Abstract
Saccharomyces cerevisiae and Schizosaccharomyces pombe are the most commonly studied yeast model systems, yet comparisons of global proteome remodeling between these yeast species are scarce. Here, we profile the proteomes of S. cerevisiae and S. pombe cultured with either glucose or pyruvate as the sole carbon source to define common and distinctive alterations in the protein landscape across species. In addition, we develop an updated streamlined-tandem mass tag (SL-TMT) strategy that substitutes chemical-based precipitation with more versatile bead-based protein aggregation method (SP3) prior to enzymatic digestion and TMT labeling. Our new workflow, SL-SP3-TMT, allow for near-complete proteome profiles in a single experiment for each species. The data reveal expected alterations in protein abundance and differences between species, highlighted complete canonical biochemical pathways, and provided insight into previously uncharacterized proteins. The techniques used herein, namely SL-SP3-TMT, can be applied to virtually any experiment aiming to study remodeling of the proteome using a high-throughput, comprehensive, yet streamlined mass spectrometry-based strategy.
Keywords: TMT, isobaric, pyruvate, SPS-MS3, pombe, cerevisiae
INTRODUCTION
The two most commonly studied yeast species in molecular biology laboratories are Saccharomyces cerevisiae and Schizosaccharomyces pombe [1]. S. cerevisiae is also referred to as baker’s/brewer’s yeast after its industrial use, or budding yeast due to its characteristic bud formation during replication. In contrast, the rod-shaped S. pombe is also known as fission yeast in reference to the medial fission it undergoes during cell division. Although sharing phylogeny within the fungi kingdom, these single-celled eukaryotes diverged from a common ancestor over a period of 100 million years [2, 3]. As such, evolution has driven fundamental differences between the two species. Genetically, comparing S. cerevisiae to S. pombe at the most basic levels reveals differences in chromosome number (16 vs. 3), intron content (5% vs. 40%), and number of protein-encoding genes (~6000 vs. ~5000) [1]. As such, we expect different protein profile alterations between these two yeast species in response to cellular perturbations, such as altering the carbon source component of the growth media.
The regulation of cellular metabolism is essential for any living organism. Both S. cerevisiae and S. pombe are well-characterized with respect to cell growth, metabolic processes, and associated genetic components. Glucose is typically the preferred carbon source for both budding and fission yeast particularly in a laboratory environment. As such, we hypothesize large scale proteomic alterations upon the substitution of glucose with another carbon source, in this case pyruvate. Pyruvate plays a critical role at the metabolic branch point between cellular respiration and fermentation and lies at the intersection of amino acid biosynthesis and gluconeogenesis. As such we expected that switching the carbon source from glucose will extensively alter the proteomes of both yeast species, as shown previously with that of S. cerevisiae [4, 5]. Moreover, dysregulation of pyruvate transport and its catabolism has implications in diseases spanning from neurodegeneration [6, 7], diabetes [8–10], and cancer [11–13].
Isobaric tag-based proteome profiling is emerging as a leading technology for proteomic analysis of complex peptide mixtures. Multiplexing allows for improved statistical power by including replicates within a single experiment and limiting missing values which often occur across experiments. The introduction of the SL-TMT (streamlined tandem mass tag) protocol facilitated isobaric tag-based proteome profiling [5]. In the SL-TMT protocol, proteins are extracted with urea, quantified and chloroform-methanol precipitated, before digestion and TMT labeling in the same buffer. Differentially labeled samples are then combined, desalted, and fractionated via high-pH reversed-phase chromatography prior to LC-MS3 analysis. Of these steps the precipitation is the most tedious, prone to human error, and least amenable to automation. More recently, the widespread use of bead-based protein aggregation technology (SP3) has alleviated some caveats associated with chemical-based methods of protein extraction [14–17]. Common protein extraction methods, such as chloroform-methanol precipitation as outlined in SL-TMT [5], require careful aspiration of often-flocculent protein disks, and thereby are more prone to sample-to-sample variation. This caveat may be a bottleneck for the adaptation of SL-TMT for automated sample processing. The addition of SP3 to SL-TMT overcomes this hurdle enables gains throughput and the potential of automation.
Here, we profile protein abundance differences due to substituting glucose for pyruvate as the sole carbon source in S. cerevisiae and S. pombe cultures. We compare two carbon sources in triplicate for each species, using only 50 μg of protein per channel and incorporating a modified SL-TMT method that uses SP3-based protein aggregation (which we have named: SL-SP3-TMT). We investigate alterations in relative protein abundance and compare orthologous proteins between species. These data showcase the utility of the SL-SP3-TMT protocol for comprehensive and high-throughput protein profiling.
METHODS
Materials.
Tandem Mass Tag (TMT) isobaric reagents, BCA Protein Concentration Kit, Protease inhibitor tablets, and trypsin were purchased from ThermoFisher Scientific (Rockford, IL). StageTip Empore-C18 material was purchased from 3M (St. Paul, MN). Sep-Pak cartridges (50 mg) were from Waters (Milford, MA). Lys-C protease was from Fujifilm Wako (Richmond, VA). Mass spectrometry-grade water and organic solvents were from J.T. Baker (Center Valley, PA). The S. cerevisiae strain used was BY4716 from ThermoFisher Scientific (Waltham, MA). The S. pombe strain was NRRL Y-164 purchased from ATCC (Manassas, Virginia). Synthetic complete (SC) and Edinburgh Minimal Media (EMM) media was from Sunrise Science (San Diego, CA). Glucose and pyruvate were from MilliporeSigma (St. Louis, MO).
Yeast growth and protein extraction.
