Abstract
INTRODUCTION
Alzheimer’s disease (AD) is a disabling, common cause of dementia and agitation is one of the most common and distressing symptoms for patients with AD. Escitalopram for Agitation in Alzheimer’s Disease (S-CitAD) tests a novel, clinically derived therapeutic approach to treat agitation in AD patients.
METHODS
S-CitAD is a NIH-funded, investigator-initiated, randomized, multicenter clinical trial. Participants receive a structured psychosocial intervention (PSI) as standard of care. Participants without sufficient response to PSI are randomized to receive 15mg escitalopram/day or a matching placebo in addition to PSI. Primary outcome is the Modified Alzheimer’s Disease Cooperative Study - Clinical Global Impression of Change (mADCS-CGIC).
DISCUSSION
S-CitAD will provide information about a practical, immediately available approach to treating agitation in patients with AD. S-CitAD may become a model of how to evaluate and predict treatment response in patients with AD and agitation as a neuropsychiatric symptom (ClinicalTrials.gov Identifier: ).
Keywords: Alzheimer Dementia, Escitalopram, Agitation, Neuropsychiatric symptoms, Randomized trial, Psychosocial intervention
1. Introduction
Functional and cognitive impairment along with common behavioral changes (neuropsychiatric symptoms; NPS) are typical, frequent, and distressing symptoms in patients with Alzheimer’s disease (AD). Common NPS include agitation, depression, anxiety, apathy, delusions, and hallucinations [2]. Almost all patients with AD experience NPS at some point [3]. Agitation, one of the most serious and debilitating consequences of AD for patients, caregivers, and families, is characterized by emotional distress, disruptive and/or aggressive behavior, disinhibition, and increased psychomotor activity [4]. The estimated 5 year period prevalence of agitation in AD is about 45% [3]. Agitation limits options to receive and stay in care and thus burdens both patients and caregivers [5]. The CitAD trial (Citalopram for agitation in Alzheimer’s disease) reported encouraging outcomes for treatment of agitation in AD patients [4]. Racemic citalopram was effective, with 40% of citalopram-treated participants experiencing clinical improvement vs. 26% on placebo. Moreover, CitAD identified a subgroup of patients, characterized by a predominance of affective symptoms [6], which benefitted most from citalopram treatment [7]. However, citalopram was associated with cognitive worsening and prolongation of the ECG-QTc interval. In blood concentration models, cognitive and cardiac changes were associated with the R-enantiomer, while clinical improvements were primarily associated with the S-enantiomer (escitalopram) [8].
2. Methods
2.1. Recruitment, eligibility, and Institutional Review Board review
Study participants are recruited from memory clinics, geriatric psychiatry clinics, day hospital programs, Veterans Administration geriatric clinics, and Alzheimer’s Research Centers. To increase external validity and applicability of findings outside of academic centers we include private practices as recruitment sites. AD patients residing in long-term care facilities are excluded because CitAD indicated lack of efficacy in that population [7]. S-CitAD participants have Alzheimer’s dementia (AD) diagnosed clinically by the NIA and the Alzheimer’s Association (2011 NIA/AA) criteria, with Mini-Mental State Examination (MMSE) [9] scores of 5 to 28, inclusive. Participants must also meet the IPA provisional criteria for agitation in cognitive disorders [10] and have clinically significant agitation/aggression as assessed by the Neuropsychiatric Inventory (NPI).
Exclusion criteria include a major depressive episode in the past 90 days by Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) criteria, presence of another brain disease that fully explains the dementia, (e.g., extensive brain vascular disease, Parkinson's disease, dementia with Lewy bodies, traumatic brain injury, or multiple sclerosis), and contraindication to treatment with a selective serotonin reuptake inhibitor (SSRI). Supplementary Table 1 shows an overview of the study design and a detailed list of entry criteria.
S-CitAD utilizes a hybrid single Institutional Review Board (sIRB) structure. Sites are permitted to rely on a local IRB since S-CitAD preceded the mandatory sIRB policy for federally funded, multicenter studies in the United States [11].
2.2. Study flow, randomization, and masking
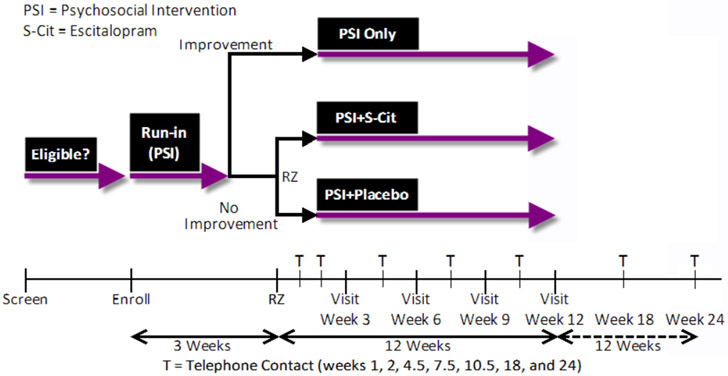
The study flow is shown in Figure 1. S-CitAD participants initially receive a non-pharmacologic psychosocial intervention (PSI) for three weeks and move on to randomization only if they do not respond to this intervention (see 2.3.). Participants who do not improve on the PSI are randomized in a 1:1 ratio to receive escitalopram or matching placebo in addition to the PSI. Participants who do improve sufficiently on the PSI (see 2.3.) are followed in an observational arm of the study to characterize the duration and course of the treatment response. The Coordinating Center (CC) generated the treatment assignment schedule with permuted blocks of varying length, stratified by clinical center (SAS/STAT software, version 9.3; SAS Institute, Cary, NC). Participants, their caregivers, and clinical center personnel are masked to treatment assignment. Masking is accomplished by use of a specifically manufactured, matching placebo.
