Prior to ART, infants in our cohort who had high sCD14 or neopterin had more severe HIV disease as characterized by low CD4% and high plasma HIV RNA level. These findings are consistent with a South African study of early treated children, in whom higher sCD14 correlated with high viremia [48]. Infants in our study had significant declines in sCD163 and neopterin following ART consistent with prior studies in adults [36, 49] and Thai/Cambodian HIV-infected children [25]. However, sCD14 levels did not decline following initiation of ART in our study, unlike prior studies of older children [50]. Upwards trajectories of sCD14 following initiation of ART have been described in adults [51].
Our study has several strengths. There have been few prospective studies of immune activation biomarkers in relation to neurodevelopmental outcomes in HIV-infected infants initiating ART, and to our knowledge, only one other study evaluated biomarkers of monocyte and macrophage activation [24]. We evaluated 3 plausible markers of poor neurodevelopment in HIV, had frequent assessment of developmental milestones, and used detailed assessments for school age neurocognitive functioning. Our study focused on early-treated infants followed for several years and thus enabled evaluation of school-age neurocognitive ability. This study was limited by small sample size and assessment of infant milestones relied partially on caregiver report. Data on gestational age of infants was not available, limiting ability to interpret age at milestone attainment and relationship between preterm birth or small for gestational age and monocyte/macrophage activation markers. The cut-offs used to define low vs high levels for each biomarker reflected medians for this cohort, however, these were pre-specified and similar to levels in relevant cohorts [25, 50, 52]. Analyses of school-age outcomes were exploratory and interpretation is limited by cohort attrition. In addition we used a US norm to scale and standardize raw scores, limiting interpretation of Z-scores due to cultural differences. Given small numbers and the exploratory nature of these analyses, it will be important to further evaluate the associations presented here in other cohorts. Our findings do align with prior studies suggesting sCD163 is an important marker for cognitive differences in HIV-infected individuals on ART and with well-controlled HIV [33, 36].
Based on our findings and prior work in adults and macaques [43, 44], we hypothesize that peripheral blood monocyte activation may signal future brain inflammation and injury in early treated HIV infected children. High soluble monocyte activation markers may reflect expansion or maturation of specific subsets of monocytes that may traffic to the brain, potentially as part of an initial protective response, that eventually results in deleterious CNS inflammation. Therapeutics that may specifically dampen monocyte activation, might offer an avenue for adjunctive treatment that may benefit cognitive outcomes in HIV-infected children.
Supplementary Material
Supplemental Digital Content 1.docx Flow chart of children included in analysis.
Supplemental Digital Content 2.docx Summary of neuropsychological tests, domains and subscales or indices used for assessment.
Supplemental Digital Content 3.docx HIV disease and growth indicators compared between infants with low and high sCD163, sCD14 and neopterin at entry (pre-ART).
Supplemental Digital Content 4.docx Univariable and multivariable analyses of Cox proportional hazards regression for low vs high sCD163 at entry and 6 months and hazard ratios for older age at neurodevelopmental milestone attainment.
Supplemental Digital Content 5.docx Kaplan-Meier analyses comparing age of attainment of milestones between infants with low vs high sCD163 at 6 months following ART initiation and stratified by 6-month plasma HIV RNA.
Acknowledgements
S.B.N. led and conceived the study, and performed and led analysis and manuscript writing. I.M. performed laboratory assays, conducted analysis and drafted the manuscript. T.L. and N.T. performed neurocognitive assessments, DW led the field site, established the clinical trial cohort and contributed to study design, K.T. provided statistical oversight, A.L. coordinated the clinical trial, contributed to study design and led collection of neurodevelopmental data, E.M.O contributed to study design, C.M. contributed to study design and immune marker studies, P.B. and M.J.B. contributed to study design and provided oversight over neurocognitive assessments, G.J.S. conceived and led the initial clinical trial and contributed to study design for the neurodevelopmental study. All authors contributed to writing or critical review of the manuscript and provided final approval of the version to be published.
We are indebted to the Optimizing Pediatric HIV Therapy study participants and their families, without whom this reseasrch would not be possible. We are grateful to the Kenya Pediatric Studies administrative, clinic, and data management staff in Nairobi, Kenya, and in Seattle, Washington for their ongoing support, commitment, and participation. We thank the Department of Paediatrics and Child Health at the University of Nairobi for thier invaluable support of this research. We thank the Kizazi Mother Infant Working Group, the UW Global Center for Integrated Health of Women, Adolescents and Children (Global WACh) and Kenya Research and Training Center (KRTC) for their thoughtful insight and helpful discussions during development of this manuscript.
