Abstract
Overdiagnosis, is defined as the diagnosis of a condition that, if unrecognized, would not cause symptoms or harm a patient during his or her lifetime, and it is increasingly acknowledged as a consequence of screening for cancer and other conditions. Because preventive care is a crucial component of primary care, which is delivered to the broad population, overdiagnosis in primary care is an important problem from a public health perspective and has far reaching implications. The scope of overdiagnosis as a result of services delivered in primary care is unclear, though overdiagnosis of indolent breast, prostate, thyroid, and lung cancers is well described and overdiagnosis of chronic kidney disease, depression, and attention-deficit/hyperactivity disorder is also recognized. However, overdiagnosis is a known consequence of all screening and can be assumed to occur in many more clinical contexts. Overdiagnosis can harm patients by leading to overtreatment (with associated potential toxicities), diagnosis related anxiety or depression, and labeling, or through financial burden. Many entrenched factors facilitate overdiagnosis, including the growing use of advanced diagnostic technology, financial incentives, a medical culture that encourages greater use of tests and treatments, limitations in the evidence that obscure the understanding of diagnostic utility, use of non-beneficial screening tests, and the broadening of disease definitions. Efforts to reduce overdiagnosis are hindered by physicians’ and patients’ lack of awareness of the problem and by confusion about terminology, with overdiagnosis often conflated with related concepts. Clarity of terminology would facilitate physicians’ understanding of the problem and the growth in evidence regarding its prevalence and downstream consequences in primary care. It is hoped that international coordination regarding diagnostic standards for disease definitions will also help minimize overdiagnosis in the future.
Introduction
Case scenario.
RG is a 48 year old woman who sees a new primary care physician. She has a body mass index of 30 and reports that her mother has diabetes. The physician is concerned about RG’s obesity and, given her age and family history, orders a hemoglobin A1C (HbA1C) test to screen for diabetes. Her HbA1C is 6.0% and the patient is told she has pre-diabetes. She is encouraged to modify her diet, increase her exercise, and consider a drug to prevent diabetes and its complications. She makes a few changes to her diet and starts taking metformin. She experiences side effects from the metformin but is happy when her next HbA1C test result is 5.8%. Has this patient experienced an early diagnosis or overdiagnosis?
In our healthcare systems, people interact with their primary care providers to engage in patient centered, comprehensive, and continuing care that includes disease prevention/health promotion, education and counseling, and diagnosis and treatment of new or ongoing problems.1 The specter of overdiagnosis, largely unrecognized and unmentioned, lurks behind much diagnostic testing performed in primary care.
Overdiagnosis is defined as the diagnosis of a condition (often subsequently treated), that would otherwise not cause symptoms or harm to a patient during his or her lifetime.2 It has long been recognized as a consequence of cancer screening but in recent years has increasingly been acknowledged as an important consequence of any diagnostic testing in the absence of symptoms.3 4 5 However, overdiagnosis remains the “elephant in the examination room” for a variety of patient, provider, and sociocultural reasons, including confusion surrounding the meaning of the term, lack of awareness outside the realm of cancer screening, and expectations about healthcare. Because primary care is delivered, at least ideally, to the entire population, the problem of primary care related overdiagnosis is of particular importance in the realm of public health.
In this review, we present an evidence based summary of the scope of overdiagnosis related to screening of asymptomatic patients in primary care and provide clinical examples of how overdiagnosis is manifest in the primary care setting. We also clarify definitions of overdiagnosis and related terms, discuss drivers and consequences, and clarify the ways in which it can be documented and studied. Lastly, we suggest future steps to guide research and the implementation of discussions about overdiagnosis in primary care.
Sources and selection criteria
We began with a review of the literature to understand the scope of the published evidence related to overdiagnosis in primary care, defining overdiagnosis as identification of a condition that would not cause clinical harm during the patient’s lifetime.6 We searched PubMed, Embase, and Web of Science on 29 August 2017 using combinations of the following terms: “over diagnosis”, overmedicaliz(s)ation, “medical overuse” [MeSH], “general practice”, “primary health care” [MeSH], and “general practice” [MeSH], with no language restriction. In addition, we hand searched references from relevant identified articles. We excluded letters and articles published only in abstract form. We excluded articles in which the definition of the term “overdiagnosis” differed from our operational definition. After reviewing 582 titles and abstracts, we identified 71 publications related to overdiagnosis in primary care, including primary studies (n=19), systematic reviews and meta-analyses (n=5), narrative reviews (n=26), editorials (n=14), guidelines (n=4), and other (n=3).
To understand the prevalence of overdiagnosis more fully, we performed a second search of the same three databases on 8 November 2017 to identify studies quantifying overdiagnosis but not limited to primary care. The second search identified 1073 papers, of which 46 quantified overdiagnosis. After reference tracking we added eight papers for a total of 54 papers.
In papers from our first search (overdiagnosis in relation to primary care), cancer was most commonly mentioned, specifically prostate (n=16) and breast (n=12), with fewer discussions of lung (n=5), colon (n=4), and thyroid (n=2) cancers (appendix table A). We also found articles about chronic kidney disease (n=5), depression (n=4), neuroblastoma (n=3), and attention-deficit/hyperactivity-disorder (n=5), with additional diseases mentioned in a single paper (appendix table B). From our search of overdiagnosis quantification, which was not limited to primary care, we found that overdiagnosis of breast cancer was most commonly measured (24 papers), followed by prostate cancer (n=10), lung cancer (n=8), and thyroid cancer (n=4). Other cancers and non-cancer problems were covered in eight studies (appendix table C). The detailed content of the identified papers informed our analysis and discussion.
Context of overdiagnosis
In the case scenario, RG’s diagnosis of pre-diabetes may represent overdiagnosis. Although it is impossible to identify overdiagnosis at the point of care with an individual patient, the potential for overdiagnosis can be understood on the basis of the evidence of benefits and harms of screening. In the case of screening for diabetes, only recently has limited direct evidence shown that screening improves clinical outcomes; in one European study of 1 900 000 patients, screening for diabetes in high risk people was associated with statistically significant reductions in all cause mortality and cardiovascular disease events at 10 years (hazard ratio 0.84 (confidence interval 0.80 to 0.89) for cardiovascular events).7 Randomized trials have shown that pre-diabetes interventions (lifestyle and drugs) can prevent or delay the progression to type 2 diabetes.8 However, a meta-analysis of prospective observational trials of progression rates from pre-diabetes to diabetes found that more than half of people with pre-diabetes do not have diabetes after 10 years, suggesting that this condition often does not progress to clinically important disease, so that treatment in this situation would fulfill the definition of overdiagnosis.9
Given the high prevalence of diabetes, the many complications of untreated disease, and the benefit of screening at a population level, screening for diabetes is recommended in high risk groups in many countries including the United Kingdom,10 Canada,11 and the United States,8 despite the potential for overdiagnosis. In RG’s case, screening was consistent with guidelines from these countries. The case of pre-diabetes illustrates the potential for overdiagnosis that exists in any screening scenario. Overdiagnosis was first described in relation to cancer screening.12 However, the potential for overdiagnosis accompanies screening for non-cancer conditions including hypertension, hyperlipidemia, and even a “screening” physical examination in an asymptomatic person. Theoretically, the potential for overdiagnosis would be eliminated if important disease that would threaten health and require treatment could be accurately identified. However, perfect tests do not exist and all diseases occur along a clinical spectrum, so overdiagnosis remains.
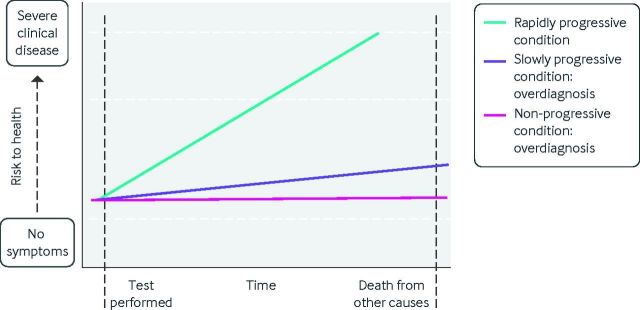
Overdiagnosis, then, is inherent to the modern practice of healthcare, which seeks to diagnose and mitigate disease before it is clinically evident. Figure 1 illustrates patterns of disease progression after diagnostic testing. The test in question could be an imaging test, a laboratory test, a component of the physical examination, or even an interview question. Tests are intended to identify clinically meaningful disease (green line), which if untreated will go on to threaten health or even become fatal. Treatment of these diseases will improve health. A subset of disease, however, will progress more slowly and will not threaten health before the patient’s death from other causes (purple line). Finally, some disease will never progress at all (pink line). The identification of patients with slowly progressive or non-progressive disease represents overdiagnosis because treatment will not improve these patients’ health will but still expose them to potential harms.
Fig 1.
