Abstract
The prognosis of non‐small‐cell lung cancer (NSCLC) patients with interstitial lung disease (ILD) is poor, and 5%‐20% of those receiving chemotherapy experience ILD exacerbation. To evaluate the safety and efficacy of nab‐paclitaxel plus carboplatin for NSCLC patients with ILD, we undertook a multicenter phase II study. Chemotherapy‐naïve patients with advanced NSCLC and mild or moderate ILD received nab‐paclitaxel (100 mg/m2, days 1, 8, and 15) plus carboplatin (area under the curve = 6, day 1) every 3 weeks for 4 cycles (maximum, 6 cycles). Interstitial lung diseases were diagnosed based on criteria for fibrosing interstitial pneumonia. The primary endpoint was the prevalence of exacerbation‐free ILD 28 days after completion of protocol treatment. Secondary endpoints were response rate, progression‐free survival, overall survival, prevalence of exacerbation‐free ILD, and toxicity. Ninety‐four patients were enrolled, and 92 patients received any protocol treatment. Median age was 70 years, and 58% had nonsquamous histology. In the primary analysis, the prevalence of exacerbation‐free ILD 28 days after protocol treatment was 95.7% (88/92; 90% confidence interval, 90.3‐98.5), which met the primary endpoint. Response rate was 51% (95% confidence interval, 40%‐62%). At the time of data cut‐off, median progression‐free survival was 6.2 months, and median overall survival was 15.4 months. The most common grade 3/4 adverse events were neutropenia (75%), leukopenia (53%), anemia (48%), and thrombocytopenia (20%). Two treatment‐related deaths (1 each of pulmonary infection and ILD exacerbation) were observed. This study showed that a combination of nab‐paclitaxel with carboplatin was tolerable in NSCLC patients with mild or moderate ILD in terms of safety. This study is registered at the University Hospital Medical Information Network (UMIN) Clinical Trial Registry (UMIN 000012989).
Keywords: carboplatin, exacerbation, interstitial lung disease, nab‐paclitaxel, non‐small‐cell lung cancer
This phase II study was to evaluate the safety for non‐small‐cell lung cancer (NSCLC) patients with interstitial lung disease (ILD). The prevalence of exacerbation‐free ILD 28 days after protocol treatment was 95.7%. Median progression‐free survival was 6.2 months, and median overall survival was 15.4 months for NSCLC patients with ILD. Nab‐paclitaxel plus carboplatin was tolerable in NSCLC patients with ILD.

Abbreviations
- AE
adverse event
- CI
confidence interval
- CT
computed tomography
- HRCT
high‐resolution computed tomography
- ILD
interstitial lung disease
- NSCLC
non‐small‐cell lung cancer
- OS
overall survival
- PFS
progression‐free survival
- UIP
usual interstitial pneumonia
1. INTRODUCTION
Lung cancer is the leading cause of cancer‐based mortality. Nevertheless, the prognosis of patients with NSCLC has been improving gradually. In lung adenocarcinoma, the development of targeted therapies for driver genes, including epidermal growth factor receptor and anaplastic large‐cell lymphoma kinase, has advanced.1, 2, 3, 4 Conversely, preexisting ILD, especially idiopathic interstitial pneumonia, has been reported to be a risk factor for drug‐related ILD.5 A large prospective cohort study of the epidermal growth factor receptor tyrosine kinase inhibitor gefitinib has shown that preexisting ILD is a strong risk factor for gefitinib‐related ILD as well as cytotoxic chemotherapy‐related ILD.6
The prognosis of NSCLC patients with ILD has been reported to be poor, and 5%‐20% of those receiving chemotherapy can experience ILD exacerbation induced by chemotherapy.7, 8, 9 Although platinum‐based chemotherapies have been considered to be the standard care for NSCLC patients, NSCLC patients with ILD have been excluded from most clinical trials.10, 11 Because NSCLC patients with ILD have few alternatives for cytotoxic chemotherapy drugs, their prognosis is not clear. Two prospective studies have been reported, but standard treatment for NSCLC patients with ILD is not known.12, 13 Some scholars have suggested that a combination of paclitaxel with carboplatin is relatively tolerable for NSCLC patients with ILD.9, 12 A large phase III study showed that a combination of nab‐paclitaxel with carboplatin improved the objective response rate significantly compared with that elicited by paclitaxel plus carboplatin for patients with advanced NSCLC, and with less neurotoxicity.14 To evaluate the safety and efficacy of nab‐paclitaxel plus carboplatin for NSCLC patients with ILD, the multicenter phase II study described here was carried out.
