Abstract
Liver cancer is a worldwide threat to human health. High expression levels of C-X-C chemokine receptor type 4 (CXCR4) have been reported to promote the migration and invasion capacities of liver cancer cells. Cordycepin, extracted from Cordyceps militaris, has anti-inflammatory, antioxidant and anticancerous properties. Therefore, in the present study, migration assays, western blotting, reverse transcription-quantitative PCR and immunofluorescence analyses were conducted to determine whether cordycepin was able to suppress the migration and invasion abilities of liver cancer cells by inhibiting CXCR4 expression. The results suggested that cordycepin notably inhibited migration and invasion, and decreased the expression of CXCR4 in a dose-dependent manner. Activation of phosphorylated (p-) NF-κB inhibitor α (IκBα) and p-P65, the principal components of the NF-κB signaling pathway, was also downregulated. In addition, cordycepin markedly suppressed the nuclear translocation of P65, but had no effect on the expression of total IκBα (t-IκBα) and total P65 (t-P65). JSH-23, an inhibitor of the NF-κB pathway, impaired the migration of liver cancer cells, and was found to act synergistically with cordycepin. Furthermore, cordycepin treatment reduced the chemotactic migration ability of liver cancer cells to stromal cell-derived factor 1 (SDF1), which was significantly enhanced following treatment with JSH-23. Collectively, the present results indicated that cordycepin inhibited the nuclear translocation of P65 by preventing p-IκBα activation; this resulted in the downregulation of CXCR4 expression, and subsequently, in the impaired migration and invasion abilities of liver cancer cells and attenuated reactivity to SDF1. The current study revealed a novel mechanism for the antimetastatic activity of cordycepin and its potential to exert positive synergistic effects with other compounds for the treatment of liver cancer.
Keywords: cordycepin, liver cancer, C-X-C chemokine receptor type 4, migration, invasion
Introduction
Liver cancer is a serious pathology with a global impact (1), and liver cancer-associated morbidity ranks third worldwide, after lung and gastric cancer (1,2). In China, the morbidity rate of liver cancer exceeds that of gastric cancer, and it ranks second among all cancer-related deaths (3-5). There is currently little indication that this condition will improve in the future, and the number of liver cancer-associated deaths is predicted to increase (5). As liver cancer exhibits no obvious or specific early stage symptoms, the majority of patients are diagnosed in the advanced stages of disease when metastasis has already occurred, resulting in poor prognosis and a high mortality rate (1,6,7).
Despite the emergence of tumor immunotherapy (8,9) and molecular targeted drug therapy (10,11), due to low efficiency and side effects, these methods are not widely used in clinical practice for the treatment of liver cancer. At present, the first-line treatment for early liver cancer is surgical resection with adjuvant radiotherapy and chemotherapy (6,12), while patients with advanced disease and metastasis often miss the opportunity to benefit from surgery (13). Additionally, the recurrence of liver cancer is associated with metastasis (13,14). Previously, it was believed that tumor metastasis was the result of tumor cell infiltration from the tumor boundary when the tumor had exceeded a certain volume (14). However, it has been confirmed that metastasis occurs at various disease stages and is closely associated with the tumor microenvironment, including immune, metabolic and inflammatory reactions (15-17). The process of tumor metastasis involves numerous factors, and there are currently no available treatments that collectively target these factors. Therefore, the development of effective therapeutic agents is critical.
The stromal cell-derived factor 1 (SDF1)/C-X-C chemo-kine receptor type 4 (CXCR4) pathway is implicated in liver cancer metastasis (18,19), and includes the elevated expression of CXCR4. The most common metastatic regions in patients with liver cancer are the lungs and bone marrow, which are both abundant in SDF1 (19-21). Mechanistically, the overexpression of CXCR4 was found to promote the migration and invasion of liver cancer cells due to the increased chemotaxis to SDF1 and activation of downstream signaling pathways. Conversely, silencing CXCR4 reduced the metastatic ability of liver cancer cells (22-24). Xia et al (25) demonstrated that the natural extract hesperidin not only induced apoptosis, but also decreased the expression of CXCR4 by regulating the activation of the PI3K/Akt signaling pathway in lung cancer cells (26). Overwhelming evidence has suggested that natural extracts can significantly inhibit the migration and invasion of liver cancer cells by regulating CXCR4 expression (27-29); this suggest that these natural extracts may have potential for preventing liver cancer metastasis by blocking the SDF1/CXCR4 pathway.
Cordycepin, extracted from Cordyceps militaris, has anti-inflammatory, antioxidant and anticancerous properties (30-32). Previous studies have shown that cordycepin inhibits the cell cycle and induces apoptosis in non-small cell lung cancer by activating AMP-activated protein kinase signaling, without affecting the proliferation of normal lung epithelial cells (33). Additionally, cordycepin significantly inhibited the proliferation and migration of gastric cancer cells in a dose-dependent manner, and induced apoptosis (34). Furthermore, cordycepin inhibited the migration and invasion abilities of human gastric cancer cells by activating the PI3K/Akt signaling pathway, and ultimately upregulated the expression of the antimetastatic factor C-type lectin-like receptor 2 (34). However, it is unclear whether cordycepin affects the expression of CXCR4 in liver cancer cells. In a previous study, a range of natural reagents were screened to highlight monomeric compounds that downregulated CXCR4, wherein cordycepin was identified (34). The aim of the present study was to investigate the role and underlying mechanisms of cordycepin-induced downregulation of CXCR4 in liver cancer cells. These findings may promote its clinical use, alone or in combination with other compounds, for the treatment of liver cancer.
Materials and methods
Reagents
DMEM and RPMI-1640 medium, as well as fetal bovine serum (FBS), were purchased from Gibco (Thermo Fisher Scientific, Inc.). PrimeScript™ RT reagent kit (cat. no. RR037A) and One Step TB Green® PrimeScript™ RT-PCR kit (cat. no. RR066A) were obtained from Takara Bio, Inc. Transwell chambers and Matrigel were purchased from BD Biosciences. Rabbit anti-human CXCR4 (cat. no. ab124824), rabbit anti-human total (t-)P65 (cat. no. ab16502), rabbit anti-human phosphorylated (p-)P65 (cat. no. ab86299) and rabbit anti-human β-actin (cat. no. ab179467) primary antibodies, as well as horseradish-peroxidase (HRP)-conjugated goat anti-rabbit (cat. no. ab6721) and HRP-conjugated goat anti-mouse (cat. no. ab6789) secondary antibodies, recombinant human SDF1 protein (cat. no. ab9798) and goat anti-rabbit IgG H&L (Alexa Fluor® 555; cat. no. ab150078) were purchased from Abcam. The primary antibodies targeting IκBα (cat. no. 9242), p-IκBα (Ser32/36; cat. no. 9246) and histone H3 (cat. no. 9715) were obtained from Cell Signaling Technology, Inc. PCR primers were synthesized by Shanghai Sangon Biotech Co., Ltd. TRIzol® reagent (cat. no. 15596026) and NE-PER™ Nuclear and Cytoplasmic Extraction Reagents (cat. no. 78833) were purchased from Thermo Fisher Scientific, Inc. The NF-κB activation inhibitor JSH-23 (cat. no. 481408-M) was obtained from Sigma-Aldrich (Merck KGaA).