Figure 1:
S-CitAD study design schematic
2.3. Psychosocial intervention
Every study participant along with a primary caregiver receives the PSI as minimal standard of care. The PSI was developed for the Clinical Antipsychotic Trials of Intervention Effectiveness Study (CATIE) [12], then modified and used successfully in the Depression in Alzheimer's Disease Study-2 (DIADS-2) and in CitAD [13]. It was designed as a practical, easy to administer intervention, and benefits both patients and caregivers. In CitAD, we observed moderate or marked improvement in agitation in 26 percent of participants receiving the PSI along with placebo, typically within 3 weeks [14]. The PSI consists of three components: 1) a 20- to 30-minute counseling session at each in-person visit, 2) the provision of educational materials, and 3) 24-hour availability for crisis management assistance, with counselling over the telephone as appropriate. PSI sessions led by a trained clinician include:
Review and potential adjustment of the patient and caregiver supportive care plan;
Opportunity to discuss feelings and emotional support;
Counseling regarding specific care-giving skills;
Assistance with problem-solving of specific issues brought up by the caregiver or study participant;
Discussion of the educational materials (The 36-Hour Day [15] and the Johns Hopkins Dementia Care Guidelines for Caregiver [16]).
Clinically significant improvement in agitation is defined as a score of either 1 or 2 (moderate improvement or marked improvement) on the Modified Alzheimer’s Disease Cooperative Study - Clinical Global Impression of Change (mADCS-CGIC) [17].
2.4. Concomitant pharmaceutical treatment
Study participants who do not show a clinically significant improvement with PSI are randomized to receive either escitalopram or matching placebo. CitAD revealed that almost all clinical response to PSI occurred by three weeks. In CitAD, patients were treated with citalopram, a racemic mixture of the enantiomers R- and S-citalopram (escitalopram). Given findings from CitAD, we believe that for AD patients escitalopram is safer than and at least as efficacious as racemic citalopram. Evaluation of its efficacy in the absence of racemic citalopram is necessary [8].
The target dose of escitalopram in S-CitAD is 15 mg/d provided as a single dose in the morning, reflecting the amount of escitalopram present in 30 mg of racemic citalopram. We allow a variety of concomitant medications to represent usual clinical practice and thereby increase external validity of the trial. Participants remain on treatments needed for medical co-morbidities. Lorazepam (up to 0.5 mg daily and up to 3 out of 7 days) and trazodone (up to 100 mg nightly) are allowed to treat episodes of more intense agitation (rescue medication) and significant sleep disturbance, respectively. Permissible and non-permissible medications are listed in Supplementary Table 2.
2.5. Main outcome measures and comparisons
The primary outcome measure is the mADCS-CGIC (Supplementary Table 1), one of the primary outcomes used in CitAD. We will assess this measure in participants of the observational as well as the interventional arms to determine whether drug or placebo offer an added benefit. The mADCS-CGIC is a systematic assessment, developed for the AD setting to assess clinically significant change in a patient’s condition over time. A trained clinician, masked to treatment assignment and other outcome measure results, uses a 7-point Likert scale to rate each patient along a continuum from marked improvement to marked worsening, based on an interview with the caregiver, an examination of the patient, and a reference to observations recorded at the initial visit. The semi-structured mADCS-CGIC requires the assessor to consider aspects of the agitation prior to providing a “global” assessment of change. These include: emotional or psychomotor agitation, verbal or physical aggression, and mood liability/distress. We will calculate the proportion of participants in each of the three study arms showing clinically significant improvement in agitation - indicated by a rating of ‘moderate’ or ‘marked’ improvement on the mADCS-CGIC - at week 12 in the intention-to-treat population. The primary analysis will use an unadjusted two-sided test of the proportions with a significance threshold of p = 0.049.
The groups will be compared using Fisher’s exact test. We will report the relative risk and 95 percent confidence intervals and will perform sensitivity analyses, including:
-
1.
A per-protocol analysis according to the treatment participants received;
-
2.
Classifying participants with missing outcome data as having significant improvement;
-
3.
Multiple imputation of mADCS-CGIC scores for missing outcomes; and
-
3.
Stratified analysis to examine possible effects of clinical site or baseline use of antipsychotics.
S-CitAD will perform a proportional odds analysis of the categorical mADCS-CGIC using ordinal logistic regression. This method has an adequate Type I error proportion under the null hypothesis and provides substantial gains in efficiency relative to a binary analysis [18].
Other important outcomes include the agitation, aggression, and dysphoria domains of the Neuropsychiatric Inventory – Clinician Rating (NPI-C) [19], domains of the Neuropsychiatric Inventory (NPI) [20], and the Alzheimer’s Disease Cooperative Study-Activities of Daily Living Scale (ADCS-ADL) [21]. The NPI-C is designed especially for clinicians to provide structured input to NPS rating [19]. It offers more granularity than the NPI since each sub-item is individually rated instead of producing one global score for a behavioral domain—as with NPI.
The NPI is the most widely-used measure of twelve NPS domains in dementia clinical trials. In S-CitAD, the ten NPI domains administered do not include agitation/aggression or dysphoria (at select visits) to avoid redundancy with the NPI-C. The frequency by severity NPI score will be used to measure changes in “other” NPS over time.
The ADCS-ADL (Supplementary Table 1) assesses functional performance in patients with AD. In a structured interview format, informants are queried whether subjects attempted each of 24 items in the inventory during the prior 3 weeks and their performance.
For continuous measures of efficacy and/or safety, analyses will estimate parameters with generalized estimating equations (GEE) using a saturated means model that includes indicators for visit and visit-by-treatment interaction and an unstructured covariance structure. If residuals are not normally distributed, violating regression assumptions, non-parametric Wilcoxon rank sum tests will be used to quantify differences. For non-continuous measures of outcome, proportions by treatment group will be tabulated and compared with a chi-square test or Fisher’s exact test.
A list of neuropsychological, neuropsychiatric, and other measures in S-CitAD are shown in Supplementary Table 1.
