Abstract
INTRODUCTION:
Metformin may be associated with reduced colorectal cancer (CRC) risk, but findings from previous studies have been inconsistent and had insufficient sample sizes to examine whether the association differs by anatomic site. This study examined whether metformin was associated with reduced CRC risk, both overall and stratified by anatomic site, in a large sample of persons with diabetes who underwent colonoscopy.
METHODS:
We performed a case-control study of US Veterans with prevalent diabetes who underwent colonoscopy between 1999 and 2014 using Department of Veterans Affairs electronic health record data. Cases were defined by presence of CRC at colonoscopy, while controls had normal colonoscopy. The primary exposure was metformin use at time of colonoscopy (yes/no). Association of metformin exposure with CRC (further stratified by proximal, distal, or rectal subsite) was examined using multivariable and multinomial logistic regression and summarized by odds ratios (ORs) with 95% confidence intervals (CIs).
RESULTS:
We included 6,650 CRC patients and 454,507 normal colonoscopy patients. CRC cases were older and had lower metformin exposure. Metformin was associated with 8% relative reduction in CRC odds (OR: 0.92, 95% CI: 0.87–0.96). By subsite, metformin was associated with a 14% statistically significant reduced rectal cancer odds (OR: 0.86, 95% CI: 0.78–0.94) but no reduced distal or proximal cancer odds.
DISCUSSION:
Metformin was associated with reduced CRC odds—particularly rectal cancer—in a large sample of persons with diabetes undergoing colonoscopy.
INTRODUCTION
Colorectal cancer (CRC) is the second leading cause of cancer and cancer-related death in the United States (1). Chemopreventive agents may reduce CRC risk. Despite interest in chemoprevention, to date, only aspirin has been recommended for CRC risk reduction, but only among individuals with increased cardiovascular disease risk (2). Additional chemoprevention strategies for risk reduction may enhance prevention efforts.
Metformin, commonly used as a blood sugar control therapy in persons with diabetes, may be a promising chemopreventive agent for CRC prevention. Previous studies examining the effect of metformin on CRC risk have had conflicting results (3–8). A recent meta-analysis of 15 studies on CRC incidence found that metformin therapy was associated with a 10% reduction in CRC incidence among patients with type 2 diabetes mellitus (hereafter referred to as diabetes or T2DM) (9). Of the studies included, however, 5 found a significant protective effect of metformin on CRC risk, whereas the other 10 showed no significant effect (9).
Variability in risk associations might be due to lumping all anatomic subsites into 1 because molecular and clinical phenotypes vary by subsite, insufficient sample size for stable estimates, and including individuals with polyps or prevalent CRC in the control group due to lack of normal colonoscopy controls (10,11). Our aim was to examine whether metformin was associated with reduced CRC risk, both overall and stratified by anatomic site, in a large sample of Veterans with diabetes who underwent colonoscopy, using cases with CRC and normal colonoscopy controls.
METHODS
We conducted a retrospective case-control study to explore the association between metformin use and CRC risk, both overall and anatomic site-specific, among US Veterans cared for by the Veterans Health Administration. The Department of Veteran Affairs (VA) is one of the largest integrated health care providers in the United States, caring for over 6 million Veterans annually (12). Since 1999, all VA sites have used an integrated electronic health record (EHR) for documentation of clinical encounters, which, along with additional health care resources, can be accessed for research. The VA Corporate Data Warehouse provides access to discrete EHR data, including demographic characteristics, administrative claims-based diagnosis and procedure codes, prescriptions (e.g., metformin), and anthropometric measures (e.g., weight and height), as well as free-text data, including procedure notes and pathology reports. CRC was ascertained by the VA Central Cancer Registry (VACCR), which has been shown to accurately identify 90% of CRC cases (13).
Study sample and selection criteria
The study population consisted of Veterans with at least 1 Current Procedural Terminology code for colonoscopy between 1999 and 2014. The analytic sample was restricted to Veterans with T2DM using previously validated methodology for defining diabetes using VA data, which included a combination of International Classification of Diseases, Ninth Revision diagnosis codes and prescription drugs (14).
