Abstract
The recall of a previously formed fear memory triggers a process through which synapses in the amygdala become “destabilized”. This labile state at retrieval may be critical for the plasticity required to modify, update, or disrupt long-term memories. One component of this process involves the rapid internalization of calcium impermeable AMPA receptors (CI-AMPAR). While some recent work has focused on the details of modifying amygdala synapses, much less is known about the environmental factors that control memory updating and the important circuit level processes. Synchrony between the hippocampus and amygdala increases during memory retrieval and stable memories can sometimes be made labile with hippocampal manipulations. Recent work shows that memory lability at retrieval is influenced by the novelty of the retrieval environment, and detection of this novelty likely relies on the dorsal hippocampus (DH). Our goal was to determine how local activity in the DH contributes to memory lability and synaptic destabilization in the amygdala during retrieval when contextual novelty is introduced. We found that contextual novelty during retrieval is necessary for alterations in amygdala activity and CI-AMPAR internalization. In the absence of novelty, suppression of local activity in the DH prior to learning allowed for retrieval-dependent CI-AMPAR internalization in the amygdala. We next tested whether the changes in AMPAR internalization were accompanied by differences in memory lability. We found that a memory was made labile when activity within the DH was disrupted in the absence of contextual novelty. These results suggest that the DH is important for encoding contextual information during learning that regulates retrieval-dependent memory modification in the amygdala.
Introduction
Maladaptive fear expression, such as heightened responding to danger cues, is commonly seen in anxiety disorders (Parsons & Ressler, 2013). Exposure-based therapies can be used as a treatment option but require multiple recall sessions to diminish fear responding and are not always effective at preventing the return of fear (Maren & Holmes, 2016). Targeting the direct modification of the original fear memory may provide a more robust and long-lasting change in behavior. When a simple fear memory is recalled it triggers a time-dependent process often called “reconsolidation” during which the memory can be modified (Misanin et al., 1968; Nader et al., 2000). Reconsolidation provides an opportunity to incorporate new information into the original memory trace, providing a unique and therapeutically relevant opportunity to modify maladaptive fear responding (Brunet et al., 2008; Hupbach et al., 2007).
Reconsolidation can be characterized based on temporary phases of synaptic destabilization and restabilization. Destabilization is essential for the induction of plasticity necessary for synaptic reorganization and memory lability, while restabilization requires protein synthesis for synaptic restrengthening in order to reflect new information included into the original memory trace (Hong et al., 2013; Li et al., 2013; Milton et al., 2013). Plasticity underlying the destabilization phase of reconsolidation is dependent on calcium influx at synapses important for memory storage, with calcium-related activity being associated with a high degree of plasticity and subsequent memory lability (Hong et al., 2013; Milton et al., 2013). The insertion of calcium impermeable-AMPARs (CI-AMPARs) into the synapse following an initial learning experience reduces calcium influx into synapses, which is associated with stability and persistence of the memory over time (Joels & Lamprecht, 2010; Migues et al., 2010; Migues et al., 2016). Shortly following a memory retrieval session, CI-AMPAR are transiently internalized to allow for the postsynaptic plasticity necessary for synaptic destabilization (Hong et al., 2013; Rao-Ruiz et al., 2011). Synaptic restabilization during reconsolidation is associated with the synthesis of new proteins, presumably replacing those that undergo reorganization and degradation during destabilization (Jarome et al., 2011; Lee et al., 2008). Inhibition of protein synthesis with amnesic agents like anisomycin has been commonly used to disrupt restabilization during reconsolidation and results in a disrupted memory trace and fewer AMPARs present in the synapse (Jarome et al., 2011; Jarome et al., 2012; Li et al., 2013; Lopez et al., 2015; Nader et al., 2000). Thus, CI-AMPARs provide essential markers for reconsolidation events that regulate memory lability.
Several factors present during learning and fear recall are known to influence memory lability and synaptic destabilization during reconsolidation. For example, multiple recall events presented in close temporal proximity have been known to alter memory lability, as measured by the necessity for protein synthesis during fear reconsolidation (Jarome et al., 2012). Additionally, the presence of new (i.e., different from those of the initial learning experience) environmental elements during fear retrieval provide additional information that can then initiate synaptic destabilization and allow for memory lability (Lee et al., 2008; Winters et al., 2009). These factors include novelty of the retrieval context (the chamber in which retrieval takes place), which is essential for reconsolidation-dependent memory lability and synaptic destabilization during reconsolidation (Jarome et al., 2015). More specifically, preexposure to the retrieval conditions is sufficient to prevent reconsolidation-dependent updating, as indicated by reduced CI-AMPAR trafficking and prevention of anisomycin-related memory impairment (Jarome et al., 2015). Brain regions encoding contextual information during fear memory formation and recall may therefore be important in influencing the process of fear memory reconsolidation.
The DH is a critical region for encoding contextual information and interacts with the amygdala during fear memory formation and retrieval (Ikegaya et al., 1995; McIntyre et al., 2005; McReynolds et al., 2010). DH plasticity has been reported in response to cued fear retrieval and may therefore be important for memory lability in the amygdala during retrieval (Sanders et al., 2003; Seidenbecher et al., 2003). Interestingly, auditory memories that are resistant to impairment with amygdalar anisomycin infusions during retrieval can be made labile with DH lesions (Wang et al., 2009). This highlights an important role for contextual information, regulated by the DH, in the long-term storage and lability of fear memories in the amygdala. While evidence suggests an interaction between the DH and amygdala during standard auditory fear memory retrieval, how the DH may influence later recall of a cued fear memory is unclear. One possibility is that contextual information processed by the DH during learning regulates the ability of an auditory fear memory to become labile during retrieval (Jarome et al., 2015).