Disease trajectory and overdiagnosis. The green line depicts the course of true disease, which would progress to threaten health if left untreated. The purple and pink lines depict the course of overdiagnosed disease, which even if left untreated would not progress to threaten health
Some level of overdiagnosis is unavoidable, owing in part to the unacknowledged trade-off between minimizing underdiagnosis and tolerating overdiagnosis. To optimize health, we seek to reduce underdiagnosis, or the failure to identify a disease that ultimately threatens a person’s health.13 Ideally, screening limits the underdiagnosis of early disease that is destined to progress. When determining the effectiveness of screening, we evaluate the balance between clinical benefit (such as improved mortality) and clinical harm (such as complications from diagnostic tests and treatment) in a screened population. Going back to our case of pre-diabetes, a few studies show that screening for diabetes leads to lower mortality and mixed evidence suggests that tighter control of type 2 diabetes leads to a reduction in some macrovascular complications.14 Although overdiagnosis of both diabetes and pre-diabetes is a consequence of screening, guideline panels believe that harms related to overdiagnosis are offset by the population level benefits of early diagnosis (and subsequent treatment) of true disease and therefore recommend risk assessment and targeted screening. Limiting screening to people at the highest risk can minimize (though not eliminate) overdiagnosis while maximizing benefit. However, to reduce overdiagnosis and its resultant harms further we must also understand the factors that drive it.
Drivers of overdiagnosis
Several factors work independently and together to encourage overdiagnosis (table 1).
Table 1.
Drivers of overdiagnosis*
| Category | Factor | Example |
|---|---|---|
| Broadening disease definitions | Lowering of diagnostic thresholds | Changes that defined CKD at a higher creatinine clearance led to diagnosis of CKD in 25-35% of people over age 65, few of whom will progress to end stage renal disease15 16 |
| Recognition of risk factors as pre-diseases | Pre-diabetes is highly prevalent (eg. prevalence nearly 36% in China); many patients are treated with drugs.17 Only about a third will progress to true diabetes over 10 years9 18 | |
| Technology | Use of advanced technology for diagnosis | Increasing use of CT, ultrasound, and MRI over time lead to a dramatic rise in the incidence of incidentally detected thyroid cancer, with no concurrent change in mortality19 |
| Use of more sensitive screening tests | Digital mammography is more sensitive than film mammography in some groups but tumors detected have better prognosis, suggesting overdiagnosis20 21 | |
| Public health interventions | Widespread screening | Population based breast cancer screening results in 1-10% of cancers; this represents overdiagnosis (in European countries)22 |
| Culture of medical care | Value of diagnosis for its own sake | Both patients and physicians feel anxious when problems are not labeled with a diagnosis23 |
| Clinician cognitive errors | Overestimation of benefit of therapy in mild or low risk disease | Widespread treatment of hyperlipidemia in patients who are otherwise at low risk of cardiovascular disease, with little potential benefit24 |
| System factors | Financial incentives for more testing | “Executive physicals” that include multiple unnecessary tests generate revenue for hospitals and are heavily marketed to companies and individuals25 |
| In the US, ownership of imaging equipment by physicians is associated with more testing and higher costs, with similar clinical outcomes26 | ||
| Evidence limitations | Lack of clarity regarding disease spectrum in studies of diagnostic accuracy | CTA is highly sensitive for diagnosing pulmonary embolism, but studies included all emboli, even small ones that may be clinically unimportant,27 leading to a near doubling of the incidence with little change in mortality and a rise in bleeding complications28 |
CDK=chronic kidney disease; CT=computed tomography; CTA= computed tomography pulmonary angiography; MRI=magnetic resonance imaging.
Broadening disease definitions
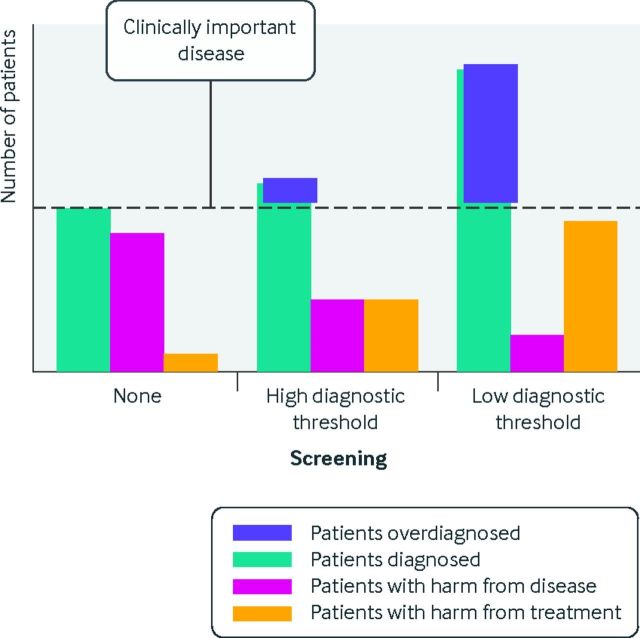
Many diseases exist on a severity spectrum. Disease cut-off points must be selected (for example, the blood pressure at which hypertension is considered) and are often chosen to minimize underdiagnosis, sometimes at the expense of high rates of overdiagnosis. Figure 2 illustrates the impact of different diagnostic thresholds on numbers of patients diagnosed, harmed from disease, and harmed from treatment. In the absence of screening, many patients have delayed diagnosis, all patients have the potential to be harmed by their disease, and few are harmed from treatment of the disease. When screening is performed with a high diagnostic threshold for disease (for example, screening for diabetes with a high glucose cut-off point), more patients are diagnosed compared with no screening, fewer have clinical harm from disease, somewhat more are potentially harmed by treatment (because more are treated), and a small amount of overdiagnosis occurs. Finally, screening with a low diagnostic threshold (for example, screening for diabetes with a low glucose cut-off point) leads to more patients being diagnosed with the disease, many patients being overdiagnosed, more being harmed from treatment, and the fewest being harmed by untreated disease.
Fig 2.
The impact of changing diagnostic thresholds on screening related overdiagnosis. Without screening, many patients with disease go undiagnosed and many experience harm from disease. Screening with a high diagnostic threshold results in more patients being diagnosed and fewer experiencing harms related to the disease, though more experience treatment related harms, and a small amount of overdiagnosis occurs. Screening with a low diagnostic threshold results in many more patients being diagnosed, among whom many are overdiagnosed, with little harm from disease but more harm related to treatment
Going back to our case, RG has been diagnosed with pre-diabetes, a relatively recently defined “disease.” Traditionally, glucose cut-off values defining diabetes were based on the risk of retinopathy.29 However, a desire to prevent other complications of diabetes led to the establishment of various diabetes precursor conditions such as impaired fasting glucose, impaired glucose tolerance, and borderline HbA1C values, with cut-off values defined on the basis of the risk of progression to frank diabetes.30 The creation of these “at risk for diabetes” categories led to the creation of the umbrella term pre-diabetes, which in effect broadened the population of patients diagnosed with diabetes in some form. However, the diagnostic tests used to identify pre-diabetic conditions have variable accuracy for predicting diabetes and different performance characteristics in different populations.31 Reliance on the fiction of a “one size fits all” test with a low diagnostic threshold to diagnose pre-diabetes (such as HbA1C of 5.7%) leads to more diagnoses of pre-diabetes with fewer of these people developing diabetes. Moreover, expanding the spectrum of diabetes to include pre-diabetes promotes the false idea that untreated pre-diabetes will universally lead to diabetes. The notion of a “pre” disease condition is not limited to diabetes; it is also discussed for conditions such as osteopenia32 and for cardiovascular risk factors such as hypertension and hyperlipidemia.33 In the case of hypertension, the expansion of guideline definitions of hypertension to include lower systolic blood pressures has identified patients with a lower risk of poor cardiovascular outcomes who have “hypertension,” thereby subjecting them to treatment that is unlikely to benefit them.
Advanced technology
The increasing availability and use of advanced technology also contributes to overdiagnosis. For example, in the eight years after high resolution computed tomography pulmonary angiography became available to diagnose pulmonary embolism, the number of cases doubled compared with the previous five years.34 In the absence of overdiagnosis, an increase in the number of cases should have led to fewer deaths from untreated pulmonary embolism. However, mortality did not change over that time period; this implies that the additional cases of pulmonary embolism were clinically insignificant and overdiagnosed.34
Incidentalomas provide another example of the impact of the widespread use of advanced imaging. About 5-15% of all abdominal imaging tests performed in asymptomatic people contain incidental findings.35 Despite recognition that most of these findings are of little clinical significance, their presence often triggers a cascade of unnecessary sequential diagnostic tests. On the rare occasion that an incidentally found abnormality appears to be clinically important, clinicians or patients may erroneously conclude that more imaging saves lives.
