Our study revealed that musculoskeletal pain, physical function, and quality of life in adolescents significantly improves and is maintained 3 years after bariatric surgery.
Abstract
OBJECTIVES:
To evaluate the longitudinal effects of metabolic and bariatric surgery (MBS) on the prevalence of musculoskeletal and lower extremity (LE) pain, physical function, and health-related quality of life.
METHODS:
The Teen Longitudinal Assessment of Bariatric Surgery study (NCT00474318) prospectively collected data on 242 adolescents undergoing MBS at 5 centers over a 3-year follow-up. Joint pain and physical function outcomes were assessed by using the Health Assessment Questionnaire Disability Index, Impact of Weight on Quality of Life – Kids, and the Short Form 36 Health Survey. Adolescents with Blount disease (n = 9) were excluded.
RESULTS:
Prevalent musculoskeletal and LE pain were reduced by 40% within 12 months and persisted over 3 years. Adjusted models revealed a 6% lower odds of having musculoskeletal pain (odds ratio = 0.94, 95% confidence interval: 0.92–0.99) and a 10% lower odds of having LE pain (odds ratio = 0.90, 95% confidence interval: 0.86–0.95) per 10% reduction of BMI. The prevalence of poor physical function (Health Assessment Questionnaire Disability Index score >0) declined from 49% to <20% at 6 months (P < .05), Physical comfort and the physical component scores, measured by the Impact of Weight on Quality of Life – Kids and the Short Form 36 Health Survey, improved at 6 months postsurgery and beyond (P < .01). Poor physical function predicted persistent joint pain after MBS.
CONCLUSIONS:
Joint pain, impaired physical function, and impaired health-related quality of life significantly improve after MBS. These benefits in patient-reported outcomes support the use of MBS in adolescents with severe obesity and musculoskeletal pain and suggest that MBS in adolescence may reverse and reduce multiple risk factors for future joint disease.
What’s Known on This Subject:
Adolescents with severe obesity have chronic musculoskeletal pain, which limits their physical function and quality of life. They are at high risk for early knee osteoarthritis and worsening obesity, which will significantly impact public health.
What This Study Adds:
There are large, sustained decreases in prevalent musculoskeletal pain and improvements in physical function after bariatric surgery. Poor physical function and clinical depressive symptoms predict musculoskeletal pain and should be addressed early in weight loss programs to ensure joint health.
Obesity in adolescents is associated with lower extremity (LE) joint pain, abnormal biomechanics, poor physical function, and cartilage abnormalities, placing them at high risk for developing degenerative joint disease.1–4 Three-quarters of adolescents with severe obesity (BMI ≥120% of the 95th percentile or BMI ≥35) report musculoskeletal joint pain and LE joint pain (hips, knee, and ankles and/or feet), which is associated with poor physical function.5
In life course studies, cumulative obesity during adolescence and adulthood was associated with symptomatic knee osteoarthritis (KOA).6 A 25-year longitudinal cohort demonstrated that for every unit increase in BMI during childhood, there is a 34% higher likelihood of knee pain while walking and 10% higher likelihood of knee stiffness in adult men.7 Rising severe obesity among adolescents8 is concerning for the development of preosteoarthritis precursor lesions that lead to irreversible joint damage.9–11 Early markers of preosteoarthritis lesions are knee pain, stiffness, limited function, bone marrow edema, and joint effusions on MRI, which have been described in adolescents who are obese.3,12–14
Metabolic and bariatric surgeries (MBSs) performed in adolescents doubled between 2003 and 2009,15 leading to improvements in comorbidities.16 In adults, musculoskeletal pain is reversed, and medial cartilage loss is mediated with weight loss, but joint damage is not reversed.17–20 Unlike adults, the adolescent muscle, bone, and joint unit is responsive to joint loading and unloading through weight and physical activity; thus, adolescence may be a crucial time to prevent preosteoarthritis.21 Currently, we know little about the persistent effects of MBS on chronic joint pain and function in adolescents with severe obesity.
We hypothesized that (1) clinically meaningful reductions in prevalent joint pain and functional improvement would follow MBS and (2) improvements in physical function and health-related quality of life (HRQOL) would be modified by improvements in LE pain. Our objectives tested (1) changes in prevalence of chronic musculoskeletal pain, LE pain, and pain intensity at weight-bearing joints of the lower back, hips, knees, and feet and/or ankles, (2) associations between MBS and improvements in joint pain, physical function, and physical HRQOL, and (3) predictors of persistent joint pain and poor physical function across 3 years after MBS in Teen Longitudinal Assessment of Bariatric Surgery (Teen-LABS) participants.
Methods
Study Design
A detailed methodology for Teen-LABS was previously described.22,23 Teen-LABS (NCT00465829) is a prospective, observational study that collects standardized data on adolescents (≤19 years of age) undergoing MBS at 5 US centers. Consecutive adolescents with a BMI >35 and comorbid disease were enrolled (n = 242) between February 28, 2007, and December 30, 2011. Data were collected at baseline (≤30 days preoperative; 0 months) and at postoperative assessments (6, 12, 24, and 36 months). The institutional review boards of each participating institution approved the study. Written assent and consent were obtained from the participants.
Participants with joint disease such as Blount disease (n = 9) or slipped capital femoral epiphyses (n = 0) were excluded. Participants who underwent laparoscopic adjustable gastric band (n = 14) were excluded because they made up a small subset of participants.24 The final analysis included 219 participants undergoing either Roux-en-Y gastric bypass or vertical sleeve gastrectomy.
