Abstract
Background:
We investigated the effects of propofol vs desflurane on ischemia and reperfusion injury (IRI)-induced inflammatory responses, especially in matrix metalloproteinase-9 (MMP-9) downregulation and heme oxygenase-1 (HO-1) upregulation, which may result in different clinical outcomes in liver transplant recipients.
Methods:
Fifty liver transplant recipients were randomized to receive propofol-based total intravenous anesthesia (TIVA group, n = 25) or desflurane anesthesia (DES group, n = 25). We then measured the following: perioperative serum cytokine concentrations (interleukin 1 receptor antagonist [IL-1RA], IL-6, IL-8, and IL-10); MMP-9 and HO-1 mRNA expression levels at predefined intervals. Further, postoperative outcomes were compared between the 2 groups.
Results:
The TIVA group showed a significant HO-1 level increase following the anhepatic phase and a significant MMP-9 reduction after reperfusion, in addition to a significant increase in IL-10 levels after the anhepatic phase and IL-1RA levels after reperfusion. Compared to DES patients, TIVA patients showed a faster return of the international normalized ratio to normal values, lower plasma alanine aminotransferase concentrations 24 hours after transplantation, and fewer patients developing acute lung injury. Moreover, compared with DES patients, TIVA patients showed a significant reduction in serum blood lactate levels. However, there were no differences in postoperative outcomes between the two groups.
Conclusion:
Propofol-based TIVA attenuated inflammatory response (elevated IL-1RA and IL-10 levels), downregulated MMP-9 response, and increased HO-1 expression with improved recovery of graft function and better microcirculation compared with desflurane anesthesia in liver transplant recipients.
Keywords: anesthetics i.v., anti-inflammation, heme oxygenase-1, ischemia-reperfusion injury, matrix metalloproteinase-9, propofol
1. Introduction
Liver transplantation (LT) has an effective therapy choice for many liver diseases including end-stage liver disease, acute liver failure, hepatocellular carcinoma, and pediatric metabolic liver disease.[1] Surgery and preservation of the allografts results in an ischemia-reperfusion injury (IRI), however, there are still no effective therapeutic approaches. It is widely recognized that the underlying mechanisms of hepatic IRI involve multiple signaling pathways, including inflammation, free radical production, and mitochondrial damage associated with the generation of oxidative stress.[2] The IRI during LT results in a more vulnerable graft through increasing immunogenicity and rejection episodes, both before and following LT,[3] and has a significant impact on the balance between successful transplantation and the occurrence of complications.[4]
Previous studies have largely focused on the effects of heme oxygenase (HO)-1 by reason of its anti-inflammatory, antioxidative, and cytoprotective properties, as well as its ability to maintain microcirculation and modulate the cell cycle.[5] Various cell types, including Kupffer cells, endothelial cells, and dendritic cells (DCs), can induce overexpression of HO-1 to prohibit from both IRI and rejection during LT.[6] Studies have shown that overexpression of HO-1 induced by transient limb ischemia may have a protective effect in hepatic IRI in rats.[7]
Matrix metalloproteinases (MMPs) have a regulatory function in immunity and inflammation by proteolytic activation or degradation of cytokines and chemokines.[8] In different MMPs, MMP-9 is an inducible enzyme primarily produced through infiltrating leukocytes following hepatic IRI,[9] and serum MMP-9 was found to be involved in the evolution of liver injury in IRI[10] and acute allograft rejection.[11] All the above evidence suggests that the increased activity of MMP-9 is directly related to hepatic ischemic insult. We have had established LT protocols over the last few years with propofol-based total intravenous anesthesia (TIVA) by use of the bispectral index (BIS) and target-controlled infusion (TCI). In our previous retrospective study, propofol-based TIVA via a TCI system was shown to potentially provide better hemodynamics and microcirculation during the anhepatic phase in LT.[12] Propofol has been revealed to ameliorate IRI in several organs through potential anti-inflammatory, antiapoptotic, or antioxidation mechanisms.[13] Increasing evidence suggests that another possible mechanism of propofol protection may be through stimulation of HO-1.[14–18] However, few studies investigate the benefit of propofol-induced upregulated HO-1 expression in IRI of LT. In brain ischemia, propofol has been shown to have an inhibitory effect on MMP-9 expression to attenuate damage to the blood brain barrier (BBB) and cephaloedema in a rat model of focal cerebral IRI.[19] In addition, propofol has been shown to alleviate intracerebral hemorrhage in rats through the inhibition of the inflammatory factor release (IL-1β and tumor necrosis factor-α) and upregulation of MMP-9 in brain.[20] However, thus far, there is no evidence available to explain whether propofol can suppress MMP-9 upregulation induced by hepatic IRI. The present study examines whether propofol-based TIVA is able to attenuate IRI-induced inflammatory responses, upregulate of MMP-9 and induce the expression of HO-1, which may result in different clinical outcomes to that of patients administered desflurane anesthesia.
2. Materials and methods
This study was approved by the Institutional Review Board of Tri-Service General Hospital (27/03/2012; TSGHIRB-2-101-05-012) and registered at the Chinese Clinical Trial Registry (09/06/2017; ChiCTR-INR-17011600). Written informed consent was obtained from each patient before the operative day; 50 LDLT recipients were enrolled from April 2012 to July 2013, and no organs were procured from prisoners. Recipients were excluded if they met the following criteria: hepatic encephalopathy, hepatorenal syndrome, or massive pleural effusion, requirements for pre-transplant endotracheal intubation with mechanical ventilation or use of vasopressors, any contraindication to fentanyl, propofol, or desflurane. Using sealed envelopes containing a patient number and assignment, recipients were randomly assigned to the TIVA with propofol (TIVA group, n = 25) or the desflurane anesthesia (DES group, n = 25) group.
