Abstract
Obesity is a risk factor for asthma, especially nonatopic asthma, and attenuates the efficacy of standard asthma therapeutics. Obesity also augments pulmonary responses to ozone, a nonatopic asthma trigger. The purpose of this study was to determine whether obesity-related alterations in gut microbiota contribute to these augmented responses to ozone. Ozone-induced increases in airway responsiveness, a canonical feature of asthma, were greater in obese db/db mice than in lean wild-type control mice. Depletion of gut microbiota with a cocktail of antibiotics attenuated obesity-related increases in the response to ozone, indicating a role for microbiota. Moreover, ozone-induced airway hyperresponsiveness was greater in germ-free mice that had been reconstituted with colonic contents of db/db than in wild-type mice. In addition, compared with dietary supplementation with the nonfermentable fiber cellulose, dietary supplementation with the fermentable fiber pectin attenuated obesity-related increases in the pulmonary response to ozone, likely by reducing ozone-induced release of IL-17A. Our data indicate a role for microbiota in obesity-related increases in the response to an asthma trigger and suggest that microbiome-based therapies such as prebiotics may provide an alternative therapeutic strategy for obese patients with asthma.
Keywords: obesity, gut microbiome, neutrophil, airway responsiveness, dietary fiber
Obesity is a risk factor for asthma, a disease characterized by airway hyperresponsiveness (AHR). Obesity increases the incidence and prevalence of asthma (1) and is associated with severe asthma (2). Importantly, weight loss reduces asthma symptoms in obese individuals with asthma, particularly those with nonatopic asthma, and also decreases AHR (3). Established asthma therapeutics such as corticosteroids are less effective in obese than in lean individuals with asthma (4). Developing effective therapeutics for this population of individuals with asthma will require improved understanding of the mechanistic basis for obese asthma.
Ozone (O3) is a nonatopic asthma trigger. In humans, exposure to O3 causes asthma symptoms, reduces lung function, causes AHR, and increases emergency room visits for asthma (5, 6). Importantly, O3-induced reductions in lung function are greater in obese than in lean human subjects (7). Pulmonary responses to O3 are also augmented in obese mice (8, 9).
The gut microbiome is altered in both human and murine obesity (10–14). Moreover, the obese microbiome appears to contribute to some obesity-related conditions, including insulin resistance (15–18). Others have reported a role for the microbiome in allergic airway responses (19). Our data also indicate a role for the microbiome in pulmonary responses to O3 in lean mice (20, 21). These data are consistent with the hypothesis that microbiota contribute to the augmented responses to O3 observed in obese mice.
To examine this hypothesis, we perturbed the gut microbiomes of obese db/db mice and lean wild-type (WT) littermates by administering a cocktail of antibiotics via the drinking water. db/db mice lack the long form of the receptor for the satiety hormone leptin and are substantially obese. In water-treated control mice, O3 caused a larger increase in airway responsiveness in db/db than in WT mice as previously described (8, 22), and in db/db mice, antibiotic treatment attenuated O3-induced AHR almost to the degree of the WT mice. To determine whether an obese gut microbiome is sufficient to augment pulmonary responses to O3, we gavaged germ-free (GF) mice with colonic contents from either WT or db/db mice. Two weeks later, O3-induced AHR was greater in mice that received colonic contents from db/db than in WT mice.
Prebiotics, including various types of dietary fiber, modify the gut microbiome of obese mice and produce beneficial effects on adiposity, insulin resistance, and hyperlipidemia (18, 23, 24). Consequently, we fed db/db and WT mice diets enriched in dietary fiber cellulose or pectin for 3 days before O3 exposure. Pectin-enriched diets attenuated obesity-related increases in the response to O3, likely by reducing IL-17A release.
Methods
Animals
The Harvard Medical Area Standing Committee on Animals approved these studies. To examine the impact of antibiotics on pulmonary responses to O3, female db/db mice on a C57BL/6J background and female control C57BL/6J mice were bred at the Harvard T.H. Chan School of Public Health specific pathogen–free facility from heterozygous db+/− parents originally purchased from The Jackson Laboratory. For other experiments, female WT C57BL/6 and db/db mice were purchased from The Jackson Laboratory. All mice, except mice used for high-fiber experiments, were fed standard mouse chow.
Protocol
Three experimental protocols were used. In the first, 8-week-old female db/db and WT mice were given a cocktail of antibiotics (ampicillin, metronidazole, neomycin, vancomycin) by addition to the drinking water as previously described (20, 21). Sucralose was added for taste. After 2 weeks of antibiotic treatment, fecal pellets were collected before mice were exposed to room air or to O3 (2 ppm for 3 h). Twenty-four hours after exposure, mice were anesthetized for the measurement of pulmonary mechanics and airway responsiveness to inhaled methacholine. After these measurements, mice were killed with an overdose of sodium pentobarbital, and BAL was performed.
For the second experiment, GF C57BL/6 female mice were maintained in gnotobiotic isolators at the Massachusetts Host-Microbiome Center and gavaged with cecal contents from female WT or db/db mice at 8–9 weeks of age. Twelve days later, fecal pellets were harvested for 16S rRNA sequencing to verify the efficacy of the cecal transfer. Recipient mice were then exposed to air or O3 and evaluated as described above.
