Abstract
Background:
Elderly Americans suffer increased mortality from sepsis. Given that beta-blockers have been shown to be cardioprotective in critical care, we investigated outpatient beta-blocker prescriptions and mortality among Medicare beneficiaries admitted for sepsis.
Methods:
We queried a 5% random sample of Medicare beneficiaries for patients admitted with sepsis. We used in-hospital and outpatient prescription drug claims to compare in-hospital and 30-day mortality based on pre-admission beta-blocker prescription and class of beta-blocker prescribed using univariate tests of comparison and multivariable logistic regression models and another class of medications for control.
Results:
Outpatient beta-blocker prescription was associated with a statistically significant decrease in in-hospital and 30-day mortality. In multivariable modeling, beta-blocker prescription was associated with 31% decrease in in-hospital mortality and 41% decrease in 30-day mortality. Both cardioselective and non-selective beta-blockers conferred mortality benefit.
Conclusions:
Our data suggests that there may be a role for preadmission beta-blockers in reducing sepsis-related mortality.
1. Introduction
More than one million Americans develop sepsis annually, a condition that is associated with up to a 50% mortality rate and is responsible for billions annually in health care expenditures.1–3 Approximately 60% of sepsis cases involve patients older than 65; increased age is also associated with higher morbidity and mortality.1,4 Thus, the incidence of sepsis and its impact on the health care system is only expected to worsen as the United States population ages.
Over-activation of the sympathetic nervous system in the setting of widespread bacterial infection is a hallmark of sepsis. The resulting high output state and impaired contractility can progress to stress-induced heart injury.5–8 For this reason, beta-blockers, whose mechanism of action is to blunt the sympathetic nervous system, are being explored for their potential therapeutic benefits in sepsis.6 To date there have been ten clinical trials, enrolling between ten and 144 patients, which have been shown to improve morbidity and mortality in patients who receive beta-blockers after diagnosis of sepsis.5,9,10 In the largest of the randomized control trials, the administration of esmolol as part of intensive care management was shown to reduce mortality by 61%.5 Other prospective studies focusing on physiological parameters in septic patients receiving intensive care have shown that beta-blockers improve cardiac function.9,11–13
Forty-two percent of patients who are admitted with sepsis present from the community; however, the role that preadmission beta-blockers play in the modulation of sepsis has yet to be elucidated.2 We investigated the relationship between outpatient beta-blocker prescriptions and mortality after admission for sepsis using Medicare beneficiary data. We hypothesized that patients who were prescribed beta-blockers would experience lower mortality.
2. Methods
We queried a random 5% national sample of Medicare beneficiaries (Medicare Provider and Analysis Review [MEDPAR] data) from 2009 to 2011for patients admitted acutely for sepsis. Patients admitted with an urgent/emergent hospital admission code, requiring intensive care upon admission, and carrying a primary diagnosis of sepsis or systemic inflammatory response syndrome (SIRS) by ICD9 diagnosis codes (038.9, 995.91, and 995.92 for sepsis; 995.90, 995.93, and 995.94 for SIRS; or 785.52 for septic shock [requires additional ICD9 code for sepsis or SIRS]) were eligible for analysis. Any patients whose in-hospital claims did not indicate that they were taken to the intensive care unit (ICU) were excluded, as this gave rise to a possible miscoding of sepsis. Patients were included in the study if they were age 65 or older as of January 1, 2009 with one year of continuous Part A and B coverage with Part D (prescription drug coverage) enrollment. Patients with Part C enrollment (coverage through healthcare maintenance organizations) were excluded to ensure completeness of data. Patients were also excluded if they had codes for asthma (493.xx) or heart block (426.xx), as beta-blockers are often contraindicated in these patients.
Medicare denominator files were used for demographic factors (e.g., age, sex, and race). MEDPAR files were used to obtain variables related to hospitalization (e.g., associated diagnoses) and to calculate each patient’s Elixhauser comorbidity index.14 Outcomes of interest identified through MEDPAR data included in-hospital and 30-day mortality.
Part D claims data were utilized as a proxy for exposure to beta-blockers. Part D files provided the name and date of any beta-blocker prescription, as well as how many days were supplied for beta-blockers. Patients were divided into two groups: beta-blocker prescription with days supplied extending through date of hospital admission for sepsis (BBRx) and no beta-blocker prescription on record (NORx). Patients with beta-blocker prescriptions extending into 30 days prior to admission but not through the admission date were excluded to avoid misclassification.
