Abstract
Chronic thromboembolic pulmonary hypertension (CTEPH), a late complication of pulmonary embolism (PE), is associated with high mortality. However, whether the right ventricular (RV) echocardiographic parameters can predict – in the short- and long-term – the development of CTEPH and mortality after PE remains unknown. Herein, we aim to investigate the incidence of CTEPH after acute PE and to evaluate the risk factors of CTEPH. In this retrospective cohort, patients with PE were followed for 10 years for the onset of CTEPH. The screening was initially conducted through echocardiography and confirmed by right heart catheterization. Also, transient and permanent risk factors were identified. Among 358 patients with PE, 8 patients (4%) were subsequently diagnosed with CTEPH at a median time of 36 months and 47 died during the follow-up period. Notably, both short- and long-term RV dilatation, hypertrophy, and increased pulmonary pressure increased the incidence of CTEPH. However, RV echocardiographic parameters failed to differentiate survivors from non-survivors. Instead, malignancy, respiratory, or chronic heart failure was strongly associated with post PE mortality in the multivariable analysis. According to our findings, post PE screening of CTEPH may facilitate early diagnosis and intervention for patients at high risk of developing CTEPH. Also, RV echocardiographic parameters are associated with subsequent CTEPH, but mortality is mainly dependent on underlying comorbidities.
Keywords: chronic thromboembolic pulmonary hypertension, pulmonary embolism
1. Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) is a distinct pulmonary vascular disease caused by chronic obstruction of major pulmonary arteries. CTEPH is a dual vascular disorder, with major-vessel vascular obliteration and a peripheral pulmonary arteriopathy resembling classic pulmonary arterial hypertension (PAH). CTEPH is also considered as a late complication of acute pulmonary embolism (PE).[1,2] While a substantial resolution of the embolus occurs in most patients with PE, in a minority of patients the embolus persists. A residual thrombus that has been in the body for a while can undergo fibrous organization, after which dissolution by endogenous or exogenous fibrinolytics is no longer possible. The organized fibrous thrombus and subsequent pulmonary arterial remodeling can lead to chronic elevation of pulmonary pressure.
CTEPH is characterized by elevated pressure in the artery with persistent pulmonary perfusion defects.[3] The constant, elevated pulmonary pressure is associated with a progressive increase in right ventricle (RV) afterload, resulting in RV dysfunction and eventually heart failure and death.[1] Ideally, patients with PE should be proactively and appropriately managed in the acute phase to prevent progression to CTEPH.
The cumulative incidence of CTEPH within 2 years of asymptomatic PE event has been reported as 0.1% to 9.1%.[4] The actual proportion of patients with PE that progress to CTEPH is still unclear, and also varies relative to how the studied patient population was selected.[5] Early diagnosis of CTEPH remains a significant clinical challenge, while the first incidence is usually reported within 2 years.[6,7] According to the 2015 ESC/ESR guidelines for the diagnosis and treatment of pulmonary hypertension, requirements for routine screening for CTEPH after PE are currently under debate, as a significant number of CTEPH cases develop without a previous acute PE event.[8] Nevertheless, assessment of the risk factors for CTEPH will be essential to facilitate the early detection of patients at risk after PE.
To date, the incidence of CTEPH after acute PE in the Taiwanese population is not known. Therefore, this study aimed to assess the incidence of CTEPH after acute PE and to evaluate the risk factors associated with CTEPH. Also, we studied the contributing factors to mortality post-acute PE.
2. Methods
2.1. Study design
This is a retrospective chart review that took place at the National Cheng Kung University Hospital between January 1, 2006, and May 31, 2017. The ethics committee of National Cheng Kung University Hospital approved this study (ethics number: B-ER-105–136).
2.2. Patients
Patients with a confirmed diagnosis of acute PE between January 1, 2006, and March 31, 2016, were included in the study. Medical records were retrospectively reviewed, and eligible patients were identified using the ICD-9 and ICD-10 codes for PE. Patients were categorized relative to PE associations with transient and permanent risk factors.[9] Transient risk factors included recent surgery, trauma (with or without bone fracture), prolonged immobilization due to medical reasons (i.e., lasting more than 7 days), deep venous thrombosis, pregnancy or recent childbirth, and the use of oral contraceptives or hormone-replacement therapy. Permanent risk factors included chronic heart or respiratory failure, cerebrovascular disease, thrombophilia, active cancer, obesity, and varicose veins. Patients with PE in the absence of risk factors were considered as having idiopathic (unprovoked) PE. Under the clinical impression of PE, the diagnosis was confirmed by examining CT angiography or ventilation/perfusion (V/Q) scans. Given the echocardiography derived high PAP and RV failure signs, patients with suspected CTEPH were referred for further evaluation. We used an RHC and V/Q scan to arrive at a definite diagnosis of CTEPH.
2.3. Treatment of PE
Anticoagulation agents were initially used to treat hemodynamically stable acute PE using either unfractionated heparin or low molecular weight heparin. Thrombolytic agents, including streptokinase, urokinase, or recombinant tissue plasminogen activator, were initially administered to treat hemodynamically unstable acute PE.[9] After the initial treatment, patients were administered anticoagulant therapy. We assessed the need to extend anticoagulant treatment beyond 6 months using risk stratification of PE recurrence.[10]
2.4. Echocardiography
Echocardiography was initially performed during the admission of acute PE and afterward, depending on patients’ clinical symptoms. These symptoms included dyspnea, palpitations, and peripheral edema. The median follow-up duration was 6 months; up to 24 months. Standard imaging was performed (iE33, Philips) with a 3.5-MHz multiphase-array probe per the recommendations of the American Society of Echocardiography.[11] LVEF was measured using the biplane Simpson's method. As described previously,[11] RV dimensions, including mid-cavity diameters and wall thickness, were measured at the mid-cavity in diastole at the parasternal long-axis view. Thickness >5 mm indicates RV hypertrophy and may suggest RV pressure overload. RV dilatation is defined based on the right ventricle appearing larger than the left ventricle. Applying the Bernoulli equation, RV pressure was calculated according to the trans-tricuspid flow velocity. All the analyzed images were acquired in three consecutive cardiac cycles and stored digitally with a frame rate of 50–90 frames per second.
