Abstract
Aphasias are caused by disruption in structural integrity and interconnectivity within a large-scale distributed language network. We investigated the distribution and behavioral consequences of altered functional connectivity in three variants of primary progressive aphasia (PPA). The goal was to clarify relationships among atrophy, resting connectivity, and the resulting behavioral changes in 73 PPA and 33 control participants. Three core regions of the left perisylvian language network: the inferior frontal gyrus (IFG), middle temporal gyrus (MTG), and anterior temporal lobe (ATL) were evaluated in agrammatic (PPA-G), logopenic (PPA-L), and semantic (PPA-S) PPA variants. All PPA groups showed decreased connectivity between IFG and MTG. The PPA-S group also showed additional loss of connectivity strength between ATL and the other language regions. Decreased connectivity between the IFG and MTG nodes in PPA-G remained significant even when controlled for the effect of atrophy. In the PPA group as a whole, IFG-MTG connectivity strength correlated with repetition and grammar scores, whereas MTG-ATL connectivity correlated with picture naming and single-word comprehension. There was no significant change in the connectivity of homologous regions in the right hemisphere. These results show that language impairments in PPA are associated with perturbations of functional connectivity within behaviorally concordant components of the language network. Altered connectivity in PPA may reflect not only the irreversible loss of cortical components indexed by atrophy, but also the dysfunction of remaining neurons.
Search Terms: Aphasia, Language impairment, Primary Progressive Aphasia, Resting state fMRI, Neuroimaging
1. INTRODUCTION
The classic Wernicke-Lichtheim-Geschwind language network model was established almost exclusively on the basis of stroke aphasia where all neural elements at the core of the lesion are irreversibly destroyed (Geschwind, 1965). However, in primary progressive aphasia (PPA), neurodegenerative processes (Alzheimer disease or frontotemporal lobar degeneration) cause a gradual loss of neurons. The distribution of neuronal loss and its progression can be assessed in vivo by mapping focal cortical thinning (atrophy) (Collins et al., 2017; Mandelli et al., 2016; Rogalski et al., 2014) or hypometabolism (Teichmann et al., 2013). However, even areas of major atrophy can have many residual neurons (M. M. Mesulam et al., 2014). Clinicoanatomical correlations in PPA are therefore more complicated than in aphasias caused by stroke. For example, task-based functional magnetic resonance imaging (fMRI) showed that remaining neurons within sites of atrophy may surprisingly show nearly normal activation in language tasks and that the language impairment may reflect altered connectivity among components of the language network (M. Mesulam, Weintraub, Parrish, & Gitelman, 2005; Sonty et al., 2003; Sonty et al., 2007).
Three different PPA subtypes have been described: non-fluent/agrammatic (PPA-G), logopenic (PPA-L), and semantic (PPA-S) (Gorno-Tempini et al., 2011). PPA-G patients have difficulty with production of grammatical sentences, PPA-L is distinguished by impairment of word finding and repetition, and individuals with PPA-S have impairment in naming and single word comprehension (Gorno-Tempini et al., 2011). Imaging studies using morphometric, metabolic, tractographic, and functional approaches have helped define the location of left-hemisphere language network atrophy in PPA subtypes. In PPA-G atrophy and hypometabolism is more prominent in the inferior frontal gyrus (IFG) and prefrontal/premotor regions and to a lesser extent in the posterior temporal regions. In PPA-L the main location for atrophy/hypometabolism is in the posterior temporal lobe. In PPA-S, atrophy is more prominent in the anterior parts of the temporal lobe (ATL) (Gorno-Tempini et al., 2004; Rabinovici et al., 2008). It should, however, be noted that light boundaries between different PPA subtypes (e.g. PPA-G and PPA-L) and the presence of mixed PPA subtypes limit this simplistic view (Bisenius et al., 2017; M. M. Mesulam, Wieneke, Thompson, Rogalski, & Weintraub, 2012).
Tractographic and functional imaging techniques have shown patterns of language network abnormalities which follow the dual stream model of language network (Hickok & Poeppel, 2007). In PPA-G and PPA-L the neurodegenerative pathology affects the dorsal language stream, which connects posterior temporal cortex with the IFG and prefrontal regions through the arcuate and superior longitudinal fasciculi. In contrast, the pathology in PPA-S preferentially affects the ventral stream of language along the inferior longitudinal fasciculus and extreme capsule (Galantucci et al., 2011).
Resting state fMRI (rsfMRI) is sensitive to polysynaptic pathways in the brain and provides an ideal way to explore the anatomy of neurocognitive networks. Our previous work (Bonakdarpour, Hurely, & Mesulam, 2013; Hurley, Bonakdarpour, Wang, & Mesulam, 2015) showed that core regions of the language network, including inferior frontal gyrus (IFG), middle temporal gyrus (MTG), and anterior temporal lobe (ATL), are asymmetrically interconnected in the left hemisphere. We then showed that the left perisylvian connections weaken in early stage PPA-G before atrophy is prominent, suggesting that resting state functional connectivity changes are a leading rather than lagging indicator in the disease process (Bonakdarpour et al., 2017a). The current study extends those findings by examining larger samples of all variants of PPA. Methodological steps are taken to account for relationships between structural and functional changes in the brain, i.e. between resting connectivity and structural MRI. Based on previous research (Bonakdarpour et al., 2017b; Guo et al., 2013; Whitwell et al., 2015) and the dual stream framework of the language network, we hypothesized that intrahemispheric connectivity strength of IFG and MTG would be reduced in PPA-G and PPA-L. In PPA-S we expected the connectivity strength of the ATL to be reduced. We also predicted that the connectivity between MTG and IFG would correlate with measures of grammar and repetition whereas the connectivity of ATL would correlate with measures of naming and comprehension.
2. METHODS
We report how we determined our sample size, all data exclusions (if any), all inclusion/exclusion criteria, whether inclusion/exclusion criteria were established prior to data analysis, all manipulations, and all measures in the study. The conditions of our ethics approval do not permit public archiving of anonymised study data. Images used for this study can be obtained through Northwestern University Mesulam Center collaborative request online at https://www.brain.northwestern.edu/scientists-students/collaborative-request.html No part of the study procedures or analyses pre-registered in a time-stamped, institutional registry prior to the research being conducted.
2.1. Participants
2.1.1. PPA participants
Participants of this study were chosen from a longitudinal investigation at the Northwestern University Mesulam Center for Cognitive Neurology and Alzheimer Disease. Clinical diagnosis of PPA was made based on criteria of a progressive language decline, which was not accompanied by significant impairment in other cognitive domains, and was caused by neurodegenerative disease. All patients were evaluated by a comprehensive battery of neuropsychological and language tests and the final diagnosis of PPA and subtypes were made based on a group consensus. The Western Aphasia Battery (WAB) (Kertesz, Western aphasia battery stimulus,. Raven,, & Coloured progressive, 2007) was used as a general measure of language function, and specific domains of language relevant to PPA were assessed via well-established tests of picture naming (Boston Naming Test, or BNT), grammar (a composite measure of grammatical performance [Northwestern Anagram Test (Weintraub et al., 2009) and Northwestern Assessment of Verbs and Sentences: NAT-NAVS] (Cho-Reyes & Thompson, 2012; M. M. Mesulam et al., 2012)), repetition (a subset of the 6 most difficult items from the repetition subtest from the WAB, [REP66])(M. M. Mesulam et al., 2012), and single-word comprehension (36-point score from the Peabody picture vocabulary test [PPVT])(Dunn, 2007). Patients were accordingly classified into three known variants of PPA: agrammatic (PPA-G), logopenic (PPA-L), and semantic (PPA-S), respectively(M. Mesulam et al., 2009).
Based on our power analysis we needed a minimum sample size of 14 PPA patients, for each subtype, to have 80% power to detect a mean difference in connectivity of 0.12, assuming a standard deviation of 0.14 and a two-tailed test at a Type I error rate of 5%. Seventy three individuals with PPA (mean age= 65.6 ± 6, 32 women, 41 men), who were recruited between 2009 and 2016 had both resting state fMRI and structural MRI scans. Their average WAB aphasia quotient (WAB-AQ) was 85.44 ± 7.7 (>65). Thirty six individuals with PPA were in the PPA-G group, 20 in the PPA-L group, and 17 in the PPA-S group. Demographic and language measures for PPA participants are displayed in Table 1.
