Abstract
Background
People with head and neck cancer (HNC) have higher comorbidity levels but it remains unclear if pre-treatment comorbidity is an independent prognosticator in HNC.
Methods
Survival analyses were performed using data from participants in a UK multicentre cohort study with cancers of the oral cavity (n = 668), oropharynx (n = 1,074) and larynx (n = 530). Survival analyses were incrementally adjusted for age, gender, marital status, income, education, stage, alcohol and smoking.
Results
After adjusting for demographic, clinical and behavioural confounders, higher baseline comorbidity was associated with reduced overall survival (mild comorbidity HR 1.4, 95% CI 1.1, 1.7; moderate comorbidity HR 1.7, 95% CI 1.3, 2.2; severe comorbidity HR 2.8, 95% CI 1.9, 4.; p-trend<.001).
Conclusions
Our findings suggest that comorbidity is an independent prognosticator for overall survival in HNC. Comorbid illnesses should be considered in the assessment and treatment planning of people with HNC.
Keywords: comorbidity, ACE 27, survival, head and neck cancer, human papillomavirus
Introduction
Head and neck cancers (HNC) comprise a group of heterogeneous malignancies affecting the oral cavity, pharynx, larynx, paranasal sinuses, salivary glands and thyroid. Combined HNC is the sixth most common cancer in Europe (1). The disease has a poor prognosis with overall 5-year survival rates around 66% (2).
Comorbidity, the presence of coexistent medical conditions that are unrelated to the index disease (3), may independently affect health outcomes. Higher comorbidity scores have been shown to be adversely associated with overall survival (OS) (3–7), choice of treatment modality (3, 4, 8) and treatment outcomes in HNC (5, 8–11). People with HNC tend to have a higher comorbidity burden compared to the general population (3, 6). This may partially be attributable to their engagement in adverse health behaviours such as smoking and high alcohol intake that increase the risk of the development of comorbid conditions as well as HNC.
The classical risk factors for most HNCs, with exception of thyroid and salivary gland tumours, are smoking and to a lesser extent alcohol consumption. More recently, the oncogenic Human Papillomavirus (HPV) has been shown to play a role in the development of certain HNC subtypes, specifically oropharyngeal squamous cell carcinomas. HPV-positive HNCs differ from tobacco-related HPV-negative tumours in their clinical characteristics and risk factor profiles (4, 12, 13). HPV-positive cancers typically present as smaller tumours with more frequent lymph node involvement but better OS (13). These findings may be partially due to a more favourable risk factor profile. People with HPV-positive oropharyngeal tumours tend to be non-smokers, consume less alcohol, have a higher socioeconomic status and a lower comorbidity burden (4, 7, 12, 14).
Previous studies have examined the impact of comorbidities on outcomes in HNC (6, 7, 14–16). However, no prospective study has been able to adjust for HPV status, behavioural and social variables in their study populations while stratifying for HNC sub-site in the same model. Hence there is insufficient evidence to conclude whether comorbidity burden at diagnosis is an independent prognostic indicator in people with HNC or whether it constitutes a surrogate marker for health risk behaviours (4, 7).
Despite these limitations the evidence suggests that comorbidity may be a useful tool to help stratify people. Revised staging models that incorporate comorbidity index scores have been proposed (4, 7). These models suggest that comorbidity in combination with the traditional Tumour Node Metastases (TNM) stage may be more accurate in predicting survival and could play an important role in treatment planning.
Using data from Head and Neck 5000, a large UK multi-centre prospective cohort study, we examined the relationship between comorbidity and outcomes in HNC. The objectives of this study were to analyse the relationship between comorbidity at diagnosis and OS before and after adjustment for confounders and at different sites.
Materials and Methods
Study population
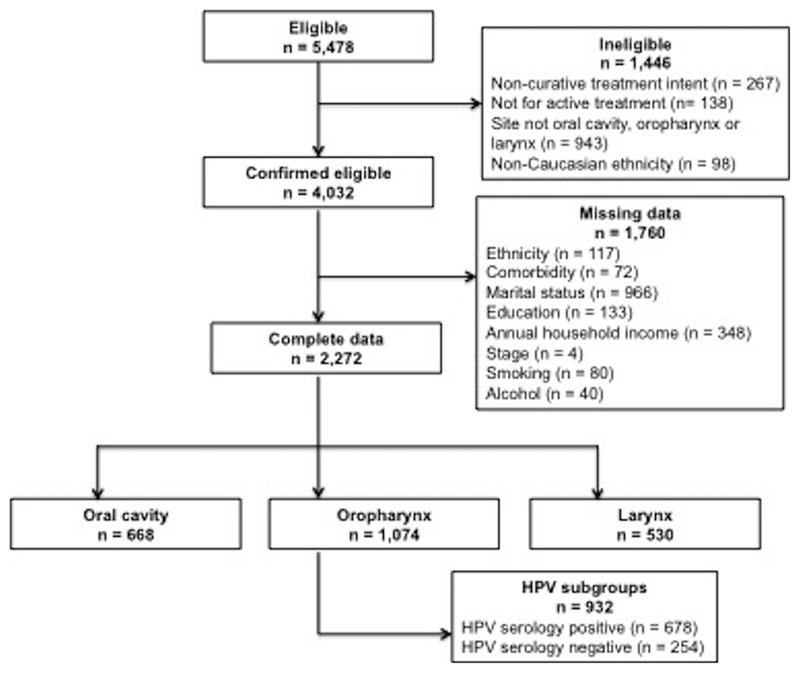
We used data from Head and Neck 5000, a large UK-based clinical cohort study that enrolled 5,478 eligible participants of 11,158 eligible people with HNC between April 2011 and December 2014 (17). Research nurses or another trained member of the research team obtained informed written consent from all participants prior to study enrolment(18). The South West – Frenchay Regional Ethics Committee granted full ethical approval (ref: 10/H0107/57) for Head and Neck 5000 in 2010. Additionally, the research and development departments of each participating NHS Trust approved the study. Study methods and recruitment rates have previously been described in detail (17, 18). For this study we included Caucasian participants with HNC of the oral cavity, oropharynx and larynx who were treated with curative intent (Figure 1).
