Enzymatic vitreolysis with ocriplasmin is more effective in patients with focal vitreomacular adhesion without epiretinal membrane and macular hole ≤400 µm (if present) at baseline. This study shows that the treatment outcomes are related to patient selection.
Key words: ocriplasmin, vitreomacular traction, macular hole, best-corrected visual acuity, vitreolysis, focal vitreomacular adhesion
Abstract
Purpose:
To evaluate the anatomical and functional outcomes with ocriplasmin in patients with vitreomacular traction (VMT) with or without macular hole (MH).
Methods:
In a Phase 4, multicenter, single-arm, open-label study, eligible patients (VMT with focal adhesion, without epiretinal membrane, and with MH ≤400 µm [if present]) received a single intravitreal injection of ocriplasmin. Nonsurgical resolution of VMT (Day 28 [primary endpoint]), best-corrected visual acuity, MH closure, vitrectomy rate, and safety were assessed through Day 180.
Results:
Overall, 466 patients were included in the full analysis set, of whom 47.4% had VMT resolution by Day 28; resolution rates in patients with VMT without MH, VMT with MH ≤250 µm, and VMT with MH >250 to ≤400 µm were 43.4%, 68.6%, and 62.7%, respectively. Macular hole closure was higher in eyes with VMT and MH ≤250 µm (57.1%) than in eyes with VMT and MH >250 to ≤400 µm (27.5%) at Day 28. Overall, 30.8% of patients with VMT resolution gained ≥10 letters in best-corrected visual acuity at Day 180. Adverse events were consistent with the known safety profile of ocriplasmin.
Conclusion:
Ocriplasmin is effective for resolution of VMT without or with MH (≤400 μm); treatment outcomes can be optimized with patient selection.
Incomplete posterior vitreous detachment and a strong and persistent adhesion of the vitreous to the macula, especially on the fovea, can exert traction, which can lead to retinal distortion and dysfunction known as vitreomacular traction (VMT). Vitreomacular traction typically manifests with decreased visual acuity, metamorphopsia, blurred vision, micropsia, photopsia, and various other ocular symptoms.1–3 Focal VMT can lead to macular holes (MHs).4 Vitreomacular traction and MHs can have a significant negative impact on patients' quality of life and their ability to perform daily tasks.5–7
Patients with VMT with or without MH have different severity of ocular symptoms, disease progression, and prognosis. The incidence and degree of metamorphopsia is reported to be higher in patients with MH and VMT than in those with VMT alone.4,8 According to the National Institute for Health and Care Excellence (NICE), United Kingdom, the presence of metamorphopsia is associated with a burden on the quality of life of patients equivalent to a 2-line decrease in visual acuity.4 Patients with VMT, especially if focal and without noticeable epiretinal membrane (ERM), are usually observed for at least 3 months before any intervention to allow for spontaneous resolution.3,9 The spontaneous resolution rate of VMT reported in studies varies depending on the examination technique, review period, and sample size.10–15 In studies using a high-resolution spectral domain optical coherence tomography (SD-OCT) technique and with a relatively large sample size (n > 100 eyes), the reported rate of spontaneous VMT resolution was 21.4% to 32.0%, with a mean follow-up period of 1 to 2 years.4,16–18 The reported spontaneous resolution rate of MH with VMT in general is lower (0 to 10%).4,19,20 Untreated MH has a poor prognosis, and approximately 75% of MHs ≤400 µm in diameter enlarge further.21–24
Pharmacological vitreolysis is a nonsurgical therapeutic approach for the treatment of VMT without or with an MH (≤400 μm).25,26 Ocriplasmin is a recombinant truncated form of human plasmin having proteolytic activity against protein components of the vitreoretinal interface (e.g., laminin, fibronectin, collagen, and others), thereby dissolving the protein matrix mediating vitreomacular adhesion (VMA).27 The efficacy and safety of ocriplasmin in the treatment of patients with VMA have been well established in clinical studies; ocriplasmin was shown to provide higher nonsurgical VMA resolution as compared to sham and placebo.28,29
This study assessed the anatomical and functional outcomes of ocriplasmin in a selective patient population diagnosed with VMT with the evidence of focal VMA (≤1,500 μm) visible on SD-OCT and absence of ERM over the central macula, when associated with MHs ≤400 μm. Ocriplasmin for VMT Intravitreal Injection Decisions (OVIID-1) is the first study to specifically investigate treatment outcomes in predefined subgroups of patients with VMT without MH, VMT with MH ≤250 µm, and VMT with MH >250 to ≤400 µm at baseline.
Methods
Study Design
OVIID-1 was a Phase 4, multicenter, prospective, single-arm, open-label interventional study conducted from April 2014 to September 2015 across 87 study sites in Europe (Italy, the Netherlands, Poland, Portugal, Spain, United Kingdom, Belgium, France, Germany, and Hungary) and Canada. This study was designed to assess the anatomical and functional outcomes of ocriplasmin treatment in patients with VMT with or without MH over a 6-month period. The study is registered at ClinicalTrials.gov (NCT02035748).
The study consisted of six visits that included a screening visit (baseline; 7–3 days before injection to assess patients' eligibility to participate in the study), an injection visit (Day 0), and four postinjection visits (Days 7, 28, 90, and 180). Unscheduled visits were at the discretion of the investigator. For each patient, only one eye (the eye with the greatest potential to benefit from the intervention in the opinion of the investigator) was included. Trained readers at a central reading center (CRC [Duke University OCT Reading Center, Durham, NC]) evaluated the SD-OCT images and approved the patient's eligibility for participation in the study. Eligible patients received a single intravitreal injection of ocriplasmin 0.125 mg in 0.1 mL volume as per the country's product label. The injection was prepared and administered as described in the label.30,31
All patients provided written informed consent before enrolling into the study. The study protocol was approved by the institutional review board of each participating center. The trial was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice (GCP) and in compliance with all federal, local, or regional requirements.