Case selection
Cases were identified by the VACCR within the 6-month period after index (first) colonoscopy and defined using International Classification of Diseases, Oncology, Third Revision (ICD-O-3) site codes for CRC (C18.0, C18.2-C18.7, C19.9, and C20.9). For identified cases, Surveillance, Epidemiology, and End Results summary stage and histology were extracted. We excluded cases with unknown Surveillance, Epidemiology, and End Results stage, diagnosed carcinoma-in-situ, or ICD-O-3 histology codes inconsistent with adenocarcinoma. If morphology code was not specified/available, we included the case as long as site, stage, and diagnosis date information were available, given that most CRCs are adenocarcinomas. Patients with history of inflammatory bowel disease or inflammatory bowel disease diagnosis at or within 6 months after baseline colonoscopy and patients with history of CRC before baseline colonoscopy were excluded. Cases were stratified by anatomic site based on the following site codes: proximal (C18.0, C18.2-C18.4), distal (C18.5-C18.7), and rectal (C19.9, C20.9).
Control selection
Controls were Veterans with normal index colonoscopy defined by presence of a Current Procedural Terminology code for diagnostic colonoscopy only (45378 or G0121) and absence of a pathology report (within 30 days of index colonoscopy). Our previous work has shown that this definition is 96.3% sensitive and 97.5% specific for normal colonoscopy and had a positive predictive value for identifying individuals with normal colonoscopy of 97% (15,16). To avoid inclusion of controls with missed CRC at baseline colonoscopy, controls with CRC diagnosed by the VACCR or International Classification of Diseases, Ninth Revision code within up to 3 years of index colonoscopy were excluded. If a candidate control had less than 3 years of follow-up (due to death or loss to follow-up at VA) based on the Veterans Health Administration Vital Status File, he/she was excluded (15).
Predictors and covariates
The primary predictor was exposure to metformin—defined by having 2 prescriptions for metformin within the 1 year period preceding baseline colonoscopy—based on outpatient pharmacy data files. Covariates included sex, race/ethnicity, age, body mass index (BMI), smoking status (current, former, or never), and aspirin use. BMI ascertainment was based on an algorithm derived from a previous study and was measured in the year before baseline colonoscopy (17). Aspirin use was defined using a previously developed and validated algorithm using structured VA medication files and free-text progress reports (18).
Statistical Analysis
Univariate analyses were conducted comparing metformin use and key covariates among cases and controls using χ2 analyses for categorical variables and Wilcoxon rank-sum tests or Kruskal-Wallis tests for continuous variables, respectively. A 0.05 level of significance in univariate analyses determined which covariates were included in multivariable models to adjust for confounding. Association of metformin exposure with CRC, both in aggregate and by anatomic subsite, was examined using multivariable logistic regression (binary and multinomial, respectively) analyses adjusted for potential confounders. Anatomic subsites were grouped as proximal (cecum through the transverse colon), distal (splenic flexure through the sigmoid colon), or rectal (rectosigmoid junction and rectum) using ICD-O-3 codes. In post hoc analyses, we tested the joint effect of metformin and aspirin exposure on site-specific CRC odds by testing for interaction. Odds ratios (ORs) and 95% confidence intervals (CIs) were used to summarize associations. The study was approved by the VA San Diego Institutional Review Board.
RESULTS
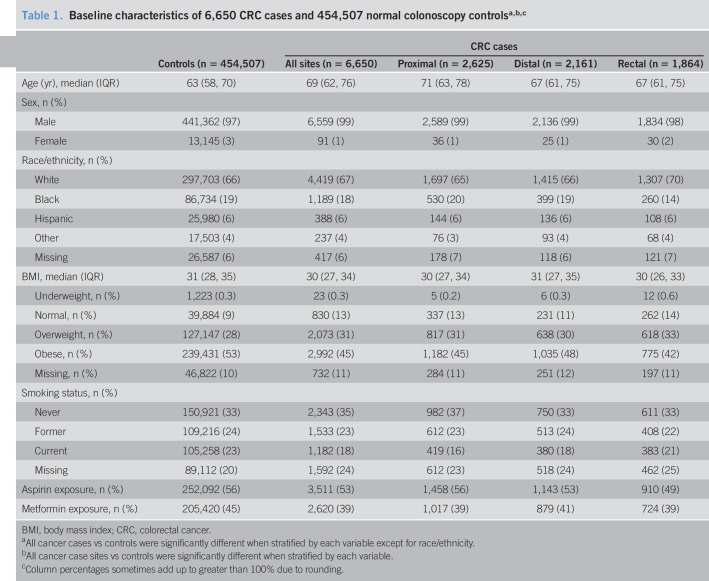
We identified 6,650 diabetic CRC cases (proximal n = 2,625; distal n = 2,161; rectal n = 1,864) and 454,507 diabetic controls with normal colonoscopy (Table 1). Duration of diabetes prevalence was a median 3 years before baseline colonoscopy for cases and controls (interquartile range: 1–5 years). Cases were older (median age 69 vs 63 years), more likely to be men (99% vs 97%), never smokers (35% vs 33%), less likely to be overweight or obese (76.2% vs 80.7%), and less likely to be exposed to aspirin (53% vs 56%). CRC cases were less likely to have been exposed to metformin than controls (39% vs 45%). Within subsites, cases with proximal cancer were older (median age 71 years for proximal vs 67 years for both distal and rectal cancer), more likely to be black (20% for proximal vs 19% for distal and 14% for rectal), more likely to be exposed to aspirin (56% for proximal vs 53% for distal and 49% for rectal), and more likely to be never smokers (37% for proximal vs 33% for both distal and rectal). Cases with rectal cancer were least likely to be exposed to aspirin (49% for rectal vs 53% for distal vs 56% for proximal) and most likely to be current smokers (21% for rectal vs 18% for distal vs 16% for proximal).