Comorbidity measure
The Adult Comorbidity Evaluation 27 (ACE 27) (19) was used to collect comorbidity data at diagnosis. The presence and severity of medical comorbidities were extracted from medical notes by local research nurses and scored by clinicians. Newly diagnosed HNCs were excluded from the score. The final ACE 27 score was derived from the highest ranked comorbidity. Participants without comorbid conditions received a score of 0. Mild comorbidity was defined as an ACE 27 grade of 1. In cases where moderate decompensation (grade 2) was present in two or more conditions affecting different organ systems, a final grade of 3 (severe decompensation) was awarded.
Measures of confounders
Confounding variables were identified based on previously reported associations with both exposure (comorbidity) and outcome (survival). Clinical information regarding diagnosis including tumour sub-site (classified using the International Classification of Diseases (ICD) 10), clinical TNM stage and planned treatment modality was taken from participants’ medical records and pathology reports. A self-administered baseline questionnaire provided data on participants’ age, gender, marital status, education and annual total household income. Health risk behaviours at diagnosis were recorded using self-report questionnaires. Smoking status was categorised as never, current or former smokers (previously smoked at least 100 cigarettes up until 1 year before diagnosis). Baseline alcohol consumption was quantified as none, moderate (men and women drinking < 14 units/week), hazardous (men consuming 14 – 50 units/week; women consuming 14 – 35 units/week) and harmful (men consuming > 50 units/week; women consuming > 35 units/week). HPV status was determined by serological testing for HPV16 E6 antibodies using a glutathione S-transferase multiplex assay with a cut-off value of ≥ 1000 Median Fluorescence Intensity units (20) at the German Cancer Research Centre (DKFZ, Heidelberg, Germany) as previously described (21).
Outcome measure
Study participants were flagged up with the UK Health and Social Care Information Centre for notification of date and cause of death. Survival time was measured from study enrolment until either death or the end of the most recent follow-up period.
Statistical analysis
Chi-squared tests were used to assess the univariate relationship between categorical variables and comorbidity. Unadjusted Kaplan-Meier graphs were plotted to estimate OS for each cancer site. Cox proportional hazards regression models were used to evaluate OS in multivariable analyses. Hazard ratios (HR) and 95% confidence intervals (CIs) were adjusted for known or suspected confounders. Using the previously described confounding variables, four a priori survival models were incrementally fitted for each cancer site. Model 1 (Minimally adjusted) was adjusted for age and gender only. Model 2 (Clinical) included the same variables as Model 1 and also adjusted for TNM stage, treatment modality, and cancer site. Model 3 (Social) included all previously used variables plus marital status, annual household income and education. Finally, Model 4 (Behavioural) was an extension of Model 3 with addition of smoking status and alcohol intake to the list of confounders. To test the proportional hazards assumption, scaled Schoenfeld residuals were applied. To investigate the association between HPV status and comorbidity stratified survival analyses were performed. Comorbidity was grouped into low (0-1) and high (≥2) ACE 27 scores to achieve a larger sample size for each category in HPV-stratified survival analyses. Similarly, treatment-stratified analyses were performed to examine the relationship between surgical and non-surgical treatment modalities and survival in people with different comorbidity burden. Surgical treatment was defined as surgery alone and surgery plus an adjunct therapy. Non-surgical treatment modalities consisted of chemoradiation and stand-alone radiotherapy.
The Head and Neck 5000 dataset version 2.2 was used for this study. All statistical analyses were performed using Stata 14.0 (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX, USA: StataCorp LP).
Results
Distribution of comorbidity in the study population
Complete data were available for 2,272 participants. HPV data were available for a subset of oropharyngeal cancer (n = 932). A total of 466 deaths were recorded in 7167.9 person-years of follow-up. Mean follow-up time was 3.2 years (standard deviation 1.2). Table 1 shows the baseline characteristics of study participants by cancer site. Almost half (52.6%) of the participants had at least one medical condition at the time of diagnosis. Participants with oropharyngeal cancers were more likely to have no comorbidity (52.5%) compared to participants with oral cavity (45.5%) or laryngeal cancers (39.5%). HPV serology was available for 86.8% (n = 932) of oropharyngeal cancer cases with HPV-positive cancers displaying a lower comorbidity burden than HPV-negative cancers. 90% of oral cavity cancer cases were managed surgically compared with only 30.6% of oropharyngeal and 29.4% of laryngeal cancers (Table 1).
Table 1. Distribution of variables by cancer sub-site.
