Abstract
Pediatric refractory solid tumors are aggressive malignant diseases, resulting in an extremely poor prognosis. KOC1, FOXM1, and KIF20A are cancer antigens that could be ideal targets for anticancer immunotherapy against pediatric refractory solid tumors with positive expression for these antigens. This nonrandomized, open‐label, phase I clinical trial evaluated the safety and efficacy of the NCCV Cocktail‐1 vaccine, which is a cocktail of cancer peptides derived from KOC1, FOXM1, and KIF20A, in patients with pediatric refractory solid tumors. Twelve patients with refractory pediatric solid tumors underwent NCCV Cocktail‐1 vaccination weekly by intradermal injections. The primary endpoint was the safety of the NCCV Cocktail‐1 vaccination, and the secondary endpoints were the immune response, as measured by interferon‐r enzyme‐linked immunospot assay, and the clinical outcomes including tumor response and progression‐free survival. The NCCV Cocktail‐1 vaccine was well tolerated. The clinical response of this trial showed that 4 patients had stable disease after 8 weeks and 2 patients maintained remission for >11 months. In 4, 8, and 5 patients, the NCCV Cocktail‐1 vaccine induced the sufficient number of peptide‐specific CTLs for KOC1, FOXM1, and KIF20A, respectively. Patients with high peptide‐specific CTL frequencies for KOC1, FOXM1, and KIF20A had better progression‐free survival than those with low frequencies. The findings of this clinical trial showed that the NCCV Cocktail‐1 vaccine could be a novel therapeutic strategy, with adequate effects against pediatric refractory solid tumors. Future large‐scale trials should evaluate the efficacy of the NCCV Cocktail‐1 vaccination.
Keywords: cytotoxic T lymphocyte, NCCV Cocktail‐1, pediatric refractory solid tumor, peptide vaccine, phase I
Kaplan‐Meier curves for progression‐free survival. We found that patients with higher numbers of cancer antigens (KOC1, FOXM1, and KIF20A) that induced peptide‐specific CTLs by vaccination had better progression‐free survival.

Abbreviations
- CR
complete response
- CT
computed tomography
- DLT
dose‐limiting toxicity
- ELISPOT
enzyme‐linked immunospot
- GPC3
glypican‐3
- HLA
human leukocyte antigen
- IFN
interferon
- IL
interleukin
- OS
overall survival
- PD
progressive disease
- PFS
progression‐free survival
- PR
partial response
- PS
performance status
- SD
stable disease
1. INTRODUCTION
Pediatric solid tumors are relatively rare, affecting approximately 100 people per million in the United States.1 In Japan, pediatric solid tumors, excluding those of the central nervous system, account for 30%‐40% of total pediatric cancer cases, with an estimated onset of 750‐1000 cases per year.2 For these tumors, multidisciplinary therapy with chemotherapy, radiation therapy, and surgery have become standard treatment and improved prognoses.3 However, for patients with pediatric refractory solid tumors, there are no standard treatments following the second therapy, and the prognosis remains extremely poor.4, 5, 6, 7, 8 Long‐term exposure to chemotherapy and radiotherapy could cause serious late complications in pediatric patients.9, 10 Therefore, novel and effective therapies are urgently required to improve their life prognoses while maintaining good quality of life.
Immunotherapy could be an effective therapeutic strategy for pediatric solid tumors. Specific tumor antigens, such as disialoganglioside, glycoprotein B7 homolog 3 protein, glycoprotein nonmetastatic B, and Wilms tumor antigen have been identified as effective targets for peptide vaccine therapy in pediatric solid tumors.11, 12, 13, 14, 15 Some previous studies reported that immunotherapeutic methods using tumor antigen‐derived peptide vaccines had an antitumor effect on refractory pediatric solid tumors.16, 17 We previously reported relatively positive clinical effects of GPC3‐derived peptide vaccine therapy in patients with refractory pediatric solid tumors, especially hepatoblastoma.18 However, to obtain more robust immunological effects and improve the prognosis of refractory pediatric solid tumors, more effective novel cancer antigens are needed.
Cancer antigens KOC1, FOXM1, and KIF20A are all associated with cancer cell proliferation, development, and response to various growth factors.19, 20, 21, 22, 23, 24, 25, 26 KOC1 has been shown to be highly expressed in esophageal cancer,19 FOXM1 in breast,20 lung,21 and colon22 cancers, and KIF20A in pancreatic,23 gastric,24 lung,25 and bladder26 cancers. In addition, previous reports have shown that each peptide‐specific CTL clones for KOC1, FOXM1, and KIF20A specifically attacks HLA‐A24‐positive cancer cell lines that endogenously express their antigens.27, 28, 29 However, there are no reports on the expression of KOC1, FOXM1, or KIF20A in pediatric solid tumors. We found that all 3 of these cancer antigens are highly expressed, with a frequency of 80% or higher, in patients with pediatric solid tumor, including neuroblastoma, Ewing sarcoma, rhabdomyosarcoma, and osteosarcoma. Therefore, KOC1, FOXM1, and KIF20A might be good targets for anticancer immunotherapy against pediatric solid tumors. We identified HLA‐A*24:02‐restricted KOC1 (s‐448403), HLA‐A*24:02‐restricted FOXM1 (OSTGC‐A24‐Fo), and HLA‐A*24:02‐restricted KIF20A (OCV‐105) as peptides with binding ability to HLA‐A24, which is the most common HLA class type in Japan.
We undertook this phase I clinical trial of the NCCV Cocktail‐1 vaccine, a cocktail of the above cancer peptides derived from KOC1, FOXM1, and KIF20A, against refractory pediatric solid tumors. This study aimed to evaluate the vaccine’s safety, tolerability, recommended phase II dose, and immunologic and clinical responses.
2. MATERIALS AND METHODS
2.1. Patient eligibility
This phase I clinical trial, carried out from March 2013 to December 2014, was approved by the Ethics Committee of the National Cancer Center, Japan (Tokyo, Japan), the Ethics Committee of Osaka City General Hospital (Osaka, Japan), and the Ethics Committee of Kyushu University Hospital (Fukuoka, Japan). This study conformed to the ethical guidelines of the 1975 Declaration of Helsinki. The trial was registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN‐CTR no. 000010149).
Patients with refractory pediatric solid tumors who provided informed written consent were enrolled. The following eligibility criteria were applied: (i) diagnosis of neuroblastoma, Ewing sarcoma, rhabdomyosarcoma, or osteosarcoma on the basis of histological examinations; (ii) no expectation of response to other therapies; (iii) age between 1 and 40 years; (iv) an ECOG‐PS of 0‐1 (2‐3 was possible if caused only by motion restriction, ie, neurological disorder or extremity loss); (v) no prior therapy within a specific period (chemotherapy within 3 weeks, radiation therapy within 2 weeks, or surgery within 1 week); (vi) life expectancy of 3 months or more; (vii) HLA‐A*24:02‐positive status as determined using commercially available genomic DNA typing tests (Mitsubishi Chemical Medicine); and (viii) establishment of the following laboratory results within 14 days: neutrophil count ≥1000/μL; platelets ≥50 000/μL; serum creatinine adjusted according to age, <0.8 mg/dL (<5 years), <1.2 mg/dL (5‐10 years), <1.5 mg/dL (10‐40 years); total bilirubin ≤1.5 mg/dL; aspartate aminotransferase ≤200 IU/L (or ≤400 IU/L with liver metastasis); and alanine aminotransferase ≤200 IU/L, (or ≤400 IU/L with liver metastasis). Patients aged less than 15 years required written informed consent from the legal guardian, those aged 16‐19 years required written informed consent from both the patient and the legal guardian, and those aged 20 years or more required written informed consent only from the patient. The following exclusion criteria were applied: (i) massive pleural effusion, massive ascites, or severe pericardial effusion; (ii) active double cancer or secondary cancer within 5 disease‐free years of primary cancer; (iii) clinically serious infection requiring systemic treatment; (iv) active gastrointestinal bleeding that requires blood transfusion; (v) severe complications, including cardiac failure, renal failure, live failure, interstitial pneumonia, paralytic ileus, or uncontrolled diabetes mellitus; (vi) severe psychiatric disorder; (vii) history of severe medical allergy; (viii) pregnancy or lactation; (ix) judged inappropriate for the trial by a responsible researcher; and (x) unsuitability for the trial based on clinical judgment.
