Abstract
Background
For the management of immune checkpoint inhibitor (ICI)-induced pneumonitis (ICI-pneumonitis), discontinuation of ICIs and high dose corticosteroid based on grade are generally recommended. The purpose of this study is to describe management and outcome of ICI-pneumonitis and explore what to consider when managing ICI-pneumonitis with or without corticosteroids in addition to grade.
Methods
We reviewed data of 706 cancer patients who were treated with ICIs and identified radiographically proven pneumonitis. The diagnosis of ICI-pneumonitis was established after excluding alternative aetiologies either by a bronchoscopy or a thorough examination of clinical features. The evaluation of the management and outcome of pneumonitis were evaluated according to the time of corticosteroid administration.
Results
ICI-pneumonitis developed in 16 patients (2.3%); nine grade 1, four grade 2 and three grade 3. Initially, 10 patients were spared from corticosteroid administration; fourpatients eventually received corticosteroid after 4 weeks of pneumonitis diagnosis due to clinical, radiographical aggravation and/or clinicians’ decision. The other sixpatients never received corticosteroid and improved or remained stable radiographically. When the four and sixpatients were compared, pneumonitis grade was similar, while the latter sixpatients had a later onset from initiation of ICIs (mean 37.48 weeksvs25.45 weeks), more prior lines of chemotherapy (median 2.5 vs 1.0 lines), higher proportion of current/ex-smokers (83.3% vs 50.0%), and fewer other accompanying immune-related adverse events (50% vs 75%). Time to improvement of pneumonitis was similar between the fourpatients who received delayed corticosteroid and fivepatients who received corticosteroid within 4 weeks(3.6 vs 2.5 weeks).
Conclusions
Our analyses provide clinical insights that stratification of the patients is important in managing ICI-pneumonitis. Along with ICI-pneumonitis grade, more factors associated with the outcome need to be unravelled in the future.
Keywords: immune checkpoint inhibitor, pneumonitis, immune-related adverse effects
Key questions.
What is already known about this subject?
Immune checkpoint inhibitor induced pneumonitis (ICI-pneumonitis) is encountered around 5% of patients receiving ICIs and may directly impact patient survival. Managements for ICI-pneumonitis include withholding ICIs and administration of corticosteroid according to grade of ICI-pneumonitis.
What does this study add?
Although grading of ICI-pneumonitis stratifies most of the patients well, it is still unknown whether and when to treat patients with corticosteroid. This retrospective case series presents the features and the outcomes of managements for ICI-pneumonitis.
How might this impact on clinical practice?
This study provides insights to clinicians who encounter patients with ICI-pneumonitis and wish to examine risks and benefits of managing ICI-pneumonitis.
Introduction
Immune checkpoint inhibitors (ICIs) have become the standard of care in the treatment of various cancer types.1–5 ICIs boost the intrinsic antitumour immune response by targeting T-cell inhibitory pathways such as the cytotoxic T-lymphocyte antigen 4 (CTLA-4) or programmed cell death 1 (PD-1) and programmed cell death ligand 1 (PD-L1) interaction.5 However, increased activity of the immune response brings autoimmune manifestations, known as immune-related adverse events (irAEs).
ICI-induced pneumonitis (ICI-pneumonitis) is a challenging irAE that ranges from ‘asymptomatic radiographic evidence only’ cases to fatal cases which led to death.6–8 In a meta-analysis of 20 published ICI trials, the overall incidence of ICI-pneumonitis was 2.7% with anti-PD-1 antibody alone and 6.6% with anti-PD-1 antibody and anti-CTLA-4 antibody combination.9 The onset of ICI-pneumonitis ranges from 9 days to 27.4 months, with a median of 1.3–2.8 months and tends to be earlier with combination therapy.6–8 Grades of pneumonitis described in literatures are largely based on the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) grading system,6 8 in which, ICI-pneumonitis is regarded as a grade 3 even if the patient required minimal oxygen therapy.10
The guideline for the management of ICI-pneumonitis was published by the National Comprehensive Cancer Network (NCCN) recently.11 The guideline recommends stratification of patients by grades first and manage according to it.11 This gives well-organised general information of what to do when clinicians encounter patients with ICI-pneumonitis. Still, there are gaps between the guideline and clinical practice in terms of exact factors that need consideration. In other words, the management beyond the wordings of the guideline depends on individual clinician’s experience and decision, weighing risks and benefits.