Table 1 –
PPA and Healthy Control Demographics
| N | Age | Gender | WAB-AQ (/100) | BNT (/60) | PPVT (/36) | NATNAVS (/15) | Rep66 (/66) | |
|---|---|---|---|---|---|---|---|---|
| HC | 33 | 63 ± 7 | 15 M/ 18 F | 98.9 ± 1.9 | 58.2 ± 1.8 | 35.1 ± 1.2 | 14.8 ± 0.4 | 65.1 ± 1.6 |
| PPA | 73 | 65 ± 6 | 41 M/32 F | 85.4 ± 7.7 | 35.9 ± 19.0 | 29.9 ± 7.3 | 10.3 ± 3.8 | 51.1 ± 10.8 |
| PPA-G | 36 | 65 ± 7 | 18 M/18F | 84.6 ± 7.7 | 44.3 ± 12.2 | 33.0 ± 2.6 | 8.4 ± 3.8 | 49.5 ± 11.3 |
| PPA-L | 20 | 66 ± 7 | 13 M/7 F | 87.1 ± 8.5 | 43.5 ± 15.8 | 33.6 ± 2.2 | 11.3 ± 2.3 | 47.4 ± 11.0 |
| PPA-S | 17 | 63 ± 6 | 10 M/7 F | 85.2 ± 6.6 | 9.1 ± 4.4 | 19.1 ± 7.5 | 13.9 ± 1.6 | 58.9 ± 4.3 |
WAB-AQ = Western Aphasia Battery Aphasia Quotient; BNT = Boston Naming Test; HC= Healthy Controls; PPVT = Peabody Picture Vocabulary Test; NATNAVS = Northwestern Anagram Test-Northwestern Assessment of Verbs and Sentences; Rep66 = WAB repetition score. PPA = Primary Progressive Aphasia; PPA-G = Agrammatic Variant of PPA; PPA-L = Logopenic Variant of PPA; PPA-S = Semantic Variant of PPA.
2.1.2. Healthy controls
Imaging results were compared to a group of 33 healthy control participants (mean age= 63.5 ± 7.0; 18 women, 15 men) from our previous publication(Hurley et al., 2015). Language measures for healthy controls are included in Table 1.
2.2. Image acquisition
MRI scans were obtained using a Siemens Trio 3T scanner. fMRI scans were acquired using a T2-weighted echo planar sequence (repetition time = 2500ms, echo time = 20ms, flip angle = 80°, field of view = 220, 3×3×3 mm voxel size) while participants were instructed to remain awake with eyes open for 10 minutes. Structural scans were acquired using a T1-weighted 3D MP-RAGE sequence (repetition time = 2300 ms, echo time = 2.91ms, flip angle = 9°, field of view = 256, 1×1×1 voxel size).
2.2.1. Selection of Regions of Interest
Three spherical left hemisphere ROIs (radius= 10 mm) in the language network were chosen for imaging analyses, as well as their homotopic counterparts in the right hemisphere. As mentioned above, nodes were selected based on our previous study (Hurley et al., 2015) in healthy individuals. The pars triangularis of the IFG (Montreal Neurologic Institute [MNI] coordinates = ±54, 24, 3) was chosen as one of the nodes, because it is a component of Broca’s area, a region that has been included as a major epicenter of the classic language network for more than 150 years, and is among the most consistently activated regions in neuroimaging studies of language (Indefrey & Levelt, 2004; Liu, Stufflebeam, Sepulcre, Hedden, & Buckner, 2009; Lohmann et al., 2010). The posterior MTG (MNI coordinates = ±66, −38, −4) was chosen as another region, because lesion mapping and functional MRI studies have shown that many of the lexico-semantic functions originally ascribed to Wernicke’s area can be linked to the integrity of this part of the temporal lobe (Baldo, Arevalo, Patterson, & Dronkers, 2013; Pascual et al., 2015; Wei et al., 2012) (Liu et al., 2009; Turken & Dronkers, 2011). It has also been shown that MTG and IFG jointly contribute to grammatical and semantic processing (Hagoort, 2005; Jefferies, 2013; Rogalsky et al., 2018), that they are more strongly interconnected during language tasks (Snijders, Petersson, & Hagoort, 2010), and that TMS in either region disrupts verbal processing (Acheson & Hagoort, 2013). The lateral portion of ATL (MNI coordinates = ±50, 11, −32) was chosen as it overlaps with peak atrophy sites in patients with word comprehension impairments as well as the functional activation sites associated with synonym identification tasks (Gitelman, Nobre, Sonty, Parrish, & Mesulam, 2005; M. M. Mesulam et al., 2013). In the left hemisphere, this lateral portion of ATL has also been shown to have ipsilateral resting functional connectivity with other language regions (Hurley et al., 2015; Pascual et al., 2015; Warren, Crinion, Lambon Ralph, & Wise, 2009).
In addition to a priori analyses in the core regions described above, we conducted exploratory analyses in two additional regions for a second level analysis: the angular gyrus (AG), and posterior superior temporal gyrus (STG). In previous studies the angular gyrus was shown to be interconnected with IFG, MTG, and ATL, although it did not show the same degree of leftward asymmetry as shown in those other components of the language network (Hurley et al., 2015). AG is, however, an important component of the Geschwind region (Catani, Jones, & ffytche, 2005) and is thought to be involved in semantically based language functions. It is also a common region of atrophy in individuals with PPA-L (M. Mesulam et al., 2009). To determine coordinates for the AG region in a method similar to what we reported in Hurley et al (2015), we first generated connectivity maps for the three core language seeds. The overlapping clusters for these maps were then determined. Cluster located within AG region of the Automated Anatomical Labeling (AAL) atlas was used to determine the location of the AG ROI. The center of the overlapping AG cluster was then used as the center of the spherical 10 mm ROI for our purposes (MNI coordinates = ±53, −61, 32).
Posterior STG was historically included as part of Wernicke’s zone (which also includes posterior MTG) (Catani et al., 2005), and was therefore thought to contributes to word comprehension. The degree of its contribution, however, has been challenged by recent studies of patients with PPA (M. M. Mesulam et al., 2019). Posterior STG has also failed to show leftward asymmetric connectivity with other components of the language network, including IFG, MTG, and ATL (Hurley et al., 2015; Liu et al., 2009; Montembeault et al., 2019). In keeping with a traditional assignment of Wernicke’s area, we placed the center of the 10 mm spherical ROI at the center of the posterior third of STG (± 62, −47, 19).
To determine specificity of our findings to the language network, in addition to measuring connectivity between the homotopic language regions, we chose two ROIs of the spatial attention network bilaterally using the following regions: the intraparietal sulci (IPS; MNI coordinates = ±23, −58, 53), and the frontal eye field (FEF = ±26, −5, 58). In previous studies by our group and others, ipsilateral connectivity between these spatial attention ROIs was shown to be of similar amplitude in both hemispheres (Hurley et al., 2015; van Daijk et al., 2010). Only gray matter voxels within each ROI were included in functional connectivity analyses (see next section).
2.2.2. Image analysis
2.2.2.1. Structural analyses
Preprocessing steps were performed using SPM12 (www.fiLion.ud.ac.uk/spm/). The T1 structural images were segmented into gray matter, white matter, and cerebrospinal fluid probability maps and warped into normalized Montreal Neurological Institute (MNI) space using the DARTEL algorithms (Ashburner, 2007). As mentioned above, in this study we used two approaches to account for the influence of atrophy on functional connectivity measurements. To limit the analysis only to the gray matter, two of the authors (RSH and AC) developed a novel SPM pipeline: the Individually-Masked Group Analysis (IMGA) toolbox (http://academic.csuohio.edu/neurocog-sys/imga/), which was used to binarize the gray matter probability maps at a threshold of 50%, thus classifying all voxels as being likely or unlikely to include valid gray matter tissue. This method ensures that Blood Oxygen Level Dependent (BOLD) signal is only queried from valid cortical voxels. The binarized maps were also used to mask out non-gray matter voxels in the fMRI volumes (see next section). As a second step, all fMRI analyses included cortical volume in each ROI as a covariate. Volume was quantified as the number of valid gray matter voxels in each ROI, normalized into quotients by including the total intracranial volume as a divisor. To evaluate the magnitude of atrophy in PPA, we compared cortical volume in each ROI against control volume values via ANOVA and Bonferroni-corrected posthoc t-tests.
2.2.2.2. fMRI analyses
2.2.2.2.1. Preprocessing
fMRI volumes were corrected for slice timing, realigned, coregistered to the structural images, and warped into MNI space. The resulting fMRI volumes were all overlaid on normalized T1 images for each subject to confirm accurate alignment, as dysmorphia due to atrophy can affect their desired coregistration. Wherever misalignments were observed they were manually corrected. Nuisance covariates, including the six affine motion parameters, global signal, white matter signal, and cerebrospinal fluid signal were regressed out of each time series. The IMGA binarized maps were then used to mask the fMRI volumes, restricting all subsequent preprocessing and resting connectivity analyses to voxels likely to contain gray matter. The volumes were then smoothed using a 6mm kernel. IMGA was used to restrict smoothing to the masked space only, preventing the dissemination of hemodynamic signal from non-gray matter voxels. The volumes were then detrended and bandpass filtered from 0.01–0.08 Hz. Using the REST toolkit (Song et al., 2011), the hemodynamic time series were extracted from and averaged across all of the unmasked voxels in each ROI.