Both yeast species were propagated in chemically-defined media. S. cerevisiae wild-type strain BY4716 was grown in SC media supplemented with 2% glucose (n=3) or 2% pyruvate (n=3) as the carbon source. Likewise, S. pombe was grown in EMM supplemented with 2% glucose (n=3) or 2% pyruvate (n=3) as the carbon source. S. cerevisiae were grown at 30°C, while S. pombe at 32°C. We harvested the cells at OD600nm=0.8. We washed the pelleted cells twice with ice cold water and cells were lysed by bead-beating in 8 M urea, 200 mM EPPS (4-(2-Hydroxyethyl)-1-piperazinepropanesulfonic acid), pH 8.5 supplemented with protease inhibitors. Protein concentration was determined with the BCA assay. The BCA assay was performed according to manufacturer’s instructions with samples that were diluted at least 1:20, to ensure that the urea was diluted below its noncompatibility limit. Samples were reduced with 5mM TCEP for 20 min, alkylated with 10 mM iodoacetamide for 20 min in the dark, and quenched with 10 mM DTT for 20 min in the dark, all at room temperature.
SP3-based protein digestion and TMT labeling.
Single- Pot Solid- Phase- enhanced Sample Preparation (SP3) as described previously [17] was used during protein isolation and digestion. In brief, 2.5 μL of each bead type were added to 50 μg of protein in 50 μL total volume, as prepared above. Neat ethanol was added to a final concentration of 50%. The beads were agitated on a vortex for 10 minutes at medium speed. The samples were centrifuged at 10,000 g for 1 min and the supernatant was aspirated. The beads (with bound protein) were washed 3 times with 80% ethanol in the same manner. For protein digestion, we added 50 μL of 200 mM EPPS (3-[4-(2-Hydroxyethyl)piperazin-1-yl]propane-1-sulfonic acid) pH 8.5 and Lys-C overnight at room temperature, followed by trypsin for 6 hr at 37°C on an orbital shaker. Both enzymes were added in the presence of beads and at a 1:100 protease-to-peptide ratio. Following digestion, the samples were centrifuged as above and held to the magnet for 2 min. Digested peptides were simply transferred into a new tube. The beads were then washed with 50 μL of 0.2M EPPS pH8.5, which was combined with the initial elution.
We added a final volume of 30% acetonitrile to the eluted peptides and labelled the 50 μg of peptide with 100 μg of TMT directly into the digestion mixture. To check mixing ratios, 2 μg of each sample were pooled, desalted, and analyzed by mass spectrometry. Using normalization factors calculated from this “label check,” samples were mixed 1:1 across all channels and desalted using a 50 mg Sep-Pak solid phase extraction column. The approximately 300 μg of peptide were fractionated with basic pH reversed-phase (BPRP) HPLC, collected in a 96-well plate and combined down to 24 fractions prior to desalting and subsequent LC-MS/MS processing [18, 19].
Mass spectrometry analysis.
Mass spectrometric data were collected on an Orbitrap Fusion Lumos mass spectrometer coupled to a Proxeon NanoLC-1200 UHPLC. The 100 μm capillary column was packed with 35 cm of Accucore 150 resin (2.6 μm, 150Å; ThermoFisher Scientific). The scan sequence began with an MS1 spectrum (Orbitrap analysis, resolution 120,000, 350–1400 Th, automatic gain control (AGC) target 5E5, maximum injection time 100 ms). The top ten precursors were then selected for MS2/MS3 analysis. MS2 analysis consisted of collision-induced dissociation (CID), quadrupole ion trap analysis, automatic gain control (AGC) 2E4, NCE (normalized collision energy) 35, q-value 0.25, maximum injection time 120 ms), and isolation window at 0.7. Following acquisition of each MS2 spectrum, we collected an MS3 spectrum in which multiple MS2 fragment ions are captured in the MS3 precursor population using isolation waveforms with multiple frequency notches. MS3 precursors were fragmented by HCD and analyzed using the Orbitrap (NCE 65, AGC 1.5E5, maximum injection time 150 ms, resolution was 50,000 at 400 Th).
Spectra were converted to mzXML via MSconvert [20]. Database searching included all entries from the Saccharomyces Genome Database (SGD; August 2017) or from Uniprot entries for S. pombe (December 2019). Each database was concatenated with one composed of all protein sequences for that database in the reversed order. Searches were performed using a 50-ppm precursor ion tolerance for total protein level profiling. The product ion tolerance was set to 0.9 Da. These wide mass tolerance windows were chosen to maximize sensitivity in conjunction with SEQUEST searches and linear discriminant analysis [21, 22]. TMT tags on lysine residues and peptide N termini (+229.163 Da) and carbamidomethylation of cysteine residues (+57.021 Da) were set as static modifications, while oxidation of methionine residues (+15.995 Da) was set as a variable modification. Peptide-spectrum matches (PSMs) were adjusted to a 1% false discovery rate (FDR) [23, 24]. PSM filtering was performed using a linear discriminant analysis, as described previously [22] and then assembled further to a final protein-level FDR of 1% [24]. Proteins were quantified by summing reporter ion counts across all matching PSMs, also as described previously [25]. Reporter ion intensities were adjusted to correct for the isotopic impurities of the different TMT reagents according to manufacturer specifications. The signal-to-noise (S/N) measurements of peptides assigned to each protein were summed and these values were normalized so that the sum of the signal for all proteins in each channel was equivalent to account for equal protein loading. Finally, each protein abundance measurement was scaled, such that the summed signal-to-noise for that protein across all channels equals 100, thereby generating a relative abundance (RA) measurement. Data analysis and visualization were performed in Microsoft Excel or R.
Data access.
RAW files will be made available upon request, in addition that data have been deposited to the ProteomeXchange Consortium via the PRIDE [26] partner repository with the dataset identifier PXD014546. Username: reviewer34024@ebi.ac.uk Password: ngb2bccT.
RESULTS AND DISCUSSION
SL-SP3-TMT sample processing enabled high proteome coverage of S. cerevisiae and S. pombe proteomes.
