Abstract
Objective
Although UK and international guidelines recommend monotherapy, antipsychotic polypharmacy in people with serious mental illness is common in clinical practice. However, empirical evidence on its effectiveness is scarce. The effectiveness of antipsychotic polypharmacy relative to monotherapy is estimated in terms of health care utilization and mortality.
Methods
Primary care data from the Clinical Practice Research Datalink, hospital data from the Hospital Episodes statistics and mortality data from the Office of National Statistics were linked to compile a cohort of patients with serious mental illness in England during the period 2000-2014. The antipsychotic prescribing profile of 17,255 adults who had at least one antipsychotic drug record during the period of observation was constructed from primary care medication records. Survival analysis models were estimated to identify the effect of antipsychotic polypharmacy on the time to the first occurrence of each of three outcomes: unplanned hospital admissions (all-cause), emergency department presentations, and mortality.
Results
Relative to monotherapy, antipsychotic polypharmacy was not associated with increased risk of an unplanned hospital admission (HR=1.14; 95% CI=0.982–1.32), emergency department presentation (HR=0.95; 95% CI=0.80–1.14) or death (HR=1.02; 95% CI=0.76–1.37). Relative to not receiving antipsychotic medication, monotherapy was associated with a reduced hazard of unplanned admissions to hospital and emergency department presentations but had no effect on mortality.
Conclusions
The study results support current guidelines for antipsychotic monotherapy in routine clinical practice. However, they also suggest that where clinicians have deemed antipsychotic polypharmacy necessary, healthcare utilization and mortality are not affected.
Keywords: Antipsychotic medication, Polypharmacy, Serious Mental Illness
Introduction
Antipsychotic drugs are a common component of the therapeutic strategy for patients with serious mental illness (https://www.rcpsych.ac.uk/mental-health/treatments-and-wellbeing/antipsychotics). Although UK and international guidelines recommend antipsychotic monotherapy (1, 2), antipsychotic polypharmacy (thereafter polypharmacy) – defined as the concurrent use of two or more different antipsychotic agents – is common in clinical practice (3, 4).
The most common rationale for polypharmacy is to improve therapeutic response when the response to monotherapy is considered inadequate (1). However, there is little empirical evidence that polypharmacy has higher efficacy than monotherapy. A Cochrane systematic review (5) of randomized controlled trials (RCTs) concluded that while polypharmacy might be superior to monotherapy in certain clinical situations, the evidence was too heterogeneous to derive firm conclusions. Significant risks associated with polypharmacy have been reported, particularly excessive dosing (6) which can in turn result in adverse effects such as metabolic syndrome (7), cognitive impairment, extrapyramidal side effects (8), and cardiovascular disorders (9). Polypharmacy efficacy and adverse effects contribute to changes in broader patient outcomes reflecting overall polypharmacy effectiveness. Whether polypharmacy is a valid therapeutic option or a ‘dirty little secret’ (10), it remains prevalent and empirical evidence on its effectiveness is needed.
Our study follows a cohort of 17,255 patients with serious mental illness over time to make inferences about polypharmacy effectiveness in terms of three outcomes: unplanned hospital admissions, emergency department (ED) presentations (A&E in the UK), and mortality. We construct the antipsychotic prescribing profile of patients from primary care records which we link to hospital and mortality data. The argument underpinning a cohort study design is that effectiveness is assessed under usual circumstances of healthcare practice rather than ideal RCT circumstances. As with all observational studies, validity relies on rigorous design and adjustment of confounding factors to minimise selection bias. Although significant progress towards this direction has been made by two studies from Denmark (11) and Finland (12) that focused on the effect of polypharmacy on mortality, studies that explored associations between polypharmacy and inpatient hospitalizations (13, 14) and ED attendances (15) suffered from important weaknesses that stem from failure (or inability due to lack of data) to model the timing of polypharmacy episodes and outcomes. The current study improves on the fundamental issue of confoundedness by employing a Cox survival analysis model that analyses time to each outcome adjusting for both time invariant confounders and time dependent polypharmacy and monotherapy (16).
Material and Methods
Data sources
Our primary data source is the Clinical Practice Research Datalink (CPRD GOLD), which includes information on individual patients from family practice records including diagnoses, referrals, laboratory results, prescriptions, and immunisations. CPRD is sourced from participating UK general practices that use the VISION software system and is broadly representative of the English population with respect to age and gender, but not region. CPRD records from English practices were linked to inpatient hospitalizations and A&E attendances from Hospital Episode Statistics, as well as mortality data from the Office of National Statistics. To preserve anonymity, the data linkages were carried out by the trusted third party NHS Digital. Information was provided by CPRD for all patients who were eligible for linkage and had an incident diagnosis of serious mental illness.