Details on study organization, data collection, treatment unmasking, and sample size and power considerations are described in the Appendix.
3. Discussion
3.1. Selective serotonin reuptake inhibitors (SSRIs) for treating agitation in patients with AD
Despite advances in our understanding of the neuroanatomy, neurochemistry, and neurophysiology of cognitive phenotypes, there has been less progress in understanding the complex NPS phenotypes of AD. An international consortium [22] proposed that NPS result from damage to frontal-subcortical or cortico-cortical networks that mediate emotional processing, and/or from loss of regulation of these circuits by ascending monoaminergic systems, including the serotonin system. Neuropathologic studies suggest that cell loss in the dorsal raphe (major site of cortical serotonin) in patients with AD is three times greater than in controls and comparable to losses in nucleus basalis and locus coeruleus, providing strong evidence that serotonergic loss is widespread in AD [23]. In fact lesions of dorsal raphe serotonergic neurons become manifest in very early AD and affect projections to cortex [24]. The role of the serotonin loss in the biology of agitation in AD patients is supported by the following convergent findings: (1) in neuropathologic studies agitation is associated with loss of serotonergic innervation to cortex [25][26], cholinergic-serotonergic imbalance [27], and reduced serotonin 5HT1A receptor binding in the temporal cortex [28]; (2) absence of serotonergic pathology is associated with absence of agitation [29]; (3) genetic studies report associations between agitation in AD and polymorphisms in genes for the 5-HT2A receptor [30] or serotonin transporter [31][32]; and (4) challenge studies, assessing the integrity of serotonergic function, suggest that agitated patients with AD have dysfunctional serotonergic systems [33][34].
SSRIs enhance functional serotonergic neurotransmission and are therefore hypothesized to compensate for the serotonin losses in AD patients. These drugs have a well characterized safety profile and are well tolerated by older patients with neurodegenerative diseases. SSRIs were initially FDA-approved for depression and some SSRI’s are also effective in patients with anxiety, panic disorders, and bulimia nervosa. CitAD [4] chose citalopram based on its neurochemical profile and success in treating agitation in AD patients in two prior RCTs [35][36]. Treatment was, however, associated with known SSRI-mediated side effects, delayed cardiac repolarization suggested by a prolongation of the ECG-QTc interval, as well as modest cognitive decline (approximately 1 point on the MMSE) of unclear clinical significance. The choice of escitalopram in S-CitAD is based on findings from racemic CitAD suggesting that the adverse effects were primarily related to R-citalopram with evidence of greater benefit with S-citalopram [8]. S-CitAD compares cognition across groups as a safety measure and excludes patients with abnormal ECG-QTc intervals (Supplementary Table 1). In case a prolonged ECG-QTc interval develops, a physician decides whether continuing on study medication is safe.
3.2. S-CitAD RCT design considerations
RCT results have been criticized for unclear clinical applicability as well as for favoring internal over external validity [37][38]. In S-CitAD, we test a realistic, immediately clinically applicable treatment sequence. Every participant receives the PSI as a first line treatment for agitation. Methodologically, the PSI lead-in phase serves to enrich the study population with initial PSI non-responders who are in need of an additional intervention. PSI responders will continue on PSI alone in the observational arm of the study. Twenty six percent of patients in the placebo arm of CitAD had a positive response on PSI largely within three weeks [39]. Should the patient not respond sufficiently, the treatment regimen is augmented by a pharmacologic treatment, escitalopram. The effect of citalopram in CitAD occurred relatively late, i.e. between weeks 6 and 9. S-CitAD consequently chose to closely follow-up participants for 12 weeks and added an extended follow-up period up to 24 months to capture sustained effects of the intervention and to characterize groups of patients who may relapse (see 3.4.).
This proposed treatment sequence thus starts with an effective, simple, non-invasive, and safe intervention and is escalated only if needed. Meanwhile the PSI is not stopped but provided continuously throughout the trial. Patients who respond well to the PSI are followed in the observational arm of the study to determine whether and how long treatment response to PSI is sustained. This arm will provide valuable data regarding the PSI that will help clinicians manage expectations and deliver the PSI effectively.
This RCT design is pragmatic because it tests the effectiveness of a treatment sequence that has considerable potential in managing agitation in patients with AD. Methodologically, we emphasize the external validity of the trial without substantially diminishing internal validity. We (i) allow for a variety of concomitant medications (Supplementary Table 2) that are very common in AD patients with agitation; only medications interfering with agitation are not allowed; (ii) support the caregiver as well as the patient with the PSI which is critical for the success of any intervention in AD patients with NPS; and (iii) aim to describe both treatment responders as well as patients at risk of relapse. Our target population is AD patients with moderate to severe agitation that is not well controlled.
3.3. Outcome assessment in S-CitAD and in future clinical trials targeting agitation: mADCS, NPI, and NPI-C
In clinical trials of interventions in AD it is important both to measure symptom severity and global psychosocial function; the latter is important to assess the clinical significance of changes in symptom severity, while the former is important for understanding the course and details of clinical response. In S-CitAD we focus on a global metric and aim to set a high standard for treatment response as we concluded that small responses were unstable and delayed the use of other therapies that might be more beneficial. As opposed to CitAD, S-CitAD did thus not include the Neurobehavioral Rating Scale (NBRS) or the Cohen-Mansfield Agitation Inventory (CMAI).