However, ICI-pneumonitis is crucial irAE that has possible association with survival outcome and must be managed properly.12 Furthermore, not only does it have variety of clinical courses, but also managements for it harbour potential significant harms to the patient. A high dose of corticosteroid, even with short-term use of less than 30 days, may cause various and possibly devastating adverse effects.13–16 Also, there are theoretical concerns that high doses of corticosteroids may counteract the anti-tumour immune responses of ICIs.17 Although these concerns have yet turned out to be innocuous,18 19 still oncological outcome of withholding ICIs is by far area of uncertainty. Evidence for re-challenging of the ICIs after irAEs has been presented only little.20 Therefore, withholding ICIs and high dose corticosteroid administration for ICI-pneumonitis deserve careful and practical consideration of the risks and benefits in real-world practice. The purpose of this retrospective case series is to describe features and outcomes of managements for ICI-pneumonitis patients.
Materials and methods
Patients
We reviewed medical records of 706 patients who were treated with either one or combination of anti-PD-1, anti-PD-L1 and/or anti-CTLA-4 monoclonal antibodies (mAbs) at Seoul National University Hospital from September 2012 to April 2018. Patients who received ICIs other than anti-PD-1/anti-PD-L1 and anti-CTLA-4 mAbs were excluded. We defined ICI-pneumonitis as new-onset radiographic evidence of pneumonitis that is likely to be caused by ICIs excluding other aetiologies considering clinical features and evaluation results. Specifically, if a bronchoalveolar lavage (BAL) was obtained, microbiological results including Pneumocystis jirovecii, tuberculosis and other pathogenic bacteria was examined. If BAL was not obtained, clinical features such as onset, symptoms, signs of infection, laboratory results and response to steroid were all examined to rule out any possible alternative diagnosis. To exclude radiation pneumonitis, we compared pneumonitis extent and previous radiation field.
We reviewed chest CT scans of the patients and identified those with new-onset lung parenchymal abnormalities, which included ground-glass opacities, consolidation and reticular opacities.
Data collection
The following information were collected: demographic features, primary cancer site, ICIs delivered, radiographic and clinical features of pneumonitis, and management methods for pneumonitis delivered. Tumour response to ICIs was recorded according to the Response Evaluation Criteria in Solid Tumors guideline, V.1.1.21 Rechallenging of ICIs was defined as any administration of ICIs after clinician recognition of possible ICI-pneumonitis. The grade of pneumonitis was assigned at the initial presentation of ICI-pneumonitis according to the NCI-CTCAE V.5.0; grade 1 if asymptomatic, grade 2 if any symptoms were present, grade 3 if oxygen demand was present, grade 4 if there was life-threatening respiratory compromise and grade 5 if death occurred (online supplementary table 1).10
esmoopen-2019-000575supp001.pdf (64.1KB, pdf)
The radiographic features and extents of pneumonitis were reviewed by an expert radiologist and a medical oncologist independently (SHY, CP) and were described as patterns according to the American Thoracic Society/European Respiratory Society classifications of idiopathic interstitial pneumonias; NSIP, non-specific interstitial pneumonia; COP, cryptogenic organising pneumonia; NOS, pneumonia not otherwise specified (online supplementary table 2).22 23 The extent of pneumonitis involvement based on the CT scan was defined according to the proportion of the lung parenchyma involved; mild if less than 25% of lung parenchyma involved; moderate if 25~50% involved; severe if more than 50% involved (online supplementary table 2). Clinical outcomes of pneumonitis were determined by radiographic evidence of either a simple chest X-ray or chest CT scan and classified into improved, stable, or aggravated.
Data analysis
To compare categorical variables, Fisher’s exact test was used. Student’s t-test was applied to compare continuous variables. All reported pvalues were two-sided, and a statistical difference was considered significant at a pvalue of less than 0.05. R V.3.4.3 software (R Development Core Team, https://www.r-project.org/) was used to perform statistical analyses.
Results
Patients
A total of 706 patients were treated with either one or a combination of anti-PD-1/anti-PD-L1 mAbs, or anti-CTLA-4 mAbs. A total of 20 patients were included for evaluation of possible ICI-pneumonitis. Among these patients, fourpatients were excluded as they turned out to have other aetiologies. Such alternative aetiologies included aspiration pneumonia, P. jirovecii pneumonia, lung cancer lymphangitic metastasis progression, and other drug-induced pneumonitis, which was diagnosed with sputum culture, BAL, thorough radiological and clinical review, respectively. Among the other 16 patients, sevenpatients had undergone a bronchoscopy with BAL, which showed no evidence of pathogenic micro-organisms including P. jirovecii, tuberculosis and bacteria. In case of the remaining ninepatients, other aetiologies including infection were excluded after thorough examination of clinical features. In particular, sevenpatients did not receive any empirical antibiotics and all patients had no sign of infection including fever. The extents of pneumonitis in threepatients who previously received radiotherapy were different from the therapeutic radiation fields.