2.5. Follow-up of CTEPH diagnosis and mortality
The primary endpoint of this study was the diagnosis of CTEPH, while the secondary endpoint was mortality. Patients selected from retrospective chart review were checked for a diagnosis of CTEPH within their medical records (before March 31, 2016) and followed up according to ESC guidelines. Patients with signs of pulmonary hypertension were investigated by using echocardiography. CTEPH was considered upon observation of a systolic pulmonary arterial pressure >40 mm Hg, with a lung perfusion scan revealing the perfusion defect.[10] Right heart catheterization was used to confirm the appearance of multiple chronic or organized thromboembolic obstructions. The final diagnosis of CTEPH was determined with the following observations of mean pulmonary arterial pressure ≥25 mm Hg at rest, pulmonary capillary wedge pressure ≤15 mm Hg, and pulmonary vascular resistance >3 Wood units.[9] Also, during the follow-up period, the subsequent mortality was recorded by the medical record.
2.6. Statistical analysis
Data are presented as means ± SD for continuous variables, and numbers and percentages for categorical variables. To investigate the risk factors for CTEPH and mortality, univariable analysis was carried out by Fisher's exact test for categorical variables when any cell in a contingency table had n < 5 or the χ2 test for all other categorical and Mann–Whitney U test for continuous variables between CTEPH and non-CTEPH groups, survivors and non-survivors, respectively. Given the limited number of CTEPH diagnosis, in the further simple and multiple Cox proportional hazard regression models, we focused on the subsequent mortality post-acute PE. Kaplan–Meier curves were plotted to differentiate the survivals in the sub-group analysis. A P-value of <.05 was considered statistically significant throughout the study. SAS software version 9.4 (SAS Inc. Cary, NC) was used for all statistical analyses.
3. Results
3.1. Patients
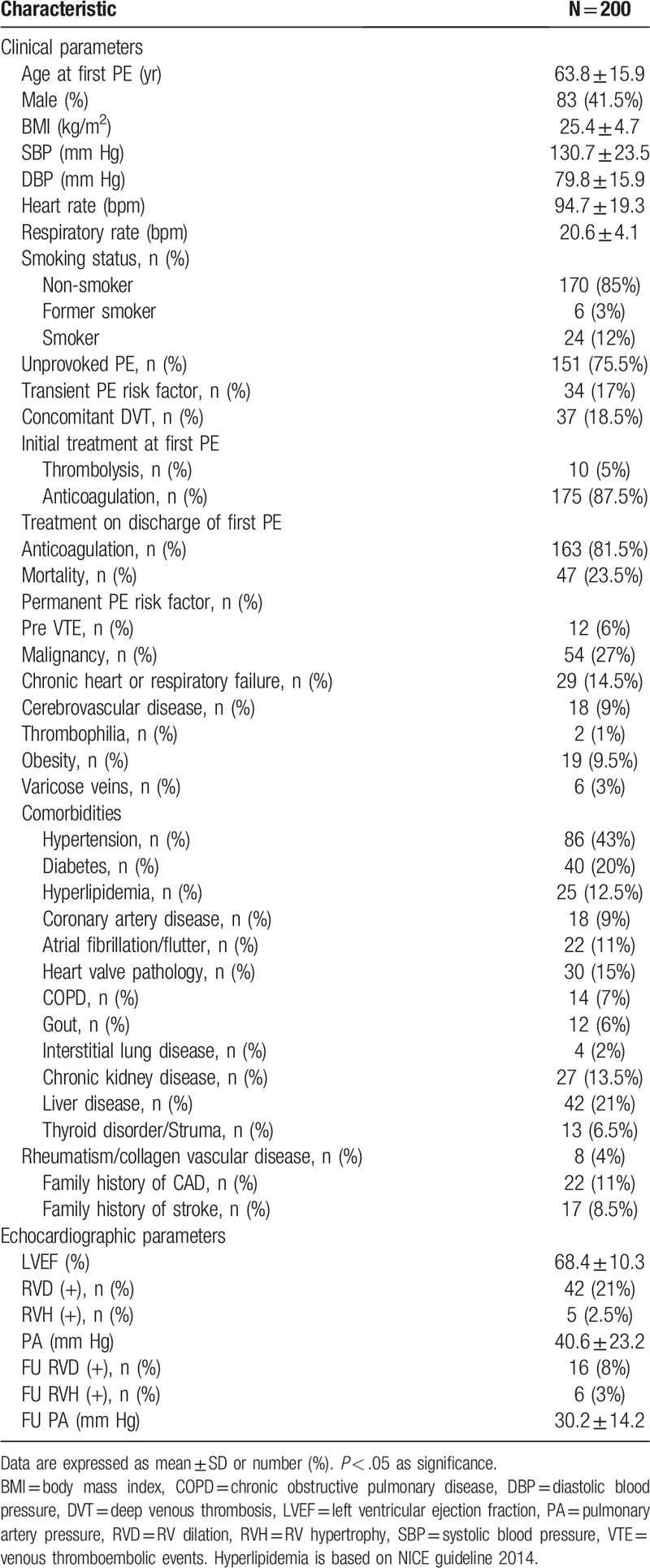
There were 358 patients diagnosed with PE from January 1, 2006 to March 31, 2016. Fifty-four patients died during the first hospitalization. After discharge, 48 patients were lost to follow-up, and 53 patients did not repeat echocardiography. Thus, the final study population consisted of 200 patients, and 8 patients were diagnosed with CTEPH (Fig. 1). Baseline characteristics are shown in Table 1. Mean age at first PE was 63.8 ± 15.9 years old. Of these, 151 (75.5%) patients had encountered an unprovoked PE. The initial treatments at first PE, 87.5% of patient were treated with anticoagulation agents, while 5% were administered thrombolytic therapy.
