Abstract
Human papillomavirus (HPV) infection is a common sexually transmitted disease. Although often transitory, persistent oncogenic HPV infection may progress to a precursor lesion and, if not treated, can further increase the risk of cancer. The purpose of this study was to investigate the relation between dietary intake and HPV persistent infection in men of a Brazilian cohort. The study population consisted of 1,248 men from the Brazilian cohort of the HIM (HPV in Men) Study, ages 18 to 70 years, who completed a quantitative food frequency questionnaire. U Mann-Whitney test was used to assess differences in median nutrient intake of selected nutrients. The association of dietary intake and persistent HPV infection was assessed in multivariate logistic models. The prevalence of any HPV infection at baseline was 66.6%. Of 1,248 participants analyzed, 1,211 (97.0%) were HPV positive at one or more times during the 4 years of follow-up and 781 (62.6%) were persistently HPV positive. Men with nonpersistent oncogenic HPV infections had higher median intake of retinol (p = 0.008), vitamin A (p < 0.001) and folate (DFE; p = 0.003) and lower median intake of energy (p = 0.005) and lycopene (p = 0.008) in comparison to men with persistent oncogenic infections. No significant association was found between selected nutrients and persistent oncogenic HPV infection. For nononcogenic persistent infections, only vitamin B12 intake was significantly associated (p = 0.003, test for trend). No association was observed between dietary intake and persistent oncogenic-type HPV infection; however, vitamin B12 intake was inversely associated with nononcogenic HPV persistence.
Keywords: diet, HPV, men, food frequency questionnaire
Human papillomavirus (HPV) infection is one of the most common sexually transmitted diseases and is generally subclinical and transitory, usually resolved spontaneously by the immune response.1–3 Although often transitory, persistent oncogenic HPV type infection may progress to a precursor lesion and, if not correctly treated, can further increase the risk of pre-cancer and cancer,4–6 contributing to diseases such as cervical, vulvar, vaginal, anal, penile and oropharyngeal cancers and genital warts in women and men.7–9 HPV prevalence is common in sexually active individuals, with male infection significantly contributing to infection and subsequent disease in women.7
Prior studies evaluated the association between diet and the risk of HPV acquisition and persistence among women.10–14
Nutrients such as folate and vitamins B6 and B12 may have a role in regulating viral integration and gene stability due to their involvement in DNA synthesis, repair and methylation.11,14 Consuming foods, particularly plant-based foods, that support normal DNA methylation, has the potential to suppress expression of viral oncogenes, promote proper signaling pathways, avoid cell transformation, and reduce the risk of cancer in humans.14–16 Antioxidant nutrients are capable of modulating immune response and decreasing viral replication and gene expression.11 Folate, vitamins A, C and E, and the active Vitamin D metabolite are reported to have the ability to inhibit cell proliferation, prevent DNA damage and enhance immunologic functions.11,14,17,18 Moreover, due to its essential role in the replication of basal and mucosal cells and in the synthesis of protein, vitamin A deficiency could lead to a higher risk of infection and metaplasia.
García-Closas et al.(2005)11 completed a systematic review of the role diet exerts on the risk of persistent HPV and cervical cancer. A possible protective effect against HPV persistence was noted for fruits, vegetables, vitamins C and E, alpha and betacarotene, lycopene, lutein/zeaxanthin and cryptoxanthin. For cervical neoplasia, evidence was probable for folate, retinol and vitamin E and possible for vegetables, vitamins B12 and C, lycopene, lutein/zeaxanthin and cryptoxanthin. However, the authors emphasized the need of more prospective studies evaluating these associations.
Chi et al. (2013),14 in a more recent review, reported that higher intake of nutrients with antioxidant and antiviral functions may prevent the progression of HPV infection to high-grade cervical intraepithelial neoplasia.
Despite the epidemiological support for a role of dietary intake and nutritional status in carcinogenic process, few take into consideration HPV infection and persistence,11 especially in men. Additionally, the association between specific dietary components and the quantity required to prevent cancer is not well established.14 Thereby a better understanding of this relation in men is an important component for the prevention of HPV infection-related diseases in both genders.
Considering the possible association of diet and risk of persistent HPV infection, the purpose of this study was to investigate the association between dietary intake of selected nutrients and HPV persistent infection in men of a Brazilian cohort.
Material and Methods
Study population
The HPV in Men (HIM) Study is a multinational prospective study that examines the natural history of HPV infection in men. Participants were eligible for participation if they were aged 18–70 years; residents of southern Florida-USA, São Paulo-Brazil or Cuernavaca-Mexico; reported no previous diagnosis of penile or anal cancers; reported no previous diagnosis of genital or anal warts; had not participated in an HPV vaccine study; reported no previous diagnosis of HIV; reported no current penile discharge or burning during urination; were not being treated for sexually transmitted infection; had not been imprisoned or homeless during the past 6 months; had not received drug treatment during the past 6 months; had no plans to relocate in the next 4 years; and were willing to comply with 10 scheduled visits every 6 months for 4 years.
In Brazil, men were recruited from “Centro de Referênda e Treinamento DST/AIDS-SP,” a large clinic in São Paulo that provides genitourinary services, and the general population through television, radio and newspaper advertisements.