Univariate tests of association (Chi-squared tests of association for categorical variables and Wilcoxon rank-sum tests for non-normally distributed continuous variables) were conducted to compare sociodemographic and clinical characteristics, as well as in-hospital and 30-day mortality, between the between BBRx and NORx groups. P-values were considered statistically significant at alpha level 0.05.
In order to examine the association of beta-blocker prescription with in-hospital and 30-day mortality, multivariable logistic regression models were created. A number of covariates were analyzed to adjust for confounding, including sociodemographic factors (age, gender, and race), cardiac history (history of myocardial infarction, ischemic heart disease, hypertension, and congestive heart failure), non-cardiac medical history (chronic obstructive pulmonary disease, renal failure, diabetes, and cancer), aggregate co-morbidity score (Elixhauser index), and treatment variables (ICU length of stay, operation). We fit the models using backwards stepwise elimination, retaining in the final models variables that were significant at 0.05 or predetermined to be clinically significant (i.e. demographics and history related to cardiovascular events).
To determine if there was a difference between exposure to cardioselective and non-selective beta-blockers, we performed subgroup analyses using the same methods. Similarly, given the expectation that mortality would be expected to increase with increasing age in our cohort, we conducted subgroup analyses by age group (65–74, 75–84, and over 85) to examine whether the mortality of any age group was particularly influenced by beta-blocker prescription. Finally, to determine if overall healthcare utilization (i.e., merely seeing a physician and receiving a prescription for any drug, and not just a beta-blocker) was in and of itself imparting a mortality benefit, we performed a parallel analysis using another commonly prescribed drug class in this age group, selective serotonin reuptake inhibitors (SSRIs). Analyses were performed using SAS 9.4 software (SAS Institute Inc, Cary, NC).
3. Results
Out of 864,604 eligible patients in our cohort of Medicare beneficiaries, we identified 6839 who were admitted with sepsis acutely. Of these patients, 2838 (41%) were BBRx and 4001 (59%) were NORx. Among BBRx, 73% were prescribed cardioselective agents while 27% were prescribed nonselective beta-blockers. Overall, the majority of the patients were white (76%), female (64%), and over 75 years old (69%). BBRx were more likely to have both cardiac and non-cardiac comorbidities, including history of myocardial infarction, ischemic heart disease, hypertension, congestive heart failure, renal failure, and diabetes (Table 1). Index hospitalization LOS and ICU LOS overall did not differ among survivors (median 7 days, 4–12; median 4 days, 2–7, respectively). However, only 24% of BBRx died during their hospitalization, compared to 31% of NORx (p < 0.0001). Similarly, the 30-day mortality rate was found to differ significantly, with a 13% mortality rate of BBRx, compared to 18% of NORx (p < 0.0001) (Table 2).
Table 1.
Demographics and clinical characteristics of medicare patients diagnosed with sepsis by beta blocker prescription (n = 6839).
| BBRxa (n = 2838) | NORxb (n = 4001) | p-value | |
|---|---|---|---|
| Female Gender, N (%) | 1836 (65) | 2517 (63) | 0.13 |
| Age group (years), N (%) | 0.11 | ||
| 65–74 | 902 (32) | 1230 (31) | |
| 75–84 | 1131 (40) | 1541 (39) | |
| ≥85 | 805 (28) | 1230 (31) | 0.01 |
| Race, N (%) | |||
| Non-Hispanic White | 2192 (77) | 2980 (74) | |
| Black | 299 (11) | 530 (13) | |
| Hispanic | 191 (6.7) | 296 (7.4) | |
| Asian | 120 (4.2) | 143 (3.6) | |
| Other | 36 (1.3) | 52 (1.3) | |
| Class of Beta-Blocker Prescribed | |||
| Cardioselective | 2051 (73) | – | |
| Nonselective | 762 (27) | – | |
| Elixhauser comorbidity index | <0.0001 | ||
| Elixhauser Index = 0 | 197 (6.9) | 470 (11.7) | |
| Elixhauser Index = 1 | 46 (1.6) | 125 (3.1) | |
| Elixhauser Index = 2 | 77 (2.7) | 216 (5.4) | |
| Elixhauser Index = 3 | 171 (6.0) | 339 (8.5) | |
| Elixhauser Index ≥ 4 | 2347 (83) | 2851 (71) | |
| Cardiac comorbidities, N (%) | |||
| History of MI | 209 (7.4) | 96 (2.4) | <0.0001 |
| Ischemic heart disease | 947 (33) | 597 (15) | <0.0001 |
| Hypertension | 1620 (57) | 1682 (42) | <0.0001 |
| Congestive heart failure | 966 (34) | 739 (18) | <0.0001 |
| Non-cardiac Comorbidities, N (%) | |||
| Chronic obstructive pulmonary disease | 799 (28) | 1006 (25) | 0.01 |
| Renal failure | 537 (19) | 464 (12) | <0.0001 |
| Diabetes Mellitus | 830 (29) | 855 (21) | <0.0001 |
| Cancer (solid and metastatic) | 208 (7.3) | 306 (7.6) | 0.622 |
All numbers are % (n) unless otherwise stated.