2.2. Study design and endpoints
This nonrandomized, open‐label, phase I clinical trial evaluated the safety and efficacy of the NCCV Cocktail‐1 vaccine in pediatric patients with refractory solid tumors. HLA‐A*24:02‐restricted KOC1 peptide (S‐488403), HLA‐A*24:02‐restricted FOXM1 peptide (OTSGC‐A24‐Fo), and HLA‐A*24:02‐restricted KIF20A peptide (OCV‐105) (all supplied by PolyPeptide Laboratories) were used in all enrolled patients with HLA‐A*24:02. These peptides were given in liquid form, emulsified with incomplete Freund’s adjuvant (Montanide ISA‐51VG; SEPPIC), by intradermal injection weekly until disease progression or recurrence. The peptides and incomplete Freund’s adjuvant were synthesized according to Good Manufacturing Practice guidelines. Administration of the two incremental doses of the peptide per patients’ body weight (less than 20 kg, total 6.0 mg for every 2.0 mg of the 3 peptides; more than 20 kg, total 3.0 mg for every 1.0 mg of the 3 peptides) was planned. The primary endpoint was the safety of the NCCV Cocktail‐1 vaccination. The secondary endpoints were immunologic response and clinical outcome, including PFS.
2.3. Evaluation of toxicity and clinical response
Enrolled patients were evaluated for signs of toxicity during and after vaccination. Adverse events were graded according to the Common Terminology Criteria for Adverse Events version 4.0. The hematological examinations were undertaken prior to each vaccination. The tumor size was evaluated by CT or MRI before vaccination, and then every 8 weeks after the first vaccination. Clinical tumor response was evaluated according to RECIST guidelines (version 1.1).
2.4. Ex vivo IFN‐γ ELISPOT assay
An ex vivo IFN‐γ ELISPOT assay was carried out to measure the antigen‐specific CTL response, as described previously.30 Briefly, a peripheral blood sample (10 mL) was obtained from each patient before the first vaccination and every month after the first vaccination, and centrifuged with a Ficoll‐Paque gradient. The PBMCs obtained from enrolled patients were frozen before immunologic analysis. All PBMCs were incubated in the same plate and simultaneously analyzed by ex vivo IFN‐γ ELISPOT assay. Noncultured PBMCs (5 × 105 per well) were added to each plate containing 1 type of the peptide antigen (10 μg/mL) and incubated for 20 hours at 37°C in 5% CO2. The peptide antigens used in this assay were HLA‐A*24:02‐restricted KOC1 peptide (S‐488403), HLA‐A*24:02‐restricted FOXM1 peptide (OTSGC‐A24‐Fo), and HLA‐A*24:02‐restricted KIF20A peptide (OCV‐105); each of these was included in each plate. The PBMCs plus HLA‐A*24:02‐restricted HIV peptide (RYLKDQQLL; ProImmune) were used as the negative control. The number of spots was automatically calculated by the Eliphoto system (Minerva Tech) using the BD ELISPOT kit (BD Biosciences). These assays were carried out in duplicate. Each peptide‐specific spot number showed the number of each peptide‐specific spot counted by subtracting the spot number in a well of HIV peptide.
2.5. In vitro IFN‐γ ELISPOT assay
We undertook an in vitro IFN‐γ ELISPOT assay to further evaluate the induction of peptide‐specific CTLs. The PBMCs obtained from enrolled patients were cultured (1 × 104 cells per well) with all 3 peptides (10 μg/mL each peptide): HLA‐A*24:02‐restricted KOC1 peptide (S‐488403), HLA‐A*24:02‐restricted FOXM1 peptide (OTSGC‐A24‐Fo), and HLA‐A*24:02‐restricted KIF20A peptide (OCV‐105) in RPMI‐1640 medium added to 10% FBS, recombinant human IL‐2 (50 IU/mL) and IL‐15 (10 ng/mL) for 14 days. Thereafter, CD8+ T cells were isolated using human CD8 microbeads (Miltenyi Biotec) from the above PBMCs that were stimulated with all 3 peptides for 14 days. The T2‐A24 cells, pulsed with each of the same 3 peptides, were prepared as target cells. The T2‐A24 cells pulsed with HLA‐A*24:02‐restricted HIV peptide (RYLKDQQLL; ProImmune) were used as negative control. The isolated CD8+ T cells were cocultured with each target cell for 20 hours at 37°C in 5% CO2, in which the ratio of effector and target cells was 0.2. The number of spots was automatically calculated by the Eliphoto system (Minerva Tech) using the BD ELISPOT kit (BD Biosciences). These assays were carried out in duplicate. Then each peptide‐specific spot number showed the number of each peptide‐specific spot counted by subtracting the spot number in a well of HIV peptide. In some patient PBMCs, an in vitro IFN‐γ ELISPOT assay was also undertaken based on another protocol, in which the PBMCs were stimulated with only 1 of each peptide per well for 14 days.
2.6. Dextramer staining and flow cytometry analysis
The PBMCs stimulated with these 3 peptides were stained with HLA‐A*24:02 Dextramer‐RPE (KOC1 [S‐488403], FOXM1 [OTSGC‐A24‐Fo], KIF20A [OCV‐105], or HIV [RYLKDQQLL]; Immudex) for 10 minutes at room temperature, and with anti‐CD8‐FITC (ProImmune) for 20 minutes at 4°C. For PBMC staining, flow cytometry was carried out using a FACSAria cell sorter (BD Biosciences).
2.7. Immunohistochemical analysis
In 9 patients from whom tumor specimens were obtained by surgery or biopsy, the immunohistochemical staining analysis was carried out before vaccination. The specimens were stained with H&E, mAbs against KOC1 (clone 69.1, dilution 1:100; Dako Cytomation), mAbs against FOXM1 (clone rabbit polyclonal, dilution 1:300; Santa Cruz Biotechnology), mAbs against KIF20A (clone rabbit polyclonal, dilution 1:1000; Bethyl Laboratories), or HLA class I (clone EMR8/5, dilution 1:2500; Hokudo) according to manufacturer protocol.
2.8. Statistical analysis
Relationships between the histological expression of cancer antigens, induction of peptide‐specific CTLs, and clinical response were analyzed using Fisher’s exact test. The PFS rates were analyzed using the Kaplan‐Meier method. Factors related to PFS were evaluated using Cox proportional hazard models. All statistical analyses were undertaken using R software version 3.1.2 (R Foundation for Statistical Computing, ://www.R-project.org). Statistical significance was defined by a P value less than .05.
3. RESULTS
3.1. Patients’ characteristics
Twelve patients were enrolled in this study (Table 1). No patient dropped out due to adverse events caused by peptide vaccination, and all patients received adequate observation to monitor toxicity. The median follow‐up period was 14.9 months (range, 0.3‐20.9 months). The average patient age was 18.0 years (range, 7‐32 years), 7 patients were male, and 11 had an ECOG‐PS of 0; only 1 (case 2) had an ECOG‐PS of 1. Of 12 patients, 3, 2, 5, and 2 patients were diagnosed with neuroblastoma, Ewing sarcoma, rhabdomyosarcoma, and osteosarcoma, respectively. Additionally, prior to vaccination, 4, 6, and 2 patients were judged as having progression, SD, and remission, respectively. All 12 patients underwent conventional chemotherapy, radiation therapy, or surgery before receiving the NCCV Cocktail‐1 vaccine therapy. All had experienced progression or relapse of the disease (1‐3 times) prior to enrollment. Two patients were judged as having remission in their clinical status before vaccination. One (case 10) received long‐term exposure to conventional chemotherapy and radiotherapy for the first recurrent lesion of rhabdomyosarcoma (several lymph node metastases and retroperitoneal tumors); biopsy and PET‐CT confirmed that the patient’s clinical status was remission. In another case (case 12), surgery and conventional chemotherapy were undertaken for the first recurrent lung metastasis of osteosarcoma, and the loss of the lesion was confirmed by CT.
Table 1.
