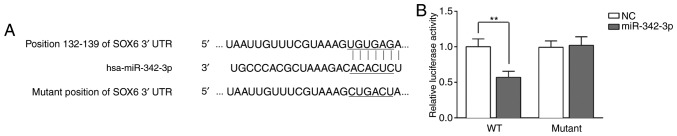Abstract
Diabetic kidney disease (DKD) is one of the major microvascular complications in patients with type 1 and/or type 2 diabetes, the first cause of end-stage renal disease (ESRD) in several countries and regions. However, the pathogenesis of DKD and the mechanisms through which it leads to ESRD remain unknown. Thus, in this study, we aimed to elucidate some of these mechanisms. The expression of microRNA (miRNA or miR)-342-3p and SRY-box 6 (SOX6) in the renal tissues of mice with DKD and mouse renal mesangial cells (MCs) was determined by RT-qPCR and western blot analysis. The diabetic kidney environment was established using high-glucose medium. SOX6 was verified as a target gene of miR-342-3p by dual-luciferase activity assay. In addition, western blot analysis was employed to determine the changes in the levels of several biomarkers of fibrosis [transforming growth factor (TGF)-β1, fibronectin (FN), collagen IV (referred to as C-IV) and phosphatase and tensin homolog (PTEN)]. Compared with THE control mice, the expression of miR-342-3p in the kidney tissues of mice with DKD was down-regulated, whereas that of SOX6 was upregulated. The same phenomenon was observed in the MCs cultured in high-glucose medium. Subsequently, miR-342-3p inhibited SOX6 expression, promoted cell proliferation and inhibited the apoptosis of MCs. Moreover, the overexpression of miR-342-3p suppressed high glucose-induced renal interstitial fibrosis. In addition, it was found that miR-342-3p inhibited SOX6 expression by binding to the 3′-UTR of SOX6. On the whole, the findings of this study demonstrate that miR-342-3p suppresses the progression of DKD by inducing the degradation of SOX6. Thus, the miR-342-3p/SOX6 axis may serve as a novel therapeutic target in the treatment of DKD.
Keywords: diabetic kidney disease, microRNA-342-3p, SOX6, renal interstitial fibrosis
Introduction
Diabetic kidney disease (DKD), one of the major microvascular complications in patients with type 1 and/or type 2 diabetes, and is the primary cause of end-stage renal disease (ESRD) in several countries (1). The use of renin/angiotensin axis inhibitors is a widely accepted treatment strategy for DKD, as it decreases the blood glucose levels and blood pressure (2). However, the pathogenesis of DKD and the mechanisms through which it leads to ESRD have not yet been fully elucidated.
MicroRNAs (miRNAs or miRs) are small endogenous, non-coding RNAs (~17-23 nucleotides in length) that bind to the 3′ non-coding region (3′-UTR) of their target genes (3). miRNAs negatively regulate the expression of their target genes by inducing the degradation of messenger RNAs (mRNAs) and/or inhibiting the translation of the proteins from the mRNAs (4). Increasing evidence has indicated that the dysregulation of miRNAs may be involved in the pathogenesis and development of DKD (5,6). Previous studies have also revealed that miR-342-3p is highly expressed in mouse kidney tissues; however, whether miR-342-3p is associated with the progression of DKD remains unknown.
SRY-box 6 (SOX6) is an important member of the Sry-related high mobility group box (Sox) family of transcription factors, which is highly conserved in various mammalian species. It has been shown that SOX6 may play an important role in cell differentiation, apoptosis and proliferation (7,8). Recent studies have demonstrated that SOX6 is involved in the mechanisms underlying diabetes mellitus. For example, Iguchi et al suggested that SOX6 downregulation induces pancreatic β-cell proliferation (9). Pleskovič et al also indicated that the SOX6 gene polymorphism rs16933090 accelerated the progression of subclinical atherosclerosis in patients with type 2 diabetes mellitus (10). However, the effects of SOX6 on the pathogenesis of DKD have not yet been fully elucidated.
In the present study, it was found that miR-342-3p expression was downregulated in the kidneys of mice with DKD, whereas SOX6 was highly expressed. In vitro experiments revealed a decreased miR-342-3p and an increased SOX6 expression in mesangial cells (MCs) cultured under high glucose conditions. Furthermore, it was found that the overexpression of miR-342-3p promoted cell proliferation, and inhibited cell apoptosis and fibrosis. It was also verified that miR-342-3p suppressed the activation of the PTEN/Akt axis by downregulating SOX6 expression. Finally, it was demonstrated that SOX6 is a target gene of miR-342-3p by prediction using bioinformatics and validation using luciferase activity assay. Overall, it was demonstrated that miR-342-3p attenuated the progression of DKD procession by targeting SOX6.
