Abstract
Our previous data shows that serum albumin can trigger natural transformation in A. baumannii. However, extracellular-matrix/basal membrane components, norepinephrine, and mucin did not have a significant effect on this process. Therefore, the effect of human products appears to be albumin specific, as both BSA and HSA have been identified as inducers of natural competence.
Acinetobacter baumannii, a member of the highly resistant ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species), has emerged over the last few decades as a severe nosocomial pathogen due to its ability to resist desiccation and nutrient starvation, and obtain novel resistance genes (1).
A. baumannii’s capacity to incorporate exogenous DNA, via horizontal genetic transfer (HGT), is contributing to its genome plasticity, as well as, to the multidrug resistance phenotype seen in a variety of clinical strains throughout the world [22,21,15]. Recently, the World Health Organization (WHO) classified this species as a priority 1 pathogen, meaning it is considered one of the critical pathogens for antibiotic research and development [28].
Natural transformation, one of the mechanisms of HGT, has been scarcely studied in a few strains among the genus Acinetobacter spp [7,16,17,20,29,19,4]. Since 2010, we have studied this process in the naturally competent clinical strain A118, which was isolated from a patient’s blood sample and was shown to be susceptible to several antibiotics [20,24], and showed that it can acquire different DNA sources [20,21,25]. Moreover, our recent publication indicates that albumin, the main protein in blood, and Ca2+ significantly enhance transformation frequency and increase expression levels of two competence genes (comEA and pilQ) in A. baumannii strains [25].
With the aim to identify other relevant host products that have an effect on competence during A. baumannii colonization/infection, and as it is known that A. baumannii can cause a wide variety of serious infections, including wound infections [6,18], and can bind to extracellular matrix/basal membrane (ECM/BM) proteins [2], we tested various human products.
Several kanamycin susceptible A. baumannii strains (A118, ATCC 17978, ATCC 19606 and A42) [1,5,8,9,11,12,23,26] were included in the present study and challenged by host human products (collagen IV, collagen I, hyaluronic acid, mucin, and norepinephrine). We decided to include additional A. baumannii strains, apart from strain A118, to observe if the effects are strain dependent. The most studied and commonly used ATCC strains were used as well as strain A42, which is a multidrug resistant clinical isolate recovered from endotracheal aspirate and which belongs to the clonal complex I [26]. Transformation assays using a plasmid (pDSredAK, conferring kanamycin resistance (KanR) or genomic DNA (from strain A. baumannii 144) known to carry a KanR determinant, were performed as previously described [20, 24]. Briefly, 50 μl of late stationary-death phase cultures of A. baumannii strains were transferred to 50 μl of sterile LB. 100 ng of plasmid DNA and/or gDNA were added and the cultures were incubated for 1 hour at 37°C followed by plating on LB agar with 10 μg/ml Kan. Transformation events were scored by counting KanR colonies, while total CFUs were assessed by plating serial dilutions on LB agar plates. Moreover, the acquisition of KanR genes was confirmed by PCR as well as by measuring the level of resistance to aminoglycosides by the gradient diffusion method (E-test method) with commercial strips (Biomerieux) [24]. Experiments were repeated at least three times and statistical analysis (Mann-Whitney test) was performed. Statistical data analysis was carried out using GraphPad Prism (GraphPad software, San Diego, CA, USA) and a P-value of <0.05 was considered significant. Human serum albumin (HSA) was also tested, as previously described, to see its effect in other A. baumannii strains.