Pain Assessment
Pain was assessed by a 0- to 10-point visual analog scale (0 indicates no pain; 10 indicates severe pain). A sample question is “How much pain have you had because of your weight in the past week?” Pain was rated at each anatomic site (lower back, hips, knees, and ankles and/or feet). The presence of musculoskeletal pain was defined as pain with a score >0 at any of the sites. The presence of LE pain was defined as pain with a score >0 at the hips, knees, or ankles and/or feet. We evaluated the whole LE instead of individual joints because all the LE joints impact stress and load across the knee. We focused on LE pain because LE pain is a risk factor for KOA. Site-specific pain was similarly defined (ie, scores >0). Site-specific pain intensity was reported as a continuous variable, excluding 0 (no pain). Back pain was included in musculoskeletal pain because it was not a primary outcome and was not associated with poor function or with the risk of developing KOA.5
Physical Functional Status Assessment
Physical function was assessed by using the self-report Health Assessment Questionnaire Disability Index (HAQ-DI), a well-validated tool that measures the impact of chronic disease on functional ability.25,26 It contains 20 items that measure physical disabilities over the past week in 8 activity categories such as dressing and grooming. Items are scored on a 4-point scale of 0 (no difficulty), 1 (some difficulty), 2 (much difficulty), and 3 (cannot do). A standard score is computed from 8 categories27 and was treated as a binary variable for which values >0 indicate poor self-reported physical function. Objective physical function was assessed by the 400-m walk test (400 MWT), measured in time (seconds) to complete the test, and ascertains mobility and endurance in populations with chronic disease.24,28
HRQOL
Two self-report HRQOL tools evaluated the effect of weight loss on physical quality of life. Weight-related quality of life was assessed by using the Impact of Weight on Quality of Life – Kids (IWQOL-Kids), an instrument for adolescents (11–19 years) with 4 subscales (physical comfort, body esteem, social life, family relations) and a total score. Raw scaled scores were transformed to a 0 to 100 scale with higher scores reflecting better weight-related quality of life.29 It has demonstrated excellent psychometric properties, including discrimination among weight status groups, and responsiveness to weight change.30,31 The Short Form 36 (SF-36) Health Survey is a measure of patient health and has been used for studies in obesity and musculoskeletal disease.32–34 It has excellent psychometric properties and is validated for respondents 14 years and older.35 It includes an 8-scale profile of scores and provides physical and mental health summary measures. We restricted analyses to the total score and the physical comfort subscale on the IWQOL-Kids and to the physical summary measures on the SF-36 to focus on associations between pain and physical well-being.
Covariates
Sex, race, age at surgery, surgical center, and percent change in BMI from baseline were evaluated as confounders. Clinical-range depressive symptoms were assessed by the Beck Depression Inventory-II (BDI-II) because it was associated with pain.5 We used the total score of ≥17 as a cut point for “clinically” depressive symptoms as a binary variable, for which ≥17 signified clinically depressive symptoms and <17 signified no clinically depressive symptoms.36 We controlled for obesity-related comorbidities (hypertension, asthma, dyslipidemia) using a composite load score, which was computed from the total number of comorbidities for each participant.37 High-sensitivity C-reactive protein (hs-CRP), a potential confounder in the relationship between obesity and pain and inflammation,38,39 was analyzed as part of a biomarker panel at a core laboratory facility.22
Statistical Analysis
We performed a secondary analysis of the Teen-LABS observational study.16 Descriptive statistics used mean (SD) or median (interquartile range [IQR]) for continuous measures and frequencies and percentages for categorical variables. Generalized estimating equations estimated the relative risk associated with each of the binary outcome variables (musculoskeletal pain [with versus without pain], LE pain [with versus without pain], physical functional status [with versus without functional disability]) and were estimated at each time point after surgery. An unstructured correlation with robust variance estimators was used for model estimates. A random intercept term was included to account for within-subject variance over time. A quantile-quantile plot was used to determine if continuous response variables (400 MWT time to completion and IWQOL-Kids score [total, physical]) were normally distributed. On the basis of the observed plot, log transformations were used to normalize the time-to-completion distribution for subsequent modeling. Linear mixed-effects models were used to determine the changes over time in physical functional status and quality-of-life parameters from the baseline. Initially, both random intercept and slope were added in the models, but slope was dropped because of the lack of statistical significance. The unstructured covariance matrix was used for covariances to be freely estimated. All models were adjusted for age at surgery, race, sex, hs-CRP, percent change in BMI from baseline, depression (BDI-II), comorbidity index, and surgical center. At baseline, the cohort consisted of 219 participants; in follow-up, the cohort was 198 at 6 months, 189 at 12 months, 182 at 24 months, and 168 at 36 months. Surgery type was entered in the initial models but was not significant and was removed from the final models. Approximately 15% of assessments had missing covariates. Multiple imputation was used for missing covariates for all models. A Markov chain Monte Carlo method that assumes multivariate normality was used for imputation of the missing covariates. A total of 50 imputed data sets were created, and each was fitted separately, with estimates pooled by using the Rubin algorithm. A sensitivity analysis was conducted, and parameter estimates were compared between the imputed and nonimputed data set. The nonimputed results were presented because results of both were similar. The statistical significance level was set at α = .05. The Bonferroni-Holm adjustment for multiple testing was used for all post hoc comparisons between time points within each hypothesis. All analyses were conducted with SAS statistical software version 9.4 (SAS Institute Inc, Cary, NC).
Results
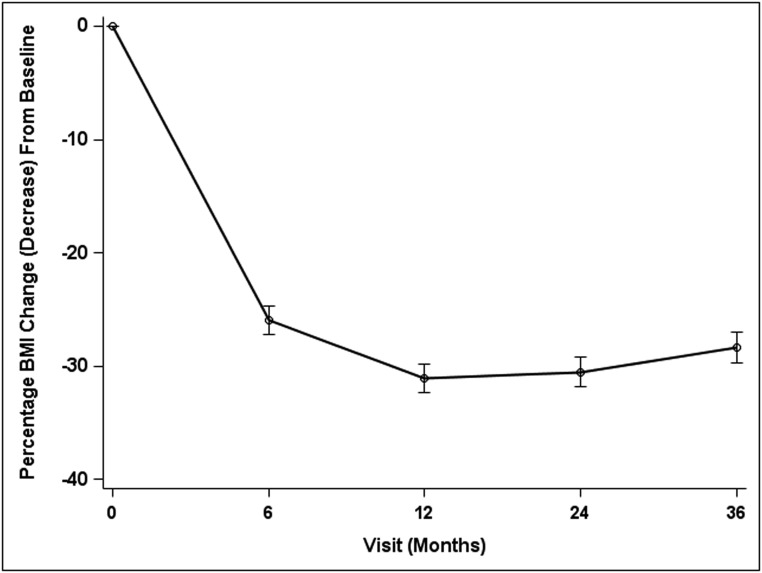
A total of 219 participants were included in the analysis, of which 70% underwent Roux-en-Y gastric bypass (n = 152) and 30% underwent vertical sleeve gastrectomy (n = 67). The mean age was 17 ± 1.6 years, and the median BMI was 50.0 (Table 1). Two-thirds of participants reported musculoskeletal and LE pain, and half reported poor physical function (HAQ-DI scores >0). The percent BMI change at 6 months was greatest at −24%, and 3 years after surgery, the overall percent BMI change was −27% (P = .02) (Fig 1).
TABLE 1.
