Abstract
Purpose
Diabetes patients must be equipped with the necessary knowledge to confidently undertake appropriate self-care activities. We prepared a diabetes self-management education (DSME) intervention and assessed how it affected patients’ self-reported levels of diabetes knowledge, self-care behaviors, and self-efficacy.
Patients and methods
A before-and-after, two-group intervention study was conducted at Jimma University Medical Centre among adult patients with type 2 diabetes. At baseline, we randomly assigned 116 participants to the DSME intervention and 104 to a comparison group. Six interactive DSME sessions supported by an illustrative handbook and fliers, experience-sharing, and take-home activities were administered to the intervention group by two nurses during a six-month period. Diabetes knowledge, self-care behaviors, and self-efficacy were measured at baseline and at nine months following the commencement of DSME intervention (endpoint) in both groups.
Results
At the endpoint, data from 78 intervention group participants and 64 comparison group participants were included in final analysis. The difference in the mean Diabetes Knowledge Scale scores before and after the DSME intervention was significantly greater in the intervention group (p = 0.044). The measured self-care behaviors included diet, exercise, glucose self-monitoring, footcare, smoking, alcohol consumption, and khat chewing. The mean number of days per week on which the intervention group participants followed general dietary recommendations increased significantly at the endpoint (p = 0.027). The intervention group followed specific dietary recommendations (p = 0.019) and performed footcare (p = 0.009) for a significantly greater number of days. There were no significant differences within or between the groups in other self-reported diabetes self-care behavior regimens or in diabetes self-efficacy.
Conclusion
Our study found significant improvements in the intervention participants’ diabetes knowledge scores and in their adherence to dietary and footcare recommendations. This demonstrates that our DSME intervention may be of clinical importance in developing countries such as Ethiopia.
Trial registration
ClinicalTrials.gov, Identifier NCT03185689, retrospectively registered on June 14, 2017: https://clinicaltrials.gov/ct2/show/NCT03185689.
Keywords: nurse-led DSME, diabetes knowledge, self-care behavior, self-efficacy
Introduction
More than 2.6 million adults in Ethiopia are living with type 1 diabetes mellitus (T1DM) or type 2 diabetes mellitus (T2DM). Ethiopia is the leading country in Africa in terms of the absolute number of individuals diagnosed with each type of diabetes.1 Especially in countries with limited resources and an increasing burden of diabetes,2 the introduction of diabetes self-management education (DSME) may play a significant role in reducing diabetes-related complications and premature deaths. Diabetes is a chronic condition for which self-management in terms of diet and exercise should complement medicines to prevent complications and to enhance favorable health outcomes.3 DSME seeks to equip diabetic patients with useful knowledge, problem-solving skills, decision-making abilities, resource utilization, and the confidence necessary to perform self-care activities.4,5
Key self-care behaviors that may prevent acute and long-term diabetes-related complications include healthy eating, regular exercise, medication management, footcare, and adaptation to psychosocial challenges.5–7 In the developed world, DSME has been proven to play a significant role in combating diabetes-related complications and premature deaths.8 However, the few studies evaluating the effectiveness of diabetes self-management programs in developing countries have shown mixed results.9–12
Diabetes knowledge is regarded as an essential precondition for effective self-care activities and favorable health outcomes.13,14 Studies from developing countries have yielded inconsistent evidence regarding such knowledge. Interventions by peers and pharmacists or supported by mobile short message service (SMS) for a duration of 3 to 6 months have been associated with significantly greater diabetes knowledge scores among diabetes patients involved in some form of diabetes education.10,11,15–18 However, research on another DSME intervention conducted by lay health promoters—lay people trained to provide patient education and counseling—over 12 months showed no significant change in overall diabetes knowledge scores at 12 months.19
Self-efficacy, one of the five constructs of Bandura’s social cognitive theory, is defined as the level of confidence a person needs to effectively perform a particular behavior within his or her ability.20 This is an important aspect of diabetes self-management, but studies from Africa reveal mixed evidence regarding the effectiveness of DSME in improving self-efficacy among T2DM patients.12,15,21
DSME is context-specific. One of the challenges associated with undertaking DSME in developing countries is the scarcity of available, culturally compatible DSME packages. The challenge is increased by the fact that many potential participants are low-literate diabetes patients. To our knowledge, there is no single, reliable diabetes self-management program that can be applied in different cultural contexts, including Africa in general and Ethiopia in particular.22 Well-structured and locally contextualized DSME intervention accommodating low-literate diabetes patients conducted in resource-constrained settings in sub-Saharan African countries are thus limited. We therefore conducted the current study with the aim of developing and testing the effectiveness of a multifaceted, nurse-led DSME program for improving diabetes knowledge, self-care activities, and self-efficacy in an Ethiopian setting.
Materials and Methods
Study Design, Setting and Period
A controlled before-and-after clinical trial design was employed to explore the effectiveness of a nurse-led DSME among adult patients with T2DM attending Jimma University Medical Centre (JUMC) in Ethiopia. The baseline survey was conducted from February 2016 to May 2016. DSME intervention was offered from November 2016 to July 2017, and the endpoint survey was conducted from August 2017 to October 2017. This study adheres to the CONSORT guidelines and includes a completed CONSORT checklist (Supplementary Material 1).
Sampling and Participant Recruitment
We calculated sample size using online Epi info_7.exe23 with the assumption that the proportion of individuals with the target HbA1c (less than or equal to 7%) would increase in the intervention group by 15% with a power of 80% and a 1:1 ratio. Based on these assumptions, the power analysis indicated a sample of 104 patients in each group. Adding a 15% contingency, 120 participants were needed for each group. We thus aimed to recruit 240 participants among the 447 adult T2DM patients being monitored at the JUMC’s diabetes clinic.