The goal of the present experiments was to directly test whether contextual novelty during auditory fear memory retrieval is necessary for memory lability, as indicated by the requirement for protein synthesis in the amygdala. Groups of rats received auditory fear conditioning and memory retrieval in either the acquisition context or a novel context. Here, we have defined contextual novelty as a context to which animals had not been exposed. (detailed description of the differences between chambers can be found in the methods section). Because internalization of CI-AMPAR is necessary for memory lability during retrieval, we wanted to determine whether contextual novelty regulates AMPAR trafficking during retrieval. We found that inactivation of the DH during training, but not during retrieval, allows for internalization of AMPAR in the amygdala when the context is not novel. We then manipulated contextual novelty in a series of behavioral experiments where infusions of anisomycin were delivered into the amygdala immediately following retrieval. Consistent with previous work, we show contextual novelty is critical for memory susceptibility to anisomycin-induced impairment following a retrieval session, and DH inactivation during training allowed for amygdala anisomycin impairments during retrieval. These results suggest contextual novelty, as processed by the hippocampus during memory retrieval, is critical for triggering memory lability and destabilization of amygdala synapses.
Methods
Subjects
Subjects were male Long-Evans rats from Envigo (n = 149; Indianapolis, IN) weighing approximately 350g at the time of arrival. Rats were individually housed with free access to water and rat chow. The animal colony was maintained at a 14:10-hr light/dark cycle with all experiments occurring under the light portion of the cycle. All experiments were approved by the University of Wisconsin-Milwaukee’s Institutional Animal Care and Use Committee.
Surgery
Immediately before surgery, rats were anesthetized with 4% isoflurane and oxygen, and after induction, isoflurane levels were maintained at 2 – 2.5% throughout the surgery. Bilateral cannulae targeted the amygdala at a 10° lateral angle (−3.0 mm posterior, +/−6.5 mm lateral, −7.6 mm ventral) and DH (−3.6 mm posterior, +/−2.6 mm lateral, −2.0 mm ventral) according to bregma (Paxinos & Watson, 2007), please see supplemental Figures 4 and 5 for cannula placements and example images. Cannula were secured to the skull with four screws and surrounded by acrylic cement. Rats were given a minimum of 7 days after surgery to recover before behavioral training and testing.
Apparatus
Auditory fear conditioning was conducted in a set of four Plexiglas and stainless steel chambers within sound-attenuating boxes (Context A). The floor contained 18 stainless steel bars connected to a shock generator (Coulbourn Instruments, Allentown, PA). Each chamber had a speaker to allow delivery of the white noise conditional stimulus (CS), overhead illumination with a 7.5 W bulb, and ventilation fans to provide a constant background noise (55 dB). The chambers were cleaned with 5% ammonium hydroxide solution between sets of rats. A set of similar chambers designated Context B served as a novel context for auditory CS testing in some conditions. Context B has several distinct features including Plexiglas flooring, lack of overhead illumination, opaque chamber top, 5% acetic acid cleaning solution, and substantially different chamber walls.
Drug preparation and infusion
Animals were adapted to transport and handling procedures for 3 days before training. This included gentle restraint and exposure to the sound of the infusion pump. Drugs were prepared on the day of infusion. Groups received bilateral microinjections of lidocaine (40 μg/μl, Sigma), or vehicle (sterile saline) at a rate of 0.5 μl/min and at a volume of 0.5 μl/hemisphere into the DH 5-min prior to training or retrieval (Chang et al., 2008; van Duuren et al., 2007). Amygdala injections occurred immediately following a retrieval session (Anisomycin: 125 μg/μl, or ACSF vehicle) (Jarome et al., 2012; Jarome et al., 2015). Drugs were infused through 33-ga injection cannulae extending 0.5–0.7 mm beyond the guide cannulae. Injectors remained in place for 90s following infusion to ensure drug diffusion.
Behavioral procedures
Rats were placed in Context A for auditory fear conditioning. During training, rats were placed into the chamber and after a 6-min BL period, four white noise presentations (72dB, 10s) that were paired with a footshock (1s, 1.0mA). The average inter-trial interval between each tone presentation was 110s, ranging from 90–130s. Rats remained in the chamber for an additional 4-min period following the final CS-UCS pairing. The total training session duration was 970-sec. Auditory CS retrieval and testing sessions took place in either Context A or B where rats received four discrete tone presentations of the CS (30-sec; 60-sec ITI) after a 4-min baseline. Aside from several distinct features, Context A and B chambers were also located in different rooms and were completely separate. Because of this, we have referred to the altered chambers in which training did not occur in as novel. Freezing was defined as the cessation of all movement excluding respiration (Fanselow, 1980) and was automatically scored in real-time with FreezeScan 1.0 detection software (Clever Sys, Inc., Reston, VA), which was calibrated to a trained human observer.
Synaptosomal membrane preparation
Animals were deeply anesthetized with isoflurane 90-min following a retrieval session. Brains were immediately removed, flash frozen with dry ice, and stored at −80°C until dissected. Crude synaptosomal fractions were obtained as previously described (Ferrara et al., 2017; Jarome et al., 2011). Amygdalae were dissected out and homogenized in TEVP buffer with 320mM sucrose and then centrifuged at 1000x g for 10-min. The supernatant was removed and centrifuged at 10,000 × g for 10-min, and the remaining pellet was denatured in lysis buffer (all in 100 ml DDH20; 0.605 g Tris-HCl, 0.25 g sodium deoxycholate, 0.876 g NaCl, 1 μg/ml PMSF, 1 μg/ml leupeptin, 1 μg/ml aprotinin, 10 ml 10% SDS). Protein levels were measured with a protein assay kit (Bio-Rad laboratories, Hercules, CA, USA).
Western blotting
Rats were sacrificed at 90-min following the retrieval session. Following synaptosomal preparation, protein levels were normalized and loaded onto an SDS/PAGE gel and then to a membrane using a transfer apparatus (Bio-Rad). Membranes were incubated in blocking buffer for 1-hr before being incubated in GluR1 (Cell Signaling, 1:1000), GluR2 (Santa Cruz, 1:500), or βactin (Cell Signaling, 1:1000) primary solutions overnight at 4 °C. Membranes were then incubated in the appropriate secondary (Santa Cruz, 1:20,000) antibody for one hour and prepped in a chemiluminescence solution for 3-min. Images were captured using a camera-based system (GBOX Chemi XT-4, Syngene) and densitometry performed using NIH Genesys.