Characteristics of 12 patients with pediatric refractory solid tumors
| No. | Age, years | Sex | PS | Clinical diagnosis | Primary lesion | Metastatic lesion | Clinical status before vaccinationa | HLA‐A | Body weight | Dose of peptideb | Prior therapy |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 19 | M | 0 | Neuroblastoma | Adrenal gland | Retroperitoneum | Stable disease | 24:02 | 85.5 | 6.0 mg | 05A1, ICE, IE, PBSCT/Hi‐MEC, Ope, RT |
| 2 | 22 | F | 1 | Ewing sarcoma | Left fibula | Bone | Progression | 24:02 | 58.5 | 6.0 mg | VDC, Ope, RT, IE, GEM/TXT |
| 3 | 32 | F | 0 | Ewing sarcoma | Right thigh | Lung | Progression | 24:02 | 42.8 | 6.0 mg | VDC/IE, Ope, TC, CPT‐11, VNR/CY, TMZ/VP, RT |
| 4 | 28 | F | 0 | Rhabdomyosarcoma | Chest wall | Abdominal wall | Progression | 24:02 | 97.0 | 6.0 mg | Ope, VAC, RT, IE, TI |
| 5 | 12 | M | 0 | Neuroblastoma | Adrenal gland | Bone | Stable disease | 24:02 | 39.2 | 6.0 mg | 05A3, PBSCT/HDC, RT, MIBG, IE |
| 6 | 14 | F | 0 | Rhabdomyosarcoma | Abdominal wall | Abdominal cavity | Stable disease | 24:02 | 56.0 | 6.0 mg | Ope, VAC, VIE, TI |
| 7 | 15 | F | 0 | Neuroblastoma | Adrenal gland | Bone | Stable disease | 24:02 | 45.9 | 6.0 mg | NewA1, PBSCT/HDC, CBSCT, Ope, RT, TC, CPT‐11/CDDP, ICE, mTOR, TMZ/CPT‐11 |
| 8 | 22 | M | 0 | Rhabdomyosarcoma | Cranial base | Lymph node | Stable disease | 24:02 | 113.0 | 6.0 mg | RT, VAC, IFO/ADR |
| 9 | 15 | M | 0 | Rhabdomyosarcoma | Cranial base | Lymph node | Stable disease | 24:02 | 41.5 | 6.0 mg | VAC, RT, Ope, PBSCT, TI, IE, V‐CPT |
| 10 | 7 | M | 0 | Rhabdomyosarcoma | Gastrocnemius | Retroperitoneum | Remission | 24:02 | 21.0 | 6.0 mg | Ope, VAC, RT, VDC, IE, CPT‐11 |
| 11 | 19 | M | 0 | Osteosarcoma | Right thigh | Lung | Progression | 24:02 | 77.0 | 6.0 mg | MAP, Ope, HD‐IFO |
| 12 | 11 | M | 0 | Osteosarcoma | Left tibia | Lung | Remission | 24:02 | 48.8 | 6.0 mg | Ope, MAP, IFO, IE, GEM/TXT |
Abbreviations: 05A1 and 05A3, cyclophosphamide‐vincristine‐pirarubicin‐cisplatin; ADR, adriamycin; CBSCT, cord blood stem cell transplantation; CDDP, cisplatin; CPT‐11, irinotecan; CY, cyclophosphamide; F, female; GEM, gemcitabine; HDC, high‐dose chemotherapy; HD‐IFO, high‐dose ifosfamide; Hi‐MEC, melphalan‐etoposide‐carboplatin; HLA, human leukocyte antigen; ICE, ifosfamide‐carboplatin‐etoposide; IE, ifosfamide‐etoposide; IFO, ifosfamide; M, male; MAP, methotrexate‐adriamycin‐cisplatin; MIBG, 3(meta)‐iodobenzylguanidine; NewA1, cyclophosphamide‐etoposide‐cisplatin‐therarubicin adriamycin; Ope, surgery; PBSCT, peripheral blood stem cell transplantation; PS, performance status; RT, radiotherapy; TC, topotecan‐cyclophosphamide; TI, topotecan‐ifosfamide; TMZ, temozolomide; TXT, docetaxel; VAC, vincristine‐actinomycin D‐cyclophosphamide; V‐CPT, vincristine‐irinotecan; VDC, vincristine‐dox‐cyclophosphamide; VIE, vincristine‐ifosfamide‐etoposide; VNR, vinorelbine; VP, VP‐16.
Progression, patient with refractory, recurrent, or progressive disease; remission, patients in remission without chance of cure; stable disease, patient with stable disease.
6.0 mg comprised 2.0 mg KOC1, 2.0 mg FOXM1, and 2.0 mg KIF20A.
3.2. Histological expression of KOC1, FOXM1, KIF20A, and HLA class I in pediatric solid tumors
In 9 patients with tissue specimen samples obtained by biopsy or surgery, we histologically evaluated the expression of KOC1, FOXM1, KIF20A, and HLA class I in pediatric solid tumors before vaccination by immunohistochemical staining analysis (Table 2). Of these 9 patients, 6 (66.7%), 6 (66.7%), and 7 (77.8%) showed positive expression for KOC1, FOXM1, and KIF20A, respectively. Additionally, in 4 (44.4%) patients, positive expression for all cancer antigens (KOC1, FOXM1, and KIF20A) was observed. As a representative sample, the staining patterns of cases 10 and 12 are shown in Figure 1A,B, respectively. As shown, the 3 antigens of KOC1, FOXM1, and KIF20A were present in the cytoplasm and cell membrane of tumor cells in both cases 10 and 12. However, there were some cases in which the expression of these cancer antigens was almost negative. The positive expression of HLA class I was observed in only 2 (22.2%) cases.
Table 2.
Histological expression in pediatric solid tumors and induction of peptide‐specific CTLs
| No. | Expression in pediatric solid tumora | Peptide‐specific CTL inductionb | Number of antigens with tumor expression that induced peptide‐specific CTLs | |||||
|---|---|---|---|---|---|---|---|---|
| KOC1 | FOXM1 | KIF20A | HLA class I | KOC1 | FOXM1 | KIF20A | ||
| 1 | + | + | + | − | + | + | + | 3 |
| 2 | NA | NA | NA | NA | NA | NA | NA | NA |
| 3 | + | + | − | + | + | + | + | 2 |
| 4 | − | − | + | − | − | − | − | 0 |
| 5 | + | − | − | − | − | + | − | 0 |
| 6 | + | + | + | − | − | + | + | 2 |
| 7 | NA | NA | NA | NA | − | − | − | NA |
| 8 | NA | NA | NA | NA | − | + | − | NA |
| 9 | − | − | + | − | − | − | − | 0 |
| 10 | + | + | + | − | + | + | + | 3 |
| 11 | − | + | + | − | − | + | − | 1 |
| 12 | + | + | + | + | + | + | + | 3 |
+, CTL frequencies ≥10 after vaccination; –, CTL frequencies <10 after vaccination.
Expression of KOC1, FOXM1, KIF20A, and human leukocyte antigen (HLA) class I was determined by immunohistochemistry. Degree of staining of tumor cells for them: −, no reactive; +, diffuse or focal reactive; NA, not analyzed.
Evaluated by ex vivo and in vitro IFN‐γ enzyme‐linked immunospot assays.
Figure 1.