In the third experiment, 8–9-week old female WT and db/db mice were fed cellulose-rich (30% cellulose and 2.4% pectin) or pectin-rich (2.4% cellulose and 30% pectin) diets for 3 days before exposure. Diets were purchased from Research Diets, Inc. (Table E1 in the data supplement). Fecal pellets were harvested, and the mice were exposed to air or O3 and evaluated as described above. Additional methodologic details are provided in the data supplement.
Statistics
Except for 16S rRNA sequencing analysis (see data supplement), the significance of differences between groups was assessed using factorial ANOVA combined with Fisher least significant difference post hoc analysis (Statistica Software) using as main effects either 1) antibiotic treatment, exposure, and genotype (antibiotic study); 2) donor and exposure (GF mice study); or 3) diet, genotype, and exposure (fiber experiments). A P value less than 0.05 (two-tailed) was considered significant. All values are expressed as mean ± SEM.
Results
Differences in Gut Microbiomes of db/db and WT Mice
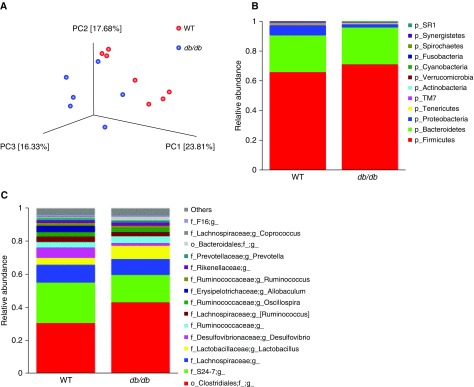
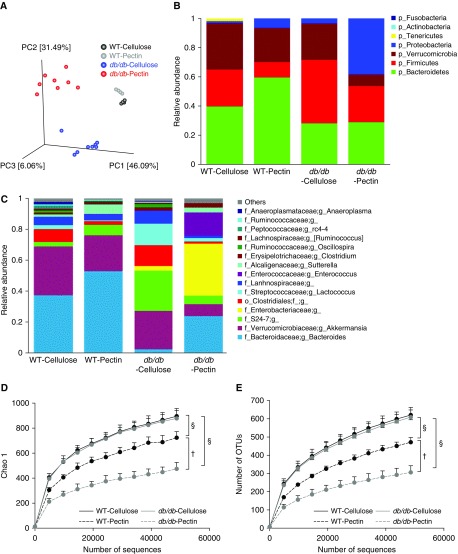
To confirm differences in the microbiomes of WT and db/db mice, we performed 16S rRNA sequencing of fecal DNA. Principal coordinate (PC) analysis of 16S rRNA sequencing data indicated differences in the gut microbiomes of WT and db/db mice that received control water (Figure 1A), consistent with reports of others (13, 25). Examination of taxon abundance at both the phylum (Figure 1B) and genus levels (Figure 1C) also indicated obesity-related differences in the gut microbiome. Statistical analysis using multivariate association with linear models (MaAsLin) (26) indicated that several taxa were significantly different in WT versus db/db mice (Figures E1A–E1J). Diversity and richness were not different (data not shown).
Figure 1.
Differences in the gut microbiomes of wild-type (WT) and db/db mice assessed by 16S rRNA sequencing of fecal DNA. Fecal pellets were collected before exposure. (A) Principal coordinate (PC) analysis calculated by the Bray-Curtis method. (B) Relative abundance of bacterial phyla. (C) Relative abundance of top 15 genus-level taxa. n = 6–7 mice per group.
Antibiotic treatment had a profound effect on the fecal microbiomes of both obese and lean mice, consistent with other reports (21, 27). Most bacterial phyla were depleted, whereas the Proteobacteria phylum was relatively enriched (data not shown).
Antibiotics Attenuate Obesity-related Increases in O3-induced AHR
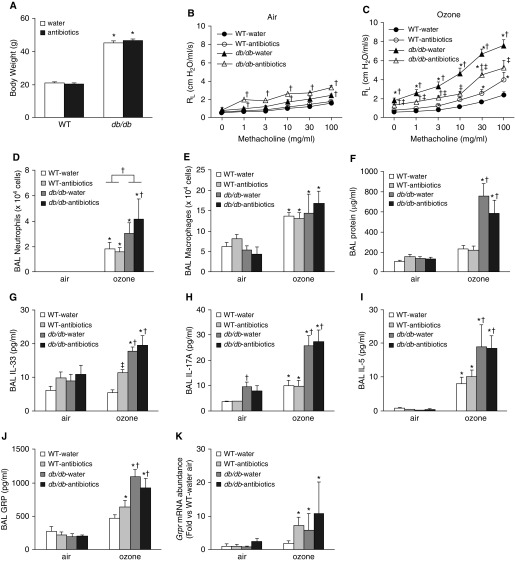
At the time of exposure, db/db mice weighed about twice as much as WT mice. Antibiotic treatment did not affect body mass in either db/db or WT mice (Figure 2A). In mice exposed to air and treated with control water, airway responsiveness was greater in db/db than in WT mice. Antibiotic treatment had no effect on airway responsiveness in air-exposed WT mice. However, in air-exposed db/db mice, airway responsiveness was significantly greater in antibiotic-treated than in water-treated mice (Figure 2B).