Immunofluorescence
Animals were deeply anesthetized with isoflurane 90-min following retrieval. Brains were immediately removed and stored at −80°C until sliced. Brains were sliced in 20-micron serial sections and were mounted onto charged slides. Tissue sections were rehydrated in wash buffer (PBS + 0.05% Tween-20) and permeabilized (PBS + 0.3% Triton X) for 15-min and incubated in blocking solution (PBS + 0.7% NGS). Slides were then incubated in zif 268/EGR1 antibody (Cell Signaling, 1:500, #4153) solution (PBS + 0.3% Triton X + 5% NGS) overnight at 4 °C. The next day, slides were incubated in secondary antibody solution for 2-hr and rinsed with wash buffer, a DAPI counterstain was applied, and slides were cover slipped.
Immunofluorescence microscopy and quantification
Specific anatomical locations were identified and verified using a rat brain atlas (Paxinos & Watson, 2007). Amygdala images were captured on an Olympus Fluoview FV1200 confocal microscope using a 20x objective lens. Serial z-stack images covered a depth of 4.55μm through five consecutive sections (0.91μm per section) and were acquired using Fluoview software (Olympus). The LUT was linear and covered the full range of data for all quantified images (0–4095). Three amygdala sections were analyzed bilaterally and were averaged for each rat (6 sections matched along the anterior-posterior axis for each rat). A series of example images can be seen in supplemental Figure 3.
Images were exported as 12-bit TIFF files and particles were quantified using ImageJ software (NIH, Bethesda, MD, USA). Images were quantified by converting them to 32-bit, difference of Gaussian filtering (sigmas of 2 and 1.5), thresholding with the triangle method, and then counting particles greater than 4 pixels in diameter within the ROI. The “Classic Watershed Analysis” provided by ImageJ was also applied to images in order to separate zif268 neurons that were close in proximity. This results in a binary image with minimal background. All particle counts were averaged, bilaterally, across animals in each condition and normalized to the slices of animals infused with control virus using the “Analyze Particles” plugin in ImageJ. Based on size and circularity, particle measures were reported as a measure of cell counts and kept consistent within each experiment.
Statistical analyses
All statistical analyses and graphing were conducted in Prism 7 software (Graphpad, San Diego, CA). Western blot samples were normalized to actin levels and expressed as a percentage of no reactivation control groups. Behavioral and western blot statistical outliers were defined as any Z score > 2 (Field, 2005) and were excluded from all subsequent analyses. The data are presented as group averages with standard error of the mean (SEM). Behavioral experiments were analyzed using a two-way Analysis of Variance (ANOVA). Non-factorial designs were used for western blot groups in order to compare the “No Reactivation” group to treatment conditions, and tissue for immunofluorescent experiments were processed in pairs based on context manipulations. Based on this, western blot and immunofluorescent experiment comparisons were analyzed using a Student’s t-tests with the exception when Levene’s test was violated and the correct Welch statistic was used in place of the uncorrected t-test value. Western blot results in Figure 1 exclude nine rats, and Figure 2 western blot results exclude three rats based on the outlier criterion reported above. For behavioral results in Figure 3 b–d, ten rats were excluded based on missed histological placements and two statistical outliers were excluded. The reported results in Figure 3 f–h exclude eight rats based on histology and three outliers.
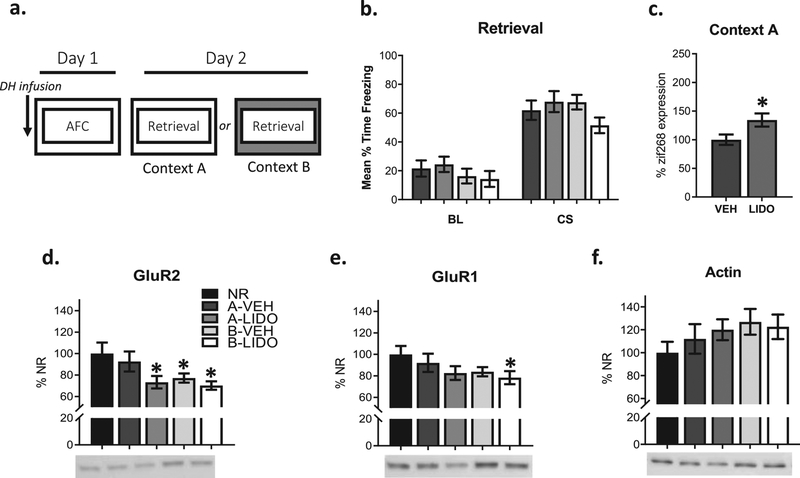
Figure 1: Training-related DH activity and contextual novelty regulate amygdala synaptic destabilization during fear retrieval.
Rats were infused with vehicle (VEH) or lidocaine (LIDO) in the DH prior to auditory fear conditioning (AFC). The next day, auditory fear retrieval occurred in the same context or a novel context to determine whether LIDO infusions into the DH during training influence cellular processes underlying synaptic destabilization, such as amygdala AMPA receptor internalization and increases in zif268 expression, in the absence of contextual novelty (a). There were no significant behavioral differences between groups during fear retrieval (b). LIDO infusions (n=3 rats) into the DH resulted in significantly greater amygdala zif28 protein expression when compared to a VEH (n=4 rats) infused group (c). A CS presentation in a novel context or DH LIDO infusions prior to training resulted in reduced GluR1 and GluR2 expression when compared to a No Reactivation (NR) control (NR n=15, Context A VEH = 15, Context A LIDO n=12; Context B VEH n=12, Context B LIDO n=11) (d-e). All protein measurements were normalized to actin and there were no significant actin differences between groups (f). *p < 0.05.