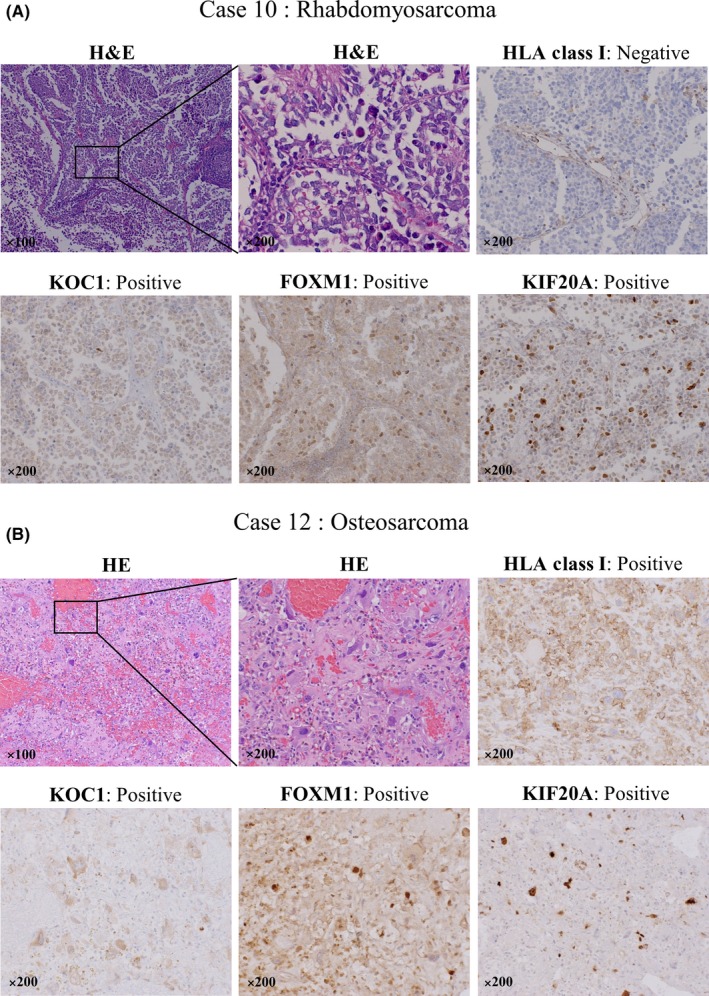
Hematoxylin‐eosin staining, and immunohistochemical staining of human leukocyte antigen (HLA) class I, KOC1, FOXM1, and KIF20A on tumor cells before vaccination in cases 10 and 12 of refractory pediatric solid tumor. A, In case 10, diagnosed with rhabdomyosarcoma, the positive expression of KOC1, FOXM1, and KIF20A (brown) are shown in the cytoplasm and the cell membrane of tumor cells as confirmed by H&E staining. The expression of HLA class I was negative in those cells. B, In case 12, diagnosed with osteosarcoma, the positive cells of HLA class I, KOC1, FOXM1, and KIF20A (brown) are shown in most of the tumor cells as confirmed by H&E staining. Magnification, ×100 or ×200
3.3. NCCV Cocktail‐1 vaccine was well tolerated
Adverse events observed in this trial are listed in Table S1. Dose‐limiting toxicity was evaluated in 10 of 12 patients, and neither DLT nor dose‐specific adverse events were observed. Of the 12 patients, adverse events related to the receipt of the NCCV Cocktail‐1 vaccine therapy during the follow‐up period were observed in 10 (83.3%) patients; 10 (83.3%) and 3 (25.0%) patients showed grade 1 and 2 adverse events, respectively. Specifically, the 3 patients who showed grade 2 adverse events were case 7 (fatigue), case 10 (injection site reaction), and case 12 (neutropenia). Additionally, 1 patient (case 1) showed an increase in alanine aminotransferase (grade 3), which could have been caused by fatty liver disease, a comorbidity in this patient. The patient was conservatively managed and improved following treatment with ursodeoxycholic and glycyrrhizic acid. No grade 4 adverse events were observed. Although other grade 3 adverse events (case 4, back pain; case 7, thrombocytopenia and anemia) were observed, they were judged to be unrelated to the vaccination. Most patients experienced immune‐related events (grade 1 or 2), such as injection site reaction, drug fever, rash or flushing, pruritus, and fatigue.
3.4. NCCV Cocktail‐1 peptide vaccine could induce peptide‐specific CTLs
To determine whether the NCCV Cocktail‐1 vaccine could induce a cancer antigen‐specific CTLs response, PBMCs obtained from 11 patients (excluding case 2) before and after vaccination were examined by ex vivo and in vitro IFN‐γ ELISPOT assay. To eliminate reactions due to impurities contained in the peptide, the difference from the number of spots against HIV peptide was counted as the number of peptide‐specific spots for KOC1, FOXM1, and KIF20A.
In case 10, in the ex vivo IFN‐γ ELISPOT assay, the number of each peptide‐specific CTLs in 5 × 105 PBMCs did not increase in KOC1, FOXM1, or KIF20A (Figure S1A). However, as shown in Figure S1B, in the in vitro IFN‐γ ELISPOT assay in case 10, the number of each peptide‐specific CTLs in 5 × 105 PBMCs increased in all 3 cancer antigens; the maximum was from 0 to 42 for KOC1, 0 to 37 for FOXM1, and 0 to 39 for KIF20A. We also undertook in vitro Dextramer analysis on case 10, and confirmed that, for FOXM1 and KIF20A, Dextramer‐positive cells of CD8+ lymphocytes were present in the PBMCs, in which the number of peptide‐specific CTLs increased in in vitro IFN‐γ ELISPOT assay (Figure S1C). For KOC1, as the results of the Dextramer analysis did not match those of the ELISPOT analysis, we judged that a nonspecific reaction was observed.
In case 12, in the ex vivo IFN‐γ ELISPOT assay, the number of each peptide‐specific CTL in 5 × 105 PBMCs increased in FOXM1 and KIF20A; the maximum was from 0 to 11 for FOXM1 and 0 to 21 for KIF20A (Figure 2A). In the in vitro IFN‐γ ELISPOT assay in case 12, the number of each peptide‐specific CTL in 5 × 105 PBMCs increased in all 3 cancer antigens; the maximum was from 5 to 15 for KOC1, 0 to 236 for FOXM1, and 0 to 12 for KIF20A (Figure 2B). In the in vitro Dextramer analysis of case 12, similar results with case 10 were obtained (Figure 2C).
Figure 2.
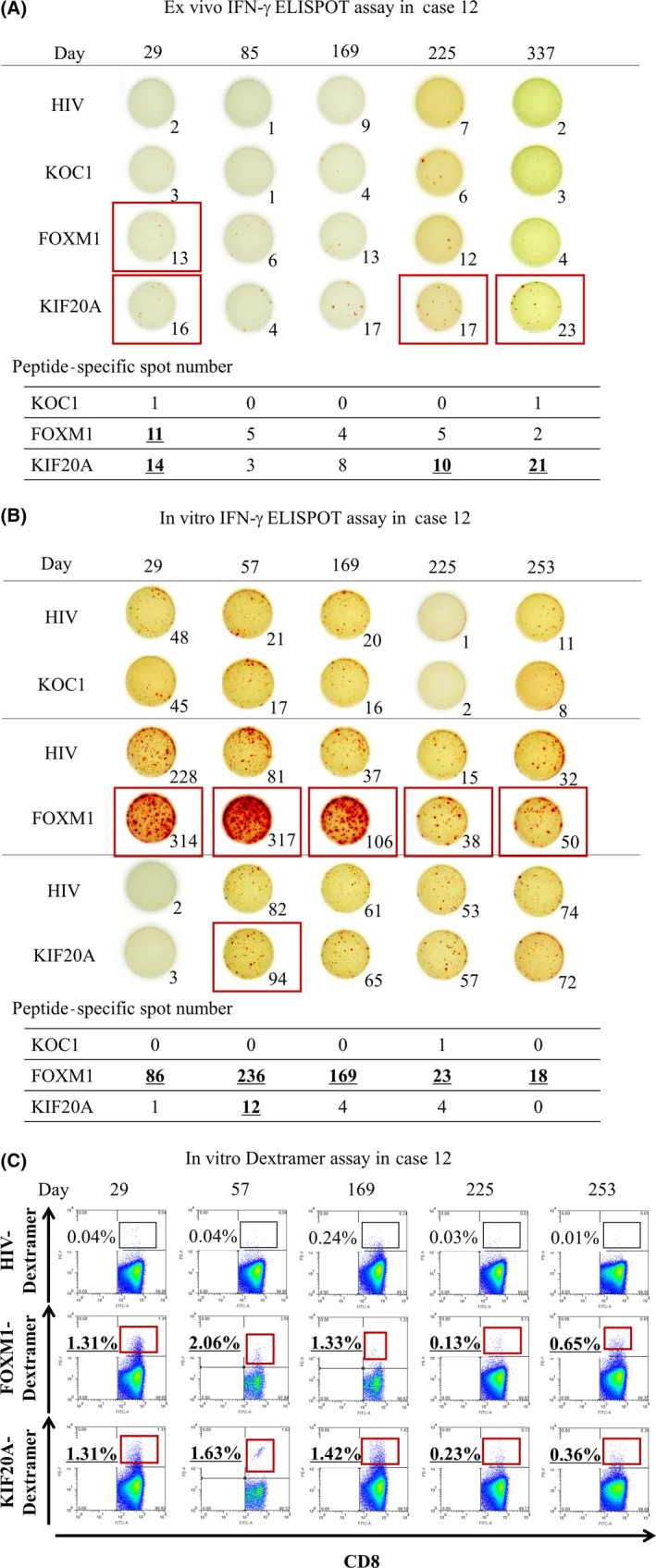
In case 12 of refractory pediatric solid tumor, ex vivo interferon (IFN)‐γ enzyme‐linked immunospot (ELISPOT) assay (A), in vitro IFN‐γ ELISPOT assay (B), and in vitro Dextramer assay (C) were carried out using PBMCs obtained after vaccination with NCCV Cocktail‐1. Each peptide‐specific spot number of KOC1, FOXM1, and KIF20A indicates the number of each peptide‐specific spot calculated by subtracting the spot number in a well of HIV peptide. When the number of IFN‐γ‐positive spots was 10 or more, it was defined that the peptide‐specific CTLs could be induced (shown by red boxes)
In cases 10 and 12, in which the induction of these peptide‐specific CTLs were obtained, the histological expression before vaccination was positive in KOC1, FOXM1, and KIF20A, and the remission status was maintained over a long period of time.