These patients were identified via Medicare Part D records to have been filling a prescription for a beta-blocker through the hospital admission date. Groups are the same for all tables.
These patients have never been on a beta-blocker per Part D records. Groups are the same for all tables.
Table 2.
Association between mortality and beta blocker prescription among medicare patients admitted with sepsis in unadjusted analyses (n = 6839).
| BBRxa (n = 2838) | NORxb (n = 4001) | p-value | |
|---|---|---|---|
| Mortality (in-hospital), N (%) | 680 (24) | 1254 (31) | <0.0001 |
| 30-day mortality, N (%) | 372 (13) | 704 (18) | <0.0001 |
All numbers are % (n) unless otherwise stated.
Number of survivors = 4905.
These patients were identified via Medicare Part D records to have been filling a prescription for a beta-blocker through the hospital admission date. Groups are the same for all tables.
These patients have never been on a beta-blocker per Part D records. Groups are the same for all tables.
Multivariable logistic regression models showed that beta-blocker prescription was associated with a 31% decrease in in-hospital mortality (aOR 0.69, CI 0.62–0.77) when adjusting for potential confounders. Being of advanced age was associated with a corresponding increase in mortality, as was having cancer or congestive heart failure; surgical procedures associated with the hospitalization more than doubled the risk of mortality (Table 3). The adjusted OR for cardioselective beta-blockers was 0.73 (CI 0.65–0.82) while for non-selective beta-blockers it was 0.59 (CI 0.49–0.71). Within the first 30 days after discharge, BBRx had a 41% decrease in mortality compared to NORx (aOR 0.59, CI 0.51, 0.68). The same trend of increasing age and mortality was observed at 30 days, as was the association with cancer, congestive heart failure, and surgical procedures and mortality. Men had a slight increase risk of mortality (Table 4). In multivariable models including drugs of a single class only, the adjusted OR for cardioselective beta-blockers at 30-days was 0.60 (CI 0.51–0.70) while for nonselective beta-blockers it was 0.55 (CI 0.43–0.71). We also compared the effect of beta-blocker class head to head in a multivariable model. The aOR of mortality with cardioselective beta-blocker vs. non-selective beta-blocker was 1.23 (1.11–1.36).
Table 3.
Adjusted odds of in-hospital mortality among medicare patients admitted for sepsis (n = 6839).
| aOR (95% CI) | |
|---|---|
| BBRxa vs. NORxb | 0.69 (0.62, 0.77) |
| Age 75–84 vs. 65–74 | 1.4 (1.2, 1.5) |
| Age ≥ 85 vs. 65–74 | 1.6 (1.4, 1.9) |
| Elix.c 1 vs Elix 0 | 1.3 (0.90, 1.9) |
| Elix. 2 vs Elix 0 | 1 (0.74, 1.4) |
| Elix. 3 vs Elix 0 | 0.95 (0.74, 1.2) |
| Elix. >=4 vs Elix 0 | 0.79 (0.65, 0.95) |
| Any surgical procedure vs. none | 2.6 (2.3, 3.0) |
| History of Congestive Heart Failure | 1.4 (1.2, 1.56) |
| History of Cancer | 1.54 (1.3, 1.9) |
c-statistic 0.64.
Hosmer-Lemeshow goodness-of-fit p-value = 0.40.
Race, diabetes, sex, renal failure, History of Myocardial Infarction, Hypertension, Ischemic heart disease and Chronic Obstructive Pulmonary disease were removed from the model based based on p-values > 0.05.
