Abstract
Purpose
To evaluate risk factors for severity of cytomegalovirus (CMV) retinitis lesion whitening (opacity), using a standardized scoring system.
Methods
We performed a cross-sectional, observational investigation of all individuals with newly diagnosed AIDS-related CMV retinitis in three randomized clinical trials and one prospective observational study. Opacity was scored by masked readers, using a prospectively defined ordinal 6-point scale. Demographic factors, laboratory data (CD4+, CD8+ T-lymphocyte counts, human immunodeficiency virus [HIV] blood levels), and lesion characteristics (location, size) were compared to the highest opacity score assigned to either eye. Among eyes with active lesions (scores ≥3), factors associated with severe opacity (scores 5, 6) were identified.
Results
There were 299 participants (401 eyes with CMV retinitis). In one or more comparisons, increased opacity was associated with lower CD4+ and lower CD8+ T-lymphocyte counts, higher HIV blood level, lack of antiretroviral therapy, male sex, race/ethnicity, and bilateral disease. In eyes with active disease, severe opacity was associated with lower CD4+ T-lymphocyte count, higher HIV blood level, older age, Karnofsky score, lesion size, and bilateral disease. No relationship was identified between opacity and lesion location.
Conclusions
Lesion border opacity (resulting from CMV activity) reflects level of immune function; as immunodeficiency becomes worse, CMV activity (and opacity) increases. The positive relationship between opacity and HIV blood level may reflect both immunodeficiency and increased CMV activity caused by transactivation of CMV by HIV. Scoring of opacity may be a useful, standard measure for continued study of CMV retinitis across different settings and populations. (Clinicaltrials.gov number for the HPMPC CMV Retinitis Trial: NCT00000142; Clinicaltrials.gov number for the Monoclonal Antibody CMV Retinitis Trial: NCT00000135; Clinicaltrials.gov number for the Ganciclovir-Cidofovir CMV Retinitis Trial: NCT0000014; Clinicaltrials.gov number for the Longitudinal Study of the Ocular Complications of AIDS: NCT00000168.)
Keywords: AIDS, cytomegalovirus, retinitis, immunodeficiency, opacity
The incidence of AIDS-related cytomegalovirus (CMV) retinitis in the United States has fallen substantially since the introduction of combination antiretroviral therapy (cART),1–4 but it remains a major public health problem in the developing world.5 A hallmark of CMV retinitis is opacification (whiteness) of lesion borders, the severity of which can vary substantially between individuals, for reasons that are not well understood.6–8 Traditionally, lesions have been described as being “fulminant/edematous” or “indolent/granular” types.7,9 Lesion type has prognostic relevance, predicting rates of lesion enlargement and vision outcomes.9–11 A study from Thailand suggested that CMV retinitis lesions in the developing world are more likely to have a fulminant/edematous appearance than cases in the West, and thus may have a poorer prognosis.5
Lesion type is defined on the basis of multiple, but probably unrelated, factors, including border opacity, fundus location, relationship to vessels, and amount of hemorrhage.7 Level of opacity is strongly related to lesion type (P < 0.001), and has been the major determinant of type designation.7 Opacity may be more useful than lesion type in clinical studies; whereas some lesions cannot be categorized by type, all lesions can be scored for opacity.7
We developed a system for scoring border opacity in the Studies of the Ocular Complications of AIDS (SOCA). Using this system, we tested the hypothesis that opacity reflects an individual's level of immunodeficiency, as reflected by CD4+ and CD8+ T-lymphocyte counts and HIV RNA blood levels. We also sought other risk factors that may influence severity of opacity.
Methods
Included in this cross-sectional investigation were participants from three prospective, multicenter, clinical trials and one prospective observational study conducted by the SOCA Research Group from 1988 to 2013 in which CMV retinitis lesion border opacity was scored by the Fundus Photograph Reading Center (FPRC, University of Wisconsin, Madison, WI, USA): “HPMPC [chemical abbreviation for cidofovir] Peripheral Cytomegalovirus Retinitis Trial” (HPCRT)12; “Monoclonal Antibody Cytomegalovirus Retinitis Trial” (MACRT)13; “Ganciclovir-Cidofovir Cytomegalovirus Retinitis Trial” (GCCRT)14; and “Longitudinal Study of the Ocular Complications of AIDS” (LSOCA).15,16 HPCRT enrolled individuals with newly diagnosed CMV retinitis, while MACRT and GCCRT enrolled individuals with either newly diagnosed or relapsed CMV retinitis. These clinical trials were performed sequentially before widespread availability of cART; data collection was completed by year 2000. LSOCA was a prospective, observational study of individuals with AIDS in the era of cART (September 1, 1998 through July 31, 2013); enrolled were individuals with histories of AIDS, as defined by the Centers for Disease Control and Prevention,17 regardless of immunologic status or presence of ocular complications of HIV disease. Among those with CMV retinitis, lesions may have been active or inactive at enrollment. Only study participants with newly diagnosed CMV retinitis were included in the current study. Approval for each study was obtained from the institutional review boards of the participating clinical centers and the three resource centers (chairman's office, coordinating center, FPRC). Written informed consent was obtained from participants. Studies were conducted in accordance with the tenets of the Declaration of Helsinki.
Data Collection
Evaluated in this investigation were all CMV retinitis lesions from each of the aforementioned studies, determined to be present at study enrollment (baseline) by the FPRC, regardless of activity level. Incident CMV retinitis that developed among LSOCA participants during follow-up were also included. Participants were included without regard to current or previous use of cART or specific anti-CMV drugs (ganciclovir, foscarnet, cidofovir, fomivirsen).
For each participant, the following data were collected: age, sex, race/ethnicity, human immunodeficiency virus (HIV) exposure risk factor (men having sex with men [MSM] only, injection drug use only, MSM and injection drug use, heterosexual contact, other), cART status, use of anti-CMV drugs, use of other antiherpetic drugs, presence of immune recovery uveitis (IRU), Karnofsky score, hemoglobin, CD4+ and CD8+ T-lymphocyte counts at study entry, and the most recent HIV RNA blood level prior to study entry. For each lesion, the following data were collected at study entry (all studies) and at development of incident CMV retinitis in LSOSA, with assessment by the FPRC: eye involved, topographic location (by zone),18 extent of lesion (percentage of fundus area), and maximum opacity score. Only those enrolled in LSOCA had data regarding HIV RNA blood levels. Information regarding cART was available only for those in GCCRT and LSOCA. Only the following information regarding cART and antiherpetic drug use was available: current use; history of use within the past 28 days, history of ever using the drugs.
Opacity was graded by masked readers at the FPRC, in accordance with a previously described protocol (Studies of the Ocular Complications of AIDS [SOCA] Research Group Cytomegalovirus Retinitis Grading Protocol. National Technical Information Service, NTIS accession no. PB97–192082, Springfield, VA 22161). Scores ranged from 1–6 (Figure). A score of 1 was assigned to inactive scars. Lesions with questionable/equivocal activity were assigned scores of 2. Scores of 3 through 6 were assigned to active lesions and represented progressively worse opacity (mild, moderate, severe, very severe). Scores were based on the most opaque portion of the border and were assigned after comparison to standard photographs of each opacity level; the segment of maximum opacity had to be at least one-half disc diameter (DD, 900 μm) in length to be scored at that level. For patient-level comparisons, participants with multiple lesions in an eye or bilateral disease were assigned the highest opacity score for any lesion in either eye. There was continuous internal quality control assessment within the reading center and lesion border opacity was one of the study variables assessed. Each image was graded by a single grader with consensus grading in difficult cases; longitudinal review of multiple visits at the time of grading; and regular quality control reviews of case samples.
