Abstract
Background
The projected rise in the incidence of type 2 diabetes mellitus (T2DM) could develop into a substantial health problem worldwide. Whether metformin can prevent or delay T2DM and its complications in people with increased risk of developing T2DM is unknown.
Objectives
To assess the effects of metformin for the prevention or delay of T2DM and its associated complications in persons at increased risk for the T2DM.
Search methods
We searched the Cochrane Central Register of Controlled Trials, MEDLINE, Scopus, ClinicalTrials.gov, the World Health Organization (WHO) International Clinical Trials Registry Platform and the reference lists of systematic reviews, articles and health technology assessment reports. We asked investigators of the included trials for information about additional trials. The date of the last search of all databases was March 2019.
Selection criteria
We included randomised controlled trials (RCTs) with a duration of one year or more comparing metformin with any pharmacological glucose‐lowering intervention, behaviour‐changing intervention, placebo or standard care in people with impaired glucose tolerance, impaired fasting glucose, moderately elevated glycosylated haemoglobin A1c (HbA1c) or combinations of these.
Data collection and analysis
Two review authors read all abstracts and full‐text articles and records, assessed risk of bias and extracted outcome data independently. We used a random‐effects model to perform meta‐analysis and calculated risk ratios (RRs) for dichotomous outcomes and mean differences (MDs) for continuous outcomes, using 95% confidence intervals (CIs) for effect estimates. We assessed the certainty of the evidence using GRADE.
Main results
We included 20 RCTs randomising 6774 participants. One trial contributed 48% of all participants. The duration of intervention in the trials varied from one to five years. We judged none of the trials to be at low risk of bias in all 'Risk of bias' domains.
Our main outcome measures were all‐cause mortality, incidence of T2DM, serious adverse events (SAEs), cardiovascular mortality, non‐fatal myocardial infarction or stroke, health‐related quality of life and socioeconomic effects.The following comparisons mostly reported only a fraction of our main outcome set. Fifteen RCTs compared metformin with diet and exercise with or without placebo: all‐cause mortality was 7/1353 versus 7/1480 (RR 1.11, 95% CI 0.41 to 3.01; P = 0.83; 2833 participants, 5 trials; very low‐quality evidence); incidence of T2DM was 324/1751 versus 529/1881 participants (RR 0.50, 95% CI 0.38 to 0.65; P < 0.001; 3632 participants, 12 trials; moderate‐quality evidence); the reporting of SAEs was insufficient and diverse and meta‐analysis could not be performed (reported numbers were 4/118 versus 2/191; 309 participants; 4 trials; very low‐quality evidence); cardiovascular mortality was 1/1073 versus 4/1082 (2416 participants; 2 trials; very low‐quality evidence). One trial reported no clear difference in health‐related quality of life after 3.2 years of follow‐up (very low‐quality evidence). Two trials estimated the direct medical costs (DMC) per participant for metformin varying from $220 to $1177 versus $61 to $184 in the comparator group (2416 participants; 2 trials; low‐quality evidence).
Eight RCTs compared metformin with intensive diet and exercise: all‐cause mortality was 7/1278 versus 4/1272 (RR 1.61, 95% CI 0.50 to 5.23; P = 0.43; 2550 participants, 4 trials; very low‐quality evidence); incidence of T2DM was 304/1455 versus 251/1505 (RR 0.80, 95% CI 0.47 to 1.37; P = 0.42; 2960 participants, 7 trials; moderate‐quality evidence); the reporting of SAEs was sparse and meta‐analysis could not be performed (one trial reported 1/44 in the metformin group versus 0/36 in the intensive exercise and diet group with SAEs). One trial reported that 1/1073 participants in the metformin group compared with 2/1079 participants in the comparator group died from cardiovascular causes. One trial reported that no participant died due to cardiovascular causes (very low‐quality evidence). Two trials estimated the DMC per participant for metformin varying from $220 to $1177 versus $225 to $3628 in the comparator group (2400 participants; 2 trials; very low‐quality evidence).
Three RCTs compared metformin with acarbose: all‐cause mortality was 1/44 versus 0/45 (89 participants; 1 trial; very low‐quality evidence); incidence of T2DM was 12/147 versus 7/148 (RR 1.72, 95% CI 0.72 to 4.14; P = 0.22; 295 participants; 3 trials; low‐quality evidence); SAEs were 1/51 versus 2/50 (101 participants; 1 trial; very low‐quality evidence).
Three RCTs compared metformin with thiazolidinediones: incidence of T2DM was 9/161 versus 9/159 (RR 0.99, 95% CI 0.41 to 2.40; P = 0.98; 320 participants; 3 trials; low‐quality evidence). SAEs were 3/45 versus 0/41 (86 participants; 1 trial; very low‐quality evidence).
Three RCTs compared metformin plus intensive diet and exercise with identical intensive diet and exercise: all‐cause mortality was 1/121 versus 1/120 participants (450 participants; 2 trials; very low‐quality evidence); incidence of T2DM was 48/166 versus 53/166 (RR 0.55, 95% CI 0.10 to 2.92; P = 0.49; 332 participants; 2 trials; very low‐quality evidence). One trial estimated the DMC of metformin plus intensive diet and exercise to be $270 per participant compared with $225 in the comparator group (94 participants; 1 trial; very‐low quality evidence).
One trial in 45 participants compared metformin with a sulphonylurea. The trial reported no patient‐important outcomes.
For all comparisons there were no data on non‐fatal myocardial infarction, non‐fatal stroke or microvascular complications.
We identified 11 ongoing trials which potentially could provide data of interest for this review. These trials will add a total of 17,853 participants in future updates of this review.
Authors' conclusions
Metformin compared with placebo or diet and exercise reduced or delayed the risk of T2DM in people at increased risk for the development of T2DM (moderate‐quality evidence). However, metformin compared to intensive diet and exercise did not reduce or delay the risk of T2DM (moderate‐quality evidence). Likewise, the combination of metformin and intensive diet and exercise compared to intensive diet and exercise only neither showed an advantage or disadvantage regarding the development of T2DM (very low‐quality evidence). Data on patient‐important outcomes such as mortality, macrovascular and microvascular diabetic complications and health‐related quality of life were sparse or missing.
Plain language summary
Metformin for prevention/delay of type 2 diabetes mellitus (T2DM) and associated complications in persons at increased risk for development of T2DM
Review question
Is the antidiabetic drug metformin able to prevent or delay the development of type 2 diabetes and its associated complications in people with moderately elevated blood sugar levels?
Background
People with moderately elevated blood sugar levels (often referred to as 'prediabetes') are said to have an increased risk for developing diabetes. Metformin is a blood sugar‐lowering medicine which has been used for a long time to treat people with type 2 diabetes. Type 2 diabetes, also known as adult‐onset diabetes, is the most common type of diabetes and prevents the body from using insulin properly (insulin resistance). Type 2 diabetes can have bad effects on health in the long term (diabetic complications), such as severe eye or kidney disease or 'diabetic feet', eventually resulting in foot ulcers.
We investigated whether metformin can also be used to prevent or delay type 2 diabetes in people at increased risk. We examined the effects of metformin on patient‐important outcomes, such as complications of diabetes, death from any cause, health‐related quality of life and side effects of the drug.
Study characteristics
To be included, people had to have blood sugar levels higher than normal, but below the levels that are used to diagnose diabetes. We found 20 randomised controlled trials (clinical studies where people are randomly put into one of two or more treatment groups) with a total of 6774 participants. The comparator group consisted of diet and exercise, intensive diet and exercise or another blood sugar‐lowering drug. One study dominated the evidence (48% of the total number of all participants). Twelve studies were performed in China. We only included studies with a treatment duration of one year or more. The treatment duration in the included studies varied from one to five years.
This evidence is up to date as of March 2019.
Key results
Fifteen studies compared metformin against diet and exercise. Eight studies compared metformin against intensive diet and exercise and three studies compared metformin plus intensive diet and exercise against intensive diet and exercise only. When compared to standard diet and exercise metformin slightly reduces or delays development of diabetes. However, when compared to intensive diet and exercise, metformin does not provide an additional benefit in reducing or delaying development of diabetes.
Seven studies compared metformin with another glucose‐lowering drug: three studies compared metformin with acarbose. Three studies compared metformin with a thiazolidinedione (such as pioglitazone). There was neither an advantage or disadvantage when comparing metformin with these drugs with respect to the development of diabetes. One study compared metformin with a sulphonylurea (glimepiride). The trial did not report patient‐important outcomes.
In general, the reporting of serious side effects was sparse. Few participants died and we did not detect a clear difference between the intervention and comparator groups. We also did not detect an advantage or disadvantage of metformin in relation to health‐related quality of life. Our included studies did not report on non‐fatal heart attacks, strokes or complications of diabetes such as kidney or eye disease. Few studies estimated the direct medical costs. When compared to diet and exercise, metformin was more expensive. When compared to intensive diet and exercise, metformin was less expensive.
We identified 11 ongoing studies which potentially could provide data for this review. These studies will add a total of 17,853 participants in future updates of our review.
Future studies should investigate more patient‐important outcomes such as complications of diabetes and especially the side effects of the drugs. We do not know whether 'prediabetes' is just a condition defined by laboratory measurements, or whether it is in fact a real risk factor for diabetes. It is also unknown whether treatment of this condition translates into better patient‐important outcomes.
Certainty of the evidence
All included studies had problems in the way they were conduced or reported.
Summary of findings
Background
Description of the condition
'Prediabetes', 'borderline diabetes', the 'prediabetic stage', 'high risk of diabetes' or 'intermediate hyperglycaemia' (WHO/IDF 2006) are often characterised by various measurements of elevated blood glucose concentrations (such as isolated impaired fasting glucose (IFG), isolated impaired glucose tolerance (IGT), isolated elevated glycosylated haemoglobin A1c (HbA1c) or combinations thereof). These elevated blood glucose levels indicating hyperglycaemia are considered too high to be normal but below the diagnostic threshold for type 2 diabetes mellitus (T2DM). Therefore, because of the continuous spectrum from the normal to the diabetic stage a sound evidence base is needed to define thresholds for conditions of 'sub‐diabetes'. It is obvious that the different terms used to describe various stages of hyperglycaemia might induce different emotional reactions, e.g. the term 'prediabetes' may imply (at least for lay persons) that the disease diabetes is unavoidable whereas (high) risk of diabetes has the positive connotation to maybe avoid the disease altogether. All of the above mentioned terms will be used throughout this systematic review, however a focus will be set on 'prediabetes' because this labelling is associated by many persons with dire consequences ‐ despite the disputable construct of intermediate health states termed prediseases (Viera 2011). On the other side, any diagnosis of 'prediabetes' might be an opportunity to review for example eating habits and physical activity levels, thus enabling 'affected' individuals to actively change their way of life.
The most commonly used criteria to define people with a high risk of developing T2DM were established by the American Diabetes Association (ADA) and the World Health Organization (WHO). The first glycaemic measurement used to define the prediabetic stage by the US National Diabetes Data Group was IGT (NDDG 1979). IGT is based on the measurement of plasma glucose two hours after ingestion of 75 g glucose. The prediabetic range is defined as a plasma glucose level between 7.8 mmol/L to 11.1 mmol/L (140 mg/dL to 200 mg/dL) two hours after the glucose load. Studies have indicated that IGT is caused by insulin resistance and defective insulin secretion (Abdul‐Ghani 2006). In 1997, the ADA and later on the WHO introduced the IFG concept to define 'prediabetes' (ADA 1997; WHO 1999). The initial definition of IFG was 6.1 mmol/L to 6.9 mmol/L (110 125 mg/dLto 125 mg/dL). Later on, the ADA reduced the lower threshold for defining IFG to 5.6 mmol/L (100 mg/dL) (ADA 2003). However, this lower cut‐off point for IFG to define 'prediabetes' was not endorsed by the WHO (WHO/IDF 2006). IFG seems to be associated with ß‐cell dysfunction (impaired insulin secretion) and an increase of the hepatic glucose output (DeFronzo 1989). More recently, HbA1c has been introduced for identifying people with a high risk of developing T2DM. In 2009, the International Expert Committee (IEC) suggested the HbA1c to identify people with a high risk of T2DM. People with HbA1c measurements between 6.0% to 6.4% fulfilled this criterion (IEC 2009). Shortly after, the ADA re‐defined this HbA1c level as 5.7% to 6.4% to identify people with a high risk of developing T2DM (ADA 2010). Unlike IFG and IGT, HbA1c reflects longer‐term glycaemic control, i.e. how the blood glucose levels have been during the previous two to three months (Inzucchi 2012).
In 2010, the International Diabetes Federation (IDF) estimated the prevalence of IGT to be 343 million, and this number is predicted to increase to 471 million by 2035 (IDF 2013). Studies have shown poor correlations between HbA1c and IFG/IGT (Gosmanov 2014; Selvin 2011). Besides, the various glycaemic tests do not seem to identify the same people (Gosmanov 2014; Selvin 2011). The risk of progression from 'prediabetes' to T2DM depends on the diagnostic criteria used to identify 'prediabetes'. Some people diagnosed with 'prediabetes' will never develop T2DM, and some will return to normoglycaemia. IGT is often accepted as the best glycaemic variable for 'prediabetes' to predict progression to T2DM. However, studies indicate that less than half of the people defined as prediabetic by means of IGT will develop T2DM in the following 10 years. IFG and HbA1c are both thought to predict a different risk spectrum for developing T2DM (Cheng 2006; Morris 2013). Most importantly, 'prediabetes' is commonly an asymptomatic condition, and naturally often remains 'undiagnosed' (Centers for Disease Control and Prevention 2015). Consequently, 'prediabetes' may exist before the diagnosis of T2DM is established.
It is still not clarified if any particular intervention, especially glucose‐lowering drugs, should be recommended for people with 'prediabetes' (Yudkin 2014). Studies have indicated that the progression from 'prediabetes' to T2DM is reduced, or maybe just delayed with 'lifestyle' interventions (increased physical activity, dietary changes or both) (Diabetes Prevention Program 2002; Diabetes Prevention Program FU 2009; Finnish Diabetes Prevention Study Group 2001). A recent meta‐analysis of 22 trials with lifestyle interventions in people with high risk of T2DM concluded that the effect of lifestyle interventions on longer‐term diabetes prevention is not clarified (Dunkley 2014).
The prescription of pharmacological glucose‐lowering interventions for the prevention of T2DM is not generally accepted among international diabetes associations and clinicians. Several groups of pharmacological glucose‐lowering interventions have been investigated in people with 'prediabetes'. Some findings indicate that the progression from 'prediabetes' to T2DM is reduced or maybe just delayed (Diabetes Prevention Program 2002; Diabetes Prevention Program FU 2009). However, the ADA recommends metformin for people with 'prediabetes' and a body mass index (BMI) > 35 kg/m², aged < 60 years, and women with prior gestational diabetes mellitus (ADA 2015).
Description of the intervention
Metformin is a biguanide originating from the plant Galega officinalis (Witters 2001). First described in 1922, it was administered to humans for the first time in France in 1957. In 1972, Canada approved its use for T2DM and later, in 1994, it received approval for use in T2DM by the US Food and Drug Administration (FDA) (Corey 2007; FDA 1994).
People with T2DM are initially advised to follow behaviour‐changing ('lifestyle') interventions including weight loss and increased physical activity (ADA 2019a). However, over time the majority of people with T2DM will require additional glucose‐lowering pharmacological interventions. Currently, metformin is the recommended first‐line, glucose‐lowering medication (ADA 2019a).
The glucose‐lowering effect increases with increasing doses of metformin, whether by the immediate‐release or prolonged‐release formulations. The maximal recommended dose of metformin is 2000 mg daily in the USA. However, the maximum recommended daily dose of metformin in Europe and in other regions is 3000 mg. The landmark study, UK Prospective Diabetes Study (UKPDS) applied a median daily dose of 2550 mg/day in people with newly diagnosed T2DM (UKPDS 1998).
Adverse effects of the intervention
The most common adverse effects of metformin are gastrointestinal disturbances, which are reported in 20% to 30% of people using this drug. However, the gastrointestinal disturbances only necessitate discontinuation of the drug in less than 5% of the affected individuals (DeFronzo 1999).
A potential complication of metformin use is lactic acidosis, a rare, but potentially fatal, metabolic condition that can occur whenever substantial tissue hypoxia exists (Kreisberg 1980). Lactic acidosis is characterised by elevated blood lactate concentrations (exceeding 5.0 mmol/L) and decreased blood pH (less than 7.35). The mortality is estimated to be about 50% (Huang 2016). A Cochrane Review found no firm evidence of metformin being associated with an increased risk of lactic acidosis or elevated lactate levels when compared to other glucose‐lowering drugs (Salpeter 2010). However, several case reports of lactic acidosis in metformin‐treated people have been published subsequently (Kalantar‐Zadeh 2013; Schousboe 2012).
How the intervention might work
The exact mechanism(s) of action of metformin are not clearly elucidated. However, metformin is known to alter carbohydrate metabolism by reducing basal hepatic glucose production (gluconeogenesis), improving insulin sensitivity in the liver and peripheral tissues, as well as increasing insulin‐stimulated glucose uptake and utilisation in peripheral tissues (AHFS 1999). It has been proposed that its prime mode of action is via activation of the 5' adenosine monophosphate‐activated protein kinase (AMPK) enzyme (Cho 2015; Duca 2015).
Why it is important to do this review
There has been an increased focus on the prevention or delay of T2DM with non‐pharmacological interventions and glucose‐lowering medications. Recently, one literature review (Moin 2018) and several systematic reviews (Haw 2017; Lily 2009; Moelands 2018; Pang 2018; Salpeter 2008) have been performed in people with elevated risk of T2DM. All these reviews have methodological short comings and applied limited search strategies. As the prevalence of intermediate hyperglycaemia is increasing, an updated review with comprehensive search and updated methodology is needed.
Objectives
To assess the effects of metformin for the prevention or delay of type 2 diabetes mellitus (T2DM) and its associated complications in persons at increased risk for the development of T2DM.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs).
Types of participants
Nondiabetic individuals at increased risk of developing T2DM, that is, diagnosed with intermediate hyperglycaemia or 'prediabetes'.
Diagnostic criteria for 'prediabetes'
To be consistent with changes in the classification of and diagnostic criteria for 'prediabetes' (impaired fasting glucose (IFG), impaired glucose tolerance (IGT) and elevated glycosylated haemoglobin A1c (HbA1c)) over the years, the diagnosis had to be established using the standard criteria valid at the time of the trial commencing (for example ADA 1997; ADA 2010; NDDG 1979; WHO 1999). Ideally, the diagnostic criteria should have been described. If necessary, we used the trial authors' definition of 'prediabetes' but contacted trial authors for additional information. Differences of glycaemic measurements used to define 'prediabetes' may introduce substantial heterogeneity. We therefore planned to subject diagnostic criteria to a subgroup analysis.
Types of interventions
We planned to investigate the following comparisons of intervention versus control/comparator.
Intervention
Metformin monotherapy (with or without diet, exercise or both).
Comparator
Placebo.
Non‐pharmacological interventions (for example diet, exercise).
Sulfonylureas (for example glibenclamide).
α‐glucosidase inhibitors (for example acarbose).
Thiazolidinediones (for example pioglitazone).
Meglitinides (for example repaglinide).
Sodium‐glucose co‐transporter 2 inhibitors (for example empagliflozin)
Glucagon‐like peptide‐1 analogues (for example liraglutide).
Dipeptidyl peptidase‐4 inhibitors (for example sitagliptin).
Insulin.
Concomitant interventions had to be the same in intervention and control groups to establish fair comparisons.
Minimum duration of intervention
We included trials with a minimum duration of intervention of one year.
Exclusion criteria
People diagnosed with the 'metabolic syndrome' because this is a special cohort of doubtful clinical usefulness and uncertain distinct disease entity (a composite of risk indicators such as elevated blood lipids, insulin resistance, obesity, high blood pressure).
We did not exclude trials because one or several of our primary or secondary outcome measures were not reported in the publication. In case none of our primary or secondary outcomes was reported, we included the trial and contacted the corresponding author for supplementary data. If no additional data were available, we planned to show these trials a supplementary table.
Types of outcome measures
Primary outcomes
All‐cause mortality.
Incidence of type 2 diabetes (T2DM).
Serious adverse events.
Secondary outcomes
Cardiovascular mortality.
Non‐fatal myocardial infarction.
Non‐fatal stroke.
Amputation of lower extremity.
Blindness or severe vision loss.
End‐stage renal disease.
Non‐serious adverse events.
Hypoglycaemia.
Health‐related quality of life.
Time to progression to T2DM.
Measures of blood glucose control.
Socioeconomic effects.
Method and timing of outcome measurement
All‐cause mortality: defined as death from any cause. Measured at the end of the intervention and the end of follow‐up.
Incidence of T2DM and time to progression to T2DM: defined according to diagnostic criteria valid at the time the diagnosis was established using the standard criteria valid at the time of the trial commencing (e.g. ADA 2008; WHO 1998). If necessary, we used the trial authors' definition of T2DM. Measured at the end of the intervention and the longest reported end of follow‐up.
Serious adverse events: defined according to the International Conference on Harmonization Guidelines as any event that leads to death, that is life‐threatening, required in‐patient hospitalisation or prolongation of existing hospitalisation, resulted in persistent or significant disability, and any important medical event which may have had jeopardised the patient or required intervention to prevent it (ICH 1997) or as reported in trials. Measured at any time of the intervention and during follow‐up.
Cardiovascular mortality, non‐fatal myocardial infarction, non‐fatal stroke, amputation of lower extremity, blindness or severe vision loss, hypoglycaemia (mild, moderate, severe/serious): defined as reported in trials. Measured at the end of the intervention and at the end of follow‐up.
End‐stage renal disease: defined as dialysis, renal transplantation or death due to renal disease. Measured at the end of the intervention and at the end of follow‐up.
Non‐serious adverse events: defined as number of participants with any untoward medical occurrence not necessarily having a causal relationship with the intervention. Measured at the end of the intervention and at the end of follow‐up.
Health‐related quality of life: defined as mental and physical health‐related quality of life as separate and combined, evaluated by a validated instrument such as Short‐Form 36. Measured at the end of the intervention and at the end of follow‐up.
Measures of blood glucose control: fasting blood glucose, blood glucose two hours after ingestion of 75 g glucose and HbA1c measurements. Measured at the end of the intervention and at the end of follow‐up.
Socioeconomic effects: for example costs of the intervention, absence from work, medication consumption. Measured at the end of the intervention and at the end of follow‐up.
Search methods for identification of studies
Electronic searches
We searched the following sources from inception of each database to 7 March 2019.
Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online (CRSO) (searched 7 March 2019).
Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Daily 1946 to March 06, 2019 (searched 7 March 2019).
Scopus (searched 7 March 2019).
ClinicalTrials.gov (searched 7 March 2019).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) Search Portal (http://apps.who.int/trialsearch/) (searched 7 March 2019).
For detailed search strategies, see Appendix 1. We continuously applied an email alert service for MEDLINE via OvidSP to identify newly published trials using the search strategy detailed in Appendix 1. We placed no restrictions on the language of publication when searching the electronic databases or reviewing reference lists of identified trials.
Searching other resources
We tried to identify additional trials by searching the reference lists of included trials, (systematic) reviews, meta‐analyses and health technology assessment reports. Additionally, we attempted to obtain additional trials by handsearching the most recent journal issues in print that were not indexed in the electronic databases as well. We also searched grey literature sources, which included internal reports and conference proceedings.
Data collection and analysis
Selection of studies
Two review authors independently scanned the abstract or title, or both, of records retrieved, to determine which trials should be assessed further (BR and BH). We investigated the full‐text articles of all potentially relevant trials. We resolved discrepancies through consensus or by recourse to another review author (MIM). If we could not resolve a disagreement, we categorised the trial as a 'study awaiting classification' and contact the trial authors for clarification. We prepared a flow diagram of the number of trials identified and excluded at each stage in accordance with the PRISMA flow diagram of trial selection (Liberati 2009; Figure 1).
1.

Trial flow diagram (as of 29.05.2017, Mim)
Data extraction and management
For trials that fulfilled inclusion criteria, two review authors (KSM and BH or YC) independently extracted key participant and intervention characteristics. We reported data on efficacy outcomes and adverse events using standard data extraction sheets from the CMED Group. We resolved any disagreements by discussion or, if required, by consultation with another review author (BR) (for details see Characteristics of included studies; Table 3; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 9; Appendix 10; Appendix 11; Appendix 12; Appendix 13).
1. Overview of trial populations.
| Trial (design) | Intervention(s) and comparator(s) | Description of power and sample size calculation | Screened/eligible (N) | Randomised (N) | Analysed (N) | Finishing trial (N) | Randomised finishing trial (%) | Follow‐up (extended follow‐up)a |
|
Alfawaz 2018 (parallel RCT) |
I: metformin | — | — | 98 | 59 | 68 | 69.4 | 1 year (1 year) |
| C1: intensive diet plus exercise | 98 | 73 | 75 | 76.5 | ||||
| C2: standard care | 98 | 85 | 94 | 95.9 | ||||
| total: | 294 | 217 | 237 | 80.6 | ||||
| PREVENT‐DM 2017 (parallel RCT) NCT02088034 | I1: metformin | Quote: "Data from a previous pilot study of the promotora‐led ILI provided estimates for participant retention at 12‐month follow‐up (90%) and 12‐month weight loss (4.9 kg, SD 4.9 kg). Based on these assumptions, the enrollment target was 30 participants per study arm in order to retain 27 in each group at 12 months. These assumptions allowed for >80% power to detect a mean weight loss difference of at least 4.9 kg (SD=4.9 kg) between groups, which was lower than that observed in DPP, at the overall 5% significance level. Power calculations adjusted for three pairwise comparisons, using a 1.7% significance level for each" | 441/197 | 29 | 27 | 27 | 93.1 | 1 year (—) |
| C1: intensive diet plus exercise | 33 | 30 | 30 | 90.9 | ||||
| C2: standard care | 30 | 28 | 28 | 93.3 | ||||
| total: | 92 | 85 | 85 | 92.4 | ||||
|
Zeng 2013 (parallel RCT) |
I: metformin | — | — | 68 | 68 | 68 | 100 | 2 years |
| C1: Standard care | 66 | 66 | 66 | 100 | ||||
| C2: pioglitazone | 70 | 70 | 70 | 100 | ||||
| total: | 204 | 204 | 204 | 100 | ||||
|
Zhao 2013 (parallel RCT) |
I: metformin plus intensive diet plus exercise | — | — | 46 | 45 | 45 | 97.8 | 1 year |
| C: intensive diet plus exercise | 46 | 46 | 46 | 97.8 | ||||
| total: | 92 | 91 | 91 | 98.9 | ||||
| Iqbal Hydrie 2012 (parallel RCT) | I1: metformin plus intensive diet and exercise | Quote: "Mean and standard deviation were reported for continuous variables and intergroup comparisons were tested by two tailed ANOVA. Comparison of proportions was by χ2 analysis. The proportion of subjects developing diabetes in each group and their comparison was by χ2 analysis. For the intervention measures, the absolute and relative risk reductions, 95% CIs of the estimates, and the number needed to treat to prevent diabetes in one person were calculated. A P value <0.05 was considered significant" | 1739/317 | 95 | 85 | 85 | 89.5 | 18 months (—) |
| C1: intensive diet and exercise | 114 | 107 | 107 | 93.9 | ||||
| C2: standard care | 108 | 82 | 82 | 75.9 | ||||
| total: | 317 | 274 | 274 | 86.4 | ||||
|
Liao 2012 (parallel RCT) |
I: metformin | — | — | 52 | 50 | 50 | 96.2 | 1 year |
| C: acarbose | 52 | 51 | 51 | 98.1 | ||||
| total: | 104 | 101 | 101 | 97.1 | ||||
|
Ji 2011 (parallel RCT) |
I1: metformin | — | — | 52 | 52 | 52 | 100 | 2 years |
| C1: intensive diet plus exercise | 60 | 60 | 60 | 100 | ||||
| C2: standard care | 64 | 64 | 64 | 100 | ||||
| total: | 176 | 176 | 176 | 100 | ||||
|
Lu 2010 (parallel RCT) |
I: metformin | — | — | 117 | 115 | 96 | 82 | 2 years |
| C: standard care | 117 | 111 | 100 | 85.5 | ||||
| total: | 234 | 226 | 196 | 83.8 | ||||
| BIGPRO1 2009b (parallel RCT) | S1 ‐ I1: metformin | Quote: "Given the number of variable to be compared, the required sample size fluctuate between 200 and 500 per group, according to the variable under consideration and allowing for multiple testing (two‐tailed test, α = β = 5%)." | S1: 457/101 S2: 457/51 | 49 | 28 | 28 | 57.1 | 1 year (—) |
| S1 ‐ C1: placebo | 52 | 36 | 36 | 69.2 | ||||
| S2 ‐ I1: metformin | 28 | 18 | 18 | 64.3 | ||||
| S2 ‐ C1: placebo | 23 | 14 | 14 | 60.9 | ||||
|
total S1: total S2: |
101 | 64 | 64 | |||||
| 51 | 32 | 32 | ||||||
|
Chen 2009 (parallel RCT) |
I: metformin | — | — | 49 | 44 | 44 | 89.8 | 2 years |
| C: standard care | 52 | 46 | 46 | 88.5 | ||||
| total: | 101 | 90 | 90 | 89.1 | ||||
|
Jin 2009 (parallel RCT) |
I: metformin | — | — | 48 | 45 | 45 | 93.8 | 3 years |
| C1: standard care | 41 | 41 | 41 | 100 | ||||
| C2: rosiglitazone | 44 | 41 | 41 | 93.2 | ||||
| total: | 133 | 127 | 127 | 95.5 | ||||
|
Li 2009 (parallel RCT) |
I: metformin | — | — | 77 | 77 | 74 | 96.1 | 3 years |
| C: intensive diet plus exercise | 83 | 83 | 79 | 95.2 | ||||
| total: | 160 | 160 | 153 | 95.6 | ||||
|
Wang 2009 (parallel RCT) |
I: metformin | — | — | 32 | 30 | 30 | 93.8 | 1 year |
| C: standard care | 32 | 32 | 32 | 100 | ||||
| total: | 64 | 62 | 62 | 96.9 | ||||
| IDPP‐1 2006 (parallel RCT) NCT00279240 | I1: metformin | Quote: "It was assumed that the cumulative incidence of diabetes in 3 years would be approximately 30% in the control group and that there would be a 50% reduction with the intervention methods. The sample size required in each of the four subgroups was 134 with a type 1 error of 5%, 80% power, and allowing for a dropout rate of 10%" | 10,839/531 | 133 | 128 | 128 | 96.2 | 3 years (—) |
| I2: metformin plus intensive diet and physical activity | 129 | 121 | 121 | 93.8 | ||||
| C1: intensive exercise plus diet | 133 | 120 | 120 | 90.2 | ||||
| C2: standard care | 136 | 133 | 133 | 97.8 | ||||
| total: | 531 | 502 | 502 | 94.5 | ||||
| Maji 2005 (parallel RCT) | I1: metformin | — | 234/234 | 48 | — | — | — | 3 years (—) |
| C1: intensive lifestyle intervention | 90 | — | — | — | ||||
| C2: rosiglitazone | 48 | — | — | — | ||||
| C3: acarbose | 48 | — | — | — | ||||
| total: | 234 | — | — | — | ||||
| Fang 2004 (parallel RCT) | I: metformin | — | 1549/178 | 48 | 44 | 44 | 91.7 | 5 years (—) |
| C1: acarbose | 50 | 45 | 45 | 90.0 | ||||
| C2: intensive exercise and diet | 40 | 36 | 36 | 90.0 | ||||
| C3: standard care | 40 | 35 | 35 | 87.5 | ||||
| total: | 178 | 160 | 160 | 89.9 | ||||
| DPP/DPPOS 2002 (parallel RCT) | I: metformin | Quote: "The principal analyses of primary and secondary outcomes will employ the "intent‐to‐treat" approach (Peduzzi, Wittes, et al., 1993). The intent‐to‐treat analyses will include all randomized participants with all participants included in their randomly assigned treatment group; treatment group assignment will not be altered based on the participant’s adherence to the assigned treatment regimen. All statistical tests will be two‐sided. The overall significance level of the primary outcome will be α = 0.05. However, because interim analyses will be conducted throughout the DPP, the significance levels used in the interim and final analyses of the primary outcome will be adjusted to account for the multiplicity of interim analyses." and "The study design provided 90 percent power to detect a 33 percent reduction from an incidence of 6.5 cases of diabetes per 100 person‐years, with a 10 percent rate of loss to follow‐up per year" | 153,183 | 1073 | — | — | — | 2.8 years (15 years) |
| C1: intensive exercise and diet | 1079 | — | — | — | ||||
| C2: placebo | 1082 | — | — | — | ||||
| total: | 3234 | 3234 | — | — | ||||
|
Lu 2002 (parallel RCT) |
I1: metformin | — | — | 80 | 75 | 75 | 93.8 | 3 years |
| C1: standard care | 72 | 64 | 64 | 88.9 | ||||
| C2: standard care plus diet instruction every 6th month | 57 | 51 | 51 | 89.5 | ||||
| C3: standard care plus fibre diet | 84 | 80 | 80 | 95.2 | ||||
| total: | 293 | 270 | 270 | 92.2 | ||||
| Li 1999 (parallel RCT) | I1: metformin | — | 29,938 | 45 | 33 | 33 | 73.3 | 1 year (—) |
| C1: placebo | 45 | 37 | 37 | 82.2 | ||||
| total: | 90 | 70 | 70 | 77.8 | ||||
|
Papoz 1978 (parallel RCT) |
I1: metformin (plus placebo) | — | — | 30 | 23 | 23 | 76.7 | 2 years (2 years) |
| C1: glibenclamide plus placebo | 28 | 22 | 22 | 78.6 | ||||
| C2: placebo | 33 | 19 | 19 | 57.6 | ||||
| total: | 91 | 64 | 64 | 71 | ||||
| Grand total | All interventions | 2426 | ||||||
| All comparators | 4348 | |||||||
| All interventions and comparators | 6774 | |||||||
—: denotes not reported
aFollow‐up under randomised conditions until end of trial or if not available, duration of intervention; extended follow‐up refers to follow‐up of participants once the original trial was terminated as specified in the power calculation bFor BIGPRO1 we evaluated two subgroups available as secondary analyses (published in 2009) from the original trial (1996), which did not meet our inclusion criteria for the population
C: comparator; I: intervention; ITT: intention‐to‐treat; RCT: randomised clinical trial.
We provided information about potentially relevant ongoing trials including trial identifier in the Characteristics of ongoing studies table and in Appendix 7 'Matrix of trial endpoint (publications and trial documents)'. For each included trial, we tried to retrieve the protocol. If not available from the search of the databases, reference screening or Internet searches, we asked authors to provide a copy of the protocol. Predefined outcomes were entered in Appendix 7.
We emailed all authors of the included trials to enquire whether they were willing to answer questions regarding their trials. We presented the results of this survey in 'Survey of trial investigators providing information on included trials' (see Appendix 14). We sought relevant missing information on the trial from the primary author(s) of the articles, if possible.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents or multiple reports of a primary trial, we maximised the information yield by collating all available data and used the most complete data set aggregated across all known publications. Duplicate publications, companion documents or multiple reports of a primary trial were listed as secondary references under the primary reference of the included, excluded trial or ongoing trial.
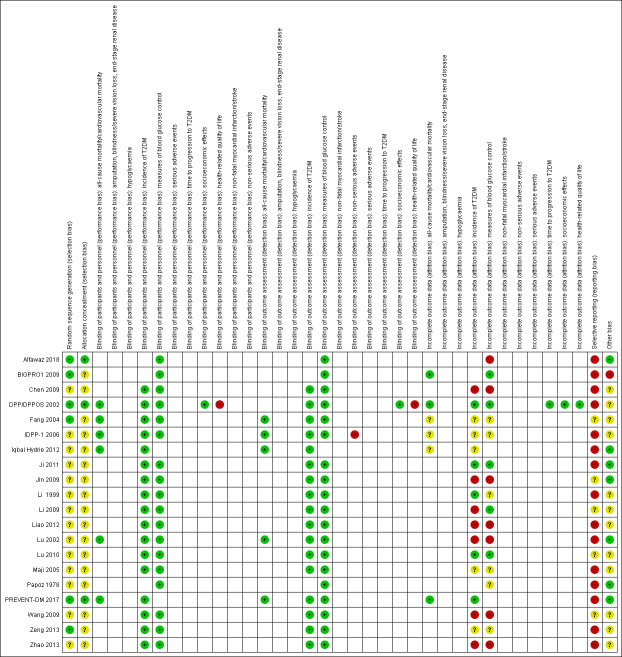
Assessment of risk of bias in included studies
Review authors (KS and BH) independently assessed the risk of bias of the included trials. Studies in Chinese were assessed by one author (YC). We resolved any disagreements by consensus, or by consultation with a third review author (BH or BR). If adequate information was not available from the trial publication, trial protocol or both, we contacted trial authors for missing data on 'Risk of bias' items.
We used the Cochrane 'Risk of bias' assessment tool (Higgins 2017) assigning assessments of low, high, or unclear risk of bias (for details, see Appendix 2; Appendix 3). We evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011 according to the criteria and associated categorisations contained therein(Higgins 2017).
Summary assessment of risk of bias
We presented a 'Risk of bias' graph and a 'Risk of bias' summary figure.
For risk of bias evaluation we grouped outcome measures as follows:
Health‐related quality of life.
Incidence of T2DM.
Macrovascular complications: non‐fatal myocardial infarction, non‐fatal stroke.
Measures of blood glucose control.
Microvascular complications: amputation of lower extremity, blindness/severe vision loss, end‐stage renal disease
Mortality: all‐cause mortality, cardiovascular mortality.
Non‐seroius adverse events (including hypoglycaemic episodes, depending on measurement).
Serious hypoglycaemic episodes (including hypoglycaemic episodes, depending on measurement).
Socioeconomic effects.
Time to progression to 2DM.
We distinguished between self‐reported, investigator‐assessed and adjudicated outcome measures.
We defined the following outcomes as self‐reported.
Non‐serious adverse events.
Hypoglycaemia, if reported by participants.
Health‐related quality of life.
Blood glucose control, if measured by trial participants.
We defined the following outcomes as investigator‐assessed:
All‐cause mortality.
Incidence of T2DM.
Time to progression to T2DM.
Serious adverse events.
Cardiovascular mortality.
Non‐fatal myocardial infarction.
Non‐fatal stroke.
Amputation of lower extremity.
Blindness or severe vision loss.
End‐stage renal disease.
Hypoglycaemia, if measured by trial personnel.
Blood glucose control, if measured by trial personnel.
Socioeconomic effects.
Summary assessment of risk of bias
Risk of bias for a trial across outcomes: some risk of bias domains, such as selection bias (sequence generation and allocation sequence concealment), affected the risk of bias across all outcome measures in a trial. Otherwise, we did not perform a summary assessment of the risk of bias across all outcomes for a trial. In case of high risk of selection bias, we excluded the trial.
Risk of bias for an outcome within a trial and across domains: we assessed the risk of bias for an outcome measure by including all entries relevant to that outcome (i.e. both trial‐level entries and outcome‐specific entries). 'Low' risk of bias was defined as low risk of bias for all key domains, 'unclear' risk of bias as unclear risk of bias for one or more key domains and 'high' risk as high risk of bias for one or more key domains.
Risk of bias for an outcome across trials and across domains: these were our main summary assessments that were incorporated in our judgements about the quality of evidence in the 'Summary of findings' tables. 'Low' risk of bias was defined as most information coming from trials at low risk of bias, 'unclear' risk of bias as most information coming from trials at low or unclear risk of bias and 'high' risk of bias as sufficient proportion of information coming from trials at high risk of bias.
Measures of treatment effect
For trials addressing the same outcome but using different outcome measure scales we planned to use standardised mean differences (SMD) with 95% CI. We planned to calculate time‐to‐event data as hazard ratio (HR) with 95% CI with the generic inverse variance method. Unadjusted hazard ratios were planned to be preferred, as adjustment could differ among the included trials.
The scales measuring health‐related quality of life may go in different directions. Some scales increase in values with improved health‐related quality of life, whereas other scales decrease in values with improved health‐related quality of life. To adjust for the different directions of the scales, scales reporting better health‐related quality of life with decreasing values were planned to be multiplied by –1.
Unit of analysis issues
We took into account the level at which randomisation occurred, such as cross‐over trials, cluster‐randomised trials and multiple observations for the same outcome. If more than one comparison from the same trial was eligible for inclusion in the same meta‐analysis, we either combined groups to create a single pair‐wise comparison or appropriately reduced the sample size so that the same participants did not contribute multiply (splitting the 'shared' group into two or more groups). While the latter approach offers some solution to adjusting the precision of the comparison, it does not account for correlation arising from the same set of participants being in multiple comparisons (Higgins 2011c).
We planned to reanalyse cluster randomised trials that did not appropriately adjust for potential clustering of participants within clusters in their analysis. The variance of the intervention effects would have been inflated by a design effect (DEFF). Calculation of a DEFF involves estimation of an intra‐cluster correlation (ICC). Estimates of ICCs were planned to be obtained through contact with authors, or imputed using estimates from other included studies that report ICCs, or using external estimates from empirical research (e.g. Bell 2013). We planned to examine the impact of clustering using sensitivity analyses.
Dealing with missing data
If possible, we obtained missing data from trial authors and carefully evaluated important numerical data such as screened, randomly assigned participants as well as intention‐to‐treat (ITT), and as‐treated and per‐protocol populations.
We investigated attrition rates (e.g. drop‐outs, losses to follow‐up, withdrawals), and critically appraised issues concerning missing data and imputation methods (e.g. last observation carried forward (LOCF)).
Where means and standard deviations (SDs) for outcomes were not reported and we could not receive the needed information from trial authors, we planned to impute these values by assuming the SDs of the missing outcome to be the average of the SDs from those trials in which this information was reported.
We planned to investigate the impact of imputation on meta‐analyses by performing sensitivity analyses.
Assessment of heterogeneity
We identified heterogeneity (inconsistency) by visually inspecting the forest plots and by using a standard Chi² test with a significance level of α = 0.1 (Deeks 2017). In view of the low power of this test, we also considered the I² statistic, which quantifies inconsistency across trials, to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003).
When we found heterogeneity, we attempted to determine possible reasons for this by examining individual trial and subgroup characteristics.
Assessment of reporting biases
Had we included 10 or more trials investigating a particular outcome, we planned to use funnel plots to assess small‐trial effects. Several explanations may account for funnel plot asymmetry, including true heterogeneity of effect with respect to trial size, poor methodological design (and hence bias of small trials) and publication bias (Sterne 2017). Therefore, we planned to interpret results carefully (Sterne 2011).
Data synthesis
We planned to undertake (or display) a meta‐analysis only if we judged participants, interventions, comparisons, and outcomes to be sufficiently similar to ensure an answer that was clinically meaningful. Unless good evidence showed homogeneous effects across trials of different methodological quality, we primarily summarised low risk of bias data using a random‐effects model (Wood 2008). We interpreted random‐effects meta‐analyses with due consideration for the whole distribution of effects and presented a prediction interval (Borenstein 2017a; Borenstein 2017b; Higgins 2011) for the outcome measures reported in the 'Summary of findings' tables. A prediction interval requires at least three trials to be calculated and specifies a predicted range for the true treatment effect in an individual trial (Riley 2011). For rare events such as event rates below 1%, we planned to use the Peto odds ratio method, provided there was no substantial imbalance between intervention and comparator group sizes, and intervention effects were not exceptionally large. In addition, we performed statistical analyses according to the statistical guidelines presented in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017).
Subgroup analysis and investigation of heterogeneity
We expected the following characteristics to introduce clinical heterogeneity, and planned to carry out subgroup analyses including investigation of interactions (Altman 2003).
Trials designed to blind participants and investigators versus open‐label trials.
Trials with long duration (≥ 2 years) versus trials with short duration (< 2 years).
Diagnostic 'prediabetes' criteria (IFG, IGT, HbA1c).
Age, depending on data.
Sex.
Ethnicity, depending on data.
Comorbid conditions, such as hypertension or obesity.
Participants with previous gestational diabetes mellitus.
Sensitivity analysis
We planned to perform sensitivity analyses to explore the influence of the following factors (when applicable) on effect sizes by restricting the analysis to:
published trials;
taking into account risk of bias, as specified in the Assessment of risk of bias in included studies section;
trials using the following filters: imputation, language of publication, source of funding (industry versus other), or country.
We also planned to test the robustness of results by repeating the analysis using different measures of effect size (RR, OR, etc) and different statistical models (fixed‐effect and random‐effects models).
Certainty of the evidence
We presented the overall quality of the certainty for each outcome specified below, according to the GRADE approach, which takes into account issues related to internal validity (risk of bias, inconsistency, imprecision, publication bias) and also to external validity, such as directness of results. Two review authors (BH and BR) independently rated the certainty of evidence for each outcome.
We included five appendices entitled 'Checklist to aid consistency and reproducibility of GRADE assessments', to help with standardisation of the 'Summary of findings' tables (Meader 2014). Alternatively, we would have used the GRADEpro Guideline Development Tool (GDT) software and presented evidence profile tables as an appendix (GRADEpro GDT 2015). We presented results for outcomes as described in the Types of outcome measures section. When meta‐analysis was not possible, we presented the results in a narrative format in the 'Summary of findings' tables. We justified all decisions to downgrade the quality of trials by using footnotes, and we made comments to aid the reader's understanding of the Cochrane Review when necessary.
'Summary of findings' tables
We presented a summary of the evidence in the Table 1 and the Table 2. This provides key information about the best estimate of the magnitude of effect, in relative terms and as absolute differences for each relevant comparison of alternative management strategies, numbers of participants and trials addressing each important outcome, and a rating of overall confidence in effect estimates for each outcome. We created the 'Summary of findings' table using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011) along with Review Manager (RevMan 5.3) table editor (RevMan 2014).
Summary of findings for the main comparison. Summary of findings table for metformin compared with diet and exercise or another antidiabetic drug.
| Metformin for prevention or delay of type 2 diabetes mellitus and its associated complications in persons at increased risk | ||||||
|
Population: people at increased risk for developing type 2 diabetes Settings: outpatients Intervention: metformin Comparison: diet and exercise or a non‐metformin blood glucose‐lowering drug | ||||||
| Outcomes | Diet and exercise or a non‐metformin blood glucose lowering drug | Metformin | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | Comments |
| All‐cause mortality (N) | ||||||
|
Placebo or diet and exercise Follow‐up: 1 to 5 years |
5 per 1000 | 5 per 1000 (2 to 14) | RR 1.11 (0.41 to 3.01) | 2833 (5) | ⊕⊝⊝⊝ very lowa | |
|
Intensive diet plus exercise Follow‐up: 1 to 5 years |
3 per 1000 | 5 per 1000 (2 to 16) | RR 1.61 (0.50 to 5.23) | 2550 (4) | ⊕⊝⊝⊝ very lowa | |
| Sulphonylurea | Not reported | |||||
|
Acarbose Follow‐up: 5 years |
See comment | 89 (1) | ⊕⊝⊝⊝ very lowb | 1/44 participants in the metformin group compared with 0/45 in the acarbose group died (Fang 2004) | ||
| Thiazolidinediones | Not reported | |||||
| Incidence of type 2 diabetes mellitus (N) | ||||||
|
Placebo or diet and exercise Diagnostic criteria:
Follow‐up: 1 to 5 years |
281 per 1000 | 141 per 1000 (107 to 183) | RR 0.50 (0.38 to 0.65) | 3632 (12) | ⊕⊕⊕⊝ moderatec | |
|
Intensive diet plus exercise Diagnostic criteria:
Follow‐up: 1 to 5 years |
167 per 1000 | 133 per 1000 (78 to 228) | RR 0.80 (0.47 to 1.37) | 2960 (7) | ⊕⊕⊕⊝ moderatec | |
| Sulphonylurea | Not reported | |||||
|
Acarbose Diagnostic criteria:
Follow‐up: 1 to 5 years |
47 per 1000 | 81 per 1000 (34 to 196) | RR 1.72 (0.72 to 4.14) | 295 (3) | ⊕⊕⊝⊝ lowd | |
|
Thiazolidinediones Diagnostic criteria:
Follow‐up: 2 to 3 years |
57 per 1000 | 56 per 1000 (23 to 136) | RR 0.99 (0.41 to 2.40) | 320 (3) | ⊕⊕⊝⊝ lowd | 1 trial reported that no participant developed T2DM (Maji 2005) |
| Serious adverse events (SAE) | ||||||
|
Placebo or diet and exercise Follow‐up: 1 to 5 years |
See comment | 309 (4) | ⊕⊝⊝⊝ very lowe | The reporting of SAE was insufficient 1 trial reported no SAE in 29 participants in the metformin group and 30 participants in the standard care group (PREVENT‐DM 2017) In 1 trial 3/45 participants in the metformin group experienced severe gastrointestinal reactions (Jin 2009) In 1 trial 1/44 participants died due to liver cancer in the metformin group compared to 0/35 participants in the standard care group (Fang 2004) In 1 trial 1/75 participants in the standard care group died due to cerebral thrombosis with pulmonary infection and 1/51 participants in the standard care plus fibre diet group experienced stomach cancer (Lu 2002) |
||
|
Intensive diet plus exercises Follow‐up: 1 to 5 years |
See comment | 139 (2) | ⊕⊝⊝⊝ very lowe | The reporting of SAE was sparse 1 trial reported no SAE in 29 participants in the metformin group and 30 participants in the standard care group (PREVENT‐DM 2017) In 1 trial 1/44 participants died due to liver cancer in the metformin group compared to 0/36 participants in the intensive exercise and diet group (Fang 2004) |
||
| Sulphonylurea | Not reported | |||||
|
Acarbose Follow‐up: 1 year |
See comment | 101 (1) | ⊕⊝⊝⊝ very lowe | In 1 trial 1/51 participants in the metformin group experienced cerebral haemorrhage, whereas 2/50 participants in the acarbose group experienced lung cancer and hepatitis, respectively (Liao 2012) | ||
|
Thiazolidinediones Follow‐up: 3 years |
See comments | 86 (1) | ⊕⊝⊝⊝ very lowe | In 1 trial 3/45 participants in the metformin group experienced severe gastrointestinal reactions (Jin 2009). No severe reactions were reported in the 41 participants in the thiazolinedione group | ||
| Cardiovascular mortality | ||||||
|
Placebo or diet and exercise Follow‐up: 2.8 to 3 years |
See comment | 2416 (2) | ⊕⊝⊝⊝ very lowf | 1 trial reported that no participant died due to cardiovascular causes (IDPP‐1 2006) 1 trial reported that 1/1073 participants in the metformin group compared with 4/1082 participants in the control group died (DPP/DPPOS 2002) |
||
|
Intensive diet plus exercise Follow‐up: 2.8 to 3 years |
See comment | 2400 (2) | ⊕⊝⊝⊝ very lowf | 1 trial reported that no participants died due to cardiovascular causes (IDPP‐1 2006) 1 trial reported that 1/1073 participants in the metformin group compared with 2/1079 participants in the intensive diet plus exercise group died from cardiovascular causes (DPP/DPPOS 2002) |
||
| Sulphonylurea | Not reported | |||||
| Acarbose | Not reported | |||||
| Thiazolidinediones | Not reported | |||||
| Non‐fatal myocardial infarction/stroke | ||||||
|
Placebo or diet and exercise Follow‐up: 2.8 to 3 years |
See comments | 2416 (2) | ⊕⊝⊝⊝ very lowf | No trial reported data exclusively on non‐fatal myocardial infarction or stroke Non‐fatal cardiovascular events occurred in 1.7% of the participants in the control group compared with 1.5% of the participants in the metformin group (DPP/DPPOS 2002) In the IDPP 2/133 participants in the diet and exercise group versus 0/128 participants in the metformin group had a cardiovascular event (IDPP‐1 2006) |
||
|
Intensive diet plus exercise Follow‐up: 2.8 to 3 years |
See comments | 2400 (2) | ⊕⊝⊝⊝ very lowf | No trial reported data exclusively on non‐fatal myocardial infarction or stroke 1 trial reported that non‐fatal cardiovascular events occurred in 1.7% of the participants in the control group compared with 1.5% of the participants in the metformin group (DPP/DPPOS 2002) 1 trial reported that 0/128 participants in the metformin group compared to 4/120 participants in the comparator group experienced cardiovascular events (IDPP‐1 2006) |
||
| Sulphonylurea | Not reported | |||||
| Acarbose | Not reported | |||||
| Thiazolidinediones | Not reported | |||||
| Health‐related quality of life | ||||||
|
Placebo or diet and exercise Description: SF‐36 to evaluate the health utility index SF‐6D (physical component summaries and mental component summaries) Minimal important difference: difference in scores between groups of at least 3% Follow‐up: 3.2 years |
See comment | 2144 (1) | ⊕⊝⊝⊝ very lowg | After a mean of 3.2 years of follow‐up there was no clear difference in any of the health‐related quality of life scores between the metformin group compared with the placebo group (DPP/DPPOS 2002) | ||
| Intensive diet plus exercise | Not reported | |||||
| Sulphonylurea | Not reported | |||||
| Acarbose | Not reported | |||||
| Thiazolidinediones | Not reported | |||||
| Socioeconomic effects | ||||||
|
Placebo or diet and exercise Description: direct medical costs Follow‐up: 2.8 to 3 years |
The mean direct medical costs of the control groups ranged from $61 to $184 | The mean direct medical costs in the metformin groups ranged from $220 to $1177 | ‐ | 2416 (2) | ⊕⊕⊝⊝ lowh | DPP: $1177 for the metformin intervention versus $184 for the placebo group (DPP/DPPOS 2002) IDPP: $220 for metformin group versus $61 in the diet and exercise group (IDPP‐1 2006) |
|
Intensive diet plus exercise Description: direct medical costs per participant Follow‐up: 2.8 to 3 years |
The mean direct medical costs of the diet plus exercise groups ranged from $225 to $3628 | The mean direct medical costs in the metformin groups ranged from $220 to $1177 | ‐ | 2400 (2) | ⊕⊕⊝⊝ lowh | DPP: $1177 for the metformin intervention versus $3628 for the intensive diet plus exercise group (DPP/DPPOS 2002) IDPP: $220 for the metformin group compared with $225 in the intensive diet plus exercise group (IDPP‐1 2006) |
| Sulphonylurea | Not reported | |||||
| Acarbose | Not reported | |||||
| Thiazolidinediones | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADA: American Diabetes Association; CI: confidence interval; DPP: Diabetes Prevention Program; FPG: fasting plasma glucose; IDDP: Indian Diabetes Prevention Program; OGTT: oral glucose tolerance test; RR: risk ratio; SAE: serious adverse event; SF‐36: Short Form 36 items questionnaire; T2DM: type 2 diabetes mellitus. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
*Assumed risk was derived from the event rates in the comparator groups
aDowngraded by three levels because of risk of bias including possible publication and other bias (early termination of studies due to benefit providing the majority of data), inconsistency and imprecision ‐ see Appendix 15; Appendix 16 bDowngraded by three levels because of risk of bias and serious risk of imprecision ‐ see Appendix 17 cDowngraded by one level because of other bias (early termination of studies due to benefit providing the majority of data) ‐ see Appendix 15 d Downgraded by two levels because of risk of bias and imprecision ‐ see Appendix 17; Appendix 18 eDowngraded by three levels because of risk of bias including very high risk of publication and other bias and imprecision ‐ see Appendix 15; Appendix 16 fDowngraded by three levels because of risk of bias including risk of publication and other bias ‐ see Appendix 15; Appendix 16 gDowngraded by three levels because of serious risk of bias (performance bias, detection bias, other bias) and imprecision ‐ see Appendix 15 hDowngraded by two levels because of risk of bias (trial stopped early for benefit providing the majority of data) and imprecision ‐ see Appendix 15; Appendix 16
Summary of findings 2. Summary of findings table for metformin plus intensive diet and exercise compared with intensive diet and exercise.
| Metformin for prevention or delay of type 2 diabetes mellitus and its associated complications in persons at increased risk | ||||||
|
Population: people at increased risk for developing type 2 diabetes Settings: outpatients Intervention: metformin plus intensive diet and exercise Comparison: intensive diet and exercise | ||||||
| Outcomes | Intensive diet plus exercise | Metformin plus intensive diet and exercise | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | Comments |
|
All‐cause mortality (N) Follow‐up: 1.5 to 3 years |
See comment | 450 (2) | ⊕⊝⊝⊝ very lowa | 1 trial reported that 1/121 participants died in the metformin plus intensive diet plus exercise group compared to 1/120 participants in the intensive diet plus exercise group (IDPP‐1 2006) 1 trial reported that 0/95 participants died in the metformin intensive diet plus exercise group compared with 0/114 participants in the intensive diet plus exercise group (Iqbal Hydrie 2012). |
||
|
Incidence of type 2 diabetes mellitus (N) Diagnostic criteria:
Follow‐up: 1 to 3 years |
289 per 1000 | 159 per 1000 (29 to 844) | RR 0.55 (0.10 to 2.92) | 332 (2) | ⊕⊝⊝⊝ very lowb | |
| Serious adverse events | Not reported | |||||
| Cardiovascular mortality | See comment | 1 trial reported that no participant (47 participants in each intervention group) died due to cardiovascular causes (IDPP‐1 2006). | ||||
| Non‐fatal myocardial infarction/stroke | Not reported | |||||
| Health‐related quality of life | Not reported | |||||
|
Socioeconomic effects Description: direct medical costs per participant Follow‐up: 3 years |
The mean direct medical costs of the intensive diet and exercise group were $225 | The mean direct medical costs in the metformin plus diet and exercise group were $270 | ‐ | 94 (1) | ⊕⊝⊝⊝ very lowc | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
*Assumed risk was derived from the event rates in the comparator groups
aDowngraded by three levels because of risk of bias and serious risk of imprecision ‐ see Appendix 19 bDowngraded by three levels because of risk of bias, inconsistency and imprecision ‐ see Appendix 19 cDowngraded by three levels because of trial stopped early for benefit (providing the majority of data), risk of bias and imprecision ‐ see Appendix 19
Interventions presented in the 'Summary of findings' tables were metformin and metformin plus intensive diet plus exercise and comparators were diet and exercise, another blood glucose lowering drug or intensive diet plus exercise.