2.2.2.2.2. Hemodynamic Correlations between regions of interest
Correlations were computed between the averaged time series of ROIs. For the language ROIs, each of the three possible correlations between pairs of ipsilateral ROIs was examined separately in the left hemisphere and the right hemisphere. The Pearson coefficients were z-transformed and averaged across participants. One-way ANOVAs were used to examine whether the magnitude of correlation coefficients differed across groups. Significant ANOVAs were followed up with post-hoc t tests, comparing each PPA group to controls, using False Discovery Rate (FDR) correction for multiple comparisons. This same procedure was also used to compare the magnitude of correlations between the two spatial attention control sites in each hemisphere.
Although IMGA was used to restrict fMRI analyses to gray matter voxels, it remains theoretically possible that connectivity and morphology are still confounded, such that those ROIs that are most disconnected are also the most atrophic. In order to further disentangle the potentially confounding influence of atrophy on connectivity, z(r) values were also subjected to analysis of co-variance (ANCOVA) including cortical volumes in each of the two relevant ROIs as covariates.
Pearson correlations were examined between the connectivity z(r) values and four language measures, which we have validated in previous studies as discussed above, i.e., picture naming (BNT), grammatical performance (NAT-NAVS), repetition performance (REP66), and single-word comprehension (PPVT). Partial correlations were also conducted including cortical volume in each ROI as a covariate to rule out contribution for cortical atrophy.
2.2.2.2.3. Whole-brain Functional Connectivity Maps
Exploratory whole-brain connectivity analyses were conducted, in order to probe for additional patterns of disconnection in PPA that may lay outside of the a priori selected ROIs. Whole-brain connectivity maps employing the three language ROIs (IFG, MTG, and ATL) as “seeds” were created using the REST-plus toolkit. The hemodynamic time series from all voxels included in the ROI were averaged and correlated with the individual time series from all other voxels in the brain. The Pearson correlation coefficients were then normalized with Fischer’s z transformation, yielding a whole-brain functional connectivity map for each subject. The whole-brain connectivity maps from each PPA group (L/G/S) were then each compared to controls, resulting in two-sample t maps. These t maps were thresholded using a false discovery rate (FDR) of p <.05, revealing areas throughout the cortex that showed significant decreased connectivity with each ROI in PPA (as compared to healthy controls).
Of note, no part of the study procedures and analyses was pre-registered prior to the research being conducted.
3. RESULTS
3.1. Cortical volume comparisons between PPA and control groups
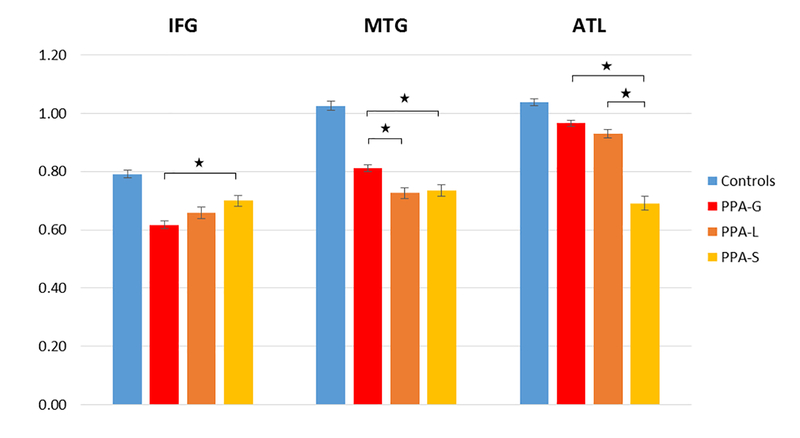
Cortical volume within each node is depicted in Figure 1. One-way ANOVAs showed a significant effect of group on cortical volume in all three left-hemispheric language ROIs (IFG: F= 28.89, p<0.0001; MTG: F= 80.39, p<0.0001; ATL: Fdf=3 = 85.88, p<0.0001). Follow up post-hoc analyses with Bonferroni correction showed that cortical volume was significantly decreased in all PPA groups compared to controls, in all 3 ROIs (p < 0.0001 for all ROIs, except for IFG in PPA-S (p = 0.001), and ATL in PPA-G (p = 0.001). Post-hoc analyses comparing cortical volume between PPA groups showed that PPA-G had the least amount of atrophy in temporal nodes (ATL and MTG), and PPA-S had the least amount of atrophy in IFG (p<0.05, Bonferoni corrected; eFigure 2). In PPA-L cortical thinning in MTG was comparable to PPA-S and both had more atrophy compared to PPA-G (p=0.02 and p= 0.01).
Figure 1. Normalized cortical volume in each ROI.
All subtypes showed reduced volume, in all ROIs, compared to the control group. Asterisks indicate significant differences in volume between PPA groups (Bonferroni corrected). IFG = Inferior Frontal Gyrus; MTG = Middle Temporal Gyrus; ATL = Anterior Temporal Lobe. PPA = Primary Progressive Aphasia; PPA-G = Nonfluent/agrammatic variant of PPA; PPA-L = Logopenic variant of PPA; PPA-S = Semantic variant of PPA.
3.2. Comparison of functional connectivity of the language ROIs in PPA and control groups
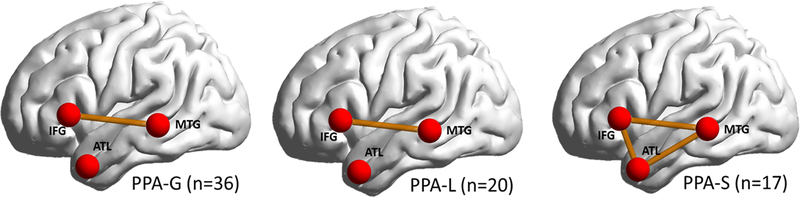
Z(r) scores for each PPA subtype is depicted in Table 2. One-way ANOVAs revealed significant group differences in the amplitude of each connection between left-hemispheric language ROIs (IFG-MTG: F=6.02, p= 0.001; MTG-ATL: F= 4.65, p= 0.004; IFG-ATL: Fdf=3=3.45, p= 0.019). Follow up t-tests with False Discovery Rate (FDR) correction showed decreased IFG-MTG connectivity in all 3 PPA groups compared to the control group (Table 2 and Figure 2). The PPA-S group showed significant decline in IFG-ATL and MTG-ATL connectivity compared to the control group. MTG-ATL connectivity in the PPA-S group was also significantly lower than the other PPA groups. Neither subgroup showed reduced connectivity in any of the homologous right-hemispheric connections compared to the control group or within PPA groups (all p>.05).
Table 2 –
Functional connectivity z(r) values in the PPA subgroups
| PPA Subtype | Node Pairs | Left Hemisphere | Right Hemisphere |
|---|---|---|---|
| PPA-G | IFG-MTG | 0.23 ± .23* | 0.16 ± .20 |
| IFG-ATL | 0.14 ± .18 | 0.06 ± .16 | |
| MTG-ATL | 0.19 ± .22 | 0.14 ± .16 | |
| PPA-L | IFG-MTG | 0.22 ± .20* | 0.23 ± .19 |
| IFG-ATL | 0.13 ± .22 | 0.03 ± .17 | |
| MTG-ATL | 0.22 ± .16 | 0.18 ± .18 | |
| PPA-S | IFG-MTG | 0.21 ± .20* | 0.18 ± .21 |
| IFG-ATL | 0.01 ± .16* | −0.01 ± .14 | |
| MTG-ATL | 0.04 ± .15* | 0.12 ± .15 | |
significantly decreased versus controls after FDR correction for multiple comparisons (p < 0.05). p values (top to bottom): p = 0.001, 0.002, 0.002, 0.001, <0.0005. ANCOVA analyses showed that the IFG-MTG connection in PPA-G continued to be significantly decreased when cortical volume was included as a covariate (p=0.036). In PPA-L patients there was only a trend towards significance (p=0.082).
Figure 2. Connections which are reduced in each PPA subtype.
Solid orange lines depict significantly lower connectivity between ROIs (red) in PPA compared to controls, corrected for multiple comparisons using Bonferroni correction. IFG = Inferior Frontal Gyrus; MTG = Middle Temporal Gyrus; ATL = Anterior Temporal Lobe. PPA = Primary Progressive Aphasia; PPA-G = Nonfluent/agrammatic variant of PPA; PPA-L = Logopenic variant of PPA; PPA-S = Semantic variant of PPA.
