Abstract
Background
We assessed the cost-effectiveness of single-inhaler fluticasone furoate (FF)/umeclidinium (UMEC)/vilanterol (VI) versus FF/VI or UMEC/VI from a Canadian public healthcare perspective, incorporating data from the IMPACT trial in chronic obstructive pulmonary disease (COPD) (NCT02164513).
Methods
Baseline inputs and treatment effects from IMPACT were populated into the validated GALAXY-COPD disease progression model. Canadian unit costs and drug costs (Canadian dollars [C$], 2017) were applied to healthcare resource utilization and treatments. Future costs and health outcomes were discounted at 1.5% annually. Analyses were probabilistic, and outputs included exacerbation rates, costs, and life years (LYs) and quality-adjusted life years (QALYs) gained.
Results
Compared with FF/VI and UMEC/VI over a lifetime horizon, the analyses predicted that treatment with FF/UMEC/VI resulted in fewer moderate and severe exacerbations, more LYs and more QALYs gained, with a small incremental cost. The base-case incremental cost-effectiveness ratio (ICER) per QALY gained was C$18,989 (95% confidence interval [CI]: C$14,665, C$25,753) versus FF/VI and C$13,776 (95% CI: C$9787, C$19,448) versus UMEC/VI. FF/UMEC/VI remained cost-effective versus both FF/VI and UMEC/VI in all sensitivity analyses, including in scenario analyses that considered different intervention and comparator discontinuation rates, and treatment effects for subsequent therapy.
Conclusion
Treatment with FF/UMEC/VI was predicted to improve outcomes and be a cost-effective treatment option for patients with symptomatic COPD and a history of exacerbations compared with FF/VI or UMEC/VI, in Canada.
Keywords: chronic obstructive pulmonary disease, cost-effectiveness, single-inhaler triple therapy, quality-adjusted life years, Canada
Introduction
Chronic obstructive pulmonary disease (COPD) is a common, progressive disease characterized by persistent airflow limitation.1 Despite the availability of current treatments, COPD is associated with substantial morbidity and mortality, and incurs significant costs due to physician visits, hospitalizations and emergency room (ER) visits.2 In Canada, COPD represents the second most common cause for hospital admissions3 and was the fifth leading cause of death in 2016,4 resulting in high healthcare resource utilization (HRU) and economic burden on the Canadian healthcare system.3,5
Pharmacological therapy in COPD aims to reduce symptoms, improve exercise tolerance and health status, and decrease exacerbation frequency.1 Guidelines from the Canadian Thoracic Society recommend a step-up to triple therapy with an inhaled corticosteroid (ICS), a long-acting muscarinic antagonist (LAMA), and a long-acting β2-agonist (LABA) in patients who remain symptomatic despite treatment with dual therapy (LAMA/LABA).6 Until recently, triple therapy could only be achieved via the use of multiple inhalers. Though the cost-effectiveness of this approach has been widely investigated (a cost-effectiveness analysis of multiple-inhaler triple therapy [MITT] with umeclidinium [UMEC] plus ICS/LABA has recently been published),7 there are very few studies exploring the cost-effectiveness of single-inhaler triple therapy (SITT). As SITT is associated with improved adherence compared with MITT,8 understanding its cost-effectiveness may be important for patients, clinicians, and healthcare payers.
Triple therapy incorporating an ICS (fluticasone furoate [FF]), a LAMA (UMEC) and a LABA (vilanterol [VI]) administered in a single dry-powder inhaler (Trelegy ELLIPTA) is the first SITT to be licensed for COPD treatment in adults in Canada.9
IMPACT (InforMing the PAthway of COPD Treatment; NCT02164513, GlaxoSmithKline plc. study CTT116855)10 was a landmark phase III study that evaluated the relative benefits and risks of triple therapy with FF/UMEC/VI (100/62.5/25 µg) compared with its component molecules, FF/VI (100/25 µg) and UMEC/VI (62.5/25 µg) in 10,355 symptomatic patients aged ≥40 years with moderate-to-severe COPD and a history of exacerbations. Treatment with FF/UMEC/VI reduced the annual rate of moderate/severe COPD exacerbations, regardless of eosinophil level, improved lung function, and improved health-related quality of life (HRQoL) versus FF/VI or UMEC/VI administered with the same delivery device at the same doses.10 Additionally, FF/UMEC/VI significantly decreased the annual rate of exacerbations leading to hospitalization compared with UMEC/VI.10 All reports of exacerbation events in the IMPACT trial were adjudicated by an independent committee, unaware of the treatment assignments.10
The IMPACT study results indicated that SITT with FF/UMEC/VI may be the most appropriate treatment option for patients with moderate-to-severe COPD who are symptomatic according to COPD Assessment Test (CAT) score and have experienced at least one exacerbation in the past 12 months.10 We conducted a cost-effectiveness analysis from a Canadian public healthcare payer perspective to compare: (1) FF/UMEC/VI versus FF/VI; and (2) FF/UMEC/VI versus UMEC/VI in the IMPACT patient population.
Materials And Methods
Cost-Effectiveness Model
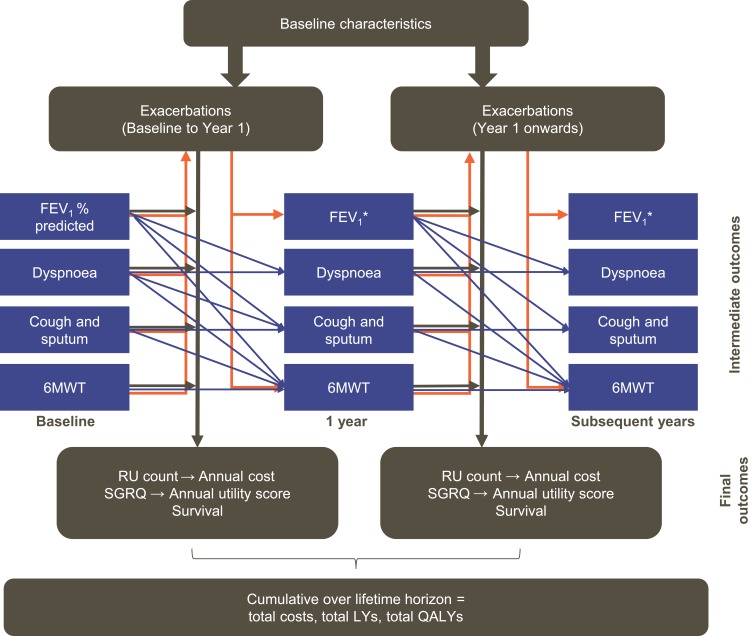
The current analysis was conducted using the GALAXY-COPD disease progression model, which has been previously published and validated.11–13 The GALAXY model incorporates associations between disease attributes, progression and outcomes,11,14,15 represented by linked-risk equations that predict disease progression in terms of decline in forced expiratory volume in 1 s (FEV1), exacerbation incidence, COPD symptoms, and HRQoL, and the associated resource utilization, survival and quality-adjusted life years (QALYs; Figure 1). The linked-risk equations for clinical outcomes are based on data from the Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE) study,16,17 with predictions of HRU based on the Towards a Revolution in COPD Health (TORCH) study.18
Figure 1.
Linked-risk equation model. Blue lines indicate the relationship between the central attributes in the different time periods. Orange lines indicate the relationship between intermediate outcomes and exacerbations. Black lines indicate the relationship between the central attributes and the final health outcomes. Adapted from Briggs AH, Baker T, Risebrough NA, et al (2017). Development of the Galaxy Chronic Obstructive Pulmonary Disease (COPD) Model Using Data from ECLIPSE: Internal Validation of a Linked-Equations Cohort Model Med Decis Making, 37(4): 469–480. https://doi.org/10.1177/0272989X16653118. Copyright © 2017 by the authors. Reprinted by permission of SAGE Publications, Inc.11 *Calculated (in mL) using the risk equation at 1 year and converted to FEV1% predicted based on the cohort profile.