Ethical approval
The study protocol was approved by the Independent Scientific Advisory Committee (protocols 14_168 and 15_213).
Sample
Our sample covers the period from 1 January 2000 to 31 March 2014. The observation period for each patient varies. The entry date to the sample is defined such that the following conditions are met on this date: i) patient has been diagnosed with serious mental illness in primary care; ii) patient is 18 years or older; iii) patient is registered with a participating practice for at least 365 days; and iv) patient is not hospitalized within the last 90 days. The latter two conditions were imposed to ensure sufficient information on patients’ medical history was available and because patients who were recently discharged from hospital are at higher risk of readmission. The observation period for each patient ends at the earliest of death date, the date registration with the practice ends and 31 March 2014. Patients were included in the sample if they had at least one antipsychotic drug record during the observation period.
Because A&E data are only available from 2007/08 the analysis of ED presentations is limited to patients with entry date after 31 March 2007.
Patient outcomes
We investigated the association between polypharmacy and the occurrence of three outcomes: unplanned hospital admissions (all-cause), ED presentations, and mortality.
Definition of polypharmacy
There is no consistent definition of polypharmacy in the literature. We define polypharmacy as the concurrent use of two or more antipsychotic substances for at least 30 days. The overlap period allows for cross-tapering between substances. A longer overlap period has a higher risk of misclassifying polypharmacy as monotherapy, while a shorter overlap may misclassify switching between substances as polypharmacy. We therefore explored overlap periods of 14, 60 and 90 days as sensitivity analyses.
We considered 33 antipsychotic substances covering first-generation antipsychotic drugs or typical antipsychotics, second-generation antipsychotics or atypical antipsychotics, and depot antipsychotics (17, 18) (see online supplement).
CPRD data provides the date a prescription was issued but the duration of prescriptions is poorly recorded. We inferred treatment duration from the total quantity (number of units) prescribed and the numeric daily dose (number of units per day). The latter is missing for 23% of prescriptions. For these prescriptions, we imputed the numeric daily dose using an imputation strategy explained in the online supplement. Less than 0.02% of prescription records were dropped from the analysis because they had implausibly large estimated duration. From the prescription dates and durations, we constructed the patient’s medication profile: times at which the patient is on any antipsychotic medication and on polypharmacy.
We calculated two measures of polypharmacy prevalence. First, the annual prevalence of polypharmacy as the number of patients with at least one polypharmacy episode in a year divided by the total number of patients observed during that year. Second, the rate of polypharmacy defined as the sum of all patients’ polypharmacy days in a year over the sum of all patients’ days at risk of polypharmacy in that year. The latter measure is an improvement over the commonly reported point estimates of polypharmacy prevalence that measure the proportion of eligible individuals on polypharmacy on a given day (see online supplement for a proof).
Covariates
We used Read codes (the diagnostic codes used in UK primary care) recorded over the entire patient’s history and our own clinical expertise to define three diagnostic categories: schizophrenia and other psychoses, bipolar disorder and affective psychoses and those who had a diagnosis from each group (codes provided in online supplement).
All other covariates were measured at the date of entry to the study sample. We controlled for: age, gender, age-gender interactions, the number of comorbid conditions as defined by Charlson et al. (19), a diagnosis of depression, alcohol consumption and smoking status, the number of GP contacts (face-to-face visits and telephone calls) in the last year, and small area deprivation profile based on patients’ residence. We approximated ability to access secondary care by the distance from the patients’ GP practice to the nearest psychiatric inpatient hospital and general hospital and by whether the practice is in a rural area. Finally, we controlled for the year in which the patient entered the sample and the time since first diagnosis. Details on the explanatory variables are provided in the online supplement.
Statistical analysis
Semi-parametric Cox hazard models (20) were applied to estimate the effect of polypharmacy on the time to the first occurrence of each of the three outcomes. The model adjusts for censoring, which may occur because i) a patient dies, ii) registration with the practice ends, or iii) the study period ends. The follow-up period – time from entry to the sample until the outcome occurs or censoring – is different from the observation period for outcomes other than death.
An individual may have multiple polypharmacy episodes. On each day during the study period the patient is in one of three states: i) receives no antipsychotic medication, ii) monotherapy (on one antipsychotic or on more than one but for less than 30 days), and iii) polypharmacy. To model this, we introduce two time-varying binary variables: ‘No antipsychotic substance’ which takes a value of 1 during periods the patient is not on an antipsychotic drug and 0 otherwise, and ‘polypharmacy’ which takes a value of 1 during periods the patient is on two or more antipsychotic substances for more than 30 days and 0 otherwise. The results are interpreted with regard to monotherapy, which is the reference category.