Figure 1.
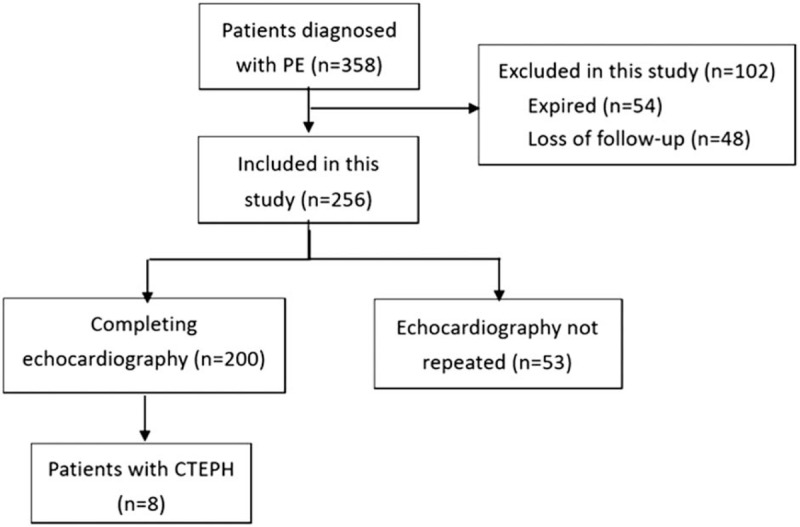
The algorithm of patient enrollment.
Table 1.
Baseline characteristics of the studied patients.
3.2. Incidence and risk factors of CTEPH
During follow-up, 8 patients (4%) were diagnosed with CTEPH after the first episode of acute PE. The median time from PE to CTEPH is 36 months (range: 3–120 months) (Table 2). The time and cumulative incidence of CTEPH are displayed in Figure 2. Interestingly, despite a higher prevalence of thrombophilia in patients who developed CTEPH, there was only 1 patient with thrombophilia in each group. Also, most of the clinical risk factors, including malignancy, chronic heart or lung disease, obesity, and varicose veins, were not associated with the subsequent diagnosis of CTEPH. In contrast, among echocardiographic risk factors during the acute stage of acute PE, the incidence of RV dilatation, RV hypertrophy, and increased pulmonary artery pressure were significantly higher in patients with CTEPH compared with patients without CTEPH. Furthermore, the follow-up RVD, RV hypertrophy, and PA pressure remained higher in those who developed CTEPH; however, the changes in PA pressure between patients with and without CTEPH were insignificant. The extended uses of anticoagulant at discharge, which implied a relatively higher risk of PE recurrence, were similar between the two groups. Nevertheless, no patients experienced recurrent PE during the follow-up period.
Table 2.
The comparison of risk factors between patients developing chronic thromboembolic pulmonary hypertension (CTEPH) or not post pulmonary embolism.
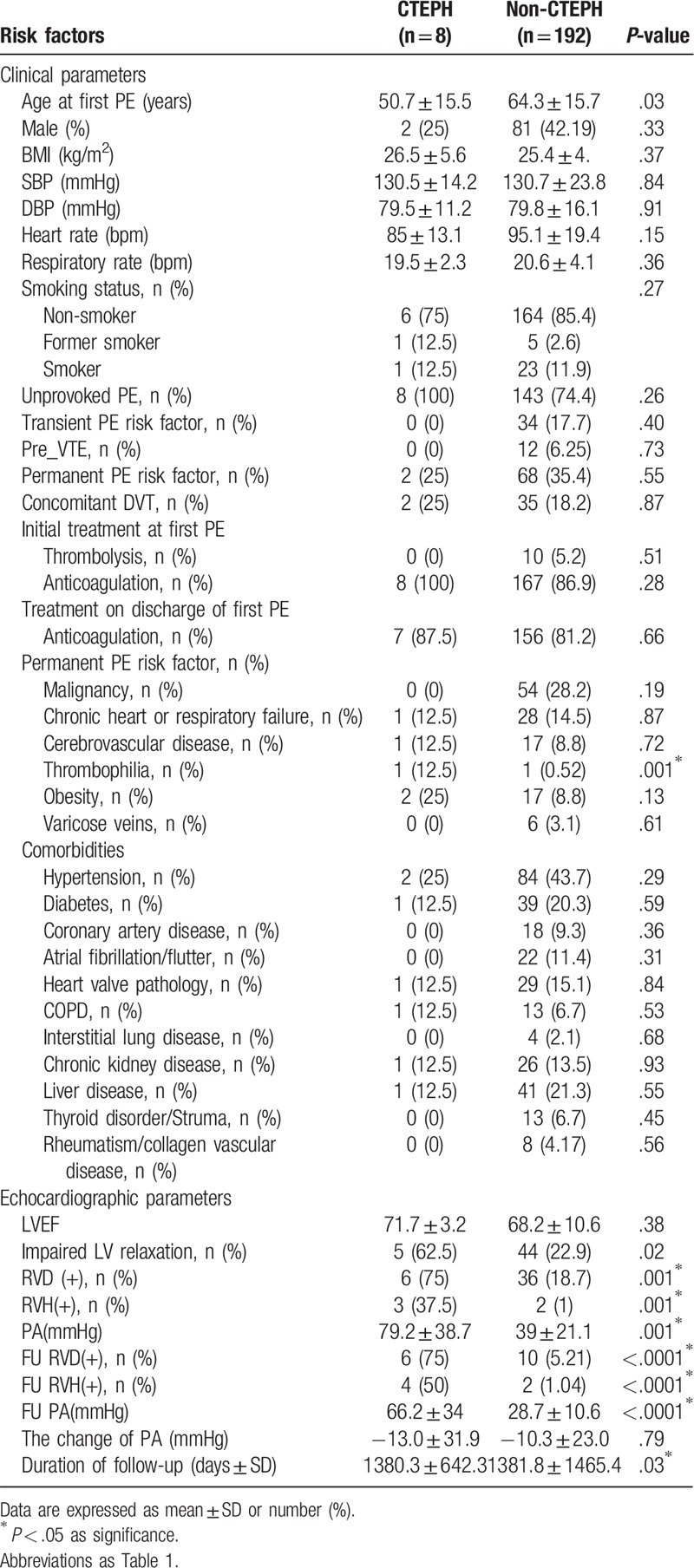
Figure 2.