Inclusion and Exclusion Criteria
Male or female patients, aged 18 years or older, diagnosed with VMT with or without MH, with evidence of focal VMA, and with no ERM over the central macula visible on SD-OCT (evaluated by the CRC) were eligible. Patients with the following conditions were excluded from the study: an active or suspected intraocular or periocular infection; the presence of ERM over the macula or broad VMT/VMA >1,500 μm at baseline or MH >400 μm diameter in the study eye (evaluated by the CRC); a history of vitrectomy or retinal detachment or laser photocoagulation in the study eye; pseudoexfoliation or Marfan syndrome or phacodonesis or any other finding that in the investigator's opinion suggests lens/zonular instability; recent ocular surgery or ocular injection within the past 3 months; comorbid conditions such as aphakia, proliferative diabetic retinopathy, ischemic retinopathies, retinal vein occlusions, exudative age-related macular degeneration, or vitreous hemorrhage; or hypersensitivity to ocriplasmin or any of the excipients. Female patients who were breastfeeding; were pregnant; had a positive urine pregnancy test at screening; or intended to become pregnant and were not willing to use adequate birth control methods for the duration of the study were also excluded.
Study Endpoints
Efficacy
The primary endpoint of this study was the proportion of patients with nonsurgical resolution of VMT at Day 28, as determined by the CRC. The assessment of resolution of VMT was based on the anatomical resolution of VMT only, that is, no resolution of the related symptoms was considered. The secondary endpoints were as follows: proportion of patients with nonsurgical resolution of VMT at Days 90 and 180; nonsurgical changes in best-corrected visual acuity (BCVA) at a distance compared with baseline at Days 28, 90, and 180; proportion of patients with nonsurgical closure of MH at Days 28, 90, and 180 (if present at baseline); proportion of patients experiencing pars plana vitrectomy at Day 180; and change in central foveal thickness at Days 28 and 180 compared with baseline.
Safety
Adverse events (AEs) were recorded throughout the study. In addition, routine ocular examinations, which included intraocular pressure (IOP), biomicroscopy/slit-lamp parameters, and dilated fundus parameters, were also measured.
Exploratory analyses
These included nonsurgical change from baseline in subretinal fluid development and resolution; proportion of patients with change in metamorphopsia assessed by the Amsler grid at Days 28, 90, and 180 compared with baseline; and metamorphopsia questionnaire at Days 28, 90, and 180 compared with baseline.
Assessments
Spectral domain OCT and BCVA measurements were performed at baseline and all postinjection visits, except on the day of injection (Visit 1/Day 0). Trained readers at a CRC evaluated the SD-OCT images obtained from a Heidelberg Spectralis SD-OCT (Heidelberg Engineering Inc, Heidelberg, Germany) or a Zeiss Cirrus high definition (HD)-OCT (Carl Zeiss Meditec AG, Oberkochen, Germany) instrument. The same OCT instrument was used for examinations throughout the study. All scans were deidentified. According to the protocol, if subjects underwent vitrectomy after Day 28, they were categorized as “no non-surgical resolution” from the time of vitrectomy (irrespective of their actual status). Best-corrected visual acuity was reported as the number of letters correctly read by the patient on an Early Treatment of Diabetic Retinopathy Study chart at 4 m. Best-corrected visual acuity testing preceded IOP measurement, the administration of eye drops to dilate or anesthetize the eye, or any examination requiring contact with the eye. The Amsler grid testing was performed at baseline and all postinjection visits, except on the day of injection (Visit 1/Day 0), to assess metamorphopsia and monitor the central visual field. Eyes were tested one at a time beginning with the right eye. The presence and/or extent of metamorphopsia was recorded at baseline and postinjection visits (Days 28, 90, and 180) using a specific metamorphopsia questionnaire provided to patients before any examination(s) and pupil dilation.
All reported AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA version 16.0) and were recorded separately for systemic and ocular events (by the study eye/fellow eye) and by severity (mild, moderate, or severe), relationship to the study drug or injection procedure, and the onset after injection. Intraocular pressure was measured by applanation tonometry at baseline, Visit 1 (within 30 minutes after the study injection), Visit 5 (Day 180), and at unscheduled visits. To minimize bias, all IOP measurements were conducted by the same operator using the same tonometer. The slit-lamp examination included evaluation of the lids, tear meniscus, cornea, iris, lens, presence or absence of phacodonesis, anterior chamber cells, and anterior chamber flare. The fundus examination included evaluation of the peripheral retina, macula, and optic nerve. As part of the fundus examination, an evaluation of the retina was performed for retinal tear/detachment, retinal hemorrhage, vitreal hemorrhage density, and vitreal cells. Slit-lamp biomicroscopy and fundus examination were performed at baseline and all postinjection visits, except on the day of injection (Visit 1/Day 0), and preceded the IOP measurement.
Statistical Analysis
With a sample size of 400 evaluable patients, the 2-sided 95% confidence interval (CI) for a single proportion using the large sample normal approximation extended ± 0.048 from the observed proportion for an expected proportion of 0.380. There was no formal statistical hypothesis; the efficacy and safety data were analyzed descriptively. For the efficacy endpoints, the number and percentage of patients and exact 2-sided 95% CIs of the proportion were calculated. Results were analyzed for the overall group and categorized by subgroups (VMT without MH, VMT with MH ≤250 μm, and VMT with MH >250 to ≤400 μm). The last observation carried forward approach was used. The efficacy analysis was performed on the full analysis set (FAS) that included all the patients who received treatment and had at least one posttreatment measurement of SD-OCT.
Safety was assessed for the safety analysis set that included all patients who received the study treatment. All statistical analyses were performed using SAS software (SAS Institute, Cary, NC).
Results
Patient Demographics and Baseline Characteristics
In total, 628 patients were enrolled; 140 patients failed screening, most commonly due to the presence of ERM over the macula at baseline (n = 54). The other reasons for screen failure were failure to meet inclusion criteria of focal VMT visible on SD-OCT (n = 43), broad VMT >1,500 µm at baseline (n = 24), and MH >400 µm (n = 24). Twenty patients discontinued before any treatment (physician decision, n = 1; withdrawal by subject, n = 18; and other, n = 1). Hence, 468 patients were treated and 448 completed the study. Primary reasons for withdrawal were AEs (1.1%), death (0.6%), lost to follow-up (0.4%), physician's decision (0.6%), progressive disease (0.2%), and withdrawal by patient (1.3%). Adverse events associated with 5 (1.1%) eyes that led to withdrawal were MH, bronchial carcinoma, esophageal carcinoma, syncope, and cerebrovascular accident. Three patients died due to sepsis, metastatic breast cancer, and intestinal obstruction; and none of these were related to the study drug. Furthermore, two more patients were excluded from the FAS due to protocol deviation. In total, 466 patients were included in the FAS and 468 were included in the safety analysis set.