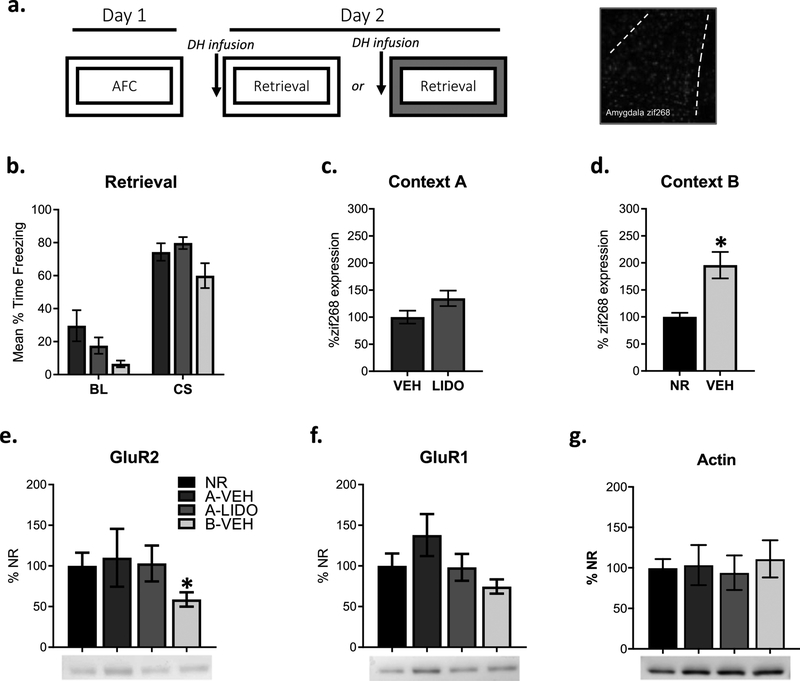
Figure 2: Amygdala synaptic destabilization during fear retrieval is regulated by contextual novelty but not DH activity.
Experimental design and representative amygdala zif268 image. Rats were trained with AFC. Groups were infused with VEH or LIDO into the DH prior to fear recall in the training context or in a novel context to determine whether a DH LIDO infusion prior to retrieval is sufficient for amygdala synaptic destabilization, evidenced by changes in zif268 expression and AMPA receptor internalization, when training and retrieval occur in the same context (a). There were no significant behavioral differences between groups during fear recall (b). There were no significant differences in zif268 expression between groups that received DH LIDO (n= 4 rats) or VEH (n= 4 rats) infusions prior to retrieval when training and retrieval occurred in the same context (c). Groups that received a VEH (n= 4 rats) infusion into the DH prior to retrieval show significantly higher zif268 expression when compared to a NR (n=4 rats) group when retrieval occurred in a novel context (d). GluR2 expression decreased from NR controls when fear recall occurred in a novel context but not when DH LIDO infusions occurred prior to fear recall in the same context (NR n=7, Context A VEH n= 6, Context A LIDO n= 7, Context B VEH n=7). (e). There were no significant differences in GluR1 or actin expression (f-g). *p < 0.05.
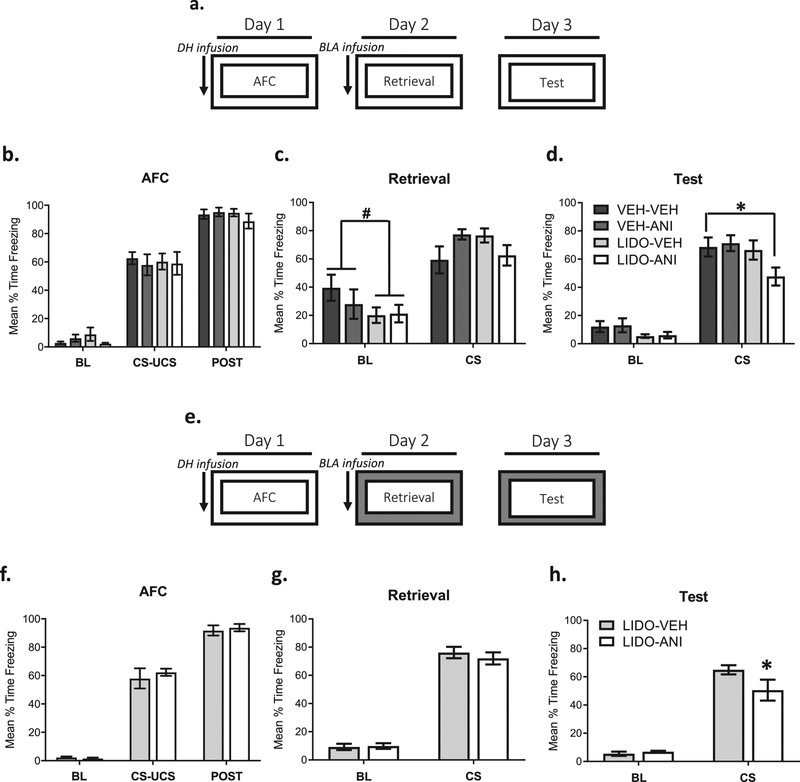
Figure 3: Memory lability at amygdala synapses is regulated by contextual novelty and DH activity during training.
Experimental design. Groups were infused with VEH or LIDO into the DH prior to AFC. Fear recall occurred in the training context, and groups received an infusion of VEH or anisomycin (ANI) into the amygdala immediately after fear recall (VEH-VEH n=9, VEH-LIDO n=9, LIDO-VEH n=8, LIDO-ANI n=7) The next day, groups were tested for fear retention in the context in which training and retrieval occurred. This will determine whether the changes in amygdala synaptic stability following retrieval are required for memory lability when the contextual conditions are manipulated (a). Groups were infused with LIDO or VEH into the DH prior to training, and there were no significant differences between groups during training (b&f). When LIDO and VEH infused groups are compared, there is a trend for a significant reduction during the baseline period but not auditory CS fear when groups received LIDO into the DH (c). Groups that received LIDO into the DH and anisomycin (ANI) into the amygdala froze significant less to the CS in comparison to the VEH-VEH condition during the CS presentation (d). Experimental design was as described in a, with the exception that all groups were infused with LIDO into the DH prior to AFC, and retrieval and testing occurred in a novel context (LIDO-VEH n=8, LIDO-ANI n=9) (e). There were no significant differences in baseline or auditory CS fear during retrieval in a novel context when groups received LIDO infusions into the DH prior to training (g). The group that received an amygdala ANI infusion immediately after retrieval froze significantly less to the CS than the VEH group during the test (h). #p = 0.06, *p < 0.05.