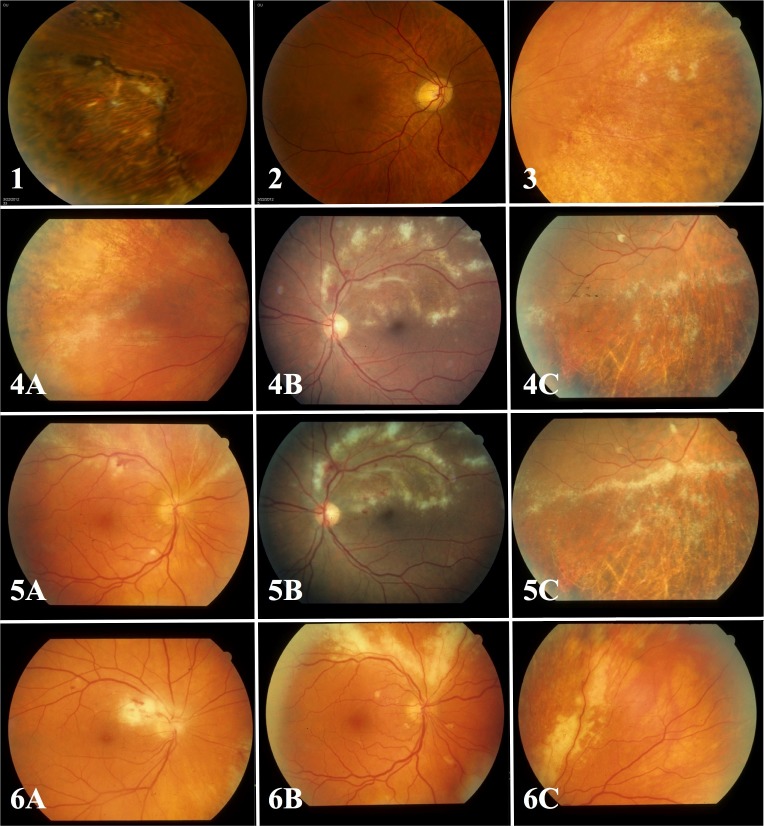
Figure.
Standard photographs used for scoring CMV retinitis lesion border opacity. Grade 1 is assigned to inactive atrophic scars (standard photograph 1, first row, left). Grade 2 is assigned to lesions with questionable or equivocal lesion opacity (standard photograph 2, first row, middle). Grade 3 is assigned to mild opacity (equivalent to or exceeding the opacity of standard photograph 3, first row, right, but less than standard photographs 4A–C, second row). Grade 4 is assigned to moderate opacity (equivalent to or exceeding the opacity of standard photographs 4A–C, but less than standard photographs 5A–C, third row). Grade 5 is assigned to severe opacity (equivalent to or exceeding the opacity of standard photographs 5A–C, but less than standard photographs 6A–C, fourth row). Grade 6 is assigned to very severe opacity (equivalent to or exceeding the opacity of standard photographs 6A–C. Lesions assigned grades 3 through 6 are considered to be active.
MACRT and HPCRT studies utilized a 7-point scale, rather than the aforementioned 6-point scale; to correct for this imbalance, we collapsed scores 2 (“questionable/equivocal”) and 3 (“possibly active”, but less opacity than the standard photograph for “mild”) from the 7-point scale into a single score 2, thus pulling all of the more severe scores down one step in the conversion.
Definitions and Conventions
Active CMV retinitis was defined as an opacity score ≥3. Lesions assigned scores of 2 were considered to be inactive for these analyses. Severe opacity was defined as scores of 5 or 6.
For purposes of analysis, cART was defined as a combination of at least three antiretroviral drugs (the definition of “highly active antiretroviral therapy” in use at the time these studies were conducted). IRU was defined as the presence of a prominent vitreous inflammatory reaction in participants with laboratory evidence of immune recovery (CD4+T-lymphocyte count >100 cells/μl).19
Data Analyses and Statistical Methods
In addition to examining aggregate data from all studies, we investigated LSOCA alone because it is the most contemporary study, and therefore the most relevant to current populations in terms of potential confounding factors.
Participant-level characteristics were compared across studies and opacity levels, using summary statistics, including mean for normally distributed data, median for data without normal distribution, and percentages for categorical data. Statistical tests include ANOVA for normally distributed data, Kruskal-Wallis test for data without normal distribution, and χ2 test for categorical data. Generalized estimating equations were used to account for within-participant correlations of two involved eyes when performing eye-specific analyses involving zone 1 comparisons.
Multiple linear regression was used to assess the independent relationship of risk factors with opacity score, modeled continuously, selecting the model with the lowest Akaike Information Criteria (AIC).20 This technique measures the trade-off between goodness-of-fit versus complexity of a model, and unlike many model selection procedures (e.g., forward and backward selection), this technique is not based on p-values, and thus, the model with lowest AIC can select variables with associated values of P > 0.05. Two models were run: one included all eligible participants in each study; the other included only participants in LSOCA. The model for all studies included a candidate set of age, sex, race/ethnicity, the HIV exposure risk factor of MSM, Karnofsky score, hemoglobin, use of antiherpetic drugs other than anti-CMV agents, CD4+ and CD8+ T-lymphocyte counts, bilateral disease, area of CMV retinitis ≥25% in either eye, and presence of a zone 1 lesion in either eye; an indicator variable for “study” was forced into the model. The model that evaluated only participants in LSOCA included the additional risk factors of HIV RNA blood level, use of cART, and presence of IRU. The variable “current use of anti-CMV drugs” was forced into the model, as it is known to influence CMV activity; it was included to reduce the possibility that other candidate variables would be chosen simply because they are surrogates for the effect of anti-CMV drug treatment. Multiple logistic regression was used to select models assessing the independent relationship of risk factors with presence of severe opacity score among participants with active disease (scores 3–6 only). Selection criteria and candidate sets were similar to the above description of multiple linear regression.
Values of P were two-sided and nominal. Statistical analyses were conducted with statistical software packages (SAS/STAT version 9.3; SAS Institute, Inc., Cary, NC, USA, and Stata version 14.1, Stata Statistical Software: Release 12; StataCorp, College Station, TX, USA).
Results
Included were 299 participants with CMV retinitis in at least one of the four SOCA Research Group studies. There were 122 eligible participants in LSOCA: 101 had prevalent CMV retinitis at study entry and 21 developed incident CMV retinitis during follow-up. Table 1 describes demographic, medical, and examination characteristics for participants grouped by SOCA Research Group study. Significant differences across studies reflect either original study design factors (inclusion criteria, use of anti-CMV drugs at study entry, presence of zone 1 lesions, size of lesions), or the evolution of the AIDS epidemic over the timeframe of the studies (demographics, available treatments). There were significant differences in opacity scores across studies, with LSOCA having a lower proportion of lesions with severe opacity.
Table 1.