2. MATERIALS AND METHODS
2.1. Study design
This prospective phase II trial was undertaken at 9 institutes in Japan. The study protocol was approved by the institutional review boards of all participating institutes, and all patients provided written informed consent. This study is registered at the University Hospital Medical Information Network Clinical Trial Registry (UMIN 000012989).
2.2. Patients
Eligible patients were aged 20 years or older with NSCLC (confirmed by histology) of clinical stage III, IV, or recurrent after surgery. Interstitial lung diseases were diagnosed by investigators based on the criteria for fibrosing interstitial pneumonia according to 2 factors: (i) HRCT findings showed UIP or “possible” UIP (“inconsistent” UIP without peribronchovascular predominance were excluded from this study) according to an official American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Association statement: idiopathic pulmonary fibrosis (2011) 15; and (ii) other than other known cause of ILD (eg, infection, drug toxicity, occupational environmental exposures, and connective tissue disease) were excluded.15, 16 Patients with “mild” or “moderate” ILD were included in this study (severe ILD was excluded) based on 3 factors: (i) forced vital capacity less than 65%; (ii) desaturation with exertion (PaO2 less than 88% at room air); and (iii) diffusing capacity of the lung for CO 50% or less.17 Mild ILD was defined as having none of these 3 factors, and moderate ILD was as having any 1 of these factors (severe ILD had 2 or 3 of these factors). To control the quality of ILD diagnosis, we undertook central evaluation of the ILD diagnosis for some patients enrolled in this study.
Patients were also required to have: an ECOG performance status of 0 or 1; no prior chemotherapy for advanced disease or thoracic radiotherapy; adequate organ function (ie, total bilirubin less than or equal to 1.5 mg/dL, aspartate aminotransferase and alanine aminotransferase less than 100 IU/L, serum creatinine less than or equal to 1.5 mg/dL, neutrophil count greater than or equal to 1500/mm3, hemoglobin greater than or equal to 9.0 g/dL, platelet count greater than or equal to 100 000/mm3, and PaO2 at room air greater than or equal to 65 Torr). Key exclusion criteria were: symptomatic brain metastases and the requirement for corticosteroid (prednisolone more than 10 mg/day) treatment.
2.3. Treatment
Patients received carboplatin (area under the curve = 6) on day 1 and 100 mg/m2 of nab‐paclitaxel on days 1, 8, and 15, every 3 weeks, up to 4 cycles (maximum, 6 cycles) if unacceptable toxicity or recurrence was not observed.
High‐resolution computed tomography of the chest was done every 2 cycles during protocol treatment, and every 2 months until disease progression after completion of protocol treatment. In addition, chest radiography was undertaken every cycle.
2.4. Statistical analyses
The primary endpoint was the prevalence of exacerbation‐free ILD within 28 days after chemotherapy, which was defined as the percentage of patients not experiencing ILD exacerbation among patients who underwent protocol treatment. Interstitial lung disease exacerbation was diagnosed on the basis of 3 factors: (i) worsening of dyspnea; (ii) HRCT findings (bilateral “ground glass” abnormality with or without focal consolidation, superimposed on the pretreatment interstitial shadow); and (iii) evidence of abnormal gas exchange.18 Patients with an apparent pulmonary infection, pulmonary embolism, or heart failure were excluded. Overall survival was determined from the date of registration to the date of death from any cause or the day of last confirmation of survival. Progression‐free survival was calculated from the date of registration to disease progression or censored at last confirmation of survival. ILD exacerbation‐free time was calculated from the date of registration to exacerbation of ILD, and censored at death or last confirmation of survival. Secondary endpoints were the response rate, PFS, OS, prevalence of exacerbation‐free ILD until data cut‐off, and toxicities. Efficacy and safety analyses were done for all patients who received at least 1 dose of the study treatment. Responses were evaluated based on RECIST version 1.1 criteria.19 Adverse events were graded according to the NCI’s Common Terminology Criteria for Adverse Events version 4.0.
Assuming a prevalence of exacerbation‐free ILD within 28 days of 90% in treated patients indicated the potential usefulness of the regimen, whereas a prevalence of 80% was at the lower limit of interest, alpha = 0.05 (1‐sided) and beta = 0.20, the estimated number of required patients was 77. The planned number of patients was 90 because the diagnosis of ILD can be difficult for physicians.
Overall survival, PFS, and ILD exacerbation‐free time curves were estimated using the Kaplan‐Meier method. Analyses were carried out using R version 3.4.0 (R Foundation for Statistical Computing, Vienna, Austria) and JMP (SAS Institute, Cary, NC, USA).
