Abstract
A reliable, accessible, and non-intrusive method for tracking respiratory and heart rate is important for improving monitoring and detection of sleep apnea. In this study, an algorithm based on motion analysis of infrared video recordings was validated in 50 adults referred for clinical overnight polysomnography (PSG). The algorithm tracks the displacements of selected feature points on each sleeping participant and extracts respiratory rate using principal component analysis and heart rate using independent component analysis. For respiratory rate estimation (mean ± standard deviation), 89.89 % ± 10.95 % of the overnight estimation was accurate within 1 breath per minute compared to the PSG-derived respiratory rate from the respiratory inductive plethysmography signal, with an average root mean square error (RMSE) of 2.10 ± 1.64 breaths per minute. For heart rate estimation, 77.97 % ± 18.91 % of the overnight estimation was within 5 beats per minute of the heart rate derived from the pulse oximetry signal from PSG, with mean RMSE of 7.47 ± 4.79 beats per minute. No significant difference in estimation of RMSE of either signal was found according to differences in body position, sleep stage, or amount of the body covered by blankets. This vision-based method may prove suitable for overnight, non-contact monitoring of respiratory rate. However, at present, heart rate monitoring is less reliable and will require further work to improve accuracy.
Keywords: Cardiopulmonary rate, noncontact, sleep disordered breathing, computer vision
Our proposed algorithm tracks the movement of selected feature points from a video of a sleeping participant, and extracts respiratory rate using principal component analysis and heart rate using independent component analysis.
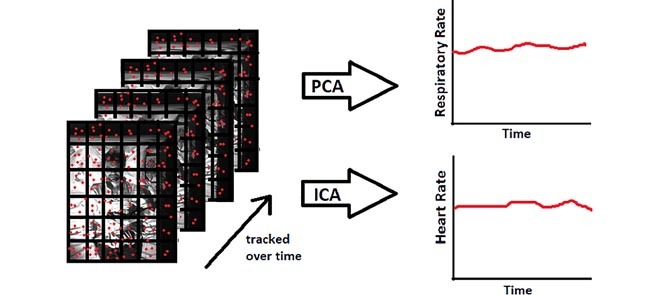
I. Introduction
Monitoring of respiratory rate, heart rate and respiratory related movement is important for evaluating the quality of sleep and the management of various sleep disorders such as sleep apnea, sleep tachycardia, bradycardia, bruxism, restless leg syndrome and periodic limb movements in sleep. Amongst sleep disorders, sleep apnea is highly prevalent, underdiagnosed, and implicated with various health conditions and excessive daytime sleepiness.
Sleep apnea is defined as the repeated intermittent complete or partial cessation of breathing for 10 seconds or more during sleep, leading to oxygen desaturation and arousals [1]. Sleep apnea disrupts sleep, often resulting in daytime fatigue, and is associated with reduced daily functions and quality of life [2]. Complete cessation of airflow during sleep defines apnea while reduced airflow or thoracoabdominal movement of 30% or more defines hypopnea when associated with ≥ 3 % drop in blood’s oxygen saturation level or arousal [1]. Apneas and hypopneas can be further categorized into central, obstructive, and mixed depending on the underlying mechanism [3].
Between 10–17% of adult men and 3–9% of women are estimated to have obstructive sleep apnea (OSA) [4]. OSA is more common in men, the elderly, and obese population [5]. Numerous studies suggest that individuals with OSA are undiagnosed [6]–[8] Other studies have linked untreated sleep apnea to approximately two fold increase in motor vehicle accidents [9] and occupational accidents [10], as well as increased risk for cardiovascular disease [11], [12], systemic hypertension [13], [14], diabetes [15], and depression [16]. Approximately 50% of patients with heart failure have sleep apnea and 20% of heart-failure related deaths occur at night [17]. The high prevalence of sleep apnea amongst those with cardiovascular complications further compels the need for an accurate, cost-effective, and non-intrusive tracking of heart and respiratory activity during sleep.
Current gold standard for sleep monitoring and assessment is through performing overnight polysomnography (PSG) at a sleep clinic. The process involves attaching over 20 sensors to the sleeper to continuously record electroencephalogram, electrocardiogram, electromyogram, oxygen saturation, nasal flow, and respiratory movement of chest and abdomen walls. Overnight clinical PSG monitoring is expensive [18], wait times are long [19], and can be inconvenient and uncomfortable. Multi-night PSG tests are rarely performed despite large night to night variance in sleep outcomes for those with OSA [20]. Consequently, numerous alternative sleep monitoring technologies have been developed to overcome the disadvantages of full night PSG recording, using reduced number of sensors and allowing for at-home recording [21]. Most consumer technologies come at a cost of reduced accuracy and cannot be used as a stand-alone solution [22], while most clinical modalities still require multiple sensors and a board-certified sleep medicine specialist [23]. Moreover, an attachment of multiple sensors could potentially be inconvenient during sleep. Various contactless technologies were explored for overnight respiratory rate and/or heart rate monitoring including applications of radar [24], WiFi [25], audio recording [26], [27], pressure sensors [28], depth cameras [29], thermal cameras [30], and infrared cameras [31].
Infrared vision based methods are very promising for a few reasons. Firstly, infrared cameras are inexpensive and readily available. Secondly, large amount of additional information can be extracted from visual data, such as sleep position, movements, and surrounding environmental factors. These additional parameters can improve sleep quality assessment and potential diagnosis of other sleep related disorders. A vision-based algorithm using near-infrared video recording for estimating respiratory rate and heart rate was previously developed and validated on healthy awake subjects while simulating sleep condition for short periods of time [31], [32]. Accurately tracked heart rate and respiratory rate can be used in future studies to diagnose sleep apnea. In this paper, we proposed improvements to our previously developed algorithm [31] to address some of the challenges associated with unwanted motion and heart rate estimation during unconstrained nocturnal sleep. We validated the algorithm on sleeping subjects who were referred for a clinical overnight sleep diagnosis.
II. Materials and Methods
A. Participants and Sleep Protocol
Subjects between the ages of 18 to 85 were recruited from those who were referred to the sleep laboratory at Toronto Rehabilitation Institute for overnight diagnostic sleep study. The protocol was approved by the ethics boards of University Health Network and the University of Toronto (Research Ethics Board approval number 13-7210-DE). All participants provided written consent before participating in the study. Those with well managed conditions such as hypertension, myasthenia gravis, and diabetes were included. During the study, subjects were not restricted by the number of pillows and blankets used and the clothes worn. No restrictions were placed on the sleep position overnight.
