Supplemental Digital Content is available in the text
Keywords: adverse outcomes, malnourished patients, vitamin D deficiency
Abstract
The impact of vitamin D deficiency on the recovery of patients with malnutrition remains undefined. Our aim was to study the prevalence of vitamin D deficiency in a well-characterized cohort of patients with malnutrition and its association with outcomes.
Within this secondary analysis of a randomized controlled trial, we examined the association of vitamin D deficiency and adverse clinical outcomes over a follow-up of 180 days in hospitalized patients at risk for malnutrition. We measured 25-hydroxyvitamin D levels upon admission and defined Vitamin D deficiency when levels were <50nmol/l. The primary endpoint was 180-day mortality.
The prevalence of vitamin D deficiency in our cohort of 828 patients was 58.2% (n = 482). Patients with vitamin D deficiency had increased 180-day mortality rates from 23.1% to 29.9% (odds ratio 1.42, 95% confidence interval [CI] 1.03–1.94, P = .03). When adjusting the analysis for demographics, comorbidities, and randomization, this association remained significant for the subgroup of patients not receiving vitamin D treatment (adjusted odds ratio 1.63, 95% CI 1.01–2.62, P = .04). There was no significantly lower risk for mortality in the subgroup of vitamin D deficient patients receiving vitamin D treatment compared to not receiving treatment (adjusted odds ratio 0.74, 95% CI 0.48–1.13, P = .15).
Vitamin D deficiency is highly prevalent in the population of malnourished inpatients and is negatively associated with long-term mortality particularly when patients are not receiving vitamin D treatment. Our findings suggest that malnourished patients might benefit from vitamin D screening and treatment in case of deficiency.
1. Introduction
Vitamin D deficiency is common worldwide[1] and associated with increased risks for cancer, cardiovascular and infectious diseases, and mortality.[2–7] For this reason, international societies including the Endocrine Society of the United States, recommend prevention and treatment of vitamin D deficiency. The required vitamin D levels are according to age groups between 600 and 800 IU/d. If patients are already vitamin D deficient, higher levels of vitamin D (50,000 IU/week for 8 weeks) are needed to correct vitamin D deficiency. This therapy is then followed by a maintenance therapy of 1500 to 2000 IU/d.[8] Although there is ongoing discussion about the optimal vitamin D serum level, there is general consensus that a 25-hydroxyvitamin D (25(OH)D) serum level of less than 50 nmol/L is considered to be deficient and levels lower than 25 nmol/L are considered to be severely deficient.[9]
Several pre-clinical and clinical studies have found vitamin D to play a key role in different pathophysiological processes. Cholecalciferol is produced in the human skin through the transformation of 7-dehydrocholesterol by the action of UV B radiation. Additionally to the endogenous production that depends significantly on sun exposure, diet can also contain vitamin D, especially in food of animal origin.[10] Vitamin D is metabolized in the liver to 25(OH)D and later activated in the kidneys to 1.25-dihydroxyvitamin (1.25(OH)2D). The activated form interacts with more than 200 genes and strongly influences several cellular processes, such as proliferation, differentiation, and apoptosis. Angiogenesis, insulin and renin production as well as bone and muscle metabolism are also influenced by 1.25(OH)2D.[11,12]
Several clinical studies have focus on the importance of vitamin D in different patient populations also including hospitalized, medical inpatients. A recently systematic review and meta-analysis[13] investigated effects of vitamin D deficiency on adverse medical outcomes across different medical inpatient populations. While several observational studies reported vitamin D deficiency to be highly prevalent in medical inpatients and strongly associated with different adverse outcomes, there were only few intervention trials demonstrating that treatment with vitamin D can actually lower the risk for these adverse outcomes. Yet, while most identified studies have focused on healthy individuals or patients with specific diseases, there is a lack of study data regarding the population of severely ill and hospitalized patients with malnutrition – a condition that puts patients at particular risk for adverse clinical outcomes.[14,15] Understanding the prevalence of vitamin D deficiency and its association with outcome in this high-risk patient population may help to design preventive strategies.
To close this gap, we conducted a secondary analysis of a prospective trial including consecutive patients with malnutrition upon hospital admission. Our aim was to study the prevalence of vitamin D deficiency in this well-characterized cohort of patients with malnutrition and its association with outcomes. We also asked the question whether vitamin D treatment may reduce the risks associated with vitamin D deficiency.
2. Methods
2.1. Study design and setting
This is a secondary analysis of the prospective effect of early nutritional support on frailty, functional outcomes and recovery of malnourished medical inpatients trial (EFFORT). EFFORT was a pragmatic, investigator-initiated, open-label, multicentre trial that was undertaken in 8 Swiss hospitals from April 2014 to February 2018. The ethical committee of the Northwestern part of Switzerland (EKNZ; 2014_001) approved the study protocol and all patients or their authorized representatives provided written informed consent. The trial was registered at ClinicalTrials.gov (https://clinicaltrials.gov/ct2/show/NCT02517476). The main aim of this trial was to assess the effects of early nutritional therapy on patient outcomes in the medical inpatients setting. The rationale for the trial, design details, and eligibility features have been published previously[16] as have the main results of the trial.[17]
2.2. Patient population and management
EFFORT enrolled consecutive patients at nutritional risk (defined by a nutritional risk score [NRS] ≥3 points) with an expected length of hospital stay (LOS) ≥5 days if they were willing to provide informed consent. NRS includes assessment of the patient's nutritional status, which is based on weight loss, body mass index and general condition or food intake and disease severity (stress metabolism), and is associated with higher risk for adverse outcomes.[18,19] Each part is scored from 0 to 3 points, and patients receive an extra point for age above 70 years. Patients were excluded if initially admitted to intensive care units or surgical units, unable to ingest oral nutrition, already receiving nutritional support on admission, with a terminal condition (ie, end-of-life situation), hospitalized because of anorexia nervosa, acute pancreatitis, acute liver failure, cystic fibrosis or stem cell transplantation, after gastric bypass surgery, or with contraindications for nutritional support, and patients previously included in the trial.