These patients were identified via Medicare Part D records to have been filling a prescription for a beta-blocker through the hospital admission date. Groups are the same for all tables.
These patients have never been on a beta-blocker per Part D records. Groups are the same for all tables.
Abbreviation for Elixhauser comorbidity score.
Table 4.
Adjusted odds of 30-day mortality in medicare patients who had survived hospitalization for sepsis (n = 4905).
| aOR (95% CI) | |
|---|---|
| BBRxa vs. NORxb | 0.59 (0.51, 0.68) |
| Male vs Female | 1.2 (1.03, 1.4) |
| Age 75–84 vs. 65–74 | 1.7 (1.42, 2.0) |
| Age ≥ 85 vs. 65–74 | 2.9 (2.4, 3.4) |
| Elix. 1 vs Elix 0 | 0.74 (0.44, 1.2) |
| Elix. 2 vs Elix 0 | 0.65 (0.43, 0.98) |
| Elix. 3 vs Elix 0 | 0.62 (0.44, 0.88) |
| Elix. >=4 vs Elix 0 | 0.73 (0.57, 0.92) |
| Black (African-American) vs. White non-Hispanic | 0.83 (0.66, 1.04) |
| Hispanic vs. White non-Hispanic | 0.73 (0.54, 0.97) |
| Asian vs. White non-Hispanic | 0.64 (0.42, 0.95) |
| Other vs. White non-Hispanic | 1.2 (0.67, 2.1) |
| Any surgical procedure | 1.5 (1.3, 1.8) |
| Congestive Heart Failure | 1.3 (1.12, 1.6) |
| Cancer | 2.5 (1.9, 3.2) |
c-statistic = 0.66.
Hosmer-Lemeshow goodness-of-fit p-value = 0.7515.
Diabetes, Chronic Obstructive Pulmonary Disease, History of Myocardial Infarction, Hypertension, Renal failure and Ischemic heart disease were removed from the model based on p-values > 0.05.
These patients were identified via Medicare Part D records to have been filling a prescription for a beta-blocker through the hospital admission date. Groups are the same for all tables.
These patients have never been on a beta-blocker per Part D records. Groups are the same for all tables.
Subgroup analyses based on age demonstrated reduced mortality in BBRx across all subgroups both in-hospital and at 30-days. For patients aged 65–74, the adjusted OR for in-hospital mortality was 0.64 (CI 0.52–0.80), compared to aOR 0.69 (CI 0.58–0.83) for those aged 75–84 and aOR 0.73 (CI 0.60–0.90) for those aged over 85. At 30 days, the adjusted OR for patients aged 65–74 was 0.48 (CI 0.35–0.65), compared to aOR 0.63, (CI 0.50–0.79) to those aged 75–84 and aOR 0.62 (CI 0.49–0.79) for those aged over 85.
In analyses measuring the effect of SSRI prescription on both in-hospital and 30-day mortality after hospitalization for sepsis, we found that there was no significant difference in risk of death between those with an SSRI prescription and those without (aOR 0.87 CI 0.72–1.04). For 30-day mortality, there was a 28% reduction in mortality in SSRI patients (aOR 0.72 CI 0.57–0.91).
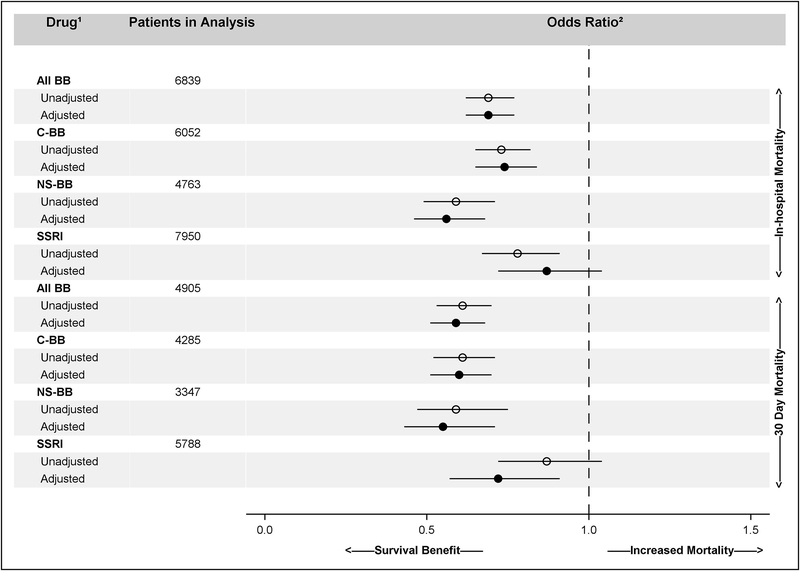
Fig.1 shows the adjusted and unadjusted odds ratios for both in-hospital mortality and 30-day mortality among all BBRx as well as among cardioselective and nonselective subgroups and those with SSRI prescriptions.