We suspected that when the number of these CTLs increased to 10 or more after vaccination by either ex vivo or in vitro ELISPOT assay, the peptide‐specific CTLs could be adequately induced by vaccination. As shown in Tables 2 and S2, we clarified that 4 (36.4%), 8 (72.7%), and 5 (45.5%) patients obtained adequate induction of the peptide‐specific CTLs from KOC1, FOXM1, and KIF20A, respectively. Four (36.4%) patients obtained adequate induction of the peptide‐specific CTLs from all 3 cancer antigens.
3.5. Clinical response
Patient clinical responses are shown in Table 3. Tumor response was evaluated 8 weeks after the first vaccination according to the RECIST guidelines (version 1.1). In 6 and 4 patients with SD and progression in clinical status before vaccination, respectively, 3 and 1 patients were judged to have SD, respectively, after 8 weeks. No patient had CR or PR. Of 2 patients with remission in clinical status before vaccination, both remained in remission (maintaining remission). The total rate of disease control (CR + PR + SD) and maintaining remission after 8 weeks was 50.0%, with a median time to tumor progression of 2.22 months. Also, some small lesions, especially the small multiple bone metastatic lesions in case 7, diminished or disappeared 8 weeks after the first vaccination. Two patients (cases 3 and 4) died during the follow‐up period, and the cause of their death was judged to be not related to vaccination therapy by the effect and safety evaluation committee.
Table 3.
Clinical response of patients with pediatric solid tumor
| No. | Tumor responsea | Sum of target lesion diametersb (mm), before/after vaccination | No. of vaccinationsc | PFS (mo) | OS (mo) |
|---|---|---|---|---|---|
| 1 | SD | 54.2/56.2 | 33 | 3.71 | >20.86 |
| 2 | PD | 51.0/NA | 1 | 0.26 | >0.26 |
| 3 | SD | 36.6/41.0 | 16 | 3.71 | 6.97 |
| 4 | PD | 137.7/223.1 | 9 | 0.95 | 7.52 |
| 5 | PD | 22.1/38.4 | 10 | 2.10 | >17.35 |
| 6 | SD | 23.2/24.6 | 13 | 2.33 | >17.15 |
| 7 | SD | 29.0/25.0 | 16 | 5.16 | >16.99 |
| 8 | PD | 20.5/47.0 | 5 | 0.95 | >16.30 |
| 9 | PD | 30.6/56.2 | 2 | 0.43 | >15.93 |
| 10 | Maintaining remission | 0.0/0.0 | 33 | >12.91 | >13.83 |
| 11 | PD | 39.1/68.3 | 8 | 0.92 | >11.86 |
| 12 | Maintaining remission | 0.0/0.0 | 31 | 11.07 | >11.40 |
Abbreviations: NA, not available; OS, overall survival; PD, progressive disease; PFS, progression‐free survival; SD, stable disease.
Evaluated 8 wk after the first vaccination according to the RECIST guideline assessment. In cases where remission status was sustained after vaccination, tumor response was defined as maintaining remission.
Evaluated before and 8 wk after the first vaccination.
Total number of injections of NCCV Cocktail‐1.
3.6. Histological expression of KOC1, FOXM1, and KIF20A in pediatric solid tumor correlated with induction of each peptide‐specific CTL
We examined the histological expression of KOC1, FOXM1, and KIF20A before vaccination in 9 patients, and evaluated the association between the histological expression and induction of peptide‐specific CTLs by these 3 antigens (Table S3). For KOC1, among 6 patients with histologically positive expression of KOC1, 4 patients showed the induction of KOC1‐specific CTLs, whereas all 3 patients with histologically negative expression of KOC1 failed to show the induction of KOC1‐specific CTLs. For FOXM1, all 6 patients with histologically positive expression of FOXM1 showed the induction of FOXM1‐specific CTLs. Among 3 patients with histologically negative expression of FOXM1, 2 patients did not show the induction of FOXM1‐specific CTLs. For KIF20A, among 7 patients with histologically positive expression of KIF20A, 4 patients showed the induction of KIF20A‐specific CTLs. Finally, among 2 patients with histologically negative expression of KIF20A, 1 patient did not show the induction of KIF20A‐specific CTLs. These results suggest that if the expression of these 3 cancer antigens was histologically positive before vaccination, each peptide‐specific CTL could be sufficiently easy to induce, and if the expression was histologically negative before vaccination, each peptide‐specific CTL might be barely induced (P = .033, Fisher’s exact test).
3.7. Induction of each peptide‐specific CTL resulted in better tumor response
Next, of 9 patients in whom the histological expression of KOC1, FOXM1, and KIF20A were assessed before vaccination, the association between the induction of peptide‐specific CTLs in these 3 cancer antigens and tumor response at 8 weeks after first vaccination was evaluated (Table S4). In KOC1, 4 patients who showed induction of KOC1‐specific CTLs were judged to have SD or maintaining remission as tumor response after 8 weeks. In contrast, among 5 patients who failed to show induction of KOC1‐specific CTLs, 4 patients had PD after 8 weeks. In KIF20A, 5 patients who showed induction of KOC1‐specific CTLs were judged to have SD or maintaining remission as clinical response after 8 weeks. In contrast, 4 patients who failed to show induction of KOC1‐specific CTLs had PD after 8 weeks. On evaluation of the relationship between these 3 cancer antigens, 4 patients who showed the induction of peptide‐specific CTLs for all cancer antigens (KOC1, FOXM1, and KIF20A) were judged to have SD or maintaining remission after 8 weeks. Among the remaining 5 patients, 4 had PD after 8 weeks. Therefore, our results suggest that adequate induction of peptide‐specific CTLs in KOC1 alone, KIF20A alone, and all 3 cancer antigens correlated significantly with tumor response after 8 weeks (P = .048, P = .008, and P = .048, respectively, Fisher’s exact test).
3.8. Progression‐free survival correlated with 3 peptide‐specific CTL frequencies
In 9 patients (except cases 2, 7, and 8) in whom the histological expression of KOC1, FOXM1, and KIF20A were assessed before vaccination, we evaluated the relationship between induction of these peptide‐specific CTLs and PFS (Figure 3). As shown in Figure 3B‐D, in each KOC1, FOXM1, and KIF20A, we compared PFS among the 3 groups; remission group in clinical status, SD/PD group with CTL induction (peptide‐specific CTL frequencies 10 or more), and SD/PD group with no CTL induction (peptide‐specific CTL frequencies less than 10). In all 3 antigens of KOC1, FOXM1, and KIF20A, we suggested that the remission group had better PFS compared to the other groups, which was a reasonable result due to background differences. In addition, in KOC1 and KIF20A, a Kaplan‐Meier analysis showed that the SD/PD group with CTL induction had significantly better PFS compared with the SD/PD group with no CTL induction (P = .040 and P = .017, respectively, log‐rank test).
Figure 3.
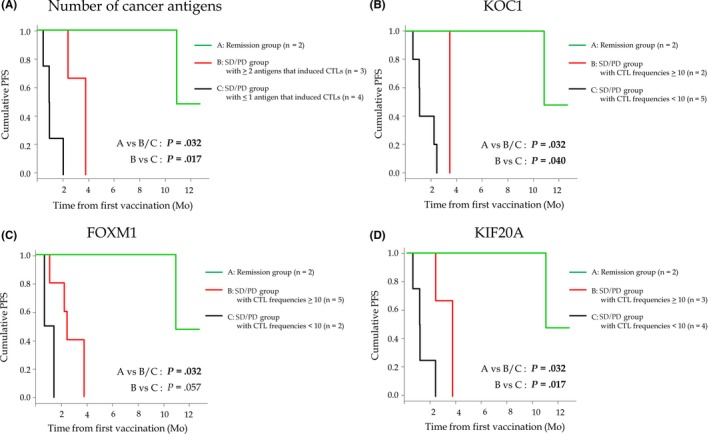
Kaplan‐Meier curves for progression‐free survival (PFS) among patients with refractory pediatric solid tumors vaccinated with NCCV Cocktail‐1. A, In the number of cancer antigens, the PFS by Kaplan‐Meier analysis was compared among 3 groups: remission group (n = 2) (green line), stable disease (SD)/progression (PD) group with ≥2 antigens that induced peptide‐specific CTLs (n = 3) (red line), and SD/PD group with ≤1 antigen that induced peptide‐specific CTLs (n = 4) (black line). B‐D, In KOC1 (B), FOXM1 (C), and KIF20A (D), the PFS by Kaplan‐Meier analysis was compared among 3 groups: remission group (green line), SD/PD group with CTL frequencies ≥10 (red line), and SD/PD group with CTL frequencies <10 (black line)
We also compared PFS among the 3 groups; remission group in clinical status (n = 2), SD/PD group with 2 or more antigens with tumor expression that induced peptide‐specific CTLs (n = 3), and SD/PD group with 1 or fewer antigen with tumor expression that induced peptide‐specific CTLs (n = 4) (Figure 3A). We found that, in SD/PD groups with clinical status (n = 7), patients with 2 or more antigens with tumor expression that induced peptide‐specific CTLs (n = 3) had sufficiently better PFS than patients with less than 1 antigen with tumor expression that induced peptide‐specific CTLs (n = 4) (P = .017, log‐rank test). In addition, in the SD/PD group with 2 or more antigens with tumor expression that induced peptide‐specific CTLs (n = 3), all had SD in tumor response. Therefore, these results suggested that patients with more antigens that induced peptide‐specific CTLs could have better clinical responses, including tumor response and PFS.