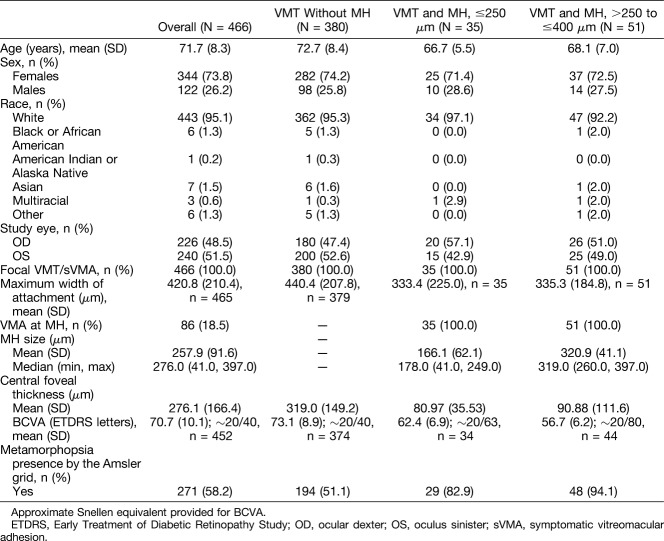
The demographic and baseline characteristics of patients, by overall cohort and subgroups, are shown in Table 1. The mean age of the study cohort was 71.7 years, and 73.8% of the patients were females (Table 1). All patients had focal VMA and no ERM over the macula with or without MH. Of the 466 patients (466 eyes), 380 (81.5%) had VMT without MH, 35 patients (7.5%) had VMT and MH ≤250 μm, and 51 patients (10.9%) had VMT and MH >250 to ≤400 μm at baseline. The mean diameter of the MH at baseline was 166 μm (range, 41 − 249 μm) in eyes with MH ≤250 μm and 321 μm (range, 260 − 397 μm) in eyes with MH >250 to ≤400 μm. The mean BCVA at baseline was 73.1 letters (∼20/40) in VMT patients without an MH, 62.4 letters (∼20/63) in the MH ≤250 μm group, and 56.7 letters (∼20/80) in the MH >250 to ≤400 μm group. Metamorphopsia presence by the Amsler grid was higher in patients with an MH (82.9% in the MH ≤250 μm group and 94.1% in the MH >250 to ≤400 μm group) than in those without an MH (51.1%; Table 1).
Table 1.
Baseline and Demographic Characteristics (FAS)
Efficacy and Exploratory Outcomes (Overall Study Cohort)
In all, 47.4% (n = 221 of 466) of patients had nonsurgical VMT resolution at Day 28 (primary endpoint; Figure 1). Analysis of the proportion of all patients achieving nonsurgical resolution of VMT at any time during the study demonstrated that 39.3% of the patients achieved resolution within the first 7 days after injection. Between Days 28 and 180, 9 additional patients had VMT resolution, and the overall resolution rate was 49.4% at Day 180. The mean (SD) gains in BCVA from baseline to Days 28, 90, and 180 were 1.7 (6.70) letters (95% CI: 1.1–2.3), 3.0 (7.07) letters (95% CI: 2.4–3.7), and 3.5 (7.77) letters (95% CI: 2.8–4.3), respectively (Figure 2). A higher proportion of patients with VMT resolution had a gain of at least ≥5 letters in BCVA at Day 28 (38.9 vs. 23.2%) and Day 180 (52.6 vs. 29.9%) compared with those without VMT resolution. Similarly, a higher proportion of patients with VMT resolution had a gain of ≥10 letters (i.e., ≥2-line improvement) in BCVA at Day 28 (17.5 vs. 8.9%) and Day 180 (30.8 vs. 9.7%). Nonsurgical closure of MH was observed in 39.5% of the patients at Day 28, and the proportions increased to 40.7% and 41.9% at Days 90 and 180, respectively (Figure 3). In total, 12.0% (n = 56) of patients underwent vitrectomy by Day 180. The mean change in central foveal thickness was −43.2 μm at Day 28 and −45.4 μm at Day 180 from a baseline value of 276.1 μm in the overall study cohort. Overall, there was a decrease in subretinal fluid thickness at Days 28, 90, and 180 with a mean (SD) change from baseline of −45.2 (119.8) µm, −48.2 (117.3) µm, and −52.8 (125.6) µm, respectively. Based on the Amsler grid assessment, there was a decrease in the proportion of patients with metamorphopsia from baseline to Day 180 (58.2 vs. 42.1%). There was an increase in the number of patients reporting no difficulties in daily activities from baseline to Day 180 (52.1 vs. 71.2%) in the metamorphopsia questionnaire. Overall, the patients reported less distortions in visual parameters (lines of crosswalk, steps of an overpass, curtain rails, window frames, bookshelves, tiles of a bathroom wall, people's faces, etc.) at Day 180 compared with baseline.
Fig. 1.
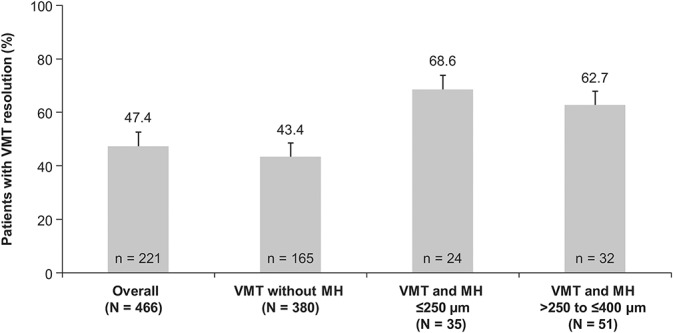
Proportion of patients with nonsurgical resolution of VMT at Day 28 by overall and subgroups (FAS). *Error bars represent 95% CI: missing data imputed using the last observation carried forward method; patients who had vitrectomy after VMT resolution were considered as “no VMT resolution.”
Fig. 2.