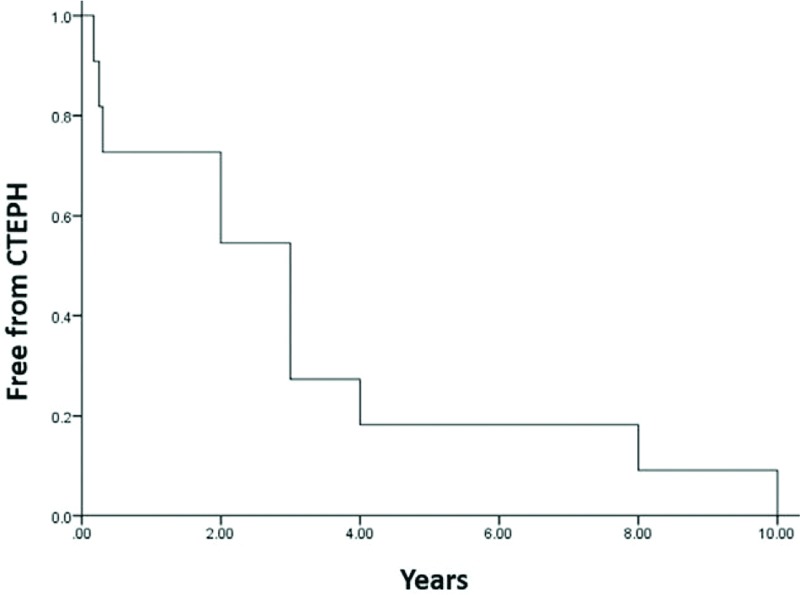
The time and cumulative incidence of chronic thromboembolic pulmonary hypertension (CTEPH).
3.3. Incidence and risk factors of mortality
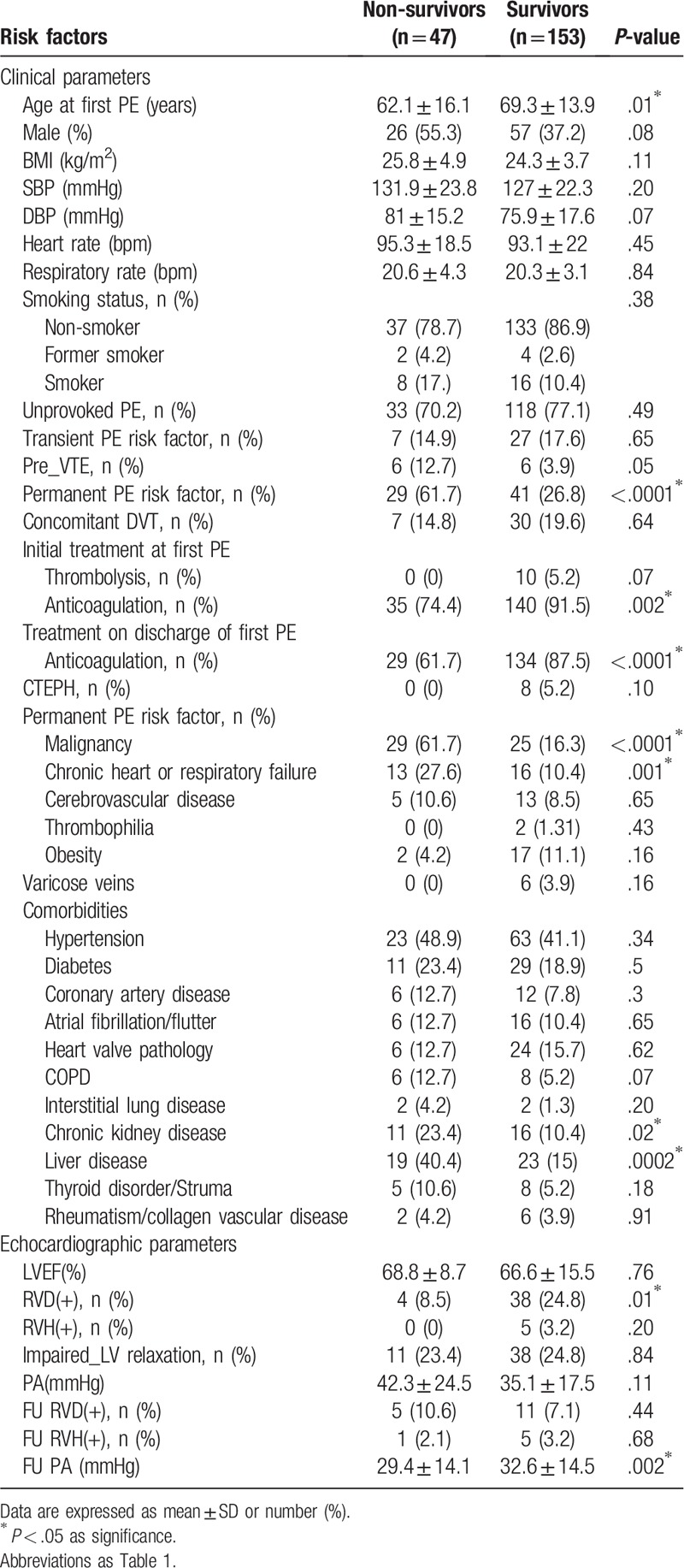
The survival rate of patients post-acute PE in the third and fifth year was 75.89% and 73.49%, respectively. Among the 200 patients with acute PE, 47 died during the follow-up period. Sixteen patients died of heart failure, 8 died of respiratory failure, 20 died of sepsis, and the causes of death of the three remaining patients were not recorded. The non-survivors tended to be younger and with permanent PE risk factors, including an underlying malignancy, liver disease, and chronic heart and lung failure. Also, the use of anticoagulants was higher in survivors compared with non-survivors. Regarding the echocardiographic parameters, only RV dilatation at the diagnosis of PE and mildly elevated PA pressure during the follow-up period were significantly different between survivors and non-survivors (Table 3).
