Abstract
Background:
The prognostic value of C-reactive protein/albumin ratio (CAR) in pancreatic cancer remains controversial. This study aimed to determine the potential role of CAR as a prognostic indicator in pancreatic cancer.
Methods:
A comprehensive literature search up to December 2018 was conducted using PubMed, Web of Science, and other databases. The hazard ratio (HR) with 95% confidence interval (CI) was employed to quantitatively assess CAR as a prognostic indicator in patients with pancreatic cancer.
Results:
Eleven studies with 2047 pancreatic cancer patients were selected for the analysis. Ten out of 11 studies included only Asian patients. The pooled results showed that a higher CAR value was significantly associated with a poor overall survival of pancreatic cancer patients (random-effects model: HR = 1.86; 95% CI = 1.53–2.26). Sensitivity analysis indicated the stability of the overall pooled results. Subgroup analysis and meta-regression analysis revealed that the country under study, cut-off value of CAR, treatment of patients, and the period of follow-up did not affect the prognostic value of CAR in pancreatic cancer patients (P > .05). No publication bias was noted across the studies (P = .933).
Conclusion:
This meta-analysis suggests that CAR is associated with the survival of pancreatic cancer patients of Asian ethnicity, and a higher CAR may be a potential prognostic indicator in pancreatic cancers.
Keywords: C-reactive protein/albumin ratio, pancreatic cancers, prognosis value
1. Introduction
One of the most lethal and aggressive digestive cancers, pancreatic cancer is the fourth leading cause of cancer-related deaths.[1] Despite great advancements in preoperative diagnosis approach, surgery techniques and chemotherapy treatment, and perioperative management, the prognosis of pancreatic cancer patients remains poor, especially for those at an advanced stage.[2,3] In addition, only 10% to 15% pancreatic cancer patients are able to undergo pancreatic resection.[4] Traditionally, TNM stage, vascular invasion, and histologic grade are considered to be the prognostic indicators of pancreatic cancer. However, these biomarkers are subject to lower reliability and rely on surgical exploration.[3,5,6] Therefore, finding reliable and easily assessable biomarkers to predict the prognosis of patients with pancreatic cancer is imperative to clinicians.
Previous studies have demonstrated that tumor-elicited inflammation plays a crucial role in various types of malignant transformation and tumor progression.[7,8] Currently, some systemic inflammation-based indicators, such as C-reactive protein (CRP), neutrophil-to-lymphocyte ratio (NLR), and lactate dehydrogenase, have been shown to be associated with the prognosis of various cancers, including pancreatic cancer.[9–11] Recently, the CRP to albumin ratio (CAR), a novel inflammation-based prognostic score, was also reported to be associated with poor outcome in various diseases, including sepsis,[12] pancreatitis,[13] and cancers.[14]
The prognostic value of CAR in pancreatic cancer patients has been explored.[15–19] However, the results remain controversial. For example, studies by Piciucchi et al[20] and Lee et al[21] failed to show the prognostic value of CAR in pancreatic cancer patients in multivariate Cox regression analysis, but other studies indicated the prognostic value in these patients. Previously, although some meta-analysis studies analyzed the prognostic value of CAR in solid tumors,[19,22] the data about pancreatic cancer was small and lacked stratified analysis. Thus, the real value of CAR in predicting the prognosis of pancreatic cancer needs to be further elucidated. Therefore, in order to derive a more precise estimation of the prognostic value of CAR in pancreatic cancer, we conducted a meta-analysis by including all the published articles.
2. Materials and methods
2.1. Literature search and study selection
This meta-analysis complied with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses criteria.[23] Two investigators independently searched for eligible studies prior to April 2018 in PubMed, EMBASE, Web of Science, Chinese National Knowledge Infrastructure, and Chinese Biomedical Database. The search strategy included the following terms: (“c-reactive protein”[MeSH Terms] or “CRP” or “albumins”[MeSH Terms or “CAR”, “ratio”[All Fields]) and (“pancreatic”[All Fields] AND “cancer”[All Fields] or “pancreatic neoplasms”[MeSH Terms]) and (“prognosis”[MeSH Terms]). Literature in all languages was searched and translated when necessary. References within the identified articles were also searched manually. The study protocol (2018-03-15) was approved by the ethics committee of the Affiliated Hospital of Guilin Medical University.