We have updated our SL-TMT protocol [5] to allow for more versatile sample processing. Specifically, we have substituted the chloroform-methanol precipitation step with SP3 sample processing which is less restrictive in required protein concentration, less prone to human error than aspiration-based precipitation techniques and more amenable to robotic processing [14, 16]. The protocol which we have established for whole proteome analysis is outlined in Figure 1. Samples were processed identically as in the original SL-TMT protocol, except protein extraction was performed with SP3 beads, on which subsequent proteolytic digestion occurs.
Figure 1: Workflow overview.
A) overnight cultures (started from agar plates) were grown to high density in the appropriate growth media (SC for S. cerevisiae and EMM for S. pombe) with 2% pyruvate or 2% glucose. These cultures were diluted back to OD 0.1. Cells were harvested at OD 0.8 and lysed via bead beating. Fifty micrograms of cleared protein lysates were reduced and alkylated. B) The lysate was then subjected to SP3 bead-based processing to aggregate, desalt, and digest protein. C) The samples were labeled with TMT, pooled, and fractionated offline via basic pH reversed-phase (BPRP)-HPLC and desalted. Concatenated fractions (24) were analyzed on an Orbitrap Fusion Lumos mass spectrometer.
Using our SL-SP3-TMT strategy, we achieved deep proteome coverage for S. cerevisiae and S. pombe with glucose and pyruvate as carbon sources. We profiled approximately 80% of the proteins present in the search database for a given species. For S. cerevisiae, we quantified 59,786 unique peptides which collapsed to 4,756 proteins, thus 79% of the entries in our S. cerevisiae search database (n=6,046). Similarly, we quantified 51,113 unique peptides for S. pombe resulting in 4,429 proteins, that compose 86% of the entries in the search database (n=5,151), as summarized in Table 1.
Table 1:
Data summary
| Proteins† | Unique peptides | Total peptides | Sig. dif. Proteins†† | Entries in DB | % of DB quantified | ||
|---|---|---|---|---|---|---|---|
| up | down | ||||||
| S. cerevisiae | 4,756 | 59,786 | 122,464 | 255 | 106 | 6,046 | 79% |
| S. pombe | 4,429 | 51,113 | 98,973 | 370 | 241 | 5,151 | 86% |
Proteins are quantified across all three replicates for each condition.
Significantly differentiated proteins are defined as those with a Benjamini-Hochberg adjusted p-value of less than 0.01 and a log2 ratio exceeding +/−1.5. DB, database.
Data generated using the SL-SP3-TMT sample processing workflow correlated well with the original SL-TMT workflow.
We developed the SL-TMT workflow to streamline TMT sample preparation [5]. Here, we improved the original workflow by replacing chloroform-methanol precipitation (CMppt) with an SP3-based step to isolate proteins for digestion. As in the current study, we previously profiled the proteomes of S. cerevisiae grown either with glucose or pyruvate as the carbon source, albeit using TMT10-plex and CMppt in place of TMT6-plex and SP3 as used here. Both sets of isobaric tagging reagents are structurally identical, differing only by the degree of multiplexing. Nonetheless, the overlap between the two sample preparation methods was strong, approaching 90% (Supplemental Figure 1A) and identical dysregulated pathways were observed in both experiments (Table 2). Specifically, 4,613 proteins were quantified in the original TMT10-plex, while 4,756 proteins were quantified in the TMT6-plex, with an overlap of 4,430. To compare further these data sets, we calculated the log2 ratio of the TMT relative abundance (RA) between the cells grown in glucose or pyruvate for each protein that was present in both data sets (n=4,430). These data were illustrated as a correlation plot for which the Pearson correlation coefficient was calculated to be 0.81 (Supplemental Figure 1B). Considering the differences between these preparations and the similarity of the results, we are confident that SP3 can be used in the SL-TMT protocol for quantitatively accurate proteome profiling.
Table 2:
KEGG pathways corresponding to proteins of higher or lower abundance when yeasts were cultured with pyruvate as the sole carbon source.
| Category | Term | Count† | % of total†† |
|---|---|---|---|
| S. pombe (lower abundance in pyruvate-containing cultured cells) | |||
| spo01100: Metabolic pathways | 28 | 20.9 | |
| spo01110: Biosynthesis of secondary metabolites | 16 | 11.9 | |
| spo01230: Biosynthesis of amino acids | 13 | 9.7 | |
| spo01130: Biosynthesis of antibiotics | 13 | 9.7 | |
| S. cerevisiae (lower abundance in pyruvate-containing cultured cells) | |||
| sce01100: Metabolic pathways | 20 | 19.0 | |
| sce01110: Biosynthesis of secondary metabolites | 11 | 10.5 | |
| sce01130: Biosynthesis of antibiotics | 8 | 7.6 | |
| S. pombe (higher abundance in pyruvate-containing cultured cells) | |||
| spo01100: Metabolic pathways | 31 | 11.9 | |
| spo00500: Starch and sucrose metabolism | 11 | 4.2 | |
| spo01200: Carbon metabolism | 8 | 3.1 | |
| S. cerevisiae (higher abundance in pyruvate-containing cultured cells) | |||
| sce01100: Metabolic pathways | 91 | 38.2 | |
| sce01200: Carbon metabolism | 36 | 15.1 | |
| sce00190: Oxidative phosphorylation | 33 | 13.9 | |
| sce00020: Citrate cycle (TCA cycle) | 22 | 9.2 | |
Count refers to the number of proteins in a given category showing statistically significant abundance differences (log2 ratio exceeding +/−1.5 and a Benjamini-Hochberg adjusted p-value <0.01).
% is the percent of those counts relative to the total number of statistically significant proteins for a given species.
SL-SP3-TMT revealed proteins with significantly altered expression profiles from both yeast species when comparing pyruvate and glucose as the carbon source.
We analyzed further our data generated using the SL-SP3-TMT method by hierarchical clustering of the TMT relative abundance values. We used the Ward clustering algorithm and the Euclidian distance metric for this analysis [27]. The dendrogram and associated heat map resulting from this analysis demonstrated the expected clustering for both S. cerevisiae (Figure 2A) and S. pombe (Figure 2B), as replicates for each treatment in both species grouped together tightly. These results showed agreement among replicates and that the protein profiles differed with the carbon source.
