Abstract
Background:
Vitamin D is a fat-soluble vitamin that is related to the health of the human body and is an indispensable nutrient for human beings. Some studies indicated that type 2 diabetes mellitus (T2DM) with diabetic peripheral neuropathy (DPN) may be associated with vitamin D deficiency, but the current understanding of this point of view remains controversial. This study aimed to evaluate the correlation between serum 25-hydroxyl vitamin D (25 [OH] D) concentration and DPN in patients with T2DM by a meta-analysis, and to provide a reference for doctors.
Methods:
Relevant studies were selected from the PubMed, Cochrane Library, China National Knowledge Infrastructure, VIP databases, and Wanfang Data Knowledge Service Platform databases dating from 2000 to December 2017. A total of 75 articles related to serum 25 (OH) D and DPN were selected from 2000 to December 2017. Based on the inclusion and exclusion criteria of the literature, a quality assessment was conducted using the Newcastle–Ottawa scale, and a meta-analysis was performed by RevMan5.3 statistical software.
Results:
Thirteen studies that involved a total of 2814 type 2 diabetic patients were finally included into the meta-analysis. Meta-analysis results, heterogeneity test showed that, P < .000 01, I2 = 92%, calculation by random effect model revealed that, the serum concentration of 25 (OH) D in T2DM combined with DPN group was lower than that in the group without DPN (weighted mean difference = −0.74, 95% confidence interval: −1.03 to −0.46)
Conclusions:
Vitamin D is associated with type 2 DPN (DPN), and vitamin D deficiency can lead to an increased risk of type 2 DPN. However, more high-quality research is needed.
Keywords: 25-hydroxyvitamin D, diabetic peripheral neuropathy, meta-analysis, Newcastle–Ottawa scale, type 2 diabetes
1. Introduction
Diabetes, a major lifestyle disease, has become a global burden. In developing countries, the prevalence of diabetes is rising rapidly. In many developing countries, China is the biggest contributor to diabetes, followed by India.[1] Type 2 diabetes (T2DM) has become a major global healthcare issue, and its incidence is reported to be an alarming increase.[2] With the rapid development of the economy and the acceleration of industrialization, the change of lifestyle and the acceleration of the aging have made the prevalence of diabetes in China rapidly rising. It has become an important chronic non-communicable disease, after cardiovascular disease and tumor, which seriously endangers the people's health. According to the World Health Organization, China's economic losses from diabetes and related cardiovascular diseases reached 557.7 billion dollars between 2005 and 2015.[3] Recently, the prevalence of diabetes in the world has risen dramatically, reaching 8.3% in 2014, which is equal to 3.87 million patients.[4] Diabetes mellitus is a progressive disease that causes complications and is broadly divided into small vessel disease, microvascular disease, and macrovascular disease. Microvascular complications affect the retina in the eyes, that is, diabetic retinopathy. Affecting the kidneys is known as diabetic nephropathy, affecting the peripheral nerve is called diabetic neuropathy.[1] However, the most common chronic complication of diabetes is neuropathy. At least 20% of patients with type 1 diabetes will have a distal symmetrical multiple neuropathy in 20 years, and 10% to 20% of newly diagnosed tT2DM with distal symmetric multiple neuropathy will increase to 50% in 10 years.[4] Indeed, the related neurological complications place a tremendous burden on the suffering patient and the whole society.[5] The peripheral nervous system is one of the organ systems that can affect diabetes.[6] In addition, peripheral neuropathy is a major cause of disability worldwide. Diabetes is the most common cause of neuropathy, accounting for 50%. More than half of diabetic patients suffer from neuropathy, and diabetic peripheral neuropathy (DPN) is a major cause of the decline in quality of life due to pain, loss of sensation, instability of gait, related injuries, foot ulcers, and amputations.[6] Furthermore, the occurrence and development of these complications can result in loss of vision and neurological function, impaired activity and cognition, reduced quality of life, limited employment and productivity, and increased costs for patients and society. Without control or treatment, irreversible damage or even death can arise.[7] A study[8] manifested that the prevalence of DPN worldwide is 2.4% to 78.8%, depending on the diagnostic method and population assessment (hospital or clinic). Risk factors included age, gender, duration of diabetes, blood sugar control, height, overweight and obesity, and insulin therapy. Another literature[9] also indicated that Peripheral neuropathy is a common chronic complication of diabetes, and the most common manifestation is the distal degenerative multiple neuropathy with sensory loss. About 20% to 30% patients may experience neuropathic pain. And the DPN affects the quality of life and life expectancy of the T2DM.[10]
How to prevent the DPN, to improve the quality of life in the patient has become the primary target for the treatment of type 2 diabetes. Relevant studies have shown that vitamin D is widely used in the human body, which is a multifunctional adrenocorticotropic hormone.[11] It is reported that vitamin D is associated with a variety of diseases, including cardiovascular disease, metabolic syndrome, cancer, multiple sclerosis, microbial infection, autoimmune disease, and Alzheimer's disease.[12] A study by Palomer X[13] elucidated that lack of vitamin D may result in poor glucose tolerance, insulin secretion change, and T2DM. Serum 25-hydroxyl vitamin D (25 [OH] D) deficiency is common in diabetic patients, and the low concentration of serum 25-(OH)D is associated with the severity of diabetic neuropathy.[14] Serum vitamin D is a steroid vitamin, mainly involved in the regulation of calcium and phosphorus metabolism. In recent years, it has been shown that serum 25-(OH)D can influence not only bones, but also chronic diseases such as T2DM, metabolic syndrome, and tumor.[15] Therefore, the study comprehensively evaluated the correlation of 25-(OH)D concentration and DPN in patients with T2DM by a meta-analysis.