3. RESULTS
3.1. Patient characteristics
Ninety‐four patients were enrolled from June 2014 and December 2016, and 92 patients received at least protocol treatment. Two patients did not receive any protocol treatment because of ILD exacerbation and infectious endocarditis before treatment start, respectively.
Table 1 summarizes the baseline characteristics of enrolled patients. The median age was 70 (range, 54‐81) years, and ~90% of patients were men. Adenocarcinoma and squamous cell carcinoma were observed in 49 (52%) and 39 (42%) patients, respectively, according to histology. Based on pretreatment HRCT of the chest, UIP and possible UIP patterns were observed in 50 (53%) and 44 (47%) patients, respectively (Figure 1). Sixty‐seven (71%) patients with mild ILD, and 27 (29%) with moderate ILD, were included in our study.
Table 1.
Characteristics of patients with non‐small‐cell lung cancer and interstitial lung disease (ILD) at baseline (n = 94)
| No. of patients | (%) | |
|---|---|---|
| Gender | ||
| Male | 84 | (89) |
| Female | 10 | (11) |
| Age, y | ||
| Median (range) | 70 (54‐81) | |
| Performance status (ECOG) | ||
| 0 | 42 | (45) |
| 1 | 52 | (55) |
| Histology | ||
| Adenocarcinoma | 49 | (52) |
| Squamous cell carcinoma | 39 | (42) |
| Adenosquamous carcinoma | 2 | (2) |
| Others | 4 | (4) |
| Clinical stage | ||
| IIIA | 15 | (16) |
| IIIB | 23 | (24) |
| IV | 47 | (50) |
| Recurrence after surgical resection | 9 | (10) |
| ILD pattern on CT findings | ||
| UIP pattern | 50 | (53) |
| Possible UIP pattern | 44 | (47) |
| Inconsistent UIP pattern | 0 | |
| Severity of ILD | ||
| Mild | 67 | (71) |
| Moderate | 27 | (29) |
| Severe | 0 | |
| Patients with moderate ILD | ||
| %DLCO ≤ 50% | 15 | (17) |
| FVC < 65% | 6 | (6) |
| SpO2 on exertion < 88% | 6 | (6) |
| % predicted FVC, percent | ||
| Median (range) | 90.1 (53.2‐140.1) | |
| FEV1/FVC ratio, percent | ||
| Median (range) | 76.2 (48.1‐114.2) | |
| % predicted DLCO, percent | ||
| Median (range) | 63.7 (21.4‐116.1) | |
| PaO2, Torr | ||
| Median (range) | 82 (65‐116) | |
| KL‐6, U/mL | ||
| Median (range) | 672 (261‐4952) | |
Abbreviations: CT, computed tomography; DLCO, diffusing capacity of the lung for CO; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; SpO2, oxygen saturation; UIP, usual interstitial pneumonia.
Figure 1.
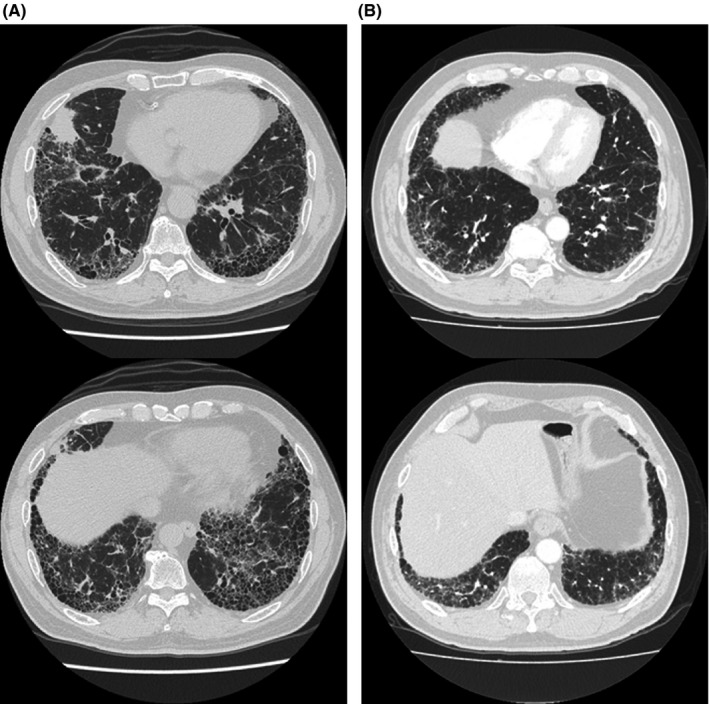
High‐resolution computed tomography (HRCT) images of the chest in patients with non‐small‐cell lung cancer and interstitial lung disease included in this study. A, Pretreatment HRCT image of the chest showing usual interstitial pneumonia pattern. B, Pretreatment HRCT image of the chest showing possible usual interstitial pneumonia pattern
3.2. Adverse events (including ILD exacerbation)
Among the 92 patients involved in the safety analysis, 4 patients experienced ILD exacerbation within 28 days after the final administration of chemotherapy: grade 5 in 1 patient, grade 3 in 1 patient, and grade 2 in 2 patients. The prevalence of exacerbation‐free ILD within 28 days after protocol treatment was 95.7% (90% CI, 90.3%‐98.5%), which met the primary endpoint (Table 2). In the subgroup analysis of CT patterns, the prevalence of exacerbation‐free ILD within 28 days was 94.0% in 50 patients with a UIP pattern, and 97.6% in 42 with a possible UIP pattern. No patient with moderate ILD had an exacerbation, and 4 patients with mild ILD had an exacerbation.