We reported the following outcomes, listed according to priority.
All‐cause mortality.
Incidence of T2DM.
Serious adverse events.
Cardiovascular mortality.
Non‐fatal myocardial infarction/stroke.
Health‐related quality of life.
Socioeconomic effects.
Results
Description of studies
For a detailed description of studies, see the 'Characteristics of included studies', 'Characteristics of excluded studies, and 'Characteristics of ongoing studies' sections.
Results of the search
The search resulted in 4289 records, which after deduplication were reduced to 3249 records. A total of 170 references were identified as potentially eligible after screening title and abstract. Of these, 49 were excluded after checking full text. Furthermore, one publication was excluded after contact with the main author (duration of intervention less than one year) (ChiCTR‐TRC‐09000548), one Japanese publication was excluded after translation (not a randomised controlled trial (RCT)) (Ishida 2005) and one Chinese publication was excluded after translation (wrong intervention) (Chen 2013). Of the remaining eligible 118 records, there were 11 ongoing trials and five trials awaiting assessment. Cross‐checking four systematic reviews (Haw 2017; Lily 2009; Moelands 2018; Salpeter 2008) revealed three additional references to already included trials. One systematic review (Pang 2018) revealed a further 10 Chinese trials to be included. At the end of the process we identified 20 trials (102 records) meeting our inclusion criteria. The flowchart of records throughout the screening process is presented in Figure 1.
Included studies
A detailed description of the characteristics of included trials is presented elsewhere (see Characteristics of included studies and Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 9; Appendix 10; Appendix 11; Appendix 12; Appendix 13). The following is a succinct overview.
Source of data
All but one trial reported data published in medical journals (Wang 2009). One trial was published as a conference proceeding (Wang 2009). One trial reported additional data in trial registers (DPP/DPPOS 2002). We contacted all authors or investigators of included trials by email (see Appendix 15). No additional data were provided.
Comparisons
Fifteen trials compared metformin with placebo or diet and exercise (Alfawaz 2018; BIGPRO1 2009; Chen 2009; DPP/DPPOS 2002; Fang 2004; IDPP‐1 2006; Ji 2011; Jin 2009; Li 1999; Lu 2002; Lu 2010; Papoz 1978; PREVENT‐DM 2017; Wang 2009; Zeng 2013). One trial compared metformin with a sulphonylurea (Papoz 1978). Three trials compared metformin with acarbose (Fang 2004; Liao 2012; Maji 2005). Three trials compared metformin with a thiazolidinediones (Jin 2009; Maji 2005; Zeng 2013). Eight trials compared metformin with intensive diet and exercise (Alfawaz 2018; DPP/DPPOS 2002; Fang 2004; IDPP‐1 2006; Ji 2011; Li 2009; Maji 2005; PREVENT‐DM 2017). Three trials compared metformin plus intensive diet and exercise with intensive diet and exercise (IDPP‐1 2006; Iqbal Hydrie 2012; Zhao 2013). Ten trials had more than two comparison groups of relevance for this review (Alfawaz 2018; DPP/DPPOS 2002; Fang 2004; IDPP‐1 2006; Ji 2011; Jin 2009; Maji 2005; Papoz 1978; PREVENT‐DM 2017; Zeng 2013).
Overview of trial populations
Five trials provided information on sample size calculation (BIGPRO1 2009; DPP/DPPOS 2002; IDPP‐1 2006; Iqbal Hydrie 2012; PREVENT‐DM 2017). Eight of the included trials reported the total number of participants screened (BIGPRO1 2009; DPP/DPPOS 2002; Fang 2004; IDPP‐1 2006; Iqbal Hydrie 2012; Li 1999; Maji 2005; PREVENT‐DM 2017). A total of 2426 participants were randomised to metformin. A total of 4348 participants were randomised to a comparator group. The number of randomised participants ranged from 28 to 1073 in the metformin groups and from 23 to 1082 in the comparator groups.
Trial design
All of the 20 included trials were parallel RCTs. Four trials performed blinding of the participants and investigators for one or more comparators (BIGPRO1 2009; DPP/DPPOS 2002; Li 1999; Papoz 1978), the same four trials applied placebo. Three trials reported a run‐in period (DPP/DPPOS 2002; Liao 2012; Maji 2005). Two trials were terminated (DPP/DPPOS 2002; IDPP‐1 2006). The duration of the intervention in the included trials varied from one year to five years. The trials were performed between the years 1969 and 2017. One trial had an extended follow‐up period after the intervention period had stopped (DPP/DPPOS 2002). Four trials were multicentre trials, defined as two or more trial centres (Alfawaz 2018; BIGPRO1 2009; DPP/DPPOS 2002; Iqbal Hydrie 2012). Twelve trials were single‐centre trials (Chen 2009; Fang 2004; Ji 2011; Jin 2009; Li 2009; Liao 2012; Lu 2010; Papoz 1978; PREVENT‐DM 2017; Wang 2009; Zeng 2013; Zhao 2013), and four trials did not provide the number of trial centres (IDPP‐1 2006; Li 1999; Lu 2002; Maji 2005). Two trials were performed in the USA ( DPP/DPPOS 2002; PREVENT‐DM 2017), two trials were performed in France (BIGPRO1 2009; Papoz 1978), two trials were performed in the Middle‐east (Alfawaz 2018; Iqbal Hydrie 2012), the remaining trials were performed in Asia. Three of the included trials stated that they had received commercial funding (BIGPRO1 2009; DPP/DPPOS 2002; IDPP‐1 2006). Six trials had received non‐commercial funding (Alfawaz 2018; Iqbal Hydrie 2012; Ji 2011; Jin 2009; Papoz 1978; PREVENT‐DM 2017). One trial stated that they had received military funding (Lu 2002). Eight trials did not report the funding source (Li 1999; Li 2009; Liao 2012; Lu 2010; Maji 2005; Wang 2009; Zeng 2013; Zhao 2013).
Settings
All included trials were performed in an outpatient setting.
Participants
Fifteen trials included only people from Asia; 12 of these Chinese (Chen 2009; Fang 2004; Ji 2011; Jin 2009; Li 1999; Li 2009; Liao 2012; Lu 2002; Lu 2010; Wang 2009; Zeng 2013; Zhao 2013); two Indian (IDPP‐1 2006; Maji 2005); one Pakistini (Iqbal Hydrie 2012). One trial included only Saudi Arabians (Alfawaz 2018). One trial only included Hispanic participants (PREVENT‐DM 2017). One trial included mainly White participants (DPP/DPPOS 2002). Two trials did not report information about ethnicity (BIGPRO1 2009; Papoz 1978) (see Appendix 5). Five trials did not report the gender of the participants in each intervention group (Iqbal Hydrie 2012; Jin 2009; Li 2009; Maji 2005; Wang 2009). One trial included only females (PREVENT‐DM 2017), and one trial included only males (Papoz 1978). For the remaining trials authors provided gender information, and both men and women were included. Four trials did not report the age of the participants (Jin 2009; Li 2009; Maji 2005; Zhao 2013). The age of the included participants varied from 41 to 65 years (see Appendix 8).
All, but five trials reported baseline fasting glucose (Iqbal Hydrie 2012; Lu 2002; Lu 2010; Wang 2009; Zhao 2013). The reported fasting glucose values at baseline varied from 5.3 mmol/L to 7.3 mmol/L. All, but four trials reported 2‐hour plasma glucose after an oral glucose tolerance test (OGTT) at baseline (Alfawaz 2018; Iqbal Hydrie 2012; Lu 2010; PREVENT‐DM 2017). The 2‐hour plasma glucose values varied from 6.4 mmol/L to 10.4 mmol/L. Seven trials reported HbA1c values at baseline (Alfawaz 2018; DPP/DPPOS 2002; IDPP‐1 2006; Li 1999; Lu 2010; Maji 2005; PREVENT‐DM 2017). HbA1c varied from 5.6% to 7.6%. One trial did not report any glycaemic variables at baseline (Iqbal Hydrie 2012). All, but three trials reported body mass index (BMI) at baseline (Chen 2009; Liao 2012; Papoz 1978). BMI varied from 24 kg/m2 to 35.6 kg/m2.
Six trials did not report exclusion criteria (Iqbal Hydrie 2012; Ji 2011; Li 2009; Lu 2002; Maji 2005; Papoz 1978). Major exclusion criteria were diagnosis of diabetes; receiving glucose‐lowering interventions and taking medications known to alter glucose tolerance; pregnant or lactating women; known renal, hepatic, pulmonary, cardiac, cerebral, mental or endocrine disease; heavy alcohol consumption.
Diagnosis
The diagnosis applied in the included trials for identifying intermediate hyperglycaemia varied. Three trials applied the World Health Organization (WHO) 1985 diagnostic criteria for the definition of impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) (fasting plasma glucose < 7.8 mmol/L and 2‐hour plasma glucose after (OGTT) ≥ 7.8 mmol/L and < 11.1 mmol/L) (Fang 2004; Li 1999; Lu 2002). Ten trials applied the WHO 1999 criteria for the definition of IFG and/or IGT (fasting plasma glucose < 7.0 mmol/L and 2‐hour plasma glucose after OGTT ≥ 7.8 mmol/L and < 11.1 mmol/L) (BIGPRO1 2009; Chen 2009; IDPP‐1 2006; Iqbal Hydrie 2012; Ji 2011; Jin 2009; Li 2009; Liao 2012; Zeng 2013; Zhao 2013). Two trials applied the diagnostic criteria for impaired glucose defined by American Diabetes Association (ADA) 1997 (fasting plasma glucose concentration of 5.3 mmol/L to 6.9 mmol/L and 2‐hour plasma glucose after OGTT ≥ 7.8 mmol/L to 11.0 mmol/L) (ADA 1997) (DPP/DPPOS 2002; Wang 2009). For the American Indian clinics in the Diabetes Prevention Program (DPP), fasting plasma glucose less then 6.9 mmol/L with no lower limit applied. Before June 1997, the criterion for plasma fasting glucose was 5.6 mmol/L to 7.7 mmol/L, or less than 7.7 mmol/L in the American Indian clinics (DPP/DPPOS 2002). A total of the 54 participants (total in all three intervention groups) included in the DPP had fasting plasma glucose above 7.0 mmol/L at baseline (DPP/DPPOS 2002). Thirteen per cent of the participants included in the DPP trial had HbA1c ≥ 6.5% at baseline (DPP/DPPOS 2002). One trial applied the diagnostic criteria for impaired glucose defined by ADA 2009 (fasting plasma glucose 5.6 mmol/L to 6.9 mmol/L or 2‐hour plasma glucose 7.8 mmol/L to 11.1 mmol/L) (Lu 2010). One trial only applied the IFG criteria defined by ADA 2009 (fasting plasma glucose 5.6 mmol/L to 6.9 mmol/L) or a HbA1c 5.7% to 6.4% (PREVENT‐DM 2017). Most of the participants were included based on an elevated HbA1c only (67%); 13% of the participants fulfilled the inclusion criteria by IFG only; the remaining participants had both IFG and intermediate elevated HbA1c (PREVENT‐DM 2017). One trial applied the diagnostic criteria for IFG defined by ADA 2017 (fasting plasma glucose 5.6 mmol/L to 6.9 mmol/L) (Alfawaz 2018). One trial applied the diagnostic criteria for impaired glucose defined by the European Diabetes Epidemiology Study Group 1970 (fasting blood glucose ≥ 5.6 mmol/L and < 7.2 mmol/L or 2‐hour blood glucose after OGTT ≥ 6.7 mmol/L and < 8.3 mmol/L; when these criteria for intermediate hyperglycaemia were fulfilled, a second test was performed: blood glucose concentrations were determined fasting at 15, 30, 60, 120, 80, 240 and 300 minutes after an oral glucose load. Eligible individuals had 2‐hour blood glucose concentrations ≥ 6.7 mmol/L but < 8.3 mmol/L or fasting blood glucose concentrations ≥ 5.6 mmol/L and < 7.2 mmol/L; blood glucose after 30 minutes ≥ 8.9 mmol/L and < 12.2 mmol/L; blood glucose after 60 minutes ≥ 8.9 mmol/L and < 12.2 mmol/L) (Papoz 1978). Another trial defined IGT as 2‐hour plasma glucose after OGTT ≥ 6.1 mmol/L and < 11.1 mmol/L and fasting plasma glucose < 6.1 mmol/L (Maji 2005). No medical associations recommend the cut‐off points applied in the study by Maji and colleagues to diagnose intermediate hyperglycaemia (Maji 2005).
In one trial, the people with IFG and IGT were only a subset of the total randomised participants (101 out of 457 (22.1%)) (BIGPRO1 2009).
Interventions
The metformin intervention varied among the included trials; one trial applied metformin 38 mg once daily with standard diet and physical activity (Zeng 2013); one trial applied metformin 375 mg to 750 mg three times a day with no concomitant intervention (Fang 2004); one trial applied metformin 250 mg twice daily with standard diet, physical activity and education (Wang 2009); two trials applied metformin 250 mg three times a day with no concomitant intervention (Li 1999; Liao 2012); three trials applied metformin 500 mg daily with standard diet and physical activity (Alfawaz 2018; Li 2009; Maji 2005); one trial randomised the participants into two different metformin groups; one metformin group receiving 500 mg twice daily with concomitant standard diet and physical activity and one metformin group receiving 500 mg twice daily with intensive diet and physical activity (IDPP‐1 2006); one trial applied metformin 500 mg twice daily with intensive diet and physical activity (Iqbal Hydrie 2012); one trial applied metformin 500 mg twice daily with standard diet, physical activity and education (Zhao 2013); two trials applied metformin 500 mg three times a day with standard diet, physical activity and education (Ji 2011; Lu 2010); one trial applied metformin 750 mg three times a day with standard diet and physical activity (Chen 2009); one trial applied metformin 750 mg three times a day with education (Lu 2002); four trials applied metformin 850 mg twice daily with standard diet and physical activity (BIGPRO1 2009; DPP/DPPOS 2002; Papoz 1978; PREVENT‐DM 2017); and one trial applied metformin 1000 mg twice or three times a day with standard diet and physical activity (Jin 2009). For details see 'Description of interventions' Appendix 4
Outcomes
Three trials had specified primary outcomes (DPP/DPPOS 2002; IDPP‐1 2006; PREVENT‐DM 2017), all of these trials were registered at ClinicalTrials.gov (Appendix 7).
Sixteen trials reported the incidence of type 2 diabetes mellitus (T2DM) (Chen 2009; DPP/DPPOS 2002; Fang 2004; IDPP‐1 2006; Ji 2011; Jin 2009; Li 1999; Li 2009; Liao 2012; Lu 2002; Lu 2010; Maji 2005; PREVENT‐DM 2017; Wang 2009; Zeng 2013; Zhao 2013). Five trials reported all‐cause mortality (DPP/DPPOS 2002; Fang 2004; IDPP‐1 2006; Lu 2002; PREVENT‐DM 2017). Sixteen trials reported 2‐hour glucose values (BIGPRO1 2009; Chen 2009; DPP/DPPOS 2002; Fang 2004; IDPP‐1 2006; Ji 2011; Jin 2009; Li 1999; Li 2009; Liao 2012; Lu 2002; Lu 2010; Papoz 1978; Wang 2009; Zeng 2013; Zhao 2013). Six trials reported HbA1c (Alfawaz 2018; DPP/DPPOS 2002; Ji 2011; Li 1999; Lu 2010; PREVENT‐DM 2017). Eighteen trials reported fasting glucose values (Alfawaz 2018; BIGPRO1 2009; Chen 2009; DPP/DPPOS 2002; Fang 2004; IDPP‐1 2006; Ji 2011; Jin 2009; Li 1999; Li 2009; Liao 2012; Lu 2002; Lu 2010; Papoz 1978; PREVENT‐DM 2017; Wang 2009; Zeng 2013; Zhao 2013).
The reporting of adverse events was lacking in most trials (see Appendix 11; Appendix 12; Appendix 13).
Source of data
Where possible, we contacted all trial authors or investigators through email. If an email address was not provided in the publication we tried to contact authors by phone (see Appendix 14).
Excluded studies
We excluded 53 articles or records after full‐text evaluation (Figure 1). These references are listed in Characteristics of excluded studies. We excluded 30 trials published in 31 references as they had a duration of the intervention less than one year (Acbay 1996; Ballon 2011; Biarnés 2005; Bulcão 2007; Caballero 2004; Chazova 2006; ChiCTR‐TRC‐09000548; Eguchi 2007; Esteghamati 2013; Flores‐Saenz 2003; Gómez‐Díaz 2012; Gore 2005; Kelly 2012; Kendall 2013; Kilic 2011; Koev 2004; Krysiak 2012; Lehtovirta 2001; Li 2009b; LIMIT‐1; Malin 2013; Morel 1999; NCT00108615; NCT02338193; RESIST; Retnakaran 2012; SLCTR/2016/026; Stroup 2013; Sultana 2012; Wan 2010). Ten trials published in 10 references were excluded due to wrong population (Celik 2012; Fleming 2002; Gram 2011; Haukeland 2008; Kato 2009; NCT03258723; Rodríguez‐Moctezuma 2005; Scheen 2009; Schuster 2004; UKPDS). Six trials published in six references were excluded due to wrong intervention (Chen 2013; Guardado‐Mendoza R 2018; Lu 2011; Pre‐DICTED; STOP‐NIDDM; Zinman 2010). Four trials, one medical letter and one narrative review published in a total of six references were excluded as they were not RCTs (CTRI/2013/02/003417; EUCTR‐000650‐21‐ES; EUCTR2008‐004497‐40‐GB; Ishida 2005; Medical letter; Vitolins 2017).
Risk of bias in included studies
For details on the risk of bias of the included trials see Characteristics of included studies.
For an overview of review authors' judgements about each risk of bias item for individual trials and across all trials see Figure 2 and Figure 3.
2.
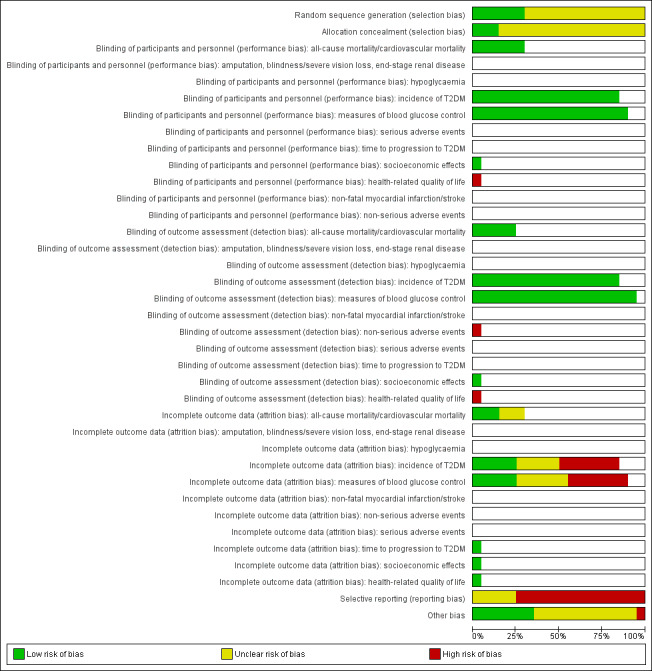
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies (blank cells indicate that the particular outcome was not measured in some trials).
3.
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study (blank cells indicate that the trial did not measure that particular outcome).
Allocation
We judged three trials to be at low risk of selection bias regarding the method of randomisation and allocation concealment (Alfawaz 2018; DPP/DPPOS 2002; PREVENT‐DM 2017). Three trials only reported the method of randomisation but not how allocation concealment was achieved (BIGPRO1 2009; Fang 2004; Zeng 2013). The remaining trials only reported that the participants were randomised but did not provide any further description. Therefore, these trials were judged as unclear risk of bias regarding randomisation and allocation concealment.
We evaluated trial baseline data for our predefined prognostic baseline variables. None of the trials reporting one or more key prognostic variables showed important differences between the intervention groups (see Appendix 5; Appendix 6)
Blinding
Four trials explicitly reported blinding of participants and investigators (BIGPRO1 2009; DPP/DPPOS 2002; Li 1999; Papoz 1978). However, one trial had a comparator group receiving placebo, which was blinded and an intensive diet and exercise group which was not blinded for the investigators and participants (DPP/DPPOS 2002).
When measured, all primary outcomes of this review were investigator‐assessed and we judged these at low risk of performance and detection bias. The trials reporting blood glucose measurements were all performed by the investigators and we judged these outcomes measures at low risk of performance and detection bias.
Incomplete outcome data
All, but two trials reported the complete number of participants randomised and completing the trial (DPP/DPPOS 2002; Maji 2005).
We judged five trials to have low risk of incomplete outcome data for all outcomes reported with relevance of our review (BIGPRO1 2009; DPP/DPPOS 2002; Ji 2011; Lu 2010; PREVENT‐DM 2017). We judged seven trials to have unclear risk of attrition bias for one or more outcomes (Fang 2004; IDPP‐1 2006; Iqbal Hydrie 2012; Li 1999; Maji 2005; Papoz 1978; Zeng 2013). The reason for unclear risk of attrition bias were unclear or missing description of how missing data were handled, unclear whether mortality status was investigated in the people lost to follow‐up and reasons for dropout were not reported. We judged eight trials to have high risk of attrition bias for one or more of the outcomes (Alfawaz 2018; Chen 2009; Jin 2009; Li 2009; Liao 2012; Lu 2002; Wang 2009; Zhao 2013). The reason for high risk of attrition bias were high dropout rate, dropout rates not balanced, reasons for dropouts not balanced, missing information on dropouts or per protocol analysis applied.
Selective reporting
We judged 15 trials as high risk of selective outcome reporting mainly because one or more outcomes of relevance for our review were likely assessed but not reported and/or the protocol were unavailable. For more details, see Figure 3, Appendix 7 and Appendix 8.
Other potential sources of bias
Seven trials appeared to be free of other potential sources of bias (Alfawaz 2018; Iqbal Hydrie 2012; Ji 2011; Jin 2009; Lu 2002; Papoz 1978; PREVENT‐DM 2017). Three of the included trials stated that they had received support from a pharmaceutical company (BIGPRO1 2009; DPP/DPPOS 2002; IDPP‐1 2006). Nine trials did not report the funding source (Chen 2009; Fang 2004; Li 1999; Li 2009; Lu 2010; Maji 2005; Wang 2009; Zeng 2013; Zhao 2013). It is known that trials receiving funding or provision of free drug or devices from a pharmaceutical company lead to more favourable results and conclusions than trials sponsored by other sources (Lundh 2017). Therefore, these trials were judged at unclear risk of bias in the 'other sources' bias‐domain.
Effects of interventions
Baseline characteristics
For details of baseline characteristics, see Appendix 5 and Appendix 6.
Metformin versus placebo or diet and exercise
Fifteen trials compared metformin with diet and exercise in combination with placebo (BIGPRO1 2009; DPP/DPPOS 2002; Li 1999; Papoz 1978) or without concomitant placebo (Alfawaz 2018; Chen 2009; Fang 2004; IDPP‐1 2006; Ji 2011; Jin 2009; Lu 2002; Lu 2010; PREVENT‐DM 2017; Wang 2009; Zeng 2013). One trial administered metformin in doses of 38 mg/day (Zeng 2013). One trial administered metformin in doses up to 500 mg/day. One trial administered metformin in doses up to 750 mg/day (Li 1999); two trials administered metformin in doses up to 1000 mg/day (Alfawaz 2018; IDPP‐1 2006); two trials administered metformin in doses up to 1500 mg/day (Ji 2011; Lu 2010); three trials administered metformin in doses up to 1700 mg/day (BIGPRO1 2009; DPP/DPPOS 2002; Papoz 1978); three trials administered metformin in doses up to 2250 mg/day (Chen 2009; Fang 2004; Lu 2002); one trial administered metformin in doses up to 2550 mg/day (PREVENT‐DM 2017); and one trial administered metformin in doses up to 3000 mg/day (Jin 2009). One trial had an extended follow‐up period (DPP/DPPOS 2002). Ten trials stated that the metformin group also received concomitant standard diet plus exercise (Alfawaz 2018; BIGPRO1 2009; Chen 2009; DPP/DPPOS 2002; Ji 2011; Jin 2009; Lu 2002; Lu 2010; Wang 2009; Zeng 2013). Nine trials included people of Chinese ethnicity (Chen 2009; Fang 2004; Ji 2011; Jin 2009; Li 1999; Lu 2002; Lu 2010; Wang 2009; Zeng 2013); one trial included mainly White people (DPP/DPPOS 2002); one trial included people of Saudi Arabian ethnicity (Alfawaz 2018); one trial included people of Indian ethnicity (IDPP‐1 2006); one trial included Hispanic people (PREVENT‐DM 2017); and two trials did not report ethnicity (BIGPRO1 2009; Papoz 1978).
Primary outcomes
All‐cause mortality
Five trials reported data on all‐cause mortality (DPP/DPPOS 2002; Fang 2004; IDPP‐1 2006; Lu 2002; PREVENT‐DM 2017).
A total of seven deaths were reported in 1353 participants in the metformin group versus seven out of 1480 participants in the comparator group (risk ratio (RR) 1.11, 95% confidence interval (CI) 0.41 to 3.01; P = 0.83; 2833 participants, 5 trials; very low‐quality evidence; Analysis 1.1). Calculation of a 95% prediction interval was not meaningful. We did not perform subgroup analyses and sensitivity analyses due to lack of data.
1.1. Analysis.
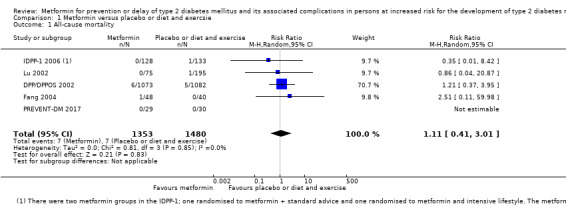
Comparison 1 Metformin versus placebo or diet and exercsie, Outcome 1 All‐cause mortality.
Incidence of type 2 diabetes (T2DM)
Twelve trials reported data on the incidence of T2DM. The definition of T2DM varied among the included trials (see Appendix 9 and Appendix 10).
A total of 324 out of 1751 participants developed T2DM in the metformin group versus 529 out of 1881 participants in the comparator group (RR 0.50, 95% CI 0.38 to 0.65; P < 0.001; 3632 participants, 12 trials; moderate‐quality evidence; Analysis 1.2). The 95% prediction interval ranged between 0.26 and 0.97.
1.2. Analysis.
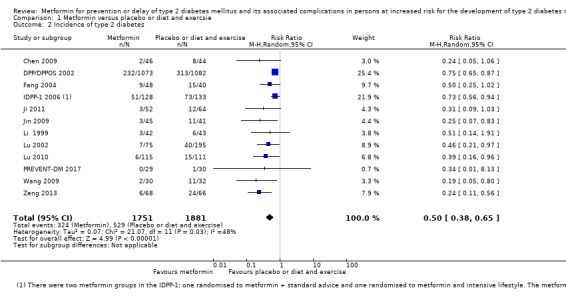
Comparison 1 Metformin versus placebo or diet and exercsie, Outcome 2 Incidence of type 2 diabetes.
One trial reported the incidence of T2DM after an extended follow‐up period (DPP/DPPOS 2002). At year 15, the cumulative incidence of T2DM was 560 participants (62%) in the former control group versus 499 participants (56%) in the former metformin group (DPP/DPPOS 2002).
The funnel plot did not show small trial effect (data not shown).
Subgroup analysis: analysing trials according to blinded versus open‐label trials showed interaction between subgroups indicating smaller effect sizes with blinded trials (P = 0.003; Analysis 1.3). Subgroup analysis according to duration of the intervention did not show interaction between subgroups (P = 0.18; Analysis 1.4). Subgroup analysis according to ethnicity showed interaction between subgroups indicating smaller effect sizes in White people (P = 0.01; Analysis 1.5). Subgroup analysis according to diagnostic criteria, age, gender, comorbid condition and previous gestational diabetes could not be performed.
1.3. Analysis.
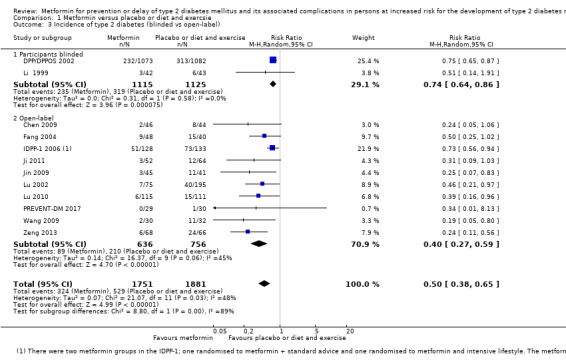
Comparison 1 Metformin versus placebo or diet and exercsie, Outcome 3 Incidence of type 2 diabetes (blinded vs open‐label).
1.4. Analysis.
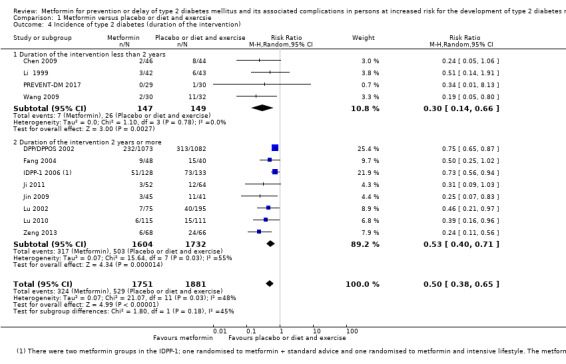
Comparison 1 Metformin versus placebo or diet and exercsie, Outcome 4 Incidence of type 2 diabetes (duration of the intervention).
1.5. Analysis.
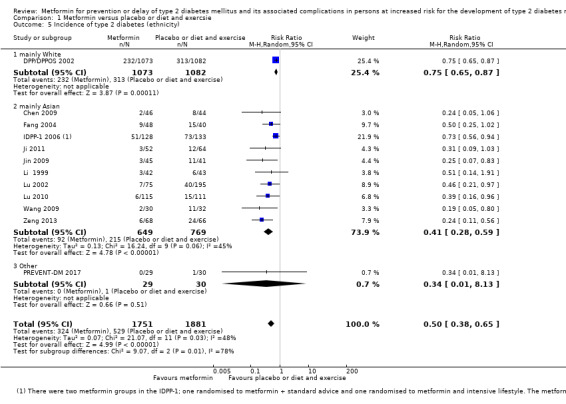
Comparison 1 Metformin versus placebo or diet and exercsie, Outcome 5 Incidence of type 2 diabetes (ethnicity).
Sensitivity analysis: sensitivity analysis according to publication status could not be performed as all included trials were published. Sensitivity analysis restricted to only trials with low risk of selection bias did not substantially change the effect estimate: RR 0.75, 95% CI 0.65 to 0.87; P < 0.001; 2155 participants, 1 trial (DPP/DPPOS 2002). Eight trials were only published in Chinese. Sensitivity analysis restricted to trials published in English did not substantially change the direction of the effect estimate: RR 0.74, 95% CI 0.65 to 0.84; P < 0.001; 2560 participants; 4 trials (DPP/DPPOS 2002; IDPP‐1 2006; Li 1999; PREVENT‐DM 2017). Sensitivity analysis excluding trials funded by a pharmaceutical company did not substantially change the direction of the effect estimate: RR 0.36, 95% CI 0.26 to 0.49; P < 0.001; 1216 participants; 10 trials (Chen 2009; Fang 2004; Ji 2011; Jin 2009; Li 1999; Lu 2002; Lu 2010; PREVENT‐DM 2017; Wang 2009; Zeng 2013).
Serious adverse events
The reporting of serious adverse events was insufficient and diverse (very low‐quality evidence). One trial reported no serious adverse events in both the intervention and comparator groups (PREVENT‐DM 2017). In one trial three out of 45 participants in the metformin group experienced severe gastrointestinal reactions (Jin 2009). In one study one out of 44 participants died due to liver cancer in the metformin group compared to 0/35 participants in the standard care group (Fang 2004). In one study one out of 75 participants in the standard care group died due to cerebral thrombosis with pulmonary infection and one out of 51 participants in the standard care plus fibre diet group experienced stomach cancer (Lu 2002).
Secondary outcomes
Cardiovascular mortality
Two trials reported cardiovascular mortality (DPP/DPPOS 2002; IDPP‐1 2006; very low‐quality evidence). One trial reported that no participant died due to cardiovascular causes (IDPP‐1 2006). One trial reported that one out of 1073 participants in the metformin group compared with four out of 1082 participants in the control group died due to cardiovascular causes (DPP/DPPOS 2002).
Non‐fatal myocardial infarction
No trial reported data exclusively on non‐fatal myocardial infarction (very low‐quality evidence). In the DPP trial non‐fatal cardiovascular events occurred in 1.7% of the participants in the control group compared with 1.5% of the participants in the metformin group (DPP/DPPOS 2002). In the Indian Diabetes Prevention Program (IDPP) trial, two out of 133 participants in the diet and exercise group versus none out of 128 participants in the metformin group had a cardiovascular event (IDPP‐1 2006).
Non‐fatal stroke
None of the trials reported on non‐fatal stroke (very low‐quality evidence).
Amputation of lower extremity
None of the trials reported on amputation of lower extremity.
Blindness or severe vision loss
None of the trials reported on blindness or severe vision loss.
End‐stage renal disease
None of the trials reported on end‐stage renal disease.
Non‐serious adverse events
Two trials reported on all non‐serious adverse events (Lu 2010; PREVENT‐DM 2017). A total of 31 out of 144 participants experienced a non‐serious adverse event in the metformin group versus 17 out of 141 participants in the comparator group (RR 3.86, 95% CI 0.18 to 83.36; P = 0.39; 285 participants, 2 trials; Analysis 1.6). Seven trials reported partially on adverse effects (DPP/DPPOS 2002; Fang 2004; IDPP‐1 2006; Jin 2009; Li 1999; Lu 2010; Wang 2009), see Appendix 11; Appendix 12; Appendix 13.
1.6. Analysis.
Comparison 1 Metformin versus placebo or diet and exercsie, Outcome 6 Non‐serious adverse events.
Hypoglycaemia
Three trials reported data on hypoglycaemia (IDPP‐1 2006; Lu 2010; Jin 2009). One trial had two intervention arms applying metformin (metformin monotherapy and metformin plus intensive diet and exercise) (IDPP‐1 2006). The number of participants with hypoglycaemia was not reported separately for each metformin group. A total of 22 out of 248 participants reported symptoms of mild hypoglycaemia. None experienced symptoms of hypoglycaemia in the comparator group (IDPP‐1 2006). One trial reported that no participant experienced hypoglycaemia (Jin 2009). One trial reported that four out of 115 participants in the metformin group experienced low blood glucose compared to two out of 111 participants in the comparator group (Lu 2010).
Health‐related quality of life
The DPP trial applied the Short Form (SF)‐36 to evaluate the health utility index (SF‐6D), physical component summaries (PCS) and mental component summaries (MCS). Minimal important difference (MID) was defined as difference in scores between groups of at least 3% (DPP/DPPOS 2002). After a mean of 3.2 years of follow‐up there was no clear difference in any of the health‐related quality of life scores between the metformin group compared with the placebo group (very low‐quality evidence).
Time to progression to T2DM
After a median of 10 years follow‐up in the DPP trial, the onset of diagnosis of T2DM was delayed by two years with metformin compared with placebo (DPP/DPPOS 2002).
Measures of blood glucose control
2‐hour glucose
Thirteen trials reported data on 2‐hour glucose after an oral glucose tolerance test (OGTT) (BIGPRO1 2009; Chen 2009; DPP/DPPOS 2002; Fang 2004; IDPP‐1 2006; Ji 2011; Jin 2009; Li 1999; Lu 2002; Lu 2010; Papoz 1978; Wang 2009; Zeng 2013). The effect estimate showed benefit in favour of metformin (mean difference (MD) ‐0.86 mmol/L; 95% CI ‐1.26 to ‐0.46; P < 0.001; 3346 participants; 13 trials; Analysis 1.7).
1.7. Analysis.
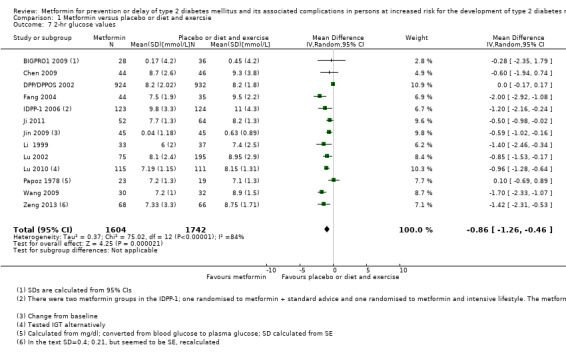
Comparison 1 Metformin versus placebo or diet and exercsie, Outcome 7 2‐hr glucose values.
Subgroup analysis: analysing trials according to blinded versus open‐label trials showed interaction between subgroups (P = 0.03; Analysis 1.8), however CIs overlapped. Subgroup analysis according to duration of the intervention did not indicate interaction between subgroups (P = 0.08; Analysis 1.9). Subgroup analysis according to ethnicity showed interaction between subgroups indicating greater effect sizes for Asian people (P < 0.001; Analysis 1.10). Subgroup analysis according to diagnostic criteria, age, gender, comorbid condition and previous gestational diabetes could not be performed.
1.8. Analysis.
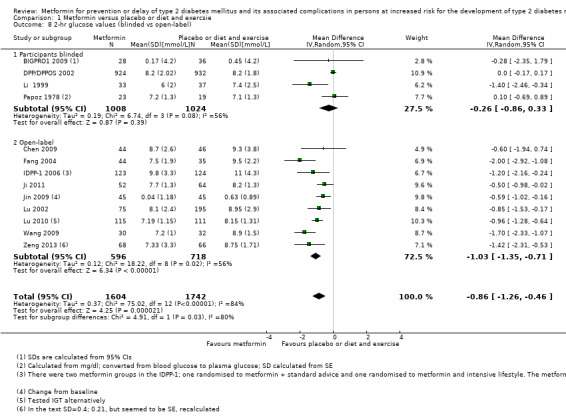
Comparison 1 Metformin versus placebo or diet and exercsie, Outcome 8 2‐hr glucose values (blinded vs open‐label).
1.9. Analysis.
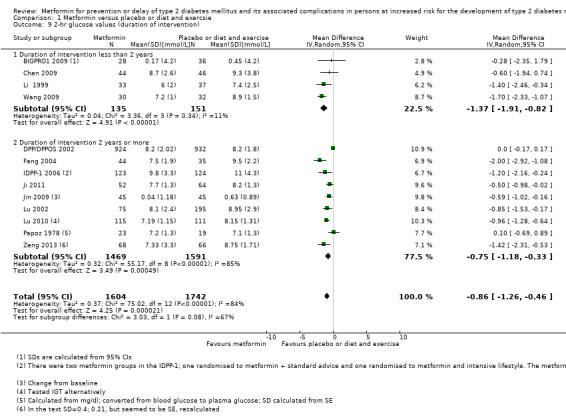
Comparison 1 Metformin versus placebo or diet and exercsie, Outcome 9 2‐hr glucose values (duration of intervention).
1.10. Analysis.
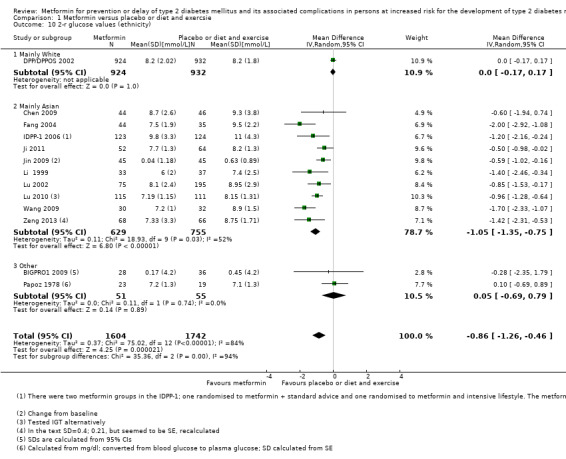
Comparison 1 Metformin versus placebo or diet and exercsie, Outcome 10 2‐r glucose values (ethnicity).
Sensitivity analysis: sensitivity analysis according to publication status could not be performed as all included trials were published. Sensitivity analysis restricted to only trials with low risk of selection bias changed the direction of the effects estimate: MD 0.00 mmol/L, 95% CI ‐0.17 to 0.17; P = 1.0; 1856 participants, 1 trial (DPP/DPPOS 2002). Eight trials were only published in Chinese. Sensitivity analysis restricted to trials published in English changed the direction of the effect estimate: MD ‐0.48 mmol/L; 95% CI ‐1.11 to 0.16; P = 0.14; 2279 participants; 5 trials (BIGPRO1 2009; DPP/DPPOS 2002; IDPP‐1 2006; Li 1999; PREVENT‐DM 2017). Sensitivity analysis excluding trials funded by a pharmaceutical company did not substantially change the direction of the effect estimate: MD ‐0.99 mmol/L, 95% CI ‐1.34 to ‐0.64; P < 0.001; 1179 participants; 10 trials (Chen 2009; Fang 2004; Ji 2011; Jin 2009; Li 1999; Lu 2002; Lu 2010; Papoz 1978; Wang 2009; Zeng 2013).
HbA1c
Six trials reported data on HbA1c (Alfawaz 2018; DPP/DPPOS 2002; Ji 2011; Li 1999; Lu 2010; PREVENT‐DM 2017) showing a MD of ‐0.08%; 95% CI ‐0.22 to 0.05; P = 0.04; 2467 participants; 6 trials; Analysis 1.11.
1.11. Analysis.
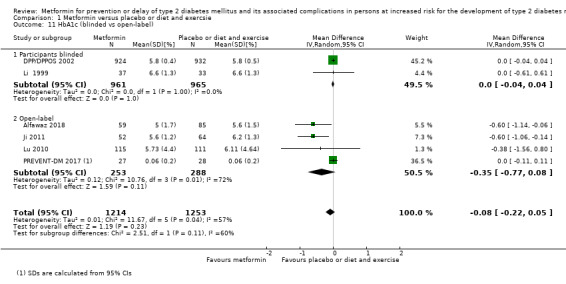
Comparison 1 Metformin versus placebo or diet and exercsie, Outcome 11 HbA1c (blinded vs open‐label).
Subgroup analysis: analysing trials according to blinded versus open‐label trials did not show interaction between subgroups (P = 0.11; Analysis 1.11 ). Subgroup analysis according to duration of the intervention did not show interaction between subgroups (P = 0.71; Analysis 1.12). Subgroup analysis according to ethnicity showed interaction between subgroups indicating greater effect sizes for Asian people (P = 0.01; Analysis 1.13). Subgroup analysis according to diagnostic criteria, age, gender, comorbid condition and previous gestational diabetes could not be performed.
1.12. Analysis.
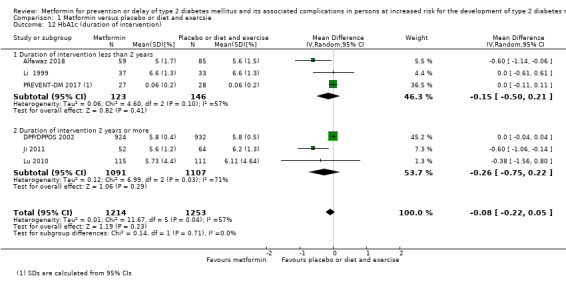
Comparison 1 Metformin versus placebo or diet and exercsie, Outcome 12 HbA1c (duration of intervention).
1.13. Analysis.
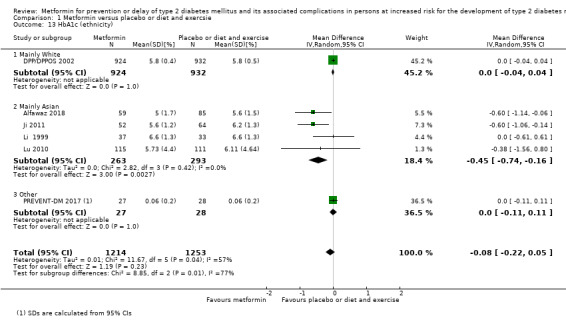
Comparison 1 Metformin versus placebo or diet and exercsie, Outcome 13 HbA1c (ethnicity).
Sensitivity analysis: sensitivity analysis according to publication status could not be performed as all included trials were published. Sensitivity analysis restricted to only trials with low risk of selection bias changed the direction of the effect estimate: MD 0.00%, 95% CI ‐0.04 to 0.04; P = 1.0; 1856 participants; 1 trial (DPP/DPPOS 2002). Two trials were only published in Chinese. Sensitivity analysis restricted to trials published in English changed the direction of the effect estimate: MD ‐0.01 %; 95% CI ‐0.13 to 0.07; P = 0.74; 2125 participants; 4 trials (Alfawaz 2018; DPP/DPPOS 2002; Li 1999; PREVENT‐DM 2017). Sensitivity analysis excluding trials funded by a pharmaceutical company did not substantially change the direction of the effect estimate: MD ‐0.29 %, 95% CI ‐0.57 to ‐0.02; P = 0.04; 611 participants; 5 trials (Alfawaz 2018; Ji 2011; Li 1999; Lu 2010; PREVENT‐DM 2017).
Fasting plasma glucose
Fifteen trials reported data on fasting plasma glucose (Alfawaz 2018; BIGPRO1 2009; Chen 2009; DPP/DPPOS 2002; Fang 2004; IDPP‐1 2006; Ji 2011; Jin 2009; Li 1999; Lu 2002; Lu 2010; Papoz 1978; PREVENT‐DM 2017; Wang 2009; Zeng 2013); random‐effects MD ‐0.28 mmol/L; 95% CI ‐0.42 to ‐0.13; P = 0.0002; 3546 participants; 15 trials; Analysis 1.14. Heterogeneity was substantial (I2 = 82%; P < 0.0001).
1.14. Analysis.
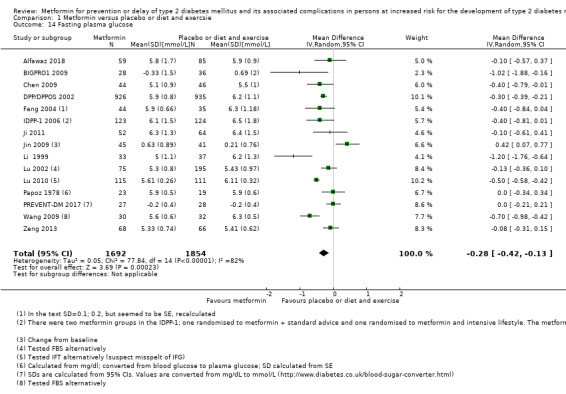
Comparison 1 Metformin versus placebo or diet and exercsie, Outcome 14 Fasting plasma glucose.
Subgroup analysis: analysing trials according to blinded versus open‐label trials did not show interaction between subgroups (P = 0.22; Analysis 1.15). Subgroup analysis according to duration of the intervention did not show interaction between subgroups (P = 0.13; Analysis 1.16). Subgroup analysis according to ethnicity did not show any interaction between subgroups (P = 0.67; Analysis 1.17). Subgroup analysis according to diagnostic criteria, age, gender, comorbid condition and previous gestational diabetes could not be performed.
1.15. Analysis.
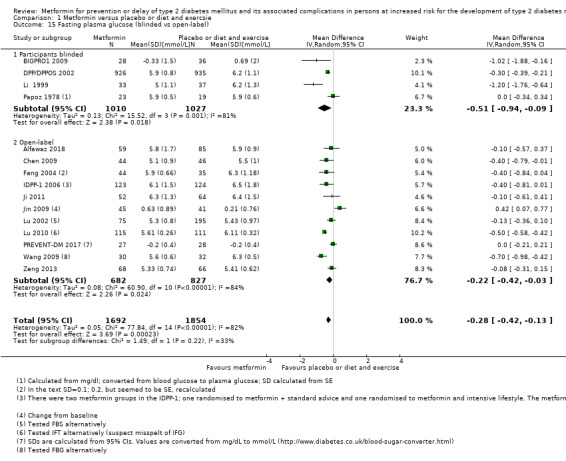
Comparison 1 Metformin versus placebo or diet and exercsie, Outcome 15 Fasting plasma glucose (blinded vs open‐label).
1.16. Analysis.
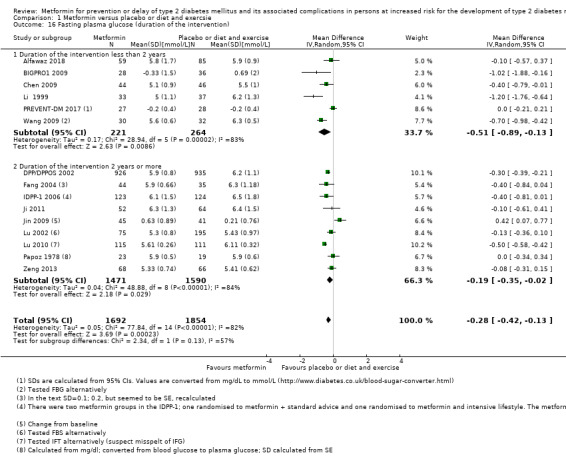
Comparison 1 Metformin versus placebo or diet and exercsie, Outcome 16 Fasting plasma glucose (duration of the intervention).
1.17. Analysis.
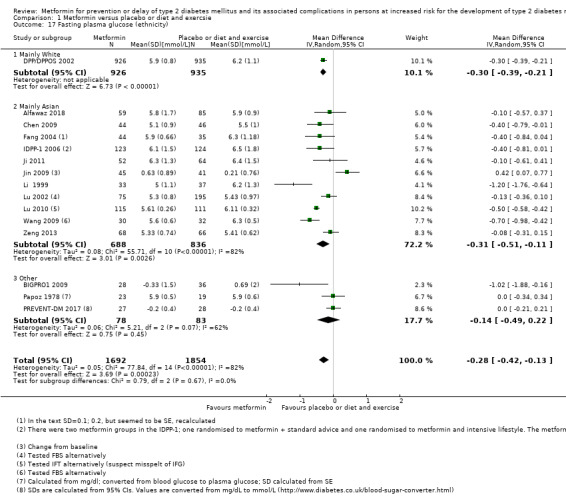
Comparison 1 Metformin versus placebo or diet and exercsie, Outcome 17 Fasting plasma glucose (ethnicity).
Sensitivity analysis: sensitivity analysis according to publication status could not be performed as all included trials were published. Sensitivity analysis restricted to only trials with low risk of selection bias changed the direction of the effect estimate; random‐effects MD ‐0.30, 95% CI ‐0.39 to ‐0.21; P < 0.001; 1861 participants; 1 trial (DPP/DPPOS 2002). Eight trials were published in Chinese. Sensitivity analysis restricted to trials published in English changed the direction of the effect estimate random‐effects MD ‐0.31 mmol/L; 95% CI ‐0.55 to ‐0.08; P = 0.009; 2483 participants; 7 trials (Alfawaz 2018; BIGPRO1 2009; DPP/DPPOS 2002; IDPP‐1 2006; Li 1999; Papoz 1978; PREVENT‐DM 2017). Sensitivity analysis excluding trials funded by a pharmaceutical company did not change the direction of the effect estimate random‐effects MD ‐0.27 mmol/L, 95% CI ‐0.48 to ‐0.06; P = 0.0002; 1374 participants; 12 trials (Alfawaz 2018; Chen 2009; Fang 2004; Ji 2011; Jin 2009; Li 1999; Lu 2002; Lu 2010; Papoz 1978; PREVENT‐DM 2017; Wang 2009; Zeng 2013).
Socioeconomic effects
During the DPP trial the metformin intervention was substantially more expensive than the placebo intervention (DPP/DPPOS 2002). Direct medical costs of the interventions during the DPP were estimated to be $1177 for the metformin intervention versus $184 for the placebo group (low‐quality evidence). By year 10, the direct medical costs of the intervention and non‐intervention‐related medical costs were lower for the metformin group than for the control group ($27,915 versus $28,237).
From the perspective of a health system (direct medical costs of the interventions plus direct medical costs of care outside the trial) costs were $99,600 per quality adjusted life years (QALY)‐gained with metformin compared with placebo. From the perspective of society (direct medical costs plus non medical costs (expenditures from medical treatments, but not involving purchase of medical services or products) plus indirect costs (costs to the society due to morbidity and mortality, e.g. absence from work due to medical treatment)) the costs were $99,200 per QALY‐gained with metformin compared with placebo (DPP/DPPOS 2002).
The IDPP trial estimated the direct medical costs of interventions over the three‐year trial period to be $220 per participant in the metformin group compared with $61 in the standard diet and physical activity group (IDPP‐1 2006).
Metformin versus intensive diet plus exercise
Eight trials compared metformin with intensive diet and exercise (Alfawaz 2018; DPP/DPPOS 2002; Fang 2004; IDPP‐1 2006; Ji 2011; Li 2009; Maji 2005; PREVENT‐DM 2017). Two trials applied metformin in doses up to 500 mg/day (Li 2009; Maji 2005); two trials applied metformin in doses up to 1000 mg/day (Alfawaz 2018; IDPP‐1 2006); one trial applied metformin in doses up to 1500 mg/day (Ji 2011); one trial applied metformin in doses up to 1700 mg/day (DPP/DPPOS 2002); one trial applied metformin in doses up to 2250 mg/day (Fang 2004); and one trial administered metformin in doses up to 2550 mg/day (PREVENT‐DM 2017). Five trials stated that the metformin group received concomitant diet and exercise (Alfawaz 2018; DPP/DPPOS 2002; Ji 2011; Jin 2009; Maji 2005).
Primary outcomes
All‐cause mortality
Four trials reported data on all‐cause mortality (DPP/DPPOS 2002; Fang 2004; IDPP‐1 2006; PREVENT‐DM 2017).
A total of seven deaths were reported in 1278 participants in the metformin group versus four out of 1272 participants in the comparator group (RR 1.61, 95% CI 0.50 to 5.23; P = 0.43; 2550 participants, 4 trials; very low‐quality of the evidence; Analysis 2.1).
2.1. Analysis.
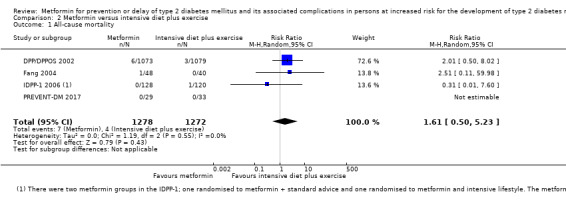
Comparison 2 Metformin versus intensive diet plus exercise, Outcome 1 All‐cause mortality.
We did not perform subgroup analyses and sensitivity analyses due to lack of data.
Incidence of type 2 diabetes (T2DM)
Seven trials reported the incidence of T2DM (DPP/DPPOS 2002; Fang 2004; IDPP‐1 2006; Ji 2011; Li 2009; Maji 2005; PREVENT‐DM 2017). The definition of T2DM varied among the included trials (see Appendix 9 and Appendix 10). Calculation of a 95% prediction interval was not meaningful.
A total of 304 out of 1455 participants developed T2DM in the metformin group versus 251 out of 1505 participants in the comparator group (RR 0.80, 95% CI 0.47 to 1.37; P = 0.42; 2960 participants, 7 trials; moderate‐quality of the evidence; Analysis 2.2). The 95% prediction interval ranged between 0.18 and 3.62.
2.2. Analysis.
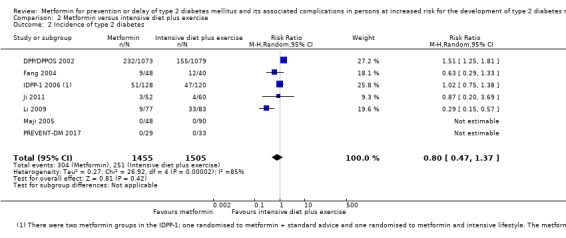
Comparison 2 Metformin versus intensive diet plus exercise, Outcome 2 Incidence of type 2 diabetes.
One trial reported the incidence of T2DM after an extended follow‐up period (DPP/DPPOS 2002). At year 15 the cumulative incidence of T2DM was 480 participants (55%) in the former intensive diet plus physical activity group versus 499 participants (56%) in the former metformin group (DPP/DPPOS 2002).
Subgroup analysis: analysing trials according to blinded versus open‐label trials could not be performed as all participants were aware of randomising to intensive diet plus exercise. Subgroup analysis according to duration of the intervention could not be performed as only one trial (without any participants developing T2DM) had a duration of intervention of less than two years (PREVENT‐DM 2017). Subgroup analysis according to ethnicity showed interaction between subgroups (P = 0.02; Analysis 2.4), however CIs overlapped. Subgroup analysis according to diagnostic criteria, age, gender, comorbid condition and previous gestational diabetes could not be performed.