Cell lines and culture
The HepG2 and Huh7 human liver cancer cell lines were purchased from the Cell Bank of Shanghai Institutes for Biological Sciences and maintained within our laboratory. HepG2 cells were cultured in DMEM containing 10% FBS and 1% streptomycin. Huh7 cells were cultured in RPMI-1640 containing 10% FBS and 1% streptomycin. Both cell lines were maintained in a humidified incubator at 37°C with 5% CO2, and passaged using 0.25% trypsin EDTA at a confluence of 80-90%. Tumor cells were treated with either cordycepin alone or in combination with the NF-κB pathway inhibitor, JSH-23. Untreated cells were used as the control.
Cordycepin and JSH-23 preparation
Cordycepin powder (Santa Cruz Biotechnology, Inc.) was dissolved in DMSO to prepare a 10 mM stock solution, which was then diluted with medium to 1, 5 and 10 µM working solutions. JSH-23 was dissolved in DMSO to produce a 1 mM stock solution, and diluted to a working concentration of 1 µM using the appropriate medium for each cell type.
Migration assay
HepG2 and Huh7 cells were seeded into a 6-well plate at a density of 8×105 cells per well. At ~50% confluency, 1, 5 or 10 µM cordycepin and/or 1 µM JSH-23 was added and the cells were further incubated for 72 h. The cells were then digested using trypsin, and resuspended in serum-free medium; 1×104 cells per well were added to the upper chamber of the Transwell insert, and 500 µl medium (with 10% FBS) was added to the lower chamber. Following a further 8 h incubation period, the upper chamber was removed and the unmigrated cells were wiped away with a cotton swab. Finally, the cells on the lower chamber were stained with 0.1% crystal violet at 37°C for 15 min, washed, air-dried and counted under an inverted light microscope prior to being photographed. All experiments were repeated three times.
Invasion assay
Matrigel working solution (100 µl; 500 ng/ml) prepared using serum-free medium was used to pre-coat the polycarbonate membrane of the upper chamber of a Transwell insert. After a 2 h incubation at 37°C, the residual basal medium was removed. The subsequent steps were performed similar to the aforementioned migration assay. Briefly, HepG2 and Huh7 cells (8×105 cells per well) were seeded into a 6-well plate and treated with cordycepin or JSH-23 for 72 h. The cells were subsequently added to the upper chamber at a density of 1×104 cells per well, and 500 µl medium (10% FBS) was added to the lower chamber. The cells were incubated for 24 h, stained and photographed as aforementioned.
Chemotaxis assay
As described in the migration assay, cells were seeded into the upper chamber of a Transwell insert at a density of 1×104 cells per well, and incubated for 12 h; 500 µl medium (serum-free) containing recombinant human SDF1 (500 ng/ml) was added to the lower chamber. After staining with 0.1% crystal violet, the cells were visualized under an inverted light microscope as aforementioned.
Reverse transcription-quantitative (RT-q) PCR
Total RNA was extracted from HepG2 and Huh7 cells treated with cordycepin and/or JSH-23 using TRIzol® reagent, according to the manufacturer's instructions. The RNA concentration and purity were measured using an ultraviolet spectrophotometer, and A260/A280 in the range of 1.8-2 was regarded as acceptable. Total RNA (1 µg) was reverse transcribed into cDNA using the PrimeScriptTM RT reagent according to the manufacturer's instructions. The reverse transcription conditions were 37°C for 15 min, followed by 85°C for 5 sec, and storage at 4°C. Subsequently, qPCR was conducted using a Bio-Rad qPCR instrument (Bio-Rad Laboratories, Inc.) with β-actin as the internal reference. The reaction procedure was as follows: pre-denaturation at 95°C for 2 min, denaturation at 95°C for 15 sec, and annealing at 60°C for 15 sec (repeated for 40 cycles). The relative expression levels of the target gene were calculated using the 2−ΔΔCq method (35). Each sample was run in triplicate and all experiments were repeated three independent times. The primer sequences were as follows: CXCR4 forward, 5′-CGGAATTCCAGCAGGTAGCAAAGTGACG-3′ and reverse, 5′-GACGCCAACATAGACCACCT-3′; and β-actin forward, 5′-CCTCGCCTTTGCCGATCC-3′ and reverse, 5′-GGATCTTCATGAGGTAGTCAGTC-3′.
Western blot analysis
HepG2 and Huh7 cells treated with cordycepin or JSH-23 were harvested for total protein extraction using cell lysis buffer for western and IP (Beyotime Institute of Biotechnology), and the protein concentration was determined using the bicinchoninic acid assay method. The protein samples (30 µg per lane) were separated by 10% SDS-PAGE, transferred onto a PVDF membrane, and blocked with 5% skim milk at room temperature for 2 h. The membranes were incubated with the primary antibodies overnight at 4°C (β-actin dilution 1:4,000; all other primary antibodies diluted 1:1,000). The membrane was then incubated with the secondary antibody at room temperature for 2-4 h (1:5,000). After washing with TBS/0.1% Tween-20, the bands were visualized using enhanced chemiluminescence solution and images were captured using a gel imager (Bio-Rad Laboratories, Inc.). The gray values were analyzed using Bio-Rad Image Lab Software 5.1 (Bio-Rad Laboratories, Inc.).
Nuclear protein extraction using NE-PER™ nuclear and cytoplasmic extraction reagents
HepG2 and Huh7 cells (5-10×106) treated with cordycepin and/or JSH-23 were collected and washed with cold PBS via centrifugation at 8,000 × g for 10 min (4°C). After discarding the supernatant, the cell pellets were resuspended in pre-cooled Cytoplasmic Extraction Reagent (CER) I, followed by a 10 min incubation on ice. The cells were then vortexed for 5 sec with pre-cooled CER II reagent, and incubated on ice for 1 min. Following centrifugation at 15,000 × g for 10 min, the supernatants (cytoplasmic fraction) were immediately transferred to a pre-chilled EP tube. The pellets were resuspended in pre-cooled Nuclear Extraction Reagent for 40 min on ice, with vortexing at 15 min intervals. Finally, the solution was centrifuged for 10 min at 15,000 × g (4°C), and the supernatants (nuclear fraction) were transferred to a clean pre-cooled EP tube. The nuclear extracts were subsequently analyzed by western blotting.
Immunofluorescence
HepG2 and Huh7 cells were cultured on sterile slides and treated with cordycepin and/or JSH-23. The slides were washed with PBS three times for 5 min each, and then fixed with 4% paraformaldehyde for 30 min at room temperature. The slides were washed for a further three times for 5 min each with PBS, followed by permeabilization with PBS/0.25% Triton for 10 min. The slides were then washed once more (PBS, three times for 5 min each) and blocked with 5% bovine serum albumin (BSA) solution for 1 h. Each sample was incubated with rabbit anti-human P65 primary antibody (1:300, diluted in 5% BSA) at 4°C for 12-24 h, washed with PBS three times, and then incubated with goat anti-rabbit Alexa Fluor® 555 secondary antibody (1:300, diluted in 5% BSA) at room temperature for 2-3 h. Following further washing with PBS, the slides were stained with DAPI solution (1:10,000) for 5-10 min. Following a final round of washing with PBS, 50% glycerol were added onto each coverslip and the cells were observed under a fluorescence microscope.