Figure 2.
An antibiotic cocktail attenuates ozone-induced airway hyperresponsiveness in db/db mice. Mice were treated with a cocktail of antibiotics (ampicillin 1 g/L, metronidazole 1 g/L, neomycin 1 g/L, vancomycin 0.5 g/L) via their drinking water for 2 weeks. Sucralose (8 g/L) was added to the water for taste. Control mice were treated with drinking water containing sucralose only. The mice were then exposed to room air or to ozone (2 ppm for 3 h) and evaluated 24 hours later. (A) Body weight. (B) Airway responsiveness of air-exposed mice. (C) Airway responsiveness of ozone-exposed mice. (D) BAL neutrophils. (E) BAL macrophages. (F) BAL protein. (G) BAL IL-33. (H) BAL IL-17A. (I) BAL IL-5. (J) BAL GRP (gastrin-releasing peptide). (K) Pulmonary mRNA expression of Grpr (receptor for gastrin-releasing peptide). Results are mean ± SE of data from six to eight mice per group. *P < 0.05 compared with air-exposed mice of same genotype and treatment, †P < 0.05 compared with WT mice of same exposure and treatment, and ‡P < 0.05 compared with water-treated mice of same exposure and genotype. RL = lung resistance.
In water-treated db/db but not WT mice, O3 exposure caused a significant increase in baseline lung resistance indicative of airway obstruction (compare PBS doses in Figures 2B and 2C). Treatment of db/db mice with antibiotics abolished O3-induced increases in baseline lung resistance. In water-treated mice, O3 caused greater increases in airway responsiveness in db/db than in WT mice, as previously reported (8, 22). Treatment with antibiotics significantly attenuated O3-induced AHR in db/db mice but increased O3-induced AHR in WT mice (Figure 2C).
O3 caused greater increases in BAL neutrophils (Figure 2D) but not macrophages (Figure 2E) in db/db than in WT mice. O3-induced increases in BAL protein, a marker of lung epithelial injury, were also greater in db/db than in WT mice (Figure 2F), consistent with previous reports (8, 22). Antibiotic treatment did not affect BAL neutrophils, macrophages, or protein in either WT or db/db mice.
The data above indicate that an intact microbiome is required for obesity-related increases in O3-induced AHR. We have reported that the cytokines IL-33 and IL-17A also contribute to obesity-related increases in O3-induced AHR (8, 22). In water-treated mice, O3 exposure caused greater increases in BAL IL-33 and BAL IL-17A in db/db than in WT mice, but antibiotics had no effect on these cytokines in db/db mice (Figures 2G and 2H). IL-33 and IL-17A exert their effects on O3-induced AHR, respectively, by causing release of type 2 cytokines (8, 9) and by increasing expression of GRPR, the receptor for gastrin-releasing peptide (GRP), a peptide found in pulmonary neuroendocrine cells that can constrict small airways (22). In water-treated mice, O3-induced increases in BAL IL-5, a type 2 cytokine, and BAL GRP were greater in db/db than in WT mice, but antibiotics had no effect on these changes (Figures 2I and 2J). Exposure to O3 increased pulmonary Grpr expression in db/db but not WT mice treated with control water (Figure 2K). Antibiotics had no effect on O3-induced increases in pulmonary Grpr expression in db/db mice but increased Grpr in WT mice. The data indicate that the microbiome does not augment O3-induced AHR in db/db mice by impacting the release of IL-33 or IL-17A within the lungs, nor does it augment it by affecting signaling events downstream of IL-33 or IL-17A.
An Obese Microbiome Augments O3-induced AHR
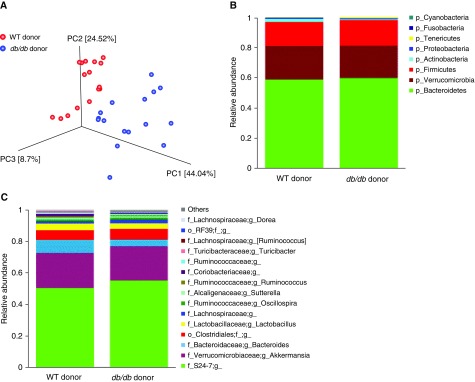
To determine whether an obese gut microbiome could augment pulmonary responses to O3, even in the absence of obesity per se, we transferred the colonic contents of WT or db/db mice into lean GF mice by gavage. 16S rRNA sequencing data confirmed differences in the taxonomic composition of the colonic contents from the WT and db/db donor mice (Figures E2 and 3). PC analysis and comparative taxonomy of 16S rRNA sequencing data from fecal DNA of recipient mice also confirmed differences in the gut microbiomes of mice that received colonic contents from WT versus db/db mice (Figure 3A), and MaAsLin analysis identified several taxa that differed significantly in abundance between the recipients of colonic contents from db/db versus WT mice (Figures E4A–E4Q), but diversity and richness were not different (data not shown).
Figure 3.