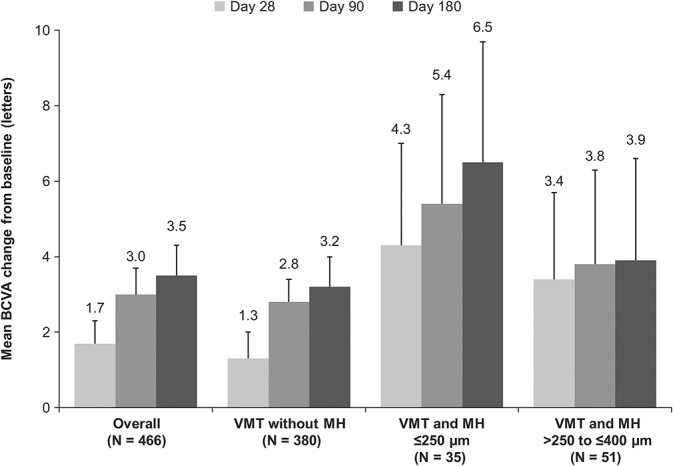
Mean change from baseline in BCVA for the study eye with ocriplasmin treatment by time points, overall, and subgroups (FAS). *Error bars represent 95% CI: missing data imputed using the last observation carried forward method; BCVA values after a vitrectomy are imputed with the last missing value before the vitrectomy.
Fig. 3.
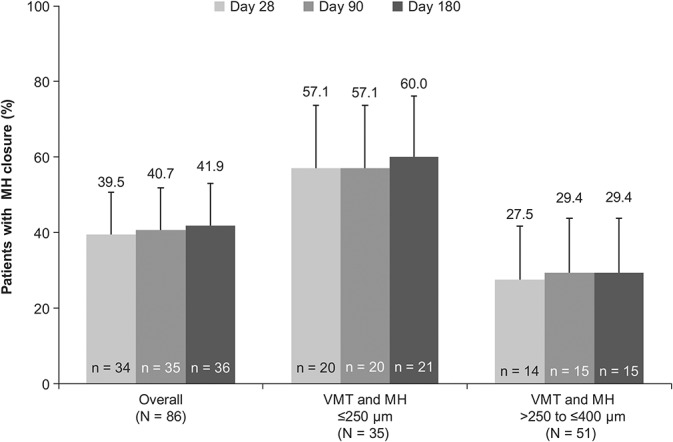
Proportion of patients with nonsurgical closure of MH by overall and subgroups (FAS). *Error bars represent 95% CI: missing data imputed using the last observation carried forward method; patients who had vitrectomy after MH closure were considered as “no MH closure” at the time point of vitrectomy.
Efficacy and Exploratory Outcomes Based on Subgroup Analysis
Vitreomacular traction without macular hole group
In these patients, the rates of nonsurgical VMT resolution were 43.4%, 46.3%, and 48.4% at Days 28, 90, and 180, respectively (Figure 1). This subgroup achieved a mean (SD) BCVA improvement of 1.3 (6.4) letters (95% CI: 0.6–2.0), 2.8 (6.8) letters (95% CI: 2.1–3.4), and 3.2 (7.5) letters (95% CI: 2.5–4.0) at Days 28, 90, and 180, respectively (Figure 2). The vitrectomy rate was 6.6% (n = 25) at Day 180. The mean change in central foveal thickness was −50.1 μm at Day 28 and −56.1 μm at Day 180 from a baseline value of 319.0 µm. In this group, the reading center found subretinal fluid at baseline in 81 of 380 patients with mean (SD) value of 168.3 (184.7) µm. The subretinal fluid thickness increased in some eyes, but overall, it was decreased at Days 28, 90, and 180 with a mean (SD) change from baseline of −95.4 (159.4) µm, −111.7 (163.9) µm, and −116.8 (172.2) µm, respectively. A similar trend was observed in the analyses of the overall group and other subgroups. The proportion of patients reporting metamorphopsia presence by the Amsler grid had decreased at Day 180 (33.4 vs. 51.1%) as compared to baseline.
Vitreomacular traction with macular hole ≤250 μm
The highest VMT resolution rate was observed in this subgroup, and 68.6% of the patients achieved nonsurgical VMT resolution at Day 28 (Figure 1). The proportion of patients achieving nonsurgical VMT resolution was 65.7% (n = 23) at Day 90 and 62.9% (n = 22) at Day 180. In the groups with an MH present at baseline, there were few patients who required vitrectomy for clinical reasons after Day 28. As per protocol, those patients were categorized as “no non-surgical resolution” from the time of vitrectomy. This led to the apparent decrease in the rate of nonsurgical VMT resolution in the MH group at Days 90 and 180. Compared with the overall group, the mean (SD) BCVA improvement was numerically higher in these patients: 4.3 (7.8) letters (95% CI: 1.5–7.0), 5.4 (8.2) letters (95% CI: 2.5–8.3), and 6.5 (9.0) letters (95% CI: 3.3–9.7) at Days 28, 90, and 180, respectively (Figure 2). In all, 57.1% of the eyes also achieved nonsurgical closure of MH by Day 28 with ocriplasmin treatment. The MH closure rates by Days 90 and 180 were 57.1% and 60%, respectively (Figure 3). In this subgroup, 28.6% of the patients had undergone vitrectomy by Day 180. The mean change in central foveal thickness was +9.9 μm at Day 28 and +30.3 μm at Day 180 from a baseline value of 80.97 µm. Compared with baseline, there was a small decrease in the proportion of patients with metamorphopsia presence by the Amsler grid at Day 180 (82.9 vs. 77.1%).