Statistical analysis
Data analysis was conducted using SPSS 21.0 (IBM Corp.) and GraphPad Prism 6.0 (GraphPad Software, Inc.). Mean comparisons among multiple groups were performed using one-way analysis of variance with Dunnett's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Cordycepin inhibits the migration and invasion abilities of HepG2 and Huh7 cells, and downregulates the expression of CXCR4
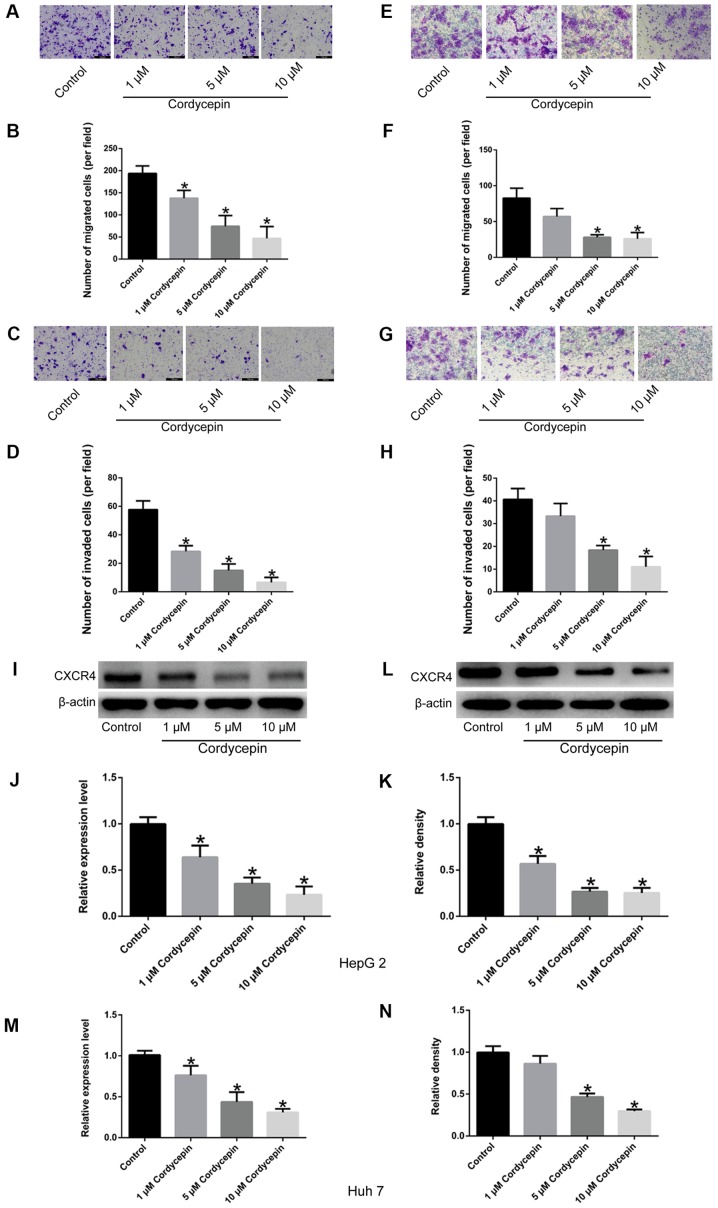
As described previously (36), relatively high concentrations of cordycepin induce apoptosis and inhibit proliferation of HepG2 cells. To determine an appropriate concentration that has no significant effect on the proliferation of HepG2 and Huh7 cells, a dose-curve experiment was performed in the present study. When the concentration of cordycepin was 1, 5 and 10 µM, no significant difference was observed in the proliferation of HepG2 and Huh7 cells by CCK-8 assay, compared with the untreated cells (Fig. 1A). Additionally, as presented in Fig. 2, the migratory and invasive abilities of HepG2 (Fig. 2A-D) and Huh7 cells (Fig. 2E-H) treated with these concentrations of cordycepin (1, 5 and 10 µM) were significantly inhibited (P<0.05) in a dose-dependent manner (with the exception of Huh7 cells treated with 1 µM cordycepin; P>0.05), compared with the untreated controls. Finally, compared with untreated cells, a significant dose-dependent downregulation of CXCR4 expression was observed by RT-qPCR and western blot assays (P<0.05; Fig. 2I-N). Therefore, three different concentrations of cordycepin (1, 5 and 10 µM) were selected for subsequent experiments, which suppressed the cell migratory and invasive capabilities, but had no significant effect on cell proliferation.
Figure 1.
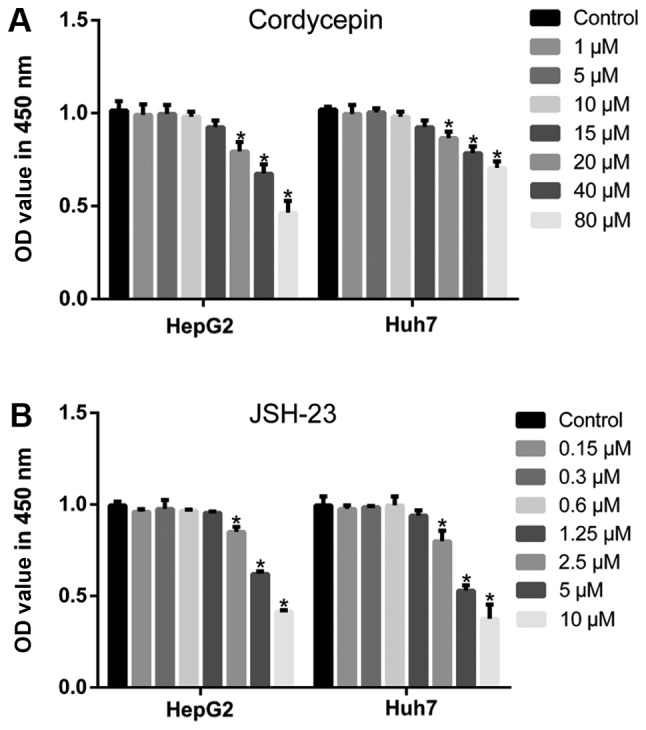
Cytotoxicity of different concentrations of cordycepin and JSH-23 to liver cancer cell lines. CCK-8 assays were used to assess cell proliferation following treatment with the indicated concentrations of (A) cordycepin and (B) JSH-23. Error bars indicate standard deviation. Experiments were performed in triplicate. *P<0.05 vs. untreated control.
Figure 2.
