Abstract
NTRK fusions are found at low frequencies (commonly <1%) in a range of common tumour types and at high frequencies (up to or greater than 90%) in rare cancer types (secretory breast carcinoma, mammary analogue secretory carcinoma and infantile fibrosarcoma). The fusions typically occur in a mutually exclusive fashion with other strong mitogenic drivers, and it is of significant importance to identify patients in order to offer transformative treatment with TRK inhibitors. Larotractinib or entrectinib have resulted in fast and durable response with few and reversible adverse events. Even though on-target resistance may occur, second generation TRK inhibitors are in development and have shown promising activity. Diagnostic strategies must be applied, considering available assays and specific tumour types.
Keywords: NTRK fusion, TRK inhibitors
Introduction
Several chromosomal rearrangements in tumour cells resulting in somatic gene fusions encoding a tyrosine kinase have been identified and such gene fusions may function as tumour drivers. A number of tyrosine kinase inhibitors (TKIs) have been approved, including TKIs targeting ABL, ALK and ROS1 fusions.1 2 Pivotal studies have confirmed that these agents are superior to chemotherapy in patient harbouring these fusions.
The neurotrophic tyrosine kinase receptors, TRKA, TRKB and TRKC function as receptors for neurotrophins, regulating several aspects of neuronal development and function.3 These receptors are encoded by the NTRK1, NTRK2 and NTRK3 genes, and oncogenic fusions may occur in any of the three NTRK genes, located on the human chromosomes 1q23.1, 9q21.33 and 15q25.3, respectively. The fusion transcripts encode a constitutive active tyrosine kinase domain, comprising the N-terminus of the fusion partner joined to the C-terminus of the TRK protein.4
TKIs targeting TRK are novel therapeutic approaches for the treatment of patients with tumours harbouring NTRK gene fusions.
Frequency of NTRK gene fusions
NTRK gene fusions are rare in common solid tumours with a frequency of <1%, but occur more frequently in some rare paediatric and adult tumour types, with a frequency of up to 100% in infantile fibrosarcoma and secretory breast cancer5 6 (table 1). The fusions are more common in the NTRK1 and NTRK3 genes, and less frequent in NTRK2, and over 60 different 5′ partner genes have been reported.5
Table 1.
Prevalence of NTRK fusion-positive cancers
| Tumour types | Prevalence (%) |
| Appendiceal cancer | 2/97 (2) |
| Cholangiocarcinoma | 1/28 (4) |
| Colorectal cancer | 13/346 (4) |
| Colorectal cancer dMMR | 10/13 (76.9) |
| Melanoma | 1/374 (0.3) |
| Glioblastoma | 3/115 (3) |
| Head and neck cancer | 2/411 (0.5) |
| Infantile fibrosarcoma | 2/4 (50) |
| Low-grade glioma | 2/461 (0.4) |
| Lung adenocarcinoma | 3/91 (3.3) |
| Mammary analogue secretory carcinoma | 2/3 (66) |
| Papillary thyroid carcinoma | 4/33 (12) |
| Paediatric high-grade glioma | 28/127 (22) |
| Polycystic astrocytoma | 3/96 (3) |
| Secretory breast cancer | 12/13 (92) |
| Spitzoid melanoma | 23/140 (16) |
Modified from Kheder and Hong.6
dMMR, deficient mismatch repair; NTRK, neurotrophic tropomyosin receptor kinase.
TRK inhibitors
Larotrectinib is a highly selective, orally administered inhibitor of TRKA, TRKB and TRKC, with IC50 values in the range of 5.3–11.5 nM.7 Safety and activity data have been obtained from patients treated with larotrectinib across three studies: an adult phase I trial, a paediatric phase I–II trial (SCOUT), and the adult and adolescent phase II basket trial (NAVIGATE). A total of 159 patients have been evaluable for response. Response rates of >75% have been reported independent of tumour type, age, NTRK gene and fusion partner. Responses are durable with a median duration of response (DoR) of 35.2 months, median progression-free survival (PFS) of 28.2 months and a median overall survival (OS) of 44.4 months.8 A total of 260 patients were included in the safety population and adverse events (AEs) were generally mild and transient. The most frequently observed AEs were fatigue, cough, elevated liver function tests, constipation, nausea and dizziness. Dose reduction due to AEs occurred in 8% of both overall patients (22/260) and dose discontinuation due to treatment-related AEs occurred in 6 (2%) patients.