Vitreomacular traction with macular hole >250 μm to ≤400 μm
In all, 62.7% (n = 32) of the patients achieved nonsurgical VMT resolution at Day 28 in this subgroup (Figure 1). However, the proportion was decreased by Days 90 and 180, with a resolution rate of 47.1% (n = 24) on both days. Patients who required vitrectomy after Day 28 were categorized as “no non-surgical resolution” from the time of vitrectomy. This led to the apparent decrease in the rate of nonsurgical VMT resolution in the MH group at Days 90 and 180. These patients had a mean (SD) change in BCVA of 3.4 (7.4) letters (95% CI: 1.1–5.7), 3.8 (8.1) letters (95% CI: 1.3–6.3), and 3.9 (8.8) letters (95% CI: 1.2–6.6) at Days 28, 90, and 180, respectively (Figure 2). The nonsurgical MH closure was observed in 27.5% of the patients at Day 28; there was a slight improvement in the closure rates by Days 90 (29.4%) and 180 (29.4%; Figure 3). Vitrectomy rate (41.2%) was highest in this subgroup of patients by Day 180. The mean change in central foveal thickness was −28.2 μm at Day 28 and −17.5 μm at Day 180 from a baseline value of 90.88 μm. There was also a decrease in the proportion of patients with metamorphopsia presence by the Amsler grid at Day 180 compared with baseline (82.4 vs. 94.1%).
Safety Outcomes
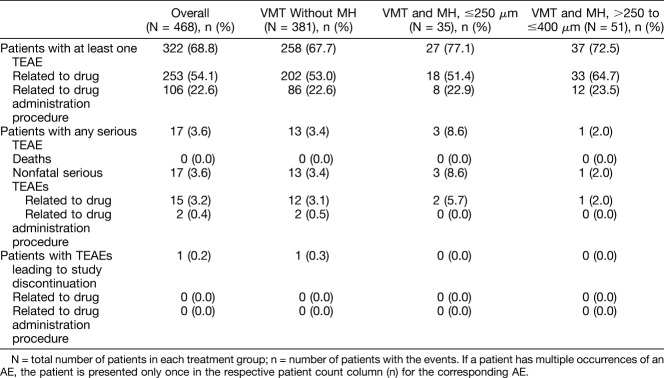
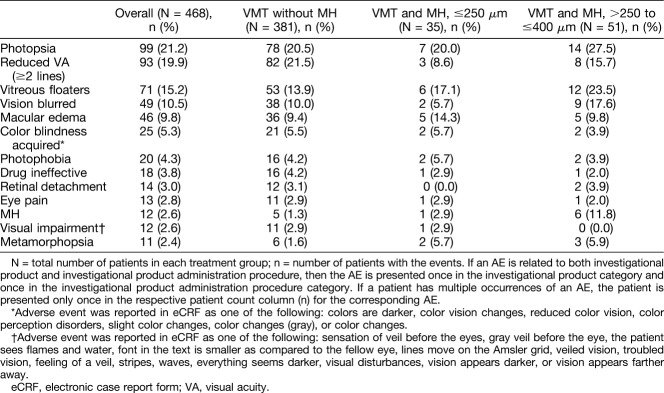
In total, 322 of the 468 patients (68.8%) who received treatment, experienced at least one ocular treatment-emergent AE (TEAE) in the study eye. The overall summary of ocular TEAEs is shown in Table 2. The most commonly reported ocular TEAEs (incidence ≥5%) in the study eyes were reduced visual acuity (≥2 lines, 26.9%), photopsia (22.0%), vitreous floaters (16.2%), vision blurred (11.5%), macular edema (11.1%), eye pain (8.5%), color blindness acquired (5.6%), MH (5.1%), and photophobia (5.1%). Of these events, eye pain was more likely to be associated with the administration procedure than the drug. Most of the AEs were mild and were predominately reported in the first 7 days. In total, 126 patients (26.9%) experienced at least one nonocular TEAE, these were generally not related to treatment. The commonly reported nonocular TEAEs were headache (3.2%) and nasopharyngitis (2.4%). In 253 patients (54.1%), the ocular TEAEs were considered to be related to the study drug (adverse drug reactions). The most commonly reported adverse drug reactions (incidence, ≥ 5%) in the study eyes included photopsia, reduced visual acuity (≥2 lines), vitreous floaters, vision blurred, macular edema, and color blindness acquired (Table 3).
Table 2.
Summary of Ocular TEAEs for the Study Eye (Safety Set)
Table 3.
Adverse Drug Reactions (≥2% in Any of the Groups) for the Study Eye (Safety Set)
No cases of intraocular infections, including endophthalmitis, were reported. In total, 18 ocular serious AEs (SAEs) were reported by 17 patients in the study eye; in 15 patients (3.2%), the SAE was considered to be related to the study drug and included an MH (1.3%), reduced visual acuity (≥2 lines, 1.1%), retinal detachment (0.9%), vitreous adhesions (0.4%), and ciliary zonular dehiscence (0.2%). Nonocular SAEs were reported in 28 patients, including 4 deaths; none of the nonocular SAEs were considered to be related to the treatment.
There were six events reported for dyschromatopsia (all in the VMT without the MH group), of which, five were in the study eye (1.1%). In addition, there were 26 patients with events for color blindness acquired (5.6%). However, these events were mild and described primarily as changes in color vision; all but four of these events (chromatopsia in one patient and color blindness acquired in three patients) had resolved without treatment by the end of the study. Most of the AEs for reduced visual acuity (≥2 lines) in the study eye were assessed as drug-related (19.9%), but most of these events resolved during the course of the study. In all, 86 patients had a decrease of ≥2 lines in BCVA, and in 73 patients, the decrease was within the first 7 days after treatment. By Day 180, BCVA had recovered to a <2-line loss in 79 patients. Late decrease in BCVA (after Day 7) was seen in 13 patients (2.8%). In 6 patients, BCVA recovered to <2-line loss, whereas 7 patients had persistent reductions in BCVA at Day 180. Of these seven subjects, as reported by investigators, four had confounding AEs (retinal pigment epitheliopathy, cataract, MH with vitrectomy, and posterior capsule opacification), which led to late reduction in visual acuity, and three subjects had an event reported that likely contributed to the reduction in visual acuity at the later time point (cataract, new MH, and uveitis).
Retinal detachment in the study eye was reported in 17 patients (3.6%), out of which, 14 (3.0%) were related to ocriplasmin. These 14 cases included 6 events (1.3%), described as serous retinal detachments (including one seroretinal detachment), one as subfoveal exudative retinal detachment, and one as exudative retinal detachment, and 4 patients (0.9%) were considered to have SAE for retinal detachment (severe decrease in visual acuity, retinal tear, or vision loss) of the study eye and 2 of these patients were reported as having vitrectomy for treatment. Three cases of retinal detachment reported as not related to drug were reported as due to intraretinal cysts because of VTM detachment, foveal detachment, and retinal detachment. In all, 2 of the 17 cases were described as rhegmatogenous retinal detachment, with none of the patients requiring vitrectomy for treatment. The incidence of retinal tear in the study eye was 1.1% (5 patients), and none of these events were serious.