We gave an individual code number to each participant. Using Excel’s random number generator, 120 patients were randomly assigned to the intervention group and 120 to the comparison group. These 240 patients were contacted, and at baseline, 116 patients in the DSME intervention group and 104 in the treatment as usual comparison group agreed to participate and provided data. At endpoint, 78 participants in the intervention group and 64 in the comparison group provided data for the final analysis. Because of the nature of our DSME intervention, we could not blind the data collectors or our study participants. Our adherence to the CONSORT flowchart has been explained in detail elsewhere.24
Inclusion and Exclusion Criteria
T2DM patients 30 years of age or older at the time of diagnosis and who had used or were presently taking oral hypoglycemic agents or insulin were eligible for inclusion in the study. Individuals with T1DM, gestational diabetes, or a severe mental or physical incapability were excluded.
Intervention
The content of the DSME intervention developed for this study was inspired by material from the International Diabetes Federation, the American Diabetes Association, the American Association of Diabetes Educators, and Diabetes UK. This, together with an extensive international literature search focusing on patients’ diabetes knowledge, self-care behaviors, and self-efficacy formed the basis of the intervention. The content and delivery of the program was then adapted to an Ethiopian cultural situation that considered low-literate diabetes patients.
The multifaceted intervention consisted of three main elements: a) six educational sessions, each lasting for 1.5 hrs on average, focusing on basic diabetic knowledge and self-care behavior; b) a colorful, well-illustrated educational handbook and fliers adapted to the local context; and c) extensive and interactive discussions with peers and take-home activities. A detailed description of our DSME intervention is available elsewhere.24
The comparison group continued their usual care during the six-month period, which included having their blood pressure and weight checked, consulting physicians, collecting medicines, and scheduling their next appointments.
Data Collection and Data Analysis
Nurses collected data directly from the patients using interview-administered questionnaires at baseline and at endpoint. None of the data collectors participated in the DSME sessions or were informed about the participants’ group assignment. The data was inputted into the EpiData entry client manager (v.4.2.0.0) and then transferred to StataSE 15 for analysis. For all outcome variables, an independent samples t-test was used. We considered p-values of less than 0.05 to be statistically significant. Intention-to-treat analysis was used to include all participants who had received the instructional handbook and were assessed at endpoint, regardless of the number of sessions they attended.
To indicate variation in engagement in the DSME sessions, we used a chi-square test. We dichotomized the knowledge and the self-efficacy scales using the mean score as the cut-off. Similarly, we used the mean number of days on which self-care behaviors were performed appropriately as the cut-off to dichotomize each self-care behavior.
Table 1 describes the data collection tools: the Simplified Michigan Diabetes Knowledge Scale (DKS),14 the Summary of Diabetes Self-Care Activity (SDSCA),25 and the Diabetes Self-Efficacy tool developed by the Stanford Self-Management Resource Center (SMRC).26 The SDSCA has two sets of items: a core set of 11 items related to diet, exercise, blood sugar testing, footcare, and smoking practices; and a more detailed set of 17 items related to general diet, specific diabetes diet, footcare, smoking, alcohol consumption, and khat chewing. For negatively stated questions about high fat intake and foot soaking, the responses were reversed prior to analysis. All the regimens assessed the frequency with which the self-care behaviors were performed during the past seven days.
Table 1.
Data Collection Tools, Content, and Coding by Outcome Variables
| Outcome Variable | Data Collection Tool | Description of Tool | Coding |
|---|---|---|---|
| Diabetes knowledge | Simplified Michigan Diabetes Knowledge Scale (DKS) – true/false version14 | Relatively brief and easy to complete; twenty items related to diet, exercise, blood glucose control, diabetes complications, footcare, insulin injection, and clinical appointments. |
|
| Diabetes self-care behavior | Revised Summary of Diabetes Self-Care Activity (SDSCA) with expanded items25 | SDSCA encompasses self-care behaviors related to diet, exercise, blood sugar testing, footcare, smoking, and alcohol intake practices. In addition, we included khat chewing. |
|
| Diabetes self-efficacy | Diabetes Self-Efficacy tool developed by Stanford Self-Management Resource Center (SMRC)26 |
|
|
All three tools were translated from English to Afan Oromo and Amharic, which are the widely used local languages. They were then translated back to English as a quality check. Prior to the actual data collection, all questionnaires were pretested on 27 T2DM patients not included in the main study. The pilot study showed a reliability coefficient α greater than 0.7 for all three tools (0.727 for DKS, 0.834 for SDSCA, and 0.921 for Diabetes Self-Efficacy). Based on the pre-test, appropriate minor modifications were made to the questionnaires.
Sociodemographic Characteristics
Of the original 116 participants in the intervention group and 104 in the comparison group included at baseline, 78 (67%) and 64 (62%), respectively, provided data at endpoint.
Of the 38 lost-to-follow-up (LTFU) or dropout patients in the intervention group, 11 (29%) were females, while 16 (40%) of the 40 LTFU patients in the comparison group were females (Table 2). A greater proportion of LTFU patients in the intervention group were 55 years and older (58%), compared to 38% of the comparison group dropouts (p = 0.03). Moreover, a greater proportion of LTFU intervention group participants (66%) reported household food insecurity at baseline, compared to 43% of the LTFU comparison group participants (p = 0.04). Household food insecurity implies that all people living together in a household do not have access to safe, sufficient, or adequate nutritious food.27 No differences were found between the groups regarding marital status, urban or rural residency, or years lived with diabetes.