Abbreviations: 6MWT, 6-min walk test; FEV1, forced expiratory volume in 1 s; LY, life year; QALY, quality-adjusted life year; RU, resource utilization; SGRQ, St. George’s Respiratory Questionnaire.
Cost-effectiveness calculations were based on population characteristics and clinical effects data from patients enrolled in IMPACT, with application of Canadian cost data to medication costs and predicted HRU.
Model Inputs
Patient Population
The study design, methods and results for IMPACT have been published previously.10 All analyses were conducted using the intent-to-treat (ITT) population (N=10,355). Since baseline characteristics were similar across treatment arms, pooled baseline characteristics across all three comparator arms were used as baseline parameter inputs for each comparator in the model (Table 1). This ensured that all treatment groups had the same starting values.
Table 1.
Summary Of Model Inputs
| Parameters | Reference Estimate | Probability Distribution | Source |
|---|---|---|---|
| Baseline characteristics | |||
| Female, % | 34 | Fixed | IMPACT Trial Supplementary Materials (GSK, 2017) |
| Mean age, years | 65.3 | Fixed | IMPACT Trial Supplementary Materials (GSK, 2017) |
| Current smoker, % | 35 | Fixed | IMPACT Trial Supplementary Materials (GSK, 2017) |
| Any CV comorbidity, % | 44 | Fixed | IMPACT Trial Supplementary Materials (GSK, 2017) |
| Any other comorbidity, % | 57 | Fixed | IMPACT Trial Supplementary Materials (GSK, 2017) |
| History of ≥1 exacerbation in the previous year, % | 100 | Fixed | IMPACT Trial Supplementary Materials (GSK, 2017) |
| BMI (kg/m2), % | |||
| BMI <21 | 17 | Fixed | IMPACT Trial Supplementary Materials (GSK, 2017) |
| BMI 21–30 | 58 | Fixed | IMPACT Trial Supplementary Materials (GSK, 2017) |
| BMI >30 | 25 | Fixed | IMPACT Trial Supplementary Materials (GSK, 2017) |
| Dyspnea, % | |||
| None | 1.7 | Fixed | Derived from a risk equation |
| Several days/week | 32.9 | Fixed | Derived from a risk equation |
| Most days/week | 65.4 | Fixed | Derived from a risk equation |
| Cough or sputum, most days/week, % | 52.2 | Fixed | Derived from a risk equation |
| Exacerbations in prior year (mean), n | |||
| Total | 1.71 | Fixed | IMPACT Trial Supplementary Materials (GSK, 2017) |
| Moderate | 1.41 | Fixed | IMPACT Trial Supplementary Materials (GSK, 2017) |
| Severe | 0.30 | Fixed | IMPACT Trial Supplementary Materials (GSK, 2017) |
| Starting FEV1% predicted | 45.5 | Fixed | IMPACT Trial Supplementary Materials (GSK, 2017) |
| Starting FEV1 predicted by the GALAXY model, mL | 1215.3 | Fixed | Based on FEV1% reported in IMPACT and formulae based in ECLIPSE to back calculate the starting FEV1 value |
| Height, cm | 167.5 | Fixed | IMPACT Trial Supplementary Materials (GSK, 2017) |
| mMRC dyspnea score ≥2, % | 37 | Fixed | % with CAT score ≥21 from IMPACT considered equivalent; IMPACT Trial Supplementary Materials (GSK, 2017) |
| Fibrinogen, μg/dL | 477.5 | Fixed | Not available from IMPACT, derived from a risk equation |
| 6MWT, m | 365.8 | Fixed | Not available from IMPACT, derived from a risk equation |
| Mean starting SGRQ total score | 50.7 | Fixed | IMPACT Trial Supplementary Materials (GSK, 2017) |
| Resulting HRQoL utility value | 0.654 | Fixed | Calculated from SGRQ-C score by algorithm19 |
| Treatment effects | |||
| FF/UMEC/VI versus FF/VI | |||
| Difference FEV1 incremental change from baseline, mL | 97 | Normal (SE=6.10) | IMPACT Trial Supplementary Materials (GSK, 2017) |
| Difference SGRQ total score change from baseline | −1.8 | Normal (SE=0.34) | IMPACT Trial Supplementary Materials (GSK, 2017), converted to SGRQ-C |
| Moderate exacerbation reduction, ratio | 0.84 | Log normal (SE=0.03) | IMPACT Trial Supplementary Materials (GSK, 2017) |
| Severe exacerbation reduction, ratio | 0.87 | Log normal (SE=0.06) | IMPACT Trial Supplementary Materials (GSK, 2017) |
| FF/UMEC/VI versus UMEC/VI | |||
| Difference FEV1 incremental change from baseline, mL | 54 | Normal (SE=7.60) | IMPACT Trial Supplementary Materials (GSK, 2017) |
| Difference SGRQ total score change from baseline | −1.8 | Normal (SE=0.42) | IMPACT Trial Supplementary Materials (GSK, 2017), converted to SGRQ-C |
| Moderate exacerbation reduction, ratio | 0.77 | Log normal (SE=0.03) | IMPACT Trial Supplementary Materials (GSK, 2017) |
| Severe exacerbation reduction, ratio | 0.66 | Log normal (SE=0.06) | IMPACT Trial Supplementary Materials (GSK, 2017) |
| Treatment discontinuation at 52 weeks | |||
| FF/UMEC/VI | 18.3% | Beta (758/4151) | IMPACT Trial Supplementary Materials (GSK, 2017) |
| FF/VI | 25.2% | Beta (1040/4134) | IMPACT Trial Supplementary Materials (GSK, 2017) |
| UMEC/VI | 27.3% | Beta (566/2070) | IMPACT Trial Supplementary Materials (GSK, 2017) |
| Drug costs | Cost per day | ||
| FF/UMEC/VI (100/62.5/25 μg) (Trelegy ELLIPTA) | C$4.41 | Fixed | Assume same as price for FF/VI + UMEC |
| UMEC (62.5 μg) (Incruse ELLIPTA) | C$1.67 | Fixed | ODB, 201720 |
| FF/VI (100/25 μg) (Breo ELLIPTA) | C$2.74 | Fixed | ODB, 201720 |
| UMEC/VI (62.5/25 μg) (Anoro ELLIPTA) | C$2.70 | Fixed | ODB, 201720 |
| Subsequent treatment cost | |||
| Average weighted cost of all treatment classes | C$4.29 | Fixed | Average cost weighted by Canadian prescription utilization in regimen class (based on IMS data)21 |
| Outpatient medication | |||
| Cost of moderate exacerbations | C$7.59 | Fixed | Calculation based on ODB, 201720 |
| Rescue medication | Cost per day | ||
| Salbutamol (Ventolin DISKUS) | C$0.03 | Fixed | ODB, 201720 |
| Occasions of rescue medication use per day | |||
| FF/UMEC/VI | 1.75 | Fixed | IMPACT Trial Supplementary Materials (GSK, 2017) |
| FF/VI | 2.03 | Fixed | IMPACT Trial Supplementary Materials (GSK, 2017) |
| UMEC/VI | 2.05 | Fixed | IMPACT Trial Supplementary Materials (GSK, 2017) |
| Hospital costs | |||
| Cost per day in ICU | C$3843.40 | Fixed | CIHI, 201622 OHIP, 201623 Statistics Canada, 201724 |
| Cost per day in general ward | C$1256.30 | Fixed | CIHI, 201622 Statistics Canada, 201724 |
| ER visit | C$76.90 | Fixed | OHIP, 201623 |
| Hospital outpatient visit – initial visit | C$157.00 | Fixed | OHIP, 201623 |
| Physician costs | |||
| Day(/night) home visit# | C$45.15 | Fixed | OHIP, 201623 |
| Visit to physician’s office (family physician) | C$77.20 | Fixed | OHIP, 201623 |
| Telephone consultation¶ | C$9.30 | Fixed | OHIP, 201623 Statistics Canada, 201724 Payscale, 201725 |
Notes: The IMPACT Trial Supplementary Materials are GlaxoSmithKline plc. internal data on file (Nov/Dec 2017). #The model assumes the same cost for home consultations whether they occur during “day” or “night” hours; OHIP 2016 schedule reports billing rates current in 2017. ¶The cost of a telephone call is based on assuming the interaction is 10 min in length and a nurse (registered nurse hourly wage C$33.01) will take the call 60% of the time and a physician the remaining 40%. Physician code H101 (C$15.00) was selected as a substitute value since there is no appropriate code for a telephone call between a patient and physician.
