Abstract
Background:
Dynamin-related protein 1 (Drp1) plays important roles in tumorigenesis, including lung cancer. However, the effect of Drp1 in lung cancer remains unclear. The present study was aimed to investigate the clinical significance and effect of Drp1 on prognosis of lung cancer.
Methods:
Oncomine and The Cancer Genome Atlas (TCGA) databases were selected to predict the differential expression levels of Drp1 in lung cancer. Then, 70 cases of lung cancer and normal tissues were collected and immunohistochemistry was used to detect the expression of Drp1. In addition, Kaplan–Meier Plotter database and TCGA database were used to verify the correlation between Drp1 expression and the clinical prognosis in lung cancer patients.
Results:
Drp1 was significantly overexpressed in lung cancer tissues based on Oncomine and TCGA databases (P < .05). Moreover, results from immunohistochemistry showed that Drp1 protein level in lung cancer was also significantly higher than that in the matched normal tissues (P < .05). Prognostic analysis from Kaplan–Meier Plotter database with the chosen probe IDs of 203105_s_at suggested that Drp1 was negatively correlated to overall survival (OS) of lung cancer patients (HR = 1.16, 95% CI: 1.02–1.31; P = .025), but not in the probe IDs of 226154_at (HR = 0.86, 95% CI: 0.73–1.01; P = .069). However, prognosis from TCGA database showed inconsistent results in which high expression of Drp1 was correlated with worse survival probability of all, male, female in lung adenocarcinoma (P < .05), but not in LUSC (P > .05).
Conclusion:
Drp1 was highly expressed in lung cancer based on bioinformatics analysis and tissue microarray, but there was a lot of inconsistency in prognosis depending on different levels of Drp1 from the bioinformatics analysis.
Keywords: Drp1, lung cancer, prognosis
1. Introduction
Lung cancer is the leading cause of cancer incidence and mortality worldwide, with 2.1 million new lung cancer cases and 1.8 million deaths predicted in 2018 according to the Global Cancer Statistics 2018.[1] Non-small cell lung cancer (NSCLC) accounts for 85% of lung cancer cases which contains various pathological types, such as non-squamous carcinoma and squamous cell carcinoma (LUSC), and 5-year survival rate for all stages is only 16%.[2] Despite advances in the level of health care, oncotherapy is still confronted with great challenges due to the delayed diagnosis, recurrence or metastasis, as well as cancer associated mutation.[3] Therefore, it is of great significance to explore tumor molecular markers which may effectively evaluate tumor screening, diagnosis, prognosis and recrudescence.
Dynamin-related protein 1 (Drp1), also named DNM1L, is a cytosolic dynamin-related GTPase belong to the dynamin protein family which was the first fission protein to be discovered.[4] Previous evidence revealed that Drp1 played an important role in various cellular biological processes, including cell growth, cell cycle, proliferation and apoptosis by regulating mitochondrial fission.[5] In addition, growing evidence found that changes in Drp1 protein levels were related to the tumorigenesis, including breast cancer, ovarian cancer, colon cancer and lung cancer.[6–9] However, the relationship between Drp1 expression levels and prognosis in patients with lung cancer remains contradictory. Kim and his colleague observed that the decrease of Drp1 expression was obvious in patients with advanced lung cancer.[6] Chiang found that higher expression of nucleolar Drp1 was associated with poor prognosis in lung adenocarcinomas (LUAC).[10]
Thus, in the current study, we further explored the expression and prognosis of Drp1 in patients with lung cancer. Oncomine and The Cancer Genome Atlas (TCGA) databases were first used to compare the different expression of Drp1 mRNA in lung cancer and normal lung tissues. In addition, tissue microarray was used to assess the expression of Drp1 protein in lung cancer and matched adjacent normal tissues. Kaplan-Meier univariate and TCGA multivariate survival analyses were then performed to explore the potentially prognostic significance of Drp1 in lung cancer patients.
2. Methods
2.1. Bioinformatics mining methods
TCGA database (https://tcga-data.nci.nih.gov/tcga/), a collection of miRNA-seq, RNA-seq, SNP array, DNA methylation, exome sequencing and more, was used for described the mRNA expression data for lung cancer.[11] Oncomine databases (https://www.oncomine.org), a cancer microarray database and web-based data mining platform, were selected to predict the expression level of Drp1 in lung cancer and normal lung tissues.[12] The search parameters used in this database were as follows; analysis type- cancer vs normal, data source- public, cancer types- selected specific cancer type, sample type- clinical specimen and, data type- mRNA. Furthermore, Kaplan–Meier Plotter database (http://kmplot.com/analysis), an online database including gene expression data and clinical data, and TCGA database were used to evaluate the prognostic significance of Drp1 in lung cancer and the overall survival (OS) of lung cancer patients.[13] Briefly, the patient samples were divided into two cohorts according to the median expression of Drp1 (high vs. low expression) and then the gene was uploaded into the database respectively to obtain the Kaplan-Meier survival plots, in which the number-at-risk was shown below the main plot. Log rank p-value and hazard ratio (HR) with 95% confidence intervals (CI) were calculated and displayed on the webpage. P < .05 was considered significant.
2.2. Tissue microarrays and immunohistochemistry
We retrospectively collected 70 cases of lung cancer and matched adjacent normal tissues from patients who underwent surgical resection in Jiangxi Provincial People's Hospital, China (from September 2016 to August 2018). Patients’ detailed clinical information, such as age, gender, tumor location, histologic differentiation and tumor node metastasis (TNM) stage was collected. The study protocol was approved by the ethical committee of Jiangxi provincial people's hospital, and the informed consent was provided from the clinicians and patients for the usage of the tissues for research.
