Abstract
Preoperative or induction chemotherapy with docetaxel, cisplatin, plus 5‐fluorouracil (DCF) is a promising regimen for advanced esophageal cancer. However, the DCF regimen is associated with a high risk of severe neutropenia or febrile neutropenia (FN). However, the current guidelines fail to recommend an optimal dosing schedule of pegfilgrastim along with the DCF regimen to prevent FN. In the present study, we assessed the efficacy and safety of giving pegfilgrastim early on day 3 during DCF therapy for esophageal cancer. In this single‐arm phase II study, patients with squamous cell carcinoma of the esophagus were recruited. They were treated with the DCF therapy on days 1‐5, with pegfilgrastim given on day 3. Primary endpoint was the occurrence of grade 4 neutropenia. Secondary endpoints included the incidence of FN, grade 3 neutropenia, dose delays/reductions, antitumor effect, and safety. Between July 2016 and December 2018, 23 patients were enrolled. The incidence of grade 4 neutropenia was 8.7% (95% confidence interval 1.1%‐28.0%). No patient experienced FN. Of the 19 patients who received two cycles of DCF, one required a dose reduction/treatment delay due to hematological toxicity in the second treatment cycle. No serious adverse events, considered relevant to pegfilgrastim, were observed. This is the first prospective study that showed an efficacious dosing schedule of pegfilgrastim for preventing hematological toxicity during DCF therapy. The results might be generalized to other similar regimens where continuous infusions of 5‐fluorouracil are used.
Keywords: docetaxel, cisplatin, plus 5‐fluorouracil therapy, esophageal cancer, neoadjuvant chemotherapy, neutropenia, pegfilgrastim
This study is a prospective clinical trial showing that giving pegfilgrastim early, as primary prophylaxis in docetaxel, cisplatin, plus 5‐fluorouracil (DCF) therapy, is effective and safe in reducing the risk of severe neutropenia and FN. We believe that our study makes a significant contribution to the literature because this is the first study to provide supporting evidence on early dosing of pegfilgrastim during DCF therapy.

Abbreviations
- ANC
absolute neutrophil count
- CF
cisplatin and 5‐fluorouracil
- DCF
docetaxel, cisplatin, plus 5‐fluorouracil
- FN
febrile neutropenia
- G‐CSF
granulocyte colony‐stimulating factor
1. INTRODUCTION
Esophageal cancer is the sixth leading cause of cancer‐related death worldwide, and its incidence is increasing globally.1 Although esophagectomy is the curative treatment for patients with locally advanced esophageal cancer, the results are unsatisfactory with a 5‐year survival rate of <40%.2 To improve treatment outcomes, several neoadjuvant therapies have been recommended. Both preoperative chemotherapy and chemoradiotherapy improve overall survival compared to surgery alone.3, 4, 5 In Western countries, neoadjuvant chemoradiotherapy is recommended as standard preoperative treatment, whereas in most Eastern countries, including Japan, neoadjuvant chemotherapy is recommended as a preoperative treatment for locally advanced esophageal cancer. However, the superiority of neoadjuvant chemotherapy or chemoradiotherapy remains controversial. Radiotherapy is expected to improve local control, whereas chemotherapy has the potential to eliminate micrometastases, thus, improving systemic control. In Japan, transthoracic esophagectomy with regional lymphadenectomy has achieved better local control.6 Therefore, systemic control with intensive neoadjuvant chemotherapy is considered more important to improve the survival of patients with locally advanced esophageal cancers.
Currently, neoadjuvant chemotherapy with CF is recommended as standard treatment for locally advanced esophageal cancers in Japan. However, overall survival with this treatment remains unsatisfactory.7 In this context, strategies to reinforce neoadjuvant chemotherapy by adding docetaxel to CF (DCF) have been explored to improve overall survival. Hara and colleagues conducted a feasibility study of the DCF regimen for locally advanced esophageal cancers and showed highly promising antitumor activity (overall response rate = 64.3%, pathological complete response = 17%) and severe myelotoxicity reactions (≥grade 3 neutropenia = 83.3%).8 Although DCF might be promising for locally advanced esophageal cancers, the adverse event of severe myelotoxicity was consistently reported in the literature. The incidence of ≥grade 3 neutropenia and febrile neutropenia (FN) reached 66.6% and 22.9%, respectively.9 Thus, although DCF has a highly promising antitumor activity, it has a very high risk of severe neutropenia or FN, which is a safety concern as preoperative treatment.
