ABSTRACT
Rheumatoid arthritis is a systemic autoimmune disease characterized by excess morbidity and mortality from cardiovascular disease. Mechanisms linking rheumatoid arthritis and cardiovascular disease include shared inflammatory mediators, post-translational modifications of peptides/proteins and subsequent immune responses, alterations in the composition and function of lipoproteins, increased oxidative stress, and endothelial dysfunction. Despite a growing understanding of these mechanisms and their complex interplay with conventional cardiovascular risk factors, optimal approaches of risk stratification, prevention, and treatment in the context of rheumatoid arthritis remain unknown. A multifaceted approach to reduce the burden posed by cardiovascular disease requires optimal management of traditional risk factors in addition to those intrinsic to rheumatoid arthritis such as increased disease activity. Treatments for rheumatoid arthritis seem to exert differential effects on cardiovascular risk as well as the mechanisms linking these conditions. More research is needed to establish whether preferential rheumatoid arthritis therapies exist in terms of prevention of cardiovascular disease. Ultimately, understanding the unique mechanisms for cardiovascular disease in rheumatoid arthritis will aid in risk stratification and the identification of novel targets for meaningful reduction of cardiovascular risk in this patient population.
Introduction
Rheumatoid arthritis is a systemic, autoimmune disease that affects approximately 0.5-1.0% of the population.1 It is characterized by a symmetrical inflammatory polyarthritis, but extra-articular features are also common and portend a poor prognosis. Because of its frequency and relevance to patients’ morbidity and survival, cardiovascular disease has been a topic of substantial research, resulting in an improved understanding of the mechanisms linking rheumatoid arthritis and cardiovascular disease and supporting improved cardiovascular risk reduction in rheumatoid arthritis patients. In this review, we describe the scientific advancements elucidating the mechanisms linking rheumatoid arthritis and cardiovascular disease, the cardiovascular risk associated with specific drugs used to treat rheumatoid arthritis, and relevant clinical management.
Sources and selection criteria
We searched PubMed for English language manuscripts published from 1 January 2006 to 1 June 2017, using the MeSH terms “rheumatoid arthritis” and “cardiovascular disease” or “cardiovascular system”, identifying 2687 reports. We then searched reference lists of articles selected through title, abstract, and full text review. We selected systematic reviews, meta-analyses, randomized controlled trials, and observational (excluding case reports and small case series (n<15)), translational, and basic science studies from these sources, prioritized by study quality and topic.
Burden of cardiovascular disease in rheumatoid arthritis
Cardiovascular disease accounts for the largest proportion of excess mortality in rheumatoid arthritis, accounting for 39.6% of deaths in a review of 50 studies that included 91 618 patients and 33 250 deaths.2 In two large meta-analyses that together contributed more than 150 000 patients, rheumatoid arthritis was associated with a 48% increased risk of cardiovascular events (relative risk 1.48, 95% confidence interval 1.36 to 1.62) and a 50% higher incidence of cardiovascular disease related mortality (standardized mortality ratio 1.50, 95% confidence interval 1.39 to 1.61) compared with the general population.3 4 Because atherosclerosis and congestive heart failure (CHF) are the most frequent manifestations of cardiovascular disease, the focus of this review reflects this.
Mechanisms linking rheumatoid arthritis and cardiovascular disease
Traditional cardiovascular risk factors
The focus of this review is on rheumatoid arthritis related factors contributing to cardiovascular risk, but it is critical to acknowledge the contribution of traditional cardiovascular risk factors in rheumatoid arthritis patients. In a prospective cohort study, hypertension, dyslipidemia, and insulin resistance were observed to be more closely related to surrogate markers of cardiovascular disease—microvascular function, endothelial function, and carotid intima media thickness—than were inflammatory markers in rheumatoid arthritis.5 Longitudinal analysis of coronary artery calcium scores showed similar incidence and progression between rheumatoid arthritis patients and controls, with traditional risk factors, rather than characteristics of rheumatoid arthritis, being most predictive.6 Traditional cardiovascular risk factors, including obesity, diabetes, smoking, and hypertension, seem to be over-represented in rheumatoid arthritis,7 8 findings that have not been universally replicated.9 10 In contrast, smoking has consistently been identified as a robust and shared risk factor for the development of cardiovascular disease and rheumatoid arthritis.11
Disease activity and inflammation
Mounting evidence suggests a central role of the immune system in the pathogenesis of cardiovascular disease, and pro-inflammatory cytokines implicated in rheumatoid arthritis also enhance atherogenesis.12 Thus, the development of “risk calculators” that adequately account for disease activity will be critical to improve cardiovascular risk assessment because conventional risk factors do not fully explain the excess burden of cardiovascular disease in rheumatoid arthritis.13 Supporting this contention, several observational studies have identified associations between higher disease activity in rheumatoid arthritis and cardiovascular outcomes. In a Swedish nested case-control study of incident rheumatoid arthritis, higher disease activity was associated with increased odds of acute coronary syndrome (ACS) based on acute phase response as well as different composite measures of disease activity (odds ratio 1.32 per unit increase in 28 joint disease activity score (DAS28); odds ratio 1.61 for moderate and 2.59 for high European Union League Against Rheumatism (EULAR) disease activity score compared with to low disease activity) .14 Similar results were observed in a US cohort study, in which time averaged disease activity in the remission range was associated with a 53% lower risk of cardiovascular events than in high disease activity, independent of traditional cardiovascular risk factors and rheumatoid arthritis treatments.15 These observations were further corroborated in the Nijmegen early rheumatoid arthritis cohort, in which a 1 unit increase in time averaged DAS28, but not disease duration, was associated with a 33% higher risk of cardiovascular disease.16 Likewise, higher C reactive protein (CRP) and erythrocyte sedimentation rates (ESR) were associated with a significantly increased risk of cardiovascular disease in two large US studies and a Spanish rheumatoid arthritis cohort.17 18 19 A population based cohort study of incident rheumatoid arthritis showed that each six week flare was associated with a 7% increase in risk of cardiovascular disease, whereas patients in remission had a risk similar to that of non-rheumatoid arthritis controls.20
Disease activity and cardiac function
Rheumatoid arthritis disease activity is not only predictive of cardiovascular events but also strongly correlates with cardiac function. Using speckle-tracking echocardiography, greater left ventricular strain was observed in rheumatoid arthritis patients than in controls and was associated with disease activity.21 Similarly, higher rheumatoid arthritis disease activity is positively associated with left ventricular global longitudinal strain,22 as well as left ventricular wall thickness.23 Diastolic dysfunction is also over-represented in rheumatoid arthritis patients and is associated with higher circulating interleukin (IL)-6 concentrations.24
Recognizing the aforementioned associations of disease activity, rheumatoid arthritis treatments that target remission might be necessary to optimally preserve cardiac function. Supporting this hypothesis, rheumatoid arthritis patients in remission (Simplified Disease Activity Index <3.3) showed improved left ventricular function as assessed by stress corrected mid-wall shortening and global longitudinal strain compared with those with low, moderate, or high disease activity.25 Differences in left ventricular function were independent of traditional cardiovascular risk factors.25 Moreover, no differences in left ventricular function were seen between healthy controls and rheumatoid arthritis patients in remission.
Disease activity and surrogate markers
To better understand the contributions of inflammation, several observational studies have examined the relation of rheumatoid arthritis disease activity with surrogate markers of cardiovascular disease. In a cross sectional study using computed tomography-angiography, rheumatoid arthritis patients had higher coronary artery plaque burden than controls and higher disease activity was associated with the presence of unstable plaque,26 suggesting that disease related inflammation contributes not only to development of plaques but also to their vulnerability and rupture. Both swollen joint counts and CRP were associated with the carotid plaque progression in rheumatoid arthritis,27 and circulating IL-6 and tumor necrosis factor α (TNF-α) concentrations were independently associated with coronary artery calcification.28 Additionally, multivariable analyses accounting for IL-6 and CRP concentrations attenuated the association between rheumatoid arthritis and coronary artery calcification, suggesting that inflammation mediates pathophysiologic processes of stable and unstable plaque.29
Exploring how traditional and non-traditional risk factors together contribute to risk of cardiovascular disease, a cohort study of rheumatoid arthritis patients undergoing serial carotid ultrasonography over a span of three years showed that systemic inflammation and traditional risk factors both predicted progression of atherosclerosis.30 Importantly, risk related to systemic inflammation seemed to be dependent on the number of traditional risk factors present. Specifically, ESR values predicted atherosclerosis progression only in rheumatoid arthritis patients with two or more conventional risk factors, suggesting an interaction between traditional and rheumatoid arthritis related cardiovascular risk factors.
Cellular mechanisms
Ex vivo and in vitro studies have begun to elucidate cellular mechanisms by which rheumatoid arthritis related inflammation drives cardiovascular disease. Aortic adventitia from rheumatoid arthritis patients undergoing coronary artery bypass grafting showed increased TNF-α and IL-18 expression compared with controls, as well as increased IL-33 and IL-33 ligand expression in endothelial cells of the aortic adventitia vasa vasora.31 These findings suggest the existence of a unique pro-inflammatory adventitial microenvironment in rheumatoid arthritis that fosters atherogenesis through linkage of innate and adaptive immune responses, key roles of IL-18 and IL-33. A cross sectional analysis of circulating peripheral blood mononuclear cells taken from rheumatoid arthritis patients recently identified cell subsets strongly associated with coronary artery calcification.32 Independent of traditional cardiovascular risk factors and other features of rheumatoid arthritis, unique effector memory CD4 T cell and monocyte subsets were associated with coronary artery calcification, suggesting a potential intrinsic link between immune subsets that are characteristic of rheumatoid arthritis and cardiovascular disease. In vitro, the addition of IL-17 and TNF-α to endothelial cells induced a pro-inflammatory, pro-thrombotic, and pro-coagulant state.33 TNF-α also cleaves VE-cadherin,34 an endothelium specific adhesion molecule important in maintaining endothelial tight junctions. Cleavage of this molecule could result in increased permeability, interstitial edema, and hemorrhage in vivo. Despite these intriguing findings, much more work is needed to elucidate the pro-inflammatory networks bridging rheumatoid arthritis and cardiovascular disease.