Results
DH activity during training regulates amygdala AMPA receptor trafficking and zif268 expression during reconsolidation in the absence of contextual novelty.
The trafficking of AMPA receptors at synapses in the amygdala following retrieval as well as increases in cellular activity and altered gene expression have been linked to memory lability and modification (Hong et al., 2013; Lee et al., 2008, Mamiya et al., 2009). Previous work shows a transient reduction in the presence of GluR2 subunits in the amygdala at 90-min following a retrieval session, and around this time point they are thought to be replaced with GluR2 lacking (GluR1) AMPA receptors (Jarome et al., 2015; Jarome 2012; Maddox et al., 2011; Plant et al., 2006). Based on this, we hypothesized that GluR2 expression in synaptosomal fractions would be reduced following memory recall in a novel context. Because changes in GluR1 expression are dependent on the exchange of CI-AMPAR and the chosen time point may not be best suited for detecting changes in GluR1 expression, these results may be more variable. Increases in zif268 expression have also been reported following fear retrieval and zif268 changes in the amygdala have been linked to memory lability following retrieval; therefore, zif268 was chosen as a measure for amygdala activity following recall for the following studies (Espejo et al., 2016; Ferrara et al., 2019; Lee 2008). Further, changes in AMPAR trafficking do not occur in the absence of contextual novelty (Jarome et al., 2015).
Because the DH encodes contextual information during auditory fear conditioning, and contextual novelty is required for CI-AMPAR internalization in the amygdala, we inactivated the DH region prior to learning to determine whether DH activity in the absence of contextual novelty regulates amygdala AMPA receptor trafficking during fear recall (Fig 1a). All groups showed similar levels of behavioral reactivation regardless of context as well as actin levels, which can be used as an accurate loading control when 2μg of total protein or less are used (Chen & Xu, 2015) (Fig 1b, 1f). Consistent with previous work (Jarome et al., 2015), we found reductions in amygdala GluR2 receptor subunits in synaptic fractions in comparison to a no reactivation (NR) control when the context was novel regardless of vehicle (t (25) = 2.256, p < 0.05) or lidocaine (t (24) = 2.696, p < 0.05) infusion into the DH (groups B-VEH and B-LIDO; Fig 1d). We also found a decrease in GluR1 synaptic expression when the context was novel and the DH was inactivated (t (25) = 2.134, p < 0.05; Fig 1e) and strong trend towards a decrease when vehicle was infused into the DH (t (25) = 1.774, p < 0.09; Fig 1e).
When the context remained the same between training and retrieval, there was no difference between the reactivation (A-VEH) and no reactivation (NR) group (t (28) = 0.532, p > 0.05; Fig 1d), supporting the idea that retrieval in a novel context is necessary for reductions in amygdala GluR2 synaptic expression during reconsolidation. However, when the DH was inactivated during training and the context was not changed between training and retrieval, there was a significant reduction in GluR2 (t (25) = 2.256, p < 0.05; Fig 1d). Internalization of CI-AMPARs as well as memory retrieval have also been associated with increased zif268 immediate early gene (IEG) expression (Ferrara et al., 2019; Gardner et al., 2005; Jarome et al., 2012; Lee et al., 2008). To determine whether our changes in AMPAR trafficking were also accompanied by changes in IEG expression, we used immunofluorescence to quantify zif268 protein in the amygdala. We found a significant increase in amygdala zif268 expression (t (19) = 2.379, p < 0.05; Fig 1c). Together, these results demonstrate that changes in the context contribute to amygdala CI-AMPAR internalization following retrieval. This essential contextual information is likely dependent on activity within the DH region, as LIDO infusions in the absence of contextual novelty also allow for CI-AMPAR internalization and increased zif268 expression in the amygdala.
DH activity during memory retrieval in the absence of a novel context does not regulate amygdala AMPA receptor internalization or zif268 expression.
The first experiment suggests that DH-activity during training is essential for synaptic destabilization in the amygdala during fear recall in the absence of contextual novelty. We next wanted to determine whether DH activity during retrieval could influence amygdala AMPAR trafficking following a retrieval session. Here, we only used a vehicle group for our novel context condition because we did not find significant differences between groups infused with lidocaine or vehicle prior to conditioning but infused lidocaine or vehicle into the DH prior to retrieval in conditions where the context was not novel (Fig 2a). DH inactivation had no effect on auditory CS fear or actin levels (Fig 2b, 2g). Amygdala zif268 expression was not increased in the absence of contextual novelty when the DH was inactivated (t (22) = 1.869, p > 0.05 Fig 2c). Similar to zif268 expression, GluR2 expression levels did not differ between groups that did not receive a reactivation session and groups that received training and retrieval in the same context regardless of vehicle (t (11) = 0.791, p > 0.70; Fig 2e) or lidocaine infusions (t (12) = 0.110, p > 0.10; Fig 2e). To ensure infusions into the DH alone were not preventing increases in amygdala zif268 expression and AMPAR trafficking following retrieval, we included a group that received training and retrieval in different contexts and vehicle infusions prior to retrieval. When comparing a novel context group to a no reactivation group, there was significantly more zif268 positive cells (t (22) = 3.751, p < 0.01; Fig 2d) and significantly less synaptic expression of GluR2 (t (12) = 2.230, p < 0.05; Fig 2e) in the amygdala, suggesting DH infusions prior to retrieval alone do not impact amygdala cellular activity and CI-AMPAR internalization. These results suggest that DH inactivation prior to retrieval does not result in synaptic destabilization required for memory lability and modification.
Memory lability in the amygdala is regulated by contextual novelty and DH activity.