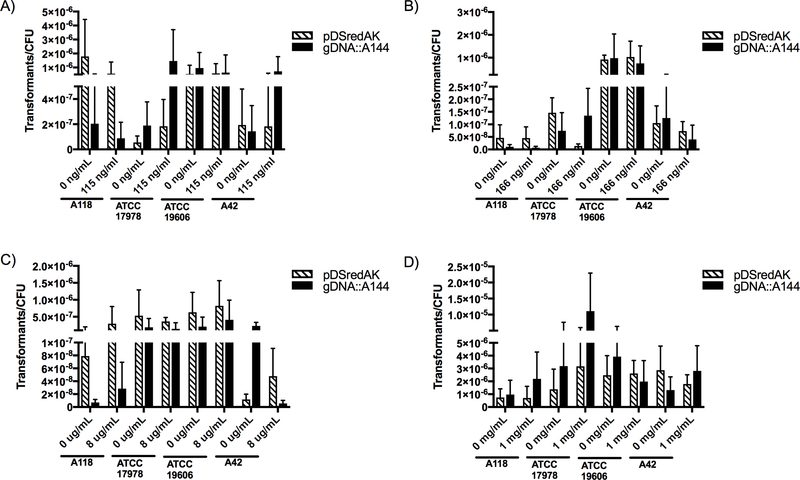
The effect of two types of collagen, network-forming collagen (type IV) and fibrillar collagen (type I), were tested. Collagen IV was tested in two physiological concentrations, 115 and 166 ng/ml, which correspond to serum collagen IV levels in healthy and sick patients, respectively [14]. Collagen I was tested at 8 ug/ml which represents approximately 3% of concentration found in adult human skin [27]. Neither collagen IV nor collagen I had a statistically significant effect on transformation frequencies in any of the four strains used (Fig. 1 a, b and c). The effect of both collagen type IV and type I appeared to be both strain and DNA-type dependent and produced varied results. Interestingly, 166 ng/mL of collagen IV decreased both the transformation frequency of strain ATCC 17978 by 10.7-fold, when transformed with plasmid DNA, as well as the transformation frequency of strain A42 by 3.13-fold, when transformed with genomic DNA. In contrast, 115 ng/mL of collagen IV increased the transformation frequency of strain 17978 by 7.74 fold when transformed with genomic DNA and by 3.35-fold when transformed with plasmid DNA. Furthermore, collagen I decreased the transformation frequency of strain A42 by 41-fold when transformed with genomic DNA and increased it by 4.07-fold when transformed with plasmid DNA. In addition, we tested hyaluronic acid, a simple polysaccharide with a relevant role in the organization and maintenance of the extracellular matrix that is also present in blood serum, synovial fluid and thoracic lymph fluid [3]. As A. baumannii frequently leads to skin and blood infections, we choose to examine the effect of hyaluronic acid at concentrations found in those regions. We chose a concentration of 1 mg/mL for our transformation assays to ensure that the experimental concentration was equal to or higher than that found in the human body [3]. Following the same trend we observed for both collagen types, there was no statistically significant effect on transformation frequencies in any of the four strains used (Fig. 1 d).
Figure 1. Natural transformation frequencies with extracellular matrix/basal membrane proteins.
Transformation assays were performed in LB broth with A) 115 ng/mL collagen IV, B) 166 ng/mL collagen IV, C) 8 μg /mL collagen I or D) 1 mg/mL hyaluronic acid. Cultures were transformed with plasmid DNA (striped) or genomic DNA (black) and plated on LB agar supplemented with 10 μg/mL kanamycin while CFUs were plate on LB agar. Data are presented as the mean and the errors bars represent the standard deviation. At least three independent replicates were performed and p <0.05 was considered significant (Mann Whitney t test, n=3 to 9).
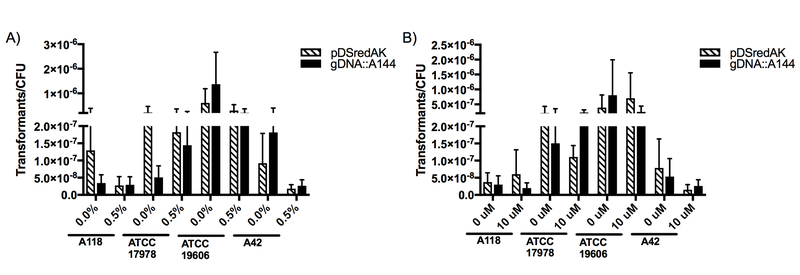
Similarly, and considering A. baumannii’s role as one of the most important pathogens in ventilator-associated pneumonia (VAP), mucin, a mucopolysaccharide that is the main component of mucus, was tested. A concentration of 0.5% was used, as this concentration had been used in a previous study [13]. The results showed that mucin decreased transformation frequencies in all strains when transformed with plasmid DNA and in all but one strain (ATCC 17978) when transformed with genomic DNA (Fig. 2.a). Strain A42 had a greater than 5-fold decrease in transformation frequencies with both genomic and plasmid DNA. However, none of the effects observed were statistically significant.