2.2. Inclusion and exclusion criteria
Studies were included if they met the following criteria:
-
(1)
pancreatic cancers were histologically diagnosed;
-
(2)
prognostic value of pretreatment CAR was evaluated;
-
(3)
hazard ratio (HR) for overall survival (OS) was evaluated with multivariate analysis using the Cox proportional hazards model;
-
(4)
a definite cut-off value of CAR was provided.
The exclusion criteria were as follows:
-
(1)
studies that were letters, reviews, or case reports;
-
(2)
other pancreatic lesions, such as pancreatic neuroendocrine tumors, pancreatic cysts;
-
(3)
insufficient information available for data extraction;
-
(4)
for studies with duplicate data, the most recent publication was chosen.
2.3. Data extraction and quality assessment
The extracted data from each study included: first author, year of publication, country where the study was conducted, age and gender of patients, total number of patients, design of the study, cut-off value of CAR, cut-off selection methods, treatment strategy, follow-up of the patients, the HRs for OS and disease-free survival (DFS), as well as their 95% confidence intervals (CIs). The qualities of the included studies were estimated using the Newcastle–Ottawa Quality Assessment Scale (NOS).[24] The NOS scores of ≥7 were defined as high-quality studies.[25] The data from all eligible studies were independently reviewed and extracted by 2 investigators. Any disagreement was resolved by discussion.
2.4. Statistical analysis
The HRs and their 95% CIs were extracted from each study to calculate pooled HRs. The heterogeneity of the pooled results was measured using Cochran Q test and Higgins I-squared (I2) statistic. Significant heterogeneity was defined as P < .1 in Cochran Q test. I2 values <25% were taken as indicators of mild heterogeneity, 25% to 50% corresponded to moderate heterogeneity, and values >50% corresponded to severe heterogeneity. The random-effects model (DerSimonian-Laird method) was used to analyze the pooled HRs when heterogeneity was significant; otherwise, the fixed-effects model (Mantel-Haenszel method) was applied. Publication bias was assessed using the Begg and Egger tests. Sensitivity analysis was used to examine the stability of the pooled results. Subgroup analysis was performed on the basis of country, cut-off value, treatment method, and follow-up. The differences between the subgroups were assessed using meta-regression analysis. The above statistical analyses were performed using STATA version 11.0 (Stata Corp LP, TX). Furthermore, linear regression analysis was performed to evaluate the correlation of the CAR cut-off value and log (CAR cut-off value) with the HR for OS using GraphPad Prism Software 6 (GraphPad Software Inc., San Diego, CA).
3. Results
3.1. Study selection process
The selection process is illustrated in Figure 1. Based on the search terms, 88 relevant studies were identified from the primary retrieval. After screening the titles and abstracts, 32 potential studies were selected while 56 were excluded because they were either reviews, animal studies, case reports, or irrelevant to the current meta-analysis. By reading the full text of the selected 32 studies, 20 were excluded due to insufficient data of HR value or lack of analysis of the prognostic value of CAR in pancreatic cancer. One study used the high sensitivity CRP/Alb (hs-CRP/Alb) ratio instead of the CRP/Alb ratio to explore the prognostic value.[26] Finally, eleven eligible studies[16–18,20–22,27–30] with 2047 pancreatic cancer patients were selected based on the inclusion criteria in this meta-analysis.
Figure 1.