Anesthesia was induced with intravenous fentanyl (1–2 μg kg−1) and lidocaine (2%, 1 mg kg−1), and propofol (1–2 mg kg-1). Rocuronium bromide (0.6 mg kg-1) was administered following the loss of eyelash reflex, and the endotracheal tube was intubated 90 seconds later. The desflurane concentration or effect-site concentration (Ce) of propofol using the Schneider kinetic model of Target-Controlled Infusion (TCI, Fresenius Orchestra Primea; Fresenius Kabi AG, Bad Homburg, Germany) was reduced or increased by 2% and 0.5 μg ml−1, respectively, in order to keep the BIS value between 40 and 60 throughout the operation, confirmed using the BIS monitor Vista with Sensor BIS Quatro (Aspect Medical System, Norwood, MA). Ventilation rate and maximum airway pressure were adjusted to maintain the end-tidal carbon dioxide at 35 to 45 mmHg. Attempts were made to keep the core body temperature higher than 36°C by warming all intravenous fluids and blood products, and using a convectional warming device.
Anesthetic use and surgical management were in accordance with the institute's standard protocol of LT by the same team of anesthesiologists and surgeons who managed the experiments. Our perioperative monitoring was in accordance with our institution standards, including measuring pulse oximetry, electrocardiography, invasive blood pressure, central venous pressure, and the PiCCOplusTM system. Coagulation function was guided by thromboelastometry (ROTEM). After reperfusion, a bolus dose of norepinephrine was used on occasion to maintain the mean arterial pressure above 60 mmHg, or within 30% of pre-reperfusion systolic arterial pressure. After the surgery, the patient was sent to the surgical intensive care unit (ICU) for further management.
Surgery related variables (total anesthesia time, operation time, transfused blood volume, estimated blood loss) and graft related variables (donor age, graft recipient weight ratio, cold and warm ischemic time) were recorded.
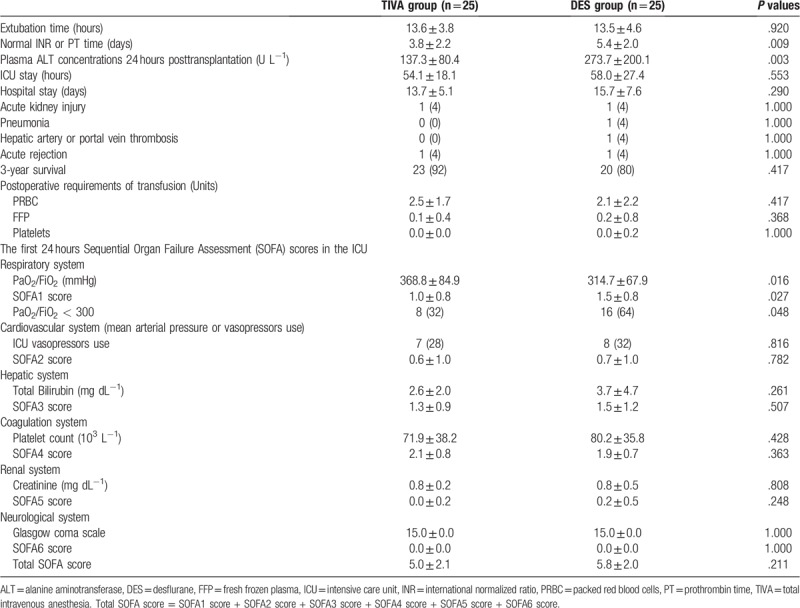
Postoperative outcomes, including extubation time, time to reach normal international normalized ratio (INR) of prothrombin time (PT), alanine aminotransferase (ALT) concentration at 24 hours after LT, postoperative acute kidney injury, pneumonia, hepatic artery or portal vein thrombosis, and acute rejection, ICU stay, days in hospital, and 3-year survival rate were compared. The Sequential Organ Failure Assessment (SOFA) scores in the ICU were also compared for the first 24 hours.
2.1. The cytokine and MMP-9 assay
Venous blood was sampled at 10 minutes postintubation (T0, baseline) and during intraoperative liver transplantation—60 min (T1) and 120 min (T2) after the start of the dissection phase; 10 minutes after the start of the anhepatic phase (T3); 10 minutes before reperfusion (T4); 10 minutes (T5), 50 minutes (T6), and 90 minutes (T7) after reperfusion; and end of surgery (T8). Plasma samples were separated by centrifugation (3500 rpm/10 minutes) immediately and stored at –80°C until analysis, and performed within 1 week. We used the MSD MULTI-SPOT Human Cytokine Multiplex Assay Kit and Human Matrix Metalloproteinase Assay Kit (Meso Scale Discovery, Gaithersburg, MD) to measure the serum concentrations of the cytokines (interleukin-1 receptor antagonist [IL-1RA], IL-6, IL-8, and IL-10) and MMP-9 in accordance with the manufacturer's instructions.
2.2. HO-1 mRNA expression level in peripheral whole blood
The mRNA expression of HO-1 was determined by reverse transcription polymerase chain reaction (PCR). Whole blood (2.5 ml) was collected at T0, T3, T4, T5, T7, and during the postoperative intensive care period at 6 hours (T9), 12 h (T10), and 24 hours (T11), following reperfusion into a TempusTM Blood RNA Tube (Applied Biosystems, Foster City, CA), containing a proprietary solution that reduces RNA degradation and gene induction. Samples were stored at – 80°C until RNA isolation.
2.3. RNA isolation and cDNA synthesis
RNA from peripheral whole blood samples was extracted using a TempusTM Spin Isolation Reagent Kit (Applied Biosystems), as previously described.[21] In brief, all RNA samples were handled with RNase-free DNase (Qiagen) and reverse transcribed to cDNA using a high-capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA).