2. Methods
This is a systematic review and ethical approval was not necessary.
2.1. Article search strategy
All published articles from 2000 to December 2017 and relevant to serum 25 (OH) D and DPN were searched on the PubMed, Cochrane Library, China National Knowledge Infrastructure (CNKI), VIP databases (VIP), and Wanfang Data Knowledge Service Platform databases (WANFANG). Simultaneously, we searched the bibliographies of the retrieved studies, narrative reviews, and meta-analyses to identify further relevant articles. The following terms were used as keywords: DPN, diabetic peripheral neuropathy, T2DM, type 2 diabetes, 25-(OH)D, 25-hydroxy vitamin D.
2.2. The inclusion criteria
To be included into the meta-analysis, the eligible study must meet the following inclusion criteria:
-
(1)
Case-control studies;
-
(2)
The patient was consistent with the diagnostic criteria for T2DM with DPN;
-
(3)
Literatures reviewed the relationship between serum 25(OH)D concentration and T2DM with DPN;
-
(4)
The literature directly or indirectly provided the mean ± standard deviation (X ± S) of serum 25(OH)D.
2.3. The exclusion criteria
-
(1)
Review of literatures;
-
(2)
Case reports;
-
(3)
Case-only studies;
-
(4)
There were short of data in documents;
-
(5)
Repeated references.
2.4. The quality assessment
According to Cochrane Evidence-based Practice Manual 5.1.0, each retrieve record was evaluated by the 2 researchers separately, if controversial, please another researcher to discuss and make a decision.[16] The study quality was assessed according to the Newcastle–Ottawa quality assessment scale (NOS):
-
(1)
selection;
-
(2)
comparability;
-
(3)
exposure.[17]
Among them, the selection part mainly evaluates 4 parts:
-
(1)
Is the case definition adequate?
-
(2)
Representativeness of the cases.
-
(3)
Selection of controls.
-
(4)
Definition of controls.
The comparability part mainly estimates 1 part: comparability of cases and controls on the basis of design or analysis. The exposure part mainly appraises 3 parts:
-
(1)
Ascertainment of exposure.
-
(2)
Same method of ascertainment for cases and controls.
-
(3)
Nonresponse rate.
The quality of the literature included was high. Every study was independently extracted and evaluated by 2 researchers, and the results were disputed. The third researcher was invited to participate in the study, and then the decision was made after discussion.
2.5. Statistical analysis
All statistical analyses were performed using Review Manager (version5.3).[18] Risk ratio (RR) was used for count data while standardized mean difference (SMD) was adopted for continuous variables as effect size, respectively, both of which were demonstrated with effect size and 95% confidence intervals (CI). If there was no heterogeneity among the studies, that is, a P-value greater than .10 or I2 less than 50%, it explained that the heterogeneity of the research was small, and the fixed effect model was used to analyze. A P-value less than .10 or I2 greater than 50% suggested that there was obvious heterogeneity among the included studies, the random effect model was used to combine the effect volume. The bias of the study was analyzed by funnel plot.
3. Results
3.1. Study selection
A total of 75 studies that were associated with serum 25 (OH) D and DPN were identified through the PubMed, Cochrane Library, CNKI, VIP, and WANFANG, 9 of which were duplicated. Of the remaining 66 studies, 48 were generally excluded based on the inclusion criteria. Finally, after reading the full manuscripts of the remaining 18 studies and conducting manual search of the reference lists of primary studies, 13 articles[12,14,15,19–28] were included in this study as they fulfilled our eligibility criteria. The study selection procedure is outlined in Figure 1.