Oxidative stress
Oxidative stress, an imbalance of reactive oxygen species (ROS) and antioxidants, is typically increased in the context of inflammation and contributes to the development of cardiovascular disease and rheumatoid arthritis,35 36 representing another potential shared pathway between the diseases. Concentrations of malondialdehyde and nitrotyrosine, two nitro-oxidative stress parameters, are associated with increased myocardial strain in rheumatoid arthritis.37 Myeloperoxidase, a source of oxidants, is increased in the serum of rheumatoid arthritis patients relative to controls. Moreover, myeloperoxidase derived oxidants seem to target and impair the function of high density lipoproteins (HDL).38 Advanced glycosylation end products, generated in the setting of hyperglycemia and oxidative stress, are increased in rheumatoid arthritis relative to controls and inversely associated with endothelial function.39
Serum and left ventricular tissues from rats with adjuvant induced arthritis (AIA), an animal model of rheumatoid arthritis, had a higher concentration of oxidative stress related aldehyde than controls.40 NADPH and glutathione pools were 30% lower in left ventricular tissues of AIA rats than controls, suggesting depletion of key antioxidant system reserves related to inflammatory arthritis. Showing both the complexity and the interconnectedness of systems, renin-angiotensin activation in the AIA mouse exacerbated vascular hypertrophy and oxidative stress, whereas angiotensin II type-1 receptor blockade reduced NADPH oxidase activity and oxidative stress in aortic tissues and improved endothelial function.41
Endothelial dysfunction
The endothelium regulates vascular tone and homeostasis, and its dysregulation is integral to atherogenesis. Rheumatoid arthritis has been characterized by endothelial dysfunction that seems to evolve throughout its natural course. A longitudinal study observed no differences in endothelial function measured by endothelial dependent, flow mediated dilatation in newly diagnosed rheumatoid arthritis cases compared with controls, although cases showed increased carotid intima media thickness over time.42 A case-control study found impaired coronary flow reserve in early rheumatoid arthritis that was inversely associated with asymmetric dimethylarginine, an inhibitor of endogenous nitric oxide synthase (eNOS),43 which is essential in regulating vascular tone, leukocyte adhesion, and platelet aggregation. Reports on the role of disease activity in endothelial function have been conflicting. Effective disease modifying anti-rheumatic drugs (DMARDs) can improve microvascular endothelial function,44 and in the AIA model circulating IL-1β, TNF-α, and MIP-1α concentrations were negatively associated with endothelial function.45 In contrast, endothelial function was not associated with rheumatoid arthritis disease activity in at least one cross sectional study but was associated with composite cardiovascular risk scores.46 Critical to endothelial function, repair, and neovascularization are endothelial progenitor cells (EPCs) and recently identified angiogenic T cells (Tang). Relative to controls, EPCs and Tang are reduced in rheumatoid arthritis, with the lowest Tang counts noted among patients with comorbid cardiovascular disease.47 48 Their connection to rheumatoid arthritis is further established through inverse associations with disease activity and improved EPC counts after treatment with TNF inhibitors.47 48
The complex interplay of mechanisms is further highlighted by the fact that endothelial function is also influenced by oxidative stress. In vitro experiments using endothelial cells from rheumatoid arthritis patients showed that endogenous ligands and phospholipid oxidation products activate endothelial cells through NFkB pathways via toll-like receptor 4 signaling.49 The AIA model was used to characterize downstream consequences of systemic inflammation in rheumatoid arthritis, showing that dysregulation of NADPH oxidases and eNOS occurred and resulted in enhanced lipid peroxidation, generation of vascular ROS, and impairment of aortic endothelial function.50
Post-translational modifications and autoantibodies
Post-translational protein modifications, including citrullination—the enzymatic conversion of the amino acid arginine to citrulline—are hypothesized to contribute to development of rheumatoid arthritis. Anti-citrullinated protein antibodies (ACPA) are highly disease specific, are detectable in most rheumatoid arthritis patients, and portend more severe arthritis. Epidemiologic studies have reported varying results specific to autoantibody associations with cardiovascular disease. Anti-cyclic citrullinated peptide (CCP; a commercial ACPA measure) and rheumatoid factor positivity have been linked to increased risk of ischemic heart disease and incident CHF.17 51 52 In separate nested case-control and cohort studies, however, seropositivity was not associated with risk of ACS or with cardiovascular disease related morbidity or mortality.14 53 Supporting a possible pathogenic role of these autoantibodies, seropositivity was associated with risk of cardiovascular disease even in the absence of rheumatoid arthritis in the Multi-Ethnic Study of Atherosclerosis (rheumatoid factor and anti-CCP) and Northwick Park Heart Study (anti-CCP).54 55
Several observational studies have characterized associations between autoantibodies and cardiac function. Anti-CCP antibody was associated with left ventricular global longitudinal strain, impaired left ventricular relaxation, and lower left ventricular mass, stroke volume, and end diastolic volume by cardiac magnetic resonance imaging in rheumatoid arthritis.22 56 57 These findings prompted investigation of the myocardium for the presence of citrullinated antigens acting as putative targets for pathogenic autoantibodies. Compared with control specimens, left ventricular tissues from rheumatoid arthritis patients showed increased staining for citrullinated proteins as well as peptidyl arginine deiminases (enzymes catalyzing citrullination).58
ACPA recognize a variety of citrullinated (cit-) peptides, with data suggesting that antigen specificity could influence pathogenicity. For instance, higher circulating concentrations of anti-cit-fibrinogen and anti-cit-vimentin antibody were associated with increased left ventricular mass in independent rheumatoid arthritis cohorts free of cardiovascular disease.59 This was not observed for antibodies recognizing non-citrullinated (native) peptides/proteins. In the same cohorts, higher concentrations of anti-cit-histone H2B antibody (but not antibody to other citrullinated or native antigens) were associated with coronary artery calcium scores but not with coronary artery calcium progression.60 In contrast, among rheumatoid arthritis patients with comorbid cardiovascular disease, investigators observed no evidence of associations between rheumatoid factor, anti-cit-fibrinogen, anti-cit-Fibβ 36-52, and anti-CCP antibody, or of rheumatoid factor with carotid intima media thickness, carotid plaque, or coronary artery calcium scores.61
A structural analog of citrulline and a result of carbamylation, homocitrulline is the antigenic target of anti-carbamylated protein antibodies. These antibodies were recently identified in rheumatoid arthritis patients and were associated with subclinical cardiovascular disease.62 63 In addition, carbamylation has been shown to be pro-atherogenic in other disease states.64 Malondialdehyde-acetaldehyde adducts represent an alternative post-translational modification postulated to contribute to both cardiovascular disease and rheumatoid arthritis. Malondialdehyde-acetaldehyde adducts are generated under oxidative stress and lipid peroxidation, are highly immunogenic, and unlike malondialdehyde are highly stable.65 66 Circulating anti-malondialdehyde-acetaldehyde antibody concentrations are increased in rheumatoid arthritis compared with controls and correlate with disease activity, and IgG isotypes are associated with comorbid cardiovascular disease.67 Moreover, anti-malondialdehyde-acetaldehyde antibody concentrations are higher among non-rheumatoid arthritis patients presenting for cardiac care than in healthy controls, isotypes vary markedly depending on coronary artery disease phenotype, and malondialdehyde-acetaldehyde adducts have been detected in atherosclerotic human aortic tissue.68
Lipid perturbations
Quantitative lipoprotein alterations
In the general population, higher total cholesterol and low density lipoprotein (LDL) concentrations confer increased risk of cardiovascular disease. In rheumatoid arthritis, however, this interpretation is overly simplistic. For example, rheumatoid arthritis patients with moderate LDL cholesterol concentrations (70-130 mg/dL) have been observed to have the lowest risk of myocardial infarction, with low and high LDL concentrations corresponding to increased risk.18 Lower total cholesterol and LDL were associated with increased cardiovascular events in a separate rheumatoid arthritis cohort 69; and in a study of US Veterans with rheumatoid factor, LDL was not associated with either myocardial infarction or stroke.17 These findings have prompted the description of a “lipid paradox” in rheumatoid arthritis,69 which is not a universal finding as higher LDL concentrations have portended higher risk of cardiovascular disease in at least one large claims based study.70 In contrast to reports for total cholesterol and LDL, associations between HDL and cardiovascular events have been relatively consistent and similar to the general population, with higher HDL concentrations yielding lower cardiovascular risk.17 18 70
Evidence suggesting the existence of this lipid paradox in rheumatoid arthritis has led to several studies examining the effects of inflammation on lipids. In an observational study of rheumatoid arthritis patients with visits one year apart, reductions in high sensitivity (hs)CRP of more than 10 mg/dL were strongly associated with increases in LDL, apolipoprotein A1, HDL, and HDL cholesterol efflux capacity.71 No associations were seen with changes in hsCRP and apolipoprotein B or atherogenic indices (total cholesterol/HDL and apoliporotein B/A1). Others have similarly found lipid ratios (such as total cholesterol/HDL or LDL/HDL) to be less influenced by systemic inflammation and thus a preferred measure for cardiovascular risk stratification in rheumatoid arthritis.72 Further supporting a link with inflammation are the changes in lipids that occur as a consequence of rheumatoid arthritis treatments. In the Treatment of Early RA (TEAR) trial, a two year, randomized, placebo controlled, four arm trial in 755 patients with early rheumatoid arthritis, starting DMARD treatment resulted in significant increases in total cholesterol, HDL, and LDL that correlated with improvements in CRP.73
Functional lipoprotein alterations
Although lipid concentrations are most often leveraged for cardiovascular risk stratification, assessments of lipoprotein function may be more relevant in the context of rheumatoid arthritis. HDL acts as an antioxidant and regulates cholesterol efflux. Under inflammatory conditions, changes in HDL composition occur and lead to impaired function.74 Rheumatoid arthritis disease activity and erosions were independently associated with the presence of pro-inflammatory HDL.74 Likewise, HDL function was impaired in rheumatoid arthritis patients relative to controls, with the lowest function in those with pro-inflammatory HDL. Proteomic profiling of HDL from patients with rheumatoid arthritis showed that inflammatory HDL contained increased acute phase proteins.75 The primary component responsible for the antioxidant properties of HDL is paroxonase-1. Paranoxase-1 activity is decreased in rheumatoid arthritis compared with controls,76 which is highly relevant as lower paranoxase-1 activity is associated with increased carotid plaque burden.77 Changes to lipid composition in rheumatoid arthritis are not limited to HDL. LDL from rheumatoid arthritis patients had higher levels of glycated end products and was more oxidized than LDL from controls.78 In vitro, LDL from rheumatoid arthritis patients underwent enhanced phagocytosis by macrophages, had higher fatty acid accumulation, and induced approximately threefold greater oxidation.
HDL antioxidant function and cholesterol efflux capacity are only moderately correlated, suggesting that these properties might be influenced differentially in rheumatoid arthritis. HDL participates in reverse cholesterol transport by removing cholesterol from cells in a process termed “cholesterol efflux.” HDL from rheumatoid arthritis patients with high disease activity had reduced cholesterol efflux capacity in vitro compared with HDL from patients with low disease activity.79 Serum from rheumatoid arthritis patients showed impaired ATP binding cassette transporter G1 (ABCG-1) mediated cholesterol efflux capacity compared with controls, which inversely correlated with disease activity.80 Other mechanisms of cholesterol efflux examined (ABCA-1, aqueous diffusion, scavenger receptor B1) did not differ from controls. Naive THP-1 macrophages exposed to plasma from rheumatoid arthritis patients downregulated cholesterol efflux proteins (ABCA-1, ABCG-1, 27-hydroxylase) and upregulated scavenger receptors (CD36, LOX1, CXCL16) resulting in increased uptake of oxidized LDL and foam cell formation.81 In vitro, serum from rheumatoid arthritis patients increased mRNA expression of scavenger receptors (SR-A, LOX-1, CD36) in human arterial endothelial cells and increased oxidized LDL induced monocyte chemoattractant protein-1 (MCP-1) production, and IL-6 and TNF-α upregulated oxidized LDL uptake through activation of SR-A and LOX-1, transforming macrophages into foam cells.82 83 These processes were attenuated with inhibition of IL-6 or TNF-α.
The collagen induced arthritis (CIA) animal model of rheumatoid arthritis, which has a pro-atherogenic lipid profile with lower HDL and higher oxidized LDL, has also aided in defining lipid mechanisms.84 After exposing murine peritoneal macrophages to serum from CIA mice, accelerated cholesterol accumulation occurred without changes in apolipoprotein A1 or HDL mediated intracellular cholesterol efflux. CD36 expression, a robust regulator of lipid influx, was increased after exposure to CIA serum. MCP-1, a pro-inflammatory cytokine upregulated in CIA, did not affect lipid accumulation or CD36 expression. However, serum with higher oxidized LDL and lower HDL concentrations upregulated CD36 mRNA expression, an effect that was reduced with administration of simvastatin. Together, these findings suggest that the inflammation of rheumatoid arthritis alters lipid composition/function, enhances scavenger receptor expression, and amplifies cholesterol influx and foam cell formation.
Interaction of traditional and rheumatoid arthritis related cardiovascular risk factors
The relation between conventional risk factors and rheumatoid arthritis related mechanisms driving excess cardiovascular disease burden is complex and often bidirectional (fig 1). Oxidative stress, for instance, characterizes systemic inflammation in rheumatoid arthritis and promotes insulin resistance, which in turn further exacerbates ROS-antioxidant imbalance. Although obesity is a recognized cardiovascular risk factor, an “obesity paradox” has been observed in rheumatoid arthritis whereby greater body mass is protective of cardiovascular disease mortality.85 Proposed hypotheses include the inadequacy of commonly used body composition measures, epidemiologic phenomena such as index event bias,86 confounding by comorbidity,85 and lack of consideration of weight trajectories.87 88 Adiposity itself may contribute to systemic inflammation, insulin resistance, and cardiovascular disease risk through the release of adipokines or other pro-inflammatory mediators.89 90 91 92 93
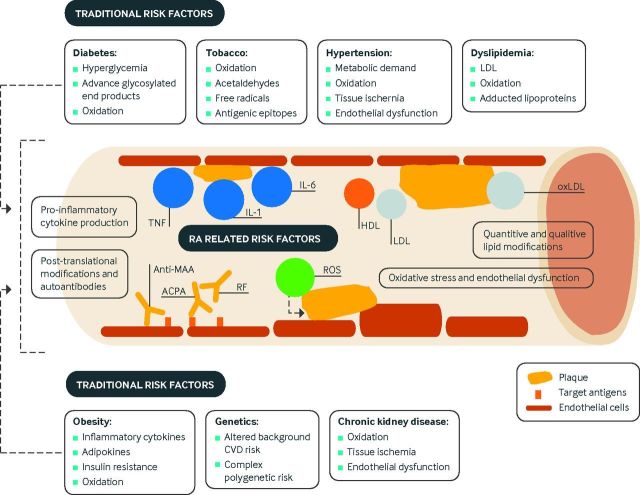
Fig 1.
Overview of mechanisms of cardiovascular disease (CVD) in rheumatoid arthritis (RA). Several mechanisms interact to amplify risk of CVD in RA. Higher RA disease activity contributes to systemic inflammation and pro-inflammatory cytokine production. This inflammation causes quantitative and qualitative lipid modifications resulting in perturbations of cholesterol transport and enhanced foam cell formation. Post-translational modifications of proteins serve as targets of RA autoantibodies that may have deleterious effects on the cardiovascular system and enhance systemic/local inflammation. Oxidative stress, which results from inflammation, contributes to post-translational modifications of proteins and directly affects endothelial function. These RA related mechanisms exacerbate the pathogenicity of traditional CVD risk factors such as tobacco use, diabetes, hypertension, dyslipidemia, obesity, and chronic kidney disease. ACPA=anti-citrullinated protein antibodies; HDL=high density lipoprotein; IL=interleukin; LDL=low density lipoprotein; oxLDL=oxidized LDL; MAA=malondialdehyde-acetaldehyde; RF=rheumatoid factor; ROS=reactive oxygen species
Cardiovascular disease risk and RA treatments
Recent advances in rheumatoid arthritis management have been accompanied by growing interest in understanding how these therapies might affect risk of cardiovascular disease. In the following section and figure 2, we review clinical data on cardiovascular risk with rheumatoid arthritis treatment, as well as studies providing insight into mechanistic pathways.
Fig 2.