Characteristics of Participants Before MBS Weight Loss Surgery (N = 219)
| Mean (SD), Median (IQR),a or Frequency (%) | |
|---|---|
| Age at operation, y, mean (SD) | 17.0 (1.6) |
| BMI,a median (IQR) | 50.0 (45.2–57.4) |
| Wt, kg,a median (IQR) | 143.2 (127.2–163.5) |
| Sex, n (%) | |
| Male | 52 (23) |
| Female | 167 (76) |
| Race, n (%) | |
| White | 161 (73) |
| African American | 44 (21) |
| Asian American | 1 (0.4) |
| Multiracial | 13 (5) |
| Surgical type, n (%) | |
| Gastric bypass | 152 (70) |
| Sleeve gastrectomy | 67 (30) |
| Musculoskeletal pain,b n (%) | |
| Yes | 136 (63) |
| LE pain,c n (%) | |
| Yes | 135 (63) |
| HAQ-DI scorea, median (IQR) | 0 (0–0.4) |
| Musculoskeletal pain, median | 0.1 |
| No musculoskeletal pain, median | 0 |
| IWQOL-Kids score, mean (SD) | |
| Total | 62.3 (17.9) |
| Physical comfort subscale | 52.8 (25.1) |
| SF-36 score, mean (SD) | |
| Physical component summary | 44.2 (8.5) |
| BDI-II total score,a median (IQR) | 6.0 (2–12) |
| Depressive symptoms, n (%) | |
| BDI-II of ≥17 | 32 (15) |
| BDI-II <17 | 85 (85) |
| hs-CRP, mg/Dl,a median (IQR) | 0.6 (0.3–1.1) |
The HAQ-DI is scored on a 4-point scale comprising 0 (no difficulty), 1 (some difficulty), 2 (much difficulty), and 3 (cannot do) in which values >0 indicate poor self-reported physical function; the HAQ-DI scores are further stratified by participants who report musculoskeletal pain and those who did not report musculoskeletal pain. The IWQOL-Kids is an instrument for adolescents (11–19 y) with 4 subscales (physical comfort, body esteem, social life, family relations) and a total score. Higher scores indicate better weight-related quality of life.
Median IQR reported.
Musculoskeletal joint pain includes any reported level of lower back, hip, knee, or ankle and/or foot pain.
LE joint pain includes any reported level of hip, knee, or ankle and/or foot pain.
FIGURE 1.
Percentage change in BMI over 36 months.
Joint Pain After MBS
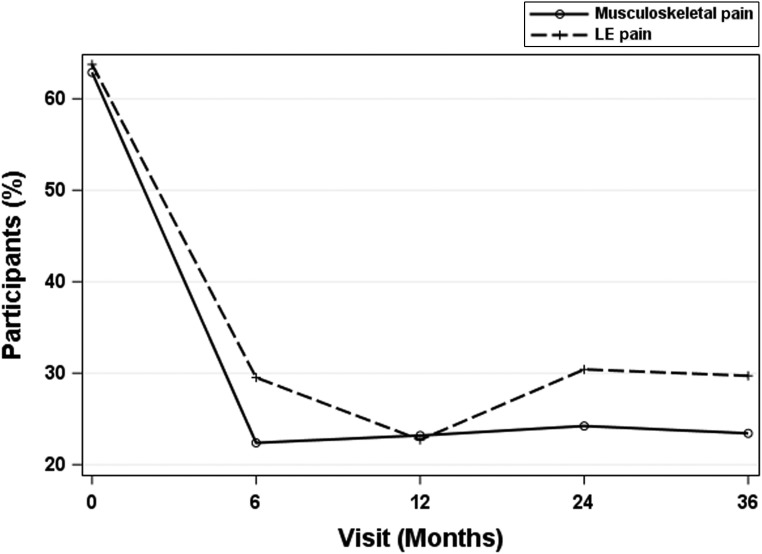
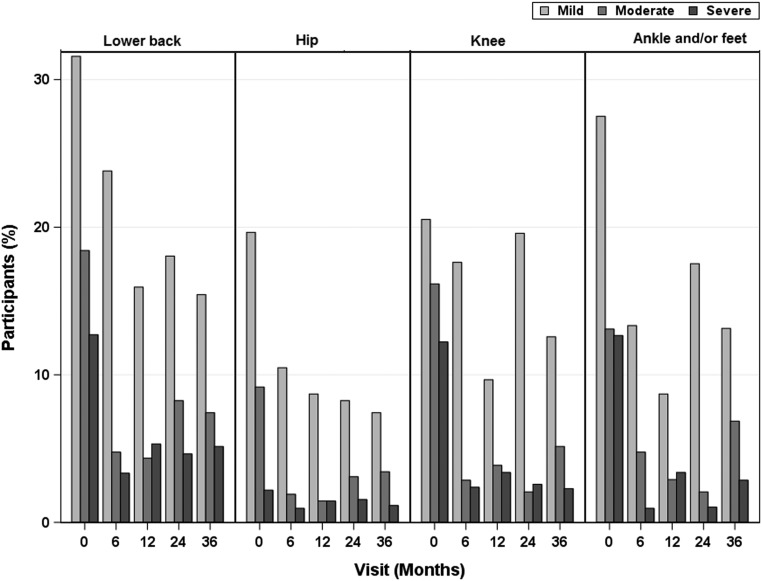
After MBS, both musculoskeletal and LE pain decreased at 6 to 12 months (P < .05) to <30% prevalence, which was maintained over 3 years (Fig 2). Pain intensity at specific joints was markedly reduced in the first 6 months and was maintained (Fig 3).
FIGURE 2.
Significant reductions in musculoskeletal and LE pain after MBS. Musculoskeletal joint pain was defined as any reported level of lower back, hip, knee, or ankle and/or foot pain. LE joint pain was defined as any reported level of hip, knee, or ankle and/or foot pain.
FIGURE 3.
Pain intensity by joint-specific site after MBS. The pain was reported per site by using a 0- to 10-point visual analog scale. Pain was categorized as mild (score of 1–3), moderate (score of 4–6), and severe (score of 7–10). Pain scores of 0 are not included.