Figure 1.
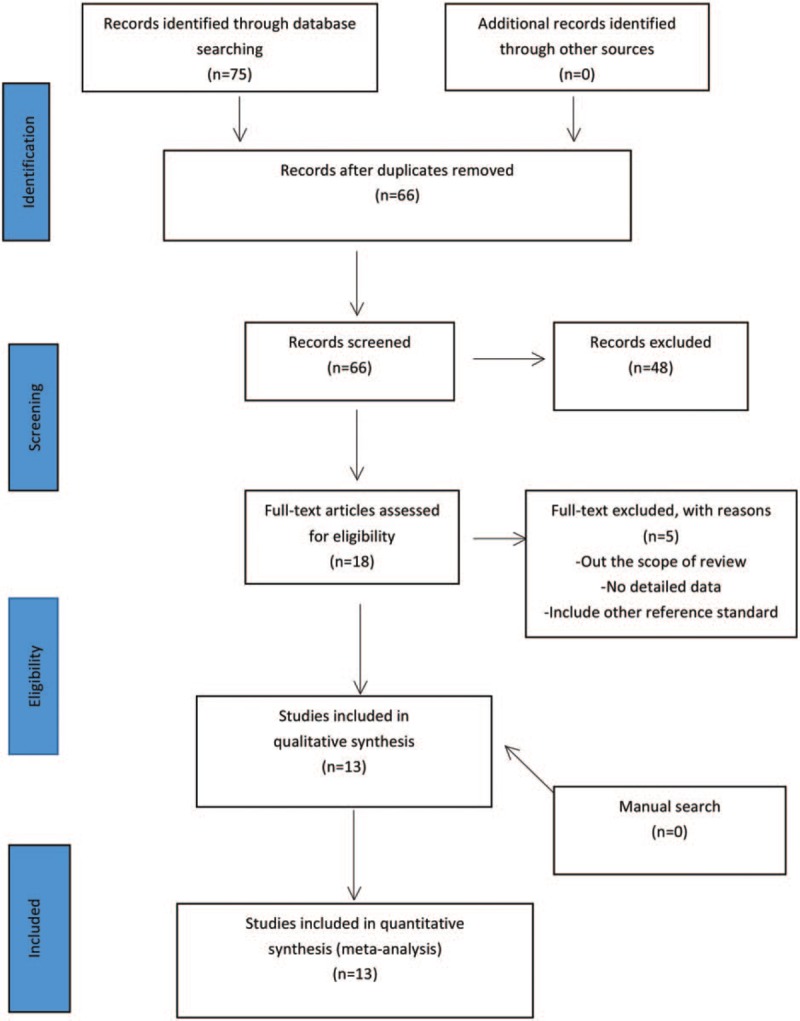
Study selection procedure.
3.2. Study characteristics and quality
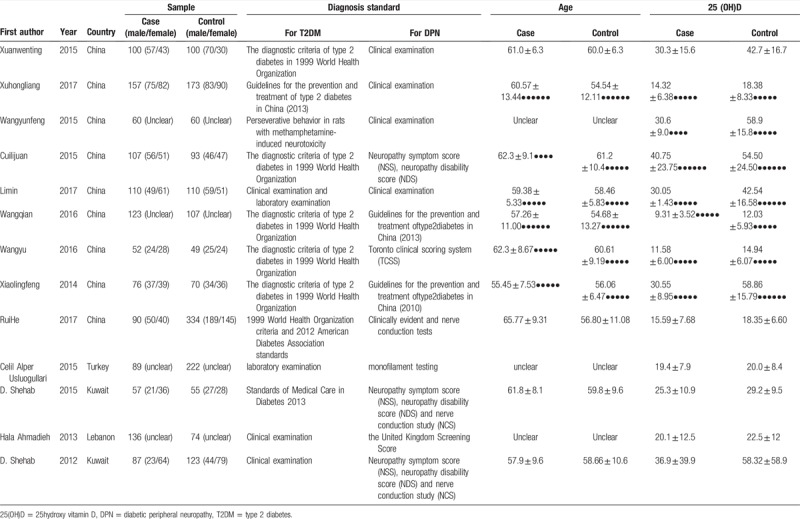
The 13 studies (8 articles were in Chinese[15,19–25] and 5 articles were in English[12,14,26–28]) comprised 2814 patients with type 2 diabetes, Eventually, they were all included in the meta-analysis study. Their principal characteristics were summarized in Table 1. There were 3 literatures[12,20,25] published in 2017, there were 2 articles[21,22] published in 2016, there were 5 studies[14,15,19,23,28] published in 2015, there was 1 document[24] published in 2014, there was 1 study[26] published in 2013, there was 1 article[27] published in 2012.