Funding statement: This work was supported the National Institute of Neurological Disorders and Stroke [K01 NS080637 to SBN] and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) [R01 HD023412 to GJS]. SBN was supported by the University of Washington International and Biometrics Cores of the Center for AIDS Research (CFAR), an National Institutes of Health (NIH) funded program [P30 AI027757], supported by the following NIH Institutes and Centers: National Institute of Allergy and Infectious Diseases (NIAID); National Cancer Institute; National Institute of Mental Health; National Institute on Drug Abuse; NICHD; National Heart, Lung, and Blood Institute; and National Center for Complementary and Alternative Medicine. Field site and biostatistical support were also provided by CFAR. GJS was supported by the NIH [K24 HD054314]. REDCap at the Institute of Translational Health Science (ITHS) was supported by National Center for Research Resources/NIH [UL1 RR025014].
Footnotes
Conflicts of interest: All authors have no conflicts of interest relevant to this work.
Prior presentation: Eighth International Workshop on HIV Pediatrics. Durban, July 2016.
References
- 1.World Health Organization. AIDS Free Framework to accelerate paediatric and adolescent HIV treatment. . In. Geneva, Switzerland; 2018. [Google Scholar]
- 2.Chase C, Ware J, Hittelman J, Blasini I, Smith R, Llorente A, et al. Early cognitive and motor development among infants born to women infected with human immunodeficiency virus. Women and Infants Transmission Study Group. Pediatrics 2000; 106(2):E25. [DOI] [PubMed] [Google Scholar]
- 3.Phillips N, Amos T, Kuo C, Hoare J, Ipser J, Thomas KG, et al. HIV-Associated Cognitive Impairment in Perinatally Infected Children: A Meta-analysis. Pediatrics 2016; 138(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laughton B, Cornell M, Boivin M, Van Rie A. Neurodevelopment in perinatally HIV-infected children: a concern for adolescence. J of the Int AIDS Soc 2013; 16:18603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koekkoek S, Eggermont L, De Sonneville L, Jupimai T, Wicharuk S, Apateerapong W, et al. Effects of highly active antiretroviral therapy (HAART) on psychomotor performance in children with HIV disease. J of Neurol 2006; 253(12):1615–1624. [DOI] [PubMed] [Google Scholar]
- 6.Puthanakit T, Ananworanich J, Vonthanak S, Kosalaraksa P, Hansudewechakul R, van der Lugt J, et al. Cognitive function and neurodevelopmental outcomes in HIV-infected Children older than 1 year of age randomized to early versus deferred antiretroviral therapy: the PREDICT neurodevelopmental study. Pediatr Infect Dis J 2013; 32(5):501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitehead N, Potterton J, Coovadia A. The neurodevelopment of HIV-infected infants on HAART compared to HIV-exposed but uninfected infants. AIDS Care 2014; 26(4):497–504. [DOI] [PubMed] [Google Scholar]
- 8.Spudich S, Gonzalez-Scarano F. HIV-1-related central nervous system disease: current issues in pathogenesis, diagnosis, and treatment. Cold Spring Harb Perspect Med 2012; 2(6):a007120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersson LM, Hagberg L, Fuchs D, Svennerholm B, Gisslen M. Increased blood-brain barrier permeability in neuro-asymptomatic HIV-1-infected individuals--correlation with cerebrospinal fluid HIV-1 RNA and neopterin levels. J Neurovirol 2001; 7(6):542–547. [DOI] [PubMed] [Google Scholar]
- 10.Thompson KA, Cherry CL, Bell JE, McLean CA. Brain cell reservoirs of latent virus in presymptomatic HIV-infected individuals. Am J Pathol 2011; 179(4):1623–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiley CA, Schrier RD, Nelson JA, Lampert PW, Oldstone MB. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proceedings of the National Academy of Sciences of the United States of America 1986; 83(18):7089–7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giulian D, Vaca K, Noonan CA. Secretion of neurotoxins by mononuclear phagocytes infected with HIV-1. Science 1990; 250(4987):1593–1596. [DOI] [PubMed] [Google Scholar]
- 13.Pulliam L, Herndier BG, Tang NM, McGrath MS. Human immunodeficiency virus-infected macrophages produce soluble factors that cause histological and neurochemical alterations in cultured human brains. J Clin Invest 1991; 87(2):503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahl V, Peterson J, Fuchs D, Gisslen M, Palmer S, Price RW. Low levels of HIV-1 RNA detected in the cerebrospinal fluid after up to 10 years of suppressive therapy are associated with local immune activation. AIDS 2014; 28(15):2251–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peluso MJ, Meyerhoff DJ, Price RW, Peterson J, Lee E, Young AC, et al. Cerebrospinal fluid and neuroimaging biomarker abnormalities suggest early neurological injury in a subset of individuals during primary HIV infection. J Infect Dis 2013; 207(11):1703–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jespersen S, Pedersen KK, Anesten B, Zetterberg H, Fuchs D, Gisslen M, et al. Soluble CD14 in cerebrospinal fluid is associated with markers of inflammation and axonal damage in untreated HIV-infected patients: a retrospective cross-sectional study. BMC Infect Dis 2016; 16:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGuire JL, Gill AJ, Douglas SD, Kolson DL, group CHA-RTER. Central and peripheral markers of neurodegeneration and monocyte activation in HIV-associated neurocognitive disorders. J Neurovirol 2015; 21(4):439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamat A, Lyons JL, Misra V, Uno H, Morgello S, Singer EJ, et al. Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. J Acquir Immune Defic Syndr 2012; 60(3):234–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pulliam L, Gascon R, Stubblebine M, McGuire D, McGrath MS. Unique monocyte subset in patients with AIDS dementia. Lancet 1997; 349(9053):692–695. [DOI] [PubMed] [Google Scholar]
- 20.Fischer-Smith T, Croul S, Sverstiuk AE, Capini C,L’Heureux D, Regulier EG, et al. CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol 2001; 7(6):528–541. [DOI] [PubMed] [Google Scholar]
- 21.Shiramizu B, Ananworanich J, Chalermchai T, Siangphoe U, Troelstrup D, Shikuma C, et al. Failure to clear intra-monocyte HIV infection linked to persistent neuropsychological testing impairment after first-line combined antiretroviral therapy. J Neurovirol 2012; 18(1):69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kallianpur KJ, Kirk GR, Sailasuta N, Valcour V, Shiramizu B, Nakamoto BK, et al. Regional cortical thinning associated with detectable levels of HIV DNA. Cereb cortex 2012; 22(9):2065–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valcour VG, Ananworanich J, Agsalda M, Sailasuta N, Chalermchai T, Schuetz A, et al. HIV DNA reservoir increases risk for cognitive disorders in cART-naive patients. PloS one 2013; 8(7):e70164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez-Ramon S, Bellon JM, Resino S, Canto-Nogues C, Gurbindo D, Ramos JT, et al. Low blood CD8+ T-lymphocytes and high circulating monocytes are predictors of HIV-1-associated progressive encephalopathy in children. Pediatrics 2003; 111(2):E168–175. [DOI] [PubMed] [Google Scholar]
- 25.Ananworanich J, Kerr SJ, Jaimulwong T, Vibol U, Hansudewechakul R, Kosalaraksa P, et al. Soluble CD163 and monocyte populations in response to antiretroviral therapy and in relationship with neuropsychological testing among HIV-infected children. J Virus Erad 2015; 1(3):196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weaver LK, Pioli PA, Wardwell K, Vogel SN, Guyre PM. Up-regulation of human monocyte CD163 upon activation of cell-surface Toll-like receptors. J Leukoc Biol 2007; 81(3):663–671. [DOI] [PubMed] [Google Scholar]
- 27.Moller HJ. Soluble CD163. Scan J Clin and Lab Inv 2012; 72(1):1–13. [DOI] [PubMed] [Google Scholar]
- 28.Buechler C, Ritter M, Orso E, Langmann T, Klucken J, Schmitz G. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J Leukoc Biol 2000; 67(1):97–103. [PubMed] [Google Scholar]
- 29.Miller SI, Ernst RK, Bader MW. LPS, TLR4 and infectious disease diversity. Nat Rev Microbiol 2005; 3(1):36–46. [DOI] [PubMed] [Google Scholar]
- 30.Lyons JL, Uno H, Ancuta P, Kamat A, Moore DJ, Singer EJ, et al. Plasma sCD14 is a biomarker associated with impaired neurocognitive test performance in attention and learning domains in HIV infection. JAIDS 2011; 57(5):371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Royal W 3rd Cherner M, Burdo TH, Umlauf A, Letendre SL, Jumare J, et al. Associations between Cognition, Gender and Monocyte Activation among HIV Infected Individuals in Nigeria. PloS one 2016; 11(2):e0147182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burdo TH, Weiffenbach A, Woods SP, Letendre S, Ellis RJ, Williams KC. Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS 2013; 27(9):1387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imp BM, Rubin LH, Tien PC, Plankey MW, Golub ET, French AL, et al. Monocyte Activation is Associated with Worse Cognitive Performance in Virologically Suppressed HIV-Infected Women. J Infect Dis 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryan LA, Zheng J, Brester M, Bohac D, Hahn F, Anderson J, et al. Plasma levels of soluble CD14 and tumor necrosis factor-alpha type II receptor correlate with cognitive dysfunction during human immunodeficiency virus type 1 infection. J Infect Dis 2001; 184(6):699–706. [DOI] [PubMed] [Google Scholar]
