Abstract
Near‐infrared photoimmunotherapy (NIR‐PIT) is a molecularly targeted cancer phototherapy that is based on injecting a conjugate of a silicon‐phthalocyanine derivative, IRdye 700DX (IR700), and a monoclonal antibody that targets an expressed antigen on the cancer cell surface. Subsequent local exposure to NIR light results in the rapid and highly selective immunogenic cell death of targeted cancer cells. Because many cancers grow in bones through which light does not penetrate well, the goal of this study was to determine if NIR‐PIT can effectively treat cancers in bone. A bovine rib was used as a bone sample. Because the sample’s NIR light transmittance was shown to be approximately 4.52% in preliminary tests, it was hypothesized that a maximum radiation dosage of 128 and 1500 J/cm2 would be sufficient to induce cell death in in vitro target cells and in vivo mouse tumor models, respectively. Cell viability was measured through bioluminescence studies comparing relative luciferase activity, as well as a cytotoxicity assay. In the in vitro model, tumor cell viability was significantly decreased after 64 and 128 J/cm2 NIR light irradiation through the bone. An in vivo mouse tumor model also showed that 1500 J/cm2 NIR light irradiation through the bone significantly reduced tumor viability at both 24 and 48 hours posttreatment compared to the control group (P = .026 and .040 respectively). Therefore, despite limitations in light transmission, NIR‐PIT nevertheless is capable of effectively treating cancers within bone.
Keywords: bone, cancer, light penetration, metastasis, near‐infrared photoimmunotherapy
Tumors were successfully treated through a bovine bone sample.
Abbreviations
- CT
computed tomography
- EGFR
epidermal growth factor receptor
- NIR
near‐infrared
- NIR‐PIT
near‐infrared photoimmunotherapy
- PI
propidium iodide
- RLU
relative light units
1. INTRODUCTION
Near‐infrared photoimmunotherapy is a novel cancer therapy that targets cells overexpressing cancer antigens using a monoclonal antibody (mAb) conjugated with a photoabsorber, a silicon‐phthalocyanine derivative, IRdye 700DX (IR700), which absorbs 690 nm of light in the NIR range. The Ab‐photoabsorber conjugate is given to the subject i.v. and binds to cancer cells at tumor sites over a period of hours. When NIR light is applied, the IR700 molecule absorbs the energy, causing a conformational change in the Ab. This leads to functional damage to the tumor cell membrane, resulting in rapid swelling and microperforations leading to cell blebbing and necrosis. This unique form of cell death is highly immunogenic. The release of the contents of the cells, including cancer‐specific antigens, induces dendritic cell maturation followed by education and priming of cytotoxic T cells, leading to an amplified immune response.1 An advantage of NIR‐PIT is that minimal damage is seen in normal cells surrounding the tumors; therefore, the method has a high selectivity for cancer.2 Following successful phase I and II studies, NIR‐PIT has recently entered phase III clinical trials worldwide in patients with advanced head and neck cancers.
Although the method has been successful in multiple soft tissue tumors, no studies have investigated its effects on cancers within bones, either primary or secondary. Bone metastases are a major cause of morbidity in patients with cancer. It has been assumed that bone is impervious to light and therefore not amenable to NIR‐PIT.3 However, this assumption has not been extensively tested experimentally in the NIR range.
This experiment is designed to determine whether NIR‐PIT is feasible in tissues shielded by bone tissues. Specifically, A431‐luc tumor cells were located behind a bovine rib and were evaluated for viability at increasing light doses in vitro. We also tested NIR‐PIT in vivo in mouse tumor models with bone tumors.
2. MATERIALS AND METHODS
2.1. Cell lines and culture
A431 luciferase (A431‐luc) cells expressing epidermal growth factor receptor (EGFR) were grown in RPMI‐1640 supplemented with 10% FBS and 1% penicillin‐streptomycin in tissue culture flasks in a humidified incubator at 37°C in an atmosphere of 95% air and 5% carbon dioxide.
2.2. Reagents
Water‐soluble, silica‐phthalocyanine derivative, IRDye 700DX NHS ester (IR700; C74H96N12Na4O27S6Si3, molecular weight of 1954.22) was obtained from LI‐COR Bioscience. Panitumumab, a fully humanized IgG2 mAb directed against EGFR, was purchased from Amgen. All other chemicals were of reagent grade.