Table 1.
Baseline characteristics of 6,650 CRC cases and 454,507 normal colonoscopy controlsa,b,c
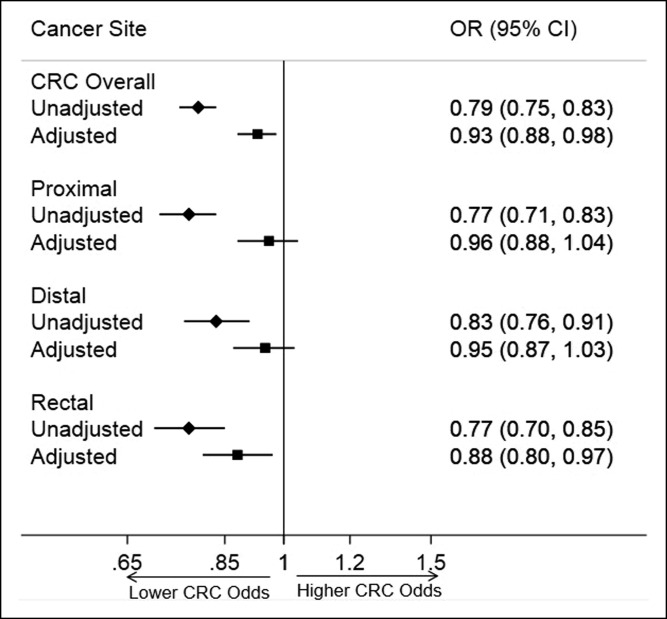
Multivariable and multinomial logistic regression analysis results are shown in Figure 1. Comparing all CRC cases with normal colonoscopy controls, metformin was associated with an 8% relative reduction in CRC odds in analyses adjusted for age, sex, race/ethnicity, BMI, smoking history, and aspirin exposure (OR: 0.92, 95% CI: 0.87–0.96). On subsite-specific adjusted multinomial analyses, metformin was associated with a statistically significant 14% relative reduction in rectal cancer odds (OR: 0.86, 95% CI: 0.78–0.94), but no statistically significant reduced odds for proximal (OR: 0.95, 95% CI: 0.88–1.03) or distal colon cancer (OR: 0.94, 95% CI: 0.86–1.02). In post hoc analyses, we found no significant interaction between metformin and aspirin exposure on CRC odds.
Figure 1.
Association of metformin exposure with colorectal cancer risk. The forest plot depicts ORs for CRC unadjusted, as well as adjusted for age, sex, race/ethnicity, body mass index, smoking status, and aspirin exposure, for all sites overall, as well as stratified by anatomic site. CRC, colorectal cancer; OR, odds ratio.
DISCUSSION
We found metformin was associated with an 8% relative reduction in CRC odds in a large case-control study of 6,650 diabetic CRC cases and 454,507 diabetic normal colonoscopy controls. Subsite-specific analyses revealed a 14% relative reduction in rectal cancer odds, but no reduced proximal or distal cancer odds, suggesting benefit might be restricted to rectal cancer, and underscoring importance of subsite-specific analyses. Our findings confirm and extend previous research on the potential association between metformin and CRC incidence, providing new context to the association between metformin use and site-specific CRC incidence.
Multiple systematic reviews and meta-analyses of observational studies have demonstrated that metformin exposure is associated with reduced CRC risk (3,9,19,20). The largest and most recent review conducted by He et al. (9) found metformin associated with a pooled 10% relative reduction in CRC risk compared to persons without diabetes (OR: 0.90, 95% CI: 0.85–0.96). Our study findings were similar even while having a potentially higher CRC risk diabetic control group, demonstrating the persistent protective association of metformin use on CRC risk across populations.