Figure 2: Hierarchical clustering and volcano plots illustrating proteins with carbon source-induced differences in abundance in both yeast species.
Heat maps and associated dendrograms showing tight clustering among replicates for both the A) S. cerevisiae and B) S. pombe data sets. Volcano plots illustrate the −log10 value of the Benjamini-Hochberg corrected p-value versus the fold-change in TMT relative abundance due to the carbon source (log2 (pyruvate/glucose)) for both the C) S. cerevisiae and D) S. pombe data sets. Highlighted in C) and D) in green are proteins with undetermined biological roles that show significant difference in abundance with respect to carbon source, as well as known glucose receptors highlighted in yellow.
To extract statistically significant data, we calculated the p-value and the log2 of the ratio of pyruvate to glucose for both species independently. We set our significant difference threshold to include only those proteins with a Benjamini-Hochberg-corrected p-value <0.01 and a log2 ratio exceeding +/− 1.5. For S. cerevisiae, 370 and 241 proteins demonstrated an increase or decrease in abundance, respectively, while these values were 255 and 106, respectively, for S. pombe (Table 1). The number of significantly altered proteins represented 7–10% of the annotated proteomes of each species. We queried significantly dysregulated proteins to extract enriched KEGG pathways for both yeast species using the DAVID bioinformatics server (Table 2). The pathways with the highest numbers of dysregulated proteins were mostly identical in both species. As expected for proteins with significant changes in abundance, metabolic pathways were the predominate category in both species, representing 12–38% of the total proteins with fold-changes exceeding +/−1.5. In addition, proteins involved in the biosynthesis of antibiotics were lower in abundance for both species when grown on pyruvate. All other categories for proteins exhibiting changes in abundance were metabolism-related, as expected.
In addition, we constructed volcano plots for both S. cerevisiae (Figure 2C) and S. pombe (Figure 2D) using the p-value and the log2 of the ratio of pyruvate to glucose. On these volcano plots, we highlighted (with a yellow circle) well-studied glucose transporters whose expression were known to change with glucose repression. Specifically, we noted HXT1 (S. cerevisiae) and ght8 (S. pombe) that are repressed, as well as HXT5 and HXT6 (S. cerevisiae) plus ght3 and ght6 (S. pombe) which were induced when glucose is limiting. Aside from highlighting common biological pathways, differential protein analysis may provide evidence for the functions of uncharacterized proteins. As such we also highlighted several proteins of unknown or putative function that were significantly differentially expressed when glucose or pyruvate was used as the carbon source for S. cerevisiae (Figure 2C) and S. pombe (Figure 2D). In total, 30 and 13 proteins of unknown function exhibited an increase or decrease in abundance, respectively, in S. cerevisiae, while 79 and 51 showed higher or lower abundance, respectively, in S. pombe (for which protein annotation is less comprehensive). YHR033W had been reported to be negatively regulated by cAMP and to be similar to PRO1 [28], the first enzyme of the proline biosynthesis pathway. Using pyruvate as the sole carbon source, the PRO1 gene was repressed. Coincidently, the duplicated gene, YHR033W, may increase its expression to maintain pathway activity as a mechanism of compensation. Likewise, YKL187C, had been shown to participate in the uptake of fatty acids and to be localized on the plasma membrane and the mitochondria [29]. Cells grown on pyruvate had increased expression of the beta-oxidation pathway proteins. It follows that, YKL187C (now known as FAT3) may be responsible for the uptake of fatty acids to feed the beta-oxidation pathway. Similar hypotheses may be drawn for other proteins currently classified as those of uncharacterized function from the data included in this study.
Abundance alterations resulting from the switch to pyruvate as the carbon source were not highly correlated for orthologous proteins between yeast species.
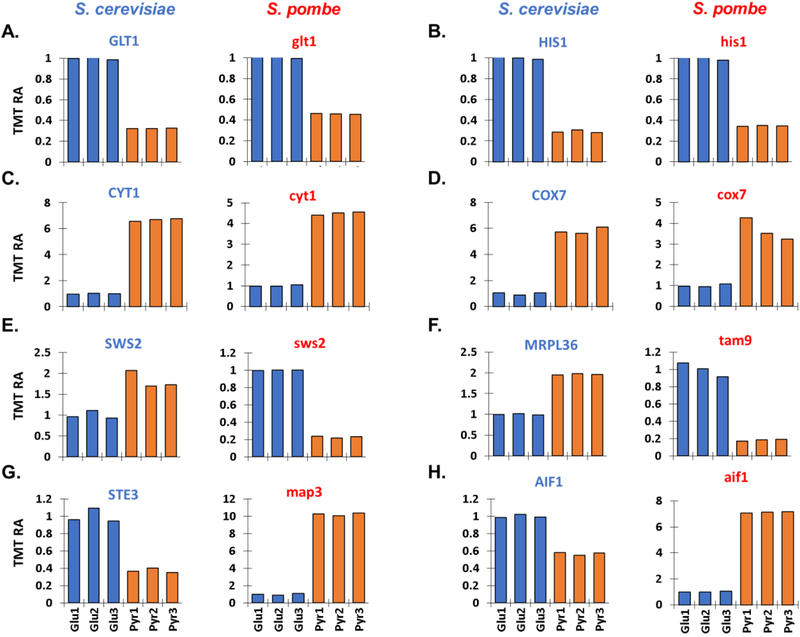
When comparing the proteins with altered abunance, we observed several orthologs that were among the most altered proteins. We highlighted several orthologous proteins that showed comparable, as well as opposite, expression patterns between species. For example, GLT1 (NAD(+)-dependent glutamate synthase) and HIS1 (an ATP phosphoribosyltransferase) were both of lower abundance in the pyruvate-treated cultures. Both proteins had roles in cellular amino acid biosynthesis (Figure 3A and B). Conversely, COX7 (Subunit VII of cytochrome c oxidase) and CYT1 (Cytochrome c1), both involved in cellular respiration, were in higher abundance in pyruvate-cultured cells (Figure 3C and D). We also noted several proteins that demonstrated opposite expression profiles in S. cerevisiae and S. pombe. For example, SWS2 (a mitochondrial small subunit ribosomal protein) and MRPL36 (a mitochondrial large subunit ribosomal protein, also known as tan9 in S. pombe) were of elevated abundance in pyruvate-grown S. cerevisiae, but down in S. pombe (Figure 3 E and F). However, the opposite patterns were observed for SWS2 (a pheromone receptor, also known as map3 in S. pombe) and AIF1 (apoptosis-inducing factor 1) (Figure 3 G and H). We attribute these differences in part to the evolutionary divergence between these species.