Eligible participants provided written informed consent, and study protocols were approved by institutional review boards at each study site. More detailed description of the study design and population is reported elsewhere.8,19,20
Men who provided consent underwent a clinical examination at a visit 2 weeks before the enrolment visit and every 6 months thereafter, for 4 years. To encourage compliance with follow-up, men were given compensation for transport and alimentation for their participation. For this study, only the Brazilian cohort was considered.
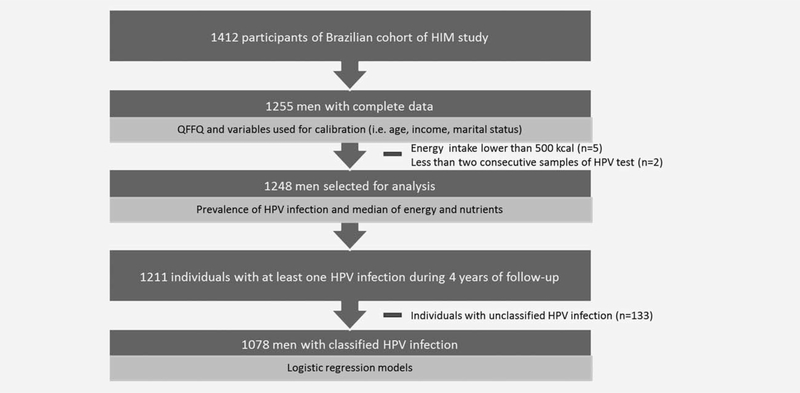
The selection of the study sample to test the hypothesis that dietary intake of selected nutrients is associated with persistent HPV infection is described in detail below (Fig. 1).
Figure 1.
Selection of sample for statistical analysis. São Paulo, 2015.
HPV DNA detection
Samples of penile and scrotal cells were obtained at each visit occurring every 6 months for a median of 4 years of follow-up. DNA was extracted from samples using the QIAamp Mini kit (Qiagen), according to the manufacturer’s instructions. The polymerase chain reaction (PCR) was used to amplify a fragment of the HPV L1 gene. HPV genotyping was conducted with the linear array method on all samples irrespective of the HPV PCR result (Roche Molecular Diagnostics, Alameda, CA) and were categorized as oncogenic (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 66) and nononcogenic HPV types [6, 11, 26, 40, 42, 44, 53, 54, 61, 62, 64, 67, 68, 69, 70, 71, 72, 73, 81, 82, 83, 84, IS39 (a sub-type of 82)] and CP6108 (type 89).
A sample was considered HPV positive if HPV DNA was detected by PCR or if it tested positive for at least 1 of the 37 HPV genotypes. Samples that amplified HPV DNA by PCR but did not test positive for a specific HPV genotype were considered unclassified infections. Individuals who had at least two positive and consecutive tests for the same HPV type were considered as having persistent infection.21
Study questionnaires
At each study visit, participants completed a computer assisted self-interview questionnaire to collect information such as age, alcoholic consume, smoking habit, sociodemographic characteristics and HPV risk factors. Physical activity was assessed using a separate questionnaire (International Physical Activity Questionnaire). Weight and height were measured by nursing assistants. Participants were classified according to Body Mass Index (BMI).22
Diet questionnaire
Dietary intake was ascertained using a previously validated Quantitative Food Frequency Questionnaire (QFFQ)23 for the Brazilian population, which provides information regarding usual dietary intake through frequency and consumed portion data.
The questionnaire is composed of 54 food items and was developed based on the dietary intake of a representative sample of the city of São Paulo that had been identified in a population-base study.24 The detailed methodology for developing the QFFQ used in the HIM Study in Brazil is available in a prior publication.23 For each food item listed, participants indicated their frequency of consumption (from 0 to 10 times a day, week, month or year) and the portion consumed (small, medium, large or extra large). A spreadsheet containing the nutritional composition of each food item, created using the Nutrition Data System for Research (NDSR, version 2.0, 2007 - University of Minnesota, Minneapolis), was used for calculating the energy and nutrient intake. The QFFQ was validated with correlation coefficients varied from 0.25 to 0.76.25 For this study, only the first QFFQ applied on the HIM Study in Brazil was considered.
Statistical analysis
From at least two 24 hr-recalls (24-HR) applied in a subsample (n = 121) of the Brazilian cohort, administered through a face-to-face interview, and using the Multiple Pass Method,26 it was possible to estimate calibration equations for FFQ dietary data, reducing measurement errors. Data from the 24HR were entered into the Nutrition Data System for Research and were converted into energy and nutrients. The Multiple Source Method (MSM), a statistical modeling technique, which calculates usual dietary intake, was used to remove within person variation that would otherwise inflate the distribution.27,28 The 24-HR values were regressed on the intake values from the main dietary questionnaires. BMI, income, education, age, physical activity, among other variables, were included as additional covariates to minimize effects in dietary intake.29–31 Data from 24-HR and FFQ were previously adjusted for energy intake using the residual method.32 Dietary folate equivalents values were adjusted according to Brazilian legislation of compulsory enrichment of wheat and corn flours.
Of 1,412 participants in the Brazilian cohort, 1,255 had complete dietary data. Individuals with energy intake lower than 500 kcal (n = 5) and individuals without at least two consecutive samples of HPV collected (n = 2) were excluded. As a result, 1248 men were assessed regarding HPV infection prevalence (Fig. 1). Median dietary intake of energy and nutrients was described for men with persistent infections (oncogenic and nononcogenic), nonpersistent infections, and among those with no HPV infection. As previously defined, persistence was considered as at least two positive consecutive tests for the same HPV type. U Mann Whitney test was used to assess differences in median nutrient intakes by HPV status group.