Table 2.
Characteristics of Lost-To-Follow-Up Participants
| Variables | Intervention | Comparison | Chi-square |
|---|---|---|---|
| Gender | |||
| Male | 27 (71%) | 24 (60%) | chi2 = 1.0518 |
| Female | 11 (29%) | 16 (40%) | p = 0.305 |
| Age in years | |||
| <45 years | 4 (10%) | 10 (25%) | chi2 = 8.8886 |
| 45–54 years | 12 (32%) | 15 (38%) | p = 0.031 |
| 55–64 years | 14 (37%) | 4 (10%) | |
| 65+ years | 8 (21%) | 11 (27%) | |
| Marital status | |||
| Married | 32 (84%) | 36 (90%) | chi2 = 0.584 |
| Unmarried | 6 (16%) | 4 (10%) | p = 0.445 |
| Residence | |||
| Urban | 26 (68%) | 33 (82%) | chi2 = 2.096 |
| Rural | 12 (32%) | 7 (18%) | p = 0.148 |
| Reported household food security status | |||
| Secure | 13 (34%) | 23 (57%) | chi2 = 4.253 |
| Insecure | 25 (66%) | 17 (43%) | p = 0.039 |
| Years lived with diabetes | |||
| <5 years | 10 (26%) | 5 (12%) | chi2 = 2.395 |
| 5–10 years | 8 (21%) | 10 (25%) | p = 0.302 |
| 10+ years | 20 (53%) | 25 (63%) | |
Note: Bold values: p-value < 0.05.
The mean (SD) age of both the intervention and comparison group participants at the diagnosis of diabetes was 47 (10) years. The reported average number of years (SD) of having lived with diabetes was 10 (6) years and 12 (7) years, respectively, for the intervention and comparison groups.
At baseline, there were no significant differences in sociodemographic or clinical characteristics between the intervention and comparison groups. Similarly, at endpoint, there were no significant differences except in the source of finance for healthcare. Compared to the baseline, the proportion of intervention group participants who paid out of pocket for healthcare at the endpoint remained nearly the same, while the proportion increased significantly by 9% in the comparison group.
The six educational sessions of the DSME program exposed the participants to different aspects of T2DM care. Together, the educational components constituted the basis of the intervention package. In total, 6 of the 78 intervention participants (8%) with endpoint data did not attended any DSME sessions but had taken a DSME teaching handbook, 17 (22%) attended one or two sessions, 25 (31%) attended three or four sessions, and 30 (39%) attended five or all six DSME sessions.
Results
The results of the study reported here include diabetes knowledge, self-care behavior regimens, and diabetes self-efficacy. Clinical outcome-related findings, including those related to glycated hemoglobin (HbA1c), are reported elsewhere.24
Diabetes Knowledge
At baseline, there was no statistically significant mean score difference on the DKS when comparing the intervention and comparison groups. At endpoint, the intervention group had a greater mean diabetes knowledge score, 11.33 out of 20, compared to that of the comparison group, 10.61 out of 20 (p = 0.050) (Table 3).
Table 3.
The Mean Score Differences on the Diabetes Knowledge Scale Between the Groups Before and After DSME Intervention
| Group | Baseline | Endpoint | ||||
|---|---|---|---|---|---|---|
| n | Mean | Std. Err. | n | Mean | Std. Err. | |
| Intervention | 116 | 10.41 | 0.21 | 78 | 11.33 | 0.25 |
| Comparison | 104 | 10.52 | 0.24 | 64 | 10.61 | 0.27 |
| Difference | −0.11 | 0.32 | 0.72 | 0.72 | ||
| Significance level | 0.742 | 0.050 | ||||
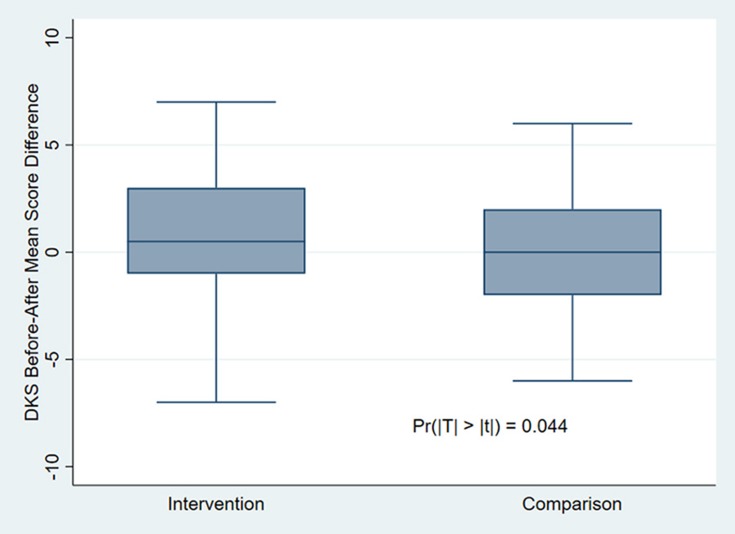
The mean DKS score significantly increased by 0.76 in the intervention group and decreased by 0.16 in the comparison group from baseline to endpoint (p = 0.044) (Figure 1).
Figure 1.
Diabetes Knowledge Scale score mean changes within groups’ before-and-after DSME intervention.
Self-Care Behavior
Diet
At endpoint, the intervention group participants followed general dietary recommendations for 5.06 days per week, which is statistically significantly greater than the 4.44 days reported by the comparison group (p = 0.027) (Table 4).