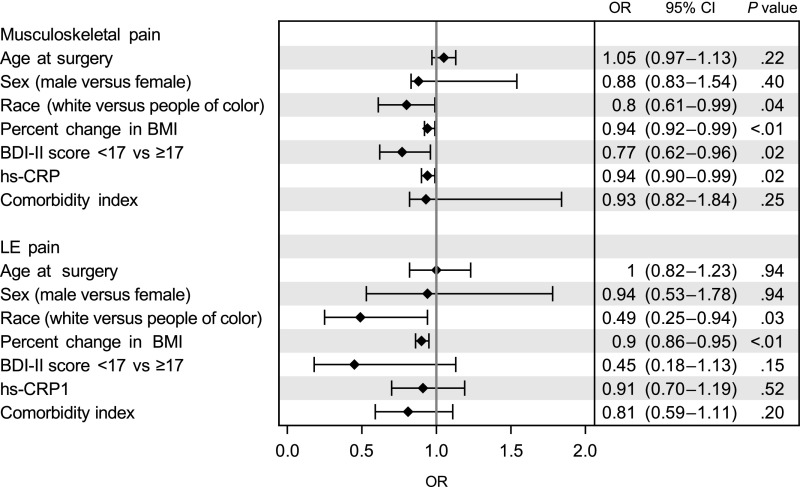
The association between percent change in BMI and musculoskeletal and LE pain in adjusted models revealed 6% lower odds of having musculoskeletal pain (odds ratio [OR] = 0.94; 95% confidence interval [CI]: 0.92–0.99) and 10% lower odds of having LE pain (OR = 0.90; 95% CI: 0.86–0.95) for every 10% reduction in BMI (Fig 4). There was a lower likelihood of having musculoskeletal and LE pain for white subjects.
FIGURE 4.
Associations between joint pain and MBS. Percent change in BMI indicates a 10% reduction in BMI. Depressive symptoms were defined as clinical-range depressive symptoms by using a suggested total score of >17 as a conservative cut point on the BDI-II. Comorbidities included hypertension, dyslipidemia, fatty liver disease, obstructive sleep apnea, chronic kidney disease, pseudotumor cerebri, polycystic ovary syndrome, asthma, gastroesophageal reflux disease, and stress urinary incontinence; a composite load score was computed from the total number for comorbidities for each participant. Musculoskeletal joint pain was defined as any reported level of lower back, hip, knee, or ankle and/or foot pain. LE joint pain was defined as any reported level of hip, knee, or ankle and/or foot pain.
Physical Function After MBS
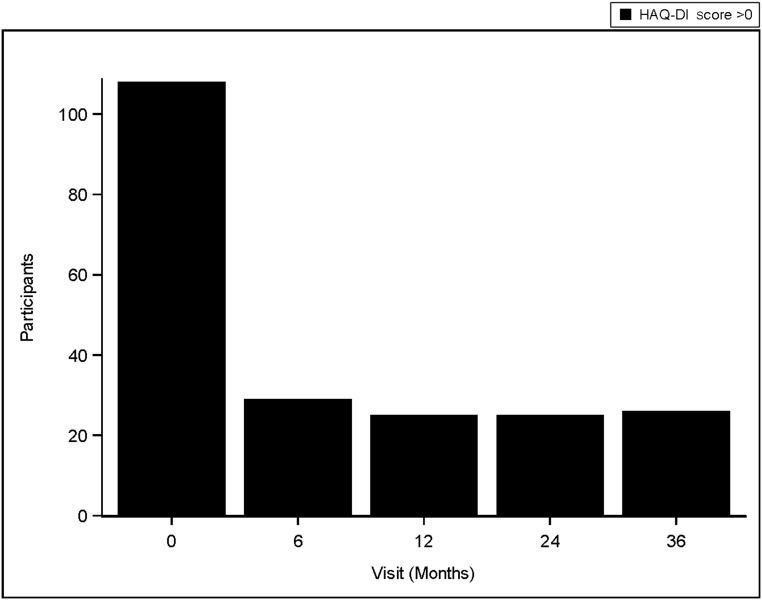
After MBS, the prevalence of poor physical function (HAQ-DI score >0) declined from 49% to <20% at 6 months (P < .05) and remained constant over 3 years (Fig 5).
FIGURE 5.
Participants reporting poor physical function decreases after MBS. Poor function status was assessed by the HAQ-DI in which poor physical function represents a score of >0 and good physical function represents a score of 0.
There was no independent association between change in BMI and poor physical function (HAQ-DI score >0) over 3 years (Table 2). However, there was a 21% greater odds of poor physical function (OR: 1.21; 95% CI: 1.08–1.52) for participants with LE pain compared with those without LE pain (Table 2). Clinically depressive symptoms were associated with greater odds of poor physical function (Table 2).
TABLE 2.
Association of Pain and Depression With Poor Self-reported Physical Function After MBS Over 3 Years
| Variables | Physical Function | |
|---|---|---|
| OR (95% CI) | P | |
| Age at surgery | 1.01 (0.97–1.05) | .48 |
| Male versus female | 0.92 (0.83–1.04) | .19 |
| White versus people of color | 1.08 (0.99–1.22) | .26 |
| Percent change in BMI | 1.00 (0.99–1.01) | .38 |
| BDI-II score ≥17 vs <17 | 1.30 (1.10–1.52) | <.01 |
| Comorbidity index | 0.97 (0.94–1.04) | .55 |
| LE pain versus no pain | 1.21 (1.08–1.52) | <.01 |
Percent change in BMI indicates an improvement in the BMI. Depressive symptoms were defined as clinical-range depressive symptoms by using a suggested total score of >17 as a conservative cut point on the BDI-II. Comorbidities included hypertension, dyslipidemia, fatty liver disease, obstructive sleep apnea, chronic kidney disease, pseudotumor cerebri, polycystic ovary syndrome, asthma, gastroesophageal reflux disease, and stress urinary incontinence; a composite load score was computed from the total number for comorbidities for each participant.
Objectively measured functioning, assessed by the 400 MWT, improved from 380 seconds (95% CI: 369–392) to 351 seconds (95% CI: 340–361) completion time at 6 months postsurgery (P < .01) and was maintained. The mean walking times did not differ on the basis of LE pain status.
HRQOL After MBS
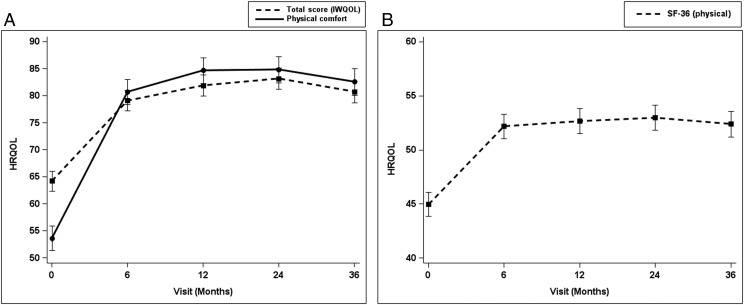
Physical comfort, total HRQOL scores (based on the IWQOL-Kids), and the physical component score on the SF-36 improved by 6 months postsurgery (P < .01) and remained constant over time (Fig 6). These scores did not differ by LE pain status.