To test the hypothesis that dietary intake of selected nutrients is associated with persistent HPV infection, two independent multivariate logistic regression models were developed to assess the risk of both oncogenic and nononcogenic persistence using backwards stepwise elimination. Non-nutrient potential confounding factors were evaluated and retained in models (p ≤0.15). For the oncogenic HPV model, the variables identified by this process were age, marital status, smoking, number of lifetime female sex partners, alcohol consumption in the previous month, and HPV status at enrollment. For non-oncogenic HPV model, variables retained were age, smoking, number of lifetime female sex partners, physical activity, alcohol consumption in the previous month and HPV status at enrollment. Treating categorical nutrient variables as continuous in multivariate logistic regression models allowed the assessment of linear trends. Individuals with no HPV infection and no classified HPV infections were withdrawn from the final logistic models (n = 170). All statistical analysis were performed using Stata 12 (Stata Corp., College Station, USA).
Results
The majority of participants (66.6%) were HPV positive at study enrolment: 409 (32.8%) positive for oncogenic HPV, 595 (47.7%) positive for nononcogenic HPV, 268 (21.5%) coinfected with both oncogenic and nononcogenic HPV and 161 (12.9%) positive for nonclassified HPV types (positive for PCR but negative in genotyping). Mean age of study participants at enrolment was 34 years old.
Among the 1,248 men, 1211 (97.0%) had at least one type of HPV infection during the 4 years of follow-up. Persistent HPV infection was observed in 781 (62.6%) individuals, among which 458 (36.7%) had persistent oncogenic infections and 636 (51.0%) persistent nononcogenic infections. The most frequently detected persistent HPV types were 62 (13.2%), 84 (10.4%), CP6108 (9.4%) and 16 (9.1%).
From the potential cofounders initially identified, only increasing age (OR = 0.43 [0.26–0.72]), higher no. of female sex partners in lifetime (OR = 2.5 [1.34–4.67]) and positive HPV status at enrollment (OR= 2.04 [1.49–2.79]) remained statistically significant in the final logistic model for persistent oncogenic HPV. For the persistent nononcogenic HPV model, only higher no. of female sex partners in lifetime (OR = 2.1 [1.12—3.94]), alcohol consumption (OR = 0.7 [0.51–0.94]) and positive HPV status at enrolment (OR = 3.2 [2.38–4.30]) presented significant association in the final model (data not shown).
Median nutrient intake and associations with participant demographic and lifestyle characteristics are reported in Table 1. With the exception of folate, the median intake of the majority of nutrients appears higher among white men, those with >12 years of education, and men with a higher monthly income.
Table 1.
Distribution of median intake of nutrients according to demographic and lifestyle variables (São Paulo, 2015)
| Variable | No | Retinol (μg) | Lycopene (μg) | Vitamin A (μg) | α-Caroteno (μg) | β-Carotene (μg) | β-Cryptoxanthin (μg) | Lutein + zeaxanthin (μg) | Folate (DFE)1 (μg) | Vitamin D (μg) | Vitamin E (mg) | Vitamin B12 (μg) | Vitamin C (mg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ethnicity | |||||||||||||
| White | 773 | 406.462 | 2629.452 | 705.992 | 393.722 | 2555.392 | 244.422 | 1806.772 | 711.742 | 3.67 | 7.282 | 5.70 | 92.782 |
| Nonwhite | 475 | 396.08 | 2564.78 | 682.97 | 373.78 | 2375.88 | 171.73 | 1741.09 | 725.90 | 3.63 | 7.22 | 5.67 | 71.12 |
| Education | |||||||||||||
| ≤12 years | 719 | 395.162 | 2557.662 | 681.972 | 369.232 | 2252.362 | 199.422 | 1689.412 | 733.192 | 3.602 | 7.24 | 5.70 | 79.482 |
| >12 years | 526 | 412.01 | 2655.35 | 716.46 | 424.57 | 2966.77 | 254.55 | 1948.48 | 696.94 | 3.73 | 7.27 | 5.69 | 93.12 |
| Monthly Income, R$ (US$) | |||||||||||||