Primary prophylaxis with pegfilgrastim, a long‐acting pegylated form of G‐CSF, is recommended for the prevention of FN in patients receiving high‐risk chemotherapy regimens such as DCF. According to the guidelines,10, 11 pegfilgrastim should be given at least 24 hours after the completion of chemotherapy (ie, 1‐3 days [American Society of Clinical Oncology, ASCO] or 1‐4 days [National Comprehensive Cancer Network, NCCN] after chemotherapy). However, there is no prospective study on the DCF regimen that validates the efficacy and safety of pegfilgrastim in the prevention of FN, and there are only two retrospective studies.12, 13 One study was on head and neck cancer and the other was our study on esophageal cancer. Our previous retrospective study evaluated the efficacy of giving pegfilgrastim on day 7, which was 24 hours after the completion of DCF therapy. ANC nadir was observed from day 7, and the incidence rates of ≥grade 3 neutropenia and FN were 50.0% and 22.7%, respectively.13 Thus, timing (day 7) of the dose was not appropriate as the ANC nadir appeared early. Until now, the optimal dosing schedule of pegfilgrastim along with regimens involving continuous infusions of 5‐fluorouracil remains unclear. No prospective trial has evaluated the timing of giving prophylactic G‐CSF along with DCF therapy. Therefore, we conducted this prospective study to assess the efficacy and safety of early dosing (day 3) of pegfilgrastim during DCF therapy for patients with advanced squamous cell carcinoma of the esophagus.
2. PATIENTS AND METHODS
2.1. Study design and patients
We conducted a single‐arm, phase II study to evaluate the efficacy and safety of early dosing (day 3) of pegfilgrastim during DCF therapy for patients with squamous cell carcinoma of the esophagus.
Participants with pathologically diagnosed primary squamous cell carcinoma of the esophagus were identified at University Hospital, Kyoto Prefectural University of Medicine and selected based on the following eligibility criteria.
2.1.1. Key inclusion criteria
Histologically confirmed squamous cell carcinoma of the esophagus; clinical stage II, III or IV (International Union Against Cancer TNM classification system, 7th edition); patients over 20 years old who could be treated with DCF therapy without dose reduction; no prior history of systemic chemotherapy or radiotherapy; ECOG performance status 0 or 1; and adequate organ function (leukocyte count ≥4000 and <12 000/μL, neutrophil count ≥1500/μL, hemoglobin level ≥10.0 g/dL, platelet count ≥100 000/μL, AST and ALT ≤100 IU/L, total bilirubin ≤1.5 mg/dL, serum creatinine ≤1.5 mg/dL, creatinine clearance ≥60 mL/min).
2.1.2. Key exclusion criteria
Malignancies other than carcinoma in situ or mucosal carcinoma; evidence of any other serious disease; active local or systemic infection; history of severe allergic reactions.
This study was approved by the ethics committee of the Kyoto Prefectural University of Medicine (approval no. ERB‐C‐601). The trial was designed and conducted in line with the Helsinki Declaration and the Ethical Guidelines for Clinical Research (the Ministry of Health, Labor and Welfare, Japan). All participants provided written informed consent before study enrolment. This trial was registered at the University Hospital Medical Information Network (UMIN) Clinical Trial Registry (ID: UMIN000023393).