A parallel tissue microarray was constructed using a tissue array (Beecher instruments, silver spring, MD) in this study. The expression of Drp1 was measured by immunohistochemical staining according to the protocol instruction.[14] Briefly, tissue sections were cut with a thickness of 8 um, deparaffinized by xylene and rehydrated in decreasing concentration of ethanol. Antigen retrieval was achieved by boiling sections on 10 mM citrate buffer for 20 minutes. After blocking of endogenous peroxidase with 3% hydrogen peroxidase in methanol, sections were incubated with Background Sniper (Biocare medical) at room temperature for 30 minutes. The sections were then incubated with primary antibody of Drp1 (Cell signaling, USA) with a working concentration of 1: 100 at 4°C overnight. The results of immunohistochemical staining were calculated according to the immunoreaction score (IRS) and evaluated by 2 pathologists in a double-blind way as described previously.[15] Briefly, the range of IRS score was from 0 to 12, which was produced by staining intensity (SI) × percentage of positive cells (PP). High expression of Drp1 was considered as IRS > 4, while low expression of Drp1 was considered as IRS ≤ 4.
2.3. Statistical analysis
Oncomine and TCGA databases were analyzed by the independent samples Student t test to compare the differential expression levels of Drp1 mRNA between the lung cancer group and the normal lung tissues group. Kaplan–Meier univariate and TCGA survival analyses were done to investigate the prognostic significance of Drp1 in lung cancer patients. SPSS 19.0 software (SPSS, Inc., Chicago, IL) was used to analyze the experimental data. The expression of Drp1 in lung cancer and matched normal tissue was evaluated by Student t test and chi square tests. P < .05 was considered statistically significant.
3. Results
Oncomine was used to investigate the differences in mRNA expression of Drp1 between tumor and normal tissue among multiple cancers in (Fig. 1). The database contained a total of 352 unique analyses for Drp1expression in tumor. There were 18 studies showing a significant statistical difference for Drp1, of which 13 showed that mRNA expression level of Drp1 was increased in tumor than normal tissues in nine kinds of cancers, including bladder cancer, breast cancer, cervical cancer, head and neck cancer, lung cancer, lymphoma, myeloma, sarcoma and other cancer, while five regarding brain and central nervous system (CNS) cancer, breast cancer, colorectal cancer and lymphoma showed an opposite result. Moreover, the most notable among these are lung cancer and sarcoma wherein Drp1 mRNA levels were significantly increasing in tumor cases in a total of three and four unique analyses, respectively. As lung cancer is the leading cause of cancer incidence and mortality worldwide,[1] we further analyzed the differential expression in lung cancer and its subtypes.
Figure 1.
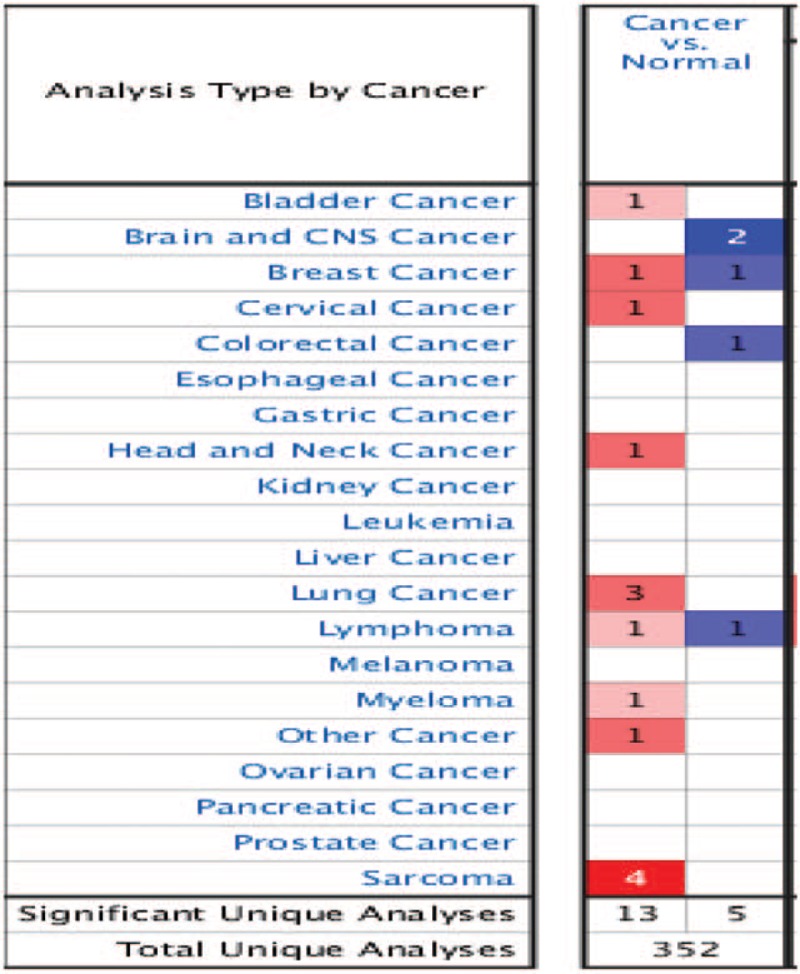
Oncomine analysis of expression levels of Drp1 across different cancers. Differences in expression levels of the genes between tumor and normal tissue are summarized in the Fig. The number of unique analyses satisfying the thresholds (P ≤ .01; fold change ≥2; gene rank ≤10%; data type: mRNA) are indicated in the colored cells. Red cells represent overexpression of the target gene, in tumor tissues compared to normal, whereas blue cells indicate downregulation of the same. Gene rank is depicted by the color depth in the cells. CNS = central nervous system.
3.1. Expression levels of Drp1 in lung cancer
There are three main types of lung cancer; non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC) and lung carcinoid tumor. Among these 3, NSCLC is the most common type of lung cancer (85%) followed by SCLC (10 ± 15%) and lung carcinoid tumor (5%).[16,17] In addition, NSCLC can be mainly divided into three main subtypes: lung adenocarcinoma cell carcinoma (LUAC), lung squamous cell carcinoma (LUSC) and large cell lung carcinoma (LCLC). Therefore, we explored the different expression of Drp1 in multiple types of lung cancer.
Meta-analysis of the 19 datasets from 5 studies on Drp1mRNA levels in lung cancer versus normal lung tissue were searched using Oncomine database (Fig. 2). As it was shown, the mRNA expression of Drp1 was higher in lung cancer tissues than normal lung tissues (P < .05, Fig. 2A). When subtypes analysis was performed, the results were in line with above findings showing an increasing in the mRNA expression of Drp1 in tumor versus normal tissue, including LUAC, LUSC, LCLC and LCLC (P < .05, Fig. 2B–E).