Abbreviations: 6MWT, 6-min walk test; BMI, body mass index; CAT, COPD Assessment Test; CIHI, Canadian Institute for Health Information; CV, cardiovascular; C$, Canadian dollars; ER, emergency room; FEV1, forced expiratory volume in 1 s; FF, fluticasone furoate; HRQoL, health-related quality of life; ICU, intensive care unit; IMS, IMS Brogan ODB RxDynamics Database; mMRC, modified Medical Research Council; ODB, Ontario Drug Benefit; OHIP, Ontario Health Insurance Program; SE, standard error; SGRQ, St. George’s Respiratory Questionnaire; SGRQ-C, St. George’s Respiratory Questionnaire for COPD patients; UMEC, umeclidinium; VI, vilanterol.
The risk equations in the GALAXY model included some baseline values for data not collected in IMPACT, including modified Medical Research Council (mMRC) Dyspnea Scale, 6-min walk test (6MWT) and fibrinogen, as covariates in the model. Values for these parameters (Table 1) were estimated as follows: the proportion of patients with CAT score ≥21 (from IMPACT) was assumed to be the same as the proportion with mMRC dyspnea score ≥2, based on published data26 and baseline 6MWT and fibrinogen were predicted using a risk equation developed through analysis of ECLIPSE data16,17 (Online Supplementary Tables S1 and S2).
Treatment Effects
Treatment effects of FF/UMEC/VI in the IMPACT ITT population were included as incremental effects relative to each of the comparators (FF/VI and UMEC/VI) (Table 1), and were assumed to continue until treatment discontinuation. For this reason, predicted outcomes with FF/VI or UMEC/VI, as reference treatments, appear the same in both analyses.
Improvement in FEV1 predicts St George’s Respiratory Questionnaire (SGRQ) and exacerbation treatment effects in the model. Therefore, to avoid double counting or under-prediction, the incremental magnitudes of SGRQ and exacerbation treatment effects were adjusted and entered into the model, calibrating to the 52-week trial results from IMPACT. FEV1 treatment effect was entered first, followed by exacerbations (moderate then severe) and then SGRQ total scores. This ensured that the predicted clinical inputs, as far as possible, matched and maintained the incremental benefits observed with FF/UMEC/VI over FF/VI or UMEC/VI in the IMPACT data.
Treatment discontinuation rates were based on IMPACT data and applied to the first year (Table 1). For subsequent years, in the base case, discontinuation rates were assumed to be the same as the first year for all treatments (18.3% for FF/UMEC/VI, 25.2% for FF/VI and 27.3% for UMEC/VI).
Utilities
Model-predicted SGRQ for COPD patients (SGRQ-C) scores were translated into SGRQ total score and then into an annual utility value using a published algorithm for converting SGRQ to EQ-5D-3L index scores,19 as follows:
 |
Costs
The study was conducted from the perspective of the Canadian public healthcare payer and therefore included only direct medical costs, in alignment with Canadian Agency for Drugs and Technology in Health (CADTH) guidelines for economic evaluations.27 Health service costs were estimated using unit costs from Canadian reference price lists (in Canadian dollars [C$]), inflated to 2017 equivalents where necessary, and applied to resource utilization counts for hospital days, ER visits and physician visits predicted by the model (Table 1). Drug costs were obtained from the Ontario Drug Benefit (ODB) Formulary, 201720 using dose, pack size and cost per pack (C$132.20, C$82.20 and C$81.00 for FF/UMEC/VI, FF/VI and UMEC/VI, respectively) to calculate the cost per daily dose. As the list price of FF/UMEC/VI (100/62.5/25 μg) was not available at the time of this analysis, the price of FF/UMEC/VI was assumed to be equal to the sum of the UMEC (C$50.00; 62.5 μg) and FF/VI (C$82.20; 100/25 μg) pack costs. Subsequent therapy cost was based on the four most common classes of treatments taken after discontinuation in IMPACT, pooled across all treatment arms (Table S3). The average daily cost of subsequent treatment was estimated at C$4.29, based on drug costs20 weighted by Canadian prescription utilization data for each treatment class.21 Treatment costs for moderate exacerbations and use of salbutamol rescue medication were also included in the analysis (see Online Supplementary Appendix).
Base-Case Settings And Assumptions
A lifetime horizon was used for the base-case analysis. Costs and health outcomes were discounted at 1.5% per annum, consistent with CADTH guidelines for economic evaluations.27 It was assumed that patients who discontinued their initial treatment experienced the same efficacy as the reference treatment (FF/VI or UMEC/VI) for the remainder of the analysis. All analyses were probabilistic, conducted by assigning distributions to input parameters and randomly sampling from these distributions over 5000 Monte Carlo simulations.
Model Outputs
The model outputs were total moderate and severe exacerbations, costs (drug-related and non-drug-related), QALYs and life years (LYs; undiscounted) gained, and incremental cost-effectiveness ratio (ICER) per QALY gained.
Sensitivity Analyses
Sensitivity analyses were conducted to test the robustness of the base-case results when parameters were not available from IMPACT (fibrinogen, 6MWT, mMRC dyspnea score). Cost, efficacy and utility inputs were varied to the upper and lower bounds of the range around baseline estimates (fibrinogen, 472.8–482.1 μg/dL; 6MWT, 360.4–371.2 m; mMRC dyspnea score ≥2, 27.8–46.3%). Baseline fibrinogen and 6MWT limits were varied to 95% confidence limits approximated using ECLIPSE data, and ±25% limits were used for mMRC dyspnea score, and hospital and physician costs. As estimates of HRQoL experienced by patients can vary, the sensitivity analysis also explored the impact of higher (+10%) utility values, to be comparable with those reported in the literature.28
Scenario analyses were performed with alternative model settings or assumptions to aid understanding of model drivers, and to provide estimates of ICERs under plausible, alternative real-world situations. The scenarios tested were: shorter time horizons, 0% and 3% discount rates, alternate background exacerbation rates, 0% treatment discontinuation for subsequent years, different treatment effects and costs for subsequent treatments, and replacement of FF/VI or UMEC/VI cost with a Canada-specific utilization-weighted cost for all ICS/LABAs or LAMA/LABAs, respectively.