While EFFORT included a total of 2028 patients, this secondary analysis focuses only on the 828 patients, where vitamin D was measured as part of the clinical routine, and we excluded the 1200 patients with no vitamin D measurement (see Appendix Table 5, for comparison of patients with and without Vitamin D measurement).
Upon admission, medical diagnosis according to International Statistical Classification of Disease and Related Health Problems 10th Revision (ICD) 10-codes, socio-demographic and anthropometric data, baseline muscle strength, and functional status using Barthel index was assessed in all patients based on the trial protocol. We also had a detailed history regarding vitamin D supplementation before hospital admission. After discharge, blinded study nurses contacted the patients after 30 and 180 days for a structured telephone interview. Different health-related outcomes and treatment history regarding vitamin D were systematically assessed at these time points.
2.3. Patient groups and endpoints
We classified patients based on their initial vitamin D level into vitamin D “deficient” (<50 nmol/L) and “not deficient” (≥50 nmol/L). Furthermore, patients were divided into “treated” and “untreated” groups for the statistical calculations (Fig. 1). For vitamin D measurement, we used a chemiluminescence immunoassay called IDS-iSYS 25-OH-Vitamin.[20] This assay detects 25(OH)D2 as well as 25(OH)D3.
Figure 1.
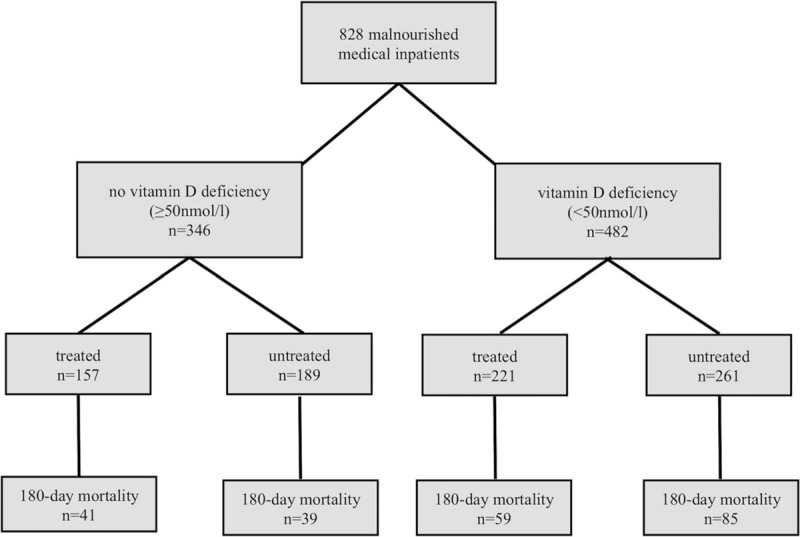
Patient classification.
Some patients had vitamin D supplementation upon hospital admission, mostly prescribed by their general practitioner or from a previous hospitalization and some patients were started on treatment during the index hospital stay. We thus classified patients as receiving vitamin D treatment (“treated patients”) if they received vitamin D supplementation before, during or after the index hospital stay and the “untreated” patients were those not receiving any kind of supplementation at any time.
The primary endpoint of this analysis is all-cause mortality within a follow-up of 180-days. Complete 180-day follow-up data were available in 95.2%. For the 4 patients lost to follow up, data carry forward from day 30 was used.
Secondary endpoints included mortality at 30-days, LOS, and functional outcomes including Barthel index decline and quality of life (QoL) each measured at 30 and 180 days.
Barthel index[17] measures performance in activities of daily living and comprises 2 groups of items, 1 related to self-care (feeding, grooming, bathing, dressing, bowel and bladder care, and toilet use), the other related to mobility (ambulation, transfers, and stair climbing). We used the German translation which has a score ranging from 100 to 0 with lower scores indicating more severe disability. Decline is defined as Barthel index reduction of 10% or more from admission.
QoL assessment is using the EuroQol group 5-dimension self-report questionnaire.[21] This included the European quality of life 5 dimensions index (values range from 0 to 1, with higher scores indicating better life quality) and the visual analog scale (EQ-5D VAS) (scores range from 0 to 100, with higher scores indicating better health status).
2.4. Statistical analysis
Patient characteristic continuous variables are expressed as medians (interquartile ranges, 25th–75th percentiles), while frequencies are reported as percentages or counts. Patient characteristics were compared using the Chi-square test and Fisher exact test for binary data, and nonparametric Mann–Whitney U test (MWU) tests for continuous data. For all binary data, logistic regression analysis was used, with results being reported with odds ratios (ORs) and 95% confident intervals (95% CI). Additionally, we adjusted our analysis for predefined possible confounders (model 1), such as demographics (ie, age and gender), comorbidities (ie, cardiovascular diseases, renal disease, and tumor), randomization, and vitamin D treatment.