Figure 2.

Meta-analysis of the 19 datasets from 5 studies on Drp1 mRNA levels in lung cancer vs normal lung tissue searched by Oncomine database. (A) Expression of Drp1in lung cancer. (B) Expression of Drp1in lung adenocarcinoma (LUAC). (C) Expression of Drp1in lung squamous cell carcinoma (LUSC). (D) Expression of Drp1in large cell lung carcinoma (LCLC). (E) Expression of Drp1in small cell lung cancer (SCLC).
Oncomine analysis of cancer vs. normal tissue in different datasets was further performed to determine differential expression of Drp1 in different subtypes of lung cancer (Fig. 3). In Bhattacharjee dataset statistics, the mRNA level of Drp1 was significantly increased in lung carcinoid tumor (fold change = 3.974), LUAC (fold change = 1.822), LUSC (fold change = 2.228) and SCLC (fold change = 2.533), respectively (Fig. 3A). Furthermore, in the analysis of the expression levels of Drp1 in Hou dataset, the subtypes of lung cancer also revealed significantly increased mRNA levels of Drp1, including LUAC (fold change = 1.456), LUSC (fold change = 2.01) and LCLC (fold change = 2.555, Fig. 3B). The mRNA level of Drp1 was also shown to be overexpressed in subtypes of lung cancer according to Garber's datasets, LUAC (fold change = 1.95), LUSC (fold change = 1.922), LCLC (fold change = 2.527) and SCLC (fold change = 2.109, Fig. 3C).
Figure 3.
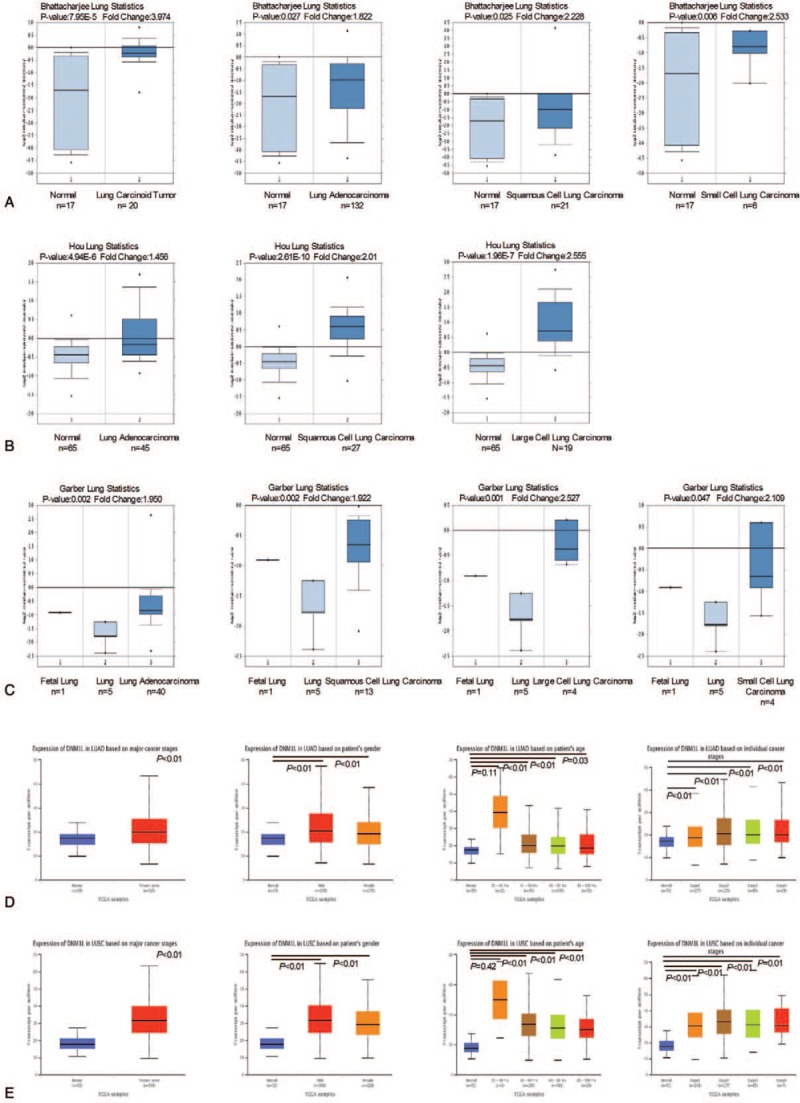
mRNA expression of Drp1 in lung cancer and subtypes using Oncomine database and The Cancer Genome Atlas (TCGA). (A) Drp1 mRNA expression in lung cancer subtypes in Bhattacharjee dataset. (B) Drp1 mRNA expression in lung cancer subtypes in Hou dataset. (C) Drp1 mRNA expression in lung cancer subtypes in Garber's datasets. X-axis of the plot represents normal vs cancer group, Y-axis represents mRNA expression in log2 median/mean centered intensity. The line in the middle represents the median value. (D) Drp1 mRNA expression in LUAC in TCGA database. (E) Drp1 mRNA expression in LUSC in TCGA database. X axis of the plot represents normal vs cancer group, Y axis represents mRNA expression in transcript per million. P < .05 were considered significant.
To further validate and cross-examine the observations made in Oncomine database, the mRNA expression data from TCGA dataset was used value the differential mRNA levels of Drp1 in tumor tissue. There was higher expression of Drp1 in both LUAC and LUSC than normal lung tissues without correlation with sex (P < .05, Fig. 3D–E). Interestingly, the overexpression of Drp1in lung cancer tissues only occurred in those people over 40 years old (P < .05, Fig. 3D–E). There was no significant difference in the expression of Drp1 between patients under 40 years old and normal one (P > .05). In addition, increased expression of Drp1 was observed in all the stages of lung cancer, but there was not any significantly difference among various tumor stages (P > .05, Fig. 3D–E). Together, these findings were in consistency with the Oncomine analysis showing an increasing in the mRNA expression of Drp1 in tumor than normal tissue.