As a result, a total of 16 patients were included. The overall incidence of ICI-pneumonitis was 2.3% (16 out of 706 patients) and eightpatients had non-small cell lung cancer of which incidence of ICI-pneumonitis was 3.65% (online supplementary table 3). The incidence of ICI-pneumonitis in patients receiving anti-PD-1 mAbs monotherapy was 2.9% (14 out of 480 patients), and there were no ICI-pneumonitis cases in patients who received anti-CTLA4 or anti-PD-L1 mAb monotherapy. The incidence of ICI-pneumonitis in combination therapy was 4.3% (two out of 47 patients). The demographic features of the 16 ICI-pneumonitis patients are shown in table 1. In addition, seven of BAL specimens had available cytological results, all of which showed lymphocytosis (Median 18%–62%) and six of them showed eosinophilia (range 2%–29%).24 T-cell subset analysis was available in five of BAL specimens which showed inverse CD4/CD8 T-cell ratio (median 0.62, range 0.11–0.88).24
Table 1.
Demographic features of patients with pneumonitis
| No. of patients (%) | |
| Gender | |
| Male | 12(75) |
| Female | 4(25) |
| ECOG PS at the start of ICI | |
| Not available | 3(19) |
| 0–1 | 13(81) |
| 2 or more | 0(0) |
| Cancer type | |
| Non-small cell lung cancer | 8(50) |
| Head-and-neck squamous cell carcinoma | 2(13) |
| Urothelial cell carcinoma | 1(6) |
| Biliary cancer | 1(6) |
| Rectal cancer | 1(6) |
| Renal cell carcinoma | 1(6) |
| Oesophageal cancer | 1(6) |
| Hodgkin’s lymphoma | 1(6) |
| Smoking status | |
| Never | 4(25) |
| Ex-smoker/current smoker | 12(75) |
| Underlying lung disease | |
| None | 14(88) |
| Chronic obstructive pulmonary disease | 1(6) |
| Combined pulmonary fibrosis and emphysema | 1(6) |
| Prior lung surgery | |
| Yes | 3(19) |
| No | 13(81) |
| Prior intrathoracic radiotherapy | |
| Yes | 3(19) |
| No | 13(81) |
| Number of prior lines of chemotherapy | |
| 0 | 2(13) |
| 1 | 5(31) |
| 2 | 5(31) |
| ≥3 | 4(25) |
| Type of ICI received | |
| Anti-PD-1 mAbs monotherapy | 14(88) |
| Anti-PD-L1 mAbs monotherapy | 0(0) |
| Anti-CTLA4 mAbs monotherapy | 0(0) |
| Combination therapy | 2(13) |
| Response to ICI at the time of ICI-pneumonitis diagnosis | |
| PR | 9(56) |
| SD | 5(31) |
| PD | 2(13) |
ECOG ECOG PS, Eastern Cooperative Oncology Group Performance Status; ICI, immune checkpoint inhibitor; mAbs, monoclonal antibodies; PD, progressive disease; PR, partial remission; SD, stable disease.
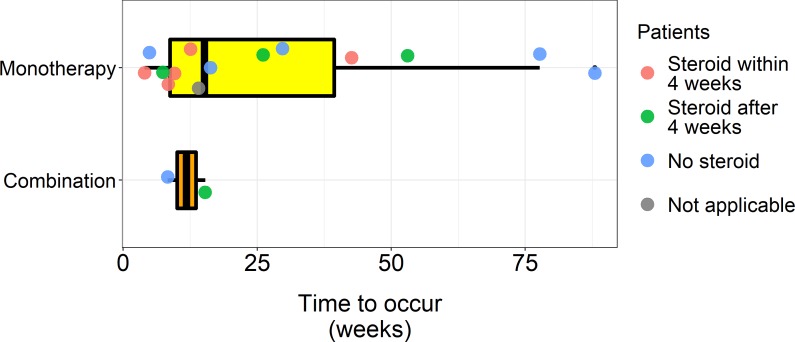
There were nine cases of grade 1, four cases of grade 2 and three cases of grade 3 pneumonitis. There were no cases of grade 4 or 5 pneumonitis. The median time to occurrence of ICI-pneumonitis after the initiation of ICI was 14.7 weeks (range 4–88 weeks, figure 1). The onset tended to be earlier in patients who received combination therapy than those who received monotherapy (11.8 weeksvs28.2 weeks, p=0.067). A total of eightpatients (n=8, 50%) had other accompanying irAEs. Thyroiditis was the most common (n=5, 31%) (online supplementary table 4). Pneumonitis grade tended to correlate with the extent of pneumonitis involvement based on CT scans (p=0.055). The COP pattern was the most commonly observed pattern on CT scans (n=7, 44%), followed by the non-specific interstitial pneumonia pattern (n=6, 38%, online supplementary table 5).