Entrectinib is an orally administered small molecule inhibitor of TRKA, TRKB, TRKC, ROS1, ALK, JAK2 and ACK1 kinases, with IC50 values for the TRK kinases of <5 nM.9 Three clinical trials have studied safety and activity of entrectinib: two phase I trials (ALKA-372–001 and STARTRK-1) and the ongoing phase II basket trial STARTRK-2. Response rates by blinded independent review was reported from 54 patients with NTRK fusions and the overall response rate (ORR) was 59.3% with complete remission in 7.4%. The median DoR was 12.9 months, median PFS was 11.8 months and the median OS was 23.9 months.10 The safety population included a total of 355 included in the three trials. The safety profile was similar to larotrectinib and most adverse events were grade 1–2 and reversible.
Both larotrectinib and entrectinib are able to penetrate the blood–brain barrier and intracranial response seems to be similar to overall response rates.
Resistance
Even though many patients experience long-term DoR treatment with TKIs may lead to acquired resistance and acquired on-target resistance mutations have been reported in patients with progression during treatment with receiving entrectinib or larotrectinib. Biopsies from such patients have identified one or more kinase domain mutations affecting the NTRK gene, leading to sterically change the drug-binding site decreasing the inhibitory properties and potency. These include amino acid substitutions involving solvent front mutations, gatekeeper mutations and mutation in the xDFG domains, which is similar to those described for resistance mutations in other classes of kinase inhibitors. Second-generation TRK inhibitors have been developed to overcome these mechanisms of resistance, like Loxo-195 and repotrectinib.
Recently, off-target mutation have been identified as genomic alterations converging to activation of the mitogen-activated protein kinase (MAPK) pathway, as cases with MAPK pathway-directed targeted therapy alone or in combination with TRK inhibition have been able to re-established disease control.11
Testing for NTRK fusion
Several approaches that may be used to identify gene fusion in patient samples, including immunohistochemistry (IHC), fluorescence in situ hybridisation (FISH), reverse-transcriptase PCR (RT-PCR) and both DNA-based and RNA-based next-generation sequencing (NGS). FISH or RT-PCR may be used when specific well-known fusions are suspected, which may be the case for ETV6-NTRK3 fusion in secretory breast cancer. However, >30 different fusions have been identified with three NTRK genes and multiple 5′ NTRK gene fusion partners have been identified. Consequently, using FISH probes for each NTRK gene would require three separate FISH assays per patient sample. IHC has been proven highly sensitive and specific12 for the detection of NTRK fusions and pan-TRK IHC is a valuable tool to identify NTRK expression. The advantages are the high sensitivity and specificity, the low cost and the fast turnaround time. However, the test may be false positive as it detects only transcribed and translated fusion proteins. DNA-based NGS is able to detect NTRK fusions; however, fusions involving NTRK2 and NTRK3 with large introns may be missed. Due to chimeric nature of the fusion transcripts, RNA sequencing is the optimal method for the de novo detection of transcribed fusion genes, either as whole transcriptome RNA sequencing or targeted RNA sequencing. Sequencing libraries targets known fusion exons in multiple oncogenes including all of the three members of the NTRK family.13
Strategy for testing
Different diagnostic strategies must be applied for certain rare tumours with a high incidence of NTRK gene fusion events and more common tumour where the incidence is <1% of cases.
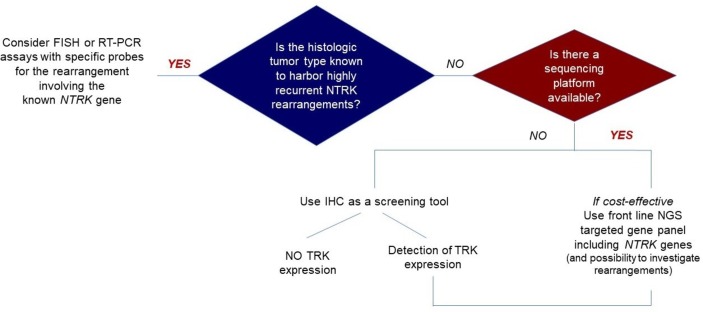
In rare cancers, NTRK fusion may be pathognomonic and be confirmed by FISH, whereas screening strategies may be used for common tumours. An ESMO working group has published a proposal using IHC to screen when NGS is not readily available, with RNA-based RNA as a reference for confirmation (figure 1).13
Figure 1.