Differences in the gut microbiomes of germ-free (GF) mice reconstituted by gavage with cecal contents from WT or db/db donors as assessed by 16S rRNA sequencing of fecal DNA from the recipient mice. Fecal pellets were harvested 12 days after gavage but before exposure. (A) PC analysis calculated by the Bray-Curtis method. (B) Relative abundance of bacterial phyla. (C) Relative abundance of top 15 genus-level taxa. n = 16 mice per group.
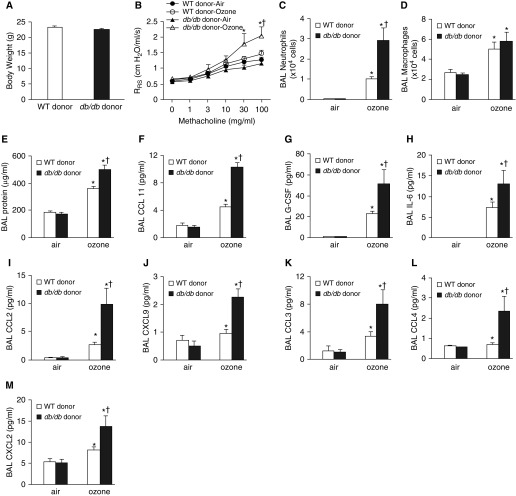
In recipients, body weight was not impacted by donor genotype (Figure 4A). In air-exposed mice, airway responsiveness was not different in mice that received colonic contents from WT versus db/db donors (Figure 4B). O3 did not cause an increase in baseline respiratory system resistance in either group of recipient mice (PBS dose in Figure 4B), nor did O3 significantly increase airway responsiveness in mice that received colonic contents from WT mice (Figure 4B). However, O3 did increase airway responsiveness in mice that received colonic contents from db/db mice, and O3-induced AHR was significantly greater in recipients of colonic contents from db/db mice than in recipients of colonic contents from WT mice (Figure 4B). O3-induced increases in BAL neutrophils and protein but not macrophages were also greater in recipients of colonic contents of db/db mice than in recipients of colonic contents from WT mice (Figures 4C–4E), as were the neutrophils and protein in db/db versus WT mice (Figures 2D–2F). O3 causes greater increases in BAL concentrations of many cytokines and chemokines in db/db mice than in WT mice (8, 22). BAL concentrations of many of the same cytokines and chemokines were also significantly greater in mice that received colonic contents of db/db than in those that received colonic contents of WT donors (Figures 4F–4M). However, BAL IL-33, IL-5, and IL-17A were not greater in recipients of db/db colonic contents than in recipients of WT colonic contents (data not shown), even though BAL concentrations of these cytokines are greater in db/db mice than in WT mice (8, 22). Taken together, the data indicate that obese microbiota reproduce many but not all aspects of the augmented responses to O3 observed in obese mice, even in the absence of obesity per se.
Figure 4.
An obese gut microbiome augments pulmonary responses to ozone. GF mice were gavaged with cecal contents from either WT or db/db mice, exposed to room air or ozone (2 ppm for 3 h) 12 days after gavage, and evaluated 24 hours after exposure. (A) Body weight. (B) Airway responsiveness. (C) BAL neutrophils. (D) BAL macrophages. (E) BAL protein. (F–M) BAL concentrations of CCL11 (F), granulocyte colony-stimulating factor (G-CSF) (G), IL-6 (H), CCL2 (I), CXCL9 (J), CCL3 (K), CCL4 (L), and CXCL2 (M). Results are mean ± SE of data from eight mice per group. *P < 0.05 compared with air-exposed mice with same donor genotype, and †P < 0.05 compared with mice that received transplanted feces from WT mice. RRS = respiratory system resistance.
A Pectin-enriched Diet Attenuates O3-induced AHR in db/db Mice
Prebiotics, including various types of dietary fiber, have the capacity to modify the gut microbiota of obese mice, leading to beneficial effects on adiposity, insulin resistance, and hyperlipidemia (18, 28, 29). To determine whether prebiotic manipulation of the gut microbiome could be used to reduce responses to O3 in obese mice, we fed db/db and WT mice diets enriched in the fermentable fiber pectin or the nonfermentable fiber cellulose for 3 days before exposure. PC analysis and comparative taxonomy of 16S rRNA sequencing data from fecal DNA indicated differences in the gut microbiomes of db/db and WT mice, regardless of which diet they consumed (Figure 5A–5C). There were also differences in the gut microbiomes of mice fed pectin-versus cellulose-enriched diets (Figure 5A–5C), although the magnitude of the diet-related difference was greater in the db/db mice (Figure 5A). Compared with a cellulose-enriched diet, a pectin-enriched diet also reduced the diversity and richness of the gut microbiome, especially in db/db mice (Figures 5D and 5E). MaAsLin analysis identified 41 taxa that differed significantly across the four groups of mice, mostly within the Firmicutes phylum (Table E2).
Figure 5.
Differences in the gut microbiomes of WT and db/db mice fed cellulose-enriched or pectin-enriched diets for 3 days as assessed by 16S rRNA sequencing of fecal DNA. (A) PC analysis calculated by the Bray-Curtis method. (B) Relative abundance of bacterial phyla. (C) Relative abundance of top 15 genus-level taxa. (D) Rarefaction curves of diversity on Chao 1 index. (E) Rarefaction curve of richness. Results for D and E are mean ± SE of data from eight mice per group. †P < 0.05 compared with WT mice fed the same diet, and §P < 0.05 compared with cellulose-fed mice of the same genotype. OTUs = operational taxonomic units.