2.4. Quantitative real-time polymerase chain reaction (RT-qPCR)
Quantification of cDNA was performed by real time quantitative PCR (ABI PRISM 7500 Real-Time PCR System; Applied Biosystems) using the TaqMan Universal PCR Master Mix II (Applied Biosystems) under standard conditions. HO-1 mRNA expression levels were quantified using the TaqMan assay HO-1 (Hs01110250/m1). For each sample, measurements were performed in triplicate, and relative expression was analyzed using the ΔΔCt method.[22] All over this method, the amounts of target gene mRNA were normalized to an endogenous control and correlated with a calibration sample using the formula RQ sample = 2 – (ΔCt sample – ΔCt calibrator). Glyceraldehyde3-phosphate dehydrogenase (GAPDH; Hs99999905/m1) was used as an endogenous control (Applied Biosystems).
2.5. Statistical analysis
All data are expressed as mean (standard deviation) or numbers with a percentage, unless otherwise indicated. Statistical analyses were performed using the Statistical Package for Social Sciences 12.0 for Windows (SPSS, Inc., Chicago, IL). To obtain a collective summary of serum biomarkers, the integral of the serum biomarker concentration-time curve was calculated as the area under the curve (AUC) for MMP-9, HO-1, IL-1RA, IL-6, IL-8, IL-10, and lactate by the trapezoidal rule. Following conversion of raw data into a logarithmic scale to achieve normality when appropriate, Student t test was used to compared the means of the two groups. Categorical variables were analyzed by the chi-squared test or Fisher exact tests. Using generalized estimating equation methods[23] with an identity link function, we modelled the changes in biomarker concentration over time by contrasting with concentration of T0 to take into account the correlated data nature. P < .05 were considered significant.
3. Results
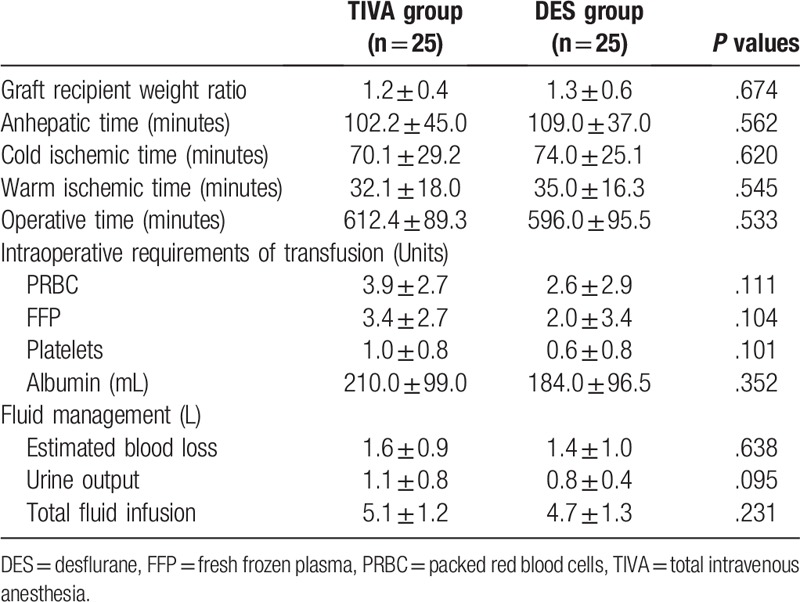
Of the 50 patients enrolled in the study, 25 patients were randomized into the TIVA group, and 25 patients were randomized into the DES group. All patients completed the study according to the protocol. There was no difference between the two groups of patients in terms of demographics, severity of liver cirrhosis, and baseline laboratory data (Table 1). The 3 main causes of LT were hepatitis B cirrhosis with hepatocellular carcinoma (42%), hepatitis B cirrhosis without hepatocellular carcinoma (22%), and alcoholic cirrhosis without hepatocellular carcinoma (18%). Variables related to anesthesia and surgery were also similar between groups (Table 2).
Table 1.
Characteristics of patients receiving TIVA or DES for liver transplantation. Values are mean ± SD or number (proportion).
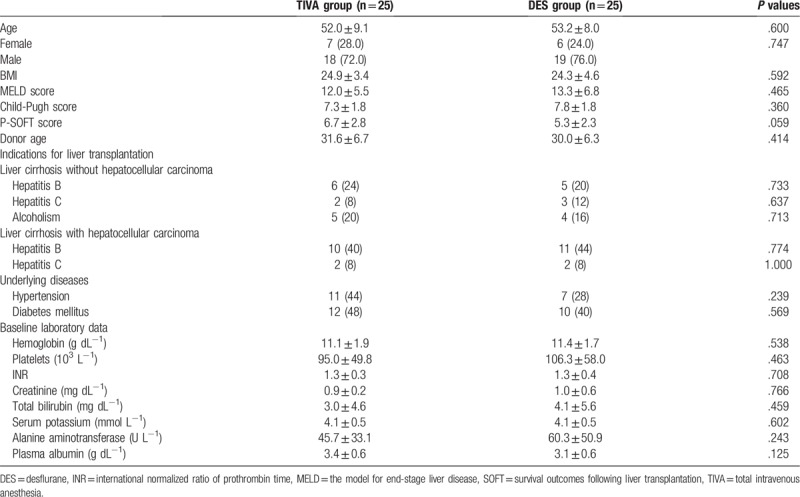
Table 2.
Variables related to anesthesia and surgery of patients receiving TIVA or DES for liver transplantation. Values are mean ± SD.