Figure 1.
Time to onset of pneumonitis from initiation of immune checkpoint inhibitors (ICIs) box plots show time to onset of pneumonitis from initiation of ICI. Median time to onset in patients who received monotherapy was 15.1 weeks, while median time to onset in patients who received combination therapy was 11.8 weeks.
Management and outcome
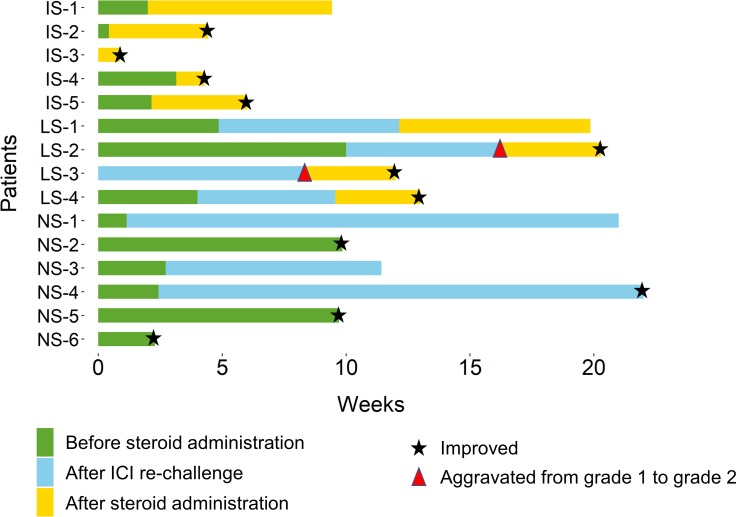
Among the 16 patients, 15 were included for the analysis of management and outcome. Individual characteristics, management outcomes, and timeline of ICI-pneumonitis patients are shown in table 2 and figure 2. A grade 3 pneumonitis patient was excluded from the analysis of management and outcome because the patient died 3 days after the diagnosis of pneumonitis and before the initiation of proper pneumonitis management. The cause of death was massive haemoptysis from tumour invasion of the trachea and tracheal stent within.
Table 2.
Individual characteristics and management outcome of immune checkpoint inhibitor (ICI)-induced pneumonitis patients
| Patient | Gender /age |
Type of cancer | ICI | BFS evaluation with BAL | Pneumonitis grade | Smoking status | Underlying lung disease | Number of prior lines of CTx | Onset from ICI initiation (weeks) |
Tumour response to ICIs | CT pattern | CT extent | Empirical antibiotics use | Improvement | Time to improvement after steroid (weeks) |
ICI rechallenge before steroid or improvement | Aggravation after ICI rechallenge |
| IS-1 | M/68 | NSCLC | Anti-PD-1 | Done | Gr 3 | Y | CPFE | 2 | 12.6 | SD | NSIP | Moderate | Y | Aggravated | – | N | – |
| IS-2 | M/70 | RCC | Anti-PD-1 | ND | Gr 2 | Y | – | 2 | 42.6 | PD | COP | Moderate | N | Y | 4.0 | N | – |
| IS-3 | M/66 | CRC | Anti-PD-1 | ND | Gr 3 | Y | – | 6 | 4.0 | PD | NSIP | Moderate | Y | Y | 0.9 | N | – |
| IS-4 | M/55 | NSCLC | Anti-PD-1 | Done | Gr 2 | Y | COPD | 1 | 9.6 | PR | NSIP | Mild | Y | Y | 1.1 | N | – |
| IS-5 | F/62 | NSCLC | Anti-PD-1 | Done | Gr 1 | N | – | 2 | 8.4 | PR | COP | Mild | N | Y | 3.9 | N | – |
| LS-1 | F/58 | NSCLC | Anti-PD-L1+Anti-CTLA-4 | ND | Gr 1 | N | – | 0 | 15.3 | PR | NSIP | Mild | N | Stable | – | Y | Y |
| LS-2 | M/65 | GBC | Anti-PD-1 | Done | Gr 1 | Y | – | 1 | 53.0 | SD | COP | Mild | N | Y | 4.0 | Y | Y |
| LS-3 | M/73 | UCC | Anti-PD-1 | Done | Gr 1 | Y | – | 1 | 26.1 | SD | NSIP | Mild | Y | Y | 3.6 | Y | Y |
| LS-4 | F/68 | HNSCC | Anti-PD-1 | Done | Gr 2 | N | – | 1 | 7.4 | PR | COP | Mild | Y | Y | 3.4 | Y | N |
| NS-1 | M/59 | NSCLC | Anti-PD-1 | ND | Gr 1 | Y | – | 1 | 4.9 | SD | NOS | Moderate | N | Stable | – | Y | N |
| NS-2 | M/67 | NSCLC | Anti-PD-1 | ND | Gr 1 | Y | – | 2 | 16.3 | PR | COP | Mild | N | Y | – | N | – |
| NS-3 | M/67 | NSCLC | Anti-PD-1+Anti-CTLA-4 | ND | Gr 1 | Y | – | 0 | 8.3 | PR | NSIP | Moderate | N | Stable | – | Y | N |
| NS-4 | M/69 | HNSCC | Anti-PD-1 | Done | Gr 1 | Y | – | 3 | 88.0 | PR | COP | Mild | N | Y | – | Y | Y |
| NS-5 | F/32 | HD | Anti-PD-1 | ND | Gr 1 | N | – | 4 | 77.7 | PR | NOS | Mild | N | Y | – | N | – |
| NS-6 | M/55 | NSCLC | Anti-PD-1 | ND | Gr 2 | Y | – | 4 | 29.7 | SD | COP | Moderate | Y | Y | – | N | – |
IS-1~IS-6 patients received corticosteroid initially within 4 weeks of pneumonitis diagnosis. LS-1~LS-4 patients received corticosteroid after 4 weeks of pneumonitis diagnosis. NS-1~NS-6 patients never received corticosteroid.