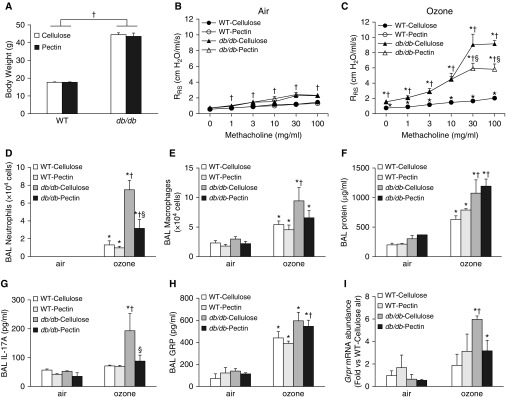
Body weight was approximately two times greater in db/db than in WT mice, but there was no difference in body weights of cellulose- versus pectin-fed mice (Figure 6A). After air exposure, airway responsiveness was greater in db/db than in WT mice, as described above (Figure 2B), but there was no effect of pectin- versus cellulose-enriched diets on this innate AHR (Figure 6B). Compared with air, acute O3 exposure increased airway responsiveness in both WT and db/db mice, but the effect was greater in db/db mice (Figure 6B). O3-induced AHR was significantly reduced in db/db but not WT mice fed pectin- versus cellulose-enriched diets (Figure 6C). In cellulose-fed mice, O3 exposure increased BAL neutrophils, macrophages, and protein to a greater extent in db/db than in WT mice (Figures 6D–6F). Compared with cellulose, db/db mice fed pectin-enriched diets had reduced BAL neutrophils but not macrophages or protein. There was no effect of dietary fiber on BAL cells or protein in WT mice.
Figure 6.
A pectin-enriched diet attenuates pulmonary responses to ozone in db/db but not WT mice. WT and db/db mice were fed either cellulose-enriched or pectin-enriched diets for 3 days. The mice were then exposed to room air or to ozone. (A) Body weight. (B) Airway responsiveness of mice exposed to room air. (C) Airway responsiveness of mice exposed to ozone. (D) BAL neutrophils. (E) BAL macrophages. (F) BAL protein. Also shown are (G) BAL concentrations of IL-17A, (H) GRP, and (I) Grpr mRNA abundance in lung. Results are mean ± SE of data from four (air) to six (ozone) mice per group. *P < 0.05 compared with air-exposed mice of the same diet and genotype, †P < 0.05 compared with WT mice of same diet and exposure, and §P < 0.05 compared with ozone-exposed cellulose-fed db/db mice.
In cellulose-fed mice, O3-induced increases in BAL IL-17A, BAL GRP, and pulmonary Grpr mRNA abundance were greater in db/db than in WT mice (Figures 6G–6I), consistent with our previous data from chow-fed mice (22). BAL GRP was not different in pectin- versus cellulose-fed db/db mice exposed to O3, but BAL IL-17A and pulmonary Grpr expression were both significantly lower in pectin- than in cellulose-fed db/db mice (Figures 6G–6I). Indeed, in pectin-fed db/db mice exposed to O3, BAL IL-17A and pulmonary Grpr expression were reduced to levels not different from those measured in WT mice (Figures 6G–6I). O3-induced increases in several other BAL cytokines and chemokines were also reduced in pectin- versus cellulose-fed db/db mice (Table E3).
Discussion
Our goal was to examine the hypothesis that obesity-related alterations in gut microbiota contribute to the augmented responses to O3 observed in obese mice. Our data support this hypothesis, at least for aspects of the response to O3. We showed that obesity-related increases in O3-induced airway obstruction and AHR, but not O3-induced injury or neutrophilic inflammation, were substantially attenuated after antibiotic treatment (Figure 2C). In addition, reconstitution of GF mice with the cecal contents of obese versus lean mice was sufficient to augment O3-induced AHR and inflammation (Figures 4B–4M). Furthermore, manipulation of the obese microbiome with prebiotics led to reductions in O3-induced AHR and inflammation (Figures 6C, 6D, and 6G and Table E4).
Our data showed reduced O3-induced airway obstruction and reduced O3-induced AHR in db/db mice treated with antibiotics (Figure 2C), indicating a role for microbiota in these events. These effects of antibiotics were likely the result of alterations in the gut microbiome, because antibiotics do not affect responses to O3 in GF mice (21). We cannot rule out the possibility that antibiotic-dependent changes in responses to O3-induced AHR were the result of changes in lung rather than gut microbiota. However, we believe this explanation is unlikely. In lean male mice, oral vancomycin causes a reduction in O3-induced AHR (21). However, oral vancomycin does not affect the lung microbiome in mice, even though vancomycin does affect the gut microbiome (30).