Materials and methods
Animal model and cell culture
This study was approved by the Animal Ethics Committee of Tianjin Medical University (Tianjin, China). All experimental mice (db/db mice, 5-6 weeks old, weighing 20-40 g, all male) were cage-fed in a specific-pathogen-free grade environment (temperature, 23±2°C; relative humidity, 55±5%) and fed standard feed and were provide with access to clean drinking water. The db/db mice fed a high-fat and sugar diet (10% fat, 20% sugars, 2.5% cholesterol, 1.0% cholate, 66.5% conventional forage; Specialty Feeds Pty, Ltd.) for 12-16 weeks were used as a model for DKD, and db/m mice fed normally served as the non-DKD controls (n=10 each group). Each strain was randomized and 3 mice were used for independent experiments. After the mice were sacrificed, the mouse kidney tissue was removed and washed with precooled normal saline for further analysis. Mouse renal MCs were provided by the National Infrastructure of Cell Line Resource of China.
Cell culture and treatment
Mouse renal mesangial cells (MCs) were cultured in low-glucose DMEM medium supplemented with 10% FBS and incubated at 37°C with 5% CO2. For high glucose induction, MCs were treated with 100 µM glucose for 12, 24 and 36 h, respectively. MCs were also treated with various concentrations of glucose (0, 50, 100 and 200 µM) for 24 h.
Plasmid and mimic transfection
miR-342-3p mimics, miRNA mimics control, plasmid SOX6 and plasmid control were synthesized by Shanghai GenePharma Co., Ltd. Lipofectamine 2000 reagent (Thermo Fisher Scientific, Inc.) was used for transient transfection, following the manufacturer's instructions. The cells were harvested at 48 h following transfection.
Total RNA extraction and reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from the mouse kidney tissues and MCs using TRIzol solution (Thermo Fisher Scientific, Inc.). The RNA sample was stored in a -80°C freezer. Reverse transcription was performed according to the instructions provided with the PrimeScript RT reagent (Takara Bio, Inc.). RT-qPCR was performed using SYBR-Green Master Mix II (Takara Bio, Inc.). The thermocycling conditions were conducted as follows: Initial denaturation 95°C for 5 min, followed by 40 cycles of denaturation at 95°C for 15 sec and annealing/elongation at 60°C for 30 sec. All miRNA samples were normalized to U6. Each experiment was repeated 2 times. The 2−ΔΔCq method (11) was used for miR-342-3p expression analysis. Specific gene primer sequences were purchased from Sangon Biotech, and the sequences of the primers are presented in Table SI.
Western blot analysis
Subconfluent cells were washed 3 times with PBS, and the proteins were then harvested with radioim-munoprecipitation assay buffer. The protein concentration was quantified using a BCATM Protein Assay kit (Pierce, Thermo Fisher Scientific, Inc.). Protein (30 µg protein/lane) from each sample was separated by 12% SDS-polyacrylamide gel electrophoresis and western blot analysis were performed following standard protocols. The separated proteins were transferred onto polyvinylidene fluoride membranes and blocked with 5% skim milk at room temperature for 1 h. The membranes were then incubated with primary antibodies at 4°C overnight, followed by incubation with anti-mouse IgG, HRP-linked antibody (cat no. 7076; dilution: 1:2,000) or anti-rabbit IgG, HRP-linked antibody (cat no. 7074; dilution: 1:2,000) (both from Cell Signaling Technology) at room temperature for 2 h. The primary antibodies used were as follows: Anti-SOX6 (cat. no. 14010-1-AP; dilution, 1:2,000), anti-transforming growth factor (TGF)-β1 (cat. no. 21898-1-AP; dilution, 1:1,000), anti-fibronectin (FN; cat. no. 15613-1-AP; dilution, 1:2,000), anti-type-Ⅳ-collagen (referred to as C-IV; cat. no. 55131-1-AP; dilution, 1:2,000), anti-phosphatase and tensin homolog (PTEN; cat. no. 22034-1-AP; dilution, 1:2,000), anti-Akt (cat. no. 10176-2-AP; dilution, 1:2,000), anti-p-Akt (cat. no. 66444-1-Ig; dilution, 1:1,000), GADPH (cat. no. 20536-1-AP; dilution, 1:2,000) (ProteinTech Group, Inc.). Protein expression levels for each sample were normal-ized to β-actin. Densitometry was performed by using the ImageJ software (version 1.38X; National Institutes of Health).
CCK-8 assay
The proliferative ability of the cells was examined using the Cell Counting kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.), as per the manufacturer's instructions. The MCs transfected with miR-342-3p mimics, miRNA mimics control, miR-342-3p mimics/plasmid SOX6 and miR-342-3p mimics/plasmid control were plated in 96-well plates. Subsequently, CCK-8 solution (10% of the medium, 10 µl) was added to each well followed by incubation for 4 h at 4°C prior to analysis. The absorbance was then measured at 450 nm using a FLUOstar® Omega Microplate Reader (BMG Labtech GmbH).