| Variables | All sites No. of people (%) = 2272 | Oral cavity No. of people (%) = 668 | Oropharynx | Larynx No. of people (%) = 530 | ||
|---|---|---|---|---|---|---|
| All cases No. of people (%) = 1074 |
HPV-negative No. of people (%) = 254 |
HPV-positive No. of people (%) = 678 |
||||
| Age, years | ||||||
| Mean (s.d) | 60.8 (10.5) | 61.2 (11.9) | 58.5 (9.1) | 59.3 (9.8) | 58.2 (8.7) | 65.0 (10.0) |
| Gender | ||||||
| Male | 1739 (76.5) | 423 (63.3) | 855 (79.6) | 195 (76.8) | 547 (80.7) | 461 (87.0) |
| ACE 27 | ||||||
| 0 | 1077 (47.4) | 304 (45.5) | 564 (52.5) | 108 (42.5) | 387 (57.1) | 209 (39.5) |
| 1 | 748 (32.9) | 208 (31.1) | 346 (32.2) | 87 (24.3) | 211 (31.1) | 194 (36.5) |
| 2 | 362 (15.9) | 119 (17.8) | 138 (12.8) | 46 (18.1) | 71 (10.5) | 105 (19.8) |
| 3 | 85 (3.7) | 37 (5.5) | 26 (2.4) | 13 (5.1) | 9 (1.3) | 22 (4.1) |
| TNM stage | 903 (39.7) | 396 (59.3) | 149 (13.9) | 63 (24.8) | 64 (9.4) | 358 (67.4) |
| I/II | 1369 (60.3) | 272 (40.7) | 925 (86.1) | 191 (75.2) | 614 (90.6) | 172 (32.6) |
| III/IV | ||||||
| Treatment | ||||||
| Surgery | ||||||
| Surgery + | 724 (31.9) | 499 (74.7) | 105 (9.8) | 39 (15.4) | 49 (7.2) | 120 (22.6) |
| ChemoRT | 361 (15.9) | 102 (15.3) | 223 (20.8) | 50 (19.7) | 152 (22.4) | 36 (6.8) |
| RT | 759 (33.4) | 35 (5.2) | 634 (59.0) | 129 (50.8) | 420 (62.0) | 90 (17.1) |
| 428 (18.8) | 32 (4.8) | 112 (10.4) | 36 (14.2) | 57 (8.4) | 284 (53.5) | |
| Marital status | ||||||
| Single | ||||||
| In relationship | 275 (12.1) | 97 (14.5) | 115 (10.7) | 40 (15.8) | 58 (8.6) | 63 (11.9) |
| Separated or widowed | 1547 (68.1) | 416 (62.3) | 780 (72.6) | 152 (59.8) | 531 (78.3) | 351 (66.3) |
| 450 (19.8) | 155 (23.2) | 179 (16.7) | 62 (24.4) | 89 (13.1) | 116 (21.8) | |
| EducationSchool | ||||||
| College | 1036 (45.6) | 299 (44.8) | 442 (41.2) | 109 (42.9) | 277 (40.9) | 295 (55.7) |
| Degree | 806 (35.5) | 218 (32.6) | 420 (39.1) | 101 (39.8) | 268 (39.5) | 168 (31.6) |
| 430 (18.9) | 151 (22.6) | 212 (19.7) | 44 (17.3) | 133 (19.6) | 67 (12.6) | |
| Annual income (£) | ||||||
| <18,000 | 1034 (45.5) | 333 (49.9) | 394 (36.7) | 128 (50.4) | 195 (28.8) | 307 (58.0) |
| 18,000-34,999 | 663 (29.2) | 187 (28.0) | 333 (31.0) | 70 (27.6) | 227 (33.5) | 143 (26.9) |
| >35,000 | 575 (25.3) | 148 (22.2) | 347 (32.3) | 56 (22.1) | 256 (37.8) | 80 (15.1) |
| Smoking status | ||||||
| Never | ||||||
| Former | 530 (23.3) | 164 (24.6) | 321 (29.9) | 43 (16.9) | 240 (35.4) | 45 (8.5) |
| Current | 1296 (57.0) | 340 (50.9) | 584 (54.4) | 110 (43.3) | 388 (57.2) | 373 (70.2) |
| 446 (19.6) | 164 (24.6) | 169 (15.7) | 101 (39.8) | 50 (7.4) | 113 (21.3) | |
| Alcohol intake | ||||||
| None | ||||||
| Moderate | 598 (26.3) | 168 (25.1) | 289 (26.9) | 65 (25.6) | 181 (28.2) | 141 (26.6) |
| Hazardous | 477 (21.0) | 141 (21.1) | 226 (21.0) | 42 (16.5) | 163 (24.0) | 110 (20.9) |
| Harmful | 862 (37.9) | 245 (36.7) | 413 (38.5) | 88 (34.7) | 262 (38.6) | 204 (38.4) |
| 335 (14.7) | 114 (17.1) | 146 (13.6) | 59 (23.2) | 62 (9.1) | 75 (14.1) | |
Abbreviations: ACE 27 = Adult Comorbidity Evaluation 27; HPV = Human Papillomavirus; s.d. = standard deviation; TNM = Tumour, node, metastasis; Surgery+ = surgery plus adjunct; ChemoRT = chemoradiotherapy; RT = radiotherapy
Relationship between covariates, comorbidity and survival
Univariate analyses showed that all covariates were individually associated with comorbidity (Table 2). Participants’ age (χ2 138.4; df(3); p<.001), treatment modality (χ2 124.3; df(9); p<.001) and annual household income (χ2 115.1; df(6); p<.001) exhibited the strongest statistical evidence of an association with comorbidity burden. To explore the relationship further treatment modality was grouped into surgical and non-surgical modalities and the highest comorbidity scores (ACE > 1) combined into one group. In this analysis observed differences were small (49% of people with ACE = 0 had surgery compared to 47% of people with ACE >1) and there was no statistical evidence to support an association between surgical treatment and co-morbidity (χ2 3.42; df(2); p = .18; Cramer’s V = .04).
Table 2. Univariate analysis of the relationship between comorbidity and all covariates.