In vivo and in vitro studies have revealed that metformin has a direct antitumor effect, which may depress tumor proliferation and induce the apoptosis, autophagy, and cell cycle arrest of tumor cells (21–23). Findings in a previous study by Paleari et al. (24) further indicate that metformin absorption in the colon is 150-fold higher than plasma, and the levels found in colonic tissue are in the range of direct antitumor effect shown in preclinical models. Although these findings at the bench were found to be robust, questions emerged whether the anticancer activity occurred at levels higher than those that are—or ever could be—achieved in humans. Although survival benefit was seen in multiple studies on those with early CRCs taking metformin, a recent large study on patients using metformin before diagnosis of stage III CRC undergoing adjuvant chemotherapy showed disease free survival, overall survival, and time-to-recurrence were comparable with those for non-T2DM patients and T2DM patients without metformin use (25). Overall, mechanisms to explain any protective effect of metformin on CRC incidence and survival require further study.
Our anatomic site-specific analyses found that risk reduction associated with metformin exposure was mainly limited to rectal cancer. We did not identify any other studies in the published literature capable of separating out site-specific effects and did not identify any mechanistic studies which could explain a site-specific effect of metformin on risk of developing rectal cancer. There is evidence that metformin use can improve treatment outcome after rectal cancer diagnosis (26–28). When used as an adjunct to neoadjuvant chemoradiation, studies on rectal cancer have shown that metformin use is associated with higher overall and disease-free survival (26,28) and higher tumor response rates to radiotherapy, particularly among patients with T2DM (metformin vs control: 74% vs 38%; P = 0.045) (27). In addition to the mechanisms described above, research conducted by Jeong et al. found that metformin can induce radiosensitivity in vitro by prolonging cell cycle arrest and inhibiting DNA repair proteins.
Despite promising findings in observational and preclinical studies, few randomized controlled trials of metformin for chemoprevention against colorectal neoplasia have been conducted (8,29,30). In a trial conducted by Higurashi et al. (8), nondiabetic patients with previously resected adenomas who received 250-mg metformin daily had lower prevalence of total polyps (metformin group vs control group: 38% vs 56.5%) and lower prevalence of adenomas on follow-up (metformin group vs placebo group: 30.6% vs 51.6%) compared with persons receiving placebo. Conversely, 2 randomized controlled trials with small sample sizes measuring the effect of metformin exposure on cancer risk experienced no notable difference in adenoma incidence compared with participants not exposed to metformin (29,30). Overall, our results extend previous work by confirming a modest risk reduction for CRC associated with metformin exposure and clarify that the risk reduction seems to be more closely associated with rectal cancer rather than colon cancer. Future trials should aim to measure the effect of a metformin intervention on site-specific CRC risk to highlight potentially important site-specific effects and also seek to identify whether any biomarkers can help select patients most likely to benefit from metformin-based chemoprevention.
Several limitations may be considered in interpreting our results. We examined metformin as a binary single time point exposure, rather than a continuous, time-varying exposure, such that duration and accumulation of exposure were not considered. Thus, our findings might underestimate the effect of metformin on CRC risk, particularly among long-term metformin users and users whose dosage or frequency of metformin use might change. Future studies should focus on duration of and accumulated exposure to metformin to better understand its role in CRC prevention. Furthermore, our ascertainment of metformin exposure was based on having a prescription for metformin, which might overrepresent actual uptake of metformin; this may have biased toward an underestimate of the protective effect of metformin on cancer risk. Metformin use based on prescriptions outside the VA health care system was not captured in our analyses, possibly leading to underascertainment of exposure; effect of such underascertainment could have been toward an underestimate of the protective effect of metformin on cancer risk as well. The study included few women, consistent with historic VA demographics, limiting ability to specifically examine sex-specific effects. In addition, residual confounding by potential confounders not well-measured in the VA EHR, such as alcohol, diet, and other lifestyle factors, could not be considered. As such, despite our current adjustment for known measurable confounders in our multivariable models, we cannot rule out the possibility that residual confounding could have impacted our findings. Strengths of this study include the large sample size, which allowed for characterization of anatomic subsite-specific cancer risk, and utilization of normal colonoscopy controls with prevalent diabetes as a comparison group, which has been performed by only 1 previous study (31).