The association between vitamin D levels and outcomes was assessed in the overall population and furthermore in a subgroup analysis for treated patients and untreated patients.
Evidence of effect modification within these subgroups was assessed by inclusion of interaction terms into the statistical models. All statistical analyses were performed using the STATA 12.1 software (Stata Corp, College Station, TX). A P-value <.05 was considered statistically significant.
3. Results
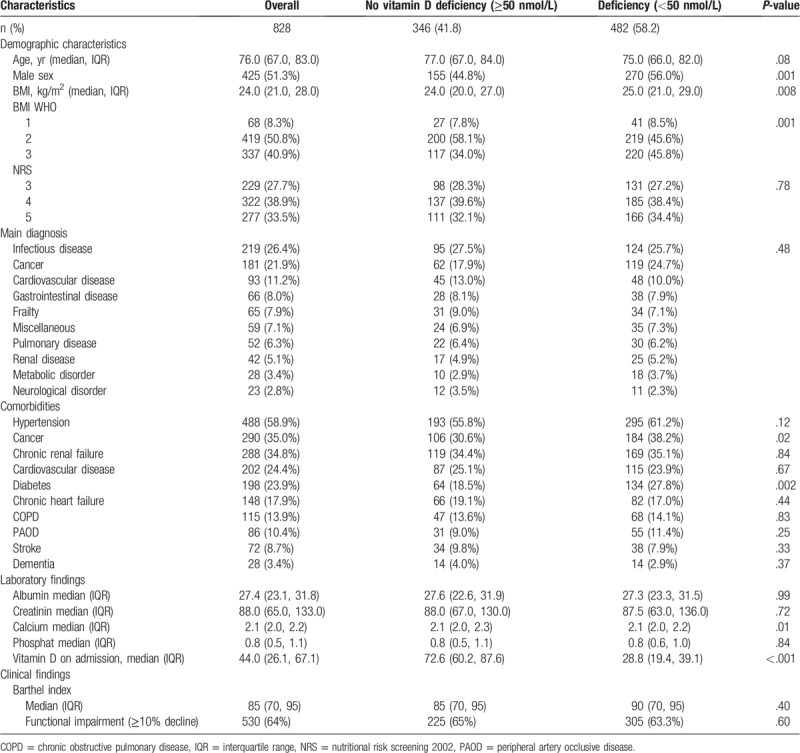
In our population of 828 malnourished inpatients, the prevalence of vitamin D deficiency was 58.2% (n = 482) including 188 patients (22.7%) who had severe deficiency with a vitamin D level of <25 nmol/L. Baseline characteristics are shown in Table 1, stratified according to their vitamin D levels. Median age of the population was 76 years and 45.7% were younger than 75 years. About half of the population was female (n = 403, 48.7%) and vitamin D deficiency was more often found in males as compared to females.
Table 1.
Baseline characteristics overall and stratified by vitamin D status.
3.1. Association of vitamin D deficiency and primary and secondary endpoints
Patients with vitamin D deficiency had an increased 180-day mortality rate of 29.9% (144/482) compared to 23.1% (80/346) in patients with no deficiency (Fig. 2A). This was also confirmed in a logistic regression analysis with an OR of 1.42 (95% CI 1.03–1.94, P = .03) for vitamin D deficiency (Table 2). Also, in a subgroup analysis, we found a significant association between vitamin D deficiency and 180-day mortality in patients, who never received vitamin D treatment (adjusted OR 1.63 [95% CI 1.01–2.62], P = .04) (Fig. 2B). In the subgroup of patients who received vitamin D treatment, there was no significant association between vitamin D deficiency and 180 days mortality (adjusted OR 0.98 [95% CI 0.60–1.61], P = .95) (Fig. 2C). This difference in subgroup effects was also found to be strong in an interaction analysis by vitamin D treatment (P for interaction .07 regarding 180-day mortality).
Figure 2.