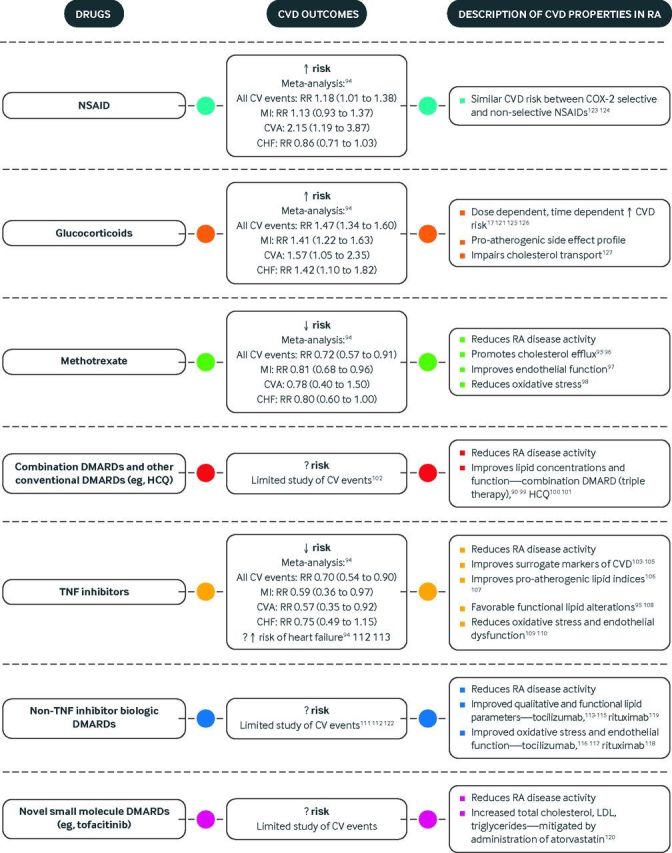
Drugs used for treatment of rheumatoid arthritis and their cardiovascular risk. CHF=congestive heart failure; COX=cyclooxygenase; CVD=cardiovascular disease; DMARDs=disease modifying anti-rheumatic drugs; HCQ=hydroxychloroquine; LDL=low density lipoprotein; MI=myocardial infarction; NSAID=non-steroidal anti-inflammatory drug; RA=rheumatoid arthritis; RR=relative risk; TNF=tumor necrosis factor17 90 94 95 96 97 98 99 100 101 102 103 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 119 120 121 122 123 124 125 126 127
Methotrexate
Methotrexate, long considered a cornerstone of rheumatoid arthritis treatment, has garnered substantial interest for its potential cardioprotective effects. In a systematic review and meta-analysis of observational and controlled trials encompassing 28 studies and 236 525 rheumatoid arthritis patients, methotrexate was associated with a 28% reduction in cardiovascular events (relative risk 0.72, 95% confidence interval 0.57 to 0.91).94 Subsequent observational studies further supported the beneficial effect of methotrexate on cardiovascular disease in rheumatoid arthritis with a 34-66% reduction in cardiovascular events.128 129 Although few studies have investigated CHF outcomes, methotrexate has looked protective in cohort and nested case-control studies.52 130
In addition to its indirect effects mediated by reductions in rheumatoid arthritis disease activity, methotrexate seems to exert other cardioprotective properties on lipids and endothelium. Sera from rheumatoid arthritis patients treated with methotrexate showed increased cholesterol efflux capacity mediated by ABCG-1 and scavenger receptor class B type I,95 an effect that was absent among those receiving adalimumab. In vitro, methotrexate inhibits foam cell formation by promoting reverse cholesterol transport through activation of adenosine A2 receptor and increases in cholesterol 27-hydroxylase and ABCA-1.96 Methotrexate has direct beneficial effects on vascular endothelium in vitro by inducing mitochondrial antioxidant enzyme production.97 Additionally, it actively scavenges superoxide radicals and prevents malondialdehyde-acetaldehyde-adduct formation in vitro, which is important because malondialdehyde-acetaldehyde modification activates the immune system, impairs endothelial function, and generates free radicals.98 Methotrexate also scavenges intracellular free radicals generated by malondialdehyde-acetaldehyde adduction.
Other conventional DMARDs and combination DMARD therapies
Post hoc analysis of the TEAR trial showed that over two years of follow-up, patients treated with triple therapy (methotrexate, hydroxychloroquine, and sulfasalazine) had improved lipid profiles compared with those treated with methotrexate monotherapy or methotrexate plus etanercept.99 Triple therapy resulted in higher HDL and lower LDL, total cholesterol, and total cholesterol/HDL ratio.99 A follow-up study from the TEAR trial showed that, whereas HDL function was similar between treatment arms, triple therapy was associated with an improved HDL inflammatory index and HDL associated haptoglobin concentrations.90 Favorable lipid changes have also been observed with hydroxychloroquine. Our group has previously reported that use of hydroxychloroquine for more than three months was associated with improved total cholesterol, LDL, triglycerides, HDL/LDL, and total cholesterol/HDL.100 Moreover, rheumatoid arthritis patients treated with hydroxychloroquine were more likely to reach recommended lipid targets. Similarly, in an observational study of incident rheumatoid arthritis, hydroxychloroquine use was associated with decreases in LDL and total cholesterol, with trends toward increased HDL and decreased triglycerides.101 Beyond favorable lipid changes, hydroxychloroquine use has been associated with a significantly reduced risk of diabetes and near 70% reduction in cardiovascular events in rheumatoid arthritis.102 131
Biologic and small molecule DMARDs
TNF inhibitors
The aforementioned systematic review and meta-analysis estimated a 30% reduction in risk of cardiovascular events with TNF inhibitors in rheumatoid arthritis (relative risk 0.70, 0.54 to 0.90), with protective associations specifically for myocardial infarction (0.59, 0.36 to 0.97) and stroke (0.57, 0.35 to 0.92).94 TNF inhibitor use might also influence outcomes after a cardiovascular event. In a propensity score adjusted observational study, mortality after acute myocardial infarction was similar between patients using TNF inhibitors and conventional DMARDs, but those who stopped TNF inhibitor more than 90 days before acute myocardial infarction had a threefold higher risk of death.132 These cardioprotective benefits may not apply to all patients receiving TNF inhibitors, as response to treatment and length of follow-up may affect risk. In a cohort study of rheumatoid arthritis patients starting TNF inhibitor treatment, EULAR good responders had similar rates of ACS to matched general population controls, whereas EULAR moderate and non-responders had 2.5-fold higher rates.133 In a comparison of methotrexate treated rheumatoid arthritis patients starting a TNF inhibitor with those receiving a non-biologic DMARD, TNF inhibitor use was marginally associated with a 20-30% reduction in composite cardiovascular events over the first six months but not by 12 months.134 Because of failed randomized controlled trials (RCTs) in CHF,135 136 concern has been raised about TNF inhibitor use in patients with this comorbidity. Not universally reported,94 137 TNF inhibitor use has been associated with an increased risk of hospital admission for CHF as well as increased mortality compared with methotrexate among older rheumatoid arthritis patients.138
TNF inhibitor use has been associated with improved surrogate markers of cardiovascular disease. In a series of 48 consecutive female patients with rheumatoid arthritis, treatment with etanercept led to significant improvements in left ventricular mass index on echocardiography over three to six months that correlated with improvements in disease activity, a finding not observed with methotrexate, leflunomide, or sulfasalazine.103 Post hoc analyses of the BeSt trial showed improved blood pressure with infliximab treatment independent of potential confounders and treatment response.104 A three month controlled trial of TNF inhibitor versus delayed treatment in 60 patients with inflammatory arthritis (rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis) found that TNF inhibitors reduced aortic stiffness (change in aortic pulse wave velocity, −0.50 v 0.05 m/s; P=0.002).105 In a substudy of the golimumab double blind RCTs, golimumab failed to yield consistent effects on carotid ultrasound indices of atherosclerosis.139
Underlying the associations between TNF inhibitors and cardiovascular disease are quantitative and functional changes in lipids as well as improvements in endothelial dysfunction and oxidative stress. In a systematic review and meta-analysis including 13 studies (n=702 patients) with lipid measurements before and after the start of TNF inhibitor treatment, long term TNF inhibitor use (22-52 weeks) was associated with increased HDL (0.27 mmol/L), total cholesterol (0.26 mmol/L), and triglycerides (0.28 mmol/L); decreased apolipoprotein B/A (0.30); and stable LDL and total cholesterol/HDL.106 In double blind RCTs of golimumab (GO-BEFORE, n=637; GO-FORWARD, n=444), increases in total cholesterol, HDL, and LDL at 14 weeks were greater for patients treated with golimumab plus methotrexate than in patients receiving methotrexate alone, although atherogenic indices (total cholesterol/HDL, apolipoprotein B/A1) were similar.107 In rheumatoid arthritis patients with active disease starting infliximab treatment, PON-1 activity increased as did in vitro HDL total antioxidative capacity.108 Early after infliximab was started, improvements correlated with inflammatory markers, a correlation that diminished after six months, suggesting direct antioxidant properties. Adalimumab inhibited cholesterol uptake in macrophages in vitro but did not affect cholesterol efflux.95 Likewise, EPCs increased after TNF inhibitor treatment and correlated with both improvements in disease activity and asymmetric dimethyl arginine reduction in a series of consecutive rheumatoid arthritis patients.109 In a small trial comparing methotrexate monotherapy with methotrexate plus infliximab in rheumatoid arthritis, both groups showed reductions in superoxide production and trends toward improved PON-1 activity.110 Combination treatment yielded additional improvements in HDL concentration and nitric oxide bioavailability from baseline, possibly related to greater improvements in disease activity.
Non-TNF biologic DMARDs and small molecule drugs
As part of regulatory trials, tocilizumab treatment was noted to increase total cholesterol, HDL, LDL, and triglycerides, prompting concern about increased cardiovascular risk.140 141 142 143 Analysis of rheumatoid arthritis patients receiving tocilizumab in subsequent safety registries and extension studies, however, suggested that lipid changes do not necessarily translate into an increase in major adverse cardiovascular events.111 Furthermore, a recent multi-database cohort study of 9218 patients starting tocilizumab propensity matched to 18 810 starting TNF inhibitor found a similar risk of cardiovascular events (hazard ratio 0.84, 0.56 to 1.26).112 Ultimately, closer inspection illustrated that lipid changes occurring in the wake of different DMARD treatments likely reflect improvement in inflammation rather than a unique pro-atherogenic drug effect.144 Although reductions in inflammation seem to have generic effects on lipid profiles, post hoc analysis of the ADACTA trial, a RCT of 326 rheumatoid arthritis patients comparing the efficacy of tocilizumab and adalimumab, showed differing effects of these biologics on lipids.113 After eight weeks of tocilizumab, greater increases were seen in total cholesterol, HDL, LDL, triglycerides, and total cholesterol/HDL compared with adalimumab. Although lipid concentrations suggested a more atherogenic profile, functional lipid modifications were actually more anti-atherogenic with tocilizumab. HDL-serum amyloid A (SAA), secretory phospholipase A2-IIa, and lipoprotein(a) all decreased to a greater extent in the tocilizumab group. In a 24 week double blind RCT of tocilizumab versus placebo in 132 rheumatoid arthritis patients with an inadequate response to methotrexate (with an 80 week, open label extension), characteristic increases in total cholesterol (12.6%), LDL (10.6%), and triglyceride (28.1%) occurred after 12 weeks, but no differences were seen in small LDL, oxidized LDL, HDL, or apolipoprotein B/A1.114 Moreover, improvements in qualitative and functional lipid parameters were again observed with tocilizumab, including HDL associated SAA (78% decrease), secretory phospholipase A2-IIa (61% decrease), lipoprotein(a) (37% decrease), and PON-1 (16% increase). In vitro, hepatic cells cultured in IL-6 showed increased LDL receptor expression, suggesting that altered hepatic clearance could represent an alternative mechanism by which tocilizumab could favorably affect lipids.115 Beyond lipid modifications, tocilizumab also seems to improve endothelial function and oxidative stress. A series of 20 rheumatoid arthritis patients treated with tocilizumab for six months after failure of conventional DMARDs showed significant improvements in endothelial function and decreased expression of vascular cell adhesion molecule.116 Leukocyte generated peroxides and peroxynitrates were also reduced with tocilizumab, and neutrophils collected after treatment generated fewer neutrophil extracellular chromatin traps. In a 24 week, open label RCT of 64 active, treatment naive, rheumatoid arthritis patients comparing the effects of monotherapy with tocilizumab, etanercept, or adalimumab on arterial stiffness, tocilizumab improved arterial stiffness to the same degree as etanercept and adalimumab.117
Evaluation of the risk of cardiovascular disease with other biologic and small molecule DMARDs has been limited. Small case series of rheumatoid arthritis patients receiving rituximab showed transient improvement in endothelial function and increased total cholesterol, triglycerides, and apolipoprotein B/A1 ratios, although lipid modulation occurred predominantly in those who responded to treatment.118 119 Proteomic characterization of HDL from responders to rituximab showed favorable qualitative changes (for example, reduced SAA content).119 In an open label study with double blinded atorvastatin, total cholesterol, LDL, and apolipoprotein B increased after the start of tofacitinib treatment, changes that were attenuated with atorvastatin in the absence of incremental toxicity.120
Few studies have compared the risk of cardiovascular events between biologic treatments in rheumatoid arthritis. The aforementioned multi-database cohort study found similar cardiovascular risk among patients starting tocilizumab and TNF inhibitors.112 In a retrospective cohort study using Medicare data, rheumatoid arthritis patients starting a TNF inhibitor (predominantly etanercept and infliximab) had a 28% increased risk of acute myocardial infarction compared with those starting abatacept.121 However, this finding was not robust to sensitivity analyses using a composite cardiovascular disease outcome. In this same study, tocilizumab was associated with a 36% lower risk than abatacept, but this finding was observed only with the composite outcome. Moreover, the number of patients starting tocilizumab was limited and the findings narrowly met statistical significance. Certainly, further pharmacovigilance studies of cardiovascular risk are needed, as the use of non-TNF inhibitor biologics and small molecule DMARDs increases.