- 35.Bryant AK, Moore DJ, Burdo TH, Lakritz JR, Gouaux B, Soontornniyomkij V, et al. Plasma soluble CD163 is associated with postmortem brain pathology in human immunodeficiency virus infection. AIDS 2017; 31(7):973–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burdo TH, Lackner A, Williams KC. Monocyte/macrophages and their role in HIV neuropathogenesis. Immunol Rev 2013; 254(1):102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hagberg L, Cinque P, Gisslen M, Brew BJ, Spudich S, Bestetti A, et al. Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res Ther 2010; 7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wamalwa D, Benki-Nugent S, Langat A, Tapia K, Ngugi E, Moraa H, et al. Treatment interruption after 2-year antiretroviral treatment (ART) initiated during acute/early HIV in infancy: a randomized trial. AIDS 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benki-Nugent S, Wamalwa D, Langat A, Tapia K, Adhiambo J, Chebet D, et al. Comparison of developmental milestone attainment in early treated HIV-infected infants versus HIV-unexposed infants: a prospective cohort study. BMC Pediatr 2017; 17(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frankenburg WK, Dodds J, Archer P, Shapiro H, Bresnick B. The Denver II: a major revision and restandardization of the Denver Developmental Screening Test. Pediatrics 1992; 89(1):91–97. [PubMed] [Google Scholar]
- 41.Garcia-Arenzana N, Navarrete-Munoz EM, Lope V, Moreo P, Vidal C, Laso-Pablos S, et al. Calorie intake, olive oil consumption and mammographic density among Spanish women. Int J Cancer 2014; 134(8):1916–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McDonald JH. Handbook of Biological Statistics. In. Baltimore, MD: Sparky House Publishing; 2014. pp. 254–260. [Google Scholar]
- 43.Burdo TH, Lentz MR, Autissier P, Krishnan A, Halpern E, Letendre S, et al. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis 2011; 204(1):154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burdo TH, Soulas C, Orzechowski K, Button J, Krishnan A, Sugimoto C, et al. Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog 2010; 6(4):e1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sugimoto C, Merino KM, Hasegawa A, Wang X, Alvarez XA, Wakao H, et al. Critical Role for Monocytes/Macrophages in Rapid Progression to AIDS in Pediatric Simian Immunodeficiency Virus-Infected Rhesus Macaques. J Virol 2017; 91(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.D’Antoni ML, Byron MM, Chan P, Sailasuta N, Sacdalan C, Sithinamsuwan P, et al. Normalization of Soluble CD163 after Institution of Antiretroviral Therapy During Acute HIV Infection Tracks with Fewer Neurological Abnormalities. J Infect Dis 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Asbjornsdottir KH, Hughes JP, Wamalwa D, Langat A, Slyker JA, Okinyi HM, et al. Differences in virologic and immunologic response to antiretroviral therapy among HIV-1-infected infants and children. AIDS 2016; 30(18):2835–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Papasavvas E, Azzoni L, Foulkes A, Violari A, Cotton MF, Pistilli M, et al. Increased microbial translocation in </= 180 days old perinatally human immunodeficiency virus-positive infants as compared with human immunodeficiency virus-exposed uninfected infants of similar age. Pediatr Infect Dis J 2011; 30(10):877–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yilmaz A, Yiannoutsos CT, Fuchs D, Price RW, Crozier K, Hagberg L, et al. Cerebrospinal fluid neopterin decay characteristics after initiation of antiretroviral therapy. J Neuroinflammation 2013; 10:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Makhubele TG, Steel HC, Anderson R, van Dyk G, Theron AJ, Rossouw TM. Systemic Immune Activation Profiles of HIV-1 Subtype C-Infected Children and Their Mothers. Mediators of Inflamm 2016; 2016:9026573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kelesidis T, Tran TT, Stein JH, Brown TT, Moser C, Ribaudo HJ, et al. Changes in Inflammation and Immune Activation With Atazanavir-, Raltegravir-, Darunavir-Based Initial Antiviral Therapy: ACTG 5260s. Clin Infect Dis 2015; 61(4):651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Totin D, Ndugwa C, Mmiro F, Perry RT, Jackson JB, Semba RD. Iron deficiency anemia is highly prevalent among human immunodeficiency virus-infected and uninfected infants in Uganda. J Nutr 2002; 132(3):423–429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1.docx Flow chart of children included in analysis.
Supplemental Digital Content 2.docx Summary of neuropsychological tests, domains and subscales or indices used for assessment.
Supplemental Digital Content 3.docx HIV disease and growth indicators compared between infants with low and high sCD163, sCD14 and neopterin at entry (pre-ART).
Supplemental Digital Content 4.docx Univariable and multivariable analyses of Cox proportional hazards regression for low vs high sCD163 at entry and 6 months and hazard ratios for older age at neurodevelopmental milestone attainment.
Supplemental Digital Content 5.docx Kaplan-Meier analyses comparing age of attainment of milestones between infants with low vs high sCD163 at 6 months following ART initiation and stratified by 6-month plasma HIV RNA.