Abstract
Cystic fibrosis is an autosomal-recessive disease that is caused by a mutant CFTR (cystic fibrosis transmembrane conductance regulator) gene and is characterized by chronic bacterial lung infections and inflammation. Complementation with functional CFTR normalizes anion transport across the airway surface. Adeno-associated virus (AAV) is a useful vector for gene therapy because of its low immunogenicity and ability to persist for months to years. However, because its episomal expression may decrease after cell division, readministration of the AAV vector may be required. To overcome this, we designed an integrating AAV-based CFTR-expressing vector, termed piggyBac (PB)/AAV, carrying CFTR flanked by the terminal repeats of the piggyBac transposon. With codelivery of the piggyBac transposase, PB/AAV can integrate into the host genome. Because of the packaging constraints of AAV, careful consideration was required to ensure that the vector would package and express its CFTR cDNA cargo. In this short-term study, PB/AAV-CFTR was aerosolized to the airways of CF pigs in the absence of the transposase. Two weeks later, transepithelial Cl− current was restored in freshly excised tracheal and bronchial tissue. Additionally, we observed an increase in tracheal airway surface liquid pH and bacterial killing in comparison with untreated CF pigs. Airway surface liquid from primary airway cells cultured from treated CF pigs exhibited increased pH correlating with decreased viscosity. Together, these results show that complementing CFTR in CF pigs with PB/AAV rescues the anion transport defect in a large-animal CF model. Delivery of this integrating viral vector system to airway progenitor cells could lead to persistent, life-long expression in vivo.
Keywords: adeno-associated virus, piggyBac transposon, CFTR, cystic fibrosis, gene therapy
Clinical Relevance
Large-animal disease models are critical tools for evaluating advances in viral vector–based delivery tools. Here, we designed a vector with the potential for lifelong expression of a therapeutic transgene and performed a short-term study that resulted in in vivo phenotypic correction of a cystic fibrosis pig. This study has widespread implications for long-term gene therapy–based corrections of monogenetic diseases.
Cystic fibrosis (CF) is an autosomal-recessive genetic disease that affects multiple organ systems; however, the leading cause of morbidity and mortality in patients with CF is chronic lung disease. CF is caused by a mutant CFTR (cystic fibrosis transmembrane conductance regulator) gene that leads to loss of a functional anion channel (1, 2). Without CFTR, viscous mucus accumulates on the airway surface and becomes a milieu for bacterial adherence (3–6). Bacterial infections and mucus accumulation lead to plugged airways and ultimately organ failure (7, 8). There are hundreds of potential disease-causing CFTR mutations (www.genet.sickkids.on.ca). Although small-molecule therapeutics provide relief for many people with CF (9–11), there is a need to treat all CFTR mutations. Ideally, a single-dose reagent would be delivered early in life to prevent the onset and progression of lung disease.
Newborn CF pigs are free from infection and inflammation at birth, but develop lung disease weeks to months after birth (12). In the absence of CFTR, the airway surface liquid pH (pHASL) is relatively acidic, and respiratory secretions exhibit a reduced bacteria-killing ability compared with that observed in non-CF pigs (13). We previously reported that complementing CFTR in CF pig airways using an adeno-associated virus (AAV) vector increased anion channel activity, increased pHASL, and improved bacteria-killing ability (14). In addition, AAV transduced ciliated and nonciliated cells in pig airways, and expressed CFTR at the apical surface (14). Efficient AAV-mediated gene therapy can potentially correct the CF airway defect and prevent lung infections.
AAV for CF gene therapy has been evaluated in five phase I and II CF clinical trials. Aerosolized AAV2 was tested in over 100 trial participants and determined to be safe, but no significant improvements in lung function were reported (15, 16). These studies relied on the weak inverted terminal repeat (ITR) promoter to drive expression from the 4.5-kb CFTR gene. However, efforts to move forward with AAV faced three limitations: a low transduction efficiency at the airway surface, a small packaging capacity, and proteasomal degradation of input virions. Directed-evolution strategies are providing new AAV capsids with enhanced tropism for airway epithelial cells, including the H22 capsid for pig airways and 2.5T for human airways (14, 17). The generation of a shortened CFTR cDNA by removing a portion of the R-domain (CFTRΔR) (18) and the development of a strong synthetic promoter and polyadenylation signal (19) fit within the packaging capacity of AAV. Delivery of AAV in the presence of doxorubicin increases AAV expression through proteasome inhibition (20, 21). Increasing transgene expression from an AAV vector through these modifications could help enable future AAV-based clinical trials.
AAV-mediated transgene expression persists for months in the lung (22). However, preclinical studies with AAV in the rabbit lung suggest that its transgene expression will wane and readministration may ultimately be required (23). If the goal is to express CFTR for the lifetime of an individual with CF after a single dose of a gene therapy reagent, genomic integration is likely required. AAV episomal expression only persists for the life of the cell (24), but an integrating vector transducing a progenitor cell population could lead to continuous repopulation of CFTR+ cells at the airway surface. The nonviral piggyBac DNA transposon is a two-part system composed of TRs flanking a gene of interest and a transposase that catalyzes integration of the gene of interest into the host genome (25). Previously, we reported that piggyBac (PB)/AAV with full-length TRs conferred persistent transgene expression in immunocompetent mouse airways in vivo (26).
In this study, we used a piggyBac with shortened TRs (27) to accommodate the size constraints of AAV and to create an integrating PB/AAV vector system for gene delivery. Between the minimal TRs lies a strong synthetic promoter, shortened CFTRΔR, and synthetic polyA. Using the gut-corrected CF pig model (28), we delivered CFTR using PB/AAV pseudotyped with an H22 capsid and observed a phenotypic correction. Thus, we confirm that the addition of the minimal TRs results in a functional vector for delivery to cells in vitro, as well as a phenotypic correction of a large-animal CF model in vivo.
