ABSTRACT
Perioperative lung injury is a major source of postoperative morbidity, excess healthcare use, and avoidable mortality. Many potential inciting factors can lead to this condition, including intraoperative ventilator induced lung injury. Questions exist as to whether protective ventilation strategies used in the intensive care unit for patients with acute respiratory distress syndrome are equally beneficial for surgical patients, most of whom do not present with any pre-existing lung pathology. Studied both individually and in combination as a package of intraoperative lung protective ventilation, the use of low tidal volumes, moderate positive end expiratory pressure, and recruitment maneuvers have been shown to improve oxygenation and pulmonary physiology and to reduce postoperative pulmonary complications in at risk patient groups. Further work is needed to define the potential contributions of alternative ventilator strategies, limiting excessive intraoperative oxygen supplementation, use of non-invasive techniques in the postoperative period, and personalized mechanical ventilation. Although the weight of evidence strongly suggests a role for lung protective ventilation in moderate risk patient groups, definitive evidence of its benefit for the general surgical population does not exist. However, given the shift in understanding of what is needed for adequate oxygenation and ventilation under anesthesia, the largely historical arguments against the use of intraoperative lung protective ventilation may soon be outdated, on the basis of its expanding track record of safety and efficacy in multiple settings.
Introduction
Roughly 230 million major surgical operations are conducted annually throughout the world.1 For many patients, postoperative recovery will unfortunately be complicated by perioperative lung injury. Between 11% and 59% of patients have a postoperative pulmonary complication (PPC), leading to a significantly higher likelihood of serious morbidity, mortality, and increased use of hospital resources.2 3 4 5 6 The most severe form of perioperative lung injury, acute respiratory distress syndrome (ARDS), has been historically reported in 0.4-3% of surgical patients at high risk, leading to a significantly greater risk of postoperative mortality.7 8 Consequently, decades of research have assessed strategies to minimize the impact of perioperative lung injury.
In the 1960s, investigators found that hyperinflation with supraphysiologic tidal volumes could reverse hypoxemia and restore pulmonary compliance during mechanical ventilation under general anesthesia.9 Partly as a result of this observation, the standard of care for intraoperative mechanical ventilation over the next 40 years largely consisted of ventilation with 10-15 mL/kg of predicted body weight (PBW).10 Later, data from landmark trials in the intensive care unit (ICU) showed that ventilation with lower tidal volumes and moderate levels of positive end expiratory pressure (PEEP) reduced mortality from ARDS.11 12 13 Although this led to a paradigm shift for mechanical ventilation in the ICU, the practice of intraoperative lung protective ventilation (IOLPV) has not yet gained wholesale acceptance in the anesthesia community. Recent observational studies have shown that higher tidal volumes and little or no PEEP are still widely prescribed.14 15 16
At the heart of the debate about the utility of IOLPV are questions about whether it is necessary for patients without pre-existing lung disease who will be exposed to only a brief period of mechanical ventilation. Furthermore, the use of PEEP and tolerance of hypercarbia are at times antithetical to the anesthesiologist’s goals of avoiding hemodynamic compromise and achieving a successful, timely postoperative extubation. Thus, legitimate concerns exist about whether a strategy used to reduce the impact of a rare condition is best suited for the perioperative population on the whole. More recently, higher quality research has helped to clarify this debate. This review will briefly describe the forms of perioperative lung injury and summarize the best available evidence on strategies to prevent it.
Sources and selection criteria
We identified studies investigating the effectiveness of perioperative lung protective ventilation through a search of PubMed (NCBI) in December 2017, updated in May 2018. The search included terms and synonyms for lung protective ventilation, positive end expiratory pressure, low tidal volumes, recruitment maneuvers, and modes of ventilation. We limited the search to studies in a surgical setting, or using general anesthesia, with specific lung injury outcomes. We included Medical Subject Headings (MeSH) when available. The full strategy is available on request. The original search returned 1446 records. We added additional references from the bibliography of select studies on the basis of relevance to the topic. We considered only articles published in English. For inclusion in this review, we prioritized prospective, randomized controlled trials (RCTs), meta-analyses of RCTs, and database studies with matching or confounder control. We excluded articles published in non-peer reviewed journals, case reports, and smaller uncontrolled studies.
Perioperative lung injury
Definitions
Perioperative lung injury can be thought of as a spectrum of disease encompassing pulmonary inflammation, impaired gas exchange, radiographic abnormalities, and respiratory failure. All have been used as surrogate endpoints to describe lung injury; however, consensus efforts have been made recently to consolidate the most clinically relevant sequelae into a composite outcome of postoperative pulmonary complications (see box 1).2 5 17 18
Box 1. Definitions of postoperative pulmonary complications and acute respiratory distress syndrome.
-
Respiratory infection
-
Antibiotic treatment for suspected pneumonia plus at least one of the following:
New/changed sputum
New/changed radiographic opacities
Temperature >38.3°C
Leukocyte count >12 000/mm3
-
-
Respiratory failure
PaO2 <60 mm Hg on room air
P:F <300
SpO2 <90% on oxygen therapy
-
Bronchospasm
Newly diagnosed wheezing treated with bronchodilators
-
Atelectasis
Radiograph showing lung opacification with a shift of anatomic structures towards the affected area and compensatory overinflation of the adjacent lung
-
Pleural effusion
Radiograph showing blunting of the costophrenic angle, loss of sharpness of the ipsilateral diaphragmatic silhouette in the upright position, displacement of the adjacent anatomical structures, or a hazy opacity with preserved vascular shadows seen in one hemithorax in the supine position
-
Pneumothorax
Air in the pleural space with no vascular bed surrounding the visceral pleura
-
Aspiration pneumonitis
Acute lung injury after inhalation of regurgitated gastric contents
-
Acute respiratory distress syndrome
Onset of findings/symptoms within 7 days of a known inciting factor
Bilateral parenchymal opacities consistent with pulmonary edema
-
Hypoxia
Mild=P:F 201-300
Moderate=P:F 101=200
Severe=P:F ≤100
PaO2=partial pressure of oxygen
P:F=ratio of PaO2 to fractional inspired oxygen concentration
SpO2=peripheral oxyhemoglobin saturation
Epidemiology
The many forms of perioperative lung injury vary widely in incidence. For example, pulmonary inflammation has been shown to occur in nearly all mechanically ventilated patients, although the degree of inflammation can be influenced by ventilator settings and anesthetic type.19 20 21 Atelectasis is also common, occurring in up to 90% of patients.22 The incidence of PPCs has been described as anywhere between 2% and 39% for major non-cardiothoracic surgery and 14-59% for thoracic surgery.2 3 23 Postoperative ARDS is rare, but it carries a nearly 50-fold increase in perioperative mortality.7 8
Pathophysiology
Atelectasis
Atelectasis develops through the compression of lung parenchyma, resorption of alveolar gas, and impaired surfactant function.24 Induction of general anesthesia and chemical paralysis alters the geometry and function of the diaphragm and chest wall, leading to increased pleural pressure and collapse of dependent lung regions.25 26 Resorption atelectasis occurs via the continued uptake of oxygen into pulmonary capillaries distal to closed airways and can be exacerbated by the administration of high concentrations of oxygen.27 Injurious mechanical ventilation practices as well as certain anesthetics can cause surfactant impairment.28 29 In addition, the sigh reflex used to mitigate atelectasis in the awake state is abolished under anesthesia.30 Atelectasis has many adverse physiologic consequences including intrapulmonary shunting, decreased compliance, increased pulmonary vascular resistance, and susceptibility to inflammatory lung injury.9 24 31 32 This susceptibility is potentially not limited solely to atelectatic lung segments, as preclinical data have shown that concurrent inflammation can occur remotely in regions with intact ventilation.33 Atelectasis can persist for as long as two days, and therefore likely contributes to development of PPCs.34
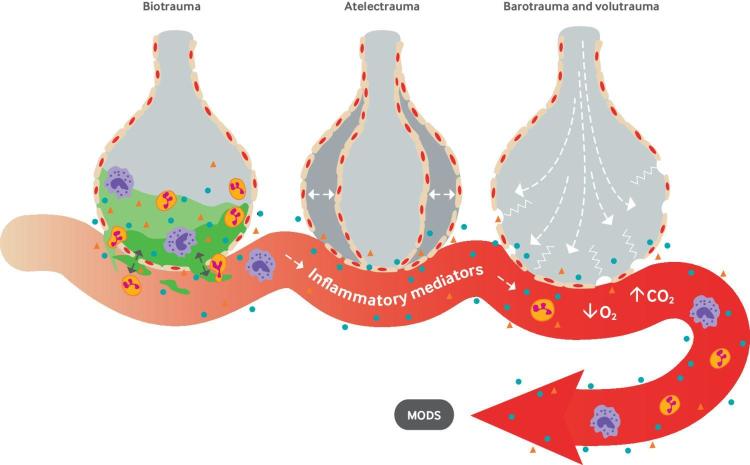
Ventilator induced lung injury
Ventilator induced lung injury occurs via volutrauma, barotrauma, atelectrauma, and biotrauma (fig 1).35 Both high tidal volumes (volutrauma) and high inspiratory pressures (barotrauma) can cause regional alveolar overdistention. Once this mechanical stress exceeds the alveolar unit’s elastic capacity, or strain, injury can occur. Subsequent fracture of the alveolar-capillary interface promotes a local inflammatory response that ultimately leads to the translocation of protein and edema into the airspace.36 During atelectrauma, repetitive and rapid alveolar opening and closure in lungs with heterogeneous regions of ventilation can damage the alveolar-capillary barrier and fragment the surrounding extracellular matrix.37 38 Biotrauma refers to inflammatory alveolar damage and the subsequent apoptotic and fibroproliferative processes that occur in response to injury, culminating in decreased pulmonary compliance, increased dead space, hypoxia, and hypercarbia.35 39 Critically, biotrauma may not be limited to the respiratory system, as the translocation of bacteria, lipopolysaccharide, and proinflammatory mediators into the systemic circulation have been postulated to contribute to multiorgan dysfunction and death.40 41
Fig 1.