Reconstitution of GF mice with the cecal contents of obese versus lean mice reproduced many of the augmented responses to O3 typically observed in obese mice (Figure 4): The greater O3-induced AHR, neutrophil recruitment, lung injury, and increases in BAL cytokines and chemokines typically observed in obese versus lean mice (8) were also observed in mice that received colonic contents from obese versus lean mice (Figures 4B–4M). These data further support a role for gut microbiota in obesity-related increases in response to O3. GF mice reconstituted with microbiota from obese versus lean mice or humans also mimic other aspects of the obese condition, including greater weight gain and insulin resistance (12, 31, 32). We did not observe greater weight gain in mice reconstituted with an obese versus lean microbiota (Figure 4A), but there may not have been time for effects of gut microbiota on weight to manifest, because we studied the mice only 2 weeks after reconstitution.
Dietary supplementation with certain prebiotics not only changed the microbiome (Figure 5) but also reduced obesity-related increases in O3-induced AHR and inflammation (Figures 6C and 6D). The amount of fiber in the cellulose- and pectin-enriched diets (30%) was high compared with the amount of fiber in normal diets, but our use of these diets provides proof of concept that prebiotics have the capacity to alter responses to O3 in obese mice. However, whereas feeding with a pectin-enriched diet caused a reduction in O3-induced AHR and inflammation in db/db mice, it had no effect in WT mice (Figures 6C–6F). The lack of effect of a pectin-enriched diet on responses to O3 in the WT mice was not a result of the prebiotic treatment failing to change the microbiota. There were significant differences in the gut microbiomes of the WT mice fed pectin- versus cellulose-enriched diets (Figure 5 and Table E2) that were similar to those reported by others using the same dietary regimen (19). However, the magnitude of this diet-related change in gut microbiota was smaller in the WT than in the db/db mice (Figure 5A). Differences in the impact of dietary fiber in the WT and db/db mice might also reflect obesity-related differences in the response to short-chain fatty acids (SCFAs). Others have reported that compared with diets rich in cellulose, diets rich in pectin increase microbial production of SCFAs (19). We have reported that exogenous administration of SCFAs does not alter responses to O3 in female WT mice (20), but whether SCFAs affect obese mice remains has not been reported.
Studies using antibodies blocking IL-17A or the IL-33 receptor ST2 have demonstrated that both IL-17A and IL-33 contribute to obesity-related increases in O3-induced AHR (8, 22). We did not observe any effect of antibiotics on O3-induced increases in BAL IL-17A or IL-33 in db/db mice (Figures 2G and H), nor were there effects of antibiotics on events downstream of IL-33 or IL-17A (Figures 2I and 2K). Similarly, there were no differences in BAL IL-17A or IL-33 in GF mice reconstituted with microbiota from obese versus lean mice (data not shown), even though BAL concentrations of many other cytokines and chemokines were impacted (Figures 4F–4M). It is possible that the IL-17A and IL-33 important for obesity-related increases in O3-induced AHR are released in some locus other than the lung and that the microbiome affects expression of these cytokines in that locus. For example, gut microbiota have been shown to regulate IL-17A+ immune cells in the small intestines (33). Expression of IL-33 in the intestines is also affected by gut microbiota (34). It is also possible that effects of the microbiome on obesity-related increases in O3-induced AHR are mediated independently of IL-17A and IL-33. However, the observations that changes in gut microbiota induced by a pectin-enriched diet in db/db mice (Figure 5 and Table E2) were associated with corresponding changes in the ability of O3 to augment BAL IL-17A and pulmonary expression of Grpr (Figures 6G and 6I), an IL-17A–dependent process (22), suggest that certain gut microbiota do have the capacity to alter pulmonary expression of IL-17A.
Among the bacterial taxa that differed significantly in Harvard T.H. Chan School of Public Health–bred db/db and WT mice (Figure E1), only one taxon also differed in the colonic contents of The Jackson Laboratory–bred db/db and WT mice that served as donors (Figure E3): The abundance of Turicibacter was lower in db/db than in WT mice, regardless of breeding facility. Notably, the abundance of Turicibacter was also lower in recipients of the colonic contents of db/db than in recipients of the colonic contents of WT mice (Figure E4F). Hence, it is conceivable that metabolic products of these bacteria normally act to counter the development of O3-induced AHR and that loss of these bacteria in obesity promotes augmented O3-induced AHR. Consistent with this hypothesis, we also observed a significantly greater abundance of Turicibacter in pectin-fed than in cellulose-fed db/db mice (Table E2), together with reduced O3-induced AHR (Figure 6C). Turicibacter was also among the bacterial taxa significantly reduced by obesity in a meta-analysis that covered many different studies of obese and lean rodents (35). Others have reported that the abundance of Turicibacter in obese mice is negatively correlated with genes in the NF-κB signaling pathway, indicating antiinflammatory effects of this taxon (36).
There are limitations to this study. First, we used female mice in this study because females dominate the obese asthma population (37) and because the impact of body mass index on O3-induced changes in pulmonary function is greater in women than in men (7). However, sex differences in the gut microbiome have been reported (20, 38). Whether microbiota also contribute to obesity-related increases in O3-induced AHR in males remains to be established. Second, we used obese db/db mice in this study. Augmented responses to O3 are also observed in other types of obese mice (8, 9, 39–41). Obese rodents exhibit alterations in their gut microbiomes regardless of the cause of their obesity (10, 13, 25, 35), but whether microbiota also contribute to the augmented responses to O3 observed in these other types of obese mice remains to be established.