Study, Study Participant, and Eye Characteristics at First Study Visit after Diagnosis of CMV Retinitis Grouped by SOCA Research Group Study
|
Characteristic |
HPCRT (1994–1996) |
MACRT (1995–1996) |
GCCRT* (1997–2000) |
LSOCA*† (1998–2013) |
Total |
P
Value |
| Study participants with newly diagnosed CMV retinitis, n | 60‡ | 72‡ | 45‡ | 122‡ | 299‡ | |
| Demographics | ||||||
| Median age, y | 38 | 39 | 41 | 39 | 39 | 0.59 |
| Race/ethnicity | 0.06 | |||||
| White, n (%) | 33 (55.0) | 38 (52.8) | 15 (33.3) | 49 (40.5) | 135 (45.3) | |
| Black, n (%) | 15 (25.0) | 19 (26.4) | 23 (51.1) | 48 (39.7) | 105 (35.2) | |
| Other, n (%) | 12 (20.0) | 15 (20.8) | 7 (15.6) | 24 (19.8) | 58 (19.5) | |
| Male sex, n (%) | 55 (91.7) | 63 (87.5) | 37 (82.2) | 87 (71.9) | 242 (81.2) | 0.004 |
| HIV exposure risk factor, n (%) | <0.0001 | |||||
| MSM only, n (%) | 49 (81.7) | 51 (70.8) | 19 (42.2) | 61 (50.4) | 180 (60.4) | |
| MSM and IDU, n (%) | 1 (1.7) | 1 (1.4) | 2 (4.4) | 4 (3.3) | 8 (2.7) | |
| IDU only, n (%) | 4 (6.7) | 3 (4.2) | 9 (20.0) | 5 (4.1) | 21 (7.0) | |
| Heterosexual, n (%) | 5 (8.3) | 12 (16.7) | 15 (33.3) | 37 (30.6) | 69 (23.2) | |
| Other risk factor, n (%) | 1 (1.7) | 5 (6.9) | 0 (0.0) | 14 (11.6) | 20 (6.7) | |
| Medical factors | ||||||
| Mean Karnofsky score | 81 | 79 | 81 | 74 | 78 | 0.0008 |
| Median hemoglobin value, g/dL | 12.4 | 11.0 | 11.9 | 11.3 | 11.5 | 0.04 |
| Treatment | ||||||
| On cART§, n (%) | 0 | 0 | 36 (80.0) | 85 (70.8) | 121 (40.7) | <0.0001 |
| Receiving anti-CMV drugs‖ in past 28 days, n (%) | 0 | 60 (83.3) | 0 | 78 (65.0) | 138 (46.5) | <0.0001 |
| Currently receiving anti-CMV drugs‖, n (%) | NA | NA | NA | 73 (60.8) | NA | NA |
| Receiving antiherpetic drugs¶, n (%) | 19 (33.9) | 21 (29.2) | 10 (22.2) | 25 (20.8) | 75 (25.6) | 0.24 |
| Laboratory values | ||||||
| Median CD4+ T-lymphocyte count (cells/μL) | 12 | 10 | 15 | 22 | 15 | <0.0001 |
| CD4+ T-lymphocyte count thresholds | ||||||
| <100 cells/μL, n (%) | 57 (96.6) | 66 (93.0) | 42 (93.3) | 102 (85.7) | 267 (90.8) | 0.08 |
| <50 cells/μL, n (%) | 54 (91.5) | 61 (85.9) | 37 (82.2) | 82 (68.9) | 234 (79.6) | 0.002 |
| Median CD8+ T-lymphocyte count (cells/μL) | 316 | 250 | 303 | 282 | 288 | 0.84 |
| CD8+ T-lymphocyte count thresholds | ||||||
| <520 cells/μL, n (%) | 36 (62.1) | 56 (78.9) | 35 (77.8) | 84 (72.4) | 211 (72.8) | 0.15 |
| <400 cells/μL, n (%) | 34 (58.6) | 49 (69.0) | 26 (57.8) | 74 (63.8) | 183 (63.1) | 0.54 |
| Mean current HIV RNA blood level (log10 copies/mL) | NA | NA | NA | 4.4 | NA | NA |
| Current HIV RNA blood level <400 copies/mL, n (%) | NA | NA | NA | 17 (15.6) | NA | NA |
| Mean maximum HIV RNA blood level (log10 copies/mL) | NA | NA | NA | 5.5 | NA | NA |
| Eye characteristics | ||||||
| Bilateral CMV retinitis, n (%) | 16 (26.7) | 27 (37.5) | 15 (33.3) | 44 (36.1) | 102 (34.1) | 0.56 |
| Zone 1 involvement in either eye, n (%) | 0 | 35 (48.6) | 21 (46.7) | 57 (46.7) | 113 (37.8) | <0.0001 |
| Extent of CMV lesion, n (percentage of individuals with ≥25% involvement in either eye) | 0 | 12 (16.9) | 10 (22.2) | 38 (31.2) | 60 (20.1) | <0.0001 |
| Immune recovery uveitis in either eye#, n (%) | NA | NA | NA | 15 (12.3) | NA | NA |
| Maximum opacity score**, n (%) | 0.008 | |||||
| 1 | 0 | 1 (1.4) | 2 (4.4) | 8 (6.6) | 11 (3.9) | |
| 2 | 1 (1.7) | 0 | 1 (2.2) | 5 (4.1) | 7 (2.3) | |
| 3 | 1 (1.7) | 1 (1.4) | 2 (4.4) | 9 (7.4) | 13 (4.4) | |
| 4 | 8 (13.3) | 23 (31.9) | 9 (20.0) | 28 (23.0) | 68 (22.7) | |
| 5 | 23 (38.3) | 22 (30.6) | 12 (26.7) | 47 (38.5) | 104 (34.8) | |
| 6 | 27 (45.0) | 25 (34.7) | 19 (42.2) | 25 (20.5) | 96 (32.1) | |
| Mean | 5.2 | 4.9 | 4.9 | 4.4 | 4.8 | 0.0002 |
There were 5 study participants enrolled in both GCCRT and LSOCA.
LSOCA included 101 individuals who were diagnosed with CMV retinitis at study enrollment and 21 individuals who developed incident CMV retinitis during follow-up.
In calculating percentages, denominators were based on the number of eyes with available data for each characteristic.
For study purposes, cART was defined as a combination of at least 3 antiretroviral drugs.
Ganciclovir, valganciclovir, foscarnet, cidofovir, or fomivirsen administered by any route.
Antiherpetic drugs other than anti-CMV agents, including acyclovir, valaciclovir, or famciclovir.
As reported by study investigators, based on the presence of a prominent vitreous inflammatory reaction in study participants with laboratory evidence of immune recovery (current CD4 + T-lymphocyte count > 100 cells/μl with a nadir < 100 cells/μl).19
The highest score assigned to any lesion in either eye by the Fundus Photograph Reading Center.
Table 2 describes demographic, medical, and examination characteristics for participants combined across all studies, grouped by opacity score. Participants with higher scores appeared less likely to be on cART and more likely to have bilateral disease. Higher scores were associated with worse immune function as reflected by lower median CD4+ T-lymphocyte counts and higher proportions of participants with CD4+ T-lymphocyte counts below thresholds of both 100 and 50 cells/μL.
Table 2.
Study Participant and Eye Characteristics Grouped by Maximum CMV Retinitis Lesion Border Opacity Score in the Worse Eye for Study Participants in HPCRT, MACRT, GCCRT, and LSOCA.