2.2. Treatment procedures
Chemotherapy consisted of an infusion of docetaxel (70 mg/m2 per day) and cisplatin (70 mg/m2 per day) on day 1, and continuous infusions of 5‐fluorouracil (750 mg/m2 per day) on days 1‐5 (DCF: 70/70/750). The regimen was repeated every 3 weeks. Pegfilgrastim was given as a single s.c. injection at a dose of 3.6 mg on day three in each cycle. Prophylactic oral antibiotics (ie, levofloxacin) on days 5‐9 in each cycle were allowed if deemed necessary by physicians.
2.3. Assessments
Blood samples were collected for complete blood count and serum chemistry on days 1, 3, 7, 8, 9, 10, and 14 of each cycle. FN is defined as an ANC lower than 1000/μL at a temperature of 38°C or higher. Safety was assessed using the incidence of adverse events. Severity of all adverse events was graded in line with the Common Terminology Criteria for Adverse Events v 4.0 (CTCAE). Tumor response was assessed using computed tomography (CT) scans in the third or fourth week of the second cycle based on the RECIST version 1.1 criteria.14 Residual tumor (R) was classified as follows: R0, no residual tumor; R1, suspicion of residual tumor or microscopic residual tumor; and R2, macroscopic residual tumor. Pathological response was evaluated in line with the Japanese Classification of Esophageal Cancer (11th edn) [23], which categorizes tumors into four response levels (grades 0‐3). Grade 3 indicates no viable cancer cells. Grade 2 indicates viable cancer cells accounting for less than 1/3 of tumor tissue while other cancer cells are severely degenerated or necrotic. Grade 1 is further classified as Grades 1a and 1b. Grade 1a indicates viable cancer cells accounting for 2/3 or more of the tumor tissue, whereas grade 1b indicates viable cancer cells accounting for 1/3 or more, but <2/3, of the tumor tissue. Grade 0 indicates no recognizable cytological or histological therapeutic effect.
2.4. Endpoints and statistical methods
Primary objective of the present study was to estimate the incidence of grade 4 neutropenia in cycle one. Secondary objectives were to estimate the incidence of FN and grade 3 neutropenia, and the depth of the ANC nadir in cycle one and the incidence of delayed chemotherapy and dose reduction of cytotoxic drugs in cycle two. Evaluation of the antitumor effect after two cycles of chemotherapy and a safety assessment during chemotherapy were also included as secondary objectives.
In the present study, we expected the incidence of grade 4 neutropenia during DCF therapy along with pegfilgrastim to be lower than that without giving prophylactic G‐CSF, and that the proportion of grade 4 neutropenia would be lower than that from the previous Japanese studies (63%‐82%).15, 16 A minimum sample size of 21 was required for one‐sided α of 0.1 and β of 0.1, with an expected 25% incidence of grade 4 neutropenia and a threshold incidence of 55%. Assuming 10% dropout cases, the target sample size was 24 patients in total. Confidence intervals for all proportions were estimated assuming binomial distribution. All statistical analyses were carried out using SPSS software (version 20) for Windows (IBM Corporation).
3. RESULTS
3.1. Patients
Between July 2016 and January 2019, 23 eligible patients were enrolled in this study. Patient characteristics are shown in Table 1. Sixteen patients were males and seven were females. Median age of all patients was 62 years (range: 44‐75). Most patients had an ECOG performance status of 0 or 1. Overall, 19 patients received two cycles of DCF therapy, and four patients discontinued the subsequent second cycle of DCF therapy. Two patients were switched to doublet chemotherapy as a result of severe toxicities (ie, hematopoietic and gastrointestinal toxicities or nephrotoxicity), and two were switched to chemoradiotherapy because of the lack of efficacy. Finally, 19 of 23 patients underwent esophagectomy (Figure 1).
Table 1.
Demographics of patients with pathologically diagnosed primary squamous cell carcinoma of the esophagus
| Clinical characteristics |
No. of patients (n = 23) n (%) |
|---|---|
| Age, y, median (range) | 62 (44‐75) |
| Gender | |
| Male/female | 16/7 |
| ECOG performance status | |
| ≤1/>1 | 22/1 |
| Tumor location | |
| Upper esophagus | 7 |
| Middle esophagus | 13 |
| Distal esophagus | 3 |
| Clinical T | |
| 1/2/3/4 | 0/1/17/5 |
| Clinical N | |
| 0/1/2/3 | 2/9/8/4 |
| Clinical M | |
| 0/1 | 23/0 |
| Clinical Stage | |
| 2/3a/3b/3c | 2/9/6/6 |
Figure 1.