The primary outcome is the mADCS-CGIC as a clinical assessment of global function by a masked clinician using a semi-structured interview. This review the patient’s clinical status with the participant and caregiver allows for an evaluation of treatment effects of a clinically meaningful magnitude. The mADCS-CGIC is a modification of the original ADCS-CGIC [21] in that the assessment is focused on changes in agitation, rather than on functioning overall. Advantages of using the mADCS-CGIC as primary outcome include: (1) The final rating is the judgment of an experienced dementia clinician masked to treatment assignment; (2) It incorporates all available data except AEs, minimizing the risk of unmasking the rater; (3) Thorough narrative coverage of a wide range of ADL and IADL function incorporating observations made by caregivers in daily life to judge the clinical significance of changes; (4) mADCS-ADL provides a single ordinal outcome with concomitant statistical advantages; (5) Comparability between studies. Disadvantages include: (1) The rating is global and thus one cannot attribute changes to specific functional areas; (2) The administration of mADCS-CGIC as implemented in S-CitAD and prior studies (CitAD) requires two experienced clinicians for each visit, one for CGIC and one for AEs; (3) The integration of observations and history from informant and participant is often a judgment call by the rater, which may result in greater inter-rater variance.
NPI-C Agitation and Aggression domains were chosen as secondary outcomes. The NPI-C has more granularity than the original NPI in that each sub-item is individually scored, as opposed to the NPI which rates only a global score for the behavioral domain. Thus the NPI-C might distinguish different types of emotional agitation or physical aggression which could be useful in understanding outcomes. Additional advantages of the NPI-C include: (1) Each domain is designed to be a free-standing scale with all relevant items addressed at each visit, and (2) The distinction between agitation and aggression is another advantage over the NPI. The major disadvantage is that the NPI-C has limited validation data. Other secondary outcomes include: (1) The NPI (excluding Agitation/Aggression) to cover NPS other than agitation and aggression with the advantage of broadly covering NPS in AD and being very widely used in trials of interventions for NPS in AD, and (2) ADCS-ADL to assess whether changes in NPS correlate with changes in specific psychosocial functions. The ADCS-ADL was designed to be used in all stages of dementia severity [21][4].
3.4. Response predictors in CitAD and S-CitAD
Building on our hypothesis-generating findings from CitAD, we will assess factors that individually, or combined into an index score [7], best characterize treatment responders, including baseline affective or executive symptoms, blood levels of escitalopram, polygenic risk scores for related conditions/traits, age, severity of cognitive impairment, and severity of agitation. If replicated, this index score might be refined and used clinically. We aim to identify subgroups most likely to benefit from treatments, and illuminate mechanisms of action involved in treatment response. We hypothesize that particularly patients with baseline symptoms that are moderate-to-high on an affective scale and low on an executive scale (‘affective group’) will respond to escitalopram treatment [6]. Based on data from CitAD [6][7], S-CitAD anticipates that at baseline 29 percent of participants will belong to an ‘affective group.’ Of these, 13 percent will show a clinically significant response during the run-in period and thus not be randomized. Of the remaining 87 percent who proceed to the RCT phase, S-CitAD anticipates the placebo response to be 14 percent and the escitalopram response to be 52 percent. In the 71 percent of participants who do not fit the definition of an ‘affective group’, S-CitAD anticipates that 34 percent will show a run-in response, and of the remaining 66 percent, S-CitAD anticipates a placebo response of 30 percent and an escitalopram response of 34 percent. Testing for an interaction between the treatment group and the ’affective group’ status, S-CitAD has an estimated 91 percent power to detect an interaction (risk ratio; RR=2.35) with a sample size of 352. We modeled these outcome predictions using 1000 Poisson regression simulations with terms for treatment group, ‘affective group’ status, and an interaction term, using a log link function.
Genetic markers that may predict treatment response will be assessed following two hypothesis-driven analytical approaches: 1) exploring the effects of single variants as in CitAD, and 2) using polygenic risk scores (PRSs) [40] to test whether the overall genetic risk for AD, depression, or response to escitalopram/citalopram for depression, predicts response to escitalopram for agitation in AD. Using existing public GWAS data on these phenotypes (dbGaP accession respectively: phs000360.v2.p1, N=18,663; phs000486.v1.p1 N= 3,540; phs000360.v2.p1, N= 529) we will determine whether an increased genetic risk for these phenotypes predicts efficacy. For example, patients who develop AD despite a low genetic risk may respond differently to escitalopram treatment. Polygenic scores are a powerful means to explore the effect of genetic risk differences at the group level (i.e. responders vs. non-responders) and do not involve multiple comparisons.
For clinical decision making it is paramount to determine groups that are likely to respond well to the PSI but also groups that are unlikely to respond to the PSI and are in need of pharmacological treatment.
3.5. Limitations
The trial was designed to test a promising therapeutic sequence that can be applied in many settings. Methodological trade-offs include the following: If the PSI is not initially effective and the participant is randomized to additional escitalopram or placebo, potential long-term effects of the PSI cannot be estimated since it is a component of both intervention arms. We accept this limitation because our primary aim is to test a practical treatment solution for AD patients with agitation. In addition, if there is a long-term effect of the PSI in both intervention arms, this might make it difficult for escitalopram to add more benefit for patients. We also cannot exclude the faint possibility that the PSI modifies the treatment effects in one intervention arm but not the other. Lastly, subgroup analyses might yield unstable or undetectable results if these subgroups are small.
4. Conclusion and expected impact of S-CitAD
S-CitAD will provide practical information about the effectiveness of an immediately available approach to treating agitation in AD. S-CitAD findings will also form the basis for later treatment development providing information on non-responders in different phases of this sequential approach, and becoming a model for how to evaluate response predictors in NPS treatment studies.