Cordycepin inhibits the migratory and invasive properties of HepG2 and Huh7 human liver cancer cells, and downregulates the expression of CXCR4. (A) Representative images (scale bar, 100 µm) and (B) quantification of migration assays in HepG2 cells. (C) Representative images (scale bar, 100 µm) and (D) quantification of invasion assays in HepG2 cells. (E) Representative images (magnification, ×200) and (F) quantification of migration assays in Huh7 cells. (G) Representative images (magnification, ×200) and (H) quantification of invasion assays in Huh7 cells. (I) Representative blots and (J) densitometry analysis of CXCR4 protein expression levels in HepG2 cells. (K) mRNA expression levels of CXCR4 in HepG2 cells. (L) Representative blots and (M) densitometry analysis of CXCR4 protein expression levels in Huh7 cells. (N) mRNA expression levels of CXCR4 in Huh7 cells. Experiments were performed in triplicate. Error bars indicate standard deviation. *P<0.05 vs. untreated control. CXCR4, C-X-C chemokine receptor type 4.
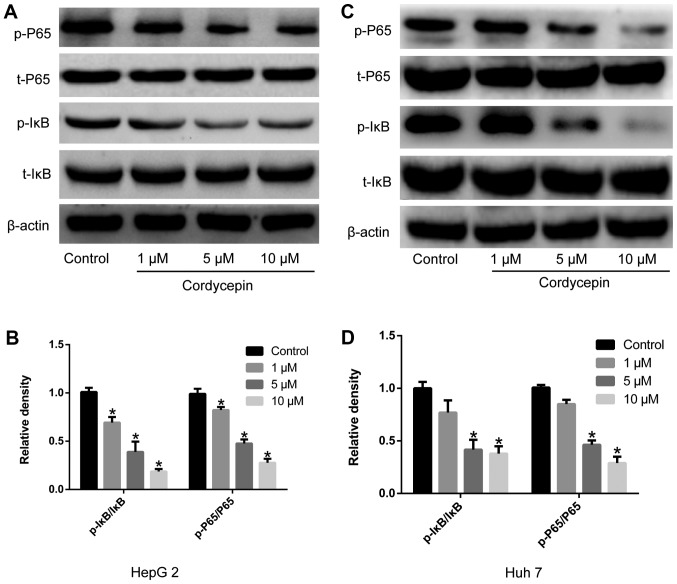
Cordycepin inhibits the activation of the NF-κB signaling pathway
Human liver cancer cells were treated with 1, 5 and 10 µM cordycepin. The expression and activation of NF-κB signaling pathway-related proteins, including t-IκBα, p-IκBα, t-P65 and p-P65, were evaluated by western blotting. As demonstrated in Fig. 3, different concentrations of cordycepin significantly downregulated the activation of IκBα and P65, and the expression levels of p-IκBα and p-P65 (P<0.05). No significant differences were observed in the expression levels of t-IκBα and t-P65.
Figure 3.
Cordycepin inhibits the activation of the NF-κB signaling pathway in HepG2 and Huh7 human liver cancer cells. Cells were treated with the indicated concentrations of cordycepin, and the phosphorylation status of IκB and P65 was analyzed by western botting. (A) Representative blots and (B) densitometry analysis in HepG2 cells. (C) Representative blots and (D) densitometry analysis in Hh7 cells. Error bars indicate standard deviation. Experiments were performed in triplicate. *P<0.05 vs. untreated control. IκBα, NF-κB inhibitor α; p-, phosphorylated; t-, total.
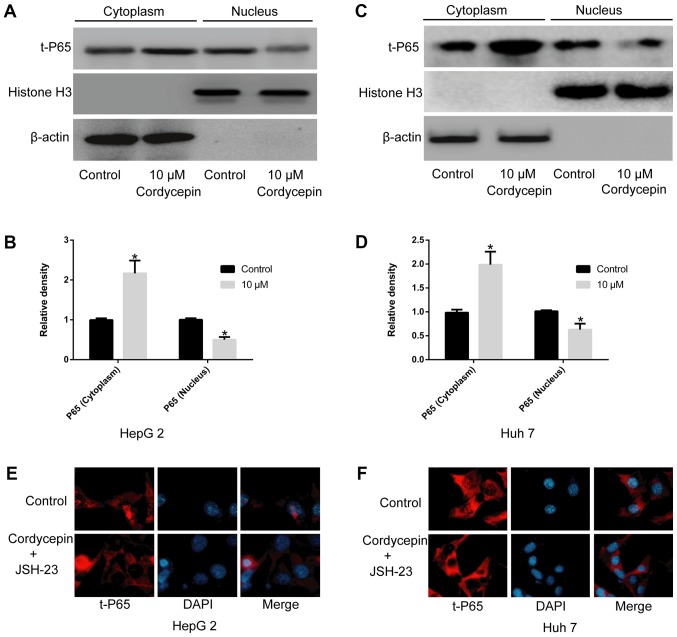
Cordycepin alone, or in combination with JSH-23, restricts the nuclear translocation of P65
To exclude the possibility of an effect of JSH-23 on the viability of HepG2 and Huh7 cells and to select a suitable drug concentration (37), a CCK-8 assay was performed. The results indicated that concentrations up to 1 µM of JSH-23 did not significantly alter cell proliferation compared with the untreated cells (Fig. 1B). As presented in Fig. 4, a significant increase in t-P65 was observed in the cytoplasm of HepG2 (Fig. 4A and B) and Huh7 (Fig. 4C and D) cells following treatment with 10 µM cordycepin (P<0.05), as well as a corresponding reduction in the nuclear fraction (P<0.05), compared with the untreated-control groups. Immunofluorescence analysis indicated that the co-administration of 1 µM JSH-23 and 10 µM cordycepin markedly inhibited the nuclear translocation of P65 in HepG2 (Fig. 4E) and Huh7 cells (Fig. 4F).
Figure 4.
Cordycepin, alone or combined with the NF-κB signaling pathway inhibitor JSH-23, inhibits the nuclear translocation of P65. (A) HepG2 cells were treated with 10 µM cordycepin and the levels of t-P65 were examined in the cytoplasmic and the nuclear fractions. Representative blots and (B) quantification is shown. (C) Huh7 cells were treated with 10 µM cordycepin and the levels of t-P65 were examined in the cytoplasmic and the nuclear fractions. Representative blots and (D) quantification is shown. (E) Immunofluorescence analysis revealed that the combination of cordycepin (10 µM) and JSH-23 (1 µM) significantly inhibited the nuclear localization of P65 in HepG2 and (F) Huh7 cells. Magnification, ×400. Error bars indicate standard deviation. *P<0.05 vs. untreated control. t-, total.