2.3. Synthesis of IR700‐conjugated panitumumab
Conjugation of dyes with mAb has been previously described.2 Briefly, panitumumab (1 mg, 6.8 nmol) was incubated with IR700 (66.8 µg, 34.2 nmol, 5 mmol/L in DMSO) in 0.1 mol/L Na2HPO4 (pH 8.5) at room temperature for 1 hour. Subsequently, the mixture was purified with a Sephadex G25 column (PD‐10; GE Healthcare). The protein concentration was determined with a Coomassie Plus protein assay kit (Pierce Biotechnology) by measuring the absorption at 595 nm (8453 Value System; Agilent Technologies). The concentration of IR700 was measured by its absorption to confirm the number of fluorophore molecules conjugated to each mAb. We abbreviate IR700‐conjugated panitumumab as pan‐IR700.
2.4. Animal model
All in vivo procedures were undertaken in compliance with the Guide for the Care and Use of Laboratory Animal Resources (1996), US National Research Council, and approved by the local Animal Care and Use Committee. Eight‐week‐old female homozygote athymic nude mice were purchased from Charles River (NCI). During procedures, mice were anesthetized with isoflurane. Tumor models were established by injecting 4 million A431‐luc cells s.c. in the right dorsum of the mice.
2.5. Bone samples
This study utilized a bovine rib bone sample that was obtained from a local butcher shop and cut in half in the axial direction. Thickness was approximately 5 mm. The sample was kept in the freezer and thawed before trials.
2.6. Computed tomography imaging study
To elucidate the internal structure of the bone specimen, a CT imaging study was carried out using the Discovery MI‐DR PET/CT with 64 slice CT (GE Healthcare).
After the CT imaging, the internal structures were analyzed using Image Works Software (GE Healthcare), and its thickness was measured.
2.7. Spectroscopic study
The transmittance of the bone specimen was obtained using a SolidSpec‐3700 integrating sphere (Shimadzu). The bone sample was placed at the entrance of the sphere, and its transmittance at the 500‐900 nm wavelengths were analyzed with a UV Probe (Shimadzu).
2.8. In vitro NIR‐PIT
In vitro NIR‐PIT trials were carried out through the bone sample with A431‐luc cells. Twenty‐four‐well plates were seeded with A431‐luc cells (1 × 105 per well) and, after 24 hours of incubation, pan‐IR700 was added to each well for a final concentration of 10 µg/mL. The cells were then incubated for an additional 3 hours at 37°C. The cells were covered by the bone sample and were irradiated with a laser (BWF5‐690‐8‐600‐0.37; B&W Tek), emitting light at 685‐695 nm wavelength at a power density of 373 mW/cm2 as measured with an optical power meter (PM100 and S310; Thorlabs). Near‐infrared light at the energy of 1, 2, 4, 8, 16, 32, 64, and 128 J/cm2 (3, 6, 11, 22, 43, 86, 172, and 344 seconds, respectively) was irradiated to each well one‐by‐one; the other wells were covered by aluminum foil.
2.9. Cytotoxicity assay
Cytotoxicity to A431‐luc cells by NIR‐PIT with pan‐IR700 was evaluated by flow cytometric PI (Life Technologies) staining, which can detect compromised cell membranes. The cells were harvested 1 hour after treatment. Propidium iodide (PI) was added in the cell suspension (final concentration 2 µg/mL) and incubated at room temperature for 30 minutes, followed by flow cytometry. Each value represents mean ± SEM of 5 experiments.
2.10. Near‐infrared PIT through bone
In all groups, 100 μg pan‐IR700 was given i.v. 5 days after the tumor cell injection. The next day, the mice were divided randomly into 4 experimental groups of 5 mice per group by different doses of NIR light exposure with lasers: (i) non‐PIT; (ii) 500 J/cm2; (iii) 1000 J/cm2; and (iv) 1500 J/cm2. Tumors were shielded by a bone specimen during NIR‐PIT.
2.11. Bioluminescence imaging studies after NIR‐PIT
D‐luciferin (15 mg/mL, 200 μL; Gold Biotechnology) was injected i.p. into mice and pipetted in the in vitro study, and analyzed with a PhotonIMAGER (Biospace Lab) for luciferase activity that is indicative of tumor cell viability. Luciferase activity was reported in relative light unit (RLU). Serial images were assessed before and after NIR light exposure, for both 24 hours (day 1) and 48 hours (day 2) posttreatment for the mice, and 1 hour after light exposure for the cells at an acquisition duration of 3 minutes. Regions of interest were placed on the entire tumor, and the RLU (photons/minute) was then calculated using M3 Vision Software (Biospace Lab).