2.4. Analysis.
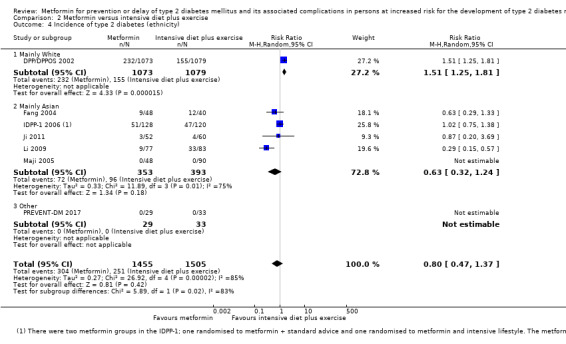
Comparison 2 Metformin versus intensive diet plus exercise, Outcome 4 Incidence of type 2 diabetes (ethnicity).
Sensitivity analysis: sensitivity analysis according to publication status could not be performed as all included trials were published. Sensitivity analysis restricted to only trials with low risk of selection bias changed the direction of the effect estimate: RR 1.51, 95% CI 1.25 to 1.81; P < 0.001; 2152 participants; 1 trial (DPP/DPPOS 2002). Three trials were published in Chinese. Sensitivity analysis restricted to trials published in English did not substantially change the direction of the effect estimate: RR 1.26, 95% CI 0.86 to 1.86; P = 0.24; 2600 participants; 4 trials (DPP/DPPOS 2002; IDPP‐1 2006; Maji 2005; PREVENT‐DM 2017). Sensitivity analysis excluding trials funded by a pharmaceutical company changed the direction of the effect estimate towards benefit of metformin therapy: RR 0.47, 95% CI 0.25 to 0.87; P = 0.02; 560 participants; 5 trials (Fang 2004; Ji 2011; Li 2009; Maji 2005; PREVENT‐DM 2017).
Serious adverse events
The reporting of serious adverse events was sparse and meta‐analysis could not be performed. One trial reported no serious adverse events in 29 participants in the metformin group and 30 participants in the standard care group (PREVENT‐DM 2017). In one trial, one out of 44 participants died due to liver cancer in the metformin group compared to zero out of 36 participants in the standard care group (Fang 2004).
Secondary outcomes
Cardiovascular mortality
Two trials reported cardiovascular mortality (DPP/DPPOS 2002; IDPP‐1 2006). One trial reported that no participants died due to cardiovascular causes (IDPP‐1 2006). One trial reported that one out of 1073 participants in the metformin group compared with two out of 1079 participants in the intensive diet plus exercise group died from cardiovascular causes (DPP/DPPOS 2002).
Non‐fatal myocardial infarction
No trial reported data exclusively on non‐fatal myocardial infarction. One trial reported that non‐fatal cardiovascular events occurred in 1.7% of the participants in the control group compared with 1.5% of the participants in the metformin group (DPP/DPPOS 2002). One trial reported that zero out of 128 participants in the metformin group compared to four out of 120 participants in the comparator group experienced cardiovascular events (IDPP‐1 2006).
Non‐fatal stroke
No trial reported data exclusively on non‐fatal stroke.
Amputation of lower extremity
None of the trials reported on amputation of lower extremity.
Blindness or severe vision loss
None of the trials reported on blindness or severe vision loss.
End‐stage renal disease
None of the trials reported on end‐stage renal disease
Non‐serious adverse events
One trial reported on all non‐serious adverse events (PREVENT‐DM 2017). Ten (34.5%) out of 29 participants in the metformin group compared with zero out of 33 participants in the comparator group experienced a non‐serious adverse event (PREVENT‐DM 2017). Three trials reported on some adverse effects (DPP/DPPOS 2002; Fang 2004; IDPP‐1 2006). In one trial, three out of 44 participants had diarrhoea in the metformin group compared to zero out of 36 participants in the comparator group (Fang 2004). One trial had two metformin groups (metformin monotherapy and metformin plus intensive diet and exercise), which were reported together. The trial reported that five out of 248 participants in the combined metformin groups experienced gastrointestinal symptoms compared to zero out of 120 participants in the comparator group (IDPP‐1 2006). In one trial, 20 events of musculoskeletal symptoms per 100 person years and 78 events of gastrointestinal symptoms per 100 person years were experienced in the metformin group compared with 24 events of musculoskeletal symptoms per 100 person years and 13 events of gastrointestinal symptoms per 100 person years in the comparator group (DPP/DPPOS 2002).
Hypoglycaemia
One trial reported data on hypoglycaemia (IDPP‐1 2006). In the two metformin groups (metformin monotherapy and metformin plus intensive diet and exercise), 22 out of 248 participants reported symptoms of mild hypoglycaemia; it was not possible to separate these data. No participant experienced symptoms of hypoglycaemia in the intensive diet plus exercise group (IDPP‐1 2006).
Health‐related quality of life
The DPP trial applied the SF‐36 to evaluate the health utility index (SF‐6D), physical component summaries (PCS) and mental component summaries (MCS). Minimal important difference (MID) was defined as scores between groups of at least 3% (DPP/DPPOS 2002). After a mean of 3.2 years of follow‐up trial authors only reported the comparison metformin versus placebo, not metformin versus diet plus exercise (DPP/DPPOS 2002).
Time to progression to T2DM
After a median of 10 years follow‐up in the DPP trial, the onset of diagnosis of T2DM was delayed by two years with metformin and four years with intensive diet and exercise compared with placebo (DPP/DPPOS 2002).
Measures of blood glucose control
2‐hour glucose
Five trials reported data on 2‐hour glucose (DPP/DPPOS 2002; Fang 2004; IDPP‐1 2006; Ji 2011; Li 2009): MD ‐0.03 mmol/L, 95% CI ‐0.26 to 0.20; P = 0.81; 2417 participants; 5 trials; Analysis 2.5.
2.5. Analysis.
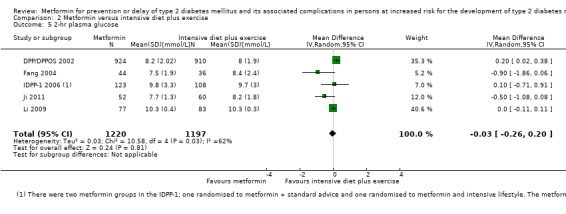
Comparison 2 Metformin versus intensive diet plus exercise, Outcome 5 2‐hr plasma glucose.
Subgroup analysis: analysing trials according to blinded versus open‐label trials could not be performed as all participants were aware of randomising to intensive diet plus exercise. Subgroup analysis according to duration of the intervention could not be performed as all trials reporting this outcome had a duration of intervention of two years or more. Subgroup analysis according to ethnicity did not show interaction between subgroups (P = 0.06; Analysis 2.6). Subgroup analysis according to diagnostic criteria, age, gender, comorbid condition and previous gestational diabetes could not be performed.
2.6. Analysis.
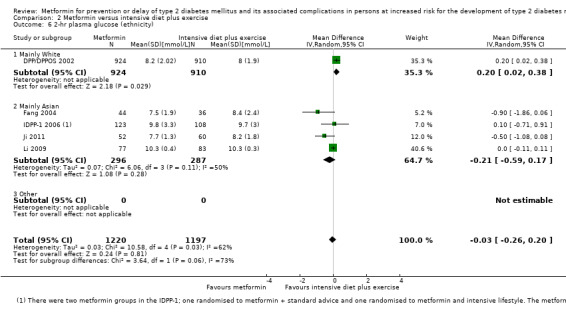
Comparison 2 Metformin versus intensive diet plus exercise, Outcome 6 2‐hr plasma glucose (ethnicity).
Sensitivity analysis: sensitivity analysis according to publication status could not be performed as all included trials were published. Sensitivity analysis restricted to only trials with low risk of selection bias changed the direction of the effect estimate: MD 0.20 mmol/L, 95% CI 0.02 to 0.38; P = 0.03; 1834 participants; 1 trial (DPP/DPPOS 2002). Three trials were only published in Chinese. Sensitivity analysis restricted to trials published in English changed the direction of the effect estimate favouring intensive diet plus exercise: MD 0.20 mmol/L, 95% CI 0.02 to 0.37; P = 0.03; 2065 participants; 2 trials (DPP/DPPOS 2002; IDPP‐1 2006). Sensitivity analysis excluding trials funded by a pharmaceutical company did not change the direction of the effect estimate: MD ‐0.32, 95% CI ‐0.83 to 0.19; P = 0.22; 352 participants; 3 trials (Fang 2004; Ji 2011; Li 2009).
HbA1c
Four trials reported data on HbA1c (Alfawaz 2018; DPP/DPPOS 2002; Ji 2011; PREVENT‐DM 2017): MD 0.01% mmol/L, 95% CI ‐0.12 to 0.14; P = 0.93; 2135 participants; 4 trials; Analysis 2.7.
2.7. Analysis.
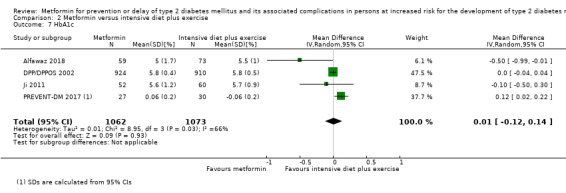
Comparison 2 Metformin versus intensive diet plus exercise, Outcome 7 HbA1c.
Subgroup analysis: analysing trials according to blinded versus open‐label trials could not be performed as all participants were aware of randomising to intensive diet plus exercise. Subgroup analysis according to duration of the intervention did not show interaction between subgroups (P = 0.65; Analysis 2.8). Subgroup analysis according to ethnicity showed interaction between subgroups (P = 0.04; Analysis 2.9). Subgroup analysis according to diagnostic criteria, age, gender, comorbid condition and previous gestational diabetes could not be performed.
2.8. Analysis.
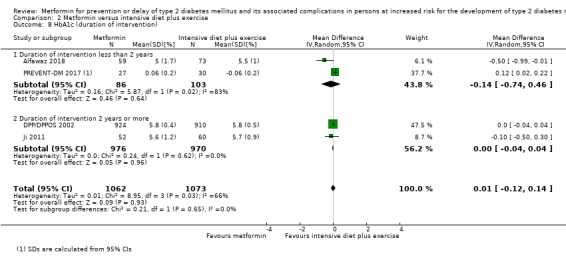
Comparison 2 Metformin versus intensive diet plus exercise, Outcome 8 HbA1c (duration of intervention).
2.9. Analysis.
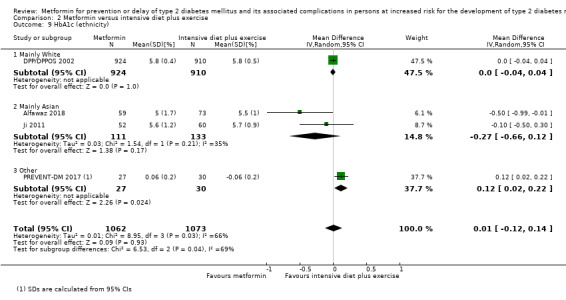
Comparison 2 Metformin versus intensive diet plus exercise, Outcome 9 HbA1c (ethnicity).
Sensitivity analysis: sensitivity analysis according to publication status could not be performed as all included trials were published. Sensitivity analysis restricted to only trials with low risk of selection bias did not substantially change the direction of the effect estimate: MD 0.00%, 95% CI ‐0.04 to 0.04; P = 1.0; 1834 participants; 1 trial (DPP/DPPOS 2002). One trial was only published in Chinese. Sensitivity analysis restricted to trials published in English did not change the direction of the effect estimate: MD 0.01%, 95% CI ‐0.13 to 0.16; P = 0.87; 2023 participants; 3 trials (Alfawaz 2018; DPP/DPPOS 2002; PREVENT‐DM 2017). Sensitivity analysis excluding trials funded by a pharmaceutical company did not change the direction of the effect estimate: MD ‐0.10%, 95% CI ‐0.44 to 0.25; P = 0.59; 301 participants; 3 trials (Alfawaz 2018; Ji 2011; PREVENT‐DM 2017).
Fasting plasma glucose
Seven trials reported data on fasting plasma glucose (Alfawaz 2018; DPP/DPPOS 2002; Fang 2004; IDPP‐1 2006; Ji 2011; Li 2009; PREVENT‐DM 2017). The MD was ‐0.26 mmol/L, 95% CI ‐0.59 to 0.07; P = 0.12; 2603 participants; 7 trials; Analysis 2.10.
2.10. Analysis.
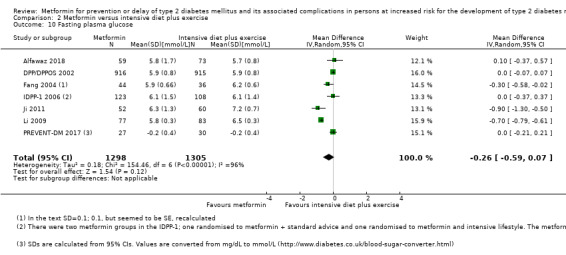
Comparison 2 Metformin versus intensive diet plus exercise, Outcome 10 Fasting plasma glucose.
Subgroup analysis: analysing trials according to blinded versus open‐label trials could not be performed as all participants were aware of randomising to intensive diet plus exercise. Subgroup analysis according to duration of the intervention did not show interaction between subgroups (P = 0.09; Analysis 2.11). Subgroup analysis according to ethnicity did not show interaction between subgroups (P = 0.10; Analysis 2.12). Subgroup analysis according to diagnostic criteria, age, gender, comorbid condition and previous gestational diabetes could not be performed.
2.11. Analysis.
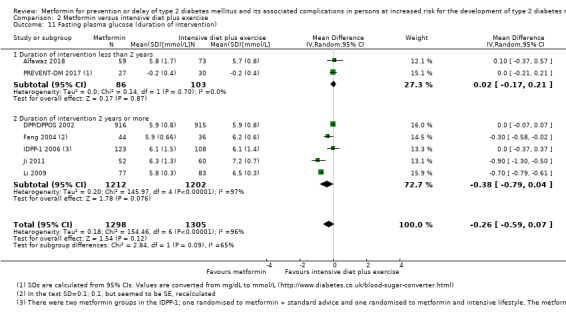
Comparison 2 Metformin versus intensive diet plus exercise, Outcome 11 Fasting plasma glucose (duration of intervention).
2.12. Analysis.
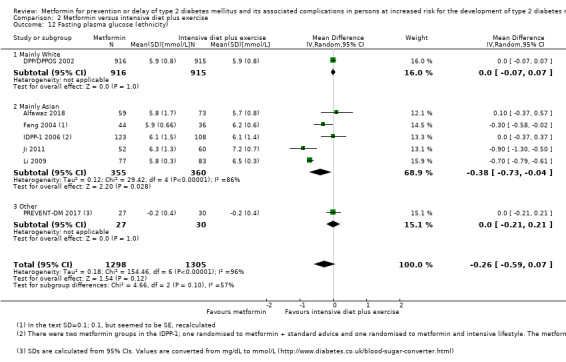
Comparison 2 Metformin versus intensive diet plus exercise, Outcome 12 Fasting plasma glucose (ethnicity).
Sensitivity analysis: sensitivity analysis according to publication status could not be performed as all included trials were published. Sensitivity analysis restricted to only trials with low risk of selection bias changed the direction of the effect estimate: MD 0.00 mmol/L, 95% CI ‐0.07 to 0.07; P = 1.0; 1831 participants; 1 trial (DPP/DPPOS 2002). Three trials were only published in Chinese. Sensitivity analysis restricted to trials published in English changed the direction of the effect estimate: MD 0.0 mmol/L, 95% CI ‐0.07 to 0.07; P = 0.95; 2251 participants; 4 trials (Alfawaz 2018; DPP/DPPOS 2002; IDPP‐1 2006; PREVENT‐DM 2017). Sensitivity analysis excluding trials funded by a pharmaceutical company changed the direction of the effect estimate towards benefit of metformin: MD ‐0.37 mmol/L, 95% CI ‐0.65 to ‐0.09; P = 0.009; 541 participants; 5 trials (Alfawaz 2018; Fang 2004; Ji 2011; Li 2009; PREVENT‐DM 2017).
Socioeconomic effects
Direct medical costs of the interventions during the DPP were estimated to be $1177 for the metformin intervention versus $3628 for the intensive diet plus physical activity group (DPP/DPPOS 2002). By year 10, the direct medical costs of the interventions and non‐intervention‐related medical costs were lower for the metformin group than for the intensive diet plus physical activity group ($27,915 versus $29,164).
The IDPP estimated direct medical costs of interventions over the 3‐year trial period to be $220 per participant in the metformin group compared with $225 in the intensive diet and physical activity group (IDPP‐1 2006).
Metformin versus insulin secretagogues
One trial compared metformin with a sulphonylurea (Papoz 1978). Metformin was administered in doses of 1700 mg/day with concomitant placebo. Glibenclamide was administered in doses of 4 mg/day with concomitant placebo. For both groups, overweight participants were recommended calorie restriction. The ethnicity of the included participants was not reported.
The trial reported 2‐hour blood glucose and fasting blood glucose in mg/100 mL. The results were converted to plasma glucose measured in mmol/L and standard errors were converted to standard deviations (SDs). For the metformin group the 2‐hour plasma glucose at the end of intervention was 7.2 mmol/L (SD 1.3) measured in 23 participants compared to 7.1 mmol/L (SD 1.3) measured in 22 participants in the glibenclamide group. For the metformin group the fasting plasma glucose at the end of intervention was 5.9 mmol/L (SD 0.5) measured in 23 participants compared to 5.6 mmol/L (SD 0.6) measured in 22 participants in the glibenclamide group.
Metformin versus acarbose
Three trials compared metformin with acarbose (Fang 2004; Liao 2012; Maji 2005). Several differences existed between these three trials. Two trials did not specify the concomitant intervention with diet and physical activity (Fang 2004; Liao 2012); the other trial specified that diet and physical activity was provided in both the metformin and the acarbose intervention groups (Maji 2005). Two trials included people with Chinese ethnicity (Fang 2004; Liao 2012), one trial included people with Indian ethnicity (Maji 2005). One trial administered metformin in doses up to 500 mg/day and acarbose in doses up to 50 mg/day (Maji 2005). One trial administered metformin in doses up to 1500 mg/day and acarbose in doses up to 300 mg/day (Liao 2012). One trial administered metformin in doses up to 2250 mg/day and acarbose in doses up to 450 mg/day (Fang 2004).
Primary outcomes
All‐cause mortality
One trial reported data on all‐cause mortality (Fang 2004). One participant out of 44 in the metformin group compared with zero out of 45 in the acarbose group died (Fang 2004).
Incidence of type 2 diabetes (T2DM)
All included trials reported data on the incidence of T2DM. A total of 12 out of 147 participants developed T2DM in the metformin group versus seven out of 148 participants in the comparator group (RR 1.72; 95% CI 0.72 to 4.14; P = 0.22; 295 participants; 3 trials; low‐quality evidence; Analysis 4.2). Calculation of a 95% prediction interval was not meaningful.
4.2. Analysis.
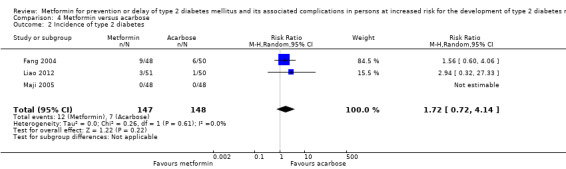
Comparison 4 Metformin versus acarbose, Outcome 2 Incidence of type 2 diabetes.
Serious adverse events
In one trial one out of 51 participants in the metformin group experienced cerebral haemorrhage, whereas two out of 50 participants in the acarbose group experienced lung cancer and hepatitis, respectively (Liao 2012).
Secondary outcomes
Cardiovascular mortality
None of the trials reported on cardiovascular mortality.
Non‐fatal myocardial infarction
None of the trials reported on non‐fatal myocardial infarction.
Non‐fatal stroke
None of the trials reported on non‐fatal stroke.
Amputation of lower extremity
None of the trials reported on amputation of lower extremity.
Blindness or severe vision loss
None of the trials reported on blindness or severe vision loss.
End‐stage renal disease
None of the trials reported on end‐stage renal disease
Non‐serious adverse events
One trial reported that in the metformin group three out of 44 participants experienced diarrhoea (Fang 2004). In the acarbose group one out of 45 participants experienced rash and one out of 45 participants experienced frequent venting (Fang 2004).
Hypoglycaemia
None of the trials reported on hypoglycaemia.
Health‐related quality of life
None of the trials reported on health‐related quality of life.
Time to progression to T2DM
None of the trials reported on time to progression to T2DM.
Measures of blood glucose control
2‐hour glucose
Both included trials reported data on 2‐hour glucose. Effects of intervention showed benefit in favour of acarbose: MD 0.49 mmol/L, 95% CI 0.09 to 0.88; P = 0.02; 190 participants; 2 trials; Analysis 4.3.
4.3. Analysis.
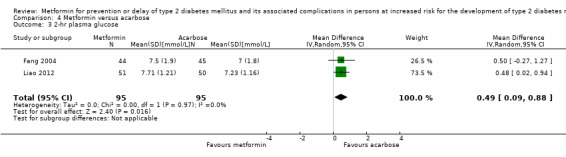
Comparison 4 Metformin versus acarbose, Outcome 3 2‐hr plasma glucose.
One trial reported a mean 2‐hour glucose of 5.9 mmol/L (SD 0.9) in the metformin group (unknown how many of the 48 initially randomised participants were included in the analysis) compared with 6.0 mmol/L (SD 0.5) in the acarbose group (unknown how many of the 48 initially randomised participants were included in the analysis) (Maji 2005).
HbA1c
One trial reported a mean HbA1c of 6.96% (SD 0.43) in the metformin group (unknown how many of the 48 initially randomised participants were included in the analysis) compared with 6.96% (SD 0.16) in the acarbose group (unknown how many of the 48 initially randomised participants were included in the analysis) (Maji 2005).
Fasting plasma glucose
Both included trials reported data on fasting plasma glucose. The MD was 0.00 mmol/L, 95% CI ‐0.35 to 0.35; P = 0.99; 190 participants; 2 trials; Analysis 4.4.
4.4. Analysis.
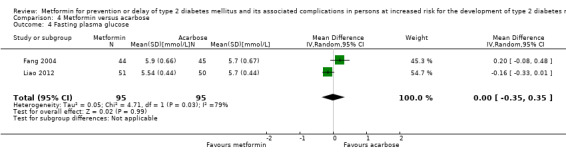
Comparison 4 Metformin versus acarbose, Outcome 4 Fasting plasma glucose.
One trial reported a mean fasting plasma glucose of 5.4 mmol/L (SD 0.3) in the metformin group (unknown how many of the 48 initially randomised participants were included in the analysis) compared with 5.5 mmol/L (SD 0.4) in the acarbose group (unknown how many of the 48 initially randomised participants were included in the analysis) (Maji 2005).
Socioeconomic effects
None of the trials reported on socioeconomic effects.
Metformin versus thiazolidinediones
Three trials compared metformin with a thiazolidinediones (Jin 2009; Maji 2005; Zeng 2013). One trial administered metformin in doses up to 38 mg/day and pioglitazone in doses up to 38 mg/day (Zeng 2013); one trial administered metformin in doses up to 500 mg/day and rosiglitazone in doses up to 2 mg/day (Maji 2005); and one trial administered metformin in doses up to 3000 mg/day and rosiglitazone in doses up to 4 mg/day (Jin 2009). All trials stated that both the intervention and comparator group received diet and exercise. Two trials included people with Chinese ethnicity (Jin 2009; Zeng 2013), and one trial included people with Indian ethnicity (Maji 2005).
Primary outcomes
All‐cause mortality
None of the trials reported on all‐cause mortality..
Incidence of type 2 diabetes (T2DM)
All included trials reported data on incidence of T2DM. A total of nine out of 161 participants developed T2DM in the metformin group versus nine out of 159 participants in the comparator group (RR 0.99, 95% CI 0.41 to 2.40; P = 0.98; 320 participants; 3 trials; low‐quality evidence; Analysis 5.1). Calculation of a 95% prediction interval was not meaningful.
5.1. Analysis.
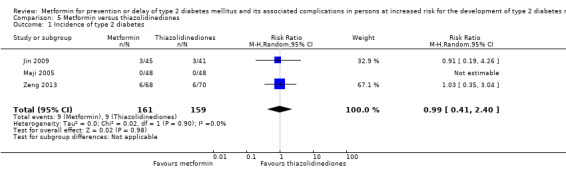
Comparison 5 Metformin versus thiazolidinediones, Outcome 1 Incidence of type 2 diabetes.
Serious adverse events
In one trial, three out of 45 participants in the metformin group experienced severe gastrointestinal reactions and no serious adverse events were reported in the 41 participants in the thiazolidinedione group (Jin 2009).
Secondary outcomes
Cardiovascular mortality
None of the trials reported on cardiovascular mortality.
Non‐fatal myocardial infarction
None of the trials reported on non‐fatal myocardial infarction.
Non‐fatal stroke
None of the trials reported on non‐fatal stroke.
Amputation of lower extremity
None of the trials reported on amputation of lower extremity.
Blindness or severe vision loss
None of the trials reported on blindness or severe vision loss.
End‐stage renal disease
None of the trials reported on end‐stage renal disease.
Non‐serious adverse events
One trial reported that in the rosiglitazone group one out of 41 participants experienced facial oedema and two out of 41 participants experienced intolerance of both lower limbs (Jin 2009).
Hypoglycaemia
One trial reported that no participant in any of the treatment arms experienced hypoglycaemia (Jin 2009).
Health‐related quality of life
None of the trials reported on health‐related quality of life.
Time to progression to T2DM
None of the trials reported on time to progression to T2DM.
Measures of blood glucose control
2‐hour glucose
All included trials reported on 2‐hour glucose. The MD was ‐0.54 mmol/L, 95% CI ‐1.80 to 0.73; P = 0.41; 224 participants; 2 trials; Analysis 5.2.
5.2. Analysis.
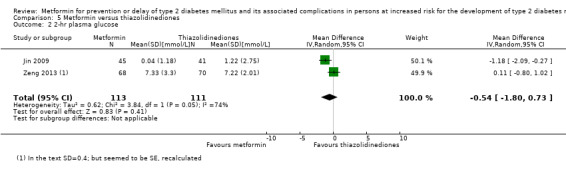
Comparison 5 Metformin versus thiazolidinediones, Outcome 2 2‐hr plasma glucose.
One trial reported a mean 2‐hour plasma glucose of 5.9 mmol/L (SD 0.9) in the metformin group (unknown how many of the 48 initially randomised participants were included in the analysis) compared with 5.8 mmol/L (SD 0.5) in the rosiglitazone group (unknown how many of the 48 initially randomised participants were included in the analysis) (Maji 2005).
HbA1c
One trial reported a mean HbA1c of 6.96% (SD 0.43) in the metformin group (unknown how many of the 48 initially randomised participants were included in the analysis) compared with 6.96% (SD 0.48) in the rosiglitazone group (unknown how many of the 48 initially randomised participants were included in the analysis) (Maji 2005).
Fasting plasma glucose
All included trials reported on fasting plasma glucose. The MD was ‐0.13 mmol/L, 95% CI ‐0.32 to 0.07; P = 0.20; 224 participants; 2 trials; Analysis 5.3.
5.3. Analysis.

Comparison 5 Metformin versus thiazolidinediones, Outcome 3 Fasting plasma glucose.
One trial reported a mean fasting plasma glucose of 5.4 mmol/L (SD 0.3) in the metformin group (unknown how many of the 48 initially randomised participants were included in the analysis) compared with 5.2 mmol/L (SD 0.4) in the rosiglitazone group (unknown how many of the 48 initially randomised participants were included in the analysis) (Maji 2005).
Socioeconomic effects
None of the trials reported on socioeconomic effects.
Metformin plus intensive diet and exercise versus intensive diet and exercise
Three trials compared metformin plus intensive diet and exercise with identical intensive diet and exercise (IDPP‐1 2006; Iqbal Hydrie 2012; Zhao 2013). All the trials administered metformin in doses up to 1000 mg/day. The ethnicity of the included people were Indian (IDPP‐1 2006), Pakistani (Iqbal Hydrie 2012), and Chinese (Zhao 2013).
Primary outcomes
All‐cause mortality
IDPP‐1 2006 reported that one out of 121 participants died in the metformin group compared to one out of 120 participants in the comparator group (very low‐quality evidence). Iqbal Hydrie 2012 trial reported that zero out of 95 participants died in the metformin group compared with zero out of 114 participants in the comparator group (Analysis 6.1).
6.1. Analysis.
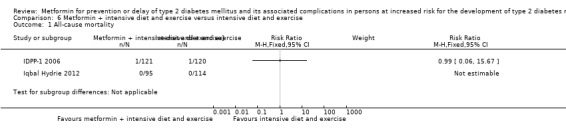
Comparison 6 Metformin + intensive diet and exercise versus intensive diet and exercise, Outcome 1 All‐cause mortality.
Incidence of type 2 diabetes (T2DM)
Two trials reported incidence of T2DM (IDPP‐1 2006; Zhao 2013). A total of 48 out of 166 participants developed T2DM in the metformin plus intensive diet and exercise compared with 53 out of 166 participants in the comparator group (RR 0.55, 95% CI 0.10 to 2.92; P = 0.49; 332 participants; 2 trials; very low‐quality evidence; Analysis 6.2). Calculation of a 95% prediction interval was not meaningful.
6.2. Analysis.
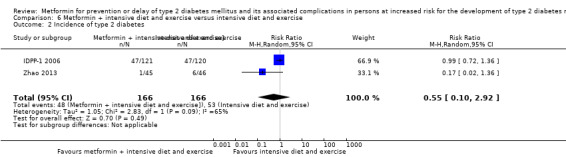
Comparison 6 Metformin + intensive diet and exercise versus intensive diet and exercise, Outcome 2 Incidence of type 2 diabetes.
Serious adverse events
None of the trials reported on serious adverse events. All included trials had a high risk of selective reporting bias regarding serious adverse events.
Secondary outcomes
Cardiovascular mortality
One trial reported that no participant died due to cardiovascular causes (IDPP‐1 2006).
Non‐fatal myocardial infarction
None of the trials reported on non‐fatal myocardial infarction.
Non‐fatal stroke
None of the trials reported on non‐fatal stroke.
Amputation of lower extremity
None of the trials reported on amputation of lower extremity.
Blindness or severe vision loss
None of the trials reported on blindness or severe vision loss.
End‐stage renal disease
None of the trials reported on end‐stage renal disease.
Non‐serious adverse events
One trial had two metformin groups (metformin monotherapy and metformin plus intensive diet and exercise) and reported that five out of 248 participants in both metformin groups experienced gastrointestinal symptoms compared to zero out of 120 participants in the comparator group (IDPP‐1 2006). One trial reported that one out of 45 participants in the metformin group experienced gastrointestinal symptoms compared with zero out of 46 participants in the comparator group (Zhao 2013).
Hypoglycaemia
One trial reported data on hypoglycaemia (IDPP‐1 2006). In the two metformin groups (metformin monotherapy and metformin plus intensive diet and exercise), 22 out of 248 participants reported symptoms of mild hypoglycaemia; it was not possible to separate these data. None experienced symptoms of hypoglycaemia in the intensive diet plus exercise group (IDPP‐1 2006).
Health‐related quality of life
None of the trials reported on health‐related quality of life.
Time to progression to T2DM
None of the trials reported on time to progression to T2DM.
Measures of blood glucose control
2‐hour glucose
Two included trials reported on 2‐hour glucose (IDPP‐1 2006; Zhao 2013). The MD was ‐0.52 mmol/L, 95% CI ‐2.08 to 1.04; P = 0.51; 316 participants; 2 trials; Analysis 6.6).
6.6. Analysis.
Comparison 6 Metformin + intensive diet and exercise versus intensive diet and exercise, Outcome 6 2‐hr glucose values.
HbA1c
None of the trials reported on HbA1c.
Fasting plasma glucose
Two trials reported data on fasting plasma glucose (IDPP‐1 2006; Zhao 2013). The MD was ‐0.26 mmol/L, 95% CI ‐0.94 to 0.43; P = 0,46; 316 participants; 2 trials; Analysis 6.7.
6.7. Analysis.
Comparison 6 Metformin + intensive diet and exercise versus intensive diet and exercise, Outcome 7 Fasting plasma glucose.
Socioeconomic effects
The IDPP estimated direct medical costs of interventions over the three‐year trial period to be $270 per participant in the metformin plus intensive diet and physical activity group compared with $225 in the intensive diet and physical activity group (IDPP‐1 2006).
Ongoing trials
We identified 11 ongoing trials which potentially could provide data of interest for this review (CTRI/2017/09/009635; JPRN‐UMIN000018995; Nadeau 2014; NCT01804049; Espinoza 2019; NCT02915198; NCT02969798; Rhee 2019; NCT03194009; ePRECIDE 2017; Ji 2019). The trials will enrol a total of 17,853 participants. All but three ongoing trials explicitly stated that they assessed one or more of the primary or secondary outcomes of interest of this review (NCT01804049; Espinoza 2019; ePRECIDE 2017). Allthough not stated in the protocol, it is very likely that the remaining trials will assess one or more outcomes of interest for this review. Two trials did not report the trial completion date (CTRI/2017/09/009635; JPRN‐UMIN000018995). One trial stated the trial completion date to be August 2018, but no trial results are available (NCT01804049). Two trials estimated the completion date to be in the year 2019 (Nadeau 2014; ePRECIDE 2017); two trials estimated the completion date to be in the year 2020 (NCT02969798; Rhee 2019)' three trials estimated the completion date to be in the year 2022 (Espinoza 2019; NCT03194009; Ji 2019); and one trial estimated the completion date to be in the year 2024 (NCT02915198).
Studies awaiting assessment
One trial was published as an abstract only; the trial concluded "No differences were seen in relative risk for diabetes by 6 years with acarbose (1.04, P = 0.81), Metformin (0.99, P = 0.94) or combination therapy (1.02, P = 0.91). In those with IGT at baseline, relative risk was reduced significantly with acarbose (0.66, P = 0.046) but not Metformin (1.09, P = 0.70) or combination therapy (0.72, P = 0.27)" (EDIT 1997). For one trial it is unclear if the trial could be included, the principal investigator was contacted and replied that the trial is neither finished nor published (NCT02409238). For two trials, it is unclear if the trials could be included: one trial we contacted the author but we did not receive a reply (ChiCTR‐IPR‐17012309), and for the other trial we could not contact the author due to lack of contact information (Polanco 2015).
Discussion
Summary of main results
This Cochrane Review investigated the effects of metformin in people at increased risk of developing type 2 diabetes mellitus (T2DM). We included 20 trials with a total of 6774 participants. We judged all trials to be unclear or high risk of bias in one or more 'Risk of bias' domains. Metformin compared with placebo or diet and exercise reduced or delayed the risk of T2DM in people at increased risk for the development of T2DM (moderate‐quality evidence). However, metformin compared to intensive diet and exercise did not reduce or delay the risk of T2DM (very low‐quality evidence). Likewise, for the combination of metformin and intensive diet and exercise compared to intensive diet and exercise only neither showed an advantage or disadvantage regarding the development of T2DM. The reporting of the incidence of T2DM for the remaining comparisons were sparse. The reporting of mortality and macrovascular and microvascular complications were sparse for all comparisons. Socioeconomic effects showed that metformin was more expensive than no treatment, however, assessment of the costs was not identical in the included trials reporting this outcome. The data on health‐related quality of life were sparse. When reported, no firm influence of metformin was found. The certainty of the evidence for these outcome measures was low or very low.
Overall completeness and applicability of evidence
The diagnosis of intermediate hyperglycaemia varied among the trials and some trials used a definition that may have included participants judged to be euglycaemic or having T2DM. Most of the trials applied the criteria established by the World Health Organization (WHO) or American Diabetes Association (ADA) (impaired fasting glucose (IFG) or impaired glucose tolerance (IGT), or both) to define intermediate hyperglycaemia. One trial applied the definition established by the European Diabetes Epidemiology Study Group 1970 (Papoz 1978). One trial applied cut‐off points not recommended by any medical association (Maji 2005). This trial defined IGT with 2‐hour plasma glucose after an oral glucose tolerance test (OGTT) ≥ 6.1 mmol/L and < 11.1 mmol/L and fasting plasma glucose < 6.1 mmol/L (Maji 2005).
Not all ethnicities were represented in the included trials; most of the trials included participants from Asia. One trial included mainly White people (DPP/DPPOS 2002) and one trial included Hispanic people only (PREVENT‐DM 2017). Two trials were performed in France, but did not report the ethnicity of the included people (BIGPRO1 2009; Papoz 1978).
Detailed information about the participants was lacking in most trials. The included trials applied different doses of metformin. A potential selection bias might exist as more healthy and motivated people may participate in a clinical trial. However, a Cochrane Review observed that clinical outcomes in people participating in randomised controlled trials (RCTs) are comparable to similar people outside trials (Vist 2008).
One of the included trials contributed with about 48% of the included participants (DPP/DPPOS 2002). Reporting of complications associated with T2DM during the intervention period was lacking.
The number of participants diagnosed with T2DM in the control groups of the included trials was higher than that estimated from observational trials (Cheng 2006; Morris 2013). This might be explained by the regular glycaemic testing of people participating in a RCT. Therefore, many of those diagnosed with T2DM in a RCT may not be diagnosed in a 'real‐world' setting.
We conducted an extensive search for trials, including publication in all languages. In total, 11 trials were published in Chinese only. We tried to contact all authors to obtain additional data, however, only two authors replied (BIGPRO1 2009; DPP/DPPOS 2002). No additional data were provided. We looked for additional data and cross‐checked our data with systematic reviews of relevance. Examination of four systematic reviews (Haw 2017; Lily 2009; Moelands 2018; Salpeter 2008) revealed three additional references. One systematic review (Pang 2018) revealed a further 10 Chinese trials to be included.
Quality of the evidence
None of the 20 included trials in our review was classified as having low risk of bias in all 'Risk of bias' domains. Only three out of 20 trials provided sufficient information on the method of randomisation and allocation concealment (Alfawaz 2018; DPP/DPPOS 2002; PREVENT‐DM 2017). Four trials explicitly reported blinding of participants and investigators (BIGPRO1 2009; DPP/DPPOS 2002; Li 1999; Papoz 1978). In all the included trials the assessment of the primary outcomes of this review and measurement of glucose values were performed by the investigators. We judged these outcomes as objective and unlikely to be influenced by lack of blinding. Only five trials provided sufficient information on incomplete outcome data (BIGPRO1 2009; DPP/DPPOS 2002; Ji 2011; Lu 2010; PREVENT‐DM 2017). Most of the trials were judged to have high risk of selective outcome reporting because one or more outcomes of relevance for our review were likely assessed but not reported and/or the protocol could not be retrieved. Three of the included trials stated that they had received funding from a pharmaceutical company (BIGPRO1 2009; DPP/DPPOS 2002; IDPP‐1 2006). It is known that trials receiving funding or provision of free drug or devices from a pharmaceutical company leads to more favourable results and conclusions than trials sponsored by other sources (Lundh 2017).
For the comparisons 'metformin versus placebo or diet and exercise' and 'metformin versus intensive diet plus exercise' outcomes were judged to be of very low, low or moderate quality of the evidence. For the remaining comparisons, outcomes were judged to be of very low‐ or low‐quality evidence.
We included trials with an intervention duration of one year or more. Trials with shorter duration could have been included, but as we were focusing on patient‐important outcomes we did not include such short‐term trials.
Potential biases in the review process
Many of the included trials were not designed or powered to detect our predefined patient‐important outcomes. For the performed meta‐analyses we investigated heterogeneity and the potential reasons for it through subgroup and sensitivity analyses. We were dealing with a substantially heterogeneous group of trials. Our meta‐analyses were limited by the inability to use individual participant data to assess whether distinct clinical characteristics may have influenced the effect estimates of the interventions. We tried to contact all trial authors for clarification if one of the bias domains was not adequately reported, however, most of the authors did not reply. We included trials with a minimum duration of one year in order to detect clinically relevant differences for the predefined outcomes. Even though we focused on long‐term trials, the reporting of clinical outcomes in the included trials was sparse. Two review authors carried out data extraction. However, the review authors extracting the data were not blinded as to which trial they were extracting data from.
Agreements and disagreements with other studies or reviews
Recently, several systematic reviews (Haw 2017; Lily 2009; Moelands 2018; Pang 2018; Salpeter 2008) have investigated strategies to prevent or delay T2DM in people at increased risk of T2DM. However, only a few of the systematic reviews have focused on metformin for prevention of T2DM in people at risk for T2DM (Lily 2009; Salpeter 2008). Both of these publications performed a search with no language restriction. One systematic review included three RCT's with a follow‐up time of at least six months investigating people with IGT or IFG (Lily 2009). This review only included trials that focused on the development of T2DM as the primary outcome and thus could have missed potential relevant data if incidence of T2DM was reported as a secondary or other outcome. The review found that metformin was effective in reducing the incidence of T2DM (fixed odds ratio (OR) 0.65, 95% confidence interval (CI) 0.55 to 0.78). Another trial included 31 RCT's with a duration of at least eight weeks (Salpeter 2008). The review included trials with people at increased risk for T2DM defined as people with obesity, polycystic ovary syndrome (PCOS), insulin resistance, impaired glucose tolerance, family history of diabetes, peripheral vascular disease or metabolic syndrome. Due to the wide inclusion criteria, the review possibly included normoglycaemic people and is therefore difficult to compare with our review. Our search did not provide any other relevant systematic review.
Authors' conclusions
Implications for practice.
There is moderate‐quality evidence that metformin compared with placebo or diet and exercise reduced or delayed the risk of type 2 diabetes mellitus (T2DM) in people with impaired glucose tolerance (IGT) and/or impaired fasting glucose (IFG). Following diet and exercise or a non‐metformin antidiabetic drug 281 per 1000 participants developed T2DM compared with 141 per 1000 participants (95% confidence interval (CI) 107 to 183) after metformin therapy.
However, metformin compared to intensive diet and exercise did not reduce or delay the risk of T2DM (moderate‐quality evidence). Following intensive diet and exercise 167 per 1000 participants developed T2DM compared with 133 per 1000 participants (95% CI 78 to 228) after metformin therapy.
Likewise, the combination of metformin and intensive diet and exercise compared to intensive diet and exercise only neither showed an advantage or disadvantage regarding the development of T2DM (very low‐quality evidence). Following intensive diet and exercise 289 per 1000 participants developed T2DM compared with 159 per 1000 participants (CI 29 to 844) after metformin combined with intensive diet and exercise.
It needs to be clarified, whether there is the same metformin effect in people with increased risk defined by other glycaemic variables, such as elevated glycosylated haemoglobin A1c (HbA1c) levels.
Data on patient‐important outcomes such as mortality, macrovascular and microvascular diabetic complications and health‐related quality of life were sparse or missing.
Implications for research.
It remains to be clarified whether the reduction or delay in the incidence of type 2 diabetes mellitus with metformin in people with IGT and/or IFG can decrease the long‐term risk of complications associated with T2DM. Future trials should also investigate the effect of metformin in people with moderately elevated HbA1c and focus on patient‐important outcomes.
Notes
Portions of the background and methods sections, the appendices, additional tables and figures 1 to 3 of this review are based on a standard template established by Cochrane Metabolic and Endocrine Disorders.
Acknowledgements
We thank Takashi Ariie for translation of a Japanese trial (Ishida 2005).
We thank Yana Daneva for translation of a Bulgarian trial (Koev 2004).
We thank Qingguo Lü and Nanwei Tong for their significant input for the first protocol version of this Cochrane Review.
The review authors and the CMED editorial base are grateful to the peer reviewer U. A. Müller, Jena University Hospital, Germany for his time and comments.
Appendices
Appendix 1. Search strategies
| Cochrane Central Register of Controlled Trials (CENTRAL) (Cochrane Register of Studies Online) |
| 1. MESH DESCRIPTOR Prediabetic state 2. MESH DESCRIPTOR Glucose Intolerance 3. (prediabet* or pre diabet*):TI,AB,KY 4. (intermediate hyperglyc?emi*):TI,AB,KY 5. ((impaired fasting ADJ2 glucose) or IFG or impaired FPG):TI,AB,KY 6. glucose intolerance:TI,AB,KY 7. ((impaired glucose ADJ (tolerance or metabolism)) or IGT):TI,AB,KY 8. ((risk or progress* or prevent* or inciden* or conversion or develop* or delay*) ADJ4 (diabetes or T2D* or NIDDM or "type 2" or "type II")):TI,AB,KY 9. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 10. MESH DESCRIPTOR Metformin 11. metformin*:TI,AB,KY 12. #10 OR #11 13. #9 AND #12 |
| MEDLINE (Ovid SP) |
| 1. Prediabetic state/ 2. Glucose Intolerance/ 3. (prediabet* or pre diabet*).tw. 4. intermediate hyperglyc?emi*.tw. 5. ((impaired fasting adj2 glucose) or IFG or impaired FPG).tw. 6. glucose intolerance.tw. 7. ((impaired glucose adj (tolerance or metabolism)) or IGT).tw. 8. ((risk or progress* or prevent* or inciden* or conversion or develop* or delay*) adj4 (diabetes or T2D* or NIDDM or "type 2" or "type II")).tw. 9. or/1‐8 10. Metformin/ 11. metformin*.tw. 12. 10 or 11 13. 9 and 12 [14‐24: Cochrane Handbook 2008 RCT filter ‐ sensitivity maximizing version] 14. randomized controlled trial.pt. 15. controlled clinical trial.pt. 16. randomi?ed.ab. 17. placebo.ab. 18. drug therapy.fs. 19. randomly.ab. 20. trial.ab. 21. groups.ab. 22. or/14‐21 23. exp animals/ not humans/ 24. 22 not 23 25. 13 and 24 26. ..dedup 25 |
| Scopus |
| 1. KEY("prediabetic state" OR "glucose intolerance" OR "impaired glucose tolerance") 2. TITLE‐ABS(prediabet* OR "pre diabet*" OR "intermediate hyperglyc?emi*") 3. TITLE‐ABS(("impaired fasting" PRE/3 glucose) OR IFG OR "impaired FPG") 4. TITLE‐ABS("glucose intolerance") 5. TITLE‐ABS(("impaired glucose" PRE/0 (tolerance OR metabolism)) OR IGT) 6. TITLE‐ABS((risk or progress* or prevent* or inciden* or conversion or develop* or delay*) W/4 (diabetes or T2D* or NIDDM or "type 2" or "type II")) 7. #1 OR #2 OR #3 OR #4 OR #5 OR #6 8. TITLE‐ABS‐KEY(Metformin) 9. #7 AND #8 10. TITLE‐ABS‐KEY(random* OR "clinical trial*" OR "double blind*" OR placebo*) 11. #9 AND #10 12. #11 AND (LIMIT‐TO (DOCTYPE, "ar") OR LIMIT‐TO (DOCTYPE, "ip")) [ar = article, ip = article in press] |
| ICTRP Search Portal (Standard search) |
| prediabet* AND metformin OR pre diabet* AND metformin OR impaired AND glucose* AND metformin OR impaired AND fasting* AND metformin OR glucose AND intoleran* AND metformin OR IFG AND metformin OR IGT AND metformin |
| ClinicalTrials.gov (Expert search) |
| (prediabetes OR prediabetic OR "pre diabetes" OR "pre diabetic" OR "impaired glucose" OR "impaired fasting" OR "glucose intolerance" OR IGT OR IFG OR ((diabetes OR "type 2" OR "type II" OR T2D OR T2DM) AND (risk OR progress OR progression OR progressed OR incident OR incidence OR conversion OR developed OR development OR develop OR delay OR delayed OR prevention OR prevent OR prevented))) [DISEASE] AND metformin [TREATMENT] |
Appendix 2. Assessment of risk of bias
| Risk of bias domains |
|
Random sequence generation (selection bias due to inadequate generation of a randomised sequence) For each included study, we described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
Allocation concealment (selection bias due to inadequate concealment of allocation prior to assignment) We described for each included study the method used to conceal allocation to interventions prior to assignment and we assessed whether intervention allocation could have been foreseen in advance of or during recruitment, or changed after assignment.
We also evaluated study baseline data to incorporate assessment of baseline imbalance into the 'Risk of bias' judgment for selection bias (Corbett 2014). Chance imbalances may also affect judgments on the risk of attrition bias. In the case of unadjusted analyses, we distinguished between studies that we rated as being at low risk of bias on the basis of both randomisation methods and baseline similarity, and studies that we judged as being at low risk of bias on the basis of baseline similarity alone (Corbett 2014). We reclassified judgements of unclear, low or high risk of selection bias as specified in Appendix 3. Blinding of participants and study personnel (performance bias due to knowledge of the allocated interventions by participants and personnel during the study) We evaluated the risk of detection bias separately for each outcome (Hróbjartsson 2013). We noted whether endpoints were self‐reported, investigator‐assessed or adjudicated outcome measures (see below).
Blinding of outcome assessment (detection bias due to knowledge of the allocated interventions by outcome assessment We evaluated the risk of detection bias separately for each outcome (Hróbjartsson 2013). We noted whether endpoints were self‐reported, investigator‐assessed or adjudicated outcome measures (see below).
Incomplete outcome data (attrition bias due to amount, nature or handling of incomplete outcome data) For each included study and/or each outcome, we described the completeness of data, including attrition and exclusions from the analyses. We stated whether the study reported attrition and exclusions, and reported the number of participants included in the analysis at each stage (compared with the number of randomised participants per intervention/comparator groups). We also noted if the study reported the reasons for attrition or exclusion and whether missing data were balanced across groups or were related to outcomes. We considered the implications of missing outcome data per outcome such as high dropout rates (e.g. above 15%) or disparate attrition rates (e.g. difference of 10% or more between study arms).
Selective reporting (reporting bias due to selective outcome reporting) We assessed outcome reporting bias by integrating the results of the appendix 'Matrix of study endpoints (publications and trial documents)' (Boutron 2014; Jones 2015; Mathieu 2009), with those of the appendix 'High risk of outcome reporting bias according to the Outcome Reporting Bias In Trials (ORBIT) classification' (Kirkham 2010). This analysis formed the basis for the judgement of selective reporting.