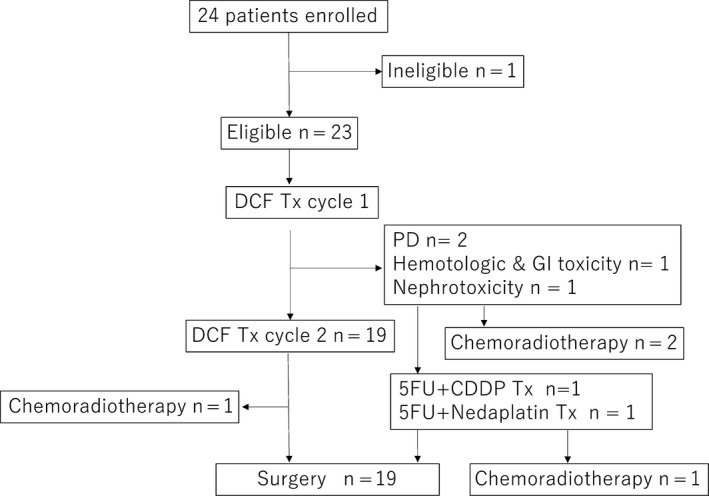
Accrual and treatment summary of patients in the present study. 5‐FU, fluorouracil; CDDP, cisplatin; DCF, docetaxel, cisplatin, plus 5‐fluorouracil therapy; GI, gastrointestinal; PD, progression of disease
3.2. Efficacy of pegfilgrastim
Incidence of grade 4 neutropenia in cycle 1 was 8.7% (2/23, 95% CI: 1.1%‐28.0%), and the confidence interval (80% CI: 2.3%‐21.5%) corresponding to one‐sided α of 0.1 showed that the incidence was statistically significantly lower than the threshold value of 55%. Six patients (26.1%) developed any grade of neutropenia (grade 3, four patients; grade 4, two patients). No patient experienced FN. Median ANC in the nadir was 2170/μL (min, 40; max, 4290). The nadir of ANC was observed on day 8 for 17 patients and on day 7 for six patients (Figure 2).
Figure 2.
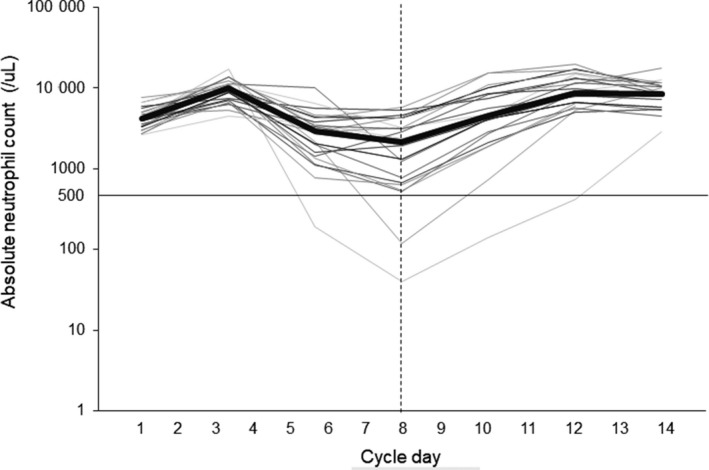
Absolute neutrophil count (ANC) trajectory of each patient in cycle 1. The bold line represents the median of all patients (n = 23). ANC values are shown on a natural logarithmic scale
Of the 19 patients who received two cycles of DCF therapy, four patients required a delay of chemotherapy in the second cycle. Reasons for treatment delay in three out of four patients included development of mild mediastinitis after the first cycle of DCF, a planned endoscopic mucosal dissection for colorectal tumor, and patient’s own preference. The other patient (5.3%) required both treatment delay and dose reduction in the second cycle as a result of hematological toxicity (Table 2).