Mechanisms of ventilator induced lung injury. MODS=multiple organ dysfunction syndrome
Acute respiratory distress syndrome
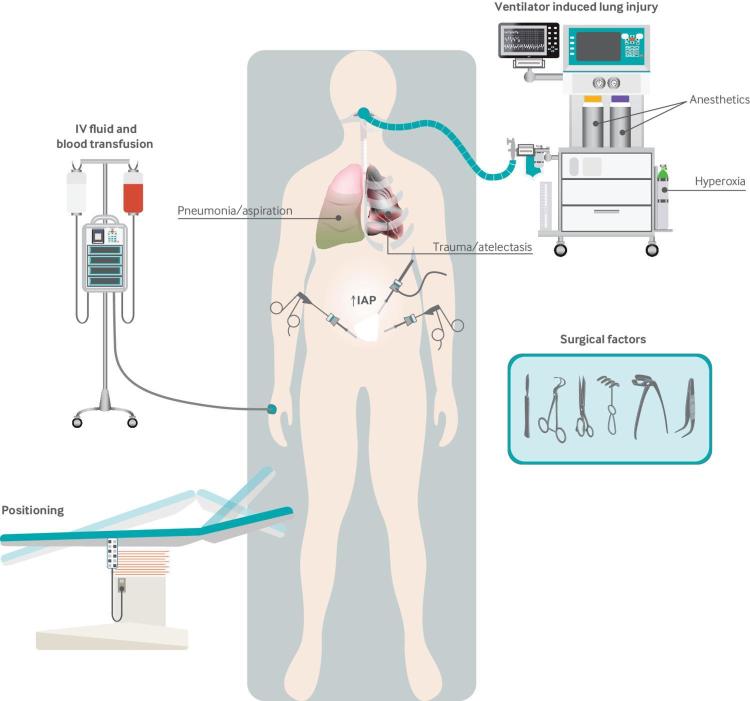
Although ARDS shares common histopathologic features with ventilator induced lung injury, it differs in that the inciting pulmonary injury is often not from mechanical ventilation itself. The salient features of ARDS are the onset of hypoxic respiratory failure within seven days of a suspected pulmonary injury and concomitant radiographic evidence of pulmonary edema.17 The Berlin definition further stratifies patients into mild, moderate, and severe categories based on the degree of hypoxemia. The pathophysiologic features of ARDS are characterized by an initial exudative phase consisting of inflammatory damage to the alveolar capillary barrier and translocation of protein rich fluid into the airspace, followed by a proliferative phase in which alveolar integrity is restored and, finally, a fibrotic phase in which the basement membrane is remodeled with fibrous protein.42 This cascade of events is incited by either direct or indirect alveolar injury, with many causes potentially occurring in the perioperative period (fig 2). Pneumonia, sepsis, and aspiration pneumonitis account for nearly 85% of cases; however, other risk factors include trauma, blood transfusion, hemorrhagic shock, burns, and inhalation injury, as well as surgical factors such as surgical trauma, retraction injury, cardiopulmonary bypass, and ischemia-reperfusion injury.42
Fig 2.
Risk factors for perioperative lung injury. IAP=intra-abdominal pressure; IV=intravenous
Evidence based strategies for perioperative lung protective ventilation
As illustrated above, the development of perioperative lung injury depends on many patient related, surgical, and anesthetic factors. Furthermore, the definition of “protective” varies considerably among studies. Accordingly, the body of literature in the field is fraught with heterogeneity and difficulties in separating what are likely synergistic treatment effects. To limit redundancy, the following sections highlight investigations comparing individual components of a protective strategy where possible, with additional attention given to studies incorporating multiple techniques (table 1).
Table 1.
Best available evidence on effect of select perioperative lung protective strategies on clinical outcomes
| Variable studied | Author, year | Study type | Population; No of patients | Intervention | Control | Results |
|---|---|---|---|---|---|---|
| Tidal volume | Sundar, 2011 | RCT | Cardiac surgery; 149 | TV 6 mL/kg; PEEP, FiO2: by table | TV 10 mL/kg; PEEP, FiO2: by table | No difference in time to extubation: median 643 (IQR 417-1032) v 450 (264-1044) min, P=0.10; higher % free from ventilation at 6 h: 37.3% v 20.3%, P=0.02; lower incidence of reintubation: 1.3% v 9.5%, P=0.03 |
| PEEP | Hemmes, 2013 | Meta-analysis | All surgery; 1669 | PEEP 3-12 cm H2O | PEEP ≤3 cm H2O | Lower incidence of postoperative lung injury (4.9% v 1.4%; RR 0.29, 95% CI 0.14 to 0.60), pulmonary infection (0.62, 0.40 to 0.96), and atelectasis (0.61, 0.41 to 0.91) |
| Hemmes, 2014 | RCT | Open abdominal surgery; 900 | TV 8 mL/kg; PEEP 12 cm H2O; RM | TV 8 mL/kg; PEEP ≤2 cm H2O; no RM | No difference in PPC incidence by POD 5 (RR 1.01, 0.86 to 1.20; P=0.86); higher incidence of intraoperative hypotension (46% v 36%; RR 1.29, 1.10 to 1.51; P=0.0016); more frequent intraoperative vasopressor use (62% v 51%; RR 1.20, 1.07 to 1.35; P=0.0016) | |
| Recruitment maneuvers | Choi, 2017 | RCT | Robotic prostatectomy; 60 | TV 6-8 mL/kg; PEEP 5 cm H2O; RM | TV 6-8 mL/kg; PEEP 5 cm H2O; no RM | Lower combined incidence of intraoperative oxygen desaturation and postoperative atelectasis: 17.8% v 43.3%, P=0.034 |
| Park, 2016 | RCT | Elective laparoscopy; 62 | TV 10 mL/kg; PEEP 0 cm H2O; RM | TV 6 mL/kg; PEEP 5 cm H2O; no RM | Higher incidence of PPCs: 47% v 14%, P=0.023 | |
| Lung protective ventilation | Futier, 2013 | RCT | Abdominal surgery; 400 | TV 6-8 mL/kg; PEEP 6-8 cm H2O; RM | TV 10-12 mL/kg; PEEP 0 cm H2O; no RM | Lower incidence of major pulmonary and non-pulmonary complications by POD 7 (10.5% v 27.5%; RR 0.40, 0.24 to 0.68; P=0.001); lower risk of needing postoperative ventilation (5.0% v 17.0%; RR 0.29, 0.14 to 0.61; P=0.001) |
| Serpa Neto, 2015 | Meta-analysis | General surgery; 2127 | TV ≤8 mL/kg; PEEP ≥5cm H2O; +/− RM | TV >8 mL/kg; PEEP <5 cm H2O; no RM | Lower incidence of PPC: 8.7% v 14.7%; RR 0.64, 0.46 to 0.88; P<0.01 | |
| Supplemental oxygen | Meyhoff, 2009 | RCT | Open abdominal surgery; 1400s | 80% FiO2 | 30% FiO2 | No difference in postoperative atelectasis (7.9% v 7.1%; OR 1.13, 0.73 to 1.72; P=0.56), postoperative pneumonia (6.0% v 6.3%; OR 0.95, 0.60 to 1.49; P=0.81), or postoperative respiratory failure (5.5% v 4.4%; OR 1.22, 0.74 to 2.03; P=0.44) |
| Postoperative NIPPV | Jaber, 2016 | RCT | Postoperative respiratory failure, abdominal surgery; 293 | IP 5-15 cm H2O; PEEP 5-10 cm H2O | Standard oxygen therapy | Lower incidence of reintubation: 33.1% v 45.5%, P=0.03; more ventilator-free days: 25.4 v 23.2 days, P=0.04; lower incidence of infection: 31.4% v 49.2%, P=0.003 |
FiO2=fraction of inspired oxygen; IP=inspiratory pressure; IQR=interquartile range; OR=odds ratio; NIPPV=non-invasive positive pressure ventilation; PEEP=positive end expiratory pressure; POD=postoperative day; PPC=postoperative pulmonary complications; RCT=randomized controlled trial; RM=recruitment maneuver; RR=relative risk; TV=tidal volume.
Tidal volume
Use of low tidal volumes is most likely the best known component of a lung protective strategy. Since the seminal work by the ARDSnet group, observational studies have shown that the median intraoperative tidal volume has decreased over time.13 43 However, anesthesiologists typically use tidal volumes greater than 6 mL/kg even when caring for hypoxic patients.16 Although use of higher tidal volumes may be influenced by inaccuracies in calculating PBW, it is also likely that a belief may continue among anesthesiologists that lower tidal volumes are harmful.14 16 However, high quality data exist to suggest the opposite.9 44 45 46 47 48 49
Contrary to the concerns raised by the anesthesiologists who first described atelectasis during general anesthesia, tidal volumes of 6 mL/kg of PBW may not lead to an increased amount of atelectasis seen on computed tomography scans compared with 10 mL/kg.9 44 In an RCT in 25 patients undergoing cardiac surgery, a tidal volume of 12 mL/kg PBW compared with 6 mL/kg resulted in poorer postoperative lung compliance (decrease of 14.9 v 5.5 mL/cm H2O; P=0.002), a higher risk of barotrauma (mean peak airway pressure increase 7.1 v 2.4 cm H2O; P<0.001), and increased intrapulmonary shunt (from 15.5% to 21.4% (P=0.021) for 12 mL group; non-significant change for 6 mL group). A retrospective study of 170 patients undergoing pneumonectomy found that patients with postoperative respiratory failure were ventilated intraoperatively with higher tidal volumes (median 8.3 (interquartile range 7.6-9.4) mL/kg versus 6.7 (6.1-7.9) mL/kg PBW; P<0.001) and that an increase in tidal volume of one additional mL/kg PBW resulted in a significantly higher likelihood of development of respiratory failure (odds ratio 1.56, 95% confidence interval 1.12 to 2.23; P=0.009).46 Another retrospective study of 1019 cases of one lung ventilation (OLV) found that tidal volume was inversely proportional to the development of respiratory complications (odds ratio 0.847, 0.739 to 0.96).47 A single center RCT of 149 patients undergoing cardiac surgery found that ventilating throughout the perioperative period with 6 mL/kg PBW compared with 10 mL/kg at the same PEEP reduced the proportion of patients needing ventilator support at six hours (37.3% v 20.3%; P=0.02) and the incidence of reintubation (1.3% v 9.5%; P=0.03).48 Lastly, in a meta-analysis of 15 studies of IOLPV, for patients receiving at least 5 cm H2O PEEP, a tidal volume of 7 mL/kg PBW or below was associated with a significantly reduced risk of development of postoperative pulmonary complications compared with a tidal volume of 10 mL/kg or above (relative risk 0.40, 0.21 to 0.78; P<0.01).49
Although the results presented above describe the evaluation of lower tidal volumes in the context of a shared PEEP strategy between groups, most investigations have included a prescription of at least 5 cm H2O PEEP. Without this level of PEEP, the protective benefit of low tidal volume ventilation is likely lost, as investigations have shown that the use of low tidal volume and zero or low PEEP leads to increased lung inflammation and 30 day mortality.50 51 Therefore, tidal volume is probably only one necessary component of IOLPV, as regimens that also incorporate moderate PEEP and recruitment maneuvers have been shown to have incremental benefits in reducing PPCs.52
Positive end expiratory pressure
PEEP has been known to be effective in preventing intraoperative atelectasis since the mid- 1980s.53 54 55 Despite this, observational studies note that 80% of today’s patients are still ventilated without PEEP.14 Potential reasons for a lack of use of PEEP by contemporary anesthesiologists may include concerns about negative hemodynamic effects and barotrauma or perhaps simply that 0 cm H2O PEEP is the default setting on many mechanical ventilators.56 However, evidence suggests a benefit for PEEP in certain patient groups.