Results
Base Case
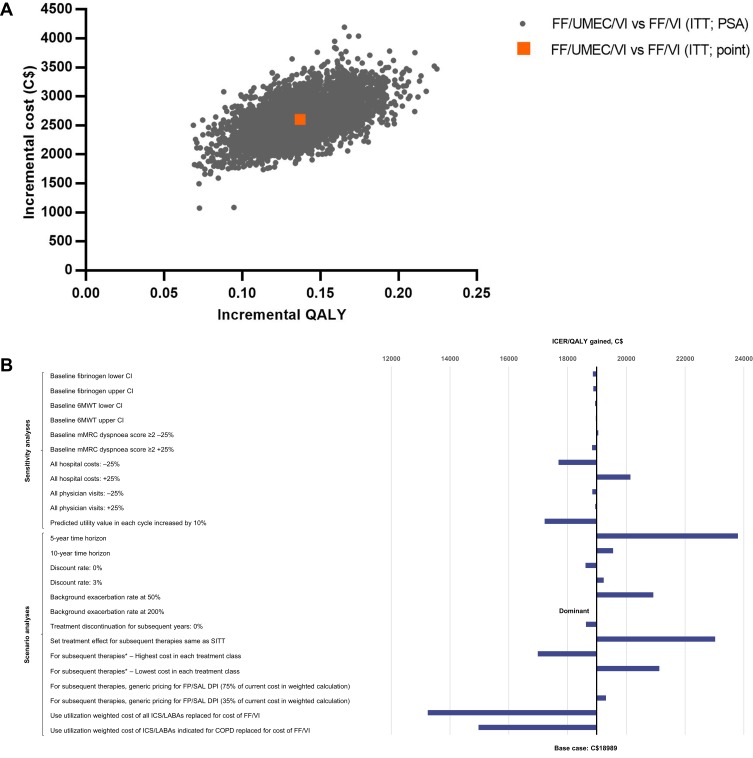
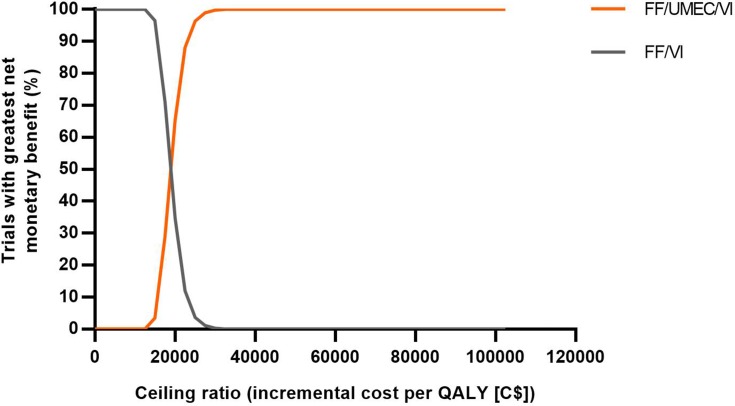
Over a lifetime horizon, FF/UMEC/VI provided an additional 0.1388 LYs (9.0524 versus 8.9136) and 0.1371 QALYs (5.0431 versus 4.9060) for an additional cost of C$2604 per patient, compared with ICS/LABA therapy, FF/VI (Table 2). Patients taking FF/UMEC/VI had fewer total exacerbations (incremental difference, 0.1050; 6% reduction) per-patient per-year (PPPY) compared with those taking FF/VI. Across all simulations, FF/UMEC/VI showed higher QALYs but was costlier compared with FF/VI (Figure 2A). The estimated ICER was C$18,989/QALY (95% CI: C$14,665, C$25,753), and at a willingness-to-pay threshold of C$50,000/QALY, the probability that FF/UMEC/VI was cost-effective versus FF/VI was 100% (Figure 3).
Table 2.
Cumulative And Incremental Lifetime Outcomes Of FF/UMEC/VI Versus FF/VI
| FF/UMEC/VI | FF/VI | Difference | ||
|---|---|---|---|---|
| Cumulative exacerbations | ||||
| Moderate exacerbations | 10.6200 | 11.2337 | −0.6136 | |
| Severe exacerbations | 3.5326 | 3.6314 | −0.0988 | |
| Total exacerbations (moderate and severe) | 14.1526 | 14.8650 | −0.7124 | |
| Moderate exacerbations PPPY | 1.1728 | 1.2605 | −0.0878 | |
| Severe exacerbations PPPY | 0.3884 | 0.4056 | −0.0172 | |
| Total exacerbations PPPY | 1.5611 | 1.6661 | −0.1050 | |
| Health outcomes | ||||
| Accumulated LYs (undiscounted) | 9.0524 | 8.9136 | 0.1388 | |
| Accumulated QALYs | 5.0431 | 4.9060 | 0.1371 | |
| Costs | ||||
| Drug costs | C$13,387 | C$11,461 | C$1926 | |
| Total non-drug costs | C$53,750 | C$53,072 | C$678 | |
| Hospital (ICU and ward) costs | C$52,506 | C$51,835 | C$672 | |
| Outpatient/ER costs | C$651 | C$646 | C$5.38 | |
| Physician visit costs | C$592 | C$591 | C$0.96 | |
| Accumulated costs total | C$67,137 | C$64,533 | C$2604 | |
| Incremental results | ||||
| Incremental costs (95% CI) | C$2604 | (C$1980, C$3285) | ||
| Incremental LYs (95% CI) undiscounted | 0.1388 | (0.068, 0.207) | ||
| Incremental QALYs (95% CI) | 0.1371 | (0.092, 0.182) | ||
| ICER/QALY gained (95% CI) | C$18,989 | (C$14,665, C$25,753) | ||
Abbreviations: CI, confidence interval; C$, Canadian dollars; ER, emergency room; FF, fluticasone furoate; ICER, incremental cost-effectiveness ratio; ICU, intensive care unit; LY, life year; QALY, quality-adjusted life year; PPPY, per patient per year; UMEC, umeclidinium; VI, vilanterol.
Figure 2.
Incremental cost-effectiveness plane for FF/UMEC/VI versus FF/VI (A), and tornado plot of sensitivity and scenario analyses (B). *Assume 100% market share of product.
Abbreviations: 6MWT, 6-min walk test; CI, confidence interval; COPD, chronic obstructive pulmonary disease; C$, Canadian dollars; DPI, dry-powder inhaler; FF, fluticasone furoate; FP, fluticasone propionate; ICER, incremental cost-effectiveness ratio; ICS, inhaled corticosteroid; ITT, intent to treat; LABA, long-acting β2-agonist; mMRC, modified Medical Research Council; PSA, probabilistic sensitivity analysis; QALY, quality-adjusted life year; SAL, salmeterol; SITT, single-inhaler triple therapy; UMEC, umeclidinium; VI, vilanterol.
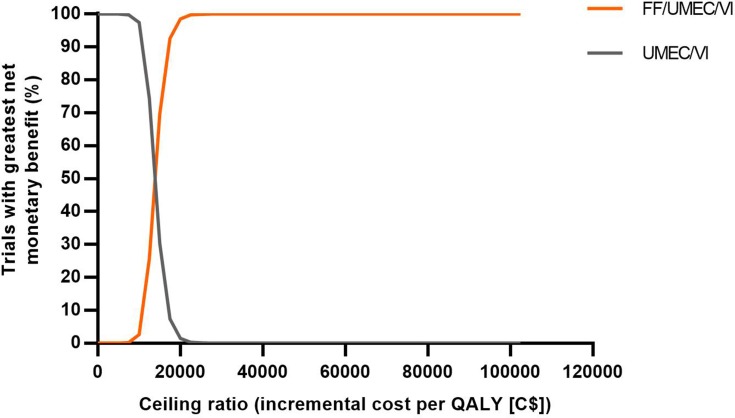
Figure 3.
Net benefit acceptability curves for FF/UMEC/VI versus FF/VI.
Abbreviations: C$, Canadian dollars; FF, fluticasone furoate; QALY, quality-adjusted life year; UMEC, umeclidinium; VI, vilanterol.