3.2. Prognostic significance of Drp1 in lung cancer
The potentially prognostic significance of Drp1 expression levels in lung cancer patients was investigated by bioinformatics analysis. Kaplan–Meier plotter analysis was first carried out to find out the correlation between Drp1 and OS, first progression (FP) and post progression survival (PPS) in lung cancer patients. The chosen probe IDs (203105_s_at and 226154_at) for Drp1 has been listed in Table 1. The analysis from ID of 203105_s_at revealed that higher expression of Drp1was associated with OS (HR = 1.16, 95% CI: 1.02–1.31; P = .025, Fig. 4A), but not with FP or PPS, in lung cancer. Drp1 expression in the probe ID of 226154_at was found to be a weak correlation correlated with OS, but not with statistically significant (HR = 0.86, 95% CI: 0.73 - 1.01; P = .069, Fig. 4A). Furthermore, higher expression of Drp1 showed a correlation with better PPS (HR = 0.55, 95% CI: 0.36 – 0.85; P < .05) in lung cancer. The similar result was occurred in male patients. Further analysis in histological subtypes showed more significant correlations between high mRNA expression of Drp1 and better OS in lung adenocarcinoma patients (HR = 0.72, 95% CI: 0.57–0.91; P < .01; HR = 0.62, 95% CI: 0.48–0.79; P < .001, Fig. 4B) but not in LUSC (HR = 0.97, 95% CI: 0.77–1.23; P = .810; HR = 1.33, 95% CI: 0.98–1.82; P = .068, Fig. 4C). Interestingly, the OS of female patients showed opposite tendency in LUAC (HR = 0.65; 95% CI: 0.45–0.95; P = .027; HR = 0.59; 95% CI: 0.39–0.9; P = .013, Fig. 4B–C) and LUSC (HR = 1.85; 95% CI: 1.04–3.29; P = 0.037; HR = 5.71; 95% CI: 2.1–15.49; P < .001, Fig. 4B–C) with increased mRNA level of Drp1. In addition, likewise, the data from the probe ID of 226154_at indicated better PPS (HR = 0.52, 95% CI: 0.32–0.85; P < .01; HR = 0.47, 95% CI: 0.24–0.91; P = .024) was occurred in all and male lung adenocarcinoma patients.
Table 1.
The correlation between Drp1 and survival outcomes in lung cancer.
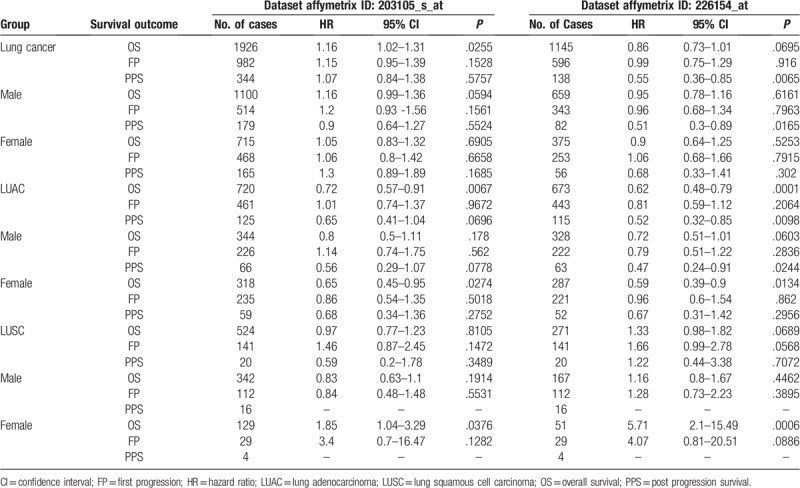
Figure 4.
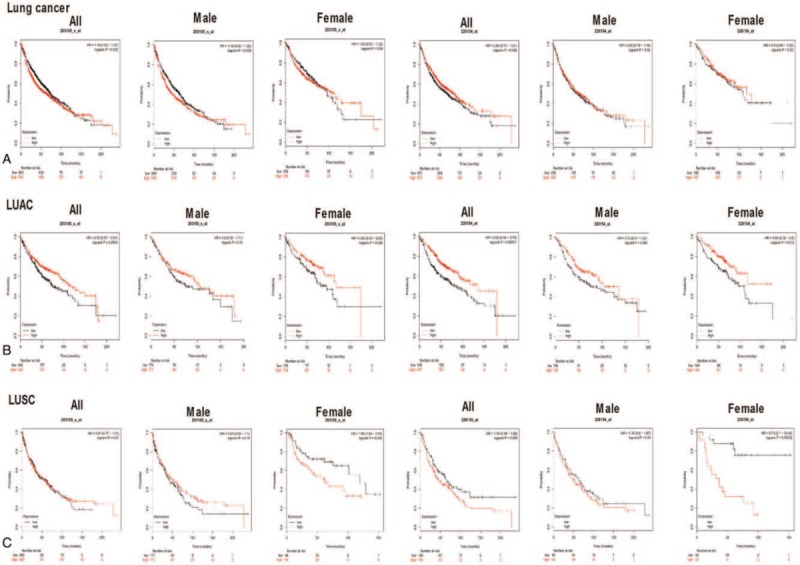
Prognostic significance of Drp1 expression in lung cancer from Kaplan–Meier plotter analysis. (A) Kaplan–Meier plots for overall survival (OS) in lung cancer patients with Drp1 expression in all, male and female. (B) Kaplan–Meier plots for OS in lung adenocarcinoma patients with Drp1 expression in all, male and female. (C) Kaplan–Meier plots for OS in lung squamous cell carcinoma patients with Drp1 expression in all, male and female. Red line represents patients with expression above the median value and in black, patients with expressions below the median value have been represented. X axis denotes number of patients at risk at specific time (in months) and Y axis shows the probability of survival. P < .05 were considered significant.
The TCGA database was next performed to verify the correlation of Drp1 expression with the survival probability of the lung cancer patients. On the contrary, high expression of Drp1was correlated with worse survival probability of all, male, female in LUAC (P < .05, Fig. 5A-B). There was not any significant difference between the expression of Drp1 and lung squamous carcinoma patient survival probability of all, male and female (P > .05, Fig. 5C-D).