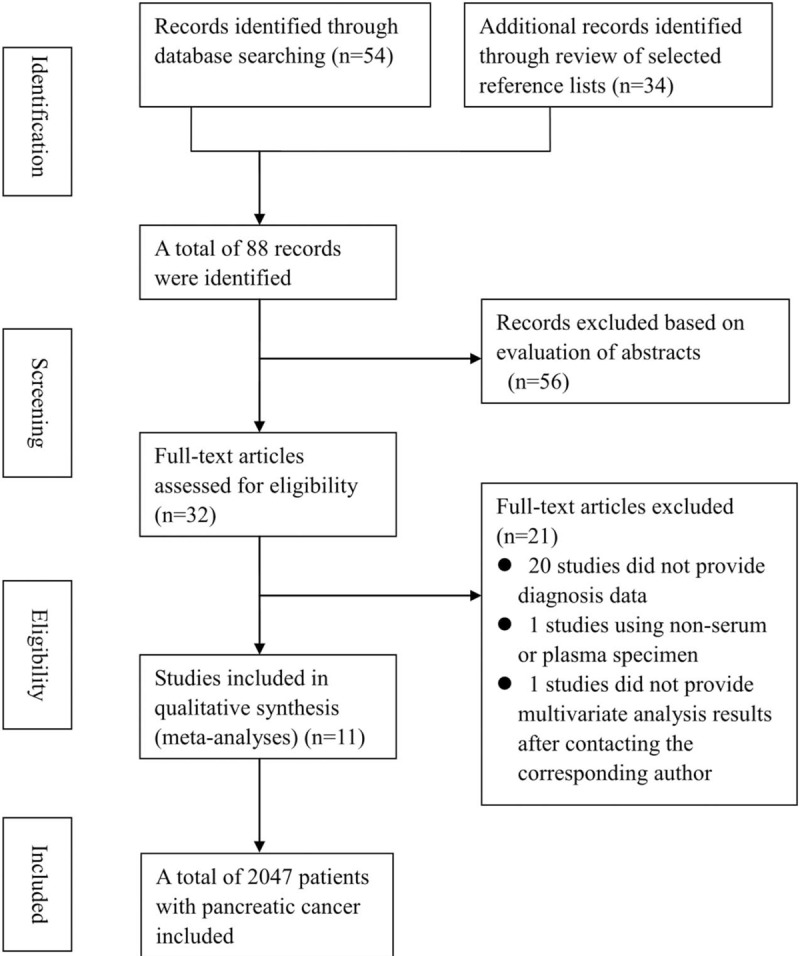
Flow chart of literature selection.
3.2. Characteristics of included studies
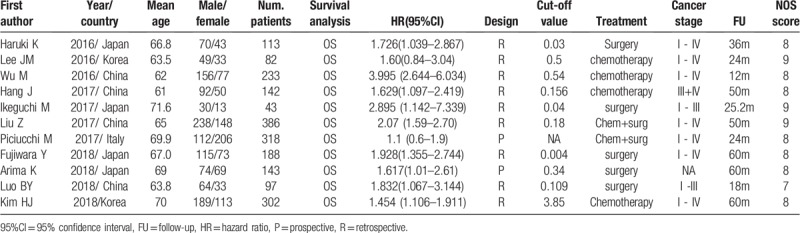
All included studies were retrospective by design. Four studies were conducted in China, 4 in Japan, 2 in Korea, and 1 in Italy. The period of follow-up varied, ranging from 12 to 60 months. The cut-off value of CAR varied across the studies, ranging from 0.004 to 3.85; we converted the units of CRP from mg/L to mg/dL to maintain uniformity across cut-off values of CAR. The quality of included studies was high, ranging from 7 to 9 based on the NOS system. The detailed characteristics of this meta-analysis are shown in Table 1.
Table 1.
Characteristic of included studies.
3.3. Overall analysis of prognostic value of CAR in pancreatic cancer
Pooled results from multivariate analysis of the data showed that patients with a high pretreatment CAR had significantly poorer OS than those with low CAR (HR = 1.86; 95% CI = 1.53–2.26; P < .001; Fig. 2). There was obvious heterogeneity across the included studies (I2 = 54.8%; P = .014). To determine whether a particular study affected the pooled HRs in our meta-analysis, a sensitivity analysis was performed by removing each study in turns. Although removal of the Wu et al[22] study reduced the heterogeneity significantly (I2 = 0, P = .548), the result remained similar to the overall results (HR, 1.72; 95% CI, 1.51–1.96; P < .001; Fig. 3). Since Piciucchi et al[20] study included Caucasian patients, we removed this study in the sensitivity analysis, and found the results to be similar to the main results. Publication bias analysis revealed no significant bias among the studies (Egger test, P = .933; Begg test, P = .876; Fig. 4)
Figure 2.