2.12. Statistical analysis
The differences between the treated and control groups were analyzed using a Student’s t‐test (2‐tailed, unpaired). P values less than .05 were considered significant. Error bars represent SEM.
3. RESULTS
3.1. Near‐infrared light penetrates bone tissue
We used bovine rib as the bone specimen to test transmittance of NIR light. To grasp the internal structure of specimens, a CT scan was first carried out for both the sample used in this study and its larger other half. The results indicated that the cortex of the bovine rib was high‐density, whereas the interior was low‐density due to the trabecular bone associated with bone marrow (Figure 1A). The sample’s thickness at the center was measured to be 4.96 mm. The bone sample’s light transmittance of various wavelengths was measured by a spectrometer. The transmittance at 690 nm, the excitation wavelength of IR700, was determined to be 4.52% of incident light (Figure 1B). Overall, the bone sample showed higher transmittance at longer wavelengths. These results suggest that a small percentage of NIR light can penetrate bone tissues and irradiate tissue underneath.
Figure 1.
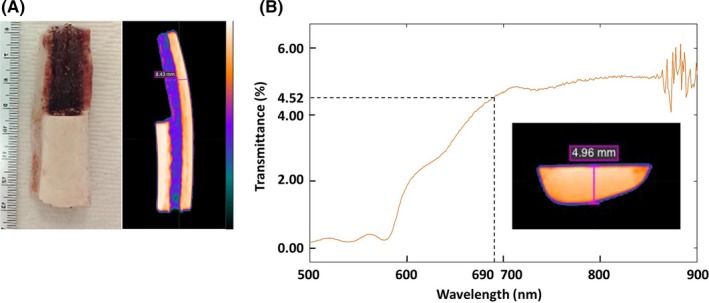
Characterization of bone sample. A, White light image and X‐ray computed tomography (CT) image of bone sample used in this study. B, Light transmittance of bone at different wavelengths, from 500 to 900 nm. The thickness of the bone samples are shown in both CT images (A, B)
3.2. Near‐infrared PIT through bone induces cell death in in vitro models
In order to test whether NIR‐PIT through bone is feasible, we undertook in vitro NIR‐PIT using A431‐luc cells by irradiating various doses of NIR light through a bovine rib (Figure 2A). Cell damage was evaluated by both a cytotoxicity assay and bioluminescence study. Cytotoxicity assay showed that the percentage of PI‐stained dead cells significantly increased after irradiation of both 64 and 128 J/cm2 light, whereas controls and groups with <32 J/cm2 dose light irradiation did not show increase of cell death (Figure 2B). Bioluminescence can also be used to determine cell viability because it depends on the presence of actively generated cellular potential, ATP. A similar result was obtained from the bioluminescence study. Compared to the absolute control group, the 64 and 128 J/cm2 groups displayed significantly decreased relative luciferase activity (P < .01), whereas the other groups showed no significant difference. The relative luciferase activity after NIR‐PIT without the bone specimen significantly decreased at NIR light doses above 1 J/cm2 (see Figure 2C,D). These results showed that NIR light transmits through bone and consequent NIR‐PIT is effective if sufficient intensities of NIR light are applied. When another bone sample was used for validating reproducibility, the results were consistent (Figure S1).
Figure 2.