Table 4.
The Mean Score Differences Regarding Self-Care Activities Between the Groups Before and After DSME Intervention
| Group | Baseline | Endpoint | ||||
|---|---|---|---|---|---|---|
| n | Mean | Std. Err. | n | Mean | Std. Err. | |
| General diet | ||||||
| Intervention | 116 | 3.91 | 0.20 | 78 | 5.06 | 0.19 |
| Comparison | 104 | 3.79 | 0.19 | 64 | 4.44 | 0.19 |
| Difference | 0.12 | 0.28 | 0.62 | 0.28 | ||
| Significance level | p = 0.654 | p = 0.027 | ||||
| Specific diet | ||||||
| Intervention | 116 | 1.39 | 0.13 | 78 | 3.98 | 0.15 |
| Comparison | 104 | 1.61 | 0.12 | 64 | 3.54 | 0.17 |
| Difference | −0.22 | 0.18 | 0.44 | 0.23 | ||
| Significance level | p = 0.222 | p = 0.057 | ||||
| Exercise | ||||||
| Intervention | 116 | 3.89 | 0.23 | 78 | 4.34 | 0.23 |
| Comparison | 104 | 3.86 | 0.24 | 64 | 3.94 | 0.26 |
| Difference | 0.03 | 0.17 | 0.40 | 0.35 | ||
| Significance level | p = 0.936 | p = 0.249 | ||||
| Footcare | ||||||
| Intervention | 116 | 5.07 | 0.12 | 78 | 5.80 | 0.13 |
| Comparison | 104 | 4.78 | 0.13 | 64 | 5.26 | 0.16 |
| Difference | 0.29 | 0.18 | 0.54 | 0.20 | ||
| Significance level | p = 0.103 | p = 0.009 | ||||
| Blood glucose monitoring | ||||||
| Intervention | 116 | 0.39 | 0.10 | 78 | 0.65 | 0.14 |
| Comparison | 104 | 0.55 | 0.11 | 64 | 0.43 | 0.09 |
| Difference | −0.16 | 0.15 | 0.22 | 0.18 | ||
| Significance level | p = 0.277 | p = 0.206 | ||||
Note: Bold values: p-value < 0.05.
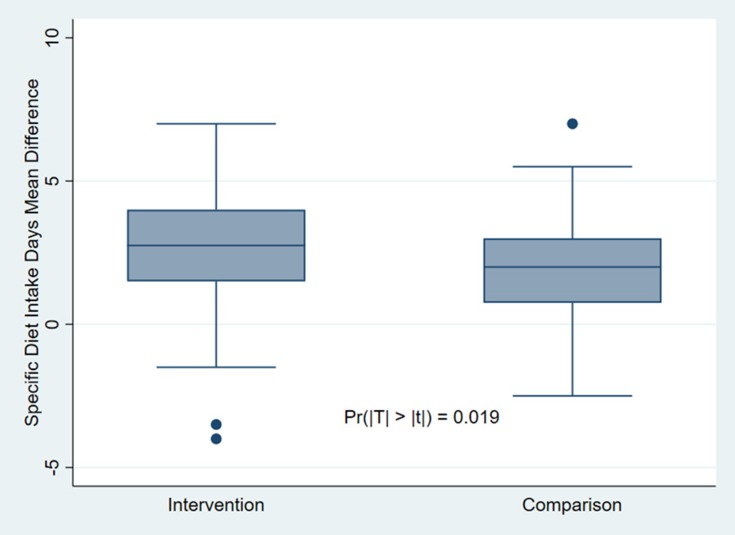
The mean number of days per week that the intervention group participants followed specific dietary recommendations significantly increased by 2.65 days from baseline to endpoint (p = 0.019) (Figure 2). The high and low outliers (black dots) are related to an unexplained and marked drop in the number of days of following the specific diet recommendations for two of the intervention group members as well as an unexplained, marked increase for one comparison group participant.
Figure 2.
Specific diet-taking behavior difference within groups before-and-after DSME intervention.
Footcare
At endpoint, the intervention group participants performed footcare for a mean of 5.80 days per week, compared to 5.26 days for the comparison group (p = 0.009). However, there was no statistically significant difference regarding change in footcare within each group (Table 4). The proportion of intervention group participants performing footcare as recommended increased significantly as the number of DSME sessions attended increased (p = 0.020).
There were no statistically significant changes within or between the two groups regarding exercise, smoking, khat chewing, or alcohol consumption at baseline or at endpoint.
Diabetes Self-Efficacy
The mean diabetes self-efficacy scores within or between the intervention and comparison groups before and after the DSME intervention were not statistically significant (Table 5).
Table 5.
The Mean Score Differences on the Diabetes Self-Efficacy Scale Between Groups Before and After DSME Intervention
| Group | Baseline | Endpoint | ||||
|---|---|---|---|---|---|---|
| n | Mean | Std. Err. | n | Mean | Std. Err. | |
| Intervention | 116 | 60.33 | 1.29 | 78 | 58.87 | 1.45 |
| Comparison | 104 | 59.32 | 1.25 | 64 | 57.31 | 1.85 |
| Difference | 1.01 | 1.81 | 1.56 | 2.31 | ||
| Significance level | p = 0.577 | p = 0.501 | ||||
Discussion
DSME plays a significant role in enabling patients to undertake self-management activities to combat their diabetes-related complications and potential premature deaths.8 We therefore developed a multifaceted, nurse-led DSME to be used in a low-resource setting. To our knowledge, this study is one of the first in sub-Saharan Africa to focus on the influence of a DSME program on patients’ diabetes knowledge, self-care activities, and self-efficacy. Our hypothesis was that a multifaceted DSME program would enhance these elements.28 The results of the study demonstrated statistically significant improvements in diabetes knowledge, adherence to dietary recommendations, and footcare practice. We did not, however, find significant differences in other self-reported activities associated with diabetes self-efficacy.