In conclusion, our data indicate a role for the microbiome in obesity-related increases in the response to an asthma trigger, O3. Obesity reduces the effectiveness of established asthma therapeutics (4). Our data demonstrating beneficial effects of the dietary fiber pectin on O3-induced AHR suggest that microbiota-based therapies such as probiotics and prebiotics may provide an alternative therapeutic strategy for obese patients with asthma. Development of these strategies will require greater understanding of the mechanistic basis for the role of microbiota in the effects of obesity on the lung and greater knowledge of the particular bacterial taxa involved.
Supplementary Material
Footnotes
Supported by National Institutes of Health grants ES-013307 (S.A.S.), ES-024032 (S.A.S.), HL007118, ES-000002, and P30-DK034854 and the Massachusetts Life Sciences Center.
Author Contributions: H.T., Y.C., D.I.K., J.D.B., and S.A.S. conceived the project. H.T., Y.C., D.I.K., J.D.B., L.B., C.H., and S.A.S. designed the experiments and interpreted the data. H.T. and S.A.S. prepared the manuscript with input from all other authors. H.T., Y.C., D.I.K., V.Y., and G.A.-A. performed the experiments and analyzed the data.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2019-0144OC on May 30, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007;175:661–666. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schatz M, Hsu JW, Zeiger RS, Chen W, Dorenbaum A, Chipps BE, et al. Phenotypes determined by cluster analysis in severe or difficult-to-treat asthma. J Allergy Clin Immunol. 2014;133:1549–1556. doi: 10.1016/j.jaci.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Dixon AE, Pratley RE, Forgione PM, Kaminsky DA, Whittaker-Leclair LA, Griffes LA, et al. Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation. J Allergy Clin Immunol. 2011;128:508–515, e1-e2. doi: 10.1016/j.jaci.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutherland ER, Goleva E, Strand M, Beuther DA, Leung DY. Body mass and glucocorticoid response in asthma. Am J Respir Crit Care Med. 2008;178:682–687. doi: 10.1164/rccm.200801-076OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foster WM, Brown RH, Macri K, Mitchell CS. Bronchial reactivity of healthy subjects: 18-20 h postexposure to ozone. J Appl Physiol (1985) 2000;89:1804–1810. doi: 10.1152/jappl.2000.89.5.1804. [DOI] [PubMed] [Google Scholar]
- 6.Gent JF, Triche EW, Holford TR, Belanger K, Bracken MB, Beckett WS, et al. Association of low-level ozone and fine particles with respiratory symptoms in children with asthma. JAMA. 2003;290:1859–1867. doi: 10.1001/jama.290.14.1859. [DOI] [PubMed] [Google Scholar]
- 7.Bennett WD, Hazucha MJ, Folinsbee LJ, Bromberg PA, Kissling GE, London SJ. Acute pulmonary function response to ozone in young adults as a function of body mass index. Inhal Toxicol. 2007;19:1147–1154. doi: 10.1080/08958370701665475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathews JA, Krishnamoorthy N, Kasahara DI, Cho Y, Wurmbrand AP, Ribeiro L, et al. IL-33 drives augmented responses to ozone in obese mice. Environ Health Perspect. 2017;125:246–253. doi: 10.1289/EHP272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams AS, Mathews JA, Kasahara DI, Chen L, Wurmbrand AP, Si H, et al. Augmented pulmonary responses to acute ozone exposure in obese mice: roles of TNFR2 and IL-13. Environ Health Perspect. 2013;121:551–557. doi: 10.1289/ehp.1205880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Mazcorro JF, Mills DA, Murphy K, Noratto G. Effect of barley supplementation on the fecal microbiota, caecal biochemistry, and key biomarkers of obesity and inflammation in obese db/db mice. Eur J Nutr. 2018;57:2513–2528. doi: 10.1007/s00394-017-1523-y. [DOI] [PubMed] [Google Scholar]
- 14.Ley RE. Obesity and the human microbiome. Curr Opin Gastroenterol. 2010;26:5–11. doi: 10.1097/MOG.0b013e328333d751. [DOI] [PubMed] [Google Scholar]
- 15.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 16.Dumas ME, Barton RH, Toye A, Cloarec O, Blancher C, Rothwell A, et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci USA. 2006;103:12511–12516. doi: 10.1073/pnas.0601056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zou J, Chassaing B, Singh V, Pellizzon M, Ricci M, Fythe MD, et al. Fiber-mediated nourishment of gut microbiota protects against diet-induced obesity by restoring IL-22-mediated colonic health. Cell Host Microbe. 2018;23:41–53, e4. doi: 10.1016/j.chom.