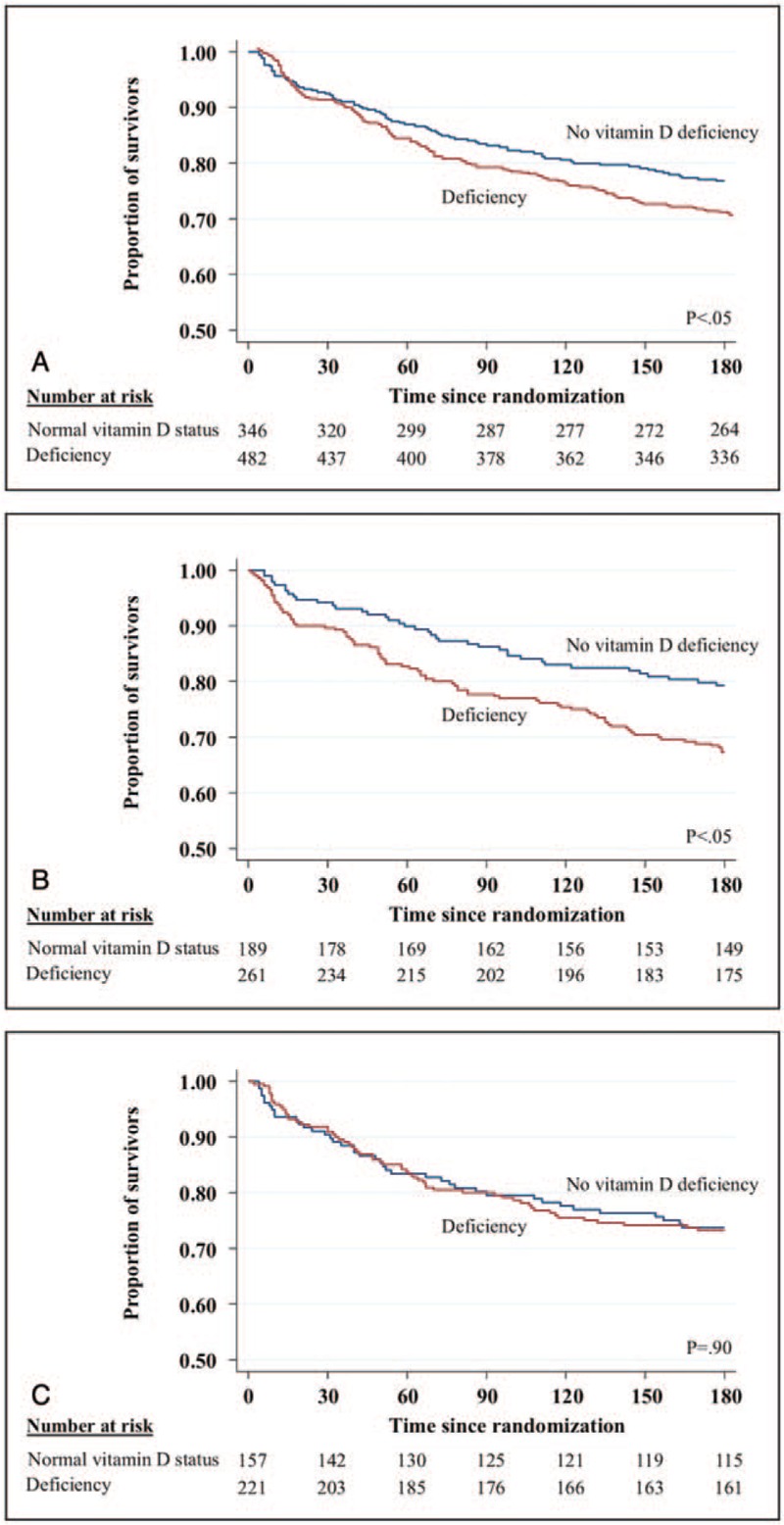
Correlation of vitamin D deficiency and survival in different patients populations. (A) 180-d mortality in the overall population; (B) 180-d mortality in untreated patients; (C) 180-d mortality in treated patients.
Table 2.
Long-term outcomes.
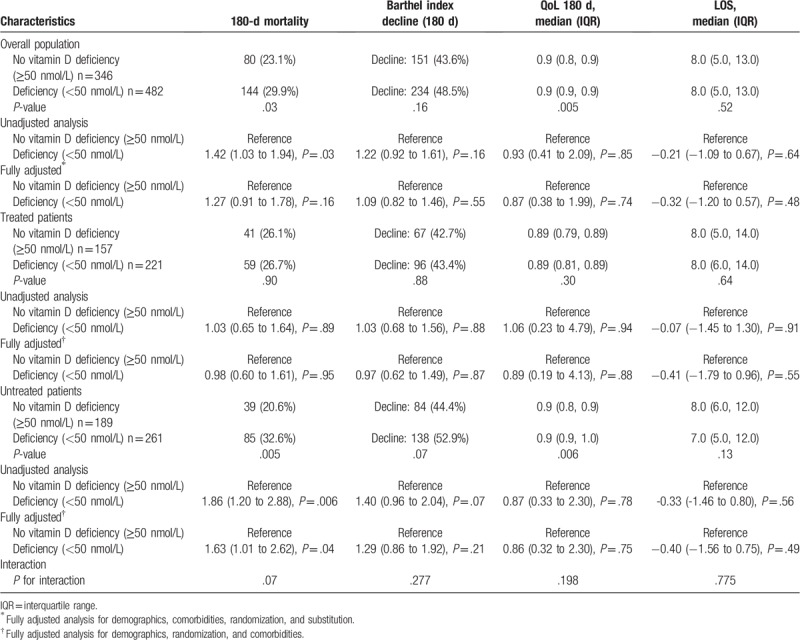
We also investigated associations of vitamin D deficiency and different short-term outcomes measured at 30 days and long-term outcomes measured at 180 days. Regarding short-term outcomes, no significant difference was found for 30-days mortality between patients with and without vitamin D deficiency. We also performed a subgroup analysis, where no significant effects were found (Table 3). Also, we did not find a significant difference in decline of Barthel Index after 30-days, neither for reduced QoL between vitamin D sufficient and deficient patients.
Table 3.
Short-term outcomes.