Other bias
|
Appendix 3. Selection bias decisions
| Selection bias decisions for studies reporting unadjusted analyses: comparison of results obtained using method details alone with results using method details and trial baseline informationa | |||
| Reported randomisation and allocation concealment methods | 'Risk of bias' judgement using methods reporting | Information gained from study characteristics data | 'Risk of bias' using baseline information and methods reporting |
| Unclear methods | Unclear risk | Baseline imbalances present for important prognostic variable(s) | High risk |
| Groups appear similar at baseline for all important prognostic variables | Low risk | ||
| Limited or no baseline details | Unclear risk | ||
| Would generate a truly random sample, with robust allocation concealment | Low risk | Baseline imbalances present for important prognostic variable(s) | Unclear riskb |
| Groups appear similar at baseline for all important prognostic variables | Low risk | ||
| Limited baseline details, showing balance in some important prognostic variablesc | Low risk | ||
| No baseline details | Unclear risk | ||
| Sequence is not truly random, or allocation concealment is inadequate | High risk | Baseline imbalances present for important prognostic variable(s) | High risk |
| Groups appear similar at baseline for all important prognostic variables | Low risk | ||
| Limited baseline details, showing balance in some important prognostic variablesc | Unclear risk | ||
| No baseline details | High risk | ||
| aTaken from Corbett 2014; judgements highlighted in bold indicate situations in which the addition of baseline assessments would change the judgement about risk of selection bias, compared with using methods reporting alone. bImbalance identified that appears likely to be due to chance. cDetails for the remaining important prognostic variables are not reported. | |||
Appendix 4. Description of interventions
| Trial ID | Intervention(s) (route, frequency, total dose/day) | Intervention(s) appropriate as applied in a clinical practice settinga | Comparator(s) (route, frequency, total dose/day) | Comparator(s) appropriate as applied in a clinical practice settinga |
| Alfawaz 2018 | Metformin 500 mg twice a day plus standard advice on diet plus exercise | Yes | C1: intensive diet and exercise | Yes |
| C2: diet plus exercise | ||||
| PREVENT‐DM 2017 | Metformin 850 mg daily for the first month, thereafter 850 mg twice daily. If side effects, then dose reduction. Titrated to the highest tolerable dose with a maximum of 850 mg three times a day | Yes | C1: intensive diet and physical activity | Yes |
| C2: diet plus exercise | ||||
| Zeng 2013 | Metformin 38 mg once daily. Diet plus exercise (no details) |
Yes | C1: diet plus exercise | Yes |
| C2: pioglitazone 38 mg once daily Diet plus exercise | ||||
| Zhao 2013 | Metformin 500 mg twice daily Education plus behaviour interventions, including diet control and increased physical activity (at least 30 minutes per day and at least 5 days per week) |
Yes | Education plus behaviour interventions, including diet control and increased physical activity (at least 30 minutes per day and at least 5 days per week) | Yes |
| Iqbal Hydrie 2012 | Metformin 500 mg twice daily plus intensive diet and physical activity | Yes | C1: intensive diet plus physical activity | Yes |
| C2: standard medical advice | ||||
| Liao 2012 | Metformin from 250 mg, three times daily, adjusting the dose according to blood glucose, with the maximum 1500 mg daily | Yes | Acarbose from 50 mg three times daily with meals, adjusting the dose according to blood glucose, with the maximum 300 mg daily | Yes |
| Ji 2011 | Metformin 500 mg, three times daily, after meals. Standard advice on diet plus exercise |
Yes | C1: Intesive diet plus exercise; based on individual dietary habits, calories are determined according to age, height, actual weight, activity intensity and season. Patients were given a low‐fat diet and a controlled diet. Patients were instructed to have a balanced diet and exercise (150 minutes per week). | Yes |
| C2: diet plus exercise | ||||
| Lu 2010 | Metformin 250 mg three times daily, according to tolerance, gradually reaching the target dose of 500 mg three times daily Lectures and leaflets were given to inform the prognosis and hazards of pre‐diabetes, and scientific diet and exercise instructions were provided for each follow‐up to promote a healthy lifestyle |
Yes | By giving lectures and sending out leaflets to inform the prognosis and hazards of pre‐diabetes, providing healthy diet and lifestyle guidance, referring to the dietary nutrition guidelines of China, and adjusting diet according to individual specific conditions to maintain a balanced nutritional status. The advice was: (1) variety of food, mainly cereals, with a combination of grains and grains; (2) eat more vegetables, fruits and potatoes; (3) daily intake of milk, beans and their preparation; (4) eat adequate amount of fish, poultry, eggs and lean meat; (5) reduce the amount of cooking oil, eat light diet with little salt, not too greasy and salty, including not too much smoke and animal oil food, daily adult salt to 6 g, eat less pickles, monosodium glutamate and other sodium‐containing food; (6) reasonable allocation of three meals, snacks should be appropriate. Reduce calorie intake to maintain the ideal weight. Patients with a BMI < 25kg/m2 were advised 30 Kcal/kg·day, with emphasis on alcohol and sugary soft drinks: patients with a BMI ≥ 25kg/m2 were encouraged to lose 0.5 g to 1.0 kg per month until ideal body weight. Initiate, encourage family members to care, supervise the completion of dietary plan. At each follow‐up, the participants were informed of dietary compliance. The exercise advice was as follows: (1) exercise prescription should consider the patient's individual factors such as gender, age, height, weight and living habits comprehensively; (2) principle of gradual progress and acting according to ability.The formulation of exercise prescription should be based on the patient's disease degree, physical condition to develop a long‐term plan, step by step, not subjective assumptions, eager for quick success and instant benefit. In the exercise prescription a clear purpose should be stated, and use the degree of realisation of this purpose to measure and modify the exercise prescription. Patients are required to engage in continuous aerobic exercise. Generally, after 30 minutes of exercise, blood glucose starts to supply energy to tissues, thus causing a drop in blood glucose. Moreover, studies have confirmed that the effect of moderate amount of exercise on blood glucose lasts for 12 months.17 hours, so people with diabetes should exercise at least once a day, no less than 30 minutes at a time. According to the principles and contents of exercise prescription, the exercise group should take appropriate physical activities and adopt various forms according to the specific conditions of each person, such as walking, jogging, playing ball games, aerobics, taijiquan, etc. It is required that the exercise program should be 1 exercise unit per day, lasting at least 30 minutes, and at least 5 days per week. At each follow‐up, participants were informed about exercise compliance and urged to stick to the prescribed exercise regimen. |
Yes |
| BIGPRO1 2009 | Metformin, 850 mg tablet twice a day; diet plus exercise | Yes | Identical placebo tablet given twice a day; diet plus exercise | Yes |
| Chen 2009 | Metformin 750 mg, three times daily All patients received behaviour changing with reference to diet and exercise therapy in the diabetes guidelines of China |
Yes | All patients received behaviour changing with reference to diet and exercise therapy in the diabetes guidelines of China. | Yes |
| Jin 2009 | Metformin 1000 mg twice or three times daily. Diet plus exercise (no details) |
Yes | C1: diet plus exercise (no details) | Yes |
| C2: rosiglitazone 4 mg, orally, once daily. Diet plus exercise (no details) | ||||
| Li 2009 | Metformin 500 mg once daily plus diet and exercise | Yes | Individualised diet and exercise and education | Yes |
| Wang 2009 | Metformin 250 mg twice daily, with or after meals. Plus standard advice on diet and exercise |
Yes | Diet plus exercise | Yes |
| IDPP‐1 2006 | I1: Metformin, 500 mg twice a day I2: Metformin, 500 mg twice a day plus intensive diet and physical activity |
Yes | C1: intensive diet and exercise | Yes |
| C2: standard care | ||||
| Maji 2005 | Metformin 500 mg once daily plus diet and physical activity | Yes | C1: intensive diet and physical activity | Yes |
| C2: rosiglitazone 2 mg daily plus diet and physical activity | ||||
| C3: acarbose 25 mg twice daily plus diet and physical activity | ||||
| Fang 2004 | Metformin 375 mg to 750 mg three times a day | Yes | C1: acarbose 75 mg to 150 mg three times a day | Yes |
| C2: intensive diet plus exercise | ||||
| C3: diet and physical activity | ||||
| DPP/DPPOS 2002 | Metformin 850 mg twice a day plus standard diet and lifestyle advice | Yes | C1: intensive diet plus exercise: consumption of a healthy low‐calorie, low‐fat diet and to engage in physical activity of moderate intensity (such as brisk walking) for at least 150 minutes/week | Yes |
| C2: placebo tablets given twice a day plus standard diet and lifestyle advice | ||||
| Lu 2002 | Metformin 750 mg three times daily. Health education (not described, assumed to be standard care) |
Yes | C1: health education (not described, assumed to be standard care) | Yes |
| C2: diet instruction (every 6 months). Health education | ||||
| C3: fibre diet, fibre (Litesse) 6 g, twice daily, take with meal. Provide fibre once a month. Health education | ||||
| Li 1999 | Metformin, 250 mg three times a day | Yes | C1: placebo administered with the same schedule as metformin | Yes |
| Papoz 1978 | Metformin 850 mg, twice daily plus placebo, twice daily; overweight participants were recommended calorie restriction | Yes | C1: glibenclamide 2.0 mg, orally, twice daily and placebo, orally, twice daily. Overweight participants were recommended calorie restriction | Yes |
| C2: placebo, orally, twice daily; overweight participants were recommended calorie restriction | ||||
|
aThe term 'clinical practice setting' refers to the specification of the intervention/comparator as used in the course of a standard medical treatment (such as dose, dose escalation, dosing scheme, provision for contraindications and other important features) BMI: body mass index; C: comparator; I: intervention. | ||||
Appendix 5. Baseline characteristics (I)
| Trial ID | Intervention(s) and comparator(s) | Duration of intervention (duration of follow‐up)a | Description of participants | Trial period (year to year) | Country | Setting | Ethnic groups (%) | Duration of being at risk for T2DM |
| Alfawaz 2018 | I: metformin | 1 year (1 year) | IFG | April 2013 ‐ March 2017 | Saudi Arabia | Outpatient | Saudi Arabian 100% | — |
| C1: intensive diet plus exercise | Saudi Arabian 100% | — | ||||||
| C2: standard care | Saudi Arabian 100% | — | ||||||
| PREVENT‐DM 2017 | I1: metformin | 12 months (12 months) | IFG and/or moderately elevated HbA1c, Hispanic | 2013 ‐ 2015 | USA | Outpatient | 100% Hispanic | — |
| C1: intensive diet plus exercise | 100% Hispanic | — | ||||||
| C2: standard care | 100% Hispanic | — | ||||||
| Zeng 2013 | I: metformin | 2 years (2 years) | IFG with or without IGT, Chinese | January 2009 ‐ March 2010 (recruitment period) 2012 (end of treatment period) | China | Outpatient | Chinese: 100 | — |
| C1: Standard care | Chinese: 100 | — | ||||||
| C2: pioglitazone | Chinese: 100 | — | ||||||
| Zhao 2013 | I: metformin plus intensive diet plus exercise | 1 year (1 year) | IFG, IGT, obese, Chinese | — | China | Outpatient | Chinese: 100 | — |
| C: intensive diet plus exercise | Chinese: 100 | — | ||||||
| Iqbal Hydrie 2012 | I1: metformin plus intensive diet and exercise | 18 months (18 months) | IGT, Asian | — | Pakistan | Outpatient | Assume 100% Asian (Pakistini) | — |
| C1: intensive diet and exercise | Assume 100% Asian (Pakistini) | — | ||||||
| C2: standard care | Assume 100% Asian (Pakistini) | — | ||||||
| Liao 2012 | I: metformin | 1 year (1 year) | IGT, Chinese | August 2009 ‐ July 2010 (recruitment period) 2011 (end of treatment period) | China | Outpatient | Chinese: 100 | — |
| C: acarbose | Chinese: 100 | — | ||||||
| Ji 2011 | I1: metformin | 2 years (2 years) | IFG and/or IGT, Chinese | September 2007 ‐ August 2008 (recruitment period) 2010 (end of treatment period) | China | Outpatient | Chinese: 100 | — |
| C1: intensive diet plus exercise | Chinese: 100 | — | ||||||
| C2: standard care | Chinese: 100 | — | ||||||
| Lu 2010 | I: metformin | 2 years (2 years) | IFG and/or IGT, Chinese | September 2007 (recruitment point) 2009 (end of treatment point) | China | Outpatient | Chinese: 100 | — |
| C: standard care | Chinese: 100 | — | ||||||
| BIGPRO1 2009 | I: metformin | 1 year (1 year) | Adults with IFG or IGT | January 1991‐ mid 1992 | France | Outpatient | — | — |
| C: placebo | — | — | ||||||
| Chen 2009 | I: metformin | 1 year (2 years) | IGT patients | — | China | Outpatient | Chinese: 100 | — |
| C: standard care | Chinese: 100 | — | ||||||
| Jin 2009 | I: metformin | 3 years (3 years) | IFG patients | January 2004 ‐ May 2006 (recruitment period) 2009 (end of treatment period) | China | Outpatient | Chinese: 100 | — |
| C1: standard care | Chinese: 100 | — | ||||||
| C2: rosiglitazone | Chinese: 100 | — | ||||||
| Li 2009 | I: metformin | 3 years (3 years) | IFG and IGT, obese, Chinese | 2004 ‐ 2005 (recruitment period) 2008 (end of treatment period) | China | Outpatient | Chinese: 100 | — |
| C: intensive diet plus exercise | Chinese: 100 | — | ||||||
| Wang 2009 | I: metformin | 1 year (1 years) | IFG, IGT or both, Chinese | January ‐ December 2008 (recruitment period) 2009 (end of treatment period) | China | Outpatient | Chinese: 100 | — |
| C: standard care | Chinese: 100 | — | ||||||
| IDPP‐1 2006 | I1: metformin | 3 years (3 years) | Participants with IGT aged 35 years to 55 years | 2001 ‐ 2005 | India | Outpatient | Asian Indian: 100 | — |
| I2: metformin plus intensive diet and physical activity | Asian Indian: 100 | — | ||||||
| C1: intensive exercise plus diet | Asian Indian: 100 | — | ||||||
| C2: standard care | Asian Indian: 100 | — | ||||||
| Maji 2005 | I1: metformin | 3 years (3 years) | IGT | Initiated 2001 | India | Outpatient | Assume 100% Indian | — |
| C1: intensive lifestyle intervention | Assume 100% Indian | — | ||||||
| C2: rosiglitazone | Assume 100% Indian | — | ||||||
| C3: acarbose | Assume 100% Indian | — | ||||||
| Fang 2004 | I: metformin | 5 years (5 years) | IFG, IGT or both, Chinese | 1998‐2003 | China | Outpatient | Assume 100% Asian (Chinese) | — |
| C1: acarbose | Assume 100% Asian (Chinese) | — | ||||||
| C2: intensive exercise and diet | Assume 100% Asian (Chinese) | — | ||||||
| C3: standard care | Assume 100% Asian (Chinese) | — | ||||||
| DPP/DPPOS 2002 | I: metformin | Mean 2.8 years (mean 15 years) | IGT and elevated fasting glucose. Overweight or obese | 1996‐1999 (recruitment period) July 2001 (end of treatment period) followed up in the DPP Outcomes Study (DPPOS 2002, to 2014) |
USA | Outpatient | White: 56 African American: 21 Hispanic: 15.1 American Indian: 4.8 Asian: 3.4 | — |
| C1: intensive exercise and diet | White: 54 African American: 19 Hispanic: 17 American Indian: 6 Asian: 5 | — | ||||||
| C2: placebo | White: 54 African American: 20 Hispanic: 16 American Indian: 6 Asian: 5 | — | ||||||
| Lu 2002 | I1: metformin | 3 year (3 years) | IGT, Chinese | — | China | Outpatient | Chinese: 100 | — |
| C1: standard care | Chinese: 100 | — | ||||||
| C2: standard care plus diet instruction every 6th month | Chinese: 100 | — | ||||||
| C3: standard care plus fibre diet | Chinese: 100 | — | ||||||
| Li 1999a | I: metformin | 12 months (12 months) | IGT, Chinese | 1992‐1994 | China | Outpatient | Assume 100% Asian (Chinese) | — |
| C: placebo | Assume 100% Asian (Chinese) | — | ||||||
| Papoz 1978 | I1: metformin (plus placebo) | 24 months (26 months) | IFG, IGT or both | Participants entered the trial from 1969 to 1971 | France | Outpatient | — | — |
| C1: glibenclamide plus placebo | — | — | ||||||
| C2: placebo | — | — | ||||||
| —: denotes not reported aBaseline data only available for the participants who completed the trial C: comparator; I: intervention; DPP: Diabetes Prevention Program; HbA1c: glycosylated haemoglobin A1c; IFG: impaired fasting glucose; IGT: impaired glucose tolerance. | ||||||||
Appendix 6. Baseline characteristics (II)
| Trial ID | Intervention(s) and comparator(s) | Sex (female %) | Age (mean/range years (SD)) | Fasting plasma glucose (mean mmol/L (SD)) | 2h‐PG (mean mmol/L (SD)) | Indicator of increased risk: elevated HbA1c (mean % (SD)) | BMI (mean kg/m² (SD)) | Comedications/Cointerventions | Comorbidities |
| Alfawaz 2018 | I: metformin | 71 | 42.6 (6.9) | 6.6 (0.5) | — | 5.6 (0.5) | 32.1 (5.7) | — | — |
| C1: intensive diet plus exercise | 70 | 43.4 (7.8) | 6.1 (0.4) | — | 5.8 (0.4) | 31.3 (6.4) | — | — | |
| C2: standard care | 75 | 42.3 (11.2) | 6.0 (0.4) | — | 5.6 (0.5) | 32.6 (5.8) | — | — | |
| PREVENT‐DM 2017 | I1: metformin | 100 | 45.8 (11.7) | 5.3 (0.6) | — | 6.0 (0.2) | 33.2 (5.5) | — | — |
| C1: intensive diet plus exercise | 100 | 45.5 (12.3) | 5.4 (0.4) | — | 5.9 (0.3) | 34.3 (7.9) | — | — | |
| C2: standard care | 100 | 44.0 (13.6) | 5.3 (0.6) | — | 5.9 (0.2) | 32.2 (5.7) | — | — | |
| Zeng 2013 | I: metformin | 44 | 47.7 (5.8) | 5.5 (0.4) | 8.75 (0.57) | — | 25.2 (1.8) | — | — |
| C1: Standard care | 42 | 48.6 (7.4) | 5.6 (0.3) | 8.9 (0.4) | — | 25.3 (2.6) | — | — | |
| C2: pioglitazone | 46 | 47.2 (4.4) | 5.7 (0.2) | 8.9 (0.6) | — | 25.2 (3.2) | — | — | |
| Zhao 2013 | I: metformin plus intensive diet plus exercise | 43 | — | — | 9.32 (1.51) | — | 28.61 (3.5) | — | — |
| C: intensive diet plus exercise | 48 | — | — | 9.13 (1.72) | — | 28.32 (3.7) | — | — | |
| Iqbal Hydrie 2012 | I1: metformin plus intensive diet and physical activity | — | 43.5 (8.4) | — | — | — | 28.1 (4.3) | Information about healthy diet and exercise | 25% had hypertension at baseline |
| C1: intensive diet and physical activity | — | 43.1 (10.1) | — | — | — | 26.1 (4.7) | — | ||
| C2: standard care | — | 44.2 (10.9) | — | — | — | 27.0 (5.7) | — | ||
| Liao 2012 | I: metformin | 46 | 50.8 (9.3) | 6.03 (0.5) | 8.2 (0.84) | — | — | — | — |
| C: acarbose | 48 | 50.5 (8.3) | 6.05 (0.51) | 8.28 (1.12) | — | — | — | — | |
| Ji 2011 | I1: metformin | 54 | 50.9 (2.7) | 7.0 (1.4) | 8.8 (1.3) | — | 24.6 (2.8) | — | — |
| C1: Intensive diet plus exercise | 47 | 52.1 (2.3) | 7.2 (0.7) | 9.0 (1.5) | — | 24.4 (1.4) | — | — | |
| C2: standard care | 53 | 53.4 (3.8) | 6.9 (1.8) | 8.7 (1.3) | — | 24.6 (2.8) | — | — | |
| Lu 2010 | I: metformin | 43 | 41 (4.6) | — | — | 5.87 (0.47) | 25.1 (2.8) | — | — |
| C: standard care | 41 | 41 (4.3) | — | — | 5.89 (0.44) | 25.5 (3.4) | — | — | |
| BIGPRO1 2009a | I: metformin | 76 | 52.6 (6.2) | 5.8 (0.6) | 8.3 (1.2) | — | 33.5 (5.9) | Diet and exercise | — |
| C: placebo | 58 | 48.9 (6.7) | 5.6 (0.8) | 8.5 (1.2) | — | 35.6 (7.5) | Diet and exercise | — | |
| Chen 2009 | I: metformin | 41 | 56.4 (2.1) | 5.4 (0.6) | 9.1 (0.8) | — | 127.2 (17.9) | — | — |
| C: standard care | 44 | 56.3 (12.8) | 5.3 (0.6) | 9.0 (0.9) | — | 125.8 (18.0) | — | — | |
| Jin 2009 | I: metformin | — | — | 6.47 (0.18) | 6.82 (0.45) | — | 23.95 (3.04) | — | — |
| C1: standard care | — | — | 6.47 (0.18) | 6.92 (0.41) | — | 24.8 (3.47) | — | — | |
| C2: rosiglitazone | — | — | 6.5 (0.19) | 6.88 (0.5) | — | 24.85 (3.97) | — | — | |
| Li 2009 | I: metformin | — | — | 6.6 (0.4) | 10.4 (0.3) | — | 28.1 (1.4) | — | — |
| C: intensive diet plus exercise | — | — | 6.6 (0.3) | 10.3 (0.4) | — | 28.2 (1.7) | — | — | |
| Wang 2009 | I: metformin | — | 49 (9) | — | 9.4 (1.6) | — | 25.0 (1.0) | — | — |
| C: standard care | — | 50 (7) | — | 9.2 (1.5) | — | 26.0 (2.0) | — | — | |
| IDPP‐1 2006 | I1: metformin | 19.5 | 45.9 (5.9) | 5.4 (0.8) | 8.5 (0.7) | 6.2 (0.6) | 25.6 (3.7) | — | 26.3% had hypertension at baseline |
| I2: metformin plus intensive diet and physical activity | 18.6 | 46.3 (5.7) | 5.4 (0.8) | 8.5 (0.7) | 6.2 (0.6) | 25.6 (3.3) | — | 37.2% had hypertension at baseline | |
| C1: intensive exercise plus diet | 21.8 | 46.1 (5.7) | 5.4 (0.7) | 8.5 (0.7) | 6.1 (0.5) | 25.7 (3.3) | — | 31.6% had hypertension at baseline | |
| C2: standard care | 23.5 | 45.2 (5.7) | 5.5 (0.8) | 8.6 (0.7) | 6.2 (0.5) | 26.3 (3.7) | — | 32.4% had hypertension at baseline | |
| Maji 2005 | I1: metformin | Only reported for all groups: 64.1 | — | 5.7 (0.8) | 8.7 (1.1) | 7.5 (0.6) | 28.2 (1.2) | Information about healthy diet and exercise | — |
| C1: intensive lifestyle intervention | 5.6 (0.9) | 8.5 (1.3) | 7.4 (0.3) | 28.6 (1.2) | — | — | |||
| C2: rosiglitazone | 5.8 (0.9) | 8.9 (0.9) | 7.6 (0.5) | 28.5 (1.2) | Information about healthy diet and exercise | — | |||
| C3: acarbose | 5.3 (0.7) | 8.8 (2.0) | 7.4 (0.6) | 28.1 (1.4) | Information about healthy diet and exercise | — | |||
| Fang 2004 | I: metformin | 48 | 50 (1) | 6.3 (2.1) | 7.48 (1.9) | — | 25.2 (0.4) | ‐ | — |
| C1: acarbose | 50 | 50 (1) | 6.5 (1.9) | 8.38 (1.9) | — | 24.9 (0.3) | ‐ | — | |
| C2: intensive exercise and diet | 40 | 49 (1) | 5.6 (2.4) | 6.99 (2.1) | — | 25.3 (0.3) | ‐ | — | |
| C3: standard care | 40 | 47 (2) | 5.7 (2.3) | 6.35 (2.2) | — | 24.8 (0.4) | ‐ | — | |
| DPP/DPPOS 2002 | I: metformin | 66.2 | 50.9 (10.3) | 5.9 (0.5) | 9.2 (0.9) | 5.9 (0.5) | 33.9 (6.6) | 17% in all treatment groups had antihypertensive treatment at baseline. 5.2% of participants reported taking pharmacologic therapy for dyslipidaemia at entry to the trial |
16% of the women in both group had previously had gestational diabetes Overall 29.6% had a history of hypertension. 34% had a history of stroke. 16% had a history of revascularization. 32% had a history of myocardial infarction |
| C1: intensive exercise and diet | 68 | 50.6 (11.3) | 5.90 (0.5) | 9.1 (0.9) | 5.91 (0.5) | 33.9 (6.8) | |||
| C2: placebo | 69 | 50.3 (10.4) | 5.92 (0.5) | 9.1 (1.0) | 5.91 (0.5) | 34.2 (6.8) | |||
| Lu 2002 | I1: metformin | 25 | 61 (9) | — | 9.0 (0.8) | — | 26.1 (2.7) | — | — |
| C1: standard care | 16 | 65 (7) | — | 9.1 (1.0) | — | 25.9 (3.3) | — | — | |
| C2: standard care plus diet instruction every 6th month | 35 | 63 (9) | — | 9.0 (0.9) | — | 26.0 (3.2) | — | — | |
| C3: standard care plus fibre diet | 29 | 64 (9) | — | 9.4 (0.9) | — | 26.2 (3.0) | — | — | |
| Li 1999b | I: metformin | 27.2 | 49 (1.3) | 6.9 (0.9) | 9.1 (0.9) | 7.4 (0.8) | 26.0 (23) | Information about healthy diet and exercise | — |
| C: placebo | 29.7 | 50 (1.1) | 7.3 (1.0) | 9.0 (1.0) | 7.3(0.8) | 26.4 (2.4) | Information about healthy diet and exercise | — | |
| Papoz 1978 | I1: metformin (plus placebo) | 0 | 44 (5.5)c | 6.7 (0.7)c,d,e | 8.2 (1.7)c,d,e | — | — | Overwieght participants were prescribed calorie restriction in order to approach their ideal body weight | — |
| C1: glibenclamide plus placebo | 0 | 43 (10.6)c | 6.7 (0.7)c,d,e | 8.8 (2.0)c,d,e | — | — | Overwieght participants were prescribed calorie restriction in order to approach their ideal body weight | — | |
| C2: placebo | 0 | 45 (5.7)c | 6.3 (0.7)c,d,e | 8.3 (2.1)c,d,e | — | — | Overwieght participants were prescribed calorie restriction in order to approach their ideal body weight | — | |
| —: denotes not reported aBaseline data only available for the people with IGT/IFG who completed the trial bBaseline data only available for the participants who completed the trial cSD calculated from standard error dGlucose concentrations were converted from mg/dL to mmol/L (diabetes.co.uk 2019a) eBlood glucose concentrations were converted to plasma glucose values (diabetes.co.uk 2019b) BMI: body mass index; C: comparator; CVD: cardiovascular disease; HbA1c: glycosylated haemoglobin A1c; I: intervention; SD: standard deviation. | |||||||||
Appendix 7. Matrix of study endpoints (publications and trial documents)
| Trial ID | |
| Alfawaz 2018 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
| Source: N/T | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): participants with metabolic syndrome Secondary outcome measure(s): individual components of metabolic syndrome Other outcome measure(s): total number of metabolic syndrome components; metabolic syndrome risk‐score | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): participants with metabolic syndrome Secondary outcome measure(s): individual components of metabolic syndrome Other outcome measure(s): — | |
| PREVENT‐DM 2017 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
|
Source:NCT02088034 Primary outcome measure(s): weight Secondary outcome measure(s): cardiometabolic markers, physical activity, dietary intake, diabetes knowledge (assessed with Spanish‐speaking Diabetes Knowledge Questionnaire) Other outcome measure(s): Trial results available in trial register: yes | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): blood glucose, insulin levels Secondary outcome measure(s): — Other outcome measure(s): weight | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): weight loss Secondary outcome measure(s): HbA1c, waist circumference Other outcome measure(s): | |
| Zeng 2013 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
|
Source: NT Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): — Trial results available in trial register: — | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): incidence of T2DM Secondary outcome measure(s): fasting blood glucose, 2‐hour plasma glucose Other outcome measure(s): fasting insulin, 2‐hour insulin, BMI, blood pressure, cholesterol, conversion to normoglycaemia | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): incidence of T2DM Secondary outcome measure(s): fasting blood glucose, 2‐hour plasma glucose Other outcome measure(s): fasting insulin, 2‐hour insulin, BMI, blood pressure, cholesterol, conversion to normoglycaemia | |
| Zhao 2013 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
|
Source: NT Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): — Trial results available in trial register: — | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): incidence of T2DM Secondary outcome measure(s): fasting blood glucose, 2‐hour plasma glucose, non‐serious adverse events Other outcome measure(s): fasting insulin, HOMA‐IR, WHR, BMI | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): — (NA) Secondary outcome measure(s): — Other outcome measure(s): — | |
| Iqbal Hydrie 2012 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
|
Source: main publication Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): — Trial results available in trial register: — | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): incidence of T2DM Secondary outcome measure(s): Other outcome measure(s): waist circumference, weight changes | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): incidence of T2DM Secondary outcome measure(s): Other outcome measure(s): | |
| Liao 2012 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
|
Source: NT Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): — Trial results available in trial register: — | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): incidence of T2DM, serious adverse events Secondary outcome measure(s): fasting plasma glucose, 2‐hour plasma glucose Other outcome measure(s): fasting insulin, HOMA‐IR, WHR, BMI, conversion to normoglycaemia | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): — Secondary outcome measure(s): fasting plasma glucose, 2‐hour plasma glucose Other outcome measure(s): — | |
| Ji 2011 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
|
Source: NT Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): — Trial results available in trial register: — | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): incidence of T2DM Secondary outcome measure(s): fasting plasma glucose, 2‐hour plasma glucose, HbA1c Other outcome measure(s): conversion to normoglycaemia, BMI, triglycerides, cholesterol, hs‐CRP, fasting insulin | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): incidence of T2DM Secondary outcome measure(s): fasting plasma glucose, 2‐hour plasma glucose, HbA1c Other outcome measure(s): conversion to normoglycaemia, BMI, triglycerides, cholesterol, hs‐CRP, fasting insulin | |
| Lu 2010 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
|
Source: NT Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): — Trial results available in trial register: — | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): incidence of T2DM, serious adverse events Secondary outcome measure(s): HbA1c (%), IGT, IFG, non‐serious adverse events Other outcome measure(s): BMI, compliance of treatment | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): incidence of T2DM, serious adverse events Secondary outcome measure(s): HbA1c (%), IGT, IFG, non‐serious adverse events Other outcome measure(s): BMI, compliance of treatment | |
| BIGPRO1 2009 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
| Source: design paper | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): not prioritised as primary or secondary: fasting plasma glucose; 2‐hour plasma glucose; plasma lipids: total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides; fasting plasma insulin; 2‐hour plasma insulin; blood pressure: systolic and diastolic blood pressure; BMI and WHR | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): not prioritised as primary or secondary: fasting plasma glucose; 2‐hour plasma glucose; plasma lipids: total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides; fasting plasma insulin; 2‐hour plasma insulin; blood pressure; BMI and WHR | |
| Chen 2009 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
|
Source: NT Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): — Trial results available in trial register: — | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): incidence of T2DM Secondary outcome measure(s): fasting plasma glucose, 2‐hour plasma glucose Other outcome measure(s): reversion to normoglycaemia | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): incidence of T2DM Secondary outcome measure(s): fasting plasma glucose, 2‐hour plasma glucose Other outcome measure(s): — | |
| Jin 2009 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
|
Source: NT Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): — Trial results available in trial register: — | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): incidence of T2DM, serious adverse event Secondary outcome measure(s): fasting plasma glucose, 2‐hour plasma glucose, non‐serious adverse event Other outcome measure(s): fasting plasma insulin, 2‐hour plasma insulin, BMI, reversion to normoglycaemia | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): incidence of T2DM Secondary outcome measure(s): fasting plasma glucose, 2‐hour plasma glucose Other outcome measure(s): fasting plasma insulin, 2‐hour plasma insulin | |
| Li 2009 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
|
Source: NT Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): — Trial results available in trial register: — | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): incidence of T2DM Secondary outcome measure(s): fasting plasma glucose, 2‐hour plasma glucose Other outcome measure(s): fasting insulin, BMI, leptin, triglycerides | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): incidence of T2DM Secondary outcome measure(s): fasting plasma glucose, 2‐hour plasma glucose Other outcome measure(s): fasting insulin, BMI, leptin, triglycerides | |
| Wang 2009 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
|
Source: NT Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): — Trial results available in trial register: — | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): incidence of T2DM Secondary outcome measure(s): 2‐hour plasma glucose, non‐serious adverse events Other outcome measure(s): fasting blood glucose | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): — (NA) Secondary outcome measure(s): — Other outcome measure(s): — | |
| IDPP‐1 2006 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
|
Source:NCT00279240 Primary outcome measure: incidence of T2DM Secondary outcome measure: benefits of the drug on anthropometric variables and biochemical parameter Other outcome measure(s): — Trial results available in trial register: yes | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): morbidity of T2DM Secondary outcome measure(s): mortality; morbidity of cardiovascular disease; fasting and 2‐hour plasma glucose; plasma lipids: total cholesterol, LDL cholesterol and HDL cholesterol; blood pressure; BMI; adverse events; costs Other outcome measure(s): — | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure: incidence of T2DM Secondary outcome measure(s): diabetes related morbidity, adverse events, all‐cause mortality, total cholesterol, LDL cholesterol and HDL cholesterol, blood pressure, BMI, socioeconomic effects Other outcome measure(s): — | |
| Maji 2005 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
|
Source: main publication Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): — Trial results available in trial register: — | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): not described whether outcomes were primary or secondary: incidence of T2DM, per cent change in glycaemic measures | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): not described whether outcomes were primary or secondary: conversion to normoglycaemia | |
| Fang 2004 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
| Source: N/T | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): incidence of T2DM, all‐cause mortality, serious adverse events Secondary outcome measure(s): fasting plasma glucose, 2‐hour plasma glucose, non‐serious adverse events Other outcome measure(s): BMI, total cholesterol, triglycerides, conversion to normoglycaemia | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): incidence of T2DM Secondary outcome measure(s): fasting plasma glucose, 2‐hour plasma glucose Other outcome measure(s): conversion to normoglycaemia | |
| DPP/DPPOS 2002 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
|
DPP: Source:NCT00004992; design article and protocol available from website (DPP/DPPOS 2002) Primary outcome measure(s): incidence of T2DM DPPOS: NCT00038727: design article and protocol available from website (DPP/DPPOS 2002) Primary outcome measure(s): incidence of T2DM; aggregate microvascular complications DPP: Secondary outcome measure(s): HbA1c; insulin and glucose; electrocardiogram; cardiovascular symptom assessment; blood pressure; carotid ultrasound; lipoproteins; fibrinolysis and clotting factors; albumin excretion; physical measurements; physical activity; nutrient intake; health‐related quality of life; resource utilisation; safety DPPOS: Secondary outcome measure(s): microvascular and cardiovascular disease risk factors; microvascular and cardiovascular disease risk factors; subclinical atherosclerosis; quality of life and economic analyses, bone density, health aging index; pulmonary function, urinary incontinence, amputation of lower extremity, hospitalisations; cardiovascular disease events Other outcome measure(s): quote: "...comparing the incidence and determinants of these health outcomes in participants with new‐onset diabetes and IGT, as well as assessing subgroups of participants in order to evaluate the effect of age, race/ethnicity, and sex on health outcomes" Trial results available in trial register: no trial results available, but references to publications at clinicaltrials.gov | |
| Endpoints quoted in publication(s)b,c | |
|
DPP: Primary outcome measure(s): incidence of T2DM DPPOS: Primary outcome measure(s): incidence of T2DM; aggregate microvascular complications DPP: Secondary outcome measure(s): HbA1c; insulin and glucose; electrocardiogram; cardiovascular symptom assessment; blood pressure; lipoproteins; fibrinolysis and clotting factors; albumin excretion; physical measurements; physical activity; nutrient intake; health‐related quality of life; resource utilisation; safety DPPOS: Secondary outcome measure(s): microvascular and cardiovascular disease risk factors; economic analyses; health aging index; urinary incontinence Other outcome measure(s): several subgroup analyses investigating the incidence of the primary outcomes | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
DPP: Primary outcome measure(s): incidence of T2DM DPPOS: Primary outcome measure(s): incidence of T2DM; aggregate microvascular complications DPP: Secondary outcome measure(s): insulin; cardiovascular symptom assessment; blood pressure; lipoproteins; fibrinolysis and clotting factors; albumin excretion; physical measurements; nutrient intake; health related quality of life; resource utilisation DPPOS: Secondary outcome measure(s): microvascular and cardiovascular disease risk factors; economic analyses; health aging index; urinary incontinence Other outcome measure(s): several subgroup analyses investigating the incidence of the primary outcomes | |
| Lu 2002 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
|
Source: NT Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): — Trial results available in trial register: — | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): incidence of T2DM, serious adverse events Secondary outcome measure(s): fasting plasma glucose, 2‐hour plasma glucose Other outcome measure(s): 1 hour PG | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): incidence of T2DM Secondary outcome measure(s): fasting blood sugar, ‐ hour plasma glucose Other outcome measure(s): 1 hour plasma glucose | |
| Li 1999 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
|
Source: main publication Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): not described whether outcomes were primary or secondary: incidence of T2DM, weight, lipids, risk factors for cardiovascular disease Trial results available in trial register: no | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): not described whether outcomes were primary or secondary: glycaemic control: fasting and 2‐hour plasma glucose, HbA1c; plasma lipids: total cholesterol and triglycerides; fasting and 2‐hour plasma insulin; blood pressure: Systolic and diastolic blood pressure; BMI and WHR; adverse events | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): not described whether outcomes were primary or secondary: incidence of T2DM, adverse events, HbA1c, total cholesterol and triglycerides, fasting plasma insulin, blood pressure, weight change | |
| Papoz 1978 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
| Source: N/T | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): blood glucose, insulin levels Secondary outcome measure(s): — Other outcome measure(s): weight | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): blood glucose, insulin levels Secondary outcome measure(s): — Other outcome measure(s): weight | |
| — denotes not reported
aTrial document(s) refers to all available information from published design papers and sources other than regular publications (e.g. FDA/EMA documents, manufacturer's websites, trial registers). bPublication(s) refers to trial information published in scientific journals (primary reference, duplicate publications, companion documents or multiple reports of a primary trial) cPrimary and secondary outcomes refer to verbatim specifications in publication/records. Unspecified outcome measures refer to all outcomes not described as primary or secondary outcome measures BMI: body mass index; CRP: C‐reactive protein; EMA: European Medicines Agency; FDA: Food and Drug Administration (US); HDL: high‐density lipoprotein; HOMA‐IR: homeostatic model assessment insulin resistance; hs‐CRP: high sensitive C‐reactive protein; IFG: impaired fasting glucose; IGT: impaired glucose tolerance; LDL: low‐density lipoprotein; NT: no trial document available; NA: no abstract available; PG: plasma glucose; T2DM: type 2 diabetes mellitus; WHR: waist‐to‐hip ratio. | |
Appendix 8. High risk of outcome reporting bias according to Outcome Reporting Bias In Trials (ORBIT) classification
| Trial ID | Outcome | High risk of bias (category A)a | High risk of bias (category D)b | High risk of bias (category E)c | High risk of bias (category G)d |
| Alfawaz 2018 | Incidence of T2DM | No | No | No | Yes |
| Adverse events | No | No | No | Yes | |
| PREVENT‐DM 2017 | Hypoglycaemia | No | No | Yes | No |
| Zeng 2013 | Incidence of T2DM | No | No | No | No |
| Measures of blood glucose control | No | No | No | No | |
| Zhao 2013 | Incidence of T2DM | No | No | No | No |
| Non‐serious adverse events | No | No | Yes | No | |
| Measures of blood glucose control | No | No | No | No | |
| Liao 2012 | Incidence of T2DM | No | No | No | No |
| Serious adverse events | No | No | No | No | |
| Measures of blood glucose control | No | No | No | No | |
| Iqbal Hydrie 2012 | Hypoglycaemia | No | Yes | No | No |
| Adverse events | No | Yes | No | No | |
| Ji 2011 | Incidence of T2DM | No | No | No | No |
| Serious adverse events | No | No | No | No | |
| Measures of blood glucose control | No | No | No | No | |
| Lu 2010 | Incidence of T2DM | No | No | No | No |
| Serious adverse events | No | No | No | No | |
| Non‐serious adverse events | No | No | No | No | |
| Measures of blood glucose control | No | No | No | No | |
| BIGPRO1 2009 | Incidence of T2DM | No | Yes | No | No |
| Hypoglycaemia | No | Yes | No | No | |
| Adverse events | No | Yes | No | No | |
| Chen 2009 | Incidence of T2DM | No | No | No | No |
| Non‐serious adverse events | No | No | Yes | No | |
| Measures of blood glucose control | No | No | No | No | |
| Jin 2009 | Incidence of T2DM | No | No | No | No |
| Serious adverse events | No | No | No | No | |
| Non‐serious adverse events | No | No | No | No | |
| Measures of blood glucose control | No | No | No | No | |
| Li 2009 | Incidence of T2DM | No | No | No | No |
| Measures of blood glucose control | No | No | No | No | |
| Wang 2009 | Incidence of T2DM | No | No | No | No |
| Non‐serious adverse events | No | No | No | No | |
| Measures of blood glucose control | No | No | No | No | |
| IDPP‐1 2006 | Serious adverse events | No | Yes | No | No |
| Non‐fatal myocardial infarction | No | Yes | No | No | |
| Stroke | No | Yes | No | No | |
| Non‐serious adverse events | No | No | Yes | No | |
| Maji 2005 | Hypoglycaemia | No | Yes | No | No |
| Adverse events | No | Yes | No | No | |
| Fang 2004 | All‐cause mortality | No | No | No | No |
| Incidence of T2DM | No | No | No | No | |
| Serious adverse events | No | No | No | No | |
| Non‐serious adverse events | No | No | No | No | |
| Measure of blood glucose control | No | No | No | No | |
| DPP 2002 | Serious adverse events | No | Yes | No | No |
| Non‐fatal myocardial infarction | No | No | Yes | No | |
| Non‐fatal stroke | No | No | Yes | No | |
| Non‐serious adverse events | No | Yes | No | No | |
| Hypoglycaemia | No | Yes | No | No | |
| Lu 2002 | Incidence of T2DM | No | No | No | No |
| Serious adverse events | No | No | No | No | |
| Measures of blood glucose control | No | No | No | No | |
| Li 1999 | Hypoglycaemia | No | Yes | No | No |
| Papoz 1978 | Adverse events | No | No | No | Yes |
|
aClear that outcome was measured and analysed; trial report states that outcome was analysed but only reports that result was not significant
(Classification 'A', table 2, Kirkham 2010)
bClear that outcome was measured and analysed; trial report states that outcome was analysed but no results reported
( Classification 'D', table 2, Kirkham 2010)
cClear that outcome was measured; clear that outcome was measured but not necessarily analysed; judgement says likely to have been analysed but not reported because of non‐significant results
(Classification 'E', table 2, Kirkham 2010)
dUnclear whether the outcome was measured; not mentioned but clinical judgement says likely to have been measured and analysed but not reported on the basis of non‐significant results
(Classification 'G', table 2, Kirkham 2010) ORBIT: Outcome Reporting Bias In Trials | |||||
Appendix 9. Definition of endpoint measurement (I)a
| Trial ID | All‐cause mortality | Development of type 2 diabetes mellitus | Serious adverse events | Cardiovascular mortality | Non‐fatal myocardial infarction | Non‐fatal stroke | Amputation of lower extremity |
| Alfawaz 2018 | NI | NI | NI | NI | NI | NI | NI |
| PREVENT‐DM 2017 | NI | Type 2 diabetes mellitus IO |
NI | NI | NI | NI | NI |
| Zeng 2013 | NR | ND IO |
NR | NR | NR | NR | NR |
| Zhao 2013 | NR | ND IO |
NR | NR | NR | NR | NR |
| Iqbal Hydrie 2012 | NI | Either fasting plasma glucose of > 125 mg/dL (6.9 mmol/L) and/or 2‐hour plasma glucose of > 199 mg/dL (11.1 mmol/L) IO |
NI | NI | NI | NI | NI |
| Liao 2012 | NR | ND IO |
NR | NR | NR | NR | NR |
| Ji 2011 | NR | ND IO |
NR | NR | NR | NR | NR |
| Lu 2010 | NR | American Diabetes Association 1997 criteria (any glucose ≥ 11.1 mmol/L or fasting plasma glucose ≥ 7.0 mmol/L). Quote: "If the patient has diabetes symptoms such as polydipsia, polyuria and polyphagy, if FPG≥ 7.0mmol/L or 2hPG> 11.1mmol/L after meal, the second FPG and/or 75 g OGTT were performed within 6 weeks. If the diabetes criteria were met, the primary study objective endpoint was determined and the study was terminated." IO |
NR | NR | NR | NR | NR |
| BIGPRO1 2009 | NI | NI | NI | NI | NI | NI | NI |
| Chen 2009 | NR | 75 g OGTT, and confirmed with a repeated test (no other data) Quote: "If the results of 75g OGTT for 2 times during the observation were diabetic, they were considered to have converted to diabetes, which was the end point of the study." IO |
NR | NR | NR | NR | NR |
| Jin 2009 | NR | ND IO |
NR | NR | NR | NR | NR |
| Li 2009 | NR | ND IO |
NR | NR | NR | NR | NR |
| Wang 2009 | NR | ND IO |
NR | NR | NR | NR | NR |
| IDPP‐1 2006 | IO | WHO 1999 (either a fasting plasma glucose ≥ 7.0 mmol/L
(≥ 126 mg/dL) and/or a 2‐hour plasma glucose concentration
≥ 11.1 mmol/L (≥ 200 mg/dL), and confirmed with a repeat test) IO |
IO | NI | NI | NI | NI |
| Maji 2005 | NI | Type 2 diabetes mellitus IO |
NI | NI | NI | NI | NI |
| Fang 2004 | NR | ND | NR | NR | NR | NR | NR |
| DPP/DPPOS 2002 | IO | American Diabetes Association criteria (fasting plasma glucose level ≥ 126 mg/dL [7.0 mmol/L] or 2‐hour plasma glucose ≥ 200 mg/dL [11.1 mmol/L] after a 75 g OGTT, and confirmed with a repeated test) IO |
Quote: "Serious adverse events have been defined to include any adverse experience occurring at any dose
that results in any of the following outcomes:
• Death
• A life‐threatening adverse experience
• Inpatient hospitalization or prolongation of existing hospitalization
• A persistent or significant disability/incapacity; or
• A congenital anomaly/birth defect" IO |
Quote:"CVD‐related deaths" IO |
NI | NI | NI |
| Lu 2002 | NR | 75 g OGTT, and confirmed with a repeated test (no other data) Quote: "If the results of 75 g OGTT at one time during the observation were diabetic, the patients were still treated according to the original regimen; if the patients were still diabetic at the next review, the patients were judged to have converted to diabetes, which was the end point of the study. If it is IGT or normal glucose tolerance, observation will be continued, and final results of each subject will be judged after review at the end of 3 years." IO |
NR | NR | NR | NR | NR |
| Li 1999 | NI | Not described, presumable WHO 1985 criteria (either a fasting plasma glucose ≥ 140 mg/dL (7.8 mmol/L) or higher or a 2‐hour plasma glucose ≥ 200 mg/dL (11.1 mmol/L) after a 75 g OGTT) IO |
NI | NI | NI | NI | NI |
| Papoz 1978 | NI | NI | NI | NI | NI | NI | NI |
|
aIn addition to definition of endpoint measurement, description who measured the outcome (AO: adjudicated outcome measurement;IO: investigator‐assessed outcome measurement; SO: self‐reported outcome measurement) 2hPG: 2‐hour plasma glucose; CVD: cardiovascular disease; FPG: fasting plasma glucose; IGT: impaired glucose tolerance; ND: not defined; NI: not investigated; NR: not reported; OGTT: oral glucose tolerance test; WHO: World Health Organization. | |||||||
Appendix 10. Definition of endpoint measurement (II)b
| Trial ID | Blindness or severe vision loss | End‐stage renal disease | Nonserious adverse events | Hypoglycaemic events | Health‐related quality of life | Time to progression to T2DM | Measures of blood glucose control | Socioeconomic effects |
| Alfawaz 2018 | NI | NI | NI | NI | NI | NI | Fasting blood glucose IO |
NI |
| PREVENT‐DM 2017 | NI | NI | Adverse events SO |
NI | NI | NI | HbA1c; fasting plasma glucose IO |
NI |
| Zeng 2013 | NR | NR | NR | NR | NR | NR | Fasting plasma glucose, 2‐hour plasma glucose; Quote:" At the end, OGTT was used to judge the number of cases of NGT, IGR and DM. Biochemical detection was conducted by Olympus automatic biochemical instrument, glucose detection by glucose oxidase method, and insulin detection by radioimmunoassay." (no details) IO |
NR |
| Zhao 2013 | NR | NR | Damage of liver, kidney function | NR | NR | NR | Fasting blood glucose, 2‐hour plasma glucose ND IO |
NR |
| Iqbal Hydrie 2012 | NI | NI | NI | NI | NI | NI | NI | NI |
| Liao 2012 | NR | NR | NR | NR | NR | NR | Fasting plasma glucose, 2‐hour plasma glucose; Quote:"At the initial visit, fasting 10‐12h overnight, venous blood was taken and plasma glucose (i.e., FPG and 2hPG) was measured after OGTT (75g glucose). The above examination was repeated every 3 months. Blood glucose was measured by hexokinase method (the biochemical instrument was automatic erab‐xl‐600)." (no more detail) IO |
NR |
| Ji 2011 | NR | NR | NR | NR | NR | NR | Fasting plasma glucose, HbA1c, 2‐hour plasma glucose Quote:"All cases were followed for 2 years, outpatient follow‐up once every 2 months, patients with glucose oxidase method is used to determination of FPG, 2h postprandial blood glucose (2 HPG), treatment before and after the treatment, test weight, height, and calculate the BMI, waist circumference, hip circumference, waist‐to‐hip ratio calculation, the determination of FPG, FINS application of chemiluminescence analysis, application of biochemical analyzer determination of TC, TG, LDL cholesterol (LDL ‐ C), immune turbidimetric method is used to test the hs CRP, glycosylated hemoglobin (HbA1c) levels, Meanwhile, ISI =1/ (determined value of FINS ×FPG) was calculated. Review OGTT at the end of treatment to determine if diabetes has developed." IO |
NR |
| Lu 2010 | NR | NR | Harmful and unexpected reactions of a drug under normal usage or dosage to prevent, diagnose, treat, or regulate physiological functions | NR | NR | NR | Fasting plasma glucose; 2‐hour plasma glucose; HbA1c Quote:"Venous blood was collected 8‐12 hours after fasting, and serum was isolated for determination of blood glucose, total cholesterol, triglyceride HDL and LDL. Blood at finger tips was used to determine glycosylated hemoglobin (DS). 5 glycosylated hemoglobin analyzer).", Quote:"Oral glucose tolerance test. On the morning of fasting venous blood sampling, 300ml sugar water containing 75g glucose was drunk within 5 minutes, and blood glucose was measured by venous blood sampling 2 hours later." IO |
NR |
| BIGPRO1 2009 | NI | NI | NI | NI | NI | NI | 2‐hour plasma glucose; fasting plasma glucose IO |
NI |
| Chen 2009 | NR | NR | NR | NR | NR | NR | Fasting plasma glucose, 2‐hour plasma glucose; ND IO |
NR |
| Jin 2009 | NR | NR | NR | NR | NR | NR | Fasting plasma glucose, 2‐hour plasma glucose; ND Quote:"Blood glucose was detected by tetokinase method" IO |
NR |
| Li 2009 | NR | NR | NR | NR | NR | NR | Fasting plasma glucose, 2‐hour plasma glucose; ND IO |
NR |
| Wang 2009 | NR | NR | ND | NR | NR | NR | Fasting blood glucose, 2‐hour plasma glucose; ND IO |
NR |
| IDPP‐1 2006 | NI | NI | NI | Hypoglycaemia SO, IO |
NI | NI | 2‐hour plasma glucose; fasting plasma glucose IO |
NI |
| Maji 2005 | NI | NI | NI | NI | NI | NI | 2‐hour plasma glucose; HbA1c; fasting plasma glucose IO |
NI |
| Fang 2004 | NR | NR | NR | NR | NR | NR | Fasting plasma glucose; 2‐hour plasma glucose; ND IO |
NR |
| DPP/DPPOS 2002 | NI | NI | NI | NI | 36‐Item Short‐
Form (SF‐36) health survey SO |
NI | 2‐hour plasma glucose; HbA1c; Fasting plasma glucose IO |
"The direct costs of medical care received outside the study and indirect costs were determined annually from patient self‐report. Direct non‐medical costs were assessed once during DPP and once during DPPOS, and costs were annualized. All costs were adjusted to 2000 or 2010 U.S. dollars using the Consumer Price Index and the Medical Consumer Price Index." IO |
| Lu 2002 | NR | NR | NR | NR | NR | NR | Fasting blood glucose, 1h plasma glucose, 2‐hour plasma glucose ND IO |
NR |
| Li 1999 | NI | NI | NI | NI | NI | NI | 2‐hour plasma glucose; HbA1c; fasting plasma glucose IO |
NI |
| Papoz 1978 | NI | NI | NI | NI | NI | NI | Fasting blood glucose, 2‐hour glucose levels IO |
NI |
|
aIn addition to definition of endpoint measurement, description who measured the outcome (AO: adjudicated outcome measurement; IO: investigator‐assessed outcome measurement; SO: self‐reported outcome measurement) BMI: body mass index; CRP: C‐reactive protein; FINS: fasting insulin; FPG: fasting plasma glucose; HbA1c: glycosylated haemoglobin A1c; ISI: insulin sensitivity index; ND: not defined;NI: not investigated; NR: not reported; OGTT: oral glucose tolerance test. | ||||||||
Appendix 11. Adverse events (I)
| Trial ID | Intervention(s) and comparator(s) | Participants included in analysis (N) | Deaths (N) | Deaths(%) | Participants with at least one adverse event (N) | Participants with at least one adverse event (%) | Participants with at least one severe/serious adverse event (N) | Participants with at least one severe/serious adverse event (%) |
| Alfawaz 2018 | I: metformin | 59 | — | — | — | — | — | — |
| C1: intensive diet plus exercise | 73 | — | — | — | — | — | — | |
| C2: standard care | 85 | — | — | — | — | — | — | |
| PREVENT‐DM 2017 | I1: metformin | 29 | 0 | 0 | 10 | 34.4 | 0 | 0 |
| C1: intensive diet plus exercise | 33 | 0 | 0 | 0 | 0 | 0 | ||
| C2: standard care | 30 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Zeng 2013 | I: metformin | 68 | — | — | — | — | — | — |
| C1: Standard care | 66 | — | — | — | — | — | — | |
| C2: pioglitazone | 70 | — | — | — | — | — | — | |
| Zhao 2013 | I: metformin plus intensive diet plus exercise | 45 | — | — | Gastrointestinal symptoms: 1 | Gastrointestinal symptoms: 2.2 | — | — |
| C: intensive diet plus exercise | 46 | — | — | Gastrointestinal symptoms: 0 | Gastrointestinal symptoms: 0 | — | — | |
| Iqbal Hydrie 2012 | I1: metformin plus intensive diet and physical activity | 95 | 0 | 0 | — | — | — | — |
| C1: intensive diet and physical activity | 114 | 0 | 0 | — | — | — | — | |
| C2: standard care | 108 | 2 | 1.9 | — | — | — | — | |
| Liao 2012 | I: metformin | 51 | — | — | — | — | Cerebral haemorrhage: 1 | Cerebral haemorrhage: 2.0 |
| C: acarbose | 50 | — | — | — | — | Lung cancer: 1 hepatitis: 1 |
Lung cancer: 2.0 hepatitis: 2.0 |
|
| Ji 2011 | I1: metformin | 52 | — | — | — | — | — | — |
| C1: Intensive diet plus exercise | 60 | — | — | — | — | — | — | |
| C2: standard care | 64 | — | — | — | — | — | — | |
| Lu 2010 | I: metformin | 115 | — | — | Diarrhoea: 11 nausea: 14 vomiting: 7 abdominal distension: 11 weak: 17 indigestion: 15 abdominal discomfort and headache: 8 abnormal defecate: 16 low blood sugar: 4 muscle pain: 2 dizzy: 7 rash: 1 sweating increases: 2 taste abnormalities: 13 chest discomfort: 2 flu symptoms: 1 weight loss, etc: 33 | Diarrhoea: 9.5 nausea: 12 vomiting: 6.1 abdominal distension: 9.5 weak: 14.8 indigestion: 13 abdominal discomfort and headache: 7.0 abnormal defecate: 13.9 low blood sugar: 3.5 muscle pain: 6.1 dizzy: 0.8 rash sweating increases: 1.7 taste abnormalities: 11.3 chest discomfort: 1.7 flu symptoms: 0.8 weight loss, etc: 28.7 | — | — |
| C: standard care | 111 | — | — | Diarrhoea: 7 nausea: 5 vomiting: 5 abdominal distension: 6 weak: 15 indigestion: 15 abdominal discomfort and headache: 3 abnormal defecate: 4 low blood sugar: 2 muscle pain: 0 dizzy: 8 rash: 0 sweating increases: 2 taste abnormalities: 1 chest discomfort: 1 flu symptoms: 2 weight loss, etc: 10 | Diarrhoea: 6.3 nausea: 4.5 vomiting: 4.5 abdominal distension: 5.4 weak: 13.5 indigestion: 13.5 abdominal discomfort and headache: 2.7 abnormal defecate: 3.6 low blood sugar: 1.8 muscle pain: 0 dizzy: 7.2 rash: 0 sweating increases: 1.8 taste abnormalities: 0.9 chest discomfort: 0.9 flu symptoms: 1.8 weight loss, etc: 9 | — | — | |
| BIGPRO1 2009 | I: metformin | 21 | — | — | — | — | — | — |
| C: placebo | 36 | — | — | — | — | — | — | |
| Chen 2009 | I: metformin | 44 | — | — | — | — | — | — |
| C: standard care | 46 | — | — | — | — | — | — | |
| Jin 2009 | I: metformin | 45 | — | — | Hypoglycaemia 0 | Hypoglycaemia 0 | Severe gastrointestinal reactions: 3 | Severe gastrointestinal reactions: 6.7 |
| C1: standard care | 41 | — | — | Hypoglycaemia 0 | Hypoglycaemia 0 | — | — | |
| C2: rosiglitazone | 41 | — | — | Facial oedema: 1
intolerance of both lower limbs: 2 hypoglycaemia: 0 |
Facial oedema: 2.4
intolerance of both lower limbs: 4.9 hypoglycaemia: 0 |
0 | 0 | |
| Li 2009 | I: metformin | 77 | — | — | — | — | — | — |
| C: intensive diet plus exercise | 83 | — | — | — | — | — | — | |
| Wang 2009 | I: metformin | 30 | — | — | Gastrointestinal symptoms: 2 | Gastrointestinal symptoms: 6.7 | — | — |
| C: standard care | 32 | — | — | — | — | — | — | |
| IDPP‐1 2006 | I1: metformin | 128 | 0 | 0 | — | 0 | — | — |
| I2: metformin plus intensive diet and physical activity | 121 | 1 | 0.8 | — | — | — | — | |
| C1: intensive exercise plus diet | 120 | 1 | 0.8 | — | — | — | — | |
| C2: standard care | 133 | 1 | 0.8 | — | — | — | — | |
| Maji 2005 | I1: metformin | — | — | — | — | — | — | — |
| C1: intensive lifestyle intervention | — | — | — | — | — | — | — | |
| C2: rosiglitazone | — | — | — | — | — | — | — | |
| C3: acarbose | — | — | — | — | — | — | — | |
| Fang 2004 | I: metformin | 44 | 1 | 2.3 | Diarrhea: 3 | Diarrhea: 6.8 | Death (liver cancer): 1 | Death (liver cancer): 2.3 |
| C1: acarbose | 45 | 0 | 0 | Abdominal distension and diarrhoea: 3 rash: 1 frequent venting: 1 |
Abdominal distension and diarrhoea: 6.7 rash: 2.2 frequent venting: 2.2 |
— | — | |
| C2: intensive exercise and diet | 36 | 0 | 0 | 0 | 0 | 0 | 0 | |
| C3: standard care | 35 | 0 | 0 | 0 | 0 | 0 | 0 | |
| DPP/DPPOS 2002 | I: metformin | 1073 | 6 | 0.6 | Musculoskeletal symptoms: 20.0 events/100 person yearsa gastrointestinal symptoms: 77.8 events/100 person years |
Musculoskeletal symptoms: — gastrointestinal symptoms: — |
||
| C1: intensive exercise and diet | 1079 | 3 | 0.3 | Musculoskeletal symptoms: 24.1 events/100 person years gastrointestinal symptoms: 12.9 events/100 person years |
Musculoskeletal symptoms: — gastrointestinal symptoms: — |
— | — | |
| C2: placebo | 1082 | 5 | 0.5 | Musculoskeletal symptoms: 21.1 events/100 person years gastrointestinal symptoms: 30.7 events/100 person years |
Musculoskeletal symptoms: — gastrointestinal symptoms: — | — | — | |
| Lu 2002 | I1: metformin | 80 | — | — | — | — | — | — |
| C1: standard care | 75 | 1 | 1.3 | — | — | Death (cerebral thrombosis with pulmonary infection): 1 | Death (cerebral thrombosis with pulmonary infection): 1.3 | |
| C2: standard care plus diet instruction every 6th month | 64 | — | — | — | — | — | — | |
| C3: standard care plus fibre diet | 51 | — | — | — | — | Stomach cancer: 1 | Stomach cancer: 2.0 | |
| Li 1999 | I: metformin | 33 | 0 | 0 | (1) Mild diarrhoea and nausea: 3 | (1) 9.1 | — | — |
| C: placebo | 37 | 0 | 0 | (1) Mild nausea: 6 (2) raised liver enzymes: 1 |
(1) 16 (2) 2.7 |
— | — | |
| Papoz 1978 | I1: metformin (plus placebo) | — | — | — | — | — | — | — |
| C1: glibenclamide plus placebo | — | — | — | — | — | — | — | |
| C2: placebo | — | — | — | — | — | — | — | |
| —: denotes not reported aAll adverse events from DPP are calculated from number of events/100 person years; some participants might have experienced more than one event. Therefore only the number of participants with an event cannot be calculated C: comparator; I: intervention. | ||||||||
Appendix 12. Adverse events (II)
| Trial ID | Intervention(s) and comparator(s) | Participants included in analysis (N) | Participants discontinuing trial due to an adverse event (N) | Participants discontinuing trial due to an adverse event (%) | Participants with at least one hospitalisation (N) | Participants with at least one hospitalisation (%) | Participants with at least one outpatient treatment (N) | Participants with at least one outpatient treatment (%) |
| Alfawaz 2018 | I: metformin | 59 | — | — | — | — | — | — |
| C1: intensive diet plus exercise | 73 | — | — | — | — | — | — | |
| C2: standard care | 85 | — | — | — | — | — | — | |
| PREVENT‐DM 2017 | I1: metformin | 29 | 1 | 3.4 | — | — | — | — |
| C1: intensive diet plus exercise | 33 | 0 | 0 | — | — | — | — | |
| C2: standard care | 30 | 0 | 0 | — | — | — | — | |
| Zeng 2013 | I: metformin | 68 | — | — | — | — | — | — |
| C1: Standard care | 66 | — | — | — | — | — | — | |
| C2: pioglitazone | 70 | — | — | — | — | — | — | |
| Zhao 2013 | I: metformin plus intensive diet plus exercise | 45 | 1 | 2.2 | — | — | — | — |
| C: intensive diet plus exercise | 46 | — | — | — | — | — | — | |
| Iqbal Hydrie 2012 | I1: metformin plus intensive diet and physical activity | 95 | 5 | 5.3 | — | — | — | — |
| C1: intensive diet and physical activity | 114 | 0 | 0 | — | — | — | — | |
| C2: standard care | 108 | 0 | 0 | — | — | — | — | |
| Liao 2012 | I: metformin | 51 | 1 | 2.0 | — | — | — | — |
| C: acarbose | 50 | 2 | 4.0 | — | — | — | — | |
| Ji 2011 | I1: metformin | 52 | — | — | — | — | — | — |
| C1: Intensive diet plus exercise | 60 | — | — | — | — | — | — | |
| C2: standard care | 64 | — | — | — | — | — | — | |
| Lu 2010 | I: metformin | 115 | 21 | 18.3 | — | — | — | — |
| C: standard care | 111 | 17 | 15.3 | — | — | — | — | |
| BIGPRO1 2009 | I: metformin | 21 | — | — | — | — | — | — |
| C: placebo | 36 | — | — | — | — | — | — | |
| Chen 2009 | I: metformin | 44 | — | — | — | — | — | — |
| C: standard care | 46 | — | — | — | — | — | — | |
| Jin 2009 | I: metformin | 45 | 3 | 6.7 | — | — | — | — |
| C1: standard care | 41 | — | 6.7 | — | — | — | — | |
| C2: rosiglitazone | 41 | 3 | 6.7 | — | — | — | — | |
| Li 2009 | I: metformin | 77 | — | — | — | — | — | — |
| C: intensive diet plus exercise | 83 | — | — | — | — | — | — | |
| Wang 2009 | I: metformin | 30 | 2 | 6.7 | — | — | — | — |
| C: standard care | 32 | — | — | — | — | — | — | |
| IDPP‐1 2006 | I1: metformin | 128 | — | — | — | — | — | — |
| I2: metformin plus intensive diet and physical activity | 121 | — | — | — | — | — | — | |
| C1: intensive exercise plus diet | 120 | — | — | — | — | — | — | |
| C2: standard care | 133 | — | — | — | — | — | — | |
| Maji 2005 | I1: metformin | — | — | — | — | — | — | — |
| C1: intensive lifestyle intervention | — | — | — | — | — | — | — | |
| C2: rosiglitazone | — | — | — | — | — | — | — | |
| C3: acarbose | — | — | — | — | — | — | — | |
| Fang 2004 | I: metformin | 44 | 4 | 9.1 | — | — | — | — |
| C1: acarbose | 45 | 5 | 11.1 | — | — | — | — | |
| C2: intensive exercise and diet | 36 | 0 | 0 | — | — | — | — | |
| C3: standard care | 35 | 0 | 0 | — | — | — | — | |
| DPP/DPPOS 2002 | I: metformin | 1073 | — | — | — | — | — | — |
| C1: intensive exercise and diet | 1079 | — | — | — | — | — | — | |
| C2: placebo | 1082 | — | — | — | — | — | — | |
| Lu 2002 | I1: metformin | 80 | — | — | — | — | — | — |
| C1: standard care | 75 | 1 | 1.3 | — | — | — | — | |