Because inactivation of the DH during auditory fear memory formation resulted in amygdala AMPAR trafficking and increases in zif268 expression when contexts were not changed between training and retrieval, we wanted to directly test the necessity of contextual novelty on auditory fear memory lability (Fig 3a). Amnesic agents like anisomycin have often been used to test restabilization and memory lability following memory retrieval (Jarome et al., 2012; Lee et al., 2008; Lopez et al., 2015; Nader et al., 2000). Here, we inactivated the DH with lidocaine during training to determine if DH activity could influence memory lability in the amygdala in the absence of contextual novelty. Because lidocaine was infused during training, we compared freezing responses, or behavioral performance, between vehicle and lidocaine groups during conditioning. There were no performance effects during training while the DH was inactivated (F(6, 58) = 0.39, p = 0.88; Fig 3b) or during the retrieval session (F(3, 29) = 2.047, p = 0.13; Fig 3c). To more closely look at an effect of DH inactivation on contextual fear during the retrieval session, lidocaine and vehicle groups were directly compared during the baseline period. There is a moderate reduction in freezing in groups that received lidocaine infusions into the DH (t(31) = 1.56, p = 0.06, Fig 3c). During the long-term retention test, there was a significant main effect of drug (F(3, 58) = 3.17, p < 0.05; Fig 3d). This main effect revealed a reduction in fear between groups that received lidocaine-anisomycin infusions in comparison to vehicle-vehicle infusions (p < 0.05). These results support the idea that contextual novelty is required for a memory to be susceptible to protein synthesis inhibition during reconsolidation (Jarome, et al 2015). Furthermore, neural activity in the DH appears to gate protein synthesis related memory lability in the amygdala.
To rule out potential confounding lidocaine/anisomycin interactions and ensure DH inactivation does not impair auditory fear memory retention, we included a condition where all groups receive lidocaine DH infusions and the retrieval/test occurs in a novel context (Fig 3e). There were no differences between groups during training (F(2, 30) = 0.33, p = 0.72; Fig 3f) or retrieval (F(1, 15) = 0.53, p = 0.48; Fig 3g). At test, there was a near statistically significant interaction (F(1, 14) = 3.29, p = 0.09), and a main effect for time (F(1, 14) = 140.80, p < 0.0001). Post hoc analysis revealed a significant reduction in freezing in groups that received anisomycin infusions immediately following retrieval (p < 0.05; Fig 3h).
Discussion
We manipulated novelty of the retrieval context to further understand the importance of the retrieval conditions during the reconsolidation of an auditory fear memory, and to determine whether the DH is critical for amygdala synaptic destabilization and memory lability during reconsolidation. We found lidocaine infusions into the DH during training as well as exposure to a novel context regulate amygdala synaptic destabilization and memory lability during retrieval. Specifically, we found that local inactivation of DH during memory formation allows for a requirement for protein synthesis in the amygdala in the absence of contextual novelty. CI-AMPAR internalization in the amygdala is necessary for synaptic destabilization during retrieval and is regulated by contextual novelty (Ferrara et al., 2019; Hong et al., 2013; Jarome et al., 2015). Lidocaine was used to inactivate the DH and may not have exclusively affected local DH principal neurons. Instead, neuronal processes, such as fibers connecting other brain regions, may have also been inhibited. Because of this we provided evidence that lidocaine infusions targeting the DH during training allow for amygdala AMPAR trafficking during reconsolidation when groups receive training and retrieval in the same context. This suggests that the DH processes contextual information during training which can subsequently regulate amygdala synaptic destabilization during auditory fear retrieval. These results support the idea that contextual novelty initiates synaptic destabilization and memory lability in the amygdala during reconsolidation, and additionally show that the contextual information that regulates later amygdala destabilization may be encoded by the DH.
Amygdala AMPA receptor trafficking during retrieval has been linked to the initiation of memory lability (Hong et al., 2013; Jarome et al., 2012; Lopez et al., 2015). Rapid internalization of CI-AMPAR during reconsolidation allows for synaptic plasticity underlying destabilization of synaptic connections to allow for the incorporation of new information into the original memory trace (Hong et al., 2013; Migues et al., 2016). The pattern of AMPAR trafficking and amount of AMPAR that return to the synapse after reconsolidation are sensitive to the cues present during retrieval (Jarome et al., 2015). Consistent with this, we demonstrate that contextual novelty during retrieval is an important factor for AMPAR internalization and this is regulated by activity in the DH during training. Specifically, when the retrieval context is different from the training context, CI-AMPAR internalize and the memory is susceptible to protein synthesis inhibition. When the context is not novel, CI-AMPAR are maintained in amygdala synapses and do not allow for memory modification. Thus, the contextual information encoded and regulating lability is dependent on activity within the hippocampal region, which then influences memory persistence in the amygdala.
Memory susceptibility to disruption has also been linked to novelty of the retrieval conditions, and specifically the retrieval context (Jarome et al., 2015). However, it is important to acknowledge that exposure to training and retrieval chambers prior to training and retrieval alone can still result in memory lability (Duvarci & Nader, 2004). To test this and target activity in the hippocampal region, we infused lidocaine into the DH prior to auditory fear conditioning. Groups received a retrieval session 24-hrs later and infusions of either vehicle or anisomycin in the amygdala immediately after retrieval. During the retrieval session, groups that received lidocaine infusions into the DH show modest reductions in context fear in comparison to groups infused with vehicle. This could be due to generally low fear responding during baseline in the vehicle conditions (i.e. less than 35% freezing), which may not be sensitive enough to detect decreases in fear. At a long-term test, groups that received lidocaine into the DH and anisomycin into the amygdala showed a deficit in CS retention, suggesting local activity in the DH during training allows for amygdala-dependent memory lability following retrieval. Collectively, this work suggests activity in the DH during training can mediate memory lability and synaptic destabilization in the amygdala during retrieval when contextual novelty is removed.
Several studies demonstrate an important role for communication between the DH and amygdala during fear memory formation (McIntyre et al., 2005; McReynolds et al., 2010; Sanders et al., 2003; Seidenbecher et al., 2003; Wang et al., 2009). During auditory fear conditioning, activity in the DH is necessary for contextual processing, and the amygdala integrates a broad spectrum of sensory information for long-term storage (Helmstetter et al., 2008). Furthermore, the plastic events occurring in the DH and amygdala impact one another, suggesting bidirectional modulation of plasticity between these regions during learning and memory (McIntyre, 2005; McReynolds et al., 2010; Richter-Levin & Akirav, 2001). For example, the amygdala and hippocampus show increased synchrony following fear learning, and inactivation of the amygdala prevents increases in hippocampal immediate early gene expression (Huff et al., 2006; Narayanan et al., 2007; Pape et al., 2005). Our results are consistent with work demonstrating an important role for the amygdala during permanent memory storage but do not attempt to rule out the necessity of other brain regions in the consolidation and retention of a fear memory. Instead, the present set of experiments emphasizes that the DH and amygdala work together during fear memory formation and recall. We show that lidocaine infusions into the DH do not prevent auditory memory formation but impacts the ability to modulate this memory with amygdala manipulation. Therefore, the DH may gate the ability to modify an auditory fear memory in the amygdala during retrieval.