Apoptosis assay
The Annexin V-fluorescein isothio-cyanate (FITC)/propidium iodide (PI) apoptosis detection kit (cat. no. C1062M; Beyotime) was used to assess cell apoptosis. The MCs were placed into 6-well plates (6×105 cells/well). At 48 h following transfection with miR-342-3p mimics, miRNA mimics control, miR-342-3p mimics/plasmid SOX6 and miR-342-3p mimics/plasmid control, flow cytometry (BDFACSCelesta; BD Biosciences) was performed to determine the apoptosis of the transfected cells by measuring the amount of Annexin V-positive and PI-negative cells. FlowJo software (version 7.2.4; FlowJo LLC) was used to analyze the data.
Dual-luciferase activity assay
Bioinformatics software TargetScan (http://www.targetscan.org/vert_72/) was used to predict the bindings sites of miR-342-3p in the 3′UTR of SOX6, and the results indicated the binding sites between the 3′UTR of SOX6 and miR-342-3p. Subsequently, to confirm the binding sites between SOX6 and miR-342-3p, dual-luciferase activity assay was performed. The 3′-UTR of SOX6, which contained the putative target site of miR-342-3p, was ligated into the pGL3 construct (Promega Corp.). The pGL3-SOX6-3′-UTR (wild-type) or pGL3-SOX6-3′-UTR (mutant) (200 ng) and pRL-TK (80 ng; Promega Corp.) constructs were co-transfected into the cells with 60 pmol miR-342-3p mimic or miRNA mimic control using Lipofectamine 2000 (Thermo Fisher Scientific, Inc.). Primers were synthesized by Genscript Nanjing Inc., and the sequences are presented in Table SI. At 24 h following transfection, the Dual Luciferase Reporter Assay System (Promega Corp.) was used to measure the luciferase activity and the luciferase activity was normalized to Renilla luciferase activity.
Statistical analysis
Statistical analysis was performed using SPSS 25.0 software (IBM Corp.). The data were analyzed using the Student's t-test or one-way ANOVA followed by Dunnett's multiple post-hoc tests. All data are presented as the means ± SD of 3 independent experiments. A value of P<0.05 was considered to indicate a statistically significant difference.
Results
High SOX6 expression is inversely associated with miR-342-3p expression in mice with DKD
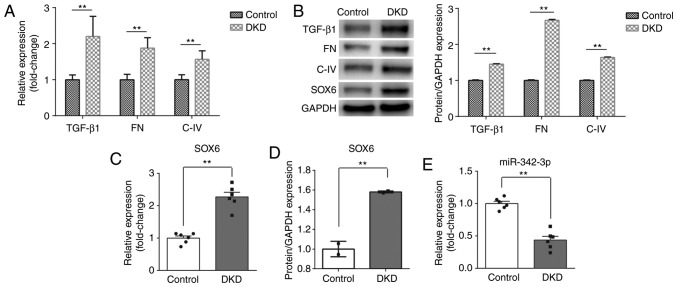
First, to confirm the successful establishment of the mouse model of DKD, RT-qPCR and western blot analysis were performed to detect the levels of biomarkers of kidney injury, including TGF-β1, FN and C-IV. As shown in Fig. 1A and B, the expression of TGF-β1, FN and C-IV was significantly increased at both the mRNA and protein level in the DKD group, as compared with the control group. Following the successful establishment of the DKD model, the SOX6 and miR-342-3p expression levels were measured in mice with DKD. As expected, the expression of SOX6 was higher than that in the control group at both the mRNA and protein level (Fig. 1B-D). However, the expression of miR-342-3p was inversely associated with that of SOX6 and was significantly decreased in the DKD group (Fig. 1E).
Figure 1.
Abnormal expression of miR-342-3p and SOX6 in the kidney tissues of mice with diabetic nephropathy. (A and B) mRNA and protein expression of TGF-β1, FN and C-IV in the control and DKD groups. (B-D) Analysis of SOX6 expression level in the control and DKD groups. A significant increase in SOX6 expression at both the mRNA and protein level was observed in the DKD group, as compared with the control group. (E) Analysis of the miR-342-3p expression level in the control and DKD groups. A notable decrease in miR-342-3p expression was observed in the DKD compared with the control group. **P<0.01. SOX6, SRY-box 6; DKD, diabetic kidney disease; TGF-β1, transforming growth factor β1; FN, fibronectin; C-IV, collagen IV.