Non-steroidal anti-inflammatory drugs and glucocorticoids
With the removal of rofecoxib and valdecoxib from the market owing to concerns about cardiovascular safety, much attention has been paid to the cardiovascular risk of other non-steroidal anti-inflammatory drugs (NSAIDs). A recent systematic review and meta-analysis of observational studies and RCTs reporting cardiovascular events among 236 525 rheumatoid arthritis patients identified an 18% increased risk of cardiovascular events with NSAIDs (relative risk 1.18, 1.01 to 1.38).94 A Danish, nationwide cohort study assessed whether NSAIDs confer similar risk in rheumatoid arthritis patients and the general population. Although the association was attenuated in rheumatoid arthritis compared with the general population, NSAIDs were still associated with a 22% increased risk of cardiovascular disease events among rheumatoid arthritis patients.122 Risk was dose dependent and primarily attributed to use of rofecoxib and diclofenac. With a previous network meta-analysis of RCTs including 116 429 patients suggesting the lowest cardiovascular risk with naproxen use among NSAIDs in the general population,145 the differential cardiovascular risk of NSAIDs (celecoxib, ibuprofen, and naproxen) was compared in a randomized, double-blind, non-inferiority trial of 24 081 osteoarthritis and rheumatoid arthritis patients at heightened cardiovascular risk with a mean duration of follow-up of 34.1 months.123 Celecoxib was non-inferior to ibuprofen (hazard ratio 0.85, 0.70 to 1.04) and naproxen (0.93, 0.67 to 1.13) for the primary outcome of time to first event meeting Antiplatelet Trialists Collaboration criteria, although only 10% of participants had rheumatoid arthritis and rheumatoid arthritis only analyses were not done. Similarly, a pooled analysis of three double blind RCTs comparing etoricoxib and non-selective diclofenac in patients with osteoarthritis (n=24 913) and rheumatoid arthritis (n=9787) showed equivalent risk of composite cardiovascular events for etoricoxib and diclofenac (hazard ratio 0.96, 0.79 to 1.16).124
Similar to NSAIDs, substantial attention has been paid to glucocorticoid use in this domain. The aforementioned meta-analysis of 236 525 rheumatoid arthritis patients reported a 47% increased risk of all cardiovascular events (relative risk 1.47, 1.34 to 1.60) as well as elevated risk for myocardial infarction (1.41, 1.22 to 1.63), CHF (1.42, 1.10 to 1.82), and stroke (1.57, 1.05 to 2.35) with prednisone use.94 An observational study examining a potential dose threshold for cardiovascular risk with prednisone use in rheumatoid arthritis observed that doses of 8 mg daily or above (and cumulative doses above 40 g) were associated with an increased risk of all cause and cardiovascular mortality independent of traditional risk factors and severity of rheumatoid arthritis.125 Other observational studies have linked even lower glucocorticoid doses with risk of cardiovascular disease. Daily prednisone doses below 7.5 mg were associated with a 23-32% increased risk for acute myocardial infarction in rheumatoid arthritis patients enrolled in Medicare and the Veterans Affairs Health System.17 121 In addition to dose, the timing of corticosteroid use contributes to risk. In a large observational study of incident rheumatoid arthritis patients, current glucocorticoid dose and cumulative duration of exposure to glucocorticoid were independently associated with increased risk of myocardial infarction.126 Complicating these analyses in observational studies is the potential confounding by indication, as disease severity prompts corticosteroid use, which is a potential explanation for why glucocorticoid use was associated with cardiovascular events only in rheumatoid factor positive patients in a population based cohort study.146 Mechanisms underlying these epidemiologic associations are not entirely clear, although known glucocorticoid side effects of weight gain, insulin resistance, dyslipidemia, and elevated blood pressure are postulated pathways. A recent cross sectional study identified a novel mechanism potentially linking glucocorticoids to cardiovascular disease in rheumatoid arthritis—impairment of reverse cholesterol transport. Cholesteryl-ester transfer protein mass and activity was reduced in rheumatoid arthritis patients taking prednisone compared with those not taking prednisone.127
Management and prevention of cardiovascular disease in rheumatoid arthritis patients
Assessment of cardiovascular disease risk
Generally, the first step in preventing cardiovascular disease is determining risk. However, traditional cardiovascular risk models including the Systematic Coronary Risk Evaluation (SCORE), Framingham risk score (FRS), Reynolds risk score (RRS), and 2013 American College of Cardiology/American Heart Association CVD Risk Score do not sufficiently stratify rheumatoid arthritis patients according to risk.147 148 149 Efforts have been made to develop novel cardiovascular disease risk models for use in rheumatoid arthritis,150 151 or to modify existing models with a correction factor for rheumatoid arthritis,152 but these novel indices failed to outperform general population models in limited external validation testing.151 153 Further assessments of these models will be critical to determining the optimal risk model for regular clinical use. In the meantime, we encourage the integration of cardiovascular risk stratification with a risk model endorsed by national guidelines into regular clinical care for rheumatoid arthritis patients.
Management of traditional cardiovascular risk factors
Despite accumulating data suggesting that rheumatoid arthritis patients have high rates of cardiovascular disease, modifiable risk factors are suboptimally managed. Assessment of adherence to cardiovascular disease quality indicators at two Canadian university clinics showed low performance rates for several measures.154 Moreover, rheumatoid arthritis patients are less likely than other patients to have lipid measurements obtained,155 156 with less than 50% of eligible rheumatoid arthritis patients in the 5% random sample of Medicare receiving appropriate lipid screening.157 Use of lipid lowering treatment for rheumatoid arthritis patients fulfilling the National Cholesterol Education Program’s Adult Treatment Panel III criteria was suboptimal, with only 27% starting therapy.155 In a separate cross sectional study, 38% of rheumatoid arthritis patients without previous cardiovascular disease were deemed at risk, and yet only 7% were receiving statins.158 The underuse of lipid lowering treatment in rheumatoid arthritis is in contrast to diabetes, for which prescription rates were over 80%.159 Although the aforementioned studies have reported suboptimal risk factor management, a recent cohort study from the UK found a higher likelihood of rheumatoid arthritis patients with hyperlipidemia and hypertension receiving drug therapies compared with matched controls,7 perhaps suggesting that efforts to increase awareness of cardiovascular risk and suboptimal screening/management of cardiovascular risk factors in rheumatoid arthritis are leading to improvements in quality of care.
Statins
Hydroxymethyl glutaryl coenzyme A reductase inhibitors (statins) lead to marked reductions in cardiovascular events in the general population and seem to have similar efficacy in patients with rheumatoid arthritis. In a large, retrospective cohort study of patients with hyperlipidemia, primary prevention of cardiovascular disease with statins was compared between rheumatoid arthritis and controls (general population and osteoarthritis).8 A reduction in LDL after the start of statin treatment was accompanied by a greater than 30% reduction in cardiovascular events in rheumatoid arthritis, similar to the risk reduction in both general and osteoarthritis controls. In a retrospective analysis of the Incremental Decrease in End Points Through Aggressive Lipid Lowering (IDEAL—an open label comparison between atorvastatin 80 mg and simvastatin 20-40 mg in 8888 post-myocardial infarction patients) and Treating to New Targets (TNT—a double blind RCT comparing atorvastatin 80 mg and 10 mg in 10 001 patients with coronary artery disease) trials, patients with inflammatory joint disease had similar decreases in atherogenic lipid indices and cardiovascular events (20% reduction with atorvastatin 80 mg) after statin treatment compared with those without inflammatory arthritis.160 Further illustrating these potential benefits, discontinuation of statin has been associated with a 67% increase in risk of acute myocardial infarction, 60% increase in risk of cardiovascular mortality, and 79% increase in risk of all cause mortality in patients with rheumatoid arthritis.161 162 Moreover, statins may improve cardiovascular disease risk in rheumatoid arthritis through mechanisms independent of lipid lowering. In an 18 month, open label study of intensive lipid management with rosuvastatin (ROsuvastat in Rheumatoid Arthritis, Ankylosing Spondylitis and other inflammatory joint diseases; RORA-AS), statin-naive patients with inflammatory arthritis (64% rheumatoid arthritis) and prevalent atherosclerosis showed improved arterial stiffness and significant reduction in carotid plaque independent of LDL reduction.163 164 In an AIA mouse model, fluvastatin reversed endothelial dysfunction and inhibited NADPH oxidases, thus reducing vascular ROS production.165 Finally, statins may reduce rheumatoid arthritis disease activity, with one non-blinded, six month trial of 30 patients with early rheumatoid arthritis reporting improved disease activity (mean DAS28 improvement 2.19 v 0.92; P<0.001) with atorvastatin 40 mg added to a regimen of methotrexate and glucocorticoids.166
Physical activity
Despite the challenges that physical activity may pose to patients with rheumatoid arthritis, the potential benefits of exercise therapy warrant consideration. Similar to the general population, physical activity is associated with a favorable cardiovascular risk profile in rheumatoid arthritis.167 In a six month trial of individualized aerobic and resistance training compared with education alone in 40 rheumatoid arthritis patients with a mean DAS28 of 3.2, exercise training significantly improved VO2 max, lipids (mean change in total cholesterol/HDL −0.4 v 0.4), and blood pressure (mean change −7.1 v 4.7 mm Hg systolic and −3.1 v 3.5 mm Hg diastolic), as well as microvascular and macrovascular endothelial function.168 169 Notably, disease activity and physical function also improved significantly in the exercise treatment group. Addressing potential safety concerns with exercise interventions, a two year RCT comparing high intensity exercise with routine care in 309 rheumatoid arthritis patients failed to find evidence of accelerated joint damage with high intensity physical activity.170 Finally, as a shared risk factor for rheumatoid arthritis and cardiovascular disease,11 smoking cessation is critical in reducing cardiovascular risk. This intervention may also pay dividends in terms of rheumatoid arthritis disease activity, as smoking has been linked to increased disease activity and inflammation.171 172 Similarly, comorbid conditions adversely affecting cardiovascular risk, including hypertension, diabetes, and chronic kidney disease, should be aggressively treated.
Management of rheumatoid arthritis
In previous work studying cause specific mortality in US veterans, we found that the rate of cardiovascular disease related mortality in rheumatoid arthritis patients in disease remission was similar to that of age and sex matched controls from the general population, whereas rheumatoid arthritis patients with low to high disease activity had increased cardiovascular mortality.173 Others have shown similar cardiovascular disease risk stratifications based on rheumatoid arthritis disease activity.174 In the RORA-AS trial, lower rheumatoid arthritis disease activity was associated with greater carotid plaque regression.164
Beyond reducing systemic inflammation, effective rheumatoid arthritis treatments could mitigate cardiovascular disease risk through other mechanisms. In the TEAR trial, for instance, reductions in rheumatoid arthritis disease activity were accompanied by improved HDL function,90 and post hoc analyses from the BeSt trial showed that rheumatoid arthritis patients in remission or low disease activity had improved blood pressures compared with those in moderate or high disease activity.104 Taken together, these studies suggest that aggressively treating rheumatoid arthritis toward remission should be the goal for cardiovascular risk reduction with the understanding that other comorbidities and patients’ preferences should be considered. We advocate a multifaceted approach to cardiovascular risk reduction in rheumatoid arthritis, targeting both traditional and rheumatoid arthritis specific cardiovascular risk factors, and both drug and non-drug treatments, and involving a multidisciplinary team of primary care providers, rheumatologists, and cardiologists (fig 3).
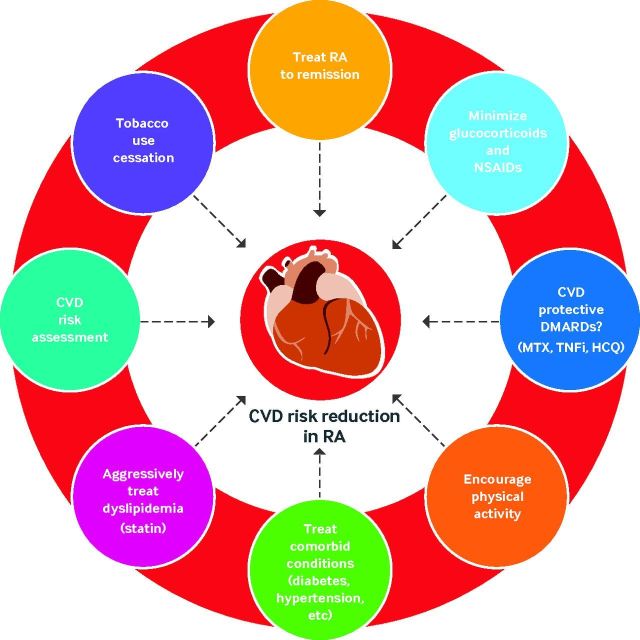
Fig 3.
Targeting cardiovascular disease (CVD) risk reduction in rheumatoid arthritis (RA). Targeting reduction of CVD in RA requires a multifaceted, team approach. Key areas to target are tobacco use cessation, treating comorbid conditions (diabetes, hypertension, chronic kidney disease), assessing cardiovascular risk, aggressively managing dyslipidemia, treating RA toward a goal of remission (or at least low disease activity), avoiding/limiting use of non-steroidal anti-inflammatory drugs (NSAIDs) and corticosteroids, and potentially selecting disease-modifying antirheumatic drugs (DMARDs) with more favorable CVD risk profiles. HCQ=hydroxychloroquine; MTX=methotrexate; TNFi=tumor necrosis factor inhibitor
Emerging treatments
Several potentially high impact RCTs investigating cardiovascular risk related to rheumatoid arthritis and/or DMARD use are ongoing or recently completed. A Food and Drug Administration mandated open label RCT comparing the risk of cardiovascular disease in 3080 rheumatoid arthritis patients receiving tocilizumab or etanercept for up to five years was recently completed (clinicialtrials.gov: NCT01331837) with preliminary results suggesting no differences between treatments in the occurrence of cardiovascular disease. An open label, 24 week RCT comparing the effect of triple therapy (methotrexate, hydroxychloroquine, and sulfasalazine) versus TNF inhibitor (etanercept or adalimumab) in 200 rheumatoid arthritis patients with inadequate response to methotrexate on change in vascular inflammation assessed by fluorodeoxyglucose positron emission tomography/computed tomography is actively recruiting (clinicaltrials.gov: NCT02374021). The Trial of Atorvastatin for the primary prevention of Cardiovascular Events in Rheumatoid Arthritis (TRACE-RA, ISRCTN41829447), a 2986 patient, double blind RCT comparing atorvastatin 40 mg daily with placebo was recently terminated early owing to a lower than expected event rate. Finally, a prospective, randomized, open label trial comparing a targeted, intensified, multidimensional intervention against conventional treatment of modifiable cardiovascular risk factors in early rheumatoid arthritis is currently enrolling patients.175
In the general population, several RCTs are under way or have recently been completed that have implications for management of rheumatoid arthritis. A six year, double blind RCT of methotrexate for the secondary prevention of cardiovascular disease in 7000 patients with type 2 diabetes mellitus or metabolic syndrome is ongoing (Cardiovascular Inflammation Reduction Trial, NCT01594333).176 In a double blind RCT targeting the secondary prevention of cardiovascular events among 10 061 patients with previous myocardial infarction and hsCRP above 2 mg/L, canakinumab (monoclonal antibody to IL-1β) 150 mg, but not the 50 mg dose, reduced cardiovascular events by 15% (hazard ratio 0.85, 0.74 to 0.98) compared with placebo.177 Whether the modest protective effect reported with canakinumab translates to anakinra, the only IL-1β targeted therapy currently approved in rheumatoid arthritis, remains unclear.