Worry from incidental findings is heightened when the incidental finding represents a potentially fatal tumor, such as thyroid cancer. Since the introduction of neck ultrasound in the 1980s, thyroid imaging has become common and the incidence of thyroid cancer has increased globally, mainly as a result of a rise in small papillary carcinomas, with little change in mortality from thyroid cancer. In this context, nearly half of thyroid cancers in men and more than 80% in women in high resource settings are estimated to represent overdiagnosis, amounting to more than 500 000 cases.36 The problem of incidental findings of unclear importance may be compounded by genetic testing, both in clinical and direct-to-consumer settings, the explosive growth of which may result in widespread overdiagnosis owing to the identification of gene carriers who may never develop disease.37
Public health screening programs
The use of screening programs as a disease prevention and control strategy is an important driver of overdiagnosis. By design, screening programs presume that a reservoir of undiagnosed disease exists and that screening will lead to a lower clinical impact of, or mortality from, that disease. However, a screening program will identify all disease along the spectrum of clinical severity and may tend to detect more indolent disease, with little to distinguish between severe and indolent disease.38 Screening therefore always results in some degree of overdiagnosis. Screening for breast cancer provides an important example. Estimated rates of overdiagnosis of breast cancer with screening vary across countries and populations, but the presence of overdiagnosis is universally accepted.39 A UK panel estimated that 11% of screen detected breast cancers represent overdiagnosis,40 and rates of overdiagnosis of breast cancer in Europe are estimated to range between 1% and 10%.22 Within the US system there is more overdiagnosis in higher income populations, which may reflect better access to care, particularly screening.41
Culture around medicine and health
In many countries there is great public enthusiasm for cancer screening and a relative lack of concern about the potential for overdiagnosis. In a study of the general US population, most adults believed that routine cancer screening is almost always a good idea, with 75% also believing that finding cancer early saves lives most or all of the time.42 A more recent survey assessed people’s tolerance of overdiagnosis in the context of effective screening. Although responses varied widely and there is no “correct” answer, participants tolerated a median of 113-150 cases of overdiagnosis per 1000 people screened to save one life.43 Notably, most survey respondents reported never having previously heard of overdiagnosis despite more than half having been screened for cancer.
System factors
Reimbursement structures may also drive overdiagnosis. In fee for service healthcare systems direct financial incentives may encourage testing regardless of clinical appropriateness. In the US, the ownership of imaging equipment by physicians is associated with more testing with similar clinical outcomes, implying that financial incentives motivate clinical behavior towards excessive testing.26 Similarly, a lower share of public health expenditure (that is, more reliance on direct payments from individuals and private health insurance) has been found to be associated with a higher incidence of thyroid cancer across different healthcare systems, with no difference in mortality from thyroid cancer,44 which again suggests profit driven testing.
Financial incentives can also operate through more complex mechanisms involving the drug and medical device industries, which may contribute to overdiagnosis in several ways. Firstly, industry may seek to expand drug markets by working to broaden disease definitions to create more patients who are eligible for certain drugs through influencing guidelines (for example, guidelines for lipid lowering), diagnostic criteria (such as in the Diagnostic and Statistical Manual of Mental Disorders (DSM)), and the content of medical education.45 46 47 48 In countries that allow direct-to-consumer advertising, drug advertising may obscure the line between disease and normal variants, driving patient requests for prescriptions. Furthermore, disease awareness campaigns may serve as proxies for efforts to increase patient requests for diagnostic testing for the disease in question and ultimately for drugs to treat it.49
Limitations in evidence application
Evidence based medicine, the application of the best medical research to the clinical care of individual patients, is understood to optimize medical decision making and improve patient outcomes.50 However, problems with physicians’ application of evidence and limitations in the evidence itself can contribute to overdiagnosis. Physicians have a poor understanding of quantitative information and test and treatment performance, which probably contributes to unnecessary testing and overdiagnosis.51 52 Several studies have examined the role of cognitive biases and heuristics on medical decision making. Doctors’ susceptibility to decision making biases such as insensitivity to known probabilities, availability, and confirmation affects their ability to interpret information, including physical examination findings and diagnostic test results.53 54 Some cognitive errors may contribute to overdiagnosis. One example is the representativeness bias, in which an individual is assumed to belong to or to be representative of a category (such as a disease) on the basis of similarity to other characteristics in the category. In practice, representativeness bias may lead a physician to overestimate the benefit of an intervention in a patient with a lower risk of disease, expecting that patient to experience similar benefit to one with more severe disease who is at higher risk of complications. Similarly, availability bias, the tendency for recent experiences to affect decision making, might lead a physician to pursue diagnostic testing aggressively because of a recent negative experience with a different patient in whom a diagnosis was missed. Many of these cognitive errors can lead physicians to overestimate the benefit of diagnosing and subsequently treating mild disease, thus pushing them to practice in a way that fosters overdiagnosis.
Important limitations with the evidence related to diagnostic test accuracy and the effectiveness of treatments also enable overdiagnosis. With regard to diagnostic testing, studies evaluating diagnostic accuracy often ignore the problem of disease spectrum, and high sensitivity tests may largely detect clinically insignificant disease,55 as seen with computed tomography pulmonary angiography, described above.28 Similar problems occur in studies evaluating the effects of treatments. Clinical trials generally report the average effect across a population; however, the average treatment effect applies poorly to individuals, with the benefit in a particular patient being related to disease severity.56 Valuing and applying the same intervention to people at lower risk of benefit to those at high risk may lead to treatment of overdiagnosed disease and unnecessary exposure of patients to potential treatment toxicities. By contrast, targeting high risk patients for intervention can maximize net benefit in the population. For example, re-analysis of data from the National Lung Screening Trial showed that using a risk of death from lung cancer based approach to screening would prevent the greatest number of deaths among those at highest risk, with very few deaths among low risk patients who would not be screened.57
Magnitude of the problem
The magnitude of overdiagnosis is unclear. The limitation in our understanding is in part attributable to the challenges of studying overdiagnosis and in part due to relative lack of attention to the problem in many clinical areas, particularly outside of cancer. Our literature review yielded few reliable estimates of rates of overdiagnosis in non-cancer clinical areas.
Overdiagnosis has been quantified for many cancers (table 2). The best evidence exists for prostate and breast cancers.2 In these two diseases, estimates of the proportion of disease that represents overdiagnosis vary widely across studies, reflecting both the challenges of quantifying overdiagnosis and the widely variable rates of overdiagnosis based on patient characteristics. Between 2.9% and 88.1% of prostate cancers have been estimated to represent overdiagnosis.
Table 2.
Estimated proportions of cancers that represent overdiagnosis*
| Disease | Context | Range of estimates |
|---|---|---|
| Breast cancer | Population based screening with mammography | 1-10% (Europe)22
12.4% (Canada, age 40-49)58 9.7% (Canada, age 50-70) |
| Prostate cancer | Screening with PSA | 2.9-88.1% based on age and characteristics59 |
| Lung cancer | Screening high risk patients with LDCT | 11.9% (US)60 |
| Thyroid cancer | Spread of imaging | 49% (men across developed countries) 83% (women across developed countries)36 |
LDCT=low dose computed tomography; PSA=prostate specific antigen.
The scope of overdiagnosis in diseases other than cancer has not been well defined.61 Although screening for chronic non-cancer conditions such as hypertension, diabetes, and depression comprises much of the routine work of primary care, there are few estimates of the magnitude of overdiagnosis of these disorders. In our literature review, we found only a few studies that quantified overdiagnosis of non-cancer conditions, namely chronic kidney disease, and abdominal aortic aneurysm (AAA). In kidney disease, authors simply reported rising rates of diagnosis and suggested that the rise probably reflected overdiagnosis without quantifying the proportion of actual overdiagnosis.62 The paper on AAA used evidence on the risk of rupture to estimate rates of overdiagnosis for screen detected AAAs of various sizes; the number of AAAs representing overdiagnosis ranged from 11.5% for >54 mm aneurysms to 87% for 26-29 mm aneurysms.63
Consequences of overdiagnosis
Overdiagnosis has many consequences, some theoretical and others more quantifiable. The effects are multi-pronged, affecting the individual, healthcare system, and society at large.
Overtreatment
Overtreatment refers to the unnecessary treatment of a condition. It occurs whenever overdiagnosed disease is treated and can affect the individual patient as well as the wider healthcare system. Overdiagnosed disease provides no opportunity for treatment benefit so the individual incurs only harms. These potential harms include direct negative consequences of the unnecessary treatment itself (such as a wound infection after thyroidectomy to treat an overdiagnosed thyroid cancer) and indirect harms related to the consequences of resultant downstream services (such as palpitations resulting from an incorrect dose of replacement levothyroxine after thyroidectomy). The individual patient is also affected by the opportunity costs related to treatment—for example, time away from usual activities while recovering from surgery.
The effects of overtreatment on the healthcare system and society are less obvious and challenging to measure. Given limited total capacity for healthcare delivery, overtreatment of one patient may limit healthcare access for another person who may truly need care, causing harm to people other than the overtreated patient, which on a broad scale amounts to societal harm.64 In addition, health system investment in overtreatment deflects resources from other pressing medical needs and represents lost opportunity to improve the health of the public.65 66
Psychological harms
It is difficult to estimate psychological harm from overdiagnosed disease because the patient often does not know the “disease” represents overdiagnosis. Patients with recognized overdiagnosed thyroid cancer who opted against aggressive care have reported anxiety and feelings of isolation and secret keeping,67 though evidence in other clinical settings is limited. However, the psychological ramifications of disease diagnoses probably apply to overdiagnosis as well as to legitimate diagnoses.68 New diagnosis of a chronic disease may require patients to adjust their life expectations and employment, owing to prognostic and functional considerations and matters related to disease treatment.69 Some may experience depression and anxiety after a new diagnosis.70 71 For example, an international study of breast cancer survivors found an increased risk of suicide up to 25 years after diagnosis,70 and patients with ductal carcinoma in situ, which may represent overdiagnosed breast cancer, often experience persistent anxiety related to fear of recurrence and death.72 73 In the context of screening, psychological harms related to unexpected diagnoses of prostate cancer and AAA have been described, though evidence for most diseases is lacking.74 75
Labeling
Patients can be affected by being “labeled” with a disease diagnosis in many adverse ways. In children, the diagnosis of a benign heart murmur can lead to unnecessary restrictions on activity.76 77 In adults the impact of labeling is more difficult to ascertain. Interestingly, patients who undergo early imaging for mild acute low back pain, which is likely to reveal insignificant anatomic abnormalities, are more likely than non-imaged patients to be out of work with disability one year later.78 This suggests that the knowledge of an identifiable abnormality may affect people’s perceptions about their own health and engagement in society.