Methods
Constructs
Single-stranded recombinant AAV (ssAAV) carrying the piggyBac transposon with minimal TRs carrying an F5Tg83 promoter driving puromycin was designed in silico and synthesized by Genscript. Human CFTRΔR cDNA was a kind gift from Lynda Ostedgaard (18) and was cloned into the PB/AAV vector with minimal TRs with the restriction enzymes SpeI and SalI. The adenovirus expressing piggyBac transposase (Adtransposase) was previously described (26, 29). In this study, we used a piggyBac transposase (iPB7) that includes seven mutations to make it hyperactive (30). ssAAV and scAAV vectors with F5Tg83 driving transposase expression were cloned using SpeI and KpnI restriction enzyme sites. AAV vectors were generated using the AAV2 genome and pseudotyped with the AAV5 capsid for in vitro studies and the AAVH22 capsid for in vivo pig studies. Ad and AAV vectors were produced as a fee for service at the University of Iowa Viral Vector Core (https://medicine.uiowa.edu/vectorcore/).
Colony Formation Assay
HeLa cells were transduced in a 24-well plate (5 × 104 cells/well) with PB/ssAAV-F5Tg83-PuroR with or without Ad- or AAV-delivered transposase as indicated. AAV multiplicities of infection (MOIs) ranged from 103 to 105, and AdGFP or Adtransposase was delivered at an MOI of 10. Twenty-four hours after transduction, the cells were trypsinized and expanded into 100-mm plates in puromycin-containing media. The media was changed three times per week. Two weeks after expansion, the cells were fixed in 2% paraformaldehyde and stained with methylene blue, and colonies were counted.
CF Pigs
All animal procedures were reviewed and approved by the University of Iowa Institutional Animal Care and Use Committee in accordance with the U.S. Department of Agriculture and National Institutes of Health guidelines. CF pigs were generated by homologous recombination in fibroblasts as previously described (31). Gut-corrected pigs were created by somatic cell nuclear transfer cloning (28). All pigs were housed at the University of Iowa animal care facility for the duration of the study. The animals were humanely killed by intravenous administration of Euthasol (90 mg/kg).
In Vivo Viral Vector Administration
Newborn (<1 wk old) gut-corrected CF pigs were sedated with inhaled 2% isoflurane for viral delivery while oxygen levels, heart rates, and respiratory rates were monitored. Viral vector was aerosolized into the trachea by passing a Penn Century Microsprayer through a 2.0 endotracheal tube. Approximately 1 × 1012 vector genomes (vg) of PB/AAVCFTRΔR formulated with 200 μM doxorubicin (Sigma Aldrich) were delivered to each pig. Vector was delivered to the nasal turbinates by a bolus dose through a 34G Teflon catheter. Two weeks later, the pigs were analyzed for phenotypic correction.
Ussing Chamber Studies
CFTR anion channel correction was measured by Ussing chamber analysis. Freshly excised tissue or well-differentiated airway epithelial cultures were mounted in Ussing chambers, and apical and basolateral chambers were maintained under a symmetrical Ringer’s solution (135 mM NaCl, 5 mM HEPES, 0.6 mM KH2PO4, 0.4 M K2HPO4, 1.2 mM MgCl2, 1.2 mM CaCl2, 5 mM dextrose). Transepithelial current was measured as previously described (32). Baseline currents were measured and the following drugs were added to inhibit ion channels: amiloride (100 μM; Sigma Aldrich) to inhibit Na+ channels, and 4,4′-dilsothiocya-no-2,2′-stilbenedifulonic acid (Sigma Aldrich) to inhibit Cl− (100 μM). After a low Cl− gradient in the apical chamber was established, the cAMP agonists forskolin (10 μM) (Cayman Chemical) and 3-isobutyl-1-methylxanthine (IBMX, 100 μM; Sigma Aldrich) were added. After the current stabilized, GlyH-101 (GlyH) was added to block CFTR-mediated Cl− current. Transepithelial currents (IT) relative to baseline measurements are reported.
In Vivo Tracheal pHASL and Bacterial Killing
Pigs were anesthetized with ketamine (20 mg/kg) and xylazine (2 mg/kg), and sedation was maintained with propofol (1 mg/kg). After a tracheal window was opened, a pH-sensitive foil was placed on the tracheal surface as previously described (13, 14, 32, 33). Bacteria-killing assays were performed as previously described (13, 14, 32). Briefly, Staphylococcus aureus isolate SA43 was conjugated to electron microscopy grids via biotin and streptavidin interaction and placed on the airway surface of the sedated pigs for 1 minute. Live/dead bacteria were imaged by confocal microscopy after SYTO and propidium iodide staining (Invitrogen). Live/dead bacteria were quantified using ImageJ.
In Vitro pHASL Measurements
The pHASL was assayed using the ratiometric pH-sensitive dye SNARF-1 as previously described (34–36). Briefly, SNARF-1 conjugated to 70 kD dextran (Molecular Probes) was applied to the apical surface of the airway cultures and fluorescence was measured with a Zeiss LSM510 inverted confocal microscope. The SNARF-1 was excited with a 510-nm argon laser and emissions were collected at 565–597 and 619–661 through an acousto-optic tunable filter. Consistent with other studies (37–40), our empirical SNARF-1 pKa of 7.39 was acid-shifted to the documented pKa of ∼7.5 at room temperature. For each cell culture, images were acquired from at least five separate regions. Then each region was averaged for each culture, and each culture was averaged for each pig, which involved three replicates for each animal. Mean pig ratios were converted to pH values in GraphPad Prism by interpolating the calibration curve. Data are represented as mean values among porcine donors.
Viscosity and Dye Immobilization
As previously described (33), FITC-conjugated dextran was applied to the surface of airway cultures. Viscosity was measured by fluorescence recovery after photobleaching, specifically, the duration of time it took for the FITC-dextran to recover in the photobleached regions of the culture. In the instances where full recovery did not occur, the proportion of the net dye loss was presented as a percentage of immobilized dye.
Statistics
All statistically significant differences were calculated using one-way ANOVA in GraphPad Prism (GraphPad Software). Unless otherwise noted, all data are presented as mean ± SE. P < 0.05 was considered statistically significant.