Table 3.
The comparison of risk factors between non-survivors and survivors post pulmonary embolism.
3.4. The univariate and multivariable analyses of mortality
According to simple Cox regression, the influence of age at first PE, sex, DBP, and permanent PE risks on the prediction of the subsequent mortality was significant (Table 4). Malignancy (HR 6.68; 95% CI 3.01–14.83; P < .0001) as well as chronic heart or respiratory failure (HR 3.6; 95% CI 1.57–8.26; P = .0025) were permanent PE risk factors that increased the incidence of death. Concerning comorbidities, chronic obstructive pulmonary disease (HR 3.4; 95% CI 1.26–9.17; P = .0158), and liver disease (HR 3.43; 95% CI 1.53–7.68; P = .0027) were associated with increased mortality. Moreover, previous venous thromboembolic events (VTE) (HR 3.57; 95% CI 1.06–12.04; P = .0403) were also potential risk factors that affected mortality. Conversely, all other echocardiographic parameters – except for RV dilatation at the diagnosis of PE – failed to indicate subsequent mortality. Multivariable analysis using multiple Cox regression confirmed that only malignancy (HR 5.43; 95% CI 2.51–11.72; P < .0001) and chronic heart or respiratory failure (HR 5.64; 95% CI 2.53–12.54; P < .0001) significantly predicted subsequent mortality (Table 5). In patients with malignancies or chronic heart/lung disease, the Kaplan–Meier plot displayed a significantly worse survival compared to patients without malignancies or chronic heart/lung disease (Fig. 3).
Table 4.
The univariate Cox regression analysis of mortality in patients post pulmonary embolism.
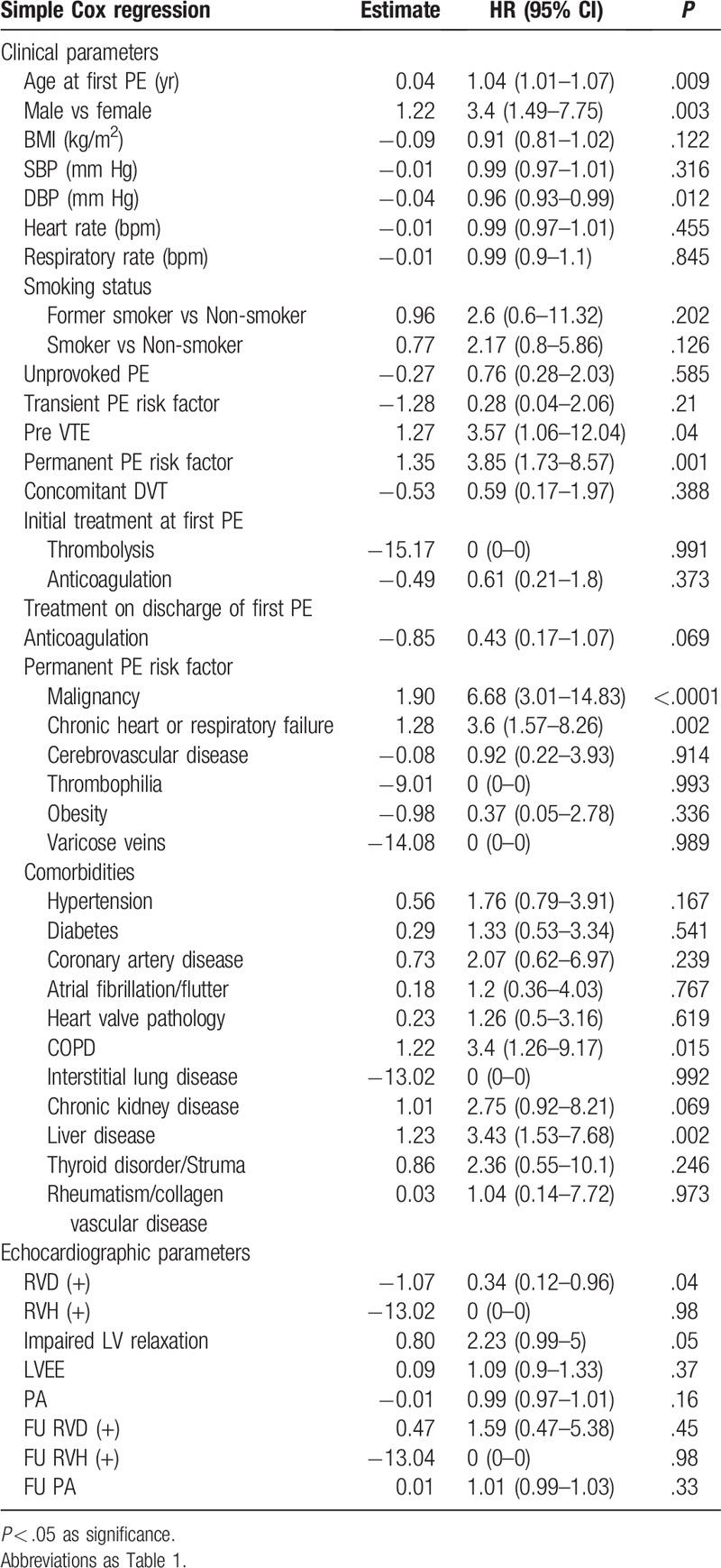
Table 5.