Table 2.
Prevalence of exacerbation‐free interstitial lung disease (ILD) within 28 days after chemotherapy and grade of ILD exacerbation in patients with non‐small‐cell lung carcinoma (n = 92)
| No. of patients | No. of patients with exacerbation‐free ILD | (%) | |
|---|---|---|---|
| Overall | 92 | 88 | 95.7 (90% CI, 90.3‐98.5) |
| Subgroup of ILD pattern | |||
| UIP pattern | 50 | 47 | 94.0 |
| Possible UIP pattern | 42 | 41 | 97.6 |
| Grade of ILD exacerbation | |||
| Grade 5 | 1 | ||
| Grade 4 | 0 | ||
| Grade 3 | 1 | ||
| Grade 2 | 2 | ||
Abbreviations: CI, confidence interval; UIP, usual interstitial pneumonia.
The most common AEs were a reduction in the white blood cell count (n = 88, 96%), reduction in the neutrophil count (n = 87, 95%), anemia (n = 87, 95%), hyponatremia (n = 80, 87%), fatigue (n = 56, 61%), reduction in the platelet count (n = 55, 60%), anorexia (n = 51, 55%), and peripheral sensory neuropathy (n = 49, 53%; Table 3). Treatment‐related AEs of grade 3/4 were a reduction in the neutrophil count (n = 69, 75%), reduction in the white blood cell count (n = 49, 54%), anemia (n = 44, 48%), reduction in the platelet count (n = 18, 20%), hyponatremia (n = 16, 17%), febrile neutropenia (n = 8, 9%), and infection (n = 6, 7%). Treatment‐related deaths occurred in 2 patients: 1 patient experienced ILD exacerbation during the fourth treatment cycle without improvement, and 1 had pulmonary infection (CT findings showed cavity) during the second treatment cycle without ILD exacerbation. During the delivery of chemotherapy, 72 patients (78%) received 4 or more cycles. Among 20 patients receiving 3 or fewer cycles of chemotherapy, treatment discontinuation was observed in 9 patients due to AEs, in 8 patients due to disease progression, and in 3 patients due to other causes.
Table 3.
Treatment‐related adverse events in patients with non‐small‐cell lung cancer and interstitial lung disease treated with nab‐paclitaxel + carboplatin (n = 92)
| Any grade | Grade 3, 4, 5 | |
|---|---|---|
| No. of patients (%) | No. of patients (%) | |
| White blood cell decreased | 88 (96) | 49 (54) |
| Neutrophil count decreased | 87 (95) | 69 (75) |
| Anemia | 87 (95) | 44 (48) |
| Hyponatremia | 80 (87) | 16 (17) |
| Fatigue | 56 (61) | 3 (3) |
| Platelet count decreased | 55 (60) | 18 (20) |
| Anorexia | 51 (55) | 2 (2) |
| Peripheral sensory neuropathy | 49 (53) | 4 (4) |
| Nausea | 36 (42) | 0 |
| AST increased | 38 (41) | 0 |
| ALT increased | 30 (32) | 1 (1) |
| Creatinine increased | 22 (24) | 0 |
| Arthralgia | 22 (24) | 1 (1) |
| Infection | 21 (23) | 6 (7) |
| Myalgia | 17 (18) | 0 |
| Diarrhea | 16 (17) | 2 (2) |
| Febrile neutropenia | 8 (9) | 8 (9) |
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase
During protocol treatment, 34 patients (37%) required 1 level dose reduction, and 7 (8%) required 2‐level dose reduction. Sixty‐six patients (72%) needed course delay, mainly due to neutropenia or thrombocytopenia.
