Abstract
Approximately 37 million people worldwide are infected with human immunodeficiency virus (HIV). One highly significant complication of HIV infection is the development of HIV-associated neurocognitive disorders (HAND) in 15 – 55% of people living with HIV (PLWH), that persists even in the antiretroviral therapy (ART) era. The entry of HIV into the central nervous system (CNS) occurs within 4 to 8 days after peripheral infection. This establishes viral reservoirs that may persist even in the presence of ART. Once in the CNS, HIV infects resident macrophages, microglia, and at low levels, astrocytes. In response to chronic infection and cell activation within the CNS, viral proteins, inflammatory mediators, and host and viral neurotoxic factors produced over extended periods of time result in neuronal injury and loss, cognitive deficits and HAND. Substance abuse is a common comorbidity in PLWH and has been shown to increase neuroinflammation and cognitive disorders. Additionally, it has been associated with poor ART adherence, and increased viral load in the cerebrospinal fluid (CSF), that may also contribute to increased neuroinflammation and neuronal injury. Studies have examined mechanisms that contribute to neuroinflammation and neuronal damage in PLWH, and how substances of abuse exacerbate these effects. This review will focus on how substances of abuse, with an emphasis on methamphetamine (meth), cocaine, and opioids, impact blood brain barrier (BBB) integrity and transmigration of HIV-infected and uninfected monocytes across the BBB, as well as their effects on monocytes/macrophages, microglia, and astrocytes within the CNS. We will also address how these substances of abuse may contribute to HIV-mediated neuropathogenesis in the context of suppressive ART. Additionally, we will review the effects of extracellular dopamine, a neurotransmitter that is increased in the CNS by substances of abuse, on HIV neuropathogenesis and how this may contribute to neuroinflammation, neuronal insult, and HAND in PLWH with active substance use. Lastly, we will discuss some potential therapies to limit CNS inflammation and damage in HIV-infected substance abusers.
Introduction
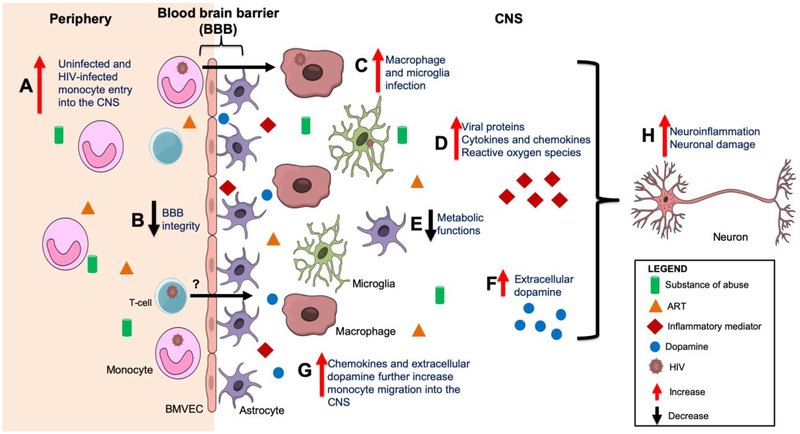
Approximately 37 million people are living with human immunodeficiency virus (HIV) worldwide, with approximately 1.8 million new infections in 2017 (1). The introduction of antiretroviral therapies (ART) has significantly reduced HIV-associated mortalities, and prolonged lifespan among people living with HIV (PLWH). HIV enters the central nervous system (CNS) within 4 to 8 days after peripheral infection, usually before a person has been diagnosed (2–4). This is mediated, in part, by the transmigration of infected peripheral blood monocytes across the blood brain barrier (BBB) (5–9). Once within the CNS, these cells can differentiate into macrophages, becoming long-lived cells and establishing a viral reservoir that persists even with effective ART (10, 11). Infected macrophages promote the infection of additional cells within the CNS including microglia, macrophages, and astrocytes, at low levels, but not neurons (12). Some studies have shown that HIV can still be detected in the cerebrospinal fluid (CSF) of PLWH on suppressive ART (4, 13–15). HIV DNA and RNA are detected in perivascular macrophages and microglia within brain tissues of HIV-infected people on ART, suggesting that these long-lived infected cells may serve as viral reservoirs in the CNS (11, 12). Additionally, viral proteins including HIV Tat, a trans activator of transcription, and HIV Nef, a negative regulatory factor, are still produced extracellularly in the CNS of PLWH, even with ART (16–18). The presence of HIV and its proteins can infect and/or activate resident CNS cells, contributing to release of viral and host inflammatory mediators and neurotoxic factors that contribute to ongoing inflammation and neuronal damage.
A significant consequence of HIV infection is HIV-associated neurocognitive disorders (HAND), with 15–55% of PLWH developing HAND even in the presence of ART (19–22). HAND is a spectrum of disorders that includes attention deficits, impaired overall executive function, motor function, working memory, learning, and speed of information processing, and dementia (23). In the pre-ART era, HIV-associated dementia was prevalent, but this form of HAND has declined significantly with the advent of ART. However, milder forms of HAND persist (23). HAND is diagnosed by a battery of neuropsychological tests and there are currently no biomarkers for diagnosis of, or treatments for, HAND. While neurons do not become infected with HIV, they are affected by viral proteins, inflammatory mediators, and neurotoxins chronically produced in the HIV-infected CNS, as well antiretrovirals, all of which contribute to neuronal damage and loss, and the development of HAND.
Substance abuse is a major comorbidity in PLWH. It may lead to the engagement of risky behaviors that facilitate HIV transmission, particularly sharing of needles, increasing the number of HIV-infected substance abusers (24). It is estimated that approximately 20% of new HIV infections are attributed to intravenous drug use, a common route of administration of substances of abuse (25). Methamphetamine (meth) and cocaine are stimulants that are abused by more than 1.5 million people in the United States (26). Additionally, there is currently a major opioid epidemic in the United States, with approximately 11 million people reported to misuse opioids in 2017 (26). Prescription opioid abuse is also on the rise and for some of these addicted individuals, difficulties in obtaining prescriptions for opioids results in their use of heroin (26). In addition, a significant number of PLWH are prescribed opioid pain medications or are addicted to heroin or prescription opioids.
Studies have shown that substances of abuse increase HIV CNS pathogenesis even in the presence of ART. Substance abuse alone, including the use of meth, cocaine, and opioids, is associated with neurocognitive impairment (NCI) (27–32). Some studies reported increased NCI in HIV-infected substance abusers when compared to infected non-substance abusers (33). However, one study reported that HAND was not associated with either cocaine or opioid use in PLWH (34). This study suggested that HAND is associated with dopamine receptor polymorphisms, indicating the importance of the dopaminergic system in HIV neuropathogenesis (34). This will be discussed in a separate section of the review. Substance abuse is also associated with poor ART adherence that may result in activation of viral reservoirs in infected individuals or HIV acquisition in people using ART for pre- and post-exposure prophylaxis (35). Some studies reported increased viral loads in the CSF of PLWH who abuse drugs that may be attributed, in part, to ART non-adherence and/or activation of HIV-infected cells (36). Serum biomarkers of inflammation including C-reactive protein are increased in PLWH who use meth and cocaine, as compared to HIV-infected non-drug users or uninfected substance abusers (37). Chronic inflammation in the periphery, mediated by translocation of microbial products, such as bacterial lipopolysaccharide (LPS), through a leaky gut epithelial barrier in PLWH, even on ART, may promote activation of peripheral monocytes and their increased migration into the CNS (38–40). A recent study reported increased monocyte activation in the peripheral blood of virally suppressed HIV-infected substance users that was not associated with ART adherence or time to ART (41). These data suggest that substance abuse contributes to increased peripheral inflammation and immune cell activation, that may result in consequent entry of monocytes, and perhaps other immune cells, into the CNS of PLWH, contributing to increased neuroinflammation, irrespective of ART. Our laboratory and others showed that a subset of peripheral mature monocytes expressing CD14, the LPS co-receptor, and CD16, the FcγRIII receptor, becomes infected with HIV, and we demonstrated that these monocytes preferentially transmigrate across a human blood brain barrier model in response to CCL2 or CXCL12, chemokines elevated in the CNS of ART treated PLWH (42–47). We also reported that CD14+CD16+ monocytes are increased in the blood of PLWH who are active substance users when compared to infected non-drug users (48). Thus, active substance use may not only increase peripheral uninfected and HIV-infected CD14+CD16+ monocytes, but also may activate this and other subgroups of monocytes, resulting in their increased transmigration across the BBB, contributing to viral entry into the CNS. This, in conjunction with reactivation of HIV in the CNS as a result of exposure of infected cells to substances of abuse, may replenish viral reservoirs and promote chronic neuroinflammation, neuronal damage and neurotoxicity in HIV-infected substance abusers, resulting in an increase in the incidence and severity of HAND.
To characterize mechanisms that facilitate HAND in HIV-infected drug users on ART, it is important to understand how substances of abuse contribute to neuronal insult. In particular, it is necessary to characterize their effects on HIV replication in the CNS and blood brain barrier (BBB) integrity, and on the entry of uninfected and HIV-infected monocytes into the brain, especially in the context of ART. The BBB tightly regulates entry of molecules and cells from the peripheral circulation into the CNS. Brain microvascular endothelial cells (BMVEC) are a major component of the BBB and these cells express surface junctional and adhesion proteins that contribute to the formation of a tight barrier and the maintenance of BBB integrity. Astrocytes are also part of the BBB and these cells extend their end feet processes to the basement membrane of BBB vessels to interact with BMVEC which is necessary to induce BBB properties, including formation of a tight barrier (49, 50). HIV enters the CNS, in part, by the transmigration of infected peripheral mature CD14+CD16+ monocytes across the BBB (51, 52). This is a multistep process that occurs in response to gradients of chemokines including CCL2 and CXCL12 (5–9). These chemokines can induce firm arrest of monocytes, followed by the crawling and diapedesis of cells across the BBB with subsequent entry into the CNS (53). Our laboratory showed that the junctional proteins, JAM-A and ALCAM, are critical for HIV-infected CD14+CD16+ monocyte transmigration across the BBB in response to CCL2 (5). These junctional proteins are increased on HIV-infected monocytes and blocking these molecules limits monocyte transmigration in vitro (8, 54). We demonstrated that this blocking occurs even in the presence of ART (Unpublished data, Rosiris Leon Rivera). Some substances of abuse decrease endothelial junctional proteins and increase BBB permeability. This may contribute to peripheral monocyte, and perhaps T-cell, transmigration across the BBB and the entry of HIV into the CNS, leading to viral reservoir seeding and chronic neuroinflammation. Additionally, the antiretroviral drug efavirenz, a non-nucleoside reverse transcriptase inhibitor, increases permeability of human brain endothelial cell monolayers, in part through decreased tight junction proteins (55). This suggests that certain antiretrovirals may impair endothelial barrier integrity, contributing to BBB permeability. Studies need to be performed to characterize the combined effects of ART, HIV, and substances of abuse on the BBB, and their contribution to the development of HAND.
Once within the CNS, HIV-infected monocytes may differentiate into macrophages and remain as long-lived infected cells (56). Studies propose that these macrophages constitute an important CNS viral reservoir even with effective ART (57). Animal models of HIV infection include HIV-infected, ART treated humanized myeloid only mice (MoM) that are generated by injecting human hematopoietic stem cells into NOD/SCID mice (58). MoM mice are reconstituted with human myeloid and B cells, but no T-cells. Thus, MoM mice can be used to characterize the effects of HIV infection of monocytes/macrophages, in the absence of T-cells, on the pathogenesis of HIV. In a study with these mice, HIV replication is sustained in the absence of T-cells and HIV-infected macrophages are detected in multiple tissues including the brain, suggesting that these infected cells are a viral reservoir in the CNS (58). Another study with MoM mice showed that ART reduces viral load and macrophage infection with HIV. Interruption of ART resulted in viral rebound in approximately 33% of the mice indicating persistent HIV infection of macrophages even with successful ART (59). Infection of mice using EcoHIV, a chimeric HIV with replacement of gp120, the HIV envelope glycoprotein, with ecotropic murine leukemia virus (MLV) gp80, establishes infection of mouse CD4+ T-cells, peripheral and CNS monocytes/macrophages, and microglia (60). Chronically EcoHIV-infected mice develop HIV associated NCI as detected by radial arm water maze, designed to test working and spatial memory (60). To confirm further the infection of macrophages, nude mice lacking T-cells were infected with EcoHIV (60). These mice have persistent productive infection in macrophages, develop NCI, and have increased monocytes/macrophages in the brains compared to uninfected animals (60). Other studies report the presence of latently infected macrophages in the brains of ART suppressed SIV-infected macaques (61, 62), highlighting the role of macrophages as viral reservoirs in the CNS even in the presence of suppressive ART. Additionally, latently infected peripheral macrophage reservoirs with virus capable of replication are detected in SIV-infected macaques treated with ART using quantitative viral outgrowth assays (QVOA) (63). Macrophages from these animals are able to release virus that could infect activated CD4 T-cells in culture (63).
T-cells may also contribute to HIV-mediated neuropathogenesis. In humanized mice containing only human T-cells, HIV is still detected in the brain, suggesting that T-cells may also contribute to the establishment of HIV infection in the CNS (64). CD8+ T-cells are increased in CSF of PLWH (65), with HIV specific CD8+ T-cells being present in that compartment (66). CD8+ T-cells are also associated with anti-HIV activity in the brain (67). Studies reported that depletion of CD8+ T-cells is associated with increased viral load in ART naïve (68) and ART treated SIV-infected macaques (69). These data suggest that CD8+ T-cells are important in controlling HIV in the CNS, and these cells may also contribute to ongoing neuroinflammation in PLWH.
Some studies characterized the effects of ART on neurocognitive outcomes. Treatment of mice in the absence of HIV with protease inhibitors, lopinavir and ritonavir, impairs cognition in these animals (70). In SIV-infected pig-tailed macaques, the combination of tenofovir, a nucleotide analog reverse transcriptase inhibitor, with protease inhibitors, atazanavir and saquinavir, and the integrase inhibitor, L-870812, causes neuronal injury measured by decreased synaptophysin compared to non-ART treated SIV-infected or uninfected animals (71). Cohort studies reported an association of NCI with efavirenz use in PLWH (72, 73). In vitro, treatment with antiretroviral drugs, meth, and gp120 cause a decrease in intracellular ATP in rat neurons, suggesting an effect on neuronal homeostasis (74). More studies need to be conducted to characterize the effect of ART on HAND development in PLWH who abuse drugs.
This review will highlight recent findings on the mechanisms by which substances of abuse, specifically meth, cocaine, and opioids, contribute to viral seeding and reseeding of the CNS, chronic neuroinflammation, and neuronal damage that characterize HAND. We will also describe the impact of increased CNS extracellular dopamine, induced by these substances of abuse, on the development of HAND. In addition, we will discuss some potential therapies to limit viral entry into the CNS, as well as chronic neuroinflammation in the context of substance abuse and HIV infection. Although this review will address many of the studies that were performed in the absence of ART, it is imperative for future studies to characterize the interactive effects of not only substances of abuse and HIV, but also of ART. New guidelines now recommend that ART be initiated as soon as possible after HIV infection. This early treatment of PLWH with ART may impact the mechanisms of HIV neuropathogenesis in the context of substance abuse. Although this manuscript does not have a separate section about ART and substances of abuse, we discuss effects of some antiretroviral drugs throughout the sections below and suggest potential areas of research to characterize the combined impact of HIV, ART, and substances of abuse.
Methamphetamine and HIV Overview
Approximately 1.6 million people were reported to abuse meth in the United States in 2017 (75). There are various routes of meth administration including oral and intravenous intake, or through smoking. Meth is a lipophilic stimulant with a long half-life of between 10 and 12 h. Meth readily crosses the plasma membrane of cells and can cross the BBB and enter the CNS, where it affects the dopamine reward pathway. Once in the CNS, meth exerts its effects by increasing extracellular dopamine by interacting with either dopamine transporters (DAT) or vesicular monoaminase transporter 2 (VMAT-2) (76, 77). Meth inhibits dopamine reuptake mediated by DAT in neurons, resulting in increased extracellular dopamine (76, 77). Meth also causes release of dopamine from storage vesicles in the cytoplasm of neurons by interacting with VMAT-2, and through reverse transport by DAT (76, 77). This further increases extracellular levels of this neurotransmitter that contributes to excitotoxicity and neuronal damage. Chronic meth use has been associated with NCI based on neurocognitive tests and imaging studies. The most frequently reported domains affected by meth use include memory, executive function, and motor function (29–31). One study showed that former meth users had reduced striatal dopamine transporter (DAT) density even after 3 years of abstinence when compared to controls, which may be related to axonal damage (78). Other studies also reported neuronal injury, and morphological and metabolic changes in some regions of the brains of meth users (79, 80). These studies emphasize the impact of meth alone on neuronal health, and below is a discussion of what has been reported about the interaction between meth and HIV-mediated neuropathogenesis.
Meth abuse is associated with a 1.5-fold increased risk of HIV acquisition (81). Studies reported that meth users have higher plasma and CSF HIV viral loads compared to non-meth users irrespective of ART adherence, indicating the role of meth in HIV CNS disease even in the presence of ART (82, 83). Among people with acute HIV infection, development of NCI is, in part, associated with meth use (84). Other cohort studies reported that HIV-infected meth users have an overall decline in neurocognitive performance compared to HIV-infected non-meth users, and uninfected meth users (85–87). Older PLWH on ART with a previous history of meth use have overall neurocognitive decline, especially in learning and memory, compared to non-meth using counterparts (88). Studies also examined the impact of meth and HIV on neuronal health to characterize further mechanisms mediating HAND in meth users. PLWH who chronically abused meth, have decreased expression of the neuronal marker N-acetylaspartate, indicating neuronal injury, compared to non-drug using PLWH or uninfected meth abusers (89). Similarly, in a separate study, HIV-infected meth users were shown to have decreased neuronal health and increased glial activation (82), suggesting that meth may contribute to neuron damage and development of HAND. However, another study concluded that neuronal injury is associated with HIV, and not with meth use (90). This study showed that in HIV-infected meth users, HIV RNA levels in the plasma correlates with neuronal injury (90). These studies highlight the complex interactions between meth abuse and HIV neuropathogenesis, and how both may be contributing to HAND even in the presence of ART.
Animal and in vitro studies also have been performed to characterize the impact of meth and HIV on neurocognition. In transgenic mice expressing HIV gp120, meth contributes to poor cognitive outcomes compared to non-meth treated mice (85, 91). In transgenic mice expressing HIV Tat, escalating doses of meth results in impaired working and spatial memory compared to non-Tat transgenic mice or non-meth treated transgenic mice, suggesting cooperative effects between Tat and meth on neurocognitive functions (92). In vitro, meth increases gp120 neurotoxic effects on rat neurons, and meth and gp120 increase IL1-β and TNF-α production by rat microglia that contributes to neuronal damage (93). This part of the review will focus on the effects of meth on HIV infection, BBB integrity, monocyte entry into the CNS, macrophage, microglia and astrocyte function, and how this may all contribute to chronic neuroinflammation and neuronal damage that lead to HAND.
Methamphetamine and HIV Infection in the CNS
Meth treatment has been linked to increased HIV infection in both in vivo and in vitro studies. In SIV-infected macaques, meth does not change plasma viral load but increases virus in the brain (94). In addition, cohort studies reporting increased viral load in PLWH using meth were addressed in the previous section highlighting the contribution of meth in HIV neuropathogenesis. In vitro, meth increases HIV co-receptor CCR5 on uninfected macaque microglia, with higher expression in SIV-infected microglia (95). Meth also increases HIV replication in human microglia through downstream signaling and activation of long terminal repeat (LTR) regions of the HIV genome (96). In addition, meth induces reactivation of HIV in latently infected human microglia (96), suggesting that it may play a role in reactivation of viral reservoirs in the CNS. Meth increases HIV replication in human monocytes, and this is in part mediated by activation of the HIV LTR (97). Meth also increases HIV infection of human monocyte derived macrophages (MDM) through increased CCR5 expression (98). Additionally, a dopamine D1 receptor (D1R) antagonist blocks this effect suggesting that the effects of meth in macrophages may be mediated, in part, by activation of D1R (98). A separate study showed that meth induces large syncytia formation, augments HIV reverse transcriptase activity, and inhibits anti-HIV activity in infected human MDM through a mechanism involving D1R (99). Additionally, treatment of human MDM with meth increases HIV production as measured by p24 antigen, and this was in part due to increased galectin-1, a protein that facilitates virus-host interactions (100). Meth treatment of monocyte derived dendritic cells also increases HIV co-receptors, CXCR4 and CCR5, through modulation of dopamine D2 receptor (D2R), which in turn increases their ability to become HIV-infected (101). Meth also induces HIV replication in human neural progenitor cells (102). These cells may differentiate into latently infected astrocytes, contributing to CNS viral reservoirs. Collectively, these studies suggest that meth use may exacerbate HIV infection of CNS resident cells and may also reactivate latent viral reservoirs through various mechanisms, contributing to ongoing neuroinflammation and neurotoxicity.
Meth treatment has also been shown to increase HIV infection of T-cells. Meth pretreatment increases HIV infection of naïve and activated CD4+ T-cells, and this is proposed to be through sigma-1 receptor (103). Another study showed increased HIV replication in CD4+ T-cells, both in vitro, and in transgenic mice, through increased activation of HIV LTR (97). In contrast, high concentrations of meth inhibit HIV replication in human T-cells through upregulation of anti-HIV miRNAs suggesting that meth effects on HIV infection of, and replication in, T-cells is concentration dependent (104). Thus, the pool of peripheral infected CD4+ T-cells available for possible transmigration across the BBB may be increased by low concentrations of meth, contributing to CNS viral seeding.