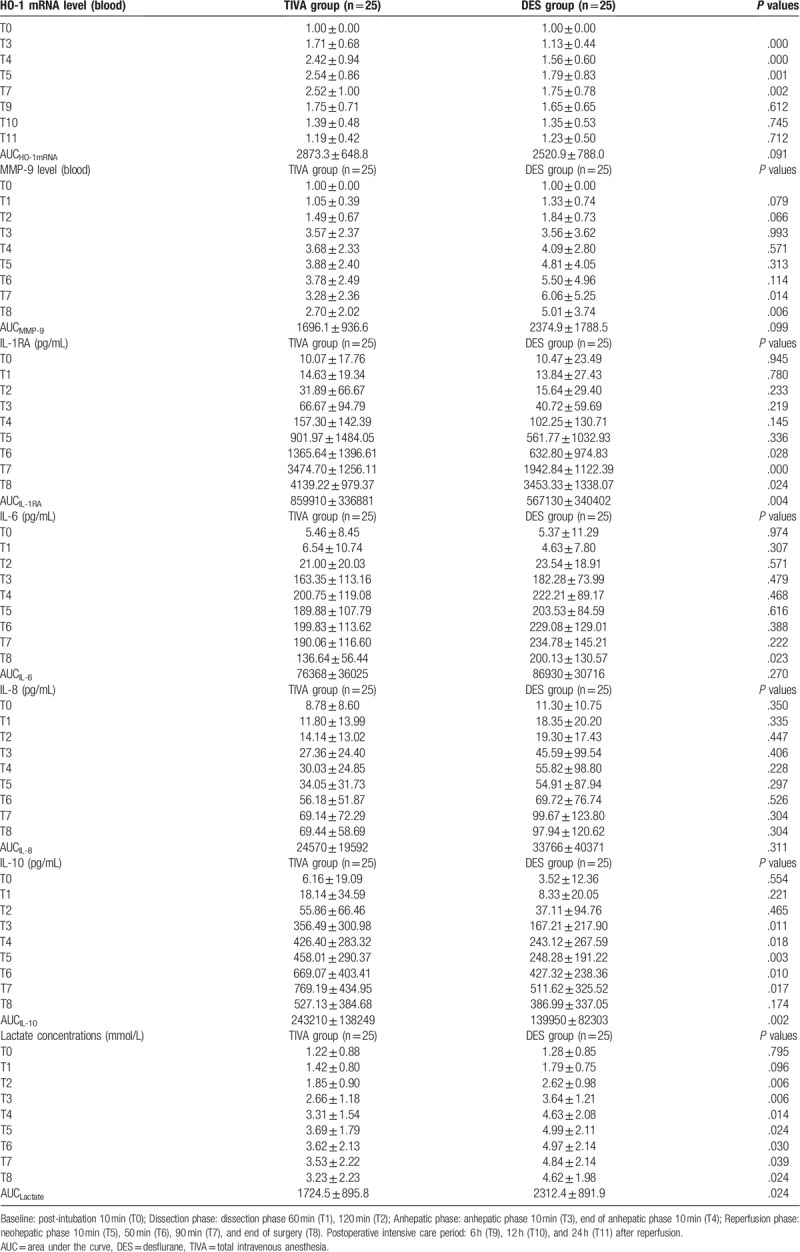
We observed a significant increase in HO-1 expression after the anhepatic phase (T3; P < .001, T4; P < .001, T5; P = .001, and T7; P = .002) in the TIVA group compared with the DES group (Table 3). The TIVA patients showed a significant reduction in serum levels of MMP-9 at T7 (P = .014), and T8 (P = .006) compared with the DES patients (Table 3). Patients in the TIVA group showed a significant increase in IL-10 (Table 3) at T3 (P = .011), T4 (P = .018), T5 (P = .003), T6 (P = .01), and T7 (P = .017), and in IL-1RA (Table 3) at T6 (P = .028), T7 (P < .001), and T8 (P = .024) compared with the DES patients. Furthermore, the mean IL-10-AUC (P = .002) and IL-1RA-AUC (P = .004) were significantly higher in the TIVA patients compared with the DES patients (Table 3). Moreover, the TIVA patients showed a significant reduction in serum levels of blood lactate (Lactate-AUC, P = .024) compared with the DES patients (Table 3).
Table 3.
Changes in heme oxygenase-1 (HO-1) mRNA level, plasma matrix metalloproteinase-9 (MMP-9), interleukin-1 receptor antagonist (IL-1RA), IL-6, IL-8, IL-10, and lactate concentration in patients receiving TIVA or DES techniques. Values are mean ± SD.
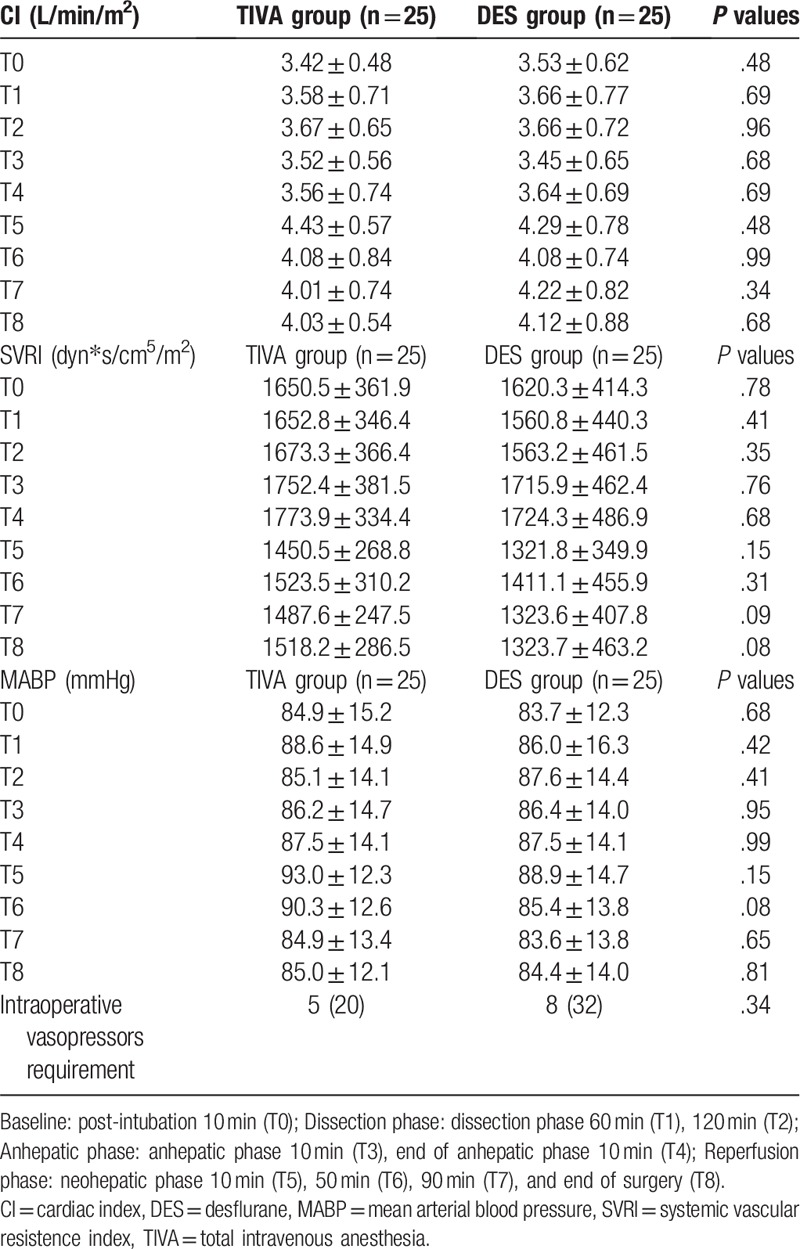
In TIVA patients, the effect concentration (Ce) of propofol at the anhepatic phase (T3, 1.38 ± 0.68; T4, 1.08 ± 0.55 μg/mL) were significant lower than those in the baseline and dissection phase (T0, 2.72 ± 0.33; T1, 2.52 ± 0.47; T2, 2.13 ± 0.64 μg/mL; P < .05). There was a trend for the Ce of propofol to increase after reperfusion. Ce of propofol in the end of anhepatic phase 10 minutes (T4, 1.08 ± 0.55 μg/mL) were significant lower than those in the neohepatic phase 90 minutes (T7, 1.42 ± 0.61 μg/mL, P < .05) and end of surgery (T8, 1.53 ± 0.67 μg/mL, P < .05). No significant difference was observed between the 2 groups with respect to the intraoperative hemodynamic parameters and the use of vasopressors (Table 5).
Table 5.
Changes in the intraoperative hemodynamics (CI, SVRI, MABP) and vasopressors requirement in patients receiving TIVA or DES techniques. Values are mean ± SD or number (proportion).
The TIVA patients showed a faster return to normal INR values (3.76 ± 2.17 vs 5.36 ± 1.98 days, P = .009), and lower plasma ALT concentration 24 h after transplantation (137.32 ± 80.42 vs 273.68 ± 200.13 U L−1, P = .003) (Table 4). The first 24 hours SOFA scores in the ICU were not significantly different between the 2 groups, except the SOFA score of the respiratory system (0.96 ± 0.84 vs 1.48 ± 0.77, P = .048) (Table 4) where fewer TIVA patients developed acute lung injury (ALI) (PaO2/FiO2 < 300, 32.0% vs 64.0%; P = .048) compared to the DES patients (Table 4).