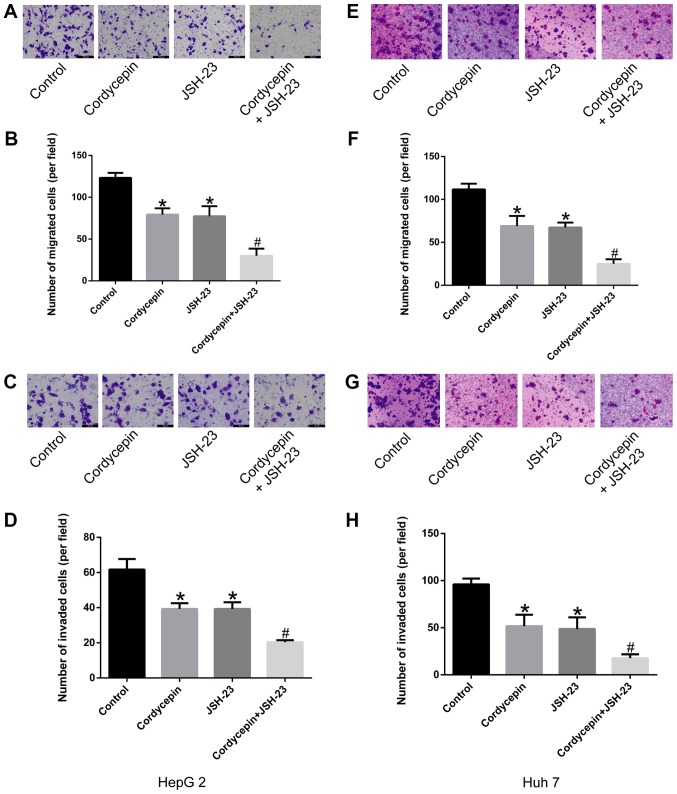
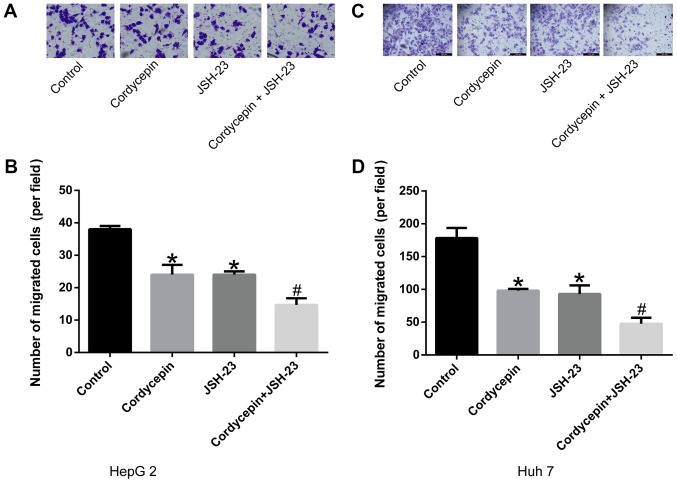
Synergistic effects of cordycepin and JSH-23 in suppressing the migration and invasion abilities of HepG2 and Huh7 human liver cancer cells
The results of the present study preliminarily suggested that cordycepin inhibited the phosphorylation of IκB, as well as the activation and nuclear localization of P65, altering the expression of its downstream gene, CXCR4. As demonstrated in Fig. 5, treatment with 10 µM cordycepin and/or 1 µM JSH-23 suppressed the migration (Fig. 5A, B, E and F) and invasion (Fig. 5C, D, G and H) capacities of liver cancer cells compared with untreated controls (P<0.05). Furthermore, the combination treatment revealed a synergistic inhibitory effect (P<0.05; Fig. 5) compared with cordycepin treatment alone.
Figure 5.
Combination treatment with cordycepin and JSH-23 inhibits the migratory and invasive capacities of human liver cancer cells. Cells were treated with 10 µM cordycepin, 1 µM JSH-23 or their combination, and analyzed for migration and invasion by Transwell assays. (A) Representative images (scale bar, 50 µm) and (B) quantification of HepG2 cell migration. (C) Representative images (scale bar, 50 μm) and (D) quantification of HepG2 cell invasion. (E) Representative images (magnification, ×100) and (F) quantification of Huh7 cell migration. (G) Representative images (magnification, ×100) and (H) quantification of Huh7 cell invasion. Error bars indicate standard deviation. Experiments were performed in triplicate. *P<0.05 vs. untreated control; and #P<0.05 vs. cordycepin alone.
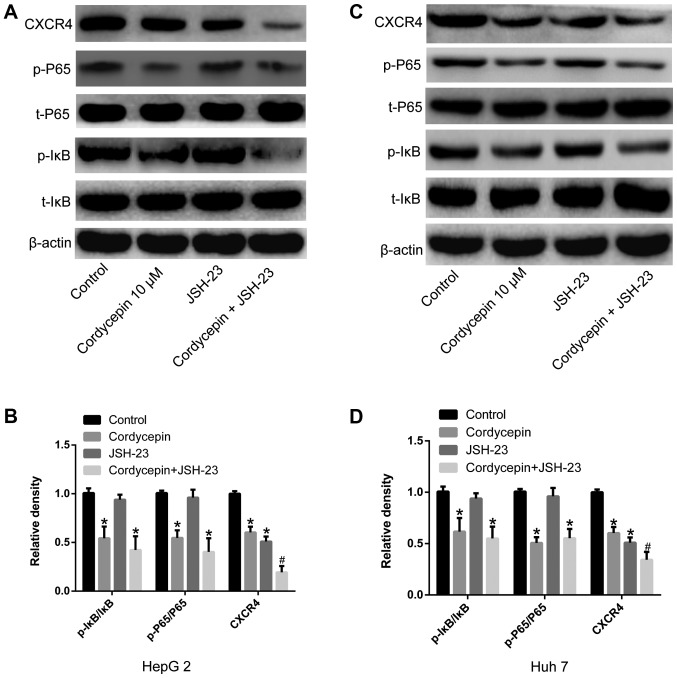
Co-treatment with cordycepin and JSH-23 inhibits the activation of the NF-κB signaling pathway, decreasing the expression of CXCR4 in human liver cancer cells
In order to understand the underlying synergistic mechanism between cordycepin and JSH-23, the expression of NF-κB signaling pathway-associated proteins (t-IκBα, p-IκBα, t-P65 and p-P65) was detected using RT-qPCR and western blotting. As shown in Fig. 6, treatment with cordycepin (10 µM) alone reduced IκBα and P65 phosphorylation in HepG2 and Huh7 human liver cancer cells (P<0.05). The co-treatment with cordycepin (10 µM) and JSH-23 (1 µM) significantly inhibited the expression of CXCR4 (P<0.05; Fig. 6), compared with cordycepin treatment alone. No significant effect on the expression levels of t-IκBα and t-P65 was observed by any of the indicated treatments (Fig. 6).
Figure 6.
Combination treatment with cordycepin and JSH-23 inhibits the activation of NF-κB signaling and downregulates CXCR4 expression. Cells were treated with 10 µM cordycepin, 1 µM JSH-23 or their combination, and the expression levels of CXCR4 and the phosphorylation status of IκB and P65 were analyzed by western blotting. (A) Representative blots and (B) densitometry analysis in HepG2 cells. (C) Representative blot and (D) densitometry analysis in Huh7 cells. Data are presented as the mean ± SD. *P<0.05 vs. untreated control; and #P<0.05 vs. cordycepin alone. CXCR4, C-X-C chemokine receptor type 4; IκBα, NF-κB inhibitor α; p-, phosphorylated; t-, total.
Cordycepin and JSH-23 treatment inhibits the chemotactic migration ability of HepG2 and Huh7 human liver cancer cells to SDF1
SDF1, a specific ligand for CXCR4, was used to detect the chemotactic ability of human liver cancer cells treated with 10 µM cordycepin and/or 1 µM JSH-23 via Transwell assays. Fig. 7 illustrates that cordycepin and JSH-23 monotherapy significantly reduced the chemotactic sensitivity of the cells to SDF1 (P<0.05), and that combination treatment further enhanced this effect compared with the control cells (P<0.05).
Figure 7.