Figure 3: Relative abundance profiles of select orthologous proteins of S. cerevisiae and S. pombe proteins.
Bar graphs illustrate the protein profiles across six channels for several proteins demonstrating significant carbon source-induced differential abundance. Proteins highlighted include: A) GLT1/glt1, B) HIS1/his1, C) CYT1/cyt1, D) COX7/cox7, E) SWS2/sws2, F) MRPL36/tam9, G) STE3/map3, and H) AIF1/aif1. For each pair of bar graphs, the graph on the left is for the S. cerevisiae ortholog and that on the right is for the corresponding S. pombe ortholog. TMT RA, tandem mass tag relative abundance.
As mentioned previously, over 100 million years of evolution separated S. pombe and S. cerevisiae [2, 3]. As such, we anticipated that the proteomes of these yeast species were diverse in function, as well as structure, and so we performed in silico digests of S. cerevisiae and S. pombe proteomes using the Protein Digestion Simulator (PNNL, https://github.com/PNNL-Comp-Mass-Spec/Protein-Digestion-Simulator). Strikingly, assuming no missed cleavages due to trypsin, only 0.3% of tryptic peptides (n=854) overlapped between the two yeast species (Supplemental Figure 2A). Similarly, if two missed cleavages were considered, the percentage of overlapping peptides dropped to 0.2% (n=2484) (Supplemental Figure 2B). At the protein level, however, approximately 2,500 orthologs were suspected between S. pombe and S. cerevisiae [30]. Considering only one-to-one (non-degenerate) orthologs, 998 were identified in our data sets. We calculated the iBAQ values (intensity Based Absolute Quantification, a protein’s total non-normalized intensity of all peptides by the number of observed peptides) [31] for all proteins in each data set using MaxQuant [32] and plotted the correlation of these values (Supplemental Figure 3A). The iBAQ values were. The iBAQ values for proteins across the two species were well correlated, with a Pearson correlation coefficient of r = 0.75. We then examined the correlation of the fold-changes (pyruvate versus glucose) for these orthologs. Notably, the protein abundance changes were not correlated between species, and the Pearson correlation coefficient was r = 4×10−6 (Supplemental Figure 3B). Although initially unexpected, these data agreed with a previous SILAC-based study of protein turnover in S. cerevisiae and S. pombe [33]. There, protein abundance between species was well correlated (Pearson r = 0.64), but protein turnover rates of orthologous proteins were not (Pearson r << 0.01).
In addition to the protein turnover study mentioned above, our data were similar to other proteomic studies comparing S. cerevisiae and S. pombe. For example, a study investigating the conservation of genetic relationships within and between complexes and pathways had shown that subset of orthologous protein complexes undergoes functional repurposing that show divergent functions and partnerships [34]. Such differences were also observed in the phosphoproteome. The analysis of over 2,000 quantitative genetic interactions in S. cerevisiae and S. pombe had demonstrated that protein kinases and transcription factors show lower than average conservation of genetic interactions with respect to rate of change of phosphorylation [35]. In addition, a cross-species protein interactome comparison of stress response pathways has shown that the interactome network of orthologous proteins in stress response and signaling proteins are not conserved between species but are “rewired” such that orthologous proteins have different binding partners in both species [36]. The notion of differences in interacting partners is also true between a given complex and other complexes which interact with it [37]. Moreover, a study that compared the interaction networks of fission yeast, budding yeast, and humans had shown further the effects of species-specific network rewiring. This study revealed that although overall genes were conserved better between the yeasts, S. pombe interactions were re significantly more conserved in human than in S. cerevisiae [38]. All these data show that interactions, pathways and associated response may be different for S. cerevisiae and S. pombe protein orthologs. We suspect that millions of years of divergent evolution had efficiently rerouted pathways as a result of cellular stress, producing dissimilar responses to a given perturbation.
Protein abundance profiles may be obtained for complete canonical pathways.