BAL, bronchoalveolar lavage; BFS, bronchofiberscopy; COP, cryptogenic organising pneumonia; COPD, chronic obstructive pulmonary disease; CPFE, combined pulmonary fibrosis and emphysema; CRC, colorectal cancer; CT, computed tomography; CTx, chemotherapy; GBC, gallbladder cancer; HD, Hodgkin’s disease; HNSCC, head-and-neck squamous cell carcinoma; ND, not done; NOS, pneumonia not otherwise specified; NSCLC, non-small cell lung cancer; NSIP, non-specific interstitial pneumonia; PD, progressive disease; PR, partial remission; RCC, renal cell carcinoma; SD, stable disease; UCC, urothelial cell carcinoma.
Figure 2.
Timeline of pneumonitis management outcome.Asterisk marks indicate the time of improvement and arrow heads indicate aggravation of ICI-pneumonitis according to grade. ICI, immune checkpoint inhibitor.
Total of fivepatients received corticosteroid within 4 weeks. These patients tended to have higher grade than the other patients (one grade 1, two grade 2 and two grade 3). Furthermore, twopatients had underlying lung diseases, which were chronic obstructive pulmonary disease and combined pulmonary fibrosis and emphysema, respectively, which might have caused clinicians to prompt steroid treatment. The patient with grade 1 pneumonitis was treated with corticosteroids within 4 weeks because the patient was enrolled in the clinical trial of anti-PD-1 antibody, which recommended administration of corticosteroids. After corticosteroid administration, fourpatients showed improvement, and time to improvement was 0.9, 1.1, 3.9 and 4.0 weeks, respectively. The only patient whose pneumonitis aggravated after corticosteroid was the patient with combined pulmonary fibrosis and emphysema.
The remaining 10 patients did not receive corticosteroids within 4 weeks. Of note, the twopatients with grade two pneumonitis did not receive corticosteroids initially because both patients had tolerable symptoms, and both patients were referred to pulmonologists for close monitoring. Among the 10 patients, twopatients experienced aggravation of ICI-pneumonitis from grade 1 to grade 2 and received corticosteroids (8.4 and 16.3 weeks after the diagnosis). Grade of ICI-pneumonitis in other twopatients remained stable, but due to radiographic evidence of aggravation for the one patient and persistent symptoms for the other patient, the twopatients subsequently received corticosteroids (9.6 and 12.1 weeks after the diagnosis). After corticosteroid administration in these fourpatients, the pneumonitis of the threepatients improved; time to improvement after corticosteroid administration was 3.4, 3.6, and 4.0 weeks, respectively. Remaining onepatient did not experience improvement but remained stable after corticosteroid administration.
Total of sixpatients (60%) never received corticosteroid. These sixpatients tended to have longer time to onset of pneumonitis from the initiation of ICIs and greater number of prior lines of chemotherapy compared with other fourpatients who received corticosteroids due to aggravation (mean 37.5 weeksvs25.5 weeks, figure 1, and median 2.5 vs 1.0 lines). Also, the proportion of current/ex-smokers was larger and that of other accompanying irAEs was smaller in the sixpatientscompared with the other fourpatients. (83.3% vs 50% and 50% vs 75%, respectively). Among the sixpatients, fourpatients experienced improvement of ICI-pneumonitis; time to improvement after the diagnosis of ICI-pneumonitis was 2.3, 9.7, 9.9 and 22.0 weeks (figure 3). ICI-pneumonitis of the other twopatients remained stable for 8.7 and 19.6 weeks, respectively.