Effects of Methamphetamine and HIV on Blood Brain Barrier Permeability
Many studies examined the effects of meth by itself on BBB integrity, with an emphasis on changes in endothelial tight junction proteins. In mice, meth induces transient BBB permeability, as quantified by the passage of albumin coupled to Evans blue dye from the peripheral circulation into the brain (105). This effect is mediated, in part, by decreased tight junction proteins occludin, zona occludens-1 (ZO-1), and claudin-5 in the hippocampus (105). In studies using confluent monolayers of human brain microvascular endothelial cells (HMBVEC) in vitro as a model of the BBB, meth not only decreases tight junction proteins, but also increases polymerization of actin, inducing occludin endocytosis and disruption of barrier properties (106, 107). These results are in agreement with studies that report that meth induced ROS contributes to decreased tight junction proteins occludin and claudin-5 in rat and human BMVEC that results in increased permeability (107, 108). Meth also increases matrix metalloproteinase-9 (MMP-9) in endothelial cells that causes a transient increase in BBB permeability through cleavage of junctional proteins that maintain the integrity of the BBB (105). The effects of meth on endothelial cells of the BBB are reported to be mediated, in part, by activation of the TNF-α/NFκB pathway in BMVEC (109). Blocking this pathway inhibits increased BBB permeability in meth treated mice, as assayed by the passage of albumin-coupled dye into the brain (109). However, another study demonstrated that meth alone does not induce BBB permeability (110). In rat astrocytes, meth increases truncation of β-dystroglycan, a transmembrane protein in astrocytic end feet processes that enables astrocytes to interact with the basal lamina (111). Such effects on both endothelial cells and astrocytes may contribute to a loss of BBB integrity.
The combination of meth and HIV or its proteins can augment BBB dysfunction. In the absence of ART, BBB permeability is increased in HIV Tat transgenic mice contributing to increased numbers of perivascular macrophages (112). These results emphasize the deleterious effect of HIV on the BBB. In the context of meth with HIV, a study showed that mice treated with meth, in combination with Tat and gp120, have reduced levels of glutathione and glutathione peroxidase in the brain, suggesting increased oxidative stress (113). These animals also exhibit increased BBB permeability and decreased brain ZO-1 and occludin (113). Antioxidant treatment blocked these effects, suggesting oxidative stress as a mechanism by which HIV proteins and meth may be mediating their effects in the brain (113). In a study using cocultured HBMVEC and human astrocytes as a model of the BBB, meth and gp120 increase permeability, as measured by decreased transendothelial electrical resistance, to a greater degree than treatment with meth or gp120 alone (114). This study also showed that treatment of HBMVEC with meth and gp120 decreases ZO-1 to a greater extent than treatment with meth or gp120 alone (114). Meth in combination with Tat reduces ZO-1 in brain endothelial cell lines (110). These studies highlight the effects of meth or HIV proteins on the BBB and how in the presence of both, BBB integrity may be compromised to a greater extent than what occurs after exposure to either factor alone.
The effects of ART on BBB integrity have also been reported. The combination of protease inhibitors, lopinavir and itonavir, causes a reduction in ZO-1 and occludin, and increases matrix metalloproteinase-2 (MMP-2) in treated mice compared to controls (70). In vitro, azidothymidine and indinavir, a nucleoside reverse transcriptase and a protease inhibitor, respectively, induce ROS production by a human brain endothelial cell line (115). These data suggest that independently, meth, ART, and HIV may all be contributing to neuropathogenesis through impairing BBB tight junction protein and metalloproteinase expression. Extensive studies need to address the combined effects of ART, HIV, and meth on BBB dysfunction, and how these may contribute to HAND.
Effects of Methamphetamine and HIV on Monocyte Migration Across the Blood Brain Barrier
Studies examined whether meth facilitates immune cell entry into the CNS as a result of an increase in BBB permeability. One study determined that meth treatment alone increases monocyte transmigration across confluent monolayers of BMVEC in response to CCL2, and antioxidant treatment inhibited this effect (107). In the context of HIV infection, treatment of a BBB model of cocultured BMVEC and astrocytes with either meth or gp120 increases its permeability and transmigration of peripheral blood mononuclear cells (PBMC) in response to CCL5, with permeability and transmigration further increased by treatment with both meth and gp120 (114). With meth treatment of this BBB model, HIV-infected PBMC transmigration across the BBB in response to CCL5 is higher than that of uninfected PBMC (114). In a separate study, meth treatment of rat BMVEC monolayers results in increased migration of T-cells (116). Additionally, increased BBB permeability in mice treated with meth increases the entry of C.neoformans into the brain (117). These studies suggest that the extent of BBB disruption is greater with exposure to both meth and HIV or its proteins, resulting in an increase in transmigration of PBMC, monocytes, as well as pathogens across the BBB into the CNS. In vivo, meth increases the population of mature CD14+CD16+ monocyte/macrophages in the brains of SIV-infected macaques. In another study with SIV-infected macaques, meth treatment increases the population of macrophages, but not T-cells, in the brain suggesting that meth may be increasing monocyte transmigration across the BBB, resulting in increased CNS macrophages (118). This highlights the contribution of meth to monocyte/macrophage migration into the CNS and a potential mechanism that may contribute to HAND in PLWH using meth. The increased immune cell transmigration across the BBB mediated by meth may also reseed viral reservoirs and increase infection/activation of other resident cells within the CNS, contributing to chronic neuroinflammation and neuronal damage, and the development of neurocognitive impairment in HIV-infected meth users.
Effects of Methamphetamine and HIV on Macrophage, Microglia and Astrocyte Function
Meth, HIV, and functions of macrophages and microglia
Meth readily crosses the BBB, and once in the CNS it can exert its effects on resident cells, contributing to HIV neuropathogenesis. Macrophages and microglia are important for clearing debris such as amyloid-beta and protein aggregates shown to be elevated in brains of PLWH (119). Meth treatment reduces phagocytosis by macrophages through alkalization of lysosomes, and also impairs phagocytosis of C. neoformans by macrophages and microglia-like cell lines (120, 121). Additionally, nucleoside reverse transcriptase inhibitors, didanosine and stavudine, impair both uninfected and HIV-infected macrophage phagocytosis (122). These data suggest that meth and some antiretroviral drugs can impair macrophage and microglia functions important for clearing debris and pathogens that may contribute to neuronal injury.
Microglia and macrophages are also involved in the CNS inflammatory response that contributes to neurodegeneration and HAND development in PLWH. In vitro, meth alone increases TNF-α in human macrophages (123), and LPS induces IL1-β, TNF-α and IL-8 in human macrophage and monocyte cell lines (123, 124). Using macrophage cell lines, meth increases IL1-β, IL-6, IL-12 and TNF-α, suggesting that it can also mediate macrophage polarization into the inflammatory M1 phenotype (125–127). In separate studies, meth alone increases TNF-α and IL-6 in a mouse microglial cell line (128), and also increases microglial activation including ROS production in mice (129). The combination of meth and gp120 increases TNF-α and IL1β by rat microglia (93). Additionally, rat microglia treated with meth and gp120 have elevated levels of nitric oxide and ROS compared to untreated microglia (93). In microglia of SIV-infected macaques treated with meth, there is increased expression of proinflammatory genes including PYCARD (Apoptosis-Associated Speck-Like Protein Containing A CARD), a component of the inflammasome, and a key mediator of inflammation (95). These studies demonstrate a contribution of meth and/or HIV in neuroinflammation. In the context of ART, one study reported increased IL1-β, IL-6, and TNF-α in brains of mice treated with lopinavir and ritonavir (70). However, raltegravir, an integrase inhibitor, in the presence of HIV, decreases TNF-α and IL-8 production below baseline in human microglia cultures (130). This suggests that some antiretrovirals may mitigate inflammatory responses in the CNS induced by HIV. More studies need to characterize the combined effects of meth, HIV and antiretrovirals on the inflammatory response by resident myeloid CNS cells.
Meth, HIV, and astrocyte function
Astrocytes are the most abundant glia cells in the CNS and are critical for maintaining brain homeostasis. The combination of meth and HIV is proposed to impair astrocyte functions, including cytokine production. Meth and HIV independently induce inflammatory cytokines in astrocytes (131, 132), and one study showed that meth or gp120 increases IL-6 in a human astrocyte cell line, with a further increase when meth and gp120 were combined (133). This suggests that the combination of these two factors may enhance neuroinflammation. In addition, meth and Tat downregulate B-catenin, a protein involved in the Wnt/B-catenin pathway, in a human astrocyte cell line (134). This effect induces senescence, and conditioned media from these astrocytes causes neuronal apoptosis due, in part, to the presence of inflammatory cytokines (135). Meth alone was shown to increase astrocyte activation, as measured by glial fibrillary acidic protein (GFAP) expression (136). Lopinavir has also been shown to increase gliosis in transgenic mice expressing HIV-1 Vpr, an accessory protein important for viral infection (137). These data suggest that meth, HIV, and/or ART may contribute to astrocyte activation, and consequent neuroinflammation. Treatment of rat astrocytes in vitro with meth induces upregulation of inflammatory genes in a cytokine/cytokine receptor functional interaction pathway, including interleukin-1 receptor antagonist (IL1RN) and interleukin-2 receptor subunit gamma (IL2RG) (138). Meth also upregulates mitogen-activated protein kinase 5 (MAP2K5), a component of the MAPK family intracellular signaling pathway, and upregulation of MAP2K5 may be neuroprotective (138). These data suggest that meth causes transcriptional changes in astrocytes that can contribute not only to inflammation, but also to neuroprotective mechanisms.
HIV and meth have been reported to impair homeostasis in astrocytes. HIV treatment of human astrocytes induces opening of mitochondrial permeability transition pores (mPTP) that could interfere with respiratory processes (139). Meth treatment, with or without HIV, has minimal effects on mPTP opening. The expression of antioxidant proteins, including glutathione reductase, is increased in astrocytes with chronic meth exposure but elevated oxidative stress is still detected (139). In a human astrocytic cell line and human primary astrocytes, meth and HIV gp120 induce cell death by oxidative stress and increased macroautophagy (140, 141). The use of some antiretrovirals has also been associated with oxidative stress. One study reported that the combination of zidovudine (azidothymidine), ritonavir, and saquinavir induces oxidative stress in rat neuroglial cultures (71). These data suggest that meth and HIV exposure, and some ART regimens, increase the oxidative burden in astrocytes that may affect their functional capacity to maintain CNS homeostasis and regulate properties of the BBB.
Glutamate uptake is an important astrocyte function that removes excess extracellular amounts of this excitatory neurotransmitter that can induce overactivation of neurons and excitotoxicity (142). HIV alone dysregulates glutamate uptake, contributing to neuronal damage (143). One study demonstrated that the combination of meth and HIV decreases the glutamate transporter, excitatory amino acid transporter-2 (EAAT-2), and glutamate uptake by human astrocytes (144). Additionally, treatment of human astrocytes with both meth and HIV induces glutamate release by a mechanism involving gap junctions and hemichannels (145). Lopinavir decreases human astrocyte EAAT-2 and increases extracellular glutamate (137). Thus, some antiretroviral drugs, meth and HIV may inhibit the ability of astrocytes to maintain glutamate levels, resulting in neuronal insult and neuropathogenesis.
Effects of Meth on T-cell function
Many studies have examined the effects of meth on HIV infection in the CNS. Few studies have reported the effects of meth on T-cell function. In one study with ART suppressed PLWH, meth use is associated with increased CD4+ T-cell proliferation, activation and exhaustion (146). In mice, meth alters the cell cycle in antigen activated T-cells, that may decrease their proliferation as indicated by a reduction in the number of CD4+ and CD8+ T-cells (147). Meth may be mediating its effects by increasing calcium influx into T-cells which in turn increases ROS production and decreases T-cell proliferative activity after stimulation (148). These effects may all impair T-cell functions in the CNS. The role of T-cells in HIV neuropathogenesis has not been well characterized.
Cocaine and HIV Overview
Cocaine is another substance of abuse that has been associated with HIV neuropathogenesis, with approximately 2.2 million users reported in the United States (26). Cocaine is a psychostimulant that elevates dopamine in the CNS by inhibiting dopamine reuptake (149–151). Some PLWH abuse cocaine, and this is associated with poor neurocognitive performance. In one cohort, NCI in PLWH who abused cocaine is associated with ART non-adherence (152), suggesting that ART nonadherence may result in increased viral load and HAND. In contrast, PLWH using cocaine were reported to have lower CD4+ T-cell counts, and an increased viral load over time irrespective of ART (36). In another study, cocaine and HIV associate independently with HAND in a cohort of PLWH, with the majority of them prescribed ART (153). A separate report demonstrated that substances of abuse, including cocaine, do not associate with HAND (154). However, other studies reported that PLWH with a history of cocaine use or with cocaine use disorder have worse neurocognition compared to uninfected people or non-cocaine using PLWH (152, 155, 156). Based on these cohort studies, irrespective of ART adherence, cocaine use is often associated with low CD4+ T-cell count, and high viremia which may contribute to increased HIV neuropathogenesis, and development of HAND. Below we discuss some of the mechanisms by which the combination of cocaine and HIV may be contributing to the development of cognitive disorders.
Cocaine and HIV Infection in the CNS
Cocaine may contribute to HIV neuropathogenesis by increasing CNS viral load. In vivo, cocaine treatment in the bone marrow, liver, thymus (BLT) humanized mouse model induces upregulation of CCR5 on human CD4+ T-cells. These animals have increased peripheral viral load compared to controls (157). This may contribute to enhanced infection in the CNS through transmigration of infected cells across the BBB, resulting in HIV neuropathogenesis. In a severe combined immunodeficiency mouse model injected with human peripheral blood leukocytes (huPBL-SCID) infected with HIV, cocaine increases CXCR4 and CCR5 as well as viral replication in peripheral blood leukocytes (158).
The effects of cocaine on viral replication have also been characterized in vitro. Cocaine enhances HIV replication in vitro in HIV-infected PBMC (159, 160). Cocaine also increases HIV production in both human and macaque MDMs (161, 162). Cocaine induces SIV activation in latently infected macaque promonocytic cells by a proposed mechanism involving increased IL-10, a cytokine that has been reported to promote HIV infection (161). These effects are proposed to be mediated by NFκB activation, resulting in interaction of this transcription factor with HIV LTR. Cocaine treatment increases HIV infection of microglia in vitro (163). In a separate study, increased HIV expression, as measured by p24, in cultured microglia treated with cocaine was blocked by inhibitors of transforming growth factor beta 1, TGF-β1, and by sigma 1 receptor, a cocaine binding receptor, antagonists (164). Cocaine also enhances HIV replication in activated primary human CD4+ T-cells by downregulating anti-HIV miRNA and increasing HIV proviral DNA integration (165, 166). Additionally, cocaine also increases HIV infection of quiescent CD4+ T-cells (167). Another study reported that cocaine upregulates HIV infection of dendritic cells, important antigen presenting cells, through miRNA-155 downregulation and downstream activation of HIV LTR (168). A separate study demonstrated that cocaine increases HIV transfer from dendritic cells to T-cells, further facilitating infection (169). The effects of cocaine on mediating HIV infection may contribute to establishment of viral reservoirs in the CNS, resulting in neuropathogenesis.
Effects of Cocaine and HIV on Blood Brain Barrier Permeability
Cocaine alone has been reported to impact BBB integrity. Cocaine treatment increases inter-endothelial gaps and decreases transendothelial electrical resistance in confluent HBMVEC monolayers suggesting that cocaine decreases BBB integrity (170). Some studies also examined the effects of cocaine on tight junction proteins and showed that cocaine decreases Z0–1 on BMVEC (171, 172) that may contribute to increased BBB permeability. ZO-1 is decreased, in part, through cocaine-mediated downstream signaling that increases platelet derived growth factor BB (PDGF-BB) (172). Tat has also been reported to increase PDGF-BB expression and migration of pericytes (173). This was confirmed in brain tissues of infected people with HIV encephalitis who had decreased pericytes (173). Pericytes support the integrity of the BBB, and migration of pericytes away from the BBB may contribute to its dysfunction. In the context of HIV infection, cocaine and Tat may increase PDGF-BB in the CNS vasculature and this may contribute not only to a decrease in tight junctions, but also to loss of pericytes, impacting BBB integrity. Cocaine also activates MMP-2, MMP-9 and prolidase, enzymes that degrade extracellular matrices that maintain BBB integrity (174). This consequently results in increased HBMVEC monolayer permeability (174). Tat and gp120 have also been reported to upregulate metalloproteinases (175, 176). Additionally, in a BBB model of HBMVEC and astrocytes, cocaine and Tat combined increase permeability as measured by transendothelial resistance and passage of FITC-dextran across the BBB, which is proposed to be mediated by decreased ZO-1 (177). The effects of the combination of HIV, cocaine, and ART on the BBB needs to be characterized further since some antiretrovirals have been shown to decrease metalloproteinases. Specifically, CCR5 antagonist maraviroc, and integrase inhibitors, raltegravir and darunavir, independently reduce metalloproteinases MMP-2 and MMP-9 in LPS treated rat astrocytes (178). This suggests a potential role of ART in alleviating some of the effects of HIV and cocaine on the BBB.
Effects of Cocaine and HIV on Monocyte Migration Across the Blood Brain Barrier
In vivo, cocaine increases monocyte transmigration across the BBB in mice (179). One of the key components in monocyte transmigration across the BBB is the adhesion of these cells to BBB endothelium. The effect of cocaine alone on adhesion and junctional proteins has been studied. In vivo, cocaine induces ALCAM upregulation in mouse brain microvessels, and increases the adhesion and transmigration of monocytes into perivascular regions of mouse brains (172). In vitro, cocaine increases ICAM-1 and VCAM-1 expression on HBMVEC (180, 181), and monocyte adhesion and transmigration across a BBB model of HBMVEC and astrocytes (181). Cocaine also induces TNF-α production from microglia (182). TNF-α causes increased expression of ICAM-1 and VCAM-1 on HBMVEC (183), facilitating increased immune cell adhesion to the BBB, and consequent entry into the CNS.
Cocaine also increases adhesion and transmigration of HIV-infected, as well as uninfected, monocytes across HBVMEC monolayers (172, 174). ALCAM is upregulated on brain endothelium in PLWH with a history of substance abuse, including the use of cocaine (172). Thus, an increase in endothelial cell ALCAM by cocaine may result in increased adhesion of monocytes and T-cells to the endothelium, and increased transmigration of infected cells across the BBB. In a BBB model of cocultured human BMVEC and astrocytes, permeability and monocyte transmigration is increased to a greater extent by Tat and cocaine as compared to Tat or cocaine alone (177). The effects of Tat and cocaine on the BBB are associated with downregulation of ZO-1 and upregulation of JAM-2 on endothelial cells (177). This suggests that cocaine-mediated effects on tight junction proteins and junctional adhesion proteins increase BBB permeability and leukocyte entry into the CNS.
Cocaine mediates chemokine release that may facilitate monocyte recruitment into the CNS. PDGF-BB, that is increased by cocaine treatment of BMVEC, induces production of CCL2 from a human astrocyte cell line (184). Our laboratory showed that Tat also increases CCL2 from human astrocytes (185). Cocaine enhances CCL2 production by an HIV-infected monocytic cell line (171). Additionally, cocaine induces CCL2 from rat microglial cells through its interaction with the sigma 1 receptor, and conditioned media from these cultures increases monocyte transmigration in vitro (179). CCL2 release mediated by cocaine and/or HIV in the brain can contribute to ongoing influx of monocytes into the CNS. In addition, cocaine increases CCR2 mRNA expression in an HIV-infected monocytic cell line (171). We showed that CCR2, the only CCL2 receptor on monocytes, is essential for CD14+CD16+ monocyte transmigration across our model of the human BBB (8). We also showed that blocking CCR2 reduced the transmigration specifically of monocytes that harbor HIV (8). This indicates the importance of CCL2 in the transmigration of monocytes that carry the virus across the BBB, and increased expression of CCR2 induced by cocaine may further facilitate HIV entry into the CNS.
Cocaine also increases the secretion of CXCL10 by pericytes and conditioned media from these cultures increases monocyte transmigration across HBMVEC monolayers in vitro (186). Additionally, this same study showed that there was an overall increase in the number of macrophages in proximity to CXCL10 expressing pericytes in brains of cocaine abusers (186). A separate study showed that in a cohort of PLWH, with some of them on ART, there is elevated CCL2 and CXCL10 in the CSF of individuals with cognitive impairment compared to those with normal cognition (187). This suggests that cocaine induced CCL2 and CXCL10 may promote neuroinflammation, and monocyte recruitment that contribute to HAND.
Effects of Cocaine and HIV on Macrophage, Microglia and Astrocyte Function
Cocaine and HIV effects on macrophages and microglia
Few studies have examined the combined effects of cocaine and HIV on macrophage and microglial functions. Sigma 1 receptor and cathepsin B expression are increased in the brains of HIV-infected cocaine users compared to non-users (188). Cathepsin B, a protease that induces neuronal death, is increased in PLWH (189). These studies suggest that cocaine and HIV independently may be increasing the production of cathepsin B. In vitro, cocaine, through sigma 1 receptor, induces cathepsin B production by HIV-infected MDM (188). This may be one of the mechanisms by which cocaine and HIV both contribute to neuronal damage, and consequent HAND development.
Cocaine alone increases TNF-α and IL-6 gene expression in BV-2 microglia cell line (190). Additionally, it induces TNF-α production in a murine microglia cell line through induction of oxidative stress and activation of the TLR2 signaling pathway (191). Cocaine treatment of mouse primary microglia induces mitochondria dysfunction and an increase in mitophagy to degrade damaged mitochondria after enclosure of these organelles within autophagosomes followed by fusion with lysosomes (192). However, cocaine inhibits the fusion of mitophagosomes with lysosomes, resulting in accumulation of damaged mitochondria (192). This defect in mitophagy contributes to cocaine induced microglial activation and production of inflammatory cytokines (192). In the context of HIV, cocaine enhances IL-6 upregulation in HIV-infected MDM (193). The combined effects of cocaine and HIV on macrophages and microglia may facilitate inflammation that may result in neuronal injury.
Oxidative stress has also been implicated in cocaine and HIV neuropathogenesis. Treatment of HIV-infected MDM with cocaine induces ROS production, and downstream activation of inflammasomes (193). Treatment with both gp120 and cocaine combined induces ROS production by a human microglia cell line (163). These effects may lead to production of cytokines such as IL-1β that may cause neuronal apoptosis. Cocaine also increases HIV gp120 induced ATP use, and decreases mitochondria oxidative phosphorylation in a human microglia cell line (163). All these processes may disrupt neuronal homeostasis, contributing to neuronal loss and neurocognitive impairment.
Cocaine, HIV and astrocyte function:
Cocaine and HIV independently and together induce astrocyte activation that may impair their functions. GFAP is increased in brain tissues of HIV-infected cocaine users, compared to either HIV-infected or uninfected non-cocaine users (194), and this is indicative of astrocyte activation. In another study in mice, cocaine treatment also caused upregulation of GFAP and changes in astrocyte morphology such as a decrease in size (195). This effect is also observed in vitro and in vivo in human and mouse astrocytes, and it is mediated either through mechanisms involving sigma 1 receptor or autophagy signaling (194, 196). Tat also increases GFAP in mouse astrocytes through Egr-1 upregulation (197).