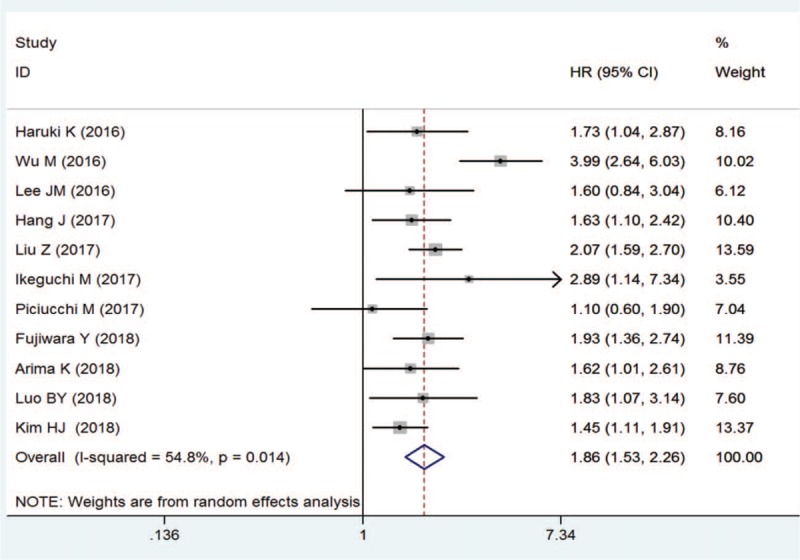
Forest plot of hazard ratio for the association of C-reactive protein/albumin ratio with overall survival in pancreatic cancers patients.
Figure 3.
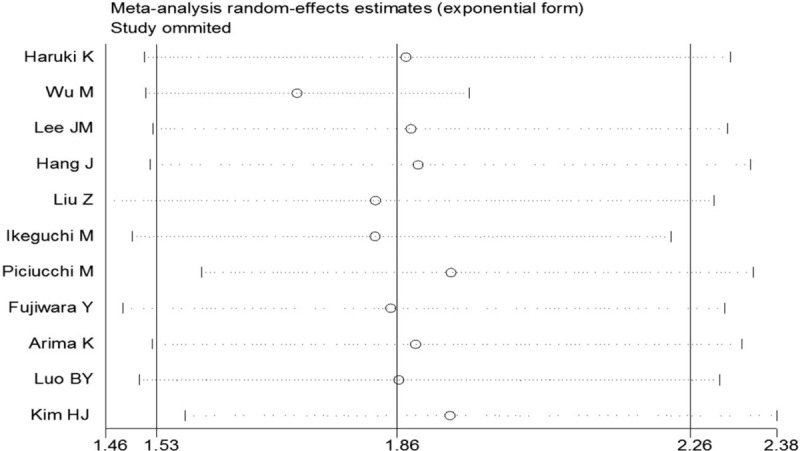
Sensitivity analysis of the relationship between C-reactive protein/albumin ratio and overall survival in pancreatic cancers patients.
Figure 4.
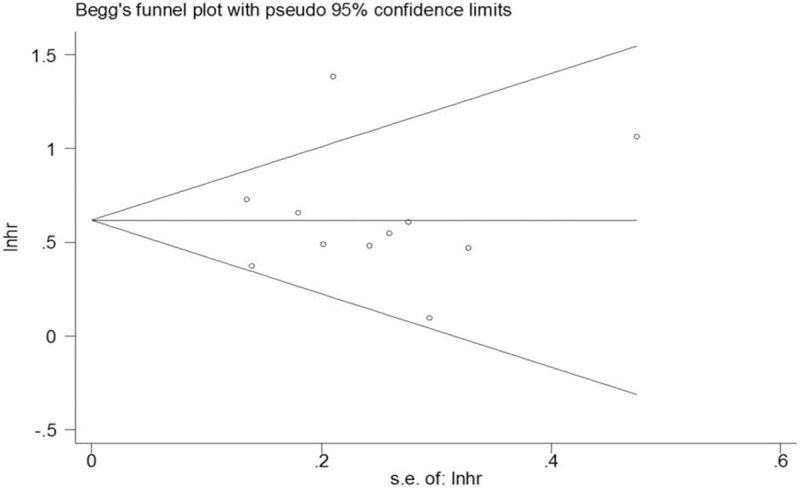
Filled funnel plots for publication bias test of overall survival.
3.4. Correlation of cut-off value and HR for OS
We performed a correlation analysis of CAR cut-off value and HR as in a previous study. First, we evaluated the correlation of cut-off value and HR for OS using linear regression analysis. The results showed no correlation between the cut-off value and HR for OS (r2 = 0.114; P = .375; Fig. 5A). In order to reduce the impact of non-normal distribution of the CAR cut-off value, the correlation of log (cut-off value) and HR for OS was analyzed, which also failed to show a significant correlation between log (cut-off value) and HR for OS (r2 = 0.032; P = .647; Fig. 5B). We therefore divided the studies into low and high cut-off groups in the following subgroup analysis, with the cut-off value as 0.1, which included three and four studies in the low and high cut-off group, respectively.