Financial harms
The potential financial harms of overdiagnosis are enormous and contribute to waste in healthcare systems.79 In the US, the cost of breast cancer overdiagnosis alone (both ductal carcinoma in situ and invasive breast cancer) in women aged 40-59 years has been estimated at $1.2bn (£0.91bn; €1.03bn) a year.80 As health systems around the world struggle to reduce unsustainably high costs,81 the elimination of overdiagnosis is an attractive way to save money without compromising, and in fact improving, public health.
Related terms
Clarity of terminology is crucial to efforts to enhance the understanding of overdiagnosis and minimize its impact. Table 3 describes terms that are related to overdiagnosis and that may be confused with true overdiagnosis.
Table 3.
Terms related to overdiagnosis
| Term | Definition | Comments |
|---|---|---|
| Overdiagnosis | The diagnosis of a condition that would not cause clinical harm during the patient’s lifetime6 | Can result from appropriate or unnecessary testing |
| Overuse (or overutilization) | The provision of health services that are more likely to harm than to benefit the patient82 | A fundamental quality problem |
| Overtreatment | A therapeutic intervention for which potential harm outweighs potential benefit83; can refer to excessive intensity of a treatment that may otherwise be appropriate84 | Can be a subcategory of overuse or represent overly aggressive treatment that may not meet the definition of overuse |
| Overmedicalization | Reinterpretation of human experiences as medical problems, without net clinical benefit4 | A social phenomenon that can lead to or result from overdiagnosis, overtreatment, and overuse |
| Misdiagnosis | An incorrect diagnosis of an illness or problem85 | A type of medical error |
| Misuse | The provision of an appropriate service where a preventable complication interferes with patient benefit82 | A fundamental quality problem related to patient safety |
| Disease mongering | Encouragement of overmedicalization by outside forces to maximize profits | A strategy pursued by the drugs industry to create or broaden drug markets |
| Low value care | The provision of health services that are wasteful or provide little or no benefit to patients86 87 | Implies cost inefficiency; term often used vaguely |
In our literature review, we noted that several of these terms are often conflated with overdiagnosis. Many articles using the term overdiagnosis were really describing misdiagnosis, defined as the diagnosis of the wrong disease.85 For example, in one cross sectional study of overdiagnosis of heart failure diagnosed by primary care practitioners, researchers found that one in six people probably did not have heart failure, but had another disorder instead.88 Although it is tempting to use the term overdiagnosis to describe the mislabeling of these patients (because the term conjures the concept of an excess of a diagnosis), this example reflects an inaccurate diagnosis and not true overdiagnosis. For patients incorrectly labeled as having heart failure, their symptoms (presumably breathlessness) were not caused by heart failure so the diagnosis was in fact a medical error.
Overdiagnosis and overmedicalization are also often conflated. The confusion regarding these terms is natural for semantic reasons, and they have overlapping themes, concepts, and drivers.4 In overmedicalization, a phenomenon that is part of the normal human experience is recontexualized as disease and (often) treated as such. Overmedicalization shares many of the same consequences of overdiagnosis (such as labeling, overtreatment, and excessive cost). For example, advances in medical technology and changes in culture have led to overmedicalization of the process of dying. This has increased the use of more intensive procedures among those who are dying, with no benefit to individuals, and has resulted in a rise in deaths in hospital.89
The term “disease mongering” is related to overmedicalization but it is used to describe situations in which outside forces, mainly the drugs industry, encourage overmedicalization for the purpose of creating new drug markets.90 91 For example, premenstrual dysphoric disorder, first mentioned in the DSM fourth edition in 1994,92 93 represents a severe form of premenstrual symptoms. A drug targeting premenstrual dysphoric disorder, Sarafem, was developed, approved by the Food and Drugs Administration, and heavily marketed to physicians and (in the US) consumers. However, Sarafem, which was approved by the FDA in 2001, was simply rebranded fluoxetine, which, in the form of Prozac, went off patent during that same year.94 Although many women experience severe menstrual symptoms,95 the relabeling of the experience as a disease is likely to have led to more widespread, and possibly unnecessary, drug treatment. Advertising is a powerful tool to facilitate disease mongering and foster overdiagnosis, as is shown in the case of Sarafem and others. In the US, direct-to-consumer advertising of testosterone replacement products was associated with testosterone testing and initiation of treatment with testosterone replacement therapy96; many of these cases probably represented overdiagnosis.
It is also important to distinguish misuse, or preventable complications of care, from overdiagnosis.82 Misuse may arise while treating an overdiagnosed disease, and some may argue that any treatment of such a disease is a case of misuse. However, given the difficulty in discerning overdiagnosis in a clinical scenario in real time, this is more of a theoretical concern. The growing emphasis on avoiding low value care is also relevant to the discussion of overdiagnosis. Low value care is defined as care that results in little benefit relative to its cost.86 87 Although care related to overdiagnosis is inherently low value, not all low value care is related to overdiagnosis.
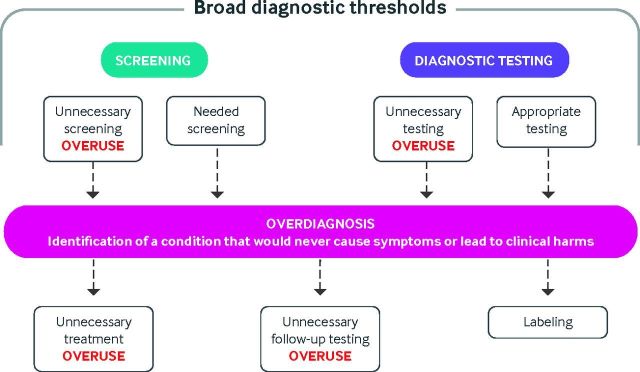
Finally, medical overuse is a term used by some authors interchangeably with overdiagnosis. Overuse is the use of unnecessary health services, either tests or treatments, for which potential harms outweigh potential benefits.82 The association between overuse and overdiagnosis is complicated (see fig 3). Overuse is broader than overdiagnosis and can be both a cause and a driver of overdiagnosis.
Fig 3.
Association between overdiagnosis and overuse of medical services
Figure 3 describes testing and screening scenarios that may lead to overdiagnosis, the consequences of overdiagnosis, and the association between overdiagnosis and the overuse of medical services (unnecessary testing and treatment). Both appropriate and inappropriate testing, as well as broadening of diagnostic thresholds, can lead to overdiagnosis, which in turn can lead to labeling and unnecessary testing and treatment.
Studying overdiagnosis
There are several approaches to quantifying overdiagnosis; each has its own set of assumptions that lead to bias and limitations. These biases in part contribute to the wide ranges of rates of overdiagnosis among studied disease, as seen in table 2.
Table 4 describes approaches to quantifying overdiagnosis. Several methods are used and, confusingly, the incidence of overdiagnosis can be expressed as a proportion of screen detected cases or as a proportion of all cases of disease.97 98 The excess incidence approach leverages long term follow-up of groups that had been exposed or unexposed to a diagnostic test, generally a screening test for cancer. Patients’ exposure to testing can occur within a randomized trial or in real world settings. After a lengthy follow-up time, the number of cancers in the screened group is compared with the number in the group that did not undergo screening; cancers in the control group would have been detected clinically or incidentally during other testing. After enough time, the number of cancers in the control group will catch up with that in the screened group—the two groups should have the same number of clinically important cancers over time but early detection as a result of screening should be associated with better prognosis in the screened group. Extra cancers in the screened group, the excess incidence, represent overdiagnosed cancers that were never destined to become clinically important. For example, a Canadian study compared rates of breast cancer over time before and after the introduction of population based screening.99 By assessing the increase in the total number of cancers after screening was introduced, the authors estimated that 5.4% of invasive breast cancers were overdiagnosed. The excess incidence approach is considered the most reliable method for estimating overdiagnosis, but it is resource intensive and requires very long follow-up.