| ≤3,000,00 (≤932) | 951 | 400.742 | 2573.342 | 686.772 | 366.262 | 2321.442 | 197.692 | 1679.292 | 732.482 | 3.632 | 7.26 | 5.672 | 79.412 |
| >3,000,00 (>932) | 297 | 409.46 | 2669.27 | 723.19 | 552.14 | 3372.93 | 390.93 | 2200.57 | 668.30 | 3.73 | 7.25 | 5.81 | 114.33 |
| Marital status | |||||||||||||
| Single | 628 | 407.392 | 2644.892 | 678.092 | 385.55 | 2513.76 | 216.09 | 1773.44 | 685.172 | 3.65 | 7.322 | 5.492 | 85.71 |
| With partner | 620 | 397.87 | 2549.21 | 713.08 | 381.66 | 2434.75 | 218.31 | 1786.60 | 759.05 | 3.65 | 7.21 | 5.96 | 84.19 |
| Smoking habit | |||||||||||||
| Current smoker | 1024 | 393.552 | 2637.69 | 685.44 | 385.13 | 2433.29 | 205.41 | 1773.92 | 677.822 | 3.602 | 7.30 | 5.64 | 66.482 |
| Nonsmoker | 224 | 404.63 | 2588.81 | 699.51 | 382.39 | 2488.56 | 220.04 | 1779.66 | 725.46 | 3.66 | 7.25 | 5.71 | 88.47 |
| No. of female sex partners during lifetime | |||||||||||||
| 0 | 111 | 407.57 | 2607.452 | 673.292 | 374.462 | 2430.462 | 213.52 | 1730.662 | 705.18 | 3.622 | 7.32 | 5.552 | 86.77 |
| 1 | 69 | 415.37 | 2661.48 | 718.24 | 387.80 | 2561.93 | 214.56 | 1882.46 | 733.99 | 3.71 | 7.39 | 5.69 | 85.34 |
| 2–9 | 361 | 400.33 | 2583.55 | 678.34 | 382.40 | 2420.29 | 215.37 | 1760.57 | 722.09 | 3.59 | 7.26 | 5.70 | 85.17 |
| 10–19 | 242 | 401.29 | 2609.00 | 695.15 | 379.73 | 2481.44 | 216.00 | 1754.56 | 720.73 | 3.69 | 7.28 | 5.69 | 83.40 |
| 20–49 | 269 | 397.73 | 2602.55 | 704.77 | 392.82 | 2511.02 | 226.46 | 1817.42 | 707.60 | 3.64 | 7.22 | 5.76 | 85.99 |
| 50+ | 103 | 408.59 | 2721.47 | 727.38 | 413.45 | 2722.78 | 233.61 | 1858.80 | 703.95 | 3.84 | 7.23 | 5.71 | 89.24 |
| No. of male sex partners during past 3 months | |||||||||||||
| 0 | 1091 | 400.812 | 2589.47 | 698.56 | 382.38 | 2465.21 | 217.70 | 1771.32 | 720.712 | 3.66 | 7.24 | 5.712 | 85.34 |
| 1 | 58 | 403.96 | 2602.10 | 703.04 | 393.87 | 2602.15 | 215.12 | 1820.73 | 694.25 | 3.58 | 7.27 | 5.63 | 85.13 |
| 2 | 38 | 420.34 | 2698.31 | 668.17 | 390.54 | 2491.14 | 215.72 | 1845.48 | 695.49 | 3.61 | 7.34 | 5.61 | 84.36 |
| ≥3 | 61 | 417.37 | 2645.79 | 689.79 | 381.02 | 2637.93 | 213.52 | 1745.14 | 697.11 | 3.74 | 7.42 | 5.54 | 84.86 |
| Alcohol consumption during last month | |||||||||||||
| Yes | 889 | 401.16 | 2614.242 | 692.382 | 384.65 | 2489.71 | 218.34 | 1793.75 | 708.652 | 3.64 | 7.25 | 5.71 | 85.13 |
| No | 358 | 406.75 | 2563.33 | 714.19 | 377.66 | 2452.94 | 215.00 | 1754.14 | 732.83 | 3.68 | 7.28 | 5.64 | 85.35 |
| Circumcision status | |||||||||||||
| Yes | 241 | 409.31 | 2633.90 | 716.992 | 390.10 | 2579.86 | 218.80 | 1810.98 | 716.37 | 3.66 | 7.32 | 5.712 | 84.94 |
| No | 985 | 400.82 | 2583.55 | 693.64 | 381.85 | 2462.37 | 216.69 | 1773.06 | 718.94 | 3.65 | 7.24 | 5.70 | 85.60 |
| Physical Activity | |||||||||||||
| Sedentary/Insuficiently active | 306 | 396.48 | 2552.842 | 674.792 | 372.472 | 2423.672 | 203.542 | 1727.532 | 718.99 | 3.66 | 7.142 | 5.772 | 81.272 |
| Active | 542 | 404.80 | 2615.48 | 702.79 | 389.69 | 2502.44 | 223.50 | 1795.84 | 716.71 | 3.63 | 7.28 | 5.68 | 87.03 |
| Very active | 400 | 404.52 | 2603.59 | 703.65 | 383.68 | 2473.88 | 218.84 | 1803.07 | 719.19 | 3.67 | 7.30 | 5.68 | 84.61 |
Values of Dietary Equivalents of folate corrected for Brazilian values.
p < 0.05, Kruskall-Wallis test.
Dietary intake values according to HPV infection status are presented in Table 2. For oncogenic HPV infections, men with persistent infections had lower median daily intakes of retinol (p = 0.0286), vitamin A* (p = 0.0002) and folate equivalents† (p = 0.0001), and higher medians of energy (p = 0.0051) and lycopene (p = 0.0237). No differences in median daily intake were found for nononcogenic infections. Individuals without any HPV infection had higher median intake of α-carotene (p = 0.0241), β-carotene (p = 0.0146) and lutein/zeaxanthin (p = 0.0403).
Table 2.