Diabetes Knowledge
Our study found a significant increase in the mean diabetes knowledge score in the intervention group and a slight decrease in the comparison group. The use of a patient-friendly and culturally sensitive information booklet, didactic teaching by clinic-based nurses, and interactive individual and group activities as part of the intervention most likely contributed to this result. Our results agree with the findings of a Cochrane review of group-based DSME studies mainly conducted in developed countries.29 It is also in line with a six-month, one-to-one diabetes education program run by pharmacists.17 Moreover, diabetes education supported by mobile SMS was found to significantly increase the mean knowledge score.15 However, a peer-led diabetes education program provided to Malian T2DM patients every three months over a one-year period was found to have no significant improvement on the mean diabetes knowledge score.19 The lengthy time interval between educational sessions (three months) may have decreased the learning effect of this peer-led intervention. The different findings of these studies are most likely related to differences in the education packages, the delivery approach, their duration and the gap between consecutive sessions, and the providers’ professional backgrounds.
Self-Care Behavior
Self-care behavior was measured by asking the participants to report which recommended activities for diabetes self-management they performed during the past seven days. These activities included their general diet, specific diet, exercise, footcare, blood glucose monitoring, smoking, alcohol consumption, and khat chewing.
Diet
In our study, the intervention group reported having followed both the general and the specific dietary recommendations for a significantly greater number of days per week during the follow-up period than the comparison group. This is in line with findings from Cameroon16 as well as a mobile SMS-supported diabetes education study conducted in Egypt.15 A home-visit diabetes management study from Brazil reported significantly increased fruit and vegetable intake in terms of both daily amount and number of days per week.30 However, a group-based diabetes education program provided by lay health promoters in South Africa reported no significant differences associated with following a meal plan.12 The various definitions and measurement tools used to monitor food consumption make comparisons between the studies difficult. They do, however, indicate a general positive direction regarding the effect of educational interventions on diabetic-related eating.21,30
In our study, we observed one high and two low outliers with regard to following specific dietary recommendations. Statistically, these are considered mild outliers that do not significantly affect the general results, and they were therefore considered in our intention-to-treat analysis. However, if these outliers had been removed from the analysis, then the significant relationship between our DSME intervention and healthy eating would have been further strengthened. The two low outlier observations in the intervention group may be related to low attendance rates, as one of the participants attended only one session and another took the DSME handbook but did not attend any sessions. This most likely contributed to the marked drop in the number of days they reported following the specific dietary recommendations. The high outlier in the comparison group may be related to the high risk of information spillover between the groups, since we recruited from the same clinic.
Footcare
Our study demonstrated a statistically significant difference between the two groups regarding footcare following the intervention. Likewise, an interactive, group-based discussion project from Morocco and a community-based, peer-supported intervention study done in Cameroon also found a significant improvement in footcare among the intervention groups.9,16 Studies conducted in other developing countries using a variety of interventions found similar impacts on footcare practice.15,31 Improved footcare is especially important in developing countries, as it can be carried out locally by the patient or by relatives and thereby reduce the risk of diabetes-related foot complications. Our study also indicated that footcare practice is positively associated with an increase in the number of DSME sessions attended. This shows that for low-literate diabetes patients, repeating DSME sessions could increase the effectiveness of DSME interventions.
Exercise
Our study did not find any significant difference between or within the two groups regarding exercise. A study from South Africa achieved the same results.25 However, these two studies are in contrast with several other diabetes education programs from developing countries that demonstrated significantly improved engagement regarding exercise among diabetes patients. The mean number of days per week,16,30 the intensity of exercise,21 and the proportion of participants performing exercise15 all increased significantly at the end of the interventions in these studies. The discrepancies in our findings may be related to local differences in accessible follow-up care and exercise centers. The most likely reason, however, is that our DSME did not target these parameters in an effective way.
Blood Glucose Monitoring
Unlike a couple of DSME studies conducted in Africa that showed a statistically significant improvement in the frequency of blood glucose testing,15,16 our study found no such relationship. These different findings may be related to the limited accessibility of glucometers and supplies for blood sugar testing within our population, as only one-fifth of our participants reported having glucometers at home. Additionally, a significant number of our patients come from districts or rural communities where laboratory facilities are few and hard to reach. Moreover, self-monitoring blood glucose requires patients to possess enhanced technical and cognitive skills,5 which may be hampered by the patient’s level of literacy and self-confidence. These factors pose a challenge to the participants of both groups with regard to testing their blood sugar levels as frequently as recommended.
The effects of smoking, alcohol consumption, and khat chewing on diabetes were problematized in the didactic sessions. However, we found no significant relationship between our DSME intervention and these reported activities. To assess these activities, we used SDSCA data collected by the nurses who conducted the interviews. The responses may have been subject to a social desirability bias for sensitive items, influencing both groups in the same way and thereby masking the possible effects of the intervention.32,33 The self-reported activities may therefore not always reflect participants’ actual behaviors.