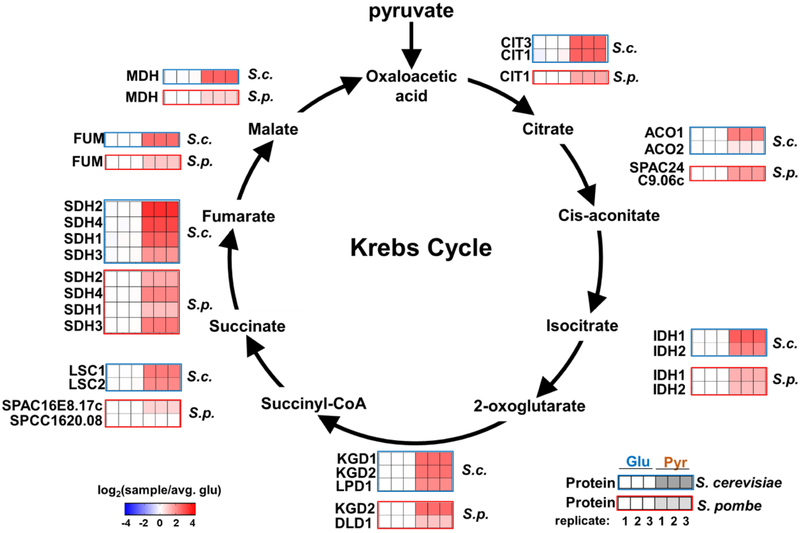
Comprehensive coverage of yeast proteomes enabled the abundance profiling of near-complete canonical pathways. As an example, we highlighted the enzymes that compose the Krebs cycle (Figure 4) and glycolysis (Supplemental Figure 4) in both yeast species. We noted that proteins associated with the pyruvate-driven Krebs cycle were increased when either species was grown with pyruvate as the sole carbon source. In addition, as expected, most glycolytic enzymes decreased or remained at constant levels in both species when the carbon source was switched to pyruvate (Supplemental Figure 4). However, a few enzymes in the glycolysis pathway showed unexpected increases. Notably, we observed a slight increase in TDH1 (glyceraldehyde-3-phosphate dehydrogenase) in S. cerevisiae, which could be a potential DNA replication stress response due to the altered carbon source [39]. We also noted an increase in ENO101 in S. pombe, which had a biological function inferred, but not independently verified, to be an enolase [40]. Further evidence will be needed to establish the role of increased ENO101 in glycolysis in relation to pyruvate in S. pombe. Nonetheless, the deep proteome coverage attained by SL-SP3-TMT can help provide a clearer picture of the proteome remodeling in and allow for the elucidation of regulation for these and other cellular pathways under any given perturbation.
Figure 4: Canonical Krebs Cycle pathway profiled in both species.
Protein abundance profiles of enzymes in the Krebs Cycle for S. cerevisiae and S. pombe are overlaid on the flow diagram.
Conclusions.
Here we presented the first study using the SL-SP3-TMT protocol to comprehensively profile relative protein abundance across two yeast species. We suggest SP3 as an alternative to precipitation methods, such as chloroform-methanol, trichloroacetic acid, or acetone precipitation for SL-TMT. Chemical precipitation methods may throttle the adoption of SL-TMT for high-throughput sample preparation due to limitations of potential variability in pellet/disk formation, difficulties in aspirating small protein amounts, and inadaptability to robotic sample processing. Using the hybrid SL-TMT × SP3 strategy, we measured relative protein abundance for over 85% of the protein encoding genes in two yeast species. Increased depth of proteome coverage allowed for a more thorough analysis of functional pathways. As such, these data can be used to elucidate the roles of proteins of unknown function. We are confident that further application of this strategy will allow for high-throughput, yet comprehensive, protein abundance profiling on a TMT-based multiplexed platform.
Supplementary Material
Supplemental Table 1: Proteins identified in the S. cerevisiae dataset. Columns include: Uniprot protein identification number (proteinID), gene symbol (Gene Symbol), protein description/name (Description), number of peptides identified per protein (peptides), the normalized summed signal-to-noise for each of the 10 channels (126 to 131), and the log2 ratios (Pyruvate/ Glucose) for the summed signal-to-noise of the TMT relative abundance values for each cell type. We also include columns for the average of replicate conditions in this experiment, the log2 fold-change between comparison groups, and the associated p-values.
Supplemental Table 2: Proteins identified in the S. pombe dataset. Column headers are identical to those in Supplemental Table 1, but for S. pombe.
Supplemental Table 3: Peptides identified in the S. cerevisiae dataset. Columns include: Uniprot protein identification number (proteinID), gene symbol (Gene Symbol), description/name (Description), redundancy, peptide sequence (peptide sequence), number of quantified peptides (num_quant), and the summed signal-to-noise for each of the 10 channels (126 to 131).
Supplemental Table 4: Peptides identified in the S. pombe dataset. Column headers are identical to those in Supplemental Table 3, but for S. pombe.
SIGNIFICANCE.
Saccharomyces cerevisiae and Schizosaccharomyces pombe are the two most widely studied yeast species in molecular biology laboratories. These single-celled eukaryotes diverged from a common ancestor over a period of 100 million years, such that evolution has driven fundamental differences between the two species. Cellular metabolism and the regulation thereof are vital for living organisms. Here, we hypothesize that large scale proteomic alterations are prevalent upon the substitution of glucose with another carbon source, in this case pyruvate. To efficiently process our samples, we developed an updated streamlined-tandem mass tag (SL-TMT) strategy with more versatile bead-based protein aggregation. The data revealed expected alterations in protein abundance and illustrated differences between species. We highlighted complete canonical biochemical pathways and provided insight into previously uncharacterized proteins.
We profiled the S. cerevisiae and S. pombe proteomes with different carbon sources
We substituted chemical-based precipitation with bead-based aggregation in SL-TMT
We measured the relative abundance of ~80% of proteins contained in yeast databases
Increased proteome coverage allowed for more thorough analysis of functional pathways
ACKNOWLEDGEMENTS
We would like to thank members of the Gygi Lab at Harvard Medical School. This work was funded in part by an NIH/NIDDK grant K01 DK098285 (J.A.P.) and GM67945 (S.P.G).
Footnotes
CONFLICTS OF INTEREST
The authors acknowledge no conflict of interest.