|
Characteristic |
Maximum Opacity Score* |
P
Value |
|||||
| 1 |
2 |
3 |
4 |
5 |
6 |
||
| Study participants, n | 11† | 7† | 13† | 68† | 104† | 96† | |
| Demographics | |||||||
| Median age, y | 34 | 36 | 42 | 40 | 39 | 38 | 0.87 |
| Race/ethnicity | 0.27 | ||||||
| White, n (%) | 5 (45.4) | 5 (71.4) | 5 (41.7) | 32 (47.1) | 51 (49.0) | 37 (38.5) | |
| Black, n (%) | 5 (45.5) | 1 (14.3) | 2 (16.7) | 27 (39.7) | 35 (33.6) | 35 (33.5) | |
| Other, n (%) | 1 (9.1) | 1 (14.3) | 5 (41.7) | 9 (13.2) | 18 (17.3) | 24 (25.0) | |
| Male sex, n (%) | 8 (72.7) | 5 (71.4) | 12 (100.0) | 52 (76.5) | 87 (83.6) | 78 (81.2) | 0.40 |
| HIV exposure risk factor | 0.77 | ||||||
| MSM only, n (%) | 4 (36.4) | 4 (57.1) | 8 (66.7) | 37 (54.4) | 70 (67.3) | 57 (59.4) | |
| MSM and IDU, n (%) | 1 (9.1) | 0 (0.0) | 0 (0.0) | 1 (1.5) | 3 (2.9) | 3 (3.1) | |
| IDU only, n (%) | 1 (9.1) | 1 (14.3) | 1 (8.3) | 5 (7.4) | 5 (4.8) | 8 (8.3) | |
| Heterosexual, n (%) | 4 (36.4) | 2 (28.6) | 1 (8.3) | 18 (26.5) | 23 (22.1) | 21 (21.9) | |
| Other risk factor, n (%) | 1 (9.1) | 0 (0.0) | 2 (16.7) | 7 (10.3) | 3 (2.9) | 7 (7.3) | |
| Medical factors | |||||||
| Mean Karnofsky score | 80 | 73 | 83 | 78 | 79 | 75 | 0.09 |
| Median hemoglobin value, g/dL | 10.8 | 12.9 | 12.5 | 11.3 | 11.6 | 11.2 | 0.67 |
| Treatment factors | |||||||
| On cART‡, n (%) | 8 (72.7) | 5 (71.4) | 9 (75.0) | 27 (39.7) | 43 (41.8) | 29 (30.2) | 0.003 |
| Receiving antiherpetic drugs§, n (%) | 3 (27.3) | 1 (14.3) | 1 (8.3) | 18 (26.5) | 22 (21.8) | 30 (31.9) | 0.40 |
| Laboratory values | |||||||
| Median CD4+ T-lymphocyte count (cells/μL) | 55 | 18 | 50 | 16 | 15 | 12 | 0.001 |
| CD4+ T-lymphocyte count thresholds | |||||||
| <100 cells/μL, n (%) | 7 (63.6) | 4 (66.7) | 11 (84.6) | 54 (80.6) | 98 (97.0) | 93 (96.9) | < 0.0001 |
| <50 cells/μL, n (%) | 5 (45.5) | 4 (66.7) | 6 (46.2) | 44 (65.7) | 86 (85.2) | 89 (92.7) | < 0.0001 |
| Median CD8+ T-lymphocyte count (cells/μL) | 541 | 379 | 354 | 274 | 310 | 204 | 0.84 |
| CD8+ T-lymphocyte count thresholds | |||||||
| <520 cells/μL, n (%) | 5 (45.5) | 4 (57.1) | 8 (61.5) | 45 (68.2) | 75 (75.0) | 74 (79.6) | 0.11 |
| <400 cells/μL, n (%) | 5 (45.4) | 4 (57.1) | 7 (53.8) | 41 (62.1) | 60 (60.0) | 66 (71.0) | 0.42 |
| Eye characteristics | |||||||
| Bilateral CMV retinitis, n (%) | 6 (54.6) | 1 (14.3) | 4 (30.8) | 11 (16.2) | 33 (31.7) | 47 (49.0) | 0.0004 |
| Zone 1 involvement in either eye, n (%) | 4 (36.4) | 1 (14.3) | 8 (61.5) | 25 (36.8) | 36 (34.6) | 39 (40.6) | 0.35 |
| Extent of CMV lesion n (percentage of individuals with ≥25% involvement in either eye) | 5 (45.6) | 1 (14.3) | 2 (15.4) | 11 (16.4) | 22 (21.2) | 19 (19.8) | 0.37 |
The highest score assigned to any lesion in either eye by the Fundus Photograph Reading Center.
In calculating percentages, denominators were based on the number of eyes with available data for each characteristic.
For study purposes, cART was defined as a combination of at least 3 antiretroviral drugs.
Antiherpetic drugs other than anti-CMV agents, including acyclovir, valaciclovir, or famciclovir.
Table 3 describes demographic, medical, and examination characteristics for participants in LSOCA. Higher opacity scores were associated with worse immune function as reflected by lower median CD4+ T-lymphocyte counts, higher proportions of participants with CD4+ T-lymphocyte counts below thresholds of 100 and 50 cells/μL, lower median CD8+ T-lymphocyte counts, and higher proportion of participants with CD8+ T-lymphocyte counts below 520 cells/μL. Higher scores were also associated with higher mean HIV RNA blood levels and a higher proportion of patients with HIV RNA blood levels above 400 copies/mL. Opacity was statistically related to male sex, and there was also a weak association with race/ethnicity.
Table 3.
Study Participants and Eye Characteristics Grouped by Maximum CMV Retinitis Lesion Border Opacity Score in the Worse Eye for Participants in LSOCA.
|
Characteristic |
Maximum Opacity Score* |
P Value | |||||
| 1 |
2 |
3 |
4 |
5 |
6 |
||
| No. of study participants | 8† | 5† | 9† | 28† | 47† | 25† | |
| Demographics | |||||||
| Median age, y | 36 | 39 | 39 | 44 | 37 | 37 | 0.78 |
| Race/ethnicity | 0.07 | ||||||
| White, n (%) | 4 (50.0) | 4 (80.0) | 2 (25.0) | 11 (39.3) | 22 (46.8) | 6 (24.0) | |
| Black, n (%) | 3 (37.5) | 1 (20.0) | 1 (12.5) | 12 (42.9) | 18 (38.3) | 13 (52.0) | |
| Other race, n (%) | 1 (12.5) | 0 (0.0) | 5 (62.5) | 5 (17.9) | 7 (14.9) | 6 (24.0) | |
| Male sex, n (%) | 7 (87.5) | 3 (60.0) | 8 (100.0) | 18 (64.3) | 38 (80.8) | 13 (52.0) | 0.03 |
| HIV exposure risk factor | 0.48 | ||||||
| MSM only, n (%) | 3 (37.5) | 3 (60.0) | 5 (62.5) | 12 (42.9) | 29 (61.7) | 9 (36.0) | |
| MSM and IDU, n (%) | 1 (12.5) | 0 (0.0) | 0 (0.0) | 1 (3.6) | 2 (4.3) | 0 (0.0) | |
| IDU only, n (%) | 1 (12.5) | 1 (20.0) | 0 | 1 (3.6) | 1 (2.1) | 1 (4.0) | |
| Heterosexual, n (%) | 2 (25.0) | 1 (20.0) | 1 (12.5) | 9 (32.1) | 12 (25.5) | 12 (48.0) | |
| Other risk factor, n (%) | 1 (12.5) | 0 (0.0) | 2 (25.0) | 5 (17.9) | 3 (6.4) | 3 (12.0) | |
| Medical factors | |||||||
| Mean Karnofsky score | 79 | 68 | 80 | 74 | 76 | 70 | 0.19 |
| Median hemoglobin value, g/dL | 10.9 | 12.0 | 12.3 | 11.6 | 11.5 | 10.8 | 0.16 |
| Treatment factors | |||||||
| On cART‡, n (%) | 6 (75.0) | 4 (80.0) | 7 (87.5) | 19 (67.9) | 33 (71.7) | 16 (64.0) | 0.84 |
| Receiving anti-CMV drugs§ in past 28 days, n (%) | 3 (37.5) | 4 (80.0) | 6 (75.0) | 16 (57.1) | 32 (69.6) | 17 (68.0) | 0.44|| |
| Currently on anti-CMV drugs§, n (%) | 3 (37.5) | 4 (80.0) | 6 (75.0) | 15 (53.6) | 30 (65.2) | 15 (60.0) | 0.51¶ |
| Receiving antiherpetic drugs#, n (%) | 3 (37.5) | 1 (20.0) | 1 (12.5) | 4 (14.3) | 6 (13.0) | 10 (40.0) | 0.08 |
| Laboratory values | |||||||
| Median CD4+ T-lymphocyte count, cells/μL | 58 | 18 | 55 | 49 | 20 | 20 | 0.006 |
| CD4+ T-lymphocyte count thresholds | |||||||
| <100 cells/μL, n (%) | 5 (62.5) | 3 (75.0) | 7 (77.8) | 20 (74.1) | 44 (95.6) | 23 (92.0) | 0.04 |
| <50 cells/μL, n (%) | 4 (50.0) | 3 (75.0) | 2 (22.2) | 14 (51.8) | 37 (80.4) | 22 (88.0) | 0.0007 |
| Median CD8+ T-lymphocyte count (cells/μL) | 576 | 331 | 523 | 271 | 311 | 141 | 0.0004 |
| CD8+ T-lymphocyte count | |||||||
| <520 cells/μL, n (%) | 3 (37.5) | 4 (80.0) | 4 (44.4) | 18 (69.2) | 35 (77.8) | 20 (87.0) | 0.04 |
| <400 cells/μL, n (%) | 3 (37.5) | 4 (80.0) | 4 (44.4) | 17 (65.4) | 27 (60.0) | 19 (82.6) | 0.14 |
| Mean current HIV RNA blood level (log10 copies/mL) | 2.4 | 4.4 | 3.8 | 4.4 | 4.7 | 4.9 | <0.0001 |
| Current HIV RNA blood level <400 copies/mL, n (%) | 4 (57.1) | 1 (20.0) | 3 (37.5) | 4 (15.4) | 4 (10.0) | 1 (4.4) | 0.009 |
| Mean maximum HIV RNA blood level (log10 copies/mL) | 5.4 | 5.4 | 5.5 | 5.4 | 5.4 | 5.8 | 0.14 |
| Eye characteristics | |||||||
| Bilateral CMV retinitis, n (%) | 4 (50.0) | 1 (20.0) | 3 (33.3) | 6 (21.4) | 16 (34.0) | 14 (56.0) | 0.14 |
| Zone 1 involvement in either eye, n (%) | 2 (25.0) | 1 (20.0) | 7 (77.8) | 11 (39.3) | 21 (44.7) | 15 (60.0) | 0.11 |
| Extent of CMV lesion, n (percentage of individuals with ≥25% involvement in either eye) | 4 (50.0) | 1 (20.0) | 2 (22.2) | 7 (25.0) | 15 (31.9) | 9 (36.0) | 0.74 |
| Immune recovery uveitis in either eye**, n (%) | 1 (12.5) | 1 (20.0) | 1 (11.1) | 5 (17.9) | 4 (8.5) | 3 (12.0) | 0.89 |
The highest score assigned to any lesion in either eye by the Fundus Photograph Reading Center.