| C2: standard care plus diet instruction every 6th month | 64 | — | — | — | — | — | — | |
| C3: standard care plus fibre diet | 51 | 1 | 2.0 | — | — | — | — | |
| Li 1999 | I: metformin | 33 | 2 | 6.1 | — | — | — | — |
| C: placebo | 37 | 1 | 2.7 | — | — | — | — | |
| Papoz 1978 | I1: metformin (plus placebo) | — | — | — | — | — | — | — |
| C1: glibenclamide plus placebo | — | — | — | — | — | — | — | |
| C2: placebo | — | — | — | — | — | — | — | |
| —: denotes not reported C: comparator; I: intervention; N: number of participants. | ||||||||
Appendix 13. Adverse events (III)
| Trial ID | Intervention(s) and comparator(s) | Participants included in analysis (N) | Participants with a specific adverse event (description) | Participants with at least one specific adverse events (N) | Participants with at least one specific adverse event (%) |
| Alfawaz 2018 | I: metformin | 59 | — | — | — |
| C1: intensive diet plus exercise | 73 | — | — | — | |
| C2: standard care | 85 | — | — | — | |
| PREVENT‐DM 2017 | I1: metformin | 29 | (1) gastrointestinal disturbances (2) dizziness/vertigo (3) headache |
(1) 9 (2) 1 (3) 1 |
(1) 27.6 (2) 3.4 (3) 3.4 |
| C1: intensive diet plus exercise | 33 | — | — | — | |
| C2: standard care | 30 | 0 | 0 | 0 | |
| Zeng 2013 | I: metformin | 68 | — | — | — |
| C1: Standard care | 66 | — | — | — | |
| C2: pioglitazone | 70 | — | — | — | |
| Zhao 2013 | I: metformin plus intensive diet plus exercise | 45 | — | — | — |
| C: intensive diet plus exercise | 46 | — | — | — | |
| Iqbal Hydrie 2012 | I1: metformin plus intensive diet and physical activity | 95 | — | — | — |
| C1: intensive diet and physical activity | 114 | — | — | — | |
| C2: standard care | 108 | — | — | — | |
| Liao 2012 | I: metformin | 51 | Cerebral haemorrhage | 1 | 2.0 |
| C: acarbose | 50 | (1) lung cancer (2) hepatitis | (1) 1 (2) 1 | (1) 2.0 (2) 2.0 | |
| Ji 2011 | I1: metformin | 52 | — | — | — |
| C1: Intensive diet plus exercise | 60 | — | — | — | |
| C2: standard care | 64 | — | — | — | |
| Lu 2010 | I: metformin | 115 | Taste abnormalities 13 | 13 | 11.3 |
| C: standard care | 111 | Taste abnormalities 1 | 1 | 0.9 | |
| BIGPRO1 2009 | I: metformin | 21 | — | — | — |
| C: placebo | 36 | — | — | — | |
| Chen 2009 | I: metformin | 44 | — | — | — |
| C: standard care | 46 | — | — | — | |
| Jin 2009 | I: metformin | 45 | Severe gastrointestinal reactions | 3 | 6.7 |
| C1: standard care | 41 | — | — | — | |
| C2: rosiglitazone | 41 | (1) facial oedema (2) intolerance of both lower limbs | (1) 1 (2) 2 | (1) 2.4 (2) 4.9 | |
| Li 2009 | I: metformin | 77 | — | — | — |
| C: intensive diet plus exercise | 83 | — | — | — | |
| Wang 2009 | I: metformin | 30 | — | — | — |
| C: standard care | 32 | — | — | — | |
| IDPP‐1 2006 | I1: metformin | 128 | (1) Cardiovascular event Only reported for both metformin groups together: (2) Hypoglycaemia (3) Gastrointestinal symptoms (4) CVD |
(1) 0 (2) 22 (3) 5 (4) 10 |
(1) 0 (2) 8.8 (3) 2.0 (4) 4.0 |
| I2: metformin plus intensive diet and physical activity | 121 | (1) Cardiovascular event Only reported for both metformin groups together: (2) Hypoglycaemia (3) Gastrointestinal symptoms (4) CVD |
(1) 5 (2) 22 (3) 5 (4) 10 |
(1) 4.1 (2) 8.8 (3) 2.0 (4) 4.0 |
|
| C1: intensive exercise plus diet | 120 | (1) Cardiovascular event (2) Hypoglycaemia (3) Gastrointestinal symptoms (4) CVD |
(1) 4 (2) 0 (3) 0 (4) 18 |
(1) 3.3 (2) 0 (3) 0 (4) 15 |
|
| C2: standard care | 133 | (1) Cardiovascular event (2) Hypoglycaemia (3) Gastrointestinal symptoms (4) CVD |
(1) 2 (2) 0 (3) 0 (4) 26 |
(1) 1.5 (2) 0 (3) 0 (4) 19.5 |
|
| Maji 2005 | I1: metformin | — | — | — | — |
| C1: intensive diet plus exercise | — | — | — | — | |
| C2: rosiglitazone | — | — | — | — | |
| C3: acarbose | — | — | — | — | |
| Fang 2004 | I: metformin | 44 | (1) Diarrhea (2) Death (liver cancer) | (1) 3 (2) 1 |
(1) 6.8 (2) 2.3 |
| C1: acarbose | 45 | (1) Abdominal distension and diarrhoea
(2) Rash (3) Frequent venting |
(1) 3 (2) 1 (3) 1 |
(1) 6.7 (2) 2.2 (3) 2.2 |
|
| C2: intensive exercise and diet | 36 | (1) Gastrointestinal side effects (2) Rashes | (1) 0 (2) 0 |
(1) 0 (2) 0 |
|
| C3: standard care | 35 | (1) Gastrointestinal side effects (2) Rashes |
(1) 0 (2) 0 |
(1) 0 (2) 0 |
|
| DPP/DPPOS 2002 | I: metformin | 1073 | — | — | — |
| C1: intensive exercise and diet | 1079 | — | — | — | |
| C2: placebo | 1082 | — | — | — | |
| Lu 2002 | I1: metformin | 80 | — | — | — |
| C1: standard care | 75 | Death (cerebral thrombosis with pulmonary infection) | 1 | 1.3 | |
| C2: standard care plus diet instruction every 6th month | 64 | — | — | — | |
| C3: standard care plus fibre diet | 51 | Stomach cancer | 1 | 2.0 | |
| Li 1999 | I: metformin | 33 | Mild diarrhoea and nausea | 3 | 9.1 |
| C: placebo | 37 | (1) Mild nausea (2) Raised liver enzymes | (1) 6 (2) 1 |
(1) 16.2 (2) 2.7 |
|
| Papoz 1978 | I1: metformin (plus placebo) | — | — | — | — |
| C1: glibenclamide plus placebo | — | — | — | — | |
| C2: placebo | — | — | — | — | |
| —: denotes not reported C: comparator; CVD: cardiovascular disease; I: intervention; N: number of participants. |
|||||
Appendix 14. Survey of authors providing information on included trials
| Trial ID | Date trial author contacted | Date trial author replied | Date trial author was asked for additional information (short summary) | Date trial author provided data (short summary) |
| Alfawaz 2018 | 7th of April 2019 | No reply | NA | NA |
| PREVENT‐DM 2017 | 11th of August 2017 | No reply | Asked if they could provide additional information on the trial | NA |
| Zeng 2013 | No contact information available | NA | NA | NA |
| Zhao 2013 | 5th of August 2019 | NA | No contact information provided in publication. Hospital was called to get contact information on first author, no one answered the phone | NA |
| Iqbal Hydrie 2012 | 9th of August 2017 | No reply | Asked if they could provide additional information on the trial including a trial protocol | NA |
| Liao 2012 | 5th of August 2019 | NA | No contact information provided in publication. Hospital was called to get contact information on first author, no one answered the phone. | NA |
| Ji 2011 | 5th of August 2019 | No reply.0 | Contacted through e‐mail | NA |
| Lu 2010 | No contact information available | NA | NA | NA |
| BIGPRO1 2009 | 14th of August 2017 | 14 August 2017 | Asked for detailed number of diabetes, deaths, CVD and adverse events. | Primary author provided contact information on the investigator possessing trial data. She was contacted 25 March 2019. No reply was given. |
| Chen 2009 | 5th of June 2019 | NA | No contact information provided in publication. Hospital was called to get contact information on first author, no one answered the phone. | NA |
| Jin 2009 | No contact information available | NA | NA | NA |
| Li 2009 | 5th of June 2019 | NA | No contact information provided in publication. Hospital was called to get contact information on first author, colleague refused to forward | NA |
| Wang 2009 | 5th of June 2019 | NA | No contact information provided in publication. Hospital was called to get contact information on first author, colleague refused to forward | NA |
| IDPP‐1 2006 | 14th of April 2015 | No reply | Asked for change of HbA1c and insulin level | NA |
| Maji 2005 | No contact information available | NA | NA | NA |
| Fang 2004 | 14th of April 2015 | No reply | Asked for change of HbA1c and insulin level and detailed number of CVD | NA |
| DPP/DPPOS 2002 | 20th of December 2014 | 23 December 2014 | Asked for detailed number of diabetes, deaths, CVD and adverse events. | NA |
| Lu 2002 | 5th of August 2019 | NA | No contact information provided in publication. Hospital was called to get contact information on first author, colleague refused to forward | NA |
| Li 1999 | 14th of April 2015 | NA | Asked for detailed number of CVD | NA |
| Papoz 1978 | 4th of May 2016 | No reply | No contact information could be identified for the first author. Contact information on one of the other authors was identified through an Internet search (Dr Eschwege) | NA |
| CVD: cardiovascular disease; HbA1c: glycosylated haemoglobin A1c; NA: not applicable. | ||||
Appendix 15. Checklist to aid consistency and reproducibility of GRADE assessments: metformin versus placebo or diet and exercise
| Items | (1) All‐cause mortality | (2) Incidence of type 2 diabetes mellitus | (3) Serious adverse events | (4) Cardiovascular mortality | (5) Non‐fatal myocardial infarction/stroke | (6) Health‐related quality of life | (7) Socioeconomic effects | |
| Trial limitations (risk of bias)a | Was random sequence generation used (i.e. no potential for selection bias)? | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Unclear |
| Was allocation concealment used (i.e. no potential for selection bias)? | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Unclear | |
| Was there blinding of participants and personnel (i.e. no potential for performance bias) or outcome not likely to be influenced by lack of blinding? | Yes | Yes | Yes | Yes | Yes | No (↓) | Yes | |
| Was there blinding of outcome assessment (i.e. no potential for detection bias) or was outcome measurement not likely to be influenced by lack of blinding? | Yes | Yes | Yes | Yes | Yes | No (↓) | Yes | |
| Was an objective outcome used? | Yes | Yes | Yes | Yes | Yes | No (↓) | Yes | |
| Were more than 80% of participants enrolled in trials included in the analysis (i.e. no potential reporting bias)?e | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | |
| Were data reported consistently for the outcome of interest (i.e. no potential selective reporting)? | Unclear | Unclear | No (↓) | Unclear | Unclear | Unclear | Yes | |
| No other biases reported (i.e. no potential of other bias)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Did the trials end up as scheduled (i.e. not stopped early)? | No (↓) | No (↓) | No (↓) | No (↓) | No (↓) | No (↓) | No (↓) | |
| Inconsistencyb | Point estimates did not vary widely? | Yes | Yes | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable |
| To what extent did confidence intervals overlap (substantial: all confidence intervals overlap at least one of the included studies point estimate; some: confidence intervals overlap but not all overlap at least one point estimate; no: at least one outlier: where the confidence interval of some of the studies do not overlap with those of most included studies)? | Substantial | Substantial | ||||||
| Was the direction of effect consistent? | No (↓) | Yes | ||||||
| What was the magnitude of statistical heterogeneity (as measured by I²) ‐ low (I²< 40%), moderate (I² 40% to 60%), high I² > 60%)? | Low | Moderate | ||||||
| Was the test for heterogeneity statistically significant (P < 0.1)? | Not statistically significant | Statistically significant (↓) | ||||||
| Indirectness | Were the populations in included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable |
| Were the interventions in the included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | |
| Was the included outcome not a surrogate outcome? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Was the outcome timeframe sufficient? | Sufficient | Sufficient | Sufficient | Sufficient | Sufficient | Sufficient | Sufficient | |
| Were the conclusions based on direct comparisons? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Imprecisionc | Was the confidence interval for the pooled estimate not consistent with benefit and harm? | No (↓) | Yes | Not applicable | Not applicable | Not applicable | N/A | N/A |
| What is the magnitude of the median sample size (high: 300 participants, intermediate: 100 to 300 participants, low: < 100 participants)?e | High | High | High | High | ||||
| What was the magnitude of the number of included studies (large: >10 studies, moderate: 5 to 10 studies, small: < 5 studies)?e | Moderate | Large | Small (↓) | Small (↓) | ||||
| Was the outcome a common event (e.g. occurs more than 1/100)? | No (↓) | Yes | Not applicable | Not applicable | ||||
| Publication biasd | Was a comprehensive search conducted? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Was grey literature searched? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Were no restrictions applied to study selection on the basis of language? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| There was no industry influence on studies included in the review? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| There was no evidence of funnel plot asymmetry? | Not applicable | Unclear | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable | |
| There was no discrepancy in findings between published and unpublished trials? | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | |
|
aQuestions on risk of bias are answered in relation to the majority of the aggregated evidence in the meta‐analysis rather than to individual trials
bQuestions on inconsistency are primarily based on visual assessment of forest plots and the statistical quantification of heterogeneity based on I² cWhen judging the width of the confidence interval it is recommended to use a clinical decision threshold to assess whether the imprecision is clinically meaningful dQuestions address comprehensiveness of the search strategy, industry influence, funnel plot asymmetry and discrepancies between published and unpublished trials eDepends on the context of the systematic review area (↓): key item for potential downgrading the quality of the evidence (GRADE) as shown in the footnotes of the 'Summary of finding' table(s); GRADE: Grading of Recommendations Assessment, Development and Evaluation. | ||||||||
Appendix 16. Checklist to aid consistency and reproducibility of GRADE assessments: metformin versus intensive diet plus exercise
| Items | (1) All‐cause mortality | (2) Incidence of type 2 diabetes mellitus | (3) Serious adverse events | (4) Cardiovascular mortality | (5) Non‐fatal myocardial infarction/stroke | (6) Health‐related quality of life | (7) Socioeconomic effects | |
| Trial limitations (risk of bias)a | Was random sequence generation used (i.e. no potential for selection bias)? | Unclear | Unclear | Yes | Unclear | Unclear | Not applicable | Unclear |
| Was allocation concealment used (i.e. no potential for selection bias)? | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | ||
| Was there blinding of participants and personnel (i.e. no potential for performance bias) or outcome not likely to be influenced by lack of blinding? | Yes | Yes | Yes | Yes | Yes | Yes | ||
| Was there blinding of outcome assessment (i.e. no potential for detection bias) or was outcome measurement not likely to be influenced by lack of blinding? | Yes | Yes | Yes | Yes | Yes | Yes | ||
| Was an objective outcome used? | Yes | Yes | Yes | Yes | Yes | Yes | ||
| Were more than 80% of participants enrolled in trials included in the analysis (i.e. no potential reporting bias)?e | Yes | Yes | Unclear | Yes | Yes | Yes | ||
| Were data reported consistently for the outcome of interest (i.e. no potential selective reporting)? | Unclear | Unclear | No (↓) | Unclear | Unclear | Yes | ||
| No other biases reported (i.e. no potential of other bias)? | Yes | Yes | Yes | Yes | Yes | Yes | ||
| Did the trials end up as scheduled (i.e. not stopped early)? | No (↓) | No (↓) | No (↓) | No (↓) | No (↓) | No (↓) | ||
| Inconsistencyb | Point estimates did not vary widely? | Yes | No (↓) | Not applicable | Not applicable | Not applicable | Not applicable | |
| To what extent did confidence intervals overlap (substantial: all confidence intervals overlap at least one of the included studies point estimate; some: confidence intervals overlap but not all overlap at least one point estimate; no: at least one outlier: where the confidence interval of some of the studies do not overlap with those of most included studies)? | Substantial | Some | ||||||
| Was the direction of effect consistent? | No (↓) | Yes | ||||||
| What was the magnitude of statistical heterogeneity (as measured by I²) ‐ low (I² < 40%), moderate (I² 40% to 60%), high I² > 60%)? | Low | High (↓) | ||||||
| Was the test for heterogeneity statistically significant (P < 0.1)? | Not statistically significant | Statistically significant (↓) | ||||||
| Indirectness | Were the populations in included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | |
| Were the interventions in the included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | ||
| Was the included outcome not a surrogate outcome? | Yes | Yes | Yes | Yes | Yes | Yes | ||
| Was the outcome timeframe sufficient? | Sufficient | Sufficient | Sufficient | Sufficient | Sufficient | Sufficient | ||
| Were the conclusions based on direct comparisons? | Yes | Yes | Yes | Yes | Yes | Yes | ||
| Imprecisionc | Was the confidence interval for the pooled estimate not consistent with benefit and harm? | No (↓) | No (↓) | Not applicable | Not applicable | Not applicable | N/A | |
| What is the magnitude of the median sample size (high: 300 participants, intermediate: 100 to 300 participants, low: < 100 participants)?e | High | High | High | |||||
| What was the magnitude of the number of included studies (large: >10 studies, moderate: 5 to 10 studies, small: < 5 studies)?e | Small (↓) | Moderate | Small (↓) | |||||
| Was the outcome a common event (e.g. occurs more than 1/100)? | No (↓) | Yes | Not applicable | |||||
| Publication biasd | Was a comprehensive search conducted? | Yes | Yes | Yes | Yes | Yes | Yes | |
| Was grey literature searched? | Yes | Yes | Yes | Yes | Yes | Yes | ||
| Were no restrictions applied to study selection on the basis of language? | Yes | Yes | Yes | Yes | Yes | Yes | ||
| There was no industry influence on studies included in the review? | Yes | Yes | Yes | Yes | Yes | Yes | ||
| There was no evidence of funnel plot asymmetry? | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable | ||
| There was no discrepancy in findings between published and unpublished trials? | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | ||
|
aQuestions on risk of bias are answered in relation to the majority of the aggregated evidence in the meta‐analysis rather than to individual trials
bQuestions on inconsistency are primarily based on visual assessment of forest plots and the statistical quantification of heterogeneity based on I² cWhen judging the width of the confidence interval it is recommended to use a clinical decision threshold to assess whether the imprecision is clinically meaningful dQuestions address comprehensiveness of the search strategy, industry influence, funnel plot asymmetry and discrepancies between published and unpublished trials eDepends on the context of the systematic review area (↓): key item for potential downgrading the quality of the evidence (GRADE) as shown in the footnotes of the 'Summary of finding' table(s); GRADE: Grading of Recommendations Assessment, Development and Evaluation. | ||||||||
Appendix 17. Checklist to aid consistency and reproducibility of GRADE assessments: metformin versus acarbose
| Items | (1) All‐cause mortality | (2) Incidence of type 2 diabetes mellitus | (3) Serious adverse events | (4) Cardiovascular mortality | (5) Non‐fatal myocardial infarction/stroke | (6) Health‐related quality of life | (7) Socioeconomic effects | |
| Trial limitations (risk of bias)a | Was random sequence generation used (i.e. no potential for selection bias)? | Yes | Unclear | Unclear | Not applicable | Not applicable | Not applicable | Not applicable |
| Was allocation concealment used (i.e. no potential for selection bias)? | Unclear | Unclear | Unclear | |||||
| Was there blinding of participants and personnel (i.e. no potential for performance bias) or outcome not likely to be influenced by lack of blinding? | Yes | Yes | Yes | |||||
| Was there blinding of outcome assessment (i.e. no potential for detection bias) or was outcome measurement not likely to be influenced by lack of blinding? | Yes | Yes | Yes | |||||
| Was an objective outcome used? | Yes | Yes | Yes | |||||
| Were more than 80% of participants enrolled in trials included in the analysis (i.e. no potential reporting bias)?e | Yes | Yes | Unclear | |||||
| Were data reported consistently for the outcome of interest (i.e. no potential selective reporting)? | Unclear | Unclear | No (↓) | |||||
| No other biases reported (i.e. no potential of other bias)? | Yes | Yes | Yes | |||||
| Did the trials end up as scheduled (i.e. not stopped early)? | Yes | Yes | Yes | |||||
| Inconsistencyb | Point estimates did not vary widely? | Not applicable | Yes | Not applicable | ||||
| To what extent did confidence intervals overlap (substantial: all confidence intervals overlap at least one of the included studies point estimate; some: confidence intervals overlap but not all overlap at least one point estimate; no: at least one outlier: where the confidence interval of some of the studies do not overlap with those of most included studies)? | Substantial | |||||||
| Was the direction of effect consistent? | Yes | |||||||
| What was the magnitude of statistical heterogeneity (as measured by I²) ‐ low (I² < 40%), moderate (I² 40% to 60%), high I² > 60%)? | Low | |||||||
| Was the test for heterogeneity statistically significant (P < 0.1)? | Not statistically significant | |||||||
| Indirectness | Were the populations in included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | ||||
| Were the interventions in the included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | |||||
| Was the included outcome not a surrogate outcome? | Yes | Yes | Yes | |||||
| Was the outcome timeframe sufficient? | Sufficient | Sufficient | Sufficient | |||||
| Were the conclusions based on direct comparisons? | Yes | Yes | Yes | |||||
| Imprecisionc | Was the confidence interval for the pooled estimate not consistent with benefit and harm? | Not applicable | Yes | Not applicable | ||||
| What is the magnitude of the median sample size (high: 300 participants, intermediate: 100 to 300 participants, low: < 100 participants)?e | Low (↓) | Intermediate | Low (↓) | |||||
| What was the magnitude of the number of included studies (large: >10 studies, moderate: 5 to 10 studies, small: < 5 studies)?e | Small (↓) | Small (↓) | Small (↓) | |||||
| Was the outcome a common event (e.g. occurs more than 1/100)? | No (↓) | Yes | Yes | |||||
| Publication biasd | Was a comprehensive search conducted? | Yes | Yes | Yes | ||||
| Was grey literature searched? | Yes | Yes | Yes | |||||
| Were no restrictions applied to study selection on the basis of language? | Yes | Yes | Yes | |||||
| There was no industry influence on studies included in the review? | No (↓) | No (↓) | No (↓) | |||||
| There was no evidence of funnel plot asymmetry? | Not applicable | Not applicable | Not applicable | |||||
| There was no discrepancy in findings between published and unpublished trials? | Unclear | Unclear | Unclear | |||||
|
aQuestions on risk of bias are answered in relation to the majority of the aggregated evidence in the meta‐analysis rather than to individual trials
bQuestions on inconsistency are primarily based on visual assessment of forest plots and the statistical quantification of heterogeneity based on I² cWhen judging the width of the confidence interval it is recommended to use a clinical decision threshold to assess whether the imprecision is clinically meaningful dQuestions address comprehensiveness of the search strategy, industry influence, funnel plot asymmetry and discrepancies between published and unpublished trials eDepends on the context of the systematic review area (↓): key item for potential downgrading the quality of the evidence (GRADE) as shown in the footnotes of the 'Summary of finding' table(s); GRADE: Grading of Recommendations Assessment, Development and Evaluation. | ||||||||
Appendix 18. Checklist to aid consistency and reproducibility of GRADE assessments: metformin versus thiazolidinediones
| Items | (1) All‐cause mortality | (2) Incidence of type 2 diabetes mellitus | (3) Serious adverse events | (4) Cardiovascular mortality | (5) Non‐fatal myocardial infarction/stroke | (6) Health‐related quality of life | (7) Socioeconomic effects | |
| Trial limitations (risk of bias)a | Was random sequence generation used (i.e. no potential for selection bias)? | Not applicable | Unclear | Unclear | Not applicable | Not applicable | Not applicable | Not applicable |
| Was allocation concealment used (i.e. no potential for selection bias)? | Unclear | Unclear | ||||||
| Was there blinding of participants and personnel (i.e. no potential for performance bias) or outcome not likely to be influenced by lack of blinding? | Yes | Yes | ||||||
| Was there blinding of outcome assessment (i.e. no potential for detection bias) or was outcome measurement not likely to be influenced by lack of blinding? | Yes | Yes | ||||||
| Was an objective outcome used? | Yes | Yes | ||||||
| Were more than 80% of participants enrolled in trials included in the analysis (i.e. no potential reporting bias)?e | Yes | Unclear | ||||||
| Were data reported consistently for the outcome of interest (i.e. no potential selective reporting)? | Unclear | No (↓) | ||||||
| No other biases reported (i.e. no potential of other bias)? | Unclear | Yes | ||||||
| Did the trials end up as scheduled (i.e. not stopped early)? | Yes | Yes | ||||||
| Inconsistencyb | Point estimates did not vary widely? | Yes | Not applicable | |||||
| To what extent did confidence intervals overlap (substantial: all confidence intervals overlap at least one of the included studies point estimate; some: confidence intervals overlap but not all overlap at least one point estimate; no: at least one outlier: where the confidence interval of some of the studies do not overlap with those of most included studies)? | Substantial | |||||||
| Was the direction of effect consistent? | Yes | |||||||
| What was the magnitude of statistical heterogeneity (as measured by I²) ‐ low (I² < 40%), moderate (I² 40% to 60%), high I² > 60%)? | Low | |||||||
| Was the test for heterogeneity statistically significant (P < 0.1)? | Not statistically significant | |||||||
| Indirectness | Were the populations in included studies applicable to the decision context? | Highly applicable | Highly applicable | |||||
| Were the interventions in the included studies applicable to the decision context? | Highly applicable | Highly applicable | ||||||
| Was the included outcome not a surrogate outcome? | Yes | Yes | ||||||
| Was the outcome timeframe sufficient? | Sufficient | Sufficient | ||||||
| Were the conclusions based on direct comparisons? | Yes | Yes | ||||||
| Imprecisionc | Was the confidence interval for the pooled estimate not consistent with benefit and harm? | Yes | Not applicable | |||||
| What is the magnitude of the median sample size (high: 300 participants, intermediate: 100‐300 participants, low: <100 participants)?e | Intermediate | Low (↓) | ||||||
| What was the magnitude of the number of included studies (large: >10 studies, moderate: 5 to 10 studies, small: < 5 studies)?e | Small (↓) | Small (↓) | ||||||
| Was the outcome a common event (e.g. occurs more than 1/100)? | Yes | Yes | ||||||
| Publication biasd | Was a comprehensive search conducted? | Yes | Yes | |||||
| Was grey literature searched? | Yes | Yes | ||||||
| Were no restrictions applied to study selection on the basis of language? | Yes | Yes | ||||||
| There was no industry influence on studies included in the review? | Unclear | Unclear | ||||||
| There was no evidence of funnel plot asymmetry? | Not applicable | Not applicable | ||||||
| There was no discrepancy in findings between published and unpublished trials? | Not applicable | Unclear | ||||||
|
aQuestions on risk of bias are answered in relation to the majority of the aggregated evidence in the meta‐analysis rather than to individual trials
bQuestions on inconsistency are primarily based on visual assessment of forest plots and the statistical quantification of heterogeneity based on I² cWhen judging the width of the confidence interval it is recommended to use a clinical decision threshold to assess whether the imprecision is clinically meaningful dQuestions address comprehensiveness of the search strategy, industry influence, funnel plot asymmetry and discrepancies between published and unpublished trials eDepends on the context of the systematic review area (↓): key item for potential downgrading the quality of the evidence (GRADE) as shown in the footnotes of the 'Summary of finding' table(s); GRADE: Grading of Recommendations Assessment, Development and Evaluation | ||||||||
Appendix 19. Checklist to aid consistency and reproducibility of GRADE assessments: metformin plus intensive diet and exercise versus intensive diet and exercise
| Items | (1) All‐cause mortality | (2) Incidence of type 2 diabetes mellitus | (3) Serious adverse events | (4) Cardiovascular mortality | (5) Non‐fatal myocardial infarction/stroke | (6) Health‐related quality of life | (7) Socioeconomic effects | |
| Trial limitations (risk of bias)a | Was random sequence generation used (i.e. no potential for selection bias)? | Unclear | Unclear | Not applicable | Not applicable | Not applicable | Not applicable | Unclear |
| Was allocation concealment used (i.e. no potential for selection bias)? | Unclear | Unclear | Unclear | |||||
| Was there blinding of participants and personnel (i.e. no potential for performance bias) or outcome not likely to be influenced by lack of blinding? | Yes | Yes | Yes | |||||
| Was there blinding of outcome assessment (i.e. no potential for detection bias) or was outcome measurement not likely to be influenced by lack of blinding? | Yes | Yes | Yes | |||||
| Was an objective outcome used? | Yes | Yes | Yes | |||||
| Were more than 80% of participants enrolled in trials included in the analysis (i.e. no potential reporting bias)?e | Yes | Yes | Yes | |||||
| Were data reported consistently for the outcome of interest (i.e. no potential selective reporting)? | Yes | Yes | Yes | |||||
| No other biases reported (i.e. no potential of other bias)? | Unclear | Unclear | Yes | |||||
| Did the trials end up as scheduled (i.e. not stopped early)? | No (↓) | No (↓) | No (↓) | |||||
| Inconsistencyb | Point estimates did not vary widely? | Not applicable | No (↓) | Not applicable | ||||
| To what extent did confidence intervals overlap (substantial: all confidence intervals overlap at least one of the included studies point estimate; some: confidence intervals overlap but not all overlap at least one point estimate; no: at least one outlier: where the confidence interval of some of the studies do not overlap with those of most included studies)? | Some | |||||||
| Was the direction of effect consistent? | No (↓) | |||||||
| What was the magnitude of statistical heterogeneity (as measured by I²) ‐ low (I² < 40%), moderate (I² 40% to 60%), high I² > 60%)? | High (↓) | |||||||
| Was the test for heterogeneity statistically significant (P < 0.1)? | Not statistically significant | |||||||
| Indirectness | Were the populations in included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | ||||
| Were the interventions in the included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | |||||
| Was the included outcome not a surrogate outcome? | Yes | Yes | Yes | |||||
| Was the outcome timeframe sufficient? | Sufficient | Sufficient | Sufficient | |||||
| Were the conclusions based on direct comparisons? | Yes | Yes | Yes | |||||
| Imprecisionc | Was the confidence interval for the pooled estimate not consistent with benefit and harm? | Not applicable | No (↓) | N/A | ||||
| What is the magnitude of the median sample size (high: 300 participants, intermediate: 100‐300 participants, low: <100 participants)?e | Intermediate | Intermediate | High | |||||
| What was the magnitude of the number of included studies (large: >10 studies, moderate: 5 to 10 studies, small: < 5 studies)?e | Small (↓) | Small (↓) | Small (↓) | |||||
| Was the outcome a common event (e.g. occurs more than 1/100)? | No | Yes | Not applicable | |||||
| Publication biasd | Was a comprehensive search conducted? | Yes | Yes | Yes | ||||
| Was grey literature searched? | Yes | Yes | Yes | |||||
| Were no restrictions applied to study selection on the basis of language? | Yes | Yes | Yes | |||||
| There was no industry influence on studies included in the review? | Unclear | Unclear | Yes | |||||
| There was no evidence of funnel plot asymmetry? | Not applicable | Not applicable | Not applicable | |||||
| There was no discrepancy in findings between published and unpublished trials? | Not applicable | Not applicable | Unclear | |||||
|
aQuestions on risk of bias are answered in relation to the majority of the aggregated evidence in the meta‐analysis rather than to individual trials
bQuestions on inconsistency are primarily based on visual assessment of forest plots and the statistical quantification of heterogeneity based on I² cWhen judging the width of the confidence interval it is recommended to use a clinical decision threshold to assess whether the imprecision is clinically meaningful dQuestions address comprehensiveness of the search strategy, industry influence, funnel plot asymmetry and discrepancies between published and unpublished trials eDepends on the context of the systematic review area (↓): key item for potential downgrading the quality of the evidence (GRADE) as shown in the footnotes of the 'Summary of finding' table(s); GRADE: Grading of Recommendations Assessment, Development and Evaluation. | ||||||||
Appendix 20. Health‐related quality of life: instruments
| Trial ID | Instrument | Dimensions (subscales) (no. of items) | Validated instrument | Answer options | Scores |
Minimum score Maximum score |
Weighting of scores | Direction of scales | Minimal important difference |
| DPP/DPPOS 2002 | SF‐36 (G) | Physical functioning (PF) (10) Role‐physical (RP) (4) Bodily pain (BP) (2) General health (GH) (5) Vitality (VT) (4) Social functioning (SF) (2) Role‐emotional (RE) (3) Mental health (MH) (5) | Yes | Likert‐scale | Scores for dimensions
Physical component summary (PCS) Mental component summary (MCS) |
Minimum scores: 0 Maximum scores:100 |
No | Higher values mean better assessment | Minimal important difference was defined as HRQoL scores between groups differed by at least 3 %; In other publication (Marrero et al) minimal important difference is defined as two points on either PCS or MCS. |
| G: generic; HRQoL: health‐related quality of life; S: specific; SF: short‐form health survey. | |||||||||
Data and analyses
Comparison 1. Metformin versus placebo or diet and exercsie.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 5 | 2833 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.41, 3.01] |
| 2 Incidence of type 2 diabetes | 12 | 3632 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.38, 0.65] |
| 3 Incidence of type 2 diabetes (blinded vs open‐label) | 12 | 3632 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.38, 0.65] |
| 3.1 Participants blinded | 2 | 2240 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.64, 0.86] |
| 3.2 Open‐label | 10 | 1392 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.27, 0.59] |
| 4 Incidence of type 2 diabetes (duration of the intervention) | 12 | 3632 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.38, 0.65] |
| 4.1 Duration of the intervention less than 2 years | 4 | 296 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.14, 0.66] |
| 4.2 Duration of the intervention 2 years or more | 8 | 3336 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.40, 0.71] |
| 5 Incidence of type 2 diabetes (ethnicity) | 12 | 3632 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.38, 0.65] |
| 5.1 mainly White | 1 | 2155 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.65, 0.87] |
| 5.2 mainly Asian | 10 | 1418 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.28, 0.59] |
| 5.3 Other | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.13] |
| 6 Non‐serious adverse events | 2 | 285 | Risk Ratio (M‐H, Random, 95% CI) | 3.86 [0.18, 83.36] |
| 7 2‐hr glucose values | 13 | 3346 | Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.26, ‐0.46] |
| 8 2‐hr glucose values (blinded vs open‐label) | 13 | 3346 | Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.26, ‐0.46] |
| 8.1 Participants blinded | 4 | 2032 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.86, 0.33] |
| 8.2 Open‐label | 9 | 1314 | Mean Difference (IV, Random, 95% CI) | ‐1.03 [‐1.35, ‐0.71] |
| 9 2‐hr glucose values (duration of intervention) | 13 | 3346 | Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.26, ‐0.46] |
| 9.1 Duration of intervention less than 2 years | 4 | 286 | Mean Difference (IV, Random, 95% CI) | ‐1.37 [‐1.91, ‐0.82] |
| 9.2 Duration of intervention 2 years or more | 9 | 3060 | Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.18, ‐0.33] |
| 10 2‐r glucose values (ethnicity) | 13 | 3346 | Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.26, ‐0.46] |
| 10.1 Mainly White | 1 | 1856 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.17, 0.17] |
| 10.2 Mainly Asian | 10 | 1384 | Mean Difference (IV, Random, 95% CI) | ‐1.05 [‐1.35, ‐0.75] |
| 10.3 Other | 2 | 106 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.69, 0.79] |
| 11 HbA1c (blinded vs open‐label) | 6 | 2467 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.22, 0.05] |
| 11.1 Participants blinded | 2 | 1926 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 11.2 Open‐label | 4 | 541 | Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.77, 0.08] |
| 12 HbA1c (duration of intervention) | 6 | 2467 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.22, 0.05] |
| 12.1 Duration of intervention less than 2 years | 3 | 269 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.50, 0.21] |
| 12.2 Duration of intervention 2 years or more | 3 | 2198 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.75, 0.22] |
| 13 HbA1c (ethnicity) | 6 | 2467 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.22, 0.05] |
| 13.1 Mainly White | 1 | 1856 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 13.2 Mainly Asian | 4 | 556 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.74, ‐0.16] |
| 13.3 Other | 1 | 55 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.11, 0.11] |
| 14 Fasting plasma glucose | 15 | 3546 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.42, ‐0.13] |
| 15 Fasting plasma glucose (blinded vs open‐label) | 15 | 3546 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.42, ‐0.13] |
| 15.1 Participants blinded | 4 | 2037 | Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐0.94, ‐0.09] |
| 15.2 Open‐label | 11 | 1509 | Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.42, ‐0.03] |
| 16 Fasting plasma glucose (duration of the intervention) | 15 | 3546 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.42, ‐0.13] |
| 16.1 Duration of the intervention less than 2 years | 6 | 485 | Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐0.89, ‐0.13] |
| 16.2 Duration of the intervention 2 years or more | 9 | 3061 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.35, ‐0.02] |
| 17 Fasting plasma glucose (ethnicity) | 15 | 3546 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.42, ‐0.13] |
| 17.1 Mainly White | 1 | 1861 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.39, ‐0.21] |
| 17.2 Mainly Asian | 11 | 1524 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.51, ‐0.11] |
| 17.3 Other | 3 | 161 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.49, 0.22] |
Comparison 2. Metformin versus intensive diet plus exercise.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 4 | 2550 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.50, 5.23] |
| 2 Incidence of type 2 diabetes | 7 | 2960 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.47, 1.37] |
| 3 Incidence of type 2 diabetes (duration of intervention) | 7 | 2960 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.47, 1.37] |
| 3.1 Duration of intervention less than 2 years | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Duration of intervention 2 years or more | 6 | 2898 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.47, 1.37] |
| 4 Incidence of type 2 diabetes (ethnicity) | 7 | 2960 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.47, 1.37] |
| 4.1 Mainly White | 1 | 2152 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.25, 1.81] |
| 4.2 Mainly Asian | 5 | 746 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.32, 1.24] |
| 4.3 Other | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 2‐hr plasma glucose | 5 | 2417 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.26, 0.20] |
| 6 2‐hr plasma glucose (ethnicity) | 5 | 2417 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.26, 0.20] |
| 6.1 Mainly White | 1 | 1834 | Mean Difference (IV, Random, 95% CI) | 0.20 [0.02, 0.38] |
| 6.2 Mainly Asian | 4 | 583 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.59, 0.17] |
| 6.3 Other | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 HbA1c | 4 | 2135 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.12, 0.14] |
| 8 HbA1c (duration of intervention) | 4 | 2135 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.12, 0.14] |
| 8.1 Duration of intervention less than 2 years | 2 | 189 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.74, 0.46] |
| 8.2 Duration of intervention 2 years or more | 2 | 1946 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.04, 0.04] |
| 9 HbA1c (ethnicity) | 4 | 2135 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.12, 0.14] |
| 9.1 Mainly White | 1 | 1834 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 9.2 Mainly Asian | 2 | 244 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.66, 0.12] |
| 9.3 Other | 1 | 57 | Mean Difference (IV, Random, 95% CI) | 0.12 [0.02, 0.22] |
| 10 Fasting plasma glucose | 7 | 2603 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.59, 0.07] |
| 11 Fasting plasma glucose (duration of intervention) | 7 | 2603 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.59, 0.07] |
| 11.1 Duration of intervention less than 2 years | 2 | 189 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.17, 0.21] |
| 11.2 Duration of intervention 2 years or more | 5 | 2414 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.79, 0.04] |
| 12 Fasting plasma glucose (ethnicity) | 7 | 2603 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.59, 0.07] |
| 12.1 Mainly White | 1 | 1831 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.07, 0.07] |
| 12.2 Mainly Asian | 5 | 715 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.73, ‐0.04] |
| 12.3 Other | 1 | 57 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.21, 0.21] |
2.3. Analysis.
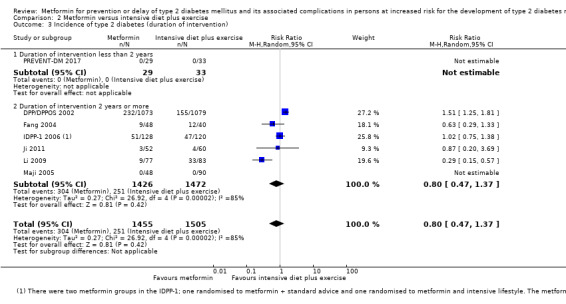
Comparison 2 Metformin versus intensive diet plus exercise, Outcome 3 Incidence of type 2 diabetes (duration of intervention).
Comparison 3. Metformin versus sulphonylurea.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 2‐hr plasma glucose | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.66, 0.86] |
| 2 Fasting plasma glucose | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.02, 0.62] |
3.1. Analysis.
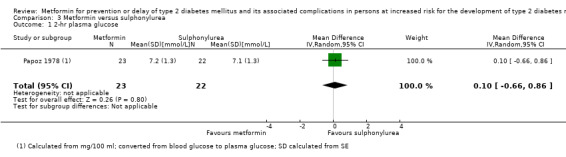
Comparison 3 Metformin versus sulphonylurea, Outcome 1 2‐hr plasma glucose.
3.2. Analysis.
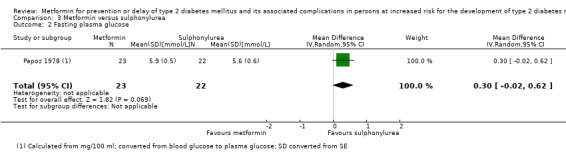
Comparison 3 Metformin versus sulphonylurea, Outcome 2 Fasting plasma glucose.
Comparison 4. Metformin versus acarbose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Incidence of type 2 diabetes | 3 | 295 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [0.72, 4.14] |
| 3 2‐hr plasma glucose | 2 | 190 | Mean Difference (IV, Random, 95% CI) | 0.49 [0.09, 0.88] |
| 4 Fasting plasma glucose | 2 | 190 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.35, 0.35] |
4.1. Analysis.
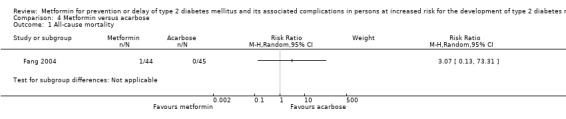
Comparison 4 Metformin versus acarbose, Outcome 1 All‐cause mortality.
Comparison 5. Metformin versus thiazolidinediones.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of type 2 diabetes | 3 | 320 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.41, 2.40] |
| 2 2‐hr plasma glucose | 2 | 224 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐1.80, 0.73] |
| 3 Fasting plasma glucose | 2 | 224 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.32, 0.07] |
Comparison 6. Metformin + intensive diet and exercise versus intensive diet and exercise.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Incidence of type 2 diabetes | 2 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.10, 2.92] |
| 3 Incidence of type 2 diabetes (blinded vs open‐label) | 3 | 524 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.53, 1.58] |
| 3.1 Participants blinded | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Open‐label | 3 | 524 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.53, 1.58] |
| 4 Incidence of type 2 diabetes (duration of the intervention) | 3 | 524 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.53, 1.58] |
| 4.1 Duration of the intervention less than 2 years | 2 | 283 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.09, 3.42] |
| 4.2 Duration of the intervention 2 years or more | 1 | 241 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.72, 1.36] |
| 5 Incidence of type 2 diabetes (ethnicity) | 3 | 524 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.53, 1.58] |
| 5.1 mainly White | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 mainly Asian | 3 | 524 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.53, 1.58] |
| 5.3 Other | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 2‐hr glucose values | 2 | 316 | Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐2.08, 1.04] |
| 7 Fasting plasma glucose | 2 | 316 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.94, 0.43] |
| 8 Fasting plasma glucose (blinded vs open‐label) | 2 | 316 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.94, 0.43] |
| 8.1 Participants blinded | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Open‐label | 2 | 316 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.94, 0.43] |
| 9 Fasting plasma glucose (duration of the intervention) | 2 | 316 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.94, 0.43] |
| 9.1 Duration of the intervention less than 2 years | 1 | 91 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.97, ‐0.23] |
| 9.2 Duration of the intervention 2 years or more | 1 | 225 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.32, 0.52] |
6.3. Analysis.
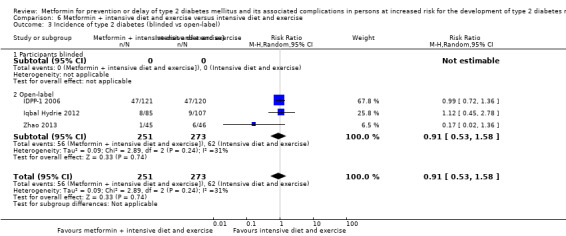
Comparison 6 Metformin + intensive diet and exercise versus intensive diet and exercise, Outcome 3 Incidence of type 2 diabetes (blinded vs open‐label).
6.4. Analysis.
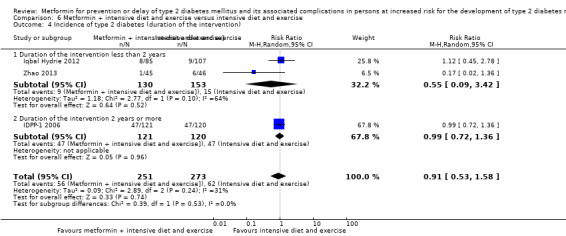
Comparison 6 Metformin + intensive diet and exercise versus intensive diet and exercise, Outcome 4 Incidence of type 2 diabetes (duration of the intervention).
6.5. Analysis.
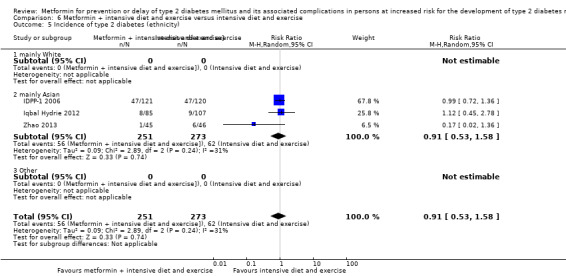
Comparison 6 Metformin + intensive diet and exercise versus intensive diet and exercise, Outcome 5 Incidence of type 2 diabetes (ethnicity).
6.8. Analysis.
Comparison 6 Metformin + intensive diet and exercise versus intensive diet and exercise, Outcome 8 Fasting plasma glucose (blinded vs open‐label).
6.9. Analysis.
Comparison 6 Metformin + intensive diet and exercise versus intensive diet and exercise, Outcome 9 Fasting plasma glucose (duration of the intervention).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alfawaz 2018.
| Methods | Parallel randomised controlled trial, randomisation ratio 1:1:1 | |
| Participants |
Inclusion criteria: fasting glucose level of 5.6 mmol/L to 6.9 mmol/L; the participants were identified through screening as recommended in guidelines by ADA 2017 Exclusion criteria: receiving glucose‐lowering intervention; pregnant or lactating women; renal, hepatic, pulmonary, cardiac complications Diagnostic criteria: ADA 2017 criteria for IFG (fasting glucose 5.6 mmol/Lto 6.9 mmol/L) |
|
| Interventions |
Number of study centres: 2 Run‐in period: none Administration‐free period before testing during trial: not reported Extension period: none |
|
| Outcomes | Composite outcome measures reported: metabolic syndrome (primary outcome) | |
| Study details | Trial terminated early: no | |
| Publication details |
Language of publication: English Funding: non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "... aimed to determine the differences in the effects of general advice (GA) on lifestyle change, intensive lifestyle modification programme (ILMP) and GA + metformin (GA + Met) in reducing the prevalence of full metabolic syndrome (MetS) in subjects with prediabetes" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "A computer‐generated serial number, randomly assigned..." Comment: adequate description of the sequence generation |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "True allocation concealment was done since the research personnel involved cannot adjust randomization" Comment: adequate description of allocation concealment |
| Blinding of participants and personnel (performance bias) measures of blood glucose control | Low risk | Comment: investigator‐assessed outcome measure, unclear if this outcome also was assessed by the blinded independent outcome committee. The outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) measures of blood glucose control | Low risk | Comment: investigator‐assessed outcome measure, unclear if this outcome also was assessed by the blinded independent outcome committee. The outcome is not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) measures of blood glucose control | High risk |
Quote from publication: "Hence, the missing data (<5% of the total data points in any variable) was dealt with the last observation carried forward (LOCF) method. However, as much as possible, the LOCF was minimized by removing the data of the subjects lost to follow up at 6‐month or 12‐month and also by removing ones with >5% missing data in any variable" Comment: in metformin plus general advise on diet and exercise group 60.2% of randomised participants were analysed. In intensive lifestyle modification programme group and general advise on diet and exercise group 74.5% and 86.7% of randomised participants were analysed, respectively. Drop‐out rates were not balanced (69.4 to 95.9% of randomised participants finished the trial). Reasons for drop‐outs were not balanced. Plausible effect size among missing outcomes enough to induce clinically‐relevant bias in observed effect size |
| Selective reporting (reporting bias) | High risk | Comment: no trial protocol available. Likely that the incidence of T2DM as well as adverse events has been collected during the trial, but not reported |
| Other bias | Low risk | Comment: the trial appeared to be free of other sources of bias |
BIGPRO1 2009.
| Methods | Parallel randomised controlled trial, randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: participants with a high waist‐to‐hip ratio ( ≥ 0.95 in men; ≥ 0.80 in women), who were considered to be non‐diabetic. Other trial inclusion criteria were age (35 to 60 years for men, 40 to 65 years for women), absence of cardiovascular diseases and no contraindications to the use of metformin Exclusion criteria: participants with Ischaemic cardiovascular disease, diabetes, psychiatric disorders, heavy chronic medical treatment, serious life‐threatening medical conditions, impaired renal function and lactic acidosis were excluded Diagnostic criteria: WHO 1999 (IFG is defined as a FPG of 110 mg/dL to 125 mg/dL (6.1 mmol/L to 6.9 mmol/L) and IGT as a 2hPG of 140 mg/dLto 199 mg/dL (7.8 mmol/L to11.0 mmol/L)) |
|
| Interventions |
Number of study centres: 33 Run‐in period: none Administration‐free period before testing during trial: NR Extension period: no |
|
| Outcomes | Composite outcome measures reported: no | |
| Study details | Trial terminated early: no | |
| Publication details |
Language of publication: English Funding: both commercial funding (Lipha Pharmaceuticals Ltd) and non‐commercial funding (INSERM, CNAMts) Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "To study the effects of 1 year of treatment with metformin versus placebo on the clinical and metabolic parameters described as part of the metabolic syndrome" | |
| Notes | Data on the people with IFG and IGT were only a subset of participants 101 out of 457 (22.1%) and reported in a post hoc analysis. Quote from publication: "Analyses were performed in the subset of trial patients who had impaired fasting glucose (IFG) or impaired glucose tolerance (IGT), or both, according to the 1999 WHO definition [13], wherein IFG is defined as a FPG of 110–125 mg/dL (6.1–6.9 mmol/L) and IGT as a 2hPG of 140–199 mg/dL (7.8–11.0 mmol/L). In addition, these analyses were repeated in another subset of subjects, defined according to inclusion criteria of the DPP [5]—namely, body mass index (BMI)≥24 kg/m2, FPG of 95–125 mg/dL (5.3–6.9 mmol/L) and 2hPG of 140–199 mg/dL (7.8–11.0 mmol/L)." "Of the 457 subjects included in the BIGPRO1 trial, 101 (22%; 49 in the metformin group and 52 in the placebo group) had IFG or IGT at baseline, with eight subjects in the metformin group and 11 in the placebo group having isolated IFG; and 51 (11%; 28 in the metformin group and 23 in the placebo group) met the DPP inclusion criteria." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Confidential balanced random lists are used to allocate to every patient's number metformin or placebo,..." Comment: adequate description of the sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of allocation concealment |
| Blinding of participants and personnel (performance bias) measures of blood glucose control | Low risk |
Quote from publication: "..., in double‐blind fashion, i.e. packages. are similar whatever their content and only identified by the patient' trial number." Comment: blinding of participants and key study personnel ensured |
| Blinding of outcome assessment (detection bias) measures of blood glucose control | Low risk |
Quote from publication: "..., in double‐blind fashion, i.e. packages. are similar whatever their content and only identified by the patient' trial number." Comment: blinding of investigators, who were outcome assessors are ensured |
| Incomplete outcome data (attrition bias) all‐cause mortality/cardiovascular mortality | Low risk | |
| Incomplete outcome data (attrition bias) measures of blood glucose control | Low risk |
Quote from publication: "All patient who definitively stop the trial treatment for any reason continue to be followed up and examined a scheduled" and "At 12 months, 37 subjects (21 metformin, 16 placebo) in the IFG/IGT subset and 19 (10 metformin, 9 placebo) in the DPP subset had dropped out. The reasons for the subjects’ absence at 12 months were roughly similar between treatment groups, with only a slight tendency to a greater influence of side effects in the metformin group and a lack of motivation in the placebo
group. To assess whether this dropout rate had any effect on the initial comparability of the two treatment groups, baseline characteristics were compared between those who missed the last visit and the remaining subjects. The only difference found was that those remaining in the trial were more often treated for hypertension than the dropouts (45% and 16%, respectively, in the IFG/IGT subset, P < 0.003; 47% and 21%, respectively, in the DPP subset, P = 0.07)." Comment: it is predefined to include all randomised participants. In the post hoc analysis of the participants with IFG and/or IGT only 63% of the participants are included in the analyses. This might introduce clinical relevant bias in effect estimates. |
| Selective reporting (reporting bias) | High risk | Comment: adverse events and incidence of T2DM were assessed in the total population, but not reported in the subset with IGT and/or IFG. However, these analyses were not predefined in the protocol. |
| Other bias | High risk | Comment: several authors have conflicts of interest, and the trial has received pharmaceutical funding |
Chen 2009.
| Methods | Parallel randomised controlled trial, randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: IGT Exclusion criteria: liver and kidney dysfunction Diagnostic criteria: WHO 1999 (2hPG of 140 mg/dL to 199 mg/dL (7.8 mmol/L to 11.0 mmol/L)) |
|
| Interventions |
Number of trial centres: 1 Run‐in period: not reported Administration‐free period before testing during trial: not specified if any study drug was taken on the testing day at the end of the intervention. However, for participants who did not convert to diabetes, the fasting and 2‐hour 75g‐OGTT blood glucose was detected at 1‐year follow‐up after drug withdrawal. Extension period: none |
|
| Outcomes | Composite outcome measures reported: none | |
| Study details | Trial terminated early: no | |
| Publication details |
Language of publication: Chinese Funding: not reported Publication status: peer‐reviewed journal, full article |
|
| Stated aim of study | Quote from publication: "To observe the effect of Shenqi Jiangtang capsule on preventing type 2 diabetes in IGT patients." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "randomised" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of allocation concealment |
| Blinding of participants and personnel (performance bias) incidence of T2DM | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding. (investigator‐assessed outcome measurement) |
| Blinding of participants and personnel (performance bias) measures of blood glucose control | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding. (investigator‐assessed outcome measurement) |
| Blinding of outcome assessment (detection bias) incidence of T2DM | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding. (investigator‐assessed outcome measurement) |
| Blinding of outcome assessment (detection bias) measures of blood glucose control | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding. (investigator‐assessed outcome measurement) |
| Incomplete outcome data (attrition bias) incidence of T2DM | High risk |
Quote from publication: "A total of 17 patients withdrew during the observation period, including 6 in the Shenqi Jiangtang capsule group, 6 in the general life intervention group and 5 in the metformin group." Comment: no reason given and PP analysis used (only reported) |
| Incomplete outcome data (attrition bias) measures of blood glucose control | High risk |
Quote from publication: "A total of 17 patients withdrew during the observation period, including 6 in the Shenqi Jiangtang capsule group, 6 in the general life intervention group and 5 in the metformin group." Comment: no reason given and PP analysis used (only reported) |
| Selective reporting (reporting bias) | High risk | Comment: protocol unavailable, non‐SAE likely to have been analysed but not reported. Outcomes stated in the methods were reported in the results (BMI, liver and kidney function) |
| Other bias | Unclear risk | Comment: unknown funding source |
DPP/DPPOS 2002.
| Methods | Parallel randomised controlled trial, randomisation ratio 1:1:1 | |
| Participants |
Inclusion criteria: ≥ 25 years, BMI ≥ 24 kg/m2 in Asians BMI ≥ 22 kg/m2, FPG 95 mg/dL to 125 mg/dL (5.3 mmol/L to 6. 9 mmol/L) and 2‐hour OGTT 140 mg/dL to 199 mg/dL (7.8 mmol/L to 11.0 mmol/L). Because of the relative higher rate of progression from IGT to diabetes in Native Americans and the small size of the population, the glucose requirement for eligibility in the Southwest American Indian Center will be fasting glucose < 126 mg/dL (7.0 mmol/L) and 2‐hour plasma glucose 140 mg/dL to 199 mg/dL (7.8 mmol/L to 11.0 mmol/L). Exclusion criteria: T2DM, participants taking medicines known to alter glucose tolerance, ever used glucose‐lowering drugs during pregnancy, illnesses that could seriously reduce their life expectancy or their ability to participate in the trial, cardiovascular disease (hospitalisation for treatment of heart disease in past 6 months; NYHA class > 2; left bundle branch block or third degree atrioventricular block; aortic stenosis; SBP > 180 mmHg or DBP > 105 mmHg); cancer requiring treatment in the past five years (unless prognosis is considered good); renal disease; gastrointestinal disease; anaemia (haematocrit < 36.0% in men or < 33.0% in women); electrolyte abnormality (serum potassium < 3.2 or > 5.5 mmol/L). Diagnostic criteria: IGT (2‐hour OGTT 140 mg/dL to 199 mg/dL (7.8 mmol/L to 11.0 mmol/L)) and elevated fasting glucose (FPG 95 mg/dL to 125 mg/dL (5.3 mmol/L to 6.9 mmol/L)) (ADA 1997). |
|
| Interventions |
Number of study centres: 27 Treatment before study: none Run‐in period: 3 weeks; during the run‐in period the participants had to fill out a daily diary and placebo pills according to a schedule Extension period: yes, an additional follow‐up with a median of 5.7 years (IQR 5.5 to 5.8) after end of the intervention period |
|
| Outcomes | Composite outcome measures reported: yes (Quote from publication: ".....a composite microvascular‐neuropathic outcome for diabetic retinopathy, nephropathy, or reduced light touch sensation in the feet. Secondary outcomes include the individual components of the composite primary outcome, cardiovascular disease, further development of diabetes, measures of glycaemia, insulin secretion, insulin sensitivity, cardiovascular disease risk factors, physical activity, nutrition, bodyweight, health‐related quality of life, and economic assessments.") | |
| Study details |
Trial terminated before regular end (for benefit/because of adverse events): the trial was stopped one year earlier than originally planned due to larger intervention effect of diet and physical activity than anticipated. Taking trial drug on glycaemic testing days: placebo and metformin was not taken on the morning of glycaemic testing |
|
| Publication details |
Language of publication: English Funding: commercial funding (Lipha (Merck‐Sante) provided medicines, and LifeScan donated materials) / non‐commercial funding (the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute of Child Health and Human Development, the National Institute on Aging, the National Eye Institute, the National Heart Lung and Blood Institute, the Office of Women’s Health, the National Center for Minority Health and Human Disease, the Centers for Disease Control and Prevention, and the American Diabetes Association) Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "The principal objective of the DPP is to prevent or delay the development of NIDDM in those persons who are at high risk for its development by virtue of having impaired glucose tolerance" | |
| Notes | Individuals who meet only one of the glucose inclusion criteria was rescreened after 6 months. Because of the relative higher rate of progression from IGT to T2DM in Native Americans and the small size of the population, the glucose requirement for eligibility in the Southwest American Indian Center differed (see above). The trial included initially four intervention groups. The troglitazone group was discontinued in 1998 because of potential liver toxicity. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "A sequence of randomization numbers within a clinical center will be constructed of the form XXYZZZ, where XX is the clinical center number, Y is a number that indicates assignment to either the intensive lifestyle intervention or pharmacological treatment, and ZZZ is a three digit sequence number within each XXY combination. The DPP Coordinating Center will prepare the master randomization list with assignments to the three treatment groups within a clinical center using the standard urn design.