REFERENCES
- [1].Forsburg SL, The yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe: models for cell biology research, Gravit Space Biol Bull 18(2) (2005) 3–9. [PubMed] [Google Scholar]
- [2].Heckman DS, Geiser DM, Eidell BR, Stauffer RL, Kardos NL, Hedges SB, Molecular evidence for the early colonization of land by fungi and plants, Science 293(5532) (2001) 1129–33. [DOI] [PubMed] [Google Scholar]
- [3].Hedges SB, The origin and evolution of model organisms, Nat Rev Genet 3(11) (2002) 838–49. [DOI] [PubMed] [Google Scholar]
- [4].Paulo JA, O’Connell JD, Everley RA, O’Brien J, Gygi MA, Gygi SP, Quantitative mass spectrometry-based multiplexing compares the abundance of 5000 S. cerevisiae proteins across 10 carbon sources, J Proteomics 148 (2016) 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Navarrete-Perea J, Yu Q, Gygi SP, Paulo JA, Streamlined Tandem Mass Tag (SL-TMT) Protocol: An Efficient Strategy for Quantitative (Phospho)proteome Profiling Using Tandem Mass Tag-Synchronous Precursor Selection-MS3, J Proteome Res 17(6) (2018) 2226–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Quansah E, Peelaerts W, Langston JW, Simon DK, Colca J, Brundin P, Targeting energy metabolism via the mitochondrial pyruvate carrier as a novel approach to attenuate neurodegeneration, Mol Neurodegener 13(1) (2018) 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ullah N, Naseer MI, Ullah I, Kim TH, Lee HY, Kim MO, Neuroprotective profile of pyruvate against ethanol-induced neurodegeneration in developing mice brain, Neurol Sci 34(12) (2013) 2137–43. [DOI] [PubMed] [Google Scholar]
- [8].Lao-On U, Attwood PV, Jitrapakdee S, Roles of pyruvate carboxylase in human diseases: from diabetes to cancers and infection, J Mol Med (Berl) 96(3–4) (2018) 237–247. [DOI] [PubMed] [Google Scholar]
- [9].Inoue T, Murakami N, Ayabe T, Oto Y, Nishino I, Goto Y, Koga Y, Sakuta R, Pyruvate Improved Insulin Secretion Status in a Mitochondrial Diabetes Mellitus Patient, J Clin Endocrinol Metab 101(5) (2016) 1924–6. [DOI] [PubMed] [Google Scholar]
- [10].Chang I, Cho N, Koh JY, Lee MS, Pyruvate inhibits zinc-mediated pancreatic islet cell death and diabetes, Diabetologia 46(9) (2003) 1220–7. [DOI] [PubMed] [Google Scholar]
- [11].Sakamoto A, Kunou S, Shimada K, Tsunoda M, Aoki T, Iriyama C, Tomita A, Nakamura S, Hayakawa F, Kiyoi H, Pyruvate secreted from patient-derived cancer-associated fibroblasts supports survival of primary lymphoma cells, Cancer Sci 110(1) (2019) 269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Elia I, Rossi M, Stegen S, Broekaert D, Doglioni G, van Gorsel M, Boon R, Escalona-Noguero C, Torrekens S, Verfaillie C, Verbeken E, Carmeliet G, Fendt SM, Breast cancer cells rely on environmental pyruvate to shape the metastatic niche, Nature 568(7750) (2019) 117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Corbet C, Stem Cell Metabolism in Cancer and Healthy Tissues: Pyruvate in the Limelight, Front Pharmacol 8 (2017) 958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hughes CS, Sorensen PH, Morin GB, A Standardized and Reproducible Proteomics Protocol for Bottom-Up Quantitative Analysis of Protein Samples Using SP3 and Mass Spectrometry, Methods Mol Biol 1959 (2019) 65–87. [DOI] [PubMed] [Google Scholar]
- [15].Hughes CS, Moggridge S, Muller T, Sorensen PH, Morin GB, Krijgsveld J, Single-pot, solid-phase-enhanced sample preparation for proteomics experiments, Nat Protoc 14(1) (2019) 68–85. [DOI] [PubMed] [Google Scholar]
- [16].Moggridge S, Sorensen PH, Morin GB, Hughes CS, Extending the Compatibility of the SP3 Paramagnetic Bead Processing Approach for Proteomics, J Proteome Res 17(4) (2018) 1730–1740. [DOI] [PubMed] [Google Scholar]
- [17].Hughes CS, Foehr S, Garfield DA, Furlong EE, Steinmetz LM, Krijgsveld J, Ultrasensitive proteome analysis using paramagnetic bead technology, Mol Syst Biol 10 (2014) 757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Paulo JA, Nicotine alters the proteome of two human pancreatic duct cell lines, JOP : Journal of the pancreas 15(5) (2014) 465–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Paulo JA, Gygi SP, Nicotine-induced protein expression profiling reveals mutually altered proteins across four human cell lines, Proteomics 17(1–2) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chambers MC, Maclean B, Burke R, Amodei D, Ruderman DL, Neumann S, Gatto L, Fischer B, Pratt B, Egertson J, Hoff K, Kessner D, Tasman N, Shulman N, Frewen B, Baker TA, Brusniak MY, Paulse C, Creasy D, Flashner L, Kani K, Moulding C, Seymour SL, Nuwaysir LM, Lefebvre B, Kuhlmann F, Roark J, Rainer P, Detlev S, Hemenway T, Huhmer A, Langridge J, Connolly B, Chadick T, Holly K, Eckels J, Deutsch EW, Moritz RL, Katz JE, Agus DB, MacCoss M, Tabb DL, Mallick P, A cross-platform toolkit for mass spectrometry and proteomics, Nat Biotechnol 30(10) (2012) 918–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Beausoleil SA, Villen J, Gerber SA, Rush J, Gygi SP, A probability-based approach for high-throughput protein phosphorylation analysis and site localization, Nature biotechnology 24(10) (2006) 1285–92. [DOI] [PubMed] [Google Scholar]
- [22].Huttlin EL, Jedrychowski MP, Elias JE, Goswami T, Rad R, Beausoleil SA, Villen J, Haas W, Sowa ME, Gygi SP, A tissue-specific atlas of mouse protein phosphorylation and expression, Cell 143(7) (2010) 1174–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Elias JE, Gygi SP, Target-decoy search strategy for mass spectrometry-based proteomics, Methods Mol Biol 604 (2010) 55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Elias JE, Gygi SP, Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry, Nat Methods 4(3) (2007) 207–14. [DOI] [PubMed] [Google Scholar]