ANCOVA analyses showed that IFG-MTG was significantly reduced in PPA-G when cortical volume was included as a co-variate. Reduced IFG-MTG connectivity in PPA-S was not significant after factoring out the effect of atrophy, and trended towards significance in PPA-L. Reduced MTG-ATL and IFG-ATL connectivity in PPA-S did not survive atrophy correction.
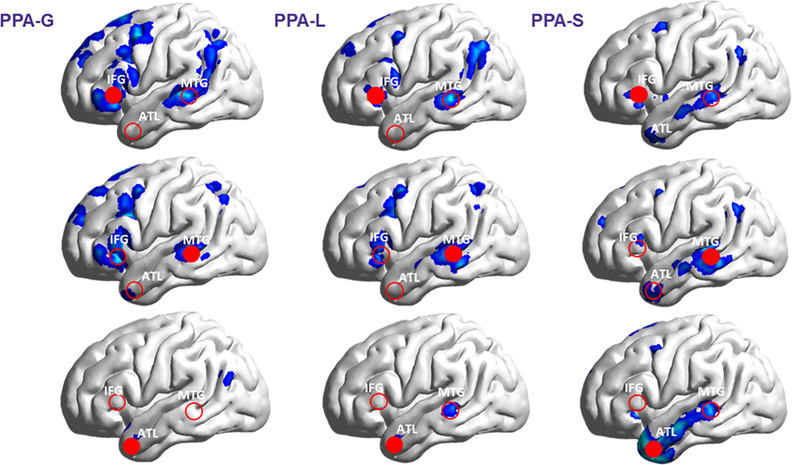
Whole brain analysis showed decreased connectivity of IFG and MTG with posterior temporoparietal and prefrontal/premotor regions (p=0.05, FDR corrected; see Figure 3). In PPA-G patients, the areas with decreased prefrontal/premotor connectivity were relatively more widespread than in PPA-L. In contrast, PPA-S patients showed decreased connectivity of MTG and ATL with lateral temporal cortex, while connectivity within dorsal perisylvian regions was mostly preserved. Correction for atrophy confirmed the same pattern we found using pair-wise seed-based analysis persisted for the IFT, MTG, and ATL regions (Supplementary Tables 1, 2 & 3). In the same manner, in PPA-G all perisylvian clusters with decreased connectivity with IFG and MTG survived correction for atrophy (Supplementary Table 1). In contrast, most of the larger perisylvian clusters observed in PPA-L and PPA-S did not survive atrophy correction (Supplementary Tables 2 & 3).
Figure 3. Reduced resting state functional connectivity in PPA compared to controls in using seed based (whole brain) analyses.
Images demonstrate areas throughout the cortex (in blue; p<0.05 FDR corrected) which are disconnected from each ROI (filled red circles) in PPA, as compared to controls. Whole-brain analyses reveals additional areas disconnected from IFG including dorsal premotor regions, manifest specifically in PPA-G. All subtypes show decreased connectivity between IFG and the temporoparietal junction. IFG = Inferior Frontal Gyrus; MTG = Middle Temporal Gyrus; ATL = Anterior Temporal Lobe. PPA = Primary Progressive Aphasia; PPA-G = Nonfluent/agrammatic variant of PPA; PPA-L = Logopenic variant of PPA; PPA-S = Semantic variant of PPA.
Additional analysis including the STG and AG ROIs using FDR correction for multiple comparisons showed decrease in IFG-AG connectivity in PPA-L and PPA-S groups (p= 0.002; p= 0.007 ) and unique decrease in connectivity in AG-MTG in PPA-S (p= 0.016). Following atrophy correction only decreased connectivity between IFG-AG in PPA-S continued to be significant (p= 0.034). There was no significant change in connectivity between STG and other ROIs.
3.3. Comparison of functional connectivity of dorsal attention network in PPA and control groups
The average z (r) score for IPS-FEF connectivity in the left and right hemispheres were respectively 0.42 ± 0.23 and 0.42 ± 0.26 for PPA-G, 0.46 ± 0.22 and 0.53 ± 0.27 for PPA-S, and 0.30 ± 0.29 and 0.44 ± 0.25 for PPA-L. Compared to the control groups (0.42 ± 0.25 and 0.40 ± 0.23), none of the PPA groups showed a significant decrease in spatial attention connectivity of the IPS-FEF node pair (p> 0.1).
Correlation between functional connectivity and language measures
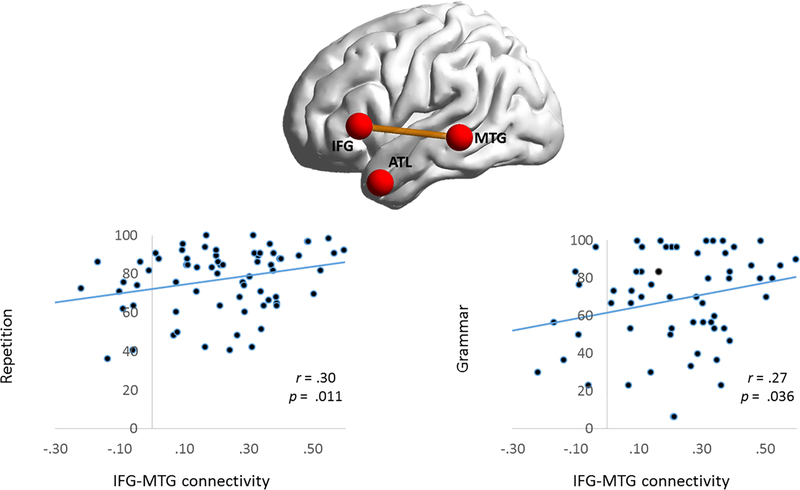
Correlations were examined between language scores and connectivity in the entire PPA sample (controls were excluded from this analysis). BNT, repetition, and PPVT scores were available for all PPA participants, while NAT-NAVS grammatical performance scores were available for N=63/73. Results were corrected for multiple comparisons using FDR. BNT and PPVT scores positively correlated with MTG-ATL connectivity, and repetition and NAT-NAVS scores positively correlated with IFG-MTG connectivity (Figure 4& 5 and Table 3). Correlations with repetition and grammar scores remained significant when cortical volume in each node was partialed out. In contrast, after partialing out cortical volume, MTG-ATL connectivity no longer correlated with PPVT and showed a trend towards significance for BNT. AG-MTG connectivity correlated with both BNT (p < 0.0001) and PPVT (p = 0.002). There was no significant correlation between IFG-AG and language measures. No language scores correlated with right hemispheric connectivity, either before or after partialing out cortical volume (all p>0.05).Table 3
Figure 4. Behavioral correlations with IFG-MTG connectivity.
Scatterplots are shown between strength of IFG-MTG connectivity and tests of repetition and grammar. Repetition and grammar scores are transformed to a 100-point scale. IFG = Inferior Frontal Gyrus; MTG = Middle Temporal Gyrus; ATL = Anterior Temporal Lobe.
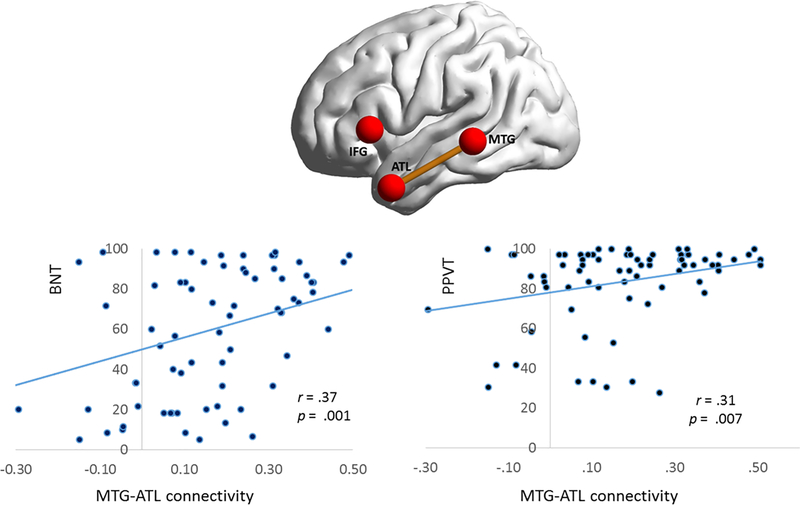
Figure 5. Behavioral correlations with MTG-ATL connectivity.
Scatterplots are shown between the strength of MTG-ATL connectivity and tests of naming (BNT) and single-word comprehension (PPVT). BNT and PPVT scores are transformed to a 100-point scale. PPA=Primary Progressive Aphasia; IFG=Inferior frontal gyrus; MTG= Middle temporal gyrus; ATL=Anterior temporal lobe.