Figure 2. Natural transformation frequencies with additional human proteins.
Transformation assays were performed in LB broth with A) 0.5% (w/v) mucin or B) 10 μM norepinephrine. Cultures were transformed with plasmid DNA (striped) or genomic DNA (black) and plated on LB agar supplemented with 10 μg/mL kanamycin while CFUs were plate on LB agar. Data are presented as the mean and the errors bars represent the standard deviation. At least three independent replicates were performed and p <0.05 was considered significant (Mann Whitney t test, n= 3 to 5).
Additionally, as it was recently shown that the host stress hormone norepinephrine (NE) upregulates the expression of efflux pump genes and increases biofilm formation in A. baumannii [10], we tested this host hormone as a potential inducer of competence. Transformation assays, using 10 uM of NE, produced varied results both within and between strains (Fig. 2.b). Strain ATCC 19606 showed a 1.80-fold increase when transformed with plasmid DNA after growth in NE and a 3.24-fold decrease when transformed with genomic DNA after growth in the same conditions. A similar trend was seen for strain A118. Strain ATCC 17978, however, showed a decrease in transformation frequency (1.9-fold decrease) when transformed with plasmid DNA and an increase (1.51-fold increase) when transformed with genomic DNA. Both genomic and plasmid DNA decreased transformation frequencies in strain A42 by 5.15-fold and 2.04-fold, respectively.
HSA’s effect on transformation was also tested in parallel using strain A42, which belong to the widespread clonal complex I, and A118 strain, which is a sporadic clone, to verify its effect. As previously observed, and in agreement with the BSA results, growth in HSA showed a statistically significant transformation frequency increase of 16.4 and 11.8 folds for A118 and A42, respectively.
Overall our results showed that extracellular-matrix/basal membrane components, mucin and the hormone NE do not impose a statistically significant effect in competence in A. baumannii. Transformation frequencies varied in each strain and, in some cases, were affected by the type of DNA used. The observed results, taken with our recent discovery that albumin proteins are inducers of natural competence in A. baumannii, led us to conclude that not all human proteins contribute to an increase in transformation frequencies and that albumins are exerting a specific effect on transformation in A. baumannii.
Acknowledgment
BQ was supported by grant MHIRT 2T37MD001368 from the National Institute on Minority Health and Health Disparities, National Institute of Health. GMT has a Post-Doctoral Fellowship from CONICET. We thank Dr. Luis Actis for his advice.