We also evaluated the clinical factors in relation to PFS for enrolled patients (n = 12) before vaccination using Cox proportional hazard models (Table S5). We have shown that in prevaccination, clinical status was the factor closely associated with PFS (P = .04). We considered that having better PFS in the remission group was a reasonable result due to background differences. Next, we evaluated the factors in relation to PFS after vaccination (Table 4). We showed that, in all patients, the number of antigens with tumor expression that induced peptide‐specific CTLs was significantly associated with PFS (P < .01). In addition, we found that in all patients, excluding 2 remission cases (cases 10 and 12), the number of antigens with tumor expression that induced peptide‐specific CTLs was the most important factor in relation to PFS (P < .05).
Table 4.
Factors relating to progression‐free survival (PFS) in patients with pediatric refractory solid tumors after vaccination with NCCV Cocktail‐1
| All patients (n = 11 for (1); n = 9 for (2), (3), and (3)’) | Excluding 2 remission cases (cases 10 and 12) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of patients | Median PFS (mo) | HR | P value | No. of patients | Median PFS (mo) | HR | P value | ||
| (1) No. of antigens that induced peptide‐specific CTLs | (1) No. of antigens that induced peptide‐specific CTLs | ||||||||
| 0 | 3 | 0.95 | 1.00 | — | 0 | 3 | 0.95 | 1.00 | — |
| 1 | 3 | 0.95 | 3.10 | .281 | 1 | 3 | 0.95 | 3.70 | .235 |
| 2 | 1 | 2.33 | 1.19 | .894 | 2 | 1 | 2.33 | 1.44 | .784 |
| 3 | 4 | 7.39 | 0.23 | .114 | 3 | 2 | 3.71 | 0.72 | .741 |
| (2) No. of antigens with tumor expression | (2) No. of antigens with tumor expression | ||||||||
| 1 | 3 | 0.95 | 1.00 | — | 1 | 3 | 0.95 | 1.00 | — |
| 2 | 2 | 2.31 | 0.24 | .250 | 2 | 2 | 2.31 | 0.20 | .213 |
| 3 | 4 | 7.39 | 0.07 | < .05 | 3 | 2 | 3.02 | 0.16 | .140 |
| (3) No. of antigens with tumor expression that induced peptide‐specific CTLs | (3) No. of antigens with tumor expression that induced peptide‐specific CTLs | ||||||||
| 0 | 3 | 0.95 | 1.00 | 0 | 3 | 0.95 | 1.00 | ||
| 1 | 1 | 0.92 | 2.45 | 1 | 1 | 0.92 | 2.45 | ||
| 2 | 2 | 3.02 | 0.00 | 2 | 2 | 3.02 | 0.00 | ||
| 3 | 3 | 11.07 | 0.00 | 3 | 1 | 3.71 | 0.00 | ||
| (3)’ No. of antigens with tumor expression that induced peptide‐specific CTLs (0,1) vs (2,3) | (3)’ No. of antigens with tumor expression that induced peptide‐specific CTLs (0,1) vs (2,3) | ||||||||
| PFS | PFS | ||||||||
| <2.33 | >2.33 | <2.33 | >2.33 | ||||||
| (0,1) | 4 | 0 | (0,1) | 4 | 0 | ||||
| (2,3) | 0 | 5 | (2,3) | 0 | 3 | ||||
| P < .01 * | P < .05 * | ||||||||
Bold indicate statistically significant values.
Abbreviations: —, reference group for P value; HR, hazard ratio.
Analyzed by Fisher’s exact test.
4. DISCUSSION
This study showed the safety and efficacy of the NCCV Cocktail‐1 vaccine, a cocktail of cancer peptides derived from KOC1, FOXM1, and KIF20A, in 12 patients with refractory pediatric solid tumors. All enrolled patients (weight, 20 kg or more) received 6.0 mg (2.0 mg of each peptide) of the NCCV Cocktail‐1 vaccination. Dose‐limiting toxicity was not observed in any patient, and all therapy‐related adverse events were grade 1 or 2, except in 1 case. Recent phase I clinical trials of other therapeutic strategies for pediatric solid tumors reported DLT and adverse events of grades 3 or 4.31, 32 In comparison, the NCCV Cocktail‐1 vaccination was well tolerated. Therefore, the peptide doses used in this study are recommended for a future clinical trial.
Previous studies reported that KOC1, FOXM1, and KIF20A showed positive expression in various malignant diseases, including esophageal, breast, lung, colon, pancreatic, stomach, and bladder cancers.19, 20, 21, 22, 23, 24, 25, 26 However, to the best of our knowledge, no study has evaluated the expression of these cancer antigens in pediatric solid tumors. Therefore, prior to this trial, we evaluated histological expressions of KOC1, FOXM1, and KIF20A in pediatric solid tumors using immunohistochemical staining analysis. Specifically, the expression of KOC1, FOXM1, and KIF20A was examined in 5 patients with neuroblastoma, 5 with Ewing sarcoma, 6 with rhabdomyosarcoma, and 5 with osteosarcoma. Thus, positive expression of KOC1 and FOXM1 was confirmed in all 21 patients with these cancers. Moreover, KIF20A expression was positive in 20 of these patients, except in 1 case of neuroblastoma (unpublished data). In the present study, in 9 patients in whom tissue specimens were available by surgery or biopsy before vaccination, histological evaluation of KOC1, FOXM1, and KIF20A expression showed positive expression in 6 (66.7%), 6 (66.7%), and 7 (77.8%) patients, respectively. Additionally, 4 (44.4%) patients had positive expression of all 3 of these cancer antigens. Furthermore, histologically positive expression of KOC1, FOXM1, and KIF20A in pediatric solid tumors was correlated with induction of each peptide‐specific CTL (Table S3). In 4 patients with positive expression for all 3 cancer antigens, the clinical responses after 8 weeks showed SD or maintaining remission. Therefore, the positive expression of these cancer antigens could play an important role in inducing peptide‐specific CTLs and obtaining better clinical response through the NCCV Cocktail‐1 vaccination. That is, KOC1, FOXM1, and KIF20A could be effective targets for immunotherapy in other malignant diseases in which the positive expression of these cancer antigens have been reported, including esophageal, breast, lung, colon, pancreatic, gastric, and bladder cancers.
We also evaluated the expression of HLA class I in this study. HLA class I expression was histologically positive in just 2 (22.2%) patients. We previously reported that, in a phase I trial of GPC3 peptide vaccine in pediatric solid tumors, only 3 (27.3%) of 11 patients with pediatric solid tumors, excluding hepatoblastoma, showed positive expression of HLA class I in primary tumors before vaccination.25 Thus, some pediatric solid tumors might show poor expression of HLA class I, which could result in the suppression of the effect of immunotherapy, including peptide vaccine therapy.33, 34, 35 However, in this study, even if the HLA class I expression was histologically negative, some cases had adequate induction of peptide‐specific CTLs and good clinical response following vaccination. Future evaluation of HLA class I in pediatric solid tumors is necessary.