Figure 5.
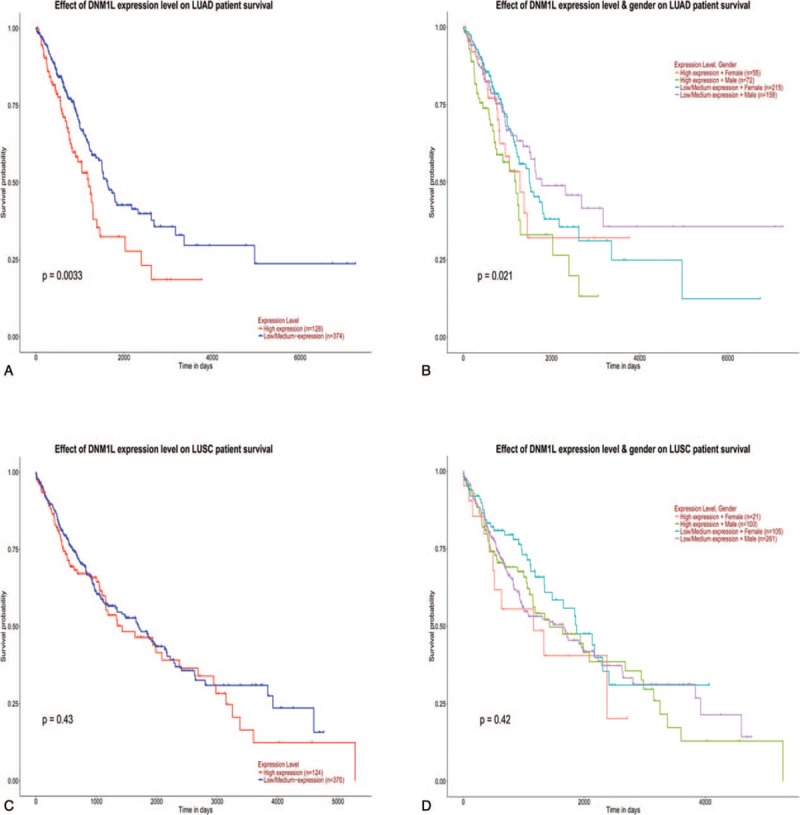
Prognostic significance of Drp1 expression in lung cancer from TCGA data. (A) TCGA data for the survival probability in lung adenocarcinoma patients with Drp1 expression. (B) TCGA data for the survival probability in lung adenocarcinoma patients with Drp1 expression according to sex. (C) TCGA data for the survival probability in lung squamous cell carcinoma patients with Drp1 expression. (D) TCGA data for the survival probability in lung squamous cell carcinoma patients with Drp1 expression according to sex.
3.3. Expression of Drp1 in a tissue microarray of lung cancer
Seventy cases of lung cancer and matched adjacent normal tissues from patients who underwent surgical resection in Jiangxi Provincial People's Hospital, China (from January 2017 to February 2018) were collected to validate the predictive results. The mean age of these lung cancer and normal lung cases was 61.2 years (46 men and 24 women), respectively. Among all of lung cancer, 67 cases were NSCLC and 3 cases were SCLC. When subgroups were made, 35 cases were LUAC, 24 cases were LUSC and 8 cases were the other rare types.
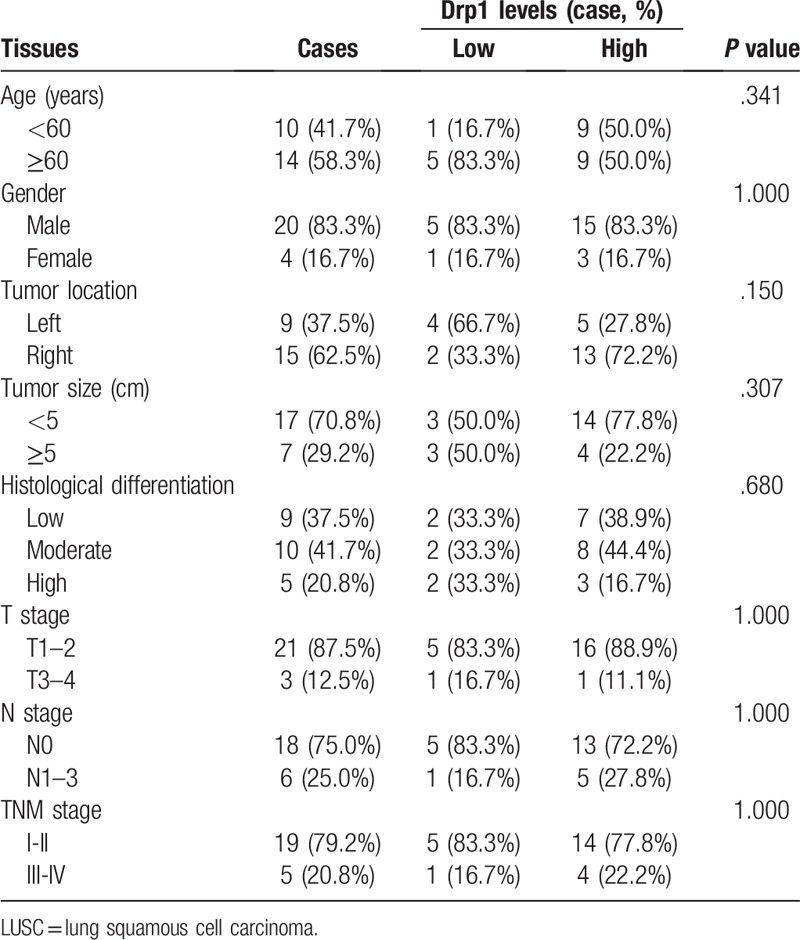
Morphological change was determined using hematoxylin/eosin (HE) staining in lung cancer and matched normal tissues (Fig. 6A-B). Then, immunohistochemistry was used to detect the expression of Drp1 in lung cancer and matched normal tissues. Results from immunohistochemistry showed that Drp1 was stained mainly located in cytoplasm of lung cancer cells (Fig. 6C-D). Compared to that in the matched normal tissues, the expression level of Drp1 protein in lung cancer was dramatically higher (P < .001, Fig. 6E). The consistent results were observed in LUAC and LUSC, respectively (P < .05, Fig. 6F-G). The detailed results were also shown in Table 2. Among all of the 70 cases of lung cancer, 50 cases were Drp1 positive (71.4%), which was significantly higher than that in the normal lung tissues (24.3%, P < .01), including LUAC and LUSC (P < .01, Table 2). In addition, we detected the expression of Drp1 in the subtypes of NSCLC based on different parameters, including age, gender, tumor location, tumor size, histological differentiation, T stage, N stage, and TNM stage (Tables 3 and 4). However, results from LUAC and LUSC both suggested that the expression of Drp1 was not related to age, gender, tumor location, tumor size, histological differentiation, T stage, N stage, and TNM stage (P > .05).