References
- 1.Aranda J, Poza M, Pardo BG, Rumbo S, Rumbo C, Parreira JR, Rodriguez-Velo P, Bou G (2010) A rapid and simple method for constructing stable mutants of Acinetobacter baumannii. BMC Microbiol 10:279. doi: 10.1186/1471-2180-10-279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bentancor LV, Routray A, Bozkurt-Guzel C, Camacho-Peiro A, Pier GB, Maira-Litran T (2012) Evaluation of the trimeric autotransporter Ata as a vaccine candidate against Acinetobacter baumannii infections. Infect Immun 80 (10):3381–3388. doi: 10.1128/IAI.06096-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dicker KT, Gurski LA, Pradhan-Bhatt S, Witt RL, Farach-Carson MC, Jia X (2014) Hyaluronan: a simple polysaccharide with diverse biological functions. Acta Biomater 10 (4):1558–1570. doi: 10.1016/j.actbio.2013.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerischer U, Ornston LN (2001) Dependence of linkage of alleles on their physical distance in natural transformation of Acinetobacter sp. strain ADP1. Arch Microbiol 176 (6):465–469. doi: 10.1007/s00203-001-0353-7 [DOI] [PubMed] [Google Scholar]
- 5.Giles SK, Stroeher UH, Eijkelkamp BA, Brown MH (2015) Identification of genes essential for pellicle formation in Acinetobacter baumannii. BMC Microbiol 15:116. doi: 10.1186/s12866-015-0440-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guerrero DM, Perez F, Conger NG, Solomkin JS, Adams MD, Rather PN, Bonomo RA (2010) Acinetobacter baumannii-associated skin and soft tissue infections: recognizing a broadening spectrum of disease. Surg Infect (Larchmt) 11 (1):49–57. doi: 10.1089/sur.2009.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harding CM, Tracy EN, Carruthers MD, Rather PN, Actis LA, Munson RS Jr. (2013) Acinetobacter baumannii strain M2 produces type IV pili which play a role in natural transformation and twitching motility but not surface-associated motility. MBio 4 (4). doi: 10.1128/mBio.00360-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hare JM, Ferrell JC, Witkowski TA, Grice AN (2014) Prophage induction and differential RecA and UmuDAb transcriptome regulation in the DNA damage responses of Acinetobacter baumannii and Acinetobacter baylyi. PLoS One 9 (4):e93861. doi: 10.1371/journal.pone.0093861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heindorf M, Kadari M, Heider C, Skiebe E, Wilharm G (2014) Impact of Acinetobacter baumannii superoxide dismutase on motility, virulence, oxidative stress resistance and susceptibility to antibiotics. PLoS One 9 (7):e101033. doi: 10.1371/journal.pone.0101033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inaba M, Matsuda N, Banno H, Jin W, Wachino JI, Yamada K, Kimura K, Arakawa Y (2016) In vitro reduction of antibacterial activity of tigecycline against multidrug-resistant Acinetobacter baumannii with host stress hormone norepinephrine. Int J Antimicrob Agents 48 (6):680–689. doi: 10.1016/j.ijantimicag.2016.09.022 [DOI] [PubMed] [Google Scholar]
- 11.Jacobs AC, Sayood K, Olmsted SB, Blanchard CE, Hinrichs S, Russell D, Dunman PM (2012) Characterization of the Acinetobacter baumannii growth phase-dependent and serum responsive transcriptomes. FEMS Immunol Med Microbiol 64 (3):403–412. doi: 10.1111/j.1574-695X.2011.00926.x [DOI] [PubMed] [Google Scholar]
- 12.Johnson TL, Waack U, Smith S, Mobley H, Sandkvist M (2015) Acinetobacter baumannii is dependent on the type II secretion system and its substrate LipA for lipid utilization and in vivo fitness. J Bacteriol. doi: 10.1128/JB.00622-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kavanaugh NL, Zhang AQ, Nobile CJ, Johnson AD, Ribbeck K (2014) Mucins suppress virulence traits of Candida albicans. MBio 5 (6):e01911. doi: 10.1128/mBio.01911-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazouni C, Arun B, Andre F, Ayers M, Krishnamurthy S, Wang B, Hortobagyi GN, Buzdar AU, Pusztai L (2008) Collagen IV levels are elevated in the serum of patients with primary breast cancer compared to healthy volunteers. Br J Cancer 99 (1):68–71. doi: 10.1038/sj.bjc.6604443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merkier AK, Catalano M, Ramirez MS, Quiroga C, Orman B, Ratier L, Famiglietti A, Vay C, Di Martino A, Kaufman S, Centron D (2008) Polyclonal spread of bla(OXA-23) and bla(OXA-58) in Acinetobacter baumannii isolates from Argentina. J Infect Dev Ctries 2 (3):235–240 [DOI] [PubMed] [Google Scholar]