Multiple small RCTs have found that patients having laparoscopic surgery who receive 5 cm H2O PEEP experience significantly better oxygenation, less postoperative atelectasis, and better pulmonary compliance than those receiving zero PEEP.57 58 59 Two small RCTs have found that 10 cm H2O PEEP may confer additional physiologic benefit.60 61 With regard to PPCs, a large retrospective study of more than 11 000 abdominal and intracranial operations showed an association between the use of at least 5 cm H2O PEEP and a significantly decreased odds of PPCs compared with less than 5 cm H2O PEEP (odds ratio 0.72, 0.61 to 0.85).62 Of note, secondary analysis did not show a benefit for craniotomy patients, suggesting that PEEP’s protective effect may not extend to patients at lower risk for atelectasis. In 2013 a meta-analysis of eight RCTs comparing intraoperative PEEP levels found that the use of PEEP between 3 and 12 cm H2O was associated with a significantly decreased risk of developing a PPC (relative risk 0.29, 0.14 to 0.60).63 These results would set the stage for a trial investigating the use of higher PEEP in patients at high risk for atelectasis.
The PROVHILO trial was a multicenter RCT investigating whether 12 cm H2O PEEP was superior to 2 cm H2O or lower PEEP for 900 patients considered to be at intermediate or greater risk for developing PPCs after open abdominal surgery.64 Of note, the incidence of PPCs in this study was higher than would be expected on the basis of the ARISCAT score. The primary outcome was incidence of PPCs developing by postoperative day 5. Median tidal volumes were 7.2 and 7.1 mL/kg PBW in each group. Notably, patients in the higher PEEP group received recruitment maneuvers after intubation and before extubation, whereas patients in the lower PEEP group did not. The incidence of the primary outcome was almost identical between groups (39% v 40%; relative risk 1.01, 0.86 to 1.20; P=0.86). In secondary analysis, a higher proportion of patients in the PEEP group experienced intraoperative hypotension (46% v 36%; P=0.0016) and needed vasopressors (62% v 51%; P=0.0016), but patients in this group were significantly less likely to need a rescue maneuver from hypoxia (relative risk 0.34, 0.18 to 0.67; P<0.008).
PROVHILO’s authors suggested that their results should prompt the adoption of a low tidal volume, low PEEP strategy for lung protection. Alternatively, one could argue that these findings suggest that an empiric high PEEP strategy is equally as ineffective as low PEEP in preventing PPCs. It is possible that the optimal PEEP level lies between these two extremes, and additional work is needed to better answer this important clinical question. Regardless, the results contribute to a body of evidence that is still inconclusive regarding the benefit of higher PEEP in the intraoperative population. For example, an updated patient level meta-analysis after PROVHILO showed no difference in incidence of PPC between patients ventilated with less than 5 cm H2O and those with 5 cm H2O or higher PEEP.49 Drawing from this conclusion and incorporating the knowledge from the ARDS literature that high PEEP may benefit only patients with more severe disease, it may be reasonable to infer that higher PEEP is not necessary for most of the intraoperative population but can be considered for patients at very high risk for atelectasis or with concomitant lung injury.65 Lastly, the risk of hemodynamic compromise needs to be taken into account when deciding the appropriate regimen for complex patients with competing physiologic objectives.
Recruitment maneuvers
Temporary hyperinflation of the lung with a recruitment maneuver, commonly defined as an inspiratory hold with 40 cm H20 of inspiratory pressure for 20-30 seconds, has been used since the 1960s to reverse atelectasis after anesthetic induction.66 Compared with the use of zero PEEP or PEEP alone, recruitment maneuvers have been shown to increase end expiratory lung volume, improve compliance, and reduce chest wall elastance during laparoscopic surgery.67 68 69 70 Investigators have shown that recruitment maneuvers lead to improved intraoperative oxygenation in abdominal surgery, thoracic surgery with OLV, post-cardiopulmonary bypass, and procedures requiring Trendelenberg positioning.71 72 73 74 75 76 77 These benefits are unfortunately short lived, as atelectasis is thought to recur within 40 minutes.78
For example, studies in multiple settings have been unable to show that temporary gains in either lung mechanics or oxygenation extend to the postoperative environment, even when the recruitment maneuver is performed shortly before extubation.70 79 80 81 82 However, the benefits of recruitment maneuvers may be enhanced or prolonged by the performance of repeated maneuvers.83 Additionally, recent data suggest that recruitment maneuvers may not confer a benefit when used solely as an adjunct to conventional ventilation, as this strategy has been shown to lead to an increased incidence of PPCs and intraoperative oxygen desaturation compared with low tidal volume, moderate PEEP ventilation during laparoscopic surgery.84 As previously noted for other techniques, the benefit of recruitment maneuvers may be most pronounced when incorporated into a package of intraoperative lung protective ventilation.
Intraoperative lung protective ventilation
Although many descriptions have been used, some consensus has recently been reached as to how IOLPV should be defined.49 For the purposes of this review, we will accordingly define IOLPV as the combination of tidal volume 8 mL/kg PBW or lower and PEEP 5 cm H2O or higher, with or without a recruitment maneuver. Conventional ventilation is then defined as tidal volume greater than 8 mL/kg PBW and less than 5 cm H2O PEEP without a recruitment maneuver. Despite the following data supporting the benefit of IOLPV, as many as 50% of patients in contemporary anesthesia practice do not receive a lung protective regimen according to this definition.85
Small RCTs have shown that IOLPV causes an attenuated release of local and systemic inflammatory mediators after cardiopulmonary bypass, major abdominal surgery, and esophagectomy.19 86 87 In open abdominal surgery, investigators have shown improvements in both intraoperative and postoperative pulmonary mechanics. An RCT in 40 patients having open abdominal surgery showed that compliance increased by 32% and airway resistance decreased by 21% for older patients undergoing open abdominal surgery with IOLPV compared with patients receiving conventional ventilation (P<0.001 and P=0.029, respectively).88 A separate RCT found that patients receiving IOLPV performed better on spirometry than those receiving conventional ventilation as late as postoperative day 5 (mean forced expiratory volume in one second 1.63 (SD 0.55) v 1.23 (0.42) L/s, P<0.001; mean forced vital capacity 2.02 (0.52) v 1.57 (0.47) L/s, P<0.001).89 More recently, several RCTs from the same group have found that IOLPV can decrease driving pressures for patients having abdominal surgery.76 90
One of the largest RCTs investigating the effect of IOLPV on clinical outcomes is the IMPROVE study, in which 400 patients undergoing abdominal surgery who were at moderate or greater risk for developing PPCs were randomized to receive either protective or conventional ventilation.91 The protective regimen consisted of a tidal volume of 6-8 mL/kg PBW, 6-8 cm H2O PEEP, and recruitment maneuvers repeated every 30 minutes for the duration of the operation. Patients in the conventional group received tidal volumes of 10-12 mL/kg PBW, zero PEEP, and no recruitment maneuvers. Protective ventilation significantly reduced the incidence of the primary outcome, a composite of major pulmonary and non-pulmonary complications (10.5% v 27.5%; relative risk 0.40, 0.24 to 0.68; P=0.001). Secondary analysis showed that this difference was largely driven by reductions in the incidence of pneumonia (1.5% v 8.0%; relative risk 0.19, 0.05 to 0.66; P=0.009), need for postoperative non-invasive positive pressure ventilation (NIPPV) (4.5% v 14.5%; 0.29, 0.13 to 0.65; P=0.002), and sepsis (6.5% v 14.5%; 0.48, 0.35 to 0.93; P=0.03). The protective benefit of avoiding postoperative mechanical ventilation extended to 13 days (relative risk 0.36, 0.19 to 0.70; P=0.003), and the cumulative probability of needing postoperative mechanical ventilation throughout the 30 day follow-up period was significantly lower for the protective group (log rank test, P<0.01). Lastly, patients in the protective ventilation group spent nearly 2.5 days less in the hospital (mean difference −2.34, 95% confidence interval −4.75 to −0.72; P=0.006).
Criticism of IMPROVE includes the use of large tidal volumes for the conventional group and the incorporation of sepsis in the primary outcome. Response to these critiques takes into account that, although it is uncommon, as many as 18% of patients are still ventilated with greater than 10 mL/kg.14 Secondly, sepsis can plausibly occur as a result of biotrauma, and it is unlikely that excluding sepsis from the primary outcome would have changed the results as contributions from pneumonia and postoperative NIPPV were also substantial. Furthermore, when the data from IMPROVE were incorporated into an updated meta-analysis of IOLPV, a significant reduction in the incidence and risk of PPCs, exclusive of sepsis, was found (8.7% v 14.7%; relative risk 0.64, 0.46 to 0.88; P<0.01).49
Recently, another multicenter RCT has added to the body of evidence supporting the use of IOLPV. The Pulmonary Surgery with Protective Ventilation (PPV) trial randomized 346 patients undergoing lung cancer surgery to either IOLPV with tidal volumes of 5 mL/kg PBW and 5-8 cm H2O PEEP or conventional ventilation with 10 mL/kg and zero PEEP. The primary outcome was the incidence of major postoperative complications within 30 days. Of note, tidal volumes were not adjusted during OLV. Although the study was stopped early because of slow enrollment, analysis showed that patients in the IOLPV group had an almost 50% reduced odds of developing a major postoperative complication (odds ratio 0.54, 0.31 to 0.95; P=0.03). Significant reductions were also found in the odds of PPCs (odds ratio 0.59, 0.38 to 0.92; P=0.02) and length of stay in hospital (median 11 (interquartile range 9-15) v 12 (9-16) days; P=0.048). Although the authors recognized that a major limitation in their study was the use of a 10 mL/kg tidal volume during OLV, the results from the PPV trial still suggest at least significant harm from hyperinflation.