In calculating percentages, denominators were based on the number of eyes with available data for each characteristic.
For study purposes, cART was defined as a combination of at least 3 antiretroviral drugs.
Ganciclovir, valganciclovir, foscarnet, cidofovir, or fomivirsen administered by any route.
P value = 0.96, excluding study participants with opacity scores of 1.
P value = 0.60, excluding study participants with opacity scores of 1.
Antiherpetic drugs other than anti-CMV agents, including acyclovir, valaciclovir, or famciclovir.
As reported by study investigators, based on the presence of a prominent vitreous inflammatory reaction in study participants with laboratory evidence of immune recovery (current CD4+T-lymphocyte count >100 cells/μL with a nadir <100 cells/μL).19
Table 4 identifies factors independently related to opacity scores adjusted for current use of anti-CMV drugs based on AIC. When all studies were considered, factors associated with higher opacity scores were nonwhite race/ethnicity, lower CD4+ T-lymphocyte count, and bilateral disease. When LSOCA alone was considered, factors associated with higher scores were nonwhite race/ethnicity and higher HIV RNA blood level.
Table 4.
Factors Associated with Opacity Score Among Study Participants Enrolled in HPCRT, MACRT, GCCRT, and LSOCA.
|
Factor* |
Difference† |
95% CI |
P
Value |
| Participants in HPCRT, MACRT, GCCRT, or LSOCA (n = 274 with complete data) | |||
| Race/ethnicity (white versus nonwhite) | −0.2 | −0.5, 0.0 | 0.11 |
| CD4+ T-lymphocyte count (per 100 cells/μL) | −0.4 | −0.6, −0.3 | <0.001 |
| Bilateral disease (yes vs. no) | 0.32 | 0.1, 0.6 | 0.02 |
| Participants in LSOCA only‡ (n = 104 with complete data) | |||
| Race/ethnicity (white vs. non-white) | −0.5 | −1.0, 0.0 | 0.05 |
| Currently on anti-CMV drugs§ (yes vs. no) | 0.2 | −0.3, 0.7 | 0.35 |
| HIV RNA blood level (per log10 copies/mL) | 0.5 | 0.3, 0.6 | <0.001 |
Factors were selected on the basis of AIC,20 from a multiple linear model regressing opacity score on candidate set of age, sex, race/ethnicity, HIV exposure risk factor, Karnofsky score, hemoglobin, use of antiherpetic drugs other than anti-CMV agents, CD4 + T-lymphocyte count, CD8+ T-lymphocyte count, bilateral CMV retinitis, area of CMV retinitis ≥25% in either eye, and zone 1 CMV retinitis in either eye; indicator variable for study was forced into the model.
Mean difference in opacity score between comparison and reference group for categorical factor or slope per one-unit increase in opacity score for continuous factors.
Additional risk factors in candidate set included HIV RNA blood level, on cART, and diagnosis of immune recovery uveitis; currently on anti-CMV drugs was forced into model.
Ganciclovir, valganciclovir, foscarnet, cidofovir, or fomivirsen administered by any route.
Table 5 identifies factors independently related to severe opacity scores among eyes with active CMV retinitis adjusted for current use of anti-CMV drugs based on AIC. When all four studies were considered, severe opacity was associated with younger age, lower Karnofsky score, lower CD4+ T-lymphocyte count, and bilateral disease. When LSOCA alone was considered, severe opacity was associated with younger age, use of antiherpetic drugs other than anti-CMV agents, lower CD4+ T-lymphocyte count, higher HIV RNA blood level, and larger lesion size.
Table 5.
Factors Associated with Severe Opacity Among Study Participants With Active CMV Retinitis Lesions* Enrolled in HPCRT, MACRT, GCCRT, and LSOCA
|
Factor† |
Odds Ratio |
95% CI |
P
Value |
| Participants in HPCRT, MACRT, GCCRT, or LSOCA (n = 259 with complete data)‡ | |||
| Age (per year) | 0.94 | 0.91, 0.98 | 0.002 |
| Karnofsky score (per 10 points) | 0.97 | 0.95, 1.00 | 0.06 |
| CD4+ T-lymphocyte count (per 100 cells/μL) | 0.53 | 0.33, 0.84 | 0.007 |
| Bilateral CMV retinitis (yes vs. no) | 2.9 | 1.5, 5.7 | 0.002 |
| Participants in LSOCA only (n = 93 with complete data)§ | |||
| Age (per year) | 0.90 | 0.84, 0.96 | 0.002 |
| Currently on anti-CMV drugs|| (yes vs. no) | 2.0 | 0.7, 5.6 | 0.21 |
| Currently on antiherpetic drugs¶ (yes vs. no) | 3.4 | 0.8, 15.3 | 0.11 |
| CD4+ T-lymphocyte count (per 100 cells/μL) | 0.54 | 0.29, 1.01 | 0.05 |
| HIV RNA blood level (per log10 copies/mL) | 1.4 | 0.9, 2.1 | 0.13 |
| Area of CMV retinitis ≥25% in either eye (yes vs. no) | 3.2 | 1.0, 10.9 | 0.06 |
Severe opacity was defined as an opacity score of 5 or 6; active CMV retinitis was defined as any opacity score from 3 through 6.
Factors were selected on the basis of AIC,20 from a multiple linear model regressing opacity score on candidate set of age, sex, race/ethnicity, HIV exposure risk factor, Karnofsky score, hemoglobin, use of antiherpetic drugs other than anti-CMV agents, CD4+ T-lymphocyte count, CD8+ T-lymphocyte count, bilateral CMV retinitis, area of CMV retinitis ≥25% in either eye, and zone 1 CMV retinitis in either eye; indicator variable for study was forced into the model.
Severe opacity was present in 185 (71%) of 259 study participants with complete data.
Additional risk factors in candidate set included HIV RNA blood level, on cART, and diagnosis of immune recovery uveitis; currently on anti-CMV drugs was forced into model. Severe opacity was present in 61 (66%) of 93 study participants with complete data.
Ganciclovir, valganciclovir, foscarnet, cidofovir, or fomivirsen administered by any route.
Antiherpetic drugs other than anti-CMV agents, including acyclovir, valaciclovir, or famciclovir.
Although current cART was associated with lower scores on univariate analysis (Table 2), it was not independently related to opacity in any analyses based on AIC. No relationships were found between opacity score and zone 1 involvement in any per-person comparisons. Because local factors might influence the relationship between zone 1 and opacity, we also performed per-eye comparisons. In these secondary analyses, zone 1 was not statistically related to opacity across all scores (P = 0.97); to severe opacity among eyes with active lesions (scores 5 and 6 vs. scores 3 and 4, P = 0.94); or to the highest score among those with active lesions (score 6 vs. scores 3–5, P = 0.58).