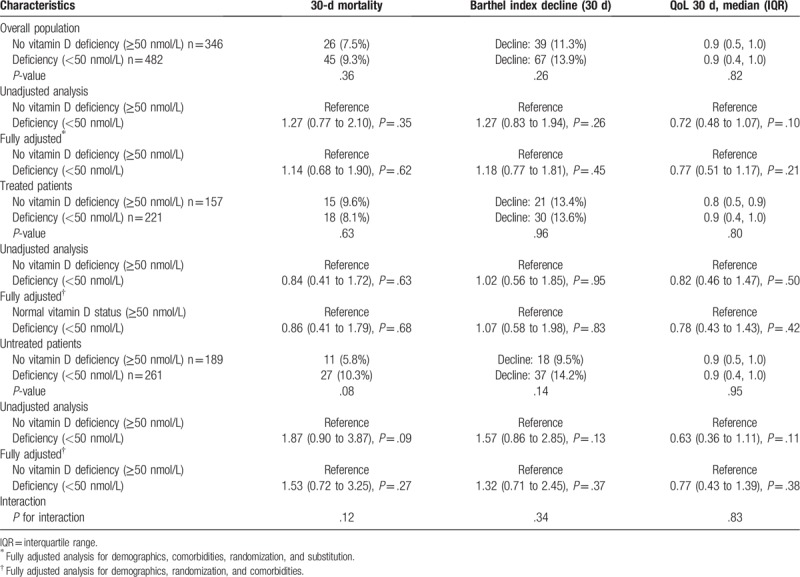
Although patients with vitamin D deficiency had worse long-term outcomes regarding functionality (Barthel index and QoL) at 180 days, the overall statistical analysis did not show any significant association of vitamin D deficiency and adverse outcomes in the overall population. The effects of vitamin D deficiency were again more pronounced in patients not treated with vitamin D, but were not statistically significant.
3.2. Association of vitamin D deficiency and severe deficiency on primary and secondary endpoints
To complement the analysis, we conducted all the calculations for 3 patient groups (no deficiency ≥50 nmol/L, deficiency ≥25 nmol–49.9 nmol/L, and severe deficiency <25nmol/L) (See Supplemental Digital Content 2–4 for long-term outcomes, short-term outcomes, and treatment as well as the baseline characteristics in Digital Content 1 and patient classification in Digital Content 5). Similar to the initial analysis, 180-day mortality was increased for vitamin D deficient and severely deficient patients (33.3% [98/294] and 24.5% [46/188], P = .01). In a logistic regression analysis, this association could only be confirmed for vitamin D deficient patients with an OR of 1.66 (95% CI 1.17–2.36, P = .004) and stayed robust after adjustment for model 1 (See supplemental Digital Content 2). In a subgroup analysis, there was also an association between vitamin D deficiency and 180-day mortality in patients who never received vitamin D treatment (adjusted OR 1.84 [95% CI 1.10–3.07], P = .02). We could not find such associations for patients with severe vitamin D deficiency nor for patients who received vitamin D treatment.
We also investigated the associations of vitamin D deficiency and severe deficiency for other short and long-term outcomes. A significant long-term outcome was decline of Barthel index after 180 days (unadjusted OR 1.42 [95% CI 1.04–1.94], P = .02). In a subgroup analysis, we could only find a significant association between Barthel index decline and vitamin D deficient patients, adjusted OR 1.70 (95% CI 1.09–2.66, P = .01) but not for severely deficient patients.
Regarding short-term outcomes, there was only a significant association for 30-days mortality for vitamin D deficient patients who never received vitamin D treatment (unadjusted OR 2.17 [95% CI 1.01–4.68], P = .04). We could not find any significant association between severely vitamin D deficient patients and any of our endpoints.
3.3. Effects of vitamin D treatment on outcomes
In an initial analysis, there was an inverse linear association of vitamin D deficiency and mortality (Fig. 3A). After dividing the population into 2 groups (“treated” and “untreated” patients) vitamin D deficiency correlated with increased mortality for patients with no vitamin D treatment. Compared to this association, the graph did not show a correlating increased mortality with low vitamin D levels on admission for patients who received vitamin D treatment (Fig. 3B). This brought us to the hypothesis that malnourished medical inpatients may benefit from vitamin D treatment in terms of long-term survival.
Figure 3.
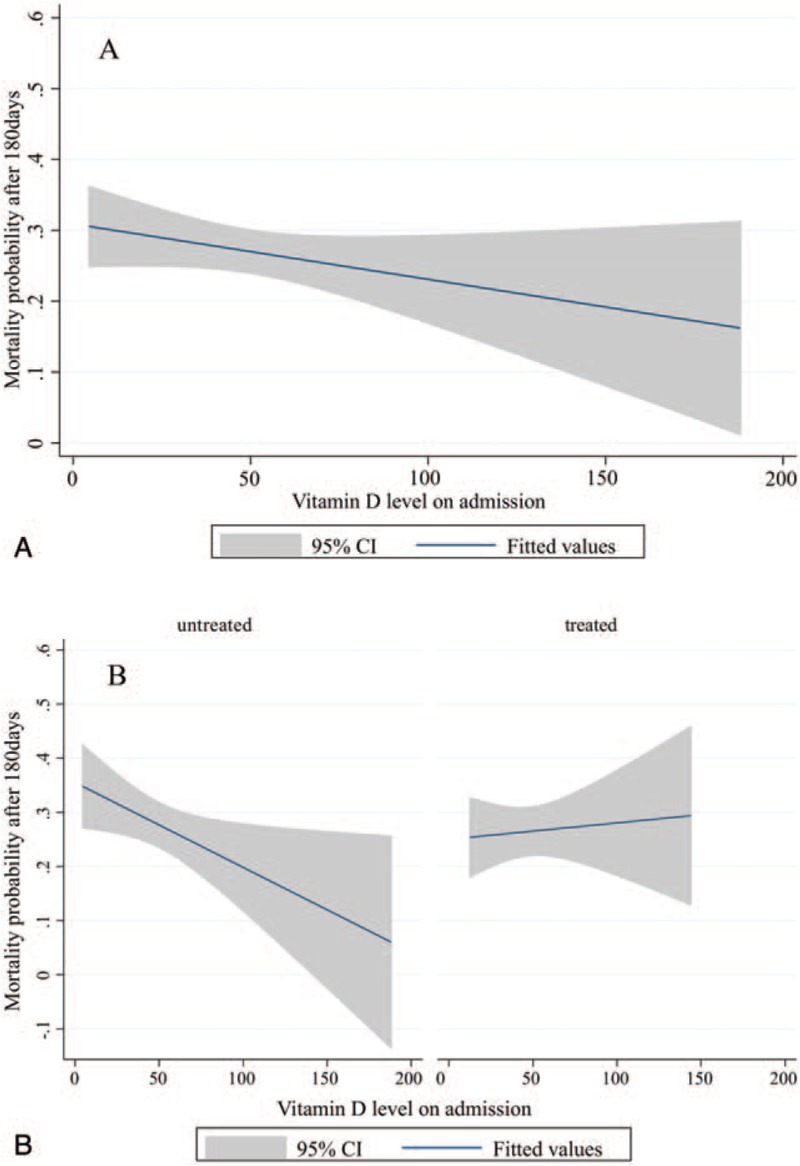
Probability of mortality according to Vitamin D status in different patients population. (A) Mortality probability and vitamin D status on admission in the overall population. (B) Mortality probability and vitamin D status on admission in treated and untreated patients.