FIGURE 6.
A, Improvement of IWQOL-Kids total and physical scores after metabolic and MBS. B, Improvement of SF-36 physical component summary scores after MBS.
Predictors of Persistent Musculoskeletal and LE Pain and Poor Self-reported Physical Function
Thirty percent of participants had persistent joint pain after MBS. Poor physical function (defined by the HAQ-DI >0) predicted greater odds of LE pain. Having better scores on the IWQOL physical comfort score predicted lower odds of having persistent musculoskeletal and LE pain (Table 3).
TABLE 3.
Predictors of Persistent Musculoskeletal Pain and LE Pain After MBS
| Variables | Musculoskeletal Pain | LE Pain | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | |||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Age at surgery | 1.08 (0.93–1.27) | .28 | 1.05 (0.89–1.24) | .53 | 1.11 (0.94–1.31) | .22 | 1.02 (0.85–1.22) | .83 |
| Male versus female | 0.67 (0.36–1.27) | .22 | 1.26 (0.61–2.67) | .54 | 0.35 (0.18–0.67) | <.01 | 0.64 (0.31–1.32) | .23 |
| White versus people of color | 0.93 (0.47–1.81) | .83 | 1.15 (0.49–2.66) | .64 | 0.90 (0.48–1.68) | .75 | 1.25 (0.55–2.87) | .59 |
| Percent change in BMI | 0.94 (0.91–0.96) | <.01 | 0.97 (0.94–0.99) | .04 | 0.94 (0.91–0.97) | <.01 | 0.97 (0.94–1.01) | .12 |
| Poor versus good self-reported physical function | 3.17 (1.72–5.84) | <.01 | 1.26 (0.48–3.27) | .63 | 7.11 (3.72–13.57) | <.01 | 2.11 (1.02–4.36) | .04 |
| IWQOL-Kids total score | 0.95 (0.93–0.97) | <.01 | 1.01 (0.97–1.05) | .56 | 0.93 (0.92–0.95) | <.01 | 0.99 (0.95–1.02) | .46 |
| IWQOL-Kids physical comfort score | 0.94 (0.93–0.96) | <.01 | 0.95 (0.92–0.98) | <.01 | 0.93 (0.91–0.95) | <.01 | 0.96 (0.92–0.99) | .01 |
| SF-36 physical score | 0.94 (0.90–0.97) | <.01 | 0.97 (0.93–1.00) | .13 | 0.91 (0.88–0.95) | <.01 | 0.97 (0.91–1.00) | .05 |
| BDI-II score ≥17 vs <17 | 3.90 (1.48–10.33) | <.01 | 2.04 (0.66–6.28) | .22 | 3.36 (1.92–9.97) | <.01 | 0.77 (0.23–2.61) | .68 |
Percent change in BMI indicates an improvement in the BMI. Poor versus good physical function status was assessed by the HAQ-DI for which poor physical function represents a score of >0 and good physical function represents a score of 0. Depressive symptoms were defined as clinical-range depressive symptoms by using a suggested total score of >17 as a conservative cut point on the BDI-II. Musculoskeletal joint pain was defined as any reported level of lower back, hip, knee, or ankle and/or foot pain. LE joint pain was defined as any reported level of hip, knee, or ankle and/or foot pain. Model adjusted for age, sex, race, depressive symptoms, comorbidities, and physical function.
Having clinical-range depressive symptoms or having musculoskeletal or LE pain predicted greater odds of persistent poor physical function (Table 4).
TABLE 4.
Predictors of Persistent Poor Physical Function (HAQ-DI >0) After MBS
| Variables | Physical Function | |||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusteda | Adjustedb | ||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Age at surgery | 1.15 (0.92–1.14) | .22 | 0.14 (0.20–0.79) | .05 | 0.56 (0.25–1.28) | .08 |
| Male versus female | 0.54 (0.24–1.22) | .14 | 1.08 (0.32–1.31) | .94 | 0.72 (0.58–1.71) | .87 |
| White versus people of color | 0.89 (0.43–1.84) | .74 | 1.92 (0.41–2.20) | .15 | 1.60 (0.78–2.89) | .27 |
| Percent change in BMI | 0.96 (0.94–0.99) | <.01 | 0.78 (0.61–1.11) | .10 | 0.77 (0.58–1.03) | .10 |
| BDI-II score ≥17 vs <17 | 3.31 (1.36–8.34) | .01 | 2.82 (1.40–3.54) | .01 | 2.98 (1.18–3.15) | .04 |
| Comorbidities | 0.61 (0.32–1.17) | .14 | 0.40 (0.12–1.36) | .14 | 0.43 (0.17–1.40) | .36 |
| Musculoskeletal pain, yes versus no | 4.05 (2.27–7.20) | <.01 | 3.81 (1.65–5.58) | <.01 | — | — |
| LE pain, yes versus no | 5.52 (3.01–10.13) | <.01 | — | — | 3.43 (2.77–6.14) | <.01 |
Percent change in BMI indicates an improvement in the BMI. Depressive symptoms were defined as clinical-range depressive symptoms by using a suggested total score of >17 as a conservative cut point on the BDI-II. Comorbidities included hypertension, dyslipidemia, fatty liver disease, obstructive sleep apnea, chronic kidney disease, pseudotumor cerebri, polycystic ovarian syndrome, asthma, gastroesophageal reflux disease, and stress urinary incontinence; a composite load score was computed from the total number for comorbidities for each participant. For the HAQ-DI, 0 represents normal function, whereas >0 represents poor function. —, not applicable.
Model adjusted for age, sex, race, depressive symptoms, comorbidities, and musculoskeletal pain.
Model adjusted for age, sex, race, depressive symptoms, comorbidities, and LE pain.
Discussion
We provide long-term evidence that joint pain and poor physical function are reversible among adolescents with severe obesity undergoing MBS. Prevalent musculoskeletal and LE pain was reduced by 40% within 6 months and lasted up to 3 years. Physical function and HRQOL measures improved and were maintained by a similar magnitude. Improvements in prevalent pain were independently associated with reductions in BMI. Improvements in physical function were independently associated with not having LE pain and not having clinical-range depressive symptoms. Persistent joint pain, after MBS, was predicted by poor physical function.