B. Data Collection
Videos were captured using Point Grey Firefly MV USB 2.0 monochromatic camera at a
resolution of 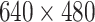 pixels at 30
frames per second. This camera was selected based on its high quantum efficiency in the
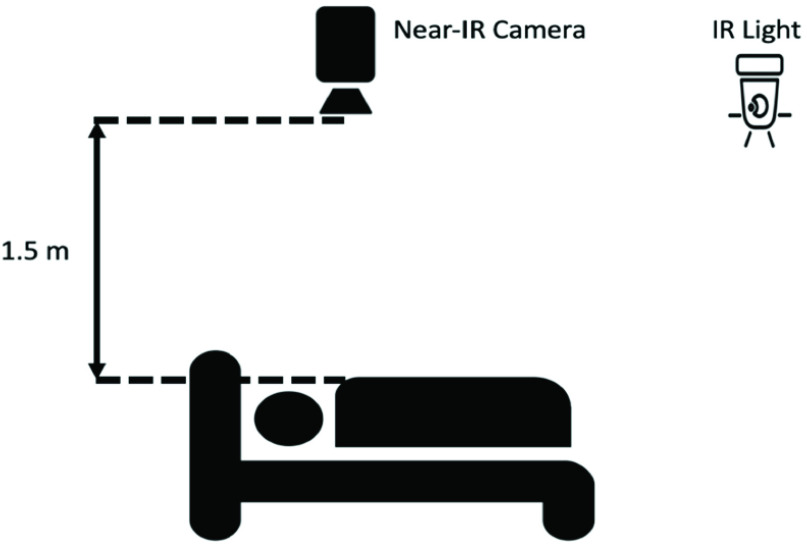
near infrared range. The camera was mounted approximately 1.5m above the bed to capture
the head and torso of the subject. A separate infrared light source (Raytec RM25-F-50),
mounted on the ceiling past the foot of the bed, was used for illumination [31]. A schematic of camera set up is shown in Fig. 1 and sample frame (anonymized) that captured by
the infrared camera is shown in Fig. 2.
pixels at 30
frames per second. This camera was selected based on its high quantum efficiency in the
near infrared range. The camera was mounted approximately 1.5m above the bed to capture
the head and torso of the subject. A separate infrared light source (Raytec RM25-F-50),
mounted on the ceiling past the foot of the bed, was used for illumination [31]. A schematic of camera set up is shown in Fig. 1 and sample frame (anonymized) that captured by
the infrared camera is shown in Fig. 2.
FIGURE 1.
Data collection setup.
FIGURE 2.

Sample frames.
Gold standard assessment of sleep was collected simultaneously using full overnight PSG (Embla s4500). Gold standard heart rate was extracted from one-lead electrocardiogram (ECG) sampled at 250 Hz. The sum of chest and abdominal movement as recorded by respiratory inductance plethysmography (RIP) was sampled at 25 Hz and used to estimate gold standard respiratory rate. Sleep stages, apneas, hypopneas, and any other conditions such as periodic limb movements in sleep, bradycardia and tachycardia were marked by trained sleep technician. Body position, head position, and amount of body covered by sheets were manually annotated by a research analyst from video, while blinded to any respiratory rate and heart rate information.
C. Data Analysis
Respiratory rate and heart rate estimates are extracted from infrared (IR) video using a computer vision motion-based algorithm previously developed by Li et al. [31]. The method tracks the deviation of selected feature points over time. Specifically, the method divides each frame into a grid of 40 pixel by 40-pixel squares. Eight distinct points (corners or textured area) are selected from each grid cell, for a maximum of 1536 points over the whole image. These points are tracked over 30 second windows. To ensure tracked motion is relevant, the maximum frame-to-frame displacement of each tracked point is calculated, and the top and bottom 25 percentile are discarded. The same set of feature points were used for both heart and respiratory rate estimation.
On sliding windows of 30 seconds with 29 seconds overlap, the method applies principal
component analysis for respiratory rate estimation and independent component analysis for
heart rate estimation. This method compared the frequency spectrum of the output signals
from independent component analysis and selected the frequency with highest harmonic
periodicity as the heart rate estimation [31].
The harmonic periodicity is determined by taking the sum of the amplitudes of the
fundamental frequency and its first two harmonics divided by the total spectral density.
However, this method can consider respiratory harmonics as the estimation for heart rate.
To overcome this challenge, we scored the top candidate frequencies, up to 12 candidates,
based on its harmonic periodicity, proximity to heart rate estimation in the previous
window, and likelihood of the frequency as a heart rate candidate. The score 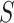 was calculated for each
candidate frequency by the following equation:
was calculated for each
candidate frequency by the following equation:
 |
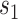 is the harmonic
periodicity of candidate frequency divided by the sum of harmonic periodicity over all
candidate frequencies,
is the harmonic
periodicity of candidate frequency divided by the sum of harmonic periodicity over all
candidate frequencies, 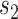 is the normalized
absolute difference between previous heart rate estimate and current candidate frequency,
and
is the normalized
absolute difference between previous heart rate estimate and current candidate frequency,
and 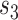 is the normalized
absolute difference between a reasonable heart rate and current candidate. For this study,
reasonable heart rate was set to 80 beats per minute (bpm), which is the midpoint of the
20 bpm to 140 bpm range considered for heart rate estimation. The candidate with highest
score was selected as the heart rate estimate.
is the normalized
absolute difference between a reasonable heart rate and current candidate. For this study,
reasonable heart rate was set to 80 beats per minute (bpm), which is the midpoint of the
20 bpm to 140 bpm range considered for heart rate estimation. The candidate with highest
score was selected as the heart rate estimate.
The gold standard heart rate and respiratory rate were calculated from ECG and RIP signals respectively. Instantaneous heart rate was calculated by detecting R-peaks from ECG signal using parabolic fitting, and then averaging 5 consecutive R-R intervals. The instantaneous heart rate was averaged over a 30-second moving window with 29 second overlap as the gold standard heart rate. The gold standard respiratory rate was extracted from the same moving window using the same method as Li et al. [31].
One of the main challenges of the vision based method [31] is accurate prediction of heart rate and respiratory rate during movements such as position change and limb movement, during which large artefacts are also seen in the gold standard measurements. In this study, the algorithm was modified such that when large spikes in displacement of the tracked feature points was detected, new reference set of feature points was sampled. Also, the 30 seconds windows including and following the large motion were labelled as ‘moving’ and excluded from analysis. Segments, in which the participant was not seen in the frame, either due to the subject moving out of bed or over exposure of the video frame, were excluded from analysis. In addition, segments where gold standard heart rate was outside the range of 20–140 bpm and gold standard respiratory rate was outside the range of 1–100 breaths per minute (br/min) were also excluded. The maximum values of the considered range were intentionally set higher than realistic values during sleep for robustness.