Results
PB/AAV Transposition In Vitro
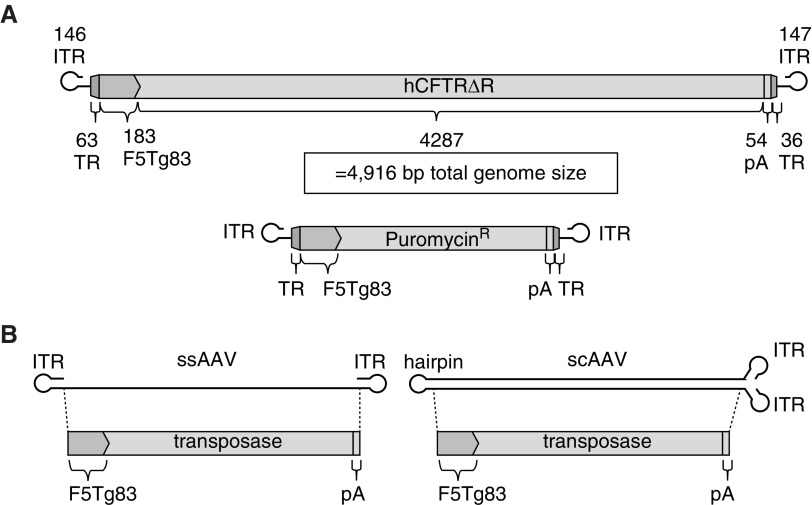
Our goal in these studies was to design an AAV vector expressing human CFTR that is flanked by the piggyBac TRs to allow for transposase-dependent genomic integration. We previously reported that AAV successfully delivered a piggyBac transposon expressing a reporter gene and integrated into the host cell genome in the presence of transposase (26). In those studies, the piggyBac transposon contained full-length TRs (5′ 308 bp and 3′ 237 bp = 545 bp total) and the cytomegalovirus promoter (520 bp). The packaging capacity of recombinant AAV2, including the prerequisite ITRs, is ∼4.9 kb (41). The full-length piggyBac TRs, cytomegalovirus promoter, and CFTR cDNA far exceed the AAV packaging capacity. To address the size constraints, we engineered an AAV2-based packaging construct with minimal piggyBac TRs (99 bp total) (27), a minimal F5Tg83 promoter (183 bp) (19), and a short synthetic polyadenylation signal (54 bp) (19) (Figure 1A). The vectors described in this study use the same promoter and polyA signal as previously described (14).
Figure 1.
Schematics of hybrid vectors. (A) A PB/adeno-associated virus (AAV) transposon was delivered by a single-stranded AAV (ssAAV). The transposon terminal repeats (TRs) flank the F5Tg83 promoter, driving expression of human CFTRΔR or puromycin resistance, followed by a shortened synthetic polyadenylation signal (pA) (19). (B) The piggyBac transposase driven by the F5Tg83 promoter followed by the short polyA is represented schematically in either the ssAAV or self-complementary AAV (scAAV) vector. bp = base pairs; hCFTR = human cystic fibrosis transmembrance conductance regulator; ITR = inverted terminal repeat; PB = piggyBac.
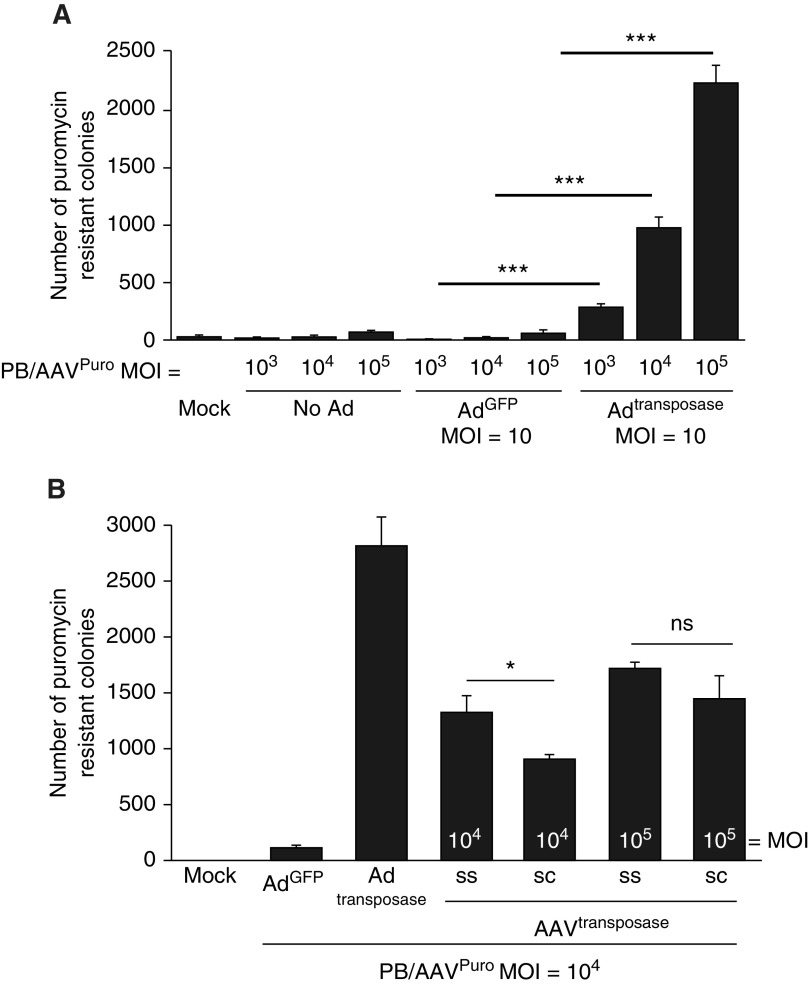
Using this packaging construct to express puromycin resistance (Figure 1A, bottom), we generated AAV with the H22 capsid, termed PB/AAVpuro. We first asked whether the minimal piggyBac TRs would confer functional transposition from the AAV genome into the cellular genome. We performed a colony formation assay as an indirect measure of transposition activity as previously described (26). Briefly, HeLa cells were cotransduced with PB/AAVpuro and adenovirus (Ad) expressing GFP (AdGFP) or Adtransposase. Transduced cells were maintained in puromycin-containing media for 2 weeks. The cells were then fixed, stained with methylene blue, and counted. We observed colony formation in a dose-dependent manner only in the presence of the transposase (Figure 2A). These results confirm that minimal TRs and a short synthetic promoter will confer persistent drug resistance in a transposase-dependent manner. Without the transposase, the number of puromycin-resistant colonies ranged from 10 to 66, which is likely the result of low-level random integration of AAV genomes (42, 43).
Figure 2.