Supplementary Material
Table 1:
Data collection and procedures by visit
| All participants | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EN | BL | T1 | T2 | F3 | T4 | F6 | T7 | F9 | T10 | F12 | T18 | T24 | |
| (Weeks from BL) | −3 | 0 | 1 | 2 | 3 | 4.5 | 6 | 7.5 | 9 | 10.5 | 12 | 18 | 24 |
| Procedure | |||||||||||||
| Consent | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Eligibility | X | X | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ |
| Psychosocial Intervention (PSI) | X | X | ≅ | ≅ | X | ≅ | X | ≅ | X | ≅ | X | ≅ | ≅ |
| Electrocardiogram (ECG), with QTcB interval | X | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ |
| Medical history | X | X | ≅ | ≅ | X | ≅ | X | ≅ | X | ≅ | X | ≅ | ≅ |
| Telephone interview (brief medical history) | ≅ | ≅ | X | X | ≅ | X | ≅ | X | ≅ | X | ≅ | X | X |
| Basic Metabolic Panel (BMP) | X | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | ||
| Vital signs and weight | X | X | ≅ | ≅ | X | ≅ | X | ≅ | X | ≅ | X | ≅ | ≅ |
| Adverse events | ≅ | X | X | X | X | X | X | X | X | X | X | X | X |
| Assessment | |||||||||||||
| DSM-V Major Depressive Episode (MDE) | X | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ |
| Modified Alzheimer's Disease Cooperative Study - Clinical Global Impression of Change (mADCS-CGIC) | X | X | ≅ | ≅ | X | ≅ | X | ≅ | X | ≅ | X | X | X |
| Neuropsychiatric Inventory – Clinician Rating (NPI-C), agitation and aggression domains | X | X | ≅ | ≅ | X | ≅ | X | ≅ | X | ≅ | X | ≅ | ≅ |
| NPI-C (dysphoria domain) | ≅ | X | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | X | ≅ | ≅ |
| NPI* | X | X | ≅ | ≅ | X | ≅ | X | ≅ | X | ≅ | X | X | X |
| Activities of Daily Living (ADL) | ≅ | X | ≅ | ≅ | X | ≅ | X | ≅ | X | ≅ | X | X | X |
| Zarit Burden Interview (ZBI) | ≅ | X | ≅ | ≅ | X | ≅ | X | ≅ | X | ≅ | X | X | X |
| Caregiver Activity Survey (CAS) | ≅ | X | ≅ | ≅ | X | ≅ | X | ≅ | X | ≅ | X | ≅ | ≅ |
| Get Up and Go Test | ≅ | X | ≅ | ≅ | X | ≅ | X | ≅ | X | ≅ | X | ≅ | ≅ |
| Neurobehavioral Rating Scale (NBRS) | ≅ | X | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ |
| Mini-Mental State Examination (MMSE) | X | X | ≅ | ≅ | X | ≅ | X | ≅ | X | ≅ | X | ≅ | ≅ |
| Number cancellation task (Alzheimer's Disease Assessment Scale-Cognitive Subscale, ADAS-cog) | ≅ | X | ≅ | ≅ | X | ≅ | X | ≅ | X | ≅ | X | ≅ | ≅ |
| Digit span | ≅ | X | ≅ | ≅ | X | ≅ | X | ≅ | X | ≅ | X | ≅ | ≅ |
| Initiation/Perseveration and Conceptualization (Dementia Rating Scale-2, DRS-2) | ≅ | X | ≅ | ≅ | X | ≅ | X | ≅ | X | ≅ | X | ≅ | ≅ |
| Facial Emotion Recognition Test | ≅ | X | ≅ | ≅ | X | ≅ | X | ≅ | X | ≅ | X | ≅ | ≅ |
| Randomized participants, additional schedule of activities† | |||||||||||||
| Procedure | |||||||||||||
| ECG, with QTcB interval | ≅ | X‡ | ≅ | ≅ | X | ≅ | X | ≅ | X | ≅ | X | ≅ | ≅ |
| Randomization | ≅ | X | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ |
| Study drug dispensing | ≅ | X | ≅ | ≅ | X | ≅ | X | ≅ | X | ≅ | ≅ | ≅ | ≅ |
| Study drug adherence | ≅ | ≅ | X | X | X | X | X | X | X | X | X | ≅ | ≅ |
| Blood for pharmacokinetics | ≅ | ≅ | ≅ | ≅ | X | ≅ | X | ≅ | X | ≅ | X | ≅ | ≅ |
| BMP | ≅ | ≅ | ≅ | ≅ | X | ≅ | ≅ | ≅ | ≅ | ≅ | X | ≅ | ≅ |
| Routine unmasking | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | ≅ | X | ≅ | ≅ |
Notes: *Only the agitation/aggression domain of the NPI is administered at EN; an abbreviated NPI is administered at BL – F12. †Participants who fail to show improvement on enrollment PSI. If study drug is terminated prior to week 12, the ECG will not occur unless deemed necessary for safety monitoring. ‡Since normal ECG results are a pre-requisite for randomization, only participants likely to be randomized will have the baseline ECG.
EN = Enrollment visit BL = Baseline visit T = Scheduled telephone contact F = Scheduled in-person follow-up visits
Acknowledgements:
S-CitAD wishes to acknowledge our patients and caregivers and their families as well as our recruitment centers across North America. We also acknowledge our funder, the National Institutes of Health/National Institute on Aging; R01AG052510.