Table 4.
| Approach | Description | Rationale | Study design | Considerations |
|---|---|---|---|---|
| Excess incidence | Compares rates of disease in a population exposed to a test with those in a non-exposed population. The difference in rates is considered overdiagnosis | Over time, disease in the control group will be diagnosed clinically, so excess diagnoses in the tested group after long term follow-up are not clinically significant and represent overdiagnosis | Long term follow-up of a randomized controlled trial comparing diagnostic or screening approaches | Applies best to cancer screening Requires very long follow-up Resource intensive Objective Least subject to bias |
| Cohort or ecological studies where patterns of test use differed between populations | Subject to bias based on population differences, secular trends, or other factors | |||
| Lead time | Uses models of the natural course of disease to compare survival time with testing or screening to what is expected in the population to generate estimates of overdiagnosis | In cases of true diagnosis, testing discovers disease earlier, resulting in longer survival. Disease that is diagnosed that would not present clinically before death from another cause represents overdiagnosis | Modeling studies, using assumptions about the natural course of disease and expected mortality | Allows adjustment for secular trends Includes many assumptions that can be flawed Can be done in the absence of long term trial data |
| Disease characteristics | Evaluates disease characteristics and considers the most benign disease to represent overdiagnosis | Characteristics on imaging or pathology over time correlate with disease progression and can be used to estimate overdiagnosis | Retrospective observational studies, autopsy studies | Simple and easy to perform Subject to multiple biases Adequate adjustment for confounders is challenging |
A second approach to estimating rates of overdiagnosis, the lead time approach, uses modeling based on estimates of expected rates of disease. Known treatment patterns, response rates, rates of disease progression, and likelihood of mortality from competing causes are used to estimate predicted disease survival times and overall survival times with screening. In these models, the proportion of patients in the screened group predicted to die of their disease beyond their overall predicted life expectancy represents overdiagnosis. Investigators have used a lead time approach to estimate rates of overdiagnosis associated with lung cancer screening. Simulating rates of lung cancer development, progression, detection, follow-up, treatment, and survival, investigators estimated that a mean of 11.9% (range 5.5-23.2%) of screen detected cancers were overdiagnosed.100
The third and least accurate method for estimating rates of overdiagnosis relies on disease characteristics. This approach uses pathologic features of screen detected disease to predict future clinical behavior; disease that is predicted to never become clinically important (or to become important only after the patient is expected to die from other causes) defines overdiagnosis. For example, a study of prostate cancer reported an increase in small and very early stage tumors over time as screening rates rose; the proportion of these low risk tumors reflected the rate of overdiagnosis with screening.101 Although this method is informed by an understanding of the association between disease characteristics and prognosis, it involves many assumptions and offers only a rough estimate of rates of overdiagnosis, though future growth in biomarkers to distinguish indolent from aggressive disease may improve its accuracy.
Minimizing and managing overdiagnosis
Because overdiagnosis is an expected consequence of screening in asymptomatic people, some degree of overdiagnosis will persist. However, the ethical obligation to avoid patient harm compels physicians to minimize the prevalence of overdiagnosis,102 which can be done by improving the understanding of overdiagnosis, optimizing disease definitions, and considering overdiagnosis when making clinical decisions. We discuss these three goals and strategies to achieve them.
Firstly, enhancing the evidence base related to overdiagnosis would improve understanding. We need better estimates of rates of overdiagnosis of non-cancer conditions for which there is widespread screening in primary care, including hypertension, mental health disorders, and hyperlipidemia. Although there are methodological concerns, even imprecise estimates of rates of overdiagnosis of these common conditions would be helpful. In part the evidence could be advanced through required reporting of overdiagnosis in studies involving changes in diagnostic thresholds and in studies of new diagnostic tests or screening approaches.
Secondly, the medical community could minimize overdiagnosis by optimizing disease definitions. Because broadening of definitions of disease can lead to overdiagnosis, any changes should use a systematic, transparent approach where benefits and harms are explicit, especially when they lead to an increase in the prevalence of disease, and broadening of definitions should require evidence of clinical benefit. International standards for defining disease and altering disease definitions already incorporate these concepts; such standards could be institutionalized by professional societies and guideline developing organizations.103 Primary care providers could minimize overdiagnosis by avoiding unnecessary screening and testing; a more consistent approach to defining disease would facilitate best clinical practice and benefit patients overall.
Thirdly, overdiagnosis must be better managed. Guidelines related to screening examinations and changes to disease definitions must acknowledge the potential for overdiagnosis and attempt to quantify it. Discussion of possible overdiagnosis is already commonly recommended in cancer screening guidelines104 105 106 and has been alluded to outside of cancer; for example, in the National Institute for Health and Care Excellence hypertension guideline.107 Broader incorporation of concerns about overdiagnosis into guidelines would be facilitated by the inclusion of such concerns into guideline development standards from organizations such as the Institute of Medicine and the Guidelines International Network.60 108
Primary care clinicians are also responsible for improving the management of overdiagnosis and can use several strategies. Firstly, clinicians must inform patients about overdiagnosis and incorporate it into clinical decision making. Currently, few patients who undergo cancer screening report discussing overdiagnosis with their physician, and it is likely that even fewer discuss overdiagnosis when they undergo screening for non-cancer conditions such as diabetes.109 110 There are challenges to discussing overdiagnosis with patients; the concept may be difficult to understand and some may not recognize overdiagnosis as a real problem.111 112 Patients who do appreciate the potential for overdiagnosis may be reluctant to raise concerns with their doctors. Patient decision aids can facilitate these discussions and enhance patient knowledge and informed screening choices.113 They are particularly suited to decisions related to population based cancer screening, where benefits and harms are similar across the population.65 Figure 4 illustrates overdiagnosis in the context of benefits and harms of prostate cancer screening.
Fig 4.
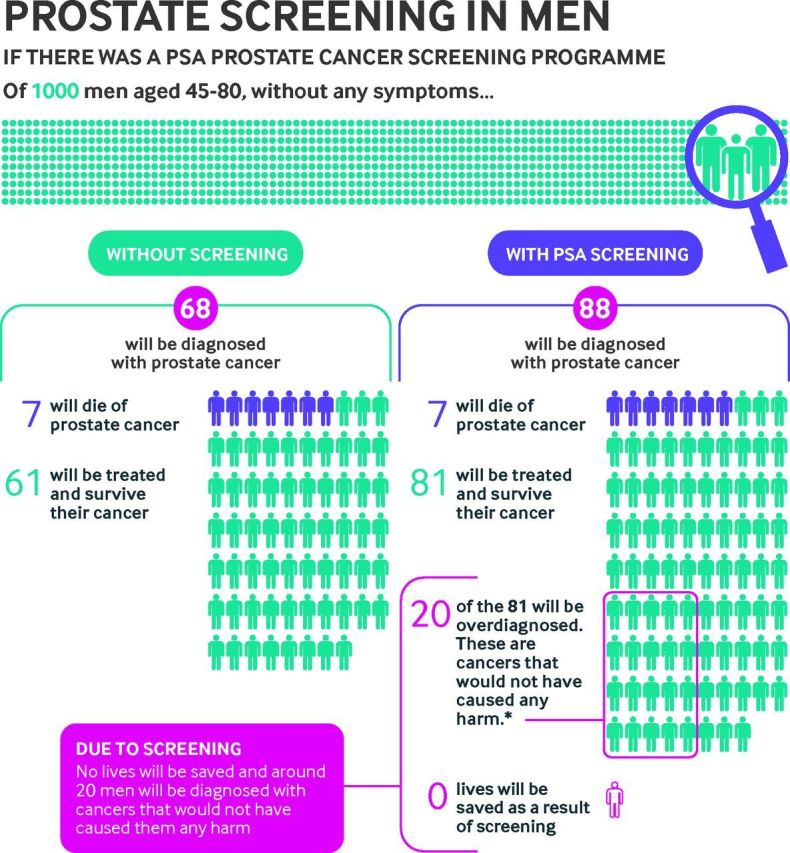
Overdiagnosis in the context of the benefits and harms of prostate cancer screening, reproduced, with permission from Cancer Research UK. PSA=prostate specific antigen
Primary care clinicians can also minimize overdiagnosis through thoughtful management approaches and referral practices. Conservative management of indolent disease that probably represents overdiagnosis can minimize harm from resultant overtreatment. Such strategies are recommended for early stage prostate and thyroid cancers,114 115 and they are increasingly discussed in the context of breast cancer.116 A recommendation for conservative management from the clinician has a big influence on the patient’s decision117 and is likely to be particularly potent in the context of an ongoing primary care relationship, empowering primary care clinicians to optimize patient care. Similarly, clinicians can selectively refer patients to specialists who are similarly committed to minimizing overdiagnosis, and these consultants also have substantial influence.118
Finally, greater clarity about the term overdiagnosis, with a broadly shared definition, would contribute to awareness of the problem and uniformity of approaches to curtail it. Lack of agreement regarding the term overdiagnosis and its conflation with related phenomena such as overuse, overtreatment, and misdiagnosis reduces practitioners’ understanding of overdiagnosis and its implications in everyday clinical practice. The dissemination of this definition will be key; it will require the cooperation of journal editors and would be enhanced by outreach in the lay press.
It is impossible to know whether the diagnosis of pre-diabetes in our case scenario will lead to clinical benefit or if it represents overdiagnosis. However, it is likely that neither the patient nor her doctor considered overdiagnosis when the initial HbA1C test was sent. Better understanding of overdiagnosis by physicians and how best to manage it, along with an appreciation of the phenomenon by patients, will be important as we try to minimize both its prevalence and its harms in primary care.
How patients were involved in the creation of this article.
To gain insight into overdiagnosis from the patient’s perspective, we solicited critical feedback from two members of the Memorial Sloan Kettering Cancer Center’s Patient and Family Advisory Council for Quality. They noted the importance of mentioning that patients may be reluctant to challenge their doctors about diagnostic testing, and this concept was incorporated into the manuscript in the Minimizing and managing overdiagnosis section.
Future research questions.
How can new technologies be harnessed to identify biomarkers of cancers and conditions that are likely to represent overdiagnosis?
At what step in the clinical decision making process should overdiagnosis be discussed and what are best communication practises?
How should overdiagnosis best be explained to clinicians and patients to optimize understanding and minimize bias?