Figure 5.
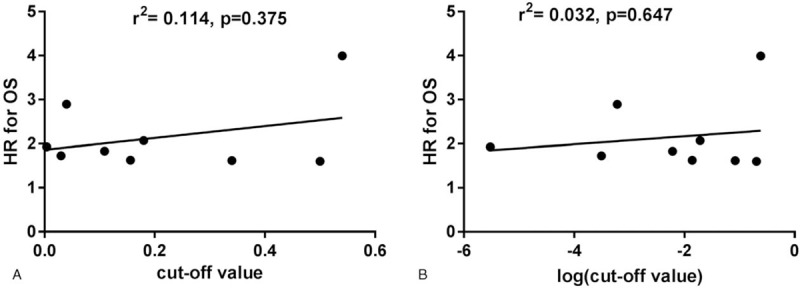
The correlation of cut-off value and log (cut-off value) of the hazard ratio for overall survival using linear regression analysis.
3.5. Subgroup analysis and meta-regression analysis
We performed subgroup analysis and meta-regression analysis of OS based on different countries, cut-off value, treatment methods, and follow-up time. The results of subgroup analysis and meta-regression analysis are illustrated in Table 2.
Table 2.
Subgroup meta-analysis and meta-regression analysis.
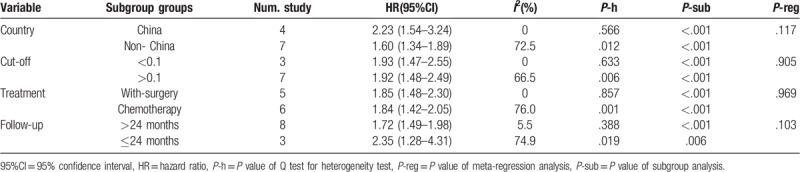
By pooling the data of different countries, the subgroup analysis results showed that higher CAR value was associated with poor OS both in China (HR, 2.23; P < .001) and in countries other than China (HR, 1.60; P < .001). However, the meta-regression did not show a significant difference between China and countries outside China regarding the prognostic value of CAR (P value for subgroup difference = .117).
The subgroup analysis for low and high cut-off group revealed that CAR was associated with poor OS both in the lower cut-off group (HR, 1.93; P < .001) and the higher cut-off group (HR, 1.92; P < .001). There was no statistically significant difference between these 2 groups by meta-regression analysis (P value for subgroup difference = .905).
When data from different treatment methods were pooled, the results indicated that elevated CAR was positively related to poor OS both in the with-surgery group (HR, 1.85; P < .001) and the no-surgery group (HR, 1.84; P < .001). No significant difference was observed between these groups (P value for subgroup difference = .969).
The included studies were divided into shorter and longer follow-up groups with 24 months as the cut-off time. A combined analysis of the subgroup analyses showed that higher CAR was associated with poor OS in both the shorter and longer follow-up groups (HR, 2.35; P = .006) and the higher cut-off group (HR, 1.72; P < .001). There was no statistically significant difference between these groups (P value for subgroup difference = .103).
4. Discussion
In the present study, we combined data from eleven eligible studies with a total of 2047 pancreatic cancer patients, and the results showed that high pretreatment CAR was significantly associated with poor OS of pancreatic cancer patients, although the heterogeneity was moderate. Subgroup analysis showed that a high CAR was related to poor OS when the patients were stratified based on the different countries, CAR cut-off value, treatment method, and follow-up, and the meta-regression suggested no significant difference between these subgroups. The sensitivity analysis showed that none of the included studies significantly affected the overall analysis. These results suggest that pretreatment CAR is an independent and significant indicator for OS in pancreatic cancer patients.