Diabetes Self-Efficacy
Self-efficacy is a core belief related to an individual’s own perceived ability to undertake or maintain certain actions in order to achieve desired outcomes.34 Our study found no statistically significant differences in diabetes self-efficacy scores between or within groups. This result may be associated with a lack of mastery in certain self-care behaviors.35 To help low-literate participants indicate their level of confidence, we used a visual, 10-step colored ladder. This was unfamiliar to many of the patients and may have influenced their answers. During our study, we experienced that questions related to diabetes self-efficacy were especially culturally sensitive and often difficult to phrase. Other studies from developing countries have found significant differences in self-efficacy between the intervention and control groups.12,15,21
The proportion of LTFU participants was high in both the intervention and comparison groups. One-third of the intervention group found it difficult to attend the DSME sessions. The proportion of the intervention-group LTFU participants who reported household food insecurity was significantly greater compared to the comparison group at baseline. Food insecure diabetic patients may be stacked with competing priorities to buy food, medicine, and medical supplies.36 This challenge is known to be associated with low adherence to diabetes self-care, poor glycemic control, and low attendance rates at diabetic clinics.36,37 A significantly greater proportion of the LTFU intervention group patients were elderly. This could be linked to practical challenges, such as securing transportation or finding someone to accompany them to the DSME sessions. An intermittent lack of drug stock at the hospital also discouraged some patients from attending their scheduled visits.
Limitations and Strengths
Our study was a controlled before-and-after clinical trial conducted in a limited-resource setting. The major strength of this study is that it was executed in a naturalistic, real-life setting. Although it has limitations and was conducted with limited resources in a less-controlled environment, it demonstrates that a multifaceted DSME can significantly improve diabetes knowledge, dietary behavior, and footcare practice. These findings possess clinical importance in facilitating self-care and most likely in preventing acute and long-term diabetes-related complications.
During the study period, challenges were posed by structural and administrative changes at the intervention site, capacity issues, intermittent shortages of test material and drugs, patient transfers to other health institutions, and breakdowns in transportation to the hospital for rural residents. These issues were handled on a day-to-day basis and may have influenced the results. There are also structural elements of the project that should be kept in mind when interpreting our results.
Case mix: The included T2DM patients were not classified according to their use of oral hypoglycemic agents or insulin. This case-mix may have introduced bias in the intervention and control groups. Patients using different medication methods could have reacted differently to the DSME sessions and in their responses to the measurement tools.
Information contamination: Since both the intervention and comparison group participants were recruited from the same hospital, there might have been information spillover. To reduce this risk, we attempted to vary the appointment dates of the participants in the two groups. However, these dates sometimes overlapped, and participants showed up when it was convenient for them (usually when transport was available). Because of the nature of DSME intervention, we could not blind the study participants and data collectors. This would also contribute to information spillover.
Low attendance rate: The study was underpowered due to the low attendance rates and LTFU participants. To increase attendance, we reminded participants who were accessible by phone and at the end of each DSME session to schedule their follow-up appointment. Free fasting blood sugar (FBS) tests were also offered during the intervention period to encourage participation.
Dose-response: According to the intention-to-treat principle, all patients that were included at baseline and at endpoint were included in the analysis, regardless of their attendance rate. Low attendance most likely diluted the effects of the intervention and may have caused statistical type II errors.
Social desirability and information bias: We recruited the nurses who conducted the data collection from different units at the same hospital and nursing school of Jimma University. This may have induced a social desirability bias among the responders. To reduce possible information biases, the data collectors were not notified about participants’ group assignments.
Short follow-up period: A longer timeframe after intervention is needed to show sustainability of the results.
Comparisons: A lack of standardized international definitions and measurement tools appropriate for developing countries make direct comparisons between studies difficult.
Conclusion
Our controlled clinical study tested a nurse-led, locally contextualized DSME program augmented with illustrative pictures, discussions, experience-sharing, take-home activities, and a clarification of previous sessions before moving onto the teaching session of each day. The study demonstrated significant short-term improvements in relevant DSME parameters such as diabetes knowledge and self-care behaviors. These findings have significant clinical and public health importance for developing diabetes self-management education projects in resource-limited settings.
In our opinion, this DSME package is important and promising in its potential to raise the self-management capacity of T2DM patients in low-resource settings but warrants further development and testing for use in developing countries.
Acknowledgments
We thank the study participants for participating in the DSME intervention, for their commitment to providing information, and for remaining in the study until its conclusion. We additionally extend our gratitude to the DSME providers and data collectors for their immense contribution. Lastly, we acknowledge the principal investigators of the SACCADE project (Prof. H. Amelak and Dr. Magnus, PIs) for supporting and funding this study under the NORHED program for capacity-building.
Funding Statement
This research project was funded by the Strategic and Collaborative Capacity Development in Ethiopia and Africa (SACCADE) project, funded under the Norwegian Programme for Capacity Development in Higher Education and Research for Development (NORHED) under the NORHED Programme, Agreement No. ETH-13/0024. The funding body had no role in the design of the study; the collection, analysis, or interpretation of data; or in writing this manuscript.
Abbreviations
CONSORT, Consolidated Standards of Reporting Trials; DKS, Diabetes Knowledge Scale; DSME, diabetes self-management education; HbA1c, glycated hemoglobin; JUMC, Jimma University Medical Centre; NORHED, The Norwegian Programme for Capacity Development in Higher Education and Research for Development; OHAs, oral hypoglycemic agents; REK, Norwegian Regional Committee for Medical and Health Research Ethics; SACCADE, Strategic and Collaborative Capacity Development in Ethiopia and Africa; SDSCA, Summary of Diabetes Self-Care Activities; SMS, short message service; SMRC, Self-Management Resource Centre; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
Ethical Approval and Consent to Participate
“This study was conducted in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.” Prior to the commencement of the study, ethical approval to conduct the study was secured from REK (Norwegian Regional Committee for Medical and Health Research Ethics) and the Jimma University Ethical Review Board. Moreover, at the recruitment stage, written informed consent was sought from each study participant, through an explanation of the study’s objective, the means of how and the reasons why they were selected for possible participation in the study, and an overview of the intervention.