Funding Source: National Institutes of Health/National Institute on Aging; R01AG052510. The funding source had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
S-CitAD Research Group: Resource centers (responsibility: study administration): Chair’s Office, Johns Hopkins School of Medicine (Bayview), Baltimore, and University of Rochester, Rochester, New York: Constantine G. Lyketsos, MD, MHS, chair; Anton P. Porsteinsson, MD, co-chair; Dimitri Avramopoulos, MD, PhD, study geneticist; Cynthia Munro, PhD, study neuropsychologist; Hochang (Ben) Lee, MD, conflict of interest officer; Nicholas Bienko, MS, lead coordinator. Coordinating Center, Johns Hopkins Bloomberg School of Public Health, Baltimore: Dave Shade, JD, director; Stephan Ehrhardt, MD, MPH, deputy director; Jennifer Jones, coordinator; Anne Shanklin Casper, MA, senior research program manager and coordinator; Constantine Frangakis, PhD, statistician; Andy Lears, data manager; Sheriza Baksh, PhD, MPH, analyst; Jamie Perin, PhD, statistician. Project Office, National Institute on Aging, Bethesda: Laurie Ryan, PhD, Alvin D. McKelvy, PhD, project officers. Clinical centers (responsibility: data collection). Johns Hopkins Bayview and Johns Hopkins School of Medicine, Baltimore: Paul Rosenberg, MD, director; Milap Nowrangi, MD, MBe, study physician; Sarah Lawrence, MS, research program manager; Meghan Schultz, RN, MSN, senior study nurse; Nimra Jamil, lead coordinator. Columbia University Medical Center, Columbia: D.P. Devanand, MD, director; Laura Simon-Pearson, lead coordinator; Gregory Pelton, MD. Roper St. Francis Research and Innovation Center: Jacobo Mintzer, MD, MBA, executive director; Olga Brawman-Mintzer, MD; Arthur Williams, study coordinator; Anthony Awkar; study coordinator. University of Rochester School of Medicine and Dentistry, Rochester: Anton P. Porsteinsson, MD, director; Melanie Keltz, BS, RN; Nancy Kowalski, RN, CNSMS, RNC; Kaitlyn Lane, BA; Kim Martin, RN; Susan Salem-Spencer, RN, MSN, lead coordinator; Asa Widman, BA. University of Toronto: Bruce G. Pollock, MD, PhD, FRCPC, director; Sanjeev Kumar, MD; Lillian Lourenco, MPH, lead coordinator; Benoit H. Mulsant, MD, MS; Kyle Lago, BA; Tarek K. Rajji, MD. University of Southern California Keck School of Medicine: Lon S. Schneider, MD, director; Maurcio Becerra, lead coordinator; Karen Dagerman, MS; Sonia Pawluczyk, MD; Liberty Teodoro, RN.
Conflicts: DPD reports consulting for Eisai, Acadia, Avanir. LSS reports grants from NIA and the State of California, within 3 years and during the conduct of the study; grants from Eli Lilly, Merck, Novartis, Roche, Biogen, UCSD and personal fees from AC Immune, Allergan, Avraham, Axovant, Boehringer Ingelheim, Cognition, Lilly Merck, Neurim, Roche, Takeda, Toyama, and vTv outside the submitted work. TKR has received research support from Brain Canada, Brain and Behavior Research Foundation, BrightFocus Foundation, Canada Foundation for Innovation, Canada Research Chair, Canadian Institutes of Health Research, Centre for Aging and Brain Health Innovation, National Institutes of Health, Ontario Ministry of Health and Long-Term Care, Ontario Ministry of Research and Innovation, and the Weston Brain Institute. ZI has received funding from Brain Canada, Canadian Institutes of Health Research, and Canadian Consortium for Neurodegeneration and Aging. Consulting fees received from Allergan, Avanir, Janssen, Lilly, Lundbeck, Otsuka, Pfizer, Sunovion. PBR reports grants from NIA, Lilly, and Alzheimer’s Association within 3 years and during the conduct of the study; consulting fees from GLG, Otsuka, Avanir, Bionomics; travel support from Avanir and Otsuka. CGL reports grants from NIMH, NIA, Associated Jewish Federation of Baltimore, Weinberg Foundation, Forest, Glaxo-Smith-Kline, Eisai, Pfizer, Astra-Zeneca, Lilly, Ortho-McNeil, Bristol-Myers, Novartis, National Football League, Elan, Functional Neuromodulation, Bright Focus Foundation. Consultant fees received from Astra-Zeneca, Glaxo-Smith Kline, Eisai, Novartis, Forest, Supernus, Adlyfe, Takeda, Wyeth, Lundbeck, Merz, Lilly, Pfizer, Genentech, Elan, NFL Players Association, NFL Benefits Office, Avanir, Zinfandel, BMS, Abvie, Janssen, Orion, Otsuka, Servier, Astellas. Honorarium or travel support from Pfizer, Forest, Glaxo-Smith Kline, Health Monitor. JM reports consulting for ACADIA Pharmaceuticals and AVANIR Pharmaceuticals. APP reports personal fees from Acadia Pharmaceuticals, personal fees from Functional Neuromodulation, personal fees from Neurim Pharmaceuticals, personal fees from Grifols, personal fees from Eisai, personal fees from Toyama, personal fees from Biogen, personal fees from Lundbeck, personal fees from Merck, grants from AstraZeneca, grants from Avanir, grants from Eisai, grants from Biogen, grants from Eli Lilly, grants from Janssen, grants from Genentech/Roche, grants from Novartis, grants from Merck, grants from Transition Therapeutics, grants from Toyama, grants from NIA, grants from NIMH, grants from DOD, personal fees from Rockpointe, outside the submitted work. CAM, SNB, DMS, LTD, DA, and SE report no conflicts of interest.