Table 1.
Characteristics of included studies.
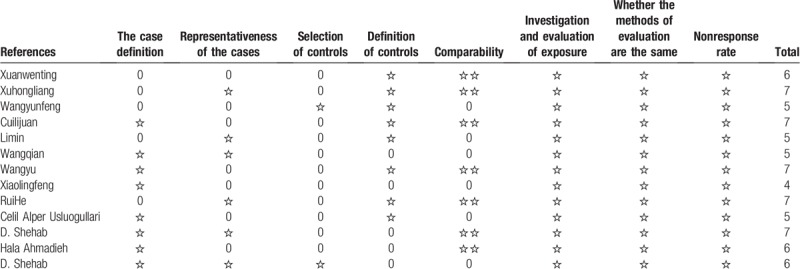
The 13 studies quality was assessed according to the NOS, The quality of the literature included was high. The results were shown in Table 2.
Table 2.
Newcastle–Ottawa Scale quality assessment in the included studies.
3.3. The concentration of serum 25-(OH)D in case group and control group
The results were shown in Figure 2. Heterogeneity test, according to the results of the meta-analysis, suggested that P < .000 01, I2 = 92%, there was a large heterogeneity between the studies, that is why the random effect model was used to calculate. The results indicated, the concentration of serum 25-(OH)D in T2DM with DPN is lower than those without DPN (SMD = −0.74, 95% CI: −1.03 to −0.46, P < .00001)
Figure 2.
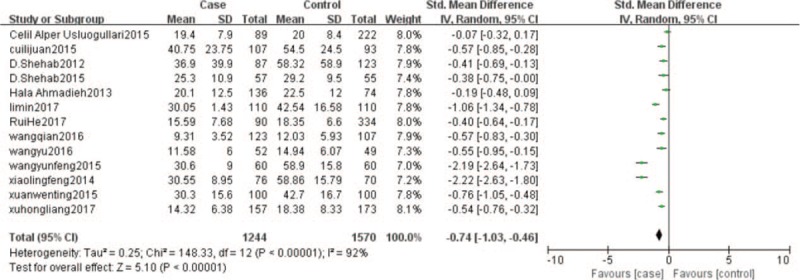
25 (OH) D in the case and control. 25 (OH) D = 25-hydroxyl vitamin D.
3.4. The number of serum 25-(OH)D deficiency in the case group and control group
The results were shown in Figure 3. Heterogeneity test, according to the results of the meta-analysis, suggested that I2 = 0%, there was a small heterogeneity between the studies, Thus, the fixed effect model was used to calculate. The results manifested that serum 25 (OH) D deficiency was a risk factor for T2DM patients with DPN (RR = 1.07, 95% CI: 0.99–1.16), the concentration of serum 25 (OH) D deficiency was positively correlated with the risk of T2DM with DPN.
Figure 3.
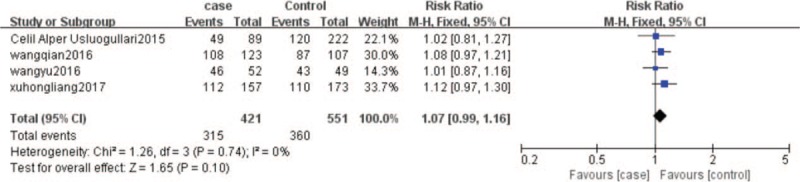
The number of 25 (OH) D deficiency in the case and control. 25 (OH) D = 25-hydroxyl vitamin D.
3.5. Funnel plot of serum 25 (OH) D concentration in case group and control group
The results were shown in Figure 4. The distribution of the funnel plot points around the estimated true value of each study was roughly symmetrical, indicating that publication bias was small.
Figure 4.