PB/AAV confers puromycin-resistant colonies only in the presence of the transposase. (A) HeLa cells were left untransduced or transduced with PB/AAVpuro at a multiplicity of infection (MOI) of 103, 104, or 105 in the absence of adenovirus (Ad), with AdGFP (MOI = 10), or with Adtransposase (MOI = 10) for 4 hours. Twenty-four hours after transduction, cells were expanded from a 24-well plate into 100-mm tissue culture dishes and grown in puromycin-containing media for 2 weeks. HeLa cells were then fixed and stained with methylene blue, and colonies were counted. (B) ssAAVtransposase and scAAVtransposase were compared for their ability to confer puromycin-resistant colonies. HeLa cells were transduced as in A with the indicated viral vectors. *P < 0.05 and ***P < 0.005; n = 4. ns = not significant.
Next, we compared the transposition activity of AAVtransposase with that of Adtransposase (26). We evaluated both a self-complementary AAV (scAAV) and a single-stranded AAV (ssAAV) for transposase delivery (Figure 1B). We hypothesized that scAAV would increase transposition activity over ssAAV because scAAV bypasses second-strand synthesis and expresses earlier than a transgene delivered by ssAAV. Unexpectedly, the transposase delivered by ssAAV led to equal or greater transposition activity as compared with scAAV (Figure 2B). We conclude that the ssAAVtransposase confers dose-dependent transposase-mediated integration of the PB/AAVpuro transposon into the genome.
Phenotypic Correction in CF Pigs by PB/AAVCFTRΔR
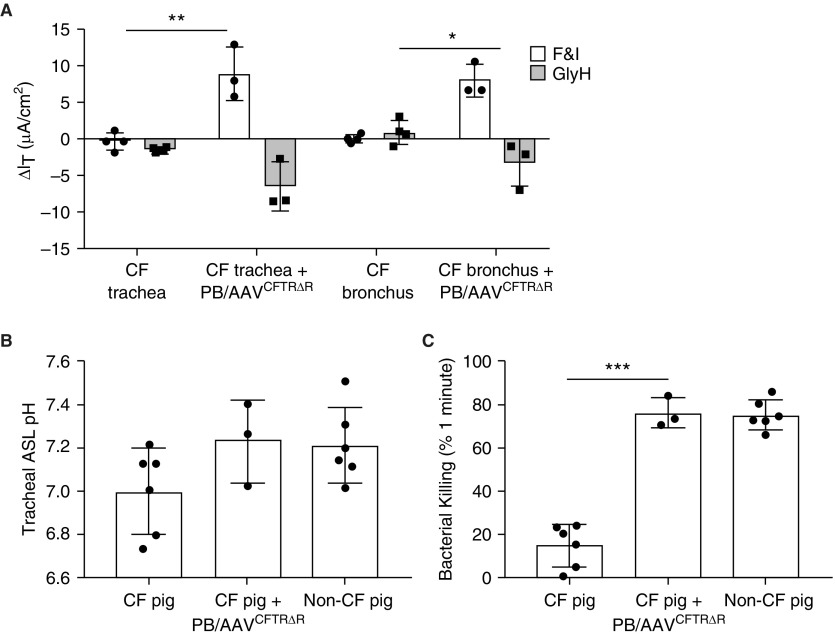
As shown in Figure 1A (top), we generated an integrating CFTR expression cassette to fit within the AAV packaging constraints. The minimal TRs of the piggyBac transposon flank an F5Tg83 promoter (19) driving CFTRΔR followed by a short polyA. In a short-term proof-of-principle study to validate the PB/AAV transposon in vivo, we aerosolized 1 × 1012 vg of PB/AAVCFTRΔR (without transposase) formulated with 250 μM doxorubicin into the trachea and lungs of newborn gut-corrected CF pigs. In addition, a bolus dose was delivered to the nasal epithelium of the same animals. Two weeks after delivery, we used a variety of metrics to measure the effects of CFTR complementation. Freshly excised tracheal and bronchial tissues were mounted in Ussing chambers to assess their bioelectric properties. The CF pigs that received PB/AAVCFTRΔR showed significantly greater changes in transepithelial current than the untreated CF pigs in response to the cAMP agonists forskolin and IBMX and the CFTR inhibitor GlyH (Figure 3A). We confirmed this in tracheal and bronchial tissue to ensure that various regions of the lung were transduced. To determine whether complementing CFTR in airway cells modified the tracheal pHASL, we used a pH optode to measure the pHASL of the tracheal surface. Tracheal pHASL levels in CF pigs treated with PB/AAVCFTRΔR were similar to those observed in non-CF pigs (Figure 3B). Furthermore, we observed a bacteria-killing ability in treated animals that mirrored non-CF levels (Figure 3C). Using a previously described protocol (44), real-time qRT-PCR was used to amplify CFTR from bronchial tissue from both transduced pigs and naive controls. CFTR RNA levels in transduced animals were 2,880-fold higher than untransduced background levels. Based on the multiple metrics, we conclude that complementing CFTR by a PB/AAV vector corrects phenotypic defects in CF pigs.
Figure 3.
PB/AAVCFTRΔR corrects the anion channel defect in vivo. Three newborn cystic fibrosis (CF) pigs received ∼1 × 1012 vg of H22 pseudotyped PB/AAVCFTRΔR via intratracheal aerosolization and nasal instillation. The AAV transposase was not included in this experiment. Two weeks after delivery, the pigs were assayed for phenotypic correction. (A) Freshly excised tracheal and bronchial tissues from untreated CF pigs or CF pigs treated with PB/AAVCFTRΔR were mounted in Ussing chambers to measure their bioelectric properties. Changes in transepithelial current were measured in response to forskolin and 3-isobutyl-1-methylxanthine (F&I) and GlyH-101 (GlyH). (B) Tracheal airway surface liquid (ASL) pH was obtained after the animal was sedated and a tracheal window was opened. A pH-sensitive foil was placed on the tracheal surface and pH was measured with a pH optode. (C) Bacterial killing was determined by placing a Staphylococcus aureus–coated electron microscopy grid on the tracheal surface for 1 minute. The grids were stained with a live/dead stain, imaged by confocal microscopy, and quantified using ImageJ. *P < 0.05, **P < 0.005, and ***P < 0.005; n = 3.