The multivariable Cox Regression analysis of mortality in patients post pulmonary embolism.
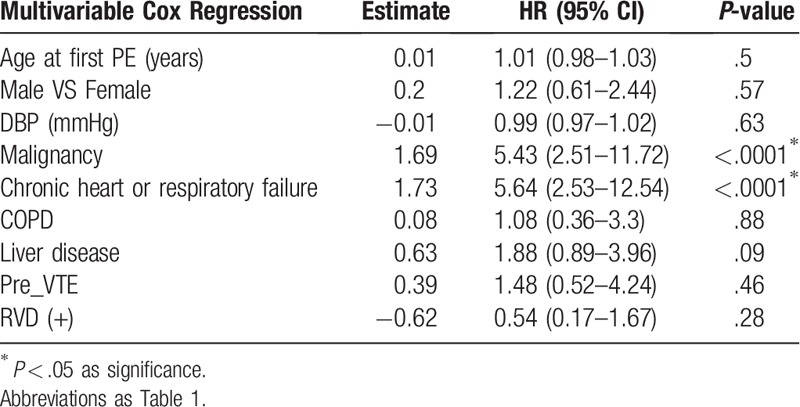
Figure 3.
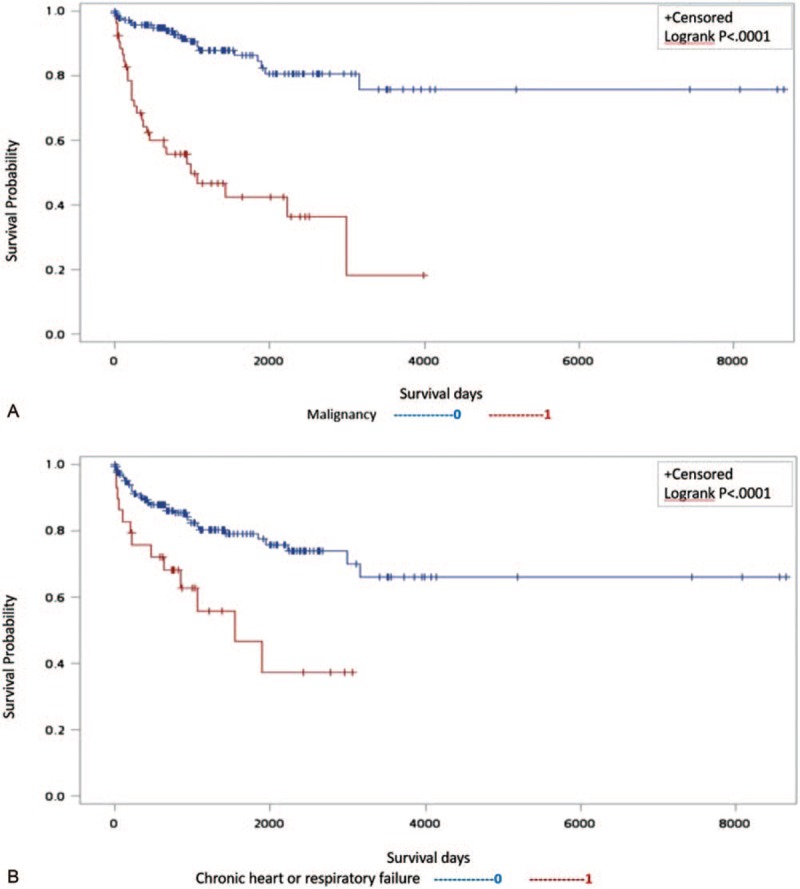
Kaplan–Meier plot representing a significantly worse survival compared with that of patients free from (A) malignancy or (B) chronic heart/lung disease.
4. Discussion
This was the first study to investigate the incidence of CTEPH and mortality in a Taiwanese population after the diagnosis of PE. The incidence of CTEPH was 4% with the median time was 36 months after the first episode of PE. Previous studies have reported that the incidence of CTEPH after symptomatic acute PE ranges from 0.1% to 9.1%.[4] With regard to the CTEPH incidence in some Asian countries, 1.7% and 6.1% were reported recently in China and Korea, respectively.[9,12] Several factors may affect the estimation of the incidence of CTEPH, such as selected populations, diagnostic methods, management of acute PE, and follow-up time.[5,9]
According to the current guidelines, patients with a history of venous thromboembolism who present with signs of right-sided heart failure should undergo a diagnostic evaluation for CTEPH.[8,12–14] However, early diagnosis of CTEPH – before the development of right heart failure – should be emphasized to enable early identification and intervention. Screening programs for CTEPH facilitate the detection of PH in specific at-risk populations. Nevertheless, it remains inconclusive whether screening should be conducted after acute PE, given the scant evidence.
Among the various modalities used to detect structural and functional effects of PH on the heart, transthoracic echocardiography is a non-invasive and simple screening tool. However, especially for patients with less-severe disease, false positive and false negative estimates are more frequent during transthoracic echocardiography compared with a right heart catheterization, which is the gold standard for PH diagnosis.[15] Therefore, the ESC guidelines do not support the routine application of echocardiography for acute PE during follow-up.[8] Conversely, it is more cost-efficient to identify patients with PE who have concomitant high risks for CTEPH.