Figure 3.
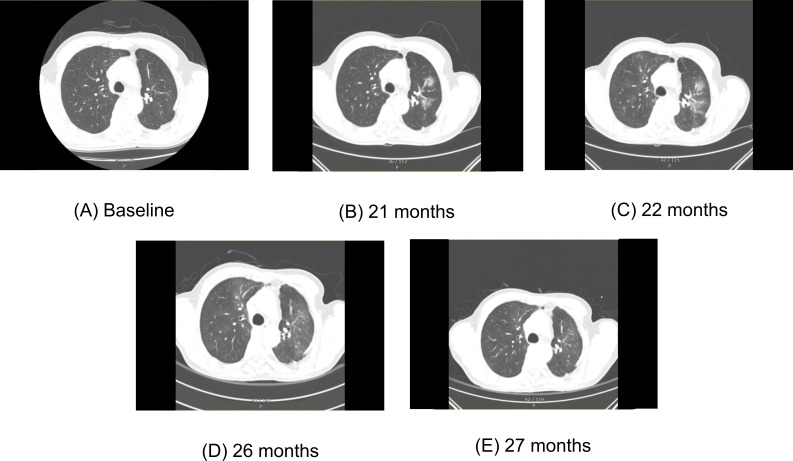
CT of a patient who improved without corticosteroid serial chest CT images of NS-4 patient who did not receive corticosteroid. (A) Chest CT before administration of immune checkpoint inhibitor (ICI) shows no definite parenchymal abnormality. (B) 21 months after initiation of ICI, ICI-pneumonitis was first recognised in this chest CT. Multiple ill-defined ground-glass opacities (GGO) and part-solid lesions consisting cryptogenic organising pneumonia (COP) pattern abnormalities were identified in bilateral upper lobes and right middle lobe. (C) ICIs were continued until 23 months after initiation of ICI. Chest CT right before last dose of ICI showed aggravation of bilateral COP pattern abnormalities. The patient did not have any symptoms, and corticosteroid was not administered. (D) Chest CT obtained 3 months after last dose of ICIs, showing overall decreased extent of multifocal ill-defined GGO and part-solid lesions in both lungs. (E) Follow-up chest CT showed further improved state of previous multifocal ill-defined GGO.
Rechallenge of ICI
Total of ninepatients were rechallenged with ICIs after median of 3.4 weeks of withholding ICIs. Among them, sixpatients experienced aggravation of ICI-pneumonitis. Among the sixpatients, fivepatients received corticosteroid before rechallenge and the remaining patient did not received any corticosteroid for ICI-pneumonitis.
Discussion
In this study, we described our experience in ICI-pneumonitis management and outcomes. Although many articles illustrated clinical features, risk factors and cancer related prognosis of ICI-pneumonitis,6–9 12 25 26 yet comprehensive description of management and outcomes have been lacking and we addressed this issue. Focusing on some of the parameters described in the results might help in designing clinical trials for ICI-pneumonitis patients.
First, about one-third of patients was observed to improve or remain stable without corticosteroid administration. As the NCCN guideline of ICI-pneumonitis management stratifies patients with pneumonitis grade,11 we also observed that most of grade 2, grade 3patients received corticosteroid for treatment of ICI-pneumonitis and improved with it. However, we might need to pay attention to the patient with grade 2 ICI-pneumonitis who improved without corticosteroid, and thus investigate factors to consider before administration of corticosteroid.
By comparing the 10 patients who did not receive corticosteroid within 4 weeks, we can peek some clues on those factors. The sixpatients who never received corticosteroid and improved or remained stable tended to have less other accompanying irAEs, a later onset of pneumonitis, more prior lines of chemotherapy and more patients with a history of smoking compared with the other fourpatients. Although the number is small that we cannot draw strong conclusion, still some of these factors have been mentioned in previous studies. A case report of patients who experienced multiple irAEs, including thyroiditis, hepatitis and pneumonitis, proposed that possible genetic susceptibility may act as a potential risk factor.27 Other studies reported that late onset irAEs, defined as an onset of later than 3 months, had better outcome than those with earlier onset after rechallenging of ICIs.28
Worrisome about delaying corticosteroid is a reasonable concern. Early administration of corticosteroids in ICI-pneumonitis is the recommendation of guidelines for patients who are expected to have a detrimental clinical course.11 29 However, we observed that corticosteroid administration may be spared for carefully selected patients with tolerable clinical features. For example, patients with ICI-pneumonitis responded to corticosteroids very well, as 78% of patients (seven out of nine) improved after corticosteroid administration, which is consistent with previous studies.6–8 We also observed that the time to improvement after corticosteroid administration was not significantly different when we compared those who received corticosteroids within 4 weeks with who received corticosteroids after 4 weeks, as demonstrated in the result and figure 2. Also, at least we haven’t observed any fatal cases for those patients who received corticosteroids after 4 weeks in this study.