D. Estimation Comparison
For each subject, percent of prediction within 1 br/min or 5 bpm of gold standard, percent of prediction within 0.5 br/min or 2.5 bpm of gold standard, root mean square error (RMSE), and mean absolute error (MAE) between estimated and gold standard respiratory rate and heart rate were calculated. Kruskal-Wallis statistical test was performed with multiple comparison test to determine whether significant difference in predictive performance can be found between various sleep-awake stages, sleep positions, amount of body covered, respiratory events, and periodic limb movement severity. P-value < 0.05 was considered significant. Pearson’s correlation between estimation error measures and age, BMI, AHI, obstructive AHI, central AHI, arousal index, movement, periodic limb movement index, number of position changes, REM sleep percentage, sleep efficiency, or variance of gold standard respiratory rate or heart rate were calculated.
The proposed method of respiratory rate and heart rate were compared against other available non-contact methods for estimating respiratory rate [33] and heart rate [34]. To determine the importance of estimating respiratory rate and heart rate variability overnight, the proposed method was also compared against mean hold method where average gold standard measurement from the first 10 minutes of recordings were used as the respiratory and heart rate estimations for the rest of the night.
III. Results
53 subjects were recruited for this study. Three subjects were excluded from analysis due
to missing video data (connection failures), leaving total of 50 subjects for analysis. 30
male and 20 female subjects were included with average age of 53 ± 15 years old and
BMI of 29.4 ± 6.4 kg/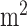 . Participant
characteristics are stratified by Apnea Hypopnea Index (AHI) and summarized in Table 1. AHI of less than 5 is defined as no sleep
apnea, 5 to 15 as mild, 15 to 30 as moderate, and greater than or equal to 30 as severe
sleep apnea. 12 subjects were not apneic, 13 were mild, 11 were moderate and 14 were severe.
48 subjects had obstructive hypopneas, 34 subjects had obstructive apneas, 19 subjects had
central hypopneas, 22 subjects had central apneas, and only 3 subjects had mixed
apneas.
. Participant
characteristics are stratified by Apnea Hypopnea Index (AHI) and summarized in Table 1. AHI of less than 5 is defined as no sleep
apnea, 5 to 15 as mild, 15 to 30 as moderate, and greater than or equal to 30 as severe
sleep apnea. 12 subjects were not apneic, 13 were mild, 11 were moderate and 14 were severe.
48 subjects had obstructive hypopneas, 34 subjects had obstructive apneas, 19 subjects had
central hypopneas, 22 subjects had central apneas, and only 3 subjects had mixed
apneas.
TABLE 1. Subject Characteristics.
| Characteristics | AHI < 5 | 5 ≤ AHI < 15 | 15 ≤ AHI < 30 | AHI ≥ 30 | All |
|---|---|---|---|---|---|
| Number (Male) | 12 (4) | 13 (9) | 11 (7) | 14 (10) | 50 (30) |
| Age, year | 45.5 ± 16.0 | 55.0 ± 14.1 | 50.1 ± 9.1 | 59.7 ± 16.1 | 53.0 ± 14.9 |
| Body mass index, kg/m2 | 28.5 ± 5.8 | 26.7 ± 4.1 | 28.0 ± 4.8 | 33.6 ± 8.1 | 29.4 ± 6.4 |
| AHI, events per hour | 3.1 ± 1.4 | 8.9 ± 2.7 | 22.3 ± 4.0 | 60.9 ± 34.3 | 25.4 ± 29.5 |
| Obstructive AHI, events per hour | 2.7 ± 1.7 | 7.6 ± 3.7 | 19.1 ± 7.9 | 55.6 ± 38.5 | 22.4 ± 29.7 |
| Central AHI, events per hour | 0.4 ± 0.9 | 2.6 ± 7.1 | 3.1 ± 6.0 | 3.8 ± 10.1 | 2.5 ± 7.0 |
| Total sleep time, hour | 5.4 ± 1.2 | 5.2 ± 1.4 | 5.3 ± 1.3 | 4.8 ± 1.6 | 5.2 ± 1.4 |
| Sleep efficiency, % | 78.2 ± 12.5 | 77.8 ± 15.6 | 76.7 ± 13.5 | 70.0 ± 24.0 | 75.5 ± 17.2 |
| REM sleep percentage | 13.6 ± 5.6 | 16.0 ± 7.2 | 17.8 ± 7.0 | 12.9 ± 8.5 | 15.0 ± 7.3 |
| Arousal index, arousals per hour | 18.1 ± 10.2 | 17.1 ± 7.7 | 24.9 ± 10.5 | 54.9 ± 33.0 | 29.6 ± 24.7 |
| Count of sleep position changes | 76 ± 21 | 77 ± 24 | 89 ± 22 | 110 ± 56 | 89 ± 37 |
| Average magnitude of movement, arbitrary units | 27.8 ± 16.4 | 32.7 ± 22.2 | 37.9 ± 22.1 | 28.1 ± 18.3 | 31.4 ± 19.7 |
| Periodic limb movement index, movements per hour | 23.7 ± 37.1 | 11.4 ± 16.3 | 11.6 ± 18.6 | 24.1 ± 34.6 | 17.9 ± 28.3 |
| Average respiratory rate, breaths per minute | 15.8 ± 3.1 | 15.2 ± 3.1 | 14.7 ± 1.1 | 17.4 ± 3.6 | 15.9 ± 3.0 |
| Variance of respiratory rate, breaths per minute squared | 3.1 ± 2.4 | 3.1 ± 2.4 | 2.4 ± 1.3 | 7.4 ± 9.1 | 4.1 ± 5.4 |
| Average heart rate, beats per minute | 68.4 ± 10.5 | 68.3 ± 8.5 | 63.0 ± 8.2 | 76.1 ± 10.7 | 69.3 ± 10.5 |
| Variance of heart rate, beats per minute squared | 21.6 ± 16.7 | 14.9 ± 8.7 | 12.2 ± 8.9 | 17.3 ± 12.5 | 16.6 ± 12.3 |
Characteristics of subjects collected from overnight polysomnography and sleep technician annotations. Data is presented in mean ± standard deviation. AHI = apnea hypopnea index, REM = rapid eye movement.