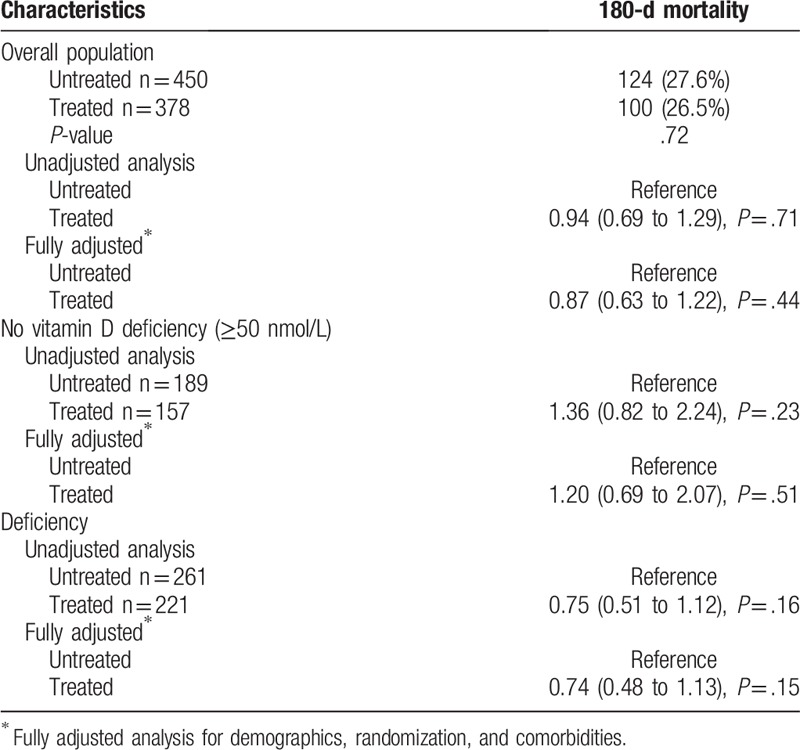
We; therefore, investigated the effects of vitamin D treatment on clinical outcomes. Overall, there was no association of vitamin D treatment with improved clinical outcomes (Table 4).
Table 4.
Treatment.
4. Discussion
The findings of our analysis regarding vitamin D deficiency in a population of malnourished medical inpatients are 3-fold. First, we found a high prevalence of vitamin D deficiency in this vulnerable patient population of almost 60% with about 20% being severely deficient. Second, we found an association between vitamin D deficiency and increased 180-day mortality. This association remained robust in multivariate analysis in patients with no treatment for vitamin D, but not in patients where vitamin D treatment was established. Third, we also noted a trend towards lower risk for mortality when vitamin D treatment was established in the subgroup of patients with vitamin D deficiency.
Despite the high probability of vitamin D deficiency in patients with malnutrition, there is lack of clinical data focusing on this particular group of patients. According to the National Health and Nutrition Examination Survey (2005/2006), the prevalence of vitamin D deficiency defined as levels <50 nmol/L was 41.6% for general adult persons.[22] According to a recent systematic review on the topic,[13] the prevalence of vitamin D deficiency in general medical inpatients was found to be around 50% using the same cut-off. Our data show an even higher number for the population of malnourished patients. This could be a consequence of the severe illness and therefore patients not being able to spend time outside. This results in reduced sun exposure and eventually in vitamin D deficiency. Another explanation could be the fact, that patients are malnourished and therefore are likely to have a diet containing low vitamin D. To evade this potential confounder, we adjusted our analysis to the randomization group of the EFFORT trial (individual nutritional support or standard nutrition). Our data provide evidence that a systematic screening for vitamin D deficiency is warranted in the population of malnourished patients although trials that prove vitamin D treatment to reduce adverse outcomes in this population are still lacking.