Astrocyte activation by cocaine and/or HIV can lead to increased cytokine production and release of neurotoxic factors such as ROS. One study reported that cocaine and gp120 induces rat astrocyte cell death in part through increased ROS production, that leads to apoptosis (198). Cocaine and HIV proteins can also affect brain homeostasis by altering the metabolic function of astrocytes. Tat and cocaine treatment reduces lactate shuttling from human astrocytes to neurons (199). This can lead to neuronal death because lactate supply to neurons is important for their aerobic metabolism. These effects on astrocytes can all contribute to neuronal insult, and subsequent neuronal loss. ART treated PLWH are reported to have dysregulated peripheral metabolic profiles (200, 201). It is thus important to characterize these profiles and their effects on the ability of astrocytes to maintain brain metabolic functions in the context of cocaine, HIV and ART exposure.
Effects of Cocaine on T-cell function
Cocaine induces apoptosis of CD4+ T-cells from both uninfected and HIV-infected people and this is mediated by increased ROS (202). Cocaine has been reported to increase activation of CD8+ T-cells but decrease the cytotoxic phenotype of these cells upon stimulation (157). These effects in T-cells may impair their immune functions such as killing of HIV-infected cells in the CNS. More studies addressing the impact of cocaine on T-cells in the context of viral reservoirs in the CNS are needed.
Opioids and HIV Overview
Opioids are used to reduce pain, and their misuse has led to opioid use disorder, a current substance abuse epidemic in the United States. The opioid epidemic is a major public health concern with misuse or addiction to opioids, including prescription pain relievers, heroin, and synthetic opioids such as fentanyl, reported in approximately 11 million people in the U.S. (26). Intravenous use of heroin is common and needle sharing by heroin abusers contributes to HIV acquisition (24, 203). Additionally, 20–50% of PLWH are prescribed opioids, and are at risk of developing opioid use disorder compared to uninfected people. A few studies have reported that opioid dependence increases neurocognitive impairment (204, 205). The use of heroin has been associated with poor working memory and recall, suggesting that opioid abuse has detrimental effects on neurocognitive performance (154). The effects of opioids including morphine, a mu opioid receptor (MOR) agonist and a heroin metabolite, on HIV/SIV infection have been contradictory with studies reporting that opioids enhance, inhibit, or have no effect on HIV/SIV pathogenesis (206–210). These results were attributed to differences in drug usage, methods of assessing infection and disease progression, and genetic, socioeconomic, and behavioral backgrounds of study subjects. One study with SIV-infected macaques treated with morphine reported a significant number of animals develop very rapid disease progression and higher mortality rates compared to untreated infected macaques (211). There is also increased macrophage localization in perivascular regions of CNS blood vessels and within the CNS parenchyma in morphine treated infected macaques, and many of these macrophages are positive for viral gp120 as detected by immunohistochemistry (211). These data suggest that morphine enhances monocyte/macrophage infection with SIV and increases the localization of these infected cells in the CNS, contributing to SIV neuropathogenesis. In an ART naïve cohort of PLWH, opioid use is associated with increased viral load and lower CD4+ T-cell counts (212). This study also reported that morphine treatment increases HIV replication in vitro in human MDM (212). Neurotoxicity of conditioned media from a human monocyte/macrophage cell line infected with HIV is enhanced with morphine treatment (213). Tat and morphine are also reported to increase synergistically murine neuronal dendritic pruning and cell death in vitro (214). Morphine enhances the production of neurotoxic factors by murine glial cells in Tat treated mixed murine glia neuronal cocultures, resulting in neuronal cell death (215). All these data suggest that opioid use can increase HIV infection and replication in macrophages and glial cells, and infected macrophage localization in the CNS perhaps by increasing the transmigration of peripheral uninfected and infected monocytes across the BBB. In addition, opioids can increase the neurotoxic effects of viral factors including Tat, and also increase the neurotoxicity of host factors produced by glial cells in response to HIV infection or exposure to Tat.
Opioids and HIV Infection in the CNS
Studies have addressed the effects of opioids on HIV replication. In a murine HIV infection model, morphine increases viral production in mouse hippocampus (84). In vitro, one study showed that morphine increases CCR5 and CXCR4 gene expression and enhances HIV infection of PBMC (216). High concentrations of morphine induce HIV replication in T-cells in vitro (217, 218). Morphine also decreases CD8+ T-cell anti-HIV activity, contributing to HIV pathogenesis (219). There are several potential mechanisms reported to contribute to increased HIV infection induced by morphine. In MDM, morphine increases the expression of galectin, a lectin that contributes to increased HIV infection by enhancing viral interaction with the cell membrane (220). Morphine increases HIV replication in MDM by downregulating the IFN pathway (221), and upregulating CCR5 (222, 223). In mouse microglia, morphine and Tat upregulate CCR5 that may contribute to an increase in infected glial cells and expansion of viral reservoirs in the CNS (224). In vitro, morphine promotes HIV replication in human macrophages by inhibiting the TLR9 pathway (212). Heroin increases HIV replication in macrophages by inhibiting anti-HIV miRNA and decreasing IFN expression (225). However, another study reported that morphine does not affect HIV replication in MDM but increases HIV-mediated inflammation (226). High concentrations of morphine induce HIV replication in a latently infected T-cell line, and antioxidant treatment attenuated this effect (217). These data suggest that there is a complex interaction between opioids and HIV infection of MDM, T-cells, and microglia, and such interactions may have different outcomes on HIV neuropathogenesis. These types of studies need to be repeated in the presence of ART.
Effects of Opioids and HIV on BBB Permeability
Studies examining the effects of opioids on BBB integrity reported that morphine alone does not change the permeability of HBMVEC monolayers in vitro, or the mouse BBB in vivo (227, 228). However, morphine induces PDGF-BB in HBMVEC, mediated by activation of ERK1/2, JNK, and p38 MAPK signaling pathways (229). Treatment of HBMVEC with morphine decreases ZO-1 and increases permeability as quantified by passage of FITC-dextran across HBMVEC monolayers (229). Decreased ZO-1 and increased permeability are mediated by morphine induced PDGF-BB (229). Tat and morphine combined increase permeability of cocultured human BMVEC and astrocytes, an effect that is mediated by increased TNF-α and IL-8, and decreased ZO-1 expression (230). This suggests that opioids with HIV or HIV proteins may contribute to loss of BBB integrity that may result in the influx of monocytes and T-cells into the CNS, and ongoing neuroinflammation in PLWH. Morphine increases BBB permeability in vivo as quantified by influx of fluorescently labeled dextran into the brains of Tat transgenic mice and non-Tat expressing control mice (231). Tat transgenic mice also exhibit BBB permeability in the absence of morphine. A diffuse pattern of cytoplasmic ZO-1 immunoreactivity in brain endothelium is induced by morphine treatment of non-Tat expressing control mice and this localization is observed in Tat transgenic mice, irrespective of morphine (231). In mice given dolutegravir, abacavir, and lamivudine, morphine treatment results in lower abacavir and dolutegravir in brains of Tat transgenic mice and non-Tat control mice which correlates with increased endothelial P-glycoprotein efflux transporter induced by morphine (231). Although morphine increases BBB permeability, the penetration of specific antiretroviral drugs into the brain is inhibited (231). These data suggest that morphine induced increases in BBB permeability may enhance influx of monocytes and T-cells into the CNS of PLWH, increasing viral seeding and neuroinflammation. Morphine may also inhibit ART mediated suppression of viral load in the CNS by inhibiting penetration of specific antiretroviral drugs into the CNS.
Effects of Opioids and HIV on Monocyte Migration Across The BBB
One study showed that morphine increases ICAM-1, VCAM-1, and ALCAM on HBMVEC and this correlates with increased PBMC adhesion (228). These data suggest that opioids increase junctional proteins and adhesion molecules on the surface of BBB endothelial cells that may contribute to further recruitment of monocytes and T-cells into the CNS. Morphine and Tat treatment of astrocytes induces CCL2 production that mediates microglial chemotaxis (232). Elevated CCL2 at the BBB can also recruit monocytes and T-cells into the CNS. Morphine treated SIV-infected macaques have increased brain perivascular macrophages, with a significant number of these cells expressing viral antigens (211). These data suggest that morphine is contributing to increased transmigration of peripheral blood monocytes into the CNS (211). In another study, SIV-infected macaques treated with morphine exhibited increased migration of dendritic cells into the brain parenchyma (233). Morphine treatment of a BBB model consisting of cocultured human astrocytes and HBMVEC increases uninfected and HIV-infected PBMC transmigration to CCL5, with uninfected PBMC transmigration exacerbated with Tat cotreatment (230). Additionally, morphine increases entry of splenocytes, into the CNS of treated mice (234). Splenocytes are comprised of various immune cells including T-cells, and their increased entry into the CNS may contribute to inflammation. Streptococcus pneumoniae infection of the CNS of mice is increased by treatment with morphine and Tat (235, 236) with increased infiltration of T-cells and inflammatory monocytes into the CNS that was proposed to assist in pathogen clearance (235). These studies suggest that one mechanism by which opioids may increase HIV associated neuroinflammation and development of HAND is by increasing uninfected and infected monocyte and T-cell influx into the CNS.
Effects of Opioids and HIV on Macrophage, Microglia, and Astrocyte Function
Opioids, HIV, and macrophage/microglia function
Morphine has been reported to increase IL-6 production in HIV-infected MDM (Dave et. al., 2012). In mouse microglia, morphine and Tat upregulate TNF-α, IL-1B, and IL-6 (224). Mouse microglia also become activated, as measured by expression of the activation marker CD11b, with meth and Tat treatment (224). Morphine by itself increases production of CCL2 by human neuronal cultures but not astrocytes or microglia (237). Another study showed that morphine alone does not increase CCL2, TNF-α, IL-6 or IL-8 production by human microglia (238). However, these cytokines are increased in HIV-infected microglia treated with morphine when compared to either HIV infection or morphine treatment alone (238). Thus, increased production of inflammatory cytokines due to opioids may exacerbate immune cell influx into the CNS, neuroinflammation, and neuronal damage that may all contribute to declines in neurocognitive performance in PLWH.
Morphine, HIV and astrocyte function
The combination of morphine and HIV or Tat was reported to induce oxidative stress through production of ROS by HIV-infected human or Tat treated mouse astrocytes, and also decrease the ability of astrocytes to uptake glutamate (215, 239). Oxidative stress and excess glutamate in the CNS may result in neuronal damage that may contribute to HIV neuropathogenesis. Morphine enhances CCL2, TNF-α, and IL-8 production by HIV-infected human astrocytes (239). Morphine was also shown to induce CCL5 production by human astrocytes (240). Some studies reported a decrease in CCL2 mRNA expression in human astrocytes after morphine treatment (241), while others have shown increased IL-6, CCL2 and CCL5 production by mouse astrocytes with Tat and morphine treatment compared to Tat alone (242). This suggests that the presence of both morphine and HIV or its proteins exacerbates neuroinflammation that may contribute to CNS pathogenesis.
Dopamine and HIV Overview
Intermittent use of drugs of abuse, including meth, cocaine and opioids, significantly increases extracellular concentrations of dopamine in the CNS (243, 244), while chronic substance abuse appears to downregulate dopamine production (245). Dopamine is a catecholamine neurotransmitter that modulates many functions of the brain, including cognition, locomotion, and reward (246, 247). Drugs of abuse can interact with presynaptic DAT, preventing reuptake of dopamine by neurons (248). This contributes to increased extracellular dopamine in the CNS, and reactive oxygen species produced by oxidation of dopamine can increase oxidative stress in CNS cells and can be neurotoxic (249, 250). Tat also targets and directly inhibits DAT function (251), which may contribute to an increase in synaptic dopamine levels during the early stages of HIV infection (252). In therapy-naïve asymptomatic HIV-infected individuals, dopamine levels are increased in the CSF when compared to uninfected controls (253). This increase during the early stages of HIV infection can contribute to synaptic damage and degeneration of dopaminergic neurons. In individuals infected with HIV for long time periods, particularly in the absence of ART, there is significant damage to dopaminergic neurons, resulting in decreased overall levels of dopamine in the CNS (254, 255). Astrocytes express dopamine receptors (256, 257) but little is known about the effects of dopamine receptor signaling on their functions, including trophic support of neurons and regulation of neuronal activity. The effects of dopamine on the ability of astrocytes to support neuronal functions in the HIV-infected CNS in the presence of ART is an area that requires further study.
Neuropathology associated with dopaminergic neuronal damage in the pre-ART era includes neuronal loss in areas of the brain innervated by dopaminergic neurons, such as the basal ganglia and the prefrontal cortex (258–260). Dopamine-rich areas of the brain in SIV-infected macaques also exhibit significant neuronal damage and loss (261). A study of brain tissues from PLWH who had HIV encephalitis and died from AIDS reported abnormal striatal dopaminergic synapses, detected by Western blot analysis of brain tissue lysates and by immunohistochemical staining of sections from the basal ganglia (262). In PLWH on suppressive ART, pathological studies on postmortem brain tissue reported less severe but detectable neuroinflammation and neuronal damage (263) and one autopsy study reported neuronal degeneration in the frontal cortex and hippocampus (264). Neuroimaging studies of brains of PLWH on suppressive ART demonstrate structural changes in frontal white matter and neuronal injury in the basal ganglia (265–267) and other studies reported structural abnormalities in white matter, increased markers of neuroinflammation, and decreased markers of neuronal integrity as detected by MR spectroscopy (MRS) (268–270). Thus, even with suppressive ART, low level chronic neuroinflammation and neuronal injury persists. Upregulation of extracellular dopamine levels in the brain of rhesus monkeys by treatment with L-DOPA or selegiline during early SIV infection increases CNS viral loads and SIV-associated neuropathology (271, 272). All of these studies suggest that abnormal dopaminergic transmission contributes to HIV neuropathogenesis in the ART era (273), and that increased extracellular dopamine during the early stages of HIV infection of the CNS can exacerbate neuronal damage.
Dopaminergic pathways in the brain, including those involved in reward processes, are targeted and damaged by HIV infection and substances of abuse (274, 275). In a mouse model in which Tat protein, under the glial fibrillary acidic protein (GFAP) promoter, is induced in the brain by doxycycline, neuropathology is similar to what is observed in PLWH (276). In this model, induced Tat expression in mouse brains decreases dopaminergic function and leads to reward deficits, contributing to increased sensitivity to subsequent meth-induced reward enhancement (277). Another study with this model reported that expression of Tat increases meth-induced locomotor sensitization, and increases microglial activation, indicative of upregulated neuroinflammation (278). Other in vitro studies demonstrated that Tat and cocaine synergistically enhance the inhibition of DAT activity, increasing extracellular dopamine to a greater degree than that induced by Tat or cocaine alone (279). These studies suggest that HIV infection may enhance the effects of substances of abuse on dopaminergic and reward pathways in the brain as well as neuroinflammation. Thus, PLWH may be more susceptible to the rewarding effects of drugs when compared to non-infected individuals. In PLWH who do become substance abusers, the levels of extracellular dopamine in the CNS, especially during the early stages of HIV infection, and neuroinflammation are further increased after active drug use, exacerbating HIV neuropathogenesis.
The mechanisms by which increased extracellular dopamine in the HIV-infected CNS contributes to chronic neuroinflammation are not completely understood. Our laboratory reported that dopamine increases uninfected CD14+CD16+ mature monocyte transmigration across our in vitro model of the human BBB, as well as their adhesion and migration (48, 280). Using PBMC from PLWH, we also showed that these mature monocytes transmigrate in increased numbers to dopamine (48).These data suggest that increased extracellular dopamine in the CNS induced by substances of abuse contributes to HIV neuropathogenesis, in part, by increasing the influx of mature monocytes into the CNS and their accumulation in dopamine rich regions of the brain, particularly during early stages of HIV infection.
HIV/SIV DNA or RNA is detected in peripheral blood CD14+CD16+ monocytes from infected people or macaques, in the presence or absence of ART (42, 281–283). Actively or latently infected CD14+CD16+ monocytes in the periphery can transmigrate across the BBB and enter the CNS over extended periods of time in PLWH on suppressive ART, replenishing viral reservoirs when some of these cells mature into macrophages, particularly in perivascular regions of the CNS. These cells can produce infectious virus in the CNS of substance abusing PLWH who are not adherent to ART, and in individuals on successful ART, Tat and Nef can still be produced. This will result in the maintenance of low level chronic neuroinflammation that results in neuronal dendritic pruning, degeneration and cell death, contributing to the development of HAND.
Dopamine and HIV Infection in the CNS
Increased extracellular dopamine in the CNS may facilitate the infection of CNS macrophages with HIV. We reported that HIV infection of human MDM is increased by DA mediated activation of D1-like and D2-like dopamine receptors, resulting in increased viral entry (284, 285). Dopamine also increases cell surface CCR5 in the human THP-1 monocyte/macrophage cell line that is mediated by D1-like and D2-like DA receptor activation, resulting in increased infection by HIV (286). These data suggest that increased extracellular dopamine in the CNS induced by substances of abuse will increase viral load in the CNS of HIV-infected substance abusers.
Effects of Dopamine and HIV on Monocyte Transmigration Across the BBB
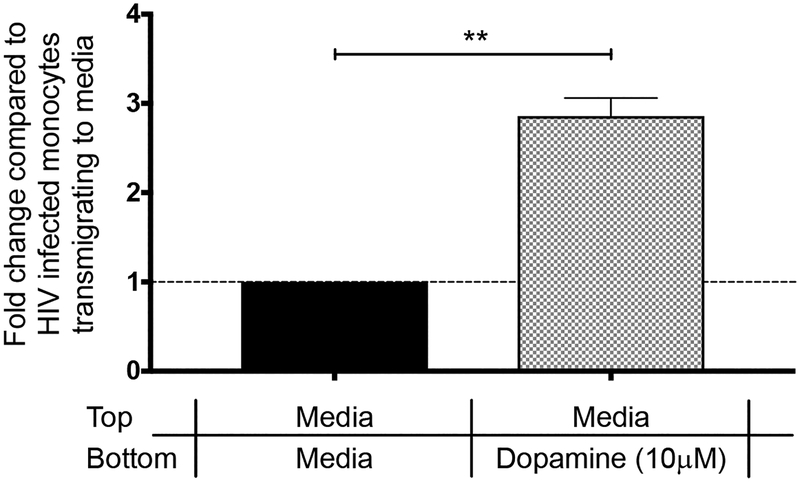
Peripheral blood monocytes in healthy individuals are heterogeneous with 5–10% of this population expressing CD14 and CD16 (CD14+CD16+) and the remaining 90–95% expressing only CD14 (CD14+CD16−) (287). The CD14+CD16+ mature monocyte subpopulation is increased in frequency relative to the total peripheral monocyte population in PLWH (288, 289), and we showed that this increase is significantly larger in PLWH who are active substance users (48). Our laboratory and others reported that mature CD14+CD16+ monocytes can be infected with HIV (42, 281–283, 290). We developed a method to culture human CD14+CD16− monocytes isolated from uninfected PBMC non-adherently in media with M-CSF to expand the CD14+CD16+ mature monocyte subpopulation. We used these cultured mature monocytes, both uninfected and HIV-infected in vitro, as well as PBMC from PLWH, in transmigration assays across our in vitro model of the human BBB, consisting of cocultured HBMVEC and human astrocytes on tissue culture inserts. We reported that uninfected and HIV-infected CD14+CD16+ monocytes preferentially transmigrate across the BBB in response to the chemokines CCL2 and CXCL12 when compared to CD14+CD16− monocytes (5–9). Our laboratory also showed that dopamine increases the adhesion and migration of cultured uninfected CD14+CD16+ monocytes (280) and the transmigration of these cells across our BBB model (48). Dopamine-induced transmigration is mediated by activation of D1-like dopamine receptors on CD14+CD16+ monocytes and the subsequent activation of ADAM 17 (9). The transmigration across the BBB of peripheral blood CD14+CD16+ monocytes, but not T-cells, from PLWH is also increased by dopamine (48). Our preliminary data demonstrate that cultured CD14+CD16+ monocytes infected with HIV in vitro also transmigrate across our BBB model in response to dopamine at higher levels when compared to baseline transmigration to media (Figure 1). For these experiments, cultured CD14+CD16+ monocytes (10×106 cells/mL) were infected in vitro with HIVADA (0.5 μg/mL). After 8 hours the virus was removed by centrifugation and the monocytes resuspended at 2×106 cells/mL in fresh culture media (RPMI 1640 supplemented with 10% human serum and 5% FBS) with 10 ng/mL M-CSF (PeproTech, Rocky Hill, NJ) in teflon flasks. HIV-infected monocytes were cultured for an additional two days to facilitate viral infection and replication. HIV-infected monocytes (1.5×105 cells) were added to the top of cocultured HBMVEC and human astrocytes on tissue culture inserts in 24 well plates as previously described (48, 291), with media or 10 μM dopamine (Sigma-Aldrich, St. Louis, MO) added to the bottom of the inserts. Each transmigration condition was performed with 4 replicate cocultures. After 18 hours, transmigrated monocytes were collected from the bottom, stained for CD14 (clone M5E2, BD Biosciences, 1:12.5 dilution; San Jose, CA) and CD16 (clone 3G8, 1:10 dilution; BD Biosciences, San Jose, CA). The number of CD14+CD16+ monocytes was quantified by flow cytometry and analyzed using FlowJo software (TreeStar v.9.5.3, Ashland, OR) (Figure 1). These data suggest a potential mechanism by which drugs of abuse may be contributing to HIV-associated neuroinflammation by increasing the population of CD14+CD16+ monocytes in the peripheral circulation of PLWH, and by increasing CNS extracellular dopamine that facilitates transmigration of uninfected and HIV-infected CD14+CD16+ monocytes across the BBB, and their migration to dopamine rich areas of the CNS. This would facilitate the establishment and reseeding of viral reservoirs and contribute to the chronic neuroinflammation and neuronal damage that mediates the development of HAND. More studies of dopamine and its impact on neuroinflammation need to be performed in the context of ART.