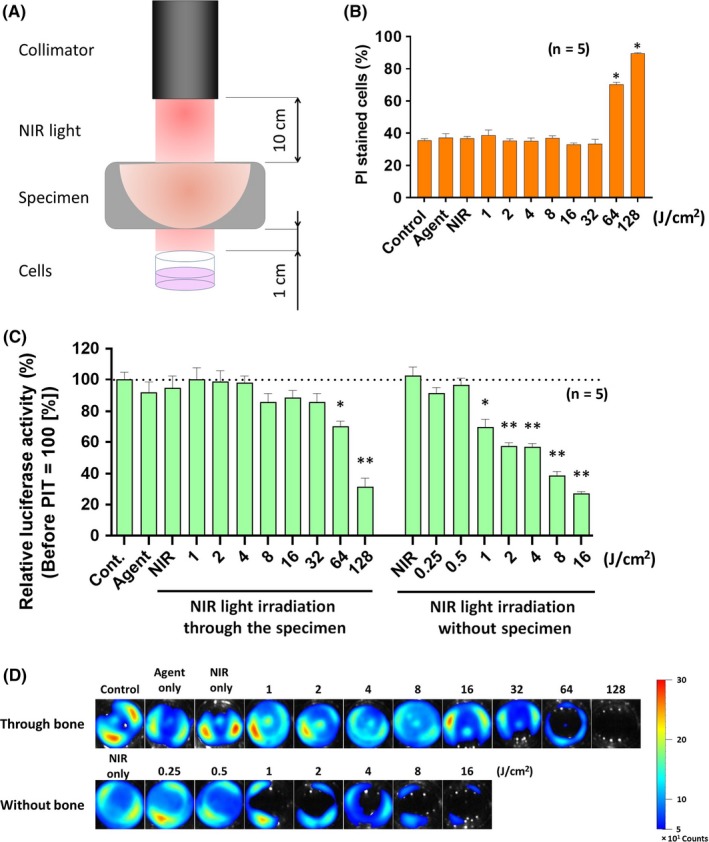
In vitro near‐infrared photoimmunotherapy (NIR‐PIT) through bone sample. A, Schema of experimental setting. B, Cell killing detected by propidium iodide (PI) staining. *P < .01 (Student's t‐test). C, Cell killing detected with bioluminescence imaging. Cont., control. *P < .05 and **P < .01, respectively (Student's t‐test). D, Bioluminescence images of NIR‐PIT treated cells through bone sample
3.3. Near‐infrared PIT reduces tumors in in vivo models
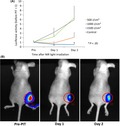
Next we tested the efficacy of NIR‐PIT through bone on in vivo models. Mice bearing A431‐luc tumors were injected with pan‐IR700 then irradiated with various doses of NIR light with bovine rib over the tumor. Bioluminescence imaging was undertaken before NIR light exposure and after 24 hours and 48 hours of treatment. There was no significant difference in luciferase activity between pretreatment and 24 hours posttreatment with the 500 and 1000 J/cm2 groups (t‐test, P = .16 and .67, respectively), or between pretreatment and 48 hours posttreatment (t‐test, P = .10 and .36, respectively). The 1500 J/cm2 treatment group, however, showed a significant reduction in luciferase activity at 24 and 48 hours posttreatment compared to the control group (P = .026 and .040, respectively; Figure 3A). Furthermore, although treatment with 1000 J/cm2 failed to produce statistically significant results, there were visibly clear reductions in tumor size after 24 hours (Figure 3B). These results suggest that, although bone significantly reduces the light penetrance, this can be overcome by using higher doses of NIR irradiation than regular NIR‐PIT. Like unshielded NIR‐PIT, bone‐shielded NIR‐PIT is effective in a dose‐dependent manner and could have therapeutic effects against bone tumors and tumors shielded by bones.
Figure 3.
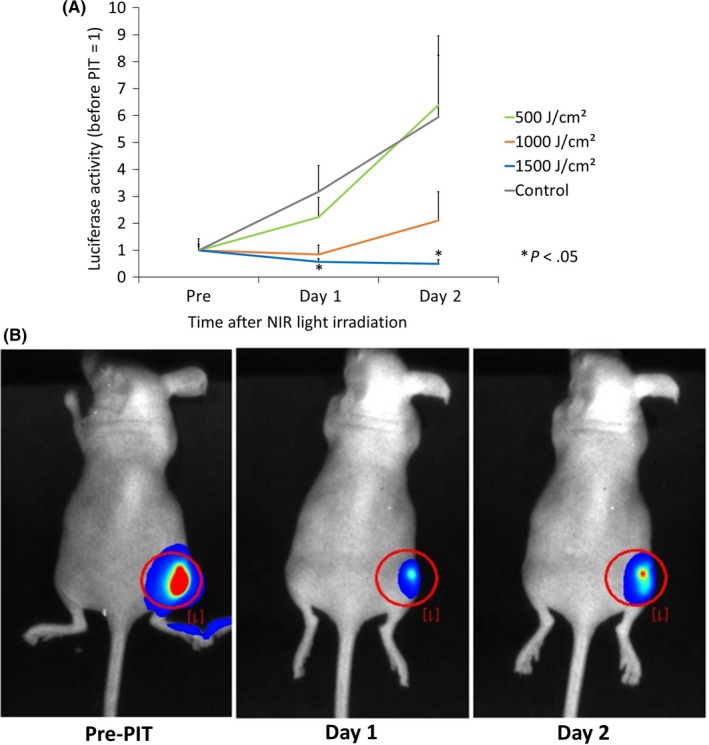
In vivo near‐infrared photoimmunotherapy (NIR‐PIT) through bone sample. A, NIR‐PIT treatment effects on mouse tumors through bone sample, measured by bioluminescence signals. B, Bioluminescence images of NIR‐PIT‐treated tumors through bone sample. *P < .05, (Student’s t‐test)
4. DISCUSSION
The primary goal of this study was to determine whether NIR‐PIT through the bone is feasible. Using A431‐luc tumor models, we showed that cell death was induced when NIR light was applied through a shielding bovine rib at increasing light doses. Based on previous reports, it was hypothesized that an irradiation dosage of 128 and 1500 J/cm2 would be sufficient to induce significant tumor death in in vitro models and in vivo mice models, respectively, and the results of this study verified both hypotheses.