Median values of nutrient intake, by HPV infection status (São Paulo, 2015)
| Persistent oncogenic HPV1 |
Persistent nononcogenic HPV1 |
HPV infection2 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes (n = 458) | No3 (n = 790) | p-Value | Yes (n = 636) | No4 (n = 612) | p-Value | Yes (n = 1211) | No (n = 37) | p-Value | |
| Energy (kcal) | 2485.96 | 2431.83 | 0.0051 | 2453.03 | 2447.79 | 0.7538 | 2451.54 | 2364.54 | 0.7136 |
| Carbohydrate (g) | 294.35 | 295.48 | 0.5759 | 294.97 | 294.36 | 0.4171 | 294.80 | 296.18 | 0.6644 |
| Protein (g) | 95.10 | 95.00 | 0.6016 | 94.93 | 95.23 | 0.0706 | 95.01 | 95.99 | 0.1117 |
| Total fat (g) | 89.21 | 88.79 | 0.0501 | 89.03 | 89.93 | 0.4605 | 89.00 | 89.11 | 0.7036 |
| Retinol (μg) | 397.79 | 403.23 | 0.0286 | 402.57 | 399.80 | 0.1979 | 402.54 | 404.17 | 0.6398 |
| Lycopene (μg) | 2641.01 | 2581.58 | 0.0237 | 2600.17 | 2615.54 | 0.7715 | 2598.33 | 2580.96 | 0.4779 |
| Vitamin A (μg) | 681.04 | 706.40 | 0.0002 | 695.65 | 695.77 | 0.5728 | 697.16 | 753.34 | 0.1701 |
| α-carotene (μg) | 382.15 | 382.40 | 0.4731 | 381.36 | 385.83 | 0.3442 | 381.85 | 412.60 | 0.0241 |
| β-carotene (μg) | 2438.44 | 2497.39 | 0.3532 | 2448.19 | 2502.44 | 0.8543 | 2835.42 | 3067.06 | 0.0146 |
| β-cryptoxanthin (μg) | 215.29 | 218.91 | 0.8217 | 216.71 | 216.29 | 0.8315 | 216.66 | 217.70 | 0.4904 |
| Lutein + zeaxanthin (μg) | 1765.61 | 1776.97 | 0.9110 | 1766.44 | 1777.78 | 0.5104 | 1770.98 | 1816.27 | 0.0403 |
| Folate (DFE) (μg) | 707.02 | 720.49 | 0.0001 | 718.12 | 713.00 | 0.9126 | 717.17 | 736.40 | 0.2094 |
| Vitamin D (μg) | 3.64 | 3.66 | 0.3364 | 3.65 | 3.66 | 0.2041 | 3.65 | 3.76 | 0.2791 |
| Vitamin E (mg) | 7.28 | 7.24 | 0.0671 | 7.26 | 7.23 | 0.3904 | 7.25 | 7.43 | 0.0502 |
| Vitamin B12 (μg) | 5.65 | 5.74 | 0.0533 | 5.68 | 5.73 | 0.0436 | 5.69 | 5.74 | 0.4458 |
| Vitamin C (mg) | 84.68 | 85.51 | 0.5029 | 84.91 | 85.52 | 0.3740 | 85.13 | 87.77 | 0.3591 |
Only classified infections considered.
Including individuals with nonclassified infections.
Includes individuals with transitory infection for oncogenic HPV and no infection for oncogenic HPV, but at least one positive result for other HPV types.
Includes individuals with transitory infection for nononcogenic HPV and no infection for nononcogenic HPV, but at least one positive result for other HPV types.
Table 3 presents the association between specific dietary nutrients and HPV persistence. No significant associations were observed between oncogenic HPV persistence and dietary intake in multivariable adjusted models. Higher intakes of vitamin B12 were significantly associated with lower risk of persistent nononcogenic HPV infections (OR = 0.55 [0.38–0.81]).
Table 3.
Association between energy-adjusted dietary intake and persistent HPV (São Paulo, 2015)
| Infection no. |
||||
|---|---|---|---|---|
| Nutrient | Transient | Persistent | Crude OR (95% CI) | Adjusted OR (95% CI)1 |
| Oncogenic HPV | ||||
| Vitamin A, (μgRAE)2 | ||||
| Quartile 1 | 143 | 147 | 1.00 | 1.00 |
| Quartile 2 | 157 | 116 | 0.72 (0.51–1.00) | 0.77 (0.52–1.15) |
| Quartile 3 | 161 | 103 | 0.62 (0.44–0.87) | 0.78 (0.49–1.24) |
| Quartile 4 | 159 | 92 | 0.56 (0.40–0.79) | 0.73 (0.42–1.25) |
| Folate equivalents (μg)3 | ||||
| Quartile 1 | 157 | 158 | 1.00 | 1.00 |
| Quartile 2 | 143 | 102 | 0.71 (0.51–0.99) | 0.96 (0.64–1.43) |
| Quartile 3 | 165 | 122 | 0.74 (0.53–1.01) | 1.24(0.80–1.92) |
| Quartile 4 | 155 | 76 | 0.49 (0.34–0.69) | 0.95 (0.54–1.69) |
| Lycopene (μg)4 | ||||
| Quartile 1 | 143 | 93 | 1.00 | 1.00 |
| Quartile 2 | 165 | 106 | 0.99 (0.69–1.41) | 0.96 (0.64–1.44) |
| Quartile 3 | 154 | 109 | 1.09 (0.76–1.56) | 1.08 (0.71–1.64) |
| Quartile 4 | 158 | 150 | 1.46(1.03–2.06) | 1.33 (0.87–2.01) |
| Vitamin B12 (μg)5 | ||||
| Quartile 1 | 190 | 159 | 1.00 | 1.00 |
| Quartile 2 | 147 | 123 | 1.00 (0.73–1.37) | 0.94 (0.65–1.37) |
| Quartile 3 | 154 | 92 | 0.71 (0.51–0.99) | 0.72 (0.47–1.09) |
| Quartile 4 | 129 | 84 | 0.78 (0.55–1.10) | 0.98 (0.61–1.58) |
| Retinol (μg)6 | ||||
| Quartile 1 | 148 | 128 | 1.00 | 1.00 |
| Quartile 2 | 160 | 115 | 0.83 (0.59–1.16) | 0.76 (0.52–1.12) |
| Quartile 3 | 150 | 117 | 0.90 (0.64–1.26) | 0.86 (0.58–1.28) |
| Quartile 4 | 162 | 98 | 0.70 (0.49–0.99) | 0.81 (0.53–1.23) |
| Vitamin E (mg)7 | ||||
| Quartile 1 | 153 | 98 | 1.00 | 1.00 |
| Quartile 2 | 137 | 100 | 1.14(0.79–1.64) | 1.01 (0.73–1.65) |