Table 4.
Postoperative outcomes in patients receiving TIVA or DES for liver transplantation. Values are mean ± SD or number (proportion).
4. Discussion
The main finding of our study was that, under IRI, propofol-based TIVA exhibited its cytoprotective properties causing an anti-inflammatory response (reduced level of MMP-9 and elevated levels of IL-1RA and IL-10) and antioxidative stress (upregulated HO-1 expression) with improved recovery of graft function and better microcirculation compared with desflurane anesthesia in LT recipients. However, there was no impact on long term outcome.
4.1. The protective effect of propofol vs desflurane on the post-reperfusion injury and graft function
IRI during LT can result in significant graft dysfunction in the postoperative period. ALT is commonly used to assess graft injury after LT, and factors such as ischemic time and vascular anastomosis steatosis may also contribute to the level of ALT.[24] Moreover, normal INR or PT is also used to assess the graft function after LT. It has been hypothesized that according to anti-IRI characteristics, volatile anesthetics might be the more appropriate choice in patients undergoing liver surgery with occlusion.[25] A recent randomized controlled trial showed sevoflurane may provide better protective effects in the clinical setting of transplantation than desflurane[26] and this protective effects of sevoflurane have been described in a mouse IR-model.[27] In our study, the TIVA group showed a faster return to a normal INR, and lower plasma ALT concentrations 24 hours after LT than the DES group; this suggested that propofol anesthesia was exerting a better protective effect on the post-reperfusion injury and graft function than desflurane anesthesia. Lactate has been shown to monitor the microcirculation conditions during anesthesia. In our study, we found that TIVA patients showed a significant reduction in serum levels of blood lactate compared with the DES patients; it was consistent with our previous findings TIVA produced a better microcirculation than the desflurane anesthesia during the ischemia-reperfusion phase in LT patients.[12] Further studies are required to see if the difference between propofol and desflurane anesthesia still holds between propofol and sevoflurane anesthesia.