The average duration of sleep with normal breathing among all subjects was 215.2 ± 99.2 minutes. The average durations of respiratory event segments among all subjects were 50.4 ± 53.7 min for obstructive hypopneas, 11.2 ± 20.0 min for obstructive apneas, 6.4 ± 8.3 min for central hypopneas, 3.1 ± 5.0 min for central apneas, and 14.0 ± 21.4 min for mixed apneas.
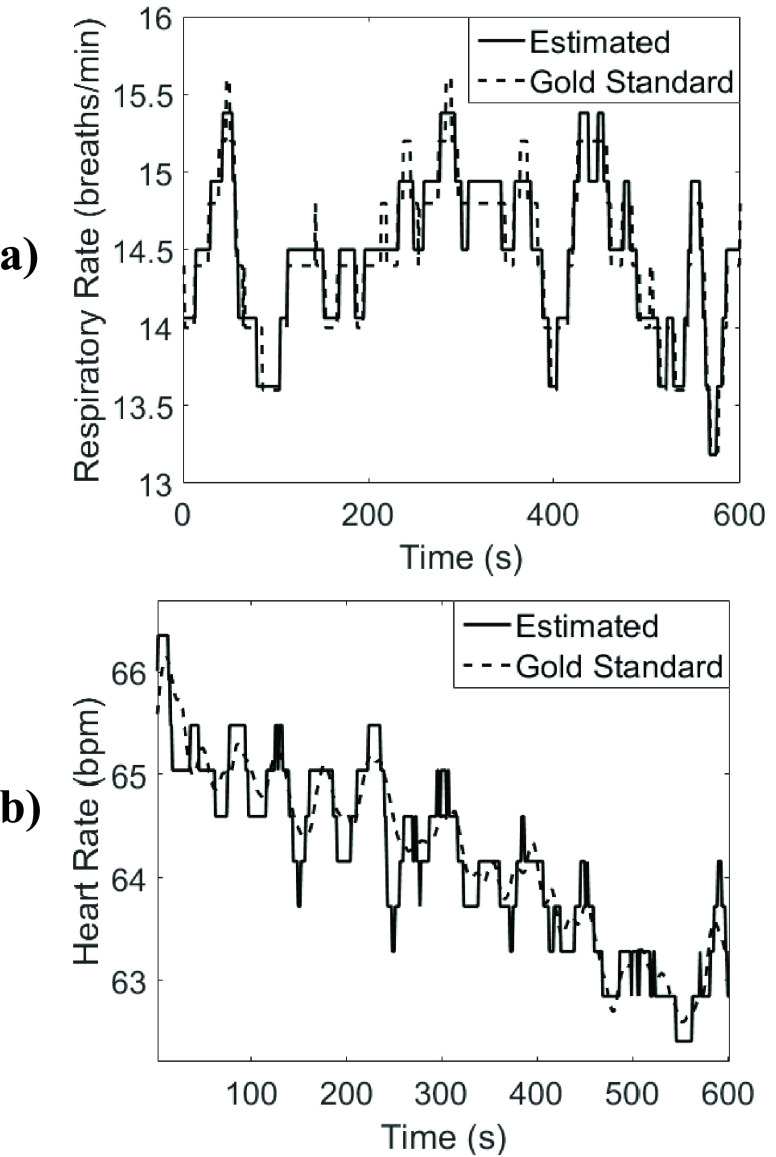
All subjects except four had full sleep cycle including NREM and REM sleep stages. Out of the 50 subjects included for respiratory rate analysis, 5 were excluded from heart rate analysis due to frequent irregular heart activities, such as premature ventricular contractions, atrial fibrillations, or atrioventricular block type II, as indicated by the sleep technician based on the PSG. The average percent of recorded duration excluded from analysis over all participants was 15.46 ± 13.05%; 12.83 ± 9.79% of which were segments with movement, 1.03 ± 1.78% were segments with error in gold standard measurements or participant was not visible in the frame of the video, and 1.61 ± 3.81% were when the algorithm did not detect any feature point. An example of the gold standard and predicted heart rate and respiratory rate are shown in Fig. 3.
FIGURE 3.
Example segment of estimated a) respiratory rate, and b) heart rate compared to gold standard rates from the sum of respiratory inductance plethysmography signal and from electrocardiogram signal respectively. (Subject 16, during uncovered right lateral NREM sleep).
Table 2 summarizes the results of respiratory rate and heart rate estimation during wakefulness, NREM or REM sleep with normal breathing, and during segments with apneas or hypopneas. For respiratory rate estimation, 89.89 ± 10.95 % of the estimated values were within 1 br/min of the gold standard, 83.65 ± 13.91 % were within 0.5 br/min, average RMSE was 2.10 ± 1.64 br/min, and average MAE was 0.82 ± 0.89 br/min. For heart rate estimation, 77.97 ± 18.91 % of the estimated segments were within 5 bpm of the gold standard, 67.60 ± 20.73 % were within 2.5 bpm, average RMSE was 7.47 ± 4.79 bpm, and average MAE was 4.36 ± 3.69 bpm. RMSE for estimating respiratory rates were significantly different between awake segments and sleep segments with normal breathing (p < 0.0001) and between sleep segments with normal breathing and respiratory events (p < 0.0001). RMSE for estimating respiratory rates were similar between NREM and REM sleep and between awake and respiratory event segments. No significant difference in median heart rate estimation RMSE was found for any pairwise comparison between awake, REM sleep with normal breathing, NREM sleep with normal breathing, and sleep with respiratory events.
TABLE 2. Respiratory and Heart Rate Estimation Error.