Table 3 –
Correlation between functional connectivity and language measures in all PPA patients
| Node Pairs | Hemisphere | BNT | PPVT | NAT-NAVS | Rep66 |
|---|---|---|---|---|---|
| IFG-MTG | L | 0.22 | 0.07 | 0.27* | 0.30* |
| R | 0.07 | 0.05 | −0.04 | 0.06 | |
| IFG-ATL | L | 0.16 | 0.22 | −0.01 | −0.05 |
| R | 0.17 | 0.08 | −0.12 | −0.08 | |
| MTG-ATL | L | 0.38* | 0.31* | 0.01 | 0.05 |
| R | 0.15 | 0.06 | 0.03 | 0.06 | |
significant correlation p values (in order: BNT, PPVT, NATNAVS, Rep66): p = 0.05, 0.022, 0.006, 0.021, (FDR corrected). Following partial correlation, covarying the effect of cortical atrophy, correlation between NAT-NAVS and IFG-MTG (r= 0.231, p=0.026) and Rep66 and IFG-MTG (r=0.246, p=0.019) remained significant.
BNT = Boston Naming Test; PPVT= Peabody Picture Vocabulary Test; NATNAVS = Northwestern Anagram Test-Northwestern Assessment of Verbs and Sentences; Rep66 = WAB repetition score. IFGt = Inferior Frontal Gyrus; MTG = Middle Temporal Gyrus; ATL = Anterior Temporal Lobe. PPA = Primary Progressive Aphasia.
4. DISCUSSION
In the current study, we found subtype and task specific patterns of reduced connectivity strength within the left hemisphere language network. All PPA subtypes showed reduced connectivity strength between left IFG and MTG. PPA-S was also associated with decreased ATL connectivity with left MTG and IFG. In keeping with the distinction between a ventral language stream for lexicosemantic processing and a dorsal language stream for grammar and repetition (Friederici & Gierhan, 2013), naming and word comprehension selectively correlated with MTG-ATL connectivity, while grammar and repetition correlated with IFG-MTG connectivity. Seed-based, whole brain analysis further differentiated PPA-G from PPA-L patients with PPA-G having relatively more decreased connectivity in the prefrontal areas. These results were specific to the language network as we found no resting connectivity loss within the dorsal visual pathway.
4.1. Relationship to atrophy
Decreased connectivity between IFG and MTG in PPA-L and PPA-S and decreased connectivity between ATL and the other two language regions in PPA-S correlated with the degree of atrophy within these regions. A simple explanation is that decrease in resting connectivity could be related predominantly to loss of cortical components (e.g. synapses, dendrites, neurons, and myelin) captured by the atrophy. We cannot rule out the possibility that dysfunction of residual neurons and synapses could also be undermining the resting state connectivity that we measured.
Our findings that connectivity changes in PPA-L and PPA-S correlated with the magnitude of atrophy remained unchanged when either pair-wise or whole-brain voxel-wise analytical approaches were used. These results may seem to be at odds with previous studies by Guo et al. who reported that decreased connectivity between ATL and posterior temporal cortex could not be attributed to atrophy alone (Guo et al., 2013). A similar conclusion was reported by Whitwell et al, who used independent component analysis (ICA) to investigate the connectivity of the posterior temporal cortex in PPA-L (Whitwell et al., 2015). In these two studies a voxel-wise approach was used for to evaluate cortical atrophy and the details of the analytical methods used to covary the effects of atrophy were not detailed. Therefore, the nature of relationship between atrophy and decreased resting connectivity in PPA-L and PPA-S deserves further investigation.
In two fMRI studies based on tasks of grammar, Wilson et al had demonstrated altered fMRI activity within the IFG and posterior temporal areas in patients with PPA-G (Wilson et al., 2016; Wilson et al., 2010). Our results complement these findings by showing that the resting state connectivity between these two regions is also impaired. In contrast to the results obtained in PPA-L and PPA-S, we showed that the magnitude of decreased IFG-MTG connectivity in PPA-G was disproportionate to the degree of atrophy. The strength of the same connection correlated with measures of grammar and repetition even when the role of atrophy was factored out. This finding is in line with our previous results (Bonakdarpour et al., 2017b) of altered IFG-MTG connectivity in a small group of early-stage PPA-G patients who had not yet manifested prominent cortical atrophy.
There are at least three explanations of why the decreased connectivity in PPA-G is not attributable to atrophy alone: 1) Patients with agrammatism may present at earlier stages of the disease because loss of fluency is more readily identifiable than word finding difficulty and perturbations of resting state connectivity may precede identifiable tissue atrophy at initial evaluation. 2) It has been shown that PPA-G patients with more prominent agrammatism may have higher incidence of frontotemporal degeneration of the tau type (FTLD-tau), which affects white matter tracts more than gray matter structures (Caso et al., 2014). The decreased connectivity may therefore reflect white matter pathology without necessarily causing equally prominent atrophy of cortical gray matter. 3) The diseases causing PPA-G may affect cortical structures and resting connectivity in ways that are independent of each other, with a more substantial component being attributable to the dysfunction of structurally intact neurons.
Both PPA-L and PPA-S groups were found to have decreased IFG-AG connectivity. This coincides with commonly observed patterns of temporoparietal atrophy in PPA-L (Gorno-Tempini et al., 2004; M. Mesulam et al., 2009). Following atrophy correction this finding was no longer significant, suggesting a degree of network disruption which was proportionate to the degree of atrophy. In the case of PPA-S, however, decreased IFG-AG connectivity survived atrophy correction. Since the main location of cortical thinning in PPA-S is in the left temporal cortex, and both IFG and AG regions were less atrophic compared to other subtypes. Decreased IFG-AG connectivity in PPA-S could be predictive of late emerging dorsal atrophy in PPA-S, which has been observed in longitudinal studies (Rogalski et al., 2014). Decreased IFG-AG connectivity sets the non-agrammatic PPA subtypes apart from PPA-G.The AG-MTG node pair is another connection along a polysynaptic pathway that stretches ATL to AG as part of the ventral language stream (Hickok & Poeppel, 2007). Decreased connectivity in AG-MTG and its correlation with both PPVT and BNT supports the importance of this pathway in lexical/semantic processing (Catani et al., 2005).
4.2. Relationship between resting connectivity changes and aphasic symptoms
We found IFG-MTG connectivity correlated with measures of repetition and grammar. These relationships remained significant after cortical volume was covaried. In one related study(Whitwell et al., 2015) of PPA-L patients, repetition was measured using the WAB, and a trend was found towards a positive correlation between repetition and the network connecting posterior IFG to inferior parietal lobule (IPL). The importance of the IFG (where Broca’s area is located) to grammar is widely accepted (Indefrey & Levelt, 2004). There is also evidence that the MTG is involved in processing of grammatical aspects of sentences (Rogalsky et al., 2018). In patients with stroke aphasia, for example, the size of the lesion in this area correlates with agrammatism(Rogalsky et al., 2018). As for repetition, our findings are also in line with dual stream language processing, with repetition relying on the dorsal language stream (Friederici & Gierhan, 2013).
The role of posterior MTG and ATL in picture/object naming and word comprehension has been shown in vivo in both fMRI and rTMS studies of healthy individuals (Pobric, Jefferies, & Ralph, 2007; Price, Devlin, Moore, Morton, & Laird, 2005). Prior rsfMRI studies of PPA-S had shown abnormalities of connectivity strength in the temporal lobe and a correlation between PPVT and fractional amplitude of low frequency fluctuation (Agosta et al., 2014; Guo et al., 2013; Reyes et al., 2018). Also, damage along the ventral stream of language network encompassing both of these regions by stroke is associated with impaired naming (Alyahya, Halai, Conroy, & Lambon Ralph, 2018). In PPA patients, atrophy symptom mapping analyses have shown a correlation between ATL, posterior MTG, and picture naming (Migliaccio et al., 2016). Cortical atrophy within the left ATL has also been shown to correlate negatively with word comprehension (Guo et al., 2013; Hurley, Paller, Rogalski, & Mesulam, 2012; M. M. Mesulam et al., 2013; Rogalski et al., 2011). In keeping with these results, we found that the impaired connectivity between ATL and MTG correlated with performance on tests of picture naming (BNT) and word comprehension (PPVT). Collectively, these findings are in keeping with the divergent specialization of the dorsal language stream for sentence processing and articulatory sequencing as opposed to the specializations of the ventral stream for lexicosemantic tasks.