| Variables, No. of people (%) | ACE 27 score 0 No. of people (%) | ACE 27 score 1 No. of people (%) | ACE 27 score 2 No. of people (%) | ACE 27 score >2 No. of people (%) | χ2 | df | p |
|---|---|---|---|---|---|---|---|
| Age (years) | |||||||
| <65 | 820 (76.1) | 417 (55.8) | 180 (49.7) | 37 (43.5) | 138.4 | 3 | <.001 |
| ≥65 | 257 (23.9) | 331 (44.3) | 182 (50.3) | 48 (56.5) | |||
| Gender | |||||||
| Male | 799 (74.2) | 576 (77.0) | 291 (80.4) | 73 (76.5) | 10.5 | 3 | .015 |
| Female | 278 (25.8) | 172 (23.0) | 71 (19.6) | 12 (23.5) | |||
| Cancer site | |||||||
| Oral cavity | 304 (28.2) | 208 (27.8) | 119 (32.9) | 37 (43.5) | 40.6 | 6 | <.001 |
| Oropharynx | 564 (52.4) | 346 (46.3) | 138 (38.1) | 26 (30.6) | |||
| Larynx | 209 (19.4) | 194 (25.9) | 105 (29.0) | 22 (25.9) | |||
| TNM Stage | |||||||
| I/II | 396 (36.8) | 301 (40.2) | 173 (47.8) | 33 (38.8) | 13.9 | 3 | .003 |
| III/IV | 681 (63.2) | 447 (59.8) | 189 (52.2) | 52 (61.2) | |||
| Treatment modality | |||||||
| Surgery | 329 (30.6) | 240 (32.1) | 115 (31.8) | 40 (47.1) | 124.3 | 9 | <.001 |
| Surgery + | 206 (19.1) | 99 (13.2) | 44 (12.2) | 12 (14.1) | |||
| ChemoRT | 419 (38.9) | 243 (32.5) | 86 (23.8) | 11 (12.9) | |||
| RT | 123 (11.4) | 166 (22.2) | 117 (32.3) | 22 (25.9) | |||
| Marital status | |||||||
| Single | 134 (12.4) | 74 (9.9) | 54 (14.9) | 13 (15.3) | 32.5 | 6 | <.001 |
| In relationship | 775 (72.0) | 497 (66.4) | 228 (63.0) | 47 (55.3) | |||
| Separated or widowed | 168 (15.6) | 177 (23.7) | 80 (22.1) | 25 (29.4) | |||
| Education | |||||||
| School | 442 (41.0) | 357 (47.7) | 197 (54.4) | 40 (47.1) | 26.2 | 6 | <.001 |
| College | 411 (38.2) | 245 (32.8) | 117 (32.3) | 33 (38.8) | |||
| Degree | 224 (20.8) | 146 (19.5) | 48 (13.3) | 12 (14.1) | |||
| Annual income (£) | |||||||
| <18,000 | 382 (35.5) | 373 (49.8) | 226 (62.4) | 53 (62.4) | 115.1 | 6 | <.001 |
| 18,000-34,999 | 344 (31.9) | 207 (62.4) | 92 (25.4) | 20 (23.5) | |||
| ≥35,000 | 351 (32.6) | 168 (62.4) | 44 (12.2) | 12 (14.1) | |||
| Smoking status | |||||||
| Never | 291 (27.0) | 162 (21.7) | 67 (18.5) | 10 (11.8) | 28.1 | 6 | <.001 |
| Former | 604 (56.1) | 435 (58.2) | 206 (56.9) | 51 (60.0) | |||
| Current | 182 (16.9) | 151 (20.2) | 89 (25.6) | 24 (28.2) | |||
| Alcohol intake | |||||||
| None | 242 (22.5) | 217 (29.0) | 110 (30.4) | 29 (34.1) | 31.8 | 9 | <.001 |
| Moderate | 251 (23.3) | 147 (19.7) | 66 (18.2) | 13 (15.3) | |||
| Hazardous | 440 (40.0) | 278 (37.2) | 117 (32.3) | 27 (31.8) | |||
| Harmful | 144 (13.4) | 106 (14.2) | 69 (19.1) | 16 (18.8) | |||
| HPV status (oropharyngeal subgroup only) | |||||||
| HPV negative | 108 (21.8) | 87 (29.2) | 46 (39.3) | 13 (59.1) | 27.8 | 3 | <.001 |
| HPV positive | 387 (78.2) | 211 (70.8) | 71 (60.7) | 9 (40.9) | |||
Abbreviations: ACE 27 = Adult Comorbidity Evaluation 27; df = degrees of freedom; HPV = Human Papillomavirus; TNM = Tumour, node, metastasis; surgery+ = surgery plus adjunct; ChemoRT = chemoradiotherapy; RT = radiotherapy
With the exception of gender, education level and treatment modality, all covariates were also individually associated with OS (Supplementary Table 1).
Survival analyses
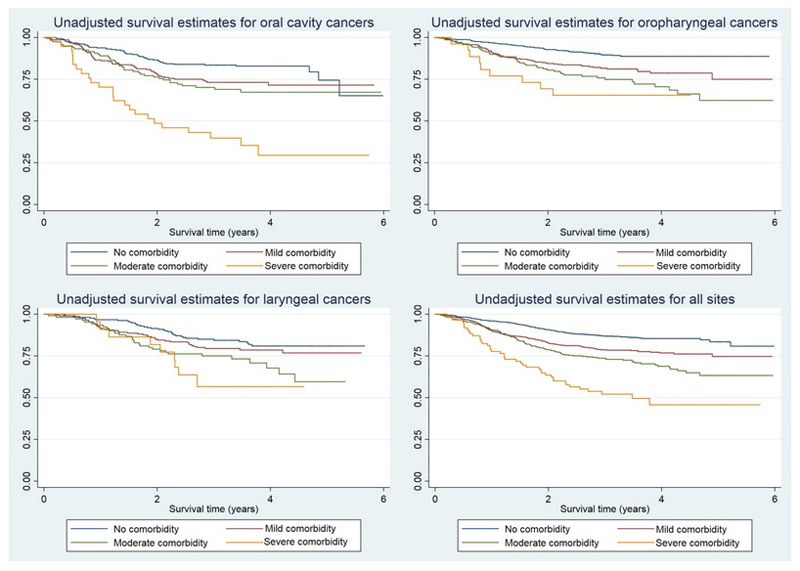
In a minimally adjusted Cox regression model comorbidity displayed a dose-dependent relationship with OS across all three HNC sites (Table 3). After full adjustment for demographic, clinical, social and behavioural factors, this trend remained strong with HRs of 1.4 (95% CI = 1.1, 1.7), 1.7 (95% CI = 1.3, 2.2) and 2.8 (95% CI = 1.9, 4.0) for mild, moderate and severe comorbidity, respectively (p-trend<.001). In unadjusted Kaplan-Meier analyses, participants with oropharyngeal and oral cavity cancers who did not have comorbid conditions displayed the best survival, while for laryngeal cancers no clear survival benefit between participants without comorbidity and those with mild decompensation (Figure 2). The association between survival and comorbidity did not differ between HPV-positive and HPV-negative oropharyngeal cancers after adjusting for all covariates (Table 3).