The primary endpoint of this study was safety of the vaccination. However, our results also indicated that cancer antigen‐specific CTLs had a crucial role in immunotherapy against KOC1, FOXM1, and KIF20A. A correlation between immune response and OS was not mentioned in a previous report of WT1 peptide vaccine in pediatric solid tumors.17, 36 Recently, Tsuchiya et al reported that GPC3‐specific CTL frequency was significantly correlated with PFS and OS in a phase I trial of GPC3 peptide vaccine therapy for pediatric solid tumors.18 Similarly, clinical trials of a GPC3‐derived peptide vaccine undertaken in adult patients with hepatocellular and ovarian clear‐cell carcinomas have confirmed its safety and indicated correlations between frequency of GPC3‐specific CTLs and OS.37, 38, 39, 40 In this study, we found that patients with a higher number of cancer antigens (KOC1, FOXM1, and KIF20A) that induced peptide‐specific CTLs by vaccination had better PFS. However, the results of this study suggested that sufficient clinical response could not be obtained in patients with refractory pediatric solid tumors who had progression before vaccination. Therefore, significantly effective therapy in refractory pediatric solid tumors with the NCCV Cocktail‐1 vaccine alone could be challenging. In the future, novel immunotherapies, including those using immunomodulatory Abs and chimeric antigen receptor T cells, could show better clinical response and improved survival rates.41, 42
The presence of peptide‐specific CTLs of KOC1, FOXM1, and KIF20A was clearly evident in peripheral blood, providing proof‐of‐concept for immunotherapy using cancer antigen‐specific CTLs. However, we did not confirm whether the peptide‐specific CTLs infiltrated tumor cells after vaccination. A future study should determine the infiltration of peptide‐specific CTLs following vaccination by collecting tumor tissue specimens before and after vaccination and comparing tumor‐infiltrating lymphocytes. Additionally, by establishing peptide‐specific T‐cell clones with high affinity from the infiltrated tumor tissues after vaccination, more effective immunotherapy might be developed, including T‐cell receptor engineered T cell therapy using T‐cell receptors obtained from these CTL clones.
It is well known that patients with metastasis or refractory pediatric solid tumors, including neuroblastoma, Ewing sarcoma, rhabdomyosarcoma, and osteosarcoma, have very poor prognosis.4, 5, 6, 7, 8 No standard therapy following the second therapy for these refractory pediatric solid tumors has been established. Molecular targeted drugs, including human anaplastic lymphoma kinase inhibitors such as crizotinib,43, 44, 45 insulin‐like growth factor‐1 receptor inhibitor,46 and Hedgehog inhibitors such as forskolin,47 have recently attracted attention for their potential use in patients with refractory pediatric solid tumors. The Children’s Oncology Group (United States) reported that the anti‐GD2 Ab, in combination with granulocyte macrophage colony‐stimulating factor and IL‐2, resulted in better clinical effects compared with standard treatment in patients with high‐risk neuroblastoma.48 Moreover, the Group reported that mifamurtide combined with chemotherapy improved OS and PFS in patients with osteosarcoma.49 None of these novel therapies provide a dramatic antitumor effect sufficient for establishing a standard therapy. Furthermore, to our knowledge, no clinical trial of peptide vaccine alone in patients with pediatric solid tumors has shown adequate antitumor efficacy. It is possible that, in refractory pediatric solid tumors, only 1 cancer antigen alone might be insufficient to induce an antitumor response and incapable of evading the immune escape mechanisms of cancer. We produced the NCCV Cocktail‐1 vaccine, combining KOC1, FOXM1, and KIF20A, to obtain a more robust antitumor effect. Other recent reports have also shown that specific cocktail peptide vaccine therapies or peptide therapies combined with chemotherapy could result in adequate antitumor effects.50, 51, 52 Although our results did not reveal the desired clinical efficacy against refractory pediatric solid tumors, they indicate that the NCCV Cocktail‐1 vaccine might be an effective adjuvant therapy in these patients.
In conclusion, the results of this phase I trial of the NCCV Cocktail‐1 vaccine suggest that the vaccine is safe and can induce adequate immunological responses in patients with refractory pediatric solid tumors. Additionally, we found that the induction of peptide‐specific CTLs by this vaccination could produce better PFS. Thus, the NCCV Cocktail‐1 vaccine could result in good clinical benefits for patients with refractory pediatric solid tumors. Currently, a phase I/IIa clinical trial of the NCCV Cocktail‐1 vaccine in patients with pediatric central nervous system tumors is also ongoing.
DISCLOSURE
The authors declare no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
We wish to thank OncoTherapy Science, SHIONOGI & Co., and Otsuka Pharmaceutical Co., which approved the use of KOC1, FOXM1, and KIF20A in this trial and provided a summary of investigational drugs for each peptide vaccine. We would also like to thank the members of the clinical trial support department of the National Cancer Hospital East, including A.S., M.W., and M.F., which coordinated this clinical trial. In addition, we thank Kenichi Yoshimura, who belongs to the Innovative Clinical Research Center, Kanazawa University, for supporting the case calculation for this trial. Finally, we would like to thank Editage for English language editing.
Akazawa Y, Hosono A, Yoshikawa T, et al. Efficacy of the NCCV Cocktail‐1 vaccine for refractory pediatric solid tumors: A phase I clinical trial. Cancer Sci. 2019;110:3650–3662. 10.1111/cas.14206
Akazawa and Hosono contributed equally to this work.
Clinical Trial Registration Number: Unique ID issued by UMIN: UMIN000010149. Scientific Title: Phase 1 study of peptide cocktail vaccine for patients with refractory pediatric sarcoma. The name of the trial register: Ako Hosono.
REFERENCES
- 1. Rodriguez‐Galindo C, Friedrich P, Alcasabas P, et al. Toward the cure of all children with cancer through collaborative efforts: pediatric oncology as a global challenge. J Clin Oncol. 2015;33(27):3065‐3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ishihara H, Ohno Y, Fujii M, et al. Epidemiological analysis of childhood cancer in Japan based on population‐based cancer registries, 1993–2009. Jpn J Clin Oncol. 2017;47(7):660‐663. [DOI] [PubMed] [Google Scholar]
- 3. Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348(8):694‐701. [DOI] [PubMed] [Google Scholar]
- 4. Pinto NR, Applebaum MA, Volchenboum SL, et al. Advances in risk classification and treatment strategies for neuroblastoma. J Clin Oncol. 2015;33(27):3008‐3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oberlin O, Rey A, Lyden E, et al. Prognostic factors in metastatic rhabdomyosarcomas: results of a pooled analysis from United States and European cooperative groups. J Clin Oncol. 2008;26(14):2384‐2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Janeway KA, Barkauskas DA, Krailo MD, et al. Outcome for adolescent and young adult patients with osteosarcoma: a report from the Children’s Oncology Group. Cancer. 2012;118(18):4597‐4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leavey PJ, Mascarenhas L, Marina N, et al. Prognostic factors for patients with Ewing sarcoma (EWS) at first recurrence following multi‐modality therapy: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2008;51(3):334‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Armstrong GT, Kawashima T, Leisenring W, et al. Aging and risk of severe, disabling, life‐threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol. 2014;32(12):1218‐1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reulen RC, Winter DL, Frobisher C, et al. British Childhood Cancer Survivor Study Steering Group. Long‐term cause‐specific mortality among survivors of childhood cancer. JAMA. 2010;304(2):172‐179. [DOI] [PubMed] [Google Scholar]
- 10. Mertens AC, Yong J, Dietz AC, et al. Conditional survival in pediatric malignancies: analysis of data from the Childhood Cancer Survivor Study and the Surveillance, Epidemiology, and End Results Program. Cancer. 2015;121(7):1108‐1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roth M, Linkowski M, Tarim J, et al. Ganglioside GD2 as a therapeutic target for antibody‐mediated therapy in patients with osteosarcoma. Cancer. 2014;120(4):548‐554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dobrenlow K, Ostrovnaya I, Gu J, et al. Oncotarget GD2 and GD3 are highly expressed in sarcomas of children, adolescents, and young adults. Pediatr Blood Cancer. 2016;63:1780‐1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang L, Ostrovnaya I, Gu J, et al. B7‐H3‐mediated tumor immunology: friend or foe? Int J Cancer. 2014;134(10):2764‐2771. [DOI] [PubMed] [Google Scholar]