Figure 6.
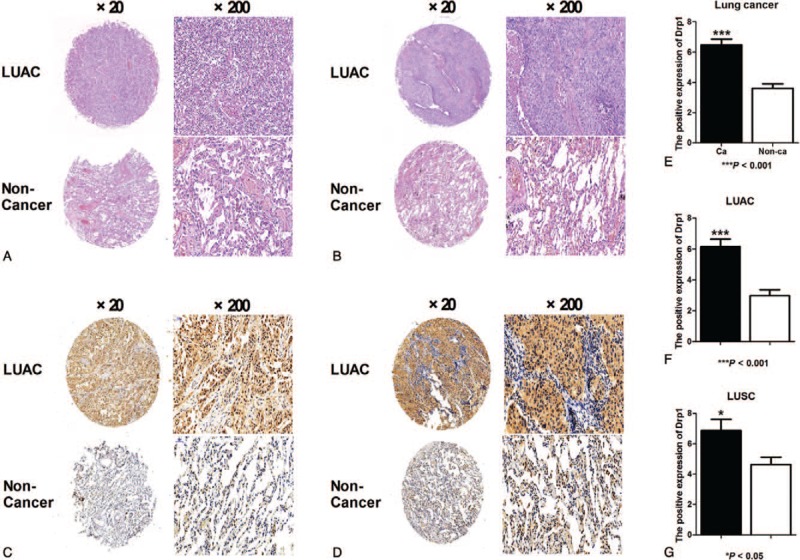
Expression of Drp1 in lung cancer and subtypes. (A) Hematoxylin/eosin (HE) staining of LUAC tissues with Drp1 expression (original magnification ×2 and ×20). (B) HE staining of LUSC with Drp1 expression (original magnification ×2 and ×20). (C) Immunohistochemical staining for Drp1 in LUAC (original magnification ×2 and ×20). (D) Immunohistochemical staining for Drp1 in LUSC (original magnification ×2 and ×20). (E) Quantification of positive staining for Drp1 in lung cancer and normal tissue. (F) Quantification of positive staining for Drp1 in LUAC and normal tissue. (G) Quantification of positive staining for Drp1 in LUSC and normal tissue. ∗P < .05; ∗∗∗P < .001.
Table 2.
Expression of Drp1 protein in lung cancer and normal lung.
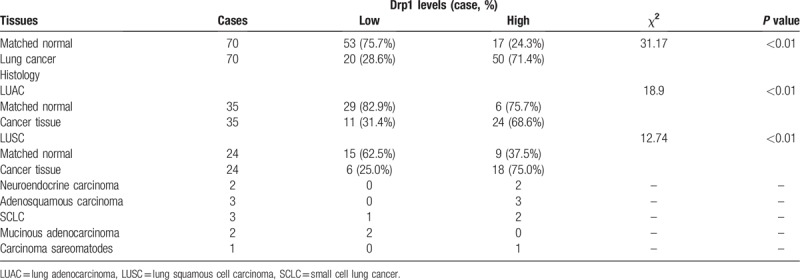
Table 3.
Differential expression of Drp1 protein and other clinicopathological parameters in LUAC.
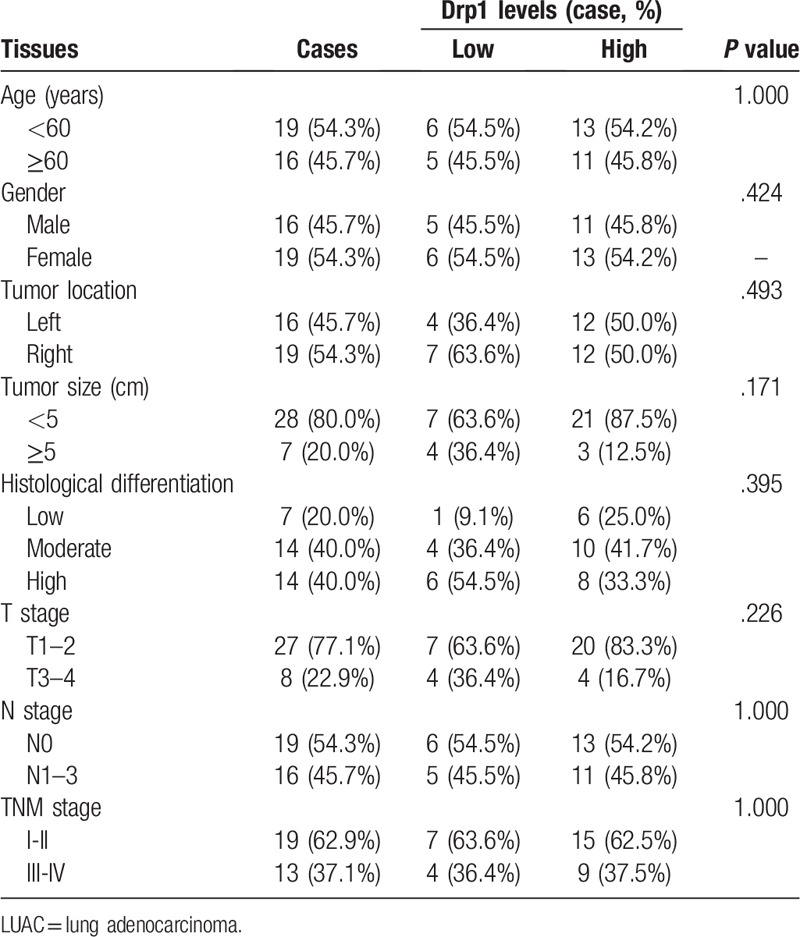
Table 4.