In conclusion, we found that metformin exposure was associated with a modest reduction in CRC risk among persons with diabetes. When evaluated by anatomic subsite, risk was modestly reduced for rectal cancer but not for proximal or distal colon cancer. Taken together with previous clinical and preclinical studies, our work supports consideration of metformin for further study as a chemopreventive agent to reduce risk of CRC, particularly rectal cancer. More studies are needed to understand the potential mechanisms that may drive subsite-specific risk reduction and patients most likely to benefit from metformin exposure.
CONFLICTS OF INTEREST
Guarantor of the article: Samir Gupta, MD.
Specific author contributions: Joshua Demb and Armaan Yaseyyedi are shared first authors. Concept and design: J.D., A.Y., L.L., R.B., A.E., P.G., J.S.G., A.J.G., T.R.K., M.E.M., and S.G. Analysis and interpretation of data: J.D., A.Y., L.L., R.B, A.E., P.G., J.S.G., M.E.M., and S.G. Drafting of manuscript: J.D., A.Y., L.L., R.B, A.E., M.E.M., and S.G. Critical revision of the manuscript for important intellectual content: J.D., A.Y., L.L., R.B, A.E., P.G., J.S.G., A.J.G., T.R.K., M.E.M., and S.G. Statistical analysis: J.D., A.Y., R.B, L.L., M.E.M., and S.G. Obtained funding: J.D. and S.G.
Financial support: This research was supported by Grant # 1I01HX001574-01A1 (PI: S.G.) from VA Health Services Research and Development; Grant #: 1R37CA 222866-01 (PI: S.G.) from the National Cancer Institute/National Institutes of Health; Grant #: 1F32CA23960-01 (PI: J.D.) from the National Cancer Institute/National Institutes of Health; and Grant #: 2P30CA023100-33 (PI: Lippman) from the National Cancer Institute/National Institutes of Health.
Potential competing interests: We have read and understood the ICMJE policy on declaration of conflicts of interests and declare we have no conflicts of interest.
Study Highlights.
WHAT IS KNOWN
✓ Metformin may be associated with reduced CRC risk.
✓ Previous studies have had insufficient sample sizes to examine associations by anatomic site.
WHAT IS NEW HERE
✓ Metformin prescription was associated with reduced CRC risk, particularly rectal cancer risk.
TRANSLATIONAL IMPACT
✓ Metformin could be a chemopreventive agent to reduce CRC risk.
✓ More granular studies of CRC within anatomic subsites could better explain possible mechanisms by which metformin impacts cancer pathogenesis.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Bibbins-Domingo K; U.S. Preventive Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive services task force recommendation statement. Ann Intern Med 2016; 164: 836. [DOI] [PubMed] [Google Scholar]
- 3.Liu F, Yan L, Wang Z, et al. Metformin therapy and risk of colorectal adenomas and colorectal cancer in type 2 diabetes mellitus patients: A systematic review and meta-analysis. Oncotarget 2017;8:16017–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nie Z, Zhu H, Gu M. Reduced colorectal cancer incidence in type 2 diabetic patients treated with metformin: A meta-analysis. Pharm Biol 2016;54:2636–42. [DOI] [PubMed] [Google Scholar]
- 5.Zhang ZJ, Zheng ZJ, Kan H, et al. Reduced risk of colorectal cancer with metformin therapy in patients with type 2 diabetes: A meta-analysis. Diabetes Care 2011;34:2323–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sehdev A, O'Neil BH. The role of aspirin, vitamin D, exercise, diet, statins, and metformin in the prevention and treatment of colorectal cancer. Curr Treat Options Oncol 2015;16:43. [DOI] [PubMed] [Google Scholar]
- 7.Cardel M, Jensen SM, Pottegård A, et al. Long-term use of metformin and colorectal cancer risk in type II diabetics: A population-based case-control study. Cancer Med 2014;3:1458–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higurashi T, Hosono K, Takahashi H, et al. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: A multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncol 2016;17:475–83. [DOI] [PubMed] [Google Scholar]
- 9.He X, Su T, Si J, et al. Metformin is associated with slightly reduced risk of colorectal cancer and moderate survival benefits in diabetes mellitus. Medicine (Baltimore) 2016;95:e2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogino S, Chan AT, Fuchs CS, et al. Molecular pathological epidemiology of colorectal neoplasia: An emerging transdisciplinary and interdisciplinary field. Gut 2011;60:397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lochhead P, Chan AT, Nishihara R, et al. Etiologic field effect: Reappraisal of the field effect concept in cancer predisposition and progression. Mod Pathol 2015;28:14–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.United States Department of Veterans Affairs. Veteran population. National Center for Veterans Analysis and Statistics. (http://www.va.gov/vetdata/veteran_population.asp) (2018). Accessed October 18, 2019. [Google Scholar]