4.3. Role of white matter changes
Diffusion tractography in PPA has revealed changes of structural connectivity that could potentially underlie the altered functional connectivity patterns that we found. For example, decreased integrity of the arcuate fasciculus (AF), which interconnects the posterior temporal lobe (including its MTG sector) with the IFG, has been reported in PPA (Bonakdarpour, 2014; Catani & Mesulam, 2008; Galantucci et al., 2011) and is known to contribute to worsened grammar and repetition measures (Saur et al., 2008; Wilson et al., 2011). Furthermore, integrity of the inferior longitudinal fasciculus (ILF), which connects MTG and ATL, is decreased in patients with PPA (Galantucci et al., 2011)and could underlie the altered functional connectivity we detected and its impact on naming and word comprehension. The altered ATL-IFG functional connectivity we showed in PPA-S may reflect the impaired structural integrity of the uncinate fasciculus, which is likely to link these two nodes to each other (Guo et al., 2013) (D’Anna et al., 2016; Galantucci et al., 2011). Additional studies are needed to explore the contribution of white matter abnormalities to perturbations of connectivity and behavior.
4.4. Dual role of MTG
All PPA subtypes had decreased connectivity in the IFG-MTG node pair. Investigations on PPA have questioned the role of Wernicke’s area as the second major hub of the language network (M.-M. Mesulam, Thompson, Weintraub, & Rogalski, 2015; M. M. Mesulam et al., 2018). Decreased IFG-MTG connectivity is the only shared feature of all PPA subtypes in this study and therefore raises the possibility that the second major hub of the language network is located in the posterior parts of the MTG rather than in the temporoparietal junction, where Wernicke’s area is traditionally sited. This is further supported by the lack of significant connectivity difference between posterior STG and the other core language ROIs supports in our exploratory analyses.
4.5. Limitations
Results of this study are based on group level analyses, therefore, using our current methodology, findings can be generalized to many but not all single participants. Optimization of rsfMRI analysis to enhance single subject results is an ongoing field of research and represent an important future direction which may be facilitated with addition of machine learning methodologies (Hacker et al., 2013).
As noted above, tractographic information could have revealed the structural foundations of the altered connectivity patterns that we found. Future investigations will benefit from joint analyses of functional and structural connectivity and their interactive impact on language function.
Finally, this project used a cross sectional approach to study functional and structural changes in PPA compared to healthy individuals. Therefore, we are not able to comment on temporal changes and the role of plasticity. Our future research will focus on longitudinal functional and structural brain changes in PPA which will allow us to answer questions regarding longitudinal trajectories in subtypes of PPA.
5. CONCLUSION
In this study we demonstrated overlapping and unique patterns of resting connectivity perturbations in three subtypes of PPA. Our results also showed that language impairments in PPA are associated with perturbations of functional connectivity within behaviorally concordant components of the language network. Analysis of resting state data in light of cortical atrophy demonstrated that altered connectivity in PPA may reflect not only the irreversible loss of cortical components indexed by atrophy, but also the dysfunction of remaining neurons which could be a target for therapeutic interventions.
Supplementary Material
Supplementary Figure. Connections which are reduced in each PPA subtype including two additional ROIs. Solid orange lines depict significantly lower connectivity between ROIs (red) in PPA compared to controls, corrected for multiple comparisons using Bonferroni correction. IFG = Inferior Frontal Gyrus; MTG = Middle Temporal Gyrus; ATL = Anterior Temporal Lobe; STG= Superior Temporal Gyrus; AG= Angular Gyrus. PPA = Primary Progressive Aphasia; PPA-G = Nonfluent/agrammatic variant of PPA; PPA-L = Logopenic variant of PPA; PPA-S = Semantic variant of PPA.
ACKNOWLEDGEMENT
Authors hereby thank Ben Rader for his help with PPA data management, and Dr. Fred Rademaker for his helpful suggestions regarding statistical analyses.
STUDY FUNDING
Supported by the Northwestern University Alzheimer Disease Center Rosenstone fellowship fund, pilot grant NIA P30 AG13854, NIDCD K23 DC014303-01A1 (Bonakdarpour), NIDCD R01 DC008552 (Mesulam), NIDCD DC013386 grants (Hurley), and NINDS NS075075 AG056258 (Rogalski)
DISCLOSURE
The authors report no disclosures relevant to the manuscript.
Dr. Bonakdarpour is funded by NIH K23 DC014303-01A1, and Northwestern Cognitive Neurology and Alzheimer Disease Center pilot grant (P30 AG13854).
Dr. Rogalski is funded by NIH grants R01AG045571, R01 DC008552, R03 DC013386, R01NS075075, and receives research support from the Alzheimer’s Association.
Mr. Wang, Mr. Chatrathi, Mr. Fereira, Ms. Basu and Ms. Guillaume report no disclosures.
Dr. Mesulam is on the medical advisory council for the Association for Frontotemporal Degeneration, the Scientific Advisory Board of the Organization for Human Brain Mapping, is funded by NIH grants P30 AG13854 and R01 DC008552 and R01 AG045571, U01 AG016976 Dr. Hurley is funded by NIH grants NIDCD DC013386 and NIDCD DC008552.
GLOSSARY
- ATL
Anterior Temporal Lobe
- BNT
Boston Naming Test
- FEF
frontal eye field
- IFG
inferior frontal gyrus
- IPS
Inferior parietal sulcus
- MRI
magnetic resonance imaging
- MTG
middle temporal gyrus
- MNI
Montreal Neurological Institute
- NAT
Northwestern Anagram Test
- NAVS
Northwestern assessment of verbs and sentences
- PPA
primary progressive aphasia
- PPA-G
nonfluent/agrammatic variant of PPA
- PPA-L
logopenic variant of PPA
- PPA-S
semantic variant of PPA
- PPVT
Peabody Picture Vocabulary Test
- ROI
region of interest
- rsf-MRI
resting state functional magnetic resonance imaging
- SPM
Statistical Parametric Mapping
- TMS
Transcranial Magnetic Stimulation
- WAB
Western Aphasia Battery
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Borna Bonakdarpour, Mesulam Center for Cognitive Neurology & Alzheimer Disease, Northwestern University Feinberg School of Medicine, Chicago, IL; Department of Neurology, Northwestern University Feinberg School of Medicine, Chicago, IL.
Robert Stephen Hurley, Mesulam Center for Cognitive Neurology & Alzheimer Disease, Northwestern University Feinberg School of Medicine, Chicago, IL; Department of Neurology, Northwestern University Feinberg School of Medicine, Chicago, IL; Department of Psychology, Cleveland State University, Cleveland, OH.
Allan Rehn Wang, Mesulam Center for Cognitive Neurology & Alzheimer Disease, Northwestern University Feinberg School of Medicine, Chicago, IL.
Hernando Rafael Fereira, Mesulam Center for Cognitive Neurology & Alzheimer Disease, Northwestern University Feinberg School of Medicine, Chicago, IL.
Anisha Basu, Mesulam Center for Cognitive Neurology & Alzheimer Disease, Northwestern University Feinberg School of Medicine, Chicago, IL.
Arjuna Chatrathi, Mesulam Center for Cognitive Neurology & Alzheimer Disease, Northwestern University Feinberg School of Medicine, Chicago, IL.
Kyla Guillaume, Mesulam Center for Cognitive Neurology & Alzheimer Disease, Northwestern University Feinberg School of Medicine, Chicago, IL.
Emily Joy Rogalski, Mesulam Center for Cognitive Neurology & Alzheimer Disease, Northwestern University Feinberg School of Medicine, Chicago, IL.
M-Marsel Mesulam, Mesulam Center for Cognitive Neurology & Alzheimer Disease, Northwestern University Feinberg School of Medicine, Chicago, IL; Department of Neurology, Northwestern University Feinberg School of Medicine, Chicago, IL.