Finally, a discussion of the potential benefits of lung protective ventilation would be incomplete without a consideration of driving pressure. Driving pressure is defined as the difference between plateau pressure and PEEP (plateau pressure minus PEEP) and has been described as a means of normalizing tidal volume to functional lung size, as it accounts for static compliance.92 Increasing driving pressure has been identified as a significant predictor of mortality in a secondary analysis of the ARDSnet trial.93 In 2016 a patient level meta-analysis of RCTs involving IOLPV found that increasing driving pressure was also a significant predictor of PPCs (odds ratio 1.16, 1.13 to 1.19; P<0.0001).94 A mediation analysis showed that reduction in driving pressure resulting from being randomized to a lung protective strategy was responsible for a significantly reduced odds of developing PPCs, whereas lowering tidal volume and increasing PEEP were not. Furthermore, increases in PEEP that resulted in an increase in driving pressure were associated with a substantially increased odds of PPCs (odds ratio 3.11, 1.39 to 6.96; P=0.006). These interesting findings suggest that, even in patients with healthy lungs, changes in driving pressure may place patients at risk for ventilator induced lung injury and PPCs even within the confines of a lung protective strategy. Clearly, further work is needed to confirm these findings in a prospective study to add to our understanding of practices that truly provide lung protection.
Additional considerations
Inspired oxygen concentration
Administration of a high fraction of inspired oxygen (FiO2) is common practice in anesthesia, especially before airway instrumentation and extubation.95 In addition, the World Health Organization has recommended an FiO2 of 80% or above to reduce surgical site infections, on the basis of mixed results from moderate quality trials.96 97 98 99 However, the use of a higher FiO2 (>80%) can also cause resorption atelectasis and worsen inflammatory lung injury.27 100 Anesthesiologists looking to balance the benefits and risks of a high FiO2 are therefore faced with a clinical dilemma, although a few studies have been able to provide meaningful guidance.
For anesthetic induction, multiple small RCTs have shown a reduction in atelectasis when preoxygenation was performed with 30% oxygen as opposed to 100%.78 101 An RCT in 36 patients found that post-induction atelectasis showed a dose dependent increase with higher FiO2.102 However, the use of moderate PEEP may be sufficient to prevent atelectasis that may occur after induction with a high FiO2.103 Before extubation, the use of higher FiO2 may similarly predispose to atelectasis, as an RCT investigating the use of 100% FiO2 alone compared with a recruitment maneuver with either 100% or 40% FiO2 10 minutes before extubation found a significantly larger percentage of atelectatic lung for both groups receiving 100% FiO2 (mean 8.3% (SD 6.2%), 6.8% (3.4%), and 2.6% (1.1%) respectively; P<0.05 across comparisons).104
As for the optimal intraoperative oxygen concentration administered between induction and emergence, the only data on the effect on PPCs comes from secondary analysis of RCTs investigating FiO2 and postoperative wound infection. The largest such trial, PROXI in 2009, compared the incidence of surgical site infection for 1400 patients undergoing laparotomy who were randomized to an intraoperative FiO2 of either 80% or 30%.105 Secondary analysis did not show any significant differences between groups in the odds of atelectasis (odds ratio 1.11, 0.75 to 1.66; P=0.60), pneumonia (0.95, 0.61 to 1.48; P=0.82), or respiratory failure (1.27, 0.78 to 2.07; P=0.34). Additional prospective trials are needed to determine the proper risk/benefit ratio of a higher FiO2, as a recent large database study of 74 000 surgical patients associated an intraoperative FiO2 of 80% with a doubling of the odds of major pulmonary complications (odds ratio 1.99, 1.72 to 2.31; P=0.05).106 Given these results, use of a high FiO2 only as necessary to correct hypoxia may be prudent, as lower concentrations may help to prevent atelectasis.
Pressure controlled versus volume controlled ventilation
The proposed advantages of pressure controlled ventilation (PCV) over volume controlled ventilation are a rapid delivery of tidal volume while avoiding barotrauma through a set maximum inflation pressure.107 Investigations into the superiority of PCV over volume controlled ventilation in the intraoperative setting to date have had marginal or conflicting results. For example, a recent meta-analysis of six RCTs of thoracic surgery patients reported that the use of PCV during OLV led to lower peak inspiratory pressures (weighted mean difference −4.91 cm H2O; P<0.0001) and a higher ratio of partial pressure of oxygen to fractional inspired oxygen concentration (weighted mean difference 11.04; P=0.04), although these differences are unlikely to be clinically significant.108 Small RCTs in laparoscopic bariatric surgery have yielded similar results.109 110 No data from prospective trials are available to evaluate whether these benefits translate to improved clinical outcomes; however, a large database study involving more than 100 000 patients having elective surgery reported that use of PCV was associated with increased odds of developing a PPC (odds ratio 1.29, 1.21 to 1.37; P<0.001).111 In this study, use of PCV predisposed to higher tidal volumes and driving pressures, a possible mechanism for the findings.
Postoperative non-invasive positive pressure ventilation
Positive pressure ventilation applied via facemask as either continuous positive airway pressure (CPAP) or bi-level positive airway pressure is otherwise known as non-invasive positive pressure ventilation. NIPPV has been shown in various populations to both prevent and treat respiratory failure effectively.112 113 In the perioperative arena, the use of NIPPV is an accepted preventive measure in patients with known or suspected obstructive sleep apnea.114 In addition, NIPPV may be able to prevent or treat postoperative respiratory failure by reducing atelectasis.115 This mechanism was reported as the likely contributing factor behind the results of two large RCTs evaluating the use of NIPPV in postoperative hypoxic respiratory failure following major abdominal surgery.116 117 The first RCT in 2005 was stopped early owing to efficacy, as the intervention group had a 10-fold reduction in the incidence of reintubation compared with those receiving standard oxygen therapy (relative risk 0.10, 0.01 to 0.76; P=0.005), as well as a significant reduction in the risk of pneumonia (0.91, 0.04 to 0.88; P=0.02). The second RCT reproduced these findings in 2016, as patients receiving NIPPV for postoperative hypoxic respiratory failure had a significantly lower incidence of reintubation within seven days (45.5% v 33.1%; absolute difference −12.4%, −23.5% to −1.3%; P=0.03), and pneumonia (22.1% v 10.1%; absolute difference −11.9%, −20.9 to −2.9; P=0.005) compared with those receiving standard oxygen therapy.
The results from these two studies are encouraging, but they differ substantially from a large RCT evaluating NIPPV for post-extubation respiratory failure in the ICU, which suggested no difference in reintubation rates and an increase in mortality likely from a delay in eventual reintubation.118 In this study, only roughly 20% of patients had postoperative respiratory failure, but it was the second leading cause of acute respiratory failure within the cohort. The divergent findings from these three trials suggest that the use of postoperative NIPPV should take into account the cause of the patient’s respiratory failure, as patients with atelectasis may benefit more than those with pneumonia, trauma, or ARDS.
High flow nasal cannula
Finally, although not technically considered a form of PPV, high flow nasal cannula (HFNC) therapy has been increasingly used for hypoxic respiratory failure and warrants inclusion in the discussion of postoperative respiratory care. HFNC consists of humidified, high flow (up to 60 L/min) oxygen with a maximum FiO2 of 100%. At high flow rates, HFNC is generally considered to provide 4-6 cm H2O of added inspiratory pressure.119 Prospective RCTs in both the ICU and the postoperative period have found that HFNC is non-inferior to NIPPV in terms of preventing respiratory failure but is better tolerated in terms of patients’ comfort.120 121 One of the largest trials to examine the potential role for HFNC in preventing postoperative respiratory failure is OPERA, in which 220 post-abdominal surgery patients were randomized to receive either HFNC or standard oxygen therapy.122 No significant differences were found between groups in the absolute reduction in risk of hypoxemia or in the incidence of PPCs. Although OPERA may have been underpowered to detect a significant difference in these outcomes, its findings may suggest that HFNC might not be an adequate preventive measure to combat atelectasis, as the amount of inspiratory pressure needed to re-inflate collapsed lung segments may be greater than HFNC can provide. Additional research is needed to clarify the potential role for HFNC in either the treatment or prevention of postoperative respiratory failure.
Emerging treatments
As is the trend for many aspects of medicine, the future of perioperative mechanical ventilation will likely involve personalization. Personalization of tidal volumes on the basis of PBW has already been adopted to some degree, but much of the emerging work in the field centers around determining the “best PEEP” to balance the maintenance of end expiratory lung volume while avoiding hyperinflation. Classically, this involves selecting the PEEP level that maximizes static pulmonary compliance or that places the patient on the lower inflection point of the pressure-volume curve.123
A recent physiologic study found that the best PEEP approach as described above can lead to improvements in oxygenation and lung mechanics with little hemodynamic effects during laparoscopy.124 Two ongoing trials are evaluating the effect of best PEEP on outcomes during laparoscopy and thoracic surgery with OLV (NCT01532245, NCT02931409). Additionally, estimation of pleural pressure via esophageal manometry can facilitate titration of PEEP to optimize transpulmonary pressure.125 Two other ongoing trials are evaluating whether PEEP titration using this technique can improve oxygenation and reduce complications in laparoscopic surgery (NCT03153592, NCT03256396). As the use of ultrasonography of the lung has expanded in recent years, investigators have begun to investigate whether this technique can also be used as an effective means of optimizing intraoperative PEEP (NCT03211936).
A large multicenter prospective RCT published in 2018 evaluated the potential benefits of personalized perioperative mechanical ventilation.90 The iPROVE trial randomized 967 adults undergoing abdominal surgery estimated to be at moderate or greater risk for PPCs to one of four groups. The first group received an open lung, individualized CPAP protocol, including a stepwise recruitment maneuver with a best PEEP titration for intraoperative ventilation, followed by postoperative CPAP if needed for oxygen desaturation (OLA-iCPAP group). A second group had the same intraoperative OLA protocol but received mandatory postoperative CPAP for three hours (OLA-CPAP group). The third and fourth groups had standard intraoperative ventilation with 5 cm H2O PEEP and then received either mandatory postoperative CPAP or standard oxygen therapy (STD-CPAP group and STD-O2 group). Patients in all groups were ventilated with 8 mL/kg PBW tidal volume. Notably, iPROVE is the first trial to use an IOLPV strategy as the control group. The study found no difference between groups in the primary outcome, a composite of respiratory and systemic complications by hospital day 7. However, secondary and exploratory analyses showed a benefit for the OLA-iCPAP regimen in terms of respiratory complications (relative risk 0.80, 0.65 to 0.99; P=0.047) and infectious complications (0.52, 0.31 to 0.06; P=0.014) compared with the STD-O2 regimen. Although the authors caution that these results should not be interpreted as conclusive, their data suggest that additional prospective studies in this emerging field have the potential to improve perioperative ventilation past the point of what we now consider a lung protective strategy.