In a recent meta-analysis which enrolled 772 PE-survivors generated a clinical prediction score for the diagnosis of CTEPH after PE.[16] Prediction factors included patients with unprovoked PE, known hypothyroidism, symptom onset weeks before PE diagnosis, right ventricular dysfunction, and diabetes mellitus.[16] In another study, patients who developed CTEPH tended to be older, had previous venous thromboembolic events, and more proximal PE than those without CTEPH.[6] Other reports indicated that medical conditions associated with an increased risk of CTEPH include male gender, a history of splenectomy, cancer, ventriculoatrial shunt, chronic inflammatory disease, and an increased body mass index.[13] Notably, in our study we also found that patients with risk factors such as unprovoked PE tended to develop CTEPH; however, most of the clinical risk factors were not associated with the subsequent diagnosis of CTEPH. Instead, echocardiographic parameters indicating RV dysfunction significantly predicted the development of CTEPH. Conversely, as we focused on the subsequent mortality post-acute PE, in multivariable Cox regression, the only clinical risk factors that significantly differentiated survivors from non-survivors were malignancy and chronic heart or respiratory failure.
Regarding the impact of low diastolic blood pressure, in the condition of geometric effects of RV enlargement and left ventricle (LV) chamber distortion in patients with CTEPH, the low LV preload, and the relative underfilling may result in the abnormal E/A ratio as well as the diastolic pressure.[17,18] Interestingly, female sex is a risk factor for the development of PAH, but the mortality rate was higher in male patients.[19,20] The sex paradox phenomenon was believed driven by the complex interaction between sex hormones and the pulmonary vasculature as well as RV dysfunction.[20] Correspondingly, we found the same condition in these patients with poor outcomes. Also, in the International Cooperative Pulmonary Embolism Registry, patients with chronic heart failure and chronic obstructive pulmonary disease reportedly demonstrated prognostic factors.[21] In our study, we further identified those risk factors, not only for their negative impact on outcomes of patients with PE, but also because of potential links to subsequent mortality. Similarly, patients with concomitant PE and liver disease may exhibit co-existing RV dysfunction while growing evidence supports the hypothesis that CTEPH is often misclassified as acute PE.[4,8] This reflected our findings that none of the patients diagnosed with CTEPH had a history of acute PE while CTEPH may be under-diagnosed or misclassified as acute PE.[22] Collectively, the early screening of echocardiography may help in predicting the development of CTEPH, but the clinical risk factors decide the subsequent mortality.
4.1. Study limitations
There are some limitations to this study. First, only 4% of patients were finally diagnosed with CTEPH and this small population may attenuate the statistic power. Nevertheless, according to the studies mentioned above, the incidence of CTEPH is not as high as the other types of PH.[4,9,12] Compared with the other major CTEPH registries consisting of hundreds of patients, our study also enrolled 358 patients with PE, which is fewer than others. Notably, in this study, patients with suspected CTEPH were referred for further evaluation, including RHC and V/Q scan. However, only some patients received RHC, while some patients refused RHC. This may have resulted in under-estimation of the incidence of CTEPH. Second, as a retrospective study, the echocardiographic parameters may not be measured consistently or in sufficient detail. Given that RV hypertrophy is a marker of long-standing pulmonary hypertension, the dilated and hypertrophic RV observed at baseline may imply a subclinical CTEPH before the diagnosis of PE. Also, the changes in PA pressure between patients with and without CTEPH were insignificant. Thus, we cannot know whether patients diagnosed with acute PE may also have under-diagnosed CTEPH. In addition to RV dimension and PA pressure, other important signs that suggested PH included flattening of the interventricular septum, right ventricular outflow Doppler acceleration time, early diastolic pulmonary regurgitation velocity, and inferior cava diameter.[8] Given the possible under-estimation of echo derived PA pressure in a chamber dilated RV, the gold standard test to diagnose CTEPH remains right heart catheterization, which was also performed in our study. In another perspective, using novel techniques, including speckle tracking, may also facilitate in early detection of RV dysfunction in patients post PE beyond the traditional echocardiography.[23,24]
Lastly, some clinical information which may be associated with CTEPH could have been missing from the medical records. Such information could include exercise, alcohol intake, high-sensitivity C-reactive protein, and depression.
4.2. Future directions
Despite the moderate incidence of CTEPH post PE, our finding suggests an urgent need to increase awareness of CTEPH, especially in patients with predisposing factors, to avoid under-diagnosis and under-treatment. Screening echocardiography post PE could be a feasible method for facilitating early diagnosis of subsequent CTEPH. However, larger scale prospective studies are required to further weigh the cost-efficiency of screening programs for early detection of CTEPH.
5. Conclusions
According to our findings, post-acute PE screening of CTEPH may facilitate early diagnosis and intervention, especially for those at high risk for developing CTEPH. Though the subsequent CTEPH is associated with RV echocardiographic parameters, the mortality is mainly dependent on underlying comorbidities. Therefore, optimal risk stratifications and managements of comorbidities are also crucial to improve patients’ outcomes.
Author contributions
Conceptualization: Chih-Hsin Hsu, Wei-Ting Li.
Data curation: Chih-Hsin Hsu, Wei-Ting Li, Hsien-Yuan Chang.
Formal analysis: Chih-Hsin Hsu, Wei-Ting Chang.
Funding acquisition: Chih-Hsin Hsu.
Investigation: Chih-Chan Lin, Hsien-Yuan Chang.
Methodology: Wei-Ting Chang.
Supervision: Chih-Hsin Hsu, Chih-Chan Lin, Wei-Ting Li, Hsien-Yuan Chang.
Validation: Chih-Chan Lin, Wei-Ting Li, Hsien-Yuan Chang, Wei-Ting Chang.
Visualization: Wei-Ting Li, Hsien-Yuan Chang, Wei-Ting Chang.