Figure 1:
Dopamine increases the transmigration across the BBB of cultured CD14+CD16+ monocytes infected in vitro with HIV. Transmigration experiments were performed with CD14+ peripheral blood monocytes cultured non-adherently with M-CSF to generate mature CD14+CD16+ monocytes. These cells were infected in vitro with HIV and added to the top of our human BBB model consisting of cocultured HBMVEC and human astrocytes on tissue culture inserts in 24 well plates. Media or 10 μM dopamine was added to the bottom of the wells. After 18 hr, cells that transmigrated across the BBB were collected from the bottom of the wells and CD14+CD16+ monocytes were quantified by flow cytometry. Monocyte transmigration was expressed as fold increase over baseline (media alone), which was set to one (dotted line) for each independent donor. Data are expressed as mean ± SEM. Significance was determined by two-tailed paired Student’s t test. **p < 0.005 (Dopamine compared to baseline); n=4 each.
Effects of Dopamine and HIV on Macrophage and Microglia Function
Dopamine, HIV, and macrophage function
RNA for all five dopamine receptors are detected by qRT-PCR in human MDM, with D1R and D5R RNA expressed at higher levels than the D2-like DR receptors (292). Additionally, in uninfected cells, dopamine treatment with or without LPS, increases IL-6, IL-8, CCL2, CXCL9, and CXCL10 proteins (292). Dopamine also inhibits IL-10, an anti-inflammatory cytokine, and has no effect on TNF-α (292). In addition, dopamine induced cytokine production in MDM cultures derived from peripheral blood monocytes from PLWH on ART is similar to that of uninfected MDM (292). In mouse peritoneal macrophages, dopamine increases LPS induced IL-6, IL-10 and CXCL1, and has no effect on TNF-α (293). Dopamine-mediated activation of D1R inhibits LPS induced TNF-α and MIP-2 in mouse peritoneal macrophages (294), and also inhibits NLRP3 inflammasome activation in mouse bone marrow derived macrophages (295). In mouse peritoneal macrophages, a D1-like dopamine receptor antagonist inhibits LPS induced TNF-α and a D2-like dopamine receptor antagonist inhibits LPS induced IL-6 (296). These data suggest that extracellular dopamine in the CNS increases the inflammatory phenotype of uninfected and HIV-infected macrophages, with increased cytokine production dependent on the activation of specific dopamine receptors on these cells.
Dopamine, HIV, and microglia function
In the murine microglial BV-2 cell line and in primary murine microglia, D1R and D5R mRNA and protein are expressed at higher levels than D2R, D3R, and D4R (297). Dopamine alone does not change microglial production of IL-6 or TNF-α in the presence or absence of LPS (297). However, LPS and IFN-γ induced nitric oxide (NO) is inhibited by dopamine, and D1R-like agonists inhibit LPS induced NO (297). LPS induced phagocytic activity in BV-2 cells and primary murine microglia is inhibited by dopamine (298). Thus, dopamine may inhibit microglial production of NO, a neurotoxic factor, and microglial phagocytosis during an inflammatory response while having no effect on cytokine production by this cell type.
Potential Therapeutic Interventions to Limit HAND in the Context of ART and Substances of Abuse
HIV-infected substance users are still at a highly significant risk of developing HAND despite effective ART. There are currently no available therapies for treating meth and cocaine use disorders. Opioid substitution therapies such as methadone, a MOR full agonist, and buprenorphine, a MOR partial agonist and full agonist of kappa opioid receptor, are being used to treat opioid addiction. Very few studies have been performed to determine the effects of these drugs on HIV-mediated neuropathogenesis. It was reported that individuals taking buprenorphine have improved neurocognitive performance compared to baseline or methadone treated drug users (299–301), suggesting that this molecule may be a potential therapy for HAND in HIV-infected opioid users. Our laboratory showed that, in vitro, buprenorphine decreases adhesion of uninfected CD14+CD16+ monocytes to ICAM-1 on BMVEC, as well as their chemotaxis (302, 303). This suggests that buprenorphine may reduce monocyte recruitment into the CNS, and mitigate monocyte induced inflammation. Additionally, our unpublished data demonstrate that prophylactic treatment of EcoHIV-infected mice with buprenorphine before infection prevents the development of NCI. Additionally, these mice have decreased numbers of inflammatory monocytes in the brains (Unpublished data, Drs. Matias Jaureguiberry-Bravo & Jennifer Kelschenbach). These data suggest that in addition to its role as an opioid substitution therapy, buprenorphine can serve as potential treatment for cognitive deficits in PLWH regardless of opioid use.
A recent study used liposomal magnetic nanoformulations to deliver antiretrovirals, latency reactivating agents, and meth antagonists to latently infected human astrocytes, to target both HIV infection and meth induced effects in these cells (304). In this study, the nanoparticles did not alter the permeability of a BBB model comprised of HBMVEC and astrocytes (304). These nanoparticles crossed the mouse BBB in vivo, suggesting a potential therapeutic intervention for drug delivery into the CNS (304). Another study showed that magnetic nanoparticles containing azidothymidine 5′-triphosphate (AZTTP), the active form of zidovudine, crosses a human BBB model more effectively compared to free drug, without affecting permeability (305). These nanoparticles can also be taken up by monocytes and these cells can then transmigrate across a BBB model in the presence of a magnetic field (305). This suggests that nanoparticles may be serve as a potential intervention to increase the delivery of ART across the BBB, reducing viral reservoirs in the CNS. Due to the low penetrance of currently used antiretrovirals into the CNS, long lived HIV-infected monocyte/macrophages remain as viral reservoirs despite successful ART. In addition, low levels of ART have been detected intracellularly in these cells due to the activity of drug efflux transporters and metabolic enzymes that degrade the antiretrovirals (306). To ensure effective ART delivery into these cells, nanoparticles containing integrase inhibitor, elvitegravir, were used (306). These nanoparticles suppressed HIV replication in monocyte derived macrophages more effectively than elvitegravir alone (306). Nanoparticles may thus improve drug delivery across the BBB, as well as into macrophages, providing a potential mechanism for drug delivery, and the targeting of long-lived CNS viral reservoirs.
Our laboratory showed that adhesion molecules, JAM-A and ALCAM, are increased on CD14+CD16+ monocytes infected in vitro when compared to uninfected cells, and also on CD14+CD16+ monocytes from PLWH (7). These molecules are further increased on monocytes that specifically harbor the virus compared to those that are only exposed to, but are not infected by, the virus. These proteins mediate transmigration of uninfected and HIV-infected mature monocytes and blocking antibodies against these molecules reduce the number of monocytes that cross a BBB model, and specifically the cells carrying HIV. Cocaine has been shown to induce ALCAM on endothelial cells. Thus, ALCAM blocking antibodies can be studied to determine whether they will limit cocaine and HIV-mediated monocyte transmigration, reducing inflammation within the CNS and viral seeding. Natalizumab, an α4-integrin antibody, limits the infiltration of monocytes into the CNS of SIV-infected macaques, resulting in decreased inflammation and neuropathogenesis (307, 308). The use of blocking antibodies can thus be a potential therapy for reducing monocyte entry into the CNS that is augmented by substances of abuse. However, caution must be taken because long term use of blocking antibodies can inhibit immune surveillance in the CNS and contribute to the development of progressive multifocal leukoencephalopathy (PML), and thus the use of these drugs continuously may be detrimental and must be monitored closely.
Cocaine and meth are associated with an increase in HIV infection that is mediated by the upregulation of the CCR5 receptor. Receptor antagonists such as maraviroc, a CCR5 inhibitor, and cenicriviroc (CVC), a dual CCR2 and CCR5 inhibitor, can be used as potential therapeutics to limit HIV infection of cells within the CNS. CVC reduced chemotaxis and transmigration of monocytes from HIV-infected donors across human aortic endothelial cells (309). Additionally, our laboratory demonstrated that CVC decreased HIV-infected CD14+CD16+ monocyte transmigration across a BBB model (8). Thus, CVC can be used not only to prevent HIV infection of monocytes by blocking CCR5, but also to reduce transmigration of these cells into the CNS by targeting CCR2. Pilot studies demonstrated improved cognitive function in PLWH who are treated with ART and maraviroc combined compared to those who only receive ART (310, 311). A single arm clinical study also reported that CVC treatment of virally suppressed PLWH is associated with improved neurocognitive performance (312).
Sigma 1 receptor is a potential target for limiting cocaine or meth-mediated effects. Cocaine and meth have been reported to interact with sigma 1 receptor on various cell types of the CNS including pericytes, astrocytes, microglia and macrophages (129, 186, 188, 313, 314). Upon interaction with sigma 1 receptor, cocaine and meth initiate inflammatory responses (314). This results in release of cytokines such as TNF-α, IL1-β, and IL6. In vitro and in vivo studies discussed in above sections showed that sigma 1 receptor antagonists attenuate these effects, and this can be one of the potential therapies for reducing the neuroinflammation and neuronal damage mediated by meth and/or cocaine. To reduce cytokines that contribute to immune cell entry into the CNS as well as neuroinflammation, antioxidants can also be examined further to reduce the effects of cocaine or meth induced ROS. Using peripheral blood from meth or cocaine users, it was reported that drug use Is associated with lower antioxidant capacities compared to non-substance users (315). This suggests that these individuals are susceptible to oxidative stress, leading to production of ROS that can damage the BBB, contributing to CNS damage. The use of antioxidants is another potential intervention to mitigate this effect. Antioxidant use such as dimethyl fumarate has been proposed to suppress HIV infection of macrophages, reduce neurotoxicity mediated by HIV-infected macrophages, as well as CCL2-mediated chemotaxis of human monocytes, in vitro (316). In a rat astroglia-like cell line, the antioxidant N-acetyl cysteine, reduces cocaine induced ROS production and cytotoxicity (317). Drugs such as fingolimod, a sphingosine-1-phosphate receptor modulator, are used to treat relapsing multiple sclerosis and fingolimod has been shown to have some inhibitory effect on astrocyte-mediated neuroinflammation (318).
Conclusion
Substances of abuse, in the context of HIV infection, contribute to BBB dysfunction, and increased endothelial adhesion protein expression, that may facilitate immune cell entry into the CNS (Figure 2A–B). Substances of abuse also facilitate HIV infection and replication that can lead to establishment and replenishment of viral reservoirs in the CNS that are difficult to eliminate (Figure 2C). Some of the functions of macrophages, microglia, and astrocytes that are important for CNS homeostasis are also impaired by HIV and substances of abuse (Figure 2D–E). Additionally, use of illicit drugs and HIV infection in the CNS can result in oxidative stress and neuroinflammation, that contribute to neuronal pruning and loss (Figure 2F–H). All of these effects may play a role in the development of HAND. Despite our understanding of the mechanisms by which substances of abuse may be affecting CNS cells, more studies need to be carried out to determine the impact of these drugs in the context of HIV and ART. Current guidelines recommend that PLWH be prescribed ART soon after diagnosis. Thus, more cohort studies need to be performed to characterize the effects of early ART initiation, HIV, and substances of abuse in these individuals. Collectively, these studies will indicate potential therapies for limiting viral seeding and neuropathogenesis mediated by substances of abuse.
Figure 2:
Substances of abuse impact several processes of HIV neuropathogenesis leading to cognitive impairment. Methamphetamine, cocaine, and opioids contribute to HAND, in part by: A. Facilitating infected monocyte and perhaps T-cell transmigration across the BBB; B. Disrupting BBB integrity through decreased tight junction proteins, and increased metalloproteinases and adhesion molecules; C. Increasing HIV infection and replication in macrophages and microglia, contributing to increased viral load in the CNS; D. Releasing virus and/or viral proteins, and other inflammatory mediators including reactive oxygen species and cytokines; E. Impairing astrocyte metabolic processes and functioning that are essential for brain homeostasis; F. Increasing extracellular dopamine through impaired dopamine reuptake and storage mechanisms; G. Further recruiting monocytes due to increased chemokines and extracellular dopamine in the CNS. H. All of these processes (A-G) may contribute to neuronal injury, and loss, resulting in HAND.
We describe studies demonstrating that substances of abuse exacerbate HIV neuropathogenesis
We suggest areas for research
We discuss potential therapies
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.UNAIDS. 2017 GLOBAL HIV STATISTICS. [Website]. 2018. Available from: http://aidsinfo.unaids.org/.
- 2.Witwer KW, Gama L, Li M, Bartizal CM, Queen SE, Varrone JJ, Brice AK, Graham DR, Tarwater PM, Mankowski JL, Zink MC, Clements JE. Coordinated regulation of SIV replication and immune responses in the CNS. PLoS One. 2009;4(12):e8129 10.1371/journal.pone.0008129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valcour V, Chalermchai T, Sailasuta N, Marovich M, Lerdlum S, Suttichom D, Suwanwela NC, Jagodzinski L, Michael N, Spudich S, van Griensven F, de Souza M, Kim J, Ananworanich J, Group RSS. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis. 2012;206(2):275–82. 10.1093/infdis/jis326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dahl V, Peterson J, Fuchs D, Gisslen M, Palmer S, Price RW. Low levels of HIV-1 RNA detected in the cerebrospinal fluid after up to 10 years of suppressive therapy are associated with local immune activation. AIDS. 2014;28(15):2251–8. 10.1097/QAD.0000000000000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams DW, Calderon TM, Lopez L, Carvallo-Torres L, Gaskill PJ, Eugenin EA, Morgello S, Berman JW. Mechanisms of HIV entry into the CNS: increased sensitivity of HIV infected CD14+CD16+ monocytes to CCL2 and key roles of CCR2, JAM-A, and ALCAM in diapedesis. PLoS One. 2013;8(7):e69270 10.1371/journal.pone.0069270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams DW, Byrd D, Rubin LH, Anastos K, Morgello S, Berman JW. CCR2 on CD14(+)CD16(+) monocytes is a biomarker of HIV-associated neurocognitive disorders. Neurology(R) neuroimmunology & neuroinflammation. 2014;1(3):e36 10.1212/nxi.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams DW, Veenstra M, Gaskill PJ, Morgello S, Calderon TM, Berman JW. Monocytes mediate HIV neuropathogenesis: mechanisms that contribute to HIV associated neurocognitive disorders. Current HIV research. 2014;12(2):85–96. Epub 2014/05/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veenstra M, Leon-Rivera R, Li M, Gama L, Clements JE, Berman JW. Mechanisms of CNS Viral Seeding by HIV(+) CD14(+) CD16(+) Monocytes: Establishment and Reseeding of Viral Reservoirs Contributing to HIV-Associated Neurocognitive Disorders. MBio. 2017;8(5). 10.1128/mBio.01280-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veenstra M, Williams DW, Calderon TM, Anastos K, Morgello S, Berman JW. Frontline Science: CXCR7 mediates CD14(+)CD16(+) monocyte transmigration across the blood brain barrier: a potential therapeutic target for NeuroAIDS. J Leukoc Biol. 2017;102(5):1173–85. 10.1189/jlb.3HI0517-167R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alexaki A, Liu Y, Wigdahl B. Cellular reservoirs of HIV-1 and their role in viral persistence. Current HIV research. 2008;6(5):388–400. Epub 2008/10/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ko A, Kang G, Hattler JB, Galadima HI, Zhang J, Li Q, Kim WK. Macrophages but not Astrocytes Harbor HIV DNA in the Brains of HIV-1-Infected Aviremic Individuals on Suppressive Antiretroviral Therapy. J Neuroimmune Pharmacol. 2019;14(1):110–9. 10.1007/s11481-018-9809-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Churchill MJ, Gorry PR, Cowley D, Lal L, Sonza S, Purcell DF, Thompson KA, Gabuzda D, McArthur JC, Pardo CA, Wesselingh SL. Use of laser capture microdissection to detect integrated HIV-1 DNA in macrophages and astrocytes from autopsy brain tissues. J Neurovirol. 2006;12(2):146–52. 10.1080/13550280600748946. [DOI] [PubMed] [Google Scholar]
- 13.Oliveira MF, Chaillon A, Nakazawa M, Vargas M, Letendre SL, Strain MC, Ellis RJ, Morris S, Little SJ, Smith DM, Gianella S. Early Antiretroviral Therapy Is Associated with Lower HIV DNA Molecular Diversity and Lower Inflammation in Cerebrospinal Fluid but Does Not Prevent the Establishment of Compartmentalized HIV DNA Populations. PLOS Pathogens. 2017;13(1):e1006112. doi: 10.1371/journal.ppat.1006112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edén A, Fuchs D, Hagberg L, Nilsson S, Spudich S, Svennerholm B, Price RW, Gisslén M. HIV-1 viral escape in cerebrospinal fluid of subjects on suppressive antiretroviral treatment. J Infect Dis. 2010;202(12):1819–25. 10.1086/657342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gisolf EH, van Praag RM, Jurriaans S, Portegies P, Goudsmit J, Danner SA, Lange JM, Prins JM. Increasing cerebrospinal fluid chemokine concentrations despite undetectable cerebrospinal fluid HIV RNA in HIV-1-infected patients receiving antiretroviral therapy. J Acquir Immune Defic Syndr. 2000;25(5):426–33. Epub 2001/01/05. [DOI] [PubMed] [Google Scholar]
- 16.Mediouni S, Darque A, Baillat G, Ravaux I, Dhiver C, Tissot-Dupont H, Mokhtari M, Moreau H, Tamalet C, Brunet C, Paul P, Dignat-George F, Stein A, Brouqui P, Spector SA, Campbell GR, Loret EP. Antiretroviral therapy does not block the secretion of the human immunodeficiency virus tat protein. Infectious disorders drug targets. 2012;12(1):81–6. Epub 2012/01/28. [DOI] [PubMed] [Google Scholar]
- 17.Bachani M, Sacktor N, McArthur JC, Nath A, Rumbaugh J. Detection of anti-tat antibodies in CSF of individuals with HIV-associated neurocognitive disorders. J Neurovirol. 2013;19(1):82–8. 10.1007/s13365-012-0144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamers SL, Poon AF, McGrath MS. HIV-1 nef protein structures associated with brain infection and dementia pathogenesis. PLoS One. 2011;6(2):e16659 10.1371/journal.pone.0016659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cysique LA, Brew BJ. Prevalence of non-confounded HIV-associated neurocognitive impairment in the context of plasma HIV RNA suppression. J Neurovirol. 2011;17(2):176–83. 10.1007/s13365-011-0021-x. [DOI] [PubMed] [Google Scholar]
- 20.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, Group C, Group H. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17(1):3–16. 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heaton RK, Franklin DR Jr., Deutsch R, Letendre S, Ellis RJ, Casaletto K, Marquine MJ, Woods SP, Vaida F, Atkinson JH, Marcotte TD, McCutchan JA, Collier AC, Marra CM, Clifford DB, Gelman BB, Sacktor N, Morgello S, Simpson DM, Abramson I, Gamst AC, Fennema-Notestine C, Smith DM, Grant I, Group C. Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. Clin Infect Dis. 2015;60(3):473–80. 10.1093/cid/ciu862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harezlak J, Buchthal S, Taylor M, Schifitto G, Zhong J, Daar E, Alger J, Singer E, Campbell T, Yiannoutsos C, Cohen R, Navia B. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. Aids. 2011;25(5):625–33. 10.1097/QAD.0b013e3283427da7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, Mankowski JL, Brown A, Volsky DJ, McArthur JC. HIV-associated neurocognitive disorder--pathogenesis and prospects for treatment. Nat Rev Neurol. 2016;12(4):234–48. 10.1038/nrneurol.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spiller MW, Broz D, Wejnert C, Nerlander L, Paz-Bailey G. HIV infection and HIV-associated behaviors among persons who inject drugs--20 cities, United States, 2012. MMWR Morbidity and mortality weekly report. 2015;64(10):270–5. Epub 2015/03/20. [PMC free article] [PubMed] [Google Scholar]
- 25.Drug and Viral Infections. NIDA. 2019. [Google Scholar]
- 26.Administration SAaMHS. Key substance use and mental health indicators in the United States: Results from the 2017 National Survey on Drug Use and Healthh (HHS Publication No. SMA 18–5068, NSDUH Series H-53). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 2018. [cited 2019 May 31]. Available from: https://www.samhsa.gov/data/. [Google Scholar]