Guidelines
EULAR commissioned a task force to propose recommendations for management of cardiovascular risk in inflammatory arthritis in 2009,152 and a 2015/16 update was recently published.178 Three overarching principles and 10 specific recommendations were provided, but the level of evidence (range 2a-4) and strength of recommendations (range B-D) were generally low.
Conclusions
Patients with rheumatoid arthritis are at increased risk of cardiovascular disease. Several mechanisms beyond traditional cardiovascular risk factors confer this risk, including heightened systemic inflammation and inflammatory mediators, post-translational modifications of peptides/proteins and immune responses against these antigens, oxidative stress, endothelial dysfunction, and unique quantitative and qualitative lipid alterations. Treating rheumatoid arthritis aggressively with DMARDs, limiting use of corticosteroids and NSAIDs, and aggressively managing traditional cardiovascular risk factors are essential to reduce the substantial burden posed by this common comorbidity.
Research questions.
How does rheumatoid arthritis related inflammation interact with other cardiovascular risk factors to propagate atherogenesis?
How do post-translational modifications and subsequent immune responses influence cardiovascular risk in rheumatoid arthritis?
What is the optimal goal of treatment in rheumatoid arthritis for reduction of risk of cardiovascular disease?
Should select rheumatoid arthritis immunomodulatory therapies be preferentially used to prevent cardiovascular disease in rheumatoid arthritis patients?
Glossary of abbreviations.
ABCA-1—aqueous diffusion, scavenger receptor B
ABCG-1—ATP binding cassette transporter G1
ACPA—anti-citrullinated protein antibodies
ACS—acute coronary syndrome
AIA—adjuvant induced arthritis
CCP—anti-cyclic citrullinated peptide
CHF—congestive heart failure
CIA—collagen induced arthritis
CRP—C reactive protein
DAS28—28 joint disease activity score
DMARD—disease modifying anti-rheumatic drug
eNOS—endogenous nitric oxide synthase
EPCs—endothelial progenitor cells
ESR—erythrocyte sedimentation rate
EULAR—European Union League Against Rheumatism
HDL—high density lipoprotein
hsCRP—high sensitivity C reactive protein
IL—interleukin
LDL—low density lipoprotein
MCP-1—monocyte chemoattractant protein-1
NSAID—non-steroidal anti-inflammatory drug
RCT—randomized controlled trial
RORA-AS—ROsuvastat in Rheumatoid Arthritis, Ankylosing Spondylitis and other inflammatory joint diseases (trial)
ROS—reactive oxygen species
SAA—serum amyloid A
Tang—angiogenic T cells
TEAR—Treatment of Early RA (trial)
TNF-α—tumor necrosis factor α
Contributors: BRE did the literature review and prepared the initial draft of the manuscript. All authors were involved in the conception, drafting, and editing of the manuscript. TRM is the guarantor.
Funding: BRE is supported by a University of Nebraska Medical Center (UNMC) internal medicine scientist development award, the UNMC Mentored Scholars Program, and the UNMC College of Medicine Physician-Scientist Training Program. TRM is supported by grants from the National Institutes of Health (NIH)/National Institute of Arthritis and Musculoskeletal and Skin Diseases (P50AR060772), National Institute of Alcohol Abuse and Alcoholism (R25AA020818), National Institute of General Medical Sciences (U54GM115458), Veterans Affairs Office of Research and Development (Merit Award, CX000896), Bristol Myers Squibb, and Ironwood Pharmaceuticals. DRA is supported by the Harry R and Sarah H Caspersen Coronary Artery Disease Research Fund and the Division of Cardiovascular Medicine, Department of Internal Medicine, UNMC. GMT is supported by grants from the Rheumatology Research Foundation and a Bristol Myers Squibb investigator initiated grant.
Competing interests: We have read and understood the BMJ policy on declaration of interests and declare the following interests: TRM serves as a consultant for Pfizer.
Provenance and peer review: Commissioned; externally peer reviewed.
Patient involvement: No patients were involved in the creation of this article.
Series explanation: State of the Art Reviews are commissioned on the basis of their relevance to academics and specialists in the US and internationally. For this reason they are written predominantly by US authors
References
- 1. Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ 2003;81:646-56. [PMC free article] [PubMed] [Google Scholar]
- 2. Sokka T, Abelson B, Pincus T. Mortality in rheumatoid arthritis: 2008 update. Clin Exp Rheumatol 2008;26(Suppl 51):S35-61. [PubMed] [Google Scholar]
- 3. Avina-Zubieta JA, Thomas J, Sadatsafavi M, Lehman AJ, Lacaille D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis 2012;71:1524-9. 10.1136/annrheumdis-2011-200726 [DOI] [PubMed] [Google Scholar]
- 4. Aviña-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum 2008;59:1690-7. 10.1002/art.24092 [DOI] [PubMed] [Google Scholar]
- 5. Sandoo A, Chanchlani N, Hodson J, Smith JP, Douglas KM, Kitas GD. Classical cardiovascular disease risk factors associate with vascular function and morphology in rheumatoid arthritis: a six-year prospective study. Arthritis Res Ther 2013;15:R203. 10.1186/ar4396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chung CP, Giles JT, Kronmal RA, et al. Progression of coronary artery atherosclerosis in rheumatoid arthritis: comparison with participants from the Multi-Ethnic Study of Atherosclerosis. Arthritis Res Ther 2013;15:R134. 10.1186/ar4314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jafri K, Bartels CM, Shin D, Gelfand JM, Ogdie A. Incidence and Management of Cardiovascular Risk Factors in Psoriatic Arthritis and Rheumatoid Arthritis: A Population-Based Study. Arthritis Care Res (Hoboken) 2017;69:51-7. 10.1002/acr.23094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. An J, Alemao E, Reynolds K, et al. Cardiovascular Outcomes Associated with Lowering Low-density Lipoprotein Cholesterol in Rheumatoid Arthritis and Matched Nonrheumatoid Arthritis. J Rheumatol 2016;43:1989-96. 10.3899/jrheum.160110 [DOI] [PubMed] [Google Scholar]
- 9. Gonzalez A, Maradit Kremers H, Crowson CS, et al. Do cardiovascular risk factors confer the same risk for cardiovascular outcomes in rheumatoid arthritis patients as in non-rheumatoid arthritis patients? Ann Rheum Dis 2008;67:64-9. 10.1136/ard.2006.059980 [DOI] [PubMed] [Google Scholar]
- 10. Solomon DH, Karlson EW, Rimm EB, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation 2003;107:1303-7. 10.1161/01.CIR.0000054612.26458.B2 [DOI] [PubMed] [Google Scholar]
- 11. Sugiyama D, Nishimura K, Tamaki K, et al. Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis 2010;69:70-81. 10.1136/ard.2008.096487 [DOI] [PubMed] [Google Scholar]
- 12. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352:1685-95. 10.1056/NEJMra043430 [DOI] [PubMed] [Google Scholar]
- 13. Solomon DH, Kremer J, Curtis JR, et al. Explaining the cardiovascular risk associated with rheumatoid arthritis: traditional risk factors versus markers of rheumatoid arthritis severity. Ann Rheum Dis 2010;69:1920-5. 10.1136/ard.2009.122226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mantel Ä, Holmqvist M, Nyberg F, et al. Risk factors for the rapid increase in risk of acute coronary events in patients with new-onset rheumatoid arthritis: a nested case-control study. Arthritis Rheumatol 2015;67:2845-54. 10.1002/art.39267 [DOI] [PubMed] [Google Scholar]
- 15. Solomon DH, Reed GW, Kremer JM, et al. Disease activity in rheumatoid arthritis and the risk of cardiovascular events. Arthritis Rheumatol 2015;67:1449-55. 10.1002/art.39098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arts EE, Fransen J, den Broeder AA, Popa CD, van Riel PL. The effect of disease duration and disease activity on the risk of cardiovascular disease in rheumatoid arthritis patients. Ann Rheum Dis 2015;74:998-1003. 10.1136/annrheumdis-2013-204531 [DOI] [PubMed] [Google Scholar]
- 17. Navarro-Millán I, Yang S, DuVall SL, et al. Association of hyperlipidaemia, inflammation and serological status and coronary heart disease among patients with rheumatoid arthritis: data from the National Veterans Health Administration. Ann Rheum Dis 2016;75:341-7. 10.1136/annrheumdis-2013-204987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang J, Chen L, Delzell E, et al. The association between inflammatory markers, serum lipids and the risk of cardiovascular events in patients with rheumatoid arthritis. Ann Rheum Dis 2014;73:1301-8. 10.1136/annrheumdis-2013-204715 [DOI] [PubMed] [Google Scholar]
- 19. Gonzalez-Gay MA, Gonzalez-Juanatey C, Lopez-Diaz MJ, et al. HLA-DRB1 and persistent chronic inflammation contribute to cardiovascular events and cardiovascular mortality in patients with rheumatoid arthritis. Arthritis Rheum 2007;57:125-32. 10.1002/art.22482 [DOI] [PubMed] [Google Scholar]
- 20. Myasoedova E, Chandran A, Ilhan B, et al. The role of rheumatoid arthritis (RA) flare and cumulative burden of RA severity in the risk of cardiovascular disease. Ann Rheum Dis 2016;75:560-5. 10.1136/annrheumdis-2014-206411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fine NM, Crowson CS, Lin G, Oh JK, Villarraga HR, Gabriel SE. Evaluation of myocardial function in patients with rheumatoid arthritis using strain imaging by speckle-tracking echocardiography. Ann Rheum Dis 2014;73:1833-9. 10.1136/annrheumdis-2013-203314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Løgstrup BB, Deibjerg LK, Hedemann-Andersen A, Ellingsen T. Left ventricular function in treatment-naive early rheumatoid arthritis. Am J Cardiovasc Dis 2014;4:79-86. [PMC free article] [PubMed] [Google Scholar]
- 23. Midtbø H, Gerdts E, Kvien TK, et al. Disease activity and left ventricular structure in patients with rheumatoid arthritis. Rheumatology (Oxford) 2015;54:511-9. 10.1093/rheumatology/keu368 [DOI] [PubMed] [Google Scholar]
- 24. Liang KP, Myasoedova E, Crowson CS, et al. Increased prevalence of diastolic dysfunction in rheumatoid arthritis. Ann Rheum Dis 2010;69:1665-70. 10.1136/ard.2009.124362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Midtbø H, Semb AG, Matre K, Kvien TK, Gerdts E. Disease activity is associated with reduced left ventricular systolic myocardial function in patients with rheumatoid arthritis. Ann Rheum Dis 2017;76:371-6. 10.1136/annrheumdis-2016-209223 [DOI] [PubMed] [Google Scholar]
- 26. Karpouzas GA, Malpeso J, Choi TY, Li D, Munoz S, Budoff MJ. Prevalence, extent and composition of coronary plaque in patients with rheumatoid arthritis without symptoms or prior diagnosis of coronary artery disease. Ann Rheum Dis 2014;73:1797-804. 10.1136/annrheumdis-2013-203617 [DOI] [PubMed] [Google Scholar]
- 27. Giles JT, Post WS, Blumenthal RS, et al. Longitudinal predictors of progression of carotid atherosclerosis in rheumatoid arthritis. Arthritis Rheum 2011;63:3216-25. 10.1002/art.30542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rho YH, Chung CP, Oeser A, et al. Inflammatory mediators and premature coronary atherosclerosis in rheumatoid arthritis. Arthritis Rheum 2009;61:1580-5. 10.1002/art.25009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Giles JT, Szklo M, Post W, et al. Coronary arterial calcification in rheumatoid arthritis: comparison with the Multi-Ethnic Study of Atherosclerosis. Arthritis Res Ther 2009;11:R36. 10.1186/ar2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. del Rincón I, Polak JF, O’Leary DH, et al. Systemic inflammation and cardiovascular risk factors predict rapid progression of atherosclerosis in rheumatoid arthritis. Ann Rheum Dis 2015;74:1118-23. 10.1136/annrheumdis-2013-205058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ahmed A, Hollan I, Curran SA, et al. Brief Report: Proatherogenic Cytokine Microenvironment in the Aortic Adventitia of Patients With Rheumatoid Arthritis. Arthritis Rheumatol 2016;68:1361-6. 10.1002/art.39574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Winchester R, Giles JT, Nativ S, et al. Association of Elevations of Specific T Cell and Monocyte Subpopulations in Rheumatoid Arthritis With Subclinical Coronary Artery Atherosclerosis. Arthritis Rheumatol 2016;68:92-102. 10.1002/art.39419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hot A, Lenief V, Miossec P. Combination of IL-17 and TNFα induces a pro-inflammatory, pro-coagulant and pro-thrombotic phenotype in human endothelial cells. Ann Rheum Dis 2012;71:768-76. 10.1136/annrheumdis-2011-200468 [DOI] [PubMed] [Google Scholar]
- 34. Sidibé A, Mannic T, Arboleas M, et al. Soluble VE-cadherin in rheumatoid arthritis patients correlates with disease activity: evidence for tumor necrosis factor α-induced VE-cadherin cleavage. Arthritis Rheum 2012;64:77-87. 10.1002/art.33336 [DOI] [PubMed] [Google Scholar]
- 35. Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol 2003;91(3A):7-11A. 10.1016/S0002-9149(02)03144-2 [DOI] [PubMed] [Google Scholar]