Growing evidence has demonstrated that, in addition to the intrinsic properties of tumor cells, the systemic and local inflammatory reactions of the host play a critical role in the pathogenesis and progression of cancer.[31] To date, certain inflammatory factors and cancer-related inflammatory prognostic scores, such as NLR, platelet-lymphocyte ratio (PLR), modified Glasgow Prognostic Score (mGPS), and CAR, have been found to be closely correlated with the survival outcomes in various cancers, including pancreatic cancer.[32–35] CRP is a common marker of host systemic inflammation, and albumin reflects the nutrition status of the host. Hypoalbuminemia, an indicator for chronic malnutrition, is a common complication for advanced cancer patients.[36] Therefore, CAR, a combined pattern of both CRP and albumin, may reveal the outcome of diseases in a better way than either one would individually. The prognostic value of CAR has been implicated in several diseases, including various cancers. Compared with other peripheral blood cell count-based indicators, such as NLR or PLR, CAR has a better prognostic value in pancreatic cancer according to some reports.[16,17,28]
Previously, several studies have reported the prognostic value of CAR in pancreatic cancer but with inconsistent results. Haruki et al[17] found that pancreatic cancer with advanced TNM stage presented a higher CAR value than early TNM stage and that CAR was an independent prognostic factor in multivariate regression analysis. Similar results were reported by Hang et al[16] and Liu et al.[26] However, inconsistent results were reported in the Lee et al[21] study, which showed that CAR was not an independent indicator to the prognosis of pancreatic cancer patients. Moreover, Piciucchi et al[20] study showed that CAR was not associated with survival of pancreatic cancer patients in an Italian cohort. We speculated at least 2 reasons contributing to this discrepancy. First is the treatment of patients, because some patients underwent surgery only, other patients were given chemotherapy. Second, the cut-off value of CAR might have led to inconsistent results, as shown in Table 2, since it varied greatly among the included studies, which might have affected the prognostic value of CAR in patients with pancreatic cancer.
It well known that the prognosis of patients with cancer is associated with the clinical stage of cancer. In this study, 3 of the included published studies[15–17] reported that the clinical stage was an independent indicator of the prognosis of patients with pancreatic cancer. Liu et al[28] study showed that a high CAR value was associated with prognosis in patients at stage III and IV, but not stage I and II. Hang et al[16] study revealed that the proportion of patients with high CAR was significantly higher in stage IV compared to stage III, suggesting that CAR increased with the progression of cancer, and high CAR indicated a worse prognosis in patients with pancreatic cancer. However, due to the limited data available, we failed to analyze the effect of CAR in different stages of pancreatic cancer in patients, and we did not know the cut-off value of CAR between patients at different stages, which might influence the prognostic value. Furthermore, whether testing of CAR in patients in early stages is superior to advanced stages as well as whether testing before treatment is superior to after treatment remains uncertain. Thus, future studies need to address these issues.
Although 2 studies[14,19] reported the prognostic value of CAR in pancreatic cancer using meta-analysis, both included only 4 articles for the analysis. Further, the sample size was small, and the studies lacked subgroup analysis based on the different variables of the studies included in them. Compared with previous studies, the present study included a much larger patient population and thus could increase the robustness of the results. In addition, we compared the difference in prognostic values of CAR by dividing the evaluations into different subgroups using subgroup analysis and meta-regression analysis. Our results indicated that different countries, cut-off value, treatment, and follow-up did not show a significant difference between each subgroup. Moreover, all the data used for meta-analysis was adjusted by confounder factors, that is from multivariate analysis, which could greatly eliminate the influence of potential confounder factors. Finally, the sensitivity analysis and publication bias results indicated the robustness of the overall meta-analysis.
There are several limitations in this meta-analysis that need to be noted. First, there was significant heterogeneity across the studies in the overall meta-analysis, which tended to reduce the robustness of the results, although the sensitivity analysis identified only 1 study that led to significant heterogeneity. Second, most of the included studies were retrospective by design, which indicated an inferior level of evidence compared with prospective studies. In addition, studies with a retrospective design are prone to recall bias or misclassification bias, and the results are subject to confounding factors,[37] which influence the reliability of results. Third, although no significant publication bias was detected, most of the included studies published positive results; thus, some publication bias was inevitably latent. Fourth, all but one of the included studies were from Asian countries, and hence the results need to be interpreted with caution when extrapolating to other ethnicities. Therefore, a future study that addresses the above-mentioned limitations is warranted in order to confirm the prognostic value of CAR in pancreatic cancer. Due to the limitations of CAR in predicting pancreatic cancer, common indicators, such as TNM stage, tumor size, PLR and NLR, continue to act as necessary alternative methods in clinical practice.
5. Conclusion
This study demonstrated that CAR was closely related to pancreatic cancer in individuals of Asian ethnicity, and pretreatment CAR could be a promising independent prognostic biomarker for OS in pancreatic cancer patients.