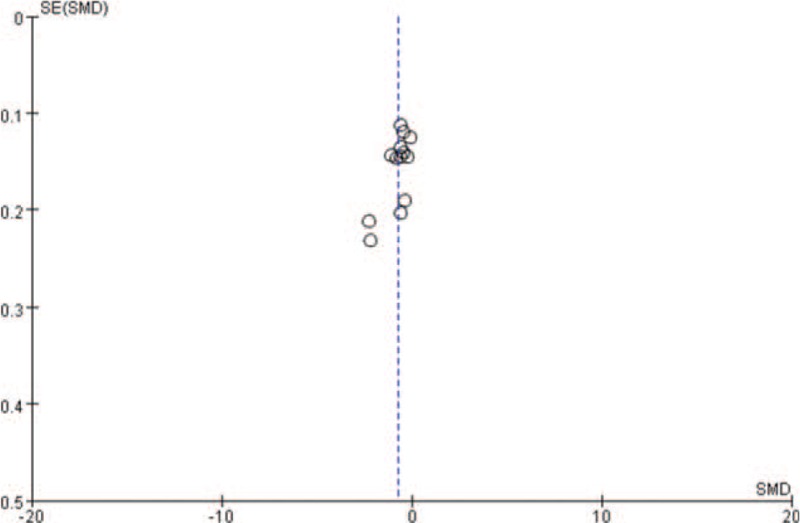
Funnel plot.
4. Discussions
4.1. Analysis of the relationship between vitamin D (VD) and T2DM combined with DPN
This study found that the serum concentration of 25 (OH) D in T2DM combined with DPN group was lower than that in non-DPN group (SMD = −0.74, 95% CI: −1.03 to −0.46, P < .00001; Fig. 2). Serum 25 (OH) D deficiency was a risk factor for T2DM patients with DPN (RR = 1.07, 95% CI: 0.99–1.16; Fig. 3), the concentration of serum 25 (OH) D deficiency was positively related to the risk of T2DM combined with DPN.
4.2. Limitations of this study
Although the 13 articles included in this Meta-analysis were consistent with the inclusion and exclusion criteria, they still had some limitations: First, the sample size of raw data was small, which would reduce the availability of data and generate bias; Second, there may be some deviations in the measurement of serum 25 (OH) D, which may lead to methodological bias; in addition, in this paper, we studied the relationship between the serum 25 (OH) D concentration and T2DM patients with DPN, the serum 25 (OH) D here referred to the measured value of clinical examination, and it was the main storage form of vitamin D in human body. Vitamin D needs to be appropriately supplemented for T2DM patients with DPN; however, we did not conduct in-depth and detailed research and introduction to vitamin D intake.
The reasons for the heterogeneity between studies may come from the following aspects:
-
(1)
subject characteristics,
-
(2)
research environment,
-
(3)
different diagnostic criteria,
-
(4)
the difference of research methods,
-
(5)
statistical methods.
4.3. The role of VD in the clinical prevention of T2DM with DPN
The results of meta-analysis in this study displayed that vitamin D was associated with T2DM patients with DPN, and vitamin D deficiency could lead to an increase in the risk of T2DM patients with DPN. The concentration of vitamin D has become an indicator for the treatment and prevention of T2DM patients with DPN.
Diabetic patients are easy to lack B vitamins, vitamin C, vitamin D, and many micronutrients, such as chromium, zinc, selenium, magnesium, iron, manganese, which can be supplemented according to the results of nutritional assessment.[3] The concentration of serum 25 (OH) D is considered to be an indicator of vitamin D levels.[29] Another study also suggested that serum 25 (OH) D was measured in blood and was a circulating form of vitamin D in the blood. Circulating serum 25 (OH) D is considered to be a nutritional status indicator of vitamin D and is associated with the onset of secondary hyperparathyroidism and cancer.[30] It has been shown that vitamin D deficiency will increase the risk of cancer, to avoid vitamin deficiency and to supply vitamin D may be an economical and safe way to reduce the incidence of cancer and to improve the prognosis and outcome of cancer.[31] According to epidemiological studies, vitamin D deficiency is associated with high prevalence of type 1 and type 2 diabetes, in animal models, vitamin D deficiency will cause diabetes, while Vitamin D supplements can prevent diabetes.[32] A study[33] confirmed that Vitamin D deficiency was an independent risk factor for DPN, which also opened a new way to prevent DPN. Studies showed that low concentration of vitamin D were a risk factor for type 2 diabetes. Low concentration of 25 (OH) D was associated with glucose metabolism disorder and insulin metabolism.[11,34] In addition, DPN is related to both inflammation and hyperglycemia.[35,36] The lower concentration of circulating 25 (OH) D, the greater the risk of major fiber neuropathy in the type 2 diabetics.[37] A study by Ahmadieh et al[26] explained that low concentration of 25 (OH) D is an independent risk factor for DPN. Studies on type 2 diabetes confirmed that vitamin D deficiency was associated with neuropathy. Recent reports also suggested that vitamin D deficiency was associated with diabetic foot ulcers.[38]
Low concentration of 25 (OH) D is a risk factor for DPN, thus proper supplementation of vitamin D may prevent the occurrence and development of DPN. A study by Basit et al[39] displayed that an intramuscular injection, 600,000 IU of vitamin D, can obviously improve the pain of patients with DPN. A study by Shehab et al[14] showed that the mechanisms of neuropathy and pathophysiology of neuropathy were related to hyperglycemia caused by microvascular injury or direct neuronal metabolism injury. Short-term oral vitamin D can improve the symptoms of peripheral neuropathy in patients with type 2 diabetes, and compared with the placebo group can significantly improve the concentration of 25 (OH) D in patients with DPN.