Acknowledgments
Web Extra material supplied by the author
Appendix 1
file: kord043352.ww1
We acknowledge the contributions from Brooke Barrow and Antonio DeRosa.
Contributors: MSK and DK substantially contributed to the conception, analysis, data interpretation, manuscript drafting, and critical revision of this article. Both authors gave final approval of this version of the manuscript for publication. Both authors agree to be accountable for all aspects of the work to ensure that questions related to the accuracy and integrity of any part of the work are appropriately investigated and resolved.
Funding: This study was not funded. MSK is funded by a career development award (K07CA187071) from the National Cancer Institute of the National Institutes of Health. DK’s work on this project was supported in part by a Cancer Center Support Grant to Memorial Sloan Kettering Cancer Center (P30 CA0087848). MSK’s work was supported in part by an NIH grant (NCI K07CA187071).
Competing interests: The authors have read and understood BMJ policy on declaration of interests and declare that we have no interests.
Provenance and peer review: Commissioned; externally peer reviewed.
Series explanation: State of the Art Reviews are commissioned on the basis of their relevance to academics and specialists in the US and internationally. For this reason they are written predominantly by US authors
References
- 1.WHO. The world health report 2008: primary health care now more than ever. WHO, 2008. http://www.who.int/whr/2008/en/
- 2. Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst 2010;102:605-13. 10.1093/jnci/djq099. [DOI] [PubMed] [Google Scholar]
- 3. Black WC. Overdiagnosis: An underrecognized cause of confusion and harm in cancer screening. J Natl Cancer Inst 2000;92:1280-2. 10.1093/jnci/92.16.1280 [DOI] [PubMed] [Google Scholar]
- 4. Carter SM, Rogers W, Heath I, Degeling C, Doust J, Barratt A. The challenge of overdiagnosis begins with its definition. BMJ 2015;350:h869. 10.1136/bmj.h869. [DOI] [PubMed] [Google Scholar]
- 5. Llor C. Reducing overdiagnosis in primary care is needed. Eur J Gen Pract 2017;23:215-6. 10.1080/13814788.2017.1365836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morgan DJ, Brownlee S, Leppin AL, et al. Setting a research agenda for medical overuse. BMJ 2015;351:h4534. 10.1136/bmj.h4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Simmons RK, Griffin SJ, Lauritzen T, Sandbæk A. Effect of screening for type 2 diabetes on risk of cardiovascular disease and mortality: a controlled trial among 139 075 individuals diagnosed with diabetes in Denmark between 2001 and 2009. Diabetologia 2017;60:2192-9. 10.1007/s00125-017-4299-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Selph S, Dana T, Blazina I, Bougatsos C, Patel H, Chou R. Screening for type 2 diabetes mellitus: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2015;162:765-76. 10.7326/M14-2221. [DOI] [PubMed] [Google Scholar]
- 9. Morris DH, Khunti K, Achana F, et al. Progression rates from HbA1c 6.0-6.4% and other prediabetes definitions to type 2 diabetes: a meta-analysis. Diabetologia 2013;56:1489-93. 10.1007/s00125-013-2902-4. [DOI] [PubMed] [Google Scholar]
- 10.National Institute for Health and Care Excellence. Type 2 diabetes: prevention in people at high risk. 2012. https://www.nice.org.uk/guidance/ph38/chapter/Recommendations.
- 11. Pottie K, Jaramillo A, Lewin G, et al. Canadian Task Force on Preventive Health Care Recommendations on screening for type 2 diabetes in adults. CMAJ 2012;184:1687-96. 10.1503/cmaj.120732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fox MS. On the diagnosis and treatment of breast cancer. JAMA 1979;241:489-94. 10.1001/jama.1979.03290310029009 [DOI] [PubMed] [Google Scholar]
- 13. Zwaan L, Singh H. The challenges in defining and measuring diagnostic error. Diagnosis (Berl) 2015;2:97-103. 10.1515/dx-2014-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rodríguez-Gutiérrez R, Montori VM. Glycemic control for patients with type 2 diabetes mellitus: our evolving faith in the face of evidence. Circ Cardiovasc Qual Outcomes 2016;9:504-12. 10.1161/CIRCOUTCOMES.116.002901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Winearls CG, Glassock RJ. Classification of chronic kidney disease in the elderly: pitfalls and errors. Nephron Clin Pract 2011;119(Suppl 1):c2-4. 10.1159/000328013. [DOI] [PubMed] [Google Scholar]
- 16. Moynihan R, Glassock R, Doust J. Chronic kidney disease controversy: how expanding definitions are unnecessarily labelling many people as diseased. BMJ 2013;347:f4298. 10.1136/bmj.f4298. [DOI] [PubMed] [Google Scholar]
- 17. Wang L, Gao P, Zhang M, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA 2017;317:2515-23. 10.1001/jama.2017.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van den Bruel A. The triumph of medicine: how overdiagnosis is turning healthy people into patients. Fam Pract 2015;32:127-8. 10.1093/fampra/cmv008. [DOI] [PubMed] [Google Scholar]
- 19. Topstad D, Dickinson JA. Thyroid cancer incidence in Canada: a national cancer registry analysis. CMAJ Open 2017;5:E612-6. 10.9778/cmajo.20160162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Gelder R, Fracheboud J, Heijnsdijk EA, et al. Digital mammography screening: weighing reduced mortality against increased overdiagnosis. Prev Med 2011;53:134-40. 10.1016/j.ypmed.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 21. Depypere H, Desreux J, Pérez-López FR, et al. EMAS position statement: individualized breast cancer screening versus population-based mammography screening programmes. Maturitas 2014;79:481-6. 10.1016/j.maturitas.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 22. Puliti D, Duffy SW, Miccinesi G, et al. EUROSCREEN Working Group Overdiagnosis in mammographic screening for breast cancer in Europe: a literature review. J Med Screen 2012;19(Suppl 1):42-56. 10.1258/jms.2012.012082. [DOI] [PubMed] [Google Scholar]
- 23. Wiener RS, Gould MK, Woloshin S, Schwartz LM, Clark JA. ‘The thing is not knowing’: patients’ perspectives on surveillance of an indeterminate pulmonary nodule. Health Expect 2015;18:355-65. 10.1111/hex.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Herzstein J, Ebell M. Improving quality by doing less: overtreatment. Am Fam Physician 2015;91:289-91. [PubMed] [Google Scholar]
- 25. Rank B. Executive physicals—bad medicine on three counts. N Engl J Med 2008;359:1424-5. 10.1056/NEJMp0806270. [DOI] [PubMed] [Google Scholar]
- 26. Hughes DR, Bhargavan M, Sunshine JH. Imaging self-referral associated with higher costs and limited impact on duration of illness. Health Aff (Millwood) 2010;29:2244-51. 10.1377/hlthaff.2010.0413. [DOI] [PubMed] [Google Scholar]
- 27. Lord SJ, Staub LP, Bossuyt PM, Irwig LM. Target practice: choosing target conditions for test accuracy studies that are relevant to clinical practice. BMJ 2011;343:d4684. 10.1136/bmj.d4684. [DOI] [PubMed] [Google Scholar]
- 28. Prasad V, Rho J, Cifu A. The diagnosis and treatment of pulmonary embolism: a metaphor for medicine in the evidence-based medicine era. Arch Intern Med 2012;172:955-8. 10.1001/archinternmed.2012.195. [DOI] [PubMed] [Google Scholar]
- 29. Engelgau MM, Thompson TJ, Herman WH, et al. Comparison of fasting and 2-hour glucose and HbA1c levels for diagnosing diabetes. Diagnostic criteria and performance revisited. Diabetes Care 1997;20:785-91. 10.2337/diacare.20.5.785 [DOI] [PubMed] [Google Scholar]
- 30. Yudkin JS, Montori VM. The epidemic of pre-diabetes: the medicine and the politics. BMJ 2014;349:g4485. 10.1136/bmj.g4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Herman WH, Cohen RM. Racial and ethnic differences in the relationship between HbA1c and blood glucose: implications for the diagnosis of diabetes. J Clin Endocrinol Metab 2012;97:1067-72. 10.1210/jc.2011-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alonso-Coello P, García-Franco AL, Guyatt G, Moynihan R. Drugs for pre-osteoporosis: prevention or disease mongering? BMJ 2008;336:126-9. 10.1136/bmj.39435.656250.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fuchs FD, Fuchs SC, Poli-de-Figueiredo CE, et al. Effectiveness of low-dose diuretics for blood pressure reduction to optimal values in prehypertension: a randomized clinical trial. J Hypertens 2018;36:933-8. 10.1097/HJH.0000000000001624. [DOI] [PubMed] [Google Scholar]
- 34. Wiener RS, Schwartz LM, Woloshin S. When a test is too good: how CT pulmonary angiograms find pulmonary emboli that do not need to be found. BMJ 2013;347:f3368. 10.1136/bmj.f3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Berland LL, Silverman SG, Gore RM, et al. Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee. J Am Coll Radiol 2010;7:754-73. 10.1016/j.jacr.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 36. Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, Dal Maso L. Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N Engl J Med 2016;375:614-7. 10.1056/NEJMp1604412. [DOI] [PubMed] [Google Scholar]
- 37. Diamandis EP, Li M. The side effects of translational omics: overtesting, overdiagnosis, overtreatment. Clin Chem Lab Med 2016;54:389-96. 10.1515/cclm-2015-0762. [DOI] [PubMed] [Google Scholar]
- 38. Gates TJ. Screening for cancer: concepts and controversies. Am Fam Physician 2014;90:625-31. [PubMed] [Google Scholar]