- 36. Quiñonez-Flores CM, González-Chávez SA, Del Río Nájera D, Pacheco-Tena C. Oxidative Stress Relevance in the Pathogenesis of the Rheumatoid Arthritis: A Systematic Review. Biomed Res Int 2016;2016:6097417. 10.1155/2016/6097417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ikonomidis I, Tzortzis S, Lekakis J, et al. Lowering interleukin-1 activity with anakinra improves myocardial deformation in rheumatoid arthritis. Heart 2009;95:1502-7. 10.1136/hrt.2009.168971 [DOI] [PubMed] [Google Scholar]
- 38. Vivekanandan-Giri A, Slocum JL, Byun J, et al. High density lipoprotein is targeted for oxidation by myeloperoxidase in rheumatoid arthritis. Ann Rheum Dis 2013;72:1725-31. 10.1136/annrheumdis-2012-202033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. de Groot L, Hinkema H, Westra J, et al. Advanced glycation endproducts are increased in rheumatoid arthritis patients with controlled disease. Arthritis Res Ther 2011;13:R205. 10.1186/ar3538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shi Q, Abusarah J, Baroudi G, Fernandes JC, Fahmi H, Benderdour M. Ramipril attenuates lipid peroxidation and cardiac fibrosis in an experimental model of rheumatoid arthritis. Arthritis Res Ther 2012;14:R223. 10.1186/ar4062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sakuta T, Morita Y, Satoh M, Fox DA, Kashihara N. Involvement of the renin-angiotensin system in the development of vascular damage in a rat model of arthritis: effect of angiotensin receptor blockers. Arthritis Rheum 2010;62:1319-28. 10.1002/art.27384 [DOI] [PubMed] [Google Scholar]
- 42. Södergren A, Karp K, Boman K, et al. Atherosclerosis in early rheumatoid arthritis: very early endothelial activation and rapid progression of intima media thickness. Arthritis Res Ther 2010;12:R158. 10.1186/ar3116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Turiel M, Atzeni F, Tomasoni L, et al. Non-invasive assessment of coronary flow reserve and ADMA levels: a case-control study of early rheumatoid arthritis patients. Rheumatology (Oxford) 2009;48:834-9. 10.1093/rheumatology/kep082 [DOI] [PubMed] [Google Scholar]
- 44. Galarraga B, Belch JJ, Pullar T, Ogston S, Khan F. Clinical improvement in rheumatoid arthritis is associated with healthier microvascular function in patients who respond to antirheumatic therapy. J Rheumatol 2010;37:521-8. 10.3899/jrheum.090417 [DOI] [PubMed] [Google Scholar]
- 45. Totoson P, Maguin-Gaté K, Nappey M, Wendling D, Demougeot C. Endothelial Dysfunction in Rheumatoid Arthritis: Mechanistic Insights and Correlation with Circulating Markers of Systemic Inflammation. PLoS One 2016;11:e0146744. 10.1371/journal.pone.0146744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sandoo A, Kitas GD, Carroll D, Veldhuijzen van Zanten JJ. The role of inflammation and cardiovascular disease risk on microvascular and macrovascular endothelial function in patients with rheumatoid arthritis: a cross-sectional and longitudinal study. Arthritis Res Ther 2012;14:R117. 10.1186/ar3847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Park YJ, Kim JY, Park J, Choi JJ, Kim WU, Cho CS. Bone erosion is associated with reduction of circulating endothelial progenitor cells and endothelial dysfunction in rheumatoid arthritis. Arthritis Rheumatol 2014;66:1450-60. 10.1002/art.38352 [DOI] [PubMed] [Google Scholar]
- 48. Rodríguez-Carrio J, Alperi-López M, López P, Alonso-Castro S, Ballina-García FJ, Suárez A. Angiogenic T cells are decreased in rheumatoid arthritis patients. Ann Rheum Dis 2015;74:921-7. 10.1136/annrheumdis-2013-204250 [DOI] [PubMed] [Google Scholar]
- 49. Menghini R, Campia U, Tesauro M, et al. Toll-like receptor 4 mediates endothelial cell activation through NF-κB but is not associated with endothelial dysfunction in patients with rheumatoid arthritis. PLoS One 2014;9:e99053. 10.1371/journal.pone.0099053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Haruna Y, Morita Y, Komai N, et al. Endothelial dysfunction in rat adjuvant-induced arthritis: vascular superoxide production by NAD(P)H oxidase and uncoupled endothelial nitric oxide synthase. Arthritis Rheum 2006;54:1847-55. 10.1002/art.21891 [DOI] [PubMed] [Google Scholar]
- 51. López-Longo FJ, Oliver-Miñarro D, de la Torre I, et al. Association between anti-cyclic citrullinated peptide antibodies and ischemic heart disease in patients with rheumatoid arthritis. Arthritis Rheum 2009;61:419-24. 10.1002/art.24390 [DOI] [PubMed] [Google Scholar]
- 52. Myasoedova E, Crowson CS, Nicola PJ, et al. The influence of rheumatoid arthritis disease characteristics on heart failure. J Rheumatol 2011;38:1601-6. 10.3899/jrheum.100979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mackey RH, Kuller LH, Deane KD, et al. Rheumatoid Arthritis, Anti-Cyclic Citrullinated Peptide Positivity, and Cardiovascular Disease Risk in the Women’s Health Initiative. Arthritis Rheumatol 2015;67:2311-22. 10.1002/art.39198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Majka DS, Vu TT, Pope RM, et al. Association of Rheumatoid Factors With Subclinical and Clinical Atherosclerosis in African American Women: The Multiethnic Study of Atherosclerosis. Arthritis Care Res (Hoboken) 2017;69:166-74. 10.1002/acr.22930 [DOI] [PubMed] [Google Scholar]
- 55. Cambridge G, Acharya J, Cooper JA, Edwards JC, Humphries SE. Antibodies to citrullinated peptides and risk of coronary heart disease. Atherosclerosis 2013;228:243-6. 10.1016/j.atherosclerosis.2013.02.009 [DOI] [PubMed] [Google Scholar]
- 56. Marasovic-Krstulovic D, Martinovic-Kaliterna D, Fabijanic D, Morovic-Vergles J. Are the anti-cyclic citrullinated peptide antibodies independent predictors of myocardial involvement in patients with active rheumatoid arthritis? Rheumatology (Oxford) 2011;50:1505-12. 10.1093/rheumatology/ker121 [DOI] [PubMed] [Google Scholar]
- 57. Giles JT, Malayeri AA, Fernandes V, et al. Left ventricular structure and function in patients with rheumatoid arthritis, as assessed by cardiac magnetic resonance imaging. Arthritis Rheum 2010;62:940-51. 10.1002/art.27349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Giles JT, Fert-Bober J, Park JK, et al. Myocardial citrullination in rheumatoid arthritis: a correlative histopathologic study. Arthritis Res Ther 2012;14:R39. 10.1186/ar3752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Geraldino-Pardilla L, Russo C, Sokolove J, et al. Association of anti-citrullinated protein or peptide antibodies with left ventricular structure and function in rheumatoid arthritis. Rheumatology (Oxford) 2017;56:534-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Geraldino-Pardilla L, Giles JT, Sokolove J, et al. Association of Anti-Citrullinated Peptide Antibodies With Coronary Artery Calcification in Rheumatoid Arthritis. Arthritis Care Res (Hoboken) 2017;69:1276-81. 10.1002/acr.23106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Montes A, Corrales A, Calaza M, et al. Brief report: lack of replication of an association between anti-citrullinated fibrinogen and subclinical atherosclerosis in patients with rheumatoid arthritis. Arthritis Rheumatol 2015;67:2861-5. 10.1002/art.39302 [DOI] [PubMed] [Google Scholar]
- 62. Shi J, Knevel R, Suwannalai P, et al. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proc Natl Acad Sci U S A 2011;108:17372-7. 10.1073/pnas.1114465108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Spinelli FR, Pecani A, Ciciarello F, et al. Association between antibodies to carbamylated proteins and subclinical atherosclerosis in rheumatoid arthritis patients. BMC Musculoskelet Disord 2017;18:214. 10.1186/s12891-017-1563-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Verbrugge FH, Tang WH, Hazen SL. Protein carbamylation and cardiovascular disease. Kidney Int 2015;88:474-8. 10.1038/ki.2015.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Thiele GM, Tuma DJ, Willis MS, et al. Soluble proteins modified with acetaldehyde and malondialdehyde are immunogenic in the absence of adjuvant. Alcohol Clin Exp Res 1998;22:1731-9. 10.1111/j.1530-0277.1998.tb03973.x [DOI] [PubMed] [Google Scholar]
- 66. Tuma DJ, Kearley ML, Thiele GM, et al. Elucidation of reaction scheme describing malondialdehyde-acetaldehyde-protein adduct formation. Chem Res Toxicol 2001;14:822-32. 10.1021/tx000222a [DOI] [PubMed] [Google Scholar]
- 67. Thiele GM, Duryee MJ, Anderson DR, et al. Malondialdehyde-acetaldehyde adducts and anti-malondialdehyde-acetaldehyde antibodies in rheumatoid arthritis. Arthritis Rheumatol 2015;67:645-55. 10.1002/art.38969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Anderson DR, Duryee MJ, Shurmur SW, et al. Unique antibody responses to malondialdehyde-acetaldehyde (MAA)-protein adducts predict coronary artery disease. PLoS One 2014;9:e107440. 10.1371/journal.pone.0107440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Myasoedova E, Crowson CS, Kremers HM, et al. Lipid paradox in rheumatoid arthritis: the impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis 2011;70:482-7. 10.1136/ard.2010.135871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Liao KP, Liu J, Lu B, Solomon DH, Kim SC. Association between lipid levels and major adverse cardiovascular events in rheumatoid arthritis compared to non-rheumatoid arthritis patients. Arthritis Rheumatol 2015;67:2004-10. 10.1002/art.39165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Liao KP, Playford MP, Frits M, et al. The association between reduction in inflammation and changes in lipoprotein levels and HDL cholesterol efflux capacity in rheumatoid arthritis. J Am Heart Assoc 2015;4:e001588. 10.1161/JAHA.114.001588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Toms TE, Panoulas VF, Douglas KM, et al. Are lipid ratios less susceptible to change with systemic inflammation than individual lipid components in patients with rheumatoid arthritis? Angiology 2011;62:167-75. 10.1177/0003319710373749 [DOI] [PubMed] [Google Scholar]
- 73. Navarro-Millán I, Charles-Schoeman C, Yang S, et al. Changes in lipoproteins associated with methotrexate or combination therapy in early rheumatoid arthritis: results from the treatment of early rheumatoid arthritis trial. Arthritis Rheum 2013;65:1430-8. 10.1002/art.37916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Charles-Schoeman C, Watanabe J, Lee YY, et al. Abnormal function of high-density lipoprotein is associated with poor disease control and an altered protein cargo in rheumatoid arthritis. Arthritis Rheum 2009;60:2870-9. 10.1002/art.24802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Watanabe J, Charles-Schoeman C, Miao Y, et al. Proteomic profiling following immunoaffinity capture of high-density lipoprotein: association of acute-phase proteins and complement factors with proinflammatory high-density lipoprotein in rheumatoid arthritis. Arthritis Rheum 2012;64:1828-37. 10.1002/art.34363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rodríguez-Carrio J, López-Mejías R, Alperi-López M, et al. Paraoxonase 1 Activity Is Modulated by the rs662 Polymorphism and IgG Anti-High-Density Lipoprotein Antibodies in Patients With Rheumatoid Arthritis: Potential Implications for Cardiovascular Disease. Arthritis Rheumatol 2016;68:1367-76. 10.1002/art.39609 [DOI] [PubMed] [Google Scholar]
- 77. Charles-Schoeman C, Lee YY, Shahbazian A, et al. Association of paraoxonase 1 gene polymorphism and enzyme activity with carotid plaque in rheumatoid arthritis. Arthritis Rheum 2013;65:2765-72. 10.1002/art.38118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kim JY, Lee EY, Park JK, Song YW, Kim JR, Cho KH. Patients with Rheumatoid Arthritis Show Altered Lipoprotein Profiles with Dysfunctional High-Density Lipoproteins that Can Exacerbate Inflammatory and Atherogenic Process. PLoS One 2016;11:e0164564. 10.1371/journal.pone.0164564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Charles-Schoeman C, Lee YY, Grijalva V, et al. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann Rheum Dis 2012;71:1157-62. 10.1136/annrheumdis-2011-200493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ronda N, Favari E, Borghi MO, et al. Impaired serum cholesterol efflux capacity in rheumatoid arthritis and systemic lupus erythematosus. Ann Rheum Dis 2014;73:609-15. 10.1136/annrheumdis-2012-202914 [DOI] [PubMed] [Google Scholar]
- 81. Voloshyna I, Modayil S, Littlefield MJ, et al. Plasma from rheumatoid arthritis patients promotes pro-atherogenic cholesterol transport gene expression in THP-1 human macrophages. Exp Biol Med (Maywood) 2013;238:1192-7. 10.1177/1535370213503262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hashizume M, Mihara M. Blockade of IL-6 and TNF-α inhibited oxLDL-induced production of MCP-1 via scavenger receptor induction. Eur J Pharmacol 2012;689:249-54. 10.1016/j.ejphar.2012.05.035 [DOI] [PubMed] [Google Scholar]