The sequence of pharmacological randomization numbers within a clinical center with the specific pharmacological treatment assignment (i.e., metformin or placebo) will be forwarded, in confidence, to the drug distribution center for drug labelling and distribution. Pharmacological treatment assignment to the sequence of pharmacological randomization numbers will be known only by the staff of the DPP Coordinating Center and the drug distribution center." Comment: adequate description of the sequence generation |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "A sequence of randomization numbers within a clinical center will be constructed of the form XXYZZZ, where XX is the clinical center number, Y is a number that indicates assignment to either the intensive lifestyle intervention or pharmacological treatment, and ZZZ is a three digit sequence number within each XXY combination. The DPP Coordinating Center will prepare the master randomization list with assignments to the three treatment groups within a clinical center using the standard urn design.
The sequence of pharmacological randomization numbers within a clinical center with the specific pharmacological treatment assignment (i.e., metformin or placebo) will be forwarded, in confidence, to the drug distribution center for drug labeling and distribution. Pharmacological treatment assignment to the sequence of pharmacological randomization numbers will be known only by the staff of the DPP Coordinating Center and the drug distribution center." Comment: adequate allocation concealment ensured |
| Blinding of participants and personnel (performance bias) all‐cause mortality/cardiovascular mortality | Low risk |
Quote from publication: "Masking intensive lifestyle intervention assignment to the participants is not possible and masking the investigators is not practical." and "Assignments to metformin and placebo were double‐blinded." Comment: no blinding for the comparison of metformin with intensive diet and physical activity, but judged that the outcome is not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) incidence of T2DM | Low risk |
Quote from publication: "Masking intensive lifestyle intervention assignment to the participants is not possible and masking the investigators is not practical." and "Assignments to metformin and placebo were double‐blinded." and "Primary outcome data (OGTT and FPG results) measured centrally will remain masked to the investigators and to the participants until confirmed progression from IGT to diabetes" Comment: assessed centrally unblinded, the outcome is not likely to be influenced by lack of blinding. Participants and investigators blinded to until progression to T2DM |
| Blinding of participants and personnel (performance bias) measures of blood glucose control | Low risk |
Quote from publication: "Masking intensive lifestyle intervention assignment to the participants is not possible and masking the investigators is not practical." and "Assignments to metformin and placebo were double‐blinded." and "Primary outcome data (OGTT and FPG results) measured centrally will remain masked to the investigators and to the participants until confirmed progression from IGT to diabetes" and Plasma lipid levels and HbA1c measured
centrally will remain masked to the investigators and to the participants during the study." Comment: assessed centrally unblinded, the outcome is not likely to be influenced by lack of blinding. Participants and investigators blinded to until progression to T2DM |
| Blinding of participants and personnel (performance bias) socioeconomic effects | Low risk |
Quote from publication: "Masking intensive lifestyle intervention assignment to the participants is not possible and masking the investigators is not practical." and "Assignments to metformin and placebo were double‐blinded." Comment: no blinding for the comparison of metformin with intensive diet and physical activity, but judged that the outcome is not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) health‐related quality of life | High risk |
Quote from publication: "Masking intensive lifestyle intervention assignment to the participants is not possible and masking the investigators is not practical." and "Assignments to metformin and placebo were double‐blinded." Comment: no blinding for the comparison of metformin with intensive diet and physical activity and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) incidence of T2DM | Low risk |
Quote from publication: "Masking intensive lifestyle intervention assignment to the participants is not possible and masking the investigators is not practical." and "Assignments to metformin and placebo were double‐blinded." and "Primary outcome data (OGTT and FPG results) measured centrally will remain masked to the investigators and to the participants until confirmed progression from IGT to diabetes" Comment: assessed centrally unblinded, the outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) measures of blood glucose control | Low risk |
Quote from publication: "Masking intensive lifestyle intervention assignment to the participants is not possible and masking the investigators is not practical." and "Assignments to metformin and placebo were double‐blinded." and "Primary outcome data (OGTT and FPG results) measured centrally will remain masked to the investigators and to the participants until confirmed progression from IGT to diabetes" and "Plasma lipid levels and HbA1c measured centrally will remain masked to the investigators and to the participants during the study." Comment: assessed centrally unblinded, the outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) socioeconomic effects | Low risk |
Quote from publication: "Masking intensive lifestyle intervention assignment to the participants is not possible and masking the investigators is not practical." and "Assignments to metformin and placebo were double‐blinded." Comment: no blinding for the comparison of metformin with intensive diet and physical activity but judged that the outcome is not likely to be influenced by lack of blinding, investigator‐assessed outcome measurement |
| Blinding of outcome assessment (detection bias) health‐related quality of life | High risk |
Quote from publication: "Masking intensive lifestyle intervention assignment to the participants is not possible and masking the investigators is not practical." and "Assignments to metformin and placebo were double‐blinded." Comment: no blinding for the comparison of metformin with intensive diet and physical activity and the outcome is likely to be influenced by lack of blinding, self‐reported outcome measurement |
| Incomplete outcome data (attrition bias) all‐cause mortality/cardiovascular mortality | Low risk |
Quote from publication: "At the close of the study, 99.6 percent of the participants were alive, of whom 92.5 percent had attended a scheduled visit within the previous five months" Comment: not stated how many participants who had known vital status in each intervention group at the end of follow‐up for the DPP trial. However, at inception of the number with unknown mortality status are relatively low. At inception of the DPPOS the number between the intervention groups we balanced. |
| Incomplete outcome data (attrition bias) incidence of T2DM | Low risk |
Quote from publication: "At the close of the study, 99.6 percent of the participants were alive, of whom 92.5 percent had attended a scheduled visit within the previous five months" Comment: not stated how many participants who had known vital status at the end of follow‐up for the DPP trial. However, at inception of the DPPOS a relatively low and balanced number of participants in the intervention groups could not be included. |
| Incomplete outcome data (attrition bias) measures of blood glucose control | Low risk | Comment: not stated how many participants who had known vital status at the end of follow‐up for the DPP trial. However, at inception of the DPPOS a relatively low and balanced number between the intervention groups could not be included. |
| Incomplete outcome data (attrition bias) time to progression to T2DM | Low risk | Comment: "At the close of the study, 99.6 percent of the participants were alive, of whom 92.5 percent had attended a scheduled visit within the previous five months" |
| Incomplete outcome data (attrition bias) socioeconomic effects | Low risk | Comment: not clearly described how many participants included in the costs analyses, but as the study have a high follow‐up rate, we assume that nearly all participants are included. |
| Incomplete outcome data (attrition bias) health‐related quality of life | Low risk |
Quote from publication: " the current reports and analyses includes 3,234 participants seen at baseline, who were randomly assigned to one of the three treatment arms investigated." Comment: article reporting health related quality of life do not report the number of participants with available data at follow‐up |
| Selective reporting (reporting bias) | High risk | Comment: several outcome are likely to be measured and analysed, but not reported, e.g. hypoglycaemia, non‐serious adverse events. Outcomes published in many different publications. Several outcomes are reported incompletely so that they cannot be included in meta‐analysis |
| Other bias | Unclear risk | Comment: received funding from a pharmaceutical company |
Fang 2004.
| Methods | Parallel randomised controlled trial, randomisation ratio 1:1:1:1 | |
| Participants |
Inclusion criteria: participants in accordance with the diagnostic criteria of IGT Exclusion criteria: participants with severe somatological disease, mental disease or history of mental disease, severe intellectual or cognitive disorders, drug or alcohol dependence Diagnostic criteria: IGT |
|
| Interventions |
Number of study centres: 1 Run‐in period: not described Administration‐free period before testing during trial: not reported Extension period: none |
|
| Outcomes | Composite outcome measures reported: none | |
| Study details |
Trial terminated before regular end (for benefit/because of adverse events): no Taking trial drug on glycaemic testing days: not specified if any study drug was taken on the testing day at the end of the intervention. |
|
| Publication details |
Language of publication: Chinese Funding: not described Publication status: full article (Chinese Journal of Clinical Rehabilitation) |
|
| Stated aim of study | Quote from publication: "To observe influence of medicine intervention and non‐medicine intervention on the outcomes of the crowd with IGT and explore which intervention can prevent IGT from developing to diabetes mellitus more effectively." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Patients were randomly allocated by random number table." Comment: adequate description of the sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of allocation concealment |
| Blinding of participants and personnel (performance bias) all‐cause mortality/cardiovascular mortality | Low risk | Comment: no description of blinding, but according to the intervention arms in the trial, neither the participants or the personnel were blinded. The outcome is not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) incidence of T2DM | Low risk | Comment: no description of blinding, but according to the intervention arms in the trial, neither the participants or the personnel were blinded. The outcome is not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) measures of blood glucose control | Low risk | Comment: no description of blinding, but according to the intervention arms in the trial, neither the participants or the personnel were blinded. The outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) all‐cause mortality/cardiovascular mortality | Low risk | Comment: unclear whether outcome assessors were blinded, but the outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) incidence of T2DM | Low risk | Comment: unclear whether outcome assessors were blinded, but the outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) measures of blood glucose control | Low risk | Comment: unclear whether outcome assessors were blinded, but the outcome is not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) all‐cause mortality/cardiovascular mortality | Unclear risk |
Quote from publication: "160/178 (90%) were analysed" Comment: unclear description on how missing data were handled |
| Incomplete outcome data (attrition bias) incidence of T2DM | Unclear risk |
Quote from publication: "160/178 (90%) were analysed" Comment: unclear description on how missing data were handled |
| Incomplete outcome data (attrition bias) measures of blood glucose control | Unclear risk |
Quote from publication: "160/178 (90%) were analysed" Comment: unclear description on how missing data were handled |
| Selective reporting (reporting bias) | Unclear risk | Comment: no trial protocol available |
| Other bias | Unclear risk | Comment: unclear funding source |
IDPP‐1 2006.
| Methods | Parallel randomised controlled trial, randomisation ratio 1:1:1:1 | |
| Participants |
Inclusion criteria: IGT (mean 2‐hour plasma glucose after OGTT 140 mg/dL to 199 mg/dL (7.8 mmol/L to 11.0 mmol/L) and FPG < 126 mg/dL (7.0 mmol/L)) (WHO 1999); no major illness; 35 to 55 years Exclusion criteria: diagnosis of T2DM during recruitment; pregnancy Diagnostic criteria: IGT (WHO 1999) |
|
| Interventions |
Number of study centres: ‐ Run‐in period: none Administration‐free period before testing during trial: not reported Titration period: none |
|
| Outcomes | Composite outcome measures reported: yes (cardiovascular disease) | |
| Study details |
Trial terminated before regular end (for benefit/because of adverse events): yes; Quote from publication: "After a median follow‐up period of 30 months, because there were
significant differences in the outcome measure between the control and intervention groups, the committee recommended the termination of the study in December 2004" Taking trial drug on glycaemic testing days: not specified |
|
| Publication details |
Language of publication: English Funding: commercial (M/S US Vitamins) Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "In a prospective community‐based study, we tested whether the progression to diabetes could be influenced by interventions in native Asian Indians with IGT who were younger, leaner and more insulin resistant than the above populations" | |
| Notes | Two more intervention groups existed that were not included in this review; 1) metformin and 2) diet plus physical activity combined with metformin | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "A randomised, controlled clinical trial was performed in subjects who were......" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of allocation concealment |
| Blinding of participants and personnel (performance bias) all‐cause mortality/cardiovascular mortality | Low risk |
Quote from publication: "Masking: Open Label" and "However, the principal investigators were blinded to the outcome until they were asked to close the study by the international data monitoring committee." Comment: outcome evaluated by an independent outcome committee. Outcome unlikely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) incidence of T2DM | Low risk |
Quote from publication: "Masking: Open Label" "However, the principal investigators were blinded to the outcome until they were asked to close the study by the international data monitoring committee." Comment: outcome evaluated by an independent outcome committee and investigator‐assessed outcome measure |
| Blinding of participants and personnel (performance bias) measures of blood glucose control | Low risk |
Quote from publication: "Masking: Open Label" and ""However, the principal investigators were blinded to the outcome until they were asked to close the study by the international data monitoring committee." Comment: investigator‐assessed outcome measure, unclear if this outcome also was assessed by the blinded independent outcome committee. The outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) all‐cause mortality/cardiovascular mortality | Low risk |
Quote from publication: "However, the principal investigators were blinded to the outcome until they were asked to close the study by the international data monitoring committee." Comment: outcome evaluated by an independent outcome committee |
| Blinding of outcome assessment (detection bias) incidence of T2DM | Low risk |
Quote from publication: "However, the principal investigators were blinded to the outcome until they were asked to close the study by the international data monitoring committee." Comment: outcome evaluated by an independent outcome committee and investigator‐assessed outcome measure |
| Blinding of outcome assessment (detection bias) measures of blood glucose control | Low risk |
Quote from publication: "However, the principal investigators were blinded to the outcome until they were asked to close the study by the international data monitoring committee." Comment: investigator‐assessed outcome measure, unclear if this outcome also was assessed by the blinded independent outcome committee. The outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) non‐serious adverse events | High risk | |
| Incomplete outcome data (attrition bias) all‐cause mortality/cardiovascular mortality | Unclear risk | Comment: unknown whether mortality status was known on the participants lost to follow‐up. The proportion of missing outcomes compared with observed event risk may have a clinically relevant impact on the intervention effect estimate |
| Incomplete outcome data (attrition bias) incidence of T2DM | Unclear risk | Comment: the proportion of missing outcomes compared with observed event risk is not enough to have a clinically relevant impact on the intervention effect estimate |
| Incomplete outcome data (attrition bias) measures of blood glucose control | Unclear risk | Comment: Insufficient information to assess whether missing data in combination with the method used to handle missing data were likely to induce bias |
| Selective reporting (reporting bias) | High risk |
Quote from publication: "An internal safety committee monitored the adverse events and safety of study protocol. The data and final outcome measures were monitored by the international monitoring committee who had looked at the results three times, i.e. when 500 subjects had completed the follow‐up assessments at 12, 24 and 30 months. The principal investigators were blinded to the interim results." Comment: several outcomes with relevance for this review are not reported or only reported in a format which makes them unsuitable for meta‐analyses, e.g. adverse events |
| Other bias | Unclear risk | Comment: role of funding source not described |
Iqbal Hydrie 2012.
| Methods | Parallel randomised controlled trial, randomisation ratio 1:1:1 | |
| Participants |
Inclusion criteria: IGT, > 30 years Exclusion criteria: NS Diagnostic criteria: WHO 1999 criteria (FPG < 7.0 mmol/L and 2‐hour plasma glucose after OGTT ≥ 7.8 mmol/L and < 11.1 mmol/L) |
|
| Interventions |
Number of study centres: multicentre, but number of centres not reported Run‐in period: none Administration‐free period before testing during trial: not reported Extension period: no |
|
| Outcomes | Composite outcome measures reported: no | |
| Study details |
Trial terminated before regular end (for benefit/because of adverse events): no Taking trial drug on glycaemic testing days: not specified |
|
| Publication details |
Language of publication: English Funding: non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "To observe the rate of conversion from impaired glucose tolerance (IGT) to diabetes following lifestyle modification (LSM) or a combination of lifestyle and metformin compared to a control population with 18‐month followup" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "After taking informed consent, the participants were randomized by age strata (31–40 years, 41–50 years, 51–60 years, and >60 years) into three different arms" Comment: insufficient information about the sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of allocation concealment |
| Blinding of participants and personnel (performance bias) all‐cause mortality/cardiovascular mortality | Low risk | Comment: no blinding, but the outcome is not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) incidence of T2DM | Low risk | Comment: no blinding, but the outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) all‐cause mortality/cardiovascular mortality | Low risk | Comment: no blinding of outcome assessment, but the outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) incidence of T2DM | Low risk | Comment: no blinding of outcome assessment, but the outcome is not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) all‐cause mortality/cardiovascular mortality | Unclear risk |
Quote from publication: "Overall 44 subjects dropped out or were lost to followup. In the control group there were 2 deaths while 24 subjects dropped out during the study. In the lifestyle modification group 8 subjects refused to continue the study and dropped out. In the LSM + drug group 5 subjects stopped taking the drug either due to side effects of the drug such as gastrointestinal problems or complaining of weakness probably due to hypoglycemia while 5 subjects refused to follow due to personal reasons and were lost to followup." Comment: unknown whether mortality status was investigated in the people lost to follow‐up |
| Incomplete outcome data (attrition bias) incidence of T2DM | Unclear risk |
Quote from publication: "Overall 44 subjects dropped out or were lost to followup. In the control group there were 2 deaths while 24 subjects dropped out during the study. In the lifestyle modification group 8 subjects refused to continue the study and dropped out. In the LSM + drug group 5 subjects stopped taking the drug either due to side effects of the drug such as gastrointestinal problems or complaining of weakness probably due to hypoglycemia while 5 subjects refused to follow due to personal reasons and were lost to followup." Comment: large difference in missingness among the intervention groups. No description of how to handle missing data. |
| Selective reporting (reporting bias) | High risk | No trial protocol available. Glycaemic measures not reported, hypoglycaemia and adverse events reported in a format that make them unsuitable for meta‐analysis. |
| Other bias | Low risk | No other sources of bias identified |
Ji 2011.
| Methods | Parallel randomised controlled trial, randomisation ratio 1:1:1 | |
| Participants |
Inclusion criteria: no history of diabetes or autoimmune disease, and no acute or chronic infection within 2 weeks before enrolment, IFG and/or IGT. Exclusion criteria: not reported Diagnostic criteria: WHO 1999 (IGT (2hPG between 7.8 mmol/L and 11.0 mmol/L); or IFG (FPG between 6.1 mmoL/L and 6.9 mmol/L)) |
|
| Interventions |
Number of study centres: 1 Run‐in period: not reported Administration‐free period before testing during trial: not specified if any study drug was taken on the testing day at the end of intervention Extension period: none |
|
| Outcomes | Composite outcome measures reported: none | |
| Study details | Trial terminated early: no | |
| Publication details |
Language of publication: Chinese Funding: non‐commercial funding (governmental funding) Publication status: peer‐reviewed journal, full‐article |
|
| Stated aim of study | Quote from publication: "To observe the changes of serum, hs‐crp and insulin sensitivity index before and after metformin treatment or intensive lifestyle intervention in patients with prediabetes." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "randomised" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of allocation concealment |
| Blinding of participants and personnel (performance bias) incidence of T2DM | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding. (investigator‐assessed outcome measurement) |
| Blinding of participants and personnel (performance bias) measures of blood glucose control | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding. (investigator‐assessed outcome measurement) |
| Blinding of outcome assessment (detection bias) incidence of T2DM | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding. (investigator‐assessed outcome measurement) |
| Blinding of outcome assessment (detection bias) measures of blood glucose control | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding. (investigator‐assessed outcome measurement) |
| Incomplete outcome data (attrition bias) incidence of T2DM | Low risk |
Quote from publication: "No patients were discontinued due to adverse drug reactions." Comment: reported (no missing data) |
| Incomplete outcome data (attrition bias) measures of blood glucose control | Low risk |
Quote from publication: "No patients were discontinued due to adverse drug reactions." Comment: reported (no missing data) |
| Selective reporting (reporting bias) | High risk | Comment: protocol unavailable. Adverse events not reported. Likely to have been assessed and evaluated during the study |
| Other bias | Low risk | Comment: no other risk of bias identified |
Jin 2009.
| Methods | Parallel randomised controlled trial, randomisation ratio 1:1:1 | |
| Participants |
Inclusion criteria: IFG Exclusion criteria: severe cardiovascular and cerebrovascular diseases and obvious abnormal liver and kidney functions Diagnostic criteria: IFG diagnosed from WHO 1999 criteria (FPG between 6.1 mmol/L to 6.9 mmol /L, 2‐hour postprandial blood glucose (2hPG) < 7.8 mmol/L) |
|
| Interventions |
Number of study centres: 1 Run‐in period: not reported Administration‐free period before testing during trial: not specified if any study drug was taken on the testing day at the end of intervention Extension period: none |
|
| Outcomes | Composite outcomes measures reported: none | |
| Study details | Trial terminated early (for benefit/because of adverse events): no | |
| Publication details |
Language of publication: Chinese Funding: non‐commercial (government funding) Publication status: peer‐reviewed, full‐article |
|
| Stated aim of study | Quote from publication: "To observe the changes of islet cell function and insulin resistance (IR) in patients with impaired fasting glucose after different methods of intervention, and to explore the pathogenesis and intervention pathway of IFG." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "randomised" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of allocation concealment |
| Blinding of participants and personnel (performance bias) incidence of T2DM | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding (investigator‐assessed outcome measurement) |
| Blinding of participants and personnel (performance bias) measures of blood glucose control | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding (investigator‐assessed outcome measurement) |
| Blinding of outcome assessment (detection bias) incidence of T2DM | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding (investigator‐assessed outcome measurement) |
| Blinding of outcome assessment (detection bias) measures of blood glucose control | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding (investigator‐assessed outcome measurement) |
| Incomplete outcome data (attrition bias) incidence of T2DM | High risk |
Quote from publication: "During the treatment, 1 patient in the rosiglitazone group had facial edema and 2 patients in the rosiglitazone group had intolerance of both lower limbs and withdrew from the study. Three patients in the metformin group were withdrawn from the study due to severe gastrointestinal reactions. No significant adverse reactions or hypoglycemia were observed in other patients." Comment: PP analysis was used (reported and reasons explained) |
| Incomplete outcome data (attrition bias) measures of blood glucose control | High risk |
Quote from publication: "During the treatment, 1 patient in the rosiglitazone group had facial edema and 2 patients in the rosiglitazone group had intolerance of both lower limbs and withdrew from the study. Three patients in the metformin group were withdrawn from the study due to severe gastrointestinal reactions. No significant adverse reactions or hypoglycemia were observed in other patients." Comment: PP analysis was used (reported and reasons explained) |
| Selective reporting (reporting bias) | Unclear risk | Comment: protocol unavailable |
| Other bias | Low risk | Comment: no other risk of bias identified |
Li 1999.
| Methods | Parallel randomised controlled trial, randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: participants aged 30 years to 60 years with IGT Exclusion criteria: diabetes, a history of ischaemic heart disease or renal or hepatic disorders, and previous treatment with metformin Diagnostic criteria: WHO 1985 (IGT (2hPG 140 mg/dL to 200 mg/dL [7.8 mmol/L to 11.0 mmol/L]) and FPG <140 mg/dL (7.8 mmol/L)) |
|
| Interventions |
Number of study centres: ‐ Run‐in period: not described Administration‐free period before testing during trial: not reported Extension period: no |
|
| Outcomes | Composite outcome measures reported: none | |
| Study details |
Trial terminated early: no Taking trial drug on glycaemic testing days: not specified |
|
| Publication details |
Language of publication: English Funding: not described Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "To evaluate the effect of metformin on glucose metabolism, insulin sensitivity and rate of conversion diabetes in people with impaired glucose tolerance (IGT)." | |
| Notes | The placebo was provided by the manufacturer of metformin, Beijing Tian‐An United Pharmaceutical Co. Ltd. (Tokyo, Japan) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "People meeting the entry criteria were randomized under double‐blind conditions to receive either placebo or metformin at a dosage of 250 mg three times daily for a duration of 12 months." Comment: insufficient information about the sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of allocation concealment |
| Blinding of participants and personnel (performance bias) incidence of T2DM | Low risk |
Quote from publication: "People meeting the entry criteria were randomized under double‐blind conditions to receive either placebo or metformin at a dosage of 250 mg three times daily for a duration of 12 months." Comment: blinding of participants and key study personnel ensured |
| Blinding of participants and personnel (performance bias) measures of blood glucose control | Low risk |
Quote from publication: "People meeting the entry criteria were randomized under double‐blind conditions to receive either placebo or metformin at a dosage of 250 mg three times daily for a duration of 12 months." Comment: blinding of participants and key study personnel ensured |
| Blinding of outcome assessment (detection bias) incidence of T2DM | Low risk |
Quote from publication: "People meeting the entry criteria were randomized under double‐blind conditions to receive either placebo or metformin at a dosage of 250 mg three times daily for a duration of 12 months." Comment: possible blinding of outcome assessment, but the outcome is not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) measures of blood glucose control | Low risk |
Quote from publication: "People meeting the entry criteria were randomized under double‐blind conditions to receive either placebo or metformin at a dosage of 250 mg three times daily for a duration of 12 months." Comment: possible blinding of outcome assessment, but the outcome is not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) incidence of T2DM | Low risk |
Quote from publication: "On an intention‐to‐treat basis, excluding only five patients lost to follow‐up, 32 of the metformin treated subjects became normally glucose tolerant (76.2%) compared to 23 (53.5%) for placebo patients. Six patients on placebo converted to frank diabetes (14.0%) and this compared to three patients (7.1%) on metformin, P = 0.091, Table 3." Comment: the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate |
| Incomplete outcome data (attrition bias) measures of blood glucose control | Unclear risk |
Quote from publication: "Twelve subjects were excluded from the metformin group for the following reasons: tablet noncompliance, seven; loss to follow‐up, three; and gastrointestinal side‐effects, two. Eight subjects were excluded from the placebo group as follows: tablet noncompliance, five; loss to follow‐up, two;.and raised liver enzymes, one." Comment: insufficient information to assess whether missing data in combination with the method used to handle missing data were likely to induce bias |
| Selective reporting (reporting bias) | High risk | Comment: no trial protocol available. Likely that hypoglycaemia is measured but not reported. |
| Other bias | Unclear risk | Comment: unclear funding source |
Li 2009.
| Methods | Parallel randomised controlled trial, randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: obesity (BMI of 25 kg/m2 or more) and impaired regulation of glucose Exclusion criteria: not reported Diagnostic criteria: WHO 1999 (FPG between 6.1 mmol/L to 6.9 mmol/L, 2‐hour glucose (2hPG) between 7.8 mmol/Lto 11.0 mmol/L) |
|
| Interventions |
Number of study centres: 1 Run‐in period: not reported Administration‐free period before testing during trial: not specified if any study drug was taken on the testing day at the end of intervention Extension period: none |
|
| Outcomes | Composite outcome measures reported: none | |
| Study details | Trial terminated early (for benefit/because of adverse events): no | |
| Publication details |
Language of publication: Chinese Funding: not reported Publication status: peer‐reviewed journal, full article |
|
| Stated aim of study | Quote from publication: "In this study, metformin was used to treat obese people with impaired regulation of mixed sugars, so as to explore the methods of diabetes intervention." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "randomised" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of allocation concealment |
| Blinding of participants and personnel (performance bias) incidence of T2DM | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding (investigator‐assessed outcome measurement) |
| Blinding of participants and personnel (performance bias) measures of blood glucose control | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding (investigator‐assessed outcome measurement) |
| Blinding of outcome assessment (detection bias) incidence of T2DM | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding (investigator‐assessed outcome measurement) |
| Blinding of outcome assessment (detection bias) measures of blood glucose control | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding (investigator‐assessed outcome measurement) |
| Incomplete outcome data (attrition bias) incidence of T2DM | High risk | Comment: a total of 7 participants (3:4) lost to follow‐up, no explanation provided, PP analysis was used (only reported) |
| Incomplete outcome data (attrition bias) measures of blood glucose control | Low risk | Comment: a total of 7 participants (3:4) lost to follow‐up, no explanation provided, ITT analysis was used (only reported) |
| Selective reporting (reporting bias) | Unclear risk | Comment: protocol unavailable |
| Other bias | Unclear risk | Comment: unknown funding source |
Liao 2012.
| Methods | Parallel randomised controlled trial, randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: IGT. All participants were not treated with any glucose‐lowering drugs before inclusion, and were treated with simple diet and exercise for 3 months, with unsatisfactory results and no obvious adverse reactions. All participants had no serious diseases of gastrointestinal tract, heart, liver, kidney and other important organs Exclusion criteria: blood glucose abnormalities caused by other diseases Diagnostic criteria: WHO 1999 (FPG between 6.1 mmol/L to 6.9 mmol/L, 2hPG between 7.8 mmol/L to 11.0 mmol/L) |
|
| Interventions |
Number of study centres: 1 Run‐in period: 3 months Administration‐free period before testing during trial: not specified if any study drug was taken on the testing day at the end of intervention Extension period: none |
|
| Outcomes | Composite outcome measures reported: none | |
| Study details | Trial terminated early (for benefit/because of adverse events): no | |
| Publication details |
Language of publication: Chinese Funding: not reported Publication status: peer‐reviewed journal, full‐article |
|
| Stated aim of study | Quote from publication: "To compare the effectiveness and security of acarbose and metformin in the treatment for impaired glucose tolerance" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "randomised" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of allocation concealment |
| Blinding of participants and personnel (performance bias) incidence of T2DM | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding. (investigator‐assessed outcome measurement) |
| Blinding of participants and personnel (performance bias) measures of blood glucose control | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding. (investigator‐assessed outcome measurement) |
| Blinding of outcome assessment (detection bias) incidence of T2DM | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding. (investigator‐assessed outcome measurement) |
| Blinding of outcome assessment (detection bias) measures of blood glucose control | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding. (investigator‐assessed outcome measurement) |
| Incomplete outcome data (attrition bias) incidence of T2DM | High risk |
Quote from publication: "In the acarbose group, 2 cases were lost after 1 year of follow‐up, 1 case of lung cancer and 1 case of hepatitis. In the metformin group, 1 case of cerebral hemorrhage was lost after 1 year of follow‐up." Comment: PP analysis was used (reported and reasons explained) |
| Incomplete outcome data (attrition bias) measures of blood glucose control | High risk |
Quote from publication: "In the acarbose group, 2 cases were lost after 1 year of follow‐up, 1 case of lung cancer and 1 case of hepatitis. In the metformin group, 1 case of cerebral hemorrhage was lost after 1 year of follow‐up." Comment: PP analysis was used (reported and reasons explained) |
| Selective reporting (reporting bias) | High risk | Comment: protocol unavailable. Outcomes that were not mentioned in the method section were reported in the result section (e.g. adverse events) |
| Other bias | Unclear risk | Comment: funding source not described |
Lu 2002.
| Methods | Parallel randomised controlled trial, randomisation ratio 1:1:1:1 | |
| Participants |
Inclusion criteria: IGT Exclusion criteria: not reported Diagnostic criteria: WHO 1985 (IGT 2hPG of 140 mg/dL to 199 mg/dL (7.8 mmol/L to 11.0 mmol/L)) |
|
| Interventions |
Number of study centres: not reported (12 authors from 5 departments in 2 hospitals) Run‐in period: not reported Administration‐free period before testing during trial: did not take study‐drug on the morning of the OGTT retest Extension period: none |
|
| Outcomes | Composite outcome measures reported: none | |
| Study details | Trial terminated early (for benefit/because of adverse events): no | |
| Publication details |
Language of publication: Chinese Funding: military funding Publication status: peer‐reviewed journal, full‐article |
|
| Stated aim of study | Quote from publication: "To evaluate the efficacy of metformin and diet fibre intervention in preventing the conversion of impaired glucose tolerance (IGT) to type 2 diabetes mellitus." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "randomised" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of allocation concealment |
| Blinding of participants and personnel (performance bias) all‐cause mortality/cardiovascular mortality | Low risk | Comment: no blinding reported, however, mortality is unlikely to be influenced by lack of blinding. (adjudicated outcome measurement) |
| Blinding of participants and personnel (performance bias) incidence of T2DM | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding. (investigator‐assessed outcome measurement) |
| Blinding of participants and personnel (performance bias) measures of blood glucose control | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding. (investigator‐assessed outcome measurement) |
| Blinding of outcome assessment (detection bias) all‐cause mortality/cardiovascular mortality | Low risk | Comment: no blinding reported, however, mortality is unlikely to be influenced by lack of blinding. (adjudicated outcome measurement) |
| Blinding of outcome assessment (detection bias) incidence of T2DM | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding. (investigator‐assessed outcome measurement) |
| Blinding of outcome assessment (detection bias) measures of blood glucose control | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding. (investigator‐assessed outcome measurement) |
| Incomplete outcome data (attrition bias) incidence of T2DM | High risk |
Quote from publication: "A total of 23 patients withdrew, and the withdrawal rate was 7.8%. Among the 72 cases in the education group, 7 cases quit without definite reason, and 1 case died of cerebral thrombosis complicated with pulmonary infection. Six cases in the education + diet instruction group were lost to follow‐up. In the dietary fiber group, 3 patients lost follow‐up and 1 patient withdrew from gastric cancer. In the metformin group, 3 patients were lost to follow‐up, and 2 patients withdrew due to going abroad." Comment: reason acceptable, but PP analysis was used (reported and reasons explained) |
| Incomplete outcome data (attrition bias) measures of blood glucose control | High risk |
Quote from publication: "A total of 23 patients withdrew, and the withdrawal rate was 7.8%. Among the 72 cases in the education group, 7 cases quit without definite reason, and 1 case died of cerebral thrombosis complicated with pulmonary infection. Six cases in the education + diet instruction group were lost to follow‐up. In the dietary fiber group, 3 patients lost follow‐up and 1 patient withdrew from gastric cancer. In the metformin group, 3 patients were lost to follow‐up, and 2 patients withdrew due to going abroad." Comment: reason acceptable, but PP analysis was used (reported and reasons explained) |
| Selective reporting (reporting bias) | High risk | Comment: protocol unavailable. Outcomes not described in the method section were reported in the results (adverse events) |
| Other bias | Low risk | Comment: no other risk of bias identified |
Lu 2010.
| Methods | Parallel randomised controlled trial, randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: (1) participants with pre‐diabetes; (2) 25 to 80 years old; (3) twice increased fasting blood glucose (fasting blood glucose 5.6 mmol/L to 6.9 mmol/L); (4) postprandial blood glucose was increased (OGTT 2‐hour blood glucose 7.8 mmol/L to 11.1 mmol/L). Exclusion criteria: (1) participants with cardiovascular diseases, hepatitis, kidney diseases and other basic diseases that may increase the risk of intervention; (2) participants who may affect the process of the experiment: inability to follow up, refusal of random grouping, pregnancy and lactation, etc., (3) participants were taking drugs that could interfere with the test results, such as diuretics, beta‐blockers 13 and glucocorticoids. Diagnostic criteria: ADA 2009 (fasting blood glucose 5.6 mmol/L to 6.9 mmol/L or 2‐hour blood glucose 7.8 mmol/L to 11.1 mmol/L). |
|
| Interventions |
Number of study centres: 1 Run‐in period: not reported Administration‐free period before testing during trial: not specified if any study drug was taken on the testing day at the end of intervention Extension period: none |
|
| Outcomes | Composite outcome measures reported: none | |
| Study details |
Trial identifier: unregistered Trial terminated early (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: Chinese Funding: not reported Publication status: peer‐reviewed journal, full article |
|
| Stated aim of study | Quote from publication: "This study is a clinical demonstration study on lifestyle adjustment and metformin intervention, two commonly used measures to prevent or delay diabetes. Through the comparison and analysis of the blood glucose index changes after the implementation, the compliance of the two kinds of intervention measures, weight changes, the incidence of adverse events and other indicators, the efficacy and safety were reasonably evaluated, and the most reasonable, effective and practical measures for preventing or delaying diabetes were finally determined." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "randomised" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of allocation concealment |
| Blinding of participants and personnel (performance bias) incidence of T2DM | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding. (investigator‐assessed outcome measurement) |
| Blinding of participants and personnel (performance bias) measures of blood glucose control | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding. (investigator‐assessed outcome measurement) |
| Blinding of outcome assessment (detection bias) incidence of T2DM | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding. (investigator‐assessed outcome measurement) |
| Blinding of outcome assessment (detection bias) measures of blood glucose control | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding. (investigator‐assessed outcome measurement) |
| Incomplete outcome data (attrition bias) incidence of T2DM | Low risk |
Quote from publication: "A total of 100 patients in the lifestyle intervention group completed the study, 17 patients withdrew from the study (2 patients went abroad, 15 patients for personal reasons), and the baseline characteristics of those who did not complete the study were the same as those who completed the study. 111 patients (6 without follow‐up) entered the primary and secondary endpoint analysis. In the metformin group, 21 patients withdrew from the study (6 with gastrointestinal reactions, lO with personal reasons, 5 with other reasons), and 115 patients entered the primary and secondary endpoint analysis (2 without follow‐up)."; "last observation carried forward (LOCF) was used"; "Since our study included observational studies of compliance, our analysis showed that the LOCF did not affect the interpretation of the results of this study." Comment: reported, acceptable reason, and appropriate imputation of data (reported and reasons explained) |
| Incomplete outcome data (attrition bias) measures of blood glucose control | Low risk |
Quote from publication: "A total of 100 patients in the lifestyle intervention group completed the study, 17 patients withdrew from the study (2 patients went abroad, 15 patients for personal reasons), and the baseline characteristics of those who did not complete the study were the same as those who completed the study. 111 patients (6 without follow‐up) entered the primary and secondary endpoint analysis. In the metformin group, 21 patients withdrew from the study (6 with gastrointestinal reactions, lO with personal reasons, 5 with other reasons), and 115 patients entered the primary and secondary endpoint analysis (2 without follow‐up)."; "last observation carried forward (LOCF) was used"; "Since our study included observational studies of compliance, our analysis showed that the LOCF did not affect the interpretation of the results of this study." Comment: reported, acceptable reason, and appropriate imputation of data (reported and reasons explained) |
| Selective reporting (reporting bias) | Unclear risk | Comment: protocol unavailable |
| Other bias | Unclear risk | Comment: unknown funding source |
Maji 2005.
| Methods | Parallel randomised controlled trial, randomisation ratio 1:1:1:1 | |
| Participants |
Inclusion criteria: IGT Exclusion criteria: NR Diagnostic criteria: IGT (2hPG 110 to 200 mg/dL [6.1 mmol/L to 11.0 mmol/L]) and FPG <1 10mg/dL (6.1 mmol/L)) |
|
| Interventions |
Number of study centres: NR Run‐in period: participants with IGT were selected and given diet and lifestyle advice for three months. The participants who still had IGT were thereafter randomised Administration‐free period before testing during trial: not reported Extension period: no |
|
| Outcomes | Composite outcome measures reported: no | |
| Study details |
Trial terminated before regular end (for benefit/because of adverse events): no Taking trial drug on glycaemic testing days: NR |
|
| Publication details |
Language of publication: English Funding: NR Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "The present study of diabetes prevention programme has been started in 2001 at Ramakrishna Mission Seva Pratishthan to assess the nature and extent if interventional therapies regarding prevention of type 2 diabetes" | |
| Notes | 2‐hour OGTT; HbA1c; FPG were reported as per cent change from baseline; not possible to include data in the meta‐analysis. There were no significant change in the percent reduction of glycaemic parameters in between the three groups receiving a pharmacological intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "Those who still had their blood sugar at the IGT range were randomised into 3 groups to receive either metformin or rosiglitazone or acarbose." Comment: insufficient information about the sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of allocation concealment |
| Blinding of participants and personnel (performance bias) incidence of T2DM | Low risk |
Quote from publication: "..no person in the study group developed diabetes during this period of three years, .."" Comment: no blinding, the outcome is not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) measures of blood glucose control | Low risk | Comment: no blinding, the outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) incidence of T2DM | Low risk |
Quote from publication: "..no person in the study group developed diabetes during this period of three years, .."" Comment: no blinding, the outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) measures of blood glucose control | Low risk | Comment: no blinding, the outcome is not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) incidence of T2DM | Unclear risk | Comment: not mentioned in the publication how missing data were handled |
| Incomplete outcome data (attrition bias) measures of blood glucose control | Unclear risk | Comment: not mentioned in the publication how missing data were handled or how many included in the analyses |
| Selective reporting (reporting bias) | High risk | No trial protocol available. Glycaemic parameters reported in a format that made them unsuitable for meta‐analysis. Data on hypoglycaemia and adverse events were not reported |
| Other bias | Unclear risk | No funding source reported |
Papoz 1978.
| Methods | Parallel randomised controlled clinical trial, randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: male, 25 to 55 years, 'borderline' diabetes (see criteria in the section 'diagnostic criteria') Exclusion criteria: NR Diagnostic criteria: fasting blood glucose ≥ 5.6 mmol/L and < 7.2 mmol/L or 2‐hour blood glucose after a 75 g oral glucose challenge ≥ 6.7 mmol/L and < 8.3 mmol/L; when these criteria for intermediate hyperglycaemia were fulfilled, a second test was performed: blood glucose concentrations were determined fasting at 15, 30, 60, 120, 80, 240 and 300 minutes after an oral glucose load. Eligible individuals had 2‐hour blood glucose concentrations ≥ 6.7 mmol/L but < 8.3 mmol/L or fasting blood glucose concentrations ≥ 5.6 mmol/L and < 7.2 mmol/L; blood glucose after 30 minutes ≥ 8.9 mmol/L and < 12.2 mmol/L; blood glucose after 60 minutes ≥ 8.9 mmol/L and < 12.2 mmol/L (the European Diabetes Epidemiology Study Group 1970 criteria) |
|
| Interventions |
Number of study centres: 1 Run‐in period: none Administration‐free period before testing during trial: participants received study‐drug on the day of testing blood glucose at 2 months and 14 months. However, the last glycaemic measurements were performed 15 days after the study drug was stopped Extension period: none |
|
| Outcomes | Composite outcome measures reported: no | |
| Study details | Trial terminated early: no | |
| Publication details |
Language of publication: English Funding: non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "A double blind controlled clinical trial was undertaken to test the effectiveness of oral hypoglycaemic drugs in improving blood glucose and plasma insulin levels of borderline diabetic patients" | |
| Notes | Blood glucose values in this trial were reported as whole blood glucose. In the tables and result section all values are converted to plasma glucose values (diabetes.co.uk) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "They were randomized into 4 groups according..." Comment: method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of allocation concealment |
| Blinding of participants and personnel (performance bias) measures of blood glucose control | Low risk |
Quote from publication: "A double blind controlled clinical trial was undertaken..." Comment: investigator‐assessed, double‐blinding |
| Blinding of outcome assessment (detection bias) measures of blood glucose control | Low risk |
Quote from publication: "A double blind controlled clinical trial was undertaken..." Comment: investigator‐assessed, double‐blinding |
| Incomplete outcome data (attrition bias) measures of blood glucose control | Unclear risk |
Quote from publication: "Thirty four patients (24 during the first year, 10 during the second year of the study) were lost to follow‐up; they came equally from the four different treatment groups and exhibited similar baseline characteristics to the follow‐up patients. Their removal from the trial did not introduce any bias into the study" Comment: the number of participants lost to follow‐up are reported, but no reasons explained |
| Selective reporting (reporting bias) | High risk | Comment: likely that adverse events have been evaluated, but not reported (see Appendix 8) |
| Other bias | Low risk | Comment: the trial appeared to be free of other sources of bias |
PREVENT‐DM 2017.
| Methods | Parallel randomised controlled trial, randomisation ratio 1:1:1 | |
| Participants |
Inclusion criteria: Latinas aged 20 years or more, IFG (FPG of 100 to 125 mg/dL) and/or elevated HbA1c of 5.7% to 6.4% (39 mmol/mol to 46 mmol/mol), BMI at 23 kg/m2 or more Exclusion criteria: diabetes at baseline, were currently pregnant or planned to become pregnant, or were participating in a supervised weight loss program. Blood pressure at or above 160 mmHg/100 mmHg, contraindication to metformin, chronic conditions that could affect a participant’s ability to participate (e.g. severe osteoarthritis), medical co morbidities that could influence body weight (e.g. uncontrolled thyroid disease), or medications that could affect weight or glucose metabolism (e.g., oral corticosteroids). Diagnostic criteria: IFG (FPG of 100 mg/dL to 125 mg/dL (5.6 mmol/L to 6.9 mmol/L) and/or intermediate elevated HbA1c of 5.7% to 6.4% (39 mmol/mol to 46 mmol/mol)) |
|
| Interventions |
Number of study centres: one Run‐in period: none Administration‐free period before testing during trial: not reported Extension period: no |
|
| Outcomes | Composite outcome measures reported: no | |
| Study details | Trial terminated before regular end (for benefit/because of adverse events): no | |
| Publication details |
Language of publication: English Funding: non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "This study was designed to compare the real‐world effectiveness of ILI, metformin, and standard care among Hispanic women (Latinas) with prediabetes" | |
| Notes | Quote from publication: "Though all participants had prediabetes by HbA1c or fasting plasma glucose criteria, more participants qualified for the study based on elevated HbA1c alone (n=53, 57.6% of total participants). Among the remaining 39 participants, 12 (13.0%) qualified by having impaired fasting glucose alone, and 27 (29.3%) met both glycemic criteria for prediabetes" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "The random allocation sequence was generated independently by a statistician and concealed in individually sealed envelopes accessible only to the research coordinator, who ultimately assigned participants to the study interventions." Comment: adequate description of the sequence generation |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "The assignment for each randomized group was concealed in individually‐sealed, opaque envelopes kept in a locked filing cabinet accessible only to the research coordinator, who ultimately assigned participants to the study interventions." Comment: adequate description of the allocation concealment |
| Blinding of participants and personnel (performance bias) all‐cause mortality/cardiovascular mortality | Low risk |
Quote from publication: "The nature of the study interventions precludes blinding participants, promotoras, or the research coordinator to treatment assignments." Comment: no blinding, the outcome is not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) incidence of T2DM | Low risk |
Quote from publication: "The nature of the study interventions precludes blinding participants, promotoras, or the research coordinator to treatment assignments." Comment: no blinding, the outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) all‐cause mortality/cardiovascular mortality | Low risk |
Quote from publication: "The nature of the study interventions precludes blinding participants, promotoras, or the research coordinator to treatment assignments." Comment: no blinding, the outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) incidence of T2DM | Low risk |
Quote from publication: "The nature of the study interventions precludes blinding participants, promotoras, or the research coordinator to treatment assignments." Comment: no blinding, the outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) measures of blood glucose control | Low risk | |
| Incomplete outcome data (attrition bias) all‐cause mortality/cardiovascular mortality | Low risk |
Quote from publication: "Data will be analyzed assuming an intent‐to‐treat approach where all randomized subjects are analyzed according to their treatment assignment, regardless of adherence." Comment: the number of participants who were lost to follow‐up or excluded due to pregnancy were low (2 in metformin and standard care; three in intensive diet plus exercise group |
| Incomplete outcome data (attrition bias) incidence of T2DM | Low risk |
Quote from publication: "Data will be analyzed assuming an intent‐to‐treat approach where all randomized subjects are analyzed according to their treatment assignment, regardless of adherence." Comment: the numbers of participants who were lost to follow‐up or excluded due to pregnancy were low (2 in metformin and standard care; three in intensive diet plus exercise group |
| Selective reporting (reporting bias) | High risk | Likely hypoglycaemia was evaluated, but no data provided |
| Other bias | Low risk | No other sources of bias identified |
Wang 2009.
| Methods | Parallel randomised controlled trial, randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: IGT and/or IFG Exclusion criteria: hyperthyroidism, hypercortisolism and acromegaly; blood glucose abnormalities caused by pancreatic exocrine gland dysfunction and liver function damage Diagnostic criteria: ADA 1997 (FPG 5.6 mmol/L to 6.9 mmol/L, and/or 2hPG 7.8 mmol/L to 11.0 mmol/L) |
|
| Interventions |
Number of study centres: 1 Run‐in period: not reported Administration‐free period before testing during trial: not specified if any study drug was taken on the testing day at the end of intervention Extension period: none |
|
| Outcomes | Composite outcome measures reported: none | |
| Study details | Trial terminated early (for benefit/because of adverse events): no | |
| Publication details |
Language of publication: Chinese Funding: not reported Publication status: conference proceedings |
|
| Stated aim of study | Quote from publication: "To observe the effect of metformin on 30 patients with impaired glucose regulation (IGR), and to explore the intervention method in the prediabetes stage." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "randomised" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of allocation concealment |
| Blinding of participants and personnel (performance bias) incidence of T2DM | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding (investigator‐assessed outcome measurement) |
| Blinding of participants and personnel (performance bias) measures of blood glucose control | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding (investigator‐assessed outcome measurement) |
| Blinding of outcome assessment (detection bias) incidence of T2DM | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding (investigator‐assessed outcome measurement) |
| Blinding of outcome assessment (detection bias) measures of blood glucose control | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding (investigator‐assessed outcome measurement) |
| Incomplete outcome data (attrition bias) incidence of T2DM | High risk |
Quote from publication: "In the treatment group, 2 patients showed gastrointestinal reactions after taking the medicine, and the discomfort symptoms disappeared after stopping the medicine, which was considered as adverse drug reactions, so they were withdrawn from the study." Comment: PP analysis was used (2/30 = 6.7%); however, if the two who were absent from analysis had developed T2DM the incidence would have been doubled (4/32 = 12.5%) |
| Incomplete outcome data (attrition bias) measures of blood glucose control | High risk |
Quote from publication: "In the treatment group, 2 patients showed gastrointestinal reactions after taking the medicine, and the discomfort symptoms disappeared after stopping the medicine, which was considered as adverse drug reactions, so they were withdrawn from the study." Comment: the author did not report the number of participants included in the FBG and 2hPG analyses, possibly PP analyses were applied |
| Selective reporting (reporting bias) | Unclear risk | Comment: protocol unavailable |
| Other bias | Unclear risk | Comment: unknown funding source |
Zeng 2013.
| Methods | Parallel randomised controlled trial, randomisation ratio 1:1:1 | |
| Participants |
Inclusion criteria: impaired fasting glucose with or without IGT Exclusion criteria: liver and kidney dysfunction; severe cardiovascular and cerebrovascular diseases Diagnostic criteria: WHO 1999 (IFG FPG 6.1 to 6.9 mmol/L; IGT 2hPG ≥7.8 mmol/L to <11.1 mmol/L) |
|
| Interventions |
Number of study centres: 1 Run‐in period: NR Administration‐free period before testing during trial: not specified if any study drug was taken on the testing day at the end of intervention Extension period: none |
|
| Outcomes | Composite outcome measures reported: none | |
| Study details | Trial terminated early (for benefit/because of adverse events): no | |
| Publication details |
Language of publication: Chinese Funding: not reported Publication status: peer‐reviewed journal, full‐article |
|
| Stated aim of study | Quote from publication: "To evaluate the clinical effects of different interventions on impaired glucose regulation." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "random number table" Comment: adequate description of the sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of allocation concealment |
| Blinding of participants and personnel (performance bias) incidence of T2DM | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding (investigator‐assessed outcome measurement) |
| Blinding of participants and personnel (performance bias) measures of blood glucose control | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding (investigator‐assessed outcome measurement) |
| Blinding of outcome assessment (detection bias) incidence of T2DM | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding (investigator‐assessed outcome measurement) |
| Blinding of outcome assessment (detection bias) measures of blood glucose control | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding (investigator‐assessed outcome measurement) |
| Incomplete outcome data (attrition bias) incidence of T2DM | Unclear risk | Comment: no exclusion reported, however, unclear whether re‐inclusion applied (not reported) |
| Incomplete outcome data (attrition bias) measures of blood glucose control | Unclear risk | Comment: no exclusion reported, however, unclear whether re‐inclusion applied (not reported) |
| Selective reporting (reporting bias) | High risk | Comment: protocol unavailable. Adverse events not reported‐ likely this outcome has been evaluated |
| Other bias | Unclear risk | Comment: unknown funding source |
Zhao 2013.