Similar to a recent study looking at general medical inpatients[13] we also found an association of vitamin deficiency and higher mortality at 180 days. This association was still robust after multivariate adjustment in the subgroup of patients not receiving vitamin D treatment while in treated patients no harmful associations were found. In addition, we noted a trend of lower risks among treated patients with vitamin D deficiency compared to untreated patients. These findings from our observational study do not prove that vitamin D supplementation in this patient population would improve outcomes, but gives a strong rational to perform trials investigating the effects of vitamin D on outcomes in this patient population. Importantly, a recently published large randomized, double-blind, placebo-controlled study could not show, that high dose vitamin D (2000 IU/d) lowers the risk of cancer of any type and major cardiovascular events in generally healthy persons (hazard ratio [HR] 0.96 [95% CI 0.88–1.06]; P = .47 and HR 0.97 [95% CI 0.85–1.12]; P = .69, respectively).[23] Even though, this was a very well-conducted trial, the results are hard to compare with ours because of the 2 very diverse patient populations. The investigated patients in the study from Manson et al included young and healthy adults, mean age 67.1 years and with no history of cardiovascular diseases or cancer. Our study population was malnourished inpatients with several comorbidities and mean age is 76 years. We; therefore, think the conclusion of this trial cannot be transferred to malnourished medical inpatients. Conducting further trials focusing on vitamin D deficiency and potential benefit from treatment in malnourished medical inpatients is warranted.
Another recently published systematic review focused on the effect of vitamin D supplementation on fractures, falls and bone density.[24] It showed no effect of vitamin D supplementation to prevent fractures or falls or has significant effects on bone mineral density. They have analyzed 81 randomized controlled trials of vitamin D supplements that reported falls, fractures or bone mineral density as an outcome. Conversely, Neelemaat et al[25] could show a decrease in falls in malnourished patients who received oral nutritional intervention. The intervention group received energy and protein-enriched diet with vitamin D supplementation during the in-hospital period and showed a decreased number of individuals with falls and fall incidents compared to the control group. This association was not significant anymore, when they looked at vitamin D deficient patients only. This leads to the assumption that the positive effect is most likely due to the combined intervention and not only due to vitamin D supplementation. The inconsistent recommendations of vitamin D supplementation to reduce falls, fractures, and mineral bone density might explain why some guidelines recommend vitamin D supplementation and others do not.
Clearly, more studies are needed to confirm our findings and to establish whether vitamin D treatment has positive effects in the population of malnourished inpatients.
Our study has several strengths, including a large and well-characterized population of malnourished medical inpatients with assessment of different outcomes and rigorously adjusted analyses based on a prespecified analysis plan. Still, we are aware of several limitations. Most importantly, patients were not randomized to receive vitamin D treatment or not and there is thus possible confounding by indication (see Appendix Table 6). We classified patients as “treated” and “untreated” according to the prescribed vitamin D supplement on hospital admission. Because most patients that had vitamin D supplements on admission were discharged again with a vitamin D order, we did not further look into subgroups based on timing of vitamin D treatment. Also, we did not have any information about compliance with vitamin D treatment before and after hospitalization. Furthermore, vitamin D levels were measured only in 828 patients out of 2088 malnourished patients, which may limit generalizability. Finally, this is an observational study and the decision about vitamin D treatment in our study was up to the treating physician team. Thus our data do not prove causality of vitamin D deficiency in malnourished patients and adverse clinical outcomes. There is risk for unmeasured factors, such as dietary intake, sun exposure, genetic variants, and lifestyles, which could interfere with our analysis. To close this gap, interventional studies are needed to verify that vitamin D deficient, malnourished patients show better outcomes after substitution.
5. Conclusion
In conclusion, vitamin D deficiency is highly prevalent in the population of malnourished inpatients and is negatively associated with long-term mortality particularly when patients are not receiving vitamin D treatment. Interventional trials should investigate the potential benefit from vitamin D screening and treatment in malnourished patients with deficiency.
Acknowledgments
The authors thank all the contributing authors from the EFFORT trial, namely Rebecca Fehr, Valerie Baechli, Martina Geiser, Manuela Deiss, Filomena Gomes, Alexander Kutz, Sarah Schmid, Carmen Benz, Silvia Mattmann, and Claudia Brand for their support.
In agreement with the initial trial, all acknowledged persons agreed to be named.
Author contributions
Data curation: Pascal Tribolet, Nina Braun, Claus Hoess, Vojtech Pavlicek, Stefan Bilz, Sarah Sigrist, Michael Braendle, Christoph Henzen, Robert Thomann, Jonas Rutishauser, Drahomir Aujesky, Nicolas Rodondi, Jaques Donzé, Zeno Stanga, Beat Mueller.
Writing – original draft: Meret Merker, Aline Amsler, Renata Pereira.
Writing – review and editing: Philipp Schuetz, Rebekka Bolliger, Pascal Tribolet, Nina Braun, Claus Hoess, Vojtech Pavlicek, Stefan Bilz, Sarah Sigrist, Michael Braendle, Christoph Henzen, Robert Thomann, Jonas Rutishauser, Drahomir Aujesky, Nicolas Rodondi, Jaques Donzé, Zeno Stanga, Beat Mueller.
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, EFFORT = effect of early nutritional support on frailty, functional outcomes, and recovery of malnourished medical inpatients trial, HR = hazard ratio, LOS = length of hospital stay, NRS = nutritional risk score, OR = odds ratio, QoL = quality of life.