Table 3. Association between comorbidity and overall survival, stratified by cancer sub-site and HPV status.
| Model | Comorbidity | All sites | Oral cavity | Oropharynx | Larynx | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All cases | HPV-negative | HPV-positive | |||||||||||
| HR (95%CI) |
p-trend | HR (95%CI) |
p-trend | HR (95%CI) |
p-trend | HR (95%CI) |
p-trend | HR (95%CI) |
p-trend | HR (95%CI) |
p-trend | ||
| Minimally adjusted* | Mild | 1.5 (1.2, 2.0) | <.001 | 1.5 (1.0, 2.2) | <.001 | 1.8 (1.3-2.6) | <.001 | 2.2 (1.2, 3.6) | .02 | 1.1 (0.6-2.0) | .05 | 1.2 (0.8, 1.9) | .003 |
| Moderate | 2.0 (1.5, 2.6) | 1.8 (1.1, 2.7) | 2.5 (1.6, 3.7) | 2.0 (1.1, 3.7) | 2.0 (1.1, 3.8) | 1.8 (1.1, 2.9) | |||||||
| Severe | 3.8 (2.6, 5.4) | 4.6 (2.8, 7.6) | 3.3 (1.6, 6.7) | 2.6 (1.3, 5.5) | |||||||||
| Clinical model† | Mild | 1.5 (1.2, 1.9) | <.001 | 1.3 (0.9, 2.0) | <.001 | 1.8 (1.2, 2.5) | <.001 | 1.8 (1.0, 3.2) | .03 | 1.2 (0.7, 2.0) | .02 | 1.4 (0.9, 2.1) | .003 |
| Moderate | 1.9 (1.5, 2.5) | 1.4 (0.9, 2.2) | 2.4 (1.6, 3.7) | 1.7 (0.9, 3.3) | 2.4 (1.2, 4.5) | 2.0 (1.2, 3.3) | |||||||
| Severe | 3.3 (2.3. 4.8) | 3.5 (2.1, 5.8) | 3.3 (1.6, 6.9) | 2.2 (1.0, 4.6) | |||||||||
| Social‡ | Mild | 1.4 (1.1, 1.8) | <.001 | 1.3 (0.9, 2.0) | .001 | 1.6 (1.1, 2.3) | <.001 | 1.7 (0.9, 2.0) | .11 | 1.1 (0.6, 2.0) | .03 | 1.3 (0.8, 2.0) | .004 |
| Moderate | 1.8 (1.4, 2.3) | 1.3 (0.8, 2.0) | 2.1 (1.4, 3.3) | 1.6 (0.9, 3.2) | 2.3 (1.2, 4.3) | 2.0 (1.2, 3.4) | |||||||
| Severe | 3.0 (2.0, 4.3) | 3.0 (1.8, 5.1) | 3.0 (1.4, 6.1) | 2.2 (1.0, 4.7) | |||||||||
| Behavioural§ | Mild | 1.4 (1.1, 1.7) | <.001 | 1.3 (0.9, 1.9) | .001 | 1.6 (1.1, 2.4) | <.001 | 1.8 (0.9, 3.3) | .08 | 1.2 (0.7, 2.1) | .03 | 1.3 (0.8, 2.0) | .006 |
| Moderate | 1.7 (1.3, 2.2) | 1.2 (0.8, 1.9) | 2.1 (1.3, 3.2) | 2.0 (1.0, 3.8) | 2.4 (1.2, 4.6) | 2.0 (1.2, 3.4) | |||||||
| Severe | 2.8 (1.9, 4.0) | 2.7 (1.6, 4.6) | 3.2 (1.5, 6.8) | 2.2 (1.0, 4.8) | |||||||||
Abbreviations: HR = hazard ratio; CI = confidence interval; HPV = Human Papillomavirus
Adjusted for age and gender
Adjusted for age, gender, cancer site (“All sites” only), TNM stage and treatment modality
Adjusted for age, gender, cancer site (“All sites” only), TNM stage, treatment modality, marital status, education and annual household income
Adjusted for age, gender, cancer site (“All sites” only), TNM stage, treatment modality, marital status, education, household income per annum, smoking and alcohol intake
Finally, fully adjusted and stratified survival analyses demonstrated that higher comorbidity was associated with worse OS in both people who received surgical treatment (HR 1.6, 95% CI = 1.1, 2.2 for mild comorbidity; HR 2.0, 95% CI = 1.4, 2.9 for moderate/severe comorbidity) and those who were managed with chemoradiation or radiotherapy alone (HR 1.3, 95% CI 0.9, 1.7 for mild comorbidity; HR 1.8, 95% CI 1.3, 2.5 for moderate/severe comorbidity). There was no statistical evidence of a difference in the association between co-morbidity and survival in these two treatment groups.
Schoenfeld residuals testing confirmed the validity of the proportional hazards assumption for all variables.
Discussion
In this large contemporary clinical cohort of people with HNC in the UK we showed a dose-response relationship between comorbidity and survival that was consistent across tumour sites and independent of adjustment for lifestyle confounding factors. The dose-dependent effect of comorbid status on OS (HR 1.5, 95% CI 1.2, 2.0 for mild comorbidity; HR 3.8, 95% CI 2.6, 5.4 for severe comorbidity; p-trend<.001) was mildly attenuated after full adjustment for all covariates (HR 1.4, 95% CI 1.1, 1.7 for mild comorbidity; HR 2.8, 95% CI 1.9, 4.0 for severe comorbidity; p-trend<.001). The narrow confidence intervals and the consistency across cancer sites mean our findings are unlikely to be the result of chance.
Our findings are consistent with previous studies that identified pre-treatment comorbidity as an important independent prognosticator (5–7, 10, 14, 16, 22–25). Only a few of these previous studies were able to control for smoking and alcohol intake in their analyses (4, 22, 24, 26–28) and none adjusted for socioeconomic variables and health risk behaviours in the same model. Most of these studies only included people with oropharyngeal cancers (22, 26–28).
One large Canadian cohort study analysed data from medical record review to explore the impact of baseline comorbidity on OS across four HNC sub-sites (4). They used the claims-based Charlson Comorbidity Index (CCI) (29) to record comorbidity data retrospectively. Unlike the ACE 27 (used in our analysis), the CCI does not quantify disease severity and includes fewer comorbid conditions. Both indices are validated for use in people with cancer but data and results derived from different comorbidity indices may differ significantly (15, 28, 30, 31). In the UK, the ACE 27 remains the recommended tool for recording comorbidity data in people with cancer (32). They reported that higher comorbidity scores were associated with greater all-cause mortality in people with cancers of the oral cavity, larynx and nasopharynx. They observed marked attenuation after adjustment for confounders and as a result concluded that comorbidity was a surrogate marker for health risk behaviours rather than an independent prognostic indicator. The lack of attenuation of the association in our data may reflect the more accurate prospective collection of data on co-morbidity and lifestyle confounders.