A study by Lee and Chen[40] illuminated that, vitamin D supplementation could have beneficial effects on the neuropathic pain of type 2 diabetes. A study by Bell[41] clarified that oral vitamin D can significantly reduce the symptoms and pain of DPN. Related studies[42,43] showed that taking vitamin D can increase serum calcium in T2DM, reduce circulating free fatty acid concentration, increase insulin secretion and improve glucose tolerance. A study by Soderstrom et al[44] has shown that in the National Health and Nutrition Examination Survey, vitamin D deficiency (defined as below 30 ng/mL) in adults with diabetes is associated with symptoms (numbness, pain, loss of consciousness, and tingling in hands or feet) after statistical correction of demographic factors and glycosylated hemoglobin concentrations.
Therefore, we suggest that DPN patients should pay more attention to the concentration of serum 25 (OH) D. If the concentration of serum 25 (OH) D is lower than 30 ng/mL, timely and appropriate vitamin D supplementation may alleviate symptoms by raising serum 25 (OH) D to a certain concentration. similarly, clinicians should pay close attention to the monitoring of serum 25 (OH) D. Adequate supplementation of vitamin D may be beneficial to prevent the occurrence and development of DPN.
But how much vitamin D supplementation can meet the amount of DPN patients need? There is still a lack of research in this issue.
5. Conclusions
In conclusion, vitamin D is associated with type 2 DPN. The serum 25 (OH) D deficiency was a risk factor for T2DM patients with DPN. The concentration of serum 25 (OH) D has become an indicator for the treatment and prevention of peripheral neuropathy (DPN) in type 2 diabetes.
Acknowledgment
The authors thank Prof. Yanming Liu for advising important information.
Author contributions
Conceptualization: Wenli Zhao, Yu Hao, Hongwu Wang, Ye Zhao, Kaito Mizuno, Yiider Tseng.
Data curation: Wenli Zhao, Xueying Wang, Ye Zhao, Kaito Mizuno, Huaien Bu.
Formal analysis: Binjie Zhang, Xueying Wang, Ye Zhao, Huaien Bu.
Funding acquisition: Hongwu Wang.
Investigation: Wenli Zhao.
Methodology: Wenli Zhao, Jinli Tu, Yu Hao, Ye Zhao, Kaito Mizuno.
Resources: Ye Zhao, Huaien Bu.
Supervision: Hongwu Wang, Yiider Tseng, Huaien Bu.
Validation: Yiider Tseng.
Visualization: Huaien Bu.
Writing – original draft: Binjie Zhang, Jinli Tu, Ye Zhao, Huaien Bu.
Writing – review and editing: Jinli Tu, Ye Zhao, Yiider Tseng, Huaien Bu.
Footnotes
Abbreviations: 25-(OH)D = 25-hydroxy vitamin D, ADA = American Diabetes Association Standards, DPN = diabetic peripheral neuropathy, NCS = nerve conduction study, NDS = neuropathy disability score, NOS = Newcastle–Ottawa scale, NSS = neuropathy symptom score, T2DM = type 2 diabetes, TCSS = Toronto clinical scoring system, WHO = World Health Organization criteria.
How to cite this article: Zhang B, Zhao W, Tu J, Wang X, Hao Y, Wang H, Zhao Y, Mizuno K, Tseng Y, Bu H. The relationship between serum 25-hydroxyvitamin D concentration and type 2 diabetic peripheral neuropathy: A systematic review and a meta-analysis. Medicine. 2019;98:48(e18118).
BZ and WZ contributed equally to this study.
Data are all contained within the paper.
This project was supported by the Construction Project of Cultivate Discipline of Chinese Preventive Medicine of State Administration of Traditional Chinese Medicine (2012 [170]) and the Key Project of Comprehensive Investment in Food Hygiene and Nutrition of the Tianjin 13th Five-Year Plan. This study was also supported in part by Hangzhou DeBuYou Health Technology Co. Ltd., China.
The authors have no conflicts of interest to disclose.