3.3. Efficacy of nab‐paclitaxel plus carboplatin for NSCLC patients with ILD
Of the 92 patients enrolled in our study, 47 patients (51%; 95% CI, 40%‐62%) achieved a partial response, and 23 patients (25%; 95% CI, 16%‐36%) had stable disease. Seventeen patients (17%; 95% CI, 11%‐28%) showed progressive disease, and 5 patients had disease that could not be evaluated. Thus, 47 patients (51%; 95% CI, 40‐62) had an objective response. In 92 patients receiving protocol treatment, the median PFS and median OS were 6.2 (95% CI, 5.4‐7.1) months and 15.4 (95% CI, 12.5‐19.8) months, respectively (Figure 2).
Figure 2.
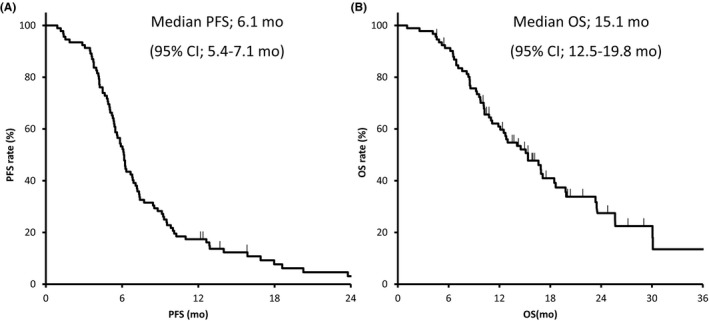
Curves showing progression‐free survival (PFS) (A) and overall survival (OS) (B) for 92 patients with non‐small‐cell lung cancer and interstitial lung disease receiving any protocol treatment. CI, confidence interval
3.4. Subsequent chemotherapy
Among the 92 patients, 49 (53%) received subsequent chemotherapy as second‐line treatment. S‐1 (n = 29), docetaxel (n = 5), and vinorelbine (n = 5) were used frequently for subsequent chemotherapy. In total follow‐up (median follow‐up, 15.6 months), 19 (20.7%) patients showed ILD exacerbation, and 15 of those did not show ILD exacerbation after 28 days after completion of protocol treatment (Figure 3). Among 15 patients experiencing ILD exacerbation after completion of protocol treatment, 10 patients received second‐line chemotherapy and 5 did not.
Figure 3.
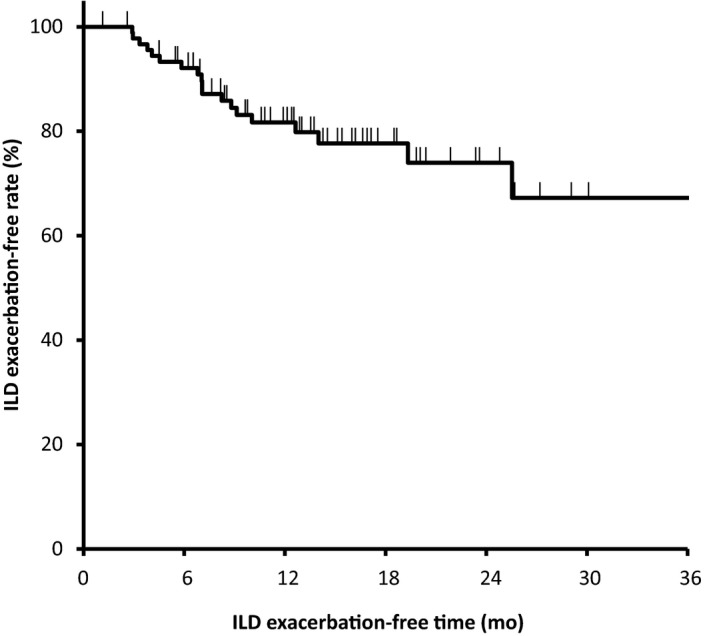
Curve showing interstitial lung disease (ILD) exacerbation‐free time for 92 patients with non‐small‐cell lung cancer and ILD receiving any protocol treatment
4. DISCUSSION
This was the largest prospective study for NSCLC patients with ILD. We found that a combination of carboplatin with nab‐paclitaxel was well tolerated in terms of safety (including the risk of ILD exacerbation). The primary endpoint of our study was met.