Differential expression of Drp1 protein and other clinicopathological parameters in LUSC.
4. Discussion
Increasing evidence reported that Drp1 plays important roles in cancer cellular processes, including mitochondrial dynamics, cell cycle progression, genome instability, cell migration and apoptosis.[8,18,19] However, the expression and prognostic effect of Drp1 in various tumors was different. In the present study, we examined the differential expression levels of Drp1 in lung cancer compared to normal lung tissues using bioinformatics and tissue microarray. Moreover, Kaplan–Meier univariate and TCGA multivariate survival analyses were performed to explore the relationship between Drp1 and prognostic significance in lung cancer.
Drp1 in various cells are different depending on cell types, physiological context or pathologic types.[20] For example, Drp1 was reported to be upregulated in breast and ovarian cancer.[8,21] However, the expression of Drp1 in colon cancer was low.[6] Interestingly, reports from studies of lung cancer showed that the expression of Drp1 was controversial. Kim and his colleague found that the level of Drp1 was decreased significantly in lung cancer tissues, however, results from Rehman and his colleague got the contrary conclusions.[22] In our study, we explored the expression of Drp1 in lung cancer using the Oncomine database, TCGA database and tissue microarray. As a result, bioinformatics prediction results based on Oncomine and TCGA databases showed that Drp1 mRNA level was significantly higher in lung cancer tissues than that in normal lung tissues and it was independent of gender, age and tumor stage. Consistently, the results from immunohistochemistry demonstrated that Drp1 was also higher in lung cancer than in normal lung tissues, but there was a not any correlation between Drp1 expression and gender, age, tumor location, tumor size, histological differentiation, T stage, N stage, and TNM stage. All these results suggested that Drp1 gene might participate in tumor formation and growth. Furthermore, Drp1 might be a molecule marker for diagnosing lung cancer.
Increasing evidences demonstrate that Drp1 protein level was associated with cell cycle progression, genome instability, cell proliferation, migration, invasion and apoptosis in different cancer cells, including lung cancer.[7,18,19] However, the prognostic effect of Drp1 in lung cancer was controversial. For example, Rehman et al. documented an increase in Drp1 expression in tissue samples from patients with LUAC and inhibition of Drp1reduced proliferation and increases apoptosis of lung cancer cells.[7,19] But, Kim and his colleague suggested that loss of Drp1 was associated with the progression of human lung cancer, and an identical pattern was observed in cultured lung cancer cell lines.[6,23] In our study, we explored the relationship between Drp1 levels and the potentially prognostic effect in lung cancer patients using bioinformatics. Kaplan-Meier plotter analysis with the chosen probe IDs of 203105_s_at demonstrated that the increased expression of Drp1 was correlated to poor OS for lung cancer patients, but not in the probe IDs of 226154_at. There was a worse prognosis in men with LUSC when the expression of Drp1 was increased. Intriguingly, lung adenocarcinoma patients with higher levels of Drp1 had better OS both the two probe IDs, especially female patients. Contrarily, the TCGA database showed that high expression of Drp1 was correlated with worse survival probability in all, male, female with LUAC, but not any relationship was observed in LUSC. These inconsistent associations of the levels of Drp1 with prognosis in lung cancer in our study and previous studies might be due to the racial difference, different population and sample size, as well as measurement methods of the expression of Drp1. More importantly, Drp1 plays its role in tumor cells process related to cell types or pathologic types. There are various pathological types of lung cancer, including LUAC, LUSC, LCLC and SCLC. All these may explain the inconsistent conclusions in our study and previous studies.
Evasion of apoptosis is implicated in almost all aspects of lung cancer, as well as treatment resistance.[24] Tumor formation in lung tissue is also associated with dysregulation of mitochondrial fusion and fission.[7,18] Drp1, a mitochondrial division protein, participating tumor cells progression mainly depends on apoptosis pathway which is regulated by mitochondrial fission and fusion.[7,18] Moreover, Drp1 may regulate cell apoptosis by different regulatory mechanisms. One side, high expression and activation of Drp1 in tumor cells promotes mitochondrial fission, ultimately initiating apoptotic ways.[4,5,23,25] On the other hand, interestingly, suppression or depletion of Drp1 also induces tumor cells apoptosis. Drp1-mediated mitochondrial fission is necessary for proper progression through the cell cycle following G1/S transition. Persistent mitochondrial hyperfusion beyond the G1/S border which was caused by Drp1 depletion increases replication stress, leading to G2/M delay, chromosomal instability and DNA damage, ultimately inhibiting cell division and triggering apoptosis.[5,7,18,26] Therefore, Drp1-induced mitochondrial fission promotes cell death, it has also been shown that Drp1-mediated fission protect against cell death.[4] These may partly explain the inconsistent prognosis related to Drp1 levels in lung cancer patients. Therefore, further research is needed to clear the potential mechanisms of Drp1in lung cancer.
There were some limitations in our present study. First, there were only 70 cases of lung cancer tissues collected in this study which was a small sample size. Therefore, a larger sample size is needed for future research. Second, prognostic effect of Drp1in lung cancer was evaluated only by bioinformatics, relevant follow up data is needed to improve accuracy. Third, this study focused on expression and prognostic significance of Drp1 in lung cancer, but its detailed molecular mechanism was not further explored. All these limitations should be improved and investigated in future studies.
5. Conclusion
In conclusion, our study demonstrated that Drp1 is highly expression in lung cancer, including SCCL and NSCLC and the upregulation of Drp1 may be act as a potential marker for the diagnosis of lung cancer. However, the prognosis effect of Drp1 in lung cancer is still controversial. Further research is needed to explore the prognostic value and potential mechanisms of Drp1in lung cancer in future.
Author contributions
Conceptualization: Lingling Yu, Zuke Xiao, Bo Tong, Shengsong Chen.