Table 2.
Incidence of neutropenia, febrile neutropenia, treatment delay and dose reduction
| No. (frequency, %) | |
|---|---|
| Neutropenia | |
| Grade 4 | 2 (8.7) |
| Grade 3 | 4 (17.4) |
| Any grade | 6 (26.1) |
| Febrile neutropenia | 0 (0) |
| Treatment delaya | 4 (21.1) |
| Due to hematotoxicity | 1 (5.3) |
| Dose reductiona | 1 (5.3) |
| Due to hematotoxicity | 1 (5.3) |
Frequency of events was calculated on the total number of patients receiving the 2nd cycle treatment (n = 19).
3.3. Safety
Overall adverse events excluding neutropenia and FN are listed in Table 3. The most common hematological toxicities above grade 3 were leukocytopenia (30.4%), thrombocytopenia (13.0%), and hyponatremia (13.0%). The most common non‐hematological adverse events above grade 3 were anorexia (17.4%) and oral mucositis (13.0%). One patient developed a treatment‐related esophageal fistula; however, it was improved by conservative treatment with antibiotics. Pegfilgrastim‐related bone pain was observed in two patients. Both cases were mild and quickly resolved. No serious adverse events possibly related to pegfilgrastim were observed.
Table 3.
Adverse events excluding neutropenia and febrile neutropenia
| ≥Grade 3 (%) | Any grade (%) | |
|---|---|---|
| Anorexia | 4 (17) | 18 (78) |
| Fatigue | 0 | 19 (83) |
| Nausea | 0 | 11 (48) |
| Vomiting | 1 (4) | 3 (13) |
| Diarrhea | 1 (4) | 11 (48) |
| Oral mucositis | 3 (13) | 8 (35) |
| Esophageal fistula | 0 | 1 (4) |
| Bone pain or back pain | 0 | 2 (9) |
| Leukopenia | 7 (30) | 11 (48) |
| Anemia | 1 (4) | 13 (57) |
| Thrombocytopenia | 3 (13) | 18 (78) |
| Aspartate aminotransferase increased | 0 | 6 (26) |
| Alanine aminotransferase increased | 1 (4) | 6 (26) |
| Creatinine increased | 0 | 3 (13) |
| Hyponatremia | 3 (13) | 18 (78) |
3.4. Treatment outcome
For efficacy assessment of DCF therapy, CT was carried out 3 weeks after the start of the second cycle. Most patients enrolled in this study received preoperative treatment and only five patients had measurable lesions by the RECIST criteria. Of the five evaluable patients, four (80%) showed partial response (PR) and one (20%) showed stable disease (SD). Of the 19 patients who underwent surgery, R0 resection was achieved in 18 patients (94.7%). Histopathological complete response CR (grade 3) was achieved in two patients (10.5%). Grade 2 and grade 1b and 1a responses were observed in seven (36.8%), 0, and 10 (52.6%) patients, respectively (Table 4).
Table 4.
Treatment outcome of early dosing (day 3) of pegfilgrastim during DCF therapy for patients with squamous cell carcinoma of the esophagus
| n (%) | |
|---|---|
| Objective response, n = 5 | |
| PR | 4 |
| SD | 1 |
| PD | 0 |
| Residual tumor (R), n = 19 | |
| R0 | 18 |
| R1 | 0 |
| R2 | 1 |
| Pathological response, n = 19 | |
| Grade 1a | 10 (52.6) |
| Grade 1b | 0 (0) |
| Grade 2 | 7 (36.8) |
| Grade 3 | 2 (10.5) |
docetaxel, cisplatin, plus 5‐fluorouracil therapy; PD, progression of disease; PR, partial response; SD, stable disease.