Collectively, these results provide insight for a role of contextual novelty during retrieval-dependent memory modification and lend further support for DH-amygdala interactions during learning and memory. This adds to existing work showing the amygdala is a critical site for memory storage, plasticity, and reconsolidation-dependent memory modification, which is gated by contextual information processed by the DH.
Supplementary Material
Supplemental figure 1: Full length gels for cropped GluR2 (99kDa), GluR1 (99kDa), and actin (45kDa) western blot images in figure 1.
Supplemental figure 2: Full length gels for cropped GluR2 (99kDa), GluR1 (99kDa), and actin (45kDa) western blot images in figure 2.
Supplemental figure 4. Histology for figure 3 when training and retrieval occurred in the same context.
Supplemental figure 5. Histology for figure 3 when retrieval occurred in a novel context.
Memories can be “destabilized” when they are retrieved, but this memory lability depends on experience with the environment, or context, in which retrieval takes place.
We found that contextual novelty at retrieval of a simple associative fear memory can control alterations in neural activity and calcium impermeable AMPA receptor internalization in the amygdala.
Activity in the dorsal hippocampus is critical for the process through which retrieval-dependent plasticity in the amygdala is controlled.
Acknowledgements:
This work was supported by National Institute of Health (NIH) grants MH112141 and AG053854 (FJH).
Footnotes
Conflict of interest: The authors declare no financial or non-financial competing interests.
References
- Brunet A, Orr SP, Tremblay J, Robertson K, Nader K, & Pitman RK (2008). Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. Journal of Psychiatric Research, 42(6): 503–506. [DOI] [PubMed] [Google Scholar]
- Chang S, Chen D, & Liang KC. (2008). Infusion of lidocaine into the dorsal hippocampus before or after the shock training phase impaired conditioned freezing in a two-phase training task of contextual fear conditioning. Neurobiology of Learning and Memory, 89: 95–105. [DOI] [PubMed] [Google Scholar]
- Chen W, & Xu WH. (2015). β-Actin as a loading control: Less than 2 μg of total protein should be loaded. Electrophoresis, 36(17): 2046–2049. [DOI] [PubMed] [Google Scholar]
- Duvarci S, & Nader K (2004). Characterization of fear memory reconsolidation. The Journal of Neuroscience, 24 (42): 9269–9275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo PJ, Ortiz V, Martijena ID, Molina VA (2016). Stress-induced resistance to the fear memory labilization/reconsolidation process. Involvement of the basolateral amygdala complex. Neuropharmacology, 109: 349–356. [DOI] [PubMed] [Google Scholar]
- Fanselow MS (1980). Conditional and unconditional components of postshock freezing. Pavlovian Journal of Biological Science, 1, 177–182. [DOI] [PubMed] [Google Scholar]
- Ferrara NC, Jarome TJ, Cullen PK, Orsi SA, Kwapis JL, Trask S, Pullins SE, & Helmstetter FJ (2019). GluR2 endocytosis-dependent protein degradation in the amygdala mediates memory updating. Scientific Reports, 9: 5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels G, & Lamprecht R (2010). Interaction between N-Ethylmaleimide-Sensitive Factor and GluR2 Is Essential for Fear Memory Formation in Lateral Amygdala. The Journal of Neuroscience, 30(47): 15981–15986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner SM, Takamiya K, Xia J, Suh J, Johnson R, Yu S, & Huganir RL (2005). Calcium-Permeable AMPA Receptor Plasticity Is Mediated by Subunit-Specific Interactions with PICK1 and NSF. Neuron, 45(6): 903–915. [DOI] [PubMed] [Google Scholar]
- Hong I, Kim J, Kim J, Lee S, Ko H, Nader K, Kaang B, Tsien R, & Choi S (2013). AMPA receptor exchange underlies transient memory destabilization on retrieval. PNAS, 110(20): 8218–8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff NC, Frank M, Wright-Hardesty K, Sprunger D, Matus-Amat P, Higgins E, & Rudy JW (2006). Amygdala regulation of immediate-early gene expression in the hippocampus induced by contextual fear conditioning. The Journal of Neuroscience, 26(5): 1616–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupbach A, Gomez R, Hardt O, & Nadel L (2007). Reconsolidation of episodic memories: A subtle reminder triggers integration of new information. Learning & Memory, 14: 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegaya Y, Saito H, & Abe K. (1995). High-frequency stimulation of the basolateral amygdala facilitates the induction of long-term potentiation in the dentate gyrus in vivo. Neurosci Res, 22: 203–207 [DOI] [PubMed] [Google Scholar]
- Jarome TJ, Ferrara NC, Kwapis JL, & Helmstetter FJ (2015). Contextual information drives the reconsolidation-dependent updating of retrieved fear memories. Neuropsychopharmacology, 40: 3044–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarome TJ, Kwapis JL, Werner CT, Parsons RG, Gafford GM, & Helmstetter FJ (2012). The timing of multiple retrieval events can alter GluR1 phosphorylation and the requirement for protein synthesis in fear memory reconsolidation. Learning and Memory, 19: 300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarome TJ, Werner CT, Kwapis JL, & Helmstetter FJ (2011). Activity Dependent Protein Degradation Is Critical for the Formation and Stability of Fear Memory in the Amygdala. PloS One, 6(9): e24349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Choi J, Lee N, Lee H, Kim J, Yu N, Choi S, Lee S, Kim H, & Kaang B (2008). Synaptic Protein Degradation Underlies Destabilization of Retrieved Fear Memory. Science, 319: 1253–1256. [DOI] [PubMed] [Google Scholar]