- 27.Ersche KD, Sahakian BJ. The neuropsychology of amphetamine and opiate dependence: implications for treatment. Neuropsychol Rev. 2007;17(3):317–36. 10.1007/s11065-007-9033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vonmoos M, Hulka LM, Preller KH, Minder F, Baumgartner MR, Quednow BB. Cognitive impairment in cocaine users is drug-induced but partially reversible: evidence from a longitudinal study. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39(9):2200–10. 10.1038/npp.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Potvin S, Pelletier J, Grot S, Hébert C, Barr AM, Lecomte T. Cognitive deficits in individuals with methamphetamine use disorder: A meta-analysis. Addictive Behaviors. 2018;80:154–60. doi: 10.1016/j.addbeh.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 30.Wang T-Y, Fan T-T, Bao Y-P, Li X-D, Liang C-M, Wang R-J, Ma J, Han Y, Meng S-Q, Wu P, Shi J, Lu L. Pattern and related factors of cognitive impairment among chronic methamphetamine users. The American Journal on Addictions. 2017;26(2):145–51. doi: 10.1111/ajad.12505. [DOI] [PubMed] [Google Scholar]
- 31.Luo Y-L, Bian J-W, Zheng Z-J, Zhao L, Han S, Sun X-H, Li J-F, Ni G-X. Effects of methamphetamine abuse on spatial cognitive function. Scientific Reports. 2018;8(1):5502. doi: 10.1038/s41598-018-23828-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernandez-Serrano MJ, Perales JC, Moreno-Lopez L, Perez-Garcia M, Verdejo-Garcia A. Neuropsychological profiling of impulsivity and compulsivity in cocaine dependent individuals. Psychopharmacology. 2012;219(2):673–83. 10.1007/s00213-011-2485-z. [DOI] [PubMed] [Google Scholar]
- 33.Levine AJ, Hardy DJ, Miller E, Castellon SA, Longshore D, Hinkin CH. The effect of recent stimulant use on sustained attention in HIV-infected adults. Journal of clinical and experimental neuropsychology. 2006;28(1):29–42. 10.1080/13803390490918066. [DOI] [PubMed] [Google Scholar]
- 34.Jacobs MM, Murray J, Byrd DA, Hurd YL, Morgello S. HIV-related cognitive impairment shows bidirectional association with dopamine receptor DRD1 and DRD2 polymorphisms in substance-dependent and substance-independent populations. Journal of neurovirology. 2013;19(5):495–504. doi: 10.1007/s13365-013-0204-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson AM, Higgins MK, Ownby RL, Waldrop-Valverde D. Changes in neurocognition and adherence over six months in HIV-infected individuals with cocaine or heroin dependence. AIDS Care. 2015;27(3):333–7. 10.1080/09540121.2014.985183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baum MK, Rafie C, Lai S, Sales S, Page B, Campa A. Crack-cocaine use accelerates HIV disease progression in a cohort of HIV-positive drug users. J Acquir Immune Defic Syndr. 2009;50(1):93–9. 10.1097/QAI.0b013e3181900129. [DOI] [PubMed] [Google Scholar]
- 37.Samikkannu T, Rao KVK, Arias AY, Kalaichezian A, Sagar V, Yoo C, Nair MPN. HIV infection and drugs of abuse: role of acute phase proteins. Journal of neuroinflammation. 2013;10:113-. doi: 10.1186/1742-2094-10-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ancuta P, Kamat A, Kunstman KJ, Kim EY, Autissier P, Wurcel A, Zaman T, Stone D, Mefford M, Morgello S, Singer EJ, Wolinsky SM, Gabuzda D. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One. 2008;3(6):e2516 10.1371/journal.pone.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nature medicine. 2006;12(12):1365–71. 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 40.Jiang W, Lederman MM, Hunt P, Sieg SF, Haley K, Rodriguez B, Landay A, Martin J, Sinclair E, Asher AI, Deeks SG, Douek DC, Brenchley JM. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis. 2009;199(8):1177–85. 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carrico AW, Cherenack EM, Roach ME, Riley ED, Oni O, Dilworth SE, Shoptaw S, Hunt P, Roy S, Pallikkuth S, Pahwa S. Substance-associated elevations in monocyte activation among methamphetamine users with treated HIV infection. AIDS. 2018;32(6):767–71. 10.1097/QAD.0000000000001751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ellery PJ, Tippett E, Chiu YL, Paukovics G, Cameron PU, Solomon A, Lewin SR, Gorry PR, Jaworowski A, Greene WC, Sonza S, Crowe SM. The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J Immunol. 2007;178(10):6581–9. 10.4049/jimmunol.178.10.6581. [DOI] [PubMed] [Google Scholar]
- 43.Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci. 2006;26(4):1098–106. 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu DT, Woodman SE, Weiss JM, McManus CM, D’Aversa TG, Hesselgesser J, Major EO, Nath A, Berman JW. Mechanisms of leukocyte trafficking into the CNS. J Neurovirol. 2000;6 Suppl 1:S82–5. Epub 2000/06/29. [PubMed] [Google Scholar]
- 45.Ellery PJ, Tippett E, Chiu Y-L, Paukovics G, Cameron PU, Solomon A, Lewin SR, Gorry PR, Jaworowski A, Greene WC, Sonza S, Crowe SM. The CD16+ Monocyte Subset Is More Permissive to Infection and Preferentially Harbors HIV-1 In Vivo. The Journal of Immunology. 2007;178(10):6581–9. doi: 10.4049/jimmunol.178.10.6581. [DOI] [PubMed] [Google Scholar]
- 46.Rostasy K, Egles C, Chauhan A, Kneissl M, Bahrani P, Yiannoutsos C, Hunter DD, Nath A, Hedreen JC, Navia BA. SDF-1α Is Expressed in Astrocytes and Neurons in the AIDS Dementia Complex: An In Vivo and In Vitro Study. Journal of Neuropathology & Experimental Neurology. 2003;62(6):617–26. doi: 10.1093/jnen/62.6.617. [DOI] [PubMed] [Google Scholar]
- 47.Dhillon NK, Williams R, Callen S, Zien C, Narayan O, Buch S. Roles of MCP-1 in development of HIV-dementia. Frontiers in bioscience : a journal and virtual library. 2008;13:3913–8. Epub 2008/05/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Calderon TM, Williams DW, Lopez L, Eugenin EA, Cheney L, Gaskill PJ, Veenstra M, Anastos K, Morgello S, Berman JW. Dopamine Increases CD14(+)CD16(+) Monocyte Transmigration across the Blood Brain Barrier: Implications for Substance Abuse and HIV Neuropathogenesis. J Neuroimmune Pharmacol. 2017;12(2):353–70. 10.1007/s11481-017-9726-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abbott NJ. Blood-brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis. 2013;36(3):437–49. 10.1007/s10545-013-9608-0. [DOI] [PubMed] [Google Scholar]
- 50.Abbott NJ. Astrocyte-endothelial interactions and blood-brain barrier permeability. Journal of anatomy. 2002;200(6):629–38. Epub 2002/08/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buckner CM, Luers AJ, Calderon TM, Eugenin EA, Berman JW. Neuroimmunity and the blood-brain barrier: molecular regulation of leukocyte transmigration and viral entry into the nervous system with a focus on neuroAIDS. J Neuroimmune Pharmacol. 2006;1(2):160–81. 10.1007/s11481-006-9017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fischer-Smith T, Croul S, Sverstiuk AE, Capini C, L’Heureux D, Regulier EG, Richardson MW, Amini S, Morgello S, Khalili K, Rappaport J. CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol. 2001;7(6):528–41. 10.1080/135502801753248114. [DOI] [PubMed] [Google Scholar]
- 53.Williams DW, Eugenin EA, Calderon TM, Berman JW. Monocyte maturation, HIV susceptibility, and transmigration across the blood brain barrier are critical in HIV neuropathogenesis. J Leukoc Biol. 2012;91(3):401–15. 10.1189/jlb.0811394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williams DW, Anastos K, Morgello S, Berman JW. JAM-A and ALCAM are therapeutic targets to inhibit diapedesis across the BBB of CD14+CD16+ monocytes in HIV-infected individuals. J Leukoc Biol. 2015;97(2):401–12. 10.1189/jlb.5A0714-347R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bertrand L, Dygert L, Toborek M. Antiretroviral Treatment with Efavirenz Disrupts the Blood-Brain Barrier Integrity and Increases Stroke Severity. Scientific reports. 2016;6:39738-. doi: 10.1038/srep39738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thompson KA, Cherry CL, Bell JE, McLean CA. Brain cell reservoirs of latent virus in presymptomatic HIV-infected individuals. Am J Pathol. 2011;179(4):1623–9. 10.1016/j.ajpath.2011.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williams KC, Corey S, Westmoreland SV, Pauley D, Knight H, deBakker C, Alvarez X, Lackner AA. Perivascular Macrophages Are the Primary Cell Type Productively Infected by Simian Immunodeficiency Virus in the Brains of Macaques. Implications for the Neuropathogenesis of AIDS. 2001;193(8):905–16. doi: 10.1084/jem.193.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Honeycutt JB, Wahl A, Baker C, Spagnuolo RA, Foster J, Zakharova O, Wietgrefe S, Caro-Vegas C, Madden V, Sharpe G, Haase AT, Eron JJ, Garcia JV. Macrophages sustain HIV replication in vivo independently of T cells. J Clin Invest. 2016;126(4):1353–66. 10.1172/JCI84456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Honeycutt JB, Thayer WO, Baker CE, Ribeiro RM, Lada SM, Cao Y, Cleary RA, Hudgens MG, Richman DD, Garcia JV. HIV persistence in tissue macrophages of humanized myeloid-only mice during antiretroviral therapy. Nature medicine. 2017;23(5):638–43. 10.1038/nm.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gu C-J, Borjabad A, Hadas E, Kelschenbach J, Kim B-H, Chao W, Arancio O, Suh J, Polsky B, McMillan J, Edagwa B, Gendelman HE, Potash MJ, Volsky DJ. EcoHIV infection of mice establishes latent viral reservoirs in T cells and active viral reservoirs in macrophages that are sufficient for induction of neurocognitive impairment. PLoS pathogens. 2018;14(6):e1007061–e. doi: 10.1371/journal.ppat.1007061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Avalos CR, Abreu CM, Queen SE, Li M, Price S, Shirk EN, Engle EL, Forsyth E, Bullock BT, Mac Gabhann F, Wietgrefe SW, Haase AT, Zink MC, Mankowski JL, Clements JE, Gama L. Brain Macrophages in Simian Immunodeficiency Virus-Infected, Antiretroviral-Suppressed Macaques: a Functional Latent Reservoir. MBio. 2017;8(4). 10.1128/mBio.01186-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gama L, Abreu C, Shirk EN, Queen SE, Beck SE, Metcalf Pate KA, Bullock BT, Zink MC, Mankowski JL, Clements JE. SIV Latency in Macrophages in the CNS. Current topics in microbiology and immunology. 2018;417:111–30. 10.1007/82_2018_89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abreu CM, Veenhuis RT, Avalos CR, Graham S, Queen SE, Shirk EN, Bullock BT, Li M, Metcalf Pate KA, Beck SE, Mangus LM, Mankowski JL, Clements JE, Gama L. Infectious virus persists in CD4+ T cells and macrophages in ART-suppressed SIV-infected Macaques. Journal of virology. 2019. 10.1128/jvi.00065-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Honeycutt JB, Liao B, Nixon CC, Cleary RA, Thayer WO, Birath SL, Swanson MD, Sheridan P, Zakharova O, Prince F, Kuruc J, Gay CL, Evans C, Eron JJ, Wahl A, Garcia JV. T cells establish and maintain CNS viral infection in HIV-infected humanized mice. J Clin Invest. 2018;128(7):2862–76. 10.1172/JCI98968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ho EL, Ronquillo R, Altmeppen H, Spudich SS, Price RW, Sinclair E. Cellular Composition of Cerebrospinal Fluid in HIV-1 Infected and Uninfected Subjects. PLoS One. 2013;8(6):e66188 10.1371/journal.pone.0066188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ganesh A, Lemongello D, Lee E, Peterson J, McLaughlin BE, Ferre AL, Gillespie GM, Fuchs D, Deeks SG, Hunt PW, Price RW, Spudich SS, Shacklett BL. Immune Activation and HIV-Specific CD8(+) T Cells in Cerebrospinal Fluid of HIV Controllers and Noncontrollers. AIDS Res Hum Retroviruses. 2016;32(8):791–800. 10.1089/aid.2015.0313.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Richards MH, Narasipura SD, Seaton MS, Lutgen V, Al-Harthi L. Migration of CD8+ T Cells into the Central Nervous System Gives Rise to Highly Potent Anti-HIV CD4dimCD8bright T Cells in a Wnt Signaling-Dependent Manner. J Immunol. 2016;196(1):317–27. 10.4049/jimmunol.1501394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marcondes MC, Morsey B, Emanuel K, Lamberty BG, Flynn CT, Fox HS. CD8+ T cells maintain suppression of simian immunodeficiency virus in the central nervous system. J Infect Dis. 2015;211(1):40–4. 10.1093/infdis/jiu401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cartwright EK, Spicer L, Smith SA, Lee D, Fast R, Paganini S, Lawson BO, Nega M, Easley K, Schmitz JE, Bosinger SE, Paiardini M, Chahroudi A, Vanderford TH, Estes JD, Lifson JD, Derdeyn CA, Silvestri G. CD8(+) Lymphocytes Are Required for Maintaining Viral Suppression in SIV-Infected Macaques Treated with Short-Term Antiretroviral Therapy. Immunity. 2016;45(3):656–68. 10.1016/j.immuni.2016.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gupta S, Knight AG, Losso BY, Ingram DK, Keller JN, Bruce-Keller AJ. Brain injury caused by HIV protease inhibitors: role of lipodystrophy and insulin resistance. Antiviral research. 2012;95(1):19–29. 10.1016/j.antiviral.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Akay C, Cooper M, Odeleye A, Jensen BK, White MG, Vassoler F, Gannon PJ, Mankowski J, Dorsey JL, Buch AM, Cross SA, Cook DR, Peña M-M, Andersen ES, Christofidou-Solomidou M, Lindl KA, Zink MC, Clements J, Pierce RC, Kolson DL, Jordan-Sciutto KL. Antiretroviral drugs induce oxidative stress and neuronal damage in the central nervous system. Journal of neurovirology. 2014;20(1):39–53. 10.1007/s13365-013-0227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ciccarelli N, Fabbiani M, Di Giambenedetto S, Fanti I, Baldonero E, Bracciale L, Tamburrini E, Cauda R, De Luca A, Silveri MC. Efavirenz associated with cognitive disorders in otherwise asymptomatic HIV-infected patients. Neurology. 2011;76(16):1403–9. 10.1212/WNL.0b013e31821670fb. [DOI] [PubMed] [Google Scholar]
- 73.Ma Q, Vaida F, Wong J, Sanders CA, Kao Y-t, Croteau D, Clifford DB, Collier AC, Gelman BB, Marra CM, McArthur JC, Morgello S, Simpson DM, Heaton RK, Grant I, Letendre SL, Group C. Long-term efavirenz use is associated with worse neurocognitive functioning in HIV-infected patients. Journal of neurovirology. 2016;22(2):170–8. 10.1007/s13365-015-0382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sanchez AB, Varano GP, de Rozieres CM, Maung R, Catalan IC, Dowling CC, Sejbuk NE, Hoefer MM, Kaul M. Antiretrovirals, Methamphetamine, and HIV-1 Envelope Protein gp120 Compromise Neuronal Energy Homeostasis in Association with Various Degrees of Synaptic and Neuritic Damage. Antimicrob Agents Chemother. 2016;60(1):168–79. 10.1128/aac.01632-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.NIDA. Methamphetamine. NIDA website 2013. [cited 2019 January 28]. Available from: https://www.drugabuse.gov/publications/research-reports/methamphetamine/what-scope-methamphetamine-abuse-in-united-states.
- 76.Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annual review of pharmacology and toxicology. 2007;47:681–98. 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- 77.Hart CL, Marvin CB, Silver R, Smith EE. Is cognitive functioning impaired in methamphetamine users? A critical review. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37(3):586–608. 10.1038/npp.2011.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McCann UD, Szabo Z, Scheffel U, Dannals RF, Ricaurte GA. Positron emission tomographic evidence of toxic effect of MDMA (“Ecstasy”) on brain serotonin neurons in human beings. The Lancet. 1998;352(9138):1433–7. doi: 10.1016/s0140-6736(98)04329-3. [DOI] [PubMed] [Google Scholar]
- 79.Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction (Abingdon, England). 2007;102 Suppl 1:16–32. 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- 80.Jan RK, Kydd RR, Russell BR. Functional and structural brain changes associated with methamphetamine abuse. Brain Sci. 2012;2(4):434–82. doi: 10.3390/brainsci2040434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Plankey MW, Ostrow DG, Stall R, Cox C, Li X, Peck JA, Jacobson LP. The relationship between methamphetamine and popper use and risk of HIV seroconversion in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2007;45(1):85–92. 10.1097/QAI.0b013e3180417c99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chana G, Everall IP, Crews L, Langford D, Adame A, Grant I, Cherner M, Lazzaretto D, Heaton R, Ellis R, Masliah E. Cognitive deficits and degeneration of interneurons in HIV+ methamphetamine users. Neurology. 2006;67(8):1486–9. 10.1212/01.wnl.0000240066.02404.e6. [DOI] [PubMed] [Google Scholar]
- 83.Carey CL, Woods SP, Rippeth JD, Gonzalez R, Heaton RK, Grant I. Additive deleterious effects of methamphetamine dependence and immunosuppression on neuropsychological functioning in HIV infection. AIDS and behavior. 2006;10(2):185–90. 10.1007/s10461-005-9056-4. [DOI] [PubMed] [Google Scholar]
- 84.Weber E, Morgan EE, Iudicello JE, Blackstone K, Grant I, Ellis RJ, Letendre SL, Little S, Morris S, Smith DM, Moore DJ, Woods SP, Group T. Substance use is a risk factor for neurocognitive deficits and neuropsychiatric distress in acute and early HIV infection. J Neurovirol. 2013;19(1):65–74. 10.1007/s13365-012-0141-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kesby JP, Heaton RK, Young JW, Umlauf A, Woods SP, Letendre SL, Markou A, Grant I, Semenova S. Methamphetamine Exposure Combined with HIV-1 Disease or gp120 Expression: Comparison of Learning and Executive Functions in Humans and Mice. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015;40(8):1899–909. 10.1038/npp.2015.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, Wolfson T, Grant I, Group H. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc. 2004;10(1):1–14. 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- 87.Gupta S, Bousman CA, Chana G, Cherner M, Heaton RK, Deutsch R, Ellis RJ, Grant I, Everall IP. Dopamine receptor D3 genetic polymorphism (rs6280TC) is associated with rates of cognitive impairment in methamphetamine-dependent men with HIV: preliminary findings. J Neurovirol. 2011;17(3):239–47. 10.1007/s13365-011-0028-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Iudicello JE, Morgan EE, Gongvatana A, Letendre SL, Grant I, Woods SP. Detrimental impact of remote methamphetamine dependence on neurocognitive and everyday functioning in older but not younger HIV+ adults: evidence for a legacy effect? J Neurovirol. 2014;20(1):85–98. 10.1007/s13365-014-0233-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chang L, Ernst T, Speck O, Grob CS. Additive effects of HIV and chronic methamphetamine use on brain metabolite abnormalities. Am J Psychiatry. 2005;162(2):361–9. 10.1176/appi.ajp.162.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Taylor MJ, Schweinsburg BC, Alhassoon OM, Gongvatana A, Brown GG, Young-Casey C, Letendre SL, Grant I. Effects of human immunodeficiency virus and methamphetamine on cerebral metabolites measured with magnetic resonance spectroscopy. J Neurovirol. 2007;13(2):150–9. 10.1080/13550280701194230. [DOI] [PubMed] [Google Scholar]