4.2. Propofol attenuation of hepatic IRI involves HO-1
HO-1 shows a protective effect in many disease models through its anti-inflammatory, antiapoptotic, and antiproliferative actions.[28] Upregulated HO-1 may be one of the critical cytoprotective mechanisms activated during ischemia, inflammation, hypoxia, or radiation,[29] and it is believed to show an important role in maintaining oxidative homeostasis during cellular damage.[30] Recent research results have brought out a redefinition of the HO pathway because there is no single antioxidant mechanism but a more complex and coordinated cytoprotective system.[5]
Propofol has been reported to inhibit the ischemic reperfusion-induced formation of lipid peroxides in LT recipients,[31] significantly decrease the leakage of liver enzymes, and markedly reduce number of lesions in histological examination of the liver in a hepatic IRI rabbit model.[32] However, there are few reports that exhibit the effect of propofol on IRI and HO-1. Acquaviva et al. exhibited that propofol utilizes its protective effects in astrocytes through upregulation of HO-1, and suggested that the stimulation of the HO-1 pathway may explain the antioxidative and anti-inflammatory properties of propofol.[15] Liang et al also exhibited that propofol exerts its postconditioning neuroprotective effect in an animal model of brain IRI in part by inducing HO-1 expression.[17] An animal study indicated that the attenuation of renal IRI by propofol also involved HO-1.[14] Under oxidative stress conditions, propofol increased both of HO-1 expression and activity in vascular endothelial cells.[16] In hyperglycemic rats, propofol also conveyed renoprotection against IRI by preserving antioxidation ability and attenuating inflammatory responses.[33] In another model, hemin pretreatment increased lung antioxidant capacity and reduced inflammatory stress, protecting the lung from orthotopic autologous LT-induced acute lung injury (ALI) during the early stage of reperfusion by enhancing HO-1 induction.[18]
Contrary to the previously mentioned protective effect of HO-1 on oxidant-induced damage, Froh et al[34] reported that overexpression of HO-1 induced by cobalt protoporphyrin increased liver damage, such as overexpression of ALT, increased cell necrosis, and fibrosis. The authors suggested that a high level of HO-1 might make cells sensitive to oxidative stress due to the accumulation of free divalent iron, thereby aggravating oxidative damage. Matsumi et al[35] investigated the clinical significance of the HO-1 gene and protein expression levels with their relationship between the exhaled carbon monoxide levels and liver injury in 29 liver allografts during living donor LT. They demonstrated that HO-1 mediated heme breakdown through IRI was associated with increased exhaled carbon monoxide levels and liver injury. The controversial effect of the HO-1 expression in cytoprotection or increased cytotoxicity in liver allografts should be studied attentively in the future. Nevertheless, in the present study, we first found that propofol also mitigated hepatic IRI (a faster return to a normal INR and lower plasma ALT concentrations 24 hours after transplantation) in LT recipients, and the upregulation of HO-1 appeared to be one of the mechanisms by which propofol protects against hepatic ischemic injury.
4.3. Propofol attenuation of hepatic IRI through inhibiting release of inflammatory factors and downregulation of MMP-9
The events that occurs during hepatic IRI manifests as an early increase in oxidative stress, liver sinusoidal endothelial cell injury, Kupffer cell activation, and advance release of reactive oxygen species, all of which by turns lead to significant tissue injury and liver remodeling.[36] MMPs are the major enzymes involved in remodeling of connective tissue; their inappropriate, prolonged, or excessive expression has deleterious consequences.[9] Therefore, increased MMPs activity may lead to liver damage, with changes of the sinusoidal cells and stromal structure remodeling. Among different MMPs, MMP-9 appears as an important mediator of leukocyte flux in liver IRI, and an inducible gelatinase expressed by leukocytes in acutely damaged livers.[9] Moreover, increased expression of hepatic MMP-9 has been reported after normothermic IRI.[37] In rat livers, MMP-9 was upregulated after 6 hours after LT,[38] and 3 hours following IRI.[39] In human orthotopic LT, MMP-9 was detected in the serum of patients minutes following reperfusion, and this is associated with acute allograft rejection.[11] The MMP-9 levels persisted elevated for several days following LT, and brought about a progression of liver damage in IRI.[10] Moreover, Shirahane et al showed that specific MMP inhibitors decrease liver damage following ischemia, which was associated with a reduction of inflammatory cytokine release.[40] Hamada et al have shown that using an anti-MMP neutralizing monoclonal antibody to target MMP-9 results in protective effect from damage following hepatic IRI.[9] Furthermore, MMP-9 inhibition has been exhibited to be beneficial in limiting post-ischemic liver injury, including in whole LT and acute “small-for-size” graft damage.[41] On the contrary, Feng et al[42] confirmed that liver damage was reduced in MMP-9-/- mice at 24 hours following reperfusion, recovery of liver following 72 hours of reperfusion was significantly delayed in MMP-9-/- mice compared to WT mice. Accordingly, MMP-9 appears to show a dual effect in hepatic IRI, which varies with time of reperfusion.[43]
However, to the best of our knowledge, this is the first report showing that propofol anesthesia attenuates hepatic IRI in LT recipients by the downregulation of MMP-9 and inhibition of the inflammatory factor release (elevated levels of IL-1RA and IL-10).
4.4. Weaknesses in study
The major limitation of this pilot study was the small number of patients selected; hence the conclusion cannot be taken as evidence. More participants are required to conduct a further exploratory study to explore the role of propofol in blunting the reperfusion injury and association with long term outcomes during liver transplant surgery. In this aspect, t more studies. Second, the only recipient pathology directly affected by graft survival associated with gender mismatch is hepatitis C-positive female recipients with male donors, female donors being independent predictor of fibrotic progression and graft loss.[44] Nevertheless, this topic does not administer to our study, and there was no significant difference in gender and indications for LT between the 2 groups. Finally, our understanding of pathophysiology in hepatic IRI is poor. Thus, more studies are needed to improve our knowledge of the mechanisms of liver cell damage, inflammation, and regeneration.
5. Conclusions
To the best of our knowledge, this is the first study to investigate the protective effect of propofol-based TIVA against IRI and graft outcome compared with desflurane anesthesia in LT recipients. Our study showed that propofol attenuates hepatic IRI with improved recovery of graft function and better microcirculation, and this protection may be through attenuating inflammation responses, downregulation of MMP-9, and enhancing HO-1 expression. However, there was no impact on long term outcome due to the small number of patients of a pilot study. Our results may shed light on the clinical application of propofol to alleviate inflammation and oxidative stress in IRI. More participants are required to conduct a further exploratory study to explore the role of propofol in blunting the reperfusion injury and association with long term outcomes during liver transplant surgery.
Author contributions
Conceptualization: Zhi-Fu Wu, Chueng-He Lu.
Data curation: Wei-Lin Lin, Nan-Kai Hung.
Formal analysis: Meei-Shyuan Lee.
Investigation: Wei-Lin Lin, Yuan-Shiou Huang, Teng-Wei Chen.
Methodology: Zhi-Fu Wu, Meei-Shyuan Lee, Chueng-He Lu.
Supervision: Teng-Wei Chen.