Rapid reduction in joint pain after MBS has been reported in adults.17,40 The greatest reductions in pain prevalence occurred in the knees, ankles/feet, hips, and lower back, and the magnitude of the reduction in prevalence of pain was ∼50%.33,41 Similarly, our data in adolescents revealed large improvements in pain prevalence across the first year, which remained stable over 3 years. Although not significant, our data revealed a return in pain intensity at the knees and ankles between 12 and 24 months. This may be explained by greater activity participation among previously deconditioned adolescents, other coexisting factors such as depression, or more insidious, existing degenerative changes and maladaptive biomechanics.42
Physical function, functional mobility, and quality-of-life measures improved with clinically meaningful differences over the 3 years.17,24,34 Specifically, the degree of change in the median HAQ-DI score of −0.125 from before to 6 months after MBS are similar to change scores between −0.08 and −0.25 for adults with rheumatic disease.43 The decrease of 30 seconds in the completion of the 400 MWT in the first 12 months is similar to a much larger adult MBS cohort.17,44,45 IWQOL-Kids total and physical comfort scores and SF-36 scores improved >10 points over the first 6 months and were maintained, also suggesting a meaningful improvement.35
Associations between weight loss, pain, and function are strong, but directionality and the modifying effects of each variable on the other is hard to prove in our observational study. Improvements in pain were independently associated with percent change in BMI, but improvements in physical function were not. However, improvements in physical function were directly associated with LE pain, suggesting that reversal of joint pain is the main factor to better daily function.
Although we hypothesized that depressive symptoms would be associated with LE pain,5 our longitudinal data did not reveal this association. However, depressive symptoms were clearly associated with poor self-reported physical function and persistent poor function. Among adults, fewer depressive symptoms before MBS and a decline in depressive symptoms were associated with improvements in pain and physical function.17 The discrepancy between the adult and our adolescent data may be a function of the larger size of the adult study being able to discriminate these associations.
Although most participants became pain free, continued joint pain after MBS was predicted by self-reported poor physical function and physical HRQOL. Furthermore, persistent poor physical function was predicted by having musculoskeletal pain, LE pain, and depressive symptoms. Many adolescents remain obese after MBS (mean BMI = 38)16 and are at a continued risk of disability and high risk for developing KOA, especially if they continue to have pain and diminished physical function.7,46 Thus, targeted intervention and prevention strategies for adolescents who are obese and have poor physical function and depressive symptoms should include a multidisciplinary approach that includes the following: (1) assessment for early joint disease; (2) treatment of pain with conservative measures including physical therapy, antiinflammatories, and gait modifications; (3) assessment for depression; and (4) mental health and physical therapy professionals embedded in treatment teams.
Mechanistically, improvements in joint pain after MBS are explained by reduced forces transmitted through the knee, such that with each pound of weight lost, there is a commensurate reduction of these forces on the knee joint.46,47 In adult studies of osteoarthritis, authors have shown how weight loss of 10% or more reduces knee compressive forces.18 Others have shown that weight loss led to reduced knee flexion, vertical ground reaction forces, and muscle forces around the knee, which all contribute to decreased knee joint loads.48
Our study had limitations. First, the study was not a randomized controlled trial, and we examined secondary outcomes on a large cohort powered to study improvements in weight- and obesity-related disease, not pain and function. However, given that this is the largest cohort on adolescents with severe obesity on this subject, our study serves as an important first step to understand the problem. Second, the data collected was self-report (except the 400 MWT), so we could not directly study the effect of weight loss on objective LE performance measures, mechanics, or joint pathology. Third, the HAQ-DI is not ideal or specific because it evaluates activities of daily living rather than knee health, and it has a ceiling effect, which does not discriminate well at low levels of physical function. Fourth, 15% of the variables had missing covariates addressed by statistical imputation, which could represent a potential error. However, we performed a sensitivity analysis of imputed and nonimputed models, which revealed similar results. Finally, the finding of racial disparity associated with joint pain, but not with function or persistent pain, is hard to interpret given the lack of generalizability from this predominantly white cohort.
Conclusions
MBS leads to large and sustained reductions in joint pain and improvements in physical function in adolescents with severe obesity over 3 years. These improvements will allow teenagers to move, be more functional, and participate in physical activity to improve their joint health and maintain their weight loss. Given that adolescents with obesity are at risk for developing osteoarthritis in midlife,11 our findings suggest that preosteoarthritis conditions exist in adolescent with severe obesity, but after MBS, preosteoarthritis risk factors and abnormal joint loads may be reversed. Adolescence represents a window of opportunity for caregivers to implement exercise and behavioral supports pre- and postsurgery to maintain long-term weight and joint health benefits. Future research should be focused on studying the relationship among weight loss, biomechanical and systemic inflammatory mechanisms, performance measures, and biomarkers to identify, target, and treat adolescents who are obese and at risk for KOA.
Acknowledgments
We acknowledge the significant contributions made by the Teen-LABS Consortium as well as our parent study, the Longitudinal Assessment of Bariatric Surgery Consortium (grant U01 DK066557). The consortium is grateful for the important work done by the adjudication committee.
Glossary
- 400 MWT
400-m walk test
- BDI-II
Beck Depression Inventory-II
- CI
confidence interval
- HAQ-DI
Health Assessment Questionnaire Disability Index
- HRQOL
health-related quality of life
- hs-CRP
high-sensitivity C-reactive protein
- IQR
interquartile range
- IWQOL-Kids
Impact of Weight on Quality of Life – Kids
- KOA
knee osteoarthritis
- LE
lower extremity
- MBS
metabolic and bariatric surgery
- OR
odds ratio
- SF-36
Short Form 36
- Teen-LABS
Teen Longitudinal Assessment of Bariatric Surgery
Footnotes
Drs Bout-Tabaku, Gupta, Jenkins, and Michalsky conceptualized and designed the study, acquired, analyzed, and interpreted the data, conducted the initial analyses, drafted the initial manuscript, and reviewed and revised the manuscript critically for important intellectual content; Drs Ryder, Baughcum, Jackson, and Inge assisted with conceptualizing and designing the study, analyzed and interpreted the data, and reviewed and revised the manuscript critically for important intellectual content; Dr Xie assisted with data acquisition, analyzed and interpreted the data, and revised the manuscript critically for important intellectual content; Drs Dixon, Helmrath, Courcoulas, Mitchell, and Harmon analyzed and interpreted the data and revised the manuscript critically for important intellectual content; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Dr Bout-Tabaku’s current affiliation is Sidra Medicine, Doha, Qatar.