Of the 24 TEAEs (5.1%) reported for an MH in the study eye, 15 were enlargements of the existing MHs present at baseline, 8 were new MHs, and 1 event was described as persistent MH. In total, 12 (2.6%) of the TEAEs for MHs were related to ocriplasmin and 6 (1.3%) of the TEAEs for MHs were considered serious by the investigator. Six patients with TEAEs for MHs required vitrectomy for treatment. The incidence of any lens opacity-related events (i.e., cataract, cortical cataract, nuclear cataract, and posterior capsule opacification) in the study eye was 3.8% (n = 18).
Ocular examinations (IOP, slit-lamp biomicroscopy, and dilated fundus examinations) revealed no clinically relevant trends across the 3 subgroups during the 180-day follow-up period.
Discussion
The OVIID-1 study evaluated the efficacy and safety profile of ocriplasmin in a predefined selected patient population, that is, patients with VMT with or without MHs, no ERM, and focal VMA. Overall, 47.4% of the study participants achieved nonsurgical resolution of VMT (primary endpoint). Also, among patients with an MH, 39.5% achieved good MH closure rate within 1 month of treatment. Furthermore, resolution in VMT or MH closure was observed to be related to a greater improvement in BCVA, and the functional improvement continued over time. During the 6-month period, the proportion of patients who needed vitrectomy was low, and there was an overall decrease in the proportion of patients with metamorphopsia (by the Amsler grid assessment). The VMT resolution rate observed in this study was higher (47.4%) than that reported in a pooled analysis of the MIVI-TRUST studies of ocriplasmin (26.5%) but similar to that observed in the OASIS trial (41.7%).28,29 Two possible reasons for the difference in VMT resolution rates could be differences in the patient populations and imaging techniques in these studies. In the MIVI-TRUST studies, the presence of ERM was not a criterion for exclusion, and CRC-OCT readings were not used for patient enrollment.29 The presence of ERM is known to reduce the rate of nonsurgical resolution.25,32–34 In the OASIS study, patients with ERM were excluded; however, a certain number of patients with broad VMA and/or ERM were enrolled as per investigator's OCT reading.28 This study excluded patients with broad VMA (>1,500 μm) and ERM. In addition, trained readers at a CRC evaluated SD-OCT images and validated the screening of patients. Thus, the present findings are consistent with and corroborate the findings of other studies, that is, a higher VMT resolution is achieved in patients with focal adhesion and the absence of ERM at baseline.28,35
The study also assessed treatment outcomes by subgroups, that is, in patients with VMT with or without MHs. In patients with VMT without an MH, the results were similar to that of the overall group, with VMT resolution of 43.4% and a moderate BCVA gain at Day 180 (3.2 letters) from baseline and decrease in metamorphopsia. In this group, the overall vitrectomy rate by the end of the study was 6.6%.
Among all the subgroups, the highest VMT resolution was observed in patients with MH ≤250 μm at baseline (62.9%) and was accompanied with a BCVA gain of 6.5 letters and the highest MH closure rate (60%) at Day 180, almost double the rate observed in patients with an MH >250 to ≤400 μm. These results are in agreement with pivotal trials and those from real-world settings that have reported a small MH (≤250 μm) at baseline to be one of the positive predictors for the resolution of VMT after ocriplasmin injection.25,28,35,36 Thus, the study results suggest that patients with VMT and an MH ≤250 μm are most likely to achieve best anatomical and functional benefits after enzymatic vitreolysis with ocriplasmin.
Patients with an MH >250 to ≤400 μm at baseline also achieved a good VMT resolution rate (47.1%) with moderate improvement in BCVA (3.9 letters) at Day 180. In total, 29.4% of the patients had MH closure by Day 180. The rate of self-closure of an MH >250 to ≤400 μm is very low (only 3–11%), with likelihood decreasing with increasing size.10,20,21,37,38 In comparison, the observed MH closure rate in our study was much higher (at least three times), suggesting potential benefits with ocriplasmin treatment in this group of patients. Closure of an MH >250 to ≤400 μm with ocriplasmin treatment has been observed in other studies as well, with the reported rate varying from 23.5% to 36.8% in clinical trials and from 12.7% to 53% in real-world studies.25,28,35,36,39 However, a direct comparison with real-world data is limited by the difference in the inclusion/exclusion criteria of enrolled patients, diagnostic methods, and sample sizes.
At present, the other alternative for management of VMT with or without MH is either “observe” for spontaneous resolution or opt for vitrectomy (pneumatic vitreolysis is an off-label procedure and not discussed here). Both options have their own limitations. Watchful waiting can negatively impact physical symptoms and vision-related quality of life due to further anatomical deterioration. Vitrectomy is an invasive surgical procedure that requires a careful risk–benefit assessment.29 Thus, enzymatic vitreolysis is a less invasive early intervention that can be considered for resolution of VMT associated without MH or with an MH ≤400 μm. This can be particularly useful in phakic patients who frequently develop nuclear sclerosis after undergoing vitrectomy for the closure of an MH. About 75% and 95% of phakic patients develop visually significant cataracts within 1 year and 2 years of vitrectomy, respectively, and thus, they have to undergo subsequent cataract surgery.40
Ocriplasmin administered as a single injection was well tolerated, and the AEs observed in the overall population and subgroups were consistent with the known safety profile of ocriplasmin and that associated with the intravitreal administration procedure. A review of AEs (i.e., dyschromatopsia, changes in color vision, visual acuity reduction, retinal detachment, and MHs) demonstrated that these events were mostly mild and transient and resolved during the course of the study. Overall, incidence rates of retinal detachment of any kind (serous or rhegmatogenous) and those considered as serious by the investigators (severe decrease in visual acuity, retinal tear, or vision loss) were 3.6% (n = 17) and 0.9% (n = 4), respectively.