- 91.Kesby JP, Markou A, Semenova S, Grant I, Ellis RJ, Letendre SL, Achim CL, Woods SP, Carr AM, Letendre SL, Ellis RJ, Schrier R, Heaton RK, Hampton Atkinson J, Cherner M, Marcotte TD, Morgan EE, Brown G, Jernigan T, Dale A, Liu T, Scadeng M, Fennema-Notestine C, Archibald SL, Achim CL, Masliah E, Lipton S, Soontornniyomkij V, Gamst AC, Cushman C, Abramson I, Vaida F, Deutsch R, Umlauf A, Hampton Atkinson J, Marquie-Beck J, Minassian A, Perry W, Geyer M, Henry B, Young J, Grethe AB, Paulus M, Ellis RJ, Morris S, Smith DM, Grant I, Semenova S, Markou A, Kesby JP, Kaul M. Cognitive deficits associated with combined HIV gp120 expression and chronic methamphetamine exposure in mice. European Neuropsychopharmacology. 2015;25(1):141–50. doi: 10.1016/j.euroneuro.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nookala AR, Schwartz DC, Chaudhari NS, Glazyrin A, Stephens EB, Berman NEJ, Kumar A. Methamphetamine augment HIV-1 Tat mediated memory deficits by altering the expression of synaptic proteins and neurotrophic factors. Brain Behav Immun. 2018;71:37–51. 10.1016/j.bbi.2018.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu J, Xu E, Tu G, Liu H, Luo J, Xiong H. Methamphetamine potentiates HIV-1gp120-induced microglial neurotoxic activity by enhancing microglial outward K(+) current. Mol Cell Neurosci. 2017;82:167–75. 10.1016/j.mcn.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Marcondes MCG, Flynn C, Watry DD, Zandonatti M, Fox HS. Methamphetamine increases brain viral load and activates natural killer cells in simian immunodeficiency virus-infected monkeys. The American journal of pathology. 2010;177(1):355–61. doi: 10.2353/ajpath.2010.090953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Najera JA, Bustamante EA, Bortell N, Morsey B, Fox HS, Ravasi T, Marcondes MC. Methamphetamine abuse affects gene expression in brain-derived microglia of SIV-infected macaques to enhance inflammation and promote virus targets. BMC Immunol. 2016;17(1):7 10.1186/s12865-016-0145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wires ES, Alvarez D, Dobrowolski C, Wang Y, Morales M, Karn J, Harvey BK. Methamphetamine activates nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kappaB) and induces human immunodeficiency virus (HIV) transcription in human microglial cells. J Neurovirol. 2012;18(5):400–10. 10.1007/s13365-012-0103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Toussi SS, Joseph A, Zheng JH, Dutta M, Santambrogio L, Goldstein H. Short communication: Methamphetamine treatment increases in vitro and in vivo HIV replication. AIDS Res Hum Retroviruses. 2009;25(11):1117–21. 10.1089/aid.2008.0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liang H, Wang X, Chen H, Song L, Ye L, Wang SH, Wang YJ, Zhou L, Ho WZ. Methamphetamine enhances HIV infection of macrophages. Am J Pathol. 2008;172(6):1617–24. 10.2353/ajpath.2008.070971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cen P, Ye L, Su QJ, Wang X, Li JL, Lin XQ, Liang H, Ho WZ. Methamphetamine inhibits Toll-like receptor 9-mediated anti-HIV activity in macrophages. AIDS Res Hum Retroviruses. 2013;29(8):1129–37. 10.1089/aid.2012.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Reynolds JL, Law WC, Mahajan SD, Aalinkeel R, Nair B, Sykes DE, Yong KT, Hui R, Prasad PN, Schwartz SA. Nanoparticle based galectin-1 gene silencing, implications in methamphetamine regulation of HIV-1 infection in monocyte derived macrophages. J Neuroimmune Pharmacol. 2012;7(3):673–85. 10.1007/s11481-012-9379-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nair MP, Saiyed ZM, Nair N, Gandhi NH, Rodriguez JW, Boukli N, Provencio-Vasquez E, Malow RM, Miguez-Burbano MJ. Methamphetamine enhances HIV-1 infectivity in monocyte derived dendritic cells. J Neuroimmune Pharmacol. 2009;4(1):129–39. 10.1007/s11481-008-9128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Skowronska M, McDonald M, Velichkovska M, Leda AR, Park M, Toborek M. Methamphetamine increases HIV infectivity in neural progenitor cells. J Biol Chem. 2018;293(1):296–311. 10.1074/jbc.RA117.000795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Prasad A, Kulkarni R, Shrivastava A, Jiang S, Lawson K, Groopman JE. Methamphetamine functions as a novel CD4(+) T-cell activator via the sigma-1 receptor to enhance HIV-1 infection. Sci Rep. 2019;9(1):958 10.1038/s41598-018-35757-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mantri CK, Mantri JV, Pandhare J, Dash C. Methamphetamine inhibits HIV-1 replication in CD4+ T cells by modulating anti-HIV-1 miRNA expression. Am J Pathol. 2014;184(1):92–100. 10.1016/j.ajpath.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Martins T, Baptista S, Goncalves J, Leal E, Milhazes N, Borges F, Ribeiro CF, Quintela O, Lendoiro E, Lopez-Rivadulla M, Ambrosio AF, Silva AP. Methamphetamine transiently increases the blood-brain barrier permeability in the hippocampus: role of tight junction proteins and matrix metalloproteinase-9. Brain Res. 2011;1411:28–40. 10.1016/j.brainres.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 106.Park M, Kim HJ, Lim B, Wylegala A, Toborek M. Methamphetamine-induced occludin endocytosis is mediated by the Arp2/3 complex-regulated actin rearrangement. J Biol Chem. 2013;288(46):33324–34. 10.1074/jbc.M113.483487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ramirez SH, Potula R, Fan S, Eidem T, Papugani A, Reichenbach N, Dykstra H, Weksler BB, Romero IA, Couraud PO, Persidsky Y. Methamphetamine disrupts blood-brain barrier function by induction of oxidative stress in brain endothelial cells. J Cereb Blood Flow Metab. 2009;29(12):1933–45. 10.1038/jcbfm.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang X, Banerjee A, Banks WA, Ercal N. N-Acetylcysteine amide protects against methamphetamine-induced oxidative stress and neurotoxicity in immortalized human brain endothelial cells. Brain Res. 2009;1275:87–95. 10.1016/j.brainres.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Coelho-Santos V, Leitao RA, Cardoso FL, Palmela I, Rito M, Barbosa M, Brito MA, Fontes-Ribeiro CA, Silva AP. The TNF-alpha/NF-kappaB signaling pathway has a key role in methamphetamine-induced blood-brain barrier dysfunction. J Cereb Blood Flow Metab. 2015;35(8):1260–71. 10.1038/jcbfm.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Patel S, Leibrand CR, Palasuberniam P, Couraud PO, Weksler B, Jahr FM, McClay JL, Hauser KF, McRae M. Effects of HIV-1 Tat and Methamphetamine on Blood-Brain Barrier Integrity and Function In Vitro. Antimicrob Agents Chemother. 2017;61(12). 10.1128/AAC.01307-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Northrop NA, Yamamoto BK. Persistent neuroinflammatory effects of serial exposure to stress and methamphetamine on the blood-brain barrier. J Neuroimmune Pharmacol. 2012;7(4):951–68. 10.1007/s11481-012-9391-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Leibrand CR, Paris JJ, Ghandour MS, Knapp PE, Kim WK, Hauser KF, McRae M. HIV-1 Tat disrupts blood-brain barrier integrity and increases phagocytic perivascular macrophages and microglia in the dorsal striatum of transgenic mice. Neurosci Lett. 2017;640:136–43. 10.1016/j.neulet.2016.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Banerjee A, Zhang X, Manda KR, Banks WA, Ercal N. HIV proteins (gp120 and Tat) and methamphetamine in oxidative stress-induced damage in the brain: potential role of the thiol antioxidant N-acetylcysteine amide. Free radical biology & medicine. 2010;48(10):1388–98. 10.1016/j.freeradbiomed.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mahajan SD, Aalinkeel R, Sykes DE, Reynolds JL, Bindukumar B, Adal A, Qi M, Toh J, Xu G, Prasad PN, Schwartz SA. Methamphetamine alters blood brain barrier permeability via the modulation of tight junction expression: Implication for HIV-1 neuropathogenesis in the context of drug abuse. Brain Res. 2008;1203:133–48. 10.1016/j.brainres.2008.01.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Manda KR, Banerjee A, Banks WA, Ercal N. Highly active antiretroviral therapy drug combination induces oxidative stress and mitochondrial dysfunction in immortalized human blood-brain barrier endothelial cells. Free radical biology & medicine. 2011;50(7):801–10. 10.1016/j.freeradbiomed.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Martins T, Burgoyne T, Kenny BA, Hudson N, Futter CE, Ambrosio AF, Silva AP, Greenwood J, Turowski P. Methamphetamine-induced nitric oxide promotes vesicular transport in blood-brain barrier endothelial cells. Neuropharmacology. 2013;65:74–82. 10.1016/j.neuropharm.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Eugenin EA, Greco JM, Frases S, Nosanchuk JD, Martinez LR. Methamphetamine alters blood brain barrier protein expression in mice, facilitating central nervous system infection by neurotropic Cryptococcus neoformans. J Infect Dis. 2013;208(4):699–704. 10.1093/infdis/jit117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bortell N, Morsey B, Basova L, Fox HS, Marcondes MC. Phenotypic changes in the brain of SIV-infected macaques exposed to methamphetamine parallel macrophage activation patterns induced by the common gamma-chain cytokine system. Front Microbiol. 2015;6:900 10.3389/fmicb.2015.00900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Achim CL, Adame A, Dumaop W, Everall IP, Masliah E. Increased accumulation of intraneuronal amyloid beta in HIV-infected patients. J Neuroimmune Pharmacol. 2009;4(2):190–9. 10.1007/s11481-009-9152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Aslanyan L, Lee HH, Ekhar VV, Ramos RL, Martinez LR. Methamphetamine Impairs IgG1-Mediated Phagocytosis and Killing of <span class=“named-content genus-species” id=“named-content-1”>Cryptococcus neoformans by J774.16 Macrophage- and NR-9640 Microglia-Like Cells. Infection and Immunity. 2019;87(2):e00113–18. doi: 10.1128/iai.00113-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tallóczy Z, Martinez J, Joset D, Ray Y, Gácser A, Toussi S, Mizushima N, Nosanchuk JD, Goldstein H, Loike J, Sulzer D, Santambrogio L. Methamphetamine inhibits antigen processing, presentation, and phagocytosis. PLoS pathogens. 2008;4(2):e28–e. 10.1371/journal.ppat.0040028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Azzam R, Lal L, Goh SL, Kedzierska K, Jaworowski A, Naim E, Cherry CL, Wesselingh SL, Mills J, Crowe SM. Adverse effects of antiretroviral drugs on HIV-1-infected and -uninfected human monocyte-derived macrophages. J Acquir Immune Defic Syndr. 2006;42(1):19–28. 10.1097/01.qai.0000214809.83218.88. [DOI] [PubMed] [Google Scholar]
- 123.Liu X, Silverstein PS, Singh V, Shah A, Qureshi N, Kumar A. Methamphetamine increases LPS-mediated expression of IL-8, TNF-α and IL-1β in human macrophages through common signaling pathways. PloS one. 2012;7(3):e33822–e. doi: 10.1371/journal.pone.0033822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tipton DA, Legan ZT, Dabbous M. Methamphetamine cytotoxicity and effect on LPS-stimulated IL-1beta production by human monocytes. Toxicol In Vitro. 2010;24(3):921–7. 10.1016/j.tiv.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 125.Burns A, Ciborowski P. Acute exposure to methamphetamine alters TLR9-mediated cytokine expression in human macrophage. Immunobiology. 2016;221(2):199–207. 10.1016/j.imbio.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang A, Wu L, Chen Z, Huang G, Lu X. Methamphetamine Causes Photoreceptor Cell Damage Through Promoting Polarization of Macrophages and Inducing Inflammatory Response. International journal of toxicology. 2017;36(5):403–9. 10.1177/1091581817718473. [DOI] [PubMed] [Google Scholar]
- 127.Li X, Wu F, Xue L, Wang B, Li J, Chen Y, Chen T. Methamphetamine causes neurotoxicity by promoting polarization of macrophages and inflammatory response. Human & experimental toxicology. 2018;37(5):486–95. 10.1177/0960327117714039. [DOI] [PubMed] [Google Scholar]
- 128.Coelho-Santos V, Gonçalves J, Fontes-Ribeiro C, Silva AP. Prevention of methamphetamine-induced microglial cell death by TNF-α and IL-6 through activation of the JAK-STAT pathway. Journal of Neuroinflammation. 2012;9(1):103. doi: 10.1186/1742-2094-9-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chao J, Zhang Y, Du L, Zhou R, Wu X, Shen K, Yao H. Molecular mechanisms underlying the involvement of the sigma-1 receptor in methamphetamine-mediated microglial polarization. Sci Rep. 2017;7(1):11540 10.1038/s41598-017-11065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tatro ET, Soontornniyomkij B, Letendre SL, Achim CL. Cytokine secretion from brain macrophages infected with human immunodeficiency virus in vitro and treated with raltegravir. BMC Infect Dis. 2014;14:386-. doi: 10.1186/1471-2334-14-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Du SH, Qiao DF, Chen CX, Chen S, Liu C, Lin Z, Wang H, Xie WB. Toll-Like Receptor 4 Mediates Methamphetamine-Induced Neuroinflammation through Caspase-11 Signaling Pathway in Astrocytes. Front Mol Neurosci. 2017;10:409 10.3389/fnmol.2017.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Serramia MJ, Munoz-Fernandez MA, Alvarez S. HIV-1 increases TLR responses in human primary astrocytes. Sci Rep. 2015;5:17887 10.1038/srep17887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shah A, Silverstein PS, Kumar S, Singh DP, Kumar A. Synergistic cooperation between methamphetamine and HIV-1 gsp120 through the P13K/Akt pathway induces IL-6 but not IL-8 expression in astrocytes. PloS one. 2012;7(12):e52060–e. doi: 10.1371/journal.pone.0052060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sharma A, Hu X-T, Napier TC, Al-Harthi L. Methamphetamine and HIV-1 Tat down regulate β-catenin signaling: implications for methampetamine abuse and HIV-1 co-morbidity. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2011;6(4):597–607. 10.1007/s11481-011-9295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yu C, Narasipura SD, Richards MH, Hu X-T, Yamamoto B, Al-Harthi L. HIV and drug abuse mediate astrocyte senescence in a β-catenin-dependent manner leading to neuronal toxicity. Aging Cell. 2017;16(5):956–65. 10.1111/acel.12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang Y, Lv X, Bai Y, Zhu X, Wu X, Chao J, Duan M, Buch S, Chen L, Yao H. Involvement of sigma-1 receptor in astrocyte activation induced by methamphetamine via up-regulation of its own expression. Journal of neuroinflammation. 2015;12:29-. doi: 10.1186/s12974-015-0250-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vivithanaporn P, Asahchop EL, Acharjee S, Baker GB, Power C. HIV protease inhibitors disrupt astrocytic glutamate transporter function and neurobehavioral performance. Aids. 2016;30(4):543–52. 10.1097/qad.0000000000000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bortell N, Basova L, Semenova S, Fox HS, Ravasi T, Marcondes MC. Astrocyte-specific overexpressed gene signatures in response to methamphetamine exposure in vitro. J Neuroinflammation. 2017;14(1):49 10.1186/s12974-017-0825-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Borgmann K, Ghorpade A. Methamphetamine Augments Concurrent Astrocyte Mitochondrial Stress, Oxidative Burden, and Antioxidant Capacity: Tipping the Balance in HIV-Associated Neurodegeneration. Neurotoxicity research. 2018;33(2):433–47. 10.1007/s12640-017-9812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shah A, Kumar S, Simon SD, Singh DP, Kumar A. HIV gp120- and methamphetamine-mediated oxidative stress induces astrocyte apoptosis via cytochrome P450 2E1. Cell death & disease. 2013;4(10):e850–e. doi: 10.1038/cddis.2013.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cao L, Fu M, Kumar S, Kumar A. Methamphetamine potentiates HIV-1 gp120-mediated autophagy via Beclin-1 and Atg5/7 as a pro-survival response in astrocytes. Cell death & disease. 2016;7(10):e2425–e. doi: 10.1038/cddis.2016.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mahmoud S, Gharagozloo M, Simard C, Amrani A, Gris D. NLRX1 Enhances Glutamate Uptake and Inhibits Glutamate Release by Astrocytes. Cells. 2019;8(5). 10.3390/cells8050400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vázquez-Santiago FJ, Noel RJ Jr., Porter JT, Rivera-Amill V. Glutamate metabolism and HIV-associated neurocognitive disorders. Journal of neurovirology. 2014;20(4):315–31. 10.1007/s13365-014-0258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cisneros IE, Ghorpade A. Methamphetamine and HIV-1-induced neurotoxicity: role of trace amine associated receptor 1 cAMP signaling in astrocytes. Neuropharmacology. 2014;85:499–507. 10.1016/j.neuropharm.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Castellano P, Nwagbo C, Martinez LR, Eugenin EA. Methamphetamine compromises gap junctional communication in astrocytes and neurons. Journal of neurochemistry. 2016;137(4):561–75. 10.1111/jnc.13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Massanella M, Gianella S, Schrier R, Dan JM, Perez-Santiago J, Oliveira MF, Richman DD, Little SJ, Benson CA, Daar ES, Dube MP, Haubrich RH, Smith DM, Morris SR. Methamphetamine Use in HIV-infected Individuals Affects T-cell Function and Viral Outcome during Suppressive Antiretroviral Therapy. Sci Rep. 2015;5:13179 10.1038/srep13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Potula R, Haldar B, Cenna JM, Sriram U, Fan S. Methamphetamine alters T cell cycle entry and progression: role in immune dysfunction. Cell Death Discov. 2018;4:44 10.1038/s41420-018-0045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Potula R, Hawkins BJ, Cenna JM, Fan S, Dykstra H, Ramirez SH, Morsey B, Brodie MR, Persidsky Y. Methamphetamine causes mitrochondrial oxidative damage in human T lymphocytes leading to functional impairment. Journal of immunology (Baltimore, Md : 1950). 2010;185(5):2867–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang W, Sonders MS, Ukairo OT, Scott H, Kloetzel MK, Surratt CK. Dissociation of high-affinity cocaine analog binding and dopamine uptake inhibition at the dopamine transporter. Molecular pharmacology. 2003;64(2):430–9. 10.1124/mol.64.2.430. [DOI] [PubMed] [Google Scholar]
- 150.Huang X, Gu HH, Zhan C-G. Mechanism for cocaine blocking the transport of dopamine: insights from molecular modeling and dynamics simulations. J Phys Chem B. 2009;113(45):15057–66. doi: 10.1021/jp900963n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Venton BJ, Seipel AT, Phillips PE, Wetsel WC, Gitler D, Greengard P, Augustine GJ, Wightman RM. Cocaine increases dopamine release by mobilization of a synapsin-dependent reserve pool. J Neurosci. 2006;26(12):3206–9. 10.1523/jneurosci.4901-04.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Meade CS, Conn NA, Skalski LM, Safren SA. Neurocognitive impairment and medication adherence in HIV patients with and without cocaine dependence. Journal of behavioral medicine. 2011;34(2):128–38. 10.1007/s10865-010-9293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Meade CS, Towe SL, Skalski LM, Robertson KR. Independent effects of HIV infection and cocaine dependence on neurocognitive impairment in a community sample living in the southern United States. Drug Alcohol Depend. 2015;149:128–35. 10.1016/j.drugalcdep.2015.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Byrd DA, Fellows RP, Morgello S, Franklin D, Heaton RK, Deutsch R, Atkinson JH, Clifford DB, Collier AC, Marra CM, Gelman B, McCutchan JA, Duarte NA, Simpson DM, McArthur J, Grant I, Group C. Neurocognitive impact of substance use in HIV infection. Journal of acquired immune deficiency syndromes (1999). 2011;58(2):154–62. doi: 10.1097/QAI.0b013e318229ba41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chang L, Wang GJ, Volkow ND, Ernst T, Telang F, Logan J, Fowler JS. Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse. Neuroimage. 2008;42(2):869–78. 10.1016/j.neuroimage.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lima DR, Goncalves PD, Ometto M, Malbergier A, Amaral RA, Dos Santos B, Cavallet M, Chaim-Avancini T, Serpa MH, Ferreira LRK, Duran FLS, Zanetti MV, Nicastri S, Busatto GF, Andrade AG, Cunha PJ. The role of neurocognitive functioning, substance use variables and the DSM-5 severity scale in cocaine relapse: A prospective study. Drug Alcohol Depend. 2019;197:255–61. 10.1016/j.drugalcdep.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 157.Kim SG, Lowe EL, Dixit D, Youn CS, Kim IJ, Jung JB, Rovner R, Zack JA, Vatakis DN. Cocaine-mediated impact on HIV infection in humanized BLT mice. Sci Rep. 2015;5:10010 10.1038/srep10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Roth MD, Whittaker KM, Choi R, Tashkin DP, Baldwin GC. Cocaine and sigma-1 receptors modulate HIV infection, chemokine receptors, and the HPA axis in the huPBL-SCID model. J Leukoc Biol. 2005;78(6):1198–203. 10.1189/jlb.0405219. [DOI] [PubMed] [Google Scholar]
- 159.Peterson PK, Gekker G, Chao CC, Schut R, Molitor TW, Balfour HH Jr., Cocaine potentiates HIV-1 replication in human peripheral blood mononuclear cell cocultures. Involvement of transforming growth factor-beta. J Immunol. 1991;146(1):81–4. Epub 1991/01/11. [PubMed] [Google Scholar]
- 160.Bagasra O, Pomerantz RJ. Human immunodeficiency virus type 1 replication in peripheral blood mononuclear cells in the presence of cocaine. J Infect Dis. 1993;168(5):1157–64. 10.1093/infdis/168.5.1157. [DOI] [PubMed] [Google Scholar]
- 161.Dhillon NK, Williams R, Peng F, Tsai YJ, Dhillon S, Nicolay B, Gadgil M, Kumar A, Buch SJ. Cocaine-mediated enhancement of virus replication in macrophages: implications for human immunodeficiency virus-associated dementia. J Neurovirol. 2007;13(6):483–95. 10.1080/13550280701528684. [DOI] [PubMed] [Google Scholar]
- 162.Swepson C, Ranjan A, Balasubramaniam M, Pandhare J, Dash C. Cocaine Enhances HIV-1 Transcription in Macrophages by Inducing p38 MAPK Phosphorylation. Front Microbiol. 2016;7:823 10.3389/fmicb.2016.00823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Samikkannu T, Atluri VS, Nair MP. HIV and Cocaine Impact Glial Metabolism: Energy Sensor AMP-activated protein kinase Role in Mitochondrial Biogenesis and Epigenetic Remodeling. Sci Rep. 2016;6:31784 10.1038/srep31784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gekker G, Hu S, Sheng WS, Rock RB, Lokensgard JR, Peterson PK. Cocaine-induced HIV-1 expression in microglia involves sigma-1 receptors and transforming growth factor-beta1. International immunopharmacology. 2006;6(6):1029–33. 10.1016/j.intimp.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 165.Mantri CK, Pandhare Dash J, Mantri JV, Dash CC. Cocaine enhances HIV-1 replication in CD4+ T cells by down-regulating MiR-125b. PLoS One. 2012;7(12):e51387 10.1371/journal.pone.0051387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Addai AB, Pandhare J, Paromov V, Mantri CK, Pratap S, Dash C. Cocaine modulates HIV-1 integration in primary CD4+ T cells: implications in HIV-1 pathogenesis in drug-abusing patients. J Leukoc Biol. 2015;97(4):779–90. 10.1189/jlb.4A0714-356R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kim SG, Jung JB, Dixit D, Rovner R Jr., Zack JA, Baldwin GC, Vatakis DN. Cocaine exposure enhances permissiveness of quiescent T cells to HIV infection. J Leukoc Biol. 2013;94(4):835–43. 10.1189/jlb.1112566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Napuri J, Pilakka-Kanthikeel S, Raymond A, Agudelo M, Yndart-Arias A, Saxena SK, Nair M. Cocaine enhances HIV-1 infectivity in monocyte derived dendritic cells by suppressing microRNA-155. PLoS One. 2013;8(12):e83682 10.1371/journal.pone.0083682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Prasad A, Kulkarni R, Jiang S, Groopman JE. Cocaine Enhances DC to T-cell HIV-1 Transmission by Activating DC-SIGN/LARG/LSP1 Complex and Facilitating Infectious Synapse Formation. Sci Rep. 2017;7:40648 10.1038/srep40648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fiala M, Eshleman AJ, Cashman J, Lin J, Lossinsky AS, Suarez V, Yang W, Zhang J, Popik W, Singer E, Chiappelli F, Carro E, Weinand M, Witte M, Arthos J. Cocaine increases human immunodeficiency virus type 1 neuroinvasion through remodeling brain microvascular endothelial cells. J Neurovirol. 2005;11(3):281–91. 10.1080/13550280590952835. [DOI] [PubMed] [Google Scholar]
- 171.Dhillon NK, Peng F, Bokhari S, Callen S, Shin SH, Zhu X, Kim KJ, Buch SJ. Cocaine-mediated alteration in tight junction protein expression and modulation of CCL2/CCR2 axis across the blood-brain barrier: implications for HIV-dementia. J Neuroimmune Pharmacol. 2008;3(1):52–6. 10.1007/s11481-007-9091-1. [DOI] [PubMed] [Google Scholar]