This trial has been registered at www.clinicaltrials.gov (identifier NCT00474318).
A complete list of nonauthor contributors appears in the Supplemental Information.
FINANCIAL DISCLOSURE: Dr Inge serves as a consultant for Standard Bariatrics, UpToDate, and Independent Medical Expert Consulting Services, which are all unrelated to this project. Dr Dixon’s research is supported through a National Health and Medical Research Council research fellowship. Dr Courcoulas received grants from Covidien and Ethicon Johnson & Johnson Health Care Systems; the other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Funded by the Rheumatology Research Foundation’s Investigator Award. The Teen Longitudinal Assessment of Bariatric Surgery Consortium was funded by cooperative agreements with the National Institute of Diabetes and Digestive and Kidney Diseases through grants U01DK072493, UM1DK072493, and UM1DK095710 (University of Cincinnati). The study was also supported by grants UL1 TR000077-04 (Cincinnati Children’s Hospital Medical Center), UL1RR025755 (Nationwide Children’s Hospital), M01-RR00188 (Texas Children’s Hospital and Baylor College of Medicine), UL1 RR024153 and UL1TR000005 (University of Pittsburgh), UL1 TR000165 (University of Alabama, Birmingham). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr Inge received honoraria and stock options from Standard Bariatrics and honoraria from UpToDate and Independent Medical Expert Consulting Services and served as a consultant for Zafgen, Inc, BioMedical Insights, and L&E Research, all outside the submitted work. Dr Harmon served on an advisory panel for Stryker Corporation from 1998 to 2015, unrelated to this project. Dr Dixon consulted for Apollo Endosurgery, Covidien, Bariatric Advantage, Nestle Health Science, Inova, and Novo Nordisk; the other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Deere KC, Clinch J, Holliday K, et al. Obesity is a risk factor for musculoskeletal pain in adolescents: findings from a population-based cohort. Pain. 2012;153(9):1932–1938 [DOI] [PubMed] [Google Scholar]
- 2.Gushue DL, Houck J, Lerner AL. Effects of childhood obesity on three-dimensional knee joint biomechanics during walking. J Pediatr Orthop. 2005;25(6):763–768 [DOI] [PubMed] [Google Scholar]
- 3.Widhalm HK, Seemann R, Hamboeck M, et al. Osteoarthritis in morbidly obese children and adolescents, an age-matched controlled study. Knee Surg Sports Traumatol Arthrosc. 2016;24(3):644–652 [DOI] [PubMed] [Google Scholar]
- 4.Gelber AC, Hochberg MC, Mead LA, et al. Body mass index in young men and the risk of subsequent knee and hip osteoarthritis. Am J Med. 1999;107(6):542–548 [DOI] [PubMed] [Google Scholar]
- 5.Bout-Tabaku S, Michalsky MP, Jenkins TM, et al. Musculoskeletal pain, self-reported physical function, and quality of life in the Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) cohort. JAMA Pediatr. 2015;169(6):552–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wills AK, Black S, Cooper R, et al. Life course body mass index and risk of knee osteoarthritis at the age of 53 years: evidence from the 1946 British birth cohort study. Ann Rheum Dis. 2012;71(5):655–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antony B, Jones G, Venn A, et al. Association between childhood overweight measures and adulthood knee pain, stiffness and dysfunction: a 25-year cohort study. Ann Rheum Dis. 2015;74(4):711–717 [DOI] [PubMed] [Google Scholar]
- 8.Ogden CL, Carroll MD, Lawman HG, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988-1994 through 2013-2014. JAMA. 2016;315(21):2292–2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deshpande BR, Katz JN, Solomon DH, et al. Number of persons with symptomatic knee osteoarthritis in the US: impact of race and ethnicity, age, sex, and obesity. Arthritis Care Res (Hoboken). 2016;68(12):1743–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Initiative US Bone and Joint The Burden of Musculoskeletal Diseases in the United States (BMUS), 3rd ed. Rosemont, IL: United States Bone and Joint Initiative; 2014 [Google Scholar]
- 11.Antony B, Jones G, Jin X, Ding C. Do early life factors affect the development of knee osteoarthritis in later life: a narrative review. Arthritis Res Ther. 2016;18(1):202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu CR, Williams AA, Coyle CH, Bowers ME. Early diagnosis to enable early treatment of pre-osteoarthritis. Arthritis Res Ther. 2012;14(3):212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roemer FW, Kwoh CK, Hannon MJ, et al. What comes first? Multitissue involvement leading to radiographic osteoarthritis: magnetic resonance imaging-based trajectory analysis over four years in the osteoarthritis initiative. Arthritis Rheumatol. 2015;67(8):2085–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duncan R, Peat G, Thomas E, Hay EM, Croft P. Incidence, progression and sequence of development of radiographic knee osteoarthritis in a symptomatic population. Ann Rheum Dis. 2011;70(11):1944–1948 [DOI] [PubMed] [Google Scholar]
- 15.Zwintscher NP, Azarow KS, Horton JD, Newton CR, Martin MJ. The increasing incidence of adolescent bariatric surgery. J Pediatr Surg. 2013;48(12):2401–2407 [DOI] [PubMed] [Google Scholar]
- 16.Inge TH, Courcoulas AP, Jenkins TM, et al. ; Teen-LABS Consortium . Weight loss and health status 3 years after bariatric surgery in adolescents. N Engl J Med. 2016;374(2):113–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King WC, Chen JY, Belle SH, et al. Change in pain and physical function following bariatric surgery for severe obesity. JAMA. 2016;315(13):1362–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Messier SP, Mihalko SL, Legault C, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310(12):1263–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teichtahl AJ, Wluka AE, Tanamas SK, et al. Weight change and change in tibial cartilage volume and symptoms in obese adults. Ann Rheum Dis. 2015;74(6):1024–1029 [DOI] [PubMed] [Google Scholar]
- 20.Hunter DJ, Beavers DP, Eckstein F, et al. The Intensive Diet and Exercise for Arthritis (IDEA) trial: 18-month radiographic and MRI outcomes. Osteoarthritis Cartilage. 2015;23(7):1090–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones G, Ding C, Glisson M, et al. Knee articular cartilage development in children: a longitudinal study of the effect of sex, growth, body composition, and physical activity. Pediatr Res. 2003;54(2):230–236 [DOI] [PubMed] [Google Scholar]