| Respiratory Rate Estimation mean (± std) | E < 1 br/min (%) | E < 0.5 br/min (%) | RMSE (br/min) | MAE |
|---|---|---|---|---|
| Awake, N = 50 | 83.32 (± 13.85) | 75.36 (± 16.34) | 2.85 (± 1.82) | 1.24 (± 1.13) |
| NREM, N = 50 | 94.63 (± 9.16) | 90.03 (± 12.30) | 1.29 (± 1.26) | 0.49 (± 0.66) |
| REM, N = 46 | 92.72 (± 11.61) | 86.79 (± 14.33) | 1.34 (± 1.44) | 0.55 (± 0.63) |
| Apnea and Hypopnea, N = 50 | 83.04 (± 13.46) | 73.40 (± 17.28) | 2.74 (± 1.89) | 1.23 (± 1.07) |
| All, N =50 | 89.89 (± 10.95) | 83.65 (± 13.91) | 2.10 9 (± 1.64) | 0.82 (± 0.89) |
| Heart Rate Estimation mean (± std) | E < 5 bpm (%) | E < 2.5 bpm (%) | RMSE (bpm) | MAE |
| Awake, N = 45 | 75.42 (± 19.20) | 64.60 (± 21.00) | 8.57 (± 5.51) | 5.13 (± 4.34) |
| NREM, N = 45 | 80.18 (± 20.81) | 71.06 (± 22.68) | 6.81 (± 5.34) | 4.05 (± 4.41) |
| REM, N = 42 | 78.66 (± 22.79) | 68.69 (± 23.95) | 6.93 (± 5.16) | 4.26 (± 4.05) |
| Apnea and Hypopnea, N = 45 | 75.34 (± 20.78) | 60.05 (± 22.92) | 7.47 (± 5.41) | 4.90 (± 4.31) |
| All, N = 45 | 77.97 (± 18.91) | 67.60 (± 20.73) | 7.47 (± 4.79) | 4.36 (± 3.69) |
Error values were calculated by averaging over all subjects. std = standard deviation, br/min = breaths per minute, bpm = beats per minute, E = percent of estimation for which the absolute error is less than specified threshold, RMSE = root mean squared error, MAE = mean absolute error, N = number of subjects, NREM = non-rapid eye movement sleep, REM = rapid eye movement sleep.
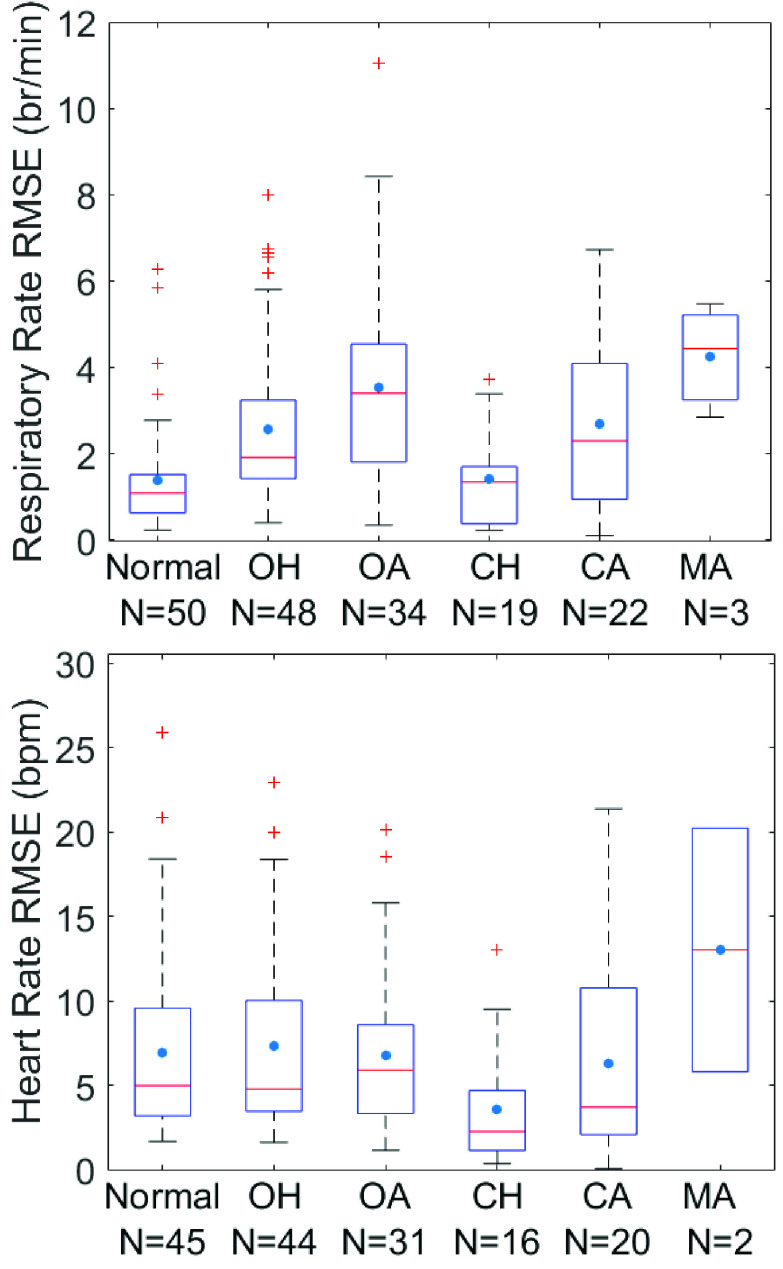
Fig. 4 shows the RMSE values for respiratory rate and heart rate estimation among different subjects during normal breathing and various respiratory events during sleep. Median (interquartile range) RMSE for respiratory rate estimation during normal breathing segments was 1.09 (0.63–1.48) br/min; similar during central hypopnea at 1.34 (0.38–1.70) br/min (p > 0.1); higher during obstructive hypopnea segments at 1.92 (1.42–3.17) br/min (p = 0.003), obstructive apnea segments at 3.39 (1.82–4.52) br/min (p < 0.0001), and central apneas at 2.30 (0.96–4.06) br/min (p = 0.036). Median heart rate estimation RMSE across subjects was 4.99 (3.27–9.52) bpm during normal breathing segments, 4.79 (3.48–9.92) bpm and 5.92 (3.35–8.58) bpm for obstructive hypopneas and apneas respectively, 2.27 (1.30–4.41) bpm and 3.72 (2.09–10.77) bpm for central hypopneas and apneas respectively, and 13.02 (9.42–16.62) bpm for mixed apneas. The median heart rate estimation RMSE was lower during central hypopneas compared to sleep segments with normal breathing, obstructive hypopneas and obstructive apneas (p < 0.01 for all).
FIGURE 4.
Quartile boxplot of root mean square error(RMSE) by subject of a) respiratory rate and b) heart rate, for normal breathing sleep segments, obstructive hypopneas(OH), obstructive apneas (OA), central hypopneas(CH), central apneas(CA), and mixed apneas(MA).