This study had several limitations. First, this study was a retrospective review based on electronic medical records and therefore management plans were not protocolised, and time of corticosteroid administration was influenced by duty clinicians’ preferences. Second, although the baseline characteristics seemed similar to those in previous studies,6–8 the number of the patients was not enough to demonstrate significant factors to consider in deciding management strategies. This is due to the relatively rare overall incidence of ICI-pneumonitis. Third, not all the patients underwent a bronchoscopy with BAL to rule out infection. To exclude alternative aetiologies in patients without BAL results, we incorporated as much available clinical features as possible. Analysis of BAL is necessary to establish definite diagnosis in patients with suspected ICI-pneumonitis. For example, microbiological results can be used to exclude infection and cytological results, such as lymphocytosis and eosinophilia in this study can be used to rule-in ICI-pneumonitis.24 However, obtaining BAL is an invasive procedure that might result in severe respiratory distress. Therefore, careful examination of risks and benefits of obtaining BAL is necessary in real world clinical practice, and in line with this, the percentage of patients with BAL results (43.8%, seven out 16) might possibly represent real world clinical practice in assessing and managing ICI-pneumonitis.
Even so, this study addresses important knowledge gap between guidelines and clinical practices in terms of sharing single-centre experience of patients and providing insights on managing ICI-pneumonitis and especially deciding on when to administer corticosteroid at the right time. Additionally, these insights may facilitate further researches on ICI-pneumonitis including designing clinical trials on ICI-pneumonitis. In conclusion, we provide our own experience on ICI-pneumonitis and propose that although stratification of patients according to current guidelines is reasonable, there are more factors to be unravelled for consideration. Potential factors include existence of other accompanying irAEs, onset of pneumonitis after initiation of ICIs, prior lines of chemotherapy and history of smoking. Further studies are necessary to confirm these findings.
Footnotes
Contributors: CP, BK and SHY substantially contributed to the conception of the work, the acquisition, analysis, or interpretation of data for the work. C-YO, SMC, MK, YSP, TMK, D-YO, D-WK, YWK, DSH and Y-JB substantially contributed to the clinical management to the patients. All the authors substantially contributed to the drafting or rewriting of the initial and/or revised manuscript.
Funding: This study was supported by the grant of the Korea Health Technology R&D Project 'Strategic CenterCentre of Cell and Bio Therapy for Heart, Diabetes & Cancer' through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare (MHW), Republic of Korea (grant number: HI17C2085).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: All data collection and analyses were conducted after the review and approval of the institutional review board (IRB approval number H-1805-025-944) and were done in compliance with the Declaration of Helsinki.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017;377:1345–56. 10.1056/NEJMoa1709684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017;376:1015–26. 10.1056/NEJMoa1613683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018;378:1277–90. 10.1056/NEJMoa1712126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carbone DP, Reck M, Paz-Ares L, et al. First-Line nivolumab in stage IV or recurrent non–small-cell lung cancer. N Engl J Med 2017;376:2415–26. 10.1056/NEJMoa1613493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Postow MA, Sidlow R, Hellmann MD. Immune-Related adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378:158–68. 10.1056/NEJMra1703481 [DOI] [PubMed] [Google Scholar]
- 6.Naidoo J, Wang X, Woo KM, et al. Pneumonitis in patients treated with Anti–Programmed Death-1/Programmed death ligand 1 therapy. JCO 2017;35:709–17. 10.1200/JCO.2016.68.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kato T, Masuda N, Nakanishi Y, et al. Nivolumab-induced interstitial lung disease analysis of two phase II studies patients with recurrent or advanced non-small-cell lung cancer. Lung Cancer 2017;104:111–8. 10.1016/j.lungcan.2016.12.016 [DOI] [PubMed] [Google Scholar]
- 8.Delaunay M, Cadranel J, Lusque A, et al. Immune-checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur Respir J 2017;50 10.1183/13993003.00050-2017 [DOI] [PubMed] [Google Scholar]
- 9.Nishino M, Giobbie-Hurder A, Hatabu H, et al. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer a systematic review and meta-analysis. JAMA Oncol 2016;2:1607–16. [DOI] [PubMed] [Google Scholar]
- 10.National Cancer Institute (NCI) Common terminology criteria for adverse events, v5.0, 2017. Available: http://www.uptodate.com/contents/common-terminology-criteria-for-adverse-events [Accessed 1 May 2018].