In keeping with previous research, our baseline data also showed a lower burden of comorbidity and better unadjusted OS in people with HPV-positive oropharyngeal cancers compared to HPV-negative cases (4, 12, 13, 16, 22, 23, 33). In HPV-stratified analyses the dose-response relationship between comorbidity and survival was less pronounced. However, point estimates indicated that greater comorbidity remained associated with worse survival, independent of HPV status. Adjustment for potential confounders resulted in only a slight attenuation of the effect size in people with HPV-positive cancer and did not markedly change the observed association in people with HPV-negative cancers.
There is some evidence to suggest that comorbidity at diagnosis may influence treatment selection and may constitute an independent risk factor for post-surgical outcomes(3, 9, 10, 34) and cause-specific mortality(35, 36) in people with HNC. However, these findings are not consistent throughout the literature with one study failing to demonstrate an association between concurrent comorbid conditions and survival in treatment-stratified analysis(37). To date no prospective study has examined potential differences in baseline comorbidity scores and OS in people treated surgically compared to those who underwent conservative management for HNC. In our large cohort the distribution of treatment modalities among cancer sub-sites reflects current standard of care(38–41). Oral cavity cancers were predominantly managed surgically while over two-thirds of oropharyngeal and laryngeal cancers received non-surgical treatment. In univariate analysis treatment modality was independently associated with comorbidity. However, the observed differences were modest suggesting that comorbid status is not an important determinant of treatment selection. When adjusted for potential confounders including cancer site, the OS of people who received surgical versus non-surgical treatment were comparable. Our robust effect size estimates suggest that comorbidity is an important independent predictor of post-treatment survival irrespective of treatment modality.
Implications for practice
In recent years research suggested that presence of comorbid conditions may have a similar prognostic significance to increasing the stage of the cancer (4, 5, 14, 42). Our results confirm the importance of comorbidity and support the use of pre-treatment comorbidity data as part of a comprehensive prognostic assessment at the time of diagnosis. We also demonstrated that treatment selection does not affect the prognostic value of comorbidity burden.. In the UK the National Cancer Intelligence Network recommends that comorbidity data is collected for all people with cancer using the ACE 27 index to optimise pre-operative assessment and risk stratification (32). More research is needed to quantify the effect of specific conditions compared to total comorbidity burden on treatment outcomes to develop a comprehensive prognostic assessment system that combines TNM stage and comorbidity to more accurately predict survival in people with HNC.
Strength and limitations
Our study has a number of strengths. First, this large prospective clinical cohort study with a sample size of 2,272 participants allowed us to produce robust effect size estimates and stratify by cancer sub-site. Second, we used the ACE 27 to document the presence and severity of comorbidity. Third, our analyses accounted for a broad range of known confounders in incrementally adjusted regression models. To date no other study on the prognostic impact of comorbidity in HNC sub-types adjusted for clinical, socioeconomic and behavioural covariates in the same model. This study therefore adds significantly to the emerging evidence on the importance of comorbidity as an independent prognostic indicator in HNC.
Our study also had some limitations. First, our list of confounding variables was extensive but it was not exhaustive. For example, anaemia at diagnosis and performance status have been reported as potential predictors of survival in people with HNC (6, 9, 16, 35, 43, 44) but were not collected in our study. Types and details of comorbid conditions were not recorded. As a result, their distribution, individual association with the chosen treatment modality could not be analysed. Second, we used self-report from questionnaires to collect information on health behaviours. Self-report may be unreliable and underestimate current behaviour and not reflect prior or subsequent lifestyle behaviour. Reassuringly the proportion of current, former and never smokers in this study was similar to that reported in other cohorts (22, 24, 45). So, though we adjusted for a wide range of confounders in our models, residual confounding by unmeasured or poorly measured factors is still a possibility. Third, cause-specific mortality data were not available so we were unable to examine the association of comorbidity with specific causes of death. Fourth, despite a mean follow-up time of 3.2 years and the large overall sample size, the number of deaths and people with severe comorbidities was low in some groups, reducing statistical power in stratified analyses. Finally, only 49.1% of eligible people (n= 5,478 of 11,158) were enrolled in the study and complete data were only available for less than half of all enrolled participants (n= 2,272) limiting generalisability of our findings.
Conclusion
Our study found that comorbidity at baseline is a strong prognostic indicator of survival in a subset of HNCs, independent of health risk behaviours and treatment selection. Our findings support the need for comorbid disease to be included in future prognostication models for HNC to further aid treatment planning and to provide more accurate survival estimates.
Supplementary Material
Table 4. Association between comorbidity and survival, stratified by treatment modality.
| Model | Comorbidity | Surgical treatment | Non-surgical treatment | ||
|---|---|---|---|---|---|
| HR (95% CI) | p-trend | HR (95% CI) | p-trend | ||
| Minimally adjusted* | Mild | 1.8 (1.3-2.5) | <.001 | 1.3 (1.0-1.8) | <.001 |
| Moderate/Severe | 2.5 (1.7-3.5) | 2.3 (1.7-3.2) | |||
| Clinical† | Mild | 1.6 (1.2-2.3) | <.001 | 1.4 (1.5-1.9) | <.001 |
| Moderate/Severe | 2.3 (1.6-3.2) | 2.1 (1.5-3.0) | |||
| Social‡ | Mild | 1.6 (1.2-2.3) | <.001 | 1.3 (0.9-1.8) | <.001 |
| Moderate/Severe | 2.1 (1.5-2.9) | 1.9 (1.4-2.7) | |||
| Behavioural§ | Mild | 1.6 (1.1-2.2) | <.001 | 1.3 (0.9-1.7) | <.001 |
| Moderate/Severe | 2.0 (1.4-2.9) | 1.8 (1.3-2.5) | |||
Abbreviations: HR = hazard ratio; CI = confidence interval
Adjusted for age and gender
Adjusted for age, gender, cancer site and TNM stage
Adjusted for age, gender, cancer site, TNM stage, marital status, education and annual household income
Adjusted for age, gender, cancer site, TNM stage, marital status, education, household income per annum, smoking and alcohol intake
Acknowledgements
We would like to thank all study participants and staff who have worked on and supported the Head and Neck 5000 study. The study was a component of independent research funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research scheme (RP-PG-0707-10034). The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. Human papillomavirus serology was supported by a Cancer Research UK Programme Grant, the Integrative Cancer Epidemiology Programme (C18281/A19169). RB is supported by a grant from the Wellcome Trust (110021/z/15/A).