Validation: Meei-Shyuan Lee, Nan-Kai Hung, Yuan-Shiou Huang, Teng-Wei Chen, Chueng-He Lu.
Visualization: Wei-Lin Lin, Nan-Kai Hung.
Writing – original draft: Zhi-Fu Wu.
Writing – review & editing: Zhi-Fu Wu, Meei-Shyuan Lee, Chueng-He Lu.
Footnotes
Abbreviations: AUC = area under the curve, HO-1 = heme oxygenase-1, IL = interleukin, IRI = ischemia and reperfusion injury, MMP-9 = matrix metalloproteinase-9, TIVA = total intravenous anesthesia.
How to cite this article: Wu ZF, Lin WL, Lee MS, Hung NK, Huang YS, Chen TW, Lu CH. Propofol vs desflurane on the cytokine, matrix metalloproteinase-9, and heme oxygenase-1 response during living donor liver transplantation: A pilot study. Medicine. 2019;98:48(e18244).
Trial registry number: Chinese Clinical Trial Registry (ChiCTR-INR-17011600).
The authors have no funding and conflicts of interests to disclose.
References
- [1].Onur A, Akbulut S, Dirican A, et al. Life-threatening or nearly life-threatening complications in living liver donors. Clin Transplant 2018;32:e13262. [DOI] [PubMed] [Google Scholar]
- [2].Abu-Amara M, Yang SY, Tapuria N, et al. Liver ischemia/reperfusion injury: processes in inflammatory networks--a review. Liver Transpl 2010;16:1016–32. [DOI] [PubMed] [Google Scholar]
- [3].Zhai Y, Petrowsky H, Hong JC, et al. Ischaemia-reperfusion injury in liver transplantation--from bench to bedside. Nat Rev Gastroenterol Hepatol 2013;10:79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Siniscalchi A, Dante A, Spedicato S, et al. Hyperdynamic circulation in acute liver failure: reperfusion syndrome and outcome following liver transplantation. Transplant Proc 2010;42:1197–9. [DOI] [PubMed] [Google Scholar]
- [5].Liu B, Qian JM. Cytoprotective role of heme oxygenase-1 in liver ischemia reperfusion injury. Int J Clin Exp Med 2015;8:19867–73. [PMC free article] [PubMed] [Google Scholar]
- [6].Yun N, Eum HA, Lee SM. Protective role of heme oxygenase-1 against liver damage caused by hepatic ischemia and reperfusion in rats. Antioxid Redox Signal 2010;13:1503–12. [DOI] [PubMed] [Google Scholar]
- [7].Lai IR, Chang KJ, Chen CF, et al. Transient limb ischemia induces remote preconditioning in liver among rats: the protective role of heme oxygenase-1. Transplantation 2006;81:1311–7. [DOI] [PubMed] [Google Scholar]
- [8].Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol 2004;4:617–29. [DOI] [PubMed] [Google Scholar]
- [9].Hamada T, Fondevila C, Busuttil RW, et al. Metalloproteinase-9 deficiency protects against hepatic ischemia/reperfusion injury. Hepatology 2008;47:186–98. [DOI] [PubMed] [Google Scholar]
- [10].Kuyvenhoven JP, Ringers J, Verspaget HW, et al. Serum matrix metalloproteinase MMP-2 and MMP-9 in the late phase of ischemia and reperfusion injury in human orthotopic liver transplantation. Transplant Proc 2003;35:2967–9. [DOI] [PubMed] [Google Scholar]
- [11].Kuyvenhoven JP, Verspaget HW, Gao Q, et al. Assessment of serum matrix metalloproteinases MMP-2 and MMP-9 after human liver transplantation: increased serum MMP-9 level in acute rejection. Transplantation 2004;77:1646–52. [DOI] [PubMed] [Google Scholar]
- [12].Lu CH, Yeh CC, Huang YS, et al. Hemodynamic and biochemical changes in liver transplantation: A retrospective comparison of desflurane and total intravenous anesthesia by target-controlled infusion under auditory evoked potential guide. Acta Anaesthesiol Taiwan 2014;52:6–12. [DOI] [PubMed] [Google Scholar]
- [13].Tsai YC, Huang CC, Chu LM, et al. Differential influence of propofol on different cell types in terms of the expression of various oxidative stress-related enzymes in an experimental endotoxemia model. Acta Anaesthesiol Taiwan 2012;50:159–66. [DOI] [PubMed] [Google Scholar]
- [14].Wang HH, Zhou HY, Chen CC, et al. Propofol attenuation of renal ischemia/reperfusion injury involves heme oxygenase-1. Acta Pharmacol Sin 2007;28:1175–80. [DOI] [PubMed] [Google Scholar]
- [15].Acquaviva R, Campisi A, Raciti G, et al. Propofol inhibits caspase-3 in astroglial cells: role of heme oxygenase-1. Curr Neurovasc Res 2005;2:141–8. [DOI] [PubMed] [Google Scholar]
- [16].Liang C, Xue Z, Wang H, et al. Propofol upregulates heme oxygenase-1 through activation of ERKs in human umbilical vein endothelial cells under oxidative stress conditions. J Neurosurg Anesthesiol 2011;23:229–35. [DOI] [PubMed] [Google Scholar]
- [17].Liang C, Cang J, Wang H, et al. Propofol attenuates cerebral ischemia/reperfusion injury partially using heme oxygenase-1. J Neurosurg Anesthesiol 2013;25:311–6. [DOI] [PubMed] [Google Scholar]
- [18].Chi X, Guo N, Yao W, et al. Induction of heme oxygenase-1 by hemin protects lung against orthotopic autologous liver transplantation-induced acute lung injury in rats. J Transl Med 2016;14:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ji FT, Liang JJ, Miao LP, et al. Propofol post-conditioning protects the blood brain barrier by decreasing matrix metalloproteinase-9 and aquaporin-4 expression and improves the neurobehavioral outcome in a rat model of focal cerebral ischemia-reperfusion injury. Mol Med Rep 2015;12:2049–55. [DOI] [PubMed] [Google Scholar]