Discussion
The most distinctive feature of CMV retinitis is an opaque lesion border. Autopsy studies have shown that opacity corresponds to disruption of normal architectural features because of edema and necrosis.21,22 Only scant inflammatory material is present in most lesions. With anti-CMV drug treatment, opacity resolves completely; thus, opacity is considered a reliable sign of CMV activity.7 Early in the AIDS epidemic, three additional factors were hypothesized to influence untreated lesion appearance: severity of immunodeficiency,23 effect of drugs used concurrently for other indications,24–26 and anatomic factors, including lesion location.7,27 We addressed each factor in our study.
We used CD4+ and CD8+ T-lymphocyte counts and blood HIV RNA levels as nonspecific markers of immune function. A lower CD4+ T-lymphocyte count was strongly associated with increased opacity scores across multiple comparisons, and was independent of other risk factors. Lowder and associates28 showed that a CD8+ T-lymphocyte count <520 cells/μL was an independent risk factor for development of CMV retinitis. Oka and associates reported that a CD8+ T-lymphocyte count <400 cells/μL predicted CMV retinitis with similar sensitivity and specificity values to a CD4+ T-lymphocyte count <50 cells/μL.29 Holbrook and associates11 identified low CD8+ T-lymphocyte count (but not low CD4+ T-lymphocyte count) as an independent risk factor for the development of second eye involvement in people with unilateral CMV retinitis. Our study showed an inverse relationship between CD8+ T-lymphocyte count and level of opacity in some comparisons, but it was not an independent risk factor.
Immune recovery with cART can inactivate CMV retinitis lesions, even without anti-CMV drugs30,31 and CMV immunity may not be reflected by CD4+ and CD8+ T-lymphocyte counts in all individuals.32 As we had no direct tests of CMV immunity, we tested the relationship between cART and opacity, to determine if cART has an effect on opacity that is independent of our laboratory measures. In univariate comparisons, cART was inversely related to opacity scores across all studies, but was not independently associated with level of opacity or severe opacity, when other factors were considered.
We found a strongly positive, independent relationship between HIV RNA blood level and opacity. While high HIV RNA blood levels reflect immunodeficiency, HIV viremia may also predispose to increased CMV activity by transactivating CMV. Coinfection of retinal cells by HIV and CMV has been reported,33 and interactions between the viruses have been described in vitro.34–36
We repeated all analyses using data only from eyes with active lesions (grades 3–6) and confirmed that relationships between opacity and immunodeficiency were not driven simply by lack of opacity (score 1) in participants whose lesions were inactive after immune recovery.
Increased opacity was also related to larger lesions and to bilateral involvement, two factors that are strongly related to each other (P = 0.007).7 Both likely reflect worse immune function; increased virus activity will result in more rapid lesion enlargement, and thus, larger lesions at diagnosis. More rapid disease progression has also been related to bilateral disease.10
We compared opacity to lesion location because of previous evidence that more opaque lesions are associated with zone 1.7,27 Henderly and associates27 reported that fulminant/edematous lesions are more common in the posterior pole and indolent/granular lesions more common in the periphery. Holland and associates7 found an association between severe opacity and zone 1, arguing that worse opacity may reflect greater retinal thickness in zone 1. In contrast, we did not find a relationship between zone 1 and any measure of opacity. The reason for this discrepancy is not clear. It might reflect different scoring systems between studies, but if a true relationship exists, the effect of location is undoubtedly small.
We found a relationship of borderline significance between opacity and race/ethnicity (P = 0.07). Among those with active lesions, black participants had more very severe scores (grade 6) than white participants or those in other racial/ethnic groups. Furthermore, on multivariate analyses, severe opacity scores were associated with nonwhite race/ethnicity across all studies and for LSOCA alone. (Black individuals constituted approximately 64% of nonwhite study participants [105 of 163 individuals] and 35% of the total study population [105 of 298 study participants] for whom race/ethnicity was recorded). Relationships between opacity and race/ethnicity may also reflect worse immune function, albeit indirectly. Wutoh and associates37 have reported that African-Americans with AIDS-related CMV retinitis are significantly more likely to have severe disease (blindness in either eye, retinal detachment, bilateral involvement, or larger lesions) at presentation than white patients. They attributed the difference possibly to problems accessing medical services or delays in seeking care. Older age and lower Karnofsky score (both associated with increased opacity scores) also probably reflect worse immune function. Hemoglobin can be low in people with severe AIDS-related immunodeficiency, but we did not find a relationship between hemoglobin and lesion border opacity in our study; hemoglobin may not have the same relationship to level of immune function as opacity scores in our study population, and it does not appear to influence opacity through other mechanisms.
We included antiherpetic drugs other than anti-CMV drugs as potential risk factors because of reports that they influenced lesion type before anti-CMV drug use was widespread,24 presumably because of their weak anti-CMV activity.38–40 Valaciclovir was shown to reduce the risk of AIDS-related CMV retinitis,41 supporting the possibility that acyclovir could reduce virus activity in eyes that were already infected, thereby reducing opacity. Instead, we found a positive relationship between antiherpetic drugs and opacity scores, perhaps attributable to confounding-by-indication; participants who are more severely immunodeficient are more likely to have herpes simplex virus or varicella-zoster virus-associated disease or to be receiving prophylaxis against these infections than those with better immune function. Because participants were not randomized to treatment, any weak effect of antiherpetic drugs on virus activity would likely be masked in this setting.
A better understanding of CMV retinitis characteristics has implications for patient care and design of future clinical studies. Lesions with severe border opacity should be treated aggressively with anti-CMV drugs, as host immune defenses are likely to be markedly compromised. Border opacity may provide prognostic information, such as the rate at which lesions will enlarge, and do so with more precision than lesion types, which are defined in part by factors unrelated to virus activity. Opacity has been proposed as an outcome measure for studies of CMV retinitis treatment.18 Future clinical trials of treatment for CMV retinitis or other comparative studies might control for opacity, especially if samples sizes are small. Opacity also provides an objective measure for comparison of lesions across different populations. From a clinical standpoint, the relationship that we identified between lesion border opacity and level of immune function will be most applicable among individuals at diagnosis of CMV retinitis, before start of specific anti-CMV drug treatment, which itself can reduce lesion activity, regardless of immune function.
Our study provides no support for the existence of a proposed “immune recovery retinitis.”42 Development of retinal lesions as an immune recovery inflammatory syndrome (IRIS) phenomenon would result in a positive relationship between opacity score and CD4+ T-lymphocyte count, the opposite of what we found. We also found no relationship between opacity and IRU.
Limitations to our results are based on study design. Although the opacity scoring system is standardized, assignment of scores depends on subjective assessment of images, and scores are categorical, limiting the precision of values. Nevertheless, we found consistent, strong relationships between opacity and levels of immunodeficiency across multiple comparisons. Scores were assigned by trained readers, whereas clinicians at points of service might grade opacity differently, even using standard photographs; however, a study by Holland and associates reported fair to good agreement between clinicians when assigning scores for change in opacity.18 Investigators have proposed that CMV strain and host immunogenetic factors influence lesion appearance in the developing world,5 but we were not able to study these potential confounders in our population. Previous studies found that CMV retinitis was related to virus strains43 and host genes,44–46 but those studies did not mention lesion opacity. Although such factors might have an additional effect on opacity, it is unlikely that they would negate the effect of immunodeficiency on opacity shown in this study. We may not have identified all confounding factors that influence lesion appearance. We had limited ability to demonstrate the effect of drugs on opacity; at study entry, participants were only asked about cART within the prior 28 days, and not about treatment duration. They were not asked about timing or duration of anti-CMV drug use, which may explain our inability to confirm the known effect of these drugs on opacity. Study participants were seen at tertiary referral centers in the United States, and may not be representative of all people with CMV retinitis, especially those in other parts of the world. Studies spanned 2 decades, and may not reflect contemporary populations; however, the diversity of participants is a strength of the study that partially addresses this concern: relationships between opacity and immunodeficiency were strong across a diverse population, indicating the robust nature of our results. Our study dealt only with individuals who had AIDS, and results may not be applicable to people with immunodeficiency from other causes who develop CMV retinitis.