- 172.Yao H, Kim K, Duan M, Hayashi T, Guo M, Morgello S, Prat A, Wang J, Su TP, Buch S. Cocaine hijacks sigma1 receptor to initiate induction of activated leukocyte cell adhesion molecule: implication for increased monocyte adhesion and migration in the CNS. J Neurosci. 2011;31(16):5942–55. 10.1523/jneurosci.5618-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Niu F, Yao H, Zhang W, Sutliff RL, Buch S. Tat 101-mediated enhancement of brain pericyte migration involves platelet-derived growth factor subunit B homodimer: implications for human immunodeficiency virus-associated neurocognitive disorders. J Neurosci. 2014;34(35):11812–25. 10.1523/jneurosci.1139-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ysrayl BB, Balasubramaniam M, Albert I, Villalta F, Pandhare J, Dash C. A Novel Role of Prolidase in Cocaine-Mediated Breach in the Barrier of Brain Microvascular Endothelial Cells. Sci Rep. 2019;9(1):2567 10.1038/s41598-018-37495-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Louboutin JP, Agrawal L, Reyes BA, Van Bockstaele EJ, Strayer DS. HIV-1 gp120-induced injury to the blood-brain barrier: role of metalloproteinases 2 and 9 and relationship to oxidative stress. Journal of neuropathology and experimental neurology. 2010;69(8):801–16. 10.1097/NEN.0b013e3181e8c96f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ju SM, Song HY, Lee JA, Lee SJ, Choi SY, Park J. Extracellular HIV-1 Tat up-regulates expression of matrix metalloproteinase-9 via a MAPK-NF-kappaB dependent pathway in human astrocytes. Experimental & molecular medicine. 2009;41(2):86–93. 10.3858/emm.2009.41.2.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gandhi N, Saiyed ZM, Napuri J, Samikkannu T, Reddy PV, Agudelo M, Khatavkar P, Saxena SK, Nair MP. Interactive role of human immunodeficiency virus type 1 (HIV-1) clade-specific Tat protein and cocaine in blood-brain barrier dysfunction: implications for HIV-1-associated neurocognitive disorder. J Neurovirol. 2010;16(4):294–305. 10.3109/13550284.2010.499891. [DOI] [PubMed] [Google Scholar]
- 178.Latronico T, Pati I, Ciavarella R, Fasano A, Mengoni F, Lichtner M, Vullo V, Mastroianni CM, Liuzzi GM. In vitro effect of antiretroviral drugs on cultured primary astrocytes: analysis of neurotoxicity and matrix metalloproteinase inhibition. J Neurochem. 2018;144(3):271–84. 10.1111/jnc.14269. [DOI] [PubMed] [Google Scholar]
- 179.Yao H, Yang Y, Kim KJ, Bethel-Brown C, Gong N, Funa K, Gendelman HE, Su TP, Wang JQ, Buch S. Molecular mechanisms involving sigma receptor-mediated induction of MCP-1: implication for increased monocyte transmigration. Blood. 2010;115(23):4951–62. 10.1182/blood-2010-01-266221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fiala M, Gan XH, Zhang L, House SD, Newton T, Graves MC, Shapshak P, Stins M, Kim KS, Witte M, Chang SL. Cocaine enhances monocyte migration across the blood-brain barrier. Cocaine’s connection to AIDS dementia and vasculitis? Advances in experimental medicine and biology. 1998;437:199–205. Epub 1998/07/17. [DOI] [PubMed] [Google Scholar]
- 181.Gan X, Zhang L, Berger O, Stins MF, Way D, Taub DD, Chang SL, Kim KS, House SD, Weinand M, Witte M, Graves MC, Fiala M. Cocaine enhances brain endothelial adhesion molecules and leukocyte migration. Clinical immunology (Orlando, Fla). 1999;91(1):68–76. 10.1006/clim.1998.4683. [DOI] [PubMed] [Google Scholar]
- 182.Lewitus GM, Konefal SC, Greenhalgh AD, Pribiag H, Augereau K, Stellwagen D. Microglial TNF-alpha Suppresses Cocaine-Induced Plasticity and Behavioral Sensitization. Neuron. 2016;90(3):483–91. 10.1016/j.neuron.2016.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.O’Carroll SJ, Kho DT, Wiltshire R, Nelson V, Rotimi O, Johnson R, Angel CE, Graham ES. Proinflammatory TNFα and IL-1β differentially regulate the inflammatory phenotype of brain microvascular endothelial cells. Journal of Neuroinflammation. 2015;12(1):131. doi: 10.1186/s12974-015-0346-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bethel-Brown C, Yao H, Callen S, Lee YH, Dash PK, Kumar A, Buch S. HIV-1 Tat-mediated induction of platelet-derived growth factor in astrocytes: role of early growth response gene 1. Journal of immunology (Baltimore, Md : 1950). 2011;186(7):4119–29. 10.4049/jimmunol.1002235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Weiss JM, Nath A, Major EO, Berman JW. HIV-1 Tat induces monocyte chemoattractant protein-1-mediated monocyte transmigration across a model of the human blood-brain barrier and up-regulates CCR5 expression on human monocytes. J Immunol. 1999;163(5):2953–9. Epub 1999/08/24. [PubMed] [Google Scholar]
- 186.Niu F, Liao K, Hu G, Sil S, Callen S, Guo ML, Yang L, Buch S. Cocaine-induced release of CXCL10 from pericytes regulates monocyte transmigration into the CNS. The Journal of cell biology. 2019;218(2):700–21. 10.1083/jcb.201712011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yuan L, Liu A, Qiao L, Sheng B, Xu M, Li W, Chen D. The relationship of CSF and plasma cytokine levels in HIV infected patients with neurocognitive impairment. Biomed Res Int. 2015;2015:506872-. 10.1155/2015/506872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lopez OV, Gorantla S, Segarra AC, Andino Norat MC, Alvarez M, Skolasky RL, Melendez LM. Sigma-1 Receptor Antagonist (BD1047) Decreases Cathepsin B Secretion in HIV-Infected Macrophages Exposed to Cocaine. J Neuroimmune Pharmacol. 2019;14(2):226–40. 10.1007/s11481-018-9807-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Cantres-Rosario Y, Plaud-Valentín M, Gerena Y, Skolasky RL, Wojna V, Meléndez LM. Cathepsin B and cystatin B in HIV-seropositive women are associated with infection and HIV-1-associated neurocognitive disorders. AIDS (London, England). 2013;27(3):347–56. doi: 10.1097/QAD.0b013e32835b3e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Guo ML, Liao K, Periyasamy P, Yang L, Cai Y, Callen SE, Buch S. Cocaine-mediated microglial activation involves the ER stress-autophagy axis. Autophagy. 2015;11(7):995–1009. 10.1080/15548627.2015.1052205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Liao K, Guo M, Niu F, Yang L, Callen SE, Buch S. Cocaine-mediated induction of microglial activation involves the ER stress-TLR2 axis. J Neuroinflammation. 2016;13:33 10.1186/s12974-016-0501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Thangaraj A, Periyasamy P, Guo ML, Chivero ET, Callen S, Buch S. Mitigation of cocaine-mediated mitochondrial damage, defective mitophagy and microglial activation by superoxide dismutase mimetics. Autophagy. 2019:1–24. 10.1080/15548627.2019.1607686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Atluri VS, Pilakka-Kanthikeel S, Garcia G, Jayant RD, Sagar V, Samikkannu T, Yndart A, Nair M. Effect of Cocaine on HIV Infection and Inflammasome Gene Expression Profile in HIV Infected Macrophages. Sci Rep. 2016;6:27864 10.1038/srep27864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yang L, Yao H, Chen X, Cai Y, Callen S, Buch S. Role of Sigma Receptor in Cocaine-Mediated Induction of Glial Fibrillary Acidic Protein: Implications for HAND. Mol Neurobiol. 2016;53(2):1329–42. 10.1007/s12035-015-9094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Fattore L, Puddu MC, Picciau S, Cappai A, Fratta W, Serra GP, Spiga S. Astroglial in vivo response to cocaine in mouse dentate gyrus: a quantitative and qualitative analysis by confocal microscopy. Neuroscience. 2002;110(1):1–6. Epub 2002/03/08. [DOI] [PubMed] [Google Scholar]
- 196.Periyasamy P, Guo ML, Buch S. Cocaine induces astrocytosis through ER stress-mediated activation of autophagy. Autophagy. 2016;12(8):1310–29. 10.1080/15548627.2016.1183844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Fan Y, Zou W, Green LA, Kim BO, He JJ. Activation of Egr-1 expression in astrocytes by HIV-1 Tat: new insights into astrocyte-mediated Tat neurotoxicity. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2011;6(1):121–9. 10.1007/s11481-010-9217-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yang Y, Yao H, Lu Y, Wang C, Buch S. Cocaine potentiates astrocyte toxicity mediated by human immunodeficiency virus (HIV-1) protein gp120. PLoS One. 2010;5(10):e13427 10.1371/journal.pone.0013427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Natarajaseenivasan K, Cotto B, Shanmughapriya S, Lombardi AA, Datta PK, Madesh M, Elrod JW, Khalili K, Langford D. Astrocytic metabolic switch is a novel etiology for Cocaine and HIV-1 Tat-mediated neurotoxicity. Cell Death Dis. 2018;9(4):415 10.1038/s41419-018-0422-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Paula AA, Falcão MCN, Pacheco AG. Metabolic syndrome in HIV-infected individuals: underlying mechanisms and epidemiological aspects. AIDS Research and Therapy. 2013;10(1):32. doi: 10.1186/1742-6405-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Willig AL, Overton ET. Metabolic Complications and Glucose Metabolism in HIV Infection: A Review of the Evidence. Current HIV/AIDS reports. 2016;13(5):289–96. doi: 10.1007/s11904-016-0330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Pandhare J, Addai AB, Mantri CK, Hager C, Smith RM, Barnett L, Villalta F, Kalams SA, Dash C. Cocaine enhances HIV-1-induced CD4(+) T-cell apoptosis: implications in disease progression in cocaine-abusing HIV-1 patients. Am J Pathol. 2014;184(4):927–36. 10.1016/j.ajpath.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.El-Bassel N, Shaw SA, Dasgupta A, Strathdee SA. Drug use as a driver of HIV risks: re-emerging and emerging issues. Current opinion in HIV and AIDS. 2014;9(2):150–5. 10.1097/coh.0000000000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Mintzer MZ, Copersino ML, Stitzer ML. Opioid abuse and cognitive performance. Drug Alcohol Depend. 2005;78(2):225–30. 10.1016/j.drugalcdep.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 205.Fernandez-Serrano MJ, Perez-Garcia M, Perales JC, Verdejo-Garcia A. Prevalence of executive dysfunction in cocaine, heroin and alcohol users enrolled in therapeutic communities. Eur J Pharmacol. 2010;626(1):104–12. 10.1016/j.ejphar.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 206.Chuang LF, Killam KF Jr., Chuang RY. Increased replication of simian immunodeficiency virus in CEM x174 cells by morphine sulfate. Biochem Biophys Res Commun. 1993;195(3):1165–73. 10.1006/bbrc.1993.2167. [DOI] [PubMed] [Google Scholar]
- 207.Donahoe RM, O’Neil SP, Marsteller FA, Novembre FJ, Anderson DC, Lankford-Turner P, McClure HH. Probable deceleration of progression of Simian AIDS affected by opiate dependency: studies with a rhesus macaque/SIVsmm9 model. J Acquir Immune Defic Syndr. 2009;50(3):241–9. 10.1097/QAI.0b013e3181967354. [DOI] [PubMed] [Google Scholar]
- 208.Donahoe RM. Multiple ways that drug abuse might influence AIDS progression: clues from a monkey model. J Neuroimmunol. 2004;147(1–2):28–32. Epub 2004/01/27. [DOI] [PubMed] [Google Scholar]
- 209.Hauser KF, Fitting S, Dever SM, Podhaizer EM, Knapp PE. Opiate drug use and the pathophysiology of neuroAIDS. Current HIV research. 2012;10(5):435–52. Epub 2012/05/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kumar R, Orsoni S, Norman L, Verma AS, Tirado G, Giavedoni LD, Staprans S, Miller GM, Buch SJ, Kumar A. Chronic morphine exposure causes pronounced virus replication in cerebral compartment and accelerated onset of AIDS in SIV/SHIV-infected Indian rhesus macaques. Virology. 2006;354(1):192–206. 10.1016/j.virol.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 211.Bokhari SM, Hegde R, Callen S, Yao H, Adany I, Li Q, Li Z, Pinson D, Yeh HW, Cheney PD, Buch S. Morphine potentiates neuropathogenesis of SIV infection in rhesus macaques. J Neuroimmune Pharmacol. 2011;6(4):626–39. 10.1007/s11481-011-9272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Liao Y, Jiang J, Liang B, Wei F, Huang J, Pan P, Su J, Zhou B, Zang N, Ye L, Liang H. Opiate use inhibits TLR9 signaling pathway in vivo: possible role in pathogenesis of HIV-1 infection. Scientific reports. 2017;7(1):13071-. doi: 10.1038/s41598-017-12066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Masvekar RR, El-Hage N, Hauser KF, Knapp PE. Morphine enhances HIV-1SF162-mediated neuron death and delays recovery of injured neurites. PLoS One. 2014;9(6):e100196 10.1371/journal.pone.0100196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Suzuki M, El-Hage N, Zou S, Hahn YK, Sorrell ME, Sturgill JL, Conrad DH, Knapp PE, Hauser KF. Fractalkine/CX3CL1 protects striatal neurons from synergistic morphine and HIV-1 Tat-induced dendritic losses and death. Molecular neurodegeneration. 2011;6:78 10.1186/1750-1326-6-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zou S, Fitting S, Hahn YK, Welch SP, El-Hage N, Hauser KF, Knapp PE. Morphine potentiates neurodegenerative effects of HIV-1 Tat through actions at mu-opioid receptor-expressing glia. Brain : a journal of neurology. 2011;134(Pt 12):3616–31. 10.1093/brain/awr281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Steele AD, Henderson EE, Rogers TJ. Mu-opioid modulation of HIV-1 coreceptor expression and HIV-1 replication. Virology. 2003;309(1):99–107. Epub 2003/05/03. [DOI] [PubMed] [Google Scholar]
- 217.Prottengeier J, Koutsilieri E, Scheller C. The effects of opioids on HIV reactivation in latently-infected T-lymphoblasts. AIDS Res Ther. 2014;11:17 10.1186/1742-6405-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Liang B, Jiang J, Pan P, Chen R, Zhuang D, Zhao F, Chen H, Huang J, Su Q, Cao C, Li J, Liang H, Ye L. Morphine Increases Lamivudine- and Nevirapine-Induced Human Immunodeficiency Virus-1 Drug-Resistant Mutations In Vitro. Microbial drug resistance (Larchmont, NY). 2017;23(3):285–93. 10.1089/mdr.2015.0347. [DOI] [PubMed] [Google Scholar]
- 219.Wang X, Tan N, Douglas SD, Zhang T, Wang YJ, Ho WZ. Morphine inhibits CD8+ T cell-mediated, noncytolytic, anti-HIV activity in latently infected immune cells. J Leukoc Biol. 2005;78(3):772–6. 10.1189/jlb.0305167. [DOI] [PubMed] [Google Scholar]
- 220.Reynolds JL, Law WC, Mahajan SD, Aalinkeel R, Nair B, Sykes DE, Mammen MJ, Yong KT, Hui R, Prasad PN, Schwartz SA. Morphine and galectin-1 modulate HIV-1 infection of human monocyte-derived macrophages. J Immunol. 2012;188(8):3757–65. 10.4049/jimmunol.1102276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wang Y, Wang X, Ye L, Li J, Song L, Fulambarkar N, Ho W. Morphine suppresses IFN signaling pathway and enhances AIDS virus infection. PLoS One. 2012;7(2):e31167 10.1371/journal.pone.0031167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Guo CJ, Li Y, Tian S, Wang X, Douglas SD, Ho WZ. Morphine enhances HIV infection of human blood mononuclear phagocytes through modulation of beta-chemokines and CCR5 receptor. Journal of investigative medicine : the official publication of the American Federation for Clinical Research. 2002;50(6):435–42. 10.1136/jim-50-06-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Li Y, Merrill JD, Mooney K, Song L, Wang X, Guo CJ, Savani RC, Metzger DS, Douglas SD, Ho WZ. Morphine enhances HIV infection of neonatal macrophages. Pediatric research. 2003;54(2):282–8. 10.1203/01.Pdr.0000074973.83826.4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Bokhari SM, Yao H, Bethel-Brown C, Fuwang P, Williams R, Dhillon NK, Hegde R, Kumar A, Buch SJ. Morphine enhances Tat-induced activation in murine microglia. J Neurovirol. 2009;15(3):219–28. 10.1080/13550280902913628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wang X, Ma T-C, Li J-L, Zhou Y, Geller EB, Adler MW, Peng J-S, Zhou W, Zhou D-J, Ho W-Z. Heroin inhibits HIV-restriction miRNAs and enhances HIV infection of macrophages. Frontiers in microbiology. 2015;6:1230-. doi: 10.3389/fmicb.2015.01230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Dave RS. Morphine affects HIV-induced inflammatory response without influencing viral replication in human monocyte-derived macrophages. FEMS immunology and medical microbiology. 2012;64(2):228–36. 10.1111/j.1574-695X.2011.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sharma HS, Ali SF. Alterations in blood-brain barrier function by morphine and methamphetamine. Ann N Y Acad Sci. 2006;1074:198–224. 10.1196/annals.1369.020. [DOI] [PubMed] [Google Scholar]
- 228.Strazza M, Pirrone V, Wigdahl B, Dampier W, Lin W, Feng R, Maubert ME, Weksler B, Romero IA, Couraud P-O, Nonnemacher MR. Prolonged Morphine Exposure Induces Increased Firm Adhesion in an in Vitro Model of the Blood-Brain Barrier. International journal of molecular sciences. 2016;17(6):916. doi: 10.3390/ijms17060916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wen H, Lu Y, Yao H, Buch S. Morphine induces expression of platelet-derived growth factor in human brain microvascular endothelial cells: implication for vascular permeability. PLoS One. 2011;6(6):e21707 10.1371/journal.pone.0021707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Mahajan SD, Aalinkeel R, Sykes DE, Reynolds JL, Bindukumar B, Fernandez SF, Chawda R, Shanahan TC, Schwartz SA. Tight junction regulation by morphine and HIV-1 tat modulates blood-brain barrier permeability. J Clin Immunol. 2008;28(5):528–41. 10.1007/s10875-008-9208-1. [DOI] [PubMed] [Google Scholar]
- 231.Leibrand CR, Paris JJ, Jones AM, Masuda QN, Halquist MS, Kim WK, Knapp PE, Kashuba ADM, Hauser KF, McRae M. HIV-1 Tat and opioids act independently to limit antiretroviral brain concentrations and reduce blood-brain barrier integrity. J Neurovirol. 2019. 10.1007/s13365-019-00757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.El-Hage N, Wu G, Wang J, Ambati J, Knapp PE, Reed JL, Bruce-Keller AJ, Hauser KF. HIV-1 Tat and opiate-induced changes in astrocytes promote chemotaxis of microglia through the expression of MCP-1 and alternative chemokines. Glia. 2006;53(2):132–46. 10.1002/glia.20262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Hollenbach R, Sagar D, Khan ZK, Callen S, Yao H, Shirazi J, Buch S, Jain P. Effect of morphine and SIV on dendritic cell trafficking into the central nervous system of rhesus macaques. J Neurovirol. 2014;20(2):175–83. 10.1007/s13365-013-0182-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Olin M, Oh S, Roy S, Peterson P, Molitor T. Morphine induces splenocyte trafficking into the CNS. J Neuroimmune Pharmacol. 2012;7(2):436–43. 10.1007/s11481-011-9307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Dutta R, Roy S. Chronic morphine and HIV-1 Tat promote differential central nervous system trafficking of CD3+ and Ly6C+ immune cells in a murine Streptococcus pneumoniae infection model. J Neuroinflammation. 2015;12:120 10.1186/s12974-015-0341-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Dutta R, Krishnan A, Meng J, Das S, Ma J, Banerjee S, Wang J, Charboneau R, Prakash O, Barke RA, Roy S. Morphine modulation of toll-like receptors in microglial cells potentiates neuropathogenesis in a HIV-1 model of coinfection with pneumococcal pneumoniae. J Neurosci. 2012;32(29):9917–30. 10.1523/jneurosci.0870-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Rock RB, Hu S, Sheng WS, Peterson PK. Morphine stimulates CCL2 production by human neurons. J Neuroinflammation. 2006;3:32 10.1186/1742-2094-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.El-Hage N, Rodriguez M, Dever SM, Masvekar RR, Gewirtz DA, Shacka JJ. HIV-1 and morphine regulation of autophagy in microglia: limited interactions in the context of HIV-1 infection and opioid abuse. Journal of virology. 2015;89(2):1024–35. 10.1128/jvi.02022-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Rodriguez M, Lapierre J, Ojha CR, Estrada-Bueno H, Dever SM, Gewirtz DA, Kashanchi F, El-Hage N. Importance of Autophagy in Mediating Human Immunodeficiency Virus (HIV) and Morphine-Induced Metabolic Dysfunction and Inflammation in Human Astrocytes. Viruses. 2017;9(8). 10.3390/v9080201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Avdoshina V, Biggio F, Palchik G, Campbell LA, Mocchetti I. Morphine induces the release of CCL5 from astrocytes: potential neuroprotective mechanism against the HIV protein gp120. Glia. 2010;58(13):1630–9. 10.1002/glia.21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Mahajan SD, Schwartz SA, Aalinkeel R, Chawda RP, Sykes DE, Nair MP. Morphine modulates chemokine gene regulation in normal human astrocytes. Clinical immunology (Orlando, Fla). 2005;115(3):323–32. 10.1016/j.clim.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 242.El-Hage N, Gurwell JA, Singh IN, Knapp PE, Nath A, Hauser KF. Synergistic increases in intracellular Ca2+, and the release of MCP-1, RANTES, and IL-6 by astrocytes treated with opiates and HIV-1 Tat. Glia. 2005;50(2):91–106. 10.1002/glia.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85(14):5274–8. 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci U S A. 1995;92(26):12304–8. 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F, Baler R. Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain’s control circuit. BioEssays : news and reviews in molecular, cellular and developmental biology. 2010;32(9):748–55. 10.1002/bies.201000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Volkow ND, Morales M. The Brain on Drugs: From Reward to Addiction. Cell. 2015;162(4):712–25. 10.1016/j.cell.2015.07.046. [DOI] [PubMed] [Google Scholar]
- 247.Solinas M, Belujon P, Fernagut PO, Jaber M, Thiriet N. Dopamine and addiction: what have we learned from 40 years of research. Journal of neural transmission (Vienna, Austria : 1996). 2019;126(4):481–516. 10.1007/s00702-018-1957-2. [DOI] [PubMed] [Google Scholar]