- 39. Barratt A. Overdiagnosis in mammography screening: a 45 year journey from shadowy idea to acknowledged reality. BMJ 2015;350:h867. 10.1136/bmj.h867. [DOI] [PubMed] [Google Scholar]
- 40. Marmot M, Altman DG, Cameron DA, et al. Independent UK Panel on Breast Cancer Screening The benefits and harms of breast cancer screening: an independent review. Lancet 2012;380:1778-86. 10.1016/S0140-6736(12)61611-0. [DOI] [PubMed] [Google Scholar]
- 41. Welch HG, Fisher ES. Income and cancer overdiagnosis—when too much care is harmful. N Engl J Med 2017;376:2208-9. 10.1056/NEJMp1615069. [DOI] [PubMed] [Google Scholar]
- 42. Schwartz LM, Woloshin S, Fowler FJ, Jr, Welch HG. Enthusiasm for cancer screening in the United States. JAMA 2004;291:71-8. 10.1001/jama.291.1.71. [DOI] [PubMed] [Google Scholar]
- 43. Van den Bruel A, Jones C, Yang Y, Oke J, Hewitson P. People’s willingness to accept overdetection in cancer screening: population survey. BMJ 2015;350:h980. 10.1136/bmj.h980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lee TJ, Kim S, Cho HJ, Lee JH. The incidence of thyroid cancer is affected by the characteristics of a healthcare system. J Korean Med Sci 2012;27:1491-8. 10.3346/jkms.2012.27.12.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Batstra L, Frances A. Diagnostic inflation: causes and a suggested cure. J Nerv Ment Dis 2012;200:474-9. 10.1097/NMD.0b013e318257c4a2. [DOI] [PubMed] [Google Scholar]
- 46. Golestaneh L, Cowan E. Hidden conflicts of interest in continuing medical education. Lancet 2017;390:2128-30. 10.1016/S0140-6736(17)32813-1. [DOI] [PubMed] [Google Scholar]
- 47. Jefferson AA, Pearson SD. Conflict of interest in seminal hepatitis C virus and cholesterol management guidelines. JAMA Intern Med 2017;177:352-7. 10.1001/jamainternmed.2016.8439. [DOI] [PubMed] [Google Scholar]
- 48. Moynihan R, Heath I, Henry D. Selling sickness: the pharmaceutical industry and disease mongering. BMJ 2002;324:886-91. 10.1136/bmj.324.7342.886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mailankody S, Prasad V. Pharmaceutical marketing for rare diseases: regulating drug company promotion in an era of unprecedented advertisement. JAMA 2017;317:2479-80. 10.1001/jama.2017.5784. [DOI] [PubMed] [Google Scholar]
- 50. Sackett DL, Rosenberg WMC, Gray JAM, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn’t. BMJ 1996;312:71-2. 10.1136/bmj.312.7023.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gigerenzer G, Gaissmaier W, Kurz-Milcke E, Schwartz LM, Woloshin S. Helping doctors and patients make sense of health statistics. Psychol Sci Public Interest 2007;8:53-96. 10.1111/j.1539-6053.2008.00033.x. [DOI] [PubMed] [Google Scholar]
- 52. Whiting PF, Davenport C, Jameson C, et al. How well do health professionals interpret diagnostic information? A systematic review. BMJ Open 2015;5:e008155. 10.1136/bmjopen-2015-008155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Klein JG. Five pitfalls in decisions about diagnosis and prescribing. BMJ 2005;330:781-3. 10.1136/bmj.330.7494.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Scott IA, Soon J, Elshaug AG, Lindner R. Countering cognitive biases in minimising low value care. Med J Aust 2017;206:407-11. 10.5694/mja16.00999 [DOI] [PubMed] [Google Scholar]
- 55. Kohn MA, Carpenter CR, Newman TB. Understanding the direction of bias in studies of diagnostic test accuracy. Acad Emerg Med 2013;20:1194-206. 10.1111/acem.12255. [DOI] [PubMed] [Google Scholar]
- 56. Kent DM, Hayward RA. Limitations of applying summary results of clinical trials to individual patients: the need for risk stratification. JAMA 2007;298:1209-12. 10.1001/jama.298.10.1209. [DOI] [PubMed] [Google Scholar]
- 57. Kovalchik SA, Tammemagi M, Berg CD, et al. Targeting of low-dose CT screening according to the risk of lung-cancer death. N Engl J Med 2013;369:245-54. 10.1056/NEJMoa1301851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nelson HD, Pappas M, Cantor A, Griffin J, Daeges M, Humphrey L. Harms of breast cancer screening: systematic review to update the 2009 US Preventive Services Task Force Recommendation. Ann Intern Med 2016;164:256-67. 10.7326/M15-0970. [DOI] [PubMed] [Google Scholar]
- 59. Adhmed O, Moran D, Daly P, et al. PSA testing: whom, by whom and how often? Ir J Med Sci 2014;183:(suppl 5)S237-38. 10.1007/s11845-014-1168-2. [DOI] [Google Scholar]
- 60. Chen Y, Yang K, Marušic A, et al. RIGHT (Reporting Items for Practice Guidelines in Healthcare) Working Group A reporting tool for practice guidelines in health care: the RIGHT statement. Ann Intern Med 2017;166:128-32. 10.7326/M16-1565. [DOI] [PubMed] [Google Scholar]
- 61. Jenniskens K, de Groot JAH, Reitsma JB, Moons KGM, Hooft L, Naaktgeboren CA. Overdiagnosis across medical disciplines: a scoping review. BMJ Open 2017;7:e018448. 10.1136/bmjopen-2017-018448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Melzer D, Tavakoly B, Winder RE, et al. Much more medicine for the oldest old: trends in UK electronic clinical records. Age Ageing 2015;44:46-53. 10.1093/ageing/afu113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Johansson M, Hansson A, Brodersen J. Estimating overdiagnosis in screening for abdominal aortic aneurysm: could a change in smoking habits and lowered aortic diameter tip the balance of screening towards harm? BMJ 2015;350:h825. 10.1136/bmj.h825. [DOI] [PubMed] [Google Scholar]
- 64. Brodersen J. How to conduct research on overdiagnosis. A keynote paper from the EGPRN May 2016, Tel Aviv. Eur J Gen Pract 2017;23:78-82. 10.1080/13814788.2017.1290795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. McCaffery KJ, Jansen J, Scherer LD, et al. Walking the tightrope: communicating overdiagnosis in modern healthcare. BMJ 2016;352:i348. 10.1136/bmj.i348. [DOI] [PubMed] [Google Scholar]
- 66. Roksund G, Brodersen J, Johnson GE, Hjörleifsson S, Laudal M, Swensen E. Overdiagnosis—Norwegian general practitioners show the way. Tidsskr Nor Laegeforen 2016;136:1903-5. 10.4045/tidsskr.16.0572. [DOI] [PubMed] [Google Scholar]
- 67. Davies L, Hendrickson CD, Hanson GS. Experience of US patients who self-identify as having an overdiagnosed thyroid cancer: a qualitative analysis. JAMA Otolaryngol Head Neck Surg 2017;143:663-9. 10.1001/jamaoto.2016.4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Doust J, Glasziou P. Is the problem that everything is a diagnosis? Aust Fam Physician 2013;42:856-9. [PubMed] [Google Scholar]
- 69. de Ridder D, Geenen R, Kuijer R, van Middendorp H. Psychological adjustment to chronic disease. Lancet 2008;372:246-55. 10.1016/S0140-6736(08)61078-8. [DOI] [PubMed] [Google Scholar]
- 70. Schairer C, Brown LM, Chen BE, et al. Suicide after breast cancer: an international population-based study of 723,810 women. J Natl Cancer Inst 2006;98:1416-9. 10.1093/jnci/djj377. [DOI] [PubMed] [Google Scholar]
- 71. Turner J, Kelly B. Emotional dimensions of chronic disease. West J Med 2000;172:124-8. 10.1136/ewjm.172.2.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. King MT, Winters ZE, Olivotto IA, et al. Patient-reported outcomes in ductal carcinoma in situ: A systematic review. Eur J Cancer 2017;71:95-108. 10.1016/j.ejca.2016.09.035. [DOI] [PubMed] [Google Scholar]
- 73. van Luijt PA, Heijnsdijk EA, Fracheboud J, et al. The distribution of ductal carcinoma in situ (DCIS) grade in 4232 women and its impact on overdiagnosis in breast cancer screening. Breast Cancer Res 2016;18:47. 10.1186/s13058-016-0705-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cotter AR, Vuong K, Mustelin L, et al. Do psychological harms result from being labelled with an unexpected diagnosis of abdominal aortic aneurysm or prostate cancer through screening? A systematic review. BMJ Open 2017;7:e017565. 10.1136/bmjopen-2017-017565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. DeFrank JT, Barclay C, Sheridan S, et al. The psychological harms of screening: the evidence we have versus the evidence we need. J Gen Intern Med 2015;30:242-8. 10.1007/s11606-014-2996-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bergman AB, Stamm SJ. The morbidity of cardiac nondisease in schoolchildren. N Engl J Med 1967;276:1008-13. 10.1056/NEJM196705042761804. [DOI] [PubMed] [Google Scholar]
- 77. Coon ER, Quinonez RA, Moyer VA, Schroeder AR. Overdiagnosis: how our compulsion for diagnosis may be harming children. Pediatrics 2014;134:1013-23. 10.1542/peds.2014-1778. [DOI] [PubMed] [Google Scholar]
- 78. Graves JM, Fulton-Kehoe D, Jarvik JG, Franklin GM. Early imaging for acute low back pain: one-year health and disability outcomes among Washington State workers. Spine (Phila Pa 1976) 2012;37:1617-27. 10.1097/BRS.0b013e318251887b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA 2012;307:1513-6. 10.1001/jama.2012.362. [DOI] [PubMed] [Google Scholar]
- 80. Ong MS, Mandl KD. National expenditure for false-positive mammograms and breast cancer overdiagnoses estimated at $4 billion a year. Health Aff (Millwood) 2015;34:576-83. 10.1377/hlthaff.2014.1087. [DOI] [PubMed] [Google Scholar]
- 81.Organisation for Economic Co-operation and Development. Fiscal sustainability of health systems. OECD Publishing, 2015. http://www.oecd.org/publications/fiscal-sustainability-of-health-systems-9789264233386-en.htm.