| Methods | Parallel randomised controlled trial, randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: IGT, IFG, BMI > 25 kg /m2, waist to hip ratio ≥ 0.9 for males and ≥ 0.85 for females Exclusion criteria: impaired liver and kidney function, severe heart and lung disease, infection, surgery and heavy alcohol consumption Diagnostic criteria: WHO 1999 (FPG between 5.6 mmol /L ˜ 6.9 mmol /L, 2hPG 7.8 mmol /L ˜ 11.0 mmol/L) |
|
| Interventions |
Number of study centres: 1 Run‐in period: not reported Administration‐free period before testing during trial: not specified if any study drug was taken on the testing day at the end of intervention Extension period: none |
|
| Outcomes | Composite outcome measures reported: none | |
| Study details | Trial terminated early (for benefit/because of adverse events): no | |
| Publication details |
Language of publication: Chinese Funding: not reported Publication status: peer‐reviewed journal, full‐article |
|
| Stated aim of study | Quote from publication: "We used metformin combined with lifestyle intervention to treat obese patients with pre‐diabetes." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "randomised" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of allocation concealment |
| Blinding of participants and personnel (performance bias) incidence of T2DM | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding. (investigator‐assessed outcome measurement) |
| Blinding of participants and personnel (performance bias) measures of blood glucose control | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding. (investigator‐assessed outcome measurement) |
| Blinding of outcome assessment (detection bias) incidence of T2DM | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding (investigator‐assessed outcome measurement) |
| Blinding of outcome assessment (detection bias) measures of blood glucose control | Low risk | Comment: no blinding reported, however, laboratory indexes are unlikely to be influenced by lack of blinding (investigator‐assessed outcome measurement) |
| Incomplete outcome data (attrition bias) incidence of T2DM | High risk |
Quote from publication: "In the treatment group, 1 patient withdrew due to gastrointestinal reaction (diarrhea); no case was lost to follow‐up in both groups." Comment: PP analysis was used (reported and reasons explained) |
| Incomplete outcome data (attrition bias) measures of blood glucose control | High risk |
Quote from publication: "In the treatment group, 1 patient withdrew due to gastrointestinal reaction (diarrhea); no case was lost to follow‐up in both groups." Comment: PP analysis was applied (reported and reasons explained) |
| Selective reporting (reporting bias) | High risk | Comment: protocol unavailable. Have only reported non‐serious adverse effects. It is likely that serious adverse effect have been collected as well, but not reported |
| Other bias | Unclear risk | Comment: funding source not reported |
2hPG: 2‐hour plasma glucose value after glucose tolerance test; ADA: American Diabetes Association; BMI: body mass index;DBP: diastolic blood pressure; FPG: fasting plasma glucose; HbA1c: glycosylated haemoglobin A1c;IGT: impaired glucose tolerance; IFG: impaired fasting glucose; ITT: intention to treat; NIDDM: non‐insulin‐dependent diabetes mellitus; NR: not reported; NYHA: New York Heart Association; OGTT: oral glucose tolerance test; PP: per protocol; RCT: randomised controlled trial; SAE: serious adverse events; SBP: systolic blood pressure; T2DM: type 2 diabetes mellitus; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Acbay 1996 | Duration of the intervention less than one year |
| Ballon 2011 | Duration of the intervention less than one year |
| Biarnés 2005 | Duration of the intervention less than one year |
| Bulcão 2007 | Duration of the intervention less than one year |
| Caballero 2004 | Duration of the intervention less than one year |
| Celik 2012 | Wrong population |
| Chazova 2006 | Duration of the intervention less than one year |
| Chen 2013 | Translated from Chinese: wrong intervention. Co‐intervention not identical. |
| ChiCTR‐TRC‐09000548 | Duration of intervention less than one year (information provided by author) |
| CTRI/2013/02/003417 | Study protocol for non‐randomised study |
| Eguchi 2007 | Duration of the intervention less than one year |
| Esteghamati 2013 | Duration of the intervention less than one year |
| EUCTR‐000650‐21‐ES | Not a RCT |
| EUCTR2008‐004497‐40‐GB | Not a RCT |
| Fleming 2002 | Wrong population |
| Flores‐Saenz 2003 | Duration of the intervention less than one year |
| Gore 2005 | Duration of the intervention less than one year |
| Gram 2011 | Wrong population |
| Guardado‐Mendoza R 2018 | Wrong intervention/comparator |
| Gómez‐Díaz 2012 | Duration of the intervention less than one year |
| Haukeland 2008 | Wrong population |
| Ishida 2005 | Not a RCT (translated from Japanese: narrative review explaining the history, mechanism and side effects of metformin) |
| Kato 2009 | Wrong population |
| Kelly 2012 | Duration of the intervention less than one year |
| Kendall 2013 | Duration of the intervention less than one year |
| Kilic 2011 | Duration of the intervention less than one year |
| Koev 2004 | Duration of the intervention less than one year |
| Krysiak 2012 | Duration of the intervention less than one year |
| Lehtovirta 2001 | Duration of the intervention less than one year |
| Li 2009b | Duration of the intervention less than one year |
| LIMIT‐1 | Duration of the intervention less than one year |
| Lu 2011 | Wrong intervention/comparator (not identical concomitant intervention) |
| Malin 2013 | Duration of the intervention less than one year |
| Medical letter | Not a RCT |
| Morel 1999 | Duration of the intervention less than one year |
| NCT00108615 | Duration of the intervention less than one year |
| NCT02338193 | Duration of the intervention less than one year |
| NCT03258723 | Wrong population |
| Pre‐DICTED | Wrong intervention/comparator |
| RESIST | Duration of the intervention less than one year |
| Retnakaran 2012 | Duration of the intervention less than one year |
| Rodríguez‐Moctezuma 2005 | Wrong population |
| Scheen 2009 | Wrong population |
| Schuster 2004 | Non‐prediabetic population |
| SLCTR/2016/026 | Duration of the intervention less than one year |
| STOP‐NIDDM | Wrong intervention (does not randomise to metformin) |
| Stroup 2013 | Duration of the intervention less than one year |
| Sultana 2012 | Duration of the intervention less than one year |
| UKPDS | Wrong population |
| Vitolins 2017 | Not a RCT |
| Wan 2010 | Duration of intervention less than one year |
| Zinman 2010 | Wrong intervention/comparator |
RCT: randomised clinical trial
Characteristics of studies awaiting assessment [ordered by study ID]
ChiCTR‐IPR‐17012309.
| Methods | Randomised, parallel, interventional study |
| Participants | Inclusion criteria: according to the classification standard of WHO glucose metabolism status (1999), IGR was diagnosed two weeks before randomisation; aged 35 to 60 years; no use of glucose‐lowering drugs (including herbal medicine for lowering blood glucose); women who are male or non‐pregnant, non‐lactating, and have no family planning for the next three years; BMI 24 kg/m2 to < 32 kg/m2 |
| Interventions | Metformin plus lifestyle intervention versus lifestyle intervention |
| Outcomes | Height, weight, blood pressure, fat, blood glucose, insulin, endothelial progenitor cells |
| Publicaton details | Trial register record:ChiCTR‐IPR‐17012309 |
| Notes | Not clarified if study can be included, duration of intervention? Author (Ping Yu) contacted for further information (06 Apil.2019). No reply. |
EDIT 1997.
| Methods | Randomised, parallel, interventional study |
| Participants | Participants 'at risk' for developing diabetes, fasting BG 5.5 to 7.7 mmol/L |
| Interventions | Metformin 500 mg three times daily plus placebo three times daily versus acarbose 50 mg three times daily plus placebo three times daily versus placebo three times daily plus placebo three times daily One of the intervention groups will not to be included in review (metformin 500 mg three times daily + acarbose 50 mg three times daily) |
| Outcomes | Incidence of T2DM, glycaemic variables |
| Publicaton details |
Trial register record:ISRCTN96631607 The study is only published as abstracts |
| Notes | Conlcusion of the trial in published abstract": "No differences were seen in relative risk for diabetes by 6 years with acarbose (1.04, P = 0.81), Metformin (0.99, P = 0.94) or combination therapy (1.02, P = 0.91). In those with IGT at baseline, relative risk was reduced significantly with acarbose (0.66, P = 0.046) but not Metformin (1.09, P = 0.70) or combination therapy (0.72, P = 0.27)." The reviewers of Van de Laar 2006 have already asked for supplemental information. |
NCT02409238.
| Methods | Randomised, parallel, interventional study |
| Participants | Prediabetes (if not diabetic):
IFG: ADA criteria: fasting plasma glucose level from 5.6 mmol/L (100 mg/dL) to 6.9 mmol/L (125 mg/dL), and/or IGT (WHO and ADA criteria: two‐hour glucose levels of 140 mg/dL to 199 mg/dL (7.8 mmol to 11.0 mmol) on the 75 g oral glucose tolerance test and/or HbA1C: 5.7% to 6.4% (ADA criteria) People with type 2 diabetes |
| Interventions | Metformin plus lifestyle interventions versus standard care |
| Outcomes | Primary efficacy endpoint: change in cerebral glucose metabolic rate Primary cognitive endpoint: change in composite z‐score of memory and multi‐domain non‐amnestic cognitive test performance using a neuropsychological assessment Secondary outcome measures: change in subjective memory and cognitive complaint, change in basic activities of daily living (ADL), change in cognitive instrumental ADL scale, change in global clinical dementia rating sum of boxes, change in mini‐mental state examination, change in the Montreal cognitive assessment scale, change in fasting plasma insulin, change in homeostatic model assessment, change in weight, change in BMI, change in waist circumference, change in FPG, change in HbA1c, change in fasting lipids |
| Publicaton details | Trial register record:NCT02409238 |
| Notes | The study includes prediabetic and diabetic people and data need to be separated for use in this review. Not clarified if study can be included. Authors (Wee Kien Han Andrew and Tan Kee Tung) contacted for information about if study are finished and published (30.03.2019). Answer: study neither finished nor published (04.04.2019). |
Polanco 2015.
| Methods | Randomised, open‐label clinical trial |
| Participants | People with prediabetes |
| Interventions | Metformin 850 mg twice daily plus lifestyle changes versus change in lifestyle |
| Outcomes | Quote: "The study was divided into two phases, with 2 intervention groups. In the first phase group 1 (52 patients) was treated with metformin 850 mg. 2 times a day, as well as changes in lifestyle and group 2 (50 subjects) only changes in lifestyle, were evaluated clinically and biochemically for a period of six years. In the second phase intervention was similar for all participants receiving combined treatment for 4 years, with an average follow‐up of 120 months (+/‐ 3.5). First phase: Group one, 75% of the subjects remained with PD; 21% developed T2DM and 3.8% showed normoglycemia with a 3.5% annual T2DM conversion. In group two, 62% remained with PD and 38% developed T2DM, with an annual incidence of 6.2%. Second phase: Group one, 57% had PD, 8 subjects developed T2DM (15.8%), with an overall incidence of 22 cases (42.3%), 4.2% cases per year. While in group two, 40% continued with PD and 22% were categorized as having T2DM, with an overall prevalence of 30 cases (60% of the population), with an annual rate of development of T2DM 6%. In the analysis of all subjects an incidence of 52 cases of T2DM (50.9%) was obtained, while the rest population remained with PD. The variables that were associated with the development of T2DM were fasting glucose levels and post challenge, HbA1c, insulin levels, HOMA IR, HOMA B, HOMA S and waist circumference (p <0.001). Early intervention with changes in lifestyle concomitant use of metformin prevents more effectively the development of T2DM in high risk subjects of Western Mexico" |
| Publicaton details | Only abstract available |
| Notes | Not possible to clarify if study meets inclusion criteria (definition of prediabetes? study published?). No contact information |
ADA: American Diabetes Association; ADL: activities of daily living; BG: blood glucose; BMI: body mass index; HbA1c: glycosylated haemoglobin A1c; IFG: impaired fasting glucose; IGR: impaired glucose regulation; IGT: impaired glucose tolerance; T2D: type 2 diabetes mellitus; WHO: World Health Organization.
Characteristics of ongoing studies [ordered by study ID]
CTRI/2017/09/009635.
| Trial name or title | A study of life style modification with and without metformin in prediabetic participants |
| Methods |
Type of trial: interventional Allocation: randomised Intervention model: parallel Masking: not reported Primary purpose: not reported |
| Participants |
Condition: IFG, IGT or HbA1c 5.7% to 6.4% Enrollment: 90 Inclusion criteria: BMI 18.5 to 29.9 kg/m2. Non diabetic individuals with either IFG (FPG > 100 mg/dL < 126 mg/dL (> 5.6 mmol/L < 7.0 mmol/L), IGT (2hPG > 140 < 200 mg/dL) (> 7.8 mmol/L < 11.1 mmol/L), HbA1c 5.7% to 6.4% Exclusion criteria: type 1 or type 2 diabetes (FPG > 126 mg/dL (7.0 mmol/L), 2hPG > 200 mg/dL (11.1 mmol/L) and HbA1c > 6.5%); contraindications to metformin (chronic kidney failure, hepatic dysfunction, renal impairment) and hypersensitivity; pregnant and lactating women |
| Interventions |
Intervention: metformin 250 mg twice daily plus life style modification Comparator: life style modification Duration of intervention: two years and six months |
| Outcomes |
Primary outcomes: conversion to normoglycaemia, IGT or IFG or intermediate elevated HbA1c, and T2DM Secondary outcomes: antioxidants Other outcomes: not reported |
| Starting date |
Study start date: 08/09/2016 Study completion date: not reported |
| Contact information | Contact: Dr Asha B, email: dr.ashareddy@gmail.com |
| Trial identifier | CTRI/2017/09/009635 |
| Notes |
ePRECIDE 2017.
| Trial name or title | Acronym: ePREDICE |
| Methods |
Type of trial: efficacy trial Allocation: randomised Intervention model: parallel assignment Masking: double‐blind Primary purpose: not specified in protocol |
| Participants |
Condition: IGT or IFG, or both Enrolment: 3000 Inclusion criteria: age 45 to 74 years; IFG (FPG 6.1‐6.9 mmol/L and 2hPG < 7.8 mmol/L) or IGT (FPG < 7.0 mmol/L and 2hPG ≥ 7.8 to < 11.1 mmol/L) or both conditions; informed consent given Exclusion criteria: T1DM; known or unknown T2DM (including screen‐detected T2DM) with or without pharmacological treatment; use of a GLP‐1 receptor agonist (exenatide or other) or pramlintide or any DPP‐4 inhibitor or metformin within the 3 months prior to enrolment; use of insulin or long‐acting insulin analogue within 3 months prior to enrolment; any previous cardiovascular or cerebrovascular clinically documented event or revascularisation procedure; clinical evidence of macrovascular complications (overt clinical cardiovascular disease) at enrolment, including angina (stable or unstable) and evidence of previous myocardial infarction in baseline electrocardiogram; current renal replacement therapy; previous diagnosis of liver cirrhosis or chronic hepatitis, or an elevation of liver enzymes (AST and or ALT) > 3 times normal ranges; previous diagnosis of chronic heart failure (NYHA class III or higher); prior solid organ transplant or awaiting solid organ transplant; malignant neoplasm requiring chemotherapy, surgery, radiation or palliative therapy in the previous 5 years. Participants with intraepithelial squamous cell carcinoma of the skin (Bowen's disease) treated with topical 5‐fluorouracil and people with basal cell skin cancer allowed to enter trial; any acute condition or exacerbation of chronic condition that would, in investigator's opinion, interfere with the initial trial visit schedule and procedures; known or suspected hypersensitivity to trial products or related products; known use of non‐prescribed narcotics or illicit drugs; simultaneous participation in any other clinical trial of an investigational agent; women of childbearing potential who are pregnant (all fertile women will be tested for before randomisation), breastfeeding or intend to become pregnant; presence of cataract that impedes the retinal evaluation of both eyes; other previously diagnosed retinal diseases; any diseases that would prevent the measurement of primary endpoints; dementia, mental disorder or evident cognitive impairment unable to give informed consent; end‐stage or metastatic cancer; institutionalisation; renal function impairment: GFR < 60 mL/minute/1.73 m².; contraindication to any of the study drugs (metformin or linagliptin). This includes: ALT > 3 times the upper limit of normal, history of cirrhosis or hepatitis, suspected renal artery stenosis, recent gastrointestinal bleeding (within last year), pregnant, breastfeeding or a female of childbearing potential not on reliable contraception and also any circumstance where ongoing medication might lead to potential adverse drug interaction with components of the trial medications; any other reason, medical condition, ongoing medication or significant disability that would prevent the participant complying with trial consent, treatment and follow‐up procedures or potentially jeopardise her/his medical care |
| Interventions |
Intervention: 2 tablets of linagliptin 5 mg + diet and physical activity Comparator (1): 2 tablets of metformin 850 mg/day + diet and physical activity Comparator (2): 2 tablets of linagliptin 2.5 mg + metformin 850 mg plus diet and physical activity Comparator (3): 2 tablets of placebo + diet and physical activity Duration of intervention: at least 3 years, and additional follow‐up to 5 years |
| Outcomes |
Primary outcome: a combined continuous variable, "the microvascular complication índex" (MCI), composed of linear combination of ETDRS score, the level of urinary albumin to creatinine ratio, and sudomotor test (SUDOSCAN) score, measured during the 36th and 60th month visits. From email correspondence: primary purpose: prevention of complications of hyperglycaemia/prevention of progression to diabetes Secondary outcomes: retinopathy score at last visit defined as 2‐steps' progression on ETDRS scale between baseline and visits at months 36 and 60; 1 SD increase in level of urinary albumin to creatinine ratio between baseline and visits at months 36 and 60; 1 SD decrease change in level of hands and feet conductance in SUDOSCAN between baseline and visits at months 36 and 60; change in microvascular endothelial function measured by EndoPAT method (in a subset); change in the Non‐Alcoholic Fatty Liver Index (in a subset); change in biomarkers of microvascular damage, endothelial function, per‐oxidation, inflammation and metabolomics (in a subset); change in the insulin secretion and β‐cell function; change in self‐perceived quality of life; change in symptoms of peripheral neuropathy; change in neuropsychological parameters: cognitive function, anxiety and depressive symptoms and indices; changes in obstructive sleep apnoea indices as measured by Somnomedics (in a subset); changes in ambulatory blood pressure monitoring (in a subset); change in the mean common carotid intimae‐media thickness (in a subset); incidence of major cardiovascular events, defined as an expanded composite of total coronary events, total stroke events, revascularisation procedures (coronary artery bypass graft, percutaneous coronary angioplasty and peripheral revascularisation), hospitalisation for heart failure, TIA and cardiovascular or cerebrovascular death. Secondary outcomes will be evaluated at 36 and 60 months Other outcome: none |
| Starting date |
Trial start date: 2015 Trial completion date: December 2019 |
| Contact information | Responsible party/principal investigator: Prof Jaakko Tuomilehto; Prof Rafael Gabriel (co‐principal investigators) |
| Trial identifier | NCT03222765; EUCTR2013‐000418‐39‐AT |
| Notes | Multinational trial with 15 clinical centres from 12 countries: Australia, Austria, Bulgaria, Germany, Greece, Italy, Lithuania, Poland, Serbia, Spain, Switzerland and Turkey. Clarified though e‐mail correspondence that the trial is double‐blind, trial start date and trial completion date |
Espinoza 2019.
| Trial name or title | Metformin for preventing frailty in high‐risk older adults |
| Methods |
Type of trial: interventional Allocation: randomised Intervention model: parallel assignment Masking: quadruple (participant, care provider, investigator, outcomes assessor) Primary purpose: prevention |
| Participants |
Condition: older prediabetic people Enrollment: 120 Inclusion criteria: men and women; all ethnic groups; age 65 and older; community‐dwelling; 2‐hour values of 140 mg/dL to 199 mg/dL after an oral glucose load, and no diagnosis of diabetes in the past 12 months; participants must have the following laboratory values: haematocrit ≥ 33%, AST < 2 X upper limit of normal,ALT < 2 X upper limit of normal, alkaline phosphatase < 2 X upper limit of normal, normal urinalysis, normal electrolytes, normal platelets, prothrombin time and partial thromboplastin time, and normal renal function for the participant´s age (defined by a serum creatinine < 1.5 mg/dL (132.6 mmol/L) in males or < 1.4 mg/dL (123.8 mmol/L) in females and creatinine clearance ≥ 60 mL/min) Exclusion criteria: characteriSed as frail, defined as the presence of 3 or more of: 1) weak hand grip strength, 2) slow walking speed, 3) low physical activity, 4) unintentional weight loss of ≥ 10 pounds over the past year, 5) self‐reported exhaustion; resident of nursing home or long‐term care facility; T2DM; taking drugs known to affect glucose sensitivity; untreated depression or geriatric depression scale score on 15‐item scale >7; diagnosis of any disabling neurologic disease Parkinson's disease, amyotrophic lateral sclerosis, multiple sclerosis, cerebrovascular accident with residual deficits (muscle weakness or gait disorder), diagnosis of dementia or mini‐mental state exam score < 18; history of moderate‐severe heart disease (NYHA Classification greater than grade II) or pulmonary disease (dyspnoea on exertion upon climbing one flight of stairs or less; abnormal breath sounds on auscultation); poorly controlled hypertension (SBP >170 mmHg, DBP >105 mmHg); systemic steroids, anabolic steroids, growth hormone or immunosuppressants within 6 months; chronic inflammatory condition, autoimmune disease, or infectious processes (e.g., active tuberculosis, human immunodeficiency virus, rheumatoid arthritis, systemic lupus erythematosus, hepatitis B or C); active tobacco use (within 6 months); active malignancy, non‐skin; disease or condition likely to cause death within 5 years; hypersensitivity to metformin or pioglitazone; donated blood within the last 2 months |
| Interventions |
Intervention: metformin up to 2000 mg Comparator: placebo Duration of intervention: two years |
| Outcomes |
Primary outcomes: frailty composite measure Secondary outcomes: gait speed, grip strength, six minute walk, short physical performance battery, body composition, frailty as defined by a deficit accumulation index Other outcomes: not reported |
| Starting date |
Study start date: April 2016 Study completion date: October 2022 |
| Contact information | Contact: Alicia Conde, M.A. 210‐617‐5197 |
| Trial identifier | NCT03222765 |
| Notes |
Ji 2019.
| Trial name or title | Efficacy of metformin in preventing diabetes in China (ChinaDPP) |
| Methods |
Type of trial: interventional Allocation: randomised Intervention model: parallel assignment Masking: open‐label Primary purpose: prevention |
| Participants |
Condition: diagnosis of IGR before the randomisation based on the 1999 WHO diagnostic and classification criteria Enrollment: 1674 Inclusion criteria: diagnosis of IGR before the randomisation based on the 1999 WHO diagnostic and classification criteria; 18 ≤age ≤70 years old; not on a treatment of anti‐diabetic agents, including Chinese traditional herbs lowering blood glucose for at least six months before screening; male or non‐pregnant, non‐breastfeeding females, females without birthing plan in next three years; BMI 21 kg/m2 ≤ BM I<32 kg/m2; written informed consent given before any trial‐related activities are carried out Exclusion criteria: administration with medications for pre‐existed diseases affect glucose metabolism (except thiazide diuretics when its daily dose ≤12.5mg); administration with anti‐obesity agents (including Chinese traditional medicine) within six months of enrolment and during intervention; administration with three or more than three types antihypertensive drugs; diabetes people (prior history of gestational diabetes will not be excluded); have any of the following cardiovascular conditions within three months prior to the screening visit: acute myocardial infarction, congestive heart failure defined as NYHA class III/IV or left ventricular ejection fraction ≤ 40%) or cerebrovascular accident; persistent uncontrolled hypertension (SBP ≥160mmHg, or DBP ≥ 100 mmHg); impaired liver function, have obvious clinical signs or symptoms of liver disease, acute or chronic hepatitis, ALT or AST levels ≥3 times the upper limit of the reference range at the screening visit; renal dysfunction (GFR < 45mL/minute); people ventilated by ventilator; hypersensitivity to metformin or to any of the excipients such as povidone K 30, magnesium stearate and hypromellose; disease which may cause tissue hypoxia (especially acute disease, or worsening of chronic respiratory disease); acute alcohol intoxication, alcoholism; severe chronic gastrointestinal disease; severe psychiatric illness; cancer requiring treatment in past five years; uncontrolled thyroid diseases; women who are pregnant or breastfeeding; participation in another clinical trial within the past 30 days; other significant disease that in the Investigator's opinion would exclude the person from the trial |
| Interventions |
Intervention: metformin 850 mg twice daily plus standard lifestyle intervention Comparator: standard lifestyle intervention Duration of intervention: 2 years |
| Outcomes |
Primary outcomes: development of T2DM Secondary outcomes: not reported Other outcomes: not reported |
| Starting date |
Study start date: April 25, 2017 Study completion date: December 31, 2022 |
| Contact information | Contact: Guangwei Li, M.D., Ph.D. guangwei_li45@126.com |
| Trial identifier | NCT03441750 |
| Notes |
JPRN‐UMIN000018995.
| Trial name or title | Metformin therapy for East Asian women with recent gestational diabetes mellitus and glucose abnormalities: a multicenter, randomised, open‐label trial |
| Methods |
Type of trial: interventional Allocation: randomised Intervention model: parallel Masking: open‐label Primary purpose: not reported |
| Participants |
Condition: women with recent GDM and glucose abnormalities, including IFG, or IGT, or both (IFG, IGT) postpartum Enrollment: 210 Inclusion criteria: women who experienced GDM in a previous singleton pregnancy in the past 5 years; postpartum metabolic abnormalities determined by a 75 g OGTT, inclusive of prior GDM with IFG, IGT, or both (IFG, IGT) postpartum; can respond to the questionnaire in Japanese; over 20 years of age; have a record of clinical data during pregnancy; own the Maternal and Child Health Handbook. Exclusion criteria: currently lactating; planning to conceive in the next two years; a history of diabetes and prior use of metformin or insulin to treat diabetes; a history of lactic acidosis; renal impairment (serum creatinine level >= 1.2 mg/dL (106 µmol/L), including dialysis patients); severe liver dysfunction (serum AST and/or ALT level exceeding more than a threefold increase in normal lab values); cardiac failure, cardiac infarction, pulmonary embolism, a high degree of failure in lung function, and hypoxaemia; excessive alcohol intake; malnutrition, or are in a state of starvation or debility, or have pituitary malfunction or adrenal insufficiency; a history of hypersensitivity reaction to metformin or other biguanides; thyroid function that is not controlled by hyperthyroidism (serum free thyroxine levels exceed normal lab values within three months); autoantibody‐positive status (e.g. GAD, IA‐2), or suspected diabetes mellitus associated with a mutation of mitochondrial DNA, maternally inherited diabetes and deafness , or maturity‐onset diabetes of the young; not considered eligible to participate in this study by the attending doctor due to other reasons. |
| Interventions |
Intervention: standard lifestyle intervention Comparator: standard lifestyle intervention plus metformin up to 1500 mg per day Duration of intervention: 24 months |
| Outcomes |
Primary outcomes: period of progression to type 2 diabetes mellitus Secondary outcomes: change in blood glucose and serum insulin levels determined by a 75 g oral glucose tolerance test (OGTT); change in index of insulin sensitivity (Matsuda index) from baseline and at study end (24 months after initiation of therapy, or the final point to be observed); change in index of insulin resistance (HOMA‐IR) from baseline and at study end (24 months after initiation of therapy, or the final point to be observed); change in index of beta‐cell function (Disposition index, Insulinogenic index) from baseline and at study end (24 months after initiation of therapy, or the final point to be observed); change in blood pressure, lipid metabolism, and body weight from baseline; improvement to normal glucose tolerance; incidence rate of adverse events Other outcomes: not stated |
| Starting date |
Study start date: November 2015 Study completion date: not stated |
| Contact information | Contact: Maki Kawasaki, email: boseinaika@ncchd.go.jp or Naoko Arata, email: boseinaika@ncchd.go.jp |
| Trial identifier | JPRN‐UMIN000018995 |
| Notes |
Nadeau 2014.
| Trial name or title | RISE adult medication study (RISE adult) |
| Methods |
Type of trial: interventional Allocation: randomised Intervention model: parallel Masking: quadruple Primary purpose: treatment |
| Participants |
Condition: people with prediabetes and early type 2 diabetes Enrollment: 267 Inclusion criteria: fasting plasma glucose 95 mg/dL to 125 mg/dL (5.3 mmol/L to 6.9 mmol/L) plus 2‐hour glucose ≥140 mg/dL (7.8 mmol/L) on 75 g OGTT plus HbA1c ≤7.0%. There is no upper limit for the 2‐hour glucose on OGTT; age 20 to 65 years; BMI ≥25 kg/m2 but ≤50 kg/m2; self‐reported diabetes < 1 year in duration; drug naive (no prior to oral glucose lowering agent(s), insulin or other injectable glucose lowering agents) Exclusion criteria: underlying disease likely to limit life span and/or increase risk of intervention or an underlying condition that is likely to limit ability to participate in outcomes assessment; an underlying disease that affects glucose metabolism other than type 2 diabetes; medications that affect glucose metabolism, or has an underlying condition that is likely to require such medications; active infections; renal disease (serum creatinine >1.4 mg/dL (123.8 µmol/L) for men; >1.3 mg/dL (114.9 µmol/L) for women) or serum potassium abnormality (<3.4 or >5.5 mmol/L); anaemia (haemoglobin <11 g/dL (6.8 mmol/L) in women, < 12 g/dL (7.4 mmol/L) in men) or known coagulopathy; cardiovascular disease, including uncontrolled hypertension; participants must be able to safely tolerate administration of intravenous fluids required during clamp studies; history of conditions that may be precipitated or exacerbated by a study drug: pancreatitis, serum ALT more than 3 times the upper limit of normal, excessive alcohol intake, suboptimally‐treated thyroid disease, medullary carcinoma of the thyroid or MEN‐2 (in participant or a family history), hypertriglyceridaemia (> 400 mg/dL despite treatment); conditions or behaviours likely to affect the conduct of the RISE Study: unable or unwilling to give informed consent, unable to adequately communicate with clinic staff, another household member is a participant or staff member in RISE, current, recent or anticipated participation in another intervention research project that would interfere with any of the interventions/outcomes in RISE, weight loss of > 5% in past three months for any reason other than postpartum weight loss, participants taking weight loss drugs or using preparations taken for intended weight loss are excluded, likely to move away from participating clinics in next two years, women of childbearing potential who are unwilling to use adequate contraception, current (or anticipated) pregnancy and lactation, major psychiatric disorder that, in the opinion of clinic staff, would impede the conduct of RISE; additional conditions may serve as criteria for exclusion at the discretion of the local site |
| Interventions |
Intervention: metformin up to 2000 mg per day Comparator (1): basal insulin glargine for 3 months followed by open‐label metformin for 9 months Comparator (2): placebo, masked to metformin alone Comparator (3): liraglutide + open‐label metformin Duration of intervention: 12 months |
| Outcomes |
Primary outcomes: ß‐cell function measured by hyperglycaemic clamp techniques Secondary outcomes: hyperglycaemic clamp and OGTT measures of ß‐cell function and glucose tolerance Other outcomes: hyperglycaemic clamp and OGTT measures of ß‐cell function and glucose tolerance |
| Starting date |
Study start date: April 2013 Study completion date: August 2019 |
| Contact information | Contact: Jesse Brown VA medical center, Chicago, Illinois, United States, 60612 |
| Trial identifier | NCT01779362 |
| Notes | Includes people with prediabetes and early type 2 diabetes. Only interesting if af subgroup analysis of the prediabetic population will be performed |
NCT01804049.
| Trial name or title | Metformin and muscle in insulin‐resistant older veterans |
| Methods |
Type of study: efficacy study Allocation: randomised Intervention model: parallel assignment Masking: double blind Primary purpose: prevention |
| Participants |
Condition: prediabetes Enrollment: 120 Inclusion criteria: participants with sedentary, weight‐stable, ambulatory veterans aged 65 years and older with prediabetes identified with fasting glucose values 100 mg/dL (5.6 mmol/L) or greater but under 126 mg/dL (7.0 mmol/L) with no use of diabetes medications Exclusion criteria: chronic medical conditions affecting muscle mass or function like active non‐skin cancer and hypogonadism; Medications affecting muscle mass or function like glucocorticoids and androgen/antiandrogens; contraindications to metformin |
| Interventions |
Intervention: metformin 850 mg orally twice daily Comparator: one placebo capsule by mouth twice daily |
| Outcomes |
Primary outcomes: change in total and appendicular lean mass Secondary outcomes: change in physical performance and muscle histologic characteristics |
| Starting date |
Study start date: February 28, 2013 Study completion date: August 2018 |
| Contact information | Michael P Davey, MD PhD VA Portland Health Care System, Portland, OR, USA Tel: 503‐273‐5125 E‐mail: michael.davey@va.gov |
| Trial identifier | NCT01804049 |
| Notes |
NCT02915198.
| Trial name or title | Investigation of metformin in prediabetes on atherosclerotic cardiovascular outcomes (VA‐IMPACT) |
| Methods |
Type of trial: interventional Allocation: randomised Intervention model: parallel Masking: double‐blind Primary purpose: treatment |
| Participants |
Condition: people with prediabetes and established atherosclerotic cardiovascular disease Enrollment: 7868 Inclusion criteria: prediabetes: this condition is fulfilled by HbA1c of at least 5.7%, but less than 6.5%, or two measurements of fasting plasma glucose (on separate days) of 100 mg/dL to 125 mg/dL (5.6 mmol/L to 6.9 mmol/L), or a 2‐hour plasma glucose level of 140 mg/dLto 199 mg/dL (7.8 mmol/L to 11.1 mmol/L) following a 75 g glucose load OGTT. At least one of these criteria must be met in the absence of diabetic treatment; established atherosclerotic cardiovascular disease: qualifying participants must have evidence of atherosclerotic disease in at least one of the following vascular beds: coronary, cerebrovascular, or peripheral arterial circulation; renal function: estimated GFR at least 45 mL/min/1.73 m2; informed consent has been fully executed, and participant agrees to study procedures Exclusion criteria: related to glucometabolic state: treatment with metformin or other antidiabetic medication within 12 months of randomisation, treatment with systemic glucocorticoids within 3 months of randomisation (due to potential effect on plasma glucose and HbA1c levels), fasting plasma glucose 140 mg/dL (7.8 mmol/L) measured between screening and randomisation visits, or any plasma glucose 200 mg/dL (11.1 mmol/L) or HbA1c 7.0% measured within 12 months of randomisation; related to safety or tolerability: metabolic acidosis (total CO2 below the local laboratory lower limit of normal on most recent blood chemistry panel), current treatment with cimetidine, vandetanib, or a systemic carbonic anhydrase inhibitor (topiramate, acetazolamide, methazolamide, dichlorphenamide, or zonisamide) (use of ophthalmic carbonic anhydrase inhibitors is not exclusionary), cirrhosis, active hepatitis, or jaundice at time of randomisation, or total bilirubin > 2 times upper limit of normal on most recent laboratory study, binge or heavy alcohol consumption within 6 months of randomisation (binge drinking is defined by consumption of 5 or more alcoholic drinks for men or 4 for women within 2 hours, heavy drinking is defined by consumption of 5 or more alcoholic drinks on one occasion, occurring 5 or more times in a month), severe anaemia (haemoglobin < 10 g/dL (6.2 mmol/L)) on screening or most recent laboratory testing, prior history of intolerance to metformin; related to likelihood of non‐modifiable events: myocardial infarction, coronary revascularisation procedure (PCI or CABG), or stroke within 1 month of randomisation, uncontrolled hypertension at screening assessment (SBP 180 mm Hg or DBP 110 mm Hg), acute or decompensated congestive heart failure; related to prognosis, reliability, ethics, or data validity: expected survival less than study duration, participants considered to be unable, unwilling, or unreliable to meet protocol requirements, impaired decision‐making capacity, defined by any history of dementia or cognitive impairment, concurrent participation in another research study involving a randomised comparison of drug or device treatments, unless specifically excepted by CSP; female participants: pregnant or intent to become pregnant during the trial, lactating, women of childbearing potential who are not using a highly effective method of contraception |
| Interventions |
Intervention: metformin up to 2000 mg per day Comparator: matching placebo |
| Outcomes |
Primary outcomes: time in days to death, non‐fatal myocardial infarction, stroke, hospitalisation for unstable angina, or symptom‐driven coronary revascularisation Secondary outcomes: time in days to cardiovascular outcomes, time in days to oncologic outcome, time in days to diabetes outcome Other outcomes: not stated |
| Starting date |
Study start date: February 2019 Study completion date: August 2024 |
| Contact information | Contact: Gregory G. Schwartz, PhD MD, telephone: (720) 723‐6070, email: Gregory.Schwartz@va.gov |
| Trial identifier | NCT02915198 |
| Notes |
NCT02969798.
| Trial name or title | Pre‐diabetes in participants with impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) |
| Methods |
Type of trial: efficacy trial Allocation: randomised Intervention model: cross‐over assignment Masking: open‐label Primary purpose: treatment |
| Participants |
Condition: IGT and IFG NGT participants will serve as controls and will be matched in age, gender, ethnicity and BMI to IGT and IFG participants Enrolment: 700 Inclusion criteria: age 18 to 65 years; FPG < 100 mg/dL (5.6 mmol/L) and 2‐h PG < 140 mg/dL (7.8 mmol/L); BMI 24 kg/m²; to 40 kg/m²; stable body weight (± 4 pounds (1.8 kg)) over the preceding 3 months; no evidence of major organ system disease as determined by physical examination, history and screening laboratory data; women of childbearing potential with a negative pregnancy test at screening and treatment visits, using contraception for the duration of participation in the study (i.e. until follow‐up 7 to 14 days after last dose) (oral contraceptive, injectable progesterone, subdermal implant, spermicidal foam/gel/film/cream/suppository, diaphragm with spermicide, copper or hormonal‐containing IUD, vasectomised male partner > 6 month predosing); signed and dated informed consent document indicating that participant has been informed of all pertinent aspects of study; willing and able to comply with scheduled visits, treatment, laboratory tests and study procedures Exclusion criteria: recent (i.e. within 3 months prior to screening) evidence or medical history of unstable concurrent disease such as: documented evidence or history of clinically significant haematological, endocrine, pulmonary, gastrointestinal, cardiovascular, hepatic, psychiatric, immunological or clinically significant neurological disease; family history of diabetes in a first‐degree relative; BMI < 24 or > 40 kg/m²; unstable body weight (change ± 4 pounds (1.8 kg) over the preceding 3 months); participating in an excessively heavy exercise programme; feeding/sleeping schedule different from a daytime feeding/night‐time sleeping schedule; receiving medications known to alter glucose metabolism (with the exception of metformin or pioglitazone, or both) or which effect brain neurosynaptic function; evidence of major organ system disease as determined by physical examination, history and screening laboratory data; pregnant or unwilling to use contraception during study; blood donation of approximately 1 pint (500 mL) within 8 weeks prior to screening; other severe acute or chronic medical or psychiatric condition or laboratory abnormality that may increase risk associated with study participation or investigational product administration or may interfere with the interpretation of study results and, in judgement of investigator, would make participant inappropriate for entry into study; people haematuria; evidence or prior history of heart failure; family history of pancreatic, bladder and breast cancer; history of pancreatitis; estimated GFR < 60 ± 5 mL/minute/1.73 m²; elevated serum creatinine (> 1.5 mg/dL for men/1.4 mg/dL for women); history of orthostatic hypotension (> 15 mmHg/10 mmHg); liver enzymes > 3‐fold above upper normal limit; history of hypersensitivity to pioglitazone, dapagliflozin or saxagliptin. |
| Interventions |
Intervention: saxagliptin 5 mg/day Comparator (1): dapagliflozin 100 mg/day Comparator (2): pioglitazone 30 mg/day Comparator (3): metformin 200 mg/day The trial will randomise participants exclusively with IGT to 1 treatment group; participants exclusively with IFG to 1 treatment group and participants with IGT plus IFG to 1 treatment group Duration of intervention: 24 months |
| Outcomes |
Primary outcomes: β‐cell function, insulin sensitivity and glucose tolerance status in people with isolated IGT; β‐cell function, insulin sensitivity and glucose tolerance status in people with isolated IFG; β‐cell function, insulin sensitivity and glucose tolerance status in people with IGT plus IFG Secondary outcomes: not stated Other outcomes: not stated |
| Starting date |
Trial start date: January 2014 Trial completion date: July 2020 |
| Contact information | Responsible party/principal investigator: Ralph A DeFronzo, The University of Texas Health Science Center at San Antonio |
| Trial identifier | NCT02969798 |
| Notes | There is a control arm with participants with NGT ‐ these will not be included in updates of our review |
NCT03194009.
| Trial name or title | Diabetes prevention via exercise, nutrition and treatment (PRuDENTE) |
| Methods |
Type of trial: interventional Allocation: randomised Intervention model: parallel assignment Masking: open‐label Primary purpose: prevention |
| Participants |
Condition: adults with FPG between 100 mg/dL and 125 mg/dL (5.6 mmol/L to 6.9 mmol/L) Enrollment: 3060 Inclusion criteria: having received primary care in the chosen health centre (ideally two or more visits to that clinic in the prior year); subscribers to "Seguro Popular" (Mexican national health insurance); BMI >= 30 kg/m2; results of FPG with values for prediabetes diagnosis (glucose between 100 mg/dL and 125 mg/dL (5.6 mmol/L to 6.9 mmol/L)) Exclusion criteria: renal insufficiency (GFR < 30 mL/minute); known hepatic impairment or altered liver enzymes (AST or ALT three times above normal values; active alcoholism or drug addiction; allergies or previous known intolerance to exercise or metformin; current pregnancy; plans to leave the area in the next three years; previous diagnosis of T2DM |
| Interventions |
Intervention: metformin plus lifestyle modifications recommendations (physical activity and diet) Comparator: lifestyle modifications recommendations (physical activity and diet) Duration of intervention: three years |
| Outcomes |
Primary outcomes: diabetes measured by HbA1c and fasting blood glucose; lifestyle modifications by decreasing adiposity indicators; caloric intake; physical activity Secondary outcomes: implementation process outcomes at the clinic level; implementation process outcomes patient level; cost‐utility of metformin Other outcomes: not reported |
| Starting date |
Study start date: August 10, 2017 Study completion date: December 31, 2022 |
| Contact information |
Contact: Luz María Sánchez‐Romero, MD, PhD luz.sanchez@insp.mx Alberto Gallardo, MD albgallardo@yahoo.com.mx |
| Trial identifier | NCT03194009 |
| Notes |
Rhee 2019.
| Trial name or title | Hospital‐based diabetes prevention study in Korea: A prospective, multicenter, randomised, open‐label, controlled study |
| Methods |
Type of trial: interventional Allocation: randomised Intervention model: parallel Masking: open‐label Primary purpose: prevention |
| Participants |
Condition: IFG, IGT Enrollment: 744 Inclusion criteria: 30 < age < 71; BMI ≥ 23 kg/m2; 75 g OGTT 2 hours after the test blood glucose 140 mg/dL ˜ 199 mg/dL (7.8 mmol/L ˜ 11.1 mmol/L) or fasting blood sugar 110 mg/dL ˜ 125 mg/dL (6.1 mmol/L ˜ 6.9 mmol/L) or HbA1c 5.7% ˜ 6.4% Exclusion criteria: diagnosed with diabetes mellitus except for maternity period or having drugs for diabetes mellitus; type 2 diabetes mellitus; fasting glucose ≥ 126 mg/dL (7.0 mmol/L); 75 g OGTT 2 hours after the blood glucose ≥ 200 mg/dL (11.1 mmol/L); HbA1c ≥ 6.5%; short life expectancy; history of severe cardiovascular disease within the last 6 months (cerebral haemorrhage, stroke, myocardial infarction, angina pectoris, heart failure, etc.); SBP >180 mmHg or DBP >105 mmHg; aortic stenosis; left bundle branch block or third degree AV block; diagnosed and treated for malignant tumours including leukaemia and lymphoma within the last 5 years; abnormal renal function (creatinine ≥ 1.4 mg/dL (123.8 mmol/L) (male) or ≥ 1.3 mg/dL (114.9 mmol/L) (female) or urine protein ≥ 2 +); anaemia (haematocrit < 36% ((male) or >< 33% (female)); cirrhosis or chronic active hepatitis (AST/ALT>3UNL); acute gastrointestinal disease (pancreatitis, infectious intestinal disease); surgery within the last 3 to 6 months or just after the surgery; chronic infection (HIV, active tuberculosis, etc.); pulmonary patients who rely on oxygen or daily bronchodilators; judged to be able to influence the clinical trial by investigator; can not communicate; psychiatric or cognitive impairment that may affect the compliance of the clinical trial; do not agree to the treatment group allocation by random assignment; participate in other studies that may interfere with the clinical trial; lost weight by more than 10% during the past 6 months, excluding weight loss after giving birth; can not have normal walking or exercise; currently pregnant or who are within the last 3 months after giving birth; planning pregnancy during the clinical trial period; have a history of drug and alcohol abuse (acute, chronic) within the last 2 years; not appropriate or unreliable for clinical trials at the discretion of the tester; taking medication or medical condition that may affect the diagnosis of diabetes (thiazide diuretics, systemic beta blockers, taking Niacin for the treatment of neutropenic depression, possibility of taking or injecting a systemic steroid preparation, taking a serotonin reuptake inhibitor (SSRI) for weight loss purpose, taking medicine for weight loss; hormone status is not appropriate during thyroid hormone replacement therapy (TSH abnormal range) (If thyroid hormone therapy is stable for more than 3 months and TSH is normal, the participant can participate in); other endocrine diseases (e.g. Cushing's syndrome, acromegaly); during treatment, fasting plasma triglyceride > 600 mg/dL (6.8 mmol/L) |
| Interventions |
Intervention: life style modification Comparator (1): conventional management Comparator (2): metformin up to 1000 mg per day Duration of intervention: 36 months |
| Outcomes |
Primary outcomes: cumulative incidence of diabetes mellitus after randomisation Secondary outcomes: change on HbA1c, fasting glucose and HOMA2%B Other outcomes: not stated |
| Starting date |
Study start date: November 2016 Study completion date: November 2020 |
| Contact information | Contact: Jeong‐Taek Woo, email: jtwoomd@khmc.or.kr or Sang Youl Rhee, email: bard95@hanmail.net |
| Trial identifier | NCT02981121 |
| Notes |
2hPG: 2‐hour glucose after an OGTT; AST: aspartate amino transferase; ALT: alanine amino transferase; BMI: body mass index; CABG: coronary artery bypass graft; DBP: diastolic blood pressure; DNA: deoxyribonucleic acid; ETDRS: Early Treatment Diabetic Retinopathy Study; FPG: fasting plasma glucose; GAD: glutamate decarboxylase; GDM: gestational diabetes mellitus; GFR: glomerular filtration rate; HbA1c: glycosylated haemoglobin A1c; IA‐2: insulin antibodies ‐ 2; IFG: impaired fasting glucose; IGR: impaired glucose regulation; IGT: impaired glucose tolerance; NGT: normal glucose tolerance; NYHA: New York Heart Association; OGTT: oral glucose tolerance test; PCI: percutaneous coronary intervention; SBP: systolic blood pressure; SD: standard deviation; TSH: thyroid stimulating hormonE; T2DM type 2 diabetes mellitus.
blood glucose mg/dL converted to mmol/L (via https://www.diabetes.co.uk/blood‐sugar‐converter.html) creatinine mg/dL converted to µmol/L (via http://www.endmemo.com/medical/unitconvert/Creatinine.php) haemoglobin g/dL converted to mmol/L (via http://unitslab.com/node/7) pounds converted to kg (via https://www.convertunits.com/from/pounds/to/kg) triglycerides mg/dL converted to mmol/L (via http://www.endmemo.com/medical/unitconvert/Triglycerides.php)
Differences between protocol and review
We changed the definition of the intervention "Metformin monotherapy" in our protocol to "Metformin monotherapy (with or without diet, exercise or both)" because most trials included some element of diet, exercise or both in the intervention groups.
In our protocol, interventions in the control group did not comprise "no antidiabetic treatment". However, we found three trials comparing metformin with this type of intervention after reading all publications. Therefore, we extracted and analysed the data from these trials.
In our protocol we stated that we planned to do subgroup analyses on 'type of comparator (active comparator or placebo/no intervention)'. This subgroup analysis was changed to 'trials designed to blind participants and investigators versus open‐labelled trials' to better address issues of risk of bias.
The corresponding author has changed.
Contributions of authors
All review authors read and approved the final review draft.
KM: acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, review draft and will be involved in future review updates.
YC: acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, review draft and will be involved in future review updates.
MIM: search strategy development and review of drafts.
BR: protocol and review draft, search strategy development, data interpretation and review of drafts.
BH: protocol and review draft, search strategy development, data interpretation and review of drafts.
Sources of support
Internal sources
West China Hospital of Sichuan University, China.
External sources
No sources of support supplied
Declarations of interest
Kasper S Madsen (KM): none known
Yuan Chi (YC): none known
Maria‐Inti Metzendorf (MM): none known
Bernd Richter (BR): none known
Bianca Hemmingsen (BH): none known
New
References
References to studies included in this review
Alfawaz 2018 {published data only}
- Alfawaz HA, Wani K, Alnaami AM, Al‐Saleh Y, Aljohani NJ, Al‐Attas OS, et al. Effects of different dietary and lifestyle modification therapies on metabolic syndrome in prediabetic Arab patients: a 12‐month longitudinal study. Nutrients 2018; Vol. 10, issue 3:pii: E383. [DOI] [PMC free article] [PubMed]
BIGPRO1 2009 {published data only}
- Charles MA, Morange P, Eschwege E, Andre P, Vague P, Juhan‐Vague I. Effect of weight change and metformin on fibrinolysis and the von willebrand factor in obese nondiabetic subjects: the BIGPRO1 Study. Biguanides and the prevention of the risk of obesity. Diabetes Care 1998. [DOI] [PubMed]
- Fontbonne A, Andre P, Eschwege E. BIGPRO (biguanides and the prevention of the risk of obesity): study design. A randomized trial of metformin versus placebo in the correction of the metabolic abnormalities associated with insulin resistance. Diabetes & Metabolism 1991;17(1 Pt 2):249‐54. [PUBMED: 1936485] [PubMed] [Google Scholar]
- Fontbonne A, Charles MA, Juhan‐Vague I, Bard JM, Andre P, Isnard F, et al. Effects of 1‐year treatment with metformin on metabolic and cardiovascular risk factors in non‐diabetic upper‐body obese subjects with mild glucose anomalies: a post‐hoc analysis of the BIGPRO1 trial. Diabetes & Metabolism 2009;35(5):385‐91. [DOI] [PubMed] [Google Scholar]
- Fontbonne A, Charles MA, Juhan‐Vague I, Bard JM, Andre P, Isnard F, et al. The effect of metformin on the metabolic abnormalities associated with upper‐body fat distribution. Diabetes Care 1996;19(9):920‐6. [DOI] [PubMed] [Google Scholar]
Chen 2009 {published data only}
- Chen C, Ye ZP, Xie M, Xu XY. Intervene effect of Shenqi Jiangtang Capsule on type 2 diabetes developed from low glucose tolerance [参芪降糖胶囊在糖耐量低减人群向2型糖尿病发展中的干预作用]. Chinese Journal of Traditional Medical Science and Technology [中国中医药科技] 2009;16(1):58‐9. [DOI: 10.3969/j.issn.1005-7072.2009.01.026] [DOI] [Google Scholar]
DPP/DPPOS 2002 {published data only}
- Diabetes Prevention Program Outcomes Study (DPPOS). https://clinicaltrials.gov/ct2/show/NCT00038727 Assessed 23rd of January 2017.
- Aroda VR, Christophi CA, Edelstein SL, Zhang P, Herman WH, Barrett‐Connor E, et al. Diabetes Prevention Program (DPP) Research Group. The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: the Diabetes Prevention Program outcomes study 10‐year follow‐up. Journal Clinical Endocrinology and Metabolism 2015;100(4):1646‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroda VR, Edelstein SL, Goldberg RB, Knowler WC, Marcovina SM, Orchard TJ, et al. Long‐term metformin use and vitamin B12 deficiency in the Diabetes Prevention Program Outcomes Study. Journal of Clinical Endocrinoligy & Metabolism 2016; Vol. 101, issue 4:1754‐61. [DOI] [PMC free article] [PubMed]
- Aroda VR, Knowler WC, Crandall JP, Perreault L, Edelstein S L, Jeffries S L, et al. Metformin for diabetes prevention: insights gained from the Diabetes Prevention Program/Diabetes Prevention Program Outcomes Study. Diabetologia 2017;60(9):1601‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carris NW, Cheng F, Kelly WN. The changing cost to prevent diabetes: a retrospective analysis of the Diabetes Prevention Program. Journal of the American Pharmaceutical Association 2017;57(6):717‐22. [DOI] [PubMed] [Google Scholar]
- Crandall J, Schade D, Ma Y, Fujimoto WY, Barrett‐Connor E, Fowler S, et al. The influence of age on the effects of lifestyle modification and metformin in prevention of diabetes. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 2006;61(10):1075‐81. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall JP, Mather K, Rajpathak SN, Goldberg RB, Watson K, Foo S, et al. Statin use and risk of developing diabetes: results from the Diabetes Prevention Program. BMJ Open Diabetes Research and Care 2017;5(1):e000438.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deboer MD, Filipp SL, Gurka MJ. Using mets severity to track risk for type 2 diabetes during intervention with lifestyle and metformin. Diabetes 2018;67:A408‐. [Google Scholar]
- Diabetes Prevention Program (DPP) Group. Protocol for the Diabetes Prevention Program (DPP). https://dppos.bsc.gwu.edu/documents/1124073/1127212/DPPPROTOCOL.PDF/807eddd1‐d9bf‐497d‐89f0‐5de15fc43d79 Assessed January 23rd 2017.
- Diabetes Prevention Program (DPP) Group. Protocol for the Diabetes prevention Program Outcomes Study (DPPOS). https://dppos.bsc.gwu.edu/documents/1124073/1127212/Version+4.2+May+1%2C+2016/14e782f2‐2da9‐47d1‐a501‐6004bcaee74e Assessed 31st of January 2017.
- Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care 2002;25(12):2165‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program: baseline characteristics of the randomized cohort. Diabetes Care 2000;23:1619‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program: design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care 1999;22:623‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research G. The 10‐year cost‐effectiveness of lifestyle intervention or metformin for diabetes prevention: an intent‐to‐treat analysis of the DPP/DPPOS. Diabetes Care 2012;35(4):723‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research, Group. Changes in albumin excretion in the Diabetes Prevention Program. Diabetes Care April 2009; Vol. 32, issue 4:720‐5. [DOI] [PMC free article] [PubMed]
- Diabetes Prevention Program Research, Group. Effects of withdrawal from metformin on the development of diabetes in the Diabetes Prevention Program. Diabetes Care April 2003; Vol. 26, issue 4:977‐80.. [DOI] [PMC free article] [PubMed]
- Florez H, Pan Q, Ackermann RT, Marrero DG, Barrett‐Connor E, Delahanty L, et al. Diabetes Prevention Program (DPP) Group. Impact of lifestyle intervention and metformin on health‐related quality of life: the diabetes prevention program randomized trial. Journal of General Internal Medicine 2012;27(12):1594‐601. [PUBMED: 22692637] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florez H, Temprosa MG, Orchard TJ, Mather KJ, Marcovina SM, Barrett‐Connor E, et al. Diabtes Prevention Program (DPP) Group. Metabolic syndrome components and their response to lifestyle and metformin interventions are associated with differences in diabetes risk in persons with impaired glucose tolerance. Diabetes, Obesity & Metabolism 2014;16(4):326‐33. [PUBMED: 24118860] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto WY, Jablonski KA, Bray GA, Kriska A, Barrett‐Connor E, Haffner S, et al. Body size and shape changes and the risk of diabetes in the diabetes prevention program. Diabetes June 2007; Vol. 56, issue 6:1680‐5. [DOI] [PMC free article] [PubMed]
- Fujimoto WY. Diabetes Prevention Program Research Group. Background and recruitment data for the U.S. Diabetes Prevention Program. Diabetes Care June 2000; Vol. 23, issue 6:876. [PMC free article] [PubMed]
- Goldberg R B, Bray G A, Marcovina S M, Mather K J, Orchard T J, Perreault L, et al. Non‐traditional biomarkers and incident diabetes in the Diabetes Prevention Program: comparative effects of lifestyle and metformin interventions. Diabetologia 2019;62(1):58‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg RB, Aroda VR, Bluemke DA, Barrett‐Connor E, Budoff M, Crandall JP, et al. Effect of long‐term metformin and lifestyle in the Diabetes Prevention Program and Its Outcome Study on coronary artery calcium. Circulation 2017;136(1):52‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg RB, Temprosa M, Haffner S, Orchard TJ, Ratner RE, Fowler SE, et al. Effect of progression from impaired glucose tolerance to diabetes on cardiovascular risk factors and its amelioration by lifestyle and metformin intervention: the Diabetes Prevention Program randomized trial by the Diabetes Prevention Program Research Group. Diabetes Care 2009;32(4):726‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg RB, Temprosa MG, Mather KJ, Orchard TJ, Kitabchi AE, Watson KE. Diabetes Prevention Program (DPP) Research Group. Lifestyle and metformin interventions have a durable effect to lower CRP and tPA levels in the diabetes prevention program except in those who develop diabetes. Diabetes Care 2014;37(8):2253‐60. [PUBMED: 24824548] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg, RB, Mather, K. Targeting the consequences of the metabolic syndrome in the Diabetes Prevention Program. Arteriosclerosis, Thrombosis and Vascular Biology September 2012; Vol. 32, issue 9:2077‐90. [DOI] [PMC free article] [PubMed]
- Haffner S, Temprosa M, Crandall J, Fowler S, Goldberg R, Horton E, et al. The Diabetes Prevention Program Research Group. Intensive lifestyle intervention or metformin on inflammation and coagulation in participants with impaired glucose tolerance. Diabetes 2005;54:1566‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamman RF, Horton E, Barrett‐Connor E, Bray GA, Christophi CA, Crandall J, et al. Diabetes Prevention Program (DPP) Research Group. Factors affecting the decline in incidence of diabetes in the Diabetes Prevention Program Outcomes Study (DPPOS). Diabetes 2015;64(3):989‐98. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman WH. The cost‐effectiveness of diabetes prevention: results from the Diabetes Prevention Program and the Diabetes Prevention Program Outcomes Study. Clinical Diabetes and Endocrinology 2015; Vol. 1, issue 9. [DOI] [PMC free article] [PubMed]
- Herman WH, Edelstein SL, Ratner RE, Montez MG, Ackermann RT, Orchard TJ, et al. Effectiveness and cost‐effectiveness of diabetes prevention among adherent participants. American Journal of Managed Care 2013; Vol. 19, issue 3:194‐202. [PMC free article] [PubMed]
- Herman WH, Hoerger TJ, Brandle M, Hicks K, Sorensen S, Zhang P, et al. The cost‐effectiveness of lifestyle modification or metformin in preventing type 2 diabetes in adults with impaired glucose tolerance. Annals of Internal Medicine March 2005; Vol. 142, issue 5:323‐32. [DOI] [PMC free article] [PubMed]
- Hernan WH, Brandle M, Zhang P, Williamson DF, Matulik MJ, Ratner RE, et al. The Diabetes Prevention Program Research Group. Costs associated with the primary prevention of type 2 diabetes mellitus in the Diabetes Prevention Program. Diabetes Care 2003;26:36‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hivert MF, Christophi CA, Franks PW, Jablonski KA, Ehrmann DA, Kahn SE, et al. Diabetes Prevention Program Research Group. Lifestyle and metformin ameliorate insulin sensitivity independently of the genetic burden of established insulin resistance variants in Diabetes Prevention Program participants. Diabetes 2016;65(2):520‐6. [PUBMED: 26525880] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaacks LM, Ma Y, Davis N, Delahanty LM, Mayer‐Davis EJ, Franks PW, et al. Diabetes Prevention Program (DPP) Research Group. Long‐term changes in dietary and food intake behaviour in the Diabetes Prevention Program Outcomes Study. Diabetic Medicine 2014;31(12):1631‐42. [PUBMED: 24824893] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Aroda VR, Goldberg RB, Younes N, Edelstein S L, Carrion‐Petersen M, et al. Androgens, irregular menses, and risk of diabetes and coronary artery calcification in the Diabetes Prevention Program. Journal of Clinical Endocrinology and Metabolism 2018;103(2):486‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Barrett‐Connor E, Aroda VR, Mather KJ, Christophi CA, Horton ES, et al. Testosterone and depressive symptoms among men in the Diabetes Prevention Program. Psychoneuroendocrinology 2016;72:63‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Dabelea D, Kalyani RR, Christophi CA, Bray GA, Pi‐Sunyer X, et al. Changes in visceral adiposity, subcutaneous adiposity, and sex hormones in the Diabetes Prevention Program. Journal of Clinical Endocrinology and Metabolism 2017;102(9):3381‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitabchi AE, Temprosa M, Knowler WC, Kahn SE, Fowler SE, Haffner SM, et al. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: effects of lifestyle intervention and metformin. Diabetes 2005;54(8):2404‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowler WC, Barrett‐Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New England Journal of Medicine 2002;346(6):393‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, et al. Diabetes Prevention Program (DPP) Research Group. 10‐year follow‐up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374(9707):2054. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowler WC, Hamman RF, Edelstein SL, Barrett‐Connor E, Ehrmann DA, Walker EA, et al. Prevention of type 2 diabetes with troglitazone in the Diabetes Prevention Program. Diabetes 2005; Vol. 54, issue 4:1150‐6. [DOI] [PMC free article] [PubMed]
- Knowler WC. Diabetes Prevention Program (DPP) Research Group. HbA1c as a predictor of diabetes and as an outcome in the diabetes prevention program: a randomized clinical trial. Diabetes Care 2015;38(1):51‐8. [PUBMED: 25336746] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachin JM, Christophi CA, Edelstein SL, Ehrmann DA, Hamman RF, Kahn SE, et al. Factors associated with diabetes onset during metformin versus placebo therapy in the diabetes prevention program. Diabetes 2007;56(4):1153‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger JA, Ma Y, Christophi CA, Florez H, Golden SH, Hazuda H, et al. Metformin, lifestyle intervention, and cognition in the Diabetes Prevention Program Outcomes Study. Diabetes Care 2017;40(7):958‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Kriska AM, Golden SH, Bray GA, Luchsinger JA, Crandall J, et al. Physical function decline in the diabetes prevention program outcome study. Diabetes 2014;63(0):A344. [Google Scholar]
- Marrero D, Pan Q, Barrett‐Connor E, Groot M, Zhang P, Percy C, et al. DPPOS Research Group. Impact of diagnosis of diabetes on health‐related quality of life among high risk individuals: the Diabetes Prevention Program Outcomes Study. Quality of Life Research 2014;23(1):75‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruthur NM, Ma Y, Delahanty LM, Nelson JA, Aroda V, White NH, et al. Early response to preventive strategies in the Diabetes Prevention Program. Journal of General Internal Medicine 2013;28(12):1629‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather KJ, Funahashi T, Matsuzawa Y, Edelstein S, Bray GA, Kahn SE, et al. Adiponectin, change in adiponectin, and progression to diabetes in the Diabetes Prevention Program. Diabetes 2008;57(4):980‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather KJ, Kim C, Christophi CA, Aroda VR, Knowler WC, Edelstein SE, et al. Steroid sex hormones, sex hormone‐binding globulin, and diabetes incidence in the Diabetes Prevention Program. Journal of Clinical Endocrinology and Metabolism 2015;100(10):3778‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffery JM, Jablonski KA, Franks PW, Delahanty LM, Aroda V, Marrero D, et al. Replication of the association of BDNF and MC4R variants with dietary intake in the Diabetes Prevention Program. Psychosomatic Medicine 2017;79(2):224‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molitch ME, Fujimoto W, Hamman RF, Knowler WC, Diabetes Prevention Program Research G. The diabetes prevention program and its global implications. Journal of the American Society of Nephrology 2003;14(7 Suppl 2):S103‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00004992. Diabetes Prevention Program. clinicaltrials.gov/ct2/show/NCT00004992 (first posted 20 March 2000).
- NCT00038727. Diabetes Prevention Program Outcomes Study (DPPOS). clinicaltrials.gov/ct2/show/NCT00038727 (first posted 5 June 2002).
- NCT03757910. Brain imaging in the Diabetes Prevention Program Outcomes Study (DPPOS‐Brain). clinicaltrials.gov/ct2/show/NCT03757910 (first posted 29 November 2018).
- Nathan DM, Barrett‐Connor E, Crandall JP, Edelstein SL, Goldberg RB, Horton ES, et al. Diabetes Prevention Program Research Group. Long‐term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15‐year follow‐up: the Diabetes Prevention Program Outcomes Study. Lancet. Diabetes & endocrinology 2015;3(11):866‐75. [PUBMED: 26377054] [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien MJ, Whitaker RC, Yu D, Ackermann RT. The comparative efficacy of lifestyle intervention and metformin by educational attainment in the Diabetes Prevention Program. Preventive Medicine 2015;77:125‐30. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchard TJ, Temprosa M, Barrett‐Connor E, Fowler SE, Goldberg RB, Mather KJ, et al. Diabetes Prevention Program Outcomes Study Research Group. Long‐term effects of the Diabetes Prevention Program interventions on cardiovascular risk factors: a report from the DPP Outcomes Study. Diabetic Medicine January 2013; Vol. 30, issue 1:46‐55. [DOI] [PMC free article] [PubMed]
- Perreault L, Kahn SE, Christophi CA, Knowler WC, Hamman RF, Diabetes Prevention Program Research G. Regression from pre‐diabetes to normal glucose regulation in the diabetes prevention program. Diabetes Care 2009;32(9):1583‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault L, Ma Y, Dagogo‐Jack S, Horton E, Marrero D, Crandall J, et al. Sex differences in diabetes risk and the effect of intensive lifestyle modification in the Diabetes Prevention Program. Diabetes Care 2008;31(7):1416‐21. [PUBMED: 18356403] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault L, Pan Q, Aroda VR, Barrett‐Connor E, Dabelea D, Dagogo‐Jack S, et al. Exploring residual risk for diabetes and microvascular disease in the Diabetes Prevention Program Outcomes Study (DPPOS). Diabetic Medicine 2017;34(12):1747‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault L, Pan Q, Mather KJ, Watson KE, Hamman RF, Kahn SE, et al. Effect of regression from prediabetes to normal glucose regulation on long‐term reduction in diabetes risk: results from the Diabetes Prevention Program Outcomes Study. Lancet 2012;379(9833):2243‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan S, Kanaya AM, Ma Y, Vittinghoff E, Barrett‐Connor E, Wing R, et al. Diabetes Prevention Program (DPP) Group. Long‐term prevalence and predictors of urinary incontinence among women in the Diabetes Prevention Program Outcomes Study. International Journal of Urology 2015;22(2):206‐12. [PUBMED: 25352018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner R, Goldberg R, Haffner S, Marcovina S, Orchard T, Fowler S, et al. The Diabetes Prevention Program Research Group. Impact of intensive lifestyle and metformin therapy on cardiovascular disease risk factors in the Diabetes Prevention Program. Diabetes Care 2005;28:888‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner RE, Diabetes Prevention Program R. An update on the Diabetes Prevention Program. Endocrine Practice 2006;12 Suppl 1:20‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin RR, Fujimoto WY, Marrero DG, Brenneman T, Charleston JB, Edelstein SL, et al. Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program: recruitment methods and results. Controlled Clinical Trials 2002;23:157‐71. [DOI] [PubMed] [Google Scholar]
- Sylvetsky AC, Edelstein SL, Walford G, Boyko EJ, Horton ES, Ibebuogu UN, et al. A high‐carbohydrate, high‐fiber, low‐fat diet results in weight loss among adults at high risk of type 2 diabetes. Journal of Nutrition 2017;147(11):2060‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Diabetes Prevention Program Research Group. Long‐term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care 2012;35(4):731‐7. [PUBMED: 22442396] [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Diabetes Prevention Program Research Group. Within‐trial cost‐effectiveness of lifestyle intervention or metformin for the primary prevention of type 2 diabetes. Diabetes Care 2003;26:2518‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West DS, Elaine Prewitt T, Bursac Z, Felix HC. Weight loss of black, white, and Hispanic men and women in the Diabetes Prevention Program. Obesity (Silver Spring, Md.) 2008;16(6):1413‐20. [PUBMED: 18421273] [DOI] [PubMed] [Google Scholar]
- Wing RR, Hamman RF, Bray GA, Delahanty L, Edelstein SL, Hill JO, et al. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obesity research 2004;12(9):1426‐34. [PUBMED: 15483207] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot M, Marrero D, Mele L, Doyle T, Schwartz F, Mather KJ, et al. Depressive symptoms, antidepressant medication use, and inflammatory markers in the Diabetes Prevention Program. Psychosomatic Medicine 2018;80(2):167‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fang 2004 {published data only}
- Fang YS, Li TY, Chen SY. Effect of medicine and non‐medicine intervention on the outcomes of patients with impaired glucose tolerance: 5‐year follow‐up [药物与非药物干预对糖耐量减低者结局的影响:5年随访]. Chinese Journal of Clinical Rehabilitation 1999;8(30):6562‐3. [Google Scholar]
IDPP‐1 2006 {published data only}
- NCT00279240. Life style modifications prevents type 2 diabetes in Asian Indians. https://clinicaltrials.gov/show/NCT00279240 (first posted 19 January 2006).
- Ramachandran A, Arun N, Shetty AS, Snehalatha C. Efficacy of primary prevention interventions when fasting and postglucose dysglycemia coexist: analysis of the Indian Diabetes Prevention Programmes (IDPP‐1 and IDPP‐2). Diabetes Care 2010;33(10):2164‐8. [PUBMED: 20519663] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V. The Indian diabetes prevention programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP‐1). Diabetologia 2006;49:289‐97. [DOI] [PubMed] [Google Scholar]
- Ramachandran A, Snehalatha C, Satyavani K, Sivasankari S, Vijay V. Metabolic syndrome does not increase the risk of conversion of impaired glucose tolerance to diabetes in Asian Indians‐‐Result of Indian diabetes prevention programme. Diabetes Research and Clinical Practice 2007;76(2):215‐8. [PUBMED: 16982107] [DOI] [PubMed] [Google Scholar]
- Ramachandran A, Snehalatha C, Yamuna A, Mary S, Ping Z. Cost‐effectiveness of the interventions in the primary prevention of diabetes among Asian Indians: within‐trial results of the Indian Diabetes Prevention Programme (IDPP). Diabetes Care 2007;30(10):2548‐52. [DOI] [PubMed] [Google Scholar]
- Snehalatha C, Mary S, Joshi VV, Ramachandran A. Beneficial effects of strategies for primary prevention of diabetes on cardiovascular risk factors: results of the Indian Diabetes Prevention Programme. Diabetes & Vascular Disease Research 2008;5(1):25‐9. [PUBMED: 18398809] [DOI] [PubMed] [Google Scholar]
- Snehalatha C, Mary S, Selvam S, Sathish Kumar CK, Shetty SB, Nanditha A, et al. Changes in insulin secretion and insulin sensitivity in relation to the glycemic outcomes in subjects with impaired glucose tolerance in the Indian Diabetes Prevention Programme‐1 (IDPP‐1). Diabetes Care 2009;32(10):1796‐801. [PUBMED: 19587369] [DOI] [PMC free article] [PubMed] [Google Scholar]
Iqbal Hydrie 2012 {published data only}
- Iqbal Hydrie MZ, Basit A, Shera AS, Hussain A. Effect of intervention in subjects with high risk of diabetes mellitus in Pakistan. Journal of Nutrition and Metabolism 2012;2012:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ji 2011 {published data only}
- Ji XZ, Guan CR, Du XM, Chen RQ. Effects of metformin on serum c‐reactive protein and insulin sensitivity index in prediabetic patients [二甲双胍对糖尿病前期患者血清C反应蛋白及胰岛素敏感指数的影响]. Chinese Journal of Clinical Pharmacology and Therapeutics [中国临床药理学与治疗学] 2011;16(8):920‐4. [Google Scholar]
Jin 2009 {published data only}
- Jin GX, Wang YM. Changes in insulin resistance and islet beta cell function in patients with impaired fasting glucose after intervention [空腹血糖受损人群干预后胰岛素抵抗与胰岛β细胞功能的改变]. Journal of Bengbu Medical College [蚌埠医学院学报] 2009;34(11):969‐71. [Google Scholar]
Li 1999 {published data only}
- Li C L, Pan CY, Lu JM. Effect of metformin on patients with impaired glucose tolerance. Diabetic Medicine 1999;16(6):477‐81. [DOI] [PubMed] [Google Scholar]
Li 2009 {published data only}
- Li JJ, Tao DB, Zhang XH, Yin GZ, Chen XY. Intervention of participants with composite impaired glucose regulation and obesity by Metformin [二甲双胍对肥胖合并混合糖调节受损的干预研究]. Central China Medical Journal [华中医学杂志] 2009;33(5):254‐6. [Google Scholar]
Liao 2012 {published data only}
- Liao XM. Clinical observation of acarbose and metformin in patients with impaired glucose tolerance [阿卡波糖和二甲双胍治疗糖耐量受损患者的临床观察]. Contemporary Medicine [当代医学] 2012;18(11):145‐6. [Google Scholar]
Lu 2002 {published data only}
- Lu JM, Pan CY, Tian H, Li CL, Yang GQ, Zhang XL, et al. Intervention of metformin and dietary fiber in the development of type 2 diabetes in people with low glucose tolerance [二甲双胍和食物纤维在糖耐量低减人群向2型糖尿病发展中的干预作用]. Chinese Journal of Diabetes [中国糖尿病杂志] 2002;‐(6):21‐4. [Google Scholar]
Lu 2010 {published data only}
- Lu YM. Effects comparison between pre‐diabetes lifestyle intervention and drug intervention [糖尿病前期生活方式干预与药物干预的效果比较]. Zhengzhou University Master Thesis. Zhengzhou University, 2010.
Maji 2005 {published data only}
- Maji D, Roy RU, Das S. Prevention of type 2 diabetes in the prediabetic population. Journal of the Indian Medical Association 2005;103(11):609‐11. [PubMed] [Google Scholar]
Papoz 1978 {published data only}
- Eschwege E. French Study Group for Diabetes Epidemiology (A.F.E.D.I.A.). Short term effects (2 years) of oral treatment for diabetes in the borderline impairment of oral glucose tolerance test. Diabetologia 1974;10(363):Abstract 31. [Google Scholar]
- Papoz L, Job D, Eschwege E, Aboulker JP, Cubeau J, Pequignot G, et al. Effect of oral hypoglycaemic drugs on glucose tolerance and insulin secretion in borderline diabetic patients. Diabetologia 1978;15(5):373‐80. [PUBMED: 104897] [DOI] [PubMed] [Google Scholar]
PREVENT‐DM 2017 {published data only}
- Alhalel N, Schueller S M, O'Brien M J. Association of changes in mental health with weight loss during intensive lifestyle intervention: does the timing matter?. Obesity Science and Practice 2018;4(2):153‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02088034. Intervention to promote weight loss in Latinas At‐risk for Diabetes. clinicaltrials.gov/ct2/show/NCT02088034 (first posted 14 March 2014).
- O'Brien MJ, Perez A, Scanlan AB, Alos VA, Whitaker RC, Foster GD, et al. PREVENT‐DM comparative effectiveness trial of lifestyle intervention and metformin. American Journal of Preventive Medicine 2017;52(6):788‐97. [PUBMED: 28237635] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez A, Alos VA, Scanlan A, Maia CM, Davey A, Whitaker RC, et al. The rationale, design, and baseline characteristics of PREVENT‐DM: A community‐based comparative effectiveness trial of lifestyle intervention and metformin among Latinas with prediabetes. Contemporary Clinical Trials 2015;45(Pt B):320‐7. [PUBMED: 26597415] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2009 {published data only}
- Wang BL, Zhang LL. Clinical observation of metformin in the treatment of prediabetes [二甲双胍治疗糖尿病前期的临床观察]. Collected papers of the 7th endocrine academic conference of Shanxi Medical Association [山西省医学会第七届内分泌学术会议论文集]. Tai Yuan [太原]: Shanxi Medical Association [山西省医学会], 2009:38‐9.
Zeng 2013 {published data only}
- Zeng ZH, Cai F, Qiu JF, Liu YQ, Zhou WZ, Weng LH. Observation on the clinical effect of different intervention methods in patients with impaired glucose regulation [糖调节受损人群不同干预方法的临床效果观察]. Health Research [健康研究] 2013;33(5):370‐2+5. [Google Scholar]
Zhao 2013 {published data only}
- Zhao XY, Sun JZ. Clinical observation of metformin in the treatment of obesity with prediabetes (46 cases report attached) [二甲双胍治疗肥胖合并糖尿病前期的临床观察(附46例报告)]. Journal of Hubei University of Science and Technology (Medical Sciences) [湖北科技学院学报(医学版)] 2013;27(1):27‐9. [Google Scholar]
References to studies excluded from this review
Acbay 1996 {published data only}
- Acbay O, Gundogdu S. The efficacy of metformin in the treatment of hypertensive subjects with impaired glucose tolerance: Its effects on insulin sensitivity, blood pressure, carbohydrate and lipid metabolism. Klinik Gelisim 1996;9(1):4019‐25. [Google Scholar]
Ballon 2011 {published data only}
- Ballon JS, Hamer RM, Catellier DJ, Stewart D, Lavange L, Golden L, et al. Metformin and impaired glucose tolerance in overweight persons with schizophrenia. Oxford University Press 2011;37:24. [Google Scholar]
Biarnés 2005 {published data only}
- Biarnés J, Fernández‐Real JM, Fernández‐Castañer M, Mar García M, Soler J, Ricart W. Differential regulation of insulin action and tumor necrosis factor alpha system activity by metformin. Metabolism 2005;54(2):235‐9. [DOI] [PubMed] [Google Scholar]
Bulcão 2007 {published data only}
- Bulcão C, Ribeiro‐Filho FF, Sañudo A, Roberta Ferreira SG. Effects of simvastatin and metformin on inflammation and insulin resistance in individuals with mild metabolic syndrome. American Journal of Cardiovascular Drugs 2007;7(3):219‐24. [DOI] [PubMed] [Google Scholar]
Caballero 2004 {published data only}
- Caballero AE, Delgado A, Aguilar‐Salinas CA, Herrera AN, Castillo JL, Cabrera T, et al. The differential effects of metformin on markers of endothelial activation and inflammation in subjects with impaired glucose tolerance: a placebo‐controlled, randomised clinical trial. Journal of Clinical Endocrinology and Metabolism 2004;89(8):3943‐8. [DOI] [PubMed] [Google Scholar]
Celik 2012 {published data only}
- Celik O, Acbay O, Celik O, Acbay O. Effects of metformin plus rosuvastatin on hyperandrogenism in polycystic ovary syndrome patients with hyperlipidaemia and impaired glucose tolerance. Journal of Endocrinological Investigation 2012;35(10):905‐10. [DOI] [PubMed] [Google Scholar]
Chazova 2006 {published data only}
- Chazova I, Almazov VA, Shlyakhto E. Moxonidine improves glycaemic control in mildly hypertensive, overweight patients: a comparison with metformin. Diabetes, Obesity & Metabolism 2006;8(4):456‐65. [DOI] [PubMed] [Google Scholar]
Chen 2013 {published data only}
- Chen Y, Su DD, Feng JG. Metformin combined with lifestyle guidance in the treatment of 34 patients with obesity prediabetes [二甲双胍联合生活方式指导治疗34例肥胖型糖尿病前期患者]. Journal of Practical Diabetology [实用糖尿病杂志] 2013;9(5):48‐9. [Google Scholar]
ChiCTR‐TRC‐09000548 {published data only}
- ChiCTR‐TRC‐09000548. A randomized controlled trial for investigating the protective effects of fenofibrate on prediabetes with hypertriglyceridemia. apps.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR‐TRC‐09000548 (accessed 30 March 2019).
CTRI/2013/02/003417 {published data only}
- CTRI/2013/02/003417. Restudy of the PURSE HIS Population for the prevalence of risk factors causing blood vessel damage to heart muscle, brain tissue or peripheral tissue and to give advice on diet, exercise and if necessary small dose of drug to prevent conversion of Pre‐Diabetes to Diabetes. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2013/02/003417 (accessed 30 March 2019).
Eguchi 2007 {published data only}
- Eguchi K. Comparison of the effects of pioglitazone and metformin on insulin resistance and hormonal markers in patients with impaired glucose tolerance and early diabetes. Hypertension Research 2007;30(1):23‐30. [DOI] [PubMed] [Google Scholar]
Esteghamati 2013 {published data only}
- Esteghamati A, Noshad S, Rabizadeh S, Ghavami M, Zandieh A, Nakhjavani M. Comparative effects of metformin and pioglitazone on omenti and leptin concentrations in patients with newly diagnosed diabetes: a randomised clinical trial. Regulatory Peptides 2013;182:1‐6. [DOI] [PubMed] [Google Scholar]
EUCTR‐000650‐21‐ES {published data only}
- EUCTR2018‐000650‐21‐ES. Clinical trial to evaluate biomarkers of vascular aging. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2018‐000650‐21‐ES (accessed 16 September 2019).
EUCTR2008‐004497‐40‐GB {published data only}
- EUCTR2008‐004497‐40‐GB. The effect of metformin on weight and cardiovascular risk markers in abdomenally obese subjects with impaired fasting glucose previously treated for 12 months with either rimonabant or placebo ‐ use of metformin following weight loss and improvement in CV risk. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2008‐004497‐40‐GB (accessed 20 March 2019).
Fleming 2002 {published data only}
- Fleming R, Hopkinson ZE, Michael Wallace A, Greer IA, Sattar N. Ovarian function and metabolic factors in women with oligomenorrhoea treated with metformin in a randomised double blind placebo‐controlled trial. Journal of Clinical Endocrinology and Metabolism 2002;87(2):569‐74. [DOI] [PubMed] [Google Scholar]
Flores‐Saenz 2003 {published data only}
- Flores‐Saenz JL, Trujillo‐Arriaga HM, Rivas‐Vilchis JF, Mendez‐Francisco JD, Alarcon‐Aguilar FJ, Roman‐Ramos R. Crossover and double blind study with metformin and rosiglitazone in impaired glucose tolerance subjects. Proceedings of the Western Pharmacology Society 2003;46:143‐7. [PubMed] [Google Scholar]
Gómez‐Díaz 2012 {published data only}
- Gómez‐Díaz RA, Talavera JO, Pool EC, Ortiz‐Navarrete FV, Solórzano‐Santos F, Mondragón‐González R, et al. Metformin decreases plasma resistin concentrations in pediatric patients with impaired glucose tolerance: a placebo‐controlled randomised clinical trial. Metabolism 2012;61(9):1247‐55. [DOI] [PubMed] [Google Scholar]
Gore 2005 {published data only}
- Gore DC, Wolf SE, Sanford A, Herndon DN, Wolfe RR. Influence of metformin on glucose intolerance and muscle catabolism following severe burn injury. Annals of Surgery 2005;241(2):334‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gram 2011 {published data only}
- Gram J, Henriksen JE, Grodum E, Juhl H, Hansen TB, Christiansen C, et al. Pharmacological treatment of the pathogenetic defects in type 2 diabetes: The randomised multicenter South Danish diabetes study. Diabetes Care 2011;34(1):27‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guardado‐Mendoza R 2018 {published data only}
- Guardado‐Mendoza R, Jimenez‐Ceja L, Farfan D, Alvarez‐Canales M, Salazar‐Lopez S, Montes De Oca M, et al. Diabetes prevention with lifestyle, linagliptin and metformin in patients with prediabetes: The PRELLIM project. Diabetologia 2018;61:S22‐3. [Google Scholar]
Haukeland 2008 {published data only}
- Haukeland JW, Konopski Z, Loeberg EM, Haaland TK, Volkmann HL, Raschpichler G. A randomised, placebo controlled trial with metformin in patients with NAFLD. Hepatology 2008;48(4):334‐9. [Google Scholar]
Ishida 2005 {published data only}
- Ishida W, Satoh J. Characteristic of metformin for treatment of impaired glucose tolerance. Nihon Rinsho 2005;63 Suppl 2:433‐7. [PubMed] [Google Scholar]
Kato 2009 {published data only}
- Kato T, Sawai Y, Kanayama H, Taguchi H, Terabayashi T, Taki F, et al. Comparative study of low‐dose pioglitazone or metformin treatment in Japanese diabetic patients with metabolic syndrome. Experimental and Clinical Endocrinology & Diabetes 2009;117(10):593‐603. [DOI] [PubMed] [Google Scholar]
Kelly 2012 {published data only}
- Kelly AS, Bergenstal RM, Gonzalez‐Campoy JM, Katz H, Bank AJ. Effects of exenatide vs. metformin on endothelial function in obese patients with pre‐diabetes: a randomised trial. Cardiovascular Diabetology 2012;11(64):1327‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kendall 2013 {published data only}
- Kendall D, Vail A, Amin R, Barrett T, Dimitri P, Ivison F, et al. Metformin in obese children and adolescents: the MOCA trial. Journal of Clinical Endocrinology and Metabolism 2013;98(1):322‐9. [DOI] [PubMed] [Google Scholar]
Kilic 2011 {published data only}
- Kilic S, Yilmaz N, Zulfikaroglu E, Erdogan G, Aydin M, Batioglu S. Inflammatory‐metabolic parameters in obese and non‐obese normoandrogenemic polycystic ovary syndrome during metformin and oral contraceptive treatment. Gynecological Endocrinology 2011;27(9):622‐9. [DOI] [PubMed] [Google Scholar]
Koev 2004 {published data only}
- Koev D, Koeva L. Treatment of subjects with obesity and impaired fasting glucose with metformin. Endokrinologya 2004;9(4):207‐13. [Google Scholar]
Krysiak 2012 {published data only}
- Krysiak R, Okopien B. Lymphocyte‐suppressing and systemic anti‐inflammatory effects of high‐dose metformin in simvastatin‐treated patients with impaired fasting glucose. Atherosclerosis 2012;225(2):403‐7. [DOI] [PubMed] [Google Scholar]
Lehtovirta 2001 {published data only}
- Lehtovirta M, Forsén B, Gullström M, Häggblom M, Eriksson JG, Taskinen MR, et al. Metabolic effects of metformin in patients with impaired glucose tolerance. Diabetic Medicine 2001;18(7):578‐83. [DOI] [PubMed] [Google Scholar]
Li 2009b {published data only}
- Li AM, Zhao J. Effect of renshen jianxin capsule for alleviating insulin resistance in patients with coronary heart disease and glucose tolerance impairment. Chinese Journal of Integrated Traditional & Western Medicine 2009;29(9):830‐3. [PubMed] [Google Scholar]
LIMIT‐1 {published data only}
- Hertog HM, Vermeer SE, Achterberg S, Algra A, Kappelle LJ, Dippel DWJ, et al. Safety and feasibility of treatment with metformin in patients with TIA or minor ischaemic stroke and impaired glucose tolerance: a randomised open‐label phase II trial. Cerebrovascular Diseases 2009;17(Suppl 6):151. [Google Scholar]
Lu 2011 {published data only}
- Lu YH, Lu JM, Wang SY, Li CL, Zheng RP, Tian H, et al. Outcome of intensive integrated intervention in participants with impaired glucose regulation in China. Advances in Therapy 2011;28(6):511‐9. [DOI] [PubMed] [Google Scholar]
Malin 2013 {published data only}
- Malin SK, Braun B. Effect of metformin on substrate utilization after exercise training in adults with impaired glucose tolerance. Applied Physiology, Nutrition, & Metabolism 2013;38(4):427‐30. [DOI] [PubMed] [Google Scholar]
Medical letter {published data only}
- Anonymous. Metformin for prediabetes. JAMA 2017;317(11):1171. [DOI] [PubMed] [Google Scholar]
Morel 1999 {published data only}
- Morel Y, Golay A, Perneger T, Lehmann T, Vadas L, Pasik C, et al. Metformin treatment leads to an increase in basal, but not insulin‐stimulated, glucose disposal in obese patients with impaired glucose tolerance. Diabetic Medicine 1999;16(8):650‐5. [DOI] [PubMed] [Google Scholar]
- Morel Y, Lehmann T, Vadas L, Golay A. Effect of metformin on insulin‐resistance in obese patients with glucose‐intolerance. Schweizerische medizinische Wochenschrift 1996;126(Suppl 74/I):12. [Google Scholar]
NCT00108615 {published data only}
- NCT00108615. Effects of insulin sensitizers in subjects with impaired glucose tolerance. clinicaltrials.gov/ct2/show/NCT00108615 (first posted 5 March 2016).
NCT02338193 {published data only}
- NCT02338193. Dapagliflozin and metformin, alone and in combination, in overweight/obese prior GDM women (DAPA‐GDM). clinicaltrials.gov/ct2/show/NCT02338193 (first posted 14 January 2015).
NCT03258723 {published data only}
- NCT03258723. Diabetes prevention with lifestyle intervention and metformin escalation (LIME). clinicaltrials.gov/ct2/show/NCT03258723 (first posted 23 August 2017).
Pre‐DICTED {published data only}
- NCT03503942. The pre‐diabetes interventions and continued tracking to ease‐out diabetes (Pre‐DICTED) program. https://clinicaltrials.gov/ct2/show/NCT03503942 Accessed 16th. [DOI] [PMC free article] [PubMed]
RESIST {published data only}
- Garnett SP, Gow M, Ho M, Baur LA, Noakes M, Woodhead HJ, et al. Optimal macronutrient content of the diet for adolescents with prediabetes; RESIST a randomised control trial. Journal of Clinical Endocrinology & Metabolism 2013;98(5):2116‐25. [DOI] [PubMed] [Google Scholar]
Retnakaran 2012 {published data only}
- Retnakaran R, Ye C, Hanley AJ, Harris SB, Zinman B. Discordant effects on central obesity, hepatic insulin resistance, and alanine aminotransferase of low‐dose metformin and thiazolidinedione combination therapy in patients with impaired glucose tolerance. Diabetes, Obesity & Metabolism 2012;14(1):91‐3. [DOI] [PubMed] [Google Scholar]
Rodríguez‐Moctezuma 2005 {published data only}
- Rodríguez‐Moctezuma JR, Robles‐López G, López‐Carmona JM, Gutiérrez‐Rosas MJ. Effects of metformin on the body composition in subjects with risk factors for type 2 diabetes. Diabetes, Obesity & Metabolism 2005;7(2):189‐92. [DOI] [PubMed] [Google Scholar]
Scheen 2009 {published data only}
- Scheen AJ, Tan MH, Betteridge DJ, Birkeland K, Schmitz O, Charbonnel B. Long‐term glycaemic effects of pioglitazone compared with placebo as add‐on treatment to metformin or sulphonylurea monotherapy in PROactive (PROactive 18). Diabetic Medicine 2009;26(12):1242‐9. [DOI] [PubMed] [Google Scholar]
Schuster 2004 {published data only}
- Schuster D, Gaillard T, Rhinesmith S, Habash D, Osei K. Impact of metformin on glucose metabolism in nondiabetic, obese African Americans: A placebo‐controlled, 24‐month randomised study. Diabetes Care 2004;27(11):2768‐9. [DOI] [PubMed] [Google Scholar]
SLCTR/2016/026 {unpublished data only}
- SLCTR/2016/026. Effects of metformin and lifestyle modifications on the progression of the atherosclerosis among individuals with impaired glucose tolerance. slctr.lk/trials/slctr‐2016‐026 (accessed 16 September 2019).
STOP‐NIDDM {published data only}
- Chiasson JL, Gomis R, Hanefeld M, Josse RG, Karasik A, Laakso M. The STOP‐NIDDM trial: An international study on the efficacy of an alpha‐ glucosidase inhibitor to prevent type 2 diabetes in a population with impaired glucose tolerance: Rationale, design, and preliminary screening data. Diabetes Care 1998;21(10):1720‐5. [DOI] [PubMed] [Google Scholar]
Stroup 2013 {published data only}
- Stroup S. Effect of metformin on weight in patients with schizophrenia with impaired fasting glucose. Neuropsychopharmacology. 2013; Vol. 38:S41.
Sultana 2012 {published data only}
- Sultana SS, Amin F, Rahman A, Afsana F. A comparative study with metformin and pioglitazone versus metformin alone in nonalcoholic fatty liver disease in newly detected glucose intolerant patients. Diabetologia 2012;55:S500. [Google Scholar]
UKPDS {published data only}
- Turner R, Rachman J, Holman R. UK Prospective Diabetes Study. Diabetes und Stoffwechsel 1996;5(3):78‐80. [Google Scholar]
Vitolins 2017 {published data only}
- Vitolins MZ, Isom SP, Blackwell CS, Kernodle D, Sydell JM, Pedley CF, et al. The healthy living partnerships to prevent diabetes and the diabetes prevention program: a comparison of year 1 and 2 intervention results. Translational Behavioral Medicine June 2017;7(2):371‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wan 2010 {published data only}
- Wan Q, Wang F, Wang F, Guan Q, Liu Y, Wang C, et al. Regression to normoglycaemia by fenofibrate in pre‐diabetic subjects complicated with hypertriglyceridaemia: a prospective randomised controlled trial. Diabetic Medicine 2010;27(11):1312‐7. [DOI] [PubMed] [Google Scholar]
Zinman 2010 {published data only}
- Zinman B, Harris SB, Neuman J, Gerstein HC, Retnakaran RR, Raboud J, et al. Low‐dose combination therapy with rosiglitazone and metformin to prevent type 2 diabetes mellitus (CANOE trial): a double‐blind randomised controlled study. Lancet 2010;376(9735):103‐11. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
ChiCTR‐IPR‐17012309 {published data only}
- ChiCTR‐IPR‐17012309. The effect of metformin on the number and function of the circulating endothelial progenitor cells in prediabetic patients. www.chictr.org.cn/showproj.aspx?proj=21001 (accessed 5 April 2019).
EDIT 1997 {published data only}
- Anonymous. Early Diabetes Intervention Trial. https://www.dtu.ox.ac.uk/EDIT/ (accessed 15 March 2019).
- Citroën HA, Tunbridge FKE, Holman RR. Possible prevention of type 2 diabetes with acarbose or metformin over three years. Diabetologia 2000;43(Suppl. 1):A73. [Google Scholar]
- Holman RR, Blackwell L, Manley SE, Tucker L, Frighi V, Stratton IM. Results from the early diabetes intervention trial. Diabetes 2003;52(Suppl. 1):A16. [Google Scholar]
- Holman RR, Blackwell L, Stratton IM, Manley SE, Tucker L, Frighi V. Six‐years results from the Early Diabetes Intervention Trial. Diabetic Medicine 2003;20(Suppl. 2):15. [Google Scholar]
- Holman RR, North BV, Tunbridge FKE. Early Diabetes Intervention Trial. Diabetes 1997;46(Suppl 1):157A. [Google Scholar]
- Holman RR, North BV, Tunbridge FKE. Early diabetes intervention trial. Diabetologia 1997; Vol. 40, issue Suppl. 1:A17.
- Holman RR, North BV, Tunbridge FKE. Possible prevention of type 2 diabetes with acarbose or metformin. Diabetes YR 2000;49(Suppl. 1):450‐P. [Google Scholar]
- ISRCTN96631607. Early diabetes intervention trial. apps.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN96631607 (accessed 30 March 2019).
- Kim JI, Stevens RJ, Holman RR. The haemoglobin glycation index is reproducible in dysglycaemic individual but is not explained by post‐challenge plasma glucose levels [Abstract]. Diabetologia 2004;47(suppl 1):A122.
NCT02409238 {published data only}
- NCT02409238. Insulin resistance and mild cognitive impairment (IRMCI) study. clinicaltrials.gov/ct2/show/NCT02409238 (first posted 6 April 2015);‐:‐. [Google Scholar]
Polanco 2015 {published data only}
- Polanco MA, Barrientos R, Godinez SA, Sanchez S, Leanos R, Plascencia S. Preventing the development of type 2 diabetes in subjects at high risk in western Mexico, using metformin and changes in lifestyle, followed 10 years. Diabetes 2015;64:A687. [Google Scholar]
References to ongoing studies
CTRI/2017/09/009635 {published data only}
- CTRI/2017/09/009635. A study of life style modification with and without metformin in prediabetic subjects. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=16184 (accessed 5 April 2019).
ePRECIDE 2017 {published and unpublished data}
- Unpublished protocol. Provided by the investigators.
- EUCTR2013‐000418‐39‐AT. Early prevention of diabetes complications in people with hyperglycaemia in Europe ‐ e‐PREDICE. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2013‐000418‐39‐AT (accessed 8 April 2016).
- EudraCT 2013‐000418‐39. Early prevention of diabetes complications in people with hyperglycaemia in Europe. www.clinicaltrialsregister.eu/ctr‐search/search?query=2013‐000418‐39 (accessed 17 March 2016).
- NCT03222765. Prevention of microvascular complications in prediabetes e‐PREDICE Study (ePREDICE). clinicaltrials.gov/ct2/show/NCT03222765 (first posted 19 July 2017).
- ePRECIDE. The project. www.epredice.eu/en/the‐project (accessed 17 March 2016).
Espinoza 2019 {published data only}
- Espinoza SE, Musi N, Wang CP, Michalek J, Orsak B, Romo T, et al. Rationale and study design of a randomized clinical trial of metformin to prevent frailty in older adults with pre‐diabetes. Journals of Gerontology Biological Sciences and Medical Sciences March 2019; Vol. pii: glz078. [DOI] [PMC free article] [PubMed]
- NCT02570672. Metformin for preventing frailty in high‐risk older adults. clinicaltrials.gov/ct2/show/NCT02570672 (first posted 7 October 2015).
Ji 2019 {published data only}
- Ji L, Sun N, Zhang Y, Zhang L, Shen S, Wang X, et al. Efficacy of metformin in preventing progression to diabetes in Chinese subjects with impaired glucose regulation: protocol for a multicenter, open‐label, randomized, controlled clinical study. Diabetes Obesity Metabolism September 2019;Epub ahead of print:No page numbers. [DOI] [PubMed] [Google Scholar]
- NCT03441750. Efficacy of metformin in preventing diabetes in China (ChinaDPP). clinicaltrials.gov/ct2/show/NCT03441750 (first posted 21 February 2018).
JPRN‐UMIN000018995 {published and unpublished data}
- JPRN‐UMIN000018995. Metformin therapy for East Asian women with recent gestational diabetes mellitus and glucose abnormalities: a multicenter, randomised, open‐label trial. upload.umin.ac.jp/cgi‐open‐bin/icdr_e/ctr_view.cgi?recptno=R000021900 (accessed 16 September 2019).
Nadeau 2014 {published and unpublished data}
- NCT01779362. RISE adult medication study (RISE Adult). clinicaltrials.gov/ct2/show/NCT01779362 (first posted 30 January 2013).
- Nadeau KJ, Mather KJ, Arslanian SA, Buchanan TA, Caprio S, Edelstein SL, et al. RISE Consortium. Restoring Insulin Secretion (RISE): design of studies of beta‐cell preservation in prediabetes and early type 2 diabetes across the life span. Diabetes Care 2014;37(3):780‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01804049 {published data only}
- NCT01804049. Metformin and muscle in insulin‐resistant older veterans (M&M). clinicaltrials.gov/ct2/show/NCT01804049 (first posted 5 March 2013).
NCT02915198 {published and unpublished data}
- NCT02915198. Investigation of metformin in pre‐diabetes on atherosclerotic cardiovascular outcomes (VA‐IMPACT). clinicaltrials.gov/ct2/show/NCT02915198 (first posted 26 September 2016).
NCT02969798 {published data only}
- NCT02969798. Pre‐diabetes in subject with impaired fasting glucose (IFG) and impaired glucose tolerance (IGT). clinicaltrials.gov/show/NCT02969798 (first received 16 March 2016).
NCT03194009 {published data only}
- NCT03194009. Prudente; diabetes prevention via exercise, nutrition and treatment (PRuDENTE). clinicaltrials.gov/ct2/show/NCT03194009 (first posted 21 June 2017).
Rhee 2019 {published and unpublished data}
- NCT02981121. Hospital‐based diabetes prevention study in Korea. clinicaltrials.gov/ct2/show/NCT02981121 (first posted 2 December 2016).
- Rhee SY, Chon S, Ahn KJ, Woo JT, Korean Diabetes Prevention Study Investigators. Hospital‐based Korean diabetes prevention study: a prospective, multi‐center, randomized, open‐label controlled study. Diabetes and Metabolism Journal 2019;43(1):49‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Abdul‐Ghani 2006
- Abdul‐Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes 2006;55(5):1430‐5. [PUBMED: 16644701] [DOI] [PubMed] [Google Scholar]
ADA 1997
- The Expert Committee on the diagnosis and classification of diabetes mellitus. Report of the Expert Committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1997;20(7):1183‐97. [DOI] [PubMed] [Google Scholar]
ADA 2003
- The Expert Committee on the diagnosis and classification of diabetes mellitus. Follow‐up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26(11):3160‐7. [DOI] [PubMed] [Google Scholar]
ADA 2008
- American Diabetes Association. Standards of medical care in diabetes ‐ 2008. Diabetes Care 2008;31(Suppl 1):S12‐54. [PUBMED: 18165335] [DOI] [PubMed] [Google Scholar]
ADA 2010
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(Suppl 1):S62‐9. [PUBMED: 20042775] [DOI] [PMC free article] [PubMed] [Google Scholar]
ADA 2015
- American Diabetes Association. Standards of medical care in diabetes‐2015. Diabetes Care 2015;38(Suppl 1):S1‐93. [PUBMED: 24357209] [PubMed] [Google Scholar]
ADA 2017
- American Diabetes Association. Standards of medical care in diabetes‐2017. Diabetes Care 2017;40:(Suppl 1). [Google Scholar]
ADA 2019a
- American Diabetes Association. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes 2019. Diabetes Care January 2019;42 (Supplement 1):S90‐S102. [DOI] [PubMed] [Google Scholar]
AHFS 1999
- American Hospital Formulary Service (AHFS). Metformin hydrochloride. American Hospital Formulary Service Drug Information. Bethesda, USA: American Society of Health‐System Pharmacists, Inc. 1999; Vol. 2755–63.
Altman 2003
- Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326(7382):219. [PUBMED: 12543843] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bell 2013
- Bell ML, McKenzie JE. Designing psycho‐oncology randomised trials and cluster randomised trials: variance components and intra‐cluster correlation of commonly used psychosocial measures. Psychooncology 2013;22:1738‐47. [DOI] [PubMed] [Google Scholar]
Borenstein 2017a
- Borenstein M, Higgins JP, Hedges LV, Rothstein HR. Basics of meta‐analysis: I² is not an absolute measure of heterogeneity. Research Synthesis Methods 2017;8(1):5‐18. [DOI] [PubMed] [Google Scholar]
Borenstein 2017b
- Borenstein M. Prediction intervals. www.meta‐analysis.com/prediction (accessed 3 July 2017).
Boutron 2014
- Boutron I, Altman DG, Hopewell S, Vera‐Badillo F, Tannock I, Ravaud P. Impact of spin in the abstracts of articles reporting results of randomized controlled trials in the field of cancer: the SPIIN randomized controlled trial. Journal of Clinical Oncology 2014;32:4120‐6. [DOI] [PubMed] [Google Scholar]
Centers for Disease Control and Prevention 2015
- http://www.cdc.gov/diabetes/data/statistics/2014statisticsreport.html. Assessed October 2015.
Cheng 2006
- Cheng C, Kushner H, Falkner BE. The utility of fasting glucose for detection of prediabetes. Metabolism: Clinical and Experimental 2006;55(4):434‐8. [PUBMED: 16546472] [DOI] [PubMed] [Google Scholar]
Cho 2015
- Cho K, Chung JY, Cho SK, Shin HW, Jang IJ, Park JW, et al. Antihyperglycemic mechanism of metformin occurs via the AMPK/LXRalpha/POMC pathway. Scientific Reports 2015;5(‐):8145. [PUBMED: 25634597] [DOI] [PMC free article] [PubMed] [Google Scholar]
Corbett 2014
- Corbett MS, Higgins JP, Woolacott NF. Assessing baseline imbalance in randomised trials: implications for the Cochrane risk of bias tool. Research Synthesis Methods 2014;5(1):79‐85. [DOI] [PubMed] [Google Scholar]
Corey 2007
- Corey EJ, Czakó B, Kürti L. Molecules and Medicine. Hoboken, NJ: Wiley, 2007. [978‐0‐470‐22749‐7] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG (editors), on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
DeFronzo 1989
- DeFronzo RA, Ferrannini E, Simonson DC. Fasting hyperglycemia in non‐insulin‐dependent diabetes mellitus: contributions of excessive hepatic glucose production and impaired tissue glucose uptake. Metabolism: Clinical and Experimental 1989;38(4):387‐95. [PUBMED: 2657323] [DOI] [PubMed] [Google Scholar]
DeFronzo 1999
- DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Annals of Internal Medicine 1999;131(4):281‐303. [DOI] [PubMed] [Google Scholar]
Diabetes Prevention Program 2002
- Knowler WC, Barrett‐Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New England Journal of Medicine 2002;346(6):393‐403. [PUBMED: 11832527] [DOI] [PMC free article] [PubMed] [Google Scholar]
Diabetes Prevention Program FU 2009
- Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, et al. Diabetes Prevention Program Research Group. 10‐year follow‐up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009 Nov 14;374(9702):1677‐86. [DOI: 10.1016/S0140‐6736(09)61457‐4; PUBMED: PMID: 19878986] [DOI] [PMC free article] [PubMed] [Google Scholar]
diabetes.co.uk 2019a
- Diabetes UK. Blood Sugar Converter. https://www.diabetes.co.uk/blood‐sugar‐converter.html Accessed 30 March 2019.
diabetes.co.uk 2019b
- Diabetes UK. Convert Whole Blood Results to Plasma Readings. https://www.diabetes.co.uk/whole‐blood‐readings‐to‐plasma‐converter.html Accessed 30 March 2019.
DPP 2002
- Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New England Journal of Medicine 2002;346(6):393‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duca 2015
- Duca FA, Cote CD, Rasmussen BA, Zadeh‐Tahmasebi M, Rutter GA, Filippi BM, et al. Metformin activates a duodenal AMPK‐dependent pathway to lower hepatic glucose production in rats. Nature Medicine 2015;21(5):506‐11. [PUBMED: 25849133] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dunkley 2014
- Dunkley AJ, Bodicoat DH, Greaves CJ, Russell C, Yates T, Davies MJ, et al. Diabetes prevention in the real world: effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations: a systematic review and meta‐analysis. Diabetes Care 2014;37(4):922‐33. [PUBMED: 24652723] [DOI] [PubMed] [Google Scholar]
FDA 1994
- FDA. FDA Approved Drug Products. https://www.accessdata.fda.gov/drugsatfda_docs/nda/pre96/020357Orig1s000rev.pdf. Assessed 20th September 2019.
Finnish Diabetes Prevention Study Group 2001
- Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne‐Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. New England Journal of Medicine 2001;344(18):1343‐50. [PUBMED: 11333990] [DOI] [PubMed] [Google Scholar]
Gosmanov 2014
- Gosmanov AR, Wan J. Low positive predictive value of hemoglobin A1c for diagnosis of prediabetes in clinical practice. American Journal of the Medical Sciences 2014;348(3):191‐4. [PUBMED: 24556928] [DOI] [PubMed] [Google Scholar]
Haw 2017
- Haw JS, Galaviz KI, Straus AN, Kowalski AJ, Magee MJ, Weber MB, et al. Long‐term sustainability of diabetes prevention approaches: a systematic review and meta‐analysis of randomized clinical trials. JAMA Internal Medicine 2017;177(12):1808‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011c
- Higgins JP, Deeks JJ, Altman DG, editor(s) on behalf of the Cochrane Statistical Methods Group. Chapter 16: Special topics in statistics. In: Higgins JT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Higgins 2017
- Higgins JP, Altman DG, Sterne JA (editors) on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017), Cochrane, 2017. Available from training.cochrane.org/handbook.
Hróbjartsson 2013
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. Canadian Medical Association Journal 2013;185(4):E201‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2016
- Huang W, Castelino RL, Peterson GM. Lactic acidosis and the relationship with metformin usage: Case reports. Medicine 2016;95(46):e4998. [PUBMED: 27861334] [DOI] [PMC free article] [PubMed] [Google Scholar]
ICH 1997
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice //1997 CFR & ICH Guidelines. PA 19063‐2043. USA: Barnett International/PAREXEL, 1997.
IDF 2013
- International Diabetes Federation. International Diabetes Federation. IDF Diabetes Atlas, 6th ed.Brussels, Belgium. International Diabetes Federation, 2013. [Google Scholar]
IEC 2009
- International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009;32(7):1327‐34. [PUBMED: 19502545] [DOI] [PMC free article] [PubMed] [Google Scholar]
Inzucchi 2012
- Inzucchi SE. Clinical practice. Diagnosis of diabetes. New England Journal of Medicine 2012;367(6):542‐50. [PUBMED: 22873534] [DOI] [PubMed] [Google Scholar]
Jones 2015
- Jones CW, Keil LG, Holland WC, Caughey MC, Platts‐Mills TF. Comparison of registered and published outcomes in randomized controlled trials: a systematic review. BMC Medicine 2015;13:282. [DOI: 10.1186/s12916-015-0520-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kalantar‐Zadeh 2013
- Kalantar‐Zadeh K, Uppot RN, Lewandrowski KB. Case records of the Massachusetts General Hospital. Case 23‐2013. A 54‐year‐old woman with abdominal pain, vomiting, and confusion. New England Journal of Medicine 2013;369(4):374‐82. [PUBMED: 23841704] [DOI] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI: 10.1136/bmj.c365] [DOI] [PubMed] [Google Scholar]
Kreisberg 1980
- Kreisberg RA. Lactate homeostasis and lactic acidosis. Annals of Internal Medicine 1980;92(2 Pt 1):227‐37. [PUBMED: 6766289] [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic and meta‐analyses of studies that evaluate interventions: explanation and elaboration. PLOS Medicine 2009;6(7):1‐28. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lily 2009
- Lily, M, Godwin, M. Treating prediabetes with metformin: systematic review and meta‐analysis. Canadian Family Physician 2009; Vol. 55, issue 4:363‐9. [PMC free article] [PubMed]
Lundh 2017
- Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2017, Issue 2. [DOI: 10.1002/14651858.MR000033.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mathieu 2009
- Mathieu S, Boutron I, Moher D, Altman DG, Ravaud P. Comparison of registered and published primary outcomes in randomized controlled trials. JAMA 2009;302:977‐84. [DOI] [PubMed] [Google Scholar]
Meader 2014
- Meader N, King K, Llewellyn A, Norman G, Brown J, Rodgers M, et al. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Systematic Reviews 2014;3:82‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moelands 2018
- Moelands SV, Lucassen PL, Akkermans RP, Grauw WJ, Laar FA. Alpha‐glucosidase inhibitors for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2018;12:CD005061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moin 2018
- Moin T, Schmittdiel JA, Flory JH, Yeh J, Karter AJ, Kruge E, et al. Review of metformin use for type 2 diabetes prevention. American Journal of Preventive Medicine 2018;55(4):565‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morris 2013
- Morris DH, Khunti K, Achana F, Srinivasan B, Gray LJ, Davies MJ, et al. Progression rates from HbA1c 6.0‐6.4% and other prediabetes definitions to type 2 diabetes: a meta‐analysis. Diabetologia 2013;56(7):1489‐93. [PUBMED: 23584433] [DOI] [PubMed] [Google Scholar]
NDDG 1979
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039‐57. [DOI] [PubMed] [Google Scholar]
Pang 2018
- Pang B, Zhao LH, Li XL, Song J, Li QW, Liao X, et al. Different intervention strategies for preventing type 2 diabetes mellitus in China: A systematic review and network meta‐analysis of randomized controlled trials. Diabetes Obesity Metabolism 2018;20(3):718‐22. [DOI] [PubMed] [Google Scholar]
Riley 2011
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta‐analyses. BMJ 2011;342:d549. [DOI] [PubMed] [Google Scholar]
Salpeter 2008
- Salpeter SR, Buckley NS, Kahn JA, Salpeter EE. Meta‐analysis: metformin treatment in persons at risk for diabetes mellitus. American Journal of Medicine 2008;121(2):149‐57. [DOI] [PubMed] [Google Scholar]
Salpeter 2010
- Salpeter SR, Greyber E, Pasternak GA, Salpeter EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.CD002967.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schousboe 2012
- Schousboe K, Fassi D, Secher EL, Elming H, Rasmussen K, Hornum M. Treatment of metformin‐associated lactate acidosis by haemodialysis [Behandling af metforminassocieret laktatacidose med haemodialyse]. Ugeskrift for Laeger 2012;174(23):1604‐6. [PUBMED: 22673381] [PubMed] [Google Scholar]
Selvin 2011
- Selvin E, Steffes MW, Ballantyne CM, Hoogeveen RC, Coresh J, Brancati FL. Racial differences in glycemic markers: a cross‐sectional analysis of community‐based data. Annals of Internal Medicine 2011;154(5):303‐9. [PUBMED: 21357907] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Sterne 2017
- Sterne JA, Egger M, Moher D, Boutron I (editors). Chapter 10: Addressing reporting biases. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
UKPDS 1998
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood‐glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352(9131):854‐65. [PubMed] [Google Scholar]
Van de Laar 2006
- Laar FA, Lucassen PL, Akkermans RP, Lisdonk EH, Grauw WJ. Alpha‐glucosidase inhibitors for people with impaired glucose tolerance or impaired fasting blood glucose. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD005061.pub2] [DOI] [PubMed] [Google Scholar]
Viera 2011
- Viera AJ. Predisease: when does it make sense?. Epidemiologic Reviews 2011;33:122‐34. [DOI] [PubMed] [Google Scholar]
Vist 2008
- Vist GE, Bryant D, Somerville L, Birminghem T, Oxman AD. Outcomes of patients who participate in randomized controlled trials compared to similar patients receiving similar interventions who do not participate. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.MR000009.pub4; PUBMED: 18677782] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1998
- Alberti KM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its compliactions. Part I: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Medicine 1998;15:539‐53. [DOI] [PubMed] [Google Scholar]
WHO 1999
- World Health Organization: definition, diagnosis, classification of diabetes mellitus, its complications. Part 1: diagnosis and classification of diabetes mellitus. Report of a World Health Organization Consultation. Geneva: World Health Organization, 1999:1‐59. [Google Scholar]
WHO/IDF 2006
- World Health Organization/ International Diabetes Federation. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a WHO/IDF consultation. http://www.idf.org/webdata/docs/WHO_IDF_definition_diagnosis_of_diabetes.pdf 2006; Vol. Assessed October 2015.
Witters 2001
- Witters LA. The blooming of the French lilac. Journal of Clinical Investigation 2001;108(8):1105‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yudkin 2014
- Yudkin JS, Montori VM. The epidemic of pre‐diabetes: the medicine and the politics. BMJ 2014;349:g4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Lü 2010
- Lü Q, Ke LQ, Tong N, Cao L, Wu T, Zhang J. Metformin for people with impaired glucose tolerance or impaired fasting blood glucose. Cochrane Database of Systematic Reviews 2010, Issue 6. [DOI: 10.1002/14651858.CD008558] [DOI] [Google Scholar]