References
- [1].Prince M, Wimo A, Guerchet M, Ali GC, Wu YT, Prina M. World Alzheimer Report 2015. The Global Impact of Dementia Alzheimer’s Disease International. Alzheimer’s Dis Int (ADI), London: 2015. [Google Scholar]
- [2].McKeith I, Cummings J. Behavioural changes and psychological symptoms in dementia disorders. Lancet Neurol 2005;4:735–42. doi: 10.1016/S1474-4422(05)70219-2. [DOI] [PubMed] [Google Scholar]
- [3].Steinberg M, Shao H, Zandi P, Lyketsos CG, Welsh-Bohmer KA, Norton MC, et al. Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry 2008;23:170–7. doi: 10.1002/gps.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Porsteinsson AP, Drye LT, Pollock BG, Devanand DP, Frangakis C, Ismail Z, et al. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA 2014;311:682–91. doi: 10.1001/jama.2014.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Porsteinsson AP, Antonsdottir IM. An update on the advancements in the treatment of agitation in Alzheimer’s disease. Expert Opin Pharmacother 2017;18:611–20. doi: 10.1080/14656566.2017.1307340. [DOI] [PubMed] [Google Scholar]
- [6].Charu V, Rosenberg PB, Schneider LS, Drye LT, Rein L, Shade D, et al. Characterizing Highly Benefited Patients in Randomized Clinical Trials. Int J Biostat 2017;13. doi: 10.1515/ijb-2016-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Schneider LS, Frangakis C, Drye LT, Devanand DP, Marano CM, Mintzer J, et al. Heterogeneity of Treatment Response to Citalopram for Patients With Alzheimer’s Disease With Aggression or Agitation: The CitAD Randomized Clinical Trial. Am J Psychiatry 2016;173:465–72. doi: 10.1176/appi.ajp.2015.15050648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ho T, Pollock BG, Mulsant BH, Schantz O, Devanand DP, Mintzer JE, et al. R- and S-citalopram concentrations have differential effects on neuropsychiatric scores in elders with dementia and agitation. Br J Clin Pharmacol 2016;82:784–92. doi: 10.1111/bcp.12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98. [DOI] [PubMed] [Google Scholar]
- [10].Cummings J, Mintzer J, Brodaty H, Sano M, Banerjee S, Devanand DP, et al. Agitation in cognitive disorders: International Psychogeriatric Association provisional consensus clinical and research definition. Int Psychogeriatrics 2015;27:7–17. doi: 10.1017/S1041610214001963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ervin A-M, Taylor HA, Ehrhardt S. NIH Policy on Single-IRB Review - A New Era in Multicenter Studies. N Engl J Med 2016;375:2315–7. doi: 10.1056/NEJMp1608766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Schneider LS, Tariot PN, Lyketsos CG, Dagerman KS, Davis KL, Davis S, et al. National Institute of Mental Health clinical antipsychotic trials of intervention effectiveness (CATIE): Alzheimer disease trial methodology. Am J Geriatr Psychiatry 2001;9:346–60. [PubMed] [Google Scholar]
- [13].Drye LT, Ismail Z, Porsteinsson AP, Rosenberg PB, Weintraub D, Marano C, et al. Citalopram for agitation in Alzheimer’s disease: design and methods. Alzheimers Dement 2012;8:121–30. doi: 10.1016/j.jalz.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Weintraub D, Drye LT, Porsteinsson AP, Rosenberg PB, Pollock BG, Devanand DP, et al. Time to Response to Citalopram Treatment for Agitation in Alzheimer Disease. Am J Geriatr Psychiatry 2015;23:1127–33. doi: 10.1016/j.jagp.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mace NL, Rabins PV. The 36-Hour Day: A Family Guide to Caring for People with Alzheimer Disease, Other Dementias, and Memory Loss in Later Life, 4th 2006. [Google Scholar]
- [16].Rabins PV, Lyketsos CG, Steele CD. Practical dementia care. OUP USA; 2006. [Google Scholar]
- [17].Schneider LS, Olin JT, Doody RS, Clark CM, Morris JC, Reisberg B, et al. Validity and reliability of the Alzheimer’s Disease Cooperative Study-Clinical Global Impression of Change. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord 1997; 11 Suppl 2:S22–32. [DOI] [PubMed] [Google Scholar]
- [18].McHugh GS, Butcher I, Steyerberg EW, Marmarou A, Lu J, Lingsma HF, et al. A simulation study evaluating approaches to the analysis of ordinal outcome data in randomized controlled trials in traumatic brain injury: results from the IMPACT Project. Clin Trials 2010;7:44–57. doi: 10.1177/1740774509356580. [DOI] [PubMed] [Google Scholar]
- [19].de Medeiros K, Robert P, Gauthier S, Stella F, Politis A, Leoutsakos J, et al. The Neuropsychiatric Inventory-Clinician rating scale (NPI-C): reliability and validity of a revised assessment of neuropsychiatric symptoms in dementia. Int Psychogeriatrics 2010;22:984–94. doi: 10.1017/S1041610210000876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cummings JL. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology 1997;48:S10–6. [DOI] [PubMed] [Google Scholar]
- [21].Galasko D, Bennett D, Sano M, Ernesto C, Thomas R, Grundman M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord 1997; 11 Suppl 2:S33–9. [PubMed] [Google Scholar]
- [22].Geda YE, Schneider LS, Gitlin LN, Miller DS, Smith GS, Bell J, et al. Neuropsychiatric symptoms in Alzheimer’s disease: past progress and anticipation of the future. Alzheimers Dement 2013;9:602–8. doi: 10.1016/j.jalz.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lyness SA, Zarow C, Chui HC. Neuron loss in key cholinergic and aminergic nuclei in Alzheimer disease: a meta-analysis. Neurobiol Aging 2003;24:1–23. [DOI] [PubMed] [Google Scholar]
- [24].Rub U, Del Tredici K, Schultz C, Thal DR, Braak E, Braak H. The evolution of Alzheimer’s disease-related cytoskeletal pathology in the human raphe nuclei. Neuropathol Appl Neurobiol 2000;26:553–67. [DOI] [PubMed] [Google Scholar]
- [25].Chen CP, Alder JT, Bowen DM, Esiri MM, McDonald B, Hope T, et al. Presynaptic serotonergic markers in community-acquired cases of Alzheimer’s disease: correlations with depression and neuroleptic medication. J Neurochem 1996;66:1592–8. [DOI] [PubMed] [Google Scholar]