- 83. Hashizume M, Mihara M. Atherogenic effects of TNF-α and IL-6 via up-regulation of scavenger receptors. Cytokine 2012;58:424-30. 10.1016/j.cyto.2012.02.010 [DOI] [PubMed] [Google Scholar]
- 84. Wen W, He M, Liang X, Gao SS, Zhou J, Yuan ZY. Accelerated transformation of macrophage-derived foam cells in the presence of collagen-induced arthritis mice serum is associated with dyslipidemia. Autoimmunity 2016;49:115-23. 10.3109/08916934.2015.1118761 [DOI] [PubMed] [Google Scholar]
- 85. Escalante A, Haas RW, del Rincón I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: role of comorbidity and systemic inflammation. Arch Intern Med 2005;165:1624-9. 10.1001/archinte.165.14.1624 [DOI] [PubMed] [Google Scholar]
- 86. Choi HK, Nguyen US, Niu J, Danaei G, Zhang Y. Selection bias in rheumatic disease research. Nat Rev Rheumatol 2014;10:403-12. 10.1038/nrrheum.2014.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. England BR, Baker JF, Sayles H, et al. Body Mass Index, Weight Loss, and Cause-Specific Mortality in Rheumatoid Arthritis. Arthritis Care Res (Hoboken) 2018;70:11-8. 10.1002/acr.23258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Baker JF, Billig E, Michaud K, et al. Weight Loss, the Obesity Paradox, and the Risk of Death in Rheumatoid Arthritis. Arthritis Rheumatol 2015;67:1711-7. 10.1002/art.39136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Rho YH, Chung CP, Solus JF, et al. Adipocytokines, insulin resistance, and coronary atherosclerosis in rheumatoid arthritis. Arthritis Rheum 2010;62:1259-64. 10.1002/art.27376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Charles-Schoeman C, Yin Lee Y, Shahbazian A, et al. Improvement of High-Density Lipoprotein Function in Patients With Early Rheumatoid Arthritis Treated With Methotrexate Monotherapy or Combination Therapies in a Randomized Controlled Trial. Arthritis Rheumatol 2017;69:46-57. 10.1002/art.39833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. George MD, Giles JT, Katz PP, et al. Impact of Obesity and Adiposity on Inflammatory Markers in Patients With Rheumatoid Arthritis. Arthritis Care Res (Hoboken) 2017;69:1789-98. 10.1002/acr.23229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Giles JT, Danielides S, Szklo M, et al. Insulin resistance in rheumatoid arthritis: disease-related indicators and associations with the presence and progression of subclinical atherosclerosis. Arthritis Rheumatol 2015;67:626-36. 10.1002/art.38986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kang Y, Park HJ, Kang MI, et al. Adipokines, inflammation, insulin resistance, and carotid atherosclerosis in patients with rheumatoid arthritis. Arthritis Res Ther 2013;15:R194. 10.1186/ar4384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Roubille C, Richer V, Starnino T, et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis 2015;74:480-9. 10.1136/annrheumdis-2014-206624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ronda N, Greco D, Adorni MP, et al. Newly identified antiatherosclerotic activity of methotrexate and adalimumab: complementary effects on lipoprotein function and macrophage cholesterol metabolism. Arthritis Rheumatol 2015;67:1155-64. 10.1002/art.39039 [DOI] [PubMed] [Google Scholar]
- 96. Reiss AB, Carsons SE, Anwar K, et al. Atheroprotective effects of methotrexate on reverse cholesterol transport proteins and foam cell transformation in human THP-1 monocyte/macrophages. Arthritis Rheum 2008;58:3675-83. 10.1002/art.24040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Thornton CC, Al-Rashed F, Calay D, et al. Methotrexate-mediated activation of an AMPK-CREB-dependent pathway: a novel mechanism for vascular protection in chronic systemic inflammation. Ann Rheum Dis 2016;75:439-48. 10.1136/annrheumdis-2014-206305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zimmerman MC, Clemens DL, Duryee MJ, et al. Direct antioxidant properties of methotrexate: Inhibition of malondialdehyde-acetaldehyde-protein adduct formation and superoxide scavenging. Redox Biol 2017;13:588-93. 10.1016/j.redox.2017.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Charles-Schoeman C, Wang X, Lee YY, et al. Association of Triple Therapy With Improvement in Cholesterol Profiles Over Two-Year Followup in the Treatment of Early Aggressive Rheumatoid Arthritis Trial. Arthritis Rheumatol 2016;68:577-86. 10.1002/art.39502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kerr G, Aujero M, Richards J, et al. Associations of hydroxychloroquine use with lipid profiles in rheumatoid arthritis: pharmacologic implications. Arthritis Care Res (Hoboken) 2014;66:1619-26. 10.1002/acr.22341 [DOI] [PubMed] [Google Scholar]
- 101. Morris SJ, Wasko MC, Antohe JL, et al. Hydroxychloroquine use associated with improvement in lipid profiles in rheumatoid arthritis patients. Arthritis Care Res (Hoboken) 2011;63:530-4. 10.1002/acr.20393 [DOI] [PubMed] [Google Scholar]
- 102. Sharma TS, Wasko MC, Tang X, et al. Hydroxychloroquine Use Is Associated With Decreased Incident Cardiovascular Events in Rheumatoid Arthritis Patients. J Am Heart Assoc 2016;5:e002867. 10.1161/JAHA.115.002867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Daïen CI, Fesler P, du Cailar G, et al. Etanercept normalises left ventricular mass in patients with rheumatoid arthritis. Ann Rheum Dis 2013;72:881-7. 10.1136/annrheumdis-2012-201489 [DOI] [PubMed] [Google Scholar]
- 104. Klarenbeek NB, van der Kooij SM, Huizinga TJ, et al. Blood pressure changes in patients with recent-onset rheumatoid arthritis treated with four different treatment strategies: a post hoc analysis from the BeSt trial. Ann Rheum Dis 2010;69:1342-5. 10.1136/ard.2009.124180 [DOI] [PubMed] [Google Scholar]
- 105. Angel K, Provan SA, Gulseth HL, Mowinckel P, Kvien TK, Atar D. Tumor necrosis factor-alpha antagonists improve aortic stiffness in patients with inflammatory arthropathies: a controlled study. Hypertension 2010;55:333-8. 10.1161/HYPERTENSIONAHA.109.143982 [DOI] [PubMed] [Google Scholar]
- 106. Daïen CI, Duny Y, Barnetche T, Daurès JP, Combe B, Morel J. Effect of TNF inhibitors on lipid profile in rheumatoid arthritis: a systematic review with meta-analysis. Ann Rheum Dis 2012;71:862-8. 10.1136/annrheumdis-2011-201148 [DOI] [PubMed] [Google Scholar]
- 107. Kirkham BW, Wasko MC, Hsia EC, et al. Effects of golimumab, an anti-tumour necrosis factor-α human monoclonal antibody, on lipids and markers of inflammation. Ann Rheum Dis 2014;73:161-9. 10.1136/annrheumdis-2012-202089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Popa C, van Tits LJ, Barrera P, et al. Anti-inflammatory therapy with tumour necrosis factor alpha inhibitors improves high-density lipoprotein cholesterol antioxidative capacity in rheumatoid arthritis patients. Ann Rheum Dis 2009;68:868-72. 10.1136/ard.2008.092171 [DOI] [PubMed] [Google Scholar]
- 109. Spinelli FR, Metere A, Barbati C, et al. Effect of therapeutic inhibition of TNF on circulating endothelial progenitor cells in patients with rheumatoid arthritis. Mediators Inflamm 2013;2013:537539. 10.1155/2013/537539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. O’Neill F, Charakida M, Topham E, et al. Anti-inflammatory treatment improves high-density lipoprotein function in rheumatoid arthritis. Heart 2017;103:766-73. 10.1136/heartjnl-2015-308953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Rao VU, Pavlov A, Klearman M, et al. An evaluation of risk factors for major adverse cardiovascular events during tocilizumab therapy. Arthritis Rheumatol 2015;67:372-80. 10.1002/art.38920 [DOI] [PubMed] [Google Scholar]
- 112. Kim SC, Solomon DH, Rogers JR, et al. Cardiovascular Safety of Tocilizumab Versus Tumor Necrosis Factor Inhibitors in Patients With Rheumatoid Arthritis: A Multi-Database Cohort Study. Arthritis Rheumatol 2017;69:1154-64. 10.1002/art.40084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Gabay C, McInnes IB, Kavanaugh A, et al. Comparison of lipid and lipid-associated cardiovascular risk marker changes after treatment with tocilizumab or adalimumab in patients with rheumatoid arthritis. Ann Rheum Dis 2016;75:1806-12. 10.1136/annrheumdis-2015-207872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. McInnes IB, Thompson L, Giles JT, et al. Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann Rheum Dis 2015;74:694-702. 10.1136/annrheumdis-2013-204345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Strang AC, Bisoendial RJ, Kootte RS, et al. Pro-atherogenic lipid changes and decreased hepatic LDL receptor expression by tocilizumab in rheumatoid arthritis. Atherosclerosis 2013;229:174-81. 10.1016/j.atherosclerosis.2013.04.031 [DOI] [PubMed] [Google Scholar]
- 116. Ruiz-Limón P, Ortega R, Arias de la Rosa I, et al. Tocilizumab improves the proatherothrombotic profile of rheumatoid arthritis patients modulating endothelial dysfunction, NETosis, and inflammation. Transl Res 2017;183:87-103. 10.1016/j.trsl.2016.12.003 [DOI] [PubMed] [Google Scholar]
- 117. Kume K, Amano K, Yamada S, Hatta K, Ohta H, Kuwaba N. Tocilizumab monotherapy reduces arterial stiffness as effectively as etanercept or adalimumab monotherapy in rheumatoid arthritis: an open-label randomized controlled trial. J Rheumatol 2011;38:2169-71. 10.3899/jrheum.110340 [DOI] [PubMed] [Google Scholar]
- 118. Hsue PY, Scherzer R, Grunfeld C, et al. Depletion of B-cells with rituximab improves endothelial function and reduces inflammation among individuals with rheumatoid arthritis. J Am Heart Assoc 2014;3:e001267. 10.1161/JAHA.114.001267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Raterman HG, Levels H, Voskuyl AE, Lems WF, Dijkmans BA, Nurmohamed MT. HDL protein composition alters from proatherogenic into less atherogenic and proinflammatory in rheumatoid arthritis patients responding to rituximab. Ann Rheum Dis 2013;72:560-5. 10.1136/annrheumdis-2011-201228 [DOI] [PubMed] [Google Scholar]
- 120. McInnes IB, Kim HY, Lee SH, et al. Open-label tofacitinib and double-blind atorvastatin in rheumatoid arthritis patients: a randomised study. Ann Rheum Dis 2014;73:124-31. 10.1136/annrheumdis-2012-202442 [DOI] [PubMed] [Google Scholar]
- 121. Zhang J, Xie F, Yun H, et al. Comparative effects of biologics on cardiovascular risk among older patients with rheumatoid arthritis. Ann Rheum Dis 2016;75:1813-8. 10.1136/annrheumdis-2015-207870 [DOI] [PubMed] [Google Scholar]
- 122. Lindhardsen J, Gislason GH, Jacobsen S, et al. Non-steroidal anti-inflammatory drugs and risk of cardiovascular disease in patients with rheumatoid arthritis: a nationwide cohort study. Ann Rheum Dis 2014;73:1515-21. 10.1136/annrheumdis-2012-203137 [DOI] [PubMed] [Google Scholar]
- 123. Nissen SE, Yeomans ND, Solomon DH, et al. PRECISION Trial Investigators Cardiovascular Safety of Celecoxib, Naproxen, or Ibuprofen for Arthritis. N Engl J Med 2016;375:2519-29. 10.1056/NEJMoa1611593 [DOI] [PubMed] [Google Scholar]
- 124. Cannon CP, Curtis SP, FitzGerald GA, et al. MEDAL Steering Committee Cardiovascular outcomes with etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the Multinational Etoricoxib and Diclofenac Arthritis Long-term (MEDAL) programme: a randomised comparison. Lancet 2006;368:1771-81. 10.1016/S0140-6736(06)69666-9 [DOI] [PubMed] [Google Scholar]
- 125. del Rincón I, Battafarano DF, Restrepo JF, Erikson JM, Escalante A. Glucocorticoid dose thresholds associated with all-cause and cardiovascular mortality in rheumatoid arthritis. Arthritis Rheumatol 2014;66:264-72. 10.1002/art.38210 [DOI] [PubMed] [Google Scholar]
- 126. Aviña-Zubieta JA, Abrahamowicz M, De Vera MA, et al. Immediate and past cumulative effects of oral glucocorticoids on the risk of acute myocardial infarction in rheumatoid arthritis: a population-based study. Rheumatology (Oxford) 2013;52:68-75. 10.1093/rheumatology/kes353 [DOI] [PubMed] [Google Scholar]
- 127. Ferraz-Amaro I, González-Gay MA, García-Dopico JA, Díaz-González F. Cholesteryl ester transfer protein in patients with rheumatoid arthritis. J Rheumatol 2013;40:1040-7. 10.3899/jrheum.121507 [DOI] [PubMed] [Google Scholar]
- 128. Davis LA, Cannon GW, Pointer LF, et al. Cardiovascular events are not associated with MTHFR polymorphisms, but are associated with methotrexate use and traditional risk factors in US veterans with rheumatoid arthritis. J Rheumatol 2013;40:809-17. 10.3899/jrheum.121012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Ajeganova S, de Faire U, Jogestrand T, Frostegård J, Hafström I. Carotid atherosclerosis, disease measures, oxidized low-density lipoproteins, and atheroprotective natural antibodies for cardiovascular disease in early rheumatoid arthritis -- an inception cohort study. J Rheumatol 2012;39:1146-54. 10.3899/jrheum.111334 [DOI] [PubMed] [Google Scholar]
- 130. Bernatsky S, Hudson M, Suissa S. Anti-rheumatic drug use and risk of hospitalization for congestive heart failure in rheumatoid arthritis. Rheumatology (Oxford) 2005;44:677-80. 10.1093/rheumatology/keh610 [DOI] [PubMed] [Google Scholar]