High glucose induces a high SOX6 and low miR-342-3p expression
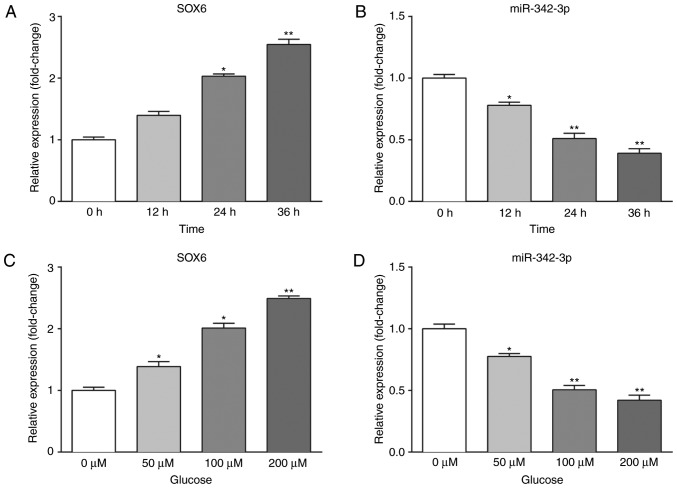
We further examined the effects of high glucose on the expression of SOX6 and miR-342-3p in vitro. MCs were placed in pre-configured high-glucose DMEM and allowed to grow to the logarithmic growth phase. To evaluate the time-dependent effects of high glucose, the MCs were cultured with 100 µM glucose and harvested at 0, 12, 24 and 36 h. To evaluate the dose-dependent effects of high glucose, the MCs were cultured with various concentrations of glucose (0, 50, 100 and 200 µM) and harvested at 24 h. Subsequently, RT-qPCR and western blot analysis were performed to measure the expression levels of SOX6 and miR-342-3p. As shown in Fig. 2, SOX6 expression was upregulated, while that of miR-342-3p was downregulated in the high-glucose environment.
Figure 2.
High glucose affects SOX6 and miR-342-3p expression in vitro in a dose- and time-dependent manner. (A and B) Expression levels of SOX6 mRNA and miR-342-3p in MCs were detected at the indicated time points (0, 12, 24 and 36 h) by RT-qPCR following culture in high-glucose medium. High glucose upregulated SOX6 and downregulated miR-342-3p expression in a time-dependent manner. (C and D) The expression of SOX6 mRNA and miR-342-3p in MCs was detected at 12 h following treatment with various concentrations of glucose (0, 50, 100 and 200 µM) by RT-qPCR. High glucose upregulated SOX6 and downregulated miR-342-3p expression in a dose-dependent manner. *P<0.05 and **P<0.01. SOX6, SRY-box 6; MCs, mesangial cells.
Confirmation of transfection efficiency in mouse MCs
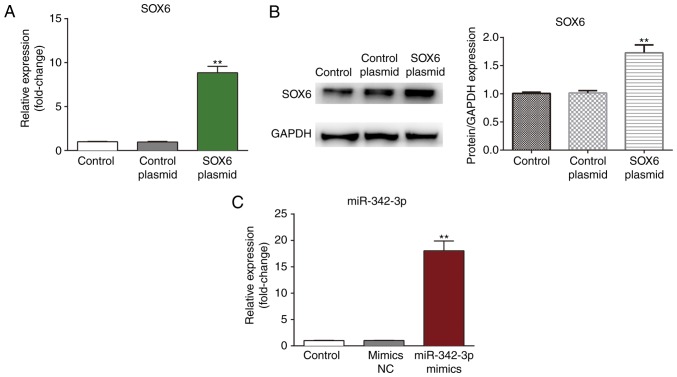
The MCs were transfected with SOX6 plasmid and miR-342-3p and the transfection efficiency was confirmed by measuring mRNA expression by RT-qPCR and protein expression by western blot analysis (Fig. 3). As shown in Fig. 3, a notable increase was observed in SOX6 expression in the MCs at both the mRNA and protein level following transfection with the SOX6 plasmid (Fig. 3A and B). In addition, the expression of miR-342-3p was significantly elevated in the MCs following transfection with the miR-342-3p mimics.
Figure 3.
Validation of the transfection efficiency in mouse MCs. (A and B) Validation of SOX6 plasmid transfection efficiency in MCs by detecting the mRNA and protein expression of SOX6 by RT-qPCR and western blot analysis, respectively. (C) Validation of miR-342-3p mimic transfection efficiency in MCs by measuring the expression of miR-342-3p by RT-qPCR. **P<0.01. MCs, mesangial cells; SOX6, SRY-box 6.