- [26].Palmer AM. Neurochemical studies of Alzheimer’s disease. Neurodegeneration 1996;5:381–91. [DOI] [PubMed] [Google Scholar]
- [27].Garcia-Alloza M, Gil-Bea FJ, Diez-Ariza M, Chen CPL-H, Francis PT, Lasheras B, et al. Cholinergic-serotonergic imbalance contributes to cognitive and behavioral symptoms in Alzheimer’s disease. Neuropsychologia 2005;43:442–9. doi: 10.1016/j.neuropsychologia.2004.06.007. [DOI] [PubMed] [Google Scholar]
- [28].Lai MKP, Tsang SWY, Francis PT, Esiri MM, Keene J, Hope T, et al. Reduced serotonin 5-HT1A receptor binding in the temporal cortex correlates with aggressive behavior in Alzheimer disease. Brain Res 2003;974:82–7. [DOI] [PubMed] [Google Scholar]
- [29].Procter AW, Francis PT, Stratmann GC, Bowen DM. Serotonergic pathology is not widespread in Alzheimer patients without prominent aggressive symptoms. Neurochem Res 1992;17:917–22. [DOI] [PubMed] [Google Scholar]
- [30].Lam LCW, Tang NLS, Ma SL, Zhang W, Chiu HFK. 5-HT2A T102C receptor polymorphism and neuropsychiatric symptoms in Alzheimer’s disease. Int J Geriatr Psychiatry 2004;19:523–6. doi: 10.1002/gps.1109. [DOI] [PubMed] [Google Scholar]
- [31].Assal F, Alarcon M, Solomon EC, Masterman D, Geschwind DH, Cummings JL. Association of the serotonin transporter and receptor gene polymorphisms in neuropsychiatric symptoms in Alzheimer disease. Arch Neurol 2004;61:1249–53. doi: 10.1001/archneur.61.8.1249. [DOI] [PubMed] [Google Scholar]
- [32].Sweet RA, Pollock BG, Sukonick DL, Mulsant BH, Rosen J, Klunk WE, et al. The 5-HTTPR polymorphism confers liability to a combined phenotype of psychotic and aggressive behavior in Alzheimer disease. Int Psychogeriatrics 2001; 13:401–9. [DOI] [PubMed] [Google Scholar]
- [33].Mintzer J, Brawman-Mintzer O, Mirski DF, Unger R, Nietert P, Meeks A, et al. Fenfluramine challenge test as a marker of serotonin activity in patients with Alzheimer’s dementia and agitation. Biol Psychiatry 1998;44:918–21. [DOI] [PubMed] [Google Scholar]
- [34].Lanctot KL, Herrmann N, Eryavec G, van Reekum R, Reed K, Naranjo CA. Central serotonergic activity is related to the aggressive behaviors of Alzheimer’s disease. Neuropsychopharmacology 2002;27:646–54. [DOI] [PubMed] [Google Scholar]
- [35].Pollock BG, Mulsant BH, Rosen J, Sweet RA, Mazumdar S, Bharucha A, et al. Comparison of citalopram, perphenazine, and placebo for the acute treatment of psychosis and behavioral disturbances in hospitalized, demented patients. Am J Psychiatry 2002;159:460–5. doi: 10.1176/appi.ajp.159.3.460. [DOI] [PubMed] [Google Scholar]
- [36].Pollock BG, Mulsant BH, Rosen J, Mazumdar S, Blakesley RE, Houck PR, et al. A double-blind comparison of citalopram and risperidone for the treatment of behavioral and psychotic symptoms associated with dementia. Am J Geriatr Psychiatry 2007;15:942–52. doi: 10.1097/JGP.0b013e3180cc1ff5. [DOI] [PubMed] [Google Scholar]
- [37].Hoertel N, Le Strat Y, Lavaud P, Dubertret C, Limosin F. Generalizability of clinical trial results for bipolar disorder to community samples: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry 2013;74:265–70. doi: 10.4088/JCP.12m07935. [DOI] [PubMed] [Google Scholar]
- [38].Susukida R, Crum RM, Ebnesajjad C, Stuart EA, Mojtabai R. Generalizability of findings from randomized controlled trials: application to the National Institute of Drug Abuse Clinical Trials Network. Addiction 2017;112:1210–9. doi: 10.1111/add.13789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rosenberg PB, Drye LT, Porsteinsson AP, Pollock BG, Devanand DP, Frangakis C, et al. Change in agitation in Alzheimer’s disease in the placebo arm of a nine-week controlled trial. Int Psychogeriatrics 2015;27:2059–67. doi: 10.1017/S1041610215001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, Sullivan PF, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 2009;460:748–52. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Davis KL, Marin DB, Kane R, Patrick D, Peskind ER, Raskind MA, et al. The Caregiver Activity Survey (CAS): development and validation of a new measure for caregivers of persons with Alzheimer’s disease. Int J Geriatr Psychiatry 1997;12:978–88. [DOI] [PubMed] [Google Scholar]
- [42].Mattis S, Jurica PJ, Leitten CL. Dementia Rating Scale-2: Professional Manual. Lutz, FL: Psychological Assessment Resources; 2001. [Google Scholar]
- [43].Gur RC, Sara R, Hagendoorn M, Marom O, Hughett P, Macy L, et al. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. J Neurosci Methods 2002;115:137–43. [DOI] [PubMed] [Google Scholar]
- [44].Mathias S, Nayak US, Isaacs B. Balance in elderly patients: the “get-up and go” test. Arch Phys Med Rehabil 1986;67:387–9. [PubMed] [Google Scholar]
- [45].Mohs RC, Knopman D, Petersen RC, Ferris SH, Ernesto C, Grundman M, et al. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer’s Disease Assessment Scale that broaden its scope. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord 1997; 11 Suppl 2:S13–21. [PubMed] [Google Scholar]
- [46].Storga D, Vrecko K, Birkmayer JG, Reibnegger G. Monoaminergic neurotransmitters, their precursors and metabolites in brains of Alzheimer patients. Neurosci Lett 1996;203:29–32. [DOI] [PubMed] [Google Scholar]
- [47].Zarit S, Orr NK, Zarit JM. The hidden victims of Alzheimer’s disease: Families under stress. NYU Press; 1985. [Google Scholar]
- [48].Bedard M, Molloy DW, Squire L, Dubois S, Lever JA, O’Donnell M. The Zarit Burden Interview: a new short version and screening version. Gerontologist 2001;41:652–7. [DOI] [PubMed] [Google Scholar]
- [49].Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA 2005;293:596–608. doi: 10.1001/jama.293.5.596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.