Writing – original draft: Chih-Hsin Hsu, Chih-Chan Lin, Wei-Ting Chang.
Writing – review and editing: Chih-Hsin Hsu, Wei-Ting Chang.
Footnotes
Abbreviations: COPD = chronic obstructive pulmonary disease, CTEPH = chronic thromboembolic pulmonary hypertension, PE = pulmonary embolism, RV = right ventricular, VTE = venous thromboembolic events.
How to cite this article: Hsu CH, Lin CC, Li WT, Chang HY, Chang WT. Right ventricular dysfunction is associated with the development of chronic thromboembolic pulmonary hypertension but not with mortality post-acute pulmonary embolism. Medicine. 2019;98:48(e17953).
Dr Chang is supported by the National Health Research Institute, Taiwan (NHRI-EX106- 10618SC).
Bayer funded this investigator-initiated study. Dr Hsu reported receiving consulting fees from Bayer, Actelion, Pfizer, and Novartis.
References
- [1].Lang IM, Dorfmuller P, Vonk Noordegraaf A. The pathobiology of chronic thromboembolic pulmonary hypertension. Ann Am Thorac Soc 2016;13: Suppl 3: S215–21. [DOI] [PubMed] [Google Scholar]
- [2].Simonneau G, Torbicki A, Dorfmüller P, et al. The pathophysiology of chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017;26:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Keogh AM, Mayer E, Benza RL, et al. Interventional and surgical modalities of treatment in pulmonary hypertension. J Am Coll Cardiol 2009;54: Suppl: S67–77. [DOI] [PubMed] [Google Scholar]
- [4].Lang IM, Pesavento R, Bonderman D, et al. Risk factors and basic mechanisms of chronic thromboembolic pulmonary hypertension: a current understanding. Eur Respir J 2013;41:462–8. [DOI] [PubMed] [Google Scholar]
- [5].Ende-Verhaar YM, Cannegieter SC, Vonk Noordegraaf A, et al. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: a contemporary view of the published literature. Eur Respir J 2017;49:1601792. [DOI] [PubMed] [Google Scholar]
- [6].Guérin L, Couturaud F, Parent F, et al. Prevalence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. Prevalence of CTEPH after pulmonary embolism. Thromb Haemost 2014;112:598–605. [DOI] [PubMed] [Google Scholar]
- [7].Pengo V, Lensing AW, Prins MH, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004;350:2257–64. [DOI] [PubMed] [Google Scholar]
- [8].Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Rev Esp Cardiol 2016;69:177. [DOI] [PubMed] [Google Scholar]
- [9].Yang S, Yang Y, Zhai Z, et al. Incidence and risk factors of chronic thromboembolic pulmonary hypertension in patients after acute pulmonary embolism. J Thorac Dis 2015;7:1927–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Giuliani L, Piccinino C, D’Armini MA, et al. Prevalence of undiagnosed chronic thromboembolic pulmonary hypertension after pulmonary embolism. Blood Coagul Fibrinolysis 2014;25:649–53. [DOI] [PubMed] [Google Scholar]
- [11].Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;685–713:786–8. [DOI] [PubMed] [Google Scholar]
- [12].Park JS, Ahn J, Choi JH, et al. The predictive value of echocardiography for chronic thromboembolic pulmonary hypertension after acute pulmonary embolism in Korea. Korean J Intern Med 2017;32:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kim NH, Lang IM. Risk factors for chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2012;21:27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bonderman D, Wilkens H, Wakounig S, et al. Risk factors for chronic thromboembolic pulmonary hypertension. Eur Respir J 2009;33:325–31. [DOI] [PubMed] [Google Scholar]
- [15].D’Alto M, Romeo E, Argiento P, et al. Accuracy and precision of echocardiography versus right heart catheterization for the assessment of pulmonary hypertension. Int J Cardiol 2013;168:4058–62. [DOI] [PubMed] [Google Scholar]
- [16].Klok FA, Dzikowska-Diduch O, Kostrubiec M, et al. Derivation of a clinical prediction score for chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. J Thromb Haemost 2016;14:121–8. [DOI] [PubMed] [Google Scholar]
- [17].Mahmud E, Raisinghani A, Hassankhani A, et al. Correlation of left ventricular diastolic filling characteristics with right ventricular overload and pulmonary artery pressure in chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol 2002;40:318–24. [DOI] [PubMed] [Google Scholar]
- [18].Gurudevan SV, Malouf PJ, Auger WR, et al. Abnormal left ventricular diastolic filling in chronic thromboembolic pulmonary hypertension: true diastolic dysfunction or left ventricular underfilling? J Am Coll Cardiol 2007;49:1334–9. [DOI] [PubMed] [Google Scholar]
- [19].Chang WT, Weng SF, Hsu CH, et al. Prognostic factors in patients with pulmonary hypertension – a nationwide cohort study. J Am Heart Assoc 2016;5:e003579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lahm T, Tuder RM, Petrache I. Progress in solving the sex hormone paradox in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2014;307:L7–26. [DOI] [PubMed] [Google Scholar]
- [21].Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 1999;9162:1386–9. [DOI] [PubMed] [Google Scholar]
- [22].Klok FA, Delcroix M, Bogaard HJ. Chronic thromboembolic pulmonary hypertension from the perspective of patients with pulmonary embolism. J Thromb Haemost 2018;16:1040–51. [DOI] [PubMed] [Google Scholar]
- [23].Li AL, Zhai ZG, Zhai YN, et al. The value of speckle-tracking echocardiography in identifying right heart dysfunction in patients with chronic thromboembolic pulmonary hypertension. Int J Cardiovasc Imaging 2018;34:1895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Patel B, Shah M, Garg L, et al. Trends in the use of echocardiography in pulmonary embolism. Medicine 2018;97:e12104. [DOI] [PMC free article] [PubMed] [Google Scholar]