- 14. Zhou LT, Liu FY, Li Y, et al. Gpnmb/osteoactivin, an attractive target in cancer immunotherapy. Neoplasma. 2012;59(1):1‐5. [DOI] [PubMed] [Google Scholar]
- 15. Oue T, Uehara S, Yamanaka H, et al. Expression of Wilms tumor 1 gene in a variety of pediatric tumors. J Pediatr Surg. 2011;46(12):2233‐2238. [DOI] [PubMed] [Google Scholar]
- 16. Kushner BH, Cheung IY, Modak S, et al. Phase I trial of a bivalent gangliosides vaccine in combination with beta‐glucan for high‐risk neuroblastoma in second or later remission. Clin Cancer Res. 2014;20(5):1375‐1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hashii Y, Sato E, Ohta H, et al. WT1 peptide immunotherapy for cancer in children and young adults. Pediatr Blood Cancer. 2010;55(2):352‐355. [DOI] [PubMed] [Google Scholar]
- 18. Tsuchiya N, Hosono A, Yoshikawa T, et al. Phase I study of glypican‐3‐derived peptide vaccine therapy for patients with refractory pediatric solid tumors. Oncoimmunology. 2017;7(1):e1377872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kono K, Mizukami Y, Daigo Y, et al. Vaccination with multiple peptides derived from novel cancer‐testis antigens can induce specific T‐cell responses and clinical responses in advanced esophageal cancer. Cancer Sci. 2009;100(8):1502‐1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7(11):847‐859. [DOI] [PubMed] [Google Scholar]
- 21. Kim IM, Ackerson T, Ramakrishna S, et al. The Forkhead Box m1 transcription factor stimulates the proliferation of tumor cells during development of lung cancer. Cancer Res. 2006;66(4):2153‐2161. [DOI] [PubMed] [Google Scholar]
- 22. Douard R, Moutereau S, Pernet P, et al. Sonic Hedgehog‐dependent proliferation in a series of patients with colorectal cancer. Surgery. 2006;139(5):665‐670. [DOI] [PubMed] [Google Scholar]
- 23. Taniuchi K, Furihata M, Saibara T. KIF20A‐mediated RNA granule transport system promotes the invasiveness of pancreatic cancer cells. Neoplasia. 2014;16(12):1082‐1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Claerhout S, Lim JY, Choi W, et al. Gene expression signature analysis identifies vorinostat as a candidate therapy for gastric cancer. PLoS ONE. 2011;6(9):e24662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fang H, Yamaguchi R, Liu X, et al. Quantitative T cell repertoire analysis by deep cDNA sequencing of T cell receptor α and β chains using next‐generation sequencing (NGS). Oncoimmunology. 2015;3(12):e968467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ho JR, Chapeaublanc E, Kirkwood L, et al. Deregulation of Rab and Rab effector genes in bladder cancer. PLoS ONE. 2012;7(6):e39469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Suda T, Tsunoda T, Daigo Y, et al. Identification of human leukocyte antigen‐A24‐restricted epitope peptides derived from gene products upregulated in lung and esophageal cancers as novel targets for immunotherapy. Cancer Sci. 2007;98(11):1803‐1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tsunoda T, Ohsawa R, Yoshimura S, et al. FOXM1 peptides and vaccines containing the same. PCT WO. 2010;2010/095428 A1.
- 29. Osawa R, Tsunoda T, Yoshimura S, et al. Identification of HLA‐A24‐restricted novel T Cell epitope peptides derived from P‐cadherin and kinesin family member 20A. J Biomed Biotechnol. 2012;2012:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yoshikawa T, Nakatsugawa M, Suzuki S, et al. HLA‐A2‐restricted glypican‐3 peptide‐specific CTL clones induced by peptide vaccine show high avidity and antigen‐specific killing activity against tumor cells. Cancer Sci. 2011;102(5):918‐925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pearson AD, Federico SM, Aerts I, et al. A phase I study of oral ridaforolimus in pediatric patients with advanced solid tumors. Oncotarget. 2016;7(51):84736‐84747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vo KT, Karski EE, Nasholm NM, et al. Phase 1 study of sirolimus in combination with oral cyclophosphamide and topotecan in children and young adults with relapsed and refractory solid tumors. Oncotarget. 2017;8(14):23851‐23861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hicklin DJ, Marincola FM, Ferrone S. HLA class I antigen downregulation in human cancer: T‐cell immunotherapy revives an old story. Mol Med Today. 1999;5(4):178‐186. [DOI] [PubMed] [Google Scholar]
- 34. Garrido F, Perea F, Bernal M, et al. The escape of cancer from T cell‐mediated immune surveillance: HLA class I loss and tumor tissue architecture. Vaccines. 2017;5(1):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garrido F, Ruiz‐Cabello F, Aptsiauri N. Rejection versus escape: the tumor MHC dilemma. Cancer Immunol Immunother. 2016;66(2):259‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sawada A, Inoue M, Kondo O, et al. Feasibility of cancer immunotherapy with WT1 peptide vaccination for solid and hematological malignancies in children. Pediatr Blood Cancer. 2016;63(2):234‐241. [DOI] [PubMed] [Google Scholar]
- 37. Sawada Y, Yoshikawa T, Nobuoka D, et al. Phase I trial of a glypican‐3‐derived peptide vaccine for advance hepatocellular carcinoma: immunologic evidence and potential for improving overall survival. Clin Cancer Res. 2012;18(13):3686‐3696. [DOI] [PubMed] [Google Scholar]
- 38. Sawada Y, Yoshikawa T, Ofuji K, et al. Phase II study of the GPC3‐derived peptide vaccine as an adjuvant therapy for hepatocellular carcinoma patients. Oncoimmunology. 2016;5(5):e1129483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Suzuki S, Sakata J, Utsumi F, et al. Efficacy of glypican‐3‐derived peptide vaccine therapy on the survival of patients with refractory ovarian clear cell carcinoma. Oncoimmunology. 2016;5(11):e1238542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tsuchiya N, Yoshikawa T, Fujinami N, et al. Immunological efficacy of glypican‐3 peptide vaccine in patients with advance hepatocellular carcinoma. Oncoimmunology. 2017;6(10):e1346764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kopp LM, Katsanis E. Targeted immunotherapy for pediatric solid tumors. Oncoimmunology. 2016;5(3):e1087637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Heczey A, Louis CU. Advances in chimeric antigen receptor immunotherapy for neuroblastoma. Discov Med. 2013;16(90):287‐294. [PMC free article] [PubMed] [Google Scholar]
- 43. Rossing C, Juergens H, Berdel WE. New targets and targeted drugs for the treatment of cancer: an outlook to pediatric oncology. Pediatr Hematol Oncol. 2011;28(7):539‐555. [DOI] [PubMed] [Google Scholar]
- 44. Schulte JH, Bachmann HS, Brockmeyer B, et al. High ALK receptor tyrosine kinase expression supersedes ALK mutation as a determining factor of an unfavorable phenotype in primary neuroblastoma. Clin Cancer Res. 2011;17(15):5082‐5092. [DOI] [PubMed] [Google Scholar]
- 45. Mosse YP, Lim MS, Voss SD, et al. Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large‐cell lymphoma: a Children’s Oncology Group phase 1 consortium study. Lancet Oncol. 2013;14(6):472‐480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pappo AS, Patel SR, Crowley J, et al. R1507, a monoclonal antibody to the insulin‐like growth factor 1 receptor, in patients with recurrent or refractory Ewing sarcoma family of tumors: result of a phase II Sarcoma Alliance for Research through Collaboration study. J Clin Oncol. 2011;29(34):4541‐4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yamanaka H, Oue T, Uehara S, et al. Forskolin, a Hedgehog signal inhibitor, inhibits cell proliferation and induces apoptosis in pediatric tumor cell lines. Mol Med Rep. 2010;3(1):133‐139. [DOI] [PubMed] [Google Scholar]
- 48. Gilman AL, Ozkaynak MF, Matthay KK, et al. Phase I study of ch14.18 with granulocyte‐macrophage colony‐stimulating factor and interleukin‐2 in children with neuroblastoma after autologous bone marrow transplantation or stem‐cell rescue: a report from the Children’s Oncology Group. J Clin Oncol. 2009;27(1):85‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Meyers PA, Schwartz CL, Krailo MD, et al. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival—a report from the Chidren’s Oncology Group. J Clin Oncol. 2008;26(4):633‐638. [DOI] [PubMed] [Google Scholar]
- 50. Gravett AM, Trautwein N, Stevanović S, Dalgleish AG, Copier J. Gemcitabine alters the proteasome composition and immunopeptidome of tumour cells. Oncoimmunology. 2018;7(6):e1438107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Walter S, Weinschenk T, Stenzl A, et al. Multipeptide immune response to cancer vaccine IMA901 after single‐dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18(8):1254‐1261. [DOI] [PubMed] [Google Scholar]
- 52. Morse MA, Secord AA, Blackwell K, et al. MHC class I‐presented tumor antigens identified in ovarian cancer by immunoproteomic analysis are targets for T‐cell responses against breast and ovarian cancer. Clin Cancer Res. 2011;17(10):3408‐3419. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