4. DISCUSSION
As DCF therapy is associated with a high risk of infection, FN, and severe neutropenia, giving primary prophylactic G‐CSF is recommended to lower the risks. However, the guidelines do not recommend a detailed dosing plan for G‐CSF in the course of continuous chemotherapy for several days. According to the guidelines,10, 11 G‐CSF should be given 24 hours after the completion of chemotherapy due to the likelihood of increased sensitivity of rapidly dividing myeloid cells to cytotoxic chemotherapy and, paradoxically, increased risks of hematological toxicity. There is a lack of data on the concomitant use of G‐CSF and chemotherapy, and the optimal dosing schedule of pegfilgrastim remained unclear along with regimens that involved continuous infusions of 5‐fluorouracil such as the DCF regimen. In the present study, we showed that giving pegfilgrastim on day 3 reduced the incidence of grade 4 neutropenia (8.7%), FN (0%), and the occurrence of dose delay or reduction of chemotherapy. In other words, giving concomitant pegfilgrastim and 5‐fluorouracil did not increase hematological toxicity, and this protocol is safe and effective for the prevention of severe neutropenia and FN. This is the first prospective study showing an effective prophylactic G‐CSF dosing schedule to prevent hematological toxicity during DCF therapy. The results might be generalized to similar regimens such as ECF (epirubicin and cisplatin plus 5‐fluorouracil) where continuous infusions of 5‐fluorouracil are used.
Based on the theoretical concern that the stimulation of bone marrow progenitors by G‐CSF might increase the pool of precursors vulnerable to cell cycle‐specific chemotherapy, giving G‐CSF is recommended 24 hours after chemotherapy. However, several reports showed that a simultaneous dose of pegfilgrastim and cytotoxic chemotherapy is feasible and safe in different chemotherapy regimens.17, 18, 19, 20 As for DCF therapy, a retrospective study investigated the efficacy and safety of early dosage of G‐CSF in patients with head and neck cancer.12 This study was conducted to compare early dosage of G‐CSF (pegfilgrastim on day 3 or filgrastim from day 3 to day 11) during chemotherapy versus pegfilgrastim on day 7 in patients treated with DCF. The incidence of grade 3‐4 neutropenia, FN, and a delay of the second cycle was significantly lower in the early G‐CSF arm compared to the day 7 G‐CSF arm (2.9% vs 20.0%, 1.4% vs 12.9%, 0% vs 12.9%, respectively). Moreover, overall survival tended to be better in the early G‐CSF arm (2‐year OS; 84.7% for the early G‐CSF vs. 77.2% for the day 7 G‐CSF arm).12
In the present study, the frequency of grade 4 neutropenia was 8.7% (80% CI: 2.3%‐21.5%), which was significantly lower than the prespecified threshold of 55%, and was also much lower than the reported frequencies (63%‐82%) for DCF therapy for esophageal cancer without prophylactic dosage of pegfilgrastim.15, 16 The frequency of grade 3 or higher neutropenia was 26.1%, which was lower than the previous reports (67%‐83%).8, 9 In terms of FN, it is reported that the incidence usually reached 22.9% in DCF therapy without preventive measures such as prophylactic use of antibiotics.9 Of note, in the present study, no patients developed FN. This result is mainly due to giving prophylactic pegfilgrastim. It may also be related to the prophylactic use of antibiotics in all patients in the current study.
With regard to adverse events, 8.7% of all patients reported pegfilgrastim‐related bone pain or back pain, all of which were mild and immediately resolved. No other adverse events related to pegfilgrastim were observed among study participants. Therefore, there was no evidence suggesting that giving early pegfilgrastim enhanced DCF therapy‐related toxicities.