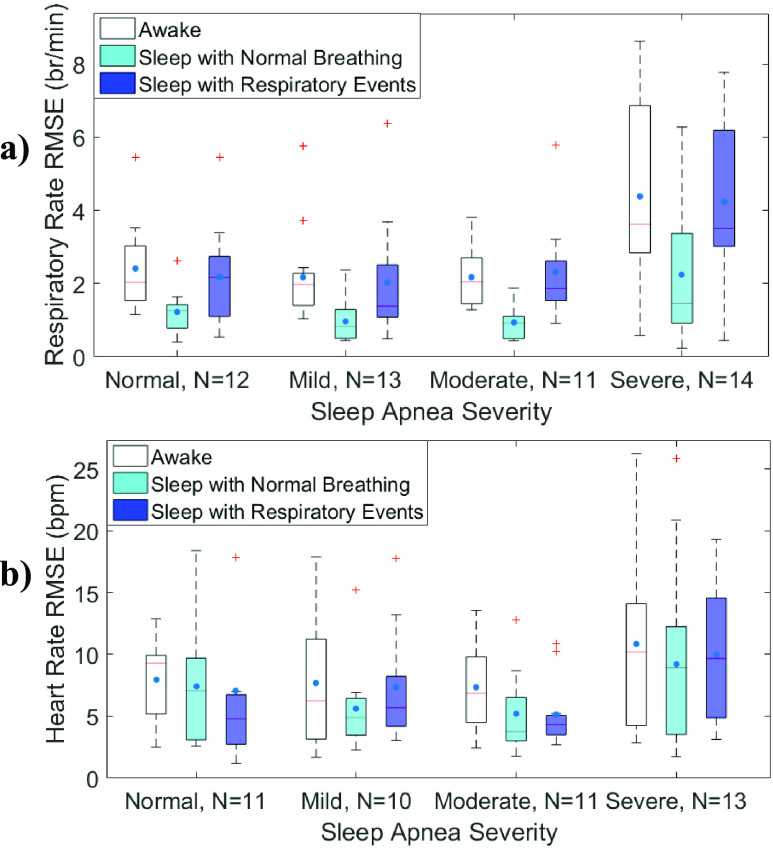
Fig. 5 shows details of RMSE during awake, sleep with normal breathing and respiratory events for participants with different severities of sleep apnea. For all sleep apnea severities, RMSE of respiratory rate estimation was significantly lower during sleep segments with normal breathing than awake segments (p < 0.02 for all) and sleep segments with respiratory event (p < 0.02, for all). While awake, the RMSE for respiratory rate estimation was significantly higher for subjects with severe sleep apnea compared to those with no, mild and moderate sleep apnea (p ≤ 0.007 for all). Similarly, during respiratory events, the RMSE for respiratory rate estimation was significantly higher for subjects with severe sleep apnea compared to those with mild or moderate sleep apnea (p ≤ 0.006 for both). RMSE of heart rate estimation was similar among subjects with different severities of sleep apnea, for awake segments, and sleep segments without and with respiratory events (p ≥ 0.3 for all).
FIGURE 5.
Quartile boxplot of root mean square error of a) respiratory rate and b) pulse for or non-apneic (AHI < 5), mild (5 ≤ AHI < 15), moderate (15 ≤ AHI < 30), and severe (AHI ≥ 30) sleep apnea subjects during awake, sleep with normal breathing, or sleep with apneas or hypopneas.
Out of the 50 subjects, all had spent time in supine posture, 48 had also slept in lateral side, and 3 had data in prone position. The RMSE for respiratory rate estimation was statistically higher while awake compared to supine and lateral positions (Fig. 6a, p ≤ 0.0001) and for various amount of blanket occlusion (Fig. 6b, p ≤ 0.0010) except for the case of completely covered. While awake, RMSE for respiratory rate estimation was significantly lower in lateral position compared to supine (p = 0.03). RMSE for heart rate estimation was similar for various sleep positions and the amount of body covered. There was no significant difference in RMSE for respiratory rate and heart rate estimation for varying severities of periodic limb movement.
FIGURE 6.

Quartile boxplot of root mean square error of respiratory rate (top) and heart rate (bottom) for each subject separated by body position (left) and amount of body occluded by blanket (right) during awake or sleep with normal breathing.
For respiratory rate estimation, RMSE was strongly correlated with variance of gold standard respiratory rate (r = 0.80, p < 0.0001). For heart rate estimation, RMSE was correlated with obstructive hypopnea and apnea index (r = 0.65, p < 0.0001).
IV. Discussion
The most important finding of this study is to modify and validate a vision-based non-contact monitoring system to estimate respiratory rate and heart rate during sleep. We achieved promising results for estimating respiratory rate in various sleeping positions and amount of blanket coverage. While the error of respiratory rate estimation was generally higher during awake periods and respiratory events during sleep, it was still less than 2.8br/min. This was expected as there are more non-periodic movements during wakefulness and obstructive apneas and hypopneas. For respiratory rate estimation, in 43 out of the 50 subjects, the error was less than 1 br/min more than 85% of the night. For heart rate estimation, in 32 out of the 45 subjects, error was less than 5 bpm more than 70% of the night. Importantly, Our method for respiratory and heart rate estimation is unaffected by the sleep position, amount of the body covered by blanket, and PLM severity during natural sleep with normal breathing.
Our proposed method for respiratory rate estimation is more accurate during normal breathing while asleep compared to wakefulness and respiratory events during sleep. There are a few factors that may have contributed to the reduced predictive accuracy and precision of respiratory rate estimation during sleep with respiratory events, and especially for subjects with severe apnea. Subjects with severe sleep apnea have frequent arousals and associated increases in head movement. The respiratory patterns of subjects with severe sleep apnea are also highly variable during sleep including: hyperventilation after an event, paradoxical breathing during obstructive events, and reduced ventilatory effort during central events. The highly variable respiratory patterns and movements during obstructive events can reduce the accuracy of our proposed method which relies on periodicity of the signal. Nonetheless, our proposed method for respiratory rate estimation outperforms the method proposed by Nakajima et al. [33] and mean-hold as seen in Table 3. Future studies could include larger sample size to increase robustness of the proposed algorithm for different breathing patterns during sleep and for various severities of sleep apnea.