Combination treatment with cordycepin and JSH-23 inhibits the chemotactic migration ability of human liver cancer cells to SDF1. Cells were treated with 10 µM cordycepin, 1 µM JSH-23 or their combination, and chemotactic migration towards SDF1 was measured by Transwell assays. (A) Representative images (scale bar, 50 µm) and (B) quantification in HepG2 cells. (C) Representative images (magnification, ×100) and (D) quantification in Huh7 cells. Data are presented as the means ± SD. *P<0.05 vs. untreated control; and #P<0.05 vs. cordycepin alone. SDF1, stromal cell-derived factor 1.
Discussion
Worldwide, liver cancer results in almost one million deaths annually, most of which are associated with metastasis (1-3,12). The treatment of liver cancer remains predominantly based on surgical resection combined with radio-, chemo- and targeted therapy, which inhibit the malignant proliferation of liver cancer cells, but do not inhibit tumor metastasis (6,12). For example, 5-fluorouracil and cisplatin primarily inhibit liver cancer cell proliferation and induce apoptosis (38-40). Sorafenib, a first-line liver cancer drug, inhibits angiogenesis of liver cancer cells, thereby cutting off the blood supply to tumors and inhibiting the proliferation of tumor cells (41,42). However, there are few reports concerning the application of targeted inhibitors of liver cancer metastasis. Cordycepin, a natural fungal compound, has been implicated in a variety of pathophysiological processes (31,32), and has been approved for clinical trials due its significant antiviral and anticancerous activities (43-46).
Using several functional experiments, the present study demonstrated that cordycepin inhibited the migration and invasion of liver cancer cells in vitro and significantly down-regulated the expression of CXCR4. Although the Huh7 cell line is derived from well-differentiated liver cancer, and the HepG2 cell line is commonly used in metabolism-related studies, these two types of cells are widely used in migration and invasion experiments (47-50). In the present study, the results suggested that cordycepin had positive effect on two different liver cancer cell lines, which suggests that it might be a valuable drug for cancer treatment. Additionally, the synergistic effect of cordycepin in combination with the NF-κB pathway inhibitor JSH-23 was demonstrated. Previous studies have also shown that CXCR4 is highly expressed in liver cancer tissues (19), and that liver cancer cells preferentially metastasize to organs and tissues with abundant expression of the CXCR4 ligand SDF1, such as the lungs (51,52) and bone marrow (53-55). In vitro studies have demonstrated that overexpression or silencing of CXCR4 in tumor cells promoted or inhibited, respectively, their migratory and invasive ability (19,26). Therefore, the present study preliminarily concluded that cordycepin inhibited the in vitro migration and invasion of liver cancer cells by down-regulating the expression of CXCR4.
Mechanistically, the present study revealed that the phosphorylation levels of P65 and IκBα in liver cancer cells changed significantly following cordycepin treatment. A previous study suggested that IκB-molecules form a conjugated complex with the P65/P50 heterodimer, inactivating P65 (56). When phosphorylated, IκB dissociated from the P65/P50 complex. Free p-IκBs undergo ubiquitination, while P65 is phosphory-lated and activated. Subsequently, p-P65 enters to nucleus, activating specific transcription factors and increasing the expression of a series of genes (57,58). In the present study, cordycepin treatment was found to significantly inhibit IκBα phosphorylation, restricting the nuclear translocation of P65 and preventing transcription factor activation, thereby regulating the expression of CXCR4. These results suggested that cordycepin may regulate the NF-κB pathway by inhibiting IκBα phosphorylation, thereby modulating its downstream targets, which include CXCR4.
JSH-23, an NF-κB pathway inhibitor (59), has similar effects to cordycepin, but does not significantly influence the phosphorylation status of IκBα. When JSH-23 was combined with cordycepin in the present study, a synergistic inhibitory effect on the migration and invasion capacities of liver cancer cells was observed.
Collectively, the results of the present study demonstrated that cordycepin inhibited the activation and nuclear translocation of P65 by inhibiting the phosphorylation of IκBα, thereby downregulating CXCR4. Furthermore, the inhibitory effects of cordycepin were significantly enhanced following combination with JSH-23, which suggested that cordycepin may have the potential to prevent liver cancer metastasis when used in combination with other therapeutic compounds.
Acknowledgments
We thank Rongmu Xia (School of Medicine, Xiamen University, Xiamen, Fujian, China) for repeating part of the experiments and his technical guidance on our experiments.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YH conceived and designed the study. ZG, WC and GD performed experiments, data acquisition and analysis. YH wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ, Estève J, et al. CONCORD Working Group Global surveillance of trends in cancer survival 2000-14 (CONCORD 3): analysis of individual records for 37,513,025 patients diagnosed with one of 18 cancers from 322 population based registries in 71 countries. Lancet. 2018;391:1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 4.Chen W, Zheng R, Zhang S, Zeng H, Zuo T, Xia C, Yang Z, He J. Cancer incidence and mortality in China in 2013: an analysis based on urbanization level. Chin J Cancer Res. 2017;29:1–10. doi: 10.21147/j.issn.1000-9604.2017.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng R, Qu C, Zhang S, Zeng H, Sun K, Gu X, Xia C, Yang Z, Li H, Wei W, et al. Liver cancer incidence and mortality in China: Temporal trends and projections to 2030. Chin J Cancer Res. 2018;30:571–579. doi: 10.21147/j.issn.1000-9604.2018.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu CY, Chen KF, Chen PJ. Treatment of Liver Cancer. Cold Spring Harb Perspect Med. 2015;5:a021535. doi: 10.1101/cshperspect.a021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shiani A, Narayanan S, Pena L, Friedman M. The Role of Diagnosis and Treatment of Underlying Liver Disease for the Prognosis of Primary Liver Cancer. Cancer control. 2017;24:1073274817729240. doi: 10.1177/1073274817729240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li S, Yang F, Ren X. Immunotherapy for hepatocellular carcinoma. Drug Discov Ther. 2015;9:363–371. doi: 10.5582/ddt.2015.01054. [DOI] [PubMed] [Google Scholar]