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20:159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 20.Cho Y, Abu-Ali G, Tashiro H, Brown TA, Osgood RS, Kasahara DI, et al. Sex differences in pulmonary responses to ozone in mice: role of the microbiome. Am J Respir Cell Mol Biol. 2019;60:198–208. doi: 10.1165/rcmb.2018-0099OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho Y, Abu-Ali G, Tashiro H, Kasahara DI, Brown TA, Brand JD, et al. The microbiome regulates pulmonary responses to ozone in mice. Am J Respir Cell Mol Biol. 2018;59:346–354. doi: 10.1165/rcmb.2017-0404OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathews JA, Krishnamoorthy N, Kasahara DI, Hutchinson J, Cho Y, Brand JD, et al. Augmented responses to ozone in obese mice require IL-17A and gastrin-releasing peptide. Am J Respir Cell Mol Biol. 2018;58:341–351. doi: 10.1165/rcmb.2017-0071OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drew JE, Reichardt N, Williams LM, Mayer CD, Walker AW, Farquharson AJ, et al. Dietary fibers inhibit obesity in mice, but host responses in the cecum and liver appear unrelated to fiber-specific changes in cecal bacterial taxonomic composition. Sci Rep. 2018;8:15566. doi: 10.1038/s41598-018-34081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyamoto J, Watanabe K, Taira S, Kasubuchi M, Li X, Irie J, et al. Barley β-glucan improves metabolic condition via short-chain fatty acids produced by gut microbial fermentation in high fat diet fed mice. PLoS One. 2018;13:e0196579. doi: 10.1371/journal.pone.0196579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beli E, Yan Y, Moldovan L, Vieira CP, Gao R, Duan Y, et al. Restructuring of the gut microbiome by intermittent fasting prevents retinopathy and prolongs survival in db/db mice. Diabetes. 2018;67:1867–1879. doi: 10.2337/db18-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, Ward DV, et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13:R79. doi: 10.1186/gb-2012-13-9-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ubeda C, Taur Y, Jenq RR, Equinda MJ, Son T, Samstein M, et al. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest. 2010;120:4332–4341. doi: 10.1172/JCI43918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang H, Zhang X, Wang S, Li H, Lu Z, Shi J, et al. Mannan-oligosaccharide modulates the obesity and gut microbiota in high-fat diet-fed mice. Food Funct. 2018;9:3916–3929. doi: 10.1039/c8fo00209f. [DOI] [PubMed] [Google Scholar]
- 29.Tan S, Caparros-Martin JA, Matthews VB, Koch H, O’Gara F, Croft KD, et al. Isoquercetin and inulin synergistically modulate the gut microbiome to prevent development of the metabolic syndrome in mice fed a high fat diet. Sci Rep. 2018;8:10100. doi: 10.1038/s41598-018-28521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barfod KK, Vrankx K, Mirsepasi-Lauridsen HC, Hansen JS, Hougaard KS, Larsen ST, et al. The murine lung microbiome changes during lung inflammation and intranasal vancomycin treatment. Open Microbiol J. 2015;9:167–179. doi: 10.2174/1874285801509010167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foley KP, Zlitni S, Denou E, Duggan BM, Chan RW, Stearns JC, et al. Long term but not short term exposure to obesity related microbiota promotes host insulin resistance. Nat Commun. 2018;9:4681. doi: 10.1038/s41467-018-07146-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Salvo C, Mattioli B, Cominelli F, Pizarro TT. P-153 induction of IL-33 by the gut microbiota in the pathogenesis of intestinal fibrosis in a spontaneous mouse model of IBD. Inflamm Bowel Dis. 2016;22:S56–S57. [Google Scholar]
- 35.Jiao N, Baker SS, Nugent CA, Tsompana M, Cai L, Wang Y, et al. Gut microbiome may contribute to insulin resistance and systemic inflammation in obese rodents: a meta-analysis. Physiol Genomics. 2018;50:244–254. doi: 10.1152/physiolgenomics.00114.2017. [DOI] [PubMed] [Google Scholar]
- 36.Liu W, Crott JW, Lyu L, Pfalzer AC, Li J, Choi SW, et al. Diet- and genetically-induced obesity produces alterations in the microbiome, inflammation and Wnt pathway in the intestine of Apc+/1638N mice: comparisons and contrasts. J Cancer. 2016;7:1780–1790. doi: 10.7150/jca.15792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akinbami LJ, Fryar CD. Current asthma prevalence by weight status among adults: United States, 2001–2014. NCHS Data Brief. 2016;(239):1–8. [PubMed] [Google Scholar]
- 38.Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 39.Williams AS, Mathews JA, Kasahara DI, Wurmbrand AP, Chen L, Shore SA. Innate and ozone-induced airway hyperresponsiveness in obese mice: role of TNF-α. Am J Physiol Lung Cell Mol Physiol. 2015;308:L1168–L1177. doi: 10.1152/ajplung.00393.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnston RA, Theman TA, Lu FL, Terry RD, Williams ES, Shore SA. Diet-induced obesity causes innate airway hyperresponsiveness to methacholine and enhances ozone-induced pulmonary inflammation. J Appl Physiol (1985) 2008;104:1727–1735. doi: 10.1152/japplphysiol.00075.2008. [DOI] [PubMed] [Google Scholar]
- 41.Shore SA, Rivera-Sanchez YM, Schwartzman IN, Johnston RA. Responses to ozone are increased in obese mice. J Appl Physiol (1985) 2003;95:938–945. doi: 10.1152/japplphysiol.00336.2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.