Data curation: Hongying Tub Tu.
Formal analysis: Lingling Yu, Bo Tong, Shengsong Chen.
Methodology: Lingling Yu.
Supervision: Shengsong Chen.
Validation: Shengsong Chen.
Visualization: Shengsong Chen.
Writing – original draft: Lingling Yu, Bo Tong, Shengsong Chen.
Writing – review & editing: Lingling Yu, Shengsong Chen.
Footnotes
Abbreviations: Drp1 = dynamin-related protein 1, FP = first progression, LUAC = lung adenocarcinomas, LUSC = lung squamous cell carcinoma, NSCLC = non-small cell lung cancer, OS = overall survival, PPS = post progression survival, TCGA = The Cancer Genome Atlas.
How to cite this article: Yu L, Xiao Z, Tu H, Tong B, Chen S. The expression and prognostic significance of Drp1 in lung cancer: A bioinformatics analysis and immunohistochemistry. Medicine. 2019;98:48(e18228).
LY, ZX, and BT contributed equally to this work and are co-first authors.
The authors have no funding and conflicts of interests to disclose.
References
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].Ge C, Li R, Song H, et al. Phase I clinical trial of a novel autologous modified-DC vaccine in patients with resected NSCLC. BMC Cancer 2017;17:884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hong QY, Wu GM, Qian GS, et al. Prevention and management of lung cancer in China. Cancer 2015;121: Suppl 17: 3080–8. [DOI] [PubMed] [Google Scholar]
- [4].Jahani-Asl A, Slack RS. The phosphorylation state of Drp1 determines cell fate. Embo Rep 2007;8:912–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hu C, Huang Y, Li L. Drp1-dependent mitochondrial fission plays critical roles in physiological and pathological progresses in mammals. Int J Mol Sci 2017;18:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kim YY, Yun SH, Yun J. Downregulation of Drp1, a fission regulator, is associated with human lung and colon cancers. Acta Biochim Biophys Sin (Shanghai) 2018;50:209–15. [DOI] [PubMed] [Google Scholar]
- [7].Shen F, Gai J, Xing J, et al. Dynasore suppresses proliferation and induces apoptosis of the non-small-cell lung cancer cell line A549. Biochem Biophys Res Commun 2018;495:1158–66. [DOI] [PubMed] [Google Scholar]
- [8].Zhao J, Zhang J, Yu M, et al. Mitochondrial dynamics regulates migration and invasion of breast cancer cells. Oncogene 2013;32:4814–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tanwar DK, Parker DJ, Gupta P, et al. Crosstalk between the mitochondrial fission protein, Drp1, and the cell cycle is identified across various cancer types and can impact survival of epithelial ovarian cancer patients. Oncotarget 2016;7:60021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chiang Y, Chen S, Hsiao Y, et al. Nuclear expression of dynamin-related protein 1 in lung adenocarcinomas. Modern Pathol 2009;22:1139–50. [DOI] [PubMed] [Google Scholar]
- [11].Bornstein S, Schmidt M, Choonoo G, et al. IL-10 and integrin signaling pathways are associated with head and neck cancer progression. BMC Genomics 2016;17:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rhodes DR, Yu J, Shanker K, et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia 2004;6:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yu L, Chen S, Bao H, et al. The role of lncRNA CASC2 on prognosis of malignant tumors: a meta-analysis and bioinformatics. Onco Targets Ther 2018;11:4355–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dadhania V, Zhang M, Zhang L, et al. Meta-analysis of the luminal and basal subtypes of bladder cancer and the identification of signature immunohistochemical markers for clinical use. EBioMedicine 2016;12:105–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hu DD, Li PC, He YF, et al. Overexpression of coiled-coil domain-containing protein 34 (CCDC34) and its Correlation with angiogenesis in esophageal squamous cell carcinoma. Med Sci Monit 2018;24:698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yang RF, Yu B, Zhang RQ, et al. Bevacizumab and gefitinib enhanced whole-brain radiation therapy for brain metastases due to non-small-cell lung cancer. Braz J Med Biol Res 2017;51:e6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ettinger DS, Akerley W, Borghaei H, et al. Non-small cell lung cancer. J Natl Compr Canc Netw 2012;10:1236–71. [DOI] [PubMed] [Google Scholar]
- [18].Qian W, Wang J, Van Houten B. The role of dynamin-related protein 1 in cancer growth: a promising therapeutic target? Expert Opin Ther Targets 2013;17:997–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lima AR, Santos L, Correia M, et al. Dynamin-related protein 1 at the crossroads of cancer. Genes (Basel) 2018;9:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hu C, Huang Y, Li L. Drp1-dependent mitochondrial fission plays critical roles in physiological and pathological progresses in mammals. Int J Mol Sci 2017;18:pii: E144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang J, Hansen K, Edwards R, et al. Mitochondrial division inhibitor 1 (mdivi-1) enhances death receptor-mediated apoptosis in human ovarian cancer cells. Biochem Biophys Res Commun 2015;456:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rehman J, Zhang HJ, Toth PT, et al. Inhibition of mitochondrial fission prevents cell cycle progression in lung cancer. Faseb J 2012;26:2175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Thomas KJ, Jacobson M. Defects in mitochondrial fission protein dynamin-related protein 1 are linked to apoptotic resistance and autophagy in a lung cancer model. PLoS One 2012;7:e45319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Glinsky GV, Glinsky VV, Ivanova AB, et al. Apoptosis and metastasis: increased apoptosis resistance of metastatic cancer cells is associated with the profound deficiency of apoptosis execution mechanisms. Cancer Lett 1997;115:185–93. [DOI] [PubMed] [Google Scholar]
- [25].Cho B, Choi SY, Cho HM, et al. Physiological and pathological significance of dynamin-related protein 1 (Drp1)-Dependent Mitochondrial Fission in the Nervous System. Exp Neurobiol 2013;22:149–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nakamura T, Cieplak P, Cho D, et al. S-Nitrosylation of Drp1 links excessive mitochondrial fission to neuronal injury in neurodegeneration. Mitochondrion 2010;10:573–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