Fig.1.
Odds of In-hospital and 30-day Mortality Among Medicare Beneficiaries Hospitalized with Sepsis Based on Part-D Prescription Drug Claims. 1. All BB = all classes of beta-blockers; C-BB = cardioselective beta-blockers; NS-BB = nonselective beta-blockers; SSRI = all classes of selective serotonin release inhibitors. 2. Odds of those with the drug of interest prescribed through the date of admission for sepsis dying versus those without such a prescription.
4. Discussion
In this study of a national cohort of elderly patients admitted for sepsis we found that patients who were prescribed beta-blockers prior to admission, despite having more co-morbid conditions, had a statistically significant decrease in both in-hospital and 30-day mortality. While most of the prior literature has focused on initiating short-acting beta-blockers in the intensive care setting, the question of whether preadmission outpatient beta-blockers may also improve morbidity and mortality has arisen. Our findings, using beta-blocker prescription through the date of admission for sepsis as a proxy for exposure to the cardioprotective effects of these drugs prior to the onset of acute illness, suggest this may be true.
Beta-blockers have historically been used to treat ischemic heart disease, heart failure, as well as for secondary prevention of myocardial infarction.7,15–17 Given their cardioprotective effects, this class of drugs is recognized as being beneficial in the treatment of sepsis. Though the exact mechanism has yet to be fully elucidated, it is thought to be due to their modulation of the sympathetic cytokine storm in the setting of sepsis and subsequent amelioration of myocardial injury.16,18–20 Somewhat surprisingly, we found that patients prescribed nonselective beta-blockers experienced a greater mortality benefit than patients prescribed cardioselective beta-blockers (compared to no beta-blocker prescribed). These findings suggest that the overall blunting of the effects of an overstimulated sympathetic nervous system in the setting of the cytokine storm induced by sepsis may play a bigger role in improving mortality than cardioprotection. However, it may be possible that patients prescribed cardioselective beta-blockers have more baseline cardiac risk placing them at higher mortality as suggested by the 23% higher mortality among patients on cardioselective beta-blockers compared to non-selective agents. Taken together, these findings have implications for selecting beta-blockers when treating sepsis acutely in critical care settings.
Most of the focus on beta-blocker treatment for sepsis in the intensive care setting has been centered on short acting beta-blockers, in particular the cardioselective drug esmolol. The large trial by Morelli and colleagues randomized patients to esmolol due to its brief half-life, thereby allowing providers to titrate the drug with great control over a shorter period of time. Patients treated with a beta-blocker in that study experienced a 61% reduction in inpatient mortality (adjusted hazard ratio 0.39 CI 0.26e0.59).5,21 Subsequent studies followed suit in their choice of a short acting beta-blocker. These studies did not report mortality benefit; rather, they found that critically ill patients with sepsis treated with beta-blockers experienced improved tissue perfusion, hemodynamics, fluid requirement, and cardiac function.22–25 While our data did not allow us to measure which of our patients may have been treated with short acting beta-blockers during hospitalization, our data indicates that baseline modulation of both cardiac drive and vascular constriction plays a role is improving mortality in older patients admitted with sepsis.
Sepsis confers increasing mortality with increasing age.26 We also found increasing odds of death in increasingly older age groups in the present study. A study from Italy encompassing patients age 40 and older who were on beta-blockers prior to admission for sepsis found an overall 22% reduction in 28-day mortality.21 However, we found a nearly double protective effect with a 41% improvement in 30-day mortality within our older cohort. While this might therefore imply an increasingly beneficial relationship between beta-blocker usage and age, we found the opposite in our cohort limited to those older than 65 regarding in-hospital mortality. In subgroup analysis by age, we found that the youngest age group (65–74 years old) experienced the highest mortality benefit in association with beta-blocker followed by the middle age group (75–84 years old) and then by those above age 85. However, this linear decrease did not hold for 30-day mortality, although patients age 65–74 still benefited the greatest. Importantly still, we found that every age group benefited from a beta-blocker prescription after they were admitted with a primary diagnosis of sepsis.