Author contributions
Conceptualization: Jihong Bai.
Data curation: Yanjun Fu.
Formal analysis: Yanjun Fu.
Funding acquisition: Zhiqing Liang.
Methodology: Yanjun Fu, Kezhi Li.
Supervision: Zhiqing Liang.
Writing – original draft: Yanjun Fu, Kezhi Li.
Writing – review & editing: Jihong Bai.
Footnotes
Abbreviations: CI = confidence interval, HR = hazard ratio, ICU = intensive care unit, NLR = neutrophil-to-lymphocyte ratio, SMD = standard mean differences.
How to cite this article: Fu YJ, Li KZ, Bai JH, Liang ZQ. C-reactive protein/albumin ratio is a prognostic indicator in Asians with pancreatic cancers: A meta-analysis. Medicine. 2019;98:48(e18219).
This work was supported by grants from the National Natural Science Foundation of China (No.81260078); Guangxi Natural Science Foundation Grant (2014GXNSFAA118152). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interests to disclose.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Okabayashi T, Shima Y, Iwata J, et al. S-1 vs. gemcitabine as an adjuvant therapy after surgical resection for ductal adenocarcinoma of the pancreas. World J Surg 2014;38:2986–93. [DOI] [PubMed] [Google Scholar]
- [3].Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg 2006;10:1199–210. discussion 1210-1191. [DOI] [PubMed] [Google Scholar]
- [4].Bilimoria KY, Bentrem DJ, Ko CY, et al. National failure to operate on early stage pancreatic cancer. Ann Surg 2007;246:173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jamieson NB, Denley SM, Logue J, et al. A prospective comparison of the prognostic value of tumor- and patient-related factors in patients undergoing potentially curative surgery for pancreatic ductal adenocarcinoma. Ann Surg Oncol 2011;18:2318–28. [DOI] [PubMed] [Google Scholar]
- [6].van Roest MH, Gouw AS, Peeters PM, et al. Results of pancreaticoduodenectomy in patients with periampullary adenocarcinoma: perineural growth more important prognostic factor than tumor localization. Ann Surg 2008;248:97–103. [DOI] [PubMed] [Google Scholar]
- [7].Murata M. Inflammation and cancer. Environ Health Prev Med 2018;23:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shadhu K, Xi C. Inflammation and pancreatic cancer: an updated review. Saudi J Gastroenterol 2018;25:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Stevens L, Pathak S, Nunes QM, et al. Prognostic significance of pre-operative C-reactive protein and the neutrophil-lymphocyte ratio in resectable pancreatic cancer: a systematic review. HPB (Oxford) 2015;17:285–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ventriglia J, Petrillo A, Huerta Alvaro M, et al. Neutrophil to lymphocyte ratio as a predictor of poor prognosis in metastatic pancreatic cancer patients treated with nab-paclitaxel plus gemcitabine: a propensity score analysis. Gastroenterol Res Pract 2018;2018:2373868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yu SL, Xu LT, Qi Q, et al. Serum lactate dehydrogenase predicts prognosis and correlates with systemic inflammatory response in patients with advanced pancreatic cancer after gemcitabine-based chemotherapy. Sci Rep 2017;7:45194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ranzani OT, Zampieri FG, Forte DN, et al. C-reactive protein/albumin ratio predicts 90-day mortality of septic patients. PLoS One 2013;8:e59321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yilmaz EM, Kandemir A. Significance of red blood cell distribution width and C-reactive protein/albumin levels in predicting prognosis of acute pancreatitis. Ulus Travma Acil Cerrahi Derg 2018;24:528–31. [DOI] [PubMed] [Google Scholar]
- [14].Wu J, Tan W, Chen L, et al. Clinicopathologic and prognostic significance of C-reactive protein/albumin ratio in patients with solid tumors: an updated systemic review and meta-analysis. Oncotarget 2018;9:13934–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fujiwara Y, Haruki K, Shiba H, et al. C-reactive protein-based prognostic measures are superior at predicting survival compared with peripheral blood cell count-based ones in patients after curative resection for pancreatic cancer. Anticancer Res 2018;38:6491–9. [DOI] [PubMed] [Google Scholar]
- [16].Hang J, Xue P, Yang H, et al. Pretreatment C-reactive protein to albumin ratio for predicting overall survival in advanced pancreatic cancer patients. Sci Rep 2017;7:2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Haruki K, Shiba H, Shirai Y, et al. The C-reactive protein to albumin ratio predicts long-term outcomes in patients with pancreatic cancer after pancreatic resection. World J Surg 2016;40:2254–60. [DOI] [PubMed] [Google Scholar]