Overexpression of miR-342-3p promotes cell proliferation and inhibits cell apoptosis
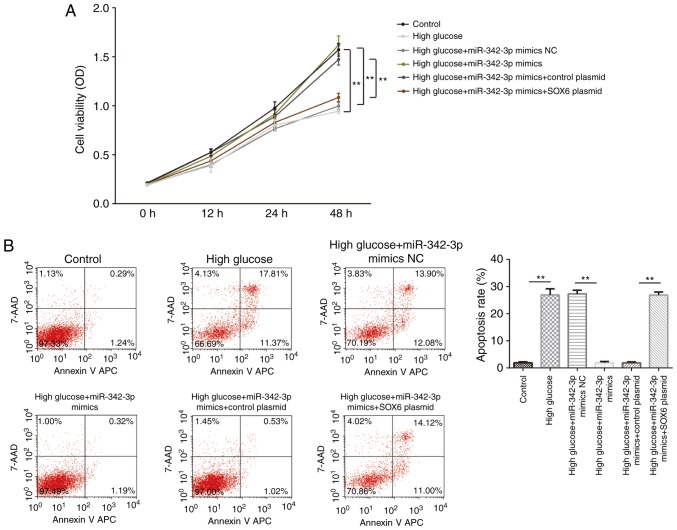
We then evaluated the role of miR-342-3p in the cellular behavior of MCs. The results of CCK-8 assay revealed that miR-342-3p overexpression suppressed high glucose-induced cell proliferation, and this effect was reversed by the exogenous expression of SOX6 (Fig. 4A). Furthermore, miR-342-3p overexpression suppressed high glucose-induced cell apoptosis, and again, this effect was partly reversed by SOX6 overexpression (Fig. 4B). Collectively, these findings suggested that high glucose-induced cellular behavioral changes were suppressed by miR-342-3p overexpression in the MCs.
Figure 4.
miR-342-3p promotes the proliferation and inhibits the apoptosis of MCs treated with high glucose. (A) miR-342-3p overexpression promoted the high glucose-induced proliferation of MCs. Exogenous SOX6 expression partially reversed this effect. (B) miR-342-3p overexpression inhibited the high glucose-induced apoptosis of MCs. Exogenous SOX6 partially reversed this effect. **P<0.01. MCs, mesangial cells; SOX6, SRY-box 6.
miR-342-3p overexpression suppresses high glucose-induced renal interstitial fibrosis
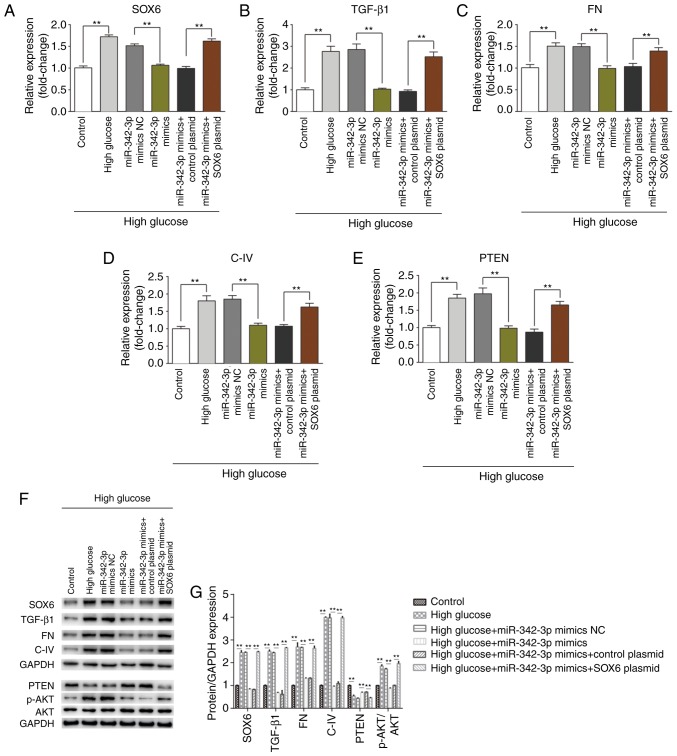
Since miR-342-3p expression was downregulated in mice with DKD and in the MCs cultured under high glucose conditions, and the overexpression of miR-342-3p inhibited high glucose-induced cellular behavioral changes, we hypothesized that miR-342-3p may suppress renal interstitial fibrosis, a crucial procedure in high glucose-induced kidney injury. RT-qPCR and western blot analysis were performed to detect the expression levels of biomarkers of fibrosis (TGF-β1, FN, C-IV and PTEN). As expected, miR-342-3p overexpression notably suppressed the expression of TGF-β1, FN, C-IV, and enhanced PTEN expression at both the mRNA and protein level (Fig. 5); however, SOX6 overexpression partly impaired the miR-342-3p-induced suppression of renal interstitial fibrosis, as the levels of the above-mentioned biomarkers increased following SOX6 overexpression. Overall, these data suggested that miR-342-3p suppressed renal interstitial fibrosis that involves the downregulation of SOX6.