| Quartile 3 | 174 | 130 | 1.17 (0.83–1.64) | 1.14(0.76–1.70) |
| Quartile 4 | 156 | 130 | 1.30 (0.92–1.83) | 1.29 (0.82–2.02) |
| Nononcogenic HPV | ||||
| Vitamin B12 (μg)8 | ||||
| Quartile 1 | 133 | 216 | 1.00 | 1.00 |
| Quartile 2 | 108 | 162 | 0.92 (0.67–1.28) | 0.77 (0.54–1.10) |
| Quartile 3 | 100 | 146 | 0.90 (0.64–1.25) | 0.70 (0.49–1.02) |
| Quartile 4 | 101 | 112 | 0.68 (0.48–0.96) | 0.55 (0.38–0.81) |
NOTE. CI, confidence interval; OR, odds ratio.
For oncogenic HPV: Logistic-regression model adjusted simultaneously for energy intake (kcal), age, marital status, smoking, no. of female sex partners in lifetime, and HPV status at enrollment. For nononcogenic HPV: Logistic-regression model adjusted simultaneously for energy intake (kcal), physical activity, age, marital status and HPV status at enrollment.
p = 0.250, test for trend.
p = 0.821, test for trend.
p = 0.137, test for trend.
P = 0.703, test for trend.
p = 0.471, test for trend.
p = 0.319, test for trend.
p = 0.003, test for trend.
Discussion
Persistent HPV infection is an important risk factor for the development of cervical, anal, penile and oropharyngeal cancers.4–9 In previous studies, carotenoids, retinol, vitamin C, vitamin E and nutrients involved in DNA methylation, such as folate, have been investigated as potential modifiers of HPV persistence and cancer risk among women.4,11,13,14,33 To our knowledge, no previous studies evaluated the association between HPV persistence and dietary intake in men. Our findings suggest that in this population of Brazilian men dietary vitamin B12 may be protective against nononcogenic HPV persistence. However, no significant association was observed for dietary intake of selected nutrients and oncogenic HPV persistence.
In a comprehensive review, antioxidant nutrients such as carotenoids, retinol and tocopherols were suggested to be protective against cervical dysplasia as they could modulate immune response and decrease viral replication and gene expression. However, there was a lack of prospective studies adequately controlling for HPV infection.11 A more recent review of dietary prevention of HPV infection and cervical cancer suggested that circulating antioxidants, at high levels, can enhance the clearance of high-risk HPV infections.14
Prior studies conducted in women and taking HPV infection and confounders into consideration showed inconsistent results. In a nested case-control study of Brazilian women, β-cryptoxanthin, lutein/zeaxanthin and vitamin C intakes were associated with decreased risk of type specific HPV persistence, but no associations were observed for α and β-carotene.10 A marginally significant association between median dietary lutein intake and HPV persistence was observed in a cohort of young woman.12 In contrast, inconsistent associations were observed when individual nutrients were examined.13
Although no association was observed with dietary folate and vitamin B12, plasma vitamin B12 levels were associated with a reduced risk of HPV persistence in a cohort of young women in Arizona.4 Methylation of the regulatory region of HPV has been shown to prevent transcription in vitro, suggesting that methylation can decrease viral proliferation and prevent maintenance of HPV infection.11,34 Vitamin B12, as well as folate and vitamin B6, may prevent carcinogenesis through their role in DNA methylation, as they contribute to the synthesis of S-adenosylmethionine.35,36 Despite relevant biochemical evidence for a role of vitamin B12, the epidemiological literature has not provided consistent evidence of an association with HPV persistence. In our study, dietary vitamin B12 was inversely associated with non-oncogenic HPV infection persistence only, which possibly indicates that other factors may have more influence in the persistence of oncogenic HPV infections. Studies have found an association between viral persistence and multiplicity of HPV infection, as it seems plausible that an underlying immune condition may increase susceptibility to multiple and persistent infections.5,21
As there are no prior studies that evaluated the relationship between dietary factors and HPV persistence in men, it is unclear if the results reported here are unique to the study population. As such, more research among men is needed to further elucidate the association between diet and HPV persistence in men.