- 11.Thompson JA, Schneider BJ, Brahmer J, et al. NCCN guidelines version 1.2018. Management of Immunotherapy-Related Toxicities, 2018. [Google Scholar]
- 12.Suresh K, Psoter KJ, Voong KR, et al. Impact of checkpoint inhibitor pneumonitis on survival in NSCLC patients receiving immune checkpoint immunotherapy. Journal of Thoracic Oncology 2019;14:494–502. 10.1016/j.jtho.2018.11.016 [DOI] [PubMed] [Google Scholar]
- 13.Narum S, Westergren T, Klemp M. Corticosteroids and risk of gastrointestinal bleeding: a systematic review and meta-analysis. BMJ Open 2014;4:e004587–9. 10.1136/bmjopen-2013-004587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Youssef J, Novosad SA, Winthrop KL. Infection risk and safety of corticosteroid use. Rheumatic Disease Clinics of North America 2016;42:157–76. 10.1016/j.rdc.2015.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waljee AK, Rogers MAM, Lin P, et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ 2017;357 10.1136/bmj.j1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamez-Pérez HE, et al. Steroid hyperglycemia: prevalence, early detection and therapeutic recommendations: a narrative review. World J Diabetes 2015;6:1073–81. 10.4239/wjd.v6.i8.1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Min L, Hodi FS, Kaiser UB. Corticosteroids and immune checkpoint blockade. Aging 2015;7:521–2. 10.18632/aging.100797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garant A, Guilbault C, Ekmekjian T, et al. Concomitant use of corticosteroids and immune checkpoint inhibitors in patients with hematologic or solid neoplasms: a systematic review. Crit Rev Oncol Hematol 2017;120:86–92. 10.1016/j.critrevonc.2017.10.009 [DOI] [PubMed] [Google Scholar]
- 19.Ricciuti B, Dahlberg SE, Adeni A, et al. Immune checkpoint inhibitor outcomes for patients with Non–Small-Cell lung cancer receiving baseline corticosteroids for palliative versus Nonpalliative indications. JCO 2019;37:1927–34. 10.1200/JCO.19.00189 [DOI] [PubMed] [Google Scholar]
- 20.Simonaggio A, Michot JM, Voisin AL, et al. Evaluation of Readministration of immune checkpoint inhibitors after immune-related adverse events in patients with cancer. JAMA Oncol 2019;5:1310–8. 10.1001/jamaoncol.2019.1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 22.Travis WD, Costabel U, Hansell DM, et al. An official American thoracic Society/European respiratory Society statement: update of the International multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733–48. 10.1164/rccm.201308-1483ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Society ATSR American thoracic Society American thoracic Society / European respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2002;166:518–626.12186831 [Google Scholar]
- 24.Meyer KC, Raghu G, Baughman RP, et al. An official American thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med 2012;185:1004–14. 10.1164/rccm.201202-0320ST [DOI] [PubMed] [Google Scholar]
- 25.Gounant V, Brosseau S, Naltet C, et al. Nivolumab-induced organizing pneumonitis in a patient with lung sarcomatoid carcinoma. Lung Cancer 2016;99:162–5. 10.1016/j.lungcan.2016.07.010 [DOI] [PubMed] [Google Scholar]
- 26.Cui P, Liu Z, Wang G, et al. Risk factors for pneumonitis in patients treated with anti-programmed death-1 therapy: a case-control study. Cancer Med 2018;7:4115–20. 10.1002/cam4.1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diamantopoulos PT, Gaggadi M, Kassi E, et al. Late-Onset nivolumab-mediated pneumonitis in a patient with melanoma and multiple immune-related adverse events. Melanoma Res 2017;27:391–5. 10.1097/CMR.0000000000000355 [DOI] [PubMed] [Google Scholar]
- 28.Santini FC, Rizvi H, Wilkins O, et al. Safety of retreatment with immunotherapy after immune-related toxicity in patients with lung cancers treated with anti-PD(L)-1 therapy. JCO 2017;35:9012 10.1200/JCO.2017.35.15_suppl.9012 [DOI] [Google Scholar]
- 29.Haanen JBAG, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol 2017;28:iv119–42. 10.1093/annonc/mdx225 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2019-000575supp001.pdf (64.1KB, pdf)