Two prospective studies in Japan evaluated the safety and efficacy of platinum‐based chemotherapy for NSCLC patients with ILD. Minegishi et al evaluated weekly paclitaxel in combination with carboplatin for advanced NSCLC with idiopathic interstitial pneumonia (n = 18), and 1 patient (5.6%; 95% CI, 0%‐17%) showed acute exacerbation of idiopathic interstitial pneumonia.12 Sekine et al reported that acute exacerbation of ILD occurred in 2 of 21 NSCLC patients with ILD (10%) receiving S‐1 plus carboplatin.13 However, the study cohorts in those studies were small, so they were not powered sufficiently to evaluate the safety of chemotherapy for NSCLC patients with ILD.
There are few data on ILD exacerbation in NSCLC patients not receiving chemotherapy. In two randomized phase III trials undertaken to evaluate the efficacy of nintedanib in patients with idiopathic pulmonary fibrosis, the proportion of patients with acute exacerbation of idiopathic pulmonary fibrosis within 1 year was 3.6%‐9.6%.20 Based on those reports, a combination of carboplatin with nab‐paclitaxel could be considered a feasible chemotherapy regimen to lower the risk of ILD exacerbation. A lower baseline forced vital capacity could be a predictive marker of acute exacerbation of preexisting ILD during lung cancer treatment.21 Considering late toxicities of cytotoxic chemotherapy, optimal duration of evaluating ILD exacerbation after protocol treatment is unclear. However, treatment‐related AEs within 28 days after chemotherapy have been evaluated in many clinical trials evaluating cytotoxic chemotherapy, excluding immune‐checkpoint inhibitors. Therefore, ILD exacerbation within 28 days after chemotherapy was defined as a treatment‐related AE in this study.
The present phase II study showed that the median PFS was 6.1 months. In 2 phase II studies on NSCLC patients with ILD, the median PFS was 4.2‐5.3 months.12, 13 A phase III study evaluating a combination of nab‐paclitaxel with carboplatin for patients with advanced NSCLC showed a median PFS of 6.3 months, similar to our study result.14 Conversely, median OS (15.1 months) tended to be longer than that recorded in other phase II studies (10.4 and 10.6 months, respectively). Several retrospective studies have reported median survival of 5.4‐11.4 months for NSCLC patients with ILD treated with chemotherapy.6, 7, 22, 23, 24 In addition, 2 small retrospective studies evaluating a combination of nab‐paclitaxel with carboplatin for NSCLC patients with ILD (median OS, 11.8 and 14.9 months, respectively) supported the survival data of the present study.25, 26
Our phase II study has 2 main limitations. First, this was a single‐arm phase II (not randomized) study with bias in patient selection. As described above, only 2 small prospective studies have evaluated chemotherapy for NSCLC with ILD. Therefore, a single‐arm, phase II study involving 94 patients would be important. Our study also included 50 patients showing a UIP pattern on CT, which has been reported to increase the risk of ILD exacerbation.27 Second, the diagnosis of ILD and exacerbation of ILD were based on CT findings and not on histology. However, a clinical diagnosis of interstitial pulmonary fibrosis according to CT findings is described in the American Thoracic Society/European Respiratory Society consensus statement.15 In clinical settings, it is often difficult to diagnose lung cancer and ILD by pathology. In patients with primary lung adenocarcinoma plus UIP, an invasive mucinous‐predominant subtype and KRAS mutation are observed more frequently compared with patients with lung adenocarcinoma but without a UIP pattern.28 Therefore, it remains important to develop treatment for lung cancer with ILD because it might have biologic differences from lung cancer without ILD. In Japan, a randomized study of carboplatin plus nab‐paclitaxel with or without nintedanib for advanced NSCLC with interstitial pulmonary fibrosis (J‐SONIC) is ongoing.29
In conclusion, we found that a combination of nab‐paclitaxel with carboplatin was tolerable in NSCLC patients with mild or moderate ILD in terms of safety, including the risk of ILD exacerbation. As the incidence of febrile neutropenia and infection are relatively higher, careful management is needed. Although this study was single‐arm, nab‐paclitaxel plus carboplatin might be more effective compared with the other regimens mentioned in other studies.