- 16.Nielsen KM, Bones AM, Van Elsas JD (1997) Induced Natural Transformation of Acinetobacter calcoaceticus in Soil Microcosms. Appl Environ Microbiol 63 (10):3972–3977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nielsen KM, van Weerelt MD, Berg TN, Bones AM, Hagler AN, van Elsas JD (1997) Natural transformation and availability of transforming DNA to Acinetobacter calcoaceticus in soil microcosms. Appl Environ Microbiol 63 (5):1945–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez F, Hujer AM, Hujer KM, Decker BK, Rather PN, Bonomo RA (2007) Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 51 (10):3471–3484. doi: 10.1128/AAC.01464-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pour NK, Dusane DH, Dhakephalkar PK, Zamin FR, Zinjarde SS, Chopade BA (2011) Biofilm formation by Acinetobacter baumannii strains isolated from urinary tract infection and urinary catheters. FEMS Immunol Med Microbiol 62 (3):328–338. doi: 10.1111/j.1574-695X.2011.00818.x [DOI] [PubMed] [Google Scholar]
- 20.Ramirez MS, Don M, Merkier AK, Bistue AJ, Zorreguieta A, Centron D, Tolmasky ME (2010) Naturally competent Acinetobacter baumannii clinical isolate as a convenient model for genetic studies. J Clin Microbiol 48 (4):1488–1490. doi: 10.1128/JCM.01264-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramirez MS, Merkier AK, Quiroga MP, Centron D (2012) Acinetobacter baumannii is able to gain and maintain a plasmid harbouring In35 found in Enterobacteriaceae isolates from Argentina. Curr Microbiol 64 (3):211–213. doi: 10.1007/s00284-011-0052-9 [DOI] [PubMed] [Google Scholar]
- 22.Roca I, Espinal P, Vila-Farres X, Vila J (2012) The Acinetobacter baumannii Oxymoron: Commensal Hospital Dweller Turned Pan-Drug-Resistant Menace. Front Microbiol 3:148. doi: 10.3389/fmicb.2012.00148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith MG, Gianoulis TA, Pukatzki S, Mekalanos JJ, Ornston LN, Gerstein M, Snyder M (2007) New insights into Acinetobacter baumannii pathogenesis revealed by high-density pyrosequencing and transposon mutagenesis. Genes Dev 21 (5):601–614. doi: 10.1101/gad.1510307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Traglia GM, Chua K, Centron D, Tolmasky ME, Ramirez MS (2014) Whole-genome sequence analysis of the naturally competent Acinetobacter baumannii clinical isolate A118. Genome Biol Evol 6 (9):2235–2239. doi: 10.1093/gbe/evu176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Traglia GM, Quinn B, Schramm ST, Soler-Bistue A, Ramirez MS (2016) Serum Albumin and Ca2+ Are Natural Competence Inducers in the Human Pathogen Acinetobacter baumannii. Antimicrob Agents Chemother 60 (8):4920–4929. doi: 10.1128/AAC.00529-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vilacoba E, Almuzara M, Gulone L, Traglia GM, Figueroa SA, Sly G, Fernandez A, Centron D, Ramirez MS (2013) Emergence and spread of plasmid-borne tet(B)::ISCR2 in minocycline-resistant Acinetobacter baumannii isolates. Antimicrob Agents Chemother 57 (1):651–654. doi: 10.1128/AAC.01751-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Cheng RY-h, Ning Fang-gang and Zhang Guo-an* (2011) The content and ratio of type I and III collagen in skin differ with age and injury. African Journal of Biotechnology 10 (13):2524–2529 [Google Scholar]
- 28.WHO (2017) Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. News release by the WHO [Google Scholar]
- 29.Wilharm G, Piesker J, Laue M, Skiebe E (2013) DNA uptake by the nosocomial pathogen Acinetobacter baumannii occurs during movement along wet surfaces. J Bacteriol 195 (18):4146–4153. doi: 10.1128/JB.00754-13 [DOI] [PMC free article] [PubMed] [Google Scholar]