- 248.Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. 2011;69(4):628–49. 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Pedrosa R, Soares-da-Silva P. Oxidative and non-oxidative mechanisms of neuronal cell death and apoptosis by L-3,4-dihydroxyphenylalanine (L-DOPA) and dopamine. British journal of pharmacology. 2002;137(8):1305–13. 10.1038/sj.bjp.0704982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Mor DE, Daniels MJ, Ischiropoulos H. The usual suspects, dopamine and alpha-synuclein, conspire to cause neurodegeneration. Movement disorders : official journal of the Movement Disorder Society. 2019;34(2):167–79. 10.1002/mds.27607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Zhu J, Mactutus CF, Wallace DR, Booze RM. HIV-1 Tat protein-induced rapid and reversible decrease in [3H]dopamine uptake: dissociation of [3H]dopamine uptake and [3H]2beta-carbomethoxy-3-beta-(4-fluorophenyl)tropane (WIN 35,428) binding in rat striatal synaptosomes. The Journal of pharmacology and experimental therapeutics. 2009;329(3):1071–83. 10.1124/jpet.108.150144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Quizon PM, Sun WL, Yuan Y, Midde NM, Zhan CG, Zhu J. Molecular mechanism: the human dopamine transporter histidine 547 regulates basal and HIV-1 Tat protein-inhibited dopamine transport. Sci Rep. 2016;6:39048 10.1038/srep39048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Scheller C, Arendt G, Nolting T, Antke C, Sopper S, Maschke M, Obermann M, Angerer A, Husstedt IW, Meisner F, Neuen-Jacob E, Muller HW, Carey P, Ter Meulen V, Riederer P, Koutsilieri E. Increased dopaminergic neurotransmission in therapy-naive asymptomatic HIV patients is not associated with adaptive changes at the dopaminergic synapses. Journal of neural transmission (Vienna, Austria : 1996). 2010;117(6):699–705. 10.1007/s00702-010-0415-6. [DOI] [PubMed] [Google Scholar]
- 254.Sardar AM, Czudek C, Reynolds GP. Dopamine deficits in the brain: the neurochemical basis of parkinsonian symptoms in AIDS. Neuroreport. 1996;7(4):910–2. Epub 1996/03/22. [DOI] [PubMed] [Google Scholar]
- 255.Kumar AM, Fernandez JB, Singer EJ, Commins D, Waldrop-Valverde D, Ownby RL, Kumar M. Human immunodeficiency virus type 1 in the central nervous system leads to decreased dopamine in different regions of postmortem human brains. J Neurovirol. 2009;15(3):257–74. 10.1080/13550280902973952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Luo Y, Roth GS. The roles of dopamine oxidative stress and dopamine receptor signaling in aging and age-related neurodegeneration. Antioxid Redox Signal. 2000;2(3):449–60. 10.1089/15230860050192224. [DOI] [PubMed] [Google Scholar]
- 257.Kumar U, Patel SC. Immunohistochemical localization of dopamine receptor subtypes (D1R-D5R) in Alzheimer’s disease brain. Brain Res. 2007;1131(1):187–96. 10.1016/j.brainres.2006.10.049. [DOI] [PubMed] [Google Scholar]
- 258.Reyes MG, Faraldi F, Senseng CS, Flowers C, Fariello R. Nigral degeneration in acquired immune deficiency syndrome (AIDS). Acta neuropathologica. 1991;82(1):39–44. [DOI] [PubMed] [Google Scholar]
- 259.Hestad K, McArthur JH, Dal Pan GJ, Selnes OA, Nance-Sproson TE, Aylward E, Mathews VP, McArthur JC. Regional brain atrophy in HIV-1 infection: association with specific neuropsychological test performance. Acta neurologica Scandinavica. 1993;88(2):112–8. Epub 1993/08/01. [DOI] [PubMed] [Google Scholar]
- 260.Aylward EH, Henderer JD, McArthur JC, Brettschneider PD, Harris GJ, Barta PE, Pearlson GD. Reduced basal ganglia volume in HIV-1-associated dementia: results from quantitative neuroimaging. Neurology. 1993;43(10):2099–104. 10.1212/wnl.43.10.2099. [DOI] [PubMed] [Google Scholar]
- 261.Marcario JK, Manaye KF, SantaCruz KS, Mouton PR, Berman NE, Cheney PD. Severe subcortical degeneration in macaques infected with neurovirulent simian immunodeficiency virus. J Neurovirol. 2004;10(6):387–99. 10.1080/13550280490521131. [DOI] [PubMed] [Google Scholar]
- 262.Gelman BB, Spencer JA, Holzer CE 3rd, Soukup VM. Abnormal striatal dopaminergic synapses in National NeuroAIDS Tissue Consortium subjects with HIV encephalitis. J Neuroimmune Pharmacol. 2006;1(4):410–20. 10.1007/s11481-006-9030-6. [DOI] [PubMed] [Google Scholar]
- 263.Anthony IC, Bell JE. The Neuropathology of HIV/AIDS. International review of psychiatry (Abingdon, England). 2008;20(1):15–24. 10.1080/09540260701862037. [DOI] [PubMed] [Google Scholar]
- 264.Bryant AK, Moore DJ, Burdo TH, Lakritz JR, Gouaux B, Soontornniyomkij V, Achim CL, Masliah E, Grant I, Levine AJ, Ellis RJ. Plasma soluble CD163 is associated with postmortem brain pathology in human immunodeficiency virus infection. Aids. 2017;31(7):973–9. 10.1097/qad.0000000000001425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Gongvatana A, Harezlak J, Buchthal S, Daar E, Schifitto G, Campbell T, Taylor M, Singer E, Algers J, Zhong J, Brown M, McMahon D, So YT, Mi D, Heaton R, Robertson K, Yiannoutsos C, Cohen RA, Navia B. Progressive cerebral injury in the setting of chronic HIV infection and antiretroviral therapy. J Neurovirol. 2013;19(3):209–18. 10.1007/s13365-013-0162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Su T, Caan MW, Wit FW, Schouten J, Geurtsen GJ, Cole JH, Sharp DJ, Vos FM, Prins M, Portegies P, Reiss P, Majoie CB. White matter structure alterations in HIV-1-infected men with sustained suppression of viraemia on treatment. Aids. 2016;30(2):311–22. 10.1097/qad.0000000000000945. [DOI] [PubMed] [Google Scholar]
- 267.Jernigan TL, Archibald SL, Fennema-Notestine C, Taylor MJ, Theilmann RJ, Julaton MD, Notestine RJ, Wolfson T, Letendre SL, Ellis RJ, Heaton RK, Gamst AC, Franklin DR Jr., Clifford DB, Collier AC, Gelman BB, Marra C, McArthur JC, McCutchan JA, Morgello S, Simpson DM, Grant I. Clinical factors related to brain structure in HIV: the CHARTER study. J Neurovirol. 2011;17(3):248–57. 10.1007/s13365-011-0032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Alakkas A, Ellis RJ, Watson CW, Umlauf A, Heaton RK, Letendre S, Collier A, Marra C, Clifford DB, Gelman B, Sacktor N, Morgello S, Simpson D, McCutchan JA, Kallianpur A, Gianella S, Marcotte T, Grant I, Fennema-Notestine C. White matter damage, neuroinflammation, and neuronal integrity in HAND. J Neurovirol. 2019;25(1):32–41. 10.1007/s13365-018-0682-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Harezlak J, Buchthal S, Taylor M, Schifitto G, Zhong J, Daar E, Alger J, Singer E, Campbell T, Yiannoutsos C, Cohen R, Navia B, Consortium HIVN. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS. 2011;25(5):625–33. 10.1097/QAD.0b013e3283427da7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Veenstra M, Byrd DA, Inglese M, Buyukturkoglu K, Williams DW, Fleysher L, Li M, Gama L, Leon-Rivera R, Calderon TM, Clements JE, Morgello S, Berman JW. CCR2 on Peripheral Blood CD14(+)CD16(+) Monocytes Correlates with Neuronal Damage, HIV-Associated Neurocognitive Disorders, and Peripheral HIV DNA: reseeding of CNS reservoirs? J Neuroimmune Pharmacol. 2019;14(1):120–33. 10.1007/s11481-018-9792-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Czub S, Czub M, Koutsilieri E, Sopper S, Villinger F, Muller JG, Stahl-Hennig C, Riederer P, Ter Meulen V, Gosztonyi G. Modulation of simian immunodeficiency virus neuropathology by dopaminergic drugs. Acta neuropathologica. 2004;107(3):216–26. 10.1007/s00401-003-0801-3. [DOI] [PubMed] [Google Scholar]
- 272.Czub S, Koutsilieri E, Sopper S, Czub M, Stahl-Hennig C, Muller JG, Pedersen V, Gsell W, Heeney JL, Gerlach M, Gosztonyi G, Riederer P, ter Meulen V. Enhancement of central nervous system pathology in early simian immunodeficiency virus infection by dopaminergic drugs. Acta neuropathologica. 2001;101(2):85–91. Epub 2001/03/29. [DOI] [PubMed] [Google Scholar]
- 273.Gelman BB, Lisinicchia JG, Chen T, Johnson KM, Jennings K, Freeman DH Jr., Soukup VM. Prefrontal dopaminergic and enkephalinergic synaptic accommodation in HIV-associated neurocognitive disorders and encephalitis. J Neuroimmune Pharmacol. 2012;7(3):686–700. 10.1007/s11481-012-9345-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Obermann M, Kuper M, Kastrup O, Yaldizli O, Esser S, Thiermann J, Koutsilieri E, Arendt G, Diener HC, Maschke M. Substantia nigra hyperechogenicity and CSF dopamine depletion in HIV. Journal of neurology. 2009;256(6):948–53. 10.1007/s00415-009-5052-3. [DOI] [PubMed] [Google Scholar]
- 275.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35(1):217–38. 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Kim BO, Liu Y, Ruan Y, Xu ZC, Schantz L, He JJ. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am J Pathol. 2003;162(5):1693–707. 10.1016/s0002-9440(10)64304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Kesby JP, Markou A, Semenova S. The effects of HIV-1 regulatory TAT protein expression on brain reward function, response to psychostimulants and delay-dependent memory in mice. Neuropharmacology. 2016;109:205–15. 10.1016/j.neuropharm.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Kesby JP, Najera JA, Romoli B, Fang Y, Basova L, Birmingham A, Marcondes MCG, Dulcis D, Semenova S. HIV-1 TAT protein enhances sensitization to methamphetamine by affecting dopaminergic function. Brain Behav Immun. 2017;65:210–21. 10.1016/j.bbi.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Dahal S, Chitti SV, Nair MP, Saxena SK. Interactive effects of cocaine on HIV infection: implication in HIV-associated neurocognitive disorder and neuroAIDS. Front Microbiol. 2015;6:931 10.3389/fmicb.2015.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Coley JS, Calderon TM, Gaskill PJ, Eugenin EA, Berman JW. Dopamine increases CD14+CD16+ monocyte migration and adhesion in the context of substance abuse and HIV neuropathogenesis. PLoS One. 2015;10(2):e0117450 10.1371/journal.pone.0117450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Shiramizu B, Gartner S, Williams A, Shikuma C, Ratto-Kim S, Watters M, Aguon J, Valcour V. Circulating proviral HIV DNA and HIV-associated dementia. Aids. 2005;19(1):45–52. Epub 2005/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Williams K, Westmoreland S, Greco J, Ratai E, Lentz M, Kim WK, Fuller RA, Kim JP, Autissier P, Sehgal PK, Schinazi RF, Bischofberger N, Piatak M, Lifson JD, Masliah E, Gonzalez RG. Magnetic resonance spectroscopy reveals that activated monocytes contribute to neuronal injury in SIV neuroAIDS. J Clin Invest. 2005;115(9):2534–45. 10.1172/jci22953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Jaworowski A, Kamwendo DD, Ellery P, Sonza S, Mwapasa V, Tadesse E, Molyneux ME, Rogerson SJ, Meshnick SR, Crowe SM. CD16+ monocyte subset preferentially harbors HIV-1 and is expanded in pregnant Malawian women with Plasmodium falciparum malaria and HIV-1 infection. J Infect Dis. 2007;196(1):38–42. 10.1086/518443. [DOI] [PubMed] [Google Scholar]
- 284.Gaskill PJ, Calderon TM, Luers AJ, Eugenin EA, Javitch JA, Berman JW. Human immunodeficiency virus (HIV) infection of human macrophages is increased by dopamine: a bridge between HIV-associated neurologic disorders and drug abuse. Am J Pathol. 2009;175(3):1148–59. 10.2353/ajpath.2009.081067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Gaskill PJ, Yano HH, Kalpana GV, Javitch JA, Berman JW. Dopamine receptor activation increases HIV entry into primary human macrophages. PLoS One. 2014;9(9):e108232 10.1371/journal.pone.0108232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Basova L, Najera JA, Bortell N, Wang D, Moya R, Lindsey A, Semenova S, Ellis RJ, Marcondes MCG. Dopamine and its receptors play a role in the modulation of CCR5 expression in innate immune cells following exposure to Methamphetamine: Implications to HIV infection. PLoS One. 2018;13(6):e0199861 10.1371/journal.pone.0199861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Ziegler-Heitbrock HW, Fingerle G, Strobel M, Schraut W, Stelter F, Schutt C, Passlick B, Pforte A. The novel subset of CD14+/CD16+ blood monocytes exhibits features of tissue macrophages. European journal of immunology. 1993;23(9):2053–8. 10.1002/eji.1830230902. [DOI] [PubMed] [Google Scholar]
- 288.Nockher WA, Bergmann L, Scherberich JE. Increased soluble CD14 serum levels and altered CD14 expression of peripheral blood monocytes in HIV-infected patients. Clinical and experimental immunology. 1994;98(3):369–74. 10.1111/j.1365-2249.1994.tb05499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Pulliam L, Gascon R, Stubblebine M, McGuire D, McGrath MS. Unique monocyte subset in patients with AIDS dementia. Lancet (London, England). 1997;349(9053):692–5. 10.1016/s0140-6736(96)10178-1. [DOI] [PubMed] [Google Scholar]
- 290.Buckner CM, Calderon TM, Willams DW, Belbin TJ, Berman JW. Characterization of monocyte maturation/differentiation that facilitates their transmigration across the blood-brain barrier and infection by HIV: implications for NeuroAIDS. Cell Immunol. 2011;267(2):109–23. 10.1016/j.cellimm.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Eugenin EA, Berman JW. Chemokine-dependent mechanisms of leukocyte trafficking across a model of the blood-brain barrier. Methods (San Diego, Calif). 2003;29(4):351–61. Epub 2003/05/03. [DOI] [PubMed] [Google Scholar]
- 292.Nolan RA, Muir R, Runner K, Haddad EK, Gaskill PJ. Role of Macrophage Dopamine Receptors in Mediating Cytokine Production: Implications for Neuroinflammation in the Context of HIV-Associated Neurocognitive Disorders. J Neuroimmune Pharmacol. 2019;14(1):134–56. 10.1007/s11481-018-9825-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Kawano M, Takagi R, Saika K, Matsui M, Matsushita S. Dopamine regulates cytokine secretion during innate and adaptive immune responses. International immunology. 2018;30(12):591–606. 10.1093/intimm/dxy057. [DOI] [PubMed] [Google Scholar]
- 294.Bone NB, Liu Z, Pittet JF, Zmijewski JW. Frontline Science: D1 dopaminergic receptor signaling activates the AMPK-bioenergetic pathway in macrophages and alveolar epithelial cells and reduces endotoxin-induced ALI. J Leukoc Biol. 2017;101(2):357–65. 10.1189/jlb.3HI0216-068RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Yan Y, Jiang W, Liu L, Wang X, Ding C, Tian Z, Zhou R. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell. 2015;160(1–2):62–73. 10.1016/j.cell.2014.11.047. [DOI] [PubMed] [Google Scholar]
- 296.Kawano M, Takagi R, Kaneko A, Matsushita S. Berberine is a dopamine D1- and D2-like receptor antagonist and ameliorates experimentally induced colitis by suppressing innate and adaptive immune responses. J Neuroimmunol. 2015;289:43–55. 10.1016/j.jneuroim.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 297.Wang B, Chen T, Li G, Jia Y, Wang J, Xue L, Chen Y. Dopamine Alters Lipopolysaccharide-Induced Nitric Oxide Production in Microglial Cells via Activation of D1-Like Receptors. Neurochemical research. 2019;44(4):947–58. 10.1007/s11064-019-02730-7. [DOI] [PubMed] [Google Scholar]
- 298.Fan Y, Chen Z, Pathak JL, Carneiro AMD, Chung CY. Differential Regulation of Adhesion and Phagocytosis of Resting and Activated Microglia by Dopamine. Frontiers in cellular neuroscience. 2018;12:309 10.3389/fncel.2018.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Soyka M, Limmer C, Lehnert R, Koller G, Martin G, Kufner H, Kagerer S, Haberthur A. A comparison of cognitive function in patients under maintenance treatment with heroin, methadone, or buprenorphine and healthy controls: an open pilot study. The American journal of drug and alcohol abuse. 2011;37(6):497–508. 10.3109/00952990.2011.600381. [DOI] [PubMed] [Google Scholar]
- 300.Scott TM, Rivera Mindt M, Cunningham CO, Arias F, Coulehan K, Mangalonzo A, Olsen P, Arnsten JH. Neuropsychological function is improved among opioid dependent adults who adhere to opiate agonist treatment with buprenorphine-naloxone: a preliminary study. Substance abuse treatment, prevention, and policy. 2017;12(1):48 10.1186/s13011-017-0133-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Rapeli P, Fabritius C, Kalska H, Alho H. Cognitive functioning in opioid-dependent patients treated with buprenorphine, methadone, and other psychoactive medications: stability and correlates. BMC clinical pharmacology. 2011;11:13 10.1186/1472-6904-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Carvallo L, Lopez L, Che FY, Lim J, Eugenin EA, Williams DW, Nieves E, Calderon TM, Madrid-Aliste C, Fiser A, Weiss L, Angeletti RH, Berman JW. Buprenorphine decreases the CCL2-mediated chemotactic response of monocytes. J Immunol. 2015;194(7):3246–58. 10.4049/jimmunol.1302647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Jaureguiberry-Bravo M, Lopez L, Berman JW. Frontline Science: Buprenorphine decreases CCL2-mediated migration of CD14(+) CD16(+) monocytes. J Leukoc Biol. 2018;104(6):1049–59. 10.1002/jlb.3hi0118-015r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Jayant RD, Tiwari S, Atluri V, Kaushik A, Tomitaka A, Yndart A, Colon-Perez L, Febo M, Nair M. Multifunctional Nanotherapeutics for the Treatment of neuroAIDS in Drug Abusers. Sci Rep. 2018;8(1):12991 10.1038/s41598-018-31285-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Saiyed ZM, Gandhi NH, Nair MPN. Magnetic nanoformulation of azidothymidine 5’-triphosphate for targeted delivery across the blood-brain barrier. Int J Nanomedicine. 2010;5:157–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Gong Y, Chowdhury P, Midde NM, Rahman MA, Yallapu MM, Kumar S. Novel elvitegravir nanoformulation approach to suppress the viral load in HIV-infected macrophages. Biochem Biophys Rep. 2017;12:214–9. doi: 10.1016/j.bbrep.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Campbell JH, Ratai EM, Autissier P, Nolan DJ, Tse S, Miller AD, Gonzalez RG, Salemi M, Burdo TH, Williams KC. Anti-alpha4 antibody treatment blocks virus traffic to the brain and gut early, and stabilizes CNS injury late in infection. PLoS Pathog. 2014;10(12):e1004533 10.1371/journal.ppat.1004533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Lakritz JR, Thibault DM, Robinson JA, Campbell JH, Miller AD, Williams KC, Burdo TH. alpha4-Integrin Antibody Treatment Blocks Monocyte/Macrophage Traffic to, Vascular Cell Adhesion Molecule-1 Expression in, and Pathology of the Dorsal Root Ganglia in an SIV Macaque Model of HIV-Peripheral Neuropathy. Am J Pathol. 2016;186(7):1754–61. 10.1016/j.ajpath.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.D’Antoni ML, Mitchell BI, McCurdy S, Byron MM, Ogata-Arakaki D, Chow D, Mehta NN, Boisvert WA, Lefebvre E, Shikuma CM, Ndhlovu LC, Baumer Y. Cenicriviroc inhibits trans-endothelial passage of monocytes and is associated with impaired E-selectin expression. J Leukoc Biol. 2018;104(6):1241–52. 10.1002/jlb.5a0817-328rrr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Ndhlovu LC, Umaki T, Chew GM, Chow DC, Agsalda M, Kallianpur KJ, Paul R, Zhang G, Ho E, Hanks N, Nakamoto B, Shiramizu BT, Shikuma CM. Treatment intensification with maraviroc (CCR5 antagonist) leads to declines in CD16-expressing monocytes in cART-suppressed chronic HIV-infected subjects and is associated with improvements in neurocognitive test performance: implications for HIV-associated neurocognitive disease (HAND). J Neurovirol. 2014;20(6):571–82. 10.1007/s13365-014-0279-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Gates TM, Cysique LA, Siefried KJ, Chaganti J, Moffat KJ, Brew BJ. Maraviroc-intensified combined antiretroviral therapy improves cognition in virally suppressed HIV-associated neurocognitive disorder. Aids. 2016;30(4):591–600. 10.1097/qad.0000000000000951. [DOI] [PubMed] [Google Scholar]
- 312.D’Antoni ML, Paul RH, Mitchell BI, Kohorn L, Fischer L, Lefebvre E, Seyedkazemi S, Nakamoto BK, Walker M, Kallianpur KJ, Ogata-Arakaki D, Ndhlovu LC, Shikuma C. Improved Cognitive Performance and Reduced Monocyte Activation in Virally Suppressed Chronic HIV After Dual CCR2 and CCR5 Antagonism. J Acquir Immune Defic Syndr. 2018;79(1):108–16. 10.1097/qai.0000000000001752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Zhang Y, Lv X, Bai Y, Zhu X, Wu X, Chao J, Duan M, Buch S, Chen L, Yao H. Involvement of sigma-1 receptor in astrocyte activation induced by methamphetamine via up-regulation of its own expression. J Neuroinflammation. 2015;12:29 10.1186/s12974-015-0250-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Yasui Y, Su T-P. Potential Molecular Mechanisms on the Role of the Sigma-1 Receptor in the Action of Cocaine and Methamphetamine. J Drug Alcohol Res. 2016;5:235970. doi: 10.4303/jdar/235970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Walker J, Winhusen T, Storkson JM, Lewis D, Pariza MW, Somoza E, Somoza V. Total antioxidant capacity is significantly lower in cocaine-dependent and methamphetamine-dependent patients relative to normal controls: results from a preliminary study. Human psychopharmacology. 2014;29(6):537–43. 10.1002/hup.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Cross SA, Cook DR, Chi AW, Vance PJ, Kolson LL, Wong BJ, Jordan-Sciutto KL, Kolson DL. Dimethyl fumarate, an immune modulator and inducer of the antioxidant response, suppresses HIV replication and macrophage-mediated neurotoxicity: a novel candidate for HIV neuroprotection. J Immunol. 2011;187(10):5015–25. 10.4049/jimmunol.1101868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Badisa RB, Kumar SS, Mazzio E, Haughbrook RD, Allen JR, Davidson MW, Fitch-Pye CA, Goodman CB. N-acetyl cysteine mitigates the acute effects of cocaine-induced toxicity in astroglia-like cells. PLoS One. 2015;10(1):e0114285 10.1371/journal.pone.0114285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Colombo E, Di Dario M, Capitolo E, Chaabane L, Newcombe J, Martino G, Farina C. Fingolimod may support neuroprotection via blockade of astrocyte nitric oxide. Ann Neurol. 2014;76(3):325–37. 10.1002/ana.24217. [DOI] [PubMed] [Google Scholar]