Guidelines
Reflecting the lack of consensus about the benefit of the aforementioned techniques in the general intraoperative population, to date neither the European Society of Anaesthesiology nor the American Society of Anesthesiologists has published formal guidelines for intraoperative mechanical ventilation. On the basis of the available evidence, the current joint European Respiratory Society/American Thoracic Society clinical practice guidelines recommend NIPPV for postoperative respiratory failure.126
Conclusion
Perioperative lung injury is an uncommon but potentially deadly condition that can be initiated or exacerbated by injurious mechanical ventilation practices. Although groundbreaking studies from the literature on ARDS have led to incremental changes in modern anesthesia practice regarding the use of smaller tidal volumes, a significant faction remains hesitant to adopt the principles of intraoperative lung protective ventilation. The reasons for this lack of wholesale adoption are multifactorial and include concerns about negative hemodynamic consequences and hypercapnia and about whether patients with healthy lungs can benefit from lung protection to a similar degree to those with ARDS. To overcome the latter concern, we can again look to the current ICU literature, which suggests a benefit for low tidal volume ventilation even for patients without ARDS. However, application of these findings to the intraoperative population still suffers from poor comparison in terms of duration of mechanical ventilation, risk of lung injury, and severity of illness.127
At this time, the evidence supporting the use of lung protective ventilation in the general perioperative population is not definitive. To illustrate the burden of proof for this question, however, consider the following: owing to the rare occurrence of PPCs in patients at low risk (2%), the potential for a given ventilator strategy to reduce this incidence by 50% would require a sample size of more than 4000 patients.2 More conservative estimates of effect size would dictate an even larger sample that would not be feasible without a very large multicenter trial. Given the very low incidence of perioperative mortality, a trial proving that IOLPV can lead to a survival benefit is even less likely to be feasible.128 However, the findings from the major studies presented in this review have contributed to a working body of evidence that supports the use of low tidal volumes, moderate PEEP, and recruitment maneuvers to prevent significant postoperative morbidity for many at risk patient groups. Additional important work on the contributions of intraoperative oxygen supplementation, mode of ventilation, postoperative NIPPV or HFNC, and personalized mechanical ventilation is necessary and ongoing. We can look forward to the results of the PROBESE trial, which will provide additional insight into the potential benefit of higher PEEP and recruitment maneuvers in obese patients at moderate risk for development of PPCs (NCT02148692). Lastly, as the mechanical ventilators used intraoperatively become more nuanced and access to NIPPV increases, integrated strategies involving perioperative lung protective ventilation such as those used in the iPROVE trial may expand our capabilities to prevent postoperative atelectasis and the risk of PPCs that comes with it.90 129
Established and emerging data from the intraoperative arena have the potential to create a fundamental shift in the way anesthesiologists approach the proper oxygenation and ventilation of their patients. Given the track record of safety and efficacy of protective techniques in multiple settings, we will probably no longer need to consider hyperinflation as the standard of care. Despite this, many contemporary anesthesiologists are hesitant to adopt these principles into their daily practice. However, given the broader body of evidence on the potential harm that can occur through hyperinflation for any patient, one can argue that the use of protective ventilation should not have to overcome such a stringent burden of proof to replace a potentially harmful strategy that has gained its status as a historical standard with far less scrutiny.
Glossary of abbreviations.
ARDS—acute respiratory distress syndrome
CPAP—continuous positive airway pressure
FiO2—inspired oxygen
HFNC—high flow nasal cannula
ICU—intensive care unit
IOLPV—intraoperative lung protective ventilation
NIPPV—non-invasive positive pressure ventilation
OLV—one lung ventilation
PBW—predicted body weight
PCV—pressure controlled ventilation
PEEP—positive end expiratory pressure
PPC—postoperative pulmonary complication
RCT—randomized controlled trial
Research questions.
Are the benefits of intraoperative lung protective ventilation (IOLPV) seen in patients at moderate and high risk applicable to patients at low risk of postoperative pulmonary complications?
Does prophylactic post-extubation non-invasive positive pressure ventilation reduce the risk of developing subsequent respiratory failure?
Does an individualized best positive end expiratory pressure (PEEP) titration strategy confer additional benefit to empiric moderate PEEP?
Do the reductions in postoperative morbidity seen with the use of IOLPV strategies translate into reduced perioperative mortality?
Does a ventilation approach based on limiting driving pressure confer a protective benefit for intraoperative patients?
Is high flow nasal cannula (HFNC) effective in either the treatment or the prevention of postoperative hypoxic respiratory failure?
Patient involvement.
The father of a 45 year old man who underwent a craniectomy for a traumatic brain injury after major polytrauma, with a postoperative course complicated by perioperative lung injury, was given an opportunity to review this manuscript. We incorporated his suggestions to limit the use of unnecessary technical jargon and abbreviations as much as possible so as to enhance the readability of the manuscript in general and make the content easier to follow.
Contributors: BOG and DT conceptualized and generated the basic outline of the review. BOG crafted the search strategy with the aid of Paul Bain of the Harvard Countway Library, reviewed the search results for inclusion/exclusion, created tables, created figures with the aid of Jane Hayward of the Beth Israel Deaconess Medical Center’s Media Services Department, and drafted the main body of the manuscript. BOG and DT revised the final version of the manuscript. BOG is the guarantor.
Competing interests: We have read and understood the BMJ policy on declaration of interests and declare the following interests: none
Provenance and peer review: Commissioned; externally peer reviewed.
Series explanation: State of the Art Reviews are commissioned on the basis of their relevance to academics and specialists in the US and internationally. For this reason they are written predominantly by US authors
References
- 1. Weiser TG, Regenbogen SE, Thompson KD, et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet 2008;372:139-44. 10.1016/S0140-6736(08)60878-8 [DOI] [PubMed] [Google Scholar]
- 2. Canet J, Gallart L, Gomar C, et al. ARISCAT Group Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology 2010;113:1338-50. 10.1097/ALN.0b013e3181fc6e0a [DOI] [PubMed] [Google Scholar]
- 3. Fernandez-Bustamante A, Klawitter J, Repine JE, et al. Early effect of tidal volume on lung injury biomarkers in surgical patients with healthy lungs. Anesthesiology 2014;121:469-81. 10.1097/ALN.0000000000000301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shander A, Fleisher LA, Barie PS, Bigatello LM, Sladen RN, Watson CB. Clinical and economic burden of postoperative pulmonary complications: patient safety summit on definition, risk-reducing interventions, and preventive strategies. Crit Care Med 2011;39:2163-72. 10.1097/CCM.0b013e31821f0522 [DOI] [PubMed] [Google Scholar]
- 5. Fernandez-Bustamante A, Frendl G, Sprung J, et al. Postoperative pulmonary complications, early mortality, and hospital stay following noncardiothoracic surgery: A multicenter study by the perioperative research network investigators. JAMA Surg 2017;152:157-66. 10.1001/jamasurg.2016.4065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. LAS VEGAS investigators Epidemiology, practice of ventilation and outcome for patients at increased risk of postoperative pulmonary complications: LAS VEGAS - an observational study in 29 countries. Eur J Anaesthesiol 2017;34:492-507. 10.1097/EJA.0000000000000646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Milot J, Perron J, Lacasse Y, Létourneau L, Cartier PC, Maltais F. Incidence and predictors of ARDS after cardiac surgery. Chest 2001;119:884-8. 10.1378/chest.119.3.884 [DOI] [PubMed] [Google Scholar]
- 8. Kogan A, Preisman S, Levin S, Raanani E, Sternik L. Adult respiratory distress syndrome following cardiac surgery. J Card Surg 2014;29:41-6. 10.1111/jocs.12264 [DOI] [PubMed] [Google Scholar]
- 9. Bendixen HH, Hedley-Whyte J, Laver MB. Impaired Oxygenation in Surgical Patients during General Anesthesia with Controlled Ventilation. A Concept of Atelectasis. N Engl J Med 1963;269:991-6. 10.1056/NEJM196311072691901 [DOI] [PubMed] [Google Scholar]
- 10. Wilson WC, Benumof J. Anesthesia for thoracic surgery. In: Miller RD, ed. Miller’s Anesthesia. 6th ed Elsevier Churchill Livingstone, 2005: 1894-5. [Google Scholar]
- 11. Hickling KG, Walsh J, Henderson S, Jackson R. Low mortality rate in adult respiratory distress syndrome using low-volume, pressure-limited ventilation with permissive hypercapnia: a prospective study. Crit Care Med 1994;22:1568-78. 10.1097/00003246-199422100-00011 [DOI] [PubMed] [Google Scholar]
- 12. Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 1998;338:347-54. 10.1056/NEJM199802053380602 [DOI] [PubMed] [Google Scholar]