Figure 5.
miR-342-3p downregulates SOX6 and kidney injury-related biomarkers in MCs treated with high glucose. (A-E) miR-342-3p overexpression inhibited the high glucose-induced upregulation of SOX6 and kidney injury-related biomarkers (TGF-β1, FN, C-IV and PTEN) at the mRNA level in MCs. Exogenous SOX6 partially reversed this inhibition. (F and G) miR-342-3p overexpression inhibited the high glucose-induced upregulation of SOX6 and kidney injury-related biomarkers (TGF-β1, FN, C-IV and PTEN) at the protein level in MCs. Exogenous SOX6 partially reversed this inhibition. **P<0.01. SOX6, SRY-box 6; MCs, mesangial cells; TGF-β1, transforming growth factor β1; FN, fibronectin; C-IV, collagen IV.
miR-342-3p directly targets SOX6
As described above, it was found that a high SOX6 expression was inversely associated with miR-342-3p expression in the progression of DKD in vivo, and that SOX6 overexpression partly reversed the protective effects of miR-342-3p on MCs. We therefore hypothesized that miR-342-3p may directly target SOX6. The results of bioinformatics analysis using TargetScan revealed the binding sites between 3′-UTR of SOX6 and miR-342-3p (Fig. 6A). The data indicated that SOX6 was a potential target of miR-342-3p. Subsequenlty, to further verify the binding of miR-342 and SOX6, luciferase activity assay was performed by inserting the wild-type and mutant sites of SOX6 into the pGL3 lucif-erase reporter vector. The pGL3 luciferase reporter vector and miR-342-3p mimics or NC were co-transfected into the MCs. After 48 h, the fluorescence intensity of the wild-type group was notably decreased, as compared to that of the mutant type group (Fig. 6B). The above-mentioned results demonstrated that SOX6 is the target gene of miR-342-3p.
Figure 6.
miR-342-3p directly targets SOX6. (A) Putative miR-342-3p-binding sequence of SOX6 3′UTR. A mutation was constructed in the SOX6 3′UTR sequence in the complementary site for the seed region of miR-342-3p. (B) MCs were transfected with miR-342-3p mimic + SOX6 3′UTR (WT), or miR-342-3p mimic + SOX6 3′UTR (Mut). Luciferase activity was measured 48 h following transfection using a dual-luciferase reporter gene assay. **P<0.01. SOX6, SRY-box 6; MCs, mesangial cells; WT, wild-type; Mut, mutant.
Discussion
To date, multi-factor intervention controlling blood glucose, blood lipids and blood pressure, including drugs that block renin angiotensin, remains the main treatment option for diabetic nephropathy (12,13). However, with the development of molecular biology techniques, the therapeutic value of molecular targets in diabetic nephropathy is gradually becoming evident. SOX6, an important member of the SOX family of transcription factors, has been shown to limit the growth and invasion of tumor cells (14-16). However, studies focusing on the function of SOX6 in the progression of DKD are limited. Herein, a high expression of SOX6 was observed in the kidneys of mice with DKD, and SOX6 expression was found to be inversely associated with miR-342-3p expression. Cellular and molecular assays revealed that SOX6 suppressed the miR-342-3p-induced inhibition of DKD progression.
The dysregulated expression of miRNAs has been shown to be involved in various mechanisms of DKD progression (6,12,17,18). miR-342-3p has been reported to participate in the progression of DKD (19). However, the potential mechanisms of action of miR-342-3p in DKD have not been fully assessed. In the present study, it was found that the expression of miR-342-3p was significantly decreased in mice with DKD. The pathological changes of diabetic nephropathy mainly include the abnormal proliferation of MCs and the excessive accumulation of extracellular matrix (ECM) components. MCs have been widely used for the study of diabetic nephropathy in vitro (20-22). For example, Xiang et al suggested that fork-head box protein P1 (FOXP1) prevented high glucose-induced oxidative stress and ECM accumulation in MCs by inhibiting the activation of the Akt/mammalian target of rapamycin (mTOR) signaling pathway; thus, FOXP1 may be a therapeutic target for the treatment of diabetic nephropathy (20). Li et al reported that miR-379-5p agomir suppressed MC proliferation and the accumulation of ECM components by regulating the LIN28B/let-7 pathway, indicating that miR-379-5p may be a potential effective therapeutic target for DN (21). Zhang et al also indicated that long non-coding RNA Rpph1 promoted inflammation and the proliferation of MCs cells in diabetic nephropathy via an interaction with Gal-3 (22). Thus, mouse renal MCs were used for the study of diabetic nephropathy in vitro in the present study. Following the in vitro experiments performed to create a high glucose environment, it was revealed that miR-342-3p inhibited SOX6 expression, promoted cell proliferation, and inhibited the apoptosis of the MCs. Moreover, the overexpression of miR-342-3p also suppressed high glucose-induced renal interstitial fibrosis. These biochemical and molecular biological findings suggest that miR-342-3p is likely to function as an inhibitor of the progression of DKD.