NTRK fusion detection: the ESMO proposal.13 FISH, fluorescence in situ hybridisation; IHC, immunohistochemistry; NGS, next-generationsequencing; NTRK, neurotrophic tropomyosin receptor kinase; RT-PCR, reverse-transcriptasePCR
The fusions typically occur in a mutually exclusive fashion with other strong mitogenic drivers, that is, genetic alterations affecting the most common driver genes belonging to the MAPK signalling pathway (Kirsten RAt Sarcoma virus (KRAS), Neuroblastoma RAS viral oncogene homolog (NRAS) and serine/threonine-protein kinase B-Raf (BRAF)). In lung cancer, NTRK fusion may be suspected in non-smokers without other activating genomic alternation after failure of standard of care. In colorectal cancer, it has been suggested that the fusions are more frequent in patients with deficient mismatch repair (dMMR) genes without BRAF or KRAS mutations, and such patients may be less responsive to checkpoint inhibitors.14
Conclusion
Finding patients with NTRK fusion is difficult, as this molecular aberration is rare in common cancers and frequent only in rare cancers. However, it is important to consider testing patients without other activating mutations. The TRK fusions are oncogenic and patients may have failed standard of care. Treatment with emerging TRK inhibitors like larotrectinib and entrectinib may be transformative, leading to fast and durable responses and few adverse event.
Footnotes
Twitter: @ulassen
Contributors: I have written the paper alone without any other contributors.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Schram AM, Chang MT, Jonsson P, et al. . Fusions in solid tumours: diagnostic strategies, targeted therapy, and acquired resistance. Nat Rev Clin Oncol 2017;14:735–48. 10.1038/nrclinonc.2017.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoshihara K, Wang Q, Torres-Garcia W, et al. . The landscape and therapeutic relevance of cancer-associated transcript fusions. Oncogene 2015;34:4845–54. 10.1038/onc.2014.406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reichardt LF. Neurotrophin-regulated signalling pathways. Phil. Trans R Soc Lond B 2006;361:1545–64. 10.1098/rstb.2006.1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaishnavi A, Le AT, Doebele RC. TRKing down an old oncogene in a new era of targeted therapy. Cancer Discov 2015;5:25–34. 10.1158/2159-8290.CD-14-0765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stransky N, Cerami E, Schalm S, et al. . The landscape of kinase fusions in cancer. Nat Commun 2014;5:4846 10.1038/ncomms5846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kheder ES, Hong DS. Emerging Targeted Therapy for Tumors with NTRK Fusion Proteins. Clin Cancer Res 2018;24:5807–14. 10.1158/1078-0432.CCR-18-1156 [DOI] [PubMed] [Google Scholar]
- 7.Drilon A, Laetsch TW, Kummar S, et al. . Efficacy of Larotrectinib in TRK Fusion–Positive Cancers in Adults and Children. N Engl J Med 2018;378:731–9. 10.1056/NEJMoa1714448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hyman DM, Tilburg CM, Albert CM, et al. . Durability of response with larotrectinib in adult and pediatric patients with Trk fusion cancer Annals of oncology 2019;30:v159–93. [Google Scholar]
- 9.Ardini E, Menichincheri M, Banfi P, et al. . Entrectinib, a pan-Trk, ROS1, and ALK inhibitor with activity in multiple molecularly defined cancer indications. Mol Cancer Ther 2016;15:628–39. 10.1158/1535-7163.MCT-15-0758 [DOI] [PubMed] [Google Scholar]
- 10.Rolfo C, Dziadziuszko R, Doebele RC, et al. . 476PUpdated efficacy and safety of Entrectinib in patients with NTRK fusion-positive tumors: integrated analysis of STARTRK-2, STARTRK-1 and ALKA-372-001. Annals of Oncology 2019;30:v159–93. 10.1093/annonc/mdz244.038 [DOI] [Google Scholar]
- 11.Cocco E, Schram AM, Kulick A, et al. . Resistance to Trk inhibition mediated by convergent MAPK pathway activation. Nat Med 2019;25:1422–7. 10.1038/s41591-019-0542-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hechtman JF, Benayed R, Hyman DM, et al. . Pan-Trk immunohistochemistry is an efficient and reliable screen for the detection of NTRK fusions. Am J Surg Pathol 2017;41:1547–51. 10.1097/PAS.0000000000000911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marchiò C, Scaltriti M, Ladanyi M, et al. . ESMO recommendations on the standard methods to detect NTRK fusions in daily practice and clinical research. Ann Oncol 2019;30:1417–27. 10.1093/annonc/mdz204 [DOI] [PubMed] [Google Scholar]
- 14.Cocco E, Benhamida J, Middha S, et al. . Colorectal carcinomas containing hypermethylated MLH1 promoter and wild-type BRAF/KRAS are enriched for targetable kinase fusions. Cancer Res 2019;79:1047–53. 10.1158/0008-5472.CAN-18-3126 [DOI] [PMC free article] [PubMed] [Google Scholar]