The limitations of this study should also be considered when interpreting these findings. Our results could be affected by measurement error in dietary intake, a common limitation of nutritional epidemiological studies. However, the calibration of dietary intake reported in HIM Study minimizes the impact of possible systematic overestimation or underestimation in dietary intake measurements; however, measurement error of the 24-hr recall is not independent of that of dietary questionnaires. Moreover, there is no information about circulating concentrations of nutrients and it is possible that diet and circulating concentrations measure different exposures, resulting in different relationships with HPV persistence.
In conclusion, results from this study indicate that dietary intake of vitamin B12 is associated with reduced risk of non-oncogenic HPV persistence in men. No associations were found between persistent oncogenic HPV infections and dietary intake of selected nutrients. More studies evaluating the association of HPV persistence and dietary intake in men are needed to further comparisons and a better understanding of this relation.
What’s new?
Can diet influence the persistence of HPV infection? Certain B vitamins, for instance, can promote viral integration, while other nutrients hinder it. This is the first study to investigate whether diet contributes to persistent oncogenic HPV-infection in men. Looking at a Brazilian cohort of men age 18–70, these authors tested the men for HPV over a period of 4 years. At each meeting, they gave them a questionnaire, asking about their diet. Persistent nononcogenic HPV infection was associated with B12 consumption, they found, but they could link none of the nutrients to persistent oncogenic HPV infection.
Acknowledgments
Grant sponsor: Coordenação de Aperfeiçoamento de Pessoal de Nível Superior
Footnotes
Conflict of Interest/Disclosures
AR Giuliano is member of the Scientific and Advisory Boards for Merck Sharp and Dohme. LL Villa is member of the board of Merck Sharp and Dohme for the Quadrivalent HPV vaccine.
Vitamin A = mcg retinol + (mcg beta-carotene equivalents/12).
Folate equivalents = mcg natural folate + (1.7 × mcg synthetic folate).
References
- 1.Giuliano AR. Human papillomavirus vaccination in males. Gynecol Oncol 2007;107:S24–S6. [DOI] [PubMed] [Google Scholar]
- 2.Di Domenico F, Foppoli C, Coccia R, et al. Antioxidants in cervical cancer: Chemopreventive and chemotherapeutic effects of polyphenols. Biochim Biophys Acta 2012;1822:737–47. [DOI] [PubMed] [Google Scholar]
- 3.Narisawa-Saito M, Kiyono T. Basic mechanisms of high-risk human papillomavirus-induced carcinogenesis: roles of E6 and E7 proteins. Cancer Sci 2007;98:1505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sedjo RL, Inserra P, Abrahamsen M, et al. Human Papillomavirus Persistence and Nutrients Involved in the Methylation Pathway among a Cohort of Young Women. Cancer Epidemiol Biomarkers Prev 2002;11:353–9. [PubMed] [Google Scholar]
- 5.Ho GY, Bierman R, Beardsley L, et al. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med 1998;338:423–8. [DOI] [PubMed] [Google Scholar]
- 6.Moscicki AB, Hills N, Shiboski S, et al. Risks for incident human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. JAMA 2001;285: 2995–3002. [DOI] [PubMed] [Google Scholar]
- 7.Giuliano AR, Salmon D. The case for a gender neutral (universal) HPV vaccination policy in the US. Cancer Epidemiol Biomarkers Prev 2008;17: 805–8. [DOI] [PubMed] [Google Scholar]
- 8.Giuliano AR, Lazcano-Ponce E, Villa LL, et al. The Human Papillomavirus Infection in Men Study: Human Papillomavirus Prevalence and Type Distribution among Men Residing in Brazil, Mexico, and the United States. Cancer Epidemiol Biomarkers Prev 2008;17:2036–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruni L, Barrionuevo-Rosas L, Albero G, et al. ICO Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in the World. Summary Report 2015-04–08. [Google Scholar]
- 10.Giuliano AR, Siegel EM, Roe DJ, et al. Dietary Intake and Risk of Persistent Human Papillomavirus (HPV) Infection: The Ludwig-McGill HPV Natural History Study. J Infect Dis 2003;188: 1508–16. [DOI] [PubMed] [Google Scholar]
- 11.García-Closas R, Castellsagué X, Bosch X, et al. The role of diet and nutrition in cervical carcinogenesis: A review of recent evidence. Int J Cancer 2005;117:629–37. [DOI] [PubMed] [Google Scholar]
- 12.Sedjo R, Roe DJ, Abrahamsen M, et al. Vitamin A, Carotenoids, and Risk of Persistent Oncogenic Human Papillomavirus Infection. Cancer Epidemiol Biomarkers Prev 2002;11:876–84. [PubMed] [Google Scholar]
- 13.Siegel EM, Salemi JL, Villa LL, et al. Dietary Consumption of Antioxidant Nutrients and Risk of Incident Cervical Intraepithelial Neoplasia. Gynecol Oncol 2010;118:289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chi HJ, Lee AH, Colville L, et al. A review of Dietary Prevention of Human Papillomavirus-related Infection of the Cervix and Cervical Intraepithelial Neoplasia. Nutr. Cancer 2013;65: 317–28. [DOI] [PubMed] [Google Scholar]