Availability of Data and Materials
The datasets used and/or analyzed in the current study and the study protocols are available from the corresponding author upon reasonable request.
Author Contributions
All authors contributed to proposal development, the study design data analysis, and the drafting and revising of the article; provided final approval for the version that will be published; and agreed to be accountable for all aspects of the work.
Disclosure
Mr Fikadu Balcha Hailu report grants from Norwegian Programme for Capacity Development in Higher Education and Research for Development (NORHED), during the conduct of the study. The authors report no other conflicts of interest in this work.
References
- 1.IDF. International Diabetes Federation: diabetes Atlas Africa Region. 2017. Available from: http://diabetesatlas.org/resources/2017-atlas.html. Accessed February25, 2018.
- 2.Abebe N, Kebede T, Addise D. Diabetes in Ethiopia 2000-2016–prevalence and related acute and chronic complications; a systematic review. Afr J Diabetes Med. 2017;25(2). [Google Scholar]
- 3.Powers MA, Bardsley J, Cypress M, et al. Diabetes self-management education and support in type 2 diabetes: a joint position statement of the American Diabetes Association, the American Association of Diabetes Educators, and the Academy of Nutrition and Dietetics. The Diabetes Educator. 2017;43(1):40–53. [DOI] [PubMed] [Google Scholar]
- 4.Lorig KR, Holman HR. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med. 2003;26(1):1–7. doi: 10.1207/S15324796ABM2601_01 [DOI] [PubMed] [Google Scholar]
- 5.Mulcahy K, Maryniuk M, Peeples M, et al. Diabetes self-management education core outcomes measures. Diabetes Educ. 2003;29(5):768–803. [DOI] [PubMed] [Google Scholar]
- 6.IDF. Diabetes education training manual for sub-Saharan Africa In: International Diabetes Federation. Jamana Printers Ltd; 2006. Available from: http://www.worlddiabetesfoundation.org/sites/default/files/DETM%20Eng.pdf. Accessed November18, 2019. [Google Scholar]
- 7.UK D. Care connect campaign: what to do when you have type 2 diabetes: an easy read guide. Available from: https://www.changepeople.org/Change/media/Change-Media-Library/Free%20Resources/Type-2-Diabetes-CHANGE-web.pdf. Accessed March12, 2015.
- 8.Ezenwaka C, Eckel J. Prevention of diabetes complications in developing countries: time to intensify self-management education. Arch Physiol Biochem. 2011;117(5):251–253. doi: 10.3109/13813455.2011.602692 [DOI] [PubMed] [Google Scholar]
- 9.Adarmouch L, Elyacoubi A, Dahmash L, El Ansari N, Sebbani M, Amine M. Short-term effectiveness of a culturally tailored educational intervention on foot self-care among type 2 diabetes patients in Morocco. J Clin Trans Endocrinol. 2017;7:54–59. doi: 10.1016/j.jcte.2017.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dube L, Van den Broucke S, Housiaux M, Dhoore W, Rendall-Mkosi K. Type 2 diabetes self-management education programs in high and low mortality developing countries; a systematic review. Diabetes Educ. 2015;41(1):69–85. doi: 10.1177/0145721714558305 [DOI] [PubMed] [Google Scholar]
- 11.Lepard MG, Joseph AL, Agne AA, Cherrington AL. Diabetes self-management interventions for adults with type 2 diabetes living in rural areas: a systematic literature review. Curr Diab Rep. 2015;15(6):608. doi: 10.1007/s11892-015-0608-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mash R, Rhode H, Zwarenstein M, et al. Effectiveness of a group diabetes education programme in under‐served communities in South Africa: a pragmatic cluster randomized controlled trial. Diabetic Med. 2014;31(8):987–993. doi: 10.1111/dme.2014.31.issue-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruce DG, Davis WA, Cull CA, Davis TM. Diabetes education and knowledge in patients with type 2 diabetes from the community: the Fremantle Diabetes Study. J Diabetes Complications. 2003;17(2):82–89. doi: 10.1016/S1056-8727(02)00191-5 [DOI] [PubMed] [Google Scholar]
- 14.Collins G, Mughal S, Barnett A, Fitzgerald J, Lloyd C. Modification and validation of the revised diabetes knowledge scale. Diabetic Med. 2011;28(3):306–310. [DOI] [PubMed] [Google Scholar]
- 15.Abaza H, Marschollek M. SMS education for the promotion of diabetes self-management in low & middle income countries: a pilot randomized controlled trial in Egypt. BMC Public Health. 2017;17(1):962. doi: 10.1186/s12889-017-4973-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Assah FK, Atanga EN, Enoru S, Sobngwi E, Mbanya JC. Community-based peer support significantly improves metabolic control in people with Type 2 diabetes in Yaounde, Cameroon. Diabetic Med. 2015;32(7):886–889. doi: 10.1111/dme.12720 [DOI] [PubMed] [Google Scholar]
- 17.Cani CG, Lopes Lda S, Queiroz M, Nery M. Improvement in medication adherence and self-management of diabetes with a clinical pharmacy program: a randomized controlled trial in patients with type 2 diabetes undergoing insulin therapy at a teaching hospital. Clinics (Sao Paulo). 2015;70(2):102–106. doi: 10.6061/clinics [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MakkiAwouda FO, Elmukashfi TA, Al-Tom SAH. Effects of health education of diabetic patient’s knowledge at diabetic health centers, Khartoum State, Sudan: 2007–2010. Glob J Health Sci. 2014;6(2):221. doi: 10.5539/gjhs.v6n2p221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Debussche X, Besancon S, Balcou-Debussche M, et al. Structured peer-led diabetes self-management and support in a low-income country: the ST2EP randomised controlled trial in Mali. PLoS ONE. ;13(1):1–13. doi: 10.1371/journal.pone.0191262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LaMorte WW. The social congnitve theory: Boston University School of Public Health. 2016. Available from: http://sphweb.bumc.bu.edu/otlt/MPH-Modules/SB/BehavioralChangeTheories/BehavioralChangeTheories5.html. Accessed February25, 2018.