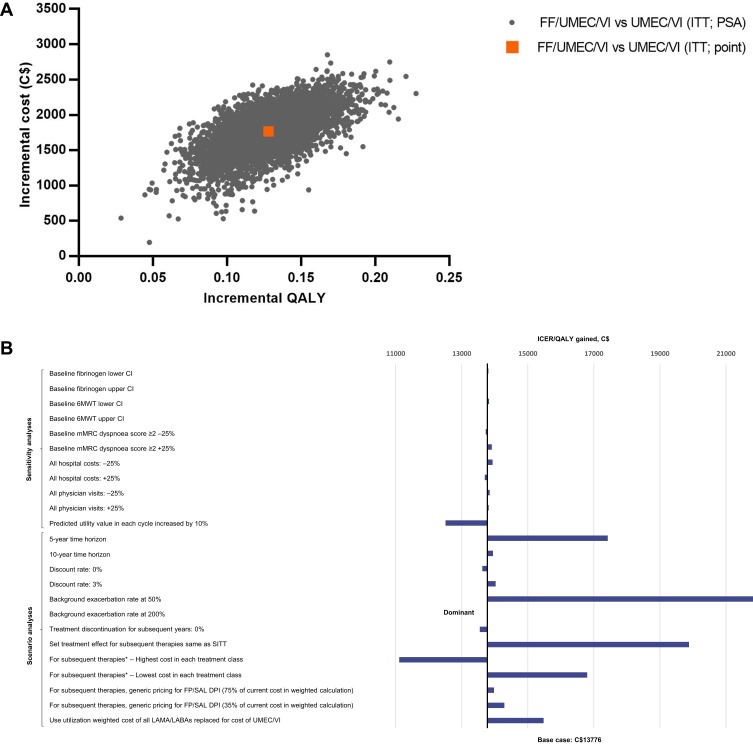
Compared with the LAMA/LABA therapy UMEC/VI, FF/UMEC/VI provided an additional 0.1177 LYs (9.0258 versus 8.9081) and 0.1282 QALYs (5.0352 versus 4.9070), at an additional cost of C$1766 per patient (Table 3). Patients receiving FF/UMEC/VI also had 10% fewer total PPPY exacerbations (incremental difference, 0.1714; 10% reduction) compared with those treated with UMEC/VI. FF/UMEC/VI showed higher QALYs and costs compared with UMEC/VI across all simulations (Figure 4A). The estimated ICER was C$13,776/QALY (95% CI: C$9787, C$19,448), and at a willingness-to-pay threshold of C$50,000/QALY, the probability that FF/UMEC/VI was cost-effective versus UMEC/VI was 100% (Figure 5).
Table 3.
Cumulative And Incremental Lifetime Outcomes Of FF/UMEC/VI Versus FF/VI
| FF/UMEC/VI | UMEC/VI | Difference | ||
|---|---|---|---|---|
| Cumulative exacerbations | ||||
| Moderate exacerbations | 10.2638 | 11.2357 | −0.9719 | |
| Severe exacerbations | 3.2631 | 3.6314 | −0.3683 | |
| Total exacerbations (moderate and severe) | 13.5269 | 14.8671 | −1.3402 | |
| Moderate exacerbations PPPY | 1.1364 | 1.2615 | −0.1251 | |
| Severe exacerbations PPPY | 0.3593 | 0.4056 | −0.0464 | |
| Total exacerbations PPPY | 1.4957 | 1.6671 | −0.1714 | |
| Health outcomes | ||||
| Accumulated LYs (undiscounted) | 9.0258 | 8.9081 | 0.1177 | |
| Accumulated QALYs | 5.0352 | 4.9070 | 0.1282 | |
| Costs | ||||
| Drug costs | C$13,346 | C$11,519 | C$1827 | |
| Total non-drug costs | C$53,065 | C$53,126 | −C$61 | |
| Hospital (ICU and ward) costs | C$51,830 | C$51,887 | −C$58 | |
| Outpatient/ER costs | C$645 | C$646 | −C$0.72 | |
| Physician visit costs | C$589 | C$592 | −C$2.67 | |
| Accumulated costs total | C$66,411 | C$64,644 | C$1766 | |
| Incremental results | ||||
| Incremental costs (95% CI) | C$1766 | (C$1167, C$2336) | ||
| Incremental LYs (95% CI) undiscounted | 0.1177 | (0.048, 0.186) | ||
| Incremental QALYs (95% CI) | 0.1282 | (0.081, 0.177) | ||
| ICER/QALY gained (95% CI) | C$13,776 | (C$9787, C$19,448) | ||
Abbreviations: CI, confidence interval; C$, Canadian dollars; ER, emergency room; FF, fluticasone furoate; ICER, incremental cost-effectiveness ratio; ICU, intensive care unit; LY, life year; QALY, quality-adjusted life year; PPPY, per patient per year; UMEC, umeclidinium; VI, vilanterol.
Figure 4.
Incremental cost-effectiveness plane for FF/UMEC/VI versus UMEC/VI (A), and tornado plot of sensitivity and scenario analyses (B). *Assume 100% market share of product.
Abbreviations: 6MWT, 6-min walk test; CI, confidence interval; COPD, chronic obstructive pulmonary disease; C$, Canadian dollars; DPI, dry-powder inhaler; FF, fluticasone furoate; FP, fluticasone propionate; ICER, incremental cost-effectiveness ratio; ITT, intent to treat; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; mMRC, modified Medical Research Council; PSA, probabilistic sensitivity analysis; QALY, quality-adjusted life year; SAL, salmeterol; SITT, single-inhaler triple therapy; UMEC, umeclidinium; VI, vilanterol.
Figure 5.
Net benefit acceptability curves for FF/UMEC/VI versus UMEC/VI.
Abbreviations: C$, Canadian dollars; FF, fluticasone furoate; QALY, quality-adjusted life year; UMEC, umeclidinium; VI, vilanterol.
Sensitivity Analyses
Across all sensitivity analyses, FF/UMEC/VI remained cost-effective versus both FF/VI and UMEC/VI at a willingness-to-pay threshold of C$50,000/QALY, with ICERs ranging from C$17,220/QALY to C$20,141/QALY versus FF/VI (Figure 2B; Table S4) and C$12,508/QALY to C$13,933/QALY versus UMEC/VI (Figure 4B; Table S5). For model parameters derived from risk equations or analogous IMPACT data (baseline fibrinogen, 6MWT and mMRC dyspnea score), sensitivity analyses showed only a minimal impact on the ICER when comparing to either FF/VI (−1 to 0% variation versus the base case) or UMEC/VI (0 to 1% variation versus the base case). Varying physician visit and hospital costs by ±25% also showed little variation (−7 to 6% versus FF/VI and −1 to 1% versus UMEC/VI). The greatest impact was observed by varying the predicted utility value in each cycle by 10%, which resulted in a 9% ICER decrease versus both dual therapies.
Results of the scenario analyses were also consistent with the base case, with FF/UMEC/VI remaining cost-effective versus FF/VI across all scenarios (Figure 2B; Table S4): the ICER ranged from dominant to C$23,800/QALY. This was also the case versus UMEC/VI (Figure 4B; Table S5), with the ICER ranging from dominant to C$21,818/QALY. In the two scenarios run for treatment discontinuation inputs, a 2% reduction in the ICER versus both comparators was observed when discontinuation was assumed as 0% after the first year. When the treatment effect for subsequent therapy was assumed to be that of FF/UMEC/VI, a 21% increase in the ICER was observed versus FF/VI (C$23,025/QALY) and a 44% increase in the ICER was observed versus UMEC/VI (C$19,880/QALY).
Varying the background exacerbation rate by 50% (of that predicted by the risk equation) resulted in a 10% increase in the ICER versus FF/VI and a 58% increase versus UMEC/VI. When the background exacerbation rate was increased by 200% (ie to approximately three exacerbations PPPY), FF/UMEC/VI was a dominant (more effective and at lower costs) option versus both comparators.