- 22.Inge TH, Zeller MH, Jenkins TM, et al. ; Teen-LABS Consortium . Perioperative outcomes of adolescents undergoing bariatric surgery: the Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study. JAMA Pediatr. 2014;168(1):47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inge TH, Zeller M, Harmon C, et al. Teen-Longitudinal Assessment of Bariatric Surgery: methodological features of the first prospective multicenter study of adolescent bariatric surgery. J Pediatr Surg. 2007;42(11):1969–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryder JR, Edwards NM, Gupta R, et al. Changes in functional mobility and musculoskeletal pain after bariatric surgery in teens with severe obesity: Teen-Longitudinal Assessment of Bariatric Surgery (LABS) study. JAMA Pediatr. 2016;170(9):871–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krishnan E, Sokka T, Häkkinen A, Hubert H, Hannonen P. Normative values for the Health Assessment Questionnaire disability index: benchmarking disability in the general population. Arthritis Rheum. 2004;50(3):953–960 [DOI] [PubMed] [Google Scholar]
- 26.Golightly YM, Hannan MT, Shi XA, et al. Association of foot symptoms with self-reported and performance-based measures of physical function: the Johnston County osteoarthritis project. Arthritis Care Res (Hoboken). 2011;63(5):654–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruce B, Fries JF. The Stanford Health Assessment Questionnaire: dimensions and practical applications. Health Qual Life Outcomes. 2003;1:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rolland YM, Cesari M, Miller ME, et al. Reliability of the 400-m usual-pace walk test as an assessment of mobility limitation in older adults. J Am Geriatr Soc. 2004;52(6):972–976 [DOI] [PubMed] [Google Scholar]
- 29.Kolotkin RL, Zeller M, Modi AC, et al. Assessing weight-related quality of life in adolescents. Obesity (Silver Spring). 2006;14(3):448–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Modi AC, Loux TJ, Bell SK, et al. Weight-specific health-related quality of life in adolescents with extreme obesity. Obesity (Silver Spring). 2008;16(10):2266–2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeller MH, Reiter-Purtill J, Ratcliff MB, Inge TH, Noll JG. Two-year trends in psychosocial functioning after adolescent Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2011;7(6):727–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dixon JB, Dixon ME, O’Brien PE. Quality of life after lap-band placement: influence of time, weight loss, and comorbidities. Obes Res. 2001;9(11):713–721 [DOI] [PubMed] [Google Scholar]
- 33.Hooper MM, Stellato TA, Hallowell PT, Seitz BA, Moskowitz RW. Musculoskeletal findings in obese subjects before and after weight loss following bariatric surgery. Int J Obes. 2007;31(1):114–120 [DOI] [PubMed] [Google Scholar]
- 34.Iossi MF, Konstantakos EK, Teel DD II, et al. Musculoskeletal function following bariatric surgery. Obesity (Silver Spring). 2013;21(6):1104–1110 [DOI] [PubMed] [Google Scholar]
- 35.Ware JE., Jr SF-36 health survey update. Spine. 2000;25(24):3130–3139 [DOI] [PubMed] [Google Scholar]
- 36.Beck AT, Steer RA, Brown G. Manual for Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996 [Google Scholar]
- 37.Zeller MH, Inge TH, Modi AC, et al. ; Teen Longitudinal Assessment of Bariatric Surgery (Teen-LABS) Consortium . Severe obesity and comorbid condition impact on the weight-related quality of life of the adolescent patient. J Pediatr. 2015;166(3):651–659.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stannus OP, Jones G, Blizzard L, Cicuttini FM, Ding C. Associations between serum levels of inflammatory markers and change in knee pain over 5 years in older adults: a prospective cohort study. Ann Rheum Dis. 2013;72(4):535–540 [DOI] [PubMed] [Google Scholar]
- 39.Shimura Y, Kurosawa H, Sugawara Y, et al. The factors associated with pain severity in patients with knee osteoarthritis vary according to the radiographic disease severity: a cross-sectional study. Osteoarthritis Cartilage. 2013;21(9):1179–1184 [DOI] [PubMed] [Google Scholar]
- 40.Vincent HK, Ben-David K, Conrad BP, et al. Rapid changes in gait, musculoskeletal pain, and quality of life after bariatric surgery. Surg Obes Relat Dis. 2012;8(3):346–354 [DOI] [PubMed] [Google Scholar]
- 41.Richette P, Poitou C, Garnero P, et al. Benefits of massive weight loss on symptoms, systemic inflammation and cartilage turnover in obese patients with knee osteoarthritis. Ann Rheum Dis. 2011;70(1):139–144 [DOI] [PubMed] [Google Scholar]
- 42.Speck RM, Bond DS, Sarwer DB, Farrar JT. A systematic review of musculoskeletal pain among bariatric surgery patients: implications for physical activity and exercise. Surg Obes Relat Dis. 2014;10(1):161–170 [DOI] [PubMed] [Google Scholar]
- 43.White DK, Wilson JC, Keysor JJ. Measures of adult general functional status: SF-36 Physical Functioning Subscale (PF-10), Health Assessment Questionnaire (HAQ), Modified Health Assessment Questionnaire (MHAQ), Katz Index of Independence in activities of daily living, Functional Independence Measure (FIM), and Osteoarthritis-Function-Computer Adaptive Test (OA-Function-CAT). Arthritis Care Res (Hoboken). 2011;63(suppl 11):S297–S307 [DOI] [PubMed] [Google Scholar]
- 44.Herring LY, Stevinson C, Davies MJ, et al. Changes in physical activity behaviour and physical function after bariatric surgery: a systematic review and meta-analysis. Obes Rev. 2016;17(3):250–261 [DOI] [PubMed] [Google Scholar]
- 45.Kwon S, Perera S, Pahor M, et al. What is a meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE-P study). J Nutr Health Aging. 2009;13(6):538–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richards C, Higginson JS. Knee contact force in subjects with symmetrical OA grades: differences between OA severities. J Biomech. 2010;43(13):2595–2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Messier SP, Gutekunst DJ, Davis C, DeVita P. Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis Rheum. 2005;52(7):2026–2032 [DOI] [PubMed] [Google Scholar]
- 48.Jakiela J, DeVita P, Beavers DP, Messier SP. Biomechanical determinants of reduced knee joint loads in patient with osteoarthritis following intensive weight loss. Medicine and Science in Sports and Exercise. 2016;48(5S suppl 1):883 [Google Scholar]