Several previous studies have investigated the penetrance and transmittance of NIR light through various bone samples. One study analyzed light penetration of bone using wavelengths from 450 to 880 nm in human postmortem samples at varying skin, skull, and brain thicknesses. Using an integrating sphere to measure transmittance, the group found that all samples transmitted light at wavelengths above 600 nm. Specific results for the skull showed that transmittance peaked at wavelengths in the range of 700‐850 nm, and penetrance ranged from 5% to 12% depending on the bone thickness.4 A similar study observed light penetrance of NIR through a sheep skull sample in order to establish the premise for traumatic brain injury treatment. They found that, at a wavelength of 810 nm, NIR of less than 1 W was unable to penetrate the 3‐cm‐thick skull; however, at 10‐15 W, a maximum of 2.9% penetrated through the sheep skull sample.5 The NIR transmittance was greater at 810 nm wavelength than at 980 nm.4 Furthermore, another study compared the penetrance of NIR light at 830 nm and red light (630 nm) through different cadaver skull locations. At a light intensity of 35.1 mW/cm2, and at skull thickness of approximately 5 mm, NIR was able to penetrate a range of 4.4%‐10.4% at the frontal and right parietal parts of the brain, respectively6. These results are comparable to the 4.5% transmittance of NIR through a bovine rib.
For the in vitro models of NIR‐PIT shielded by bone, both cytotoxicity assays and bioluminescence measurements showed significant treatment effects, with necrosis starting from exposures of 64 J/cm2 (Figure 2). In the mouse models, significant tumor death resulted from the 1500 J/cm2 NIR light irradiation, and the reduction in tumor size was seen both 24 hours and 48 hours after treatment in tumors shielded by bone. These results suggest that NIR light does indeed penetrate bone in reasonable quantities and that NIR‐PIT can be effective through bone provided sufficient intensities are delivered. Furthermore, the prolonged effects of treatment over 48 hours indicate that the treatment remains effective for longer periods even with the rapid regrowth rate of A431 cells.
There are several broader implications of this study. First, it is possible that NIR‐PIT can effectively treat lesions within bones, such as bone metastases. Such tumors can be treated with NIR‐PIT by choosing an Ab‐drug conjugate that targets tumor antigens expressed on the metastasized tumors. Additionally, primary bone tumors, such as osteosarcoma, which affects primarily young patients and accounts for nearly 20% of all primary bone tumors, can be treated provided a suitable Ab can be found. As these bone tumors generally locate intramedullary, NIR‐PIT has the potential to treat these tumors if NIR light can be transmitted through the cortex and reach the medullary tissue.7 Naturally, thickness of bone could become an issue. In this study, the bone was approximately 5 mm thick. Bone metastasis can grow virtually in any bone and thickness could exceed 5 mm. According to previous anatomical studies investigating femur bone dimensions, the medial cortical thickness of human femur bones is approximately 6.7 mm.8 When bone tumors arise in larger bones, however, tumors usually erode the cortex so it might still be possible to treat with NIR‐PIT. Thicker bone samples should be investigated in future studies.