- Lee J (2008). Memory reconsolidation mediates the strengthening of memories by additional learning. Nature Neuroscience, 11(11): 1264–1266. [DOI] [PubMed] [Google Scholar]
- Li Y, Meloni EG, Carlezon WA Jr., Milad MR, Pitman RK, Nader K, & Bolshakov VY (2013). Learning and reconsolidation implicate different synaptic mechanisms. PNAS, 110(12): 4798–4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J, Gamache K, Schneider R, & Nader K (2015). Memory Retrieval Requires Ongoing Protein Synthesis and NMDA Receptr Activity-Mediated AMPA Receptor Trafficking. The Journal of Neuroscience, 35(6): 2465–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox SA, Monsey MS, & Schafe GE (2011). Early growth response gene 1 (Egr-1) is required for new and reactivated fear memories in the lateral amygdala. Learning & Memory, 18: 24–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, & Holmes A (2016). Stress and fear extinction. Neuropsychopharmacology, 41(1): 58–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CK, Miyashita T, Setlow B, Marjon KD, Steward O, Guzowski JF, & McGaugh JL (2005). Memory-influencing intra-basolateral amygdala drug infusions modulate expression of Arc protein in the hippocampus. PNAS, 102(30): 10718–10723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds JR, Donowho K, Abdi A, McGaugh JL, Roozendaal, & McIntyre CK (2010). Memory-enhancing corticosterone treatment increases amygdala norepinephrine and Arc protein expression in hippocampal synaptic fractions. Neurobiology of Learning & Memory, 93(3): 312–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migues PV, Hardt O, Wu DC, Gamache K, Sacktor TC, Wang YT, & Nader K (2010). PKM • maintains memories by regulating GluR2-dependent AMPA receptor trafficking. Nature Neuroscience, 13(5): 630–634. [DOI] [PubMed] [Google Scholar]
- Migues PV, Liu L, Archbold GEB, Einarsson E, Wong J Bonasia K, Ko SH, Wang YT, & Hardt O (2016). Blocking synaptic removal of GluA2-containing AMPA receptors prevents the natural forgetting of long-term memories. The Journal of Neuroscience, 36(12): 3481–3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton AL, Merlo E, Ratano P, Gregory BL, Dumbreck JK, & Everitt BJ (2013). Double Dissociation of the Requirement for GluN2B- and GluN2A-Containing NMDA Receptors in the Destabilization and Restabilization of a Reconsolidating Memory. The Journal of Neuroscience, 33(3): 1109–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamiya N, Fukushima H, Suzuki A, Matsuyama Z, Homma S, Frankland PW, & Kida S (2009). Brain region-specific gene expression activation required for reconsolidation and extinction of contextual fear memory. The Journal of Neuroscience, 29(2): 402–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misanin JR, Miller RR, Lewis DJ (1968). Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science, 160 (3827): 554–555. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, & LeDoux JE (2000). Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature, 406: 722–726. [DOI] [PubMed] [Google Scholar]
- Narayanan RT, Seidenbecher T, Kluge C, Bergado J, Stork O, & Pape HC (2007). Dissociated theta phase synchronization in amygdalo- hippocampal circuits during various stages of fear memory. European Journal of Neuroscience, 25(6): 1823–1831. [DOI] [PubMed] [Google Scholar]
- Pape HC, Narayanan RT, Smid J, Stork O, & Seidenbeccher T (2005). Theta activity in neurons and networks of the amygdala related to long-term fear memory. Hippocampus, 15(7): 874–880. [DOI] [PubMed] [Google Scholar]
- Parsons RG, & Ressler KJ (2013). Implications of memory modulation for post-traumatic stress and fear disorders. Nature Neuroscience, 16(2): 146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant K, Pelkey KA, Bortolotto ZA, Morita D, Terashima A, McBain CJ, Collingridge GL, & Isaac JT (2006). Transient incorporation of native Glur2-lacking AMPA receptors during hippocampal long-term potentiation. Nature Neuroscience 9, 602–604. [DOI] [PubMed] [Google Scholar]
- Rao-Ruiz P, Rotaru DC, van der Loo RJ, Mansvelder HD, Stiedl O, Smit AB, & Spijker S (2011). Retrieval-specific endocytosis of GluA2-AMPARs underlies adaptive reconsolidation of contextual fear. Nature Neuroscience, 14: 1302–1308. [DOI] [PubMed] [Google Scholar]
- Richter-Levin G, & Akirav I (2001). Amygdala-hippocampus dynamic interaction in relation to memory. Molecular Neurobiology, 22:11–20. [DOI] [PubMed] [Google Scholar]
- Sander MJ, Wiltgen BJ, & Fanselow MS (2003). The place of the hippocampus in fear conditioning. The European Journal of Pharmacology, 463: 217–223. [DOI] [PubMed] [Google Scholar]
- Seidenbecher T, Laxmi TR, Stork O, & Pape H (2003). Amygdalar and Hippocampal Theta Rhythm Synchronization During Fear Memory Retrieval. Science, 301(5634): 846–850. [DOI] [PubMed] [Google Scholar]
- Wang S, Alvares L, & Nader K (2009). Cellular and systems mechanisms of memory strength as a constraint on auditory fear reconsolidation. Nature Neuroscience, 12: 905–912. [DOI] [PubMed] [Google Scholar]
- Winters BD, Tucci MC, DaCosta-Furtado M (2009). Older and stronger object memories are selectively destabilized by reactivation in the presence of new information. Learning & Memory, 16: 545–553. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure 1: Full length gels for cropped GluR2 (99kDa), GluR1 (99kDa), and actin (45kDa) western blot images in figure 1.
Supplemental figure 2: Full length gels for cropped GluR2 (99kDa), GluR1 (99kDa), and actin (45kDa) western blot images in figure 2.
Supplemental figure 4. Histology for figure 3 when training and retrieval occurred in the same context.
Supplemental figure 5. Histology for figure 3 when retrieval occurred in a novel context.