pHASL and Viscosity in Primary Cells Cultured from Treated CF Pigs
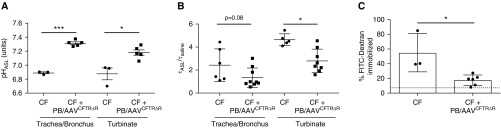
We next examined pHASL and viscosity in primary cultures of airway cells derived from untreated or PB/AAVCFTRΔR-treated CF pigs. These cells were cultured at an air–liquid interface and tested after differentiation (≥2 wk). Consistent with the in vivo measurements, pHASL in the cultured airway cells from treated pigs was increased compared with that in untreated controls (Figure 4A). In addition to decreased pHASL, previous studies demonstrated that another consequence of reduced CFTR-dependent anion transport in CF is viscous mucus (45). We next assayed the ASL viscosity in the cultured airway cells from treated pigs by applying FITC-conjugated dextran to the apical surface of primary cultures, and quantified the fluorescence recovery after photobleaching (45). Here, we observed a reduced mucus viscosity in epithelia cultured from CF pigs that received PB/AAVCFTRΔR relative to cultures from untreated CF pigs (Figure 4B). In some cases, the fluorescent dye did not fully recover after photobleaching of CF cultures. As an additional measure of mucus viscosity, we quantified immobilization of the fluorescent dye FITC-dextran. After photobleaching, the fluorescent dye diffusion was measured over time and FITC expression after photobleaching stabilized within 30 minutes. In CF cultures, the viscous mucus was partially immobilized after it was photobleached, whereas CF+AAV cultures of trachea/bronchus and turbinate recovered to near saline control levels (Figure 4C). Together, these data indicate that the PB/AAVCFTRΔR vector can rescue several CF phenotypic defects of epithelial cells cultured from trachea/bronchus and turbinates, including 1) Cl− current activity, 2) pHASL, and 3) ASL viscosity.
Figure 4.
Primary airway epithelial cells cultured from PB/AAVCFTRΔR-treated pigs exhibit increased ASL pH and decreased viscosity compared with those cultured from untreated CF pigs. Trachea/bronchus and turbinate primary airway epithelial cells were cultured from untreated CF pigs or CF pigs treated with PB/AAVCFTRΔR. (A) Airway epithelial cultures were treated with SNARF-1 and ASL pH measurements were obtained by confocal microscopy. (B) Viscosity was measured by coating primary cells with FITC-conjugated dextran and measuring the tau of ASL and saline by fluorescence recovery after photobleaching. (C) The recovery of FITC-dextran fluorescent dye after photobleaching was quantified. Increased mucus viscosity in CF cultures prevents dye from refilling the photobleached area, and therefore the dye is immobilized in the mucus. The dashed line indicates saline background. *P < 0.05 and ***P < 0.005; n = 3.
Discussion
A major limitation for using AAV as a CF gene therapy vector is its small carrying capacity. To overcome this limitation, we carefully considered the necessity of each nucleotide and designed an integrating PB/AAV vector carrying minimal TRs of piggyBac, a shortened CFTR, and a short promoter and polyadenylation signal. To validate the minimal piggyBac TRs in the context of a viral vector, we quantified transposition in vitro. In the absence of the transposase, PB/AAV behaved like a standard AAV vector; however, delivery of the transposase by a separate AAV vector led to transposase-mediated integration in a dose-dependent manner.
Using gut-corrected CF pigs, we aerosolized AAVH22 expressing CFTRΔR and rescued several phenotypic defects, similar to what was done in our previous studies (14, 19). However, the transposase was not included in this proof-of-principle experiment because the goal was to validate CFTR expression from the novel vector design, not to achieve genomic integration. We observed phenotypic correction by measuring changes in the transepithelial current, pHASL, and bacteria-killing ability. In addition, we assayed for pHASL and viscosity changes in primary airway epithelia harvested from CF pigs after PB/AAVCFTRΔR delivery. In this report, we 1) validate that minimal PB TRs functionally confer integration from a nonintegrating viral vector in vitro, and 2) demonstrate that PB/AAVCFTRΔR can correct the phenotypic defect of a CF pig model in vivo.
In our previously described persistence study in mice, PB/AAV-expressing luciferase lasted for 6 months (the duration of the experiment) in the presence of the transposase (26). Polidocanol treatment was used to accelerate cell turnover at a 3-month time point by denuding surface epithelial cells. Luciferase expression after the polidocanol treatment drastically declined in the absence of the transposase, but stabilized in the mice that received the transposase (26). These data suggest that a population of progenitor cells were transduced in vivo. These previous studies were performed in Balb/c immunocompetent mice. To our knowledge, no studies to date have specifically described the immunogenicity of the piggyBac transposase. Like any foreign protein, there is a potential for an immune response. However, considering that the piggyBac transposase is naturally endemic in moths, humans are unlikely to have preexisting immunity. Optimally, a gene therapy reagent would be delivered as a single dose, avoiding the need for repeated administration.
For our in vitro studies, we commonly use a standard MOI of 10,000. This requires a volume of 0.5 μl per well of a 24-well plate (1 × 1012 vg/ml transducing 50,000 cells/well). In our pig experiments in vivo, we delivered 1 ml of vector with a titer of 1 × 1012 vg/ml. The total number of airway epithelial cells in an 88-kg human adult has been estimated to be 1.05 × 1010 with 18× more alveolar cells than bronchial epithelial cells (46). If we extrapolate 2.4 × 108 total airway epithelial cells for a 2-kg pig, we are delivering an MOI of 4,166 in vivo. However, this MOI assumes a homogeneous delivery pattern. Based in part on the 15-μm particle size delivered by the microsprayer, we suspect that most vector deposition occurs in the conducting airways. Deposition will gradually decrease as the alveoli are reached. Based on our estimates, we suspect that the achievable MOI in vivo is comparable to that obtained in our in vitro studies; however, local MOIs will vary greatly.