This study also suggests the potential application of NIR‐PIT in treating brain tumors, such as glioblastoma, through the skull. Glioblastoma multiforme is the most common and malignant primary brain tumor in adults and is difficult to treat due to its highly invasive growth pattern, making surgical resection ineffective. A previous study investigated the effects of NIR‐PIT against glioblastoma and found that the treatment was highly effective. Specifically, targeting glioblastoma stem cells expressing the cancer stem cell marker AC133/CD133, they reported that NIR‐PIT was able to induce rapid cell death and significantly decrease s.c. and invasively growing brain tumors. Furthermore, a single dose of NIR‐PIT was able to extend the survival of mice with orthotopic gliomas by more than a factor of 2, even when the NIR light was applied through the intact skull.9
In humans, however, studies have yet to test the practicality of NIR‐PIT through the intact skull. If NIR‐PIT can be applied without the invasive process of surgical operations on the skull, not only will it be more time‐efficient, but it will also be more convenient for patients and lead to shorter recovery times with fewer side‐effects. Thus, it is of significance importance to study how to deliver sufficient amounts of NIR light to the tumor by transilluminating the skull. The thickness of the skull varies considerably and certain locations could be more amenable to NIR‐PIT than others. A previous study reported that in certain areas, such as the frontal and temporal bone, the skull is less than 4 mm thick,10 similar to the bone thickness in the current study. Additionally, although light energy for yielding sufficient therapeutic effects through the bone specimen was approximately 15‐fold greater than that without penetrating through the bone, the light fluency used in this study (up to 400 mW/cm2) does not yield adverse side‐effects to the tissue.11
This study has several limitations. Bovine not human bone was used because of its ready availability. The bovine rib was used due to its flat structure and porous composition, but it is not a perfect representation of bone in multiple sites. A previous study investigating interspecies differences in bone composition reported that human bone samples had a significantly lower bone density overall compared to bovine bone.12 This suggests that NIR‐PIT might be more effective on human bone samples.
Overall, this study showed potential for NIR‐PIT to be used as a bone cancer therapy. Despite only a 4.52% transmittance of NIR light through bone, NIR‐PIT was shown to be effective in killing cancer cells. This extends the possible uses of NIR‐PIT for treating tumors both within and behind bone in humans.
DISCLOSURE
The authors declare no conflicts of interest.
Supporting information
ACKNOWLEDGMENTS
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research (ZIA BC011513).
Nakamura YA, Okuyama S, Furusawa A, et al. Near‐infrared photoimmunotherapy through bone. Cancer Sci. 2019;110:3689–3694. 10.1111/cas.14203
REFERENCES
- 1. Ogawa M, Tomita Y, Nakamura Y, et al. Immunogenic cancer cell death selectively induced by near infrared photoimmunotherapy initiates host tumor immunity. Oncotarget. 2017;8:10425‐10436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mitsunaga M, Ogawa M, Kosaka N, Rosenblum LT, Choyke PL, Kobayashi H. Cancer cell‐selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat Med. 2011;17:1685‐1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jimenez‐Andrade JM, Mantyh WG, Bloom AP, Ferng AS, Geffre CP, Mantyh PW. Bone cancer pain. Ann N Y Acad Sci. 2010;1198:173‐181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hart NS, Fitzgerald M. A new perspective on delivery of red‐near‐infrared light therapy for disorders of the brain. Discov Med. 2016;22:147‐156. [PubMed] [Google Scholar]
- 5. Henderson TA, Morries LD. Near‐infrared photonic energy penetration: can infrared phototherapy effectively reach the human brain? Neuropsychiatr Dis Treat. 2015;11:2191‐2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jagdeo JR, Adams LE, Brody NI, Siegel DM. Transcranial red and near infrared light transmission in a cadaveric model. PLoS ONE. 2012;7:e47460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kundu ZS. Classification, imaging, biopsy and staging of osteosarcoma. Indian J Orthop. 2014;48:238‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Niimi R, Kono T, Nishihara A, et al. Cortical thickness of the femur and long‐term bisphosphonate use. J Bone Miner Res. 2015;30:225‐231. [DOI] [PubMed] [Google Scholar]
- 9. Jing H, Weidensteiner C, Reichardt W, et al. Imaging and selective elimination of glioblastoma stem cells with theranostic near‐infrared‐labeled CD133‐specific antibodies. Theranostics. 2016;6:862‐874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mahinda H, Murty OP. Variability in thickness of human skull bones and sternum – an autopsy experience. J Forensic Leg Med. 2009;16:26‐31. [Google Scholar]
- 11. Okuyama S, Nagaya T, Ogata F, et al. Avoiding thermal injury during near‐infrared photoimmunotherapy (NIR‐PIT): the importance of NIR light power density. Oncotarget. 2017;8:113194‐113201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aerssens J, Boonen S, Lowet G, Dequeker J. Interspecies differences in bone composition, density, and quality: potential implications for in vivo bone research. Endocrinology. 1998;139:663‐670. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