References
- [1].Pradeepa R, Mohan V. Prevalence of type 2 diabetes and its complications in India and economic costs to the nation. Eur J Clin Nutr 2017;71:816–24. [DOI] [PubMed] [Google Scholar]
- [2].Issa CM. Vitamin D and Type 2 Diabetes Mellitus. Adv Exp Med Biol 2017;996:193–205. [DOI] [PubMed] [Google Scholar]
- [3].Chinese Diabetes Society Guidelines for the prevention and treatment of type 2 diabetes in China (2013 edition). Chin J Endocrinol Metab 2014;30:893–941. [Google Scholar]
- [4].Maffi P, Secchi A. The burden of diabetes: emerging data. Dev Ophthalmol 2017;60:1–5. [DOI] [PubMed] [Google Scholar]
- [5].Feldman EL, Nave KA, Jensen TS, et al. New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron 2017;93:1296–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Grote CW, Wright DE. A role for insulin in diabetic neuropathy. Front Neurosci 2016;10:581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Valencia WM, Florez H. How to prevent the microvascular complications of type 2 diabetes beyond glucose control. BMJ (Clin Res Ed) 2017;356:i6505. [DOI] [PubMed] [Google Scholar]
- [8].Roman-Pintos LM, Villegas-Rivera G, Rodriguez-Carrizalez AD, et al. Diabetic polyneuropathy in type 2 diabetes mellitus: inflammation, oxidative stress, and mitochondrial function. J Diabetes Res 2016;2016:3425617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jolivalt CG, Frizzi KE, Guernsey L, et al. Peripheral neuropathy in mouse models of diabetes. Curr Protoc Mouse Biol 2016;6:223–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Argoff CE, Cole BE, Fishbain DA, et al. Diabetic peripheral neuropathic pain: clinical and quality-of-life issues. Mayo Clinic Proc 2006;81: 4 Suppl: S3–11. [DOI] [PubMed] [Google Scholar]
- [11].Lv WS, Zhao WJ, Gong SL, et al. Serum 25-hydroxyvitamin D levels and peripheral neuropathy in patients with type 2 diabetes: a systematic review and meta-analysis. J Endocrinol Investig 2015;38:513–8. [DOI] [PubMed] [Google Scholar]
- [12].He R, Hu Y, Zeng H, et al. Vitamin D deficiency increases the risk of peripheral neuropathy in Chinese patients with type 2 diabetes. Diabetes Metab Res Rev 2017;33:e2820. [DOI] [PubMed] [Google Scholar]
- [13].Palomer X, Gonzalez-Clemente JM, Blanco-Vaca F, et al. Role of vitamin D in the pathogenesis of type 2 diabetes mellitus. Diabetes Obes Metab 2008;10:185–97. [DOI] [PubMed] [Google Scholar]
- [14].Shehab D, Al-Jarallah K, Abdella N, et al. Prospective evaluation of the effect of short-term oral vitamin d supplementation on peripheral neuropathy in type 2 diabetes mellitus. Med Princ Pract 2015;24:250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Xuan wenting, Huang qiuxia The correlation between serum 25- hydroxyvitamin D level and microvascular disease in type 2 diabetic patients. Guangdong Med J 2015;36:1229–31. [Google Scholar]
- [16].Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]. Available at: http://www.cochrane- handbook.org [Accessed March 3, 2013]. [Google Scholar]
- [17].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [18].Scholten RJ, Clarke M, Hetherington J. The cochrane collaboration. Eur J Clin Nutr 2005;59: Suppl 1: S147–9. [DOI] [PubMed] [Google Scholar]
- [19].Cui lijuan, Sun kan, Chang xiangyun, Li jun Correlation between 25- hydroxyvitamin D and peripheral neuropathy in elderly patients with type 2 diabetes mellitus. Guangdong Med J 2015;36:3802–4. [Google Scholar]
- [20].Li min, Qian kuo, Fan bo, et al. The correlation between microvascular disease and serum 25-hydroxyvitamin D in adult type 2 diabetesmellitus. Diabetes New world 2017;20:44–5. [Google Scholar]
- [21].Wang qian, Yang yan, Song meihui, et al. The correlation between the level of vitamin D and diabetic peripheral neuropathy in male patients with type 2 diabetes mellitus. J Military Surg Southwest China 2016;18:109–12. [Google Scholar]
- [22].Wang Yu. The relationship between serum 25-hydroxyvitamin D level and peripheral neuropathy of type 2 diabetes mellitus. Tai Yuan Shanxi Medical university 2016;2–5. [Google Scholar]
- [23].Wang yunfeng, Guan yue, Wang deping, et al. The relationship of 25 (OH) D deficiency with diabetic peripheral neuropathy. Prog Anatom Sci 2015;21:352–4. [Google Scholar]