Changing the time horizon showed a substantial impact only when reduced to 5 years, resulting in a 25% increase in the ICER compared with the lifetime horizon versus FF/VI (C$23,800/QALY) and a 26% increase in the ICER compared with the lifetime horizon versus UMEC/VI (C$17,420/QALY). Variation in the ICER relative to the base case was minimal with a 10-year time horizon versus both comparators (3% change [C$19,551/QALY] versus FF/VI and 1% change [C$13,946/QALY] versus UMEC/VI).
Discussion
This cost-effectiveness study from the Canadian public healthcare payer perspective used the GALAXY model to predict disease progression and outcomes, based on pooled patient characteristics and relative treatment effects for FF/UMEC/VI versus FF/VI or UMEC/VI from IMPACT.10 Treatment with FF/UMEC/VI resulted in increased clinical benefit and health outcomes (reduction in exacerbations, gains in LYs and QALYs) with a moderate cost increment. Costs were greater with FF/UMEC/VI, mainly due to increased medication costs relative to both dual therapies. Compared with UMEC/VI, these were offset by lower non-drug costs with FF/UMEC/VI due to fewer severe exacerbations and associated hospital days. Non-drug costs were higher for FF/UMEC/VI versus FF/VI, partly because the exacerbation benefit for FF/UMEC/VI versus FF/VI was less than that versus UMEC/VI, but also because the greater FEV1 benefit observed with FF/UMEC/VI led to improved survival and thus a higher overall lifetime cost, due to prolonged utilization of healthcare resources. These analyses suggest that the higher acquisition cost of FF/UMEC/VI could be partially offset by savings elsewhere in the health service. Over a lifetime horizon, the probability that treatment with FF/UMEC/VI would be cost-effective versus UMEC/VI or FF/VI was 100% at a willingness-to-pay threshold of C$50,000/QALY.
FF/UMEC/VI remained cost-effective across all sensitivity and scenario analyses, suggesting that the results are robust when considering a number of uncertainties. In particular, cost-effectiveness was maintained in scenarios with higher and lower exacerbation rates, an important observation in the context of real-world populations where rates may be variable. For example, annual exacerbation rates in North American populations have been reported to range widely from 0.47 to 4.22.29 It is also important to note that the baseline values for estimated parameters such as 6MWT, fibrinogen level, and mMRC dyspnea score had a minimal influence on cost-effectiveness. Here, the mMRC dyspnea score was estimated in line with patient CAT scores from the IMPACT trial and, although there is more than one view regarding the equivalence of these parameters,1,26 the negligible deviation noted in sensitivity analyses when varying mMRC dyspnea score by ±25% suggests that the results would not have been significantly different had alternative equivalence cut-offs been used.
Data on long-term discontinuation rates were not available and the assumption in the base case that discontinuation rates for FF/UMEC/VI in the first year were maintained in subsequent years is probably an overestimate. We therefore tested alternative assumptions in scenario analyses. Against both comparators, a 2% reduction in the ICER was observed when the discontinuation rate was set to 0% in subsequent years, since maintaining the relative treatment effect for FF/UMEC/VI for the treatment duration increased the incremental LYs and QALYs. However, the impact of discontinuation on treatment outcomes and cost-effectiveness is heavily influenced by the assumed effect of subsequent therapy and its costs. In the base case, treatment effect was assumed to be similar to the reference treatment but, in IMPACT, dual therapy had higher discontinuation rates than FF/UMEC/VI,10 and most of the patients who discontinued any treatment subsequently received triple therapy [data on file]. Thus, it is clinically plausible that patients who discontinued may have derived greater benefit with subsequent therapy than was assumed in the base case analysis, which would decrease the incremental LYs and QALYs gained for FF/UMEC/VI versus FF/VI or UMEC/VI. This was tested in the scenario analysis where the effect of subsequent therapy was assumed to be similar to FF/UMEC/VI; ICERs increased versus both FF/VI (21%) and UMEC/VI (44%) but FF/UMEC/VI remained cost-effective.
It should be noted that this analysis did not examine the effects of baseline eosinophil levels on cost-effectiveness of treatment, as the annual rate of moderate or severe exacerbations was lower with triple therapy than dual therapy regardless of eosinophil level in the prespecified IMPACT analysis. Furthermore, Canadian guidelines do not currently consider eosinophil count in their recommendations.6 While FF/UMEC/VI was associated with clinical benefit irrespective of baseline eosinophil count (<150 or ≥150 cells/µL) in the IMPACT trial,10 discussions surrounding the most appropriate cut-off points for eosinophil count analyses are still evolving and warrant further investigation before baseline eosinophil count can be confidently incorporated into cost-effectiveness models.
Currently, there is little evidence available evaluating the pharmacoeconomic value of inhaled triple therapy for COPD maintenance in Canada. Although several other models are available for COPD economic analysis, these are structured around disease “states” typically described in terms of predicted FEV1 and, only in some cases, exacerbations.13,30–34 The advantage of the GALAXY model for estimating cost-effectiveness is that it incorporates a broad range of disease attributes, including patient baseline characteristics, exacerbation frequency, symptom frequency, and exercise capacity, all of which can influence long-term outcomes.11 This better encompasses COPD’s multifactorial nature.35,36 Using IMPACT data,10 which evaluated effects of all three comparators over 52 weeks, was another advantage of this analysis; this is the same duration as the cycle length used in the GALAXY model11 and provided a strong basis for extrapolation beyond the first year. Additionally, using direct evidence from the large, landmark IMPACT trial ensured the results were not subject to the uncertainties introduced by including treatment effects from differing populations, as they would have been in a network meta-analysis.
There are some limitations to this study that should be considered when interpreting its results. Firstly, it must be noted that pneumonia events are not explicitly modeled in GALAXY, although COPD-related HRU (including that related to pneumonia) is predicted by the risk equations. Although a recent analysis of IMPACT data demonstrated a favorable benefit/risk profile for FF/UMEC/VI versus FF/VI or UMEC/VI when considering the risk of pneumonia and reduction in exacerbations as a composite outcome,37 explicit exploration of the impact of pneumonia should be considered in future health economic analyses.
Secondly, it is important to clarify that the baseline utility value specific to the Canadian population was unavailable for this analysis, and it may have differed from the model-predicted value. Sensitivity analyses that represented a higher population baseline value by increasing the utility value by 10% in each model cycle, however, did not change the analysis conclusions, indicating that baseline utility was not a model driver.
Thirdly, due to COPD’s chronic nature, a lifetime horizon was used for the base case; however, considering shorter timeframes may also be appropriate, partly because discontinuation rates, like those observed in IMPACT,10 suggest that a high proportion of patients receive alternative therapies in the longer term. Analysis of real-world evidence may be prudent to gain a deeper understanding of these treatment patterns. Scenario analyses incorporating 5- and 10-year time horizons were generally consistent with the base-case results; there was minimal variation in the ICER with a 10-year time horizon. FF/UMEC/VI also remained cost-effective versus FF/VI or UMEC/VI when the time horizon was reduced to 5 years, although the ICER increased by 25% and 26% versus FF/VI and UMEC/VI, respectively, compared with the base case. This suggests that not all health effects and cost consequences were captured within this short timeframe.