6. REFERENCES
- Acheson DJ, & Hagoort P (2013). Stimulating the brain’s language network: syntactic ambiguity resolution after TMS to the inferior frontal gyrus and middle temporal gyrus. J Cogn Neurosci, 25(10), 1664–1677. doi: 10.1162/jocn_a_00430 [DOI] [PubMed] [Google Scholar]
- Agosta F, Galantucci S, Valsasina P, Canu E, Meani A, Marcone A, … Filippi M (2014). Disrupted brain connectome in semantic variant of primary progressive aphasia. NeurobiolAging, 35(11), 2646–2655. doi: 10.1016/j.neurobiolaging.2014.05.017 [DOI] [PubMed] [Google Scholar]
- Alyahya RSW, Halai AD, Conroy P, & Lambon Ralph MA (2018). Noun and verb processing in aphasia: Behavioural profiles and neural correlates. Neuroimage Clin, 18, 215–230. doi: 10.1016/j.nicl.2018.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J (2007). A fast diffeomorphic image registration algorithm. Neuroimage, 38(1), 95–113. doi: 10.1016/j.neuroimage.2007.07.007 [DOI] [PubMed] [Google Scholar]
- Baldo JV, Arevalo A, Patterson JP, & Dronkers NF (2013). Grey and white matter correlates of picture naming: evidence from a voxel-based lesion analysis of the Boston Naming Test. Cortex, 49(3), 658–667. doi: 10.1016/j.cortex.2012.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisenius S, Mueller K, Diehl-Schmid J, Fassbender K, Grimmer T, Jessen F, … group F. T. s. (2017). Predicting primary progressive aphasias with support vector machine approaches in structural MRI data. Neuroimage Clin, 14, 334–343. doi: 10.1016/j.nicl.2017.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonakdarpour B (2014). Neuroimaging in Primary Progressive Aphasia. Perspectives in Neurophysiology and Neurogenic Speech and Language Disorders, 24(4), 145–156. [Google Scholar]
- Bonakdarpour B, Hurely RS, & Mesulam M-M (2013). Temporal pole and the Language Network: Physiologic Evidence from Resting State fMRI. Paper presented at the Annual American Neurological Association meeting, October, 2013. Abstract published in Annals of Neurology, 2013 Dec; 74 (Supp.):S31. [Google Scholar]
- Bonakdarpour B, Rogalski EJ, Wang A, Sridhar J, Mesulam MM, & Hurley RS (2017a). Functional Connectivity is Reduced in Early-stage Primary Progressive Aphasia When Atrophy is not Prominent. Alzheimer Dis Assoc Disord, 31(2), 101–106. doi: 10.1097/WAD.0000000000000193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonakdarpour B, Rogalski EJ, Wang A, Sridhar J, Mesulam MM, & Hurley RS (2017b). Functional Connectivity is Reduced in Early-Stage Primary Progressive Aphasia When Atrophy is not Prominent. Alzheimer Dis Assoc Disord. doi: 10.1097/WAD.0000000000000193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caso F, Mandelli ML, Henry M, Gesierich B, Bettcher BM, Ogar J, … Gorno-Tempini ML (2014). In vivo signatures of nonfluent/agrammatic primary progressive aphasia caused by FTLD pathology. Neurology, 82(3), 239–247. doi: 10.1212/WNL.0000000000000031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Jones DK, & ffytche DH (2005). Perisylvian language networks of the human brain. Ann Neurol, 57(1), 8–16. doi: 10.1002/ana.20319 [DOI] [PubMed] [Google Scholar]
- Catani M, & Mesulam M (2008). The arcuate fasciculus and the disconnection theme in language and aphasia: history and current state. Cortex, 44(8), 953–961. doi: 10.1016/j.cortex.2008.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho-Reyes S, & Thompson CK (2012). Verb and sentence production and comprehension in aphasia: Northwestern Assessment of Verbs and Sentences (NAVS). Aphasiology, 26(10), 1250–1277. doi: 10.1080/02687038.2012.693584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins JA, Montal V, Hochberg D, Quimby M, Mandelli ML, Makris N, … Dickerson BC (2017). Focal temporal pole atrophy and network degeneration in semantic variant primary progressive aphasia. Brain, 140(Pt 2), 457–471. doi: 10.1093/brain/aww313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Anna L, Mesulam M-M, Thiebaut de Schotten M, Dell’Acqua F, Murphy D, Wieneke C, … Catani M (2016). Fronto-temporal networks and behavioral symptoms in primary progressive aphasia. Neurology, 86, 1393–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn LM, Dunn DM (2007). PPVT-4 : Peabody Picture Vocabulary Test. Form A [Visual Material;]: Minneapolis, MN: : Pearson Assessments. [Google Scholar]
- Friederici AD, & Gierhan SM (2013). The language network. Curr Opin Neurobiol, 23(2), 250–254. doi: 10.1016/j.conb.2012.10.002 [DOI] [PubMed] [Google Scholar]
- Galantucci S, Tartaglia MC, Wilson SM, Henry ML, Filippi M, Agosta F, … Gorno-Tempini ML (2011). White matter damage in primary progressive aphasias: a diffusion tensor tractography study. Brain, 134(Pt 10), 3011–3029. doi: 10.1093/brain/awr099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N (1965). Disconnexion syndromes in animals and man. II. Brain, 88(3), 585–644. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Nobre AC, Sonty S, Parrish TB, & Mesulam MM (2005). Language network specializations: an analysis with parallel task designs and functional magnetic resonance imaging. Neuroimage, 26(4), 975–985. doi: 10.1016/j.neuroimage.2005.03.014 [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, … Miller BL (2004). Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol, 55(3), 335–346. doi: 10.1002/ana.10825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, … Grossman M (2011). Classification of primary progressive aphasia and its variants. Neurology, 76(11), 10061014. doi: 10.1212/WNL.0b013e31821103e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo CC, Gorno-Tempini ML, Gesierich B, Henry M, Trujillo A, Shany-Ur T, … Seeley WW (2013). Anterior temporal lobe degeneration produces widespread network-driven dysfunction. Brain, 136(Pt 10), 2979–2991. doi: 10.1093/brain/awt222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker CD, Laumann TO, Szrama NP, Baldassarre A, Snyder AZ, Leuthardt EC, & Corbetta M (2013). Resting state network estimation in individual subjects. Neuroimage, 82, 616–633. doi: 10.1016/j.neuroimage.2013.05.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagoort P (2005). On Broca, brain, and binding: a new framework. Trends Cogn Sci, 9(9), 416–423. doi: 10.1016/j.tics.2005.07.004 [DOI] [PubMed] [Google Scholar]
- Hickok G, & Poeppel D (2007). The cortical organization of speech processing. Nat Rev Neurosci, 8(5), 393–402. doi: 10.1038/nrn2113 [DOI] [PubMed] [Google Scholar]
- Hurley RS, Bonakdarpour B, Wang X, & Mesulam MM (2015). Asymmetric connectivity between the anterior temporal lobe and the language network. J Cogn Neurosci, 27(3), 464–473. doi: 10.1162/jocn_a_00722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RS, Paller KA, Rogalski EJ, & Mesulam MM (2012). Neural mechanisms of object naming and word comprehension in primary progressive aphasia. J Neurosci, 32(14), 4848–4855. doi: 10.1523/JNEUROSCI.5984-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indefrey P, & Levelt WJ (2004). The spatial and temporal signatures of word production components. Cognition, 92(1–2), 101–144. doi: 10.1016/j.cognition.2002.06.001 [DOI] [PubMed] [Google Scholar]
- Jefferies E (2013). The neural basis of semantic cognition: converging evidence from neuropsychology, neuroimaging and TMS. Cortex, 49(3), 611–625. doi: 10.1016/j.cortex.2012.10.008 [DOI] [PubMed] [Google Scholar]
- Kertesz AKA, Western aphasia battery stimulus, b.,. Raven JC ,, & Coloured progressive, m. (2007). The Western aphasia battery [Visual Material]: San Antonio, TX: : PsychCorp. [Google Scholar]
- Liu H, Stufflebeam SM, Sepulcre J, Hedden T, & Buckner RL (2009). Evidence from intrinsic activity that asymmetry of the human brain is controlled by multiple factors. Proc Natl Acad Sci U S A, 106(48), 20499–20503. doi: 10.1073/pnas.0908073106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann G, Hoehl S, Brauer J, Danielmeier C, Bornkessel-Schlesewsky I, Bahlmann J, … Friederici A (2010). Setting the frame: the human brain activates a basic low-frequency network for language processing. Cereb Cortex, 20(6), 1286–1292. doi: 10.1093/cercor/bhp190 [DOI] [PubMed] [Google Scholar]
- Mandelli ML, Vilaplana E, Brown JA, Hubbard HI, Binney RJ, Attygalle S, … Gorno-Tempini ML (2016). Healthy brain connectivity predicts atrophy progression in non-fluent variant of primary progressive aphasia. Brain, 139(Pt 10), 2778–2791. doi: 10.1093/brain/aww195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M-M, Thompson CK, Weintraub S, & Rogalski EJ (2015). The Wernicke conundrum and the anatomy of language comprehension in primary progressive aphasia. Brain, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M, Weintraub S, Parrish T, & Gitelman D (2005). Primary progressive aphasia: reversed asymmetry of atrophy and right hemisphere language dominance. Neurology, 64(3), 556–557. doi: 10.1212/01.WNL.0000150545.46351.DE [DOI] [PubMed] [Google Scholar]