Although clinical efficacy was not a primary endpoint in our study, of the 19 patients who received two cycles of DCF therapy, tumor‐size reduction at the primary and/or metastatic sites was observed in 16 (84.2%) patients, with only one patient showing an increase in tumor size (data not shown). Although only five patients could be evaluated using the RECIST criteria, the overall response rate in these patients was 80%. Of the 19 patients who received surgery, two (10.5%) achieved histopathological CR (grade 3). The pathological response (grade 2 or higher) reached 47.3%, which was comparable with that in previous studies.8, 13 Dose‐dense chemotherapy with prophylactic pegfilgrastim was expected to improve the pathological response. However, the results of this study were not in accordance with this hypothesis possibly due to the small sample size. When applying the DCF regimen as neoadjuvant chemotherapy, both a high response rate and a low disease progression rate is important to avoid the inability to operate. In this study, there were two patients (8.6%) with no response after one cycle of DCF therapy which was higher than that in a previous study by Hara et al (2.3%).8 As the study by Hara et al was conducted to investigate the feasibility of the DCF regimen as neoadjuvant chemotherapy in patients with clinical stage II/III esophageal cancer, cT4 cases were not included in the study. In contrast, as our study included patients who received the DCF regimen as palliative chemotherapy and included 21.7% cT4 cases, there were more advanced cases in our study than in the study by Hara et al Moreover, differences in the background of patients between these studies may influence the outcome. However, it should be taken into account that giving pegfilgrastim may have attenuated the antitumor effects of DCF therapy.
The small number of patients and a short follow‐up period (42 days) are major limitations of our study. In addition, we used pegfilgrastim at a dose of 3.6 mg, which is approved by the Japanese healthcare system. However, pegfilgrastim is usually given at a dose of 6.0 mg in other countries. Therefore, it is not clear whether the study results are applicable to the 6.0‐mg pegfilgrastim treatment protocol. In this study, prophylactic antibiotics were given in all cases, and this could have potentially confounded the effect of pegfilgrastim on FN development. Recently, giving broad‐spectrum antibiotics has been reported to impair the anticancer effects of chemotherapy.21, 22 Moreover, primary antibioprophylaxis therapy for chemotherapy‐induced FN is not recommended by the European Organization for Research and Treatment of Cancer (EORTC).23 Therefore, it is necessary to examine the effect of pegfilgrastim without antibiotics.
In conclusion, the present study is the first prospective clinical trial showing that giving early pegfilgrastim as primary prophylaxis in DCF therapy is effective and safe in reducing the risk of severe neutropenia and FN. Our observations should be further confirmed by comparative trials with sufficient sample size and a longer follow‐up period. In the future, the impact on efficacy should be carefully evaluated in large‐scale studies.
DISCLOSURE
Satoshi Teramukai received statistical analysis instruction fee from Sanofi. Yuji Naito received research funding from Nippon Kayaku outside the submitted work. The other authors have no conflicts of interest to declare.
ACKNOWLEDGMENTS
The authors would like to thank the patients and families who participated in this study. We also appreciate the help and support from Saeko Tsuchiya in this study.
Ishikawa T, Yasuda T, Okayama T, et al. Early administration of pegfilgrastim for esophageal cancer treated with docetaxel, cisplatin, and fluorouracil: A phase II study. Cancer Sci. 2019;110:3754–3760. 10.1111/cas.14218
This study was registered at the UMIN Clinical Trials Registry as UMIN000023393.