TABLE 3. Respiratory and Heart Rate Estimation Performance Comparison.
| Respiratory Rate Estimation E < 1 br/min (%) mean (± std) | Li . . |
Nakajima  . [33] . [33]
|
Mean Hold |
|---|---|---|---|
| Awake, N = 50 | 83.32 (± 13.85) | 56.63 (± 17.78) | 28.72 (± 14.42) |
| Normal sleep, N = 50 | 94.20 (± 8.88) | 74.11 (± 19.42) | 32.46 (± 22.33) |
| Apnea and hypopnea, N = 50 | 83.04 (± 13.46) | 60.36 (± 20.51) | 26.12 (± 15.64) |
| All, N = 50 | 89.89 (± 10.95) | 69.10 (± 18.77) | 31.75 (± 17.37) |
| Processing time per predicted second | 0.875s (± 0.116s)* | 2.824s (± 0.229s) | N/A |
| Heart Rate Estimation E < 5 bpm (%) mean (± std) | Li . . |
Balakrishnan  . [34] . [34]
|
Mean Hold |
| Awake, N = 45 | 75.42 (± 19.20) | 38.60 (± 21.82) | 66.83 (± 20.94) |
| Normal sleep, N = 45 | 79.91 (± 20.67) | 49.91 (± 26.34) | 58.43 (± 31.49) |
| Apnea and hypopnea, N = 45 | 75.34 (± 20.78) | 46.97 (± 24.03) | 54.28 (± 30.) |
| All, N = 45 | 77.97 (± 18.91) | 47.77 (± 24.09) | 58.47 (± 28.17) |
| Processing time per predicted second | 0.875s (± 0.116s)* | 0.656s (± 0.090s) | N/A |
Performance measure compared is percentage of recorded duration for which respiratory and heart rate estimates are within 1 breath/min and 5 beats per minute respectively. Processing time was calculated by taking the average run times for the analysis of the first 15 minute segment for each subject. Values shown are the mean over all subjects.
std = standard deviation, br/min = breaths per minute, bpm = beats per minute, E = percent of estimation for which the absolute error is less than specified threshold, N = number of subjects,
Combined processing time for respiratory rate and pulse estimation
Estimating heart rate based on motion tracking is more challenging than respiratory rate estimation. Our proposed method for heart rate estimation achieved reasonable accuracy for normal breathing during sleep. One source of error in heart rate estimation is the residual respiratory motion harmonics. This is supported by the fact that RMSE for heart rate estimation was lower during central respiratory events, for which respiratory effort is minimum or zero. Overall, our proposed method for heart rate estimation outperformed the methods proposed by Balakrishnan et al. [34] and mean-hold when comparing percentage of estimation within 2.5bpm or 5bpm of gold standard as seen in Table 3. Future work could consider setting a more adaptive bound on the expected values of heart rate to improve heart rate estimation accuracy.
One limitation of estimating respiratory rate and heart rate through this vision based motion tracking was the exclusion of segments with large body movements. Our method was unable to isolate respiratory and heart beat during periods with large movements. As well, a new set of feature points needed to be selected and tracked after each body position change, which resulted in a minimum of 30 second segments with no respiratory rate and heart rate estimation on each occurrence. Participants with irregular cardiac activities were also excluded from heart rate estimation. Only 3 participants slept in the prone position for this study; the sample size would need to be increased significantly to determine the performance of our algorithm during sleep in prone position. Another limitation of this study pertains to the thin sheets and blankets used by the participants. Thicker blankets and airier covers could reduce the amount of motion captured by the camera from a distance, which could lead to reduced predictive accuracy in realistic home environments. Another limitation of this study is the limited number of baseline methods used for comparisons. Baselines used (Nakajima et al. [33] and Balakrishnan et al. [34]) were chosen based on the availability of code and also based on the ability of these approaches to process monochromatic video by analyzing motion.
V. Conclusion
Through tracking the motion of upper torso and head, algorithms developed by our group (Li et al., 2017) were experimentally validated and shown to be able to estimate respiratory rate during sleep with high accuracy. In the future, a non-contact sleep monitoring system can be developed for the home environment based on our algorithm and tested against available portable sleep monitoring devices. A non-contact solution is crucial for enabling long-term continuous monitoring and studying of various nocturnal conditions, such as monitoring for sudden infant death syndrome, nocturnal asthma, or nocturnal seizures. For diagnosis of sleep apnea, changes in respiratory volumes may be estimated through tracking changes in the magnitude of chest and abdominal movements with high accuracy. Further differentiation for central versus obstructive sleep apnea, or for positional OSA, can also be possible through motion analysis. This would significantly improve the utility of at-home sleep monitors.
Acknowledgment
The authors thank the participants and the sleep technicians at the sleep laboratory at Toronto Rehabilitation Institute whose participation and help made this study possible.
Funding Statement
This work was supported in part by the Toronto Rehabilitation Institute, University Health Network, FedDev Ontario, and BresoTEC Inc.
Contributor Information
Azadeh Yadollahi, Email: azadeh.yadollahi@uhn.ca.
Babak Taati, Email: babak.taati@uhn.ca.
References
- [1].Kapur V. K.et al. , “Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: An American academy of sleep medicine clinical practice guideline,” J. Clin. Sleep Med., vol. 13, no. 3, pp. 479–504, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bjornsdottir E.et al. , “Quality of life among untreated sleep apnea patients compared with the general population and changes after treatment with positive airway pressure,” J. Sleep Res., vol. 24, pp. 328–338, Jun. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Javaheri S.et al. , “Sleep apnea types, mechanisms, and clinical cardiovascular consequences,” J. Amer. College Cardiol., vol. 69, pp. 841–858, Feb. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Peppard P. E., Young T., Barnet J. H., Palta Hagen M. E. W., and Hla K. M., “Increased prevalence of sleep-disordered breathing in adults,” Amer. J. Epidemiology, vol. 177, no. 9, pp. 1006–1014, May 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Punjabi N. M., “The epidemiology of adult obstructive sleep apnea,” Proc. Amer. Thoracic Soc., vol. 5, pp. 136–143, Feb. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ram S., Seirawan H., Kumar S. K. S., and Clark G. T., “Prevalence and impact of sleep disorders and sleep habits in the United States,” Sleep Breathing, vol. 14, pp. 63–70, Feb. 2010. [DOI] [PubMed] [Google Scholar]