In this study, a high SOX6 expression was found to be inversely associated with miR-342-3p expression in the progression of DKD in vivo, and SOX6 overexpression was shown to partly reverse the miR-342-3p-induced protective effects on MCs. The TargetScan database (http://www.targetscan.org) indicated that SOX6 is a potential target of miR-342-3p (Fig. S1). We therefore hypothesized that SOX6 is the target gene of miR-342-3p. Based on the assumption that miR-342-3p directly targets SOX6, a dual-luciferase activity assay was carried out to confirm the targeted association between miR-342-3p and SOX6. Using dual-luciferase activity assays, SOX6 was identified as a target gene of miR-342-3p. The targeting association between miR-342-3p and SOX6 represents a key step in an unknown mechanism of DKD progression. SOX6 is a transcription factor that may bind with the promoters of TGF-β1, FN, C-IV and PTEN and regulate the transcription of the aforementioned genes (TGF-β1, FN, C-IV and PTEN). Herein, it was revealed that miR-342-3p targets SOX6 and downregulates its expression. miR-342-3p may regulate the expression of TGF-β1, FN, C-IV and PTEN via SOX6. However, in the present study, we did not examine the role of SOX6 in cell proliferation, apoptosis and extracellular matrix in MCs cultured under high glucose conditions, which would have made our conclusions more convincing. This may be a limitation of the present study.
In conclusion, the present study demonstrated that miR-342-3p exerts an inhibitory effect on the progression of DKD in vitro and in vivo. Moreover, miR-342-3p inhibits the progression of renal interstitial fibrosis by targeting SOX6. Overall, the results of the present study revealed that the miR-342-3p/SOX6 axis may serve as a potential therapeutic target for DKD. However, this study is only a preliminary study of the role of miR-342-3p in diabetic nephropathy. In order to confirm our findings on the role of miR-342-3p in diabetic nephropathy, further extensive in-depth studies are required. For example, the role of SOX6 alone in MCs cultured under high glucose conditions also warrants investigation. The role of the miR-342-3p/SOX6 axis in diabetic nephropathy in vivo also requires further analysis. Furthermore, there is a vast difference between the conditions in in vitro studies and in real-life human diabetic nephropathy. Thus, we aim to perform further studies to elucidate these issues in the future.
Supplementary Data
Acknowledgments
Not applicable.
Funding
This study was supported by grants from the National Science Foundation for Young Scholars of Tianjin Medical University (grant no. 2011KY24; Tianjin, China), the Natural Science Foundation of China (grant no. 81600628; China) and the Natural Science Foundation of Tianjin (grant no. 16JCYBJC25700; Tianjin, China).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
ZHJ contributed to the study design, data collection, statistical analysis, data interpretation and manuscript preparation. YZT HNS, MY and BL contributed to data collection and statistical analysis. CLN contributed to the study design, data collection and manuscript preparation. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the Animal Ethics Committee of Tianjin Medical University (Tianjin, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Lu Y, Stamm C, Nobre D, Pruijm M, Teta D, Cherpillod A, Halabi G, Phan O, Fumeaux Z, Bullani R, et al. Changing trends in end-stage renal disease patients with diabetes. Swiss Med Wkly. 2017;147:w14458. doi: 10.4414/smw.2017.14458. [DOI] [PubMed] [Google Scholar]
- 2.Rahimi Z. The role of renin angiotensin aldosterone system genes in diabetic nephropathy. Can J Diabetes. 2016;40:178–183. doi: 10.1016/j.jcjd.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 3.Xiong H, Yan T, Zhang W, Shi F, Jiang X, Wang X, Li S, Chen Y, Chen C, Zhu Y. miR-613 inhibits cell migration and invasion by downregulating Daam1 in triple-negative breast cancer. Cell Signal. 2018;44:33–42. doi: 10.1016/j.cellsig.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 4.He L, Hannon GJ. MicroRNAs: Small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 5.Beltrami C, Simpson K, Jesky M, Wonnacott A, Carrington C, Holmans P, Newbury L, Jenkins R, Ashdown T, Dayan C, et al. Association of elevated urinary miR-126, miR-155, and miR-29b with diabetic kidney disease. Am J Pathol. 2018;188:1982–1992. doi: 10.1016/j.ajpath.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Tang WB, Zheng L, Yan R, Yang J, Ning J, Peng L, Zhou Q, Chen L. miR302a-3p may modulate renal epithelial-mesen-chymal transition in diabetic kidney disease by targeting ZEB1. Nephron. 2018;138:231–242. doi: 10.1159/000481465. [DOI] [PubMed] [Google Scholar]