- 82. Chassin MR, Galvin RW, Institute of Medicine National Roundtable on Health Care Quality The urgent need to improve health care quality. JAMA 1998;280:1000-5. 10.1001/jama.280.11.1000 [DOI] [PubMed] [Google Scholar]
- 83.National Cancer Institute. NCI dictionary of cancer terms: overtreatment. https://www.cancer.gov/publications/dictionaries/cancer-terms /def/overtreatment
- 84. Tseng CL, Soroka O, Maney M, Aron DC, Pogach LM. Assessing potential glycemic overtreatment in persons at hypoglycemic risk. JAMA Intern Med 2014;174:259-68. 10.1001/jamainternmed.2013.12963. [DOI] [PubMed] [Google Scholar]
- 85.Dictionary OL. Misdiagnosis: Oxford Living Dictionaries. c2018. https://en.oxforddictionaries.com/definition/misdiagnosis.
- 86. Schpero WL. Limiting low-value care by “choosing wisely”. Virtual Mentor 2014;16:131-4. 10.1001/virtualmentor.2014.16.02.pfor2-1402. [DOI] [PubMed] [Google Scholar]
- 87. Schwartz AL, Landon BE, Elshaug AG, Chernew ME, McWilliams JM. Measuring low-value care in Medicare. JAMA Intern Med 2014;174:1067-76. 10.1001/jamainternmed.2014.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Valk MJ, Mosterd A, Broekhuizen BD, et al. Overdiagnosis of heart failure in primary care: a cross-sectional study. Br J Gen Pract 2016;66:e587-92. 10.3399/bjgp16X685705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Clark D. Between hope and acceptance: the medicalisation of dying. BMJ 2002;324:905-7. 10.1136/bmj.324.7342.905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ebeling M. ‘Get with the Program!’: pharmaceutical marketing, symptom checklists and self-diagnosis. Soc Sci Med 2011;73:825-32. 10.1016/j.socscimed.2011.05.054. [DOI] [PubMed] [Google Scholar]
- 91. Mintzes B. Disease mongering in drug promotion: do governments have a regulatory role? PLoS Med 2006;3:e198. 10.1371/journal.pmed.0030198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. American Psychiatric Association Diagnostic and statistical manual of mental disorders: DSM-IV. 4th ed American Psychiatric Association, 1994:866. [Google Scholar]
- 93. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry 2012;169:465-75. 10.1176/appi.ajp.2012.11081302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Petersen M. Market place; drug maker is set to ship generic prozac. New York Times 2001 Aug 2. [Google Scholar]
- 95. Yonkers KA, Simoni MK. Premenstrual disorders. Am J Obstet Gynecol 2018;218:68-74. 10.1016/j.ajog.2017.05.045. [DOI] [PubMed] [Google Scholar]
- 96. Layton JB, Kim Y, Alexander GC, Emery SL. Association between direct-to-consumer advertising and testosterone testing and initiation in the United States, 2009-2013. JAMA 2017;317:1159-66. 10.1001/jama.2016.21041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Carter JL, Coletti RJ, Harris RP. Quantifying and monitoring overdiagnosis in cancer screening: a systematic review of methods. BMJ 2015;350:g7773. 10.1136/bmj.g7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Etzioni R, Gulati R, Mallinger L, Mandelblatt J. Influence of study features and methods on overdiagnosis estimates in breast and prostate cancer screening. Ann Intern Med 2013;158:831-8. 10.7326/0003-4819-158-11-201306040-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Coldman A, Phillips N. Incidence of breast cancer and estimates of overdiagnosis after the initiation of a population-based mammography screening program. CMAJ 2013;185:E492-8. 10.1503/cmaj.121791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Han SS, Ten Haaf K, Hazelton WD, et al. The impact of overdiagnosis on the selection of efficient lung cancer screening strategies. Int J Cancer 2017;140:2436-43. 10.1002/ijc.30602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Noldus J, Graefen M, Haese A, Henke RP, Hammerer P, Huland H. Stage migration in clinically localized prostate cancer. Eur Urol 2000;38:74-8. 10.1159/000020255 [DOI] [PubMed] [Google Scholar]
- 102. Carter SM, Degeling C, Doust J, et al. A definition and ethical evaluation of overdiagnosis. J Med Ethics 2016. 10.1136/medethics-2015-102928. [DOI] [PubMed] [Google Scholar]
- 103. Doust J, Vandvik PO, Qaseem A, et al. Guidelines International Network (G-I-N) Preventing Overdiagnosis Working Group Guidance for modifying the definition of diseases: a checklist. JAMA Intern Med 2017;177:1020-5. 10.1001/jamainternmed.2017.1302. [DOI] [PubMed] [Google Scholar]
- 104. Heidenreich A, Abrahamsson PA, Artibani W, et al. European Association of Urology Early detection of prostate cancer: European Association of Urology recommendation. Eur Urol 2013;64:347-54. 10.1016/j.eururo.2013.06.051. [DOI] [PubMed] [Google Scholar]
- 105. Moyer VA, U.S. Preventive Services Task Force Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2012;156:880-91, W312. 10.7326/0003-4819-156-12-201206190-00424. [DOI] [PubMed] [Google Scholar]
- 106. Siu AL, U.S. Preventive Services Task Force Screening for Breast Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 2016;164:279-96. 10.7326/M15-2886. [DOI] [PubMed] [Google Scholar]
- 107.National Institute for Health and Care Excellence. Hypertension in adults: diagnosis and management. 2011. https://www.nice.org.uk/guidance/cg127 [PubMed]
- 108. Kung J, Miller RR, Mackowiak PA. Failure of clinical practice guidelines to meet Institute of Medicine standards: Two more decades of little, if any, progress. Arch Intern Med 2012;172:1628-33. 10.1001/2013.jamainternmed.56. [DOI] [PubMed] [Google Scholar]
- 109. Moynihan R, Nickel B, Hersch J, et al. Public opinions about overdiagnosis: a national community survey. PLoS One 2015;10:e0125165. 10.1371/journal.pone.0125165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Wegwarth O, Gigerenzer G. Less is more: Overdiagnosis and overtreatment: evaluation of what physicians tell their patients about screening harms. JAMA Intern Med 2013;173:2086-7. 10.1001/jamainternmed.2013.10363. [DOI] [PubMed] [Google Scholar]
- 111. Nagler RH, Franklin Fowler E, Gollust SE. Women’s awareness of and responses to messages about breast cancer overdiagnosis and overtreatment: results from a 2016 national survey. Med Care 2017;55:879-85. 10.1097/MLR.0000000000000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Park SH, Lee B, Lee S, et al. A qualitative study of women’s views on overdiagnosis and screening for thyroid cancer in Korea. BMC Cancer 2015;15:858. 10.1186/s12885-015-1877-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Hersch J, Barratt A, Jansen J, et al. Use of a decision aid including information on overdetection to support informed choice about breast cancer screening: a randomised controlled trial. Lancet 2015;385:1642-52. 10.1016/S0140-6736(15)60123-4. [DOI] [PubMed] [Google Scholar]
- 114. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016;26:1-133. 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Parker C, Gillessen S, Heidenreich A, Horwich A, ESMO Guidelines Committee Cancer of the prostate: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26(Suppl 5):v69-77. 10.1093/annonc/mdv222. [DOI] [PubMed] [Google Scholar]
- 116. Benson JR, Jatoi I, Toi M. Treatment of low-risk ductal carcinoma in situ: is nothing better than something? Lancet Oncol 2016;17:e442-51. 10.1016/S1470-2045(16)30367-9. [DOI] [PubMed] [Google Scholar]
- 117. Kim C, Wright FC, Look Hong NJ, et al. Patient and provider experiences with active surveillance: A scoping review. PLoS One 2018;13:e0192097. 10.1371/journal.pone.0192097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Jang TL, Bekelman JE, Liu Y, et al. Physician visits prior to treatment for clinically localized prostate cancer. Arch Intern Med 2010;170:440-50. 10.1001/archinternmed.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]