- [7].Kapur V., Strohl K. P., Redline S., Iber C., O’Connor G., and Nieto J., “Underdiagnosis of sleep apnea syndrome in U.S. Communities,” Sleep Breathing, vol. 6, no. 2, pp. 49–54, 2002. [DOI] [PubMed] [Google Scholar]
- [8].Young T., Evans L., Finn L., and Palta M., “Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women,” Sleep, vol. 20, no. 9, pp. 705–706, 1997. [DOI] [PubMed] [Google Scholar]
- [9].Tregear S., Reston J., Schoelles K., and Phillips B., “Obstructive sleep apnea and risk of motor vehicle crash: Systematic review and meta-analysis,” J. Clin. Sleep Med., vol. 5, no. 6, pp. 573–581, 2019. [PMC free article] [PubMed] [Google Scholar]
- [10].Garbarino S., Guglielmi O., Sanna A., Mancardi G. L., and Magnavita N., “Risk of occupational accidents in workers with obstructive sleep apnea: Systematic review and meta-analysis,” Sleep, vol. 39, pp. 1211–1218, Jun. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gottlieb D. J.et al. , “Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: The sleep heart health study,” Circulation, vol. 122, pp. 352–360, Jul. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yaggi H. K., Concato J., Kernan W. N., Lichtman J. H., Brass L. M., and Mohsenin V., “Obstructive sleep apnea as a risk factor for stroke and death,” England J. Med., vol. 353, pp. 2034–2041, Nov. 2005. [DOI] [PubMed] [Google Scholar]
- [13].Borsini E.et al. , “Prevalence of sleep apnea and cardiovascular risk factors in patients with hypertension in a day hospital model,” Clin. Exp. Hypertension, vol. 40, no. 3, pp. 231–237, 2018. [DOI] [PubMed] [Google Scholar]
- [14].Peppard P. E., Young T., Palta M., and Skatrud J., “Prospective study of the association between sleep-disordered breathing and hypertension,” England J. Med., vol. 342, pp. 1378–1384, May 2000. [DOI] [PubMed] [Google Scholar]
- [15].Kendzerska T., Gershon A. S., Hawker G., Tomlinson G., and Leung R. S., “Obstructive sleep apnea and incident diabetes. A historical cohort study,” Amer. J. Respiratory Crit. Care Med., vol. 190, pp. 218–225, Jul. 2014. [DOI] [PubMed] [Google Scholar]
- [16].Peppard P. E., Szklo-Coxe M., Hla K. M., and Young T., “Longitudinal association of sleep-related breathing disorder and depression,” Arch. Internal Med., vol. 166, no. 16, pp. 1709–1715, 2006. [DOI] [PubMed] [Google Scholar]
- [17].Krawczyk M.et al. , “Sleep disordered breathing in patients with heart failure,” Cardiol. J., vol. 20, no. 4, pp. 345–355, 2013. [DOI] [PubMed] [Google Scholar]
- [18].Kim R. D.et al. , “An economic evaluation of home versus laboratory-based diagnosis of obstructive sleep apnea,” Sleep, vol. 38, pp. 1027–1037, Jul. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rotenberg B. W., George C. F., Sullivan K. M., and Wong E., “Wait times for sleep apnea care in ontario: A multidisciplinary assessment,” Can. Respiratory J., vol. 17, no. 4, pp. 170–174, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Stöberl A. S.et al. , “Night-to-night variability of obstructive sleep apnea,” J. Sleep Res., vol. 26, pp. 782–788, Dec. 2017. [DOI] [PubMed] [Google Scholar]
- [21].Shelgikar A. V., Anderson P. F., and Stephens M. R., “Sleep tracking, wearable technology, and opportunities for research and clinical care,” Chest, vol. 150, pp. 732–743, Sep. 2016. [DOI] [PubMed] [Google Scholar]
- [22].Abrahamyan L.et al. , “Diagnostic accuracy of level IV portable sleep monitors versus polysomnography for obstructive sleep apnea: A systematic review and meta-analysis,” Sleep Breathing, vol. 22, pp. 593–611, Sep. 2018. [DOI] [PubMed] [Google Scholar]
- [23].Collop N. A.et al. , “Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable monitoring task force of the american academy of sleep medicine,” J. Clin. Sleep Med., vol. 3, no. 7, pp. 737–747, Dec. 2007. [PMC free article] [PubMed] [Google Scholar]
- [24].Rahman T.et al. , “DoppleSleep: A contactless unobtrusive sleep sensing system using short-range Doppler radar,” in Proc. Int. Joint Conf. Pervasive Ubiquitous Comput., Osaka, Japan, 2015, pp. 39–50. [Google Scholar]
- [25].Liu J., Chen Y., Wang Y., Chen X., Cheng J., and Yang J., “Monitoring vital signs and postures during sleep using WiFi signals,” IEEE Internet Things J., vol. 5, no. 3, pp. 2071–2084, Jun. 2018. [Google Scholar]
- [26].Kalkbrenner C., Eichenlaub M., Rüdiger S., Kropf-Sanchen C., Rottbauer W., and Brucher R., “Apnea and heart rate detection from tracheal body sounds for the diagnosis of sleep-related breathing disorders,” Med. Biol. Eng. Comput., vol. 56, pp. 671–681, Apr. 2018. [DOI] [PubMed] [Google Scholar]
- [27].Yadollahi A. and Moussavi Z. M. K., “A robust method for estimating respiratory flow using tracheal sounds entropy,” IEEE Trans. Biomed. Eng., vol. 53, no. 4, pp. 662–668, Apr. 2006. [DOI] [PubMed] [Google Scholar]
- [28].Bu N., Ueno N., and Fukuda O., “Monitoring of respiration and heartbeat during sleep using a flexible piezoelectric film sensor and empirical mode decomposition,” in Proc. 29th Annu. Int. Conf. IEEE Eng. Med. Biol. Soc., Lyon, France, Aug. 2007, pp. 1362–1366. [DOI] [PubMed] [Google Scholar]
- [29].Xia J. and Siochi R. A., “A real-time respiratory motion monitoring system using KINECT: Proof of concept,” Med. Phys., vol. 39, no. 5, pp. 2682–2685, 2012. [DOI] [PubMed] [Google Scholar]
- [30].Hu M.et al. , “Combination of near-infrared and thermal imaging techniques for the remote and simultaneous measurements of breathing and heart rates under sleep situation,” PLoS ONE, vol. 13, no. 1, 2018, Art. no. e0190466. doi: 10.1371/journal.pone.0190466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Li M. H., Yadollahi A., and Taati B., “Noncontact vision-based cardiopulmonary monitoring in different sleeping positions,” IEEE J. Biomed. Health Inform., vol. 21, no. 5, pp. 1367–1375, Sep. 2017. [DOI] [PubMed] [Google Scholar]
- [32].Li M. H., Yadollahi A., and Taati B., “A non-contact vision-based system for respiratory rate estimation,” in Proc. 36th Annu. Int. Conf. IEEE Eng. Med. Biol. Soc., Chicago, IL, USA, Aug. 2014, pp. 2119–2122. [DOI] [PubMed] [Google Scholar]
- [33].Nakajima K., Matsumoto Y., and Tamura T., “Development of real-time image sequence analysis for evaluating posture change and respiratory rate of a subject in bed,” Physiol. Meas., vol. 22, no. 3, pp. N21–N28, 2001. [DOI] [PubMed] [Google Scholar]
- [34].Balakrishnan G., Durand F., and Guttag J., “Detecting pulse from head motions in video,” in Proc. IEEE Conf. Comput. Vis. Pattern Recognit. (CVPR), Portland, OR, USA, Jun. 2013, pp. 3430–3437. [Google Scholar]