- [20].Han D, Li S, Xiong Q, et al. Effect of propofol on the expression of MMP-9 and its relevant inflammatory factors in brain of rat with intracerebral hemorrhage. Cell Biochem Biophys 2015;72:675–9. [DOI] [PubMed] [Google Scholar]
- [21].Koren A, Sodja E, Rijavec M, et al. Prognostic value of cytokeratin-7 mRNA expression in peripheral whole blood of advanced lung adenocarcinoma patients. Cell Oncol (Dordr) 2015;38:387–95. [DOI] [PubMed] [Google Scholar]
- [22].Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- [23].Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986;42:121–30. [PubMed] [Google Scholar]
- [24].Walsh TS, Garden OJ, Lee A. Metabolic, cardiovascular, and acid-base status after hepatic artery or portal vein reperfusion during orthotopic liver transplantation. Liver Transpl 2002;8:537–44. [DOI] [PubMed] [Google Scholar]
- [25].Tao KM, Yang LQ, Liu YT, et al. Volatile anesthetics might be more beneficial than propofol for postoperative liver function in cirrhotic patients receiving hepatectomy. Med Hypotheses 2010;75:555–7. [DOI] [PubMed] [Google Scholar]
- [26].Lee J, Yoo YJ, Lee JM, et al. Sevoflurane versus desflurane on the incidence of postreperfusion syndrome during living donor liver transplantation: a randomized controlled trial. Transplantation 2016;100:600–6. [DOI] [PubMed] [Google Scholar]
- [27].Granja TF, Kohler D, Schad J, et al. Adenosine receptor adora2b plays a mechanistic role in the protective effect of the volatile anesthetic sevoflurane during liver ischemia/reperfusion. Anesthesiology 2016;125:547–60. [DOI] [PubMed] [Google Scholar]
- [28].McDaid J, Yamashita K, Chora A, et al. Heme oxygenase-1 modulates the allo-immune response by promoting activation-induced cell death of T cells. FASEB J 2005;19:458–60. [DOI] [PubMed] [Google Scholar]
- [29].Brockmann JG, August C, Wolters HH, et al. Sequence of reperfusion influences ischemia/reperfusion injury and primary graft function following porcine liver transplantation. Liver Transpl 2005;11:1214–22. [DOI] [PubMed] [Google Scholar]
- [30].Liu A, Fang H, Wei W, et al. Ischemic preconditioning protects against liver ischemia/reperfusion injury via heme oxygenase-1-mediated autophagy. Crit Care Med 2014;42:e762–71. [DOI] [PubMed] [Google Scholar]
- [31].Tsai YF, Lin CC, Lee WC, et al. Propofol attenuates ischemic reperfusion-induced formation of lipid peroxides in liver transplant recipients. Transplant Proc 2012;44:376–9. [DOI] [PubMed] [Google Scholar]
- [32].Ye L, Luo CZ, McCluskey SA, et al. Propofol attenuates hepatic ischemia/reperfusion injury in an in vivo rabbit model. J Surg Res 2012;178:e65–70. [DOI] [PubMed] [Google Scholar]
- [33].Yoo YC, Yoo KJ, Lim BJ, et al. Propofol attenuates renal ischemia-reperfusion injury aggravated by hyperglycemia. J Surg Res 2013;183:783–91. [DOI] [PubMed] [Google Scholar]
- [34].Froh M, Conzelmann L, Walbrun P, et al. Heme oxygenase-1 overexpression increases liver injury after bile duct ligation in rats. World J Gastroenterol 2007;13:3478–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Matsumi J, Morimatsu H, Matsusaki T, et al. Heme breakdown and ischemia/reperfusion injury in grafted liver during living donor liver transplantation. Int J Mol Med 2012;29:135–40. [DOI] [PubMed] [Google Scholar]
- [36].Palladini G, Ferrigno A, Richelmi P, et al. Role of matrix metalloproteinases in cholestasis and hepatic ischemia/reperfusion injury: a review. World J Gastroenterol 2015;21:12114–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Moore C, Shen XD, Gao F, et al. Fibronectin-alpha4beta1 integrin interactions regulate metalloproteinase-9 expression in steatotic liver ischemia and reperfusion injury. Am J Pathol 2007;170:567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Amersi F, Shen XD, Moore C, et al. Fibronectin-alpha 4 beta 1 integrin-mediated blockade protects genetically fat Zucker rat livers from ischemia/reperfusion injury. Am J Pathol 2003;162:1229–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Cursio R, Mari B, Louis K, et al. Rat liver injury after normothermic ischemia is prevented by a phosphinic matrix metalloproteinase inhibitor. FASEB J 2002;16:93–5. [DOI] [PubMed] [Google Scholar]
- [40].Shirahane K, Yamaguchi K, Koga K, et al. Hepatic ischemia/reperfusion injury is prevented by a novel matrix metalloproteinase inhibitor, ONO-4817. Surgery 2006;139:653–64. [DOI] [PubMed] [Google Scholar]
- [41].Ma ZY, Qian JM, Rui XH, et al. Inhibition of matrix metalloproteinase-9 attenuates acute small-for-size liver graft injury in rats. Am J Transplant 2010;10:784–95. [DOI] [PubMed] [Google Scholar]
- [42].Feng M, Wang H, Wang Q, et al. Matrix metalloprotease 9 promotes liver recovery from ischemia and reperfusion injury. J Surg Res 2013;180:156–61. [DOI] [PubMed] [Google Scholar]
- [43].Ji J. Dual role of matrix metalloprotease 9 in liver ischemia and reperfusion injury. J Surg Res 2013;185:545–6. [DOI] [PubMed] [Google Scholar]
- [44].Lai JC, Verna EC, Brown RS, Jr, et al. Hepatitis C virus-infected women have a higher risk of advanced fibrosis and graft loss after liver transplantation than men. Hepatology 2011;54:418–24. [DOI] [PMC free article] [PubMed] [Google Scholar]