- 9.Yu S, Wang Y, Jing L, Claret FX, Li Q, Tian T, Liang X, Ruan Z, Jiang L, Yao Y, et al. Autophagy in the 'inflammation-carcinogenesis' pathway of liver and HCC immunotherapy. Cancer Lett. 2017;411:82–89. doi: 10.1016/j.canlet.2017.09.049. [DOI] [PubMed] [Google Scholar]
- 10.Klungboonkrong V, Das D, McLennan G. Molecular Mechanisms and Targets of Therapy for Hepatocellular Carcinoma. J Vasc Interv Radiol. 2017;28:949–955. doi: 10.1016/j.jvir.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Kudo M, Arizumi T. Transarterial Chemoembolization in Combination with a Molecular Targeted Agent: Lessons Learned from Negative Trials (Post-TACE, BRISK-TA, SPACE, ORIENTAL, and TACE-2) Oncology. 2017;93(Suppl 1):127–134. doi: 10.1159/000481243. [DOI] [PubMed] [Google Scholar]
- 12.Tohme S, Simmons RL, Tsung A. Surgery for Cancer: A Trigger for Metastases. Cancer Res. 2017;77:1548–1552. doi: 10.1158/0008-5472.CAN-16-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeeshan R, Mutahir Z. Cancer metastasis - tricks of the trade. Bosn J Basic Med Sci. 2017;17:172–182. doi: 10.17305/bjbms.2017.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 15.Ai J, Tang Q, Wu Y, Xu Y, Feng T, Zhou R, Chen Y, Gao X, Zhu Q, Yue X, et al. The role of polymeric immunoglobulin receptor in inflammation-induced tumor metastasis of human hepatocellular carcinoma. J Natl Cancer Inst. 2011;103:1696–1712. doi: 10.1093/jnci/djr360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Budhu A, Forgues M, Ye QH, Jia HL, He P, Zanetti KA, Kammula US, Chen Y, Qin LX, Tang ZY, et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10:99–111. doi: 10.1016/j.ccr.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 17.Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, Agdashian D, Terabe M, Berzofsky JA, Fako V, et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 2018;360:eaan5931. doi: 10.1126/science.aan5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song T, Dou C, Jia Y, Tu K, Zheng X. TIMP-1 activated carcinoma-associated fibroblasts inhibit tumor apoptosis by activating SDF1/CXCR4 signaling in hepatocellular carcinoma. Oncotarget. 2015;6:12061–12079. doi: 10.18632/oncotarget.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, Huang Y, Zhang J, Xing B, Xuan W, Wang H, Huang H, Yang J, Tang J. High co-expression of the SDF1/CXCR4 axis in hepatocarcinoma cells is regulated by AnnexinA7 in vitro and in vivo. Cell Commun Signal. 2018;16:22. doi: 10.1186/s12964-018-0234-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao PT, Ding GY, Yang X, Dong RZ, Hu B, Zhu XD, Cai JB, Ji Y, Shi GM, Shen YH, et al. Invasive potential of hepatocellular carcinoma is enhanced by loss of selenium-binding protein 1 and subsequent upregulation of CXCR4. Am J Cancer Res. 2018;8:1040–1049. [PMC free article] [PubMed] [Google Scholar]
- 21.Li M, Lu Y, Xu Y, Wang J, Zhang C, Du Y, Wang L, Li L, Wang B, Shen J, et al. Horizontal transfer of exosomal CXCR4 promotes murine hepatocarcinoma cell migration, invasion and lymphangiogenesis. Gene. 2018;676:101–109. doi: 10.1016/j.gene.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Liu YC, Sung YC, Ramjiawan RR, Lin TT, Chang CC, Jeng KS, Chang CF, Liu CH, Gao DY, et al. Overcoming sorafenib evasion in hepatocellular carcinoma using CXCR4-targeted nanoparticles to co-deliver MEK-inhibitors. Sci Rep. 2017;7:44123. doi: 10.1038/srep44123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, Li P, Chang Y, Xu Q, Wu Z, Ma Q, Wang Z. The SDF-1/CXCR4 axis induces epithelial-mesenchymal transition in hepatocellular carcinoma. Mol Cell Biochem. 2014;392:77–84. doi: 10.1007/s11010-014-2020-8. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Zhang W, Ding Y, Guo X, Yuan Y, Li D. CRISPR/Cas9-mediated genome engineering of CXCR4 decreases the malignancy of hepatocellular carcinoma cells in vitro and in vivo. Oncol Rep. 2017;37:3565–3571. doi: 10.3892/or.2017.5601. [DOI] [PubMed] [Google Scholar]
- 25.Xia R, Sheng X, Xu X, Yu C, Lu H. Hesperidin induces apoptosis and G0/G1 arrest in human non-small cell lung cancer A549 cells. Int J Mol Med. 2018;41:464–472. doi: 10.3892/ijmm.2017.3250. [DOI] [PubMed] [Google Scholar]
- 26.Xia R, Xu G, Huang Y, Sheng X, Xu X, Lu H. Hesperidin suppresses the migration and invasion of non-small cell lung cancer cells by inhibiting the SDF-1/CXCR-4 pathway. Life Sci. 2018;201:111–120. doi: 10.1016/j.lfs.2018.03.046. [DOI] [PubMed] [Google Scholar]
- 27.Fontanella R, Pelagalli A, Nardelli A, D'Alterio C, Ieranò C, Cerchia L, Lucarelli E, Scala S, Zannetti A. A novel antagonist of CXCR4 prevents bone marrow-derived mesenchymal stem cell-mediated osteosarcoma and hepatocellular carcinoma cell migration and invasion. Cancer Lett. 2016;370:100–107. doi: 10.1016/j.canlet.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 28.Kaemmerer D, Schindler R, Mußbach F, Dahmen U, Altendorf-Hofmann A, Dirsch O, Sänger J, Schulz S, Lupp A. Somatostatin and CXCR4 chemokine receptor expression in hepatocellular and cholangiocellular carcinomas: Tumor capillaries as promising targets. BMC Cancer. 2017;17:896. doi: 10.1186/s12885-017-3911-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mardomi A, Sabzichi M, Hussein Somi M, Shanehbandi D, Rahbarghazi R, Taj Sanjarani O, Samadi N. Trafficking mechanism of bone marrow-derived mesenchymal stem cells toward hepatocellular carcinoma HepG2 cells by modulating Endoglin, CXCR4 and TGF-β. Cell Mol Biol (Noisy-le-grand) 2016;62:81–86. [PubMed] [Google Scholar]
- 30.Chen YC, Chen YH, Pan BS, Chang MM, Huang BM. Functional study of Cordyceps sinensis and cordycepin in male reproduction: A review. Yao Wu Shi Pin Fen Xi. 2017;25:197–205. doi: 10.1016/j.jfda.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tuli HS, Sharma AK, Sandhu SS, Kashyap D. Cordycepin: A bioactive metabolite with therapeutic potential. Life Sci. 2013;93:863–869. doi: 10.1016/j.lfs.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 32.Yoon SY, Park SJ, Park YJ. The Anticancer Properties of Cordycepin and Their Underlying Mechanisms. Int J Mol Sci. 2018;19:E3027. doi: 10.3390/ijms19103027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei C, Yao X, Jiang Z, Wang Y, Zhang D, Chen X, Fan X, Xie C, Cheng J, Fu J, et al. Cordycepin Inhibits Drug-resistance Non-small Cell Lung Cancer Progression by Activating AMPK Signaling Pathway. Pharmacol Res. 2019;144:79–89. doi: 10.1016/j.phrs.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Lv Y, Liu TS, Yan WD, Chen LY, Li ZH, Piao YS, An RB, Lin ZH, Ren XS. Cordycepin suppresses cell proliferation and migration by targeting CLEC2 in human gastric cancer cells via Akt signaling pathway. Life Sci. 2019;223:110–119. doi: 10.1016/j.lfs.2019.03.025. [DOI] [PubMed] [Google Scholar]