- 13. Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A, Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301-8. 10.1056/NEJM200005043421801 [DOI] [PubMed] [Google Scholar]
- 14. Jaber S, Coisel Y, Chanques G, et al. A multicentre observational study of intra-operative ventilatory management during general anaesthesia: tidal volumes and relation to body weight. Anaesthesia 2012;67:999-1008. 10.1111/j.1365-2044.2012.07218.x [DOI] [PubMed] [Google Scholar]
- 15. Hess DR, Kondili D, Burns E, Bittner EA, Schmidt UH. A 5-year observational study of lung-protective ventilation in the operating room: a single-center experience. J Crit Care 2013;28:533.e9-15. 10.1016/j.jcrc.2012.11.014 [DOI] [PubMed] [Google Scholar]
- 16. Blum JM, Maile M, Park PK, et al. A description of intraoperative ventilator management in patients with acute lung injury and the use of lung protective ventilation strategies. Anesthesiology 2011;115:75-82. 10.1097/ALN.0b013e31821a8d63 [DOI] [PubMed] [Google Scholar]
- 17. Ranieri VM, Rubenfeld GD, Thompson BT, et al. ARDS Definition Task Force Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [DOI] [PubMed] [Google Scholar]
- 18. Jammer I, Wickboldt N, Sander M, et al. European Society of Anaesthesiology (ESA) and the European Society of Intensive Care Medicine (ESICM) European Society of Anaesthesiology. European Society of Intensive Care Medicine Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: a statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur J Anaesthesiol 2015;32:88-105. 10.1097/EJA.0000000000000118 [DOI] [PubMed] [Google Scholar]
- 19. Zupancich E, Paparella D, Turani F, et al. Mechanical ventilation affects inflammatory mediators in patients undergoing cardiopulmonary bypass for cardiac surgery: a randomized clinical trial. J Thorac Cardiovasc Surg 2005;130:378-83. 10.1016/j.jtcvs.2004.11.061 [DOI] [PubMed] [Google Scholar]
- 20. O’Gara B, Talmor D. Lung protective properties of the volatile anesthetics. Intensive Care Med 2016;42:1487-9. 10.1007/s00134-016-4429-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jabaudon M, Futier E, Roszyk L, Sapin V, Pereira B, Constantin JM. Association between intraoperative ventilator settings and plasma levels of soluble receptor for advanced glycation end-products in patients without pre-existing lung injury. Respirology 2015;20:1131-8. 10.1111/resp.12583 [DOI] [PubMed] [Google Scholar]
- 22. Lundquist H, Hedenstierna G, Strandberg A, Tokics L, Brismar B. CT-assessment of dependent lung densities in man during general anaesthesia. Acta Radiol 1995;36:626-32. 10.1177/028418519503600464 [DOI] [PubMed] [Google Scholar]
- 23. Agostini P, Cieslik H, Rathinam S, et al. Postoperative pulmonary complications following thoracic surgery: are there any modifiable risk factors? Thorax 2010;65:815-8. 10.1136/thx.2009.123083 [DOI] [PubMed] [Google Scholar]
- 24. Duggan M, Kavanagh BP. Pulmonary atelectasis: a pathogenic perioperative entity. Anesthesiology 2005;102:838-54. 10.1097/00000542-200504000-00021 [DOI] [PubMed] [Google Scholar]
- 25. Froese AB, Bryan AC. Effects of anesthesia and paralysis on diaphragmatic mechanics in man. Anesthesiology 1974;41:242-55. 10.1097/00000542-197409000-00006 [DOI] [PubMed] [Google Scholar]
- 26. Krayer S, Rehder K, Vettermann J, Didier EP, Ritman EL. Position and motion of the human diaphragm during anesthesia-paralysis. Anesthesiology 1989;70:891-8. 10.1097/00000542-198906000-00002 [DOI] [PubMed] [Google Scholar]
- 27. Joyce CJ, Baker AB, Kennedy RR. Gas uptake from an unventilated area of lung: computer model of absorption atelectasis. J Appl Physiol (1985) 1993;74:1107-16. 10.1152/jappl.1993.74.3.1107 [DOI] [PubMed] [Google Scholar]
- 28. Wollmer P, Schairer W, Bos JA, Bakker W, Krenning EP, Lachmann B. Pulmonary clearance of 99mTc-DTPA during halothane anaesthesia. Acta Anaesthesiol Scand 1990;34:572-5. 10.1111/j.1399-6576.1990.tb03147.x [DOI] [PubMed] [Google Scholar]
- 29. Nicholas TE, Barr HA. The release of surfactant in rat lung by brief periods of hyperventilation. Respir Physiol 1983;52:69-83. 10.1016/0034-5687(83)90137-8 [DOI] [PubMed] [Google Scholar]
- 30. Bendixen HHSG, Smith GM, Mead J. Pattern of Ventilation in Young Adults. J Appl Physiol 1964;19:195-8. 10.1152/jappl.1964.19.2.195 [DOI] [PubMed] [Google Scholar]
- 31. Avery ME, Mead J. Surface properties in relation to atelectasis and hyaline membrane disease. AMA J Dis Child 1959;97:517-23. [DOI] [PubMed] [Google Scholar]
- 32. Muscedere JG, Mullen JB, Gan K, Slutsky AS. Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med 1994;149:1327-34. 10.1164/ajrccm.149.5.8173774 [DOI] [PubMed] [Google Scholar]
- 33. Tsuchida S, Engelberts D, Peltekova V, et al. Atelectasis causes alveolar injury in nonatelectatic lung regions. Am J Respir Crit Care Med 2006;174:279-89. 10.1164/rccm.200506-1006OC [DOI] [PubMed] [Google Scholar]
- 34. Lindberg P, Gunnarsson L, Tokics L, et al. Atelectasis and lung function in the postoperative period. Acta Anaesthesiol Scand 1992;36:546-53. 10.1111/j.1399-6576.1992.tb03516.x [DOI] [PubMed] [Google Scholar]
- 35. Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med 2013;369:2126-36. 10.1056/NEJMra1208707 [DOI] [PubMed] [Google Scholar]
- 36. Güldner A, Kiss T, Serpa Neto A, et al. Intraoperative protective mechanical ventilation for prevention of postoperative pulmonary complications: a comprehensive review of the role of tidal volume, positive end-expiratory pressure, and lung recruitment maneuvers. Anesthesiology 2015;123:692-713. 10.1097/ALN.0000000000000754 [DOI] [PubMed] [Google Scholar]
- 37. Mead J, Takishima T, Leith D. Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol 1970;28:596-608. 10.1152/jappl.1970.28.5.596 [DOI] [PubMed] [Google Scholar]
- 38. Davidovich N, DiPaolo BC, Lawrence GG, Chhour P, Yehya N, Margulies SS. Cyclic stretch-induced oxidative stress increases pulmonary alveolar epithelial permeability. Am J Respir Cell Mol Biol 2013;49:156-64. 10.1165/rcmb.2012-0252OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Uhlig S. Ventilation-induced lung injury and mechanotransduction: stretching it too far? Am J Physiol Lung Cell Mol Physiol 2002;282:L892-6. 10.1152/ajplung.00124.2001 [DOI] [PubMed] [Google Scholar]
- 40. Slutsky AS, Tremblay LN. Multiple system organ failure. Is mechanical ventilation a contributing factor? Am J Respir Crit Care Med 1998;157:1721-5. 10.1164/ajrccm.157.6.9709092 [DOI] [PubMed] [Google Scholar]
- 41. Imai Y, Parodo J, Kajikawa O, et al. Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. JAMA 2003;289:2104-12. 10.1001/jama.289.16.2104 [DOI] [PubMed] [Google Scholar]
- 42. Thompson BT, Chambers RC, Liu KD. Acute Respiratory Distress Syndrome. N Engl J Med 2017;377:562-72. 10.1056/NEJMra1608077 [DOI] [PubMed] [Google Scholar]
- 43. Blum JM, Fetterman DM, Park PK, Morris M, Rosenberg AL. A description of intraoperative ventilator management and ventilation strategies in hypoxic patients. Anesth Analg 2010;110:1616-22. 10.1213/ANE.0b013e3181da82e1 [DOI] [PubMed] [Google Scholar]
- 44. Cai H, Gong H, Zhang L, Wang Y, Tian Y. Effect of low tidal volume ventilation on atelectasis in patients during general anesthesia: a computed tomographic scan. J Clin Anesth 2007;19:125-9. 10.1016/j.jclinane.2006.08.008 [DOI] [PubMed] [Google Scholar]
- 45. Chaney MA, Nikolov MP, Blakeman BP, Bakhos M. Protective ventilation attenuates postoperative pulmonary dysfunction in patients undergoing cardiopulmonary bypass. J Cardiothorac Vasc Anesth 2000;14:514-8. 10.1053/jcan.2000.9487 [DOI] [PubMed] [Google Scholar]
- 46. Fernández-Pérez ER, Keegan MT, Brown DR, Hubmayr RD, Gajic O. Intraoperative tidal volume as a risk factor for respiratory failure after pneumonectomy. Anesthesiology 2006;105:14-8. 10.1097/00000542-200607000-00007 [DOI] [PubMed] [Google Scholar]
- 47. Blank RS, Colquhoun DA, Durieux ME, et al. Management of One-lung Ventilation: Impact of Tidal Volume on Complications after Thoracic Surgery. Anesthesiology 2016;124:1286-95. 10.1097/ALN.0000000000001100 [DOI] [PubMed] [Google Scholar]
- 48. Sundar S, Novack V, Jervis K, et al. Influence of low tidal volume ventilation on time to extubation in cardiac surgical patients. Anesthesiology 2011;114:1102-10. 10.1097/ALN.0b013e318215e254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Serpa Neto A, Hemmes SN, Barbas CS, et al. PROVE Network Investigators Protective versus Conventional Ventilation for Surgery: A Systematic Review and Individual Patient Data Meta-analysis. Anesthesiology 2015;123:66-78. 10.1097/ALN.0000000000000706 [DOI] [PubMed] [Google Scholar]
- 50. Sato H, Nakamura K, Baba Y, Terada S, Goto T, Kurahashi K. Low tidal volume ventilation with low PEEP during surgery may induce lung inflammation. BMC Anesthesiol 2016;16:47. 10.1186/s12871-016-0209-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Levin MA, McCormick PJ, Lin HM, Hosseinian L, Fischer GW. Low intraoperative tidal volume ventilation with minimal PEEP is associated with increased mortality. Br J Anaesth 2014;113:97-108. 10.1093/bja/aeu054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yang D, Grant MC, Stone A, Wu CL, Wick EC. A Meta-analysis of Intraoperative Ventilation Strategies to Prevent Pulmonary Complications: Is Low Tidal Volume Alone Sufficient to Protect Healthy Lungs? Ann Surg 2016;263:881-7. 10.1097/SLA.0000000000001443 [DOI] [PubMed] [Google Scholar]
- 53. Brismar B, Hedenstierna G, Lundquist H, Strandberg A, Svensson L, Tokics L. Pulmonary densities during anesthesia with muscular relaxation--a proposal of atelectasis. Anesthesiology 1985;62:422-8. 10.1097/00000542-198504000-00009 [DOI] [PubMed] [Google Scholar]
- 54. Tokics L, Hedenstierna G, Strandberg A, Brismar B, Lundquist H. Lung collapse and gas exchange during general anesthesia: effects of spontaneous breathing, muscle paralysis, and positive end-expiratory pressure. Anesthesiology 1987;66:157-67. 10.1097/00000542-198702000-00009 [DOI] [PubMed] [Google Scholar]