Footnotes
Conflict of interest
None declared
References
- 1.Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18(3):581–592. doi: 10.1093/annonc/mdl498. [DOI] [PubMed] [Google Scholar]
- 2.Pulte D, Brenner H. Changes in Survival in Head and Neck Cancers in the Late 20th and Early 21st Century: A Period Analysis. The Oncologist. 2010;15(9):994–1001. doi: 10.1634/theoncologist.2009-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paleri V, Wight RG, Silver CE, et al. Comorbidity in head and neck cancer: A critical appraisal and recommendations for practice. Oral Oncol. 2010;46(10):712–719. doi: 10.1016/j.oraloncology.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Habbous S, Harland LTG, La Delfa A, et al. Comorbidity and prognosis in head and neck cancers: Differences by subsite, stage, and human papillomavirus status. Head Neck. 2014;36(6):802–810. doi: 10.1002/hed.23360. [DOI] [PubMed] [Google Scholar]
- 5.Piccirillo JF. Importance of Comorbidity in Head and Neck Cancer. Laryngoscope. 2000;110(4):593–602. doi: 10.1097/00005537-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Wang JR, Habbous S, Espin-Garcia O, et al. Comorbidity and performance status as independent prognostic factors in patients with head and neck squamous cell carcinoma. Head Neck. 2016;38(5):736–42. doi: 10.1002/hed.23947. [DOI] [PubMed] [Google Scholar]
- 7.Datema FR, Ferrier MB, van der Schroeff MP, Baatenburg de Jong RJ. Impact of comorbidity on short-term mortality and overall survival of head and neck cancer patients. Head Neck. 2010;32(6):728–736. doi: 10.1002/hed.21245. [DOI] [PubMed] [Google Scholar]
- 8.Derks W, de Leeuw RJ, Hordijk GJ. Elderly patients with head and neck cancer: the influence of comorbidity on choice of therapy, complication rate, and survival. Curr Opin Otolaryngol Head Neck Surg. 2005;13(2):92–6. doi: 10.1097/01.moo.0000156169.63204.39. [DOI] [PubMed] [Google Scholar]
- 9.Ferrier MB, Spuesens EB, Le Cessie S, Baatenburg de Jong RJ. Comorbidity as a major risk factor for mortality and complications in head and neck surgery. Arch Otolaryngol Head Neck Surg. 2005;131(1):27–32. doi: 10.1001/archotol.131.1.27. [DOI] [PubMed] [Google Scholar]
- 10.Omura G, Ando M, Saito Y, Kobayashi K, Yamasoba T, Asakage T. Comorbidity as predictor poor prognosis for patients with advanced head and neck cancer treated with major surgery. Head Neck. 2016;38(3):364–369. doi: 10.1002/hed.23897. [DOI] [PubMed] [Google Scholar]
- 11.Hu M, Ampil F, Clark C, Sonavane K, Caldito G, Nathan C-AO. Comorbid predictors of poor response to chemoradiotherapy for laryngeal squamous cell carcinoma. Laryngoscope. 2012;122(3):565–571. doi: 10.1002/lary.22489. [DOI] [PubMed] [Google Scholar]
- 12.Hess CB, Rash DL, Daly ME, et al. Competing causes of death and medical comorbidities among patients with human papillomavirus–positive vs human papillomavirus–negative oropharyngeal carcinoma and impact on adherence to radiotherapy. JAMA Otolaryngol Head Neck Surg. 2014;140(4):312–316. doi: 10.1001/jamaoto.2013.6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boscolo-Rizzo P, Del Mistro A, Bussu F, et al. New insights into human papillomavirus-associated head and neck squamous cell carcinoma. Acta Otorhinolaryngol Ital. 2013;33(2):77–87. [PMC free article] [PubMed] [Google Scholar]
- 14.Bøje CR, Dalton SO, Primdahl H, et al. Evaluation of comorbidity in 9388 head and neck cancer patients: A national cohort study from the DAHANCA database. Radiother Oncol. 2014;110(1):91–97. doi: 10.1016/j.radonc.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Lee C-C, Ho H-C, Su Y-C, Chen P-C, Yu C-H, Yang C-C. Comparison of different comorbidity measures for oral cancer patients with surgical intervention: A longitudinal study from a single cancer center. Auris Nasus Larynx. 2016;43(3):322–329. doi: 10.1016/j.anl.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Baumeister P, Rauch J, Jacobi C, et al. Impact of comorbidity and anemia in patients with oropharyngeal cancer primarily treated with surgery in the human papillomavirus era. Head Neck. 2016 doi: 10.1002/hed.24528. [DOI] [PubMed] [Google Scholar]
- 17.Ness AR, Waylen A, Hurley K, et al. Recruitment, response rates and characteristics of 5511 people enrolled in a prospective clinical cohort study: head and neck 5000. Clin Otolaryngol. 2016;41(6):804–809. doi: 10.1111/coa.12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ness AR, Waylen A, Hurley K, et al. Establishing a large prospective clinical cohort in people with head and neck cancer as a biomedical resource: head and neck 5000. BMC Cancer. 2014;14(1):973. doi: 10.1186/1471-2407-14-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piccirillo JF, Feinstein AR. Clinical symptoms and comorbidity: significance for the prognostic classification of cancer. Cancer. 1996;77(5):834–42. [PubMed] [Google Scholar]
- 20.Kreimer AR, Johansson M, Waterboer T, et al. Evaluation of Human Papillomavirus Antibodies and Risk of Subsequent Head and Neck Cancer. J Clin Oncol. 2013;31(21):2708–2715. doi: 10.1200/JCO.2012.47.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waterboer T, Sehr P, Michael KM, et al. Multiplex Human Papillomavirus Serology Based on In Situ–Purified Glutathione S-Transferase Fusion Proteins. Clin Chem. 2005;51(10):1845–1853. doi: 10.1373/clinchem.2005.052381. [DOI] [PubMed] [Google Scholar]