- Mesulam M, Wieneke C, Rogalski E, Cobia D, Thompson C, & Weintraub S (2009). Quantitative template for subtyping primary progressive aphasia. Arch Neurol, 66(12), 1545–1551. doi: 10.1001/archneurol.2009.288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Rader BM, Sridhar J, Nelson MJ, Hyun J, Rademaker A, … Rogalski EJ (2018). Word comprehension in temporal cortex and Wernicke area: A PPA perspective. Neurology. doi: 10.1212/WNL.0000000000006788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Rader BM, Sridhar J, Nelson MJ, Hyun J, Rademaker A, … Rogalski EJ (2019). Word comprehension in temporal cortex and Wernicke area: A PPA perspective. Neurology, 92(3), e224–e233. doi: 10.1212/WNL.0000000000006788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Rogalski EJ, Wieneke C, Hurley RS, Geula C, Bigio EH, … Weintraub S (2014). Primary progressive aphasia and the evolving neurology of the language network. Nat Rev Neurol, 10(10), 554–569. doi: 10.1038/nrneurol.2014.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Wieneke C, Hurley R, Rademaker A, Thompson CK, Weintraub S, & Rogalski EJ (2013). Words and objects at the tip of the left temporal lobe in primary progressive aphasia. Brain, 136(Pt 2), 601–618. doi: 10.1093/brain/aws336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Wieneke C, Thompson C, Rogalski E, & Weintraub S (2012). Quantitative classification of primary progressive aphasia at early and mild impairment stages. Brain, 135(Pt 5), 1537–1553. doi: 10.1093/brain/aws080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio R, Boutet C, Valabregue R, Ferrieux S, Nogues M, Lehericy S, … Teichmann M (2016). The Brain Network of Naming: A Lesson from Primary Progressive Aphasia. PLoS One, 11(2), e0148707. doi: 10.1371/journal.pone.0148707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montembeault M, Chapleau M, Jarret J, Boukadi M, Laforce R Jr., Wilson MA, … Brambati SM (2019). Differential language network functional connectivity alterations in Alzheimer’s disease and the semantic variant of primary progressive aphasia. Cortex, 117, 284–298. doi: 10.1016/j.cortex.2019.03.018 [DOI] [PubMed] [Google Scholar]
- Pascual B, Masdeu JC, Hollenbeck M, Makris N, Insausti R, Ding SL, & Dickerson BC (2015). Large-scale brain networks of the human left temporal pole: a functional connectivity MRI study. Cereb Cortex, 25(3), 680–702. doi: 10.1093/cercor/bht260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobric G, Jefferies E, & Ralph MA (2007). Anterior temporal lobes mediate semantic representation: mimicking semantic dementia by using rTMS in normal participants. Proc Natl Acad Sci U S A, 104(50), 20137–20141. doi: 10.1073/pnas.0707383104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Devlin JT, Moore CJ, Morton C, & Laird AR (2005). Meta-analyses of object naming: effect of baseline. Hum Brain Mapp, 25(1), 70–82. doi: 10.1002/hbm.20132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovici GD, Jagust WJ, Furst AJ, Ogar JM, Racine CA, Mormino EC, … Gorno-Tempini ML (2008). Abeta amyloid and glucose metabolism in three variants of primary progressive aphasia. Ann Neurol, 64(4), 388–401. doi: 10.1002/ana.21451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes P, Ortega-Merchan MP, Rueda A, Uriza F, Santamaria-Garcia H, Rojas-Serrano N, … Matallana D (2018). Functional Connectivity Changes in Behavioral, Semantic, and Nonfluent Variants of Frontotemporal Dementia. Behav Neurol, 2018, 9684129. doi: 10.1155/2018/9684129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski E, Cobia D, Harrison TM, Wieneke C, Thompson CK, Weintraub S, & Mesulam MM (2011). Anatomy of language impairments in primary progressive aphasia. J Neurosci, 31(9), 3344–3350. doi: 10.1523/JNEUROSCI.5544-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski E, Cobia D, Martersteck A, Rademaker A, Wieneke C, Weintraub S, & Mesulam MM (2014). Asymmetry of cortical decline in subtypes of primary progressive aphasia. Neurology, 83(13), 1184–1191. doi: 10.1212/WNL.0000000000000824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalsky C, LaCroix AN, Chen KH, Anderson SW, Damasio H, Love T, & Hickok G (2018). The Neurobiology of Agrammatic Sentence Comprehension: A Lesion Study. J Cogn Neurosci, 30(2), 234–255. doi: 10.1162/jocn_a_01200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur D, Kreher BW, Schnell S, Kummerer D, Kellmeyer P, Vry MS, … Weiller C (2008). Ventral and dorsal pathways for language. Proc Natl Acad Sci U S A, 105(46), 18035–18040. doi: 10.1073/pnas.0805234105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders TM, Petersson KM, & Hagoort P (2010). Effective connectivity of cortical and subcortical regions during unification of sentence structure. Neuroimage, 52(4), 1633–1644. doi: 10.1016/j.neuroimage.2010.05.035 [DOI] [PubMed] [Google Scholar]
- Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, … Zang YF (2011). REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One, 6(9), e25031. doi: 10.1371/journal.pone.0025031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonty SP, Mesulam MM, Thompson CK, Johnson NA, Weintraub S, Parrish TB, & Gitelman DR (2003). Primary progressive aphasia: PPA and the language network. Ann Neurol, 53(1), 35–49. doi: 10.1002/ana.10390 [DOI] [PubMed] [Google Scholar]
- Sonty SP, Mesulam MM, Weintraub S, Johnson NA, Parrish TB, & Gitelman DR (2007). Altered effective connectivity within the language network in primary progressive aphasia. J Neurosci, 27(6), 1334–1345. doi: 10.1523/JNEUROSCI.4127-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichmann M, Kas A, Boutet C, Ferrieux S, Nogues M, Samri D, … Migliaccio R (2013). Deciphering logopenic primary progressive aphasia: a clinical, imaging and biomarker investigation. Brain, 136(Pt 11), 3474–3488. doi: 10.1093/brain/awt266 [DOI] [PubMed] [Google Scholar]
- Turken AU, & Dronkers NF (2011). The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. FrontSyst Neurosci, 5, 1. doi: 10.3389/fnsys.2011.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Daijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, & Buckner RL (2010). Intrinsic Functional Connectivity As a Tool For Human Connectomics: Theory, Properties, and Optimization [DOI] [PMC free article] [PubMed]
- Warren JE, Crinion JT, Lambon Ralph MA, & Wise RJ (2009). Anterior temporal lobe connectivity correlates with functional outcome after aphasic stroke. Brain, 132(Pt 12), 3428–3442. doi: 10.1093/brain/awp270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei T, Liang X, He Y, Zang Y, Han Z, Caramazza A, & Bi Y (2012). Predicting conceptual processing capacity from spontaneous neuronal activity of the left middle temporal gyrus. J Neurosci, 32(2), 481–489. doi: 10.1523/JNEUROSCI.1953-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, Mesulam MM, Wieneke C, Rademaker A, Rogalski EJ, & Thompson CK (2009). The northwestern anagram test: measuring sentence production in primary progressive aphasia. Am J Alzheimers Dis Other Demen, 24(5), 408–416. doi: 10.1177/1533317509343104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Jones DT, Duffy JR, Strand EA, Machulda MM, Przybelski SA, … Josephs KA (2015). Working memory and language network dysfunctions in logopenic aphasia: a task-free fMRI comparison with Alzheimer’s dementia. NeurobiolAging, 36(3), 1245–1252. doi: 10.1016/j.neurobiolaging.2014.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, DeMarco AT, Henry ML, Gesierich B, Babiak M, Miller BL, & Gorno-Tempini ML (2016). Variable disruption of a syntactic processing network in primary progressive aphasia. Brain. doi: 10.1093/brain/aww218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Dronkers NF, Ogar JM, Jang J, Growdon ME, Agosta F, … Gorno-Tempini ML (2010). Neural correlates of syntactic processing in the nonfluent variant of primary progressive aphasia. J Neurosci, 30(50), 16845–16854. doi: 10.1523/JNEUROSCI.2547-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Galantucci S, Tartaglia MC, Rising K, Patterson DK, Henry ML, … Gorno-Tempini ML (2011). Syntactic processing depends on dorsal language tracts. Neuron, 72(2), 397–403. doi: 10.1016/j.neuron.2011.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure. Connections which are reduced in each PPA subtype including two additional ROIs. Solid orange lines depict significantly lower connectivity between ROIs (red) in PPA compared to controls, corrected for multiple comparisons using Bonferroni correction. IFG = Inferior Frontal Gyrus; MTG = Middle Temporal Gyrus; ATL = Anterior Temporal Lobe; STG= Superior Temporal Gyrus; AG= Angular Gyrus. PPA = Primary Progressive Aphasia; PPA-G = Nonfluent/agrammatic variant of PPA; PPA-L = Logopenic variant of PPA; PPA-S = Semantic variant of PPA.