In conclusion, our investigation shows that opacity of CMV retinitis lesion borders is greater in individuals with more severe immunodeficiency, and the relationship is independent of antiretroviral or anti-CMV drug effects. Other factors related to opacity (older age, non-white race/ethnicity, lower Karnofsky score) are probably indirect measures of immunodeficiency because they reflect general health or access-to-care issues. Because opacity reflects virus activity, more severe opacity was related to larger lesions and multiple foci of infection (bilateral involvement). In the era of cART, patients who develop CMV retinitis will be more heterogeneous in terms of prior drug exposure, and may have different levels of immune function, which would influence the course of retinal disease. Scoring of opacity may be a useful, standard measure for continued study of CMV retinitis across different settings and populations.
Acknowledgments
Supported by cooperative agreements from the National Eye Institute to David Geffen School of Medicine at UCLA (EY08057), the Johns Hopkins University School of Medicine and the Icahn School of Medicine at Mount Sinai (U10EY08052), the Johns Hopkins University School of Public Health (U10EY08057), and the University of Wisconsin, Madison (U10EY08067). Additional support was provided by the Skirball Foundation (New York, NY, USA; GNH), the Elizabeth Taylor AIDS Foundation (Beverly Hills, CA, USA) through a gift to the Herb Ritts Jr., Memorial Vision Fund at the UCLA Stein Eye Institute, and an unrestricted grant from Research to Prevent Blindness, Inc. (New York, NY, USA) to the UCLA Stein Eye Institute in support of clinical research.
Disclosure: G.N. Holland, None; M.L. Van Natta, None; D.T. Goldenberg, None; R. Ritts Jr, None; R.P. Danis, EyeKor (C), Ionis Pharmaceuticals (C), Chengdu KangHong Biopharmaceuticals (C); D.A. Jabs, None
References
- 1.Holland GN. AIDS and ophthalmology: the first quarter century. Am J Ophthalmol. 2008;145:397–408. doi: 10.1016/j.ajo.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Jabs DA. Cytomegalovirus retinitis and the acquired immunodeficiency syndrome--bench to bedside: LXVII Edward Jackson Memorial Lecture. Am J Ophthalmol. 2011;151:198–216.e1. doi: 10.1016/j.ajo.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 4.Holtzer CD, Jacobson MA, Hadley WK, et al. Decline in the rate of specific opportunistic infections at San Francisco General Hospital, 1994-1997. AIDS. 1998;12:1931–1933. [PubMed] [Google Scholar]
- 5.Ausayakhun S, Keenan JD, Ausayakhun S, et al. Clinical features of newly diagnosed cytomegalovirus retinitis in northern Thailand. Am J Ophthalmol. 2012;153:923–931.e1. doi: 10.1016/j.ajo.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jabs DA, Van Natta ML, Kempen JH, et al. Characteristics of patients with cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Am J Ophthalmol. 2002;133:48–61. doi: 10.1016/s0002-9394(01)01322-8. [DOI] [PubMed] [Google Scholar]
- 7.Holland GN, Vaudaux JD, Jeng SM, et al. Characteristics of untreated AIDS-related cytomegalovirus retinitis. I. Findings before the era of highly active antiretroviral therapy (1988 to 1994) Am J Ophthalmol. 2008;145:5–11. doi: 10.1016/j.ajo.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 8.Holland GN, Vaudaux JD, Shiramizu KM, et al. Characteristics of untreated AIDS-related cytomegalovirus retinitis. II. Findings in the era of highly active antiretroviral therapy (1997 to 2000) Am J Ophthalmol. 2008;145:12–22. doi: 10.1016/j.ajo.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 9.Holland GN, Shuler JD. Progression rates of cytomegalovirus retinopathy in ganciclovir-treated and untreated patients. Arch Ophthalmol. 1992;110:1435–1442. doi: 10.1001/archopht.1992.01080220097029. [DOI] [PubMed] [Google Scholar]
- 10.Studies of Ocular Complications of AIDS Research Group in collaboration with the AIDS Clinical Trials Group. Foscarnt-Ganciclovir Cytomegalovirus Retinitis Trial. 4. Visual Outcomes. Ophthalmology. 1994;101:1250–1261. [PubMed] [Google Scholar]
- 11.Holbrook JT, Davis MD, Hubbard LD, et al. Risk factors for advancement of cytomegalovirus retinitis in patients with acquired immunodeficiency syndrome. Studies of Ocular Complications of AIDS Research Group. Arch Ophthalmol. 2000;118:1196–1204. doi: 10.1001/archopht.118.9.1196. [DOI] [PubMed] [Google Scholar]
- 12.Studies of Ocular Complications of AIDS (SOCA) Research Group; AIDS Clinical Trials Group (ACTG) Parenteral cidofovir for cytomegalovirus retinitis in patients with AIDS: the HPMPC peripheral cytomeglovirus retinitis trial. A randomized, controlled trial. Ann Intern Med. 1997;126:264–274. doi: 10.7326/0003-4819-126-4-199702150-00002. [DOI] [PubMed] [Google Scholar]
- 13.Studies of Ocular Complications of AIDS (SOCA) Research Group; AIDS Clinical Trials Group (ACTG) MSL-109 Adjuvant therapy for cytomegalovirus retinitis in patients with acquired immunodeficiency syndrome: the Monoclonal Antibody Cytomegalovirus Retinitis Trial. Arch Ophthalmol. 1997;115:1528–1536. [PubMed] [Google Scholar]
- 14.Studies of Ocular Complications of AIDS Research Group; AIDS Clinical Trials Group. The ganciclovir implant plus oral ganciclovir versus parenteral cidofovir for the treatment of cytomegalovirus retinitis in patients with acquired immunodeficiency syndrome: The Ganciclovir Cidofovir Cytomegalovirus Retinitis Trial. Am J Ophthalmol. 2001;131:457–467. doi: 10.1016/s0002-9394(01)00840-6. [DOI] [PubMed] [Google Scholar]
- 15.Jabs DA, Van Natta ML, Holbrook JT, Kempen JH, Meinert CL, Davis MD. Longitudinal study of the ocular complications of AIDS: 1. Ocular diagnoses at enrollment. Ophthalmology. 2007;114:780–786. doi: 10.1016/j.ophtha.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Jabs DA, Van Natta ML, Holbrook JT, Kempen JH, Meinert CL, Davis MD. Longitudinal study of the ocular complications of AIDS: 2. Ocular examination results at enrollment. Ophthalmology. 2007;114:787–793. doi: 10.1016/j.ophtha.2006.07.065. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 18.Holland GN, Buhles WC, Jr, Mastre B, Kaplan HJ. A controlled retrospective study of ganciclovir treatment for cytomegalovirus retinopathy. Use of a standardized system for the assessment of disease outcome. Arch Ophthalmol. 1989;107:1759–1766. doi: 10.1001/archopht.1989.01070020841024. [DOI] [PubMed] [Google Scholar]
- 19.Kempen JH, Min YI, Freeman WR, et al. Risk of immune recovery uveitis in patients with AIDS and cytomegalovirus retinitis. Ophthalmology. 2006;113:684–694. doi: 10.1016/j.ophtha.2005.10.067. [DOI] [PubMed] [Google Scholar]
- 20.Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–723. [Google Scholar]