- 15.Gadducci A, Barsotti C, Cosio S, et al. Smoking habit, immune suppression, oral contraceptive use, and hormone replacement therapy use and cervical carcinogenesis: a review of the literature. Gynecol Endocrinol 2011;27:597–604. [DOI] [PubMed] [Google Scholar]
- 16.Reddy L, Odhav B, Bhoola KD, Natural products for cancer prevention: a global perspective. Pharmacol Ther 2003;99:1–13. [DOI] [PubMed] [Google Scholar]
- 17.Borutinskaite VV, Navakauskiene R, Magnusson K-E, Retinoic Acid and histone deacetylase inhibitor BML-210 inhibit proliferation of human cervical cancer HeLa cells. Ann N Y Acad Sci 2006; 1091:346–55. [DOI] [PubMed] [Google Scholar]
- 18.Luong KVQ, Nguyen LTH. The beneficial role of vitamin D and its analogs in cancer treatment and prevention. Crit Rev Oncol Hem 2010;73: 192–201. [DOI] [PubMed] [Google Scholar]
- 19.Giuliano AR, Lazcano E, Villa LL, et al. Circumcision and sexual behavior: factors independently associated with human papillomavirus detection among men in the HIM study. Int J Cancer 2009; 124:1251–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giuliano AR, Lee JH, Fulp W, et al. Incidence and clearance of genital human papillomavirus infection in men (HIM): a cohort study. Lancet 2011;377:932–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lajous M, Mueller M, Cruz-Valdéz A, et al. Determinants of Prevalence, Acquisition, and Persistence of Human Papillomavirus in Healthy Mexican Military Men. Cancer Epidemiol Biomarkers Prev 2005;14:1710–6. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. Diet, nutrition, and the prevention of chronic diseases Geneva: World Health Organization, 1990. (WHO Technical Reports Series 797). [Google Scholar]
- 23.Fisberg RM, Colucci ACA, Morimoto JM, et al. Questionário de freqüência alimentar para adultos com base em estudo populacional. Rev Saúde Pública 2008;42:550–4. [DOI] [PubMed] [Google Scholar]
- 24.César CLG, Carandina L, Alves MCGP, Barros MBA, Goldbaum. Saúde e condição de vida em São Paulo Inquérito multicêntrico de saúde no estado de São Paulo - ISA-SP. São Paulo: Faculdade de Saúde Pública, Universidade de São Paulo, 2005. [Google Scholar]
- 25.Teixeira JA, Baggio ML, Giuliano AR, et al. Performance of the Quantitative Food Frequency Questionnaire Used in the Brazilian Center if the Prospective Study Natural History of Human Papillomavirus Infection in Men: The HIM Study. J Am Diet Assoc 2011;111: 1045–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guenther PM, Cleveland LE, Ingwersen LA, et al. Questionnaire development and data collection procedures In: Tippett KS, Cypel YS, editors. Design and operation: the continuing survey of food intakes by individuals and the Diet and Health Knowledge Survey, 1994–1996. Beltsville, MD: United States Department of Agriculture, 1998. 42–63. (Nationwide Food Surveys Report, 96–1). [Google Scholar]
- 27.Harttig U, Haubrock J, Knueppel S, et al. The MSM program: web-based statistics package for estimating usual dietary intake using the Multiple Source Method. Eur J Clin Nutr 2011;65:S87–S91. [DOI] [PubMed] [Google Scholar]
- 28.Haubrock J, Noethlings U, Volatier JL, et al. Estimating Usual Food Intake Distributions by Using the Multiple Source Method in the EPIC-Potsdam Calibration Study. J Nutr 2011; 141:914–920. [DOI] [PubMed] [Google Scholar]
- 29.Tooze JA, Subar AF, Thompson FE, et al. Psychosocial predictors of energy underreporting in a large doubly labeled water study. Am J Clin Nutr 2004;79:795–804. [DOI] [PubMed] [Google Scholar]
- 30.Hill RJ, Davies PS. The validity of self-reported energy intake as determined using the doubly labeled water technique. Br J Nutr 2001; 85:415–30. [DOI] [PubMed] [Google Scholar]
- 31.Trabulsi J, Schoeller DA. Evaluation of dietary assessment instruments against doubly labeled water, a biomarker of habitual energy intake. Am J Physiol Endocrinol Metab 2001;281:E891–9. [DOI] [PubMed] [Google Scholar]
- 32.Willett WC, Howe GR, Kushi LW. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65:1220S–8S. [DOI] [PubMed] [Google Scholar]
- 33.Potischman N, Brinton LA, Nutrition and cervical neoplasia. Cancer Causes Control 1996;7:113–26. [DOI] [PubMed] [Google Scholar]
- 34.Selhub J, Miller JW. The pathogenesis of homocysteinemia: interruption of the coordinate regulation by S-adenosylmethionine of the remethylation and transsulfuration of homocysteine. Am J Clin Nutr 1992;55:131–8. [DOI] [PubMed] [Google Scholar]
- 35.Bailey LB, Gregory JF III. Folate metabolism and requirements. J Nutr 1999;129:779–82. [DOI] [PubMed] [Google Scholar]
- 36.Choi SW, Mason JB, Folate and carcinogenesis: an integrated scheme. J Nutr 2000;130:129–132. [DOI] [PubMed] [Google Scholar]