How to cite this article: Merker M, Amsler A, Pereira R, Bolliger R, Tribolet P, Braun N, Hoess C, Pavlicek V, Bilz S, Sigrist S, Brändle M, Thomann R, Rutishauser J, Aujesky D, Rodondi N, Donzé J, Stanga Z, Mueller B, Schuetz P. Vitamin D deficiency is highly prevalent in malnourished inpatients and associated with higher mortality: A prospective cohort study. Medicine. 2019;98:48(e18113).
MM, AA, and RP are contributed equally and first authors to this study.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Bosomworth NJ. Mitigating epidemic vitamin D deficiency: the agony of evidence. Can Fam Physician 2011;57:16–20. [PMC free article] [PubMed] [Google Scholar]
- [2].Chowdhury R, Kunutsor S, Vitezova A, et al. Vitamin D and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ 2014;348:g1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Garland CF, Kim JJ, Mohr SB, et al. Meta-analysis of all-cause mortality according to serum 25-hydroxyvitamin D. Am J Public Health 2014;104:e43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zittermann A, Prokop S. The role of vitamin D for cardiovascular disease and overall mortality. Adv Exp Med Biol 2014;810:106–19. [DOI] [PubMed] [Google Scholar]
- [5].Nnoaham KE, Clarke A. Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis. Int J Epidemiol 2008;37:113–9. [DOI] [PubMed] [Google Scholar]
- [6].Klassen KM, Fairley CK, Chen M, et al. Vitamin D deficiency may be associated with a more rapid decline in CD4 cell count to < 350 cells/microL in untreated HIV-infected adults. Curr HIV Res 2015;13:517–23. [DOI] [PubMed] [Google Scholar]
- [7].Feldman D, Krishnan AV, Swami S, et al. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer 2014;14:342–57. [DOI] [PubMed] [Google Scholar]
- [8].Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–30. [DOI] [PubMed] [Google Scholar]
- [9].Wimalawansa SJ. Vitamin D in the new millennium. Curr Osteoporos Rep 2012;10:4–15. [DOI] [PubMed] [Google Scholar]
- [10].Borel P, Caillaud D, Cano NJ. Vitamin D bioavailability: state of the art. Crit Rev Food Sci Nutr 2015;55:1193–205. [DOI] [PubMed] [Google Scholar]
- [11].DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr 2004;80: 6 Suppl: 1689S–96S. [DOI] [PubMed] [Google Scholar]
- [12].Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocrine Rev 2005;26:662–87. [DOI] [PubMed] [Google Scholar]
- [13].Graedel L, Merker M, Felder S, et al. Vitamin D deficiency strongly predicts adverse medical outcome across different medical inpatient populations: results from a prospective study. Medicine (Baltimore) 2016;95:e3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Felder S, Braun N, Stanga Z, et al. Unraveling the link between malnutrition and adverse clinical outcomes: association of acute and chronic malnutrition measures with blood biomarkers from different pathophysiological states. Ann Nutr Metab 2016;68:164–72. [DOI] [PubMed] [Google Scholar]
- [15].Felder S, Lechtenboehmer C, Bally M, et al. Association of nutritional risk and adverse medical outcomes across different medical inpatient populations. Nutrition 2015;31:1385–93. [DOI] [PubMed] [Google Scholar]
- [16].Schuetz P, Fehr R, Baechli V, et al. Design and rationale of the effect of early nutritional therapy on frailty, functional outcomes and recovery of malnourished medical inpatients (EFFORT): a pragmatic, multicenter, randomized-controlled trial. Int J Clin Trials 2018;5:77. [Google Scholar]
- [17].Schuetz P, Fehr R, Baechli V, et al. Individualised nutritional support in medical inpatients at nutritional risk: a randomised clinical trial. Lancet 2019;393(10188):2312–21. doi: 10.1016/S0140-6736(18)32776-4. Epub 2019 Apr 25. [DOI] [PubMed] [Google Scholar]
- [18].Kondrup J, Rasmussen HH, Hamberg O, et al. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr 2003;22:321–36. [DOI] [PubMed] [Google Scholar]
- [19].Reber E, Gomes F, Vasiloglou MF, et al. Nutritional risk screening and assessment. J Clin Med 2019;8:pii: E1065.doi: 10.3390/jcm8071065. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Simpson CA, Cusano AM, Bihuniak J, et al. Effect of 25(OH) vitamin D reference method procedure (RMP) alignment on clinical measurements obtained with the IDS-iSYS chemiluminescent-based automated analyzer. J Steroid Biochem Mol Biol 2015;148:41–6. [DOI] [PubMed] [Google Scholar]
- [21].Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53–72. [DOI] [PubMed] [Google Scholar]
- [22].Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res 2011;31:48–54. [DOI] [PubMed] [Google Scholar]
- [23].Manson JE, Cook NR, Lee IM, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med 2018. [Google Scholar]
- [24].Bolland MJ, Grey A, Avenell A. Effects of vitamin D supplementation on musculoskeletal health: a systematic review, meta-analysis, and trial sequential analysis. Lancet Diabetes Endocrinol 2018;6:847–58. [DOI] [PubMed] [Google Scholar]
- [25].Neelemaat F, Lips P, Bosmans JE, et al. Short-term oral nutritional intervention with protein and vitamin D decreases falls in malnourished older adults. J Am Geriatr Soc 2012;60:691–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