Like all studies utilizing administrative data, ours has some important limitations to consider. Patients with sepsis may have been misclassified due to errors in coding or upcoding for financial benefit. However, this likely affected both study groups equally. We were unable to access clinical markers, such as laboratory values and vital signs, which could serve to better characterize the severity of sepsis. Similarly, we did not have in-hospital pharmacy records. It is possible that those who were on a beta-blocker prior to admission may have been more likely to continue receiving a beta-blocker during hospitalization thereby confounding our findings. However, the confounding is likely minimal because our patient cohort was derived from 2009 to 2011 claims databefore use of beta-blockers for modulation of sepsis was widely accepted and implemented in critical care practice. We also lacked data on whether patients who were prescribed beta-blocker were actually taking them at the time of being admitted for sepsis. To minimize misclassification, we did not include any patients who did not actively fill a beta-blocker prescription within 30-days of admission using the a priori assumption that the patients who were filling their prescription at regular 30-day intervals were likely compliant with their medications. Importantly, patients who receive outpatient medical follow-up and fill prescriptions accordingly may have improved outcomes simply due to the effects of increased medical surveillance rather than effects of the medications prescribed. To assess this “healthcare utilization” effect, we conducted a parallel analysis using SSRI prescriptions that supports our assumption that the cardioprotective and vasoactive effects of beta-blockers are the reason for the observed mortality benefit, particularly in the inpatient setting. Having been prescribed an SSRI had no impact on inpatient mortality and a significantly smaller impact than beta-blockers in 30-day mortality. The latter finding suggests that utilization per se may still play some role in post-discharge outcomes. Finally, patients who rely on Part D claims for prescription drug benefits are more likely to female and of lower socioeconomic status; thus, these results may not be generalizable to other populations.27,28
Nevertheless, our analysis of this large cohort of elderly Americans admitted for sepsis suggests that there may be a significant benefit from preadmission beta-blockers in reducing mortality. While our findings support the favorable effects of beta-blockers on sepsis outcomes, they cannot support expanded indications for routine beta-blocker usage simply due to the possibility that a patient may develop sepsis. Rather, our findings do support the need for vigilance among outpatient providers to ensure that those who do meet criteria for beta-blocker treatment are on them and substantiate the use of beta-blockers after diagnosis of sepsis.29 Furthermore, sepsis mortality prediction models might be enhanced with inclusion of this outpatient medication exposure, as they appear to provide significant protection against mortality.
Acknowledgments
This research is supported by a grant from the Agency for Healthcare Research and Quality (R01HS022694) to HPS. The content represents the thoughts and opinions of the authors and not the funding agency.
Footnotes
Conflict of interest statement
We have no conflicts of interest to disclose.
References
- 1.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369(9):840–851. [DOI] [PubMed] [Google Scholar]
- 2.Novosad SA, Sapiano MR, Grigg C, et al. Vital signs: epidemiology of sepsis: prevalence of health care factors and opportunities for prevention. MMWR Morb Mortal Wkly Rep. 2016;65(33):864–869. [DOI] [PubMed] [Google Scholar]
- 3.Stoller J, Halpin L, Weis M, et al. Epidemiology of severe sepsis: 2008–2012. J Crit Care. 2016;31(1):58–62. [DOI] [PubMed] [Google Scholar]
- 4.Inoue S, Suzuki K, Komori Y, et al. Persistent inflammation and T cell exhaustion in severe sepsis in the elderly. Crit Care. 2014;18(3):R130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morelli A, Ertmer C, Westphal M, et al. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. JAMA. 2013;310(16):1683–1691. [DOI] [PubMed] [Google Scholar]
- 6.Hamzaoui O, Teboul JL. The role of beta-blockers in septic patients. Minerva Anestesiol. 2015;81(3):312–319. [PubMed] [Google Scholar]