AAV is a promising gene therapy vector for multiple diseases, most notably neuromuscular and retinal diseases. For example, AAV vectors have been assessed in neuromuscular clinical trials involving spinal muscular atrophy type 1 (SMN); intramuscular delivery of limb-girdle muscular dystrophy, type 2D (LGMD2D) (47); and Becker muscular dystrophy (BMD) (48, 49). Encouragingly, LGMD2D and BMD delivered by AAV demonstrated persistent correction of muscle function for at least 6 months in trial participants. The U.S. Food and Drug Administration recently approved a gene therapy treatment for Leber congenital amaurosis 2 (LCA2) that uses AAV to deliver RPE65, with remarkable improvements in eyesight. MERTK (Mer proto-oncogene tyrosine kinase) and REP1 (Rab-escort protein 1) are also in clinical trials for AAV-mediated gene replacement. These studies reported a safe vector delivery and gene correction, which led to improved vision in the participants (reviewed in Reference 50). AAV is a good choice of vector for these models because muscle cells have low turnover (51) and retinal cells are relatively immunoprivileged (52) and highly permissive to AAV (53, 54). It may be many years before the duration of expression from these studies is known.
We evaluated transposase delivery using Ad, ssAAV, and scAAV vectors. Although viral vectors are an efficient way to deliver the transposase, other delivery options may be considered. The transposase is required for the initial transposition into the host genome, but transient transposase expression may be preferred. Transposase delivery by mRNA is a promising way to improve transposition efficiency and the quality of integration (55, 56). Another way to deliver the transposase is to incorporate the transposase protein into integrase-deficient lentiviral particles (57). Short-term transposase expression could prevent the undesired effects of persistent transposase expression. Overall, there are several options for transient delivery of the transposase that confer persistent expression from an integrated transposon.
We provide evidence for the retention of dose-dependent transposition activity using minimal transposon TRs. We also show functional correction of CF defects in CF pigs after aerosolized delivery of a shortened CFTR by a PB/AAV vector as measured by the Cl− current, tracheal pHASL, and bacterial killing. Future studies will compare the persistence of PB/AAV in the presence and absence of the transposase. We speculate that after airway epithelial cell turnover, a population of cells containing the integrated transposon will repopulate the airway epithelium and persistently express the gene of interest. The PB/AAV transposon system is a promising vector system to efficiently and persistently complement CFTR defects in airway cells.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Linda Powers, Peter Taft, Nick Gansemer, and Mallory Stroik for their technical assistance in these studies. They also thank Xiao Xiao Tang for her technical expertise in the viscosity studies, and the members of their lab who volunteered their time to feed and care for the animals during the duration of the experiments. They thank the Iowa Office of Animal Resources and the animal caretakers. They also thank Phil Karp and the In Vitro Models and Cell Culture Core for providing the primary cell cultures, and the Viral Vector Core for vector production.
Footnotes
Supported by the National Institutes of Health (NIH) (P01 HL-51670, P01 HL-091842, R01 HL-133089, and R01 HL-105821), the Center for Gene Therapy (NIH P30 DK 054759), and the Cystic Fibrosis Foundation (SINN15XX0).
Author Contributions: A.L.C. designed and performed experiments, analyzed data, and prepared the manuscript. I.M.T., B.K.S., and V.S.S. performed experiments and analyzed data. D.A.S. and P.B.M. prepared the manuscript. J.Z. designed experiments. P.L.S. designed experiments, analyzed data, and prepared the manuscript.
Originally Published in Press as DOI: 10.1165/rcmb.2019-0006OC on June 11, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 2.Anderson MP, Gregory RJ, Thompson S, Souza DW, Paul S, Mulligan RC, et al. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 1991;253:202–205. doi: 10.1126/science.1712984. [DOI] [PubMed] [Google Scholar]
- 3.Carnoy C, Scharfman A, Van Brussel E, Lamblin G, Ramphal R, Roussel P. Pseudomonas aeruginosa outer membrane adhesins for human respiratory mucus glycoproteins. Infect Immun. 1994;62:1896–1900. doi: 10.1128/iai.62.5.1896-1900.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J, Lory S, Ramphal R, Jin S. Isolation and characterization of Pseudomonas aeruginosa genes inducible by respiratory mucus derived from cystic fibrosis patients. Mol Microbiol. 1996;22:1005–1012. doi: 10.1046/j.1365-2958.1996.01533.x. [DOI] [PubMed] [Google Scholar]
- 5.Ramphal R. The role of bacterial adhesion in cystic fibrosis including the staphylococcal aspect. Infection. 1990;18:61–64. doi: 10.1007/BF01644188. [DOI] [PubMed] [Google Scholar]
- 6.Girod S, Zahm JM, Plotkowski C, Beck G, Puchelle E. Role of the physiochemical properties of mucus in the protection of the respiratory epithelium. Eur Respir J. 1992;5:477–487. [PubMed] [Google Scholar]
- 7.Martin DW, Schurr MJ, Mudd MH, Govan JR, Holloway BW, Deretic V. Mechanism of conversion to mucoidy in Pseudomonas aeruginosa infecting cystic fibrosis patients. Proc Natl Acad Sci USA. 1993;90:8377–8381. doi: 10.1073/pnas.90.18.8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathee K, Ciofu O, Sternberg C, Lindum PW, Campbell JI, Jensen P, et al. Mucoid conversion of Pseudomonas aeruginosa by hydrogen peroxide: a mechanism for virulence activation in the cystic fibrosis lung. Microbiology. 1999;145:1349–1357. doi: 10.1099/13500872-145-6-1349. [DOI] [PubMed] [Google Scholar]