- [25].McAlister GC, Huttlin EL, Haas W, Ting L, Jedrychowski MP, Rogers JC, Kuhn K, Pike I, Grothe RA, Blethrow JD, Gygi SP, Increasing the Multiplexing Capacity of TMTs Using Reporter Ion Isotopologues with Isobaric Masses, Analytical Chemistry 84(17) (2012) 7469–7478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Perez-Riverol Y, Csordas A, Bai J, Bernal-Llinares M, Hewapathirana S, Kundu DJ, Inuganti A, Griss J, Mayer G, Eisenacher M, Perez E, Uszkoreit J, Pfeuffer J, Sachsenberg T, Yilmaz S, Tiwary S, Cox J, Audain E, Walzer M, Jarnuczak AF, Ternent T, Brazma A, Vizcaino JA, The PRIDE database and related tools and resources in 2019: improving support for quantification data, Nucleic Acids Res 47(D1) (2019) D442–D450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Murtagh F, Legendre P, Ward’s Hierarchical Clustering Method: Clustering Criterion and Agglomerative Algorithm, arXiv e-prints, 2011. [Google Scholar]
- [28].Tadi D, Hasan RN, Bussereau F, Boy-Marcotte E, Jacquet M, Selection of genes repressed by cAMP that are induced by nutritional limitation in Saccharomyces cerevisiae, Yeast 15(16) (1999) 1733–45. [DOI] [PubMed] [Google Scholar]
- [29].Jung S, Smith JJ, von Haller PD, Dilworth DJ, Sitko KA, Miller LR, Saleem RA, Goodlett DR, Aitchison JD, Global analysis of condition-specific subcellular protein distribution and abundance, Mol Cell Proteomics 12(5) (2013) 1421–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lock A, Rutherford K, Harris MA, Hayles J, Oliver SG, Bahler J, Wood V, PomBase 2018: user-driven reimplementation of the fission yeast database provides rapid and intuitive access to diverse, interconnected information, Nucleic Acids Res 47(D1) (2019) D821–D827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Schwanhausser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, Chen W, Selbach M, Global quantification of mammalian gene expression control, Nature 473(7347) (2011) 337–42. [DOI] [PubMed] [Google Scholar]
- [32].Cox J, Mann M, MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification, Nat Biotechnol 26(12) (2008) 1367–72. [DOI] [PubMed] [Google Scholar]
- [33].Christiano R, Nagaraj N, Frohlich F, Walther TC, Global proteome turnover analyses of the Yeasts S. cerevisiae and S. pombe, Cell Rep 9(5) (2014) 1959–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Frost A, Elgort MG, Brandman O, Ives C, Collins SR, Miller-Vedam L, Weibezahn J, Hein MY, Poser I, Mann M, Hyman AA, Weissman JS, Functional repurposing revealed by comparing S. pombe and S. cerevisiae genetic interactions, Cell 149(6) (2012) 1339–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Beltrao P, Trinidad JC, Fiedler D, Roguev A, Lim WA, Shokat KM, Burlingame AL, Krogan NJ, Evolution of phosphoregulation: comparison of phosphorylation patterns across yeast species, PLoS Biol 7(6) (2009) e1000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Das J, Vo TV, Wei X, Mellor JC, Tong V, Degatano AG, Wang X, Wang L, Cordero NA, Kruer-Zerhusen N, Matsuyama A, Pleiss JA, Lipkin SM, Yoshida M, Roth FP, Yu H, Crossspecies protein interactome mapping reveals species-specific wiring of stress response pathways, Sci Signal 6(276) (2013) ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Roguev A, Shevchenko A, Schaft D, Thomas H, Stewart AF, Shevchenko A, A comparative analysis of an orthologous proteomic environment in the yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe, Mol Cell Proteomics 3(2) (2004) 125–32. [DOI] [PubMed] [Google Scholar]
- [38].Vo TV, Das J, Meyer MJ, Cordero NA, Akturk N, Wei X, Fair BJ, Degatano AG, Fragoza R, Liu LG, Matsuyama A, Trickey M, Horibata S, Grimson A, Yamano H, Yoshida M, Roth FP, Pleiss JA, Xia Y, Yu H, A Proteome-wide Fission Yeast Interactome Reveals Network Evolution Principles from Yeasts to Human, Cell 164(1–2) (2016) 310–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tkach JM, Yimit A, Lee AY, Riffle M, Costanzo M, Jaschob D, Hendry JA, Ou J, Moffat J, Boone C, Davis TN, Nislow C, Brown GW, Dissecting DNA damage response pathways by analysing protein localization and abundance changes during DNA replication stress, Nat Cell Biol 14(9) (2012) 966–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wang H, Wang H, Wang M, Zhang L, Wang R, Mei Y, Shao W, Identification and refinement of two strong constitutive promoters for gene expression system of Schizosaccharomyces pombe, World J Microbiol Biotechnol 30(6) (2014) 1809–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Proteins identified in the S. cerevisiae dataset. Columns include: Uniprot protein identification number (proteinID), gene symbol (Gene Symbol), protein description/name (Description), number of peptides identified per protein (peptides), the normalized summed signal-to-noise for each of the 10 channels (126 to 131), and the log2 ratios (Pyruvate/ Glucose) for the summed signal-to-noise of the TMT relative abundance values for each cell type. We also include columns for the average of replicate conditions in this experiment, the log2 fold-change between comparison groups, and the associated p-values.
Supplemental Table 2: Proteins identified in the S. pombe dataset. Column headers are identical to those in Supplemental Table 1, but for S. pombe.
Supplemental Table 3: Peptides identified in the S. cerevisiae dataset. Columns include: Uniprot protein identification number (proteinID), gene symbol (Gene Symbol), description/name (Description), redundancy, peptide sequence (peptide sequence), number of quantified peptides (num_quant), and the summed signal-to-noise for each of the 10 channels (126 to 131).
Supplemental Table 4: Peptides identified in the S. pombe dataset. Column headers are identical to those in Supplemental Table 3, but for S. pombe.