- 13.McGinnis KA, Brandt CA, Skanderson M, et al. Validating smoking data from the Veteran's Affairs Health Factors dataset, an electronic data source. Nicotine Tob Res 2011;13:1233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the department of Veterans Affairs based on computerized patient data. Diabetes Care 2004;27(Suppl 2):B10–21. [DOI] [PubMed] [Google Scholar]
- 15.Gupta S Liu L Patterson OV. et al. A framework for leveraging “Big data” to advance epidemiology and improve quality: Design of the VA colonoscopy collaborative. EGEMS 2018;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Earles A, Liu L, Bustamante R, et al. Structured approach for evaluating strategies for cancer ascertainment using large-scale electronic health record data. JCO Clin Cancer Inform 2018;2:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noël PH, Copeland LA, Perrin RA, et al. VHA corporate data Warehouse height and weight data: Opportunities and challenges for health services research. J Rehabil Res Dev 2010;47:739–50. [DOI] [PubMed] [Google Scholar]
- 18.Bustamante R, Earles A, Murphy JD, et al. Ascertainment of aspirin exposure using structured and unstructured lar ge-scale electronic health record data. Med Care 2019;57:e60–e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Decensi A, Puntoni M, Goodwin P, et al. Metformin and cancer risk in diabetic patients: A systematic review and meta-analysis. Cancer Prev Res (Phila) 2010;3:1451–61. [DOI] [PubMed] [Google Scholar]
- 20.Noto H, Goto A, Tsujimoto T, et al. Cancer risk in diabetic patients treated with metformin: A systematic review and meta-analysis. PLoS One 2012;7:e33411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chi Thent Z, Hannim Zaidun N, Fairuz Azmi M, et al. Is metformin a therapeutic paradigm for colorectal cancer: Insight into the molecular pathway? Curr Drug Targets 2017;18:734–50. [DOI] [PubMed] [Google Scholar]
- 22.Lan B, Zhang J, Zhang P, et al. Metformin suppresses CRC growth by inducing apoptosis via ADORA1. Front Biosci (Landmark Ed) 2017;22:248–57. [DOI] [PubMed] [Google Scholar]
- 23.Najafi M, Cheki M, Rezapoor S, et al. Metformin: Prevention of genomic instability and cancer: A review. Mutat Res 2018;827:1–8. [DOI] [PubMed] [Google Scholar]
- 24.Paleari L, Burhenne J, Weiss J, et al. High accumulation of metformin in colonic tissue of subjects with diabetes or the metabolic syndrome. Gastroenterology 2018;154:1543–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh PP, Shi Q, Foster NR, et al. Relationship between metformin use and recurrence and survival in patients with resected stage III colon cancer receiving adjuvant chemotherapy: Results from North Central Cancer Treatment Group N0147 (Alliance). Oncologist 2016;21:1509–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skinner HD, Crane CH, Garrett CR, et al. Metformin use and improved response to therapy in rectal cancer. Cancer Med 2013;2:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oh BY, Park YA, Huh JW, et al. Metformin enhances the response to radiotherapy in diabetic patients with rectal cancer. J Cancer Res Clin Oncol 2016;142:1377–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gash KJ, Chambers AC, Cotton DE, et al. Potentiating the effects of radiotherapy in rectal cancer: The role of aspirin, statins and metformin as adjuncts to therapy. Br J Cancer 2017;117:210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Home PD, Kahn SE, Jones NP, et al. Experience of malignancies with oral glucose-lowering drugs in the randomised controlled ADOPT (A Diabetes Outcome Progression Trial) and RECORD (Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycaemia in Diabetes) clinical trials. Diabetologia 2010;53:1838–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim YH, Noh R, Cho SY, et al. Inhibitory effect of metformin therapy on the incidence of colorectal advanced adenomas in patients with diabetes. Intest Res 2015;13:145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanadiya MK, Gohel TD, Sanaka MR, et al. Relationship between type-2 diabetes and use of metformin with risk of colorectal adenoma in an American population receiving colonoscopy. J Diabetes Complications 2013;27:463–6. [DOI] [PubMed] [Google Scholar]