- 55. Neumann P, Rothen HU, Berglund JE, Valtysson J, Magnusson A, Hedenstierna G. Positive end-expiratory pressure prevents atelectasis during general anaesthesia even in the presence of a high inspired oxygen concentration. Acta Anaesthesiol Scand 1999;43:295-301. 10.1034/j.1399-6576.1999.430309.x [DOI] [PubMed] [Google Scholar]
- 56. Chiao SS, Colquhoun DA, Naik BI, et al. Changing Default Ventilator Settings on Anesthesia Machines Improves Adherence to Lung-Protective Ventilation Measures. Anesth Analg 2018;126:1219-22. 10.1213/ANE.0000000000002575 [DOI] [PubMed] [Google Scholar]
- 57. Karsten J, Luepschen H, Grossherr M, et al. Effect of PEEP on regional ventilation during laparoscopic surgery monitored by electrical impedance tomography. Acta Anaesthesiol Scand 2011;55:878-86. 10.1111/j.1399-6576.2011.02467.x [DOI] [PubMed] [Google Scholar]
- 58. Kim JY, Shin CS, Kim HS, Jung WS, Kwak HJ. Positive end-expiratory pressure in pressure-controlled ventilation improves ventilatory and oxygenation parameters during laparoscopic cholecystectomy. Surg Endosc 2010;24:1099-103. 10.1007/s00464-009-0734-6 [DOI] [PubMed] [Google Scholar]
- 59. Meininger D, Byhahn C, Mierdl S, Westphal K, Zwissler B. Positive end-expiratory pressure improves arterial oxygenation during prolonged pneumoperitoneum. Acta Anaesthesiol Scand 2005;49:778-83. 10.1111/j.1399-6576.2005.00713.x [DOI] [PubMed] [Google Scholar]
- 60. Talab HF, Zabani IA, Abdelrahman HS, et al. Intraoperative ventilatory strategies for prevention of pulmonary atelectasis in obese patients undergoing laparoscopic bariatric surgery. Anesth Analg 2009;109:1511-6. 10.1213/ANE.0b013e3181ba7945 [DOI] [PubMed] [Google Scholar]
- 61. Sen O, Erdogan Doventas Y. Effects of different levels of end-expiratory pressure on hemodynamic, respiratory mechanics and systemic stress response during laparoscopic cholecystectomy. Braz J Anesthesiol 2017;67:28-34. 10.1016/j.bjane.2015.08.015 [DOI] [PubMed] [Google Scholar]
- 62. de Jong MAC, Ladha KS, Vidal Melo MF, et al. Differential Effects of Intraoperative Positive End-expiratory Pressure (PEEP) on Respiratory Outcome in Major Abdominal Surgery Versus Craniotomy. Ann Surg 2016;264:362-9. 10.1097/SLA.0000000000001499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hemmes SN, Serpa Neto A, Schultz MJ. Intraoperative ventilatory strategies to prevent postoperative pulmonary complications: a meta-analysis. Curr Opin Anaesthesiol 2013;26:126-33. 10.1097/ACO.0b013e32835e1242 [DOI] [PubMed] [Google Scholar]
- 64. Hemmes SN, Gama de Abreu M, Pelosi P, Schultz MJ, PROVE Network Investigators for the Clinical Trial Network of the European Society of Anaesthesiology High versus low positive end-expiratory pressure during general anaesthesia for open abdominal surgery (PROVHILO trial): a multicentre randomised controlled trial. Lancet 2014;384:495-503. 10.1016/S0140-6736(14)60416-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Briel M, Meade M, Mercat A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA 2010;303:865-73. 10.1001/jama.2010.218 [DOI] [PubMed] [Google Scholar]
- 66. Egbert LD, Layer MB, Bendixen HH. Intermittent deep breaths and compliance during anesthesia in man. Anesthesiology 1963;24:57-60. 10.1097/00000542-196301000-00009 [DOI] [Google Scholar]
- 67. Reinius H, Jonsson L, Gustafsson S, et al. Prevention of atelectasis in morbidly obese patients during general anesthesia and paralysis: a computerized tomography study. Anesthesiology 2009;111:979-87. 10.1097/ALN.0b013e3181b87edb [DOI] [PubMed] [Google Scholar]
- 68. Cakmakkaya OS, Kaya G, Altintas F, Hayirlioglu M, Ekici B. Restoration of pulmonary compliance after laparoscopic surgery using a simple alveolar recruitment maneuver. J Clin Anesth 2009;21:422-6. 10.1016/j.jclinane.2009.08.001 [DOI] [PubMed] [Google Scholar]
- 69. Futier E, Constantin JM, Pelosi P, et al. Intraoperative recruitment maneuver reverses detrimental pneumoperitoneum-induced respiratory effects in healthy weight and obese patients undergoing laparoscopy. Anesthesiology 2010;113:1310-9. 10.1097/ALN.0b013e3181fc640a [DOI] [PubMed] [Google Scholar]
- 70. Whalen FX, Gajic O, Thompson GB, et al. The effects of the alveolar recruitment maneuver and positive end-expiratory pressure on arterial oxygenation during laparoscopic bariatric surgery. Anesth Analg 2006;102:298-305. 10.1213/01.ane.0000183655.57275.7a [DOI] [PubMed] [Google Scholar]
- 71. Pang CK, Yap J, Chen PP. The effect of an alveolar recruitment strategy on oxygenation during laparascopic cholecystectomy. Anaesth Intensive Care 2003;31:176-80. [DOI] [PubMed] [Google Scholar]
- 72. Park HP, Hwang JW, Kim YB, et al. Effect of pre-emptive alveolar recruitment strategy before pneumoperitoneum on arterial oxygenation during laparoscopic hysterectomy. Anaesth Intensive Care 2009;37:593-7. [DOI] [PubMed] [Google Scholar]
- 73. Chalhoub V, Yazigi A, Sleilaty G, et al. Effect of vital capacity manoeuvres on arterial oxygenation in morbidly obese patients undergoing open bariatric surgery. Eur J Anaesthesiol 2007;24:283-8. 10.1017/S0265021506001529 [DOI] [PubMed] [Google Scholar]
- 74. Choi ES, Oh AY, In CB, Ryu JH, Jeon YT, Kim HG. Effects of recruitment manoeuvre on perioperative pulmonary complications in patients undergoing robotic assisted radical prostatectomy: A randomised single-blinded trial. PLoS One 2017;12:e0183311. 10.1371/journal.pone.0183311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ferrando C, Mugarra A, Gutierrez A, et al. Setting individualized positive end-expiratory pressure level with a positive end-expiratory pressure decrement trial after a recruitment maneuver improves oxygenation and lung mechanics during one-lung ventilation. Anesth Analg 2014;118:657-65. 10.1213/ANE.0000000000000105 [DOI] [PubMed] [Google Scholar]
- 76. Ferrando C, Suarez-Sipmann F, Tusman G, et al. Open lung approach versus standard protective strategies: Effects on driving pressure and ventilatory efficiency during anesthesia - A pilot, randomized controlled trial. PLoS One 2017;12:e0177399. 10.1371/journal.pone.0177399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Unzueta C, Tusman G, Suarez-Sipmann F, Böhm S, Moral V. Alveolar recruitment improves ventilation during thoracic surgery: a randomized controlled trial. Br J Anaesth 2012;108:517-24. 10.1093/bja/aer415 [DOI] [PubMed] [Google Scholar]
- 78. Rothen HU, Sporre B, Engberg G, Wegenius G, Hedenstierna G. Reexpansion of atelectasis during general anaesthesia may have a prolonged effect. Acta Anaesthesiol Scand 1995;39:118-25. 10.1111/j.1399-6576.1995.tb05602.x [DOI] [PubMed] [Google Scholar]
- 79. Lumb AB, Greenhill SJ, Simpson MP, Stewart J. Lung recruitment and positive airway pressure before extubation does not improve oxygenation in the post-anaesthesia care unit: a randomized clinical trial. Br J Anaesth 2010;104:643-7. 10.1093/bja/aeq080 [DOI] [PubMed] [Google Scholar]
- 80. Scherer M, Dettmer S, Meininger D, et al. Alveolar recruitment strategy during cardiopulmonary bypass does not improve postoperative gas exchange and lung function. Cardiovasc Eng 2009;9:1-5. 10.1007/s10558-009-9063-6 [DOI] [PubMed] [Google Scholar]
- 81. Verbeek GL, Myles PS, Westall GP, et al. Intra-operative protective mechanical ventilation in lung transplantation: a randomised, controlled trial. Anaesthesia 2017;72:993-1004. 10.1111/anae.13964 [DOI] [PubMed] [Google Scholar]
- 82. Nestler C, Simon P, Petroff D, et al. Individualized positive end-expiratory pressure in obese patients during general anaesthesia: a randomized controlled clinical trial using electrical impedance tomography. Br J Anaesth 2017;119:1194-205. 10.1093/bja/aex192 [DOI] [PubMed] [Google Scholar]
- 83. Almarakbi WA, Fawzi HM, Alhashemi JA. Effects of four intraoperative ventilatory strategies on respiratory compliance and gas exchange during laparoscopic gastric banding in obese patients. Br J Anaesth 2009;102:862-8. 10.1093/bja/aep084 [DOI] [PubMed] [Google Scholar]
- 84. Park SJ, Kim BG, Oh AH, Han SH, Han HS, Ryu JH. Effects of intraoperative protective lung ventilation on postoperative pulmonary complications in patients with laparoscopic surgery: prospective, randomized and controlled trial. Surg Endosc 2016;30:4598-606. 10.1007/s00464-016-4797-x [DOI] [PubMed] [Google Scholar]
- 85. Ladha K, Vidal Melo MF, McLean DJ, et al. Intraoperative protective mechanical ventilation and risk of postoperative respiratory complications: hospital based registry study. BMJ 2015;351:h3646. 10.1136/bmj.h3646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Jabaudon M, Blondonnet R, Roszyk L, et al. Soluble Receptor for Advanced Glycation End-Products Predicts Impaired Alveolar Fluid Clearance in Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 2015;192:191-9. 10.1164/rccm.201501-0020OC [DOI] [PubMed] [Google Scholar]
- 87. Michelet P, D’Journo XB, Roch A, et al. Protective ventilation influences systemic inflammation after esophagectomy: a randomized controlled study. Anesthesiology 2006;105:911-9. 10.1097/00000542-200611000-00011 [DOI] [PubMed] [Google Scholar]
- 88. Weingarten TN, Whalen FX, Warner DO, et al. Comparison of two ventilatory strategies in elderly patients undergoing major abdominal surgery. Br J Anaesth 2010;104:16-22. 10.1093/bja/aep319 [DOI] [PubMed] [Google Scholar]
- 89. Severgnini P, Selmo G, Lanza C, et al. Protective mechanical ventilation during general anesthesia for open abdominal surgery improves postoperative pulmonary function. Anesthesiology 2013;118:1307-21. 10.1097/ALN.0b013e31829102de [DOI] [PubMed] [Google Scholar]
- 90. Ferrando C, Soro M, Unzueta C, et al. Individualized PeRioperative Open-lung VEntilation (iPROVE) Network Individualised perioperative open-lung approach versus standard protective ventilation in abdominal surgery (iPROVE): a randomised controlled trial. Lancet Respir Med 2018;6:193-203. 10.1016/S2213-2600(18)30024-9 [DOI] [PubMed] [Google Scholar]