- 22.Skillington S, Kallogjeri D, Lewis JS, Jr, Piccirillo JF. Prognostic importance of comorbidity and the association between comorbidity and p16 in oropharyngeal squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2016;142(6):568–575. doi: 10.1001/jamaoto.2016.0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ankola AA, Smith RV, Burk RD, Prystowsky MB, Sarta C, Schlecht NF. Comorbidity, human papillomavirus infection and head and neck cancer survival in an ethnically diverse population. Oral Oncol. 2013;49(9):911–917. doi: 10.1016/j.oraloncology.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duffy SA, Ronis DL, McLean S, et al. Pretreatment Health Behaviors Predict Survival Among Patients With Head and Neck Squamous Cell Carcinoma. J Clin Oncol. 2009;27(12):1969–1975. doi: 10.1200/JCO.2008.18.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL., Jr Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291(20):2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 26.Rios Velazquez E, Hoebers F, Aerts HJWL, et al. Externally validated HPV-based prognostic nomogram for oropharyngeal carcinoma patients yields more accurate predictions than TNM staging. Radiother Oncol. 2014;113(3):324–330. doi: 10.1016/j.radonc.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Rietbergen MM, Brakenhoff RH, Bloemena E, et al. Human papillomavirus detection and comorbidity: critical issues in selection of patients with oropharyngeal cancer for treatment De-escalation trials. Ann Oncol. 2013;24(11):2740–2745. doi: 10.1093/annonc/mdt319. [DOI] [PubMed] [Google Scholar]
- 28.Göllnitz I, Inhestern J, Wendt TG, et al. Role of comorbidity on outcome of head and neck cancer: a population - based study in Thuringia, Germany. Cancer Med. 2016;5(11):3260–3271. doi: 10.1002/cam4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 30.Kallogjeri D, Gaynor SM, Piccirillo ML, Jean RA, Spitznagel EL, Jr, Piccirillo JF. Comparison of Comorbidity Collection Methods. J Am Coll Surg. 2014;219(2):245–255. doi: 10.1016/j.jamcollsurg.2014.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paleri V, Wight RG. Applicability of the adult comorbidity evaluation - 27 and the Charlson indexes to assess comorbidity by notes extraction in a cohort of United Kingdom patients with head and neck cancer: a retrospective study. J Laryngol Otol. 2002;116(3):200–5. doi: 10.1258/0022215021910528. [DOI] [PubMed] [Google Scholar]
- 32.Robson A, Sturman J, Williamson P, Conboy P, Penney S, Wood H. Pre-treatment clinical assessment in head and neck cancer: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 2016;130(Suppl 2):S13–S22. doi: 10.1017/S0022215116000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwartz DSR, Yueh DB, McDougall DJK, Daling DJR, Schwartz DSM. Human Papillomavirus Infection and Survival in Oral Squamous Cell Cancer: A Population-Based Study. Otolaryngol Head Neck Sur. 2001;125(1):1–9. doi: 10.1067/mhn.2001.116979. [DOI] [PubMed] [Google Scholar]
- 34.Genther DJ, Gourin CG. Effect of comorbidity on short-term outcomes and cost of care after head and neck cancer surgery in the elderly. Head Neck. 2015;37(5):685–693. doi: 10.1002/hed.23651. [DOI] [PubMed] [Google Scholar]
- 35.Han KY, Jong-Lyel R, Sung-Bae K, Seung-Ho C, Yuhl NS, Yoon KS. Risk factors for competing non-cancer mortality after definitive treatment for advanced-stage head and neck cancer. Oral Dis. 2018;0(0) doi: 10.1111/odi.12904. [DOI] [PubMed] [Google Scholar]
- 36.Väisänen JA, Alho O-P, Koivunen PT, Läärä E. Cause-specific mortality in patients with head and neck cancer: Long-term follow-up of a population-based cohort from 1986 to 2012 accounting for competing risks. Oral Oncol. 2018;79:20–26. doi: 10.1016/j.oraloncology.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 37.Chokshi S, Ghobadi A, Athar M, Shah S, Dowell J. Impact of Comorbidity on Initial Treatment and Overall Survival in Elderly Head and Neck Cancer Patients. Anticancer Res. 2014;34(10):5543–5546. [PubMed] [Google Scholar]
- 38.Ellis MA, Graboyes EM, Wahlquist AE, et al. Primary Surgery vs Radiotherapy for Early Stage Oral Cavity Cancer. Otolaryngology–Head and Neck Surgery. 2018;158(4):649–659. doi: 10.1177/0194599817746909. [DOI] [PubMed] [Google Scholar]
- 39.Montero PH, Patel SG. Cancer of the oral cavity. Surg Oncol Clin N Am. 2015;24(3):491–508. doi: 10.1016/j.soc.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spiotto MT, Jefferson G, Wenig B, Markiewicz M, Weichselbaum RR, Koshy M. Differences in survival with surgery and postoperative radiotherapy compared with definitive chemoradiotherapy for oral cavity cancer: A national cancer database analysis. JAMA Otolaryngology–Head & Neck Surgery. 2017;143(7):691–699. doi: 10.1001/jamaoto.2017.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ryan B, Katherine H, Stacey F, CA Y. Current treatment of head and neck squamous cell cancer. J Surg Oncol. 2014;110(5):551–574. doi: 10.1002/jso.23724. [DOI] [PubMed] [Google Scholar]
- 42.Bøje CR. Impact of comorbidity on treatment outcome in head and neck squamous cell carcinoma: A systematic review. Radiother Oncol. 2013;110(1):81–90. doi: 10.1016/j.radonc.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 43.Caro JJ, Salas M, Ward A, Goss G. Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer. 2001;91(12):2214–21. [PubMed] [Google Scholar]
- 44.Sadat F, Wienke A, Dunst J, Kuhnt T. Survival of patients with head and neck cancer. Strahlenther Onkol. 2012;188(1):62–70. doi: 10.1007/s00066-011-0009-8. [DOI] [PubMed] [Google Scholar]
- 45.Ang KK, Harris J, Wheeler R, et al. Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.