- 9.Van Goor F, Hadida S, Grootenhuis PD, Burton B, Cao D, Neuberger T, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci USA. 2009;106:18825–18830. doi: 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu H, Burton B, Huang CJ, Worley J, Cao D, Johnson JP, Jr, et al. Ivacaftor potentiation of multiple CFTR channels with gating mutations. J Cyst Fibros. 2012;11:237–245. doi: 10.1016/j.jcf.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Accurso FJ, Rowe SM, Clancy JP, Boyle MP, Dunitz JM, Durie PR, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med. 2010;363:1991–2003. doi: 10.1056/NEJMoa0909825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welsh MJ, Rogers CS, Stoltz DA, Meyerholz DK, Prather RS. Development of a porcine model of cystic fibrosis. Trans Am Clin Climatol Assoc. 2009;120:149–162. [PMC free article] [PubMed] [Google Scholar]
- 13.Pezzulo AA, Tang XX, Hoegger MJ, Abou Alaiwa MH, Ramachandran S, Moninger TO, et al. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature. 2012;487:109–113. doi: 10.1038/nature11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steines B, Dickey DD, Bergen J, Excoffon KJ, Weinstein JR, Li X, et al. CFTR gene transfer with AAV improves early cystic fibrosis pig phenotypes. JCI Insight. 2016;1:e88728. doi: 10.1172/jci.insight.88728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moss RB, Rodman D, Spencer LT, Aitken ML, Zeitlin PL, Waltz D, et al. Repeated adeno-associated virus serotype 2 aerosol-mediated cystic fibrosis transmembrane regulator gene transfer to the lungs of patients with cystic fibrosis: a multicenter, double-blind, placebo-controlled trial. Chest. 2004;125:509–521. doi: 10.1378/chest.125.2.509. [DOI] [PubMed] [Google Scholar]
- 16.Moss RB, Milla C, Colombo J, Accurso F, Zeitlin PL, Clancy JP, et al. Repeated aerosolized AAV-CFTR for treatment of cystic fibrosis: a randomized placebo-controlled phase 2B trial. Hum Gene Ther. 2007;18:726–732. doi: 10.1089/hum.2007.022. [DOI] [PubMed] [Google Scholar]
- 17.Excoffon KJ, Koerber JT, Dickey DD, Murtha M, Keshavjee S, Kaspar BK, et al. Directed evolution of adeno-associated virus to an infectious respiratory virus. Proc Natl Acad Sci USA. 2009;106:3865–3870. doi: 10.1073/pnas.0813365106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ostedgaard LS, Zabner J, Vermeer DW, Rokhlina T, Karp PH, Stecenko AA, et al. CFTR with a partially deleted R domain corrects the cystic fibrosis chloride transport defect in human airway epithelia in vitro and in mouse nasal mucosa in vivo. Proc Natl Acad Sci USA. 2002;99:3093–3098. doi: 10.1073/pnas.261714599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan Z, Sun X, Feng Z, Li G, Fisher JT, Stewart ZA, et al. Optimization of recombinant adeno-associated virus-mediated expression for large transgenes, using a synthetic promoter and tandem array enhancers. Hum Gene Ther. 2015;26:334–346. doi: 10.1089/hum.2015.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan Z, Zak R, Zhang Y, Ding W, Godwin S, Munson K, et al. Distinct classes of proteasome-modulating agents cooperatively augment recombinant adeno-associated virus type 2 and type 5-mediated transduction from the apical surfaces of human airway epithelia. J Virol. 2004;78:2863–2874. doi: 10.1128/JVI.78.6.2863-2874.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grieger JC, Samulski RJ. Packaging capacity of adeno-associated virus serotypes: impact of larger genomes on infectivity and postentry steps. J Virol. 2005;79:9933–9944. doi: 10.1128/JVI.79.15.9933-9944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Afione SA, Conrad CK, Kearns WG, Chunduru S, Adams R, Reynolds TC, et al. In vivo model of adeno-associated virus vector persistence and rescue. J Virol. 1996;70:3235–3241. doi: 10.1128/jvi.70.5.3235-3241.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halbert CL, Standaert TA, Wilson CB, Miller AD. Successful readministration of adeno-associated virus vectors to the mouse lung requires transient immunosuppression during the initial exposure. J Virol. 1998;72:9795–9805. doi: 10.1128/jvi.72.12.9795-9805.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kearns WG, Afione SA, Fulmer SB, Pang MC, Erikson D, Egan M, et al. Recombinant adeno-associated virus (AAV-CFTR) vectors do not integrate in a site-specific fashion in an immortalized epithelial cell line. Gene Ther. 1996;3:748–755. [PubMed] [Google Scholar]
- 25.Wilson MH, Coates CJ, George AL., Jr PiggyBac transposon-mediated gene transfer in human cells. Mol Ther. 2007;15:139–145. doi: 10.1038/sj.mt.6300028. [DOI] [PubMed] [Google Scholar]
- 26.Cooney AL, Singh BK, Sinn PL. Hybrid nonviral/viral vector systems for improved piggyBac DNA transposon in vivo delivery. Mol Ther. 2015;23:667–674. doi: 10.1038/mt.2014.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solodushko V, Bitko V, Fouty B. Minimal piggyBac vectors for chromatin integration. Gene Ther. 2014;21:1–9. doi: 10.1038/gt.2013.52. [DOI] [PubMed] [Google Scholar]
- 28.Stoltz DA, Rokhlina T, Ernst SE, Pezzulo AA, Ostedgaard LS, Karp PH, et al. Intestinal CFTR expression alleviates meconium ileus in cystic fibrosis pigs. J Clin Invest. 2013;123:2685–2693. doi: 10.1172/JCI68867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooney AL. 2018. Integrating viral vectors as a gene therapy approach for cystic fibrosis. PhD dissertation, University of Iowa, Iowa City, Iowa. [Google Scholar]
- 30.Yusa K, Zhou L, Li MA, Bradley A, Craig NL. A hyperactive piggyBac transposase for mammalian applications. Proc Natl Acad Sci USA. 2011;108:1531–1536. doi: 10.1073/pnas.1008322108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, Taft PJ, et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321:1837–1841. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen JH, Stoltz DA, Karp PH, Ernst SE, Pezzulo AA, Moninger TO, et al. Loss of anion transport without increased sodium absorption characterizes newborn porcine cystic fibrosis airway epithelia. Cell. 2010;143:911–923. doi: 10.1016/j.cell.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blossfeld S, Gansert D. A novel non-invasive optical method for quantitative visualization of pH dynamics in the rhizosphere of plants. Plant Cell Environ. 2007;30:176–186. doi: 10.1111/j.1365-3040.2006.01616.x. [DOI] [PubMed] [Google Scholar]