Limitations of this study include the open-label design, absence of a control group, lack of reading center masking, and the descriptive analysis of the data. Another limitation was a short follow-up period to observe BCVA change over time. This was a large, prospective, global study that enrolled patients from routine clinical settings and used trained readers to evaluate SD-OCT images for efficacy evaluations. In summary, the results of this study support the efficacy of a single injection of ocriplasmin for the treatment of VMT with and without MH and provide evidence of anatomical and functional improvements in specific subgroups of patients that can be useful to optimize treatment outcomes.
Conclusions
Vitreomacular traction resolution and nonsurgical MH closure with ocriplasmin were associated with improvements in BCVA at the end of the 6-month follow-up period in patients with focal VMA ≤1,500 µm and absence of ERM at baseline, including when associated with MHs ≤400 µm. The safety findings were consistent with pivotal studies and other randomized clinical trials, which are well characterized for ocriplasmin.
Acknowledgments
Manuscript writing support was provided by Sumeet Sood and Shivani Vadapalli (Novartis Healthcare Pvt. Ltd., Hyderabad, India), which was funded by Novartis Pharma AG, in accordance with the Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Footnotes
Supported by Alcon Research Ltd, Fort Worth, TX (a Novartis company).
Presented at: 15th European Society of Retina Specialists (EURETINA) Congress 2015, Nice, France, September 17–20, 2015; 16th European Society of Retina Specialists (EURETINA) Congress 2016, Copenhagen, Denmark, September 8–11, 2016; 47th Annual Scientific Congress of the Royal Australian and New Zealand College of Ophthalmologists (RANZCO), Wellington, New Zealand, October 31–November 4, 2015; 9th Asia Pacific Vitreo-Retina Society (APVRS) Congress, Sydney, Australia, July 31–August 2, 2015; Canadian Ophthalmological Society, Annual Meeting, Victoria, Canada, June 18–21, 2015; FLOREtina 2015, Florence, Italy, December 10–13, 2015; 121st French Society of Ophthalmology Congress, Paris, France, May 9–12, 2015; European Society of Ophthalmology Congress, Vienna, Austria, June 6–9, 2015; Oxford Ophthalmological Congress, Oxford, United Kingdom, July 5–8, 2015; 15th European School for Advanced Studies in Ophthalmology (ESASO) Retina Academy, Barcelona, Spain, October 22–24, 2015; 16th European School for Advanced Studies in Ophthalmology (ESASO) Retina Academy, Estoril, Portugal, June 23–25, 2016; 2015 Annual meeting of the Association for Research in Vision and Ophthalmology (ARVO), Denver, Colorado, May 3–7, 2015; 2016 Annual meeting of the Association for Research in Vision and Ophthalmology (ARVO), Seattle, Washington, May 1–5, 2016; and 113th Congress of German Ophthalmological Society (DOG), Berlin, Germany, October 1–4, 2015.
R. Tadayoni is a consultant for Alcon, Thrombogenics, Bausch + Lomb, Zeiss, Novartis, Bayer, Allergan, Alimera, Roche, Genentech, FCI, and Thea. F. G. Holz reports grants and personal fees from Alcon, Allergan, Novartis, Bayer Healthcare, Genentech/Roche, Heidelberg Engineering, and Acucela; grants from Zeiss, Optos, and Alimera; and personal fees from Boehringer Ingelheim. C. Zech is a consultant for Alcon and FCI. X. Liu and C. Spera are Novartis employees. P. Stalmans has received a research grant from Thrombogenics and is a consultant for Bausch + Lomb, DORC, Nano-Retina, Ophtec, ReNeuron, Vitreq, and Zeiss.
References
- 1.Duker JS, Kaiser PK, Binder S, et al. The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology 2013;120:2611–2619. [DOI] [PubMed] [Google Scholar]
- 2.Bottos J, Elizalde J, Arevalo JF, et al. Vitreomacular traction syndrome. J Ophthalmic Vis Res 2012;7:148–161. [PMC free article] [PubMed] [Google Scholar]
- 3.Steel DH, Lotery AJ. Idiopathic vitreomacular traction and macular hole: a comprehensive review of pathophysiology, diagnosis, and treatment. Eye (Lond) 2013;27:S1–S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stalmans P. A retrospective cohort study in patients with tractional diseases of the vitreomacular interface (ReCoVit). Graefes Arch Clin Exp Ophthalmol 2016;254:617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirneiss C, Neubauer AS, Gass CA, et al. Visual quality of life after macular hole surgery: outcome and predictive factors. Br J Ophthalmol 2007;91:481–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knudtson MD, Klein BE, Klein R, et al. Age-related eye disease, quality of life, and functional activity. Arch Ophthalmol 2005;123:807–814. [DOI] [PubMed] [Google Scholar]
- 7.Okamoto F, Okamoto Y, Fukuda S, et al. Vision-related quality of life and visual function after vitrectomy for various vitreoretinal disorders. Invest Ophthalmol Vis Sci 2010;51:744–751. [DOI] [PubMed] [Google Scholar]
- 8.Brazier J, Hirneib C, Tangelder M, et al. Prevalence of metamorphopsia in patients with vitreomacular traction, with or without hole, and its impact on quality of life: the MeMo study. Poster presented at: ISPOR 21st Annual International Meeting; May 21–25, 2016; Washington, DC.