Additionally, as the ELLIPTA inhaler was used to administer treatment in all three study arms of the IMPACT trial (a key strength of its design), it was not possible to evaluate the impact of single versus multiple inhalers or once-daily versus more frequent dosing on treatment adherence, and so this aspect of effectiveness could not be incorporated into the economic model. SITT may lead to improved patient satisfaction and adherence, and therefore potentially improved effectiveness, versus MITT: number of inhalers has recently been demonstrated as a driver of choice for COPD treatment.38 This is an important consideration in real-world scenarios and a future economic evaluation of SITT versus MITT, incorporating the impact of differential treatment adherence, is warranted. FF/UMEC/VI is the first SITT approved for long-term, COPD maintenance therapy in Canada, in patients whose symptoms are not adequately controlled by a combination of ICS and LABA. FF/UMEC/VI enables patients to benefit from maintenance therapy with ICS/LAMA/LABA delivered in a single, once-daily inhalation.
Finally, the Canadian list price of FF/UMEC/VI was not available at the time of these analyses and so the price was set to equal the sum of the respective prices for UMEC and FF/VI. Any reduction in the price of FF/UMEC/VI relative to the sum of its components may lead to a more favorable ICER compared with the results currently presented.
This study was from the perspective of the public healthcare payer in Canada only, and the cost-effectiveness of triple therapy with FF/UMEC/VI for COPD may differ in other regions and countries. However, a UK-based economic evaluation of FULFIL data using the GALAXY model found FF/UMEC/VI to be a cost-effective option when compared with another dual therapy, budesonide/formoterol, for the treatment of symptomatic COPD.39 These results suggest that FF/UMEC/VI could be a cost-effective option across a variety of payer systems internationally, although confirmatory research in more countries is required.
Conclusion
FF/UMEC/VI is predicted to be cost-effective in Canada for the treatment of patients with symptomatic COPD and a history of exacerbations. FF/UMEC/VI was more effective than FF/VI or UMEC/VI, resulting in improved health outcomes versus both comparators. Although the cost of FF/UMEC/VI was higher than that of FF/VI or UMEC/VI, the finding that FF/UMEC/VI was cost-effective versus both comparators over a lifetime horizon, and in numerous sensitivity and scenario analyses, indicates that this may be an appropriate investment of health service funds in Canada.
Acknowledgments
An abstract of the FF/UMEC/VI versus FF/VI data from this paper was presented at the ERS 2018 conference as a poster presentation with interim findings. The poster’s abstract was published in the European Respiratory Journal: https://erj.ersjournals.com/content/52/suppl_62/PA3154. An additional abstract of the FF/UMEC/VI versus UMEC/VI data from this paper was presented at the ISPOR EU 2018 conference as a poster presentation with interim findings. The poster’s abstract was published in Value in Health: https://doi.org/10.1016/j.jval.2018.09.2451. Trademarks are the property of their respective owners. Editorial support (in the form of writing assistance, collating author comments, assembling tables/figures, grammatical editing and referencing) was provided by Molly Macpherson, BSc, of Gardiner–Caldwell Communications (Macclesfield, UK), and was funded by GlaxoSmithKline plc. This study was funded by GlaxoSmithKline plc (IMPACT ClinicalTrials.gov number, NCT02164513; CTT116855).
Abbreviations
6MWT, 6-min walk test; C$, Canadian dollars; CADTH, Canadian Agency for Drugs and Technology in Health; CAT, COPD Assessment Test; CI, confidence interval; COPD, chronic obstructive pulmonary disease; ECLIPSE, Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points; ER, emergency room; FEV1, forced expiratory volume in 1 s; FF, fluticasone furoate; HRQoL, health-related quality of life; ICER, incremental cost-effectiveness ratio; ICS, inhaled corticosteroid; IMPACT, InforMing the PAthway of COPD Treatment; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; LY, life year; mMRC, modified Medical Research Council; ODB, Ontario Drug Benefit; QALY, quality-adjusted life year; SITT, single-inhaler triple therapy; SGRQ, St George’s Respiratory Questionnaire; SGRQ-C, SGRQ for COPD patients; TORCH, Towards a Revolution in COPD Health; UMEC, umeclidinium; VI, vilanterol.
Disclosure
The authors declare the following conflicts of interest during the last three years in relation to this article: A.S. Ismaila, M. Schroeder and A. Martin are employees of, and hold shares in, GlaxoSmithKline plc.; A.S. Ismaila is also an unpaid, part-time professor at McMaster University, Canada. E.C. Goodall is an employee of GlaxoSmithKline plc. N. Risebrough, D. Shah and K. Ndirangu are employees of ICON plc., which received funding from GlaxoSmithKline plc. to conduct this study, but were not themselves paid for development of this article. G. Criner reports grants and personal fees from GlaxoSmithKline plc., Boehringer Ingelheim, Chiesi, Mereo, AstraZeneca, Pulmonx, PneumRx, Olympus, Broncus, Lungpacer, Mereo, Nuvaira, ResMed, Respironics and Patara, Pearl, and Sanofi, personal fees from Verona, BTG, EOLO and NGM, and grants from ALung, Fisher Paykel and Galapagos, outside the submitted work. M. Dransfield reports personal and other fees from GlaxoSmithKline plc. during the conduct of this study, and grants from Department of Defense, personal and other fees from Boehringer Ingelheim, GlaxoSmithKline plc., AstraZeneca and PneumRx/BTG, personal fees from Genentech, Quark Pharmaceuticals and Mereo, grants from NIH and American Lung Association and other fees from Novartis, Yungjin, Pulmonx and Boston Scientific, outside the submitted work. D.M.G. Halpin reports personal fees from AstraZeneca, Chiesi, GlaxoSmithKline plc. and Pfizer, and personal fees and non-financial support from Boehringer Ingelheim and Novartis, outside the submitted work. M.L. Han reports personal fees from GlaxoSmithKline plc. during the conduct of this study, and personal fees from GlaxoSmithKline plc., Boehringer Ingelheim, AstraZeneca, Mylan, and other fees from Novartis and Sunovion, outside of the submitted work. D.A. Lomas reports grants, personal fees and non-financial support from GlaxoSmithKline plc. during the conduct of the study, and personal fees and grants from GlaxoSmithKline plc., and personal fees from Griffols, outside of the submitted work. D.A. Lomas also chaired the Respiratory Therapy Area Board at GlaxoSmithKline plc. between 2012 and 2015. The authors report no other conflicts of interest in this work.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. [updated 2019]. Available from: https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf Accessed January14, 2019.
- 2.Ehteshami-Afshar S, FitzGerald JM, Doyle-Waters MM, et al. The global economic burden of asthma and chronic obstructive pulmonary disease. Int J Tuberc Lung Dis. 2016;20:11–23. doi: 10.5588/ijtld.15.0472 [DOI] [PubMed] [Google Scholar]
- 3.Canadian Institute for Health Information (CIHI) [homepage on the Internet]. Hospital stays in Canada. 2017. Available from: https://www.cihi.ca/en/hospital-stays-in-canada Accessed January14, 2019.
- 4.Statistics Canada. Table 13-10-0394-01: leading causes of death, total population, by age group. 2019. Available from: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310039401 Accessed January14, 2019.
- 5.Dang-Tan T, Ismaila A, Zhang S, et al. Clinical, humanistic, and economic burden of chronic obstructive pulmonary disease (COPD) in Canada: a systematic review. BMC Res Notes. 2015;8:464–487. doi: 10.1186/s13104-015-1427-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourbeau J, Bhutani M, Hernandez P, et al. CTS position statement: pharmacotherapy in patients with COPD—an update. Can J Respir Crit Care Sleep Med. 2017;1:222–241. doi: 10.1080/24745332.2017.1395588 [DOI] [Google Scholar]
- 7.Driessen M, Shah D, Risebrough N, et al. Cost-effectiveness of umeclidinium as add-on to ICS/LABA therapy in COPD: a UK perspective. Respir Med. 2018;145:130–137. doi: 10.1016/j.rmed.2018.10.024 [DOI] [PubMed] [Google Scholar]
- 8.Gaduzo S, McGovern V, Roberts J, et al. When to use single-inhaler triple therapy in COPD: a practical approach for primary health care professionals. Int J COPD. 2019;14:391–401. doi: 10.2147/COPD.S173901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Health Canada. TRELEGY ELLIPTA product monograph. April 2018. Available from: http://ca.gsk.com/media/1385746/trelegy-ellipta_pm-2018-04-03.pdf Accessed January14, 2019.