- 35.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 36.Shao LW, Huang LH, Yan S, Jin JD, Ren SY. Cordycepin induces apoptosis in human liver cancer HepG2 cells through extrinsic and intrinsic signaling pathways. Oncol Lett. 2016;12:995–1000. doi: 10.3892/ol.2016.4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lai Y, Fan L, Zhao Y, Ge H, Feng X, Wang Q, Zhang X, Peng Y, Wang X, Tao L. Cx32 suppresses extrinsic apoptosis in human cervical cancer cells via the NF-κB signalling pathway. Int J Oncol. 2017;51:1159–1168. doi: 10.3892/ijo.2017.4106. [DOI] [PubMed] [Google Scholar]
- 38.Ikeda M, Okusaka T, Sato Y, Furuse J, Mitsunaga S, Ueno H, Morizane C, Inaba Y, Kobayashi T, Arai Y. A Phase I/II trial of continuous hepatic intra-arterial infusion of 5-fluoro-uracil, mitoxantrone and cisplatin for advanced hepatocellular carcinoma. Jpn J Clin Oncol. 2017;47:512–519. doi: 10.1093/jjco/hyx038. [DOI] [PubMed] [Google Scholar]
- 39.Kumamoto T, Tanaka K, Matsuo K, Takeda K, Nojiri K, Mori R, Taniguchi K, Matsuyama R, Ueda M, Akiyama H, et al. Adjuvant hepatic arterial infusion chemotherapy with 5-Fluorouracil and interferon after curative resection of hepatocellular carcinoma: A preliminary report. Anticancer Res. 2013;33:5585–5590. [PubMed] [Google Scholar]
- 40.Zhang W, Zhong Y, Cui H, Wang L, Yang R, Su Z, Xiang B, Wei Q. Combination of calcineurin B subunit (CnB) and 5-fluoro-uracil reverses 5-fluorouracil-induced immunosuppressive effect and enhances the antitumor activity in hepatocellular carcinoma. Oncol Lett. 2017;14:6135–6142. doi: 10.3892/ol.2017.6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun T, Liu H, Ming L. Multiple Roles of Autophagy in the Sorafenib Resistance of Hepatocellular Carcinoma. Cell Physiol Biochem. 2017;44:716–727. doi: 10.1159/000485285. [DOI] [PubMed] [Google Scholar]
- 42.Zhu YJ, Zheng B, Wang HY, Chen L. New knowledge of the mechanisms of sorafenib resistance in liver cancer. Acta Pharmacol Sin. 2017;38:614–622. doi: 10.1038/aps.2017.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Clercq E. Curious (Old and New) Antiviral Nucleoside Analogues with Intriguing Therapeutic Potential. Curr Med Chem. 2015;22:3866–3880. doi: 10.2174/0929867322666150625094705. [DOI] [PubMed] [Google Scholar]
- 44.Du Y, Yu J, Du L, Tang J, Feng WH. Cordycepin enhances Epstein-Barr virus lytic infection and Epstein-Barr virus-positive tumor treatment efficacy by doxorubicin. Cancer Lett. 2016;376:240–248. doi: 10.1016/j.canlet.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 45.Lee JB, Adrower C, Qin C, Fischer PM, de Moor CH, Gershkovich P. Development of Cordycepin Formulations for Preclinical and Clinical Studies. AAPS PharmSciTech. 2017;18:3219–3226. doi: 10.1208/s12249-017-0795-0. [DOI] [PubMed] [Google Scholar]
- 46.Ryu E, Son M, Lee M, Lee K, Cho JY, Cho S, Lee SK, Lee YM, Cho H, Sung GH, et al. Cordycepin is a novel chemical suppressor of Epstein-Barr virus replication. Oncoscience. 2014;1:866–881. doi: 10.18632/oncoscience.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bai H, Weng Y, Bai S, Jiang Y, Li B, He F, Zhang R, Yan S, Deng F, Wang J, et al. CCL5 secreted from bone marrow stromal cells stimulates the migration and invasion of Huh7 hepatocellular carcinoma cells via the PI3K-Akt pathway. Int J Oncol. 2014;45:333–343. doi: 10.3892/ijo.2014.2421. [DOI] [PubMed] [Google Scholar]
- 48.Lee JH, Hur W, Hong SW, Kim JH, Kim SM, Lee EB, Yoon SK. ELK3 promotes the migration and invasion of liver cancer stem cells by targeting HIF-1α. Oncol Rep. 2017;37:813–822. doi: 10.3892/or.2016.5293. [DOI] [PubMed] [Google Scholar]
- 49.Lin XL, Liu M, Liu Y, Hu H, Pan Y, Zou W, Fan X, Hu X. Transforming growth factor β1 promotes migration and invasion in HepG2 cells: Epithelial-to-mesenchymal transition via JAK/STAT3 signaling. Int J Mol Med. 2018;41:129–136. doi: 10.3892/ijmm.2017.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xie X, Zhu H, Zhang J, Wang M, Zhu L, Guo Z, Shen W, Wang D. Solamargine inhibits the migration and invasion of HepG2 cells by blocking epithelial-to-mesenchymal transition. Oncol Lett. 2017;14:447–452. doi: 10.3892/ol.2017.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pectasides E, Miksad R, Pyatibrat S, Srivastava A, Bullock A. Spontaneous Regression of Hepatocellular Carcinoma with Multiple Lung Metastases: A Case Report and Review of the Literature. Dig Dis Sci. 2016;61:2749–2754. doi: 10.1007/s10620-016-4141-2. [DOI] [PubMed] [Google Scholar]
- 52.Yang T, Lu JH, Lin C, Shi S, Chen TH, Zhao RH, Wang Y, Wu MC. Concomitant lung metastasis in patients with advanced hepa-tocellular carcinoma. World J Gastroenterol. 2012;18:2533–2539. doi: 10.3748/wjg.v18.i20.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhatia R, Ravulapati S, Befeler A, Dombrowski J, Gadani S, Poddar N. Hepatocellular carcinoma with bone metastases: Incidence, prognostic significance, and management-single-center experience. J Gastrointest Cancer. 2017;48:321–325. doi: 10.1007/s12029-017-9998-6. [DOI] [PubMed] [Google Scholar]
- 54.Hong YM, Yoon KT, Cho M, Kang DH, Kim HW, Choi CW, Park SB, Heo J, Woo HY, Lim W, et al. Bone marrow metastasis presenting as bicytopenia originating from hepatocellular carcinoma. Clin Mol Hepatol. 2016;22:267–271. doi: 10.3350/cmh.2015.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Di Stadio CS, Altieri F, Minopoli G, Miselli G, Rippa E, Arcari P. Role of human GKN1 on APP processing in gastric cancer. Biochimie. 2017;135:149–153. doi: 10.1016/j.biochi.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 56.Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013;12:86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DiDonato JA, Mercurio F, Karin M. NF-κB and the link between inflammation and cancer. Immunol Rev. 2012;246:379–400. doi: 10.1111/j.1600-065X.2012.01099.x. [DOI] [PubMed] [Google Scholar]
- 58.Sokolova O, Naumann M. NF-κB Signaling in gastric cancer. Toxins (Basel) 2017;9:E119. doi: 10.3390/toxins9040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thiel G, Ulrich M, Mukaida N, Rössler OG. Resveratrol stimulation induces interleukin-8 gene transcription via NF-κB. Pharmacol Res. 2018;134:238–245. doi: 10.1016/j.phrs.2018.07.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.