- 91. Futier E, Constantin JM, Paugam-Burtz C, et al. IMPROVE Study Group A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med 2013;369:428-37. 10.1056/NEJMoa1301082 [DOI] [PubMed] [Google Scholar]
- 92. Bugedo G, Retamal J, Bruhn A. Driving pressure: a marker of severity, a safety limit, or a goal for mechanical ventilation? Crit Care 2017;21:199. 10.1186/s13054-017-1779-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Amato MB, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 2015;372:747-55. 10.1056/NEJMsa1410639 [DOI] [PubMed] [Google Scholar]
- 94. Neto AS, Hemmes SN, Barbas CS, et al. PROVE Network Investigators Association between driving pressure and development of postoperative pulmonary complications in patients undergoing mechanical ventilation for general anaesthesia: a meta-analysis of individual patient data. Lancet Respir Med 2016;4:272-80. 10.1016/S2213-2600(16)00057-6 [DOI] [PubMed] [Google Scholar]
- 95. Benumof JL. Preoxygenation: best method for both efficacy and efficiency. Anesthesiology 1999;91:603-5. 10.1097/00000542-199909000-00006 [DOI] [PubMed] [Google Scholar]
- 96. Greif R, Akça O, Horn EP, Kurz A, Sessler DI, Outcomes Research Group Supplemental perioperative oxygen to reduce the incidence of surgical-wound infection. N Engl J Med 2000;342:161-7. 10.1056/NEJM200001203420303 [DOI] [PubMed] [Google Scholar]
- 97. World Health Organization Global Guidelines for the Prevention of Surgical Site Infection. World Health Organization, 2016. [PubMed] [Google Scholar]
- 98. Wadhwa A, Kabon B, Fleischmann E, Kurz A, Sessler DI. Supplemental postoperative oxygen does not reduce surgical site infection and major healing-related complications from bariatric surgery in morbidly obese patients: a randomized, blinded trial. Anesth Analg 2014;119:357-65. 10.1213/ANE.0000000000000318 [DOI] [PubMed] [Google Scholar]
- 99. Kurz A, Kopyeva T, Suliman I, et al. Supplemental oxygen and surgical-site infections: an alternating intervention controlled trial. Br J Anaesth 2018;120:117-26. 10.1016/j.bja.2017.11.003 [DOI] [PubMed] [Google Scholar]
- 100. Carpagnano GE, Kharitonov SA, Foschino-Barbaro MP, Resta O, Gramiccioni E, Barnes PJ. Supplementary oxygen in healthy subjects and those with COPD increases oxidative stress and airway inflammation. Thorax 2004;59:1016-9. 10.1136/thx.2003.020768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Rothen HU, Sporre B, Engberg G, Wegenius G, Reber A, Hedenstierna G. Atelectasis and pulmonary shunting during induction of general anaesthesia--can they be avoided? Acta Anaesthesiol Scand 1996;40:524-9. 10.1111/j.1399-6576.1996.tb04483.x [DOI] [PubMed] [Google Scholar]
- 102. Edmark L, Kostova-Aherdan K, Enlund M, Hedenstierna G. Optimal oxygen concentration during induction of general anesthesia. Anesthesiology 2003;98:28-33. 10.1097/00000542-200301000-00008 [DOI] [PubMed] [Google Scholar]
- 103. Rusca M, Proietti S, Schnyder P, et al. Prevention of atelectasis formation during induction of general anesthesia. Anesth Analg 2003;97:1835-9. 10.1213/01.ANE.0000087042.02266.F6 [DOI] [PubMed] [Google Scholar]
- 104. Benoît Z, Wicky S, Fischer JF, et al. The effect of increased FIO(2) before tracheal extubation on postoperative atelectasis. Anesth Analg 2002;95:1777-81. 10.1097/00000539-200212000-00058 [DOI] [PubMed] [Google Scholar]
- 105. Meyhoff CS, Wetterslev J, Jorgensen LN, et al. PROXI Trial Group Effect of high perioperative oxygen fraction on surgical site infection and pulmonary complications after abdominal surgery: the PROXI randomized clinical trial. JAMA 2009;302:1543-50. 10.1001/jama.2009.1452 [DOI] [PubMed] [Google Scholar]
- 106. Staehr-Rye AK, Meyhoff CS, Scheffenbichler FT, et al. High intraoperative inspiratory oxygen fraction and risk of major respiratory complications. Br J Anaesth 2017;119:140-9. 10.1093/bja/aex128 [DOI] [PubMed] [Google Scholar]
- 107. Prella M, Feihl F, Domenighetti G. Effects of short-term pressure-controlled ventilation on gas exchange, airway pressures, and gas distribution in patients with acute lung injury/ARDS: comparison with volume-controlled ventilation. Chest 2002;122:1382-8. 10.1378/chest.122.4.1382 [DOI] [PubMed] [Google Scholar]
- 108. Kim KN, Kim DW, Jeong MA, Sin YH, Lee SK. Comparison of pressure-controlled ventilation with volume-controlled ventilation during one-lung ventilation: a systematic review and meta-analysis. BMC Anesthesiol 2016;16:72. 10.1186/s12871-016-0238-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Cadi P, Guenoun T, Journois D, Chevallier JM, Diehl JL, Safran D. Pressure-controlled ventilation improves oxygenation during laparoscopic obesity surgery compared with volume-controlled ventilation. Br J Anaesth 2008;100:709-16. 10.1093/bja/aen067 [DOI] [PubMed] [Google Scholar]
- 110. Gupta SD, Kundu SB, Ghose T, et al. A comparison between volume-controlled ventilation and pressure-controlled ventilation in providing better oxygenation in obese patients undergoing laparoscopic cholecystectomy. Indian J Anaesth 2012;56:276-82. 10.4103/0019-5049.98777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Bagchi A, Rudolph MI, Ng PY, et al. The association of postoperative pulmonary complications in 109,360 patients with pressure-controlled or volume-controlled ventilation. Anaesthesia 2017;72:1334-43. 10.1111/anae.14039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ferrer M, Sellarés J, Valencia M, et al. Non-invasive ventilation after extubation in hypercapnic patients with chronic respiratory disorders: randomised controlled trial. Lancet 2009;374:1082-8. 10.1016/S0140-6736(09)61038-2 [DOI] [PubMed] [Google Scholar]
- 113. Gray A, Goodacre S, Newby DE, Masson M, Sampson F, Nicholl J, 3CPO Trialists Noninvasive ventilation in acute cardiogenic pulmonary edema. N Engl J Med 2008;359:142-51. 10.1056/NEJMoa0707992 [DOI] [PubMed] [Google Scholar]
- 114. American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea Practice guidelines for the perioperative management of patients with obstructive sleep apnea: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology 2014;120:268-86. 10.1097/ALN.0000000000000053 [DOI] [PubMed] [Google Scholar]
- 115. Stock MC, Downs JB, Gauer PK, Alster JM, Imrey PB. Prevention of postoperative pulmonary complications with CPAP, incentive spirometry, and conservative therapy. Chest 1985;87:151-7. 10.1378/chest.87.2.151 [DOI] [PubMed] [Google Scholar]
- 116. Squadrone V, Coha M, Cerutti E, et al. Piedmont Intensive Care Units Network (PICUN) Continuous positive airway pressure for treatment of postoperative hypoxemia: a randomized controlled trial. JAMA 2005;293:589-95. 10.1001/jama.293.5.589 [DOI] [PubMed] [Google Scholar]
- 117. Jaber S, Lescot T, Futier E, et al. NIVAS Study Group Effect of Noninvasive Ventilation on Tracheal Reintubation Among Patients With Hypoxemic Respiratory Failure Following Abdominal Surgery: A Randomized Clinical Trial. JAMA 2016;315:1345-53. 10.1001/jama.2016.2706 [DOI] [PubMed] [Google Scholar]
- 118. Esteban A, Frutos-Vivar F, Ferguson ND, et al. Noninvasive positive-pressure ventilation for respiratory failure after extubation. N Engl J Med 2004;350:2452-60. 10.1056/NEJMoa032736 [DOI] [PubMed] [Google Scholar]
- 119. Helviz Y, Einav S. A Systematic Review of the High-flow Nasal Cannula for Adult Patients. Crit Care 2018;22:71. 10.1186/s13054-018-1990-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Hernández G, Vaquero C, Colinas L, et al. Effect of Postextubation High-Flow Nasal Cannula vs Noninvasive Ventilation on Reintubation and Postextubation Respiratory Failure in High-Risk Patients: A Randomized Clinical Trial. JAMA 2016;316:1565-74. 10.1001/jama.2016.14194 [DOI] [PubMed] [Google Scholar]
- 121. Stéphan F, Barrucand B, Petit P, et al. BiPOP Study Group High-Flow Nasal Oxygen vs Noninvasive Positive Airway Pressure in Hypoxemic Patients After Cardiothoracic Surgery: A Randomized Clinical Trial. JAMA 2015;313:2331-9. 10.1001/jama.2015.5213 [DOI] [PubMed] [Google Scholar]
- 122. Futier E, Paugam-Burtz C, Godet T, et al. OPERA study investigators Effect of early postextubation high-flow nasal cannula vs conventional oxygen therapy on hypoxaemia in patients after major abdominal surgery: a French multicentre randomised controlled trial (OPERA). Intensive Care Med 2016;42:1888-98. 10.1007/s00134-016-4594-y [DOI] [PubMed] [Google Scholar]
- 123. Suter PM, Fairley HB, Isenberg MD. Effect of tidal volume and positive end-expiratory pressure on compliance during mechanical ventilation. Chest 1978;73:158-62. 10.1378/chest.73.2.158 [DOI] [PubMed] [Google Scholar]
- 124. D’Antini D, Rauseo M, Grasso S, et al. Physiological effects of the open lung approach during laparoscopic cholecystectomy: focus on driving pressure. Minerva Anestesiol 2018;84:159-67. [DOI] [PubMed] [Google Scholar]
- 125. Talmor D, Sarge T, Malhotra A, et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med 2008;359:2095-104. 10.1056/NEJMoa0708638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Rochwerg B, Brochard L, Elliott MW, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J 2017;50:1602426. 10.1183/13993003.02426-2016 [DOI] [PubMed] [Google Scholar]
- 127. Serpa Neto A, Cardoso SO, Manetta JA, et al. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA 2012;308:1651-9. 10.1001/jama.2012.13730 [DOI] [PubMed] [Google Scholar]
- 128. Noordzij PG, Poldermans D, Schouten O, Bax JJ, Schreiner FA, Boersma E. Postoperative mortality in The Netherlands: a population-based analysis of surgery-specific risk in adults. Anesthesiology 2010;112:1105-15. 10.1097/ALN.0b013e3181d5f95c [DOI] [PubMed] [Google Scholar]
- 129. Futier E, Marret E, Jaber S. Perioperative positive pressure ventilation: an integrated approach to improve pulmonary care. Anesthesiology 2014;121:400-8. 10.1097/ALN.0000000000000335 [DOI] [PubMed] [Google Scholar]