- 10.Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378:1671–1680. doi: 10.1056/NEJMoa1713901 [DOI] [PubMed] [Google Scholar]
- 11.Briggs AH, Baker T, Risebrough NA, et al. Development of the Galaxy Chronic Obstructive Pulmonary Disease (COPD) model using data from ECLIPSE: internal validation of a linked-equations cohort model. Med Decis Making. 2017;37:469–480. doi: 10.1177/0272989X16653118 [DOI] [PubMed] [Google Scholar]
- 12.Risebrough NA, Briggs A, Baker TM, et al. Validating a model to predict disease progression outcomes in patients with COPD. Value Health. 2014;17:A560–A561. doi: 10.1016/j.jval.2014.08.1852 [DOI] [PubMed] [Google Scholar]
- 13.Hoogendoorn M, Feenstra TL, Asukai Y, et al. External validation of health economic decision models for chronic obstructive pulmonary disease (COPD): report of the third COPD modeling meeting. Value Health. 2017;20:397–403. doi: 10.1016/j.jval.2016.10.016 [DOI] [PubMed] [Google Scholar]
- 14.Exuzides A, Colby C, Briggs AH, et al. Statistical modeling of disease progression for chronic obstructive pulmonary disease using data from the ECLIPSE study. Med Decis Making. 2017;37:453–468. doi: 10.1177/0272989X15610781 [DOI] [PubMed] [Google Scholar]
- 15.Tabberer M, Gonzalez-McQuire S, Muellerova H, et al. Development of a conceptual model of disease progression for use in economic modeling of chronic obstructive pulmonary disease. Med Decis Making. 2017;37:440–452. doi: 10.1177/0272989X16662009 [DOI] [PubMed] [Google Scholar]
- 16.Vestbo J, Anderson W, Coxson HO, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE). Eur Respir J. 2008;31:869–873. doi: 10.1183/09031936.00111707 [DOI] [PubMed] [Google Scholar]
- 17.Agusti A, Calverley PM, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11:122. doi: 10.1186/1465-9921-11-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775–789. doi: 10.1056/NEJMoa063070 [DOI] [PubMed] [Google Scholar]
- 19.Starkie HJ, Briggs AH, Chambers MG, et al. Predicting EQ-5D values using the SGRQ. Value Health. 2011;14:354–360. doi: 10.1016/j.jval.2010.09.011 [DOI] [PubMed] [Google Scholar]
- 20.Ontario Drug Benefit Formulary. Comparative drug index (effective December 2017). Available from: https://www.formulary.health.gov.on.ca/formulary/ Accessed January14, 2019.
- 21.IMS Brogan. ODB RxDynamics® Database. Respiratory Therapies 2017. [data on file]. [Google Scholar]
- 22.Canadian Institute for Health Information (CIHI). Care in Canadian ICUs. 2016. Available from: https://www.cihi.ca/en/ICU_DataTables_EN.xlsx Accessed January14, 2019.
- 23.Ontario Health Insurance Plan (OHIP) – Schedule of benefits. 2016. Available from: http://www.health.gov.on.ca/en/pro/programs/ohip/sob/ Accessed January14, 2019.
- 24.Statistics Canada. Consumer price index. 2017. Available from: http://statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/cpis01a-eng.htm Accessed January14, 2019.
- 25.Payscale. Registered Nurse wage. 2017. Available from: http://www.payscale.com Accessed January14, 2019.
- 26.Jones PW, Adamek L, Nadeau G, et al. Comparisons of health status scores with MRC grades in COPD: implications for the GOLD 2011 classification. Eur Respir J. 2013;42:47–654. doi: 10.1183/09031936.00125612 [DOI] [PubMed] [Google Scholar]
- 27.Canadian Agency for Drugs and Technology in Health. Guidelines for the Economic Evaluation of Health Technologies: Canada - 4th Edition. Ottawa: CADTH; 2017. Available from: https://www.cadth.ca/sites/default/files/pdf/guidelines_for_the_economic_evaluation_of_health_technologies_canada_4th_ed.pdf. Accessed January 14, 2019. [Google Scholar]
- 28.Rutten-van Mölken MP, Oostenbrink JB, Tashkin DP, et al. Does quality of life of COPD patients as measured by the generic EuroQol five-dimension questionnaire differentiate between COPD severity stages? Chest. 2006;130:1117–1128. doi: 10.1378/chest.130.4.1117 [DOI] [PubMed] [Google Scholar]
- 29.Sadatsafavi M, Sin DD, Zafari Z, et al. The association between rate and severity of exacerbations in chronic obstructive pulmonary disease: an application of a joint frailty-logistic model. Am J Epidemiol. 2016;184:681–689. doi: 10.1093/aje/kww085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spencer M, Briggs AH, Grossmann RF, et al. Development of an economic model to assess the cost effectiveness of treatment interventions for chronic obstructive pulmonary disease. PharmacoEconomics. 2005;23:619–637. doi: 10.2165/00019053-200523060-00008 [DOI] [PubMed] [Google Scholar]
- 31.Starkie HJ, Briggs AH, Chambers MG. Pharmacoeconomics in COPD: lessons for the future. Int J Chron Obstruct Pulmon Dis. 2008;3:71–88. [PMC free article] [PubMed] [Google Scholar]
- 32.Mittmann N, Hernandez P, Mellstrom C, et al. Cost effectiveness of budesonide/formoterol added to tiotropium bromide versus placebo added to tiotropium bromide in patients with chronic obstructive pulmonary disease: Australian, Canadian and Swedish healthcare perspectives. Pharmacoeconomics. 2011;29:403–414. doi: 10.2165/11590380-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 33.Hoogendoorn M, Rutten van Mölken MO, Hoogenveen RT. Developing and applying a stochastic dynamic population model for chronic obstructive pulmonary disease. Value Health. 2011;14:1039–1047. doi: 10.1016/j.jval.2011.06.008 [DOI] [PubMed] [Google Scholar]
- 34.Hoogendoorn M, Feenstra TL, Asukai Y, et al. Cost-effectiveness models for chronic obstructive pulmonary disease: cross model comparison of hypothetical treatment scenarios. Value Health. 2014;17:525–536. doi: 10.1016/j.jval.2014.03.1721 [DOI] [PubMed] [Google Scholar]
- 35.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–1012. doi: 10.1056/NEJMoa021322 [DOI] [PubMed] [Google Scholar]
- 36.Briggs A, Spencer M, Wang H, et al. Development and validation of a prognostic index for health outcomes in chronic obstructive pulmonary disease. Arch Intern Med. 2008;168:71–79. doi: 10.1001/archinternmed.2007.37 [DOI] [PubMed] [Google Scholar]
- 37.Dransfield M, Crim C, Criner G, et al. Risk of exacerbation and pneumonia with single inhaler triple versus dual therapy in IMPACT. Thorax. 2018;73:A236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schroeder M, Lewis H, Doll H, et al. Evaluating patient preferences of maintenance therapy for the treatment of chronic obstructive pulmonary disease in the UK: a discrete choice experiment. Thorax. 2018;73:A232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schroeder M, Shah D, Risebrough N, et al. Cost-effectiveness analysis of a single-inhaler triple therapy for patients with advanced chronic obstructive pulmonary disease (COPD) using the FULFIL trial: a UK perspective. Respir Med X. 2019;1:100008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. [updated 2019]. Available from: https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf Accessed January14, 2019.