- 7.Wang Z, Wu Q, Nie X, Guo J, Yang C. Combination therapy with milrinone and esmolol for heart protection in patients with severe sepsis: a prospective, randomized trial. Clin Drug Investig. 2015;35(11):707–716. [DOI] [PubMed] [Google Scholar]
- 8.Vieillard-Baron A. Septic cardiomyopathy. Ann Intens Care. 2011;1(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanfilippo F, Santonocito C, Morelli A, Foex P. Beta-blocker use in severe sepsis and septic shock: a systematic review. Curr Med Res Opin. 2015;31(10): 1817–1825. [DOI] [PubMed] [Google Scholar]
- 10.Gutierrez J, Hossam A, Lazarezcu R, Kay E, Rundek T. Effect of beta blockers on sepsis outcome. Med Sci Monit. 2009;15(10). Cr499–503. [PubMed] [Google Scholar]
- 11.Balik M, Rulisek J, Leden P, et al. Concomitant use of beta-1 adrenoreceptor blocker and norepinephrine in patients with septic shock. Wien Klin Wochenschr. 2012;124(15–16):552–556. [DOI] [PubMed] [Google Scholar]
- 12.Yang S, Liu Z, Yang W, et al. Effects of the beta-blockers on cardiac protection and hemodynamics in patients with septic shock: a prospective study. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2014;26(10):714–717. [DOI] [PubMed] [Google Scholar]
- 13.Schmittinger CA, Dunser MW, Haller M, et al. Combined milrinone and enteral metoprolol therapy in patients with septic myocardial depression. Crit Care. 2008;12(4):R99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. [DOI] [PubMed] [Google Scholar]
- 15.Sieber FE, Barnett SR. Preventing postoperative complications in the elderly. Anesthesiol Clin. 2011;29(1):83–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mookerjee RP, Pavesi M, Thomsen KL, et al. Treatment with non-selective beta blockers is associated with reduced severity of systemic inflammation and improved survival of patients with acute-on-chronic liver failure. J Hepatol. 2016;64(3):574–582. [DOI] [PubMed] [Google Scholar]
- 17.Schumann SA, Hickner J. When not to use beta-blockers in seniors with hypertension. J Fam Pract. 2008;57(1):18–21. [PMC free article] [PubMed] [Google Scholar]
- 18.Contenti J, Occelli C, Corraze H, Lemoel F, Levraut J. Long-term beta-blocker therapy decreases blood lactate concentration in severely septic patients. Crit Care Med. 2015;43(12):2616–2622. [DOI] [PubMed] [Google Scholar]
- 19.de Montmollin E, Aboab J, Mansart A, Annane D. Bench-to-bedside review: beta-adrenergic modulation in sepsis. Crit Care. 2009;13(5):230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ince C. To beta block or not to beta block; that is the question. Crit Care. 2015;19(1):339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morelli A, Donati A, Ertmer C, et al. Microvascular effects of heart rate control with esmolol in patients with septic shock: a pilot study. Crit Care Med. 2013;41(9):2162–2168. [DOI] [PubMed] [Google Scholar]
- 22.Shang X, Wang K, Xu J, et al. The effect of esmolol on tissue perfusion and clinical prognosis of patients with severe sepsis: a prospective cohort study. Biomed Res Int. 2016;2016:1038034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei C, Louis H, Schmitt M, et al. Effects of low doses of esmolol on cardiac and vascular function in experimental septic shock. Crit Care. 2016;20(1):407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du W, Wang XT, Long Y, Liu DW, et al. Efficacy and safety of esmolol in treatment of patients with septic shock. Chin Med J Engl. 2016;129(14): 1658–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tao Y, Jingyi W, Xiaogan J, Weihua L, Xiaoju J. Effect of esmolol on fluid responsiveness and hemodynamic parameters in patients with septic shock. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2015;27(11):885–889. [PubMed] [Google Scholar]
- 26.Nasa P, Juneja D, Singh O. Severe sepsis and septic shock in the elderly: an overview. World J Crit Care Med. 2012;1(1):23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elliott RA, Majumdar SR, Gillick MR, Soumerai SB. Benefits and consequences for the poor and the disabled. N Engl J Med. 2005;353(26):2739–2741. [DOI] [PubMed] [Google Scholar]
- 28.Mott DA, Thorpe JM, Thorpe CT, Kreling DH, Gadkari AS. Effects of Medicare Part D on drug affordability and use: are seniors with prior high out-of-pocket drug spending affected more? Res Soc Adm Pharm. 2010;6(2): 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.(U.S.), N.C.f.H.S.. National Ambulatory Medical Care Survey: 2013 State and National Summary Tables. Center for Disease Control and Prevention; 2013. [Google Scholar]