- 131. Wasko MC, Hubert HB, Lingala VB, et al. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA 2007;298:187-93. 10.1001/jama.298.2.187 [DOI] [PubMed] [Google Scholar]
- 132. Low AS, Symmons DP, Lunt M, et al. British Society for Rheumatology Biologics Register for Rheumatoid Arthritis (BSRBR-RA) and the BSRBR Control Centre Consortium Relationship between exposure to tumour necrosis factor inhibitor therapy and incidence and severity of myocardial infarction in patients with rheumatoid arthritis. Ann Rheum Dis 2017;76:654-60. 10.1136/annrheumdis-2016-209784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Ljung L, Rantapää-Dahlqvist S, Jacobsson LT, Askling J. Response to biological treatment and subsequent risk of coronary events in rheumatoid arthritis. Ann Rheum Dis 2016;75:2087-94. 10.1136/annrheumdis-2015-208995 [DOI] [PubMed] [Google Scholar]
- 134. Solomon DH, Curtis JR, Saag KG, et al. Cardiovascular risk in rheumatoid arthritis: comparing TNF-α blockade with nonbiologic DMARDs. Am J Med 2013;126:730.e9-17. 10.1016/j.amjmed.2013.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT, Anti-TNF Therapy Against Congestive Heart Failure Investigators Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation 2003;107:3133-40. 10.1161/01.CIR.0000077913.60364.D2 [DOI] [PubMed] [Google Scholar]
- 136. Mann DL, McMurray JJ, Packer M, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation 2004;109:1594-602. 10.1161/01.CIR.0000124490.27666.B2 [DOI] [PubMed] [Google Scholar]
- 137. Solomon DH, Rassen JA, Kuriya B, et al. Heart failure risk among patients with rheumatoid arthritis starting a TNF antagonist. Ann Rheum Dis 2013;72:1813-8. 10.1136/annrheumdis-2012-202136 [DOI] [PubMed] [Google Scholar]
- 138. Setoguchi S, Schneeweiss S, Avorn J, et al. Tumor necrosis factor-alpha antagonist use and heart failure in elderly patients with rheumatoid arthritis. Am Heart J 2008;156:336-41. 10.1016/j.ahj.2008.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Wasko MC, Hsia EC, Kirkham B, et al. Effect of golimumab on carotid atherosclerotic disease measures and cardiovascular events in inflammatory arthritides. J Clin Rheumatol 2014;20:1-10. 10.1097/RHU.0000000000000053 [DOI] [PubMed] [Google Scholar]
- 140. Jones G, Sebba A, Gu J, et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis 2010;69:88-96. 10.1136/ard.2008.105197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Genovese MC, McKay JD, Nasonov EL, et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum 2008;58:2968-80. 10.1002/art.23940 [DOI] [PubMed] [Google Scholar]
- 142. Emery P, Keystone E, Tony HP, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis 2008;67:1516-23. 10.1136/ard.2008.092932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Kremer JM, Blanco R, Brzosko M, et al. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate: results from the double-blind treatment phase of a randomized placebo-controlled trial of tocilizumab safety and prevention of structural joint damage at one year. Arthritis Rheum 2011;63:609-21. 10.1002/art.30158 [DOI] [PubMed] [Google Scholar]
- 144. Charles-Schoeman C, Gonzalez-Gay MA, Kaplan I, et al. Effects of tofacitinib and other DMARDs on lipid profiles in rheumatoid arthritis: implications for the rheumatologist. Semin Arthritis Rheum 2016;46:71-80. 10.1016/j.semarthrit.2016.03.004 [DOI] [PubMed] [Google Scholar]
- 145. Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ 2011;342:c7086. 10.1136/bmj.c7086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Davis JM, 3rd, Maradit Kremers H, Crowson CS, et al. Glucocorticoids and cardiovascular events in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum 2007;56:820-30. 10.1002/art.22418 [DOI] [PubMed] [Google Scholar]
- 147. Arts EE, Popa C, Den Broeder AA, et al. Performance of four current risk algorithms in predicting cardiovascular events in patients with early rheumatoid arthritis. Ann Rheum Dis 2015;74:668-74. 10.1136/annrheumdis-2013-204024 [DOI] [PubMed] [Google Scholar]
- 148. Crowson CS, Matteson EL, Roger VL, Therneau TM, Gabriel SE. Usefulness of risk scores to estimate the risk of cardiovascular disease in patients with rheumatoid arthritis. Am J Cardiol 2012;110:420-4. 10.1016/j.amjcard.2012.03.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Kawai VK, Chung CP, Solus JF, Oeser A, Raggi P, Stein CM. The ability of the 2013 American College of Cardiology/American Heart Association cardiovascular risk score to identify rheumatoid arthritis patients with high coronary artery calcification scores. Arthritis Rheumatol 2015;67:381-5. 10.1002/art.38944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Solomon DH, Greenberg J, Curtis JR, et al. Derivation and internal validation of an expanded cardiovascular risk prediction score for rheumatoid arthritis: a Consortium of Rheumatology Researchers of North America Registry Study. Arthritis Rheumatol 2015;67:1995-2003. 10.1002/art.39195 [DOI] [PubMed] [Google Scholar]
- 151. Crowson CS, Rollefstad S, Kitas GD, van Riel PL, Gabriel SE, Semb AG, A Trans-Atlantic Cardiovascular Risk Consortium for Rheumatoid Arthritis (ATACC-RA) Challenges of developing a cardiovascular risk calculator for patients with rheumatoid arthritis. PLoS One 2017;12:e0174656. 10.1371/journal.pone.0174656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Peters MJ, Symmons DP, McCarey D, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis 2010;69:325-31. 10.1136/ard.2009.113696 [DOI] [PubMed] [Google Scholar]
- 153. Crowson CS, Gabriel SE, Semb AG, et al. Trans-Atlantic Cardiovascular Consortium for Rheumatoid Arthritis Rheumatoid arthritis-specific cardiovascular risk scores are not superior to general risk scores: a validation analysis of patients from seven countries. Rheumatology (Oxford) 2017;56:1102-10. 10.1093/rheumatology/kex038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Barber CE, Esdaile JM, Martin LO, et al. Gaps in Addressing Cardiovascular Risk in Rheumatoid Arthritis: Assessing Performance Using Cardiovascular Quality Indicators. J Rheumatol 2016;43:1965-73. 10.3899/jrheum.160241 [DOI] [PubMed] [Google Scholar]
- 155. Akkara Veetil BM, Myasoedova E, Matteson EL, Gabriel SE, Crowson CS. Use of lipid-lowering agents in rheumatoid arthritis: a population-based cohort study. J Rheumatol 2013;40:1082-8. 10.3899/jrheum.121302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Curtis JR, John A, Baser O. Dyslipidemia and changes in lipid profiles associated with rheumatoid arthritis and initiation of anti-tumor necrosis factor therapy. Arthritis Care Res (Hoboken) 2012;64:1282-91. 10.1002/acr.21693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Bartels CM, Kind AJ, Everett C, Mell M, McBride P, Smith M. Low frequency of primary lipid screening among medicare patients with rheumatoid arthritis. Arthritis Rheum 2011;63:1221-30. 10.1002/art.30239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Toms TE, Panoulas VF, Douglas KM, et al. Statin use in rheumatoid arthritis in relation to actual cardiovascular risk: evidence for substantial undertreatment of lipid-associated cardiovascular risk? Ann Rheum Dis 2010;69:683-8. 10.1136/ard.2009.115717 [DOI] [PubMed] [Google Scholar]
- 159. Gyberg V, De Bacquer D, De Backer G, et al. EUROASPIRE Investigators Patients with coronary artery disease and diabetes need improved management: a report from the EUROASPIRE IV survey: a registry from the EuroObservational Research Programme of the European Society of Cardiology. Cardiovasc Diabetol 2015;14:133. 10.1186/s12933-015-0296-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Semb AG, Kvien TK, DeMicco DA, et al. Effect of intensive lipid-lowering therapy on cardiovascular outcome in patients with and those without inflammatory joint disease. Arthritis Rheum 2012;64:2836-46. 10.1002/art.34524 [DOI] [PubMed] [Google Scholar]
- 161. De Vera MA, Choi H, Abrahamowicz M, Kopec J, Goycochea-Robles MV, Lacaille D. Statin discontinuation and risk of acute myocardial infarction in patients with rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis 2011;70:1020-4. 10.1136/ard.2010.142455 [DOI] [PubMed] [Google Scholar]
- 162. De Vera MA, Choi H, Abrahamowicz M, Kopec J, Lacaille D. Impact of statin discontinuation on mortality in patients with rheumatoid arthritis: a population-based study. Arthritis Care Res (Hoboken) 2012;64:809-16. 10.1002/acr.21643 [DOI] [PubMed] [Google Scholar]
- 163. Ikdahl E, Rollefstad S, Hisdal J, et al. Sustained Improvement of Arterial Stiffness and Blood Pressure after Long-Term Rosuvastatin Treatment in Patients with Inflammatory Joint Diseases: Results from the RORA-AS Study. PLoS One 2016;11:e0153440. 10.1371/journal.pone.0153440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Rollefstad S, Ikdahl E, Hisdal J, et al. Rosuvastatin-Induced Carotid Plaque Regression in Patients With Inflammatory Joint Diseases: The Rosuvastatin in Rheumatoid Arthritis, Ankylosing Spondylitis and Other Inflammatory Joint Diseases Study. Arthritis Rheumatol 2015;67:1718-28. 10.1002/art.39114 [DOI] [PubMed] [Google Scholar]
- 165. Haruna Y, Morita Y, Yada T, Satoh M, Fox DA, Kashihara N. Fluvastatin reverses endothelial dysfunction and increased vascular oxidative stress in rat adjuvant-induced arthritis. Arthritis Rheum 2007;56:1827-35. 10.1002/art.22632 [DOI] [PubMed] [Google Scholar]
- 166. El-Barbary AM, Hussein MS, Rageh EM, Hamouda HE, Wagih AA, Ismail RG. Effect of atorvastatin on inflammation and modification of vascular risk factors in rheumatoid arthritis. J Rheumatol 2011;38:229-35. 10.3899/jrheum.100582 [DOI] [PubMed] [Google Scholar]
- 167. Khoja SS, Almeida GJ, Chester Wasko M, Terhorst L, Piva SR. Association of Light-Intensity Physical Activity With Lower Cardiovascular Disease Risk Burden in Rheumatoid Arthritis. Arthritis Care Res (Hoboken) 2016;68:424-31. 10.1002/acr.22711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Metsios GS, Stavropoulos-Kalinoglou A, Veldhuijzen van Zanten JJ, et al. Individualised exercise improves endothelial function in patients with rheumatoid arthritis. Ann Rheum Dis 2014;73:748-51. 10.1136/annrheumdis-2013-203291 [DOI] [PubMed] [Google Scholar]
- 169. Stavropoulos-Kalinoglou A, Metsios GS, Veldhuijzen van Zanten JJ, Nightingale P, Kitas GD, Koutedakis Y. Individualised aerobic and resistance exercise training improves cardiorespiratory fitness and reduces cardiovascular risk in patients with rheumatoid arthritis. Ann Rheum Dis 2013;72:1819-25. 10.1136/annrheumdis-2012-202075 [DOI] [PubMed] [Google Scholar]
- 170. de Jong Z, Munneke M, Zwinderman AH, et al. Is a long-term high-intensity exercise program effective and safe in patients with rheumatoid arthritis? Results of a randomized controlled trial. Arthritis Rheum 2003;48:2415-24. 10.1002/art.11216 [DOI] [PubMed] [Google Scholar]
- 171. Sokolove J, Wagner CA, Lahey LJ, et al. Increased inflammation and disease activity among current cigarette smokers with rheumatoid arthritis: a cross-sectional analysis of US veterans. Rheumatology (Oxford) 2016;55:1969-77. 10.1093/rheumatology/kew285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Fisher MC, Hochberg MC, El-Taha M, Kremer JM, Peng C, Greenberg JD, CORRONA Investigators Smoking, smoking cessation, and disease activity in a large cohort of patients with rheumatoid arthritis. J Rheumatol 2012;39:904-9. 10.3899/jrheum.110852 [DOI] [PubMed] [Google Scholar]
- 173. England BR, Sayles H, Michaud K, et al. Cause-Specific Mortality in Male US Veterans With Rheumatoid Arthritis. Arthritis Care Res (Hoboken) 2016;68:36-45. 10.1002/acr.22642 [DOI] [PubMed] [Google Scholar]
- 174. Provan SA, Semb AG, Hisdal J, et al. Remission is the goal for cardiovascular risk management in patients with rheumatoid arthritis: a cross-sectional comparative study. Ann Rheum Dis 2011;70:812-7. 10.1136/ard.2010.141523 [DOI] [PubMed] [Google Scholar]
- 175. Svensson AL, Christensen R, Persson F, et al. Multifactorial intervention to prevent cardiovascular disease in patients with early rheumatoid arthritis: protocol for a multicentre randomised controlled trial. BMJ Open 2016;6:e009134. 10.1136/bmjopen-2015-009134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Everett BM, Pradhan AD, Solomon DH, et al. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J 2013;166:199-207.e15. 10.1016/j.ahj.2013.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Ridker PM, Everett BM, Thuren T, et al. CANTOS Trial Group Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med 2017;377:1119-31. 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- 178. Agca R, Heslinga SC, Rollefstad S, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis 2017;76:17-28. 10.1136/annrheumdis-2016-209775 [DOI] [PubMed] [Google Scholar]