REFERENCES
- 1. Global Burden of Disease Cancer C , Fitzmaurice C, Dicker D, et al. The global burden of cancer 2013. JAMA Oncol. 2015; 1: 505‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347:1662‐1669. [DOI] [PubMed] [Google Scholar]
- 3. Shapiro J, van Lanschot JJB, Hulshof M, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long‐term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090‐1098. [DOI] [PubMed] [Google Scholar]
- 4. Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long‐term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27:5062‐5067. [DOI] [PubMed] [Google Scholar]
- 5. van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074‐2084. [DOI] [PubMed] [Google Scholar]
- 6. Kitagawa Y, Ando N, Nakamura K, Shibata T, Fukuda H. The role of adjuvant chemotherapy for localized squamous cell esophageal cancer: current Japanese standard and the unending role of the drawing board. Ann Surg Oncol. 2012;19:1425‐1427. [DOI] [PubMed] [Google Scholar]
- 7. Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5‐fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 2012;19:68‐74. [DOI] [PubMed] [Google Scholar]
- 8. Hara H, Tahara M, Daiko H, et al. Phase II feasibility study of preoperative chemotherapy with docetaxel, cisplatin, and fluorouracil for esophageal squamous cell carcinoma. Cancer Sci. 2013;104:1455‐1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yokota T, Kato K, Hamamoto Y, et al. Phase II study of chemoselection with docetaxel plus cisplatin and 5‐fluorouracil induction chemotherapy and subsequent conversion surgery for locally advanced unresectable oesophageal cancer. Br J Cancer. 2016;115:1328‐1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smith TJ, Bohlke K, Lyman GH, et al. Recommendations for the Use of WBC Growth Factors: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2015;33:3199‐3212. [DOI] [PubMed] [Google Scholar]
- 11. Crawford J, Becker PS, Armitage JO, et al. Myeloid growth factors, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw JNCCN. 2017;15:1520‐1541. [DOI] [PubMed] [Google Scholar]
- 12. Linot B, Augereau P, Breheret R, Laccourreye L, Capitain O. Efficacy and safety of early G‐CSF administration in patients with head and neck cancer treated by docetaxel‐cisplatin and 5‐fluorouracil (DCF protocol): a retrospective study. Support Care Cancer. 2014;22:2831‐2837. [DOI] [PubMed] [Google Scholar]
- 13. Yasuda T, Ishikawa T, Ohta T, et al. [Impact of primary prophylaxis with pegfilgrastim on clinical outcomes in patients with advanced esophageal cancer receiving chemotherapy with docetaxel, cisplatin, and 5‐FU]. Gan to Kagaku Ryoho. Cancer Chemotherapy. 2018;45:1733‐1736. [PubMed] [Google Scholar]
- 14. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45: 228‐247. [DOI] [PubMed] [Google Scholar]
- 15. Yamasaki M, Miyata H, Tanaka K, et al. Multicenter phase I/II study of docetaxel, cisplatin and fluorouracil combination chemotherapy in patients with advanced or recurrent squamous cell carcinoma of the esophagus. Oncology. 2011;80:307‐313. [DOI] [PubMed] [Google Scholar]
- 16. Sugawara M, Katada C, Katada N, et al. Retrospective evaluation of adverse events of neoadjuvant or induction chemotherapy with docetaxel, cisplatin, and 5‐fluorouracil in esophageal squamous cell carcinoma. Esophagus. 2013;10:65‐69. [Google Scholar]
- 17. Lokich JJ. Same day pegfilgrastim and CHOP chemotherapy for non‐Hodgkin lymphoma. Am J Clin Oncol. 2006;29:361‐363. [DOI] [PubMed] [Google Scholar]
- 18. Lokich J. Same‐day pegfilgrastim and chemotherapy. Cancer Invest. 2005;23:573‐576. [DOI] [PubMed] [Google Scholar]
- 19. Eckstrom J, Bartels T, Abraham I, et al. A single‐arm, retrospective analysis of the incidence of febrile neutropenia using same‐day versus next‐day pegfilgrastim in patients with gastrointestinal cancers treated with FOLFOX or FOLFIRI. Support Care Cancer. 2019;27:873‐878. [DOI] [PubMed] [Google Scholar]
- 20. Whitworth JM, Matthews KS, Shipman KA, et al. The safety and efficacy of day 1 versus day 2 administration of pegfilgrastim in patients receiving myelosuppressive chemotherapy for gynecologic malignancies. Gynecol Oncol. 2009;112:601‐604. [DOI] [PubMed] [Google Scholar]
- 21. Iida N, Dzutsev A, Stewart CA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342:967‐970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Iida N, Mizukoshi E, Yamashita T, et al. Overuse of antianaerobic drug is associated with poor postchemotherapy prognosis of patients with hepatocellular carcinoma. Int J Cancer. 2019;145(10):2701‐2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aapro MS, Bohlius J, Cameron DA, et al. 2010 update of EORTC guidelines for the use of granulocyte‐colony stimulating factor to reduce the incidence of chemotherapy‐induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011;47:8‐32. [DOI] [PubMed] [Google Scholar]