- 7.Hamada-Kanazawa M, Ogawa D, Takano M, Miyake M. Sox6 suppression induces RA-dependent apoptosis mediated by BMP-4 expression during neuronal differentiation in P19 cells. Mol Cell Biochem. 2016;412:49–57. doi: 10.1007/s11010-015-2607-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han Y, Xu H, Cheng J, Zhang Y, Gao C, Fan T, Peng B, Li B, Liu L, Cheng Z. Downregulation of long non-coding RNA H19 promotes P19CL6 cells proliferation and inhibits apoptosis during late-stage cardiac differentiation via miR-19b-modulated Sox6. Cell Biosci. 2016;6:58. doi: 10.1186/s13578-016-0123-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iguchi H, Urashima Y, Inagaki Y, Ikeda Y, Okamura M, Tanaka T, Uchida A, Yamamoto TT, Kodama T, Sakai J. SOX6 suppresses cyclin D1 promoter activity by interacting with beta-catenin and histone deacetylase 1, and its down-regulation induces pancreatic beta-cell proliferation. J Biol Chem. 2007;282:19052–19061. doi: 10.1074/jbc.M700460200. [DOI] [PubMed] [Google Scholar]
- 10.Pleskovič A, Šantl Letonja M, Cokan Vujkovac A, Kruzliak P, Petrovič D. SOX6 gene polymorphism (rs16933090) and markers of subclinical atherosclerosis in patients with type 2 diabetes mellitus. Int Angiol. 2016;35:552–556. [PubMed] [Google Scholar]
- 11.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 12.Liu F, Zhang ZP, Xin GD, Guo LH, Jiang Q, Wang ZX. miR-192 prevents renal tubulointerstitial fibrosis in diabetic nephropathy by targeting Egr1. Eur Rev Med Pharmacol Sci. 2018;22:4252–4260. doi: 10.26355/eurrev_201807_15420. [DOI] [PubMed] [Google Scholar]
- 13.Rossing P, Persson F, Frimodt-Møller M. Prognosis and treatment of diabetic nephropathy: Recent advances and perspectives. Nephrol Ther. 2018;14(Suppl 1):S31–S37. doi: 10.1016/j.nephro.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Y, Zheng X, Chen LJ, Xu B, Jiang JT. microRNA-181b suppresses the metastasis of lung cancer cells by targeting sex determining region Y-related high mobility group-box 6 (Sox6) Pathol Res Pract. 2019;215:335–342. doi: 10.1016/j.prp.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Jiang W, Yuan Q, Jiang Y, Huang L, Chen C, Hu G, Wan R, Wang X, Yang L. Identification of Sox6 as a regulator of pancreatic cancer development. J Cell Mol Med. 2018;22:1864–1872. doi: 10.1111/jcmm.13470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurtsdotter I, Topcic D, Karlén A, Singla B, Hagey DW, Bergsland M, Siesjö P, Nistér M, Carlson JW, Lefebvre V, et al. SOX5/6/21 prevent oncogene-driven transformation of brain stem cells. Cancer Res. 2017;77:4985–4997. doi: 10.1158/0008-5472.CAN-17-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jia Y, Zheng Z, Guan M, Zhang Q, Li Y, Wang L, Xue Y. Exendin-4 ameliorates high glucose-induced fibrosis by inhibiting the secretion of miR-192 from injured renal tubular epithelial cells. Exp Mol Med. 2018;50:56. doi: 10.1038/s12276-018-0084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xue M, Cheng Y, Han F, Chang Y, Yang Y, Li X, Chen L, Lu Y, Sun B, Chen L. Triptolide attenuates renal tubular epithelial-mesenchymal transition via the MiR-188-5p-mediated PI3K/AKT pathway in diabetic kidney disease. Int J Biol Sci. 2018;14:1545–1557. doi: 10.7150/ijbs.24032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Assmann TS, Recamonde-Mendoza M, de Souza BM, Bauer AC, Crispim D. MicroRNAs and diabetic kidney disease: Systematic review and bioinformatic analysis. Mol Cell Endocrinol. 2018;477:90–102. doi: 10.1016/j.mce.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Xiang H, Xue W, Wu X, Zheng J, Ding C, Li Y, Dou M. FOXP1 inhibits high glucose-induced ECM accumulation and oxidative stress in mesangial cells. Chem Biol Interact. 2019;313:108818. doi: 10.1016/j.cbi.2019.108818. [DOI] [PubMed] [Google Scholar]
- 21.Li N, Wang LJ, Xu WL, Liu S, Yu JY. MicroRNA-379-5p suppresses renal fibrosis by regulating the LIN28/let-7 axis in diabetic nephropathy. Int J Mol Med. 2019 doi: 10.3892/ijmm.2019.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang P, Sun Y, Peng R, Chen W, Fu X, Zhang L, Peng H, Zhang Z. Long non-coding RNA Rpph1 promotes inflammation and proliferation of mesangial cells in diabetic nephropathy via an interaction with Gal-3. Cell Death Dis. 2019;10:526. doi: 10.1038/s41419-019-1765-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.