- 34.Shah VS, Meyerholz DK, Tang XX, Reznikov L, Abou Alaiwa M, Ernst SE, et al. Airway acidification initiates host defense abnormalities in cystic fibrosis mice. Science. 2016;351:503–507. doi: 10.1126/science.aad5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shah VS, Ernst S, Tang XX, Karp PH, Parker CP, Ostedgaard LS, et al. Relationships among CFTR expression, HCO3− secretion, and host defense may inform gene- and cell-based cystic fibrosis therapies. Proc Natl Acad Sci USA. 2016;113:5382–5387. doi: 10.1073/pnas.1604905113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thornell IM, Li X, Tang XX, Brommel CM, Karp PH, Welsh MJ, et al. Nominal carbonic anhydrase activity minimizes airway-surface liquid pH changes during breathing. Physiol Rep. 2018;6:e13569. doi: 10.14814/phy2.13569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buckler KJ, Vaughan-Jones RD. Application of a new pH-sensitive fluoroprobe (carboxy-SNARF-1) for intracellular pH measurement in small, isolated cells. Pflugers Arch. 1990;417:234–239. doi: 10.1007/BF00370705. [DOI] [PubMed] [Google Scholar]
- 38.Blank PS, Silverman HS, Chung OY, Hogue BA, Stern MD, Hansford RG, et al. Cytosolic pH measurements in single cardiac myocytes using carboxy-seminaphthorhodafluor-1. Am J Physiol. 1992;263:H276–H284. doi: 10.1152/ajpheart.1992.263.1.H276. [DOI] [PubMed] [Google Scholar]
- 39.Westerblad H, Bruton JD, Lännergren J. The effect of intracellular pH on contractile function of intact, single fibres of mouse muscle declines with increasing temperature. J Physiol. 1997;500:193–204. doi: 10.1113/jphysiol.1997.sp022009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ch’en FF, Dilworth E, Swietach P, Goddard RS, Vaughan-Jones RD. Temperature dependence of Na+-H+ exchange, Na+-HCO3- co-transport, intracellular buffering and intracellular pH in guinea-pig ventricular myocytes. J Physiol. 2003;552:715–726. doi: 10.1113/jphysiol.2003.051888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dong JY, Fan PD, Frizzell RA. Quantitative analysis of the packaging capacity of recombinant adeno-associated virus. Hum Gene Ther. 1996;7:2101–2112. doi: 10.1089/hum.1996.7.17-2101. [DOI] [PubMed] [Google Scholar]
- 42.Nakai H, Montini E, Fuess S, Storm TA, Grompe M, Kay MA. AAV serotype 2 vectors preferentially integrate into active genes in mice. Nat Genet. 2003;34:297–302. doi: 10.1038/ng1179. [DOI] [PubMed] [Google Scholar]
- 43.Schnepp BC, Clark KR, Klemanski DL, Pacak CA, Johnson PR. Genetic fate of recombinant adeno-associated virus vector genomes in muscle. J Virol. 2003;77:3495–3504. doi: 10.1128/JVI.77.6.3495-3504.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cooney AL, Abou Alaiwa MH, Shah VS, Bouzek DC, Stroik MR, Powers LS, et al. Lentiviral-mediated phenotypic correction of cystic fibrosis pigs. JCI Insight. 2016;1:88730. doi: 10.1172/jci.insight.88730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang XX, Ostedgaard LS, Hoegger MJ, Moninger TO, Karp PH, McMenimen JD, et al. Acidic pH increases airway surface liquid viscosity in cystic fibrosis. J Clin Invest. 2016;126:879–891. doi: 10.1172/JCI83922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mercer RR, Russell ML, Roggli VL, Crapo JD. Cell number and distribution in human and rat airways. Am J Respir Cell Mol Biol. 1994;10:613–624. doi: 10.1165/ajrcmb.10.6.8003339. [DOI] [PubMed] [Google Scholar]
- 47.Mendell JR, Rodino-Klapac LR, Rosales XQ, Coley BD, Galloway G, Lewis S, et al. Sustained alpha-sarcoglycan gene expression after gene transfer in limb-girdle muscular dystrophy, type 2D. Ann Neurol. 2010;68:629–638. doi: 10.1002/ana.22251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mendell JR, Sahenk Z, Malik V, Gomez AM, Flanigan KM, Lowes LP, et al. A phase 1/2a follistatin gene therapy trial for Becker muscular dystrophy. Mol Ther. 2015;23:192–201. doi: 10.1038/mt.2014.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al-Zaidy SA, Sahenk Z, Rodino-Klapac LR, Kaspar B, Mendell JR. Follistatin gene therapy improves ambulation in Becker muscular dystrophy. J Neuromuscul Dis. 2015;2:185–192. doi: 10.3233/JND-150083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petit L, Khanna H, Punzo C. Advances in gene therapy for diseases of the eye. Hum Gene Ther. 2016;27:563–579. doi: 10.1089/hum.2016.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rose AJ, Richter EA. Regulatory mechanisms of skeletal muscle protein turnover during exercise. J Appl Physiol (1985) 2009;106:1702–1711. doi: 10.1152/japplphysiol.91375.2008. [DOI] [PubMed] [Google Scholar]
- 52.Benhar I, London A, Schwartz M. The privileged immunity of immune privileged organs: the case of the eye. Front Immunol. 2012;3:296. doi: 10.3389/fimmu.2012.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Riaz M, Raz Y, Moloney EB, van Putten M, Krom YD, van der Maarel SM, et al. Differential myofiber-type transduction preference of adeno-associated virus serotypes 6 and 9. Skelet Muscle. 2015;5:37. doi: 10.1186/s13395-015-0064-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lei B, Zhang K, Yue Y, Ghosh A, Duan D. Adeno-associated virus serotype-9 efficiently transduces the retinal outer plexiform layer. Mol Vis. 2009;15:1374–1382. [PMC free article] [PubMed] [Google Scholar]
- 55.Bire S, Gosset D, Jégot G, Midoux P, Pichon C, Rouleux-Bonnin F. Exogenous mRNA delivery and bioavailability in gene transfer mediated by piggyBac transposition. BMC Biotechnol. 2013;13:75. doi: 10.1186/1472-6750-13-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bire S, Ley D, Casteret S, Mermod N, Bigot Y, Rouleux-Bonnin F. Optimization of the piggyBac transposon using mRNA and insulators: toward a more reliable gene delivery system. PLoS One. 2013;8:e82559. doi: 10.1371/journal.pone.0082559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cai Y, Bak RO, Krogh LB, Staunstrup NH, Moldt B, Corydon TJ, et al. DNA transposition by protein transduction of the piggyBac transposase from lentiviral Gag precursors. Nucleic Acids Res. 2014;42:e28. doi: 10.1093/nar/gkt1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.