- [18].Ikeguchi M, Hanaki T, Endo K, et al. C-reactive protein/albumin ratio and prognostic nutritional index are strong prognostic indicators of survival in resected pancreatic ductal adenocarcinoma. J Pan Cancer 2017;3:31–6. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Li N, Tian GW, Wang Y, et al. Prognostic role of the pretreatment C-reactive protein/albumin ratio in solid cancers: a meta-analysis. Sci Rep 2017;7:41298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Piciucchi M, Stigliano S, Archibugi L, et al. The neutrophil/lymphocyte ratio at diagnosis is significantly associated with survival in metastatic pancreatic cancer patients. Int J Mol Sci 2017;18:E730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lee JM, Lee HS, Hyun JJ, et al. Prognostic value of inflammation-based markers in patients with pancreatic cancer administered gemcitabine and erlotinib. World J Gastrointest Oncol 2016;8:555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wu M, Guo J, Guo L, et al. The C-reactive protein/albumin ratio predicts overall survival of patients with advanced pancreatic cancer. Tumour Biol 2016;37:12525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [25].Zhang J, Chen L, Zhou R, et al. Pretreatment lymphocyte monocyte ratio predicts long-term outcomes in patients with digestive system tumor: a meta-analysis. Gastroenterol Res Pract 2016;2016:9801063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Xu H, Hu L, Wei X, et al. The predictive value of preoperative high-sensitive C-reactive protein/albumin ratio in systemic inflammatory response syndrome after percutaneous nephrolithotomy. J Endourol 2018;33:1–8. [DOI] [PubMed] [Google Scholar]
- [27].Kim HJ, Lee SY, Kim DS, et al. Inflammatory markers as prognostic indicators in pancreatic cancer patients who underwent gemcitabine-based palliative chemotherapy. Korean J Intern Med 2018;[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liu Z, Jin K, Guo M, et al. Prognostic value of the CRP/Alb ratio, a novel inflammation-based score in pancreatic cancer. Ann Surg Oncol 2017;24:561–8. [DOI] [PubMed] [Google Scholar]
- [29].Luo BY, Yang Y, Duan YF, et al. Preoperative C-reactive protein/albumin ratio predicts the prognosis of patients with resectable pancreatic cancer. Zhonghua Wai Ke Za Zhi 2018;56:712–7. [DOI] [PubMed] [Google Scholar]
- [30].Arima K, Yamashita YI, Hashimoto D, et al. Clinical usefulness of postoperative C-reactive protein/albumin ratio in pancreatic ductal adenocarcinoma. Am J Surg 2018;216:111–5. [DOI] [PubMed] [Google Scholar]
- [31].Alifano M, Mansuet-Lupo A, Lococo F, et al. Systemic inflammation, nutritional status and tumor immune microenvironment determine outcome of resected non-small cell lung cancer. PLoS One 2014;9:e106914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Xu HJ, Ma Y, Deng F, et al. The prognostic value of C-reactive protein/albumin ratio in human malignancies: an updated meta-analysis. Onco Targets Ther 2017;10:3059–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhang J, Zhang HY, Li J, et al. PLR and PLT may predict the prognosis of patients with colorectal cancer: a systematic review and meta-analysis. Oncotarget 2017;8:68837–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zhang X, Chen X, Wu T, et al. Modified glasgow prognostic score as a prognostic factor in gastriccancer patients: a systematic review and meta-analysis. Int J Clin Exp Med 2015;8:15222–9. [PMC free article] [PubMed] [Google Scholar]
- [35].Zheng J, Cai J, Li H, et al. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio as prognostic predictors for hepatocellular carcinoma patients with various treatments: a meta-analysis and systematic review. Cell Physiol Biochem 2017;44:967–81. [DOI] [PubMed] [Google Scholar]
- [36].Dequanter D, Lothaire P. Serum albumin concentration and surgical site identify surgical risk for major post-operative complications in advanced head and neck patients. B-ENT 2011;7:181–3. [PubMed] [Google Scholar]
- [37].Song JW, Chung KC. Observational studies: cohort and case-control studies. Plast Reconstr Surg 2010;126:2234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]