DISCLOSURE
Dr Kenmotsu reports grants and personal fees from AstraZeneca, Chugai Pharmaceutical, and Boeringer Ingelheim, and personal fees from Ono Pharmaceutical, Eli Lilly, Kyowa Hakko Kirin, Bristol‐Myers Squibb, MSD, Novartis Pharma, and Taiho Pharmaceutical. Dr Yoh reports grants and personal fees from Chugai Pharma, AstraZeneca, Lilly Japan, Ono Pharmaceutical, Novartis, MSD, and Taiho Pharmaceutical, personal fees from Boehringer Ingelheim, and grants from Bayer, Pfizer, and Bristol‐Myers Squibb. Dr Ono reports other from Chugai Pharma, MSD, Taiho Pharmaceutical, Ono Pharmaceutical, Boehringer Ingelheim, and Novartis. Dr Baba reports personal fees from Ono Pharmaceutical, Bristol Myers Squibb, AstraZeneca, Boston Scientific Japan, Nippon Boehringer Ingelheim, Daiichi Sankyo, Toray Industries, Shionogi, Astellas Pharma, AMCO, and Asahi Kasei Pharma. Dr Fujiwara reports grants from Abbvie, Chugai, Daiichi‐Sankyo, Eisai, Eli Lilly, Incyte, Merck Serono, grants and personal fees from AstraZeneca, BMS, MSD, and Novartis, and personal fees from ONO, Sysmex, and Taiho. Dr Okamoto received research funds from Takeda, MSD, Ono, Astrazeneca, Merck, Chugai, Taiho, Bristol, Eli Lilly, and Daiich Sankyo. Dr Yamamoto reports grants and personal fees from Taiho Pharmaceutical. Dr Ninomiya reports personal fees from Chugai Pharmaceutical and Boehringer Ingerheim. Dr Ogura reports grants and personal fees from Nippon Boehringer Ingelheim, and personal fees from Shionogi and Eisai. Dr Kato reports grants from Kanagawa Cancer Foundation, during the conduct of the study, grants and personal fees from Abbvie, AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Chugai, Kyowa Kirin, MSD, Ono, Pfizer, Taiho, and Merck Serono, grants from Astellas, and personal fees from Novartis, Sumitomo Dainippon, and F. Hoffmann ‐ La Roche. All remaining authors have declared no conflicts of interest.
ACKNOWLEDGMENTS
The study was supported by Ministry of Health, Labor and Welfare, Japan.
We thank all the patients who participated in this study and their families. We also thank Ms Mie Yamada, Ms Chiemi Asano, and Ms Miho Watanabe for data management, and all investigators and site coordinators from the 9 sites for their contributions to this study: National Cancer Center Hospital East, National Cancer Center Hospital, Yokohama Municipal Citizen’s Hospital, Saitama Medical University, Juntendo University, Okayama University Hospital, Wakayama Medical University, Kanagawa Cardiovascular and Respiratory Center, Shizuoka Cancer Center. We thank Arshad Makhdum, PhD, from Edanz Group for editing a draft of this manuscript.
Kenmotsu H, Yoh K, Mori K, et al. Phase II study of nab‐paclitaxel + carboplatin for patients with non‐small‐cell lung cancer and interstitial lung disease. Cancer Sci. 2019;110:3738–3745. 10.1111/cas.14217
REFERENCES
- 1. Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non‐small‐cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380‐2388. [DOI] [PubMed] [Google Scholar]
- 2. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non‐small‐cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121‐128. [DOI] [PubMed] [Google Scholar]
- 3. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin‐paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947‐957. [DOI] [PubMed] [Google Scholar]
- 4. Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK‐positive lung cancer. N Engl J Med. 2013;368(25):2385‐2394. [DOI] [PubMed] [Google Scholar]
- 5. Camus P, Kudoh S, Ebina M. Interstitial lung disease associated with drug therapy. Br J Cancer. 2004;91(Suppl 2):S18‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Minegishi Y, Takenaka K, Mizutani H, et al. Exacerbation of idiopathic interstitial pneumonias associated with lung cancer therapy. Intern Med. 2009;48(9):665‐672. [DOI] [PubMed] [Google Scholar]
- 7. Watanabe N, Niho S, Kirita K, et al. Vinorelbine and cisplatin in patients with advanced non‐small cell lung cancer with interstitial pneumonia. Anticancer Res. 2015;35(3):1697‐1701. [PubMed] [Google Scholar]
- 8. Enomoto Y, Kenmotsu H, Watanabe N, et al. Efficacy and safety of combined carboplatin, paclitaxel, and bevacizumab for patients with advanced non‐squamous non‐small cell lung cancer with pre‐existing interstitial lung disease: a retrospective multi‐institutional study. Anticancer Res. 2015;35(7):4259‐4263. [PubMed] [Google Scholar]