- 21.Holland GN, Pepose JS, Pettit TH, Gottlieb MS, Yee RD, Foos RY. Acquired immune deficiency syndrome. Ocular manifestations. Ophthalmology. 1983;90:859–873. doi: 10.1016/s0161-6420(83)80009-8. [DOI] [PubMed] [Google Scholar]
- 22.Pepose JS, Holland GN, Nestor MS, Cochran AJ, Foos RY. Acquired immune deficiency syndrome. Pathogenic mechanisms of ocular disease. Ophthalmology. 1985;92:472–484. doi: 10.1016/s0161-6420(85)34008-3. [DOI] [PubMed] [Google Scholar]
- 23.Bloom JN, Palestine AG. The diagnosis of cytomegalovirus retinitis. Ann Intern Med. 1988;109:963–969. doi: 10.7326/0003-4819-109-12-963. [DOI] [PubMed] [Google Scholar]
- 24.Fay MT, Freeman WR, Wiley CA, Hardy D, Bozzette S. Atypical retinitis in patients with the acquired immunodeficiency syndrome. Am J Ophthalmol. 1988;105:483–490. doi: 10.1016/0002-9394(88)90239-5. [DOI] [PubMed] [Google Scholar]
- 25.Guyer DR, Jabs DA, Brant AM, Beschorner WE, Green WR. Regression of cytomegalovirus retinitis with zidovudine. A clinicopathologic correlation. Arch Ophthalmol. 1989;107:868–874. doi: 10.1001/archopht.1989.01070010890037. [DOI] [PubMed] [Google Scholar]
- 26.Sha BE, Benson CA, Deutsch TA, Urbanski PA, Phair JP, Kessler HA. Suppression of cytomegalovirus retinitis in persons with AIDS with high-dose intravenous acyclovir. J Infect Dis. 1991;164:777–780. doi: 10.1093/infdis/164.4.777. [DOI] [PubMed] [Google Scholar]
- 27.Henderly DE, Freeman WR, Causey DM, Rao NA. Cytomegalovirus retinitis and response to therapy with ganciclovir. Ophthalmology. 1987;94:425–434. doi: 10.1016/s0161-6420(87)33454-2. [DOI] [PubMed] [Google Scholar]
- 28.Lowder CY, Butler CP, Dodds EM, Secic M, Recillas-Gispert C. CD8+ T lymphocytes and cytomegalovirus retinitis in patients with the acquired immunodeficiency syndrome. Am J Ophthalmol. 1995;120:283–290. doi: 10.1016/s0002-9394(14)72157-9. [DOI] [PubMed] [Google Scholar]
- 29.Oka S, Nagata Y, Fujino Y, et al. CD8+ T lymphocyte counts as an adjunctive predictor of cytomegalovirus retinitis in patients with acquired immunodeficiency syndrome. Intern Med. 1997;36:461–465. doi: 10.2169/internalmedicine.36.461. [DOI] [PubMed] [Google Scholar]
- 30.Reed JB, Schwab IR, Gordon J, Morse LS. Regression of cytomegalovirus retinitis associated with protease-inhibitor treatment in patients with AIDS. Am J Ophthalmol. 1997;124:199–205. doi: 10.1016/s0002-9394(14)70784-6. [DOI] [PubMed] [Google Scholar]
- 31.Reed JB, Briggs JW, McDonald JC, Freeman WR, Morse LS. Highly active antiretroviral therapy-associated regression of cytomegalovirus retinitis: Long-term results in a small case series. Retina. 2001;21:339–343. doi: 10.1097/00006982-200108000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Sinclair E, Tan QX, Sharp M, et al. Protective immunity to cytomegalovirus (CMV) retinitis in AIDS is associated with CMV-specific T cells that express interferon- gamma and interleukin-2 and have a CD8+ cell early maturational phenotype. J Infect Dis. 2006;194:1537–1546. doi: 10.1086/508997. [DOI] [PubMed] [Google Scholar]
- 33.Skolnik PR, Pomerantz RJ, de la Monte SM, et al. Dual infection of retina with human immunodeficiency virus type 1 and cytomegalovirus. Am J Ophthalmol. 1989;10:361–372. doi: 10.1016/0002-9394(89)90659-4. [DOI] [PubMed] [Google Scholar]
- 34.Skolnik PR, Kosloff BR, Hirsch MS. Bidirectional interactions between human immunodeficiency virus type 1 and cytomegalovirus. J Infect Dis. 1988;157:508–514. doi: 10.1093/infdis/157.3.508. [DOI] [PubMed] [Google Scholar]
- 35.Ho WZ, Harouse JM, Rando RF, Gonczol E, Srinivasan A, Plotkin SA. Reciprocal enhancement of gene expression and viral replication between human cytomegalovirus and human immunodeficiency virus type 1. J Gen Virol. 1990;71:97–103. doi: 10.1099/0022-1317-71-1-97. [DOI] [PubMed] [Google Scholar]
- 36.Ho WZ, Ayyavoo V, Srinivasan A, Stinski MF, Plotkin SA, Gonczol E. Human immunodeficiency virus type 1 tat gene enhances human cytomegalovirus gene expression and viral replication. AIDS Res Human Retroviruses. 1991;7:689–695. doi: 10.1089/aid.1991.7.689. [DOI] [PubMed] [Google Scholar]
- 37.Wutoh AK, Hidalgo J, Bareta J, Rhee W, Beardsley R, Steidl S. Race and the treatment of cytomegalovirus retinitis in a cohort of patients with acquired immunodeficiency syndrome. J National Med Assoc. 1998;90:214–220. [PMC free article] [PubMed] [Google Scholar]
- 38.Meyers JD, Reed EC, Shepp DH, et al. Acyclovir for prevention of cytomegalovirus infection and disease after allogeneic marrow transplantation. N Engl J Med. 1988;318:70–75. doi: 10.1056/NEJM198801143180202. [DOI] [PubMed] [Google Scholar]
- 39.Balfour HH, Jr, Chace BA, Stapleton JT, Simmons RL, Fryd DS. A randomized, placebo-controlled trial of oral acyclovir for the prevention of cytomegalovirus disease in recipients of renal allografts. N Engl J Med. 1989;320:1381–1387. doi: 10.1056/NEJM198905253202105. [DOI] [PubMed] [Google Scholar]
- 40.Mollison LC, Richards MJ, Johnson PD, et al. High-dose oral acyclovir reduces the incidence of cytomegalovirus infection in liver transplant recipients. J Infect Dis. 1993;168:721–724. doi: 10.1093/infdis/168.3.721. [DOI] [PubMed] [Google Scholar]
- 41.Feinberg JE, Hurwitz S, Cooper D, et al. AIDS Clinical Trials Group Protocol 204/Glaxo Wellcome 123-014 International CMV Prophylaxis Study Group A randomized, double-blind trial of valaciclovir prophylaxis for cytomegalovirus disease in patients with advanced human immunodeficiency virus infection. J Infect Dis. 1998;177:48–56. doi: 10.1086/513804. [DOI] [PubMed] [Google Scholar]
- 42.Ruiz-Cruz M, Alvarado-de la Barrera C, Ablanedo-Terrazas Y, Reyes-Teran G. Proposed clinical case definition for cytomegalovirus-immune recovery retinitis. Clin Infect Dis. 2014;59:298–303. doi: 10.1093/cid/ciu291. [DOI] [PubMed] [Google Scholar]
- 43.Shepp DH, Match ME, Ashraf AB, Lipson SM, Millan C, Pergolizzi R. Cytomegalovirus glycoprotein B groups associated with retinitis in AIDS. J Infect Dis. 1996;174:184–187. doi: 10.1093/infdis/174.1.184. [DOI] [PubMed] [Google Scholar]
- 44.Deghaide NH, Rodrigues Mde L, Castelli EC, Mendes-Junior CT, Figueiredo JF, Donadi EA. Tumor necrosis factor region polymorphisms are associated with AIDS and with cytomegalovirus retinitis. AIDS. 2009;23:1641–1647. doi: 10.1097/QAD.0b013e32832e5591. [DOI] [PubMed] [Google Scholar]
- 45.Sezgin E, Jabs DA, Hendrickson SL, et al. Effect of host genetics on the development of cytomegalovirus retinitis in patients with AIDS. AIDS. 2010;202:606–613. doi: 10.1086/654814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sezgin E, van Natta ML, Ahuja A, et al. Association of host genetic risk factors with the course of cytomegalovirus retinitis in patients infected with human immunodeficiency virus. Am J Ophthalmol. 2011;151:999–1006.e4. doi: 10.1016/j.ajo.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]