- 21.Jayasuriya R, Pinidiyapathirage MJ, Jayawardena R, et al. Translational research for diabetes self-management in Sri Lanka: a randomized controlled trial. Prim Care Diabetes. 2015;9(5):338–345. doi: 10.1016/j.pcd.2015.01.014 [DOI] [PubMed] [Google Scholar]
- 22.Clark M. Diabetes self-management education: a review of published studies. Prim Care Diabetes. 2008;2(3):113–120. doi: 10.1016/j.pcd.2008.04.004 [DOI] [PubMed] [Google Scholar]
- 23.Dean AGSK, Soe MM OpenEpi: open source epidemiologic statistics for public health, version. 2013. Available from: http://www.openepi.com/Menu/OE_Menu.htm. Accessed May16, 2015.
- 24.Hailu FB, Hjortdahl P, Moen A. An intensified nurse-led diabetes self-management education improves clinical parameters in Ethiopia. Front Public Health. 2018;6:302. doi: 10.3389/fpubh.2018.00302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care. 2000;23(7):943–950. doi: 10.2337/diacare.23.7.943 [DOI] [PubMed] [Google Scholar]
- 26.SMRC. Stanford Self-Management Resource Center: self-effiacy for diabetes. Available from: https://www.selfmanagementresource.com/docs/pdfs/English_-_self-efficacy_diabetes.pdf. Accessed February23, 2018.
- 27.Coates J, Bilinsky SA. Household Food Insecurity Access Scale (HFIAS) for measurement of household food access: indicator guide (v. 3) In: Food and Nutrition Technical Assistance Project. Washington, DC: Academy for Educational Development; 2007. [Google Scholar]
- 28.Price C, Shandu D, Dedicoat M, Wilkinson D, Gill G. Long-term glycaemic outcome of structured nurse-led diabetes care in rural Africa. QJM. 2011;104(7):571–574. doi: 10.1093/qjmed/hcr005 [DOI] [PubMed] [Google Scholar]
- 29.Deakin TA, McShane CE, Cade JE, Williams R. Group based training for self‐management strategies in people with type 2 diabetes mellitus. Cochrane Lib. 2005;2:1–90. doi: 10.1002/14651858.CD003417.pub2 [DOI] [PubMed] [Google Scholar]
- 30.do Valle Nascimento TM, Resnicow K, Nery M, et al. A pilot study of a community health agent-led type 2 diabetes self-management program using motivational interviewing-based approaches in a public primary care center in Sao Paulo, Brazil. BMC Health Serv Res. 2017;17(1):32. doi: 10.1186/s12913-016-1968-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paz-Pacheco E, Sandoval MA, Ardena GJ, et al. Effectiveness of a community-based diabetes self-management education (DSME) program in a rural agricultural setting. Prim Health Care Res Dev. 2017;18(1):35–49. doi: 10.1017/S1463423616000335 [DOI] [PubMed] [Google Scholar]
- 32.Demetriou C, Ozer BU, Essau CA. Self‐report questionnaires. Encycl Clin Psychol. 2015;1–6. [Google Scholar]
- 33.Dodd-McCue D, Tartaglia A. Self-report response bias: learning how to live with its diagnosis in chaplaincy research. Chaplaincy Today. 2010;26(1):2–8. doi: 10.1080/10999183.2010.10767394 [DOI] [Google Scholar]
- 34.Bandura A. Social Cognitive Theory. W: Annals of Child Development. Vol. 6. Six Theories of Child Development. Vasta R (Red.) Greenwich: JAI Press; 1989. [Google Scholar]
- 35.Akhtar M. What is self-efficacy? Bandura’s 4 sources of efficacy beliefs. 2008. Available from: http://positivepsychology.org.uk/self-efficacy-definition-bandura-meaning/. Accessed February23, 2018.
- 36.Gucciardi E, Vahabi M, Norris N, Del Monte JP, Farnum C. The intersection between food insecurity and diabetes: a review. Curr Nutr Rep. 2014;3(4):324. doi: 10.1007/s13668-014-0104-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heerman W, Wallston K, Osborn C, et al. Food insecurity is associated with diabetes self‐care behaviours and glycaemic control. Diabetic Med. 2015;33:844–850. doi: 10.1111/dme.12896 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- IDF. International Diabetes Federation: diabetes Atlas Africa Region. 2017. Available from: http://diabetesatlas.org/resources/2017-atlas.html. Accessed February25, 2018.
Data Availability Statement
The datasets used and/or analyzed in the current study and the study protocols are available from the corresponding author upon reasonable request.