- 9. Kenmotsu H, Naito T, Mori K, et al. Effect of platinum‐based chemotherapy for non‐small cell lung cancer patients with interstitial lung disease. Cancer Chemother Pharmacol. 2015;75(3):521‐526. [DOI] [PubMed] [Google Scholar]
- 10. Hanna N, Johnson D, Temin S, et al. Systemic therapy for stage IV non‐small‐cell lung cancer: American society of clinical oncology clinical practice guideline update. J Clin Oncol. 2017;35(30):3484‐3515. [DOI] [PubMed] [Google Scholar]
- 11. Planchard D, Popat S, Kerr K, et al. Metastatic non‐small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2018;29(Supplement_4):iv192‐iv237. [DOI] [PubMed] [Google Scholar]
- 12. Minegishi Y, Sudoh J, Kuribayasi H, et al. The safety and efficacy of weekly paclitaxel in combination with carboplatin for advanced non‐small cell lung cancer with idiopathic interstitial pneumonias. Lung Cancer. 2011;71(1):70‐74. [DOI] [PubMed] [Google Scholar]
- 13. Sekine A, Satoh H, Baba T, et al. Safety and efficacy of S‐1 in combination with carboplatin in non‐small cell lung cancer patients with interstitial lung disease: a pilot study. Cancer Chemother Pharmacol. 2016;77(6):1245‐1252. [DOI] [PubMed] [Google Scholar]
- 14. Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab‐paclitaxel in combination with carboplatin versus solvent‐based paclitaxel plus carboplatin as first‐line therapy in patients with advanced non‐small‐cell lung cancer: final results of a phase III trial. J Clin Oncol. 2012;30(17):2055‐2062. [DOI] [PubMed] [Google Scholar]
- 15. Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence‐based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788‐824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733‐748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shah NR, Noble P, Jackson RM, et al. A critical assessment of treatment options for idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2005;22(3):167‐174. [PMC free article] [PubMed] [Google Scholar]
- 18. Collard HR, Moore BB, Flaherty KR, et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176(7):636‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228‐247. [DOI] [PubMed] [Google Scholar]
- 20. Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071‐2082. [DOI] [PubMed] [Google Scholar]
- 21. Enomoto Y, Inui N, Kato T, et al. Low forced vital capacity predicts cytotoxic chemotherapy‐associated acute exacerbation of interstitial lung disease in patients with lung cancer. Lung Cancer. 2016;96:63‐67. [DOI] [PubMed] [Google Scholar]
- 22. Shukuya T, Ishiwata T, Hara M, et al. Carboplatin plus weekly paclitaxel treatment in non‐small cell lung cancer patients with interstitial lung disease. Anticancer Res. 2010;30(10):4357‐4361. [PubMed] [Google Scholar]
- 23. Kinoshita T, Azuma K, Sasada T, et al. Chemotherapy for non‐small cell lung cancer complicated by idiopathic interstitial pneumonia. Oncol Lett. 2012;4(3):477‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Okuda K, Hirose T, Oki Y, et al. Evaluation of the safety and efficacy of combination chemotherapy with vinorelbine and platinum agents for patients with non‐small cell lung cancer with interstitial lung disease. Anticancer Res. 2012;32(12):5475‐5480. [PubMed] [Google Scholar]
- 25. Niwa H, Nakahara Y, Yokoba M, Mitsufuji H, Sasaki J, Masuda N. Safety and efficacy of carboplatin plus nab‐paclitaxel for treating advanced non‐small‐cell lung cancer with interstitial lung disease. Mol Clin Oncol. 2017;7(4):604‐608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yasuda Y, Hattori Y, Tohnai R, et al. The safety and efficacy of carboplatin plus nanoparticle albumin‐bound paclitaxel in the treatment of non‐small cell lung cancer patients with interstitial lung disease. Jpn J Clin Oncol. 2018;48(1):89‐93. [DOI] [PubMed] [Google Scholar]
- 27. Kenmotsu H, Naito T, Kimura M, et al. The risk of cytotoxic chemotherapy‐related exacerbation of interstitial lung disease with lung cancer. J Thorac Oncol. 2011;6(7):1242‐1246. [DOI] [PubMed] [Google Scholar]
- 28. Masai K, Tsuta K, Motoi N, et al. Clinicopathological, immunohistochemical, and genetic features of primary lung adenocarcinoma occurring in the setting of usual interstitial pneumonia pattern. J Thorac Oncol. 2016;11(12):2141‐2149. [DOI] [PubMed] [Google Scholar]
- 29. Otsubo K, Kishimoto J, Kenmotsu H, et al. Treatment rationale and design for J‐SONIC: a randomized study of carboplatin plus nab‐paclitaxel with or without nintedanib for advanced non‐small‐cell lung cancer with idiopathic pulmonary fibrosis. Clin Lung Cancer. 2018;19(1):e5‐e9. [DOI] [PubMed] [Google Scholar]