- [24].Xiao lingfeng. The relationship between 25 (OH) D deficiency and type 2 diabetic peripheral neuropathy. Chin J Diabetes 2014;22:711–4. [Google Scholar]
- [25].Xu hongliang, Lei jian. Study on correlation between serum 25- hydroxyvitamin D level and peripheral neuropathy in T2DM. Lab Med Clin 2017;14:936–8. [Google Scholar]
- [26].Ahmadieh H, Azar ST, Lakkis N, et al. Hypovitaminosis d in patients with type 2 diabetes mellitus: a relation to disease control and complications. ISRN Endocrinol 2013;2013:641098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shehab D, Al-Jarallah K, Mojiminiyi OA, et al. Does vitamin D deficiency play a role in peripheral neuropathy in Type 2 diabetes? Diabetic Med 2012;29:43–9. [DOI] [PubMed] [Google Scholar]
- [28].Usluogullari CA, Balkan F, Caner S, et al. The relationship between microvascular complications and vitamin D deficiency in type 2 diabetes mellitus. BMC Endocrine Disord 2015;15:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Heaney RP. Functional indices of vitamin D status and ramifications of vitamin D deficiency. Am J Clin Nutr 2004;80: 6 Suppl: 1706s–s1709. [DOI] [PubMed] [Google Scholar]
- [30].Hollis BW, Wagner CL. Clinical review: the role of the parent compound vitamin D with respect to metabolism and function: why clinical dose intervals can affect clinical outcomes. J Clin Endocrinol Metab 2013;98:4619–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Feldman D, Krishnan AV, Swami S, et al. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer 2014;14:342–57. [DOI] [PubMed] [Google Scholar]
- [32].Van Belle TL, Gysemans C, Mathieu C. Vitamin D and diabetes: the odd couple. Trends Endocrinol Metab 2013;24:561–8. [DOI] [PubMed] [Google Scholar]
- [33].Peltier A, Goutman SA, Callaghan BC. Painful diabetic neuropathy. BMJ (Clin Res Ed) 2014;348:g1799. [DOI] [PubMed] [Google Scholar]
- [34].Hurskainen AR, Virtanen JK, Tuomainen TP, et al. Association of serum 25-hydroxyvitamin D with type 2 diabetes and markers of insulin resistance in a general older population in Finland. Diabetes Metab Res Rev 2012;28:418–23. [DOI] [PubMed] [Google Scholar]
- [35].Verrotti A, Loiacono G, Mohn A, et al. New insights in diabetic autonomic neuropathy in children and adolescents. Eur J Endocrinol 2009;161:811–8. [DOI] [PubMed] [Google Scholar]
- [36].Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 2011;11:98–107. [DOI] [PubMed] [Google Scholar]
- [37].Alamdari A, Mozafari R, Tafakhori A, et al. An inverse association between serum vitamin D levels with the presence and severity of impaired nerve conduction velocity and large fiber peripheral neuropathy in diabetic subjects. Neurol Sci 2015;36:1121–6. [DOI] [PubMed] [Google Scholar]
- [38].Putz Z, Martos T, Nemeth N, et al. Vitamin D and neuropathy. Orvosi Hetilap 2013;154:2012–5. [DOI] [PubMed] [Google Scholar]
- [39].Basit A, Basit KA, Fawwad A, et al. Vitamin D for the treatment of painful diabetic neuropathy. BMJ Open Diabetes Res Care 2016;4:e000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lee P, Chen R. Vitamin D as an analgesic for patients with type 2 diabetes and neuropathic pain. Arch Intern Med 2008;168:771–2. [DOI] [PubMed] [Google Scholar]
- [41].Bell DS. Reversal of the symptoms of diabetic neuropathy through correction of vitamin D deficiency in a type 1 diabetic patient. Case Rep Endocrinol 2012;2012:165056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Inomata S, Kadowaki S, Yamatani T, et al. Effect of 1 alpha (OH)-vitamin D3 on insulin secretion in diabetes mellitus. Bone Miner 1986;1:187–92. [PubMed] [Google Scholar]
- [43].Alvarez JA, Ashraf A. Role of vitamin d in insulin secretion and insulin sensitivity for glucose homeostasis. Int J Endocrinol 2010;2010:351385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Soderstrom LH, Johnson SP, Diaz VA, et al. Association between vitamin D and diabetic neuropathy in a nationally representative sample: results from 2001–2004 NHANES. Diabetic Med 2012;29:50–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