- 9.Garcia-Layana A, Garcia-Arumi J, Ruiz-Moreno JM, et al. A review of current management of vitreomacular traction and macular hole. J Ophthalmol 2015;2015:809640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ezra E, Gregor ZJ. Surgery for idiopathic full-thickness macular hole: two-year results of a randomized clinical trial comparing natural history, vitrectomy, and vitrectomy plus autologous serum: Morfields Macular Hole Study Group Report no. 1. Arch Ophthalmol 2004;122:224–236. [DOI] [PubMed] [Google Scholar]
- 11.Hikichi T, Yoshida A, Trempe CL. Course of vitreomacular traction syndrome. Am J Ophthalmol 1995;119:55–61. [DOI] [PubMed] [Google Scholar]
- 12.Odrobina D, Michalewska Z, Michalewski J, et al. Long-term evaluation of traction disorder in spectral-domain optical coherence tomography. Retina 2011;31:324–331. [DOI] [PubMed] [Google Scholar]
- 13.Codenotti M, Iuliano L, Fogliato G, et al. A novel spectral-domain optical coherence tomography model to estimate changes in vitreomacular traction syndrome. Graefes Arch Clin Exp Ophthalmol 2014;252:1729–1735. [DOI] [PubMed] [Google Scholar]
- 14.Theodossiadis GP, Grigoropoulos VG, Theodoropoulou S, et al. Spontaneous resolution of vitreomacular traction demonstrated by spectral-domain optical coherence tomography. Am J Ophthalmol 2014;157:842–851. [DOI] [PubMed] [Google Scholar]
- 15.Almeida DR, Chin EK, Rahim K, et al. Factors associated with spontaneous release of vitreomacular traction. Retina 2015;35:492–497. [DOI] [PubMed] [Google Scholar]
- 16.John VJ, Flynn HW, Jr, Smiddy WE, et al. Clinical course of vitreomacular adhesion managed by initial observation. Retina 2014;34:442–446. [DOI] [PubMed] [Google Scholar]
- 17.Tzu JH, John VJ, Flynn HW, Jr, et al. Clinical course of vitreomacular traction managed initially by observation. Ophthalmic Surg Lasers Imaging Retina 2015;46:571–576. [DOI] [PubMed] [Google Scholar]
- 18.Wu L, Zas M, Berrocal MH, et al. Anatomical and functional outcomes of symptomatic idiopathic vitreomacular traction: a natural history study from the pan American collaborative retina study group. Retina 2016;36:1913–1918. [DOI] [PubMed] [Google Scholar]
- 19.Johnson MW. Perifoveal vitreous detachment and its macular complications. Trans Am Ophthalmol Soc 2005;103:537–567. [PMC free article] [PubMed] [Google Scholar]
- 20.Chew EY, Sperduto RD, Hiller R, et al. Clinical course of macular holes: the eye disease case-control study. Arch Ophthalmol 1999;117:242–246. [DOI] [PubMed] [Google Scholar]
- 21.Idiopathic Macular Hole PPP—2014. San Francisco, CA: American Academy of Ophthalmology; 2014. [Google Scholar]
- 22.Hikichi T, Yoshida A, Akiba J, et al. Prognosis of stage 2 macular holes. Am J Ophthalmol 1995;119:571–575. [DOI] [PubMed] [Google Scholar]
- 23.Hikichi T, Yoshida A, Akiba J, Trempe CL. Natural outcomes of stage 1, 2, 3, and 4 idiopathic macular holes. Br J Ophthalmol 1995;79:517–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JW, Freeman WR, Azen SP, et al. Prospective randomized trial of vitrectomy or observation for stage 2 macular holes. Vitrectomy for Macular Hole Study Group. Am J Ophthalmol 1996;121:605–614. [DOI] [PubMed] [Google Scholar]
- 25.Sharma P, Juhn A, Houston SK, et al. Efficacy of intravitreal ocriplasmin on vitreomacular traction and full-thickness macular holes. Am J Ophthalmol 2015;159:861–867. [DOI] [PubMed] [Google Scholar]
- 26.Singh RP, Li A, Bedi R, et al. Anatomical and visual outcomes following ocriplasmin treatment for symptomatic vitreomacular traction syndrome. Br J Ophthalmol 2014;98:356–360. [DOI] [PubMed] [Google Scholar]
- 27.Jetrea [Prescribing Information]. Iselin, NJ: ThromboGenics NV; 2016. [Google Scholar]
- 28.Dugel PU, Tolentino M, Feiner L, et al. Results of the 2-year ocriplasmin for treatment for symptomatic vitreomacular adhesion including macular hole (OASIS) randomized trial. Ophthalmology 2016;123:2232–2247. [DOI] [PubMed] [Google Scholar]
- 29.Stalmans P, Benz MS, Gandorfer A, et al. Enzymatic vitreolysis with ocriplasmin for vitreomacular traction and macular holes. N Engl J Med 2012;367:606–615. [DOI] [PubMed] [Google Scholar]
- 30.JETREA [Prescribing Information]. Iselin, NJ: Thrombogenics, Inc; 2017. [Google Scholar]
- 31.JETREA [Summary of Product Characteristics]. Leuven, Belgium: ThromboGenics NV; 2016. [Google Scholar]
- 32.Chatziralli I, Theodossiadis G, Parikakis E, et al. Real-life experience after intravitreal ocriplasmin for vitreomacular traction and macular hole: a spectral-domain optical coherence tomography prospective study. Graefes Arch Clin Exp Ophthalmol 2016;254:223–233. [DOI] [PubMed] [Google Scholar]
- 33.Khan MA, Haller JA. Ocriplasmin for treatment of vitreomacular traction: an update. Ophthalmol Ther 2016;5:147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stefanini FR, Maia M, Falabella P, et al. Profile of ocriplasmin and its potential in the treatment of vitreomacular adhesion. Clin Ophthalmol 2014;8:847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haller JA, Stalmans P, Benz MS, et al. Efficacy of intravitreal ocriplasmin for treatment of vitreomacular adhesion: subgroup analyses from two randomized trials. Ophthalmology 2015;122:117–122. [DOI] [PubMed] [Google Scholar]
- 36.Figueira J, Martins D, Pessoa B, et al. The Portuguese experience with ocriplasmin in clinical practice. Ophthalmic Res 2016;56:186–192. [DOI] [PubMed] [Google Scholar]
- 37.Bainbridge J, Gregor Z. Macular holes. In: Kirchhof B, Wong D, eds. Vitreo-Retinal Surgery. Berlin, Heidelberg: Springer Berlin Heidelberg; 2007:1–18. [Google Scholar]
- 38.Guyer DR, de Bustros S, Diener-West M, Fine SL. Observations on patients with idiopathic macular holes and cysts. Arch Ophthalmol 1992;110:1264–1268. [DOI] [PubMed] [Google Scholar]
- 39.Haynes RJ, Yorston D, Laidlaw DA, et al. Real world outcomes of ocriplasmin use by members of the British and Eire Association of Vitreoretinal Surgeons. Eye (Lond) 2017;31:107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lahey JM, Francis RR, Fong DS, et al. Combining phacoemulsification with vitrectomy for treatment of macular holes. Br J Ophthalmol 2002;86:876–878. [DOI] [PMC free article] [PubMed] [Google Scholar]