All coefficient estimates are reported as hazard ratios (HR) where a HR greater than 1 indicates an increase in the risk of the outcome associated with a unit change in the explanatory variable, and vice versa for a HR below 1. Details on the survival analysis are provided in the online supplement. All analyses were performed in Stata 14 (StataCorp LP, College Station, TX, US).
Results
All patients were prescribed an antipsychotic substance at some point during the observation period. Table 1 provides descriptive statistics. Unplanned admissions and mortality outcomes were studied using the same sample of 17,255 patients from 215 practices. These patients were observed for 5.7 years on average and 12.9% of them had at least one polypharmacy episode during the observation period. The average number of polypharmacy episodes per patient on polypharmacy was 5.5 and the mean polypharmacy episode length was 66 days (range: 2 to 2,340).
Table 1. Descriptive statistics.
| Analysis | |||
|---|---|---|---|
| Unplanned admissions | Death | ED presentations | |
| Full sample | |||
| N individuals | 17,255 | 17,255 | 13,247 |
| During observation perioda | |||
| Mean years | 5.7 | 5.7 | 4.1 |
| Patients with at least one polypharmacy episode | 2,228 | 2,228 | 1,548 |
| % patients with at least one polypharmacy episode | 12.9 | 12.9 | 11.7 |
| Polypharmacy episodes per patient on polyph. | 5.5 | 5.5 | 4.8 |
| Number of switches on/off polypharmacy per year | 0.96 | 0.97 | 1.15 |
| Mean polypharmacy length | 66 | 66 | 69 |
| During follow-up periodb | |||
| Mean years | 3.6 | 5.7 | 2.5 |
| Patients with at least one polypharmacy episode | 1,515 | 2,228 | 1,068 |
| % patients with at least one polypharmacy episode | 8.8 | 12.9 | 8.1 |
| Sample of patients experiencing outcome | |||
| N individuals | 8,916 | 604 | 7,523 |
| During follow-up period | |||
| Mean years | 2.6 | 2.8 | 1.8 |
| Patients with at least one polypharmacy episode | 704 | 52 | 511 |
| % patients with at least one polypharmacy episode | 7.9 | 8.6 | 6.8 |
Observation period: from entry to the sample until the earliest of death, end of registration with practice, 31 March 2014.
Follow-up period: from entry to the sample until outcome occurs or censoring (end of observation period).
For the unplanned admissions analysis the average follow-up period was shorter than the observation period (3.6 years) with 8.8% of patients having at least one polypharmacy episode during this period. Almost 52% of the patients (8,916) had an unplanned admission and of those 7.9% had at least one polypharmacy episode before the admission.
For the mortality analysis, the average time to death or censoring was 5.7 years. From the 604 patients who died (3.5%), 52 (8.6%) had received polypharmacy.
The sample for ED attendances covers a shorter period from 1 April 2007 to 31 March 2014 totalling 13,247 patients from 215 practices. Of the 7,523 patients with an ED presentation, 511 (6.8%) had received polypharmacy.
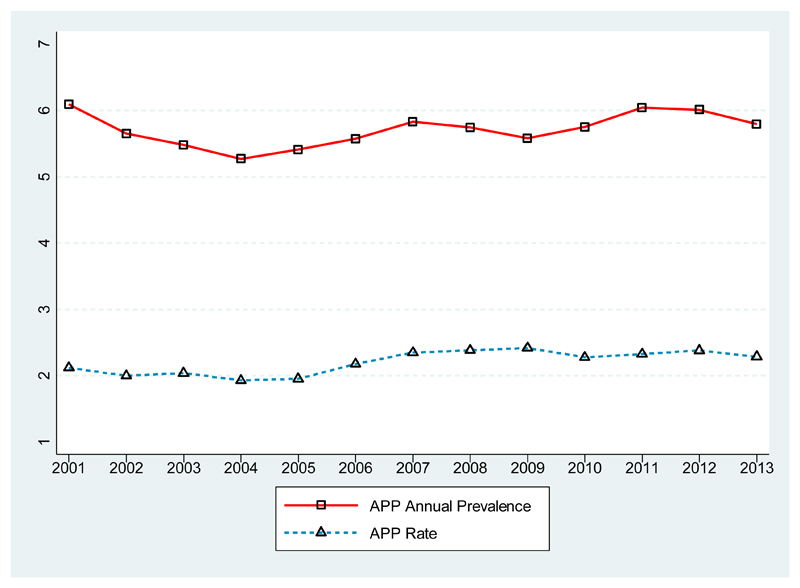
Figure 1 shows that annual prevalence of polypharmacy fluctuates between 5% and 6% while the polypharmacy rate is around 2%. Polypharmacy rate estimates are lower than the annual polypharmacy prevalence because the former reflects both whether a patient is on polypharmacy during the year and the total duration of polypharmacy episodes. Figures of the annual prevalence of polypharmacy and the polypharmacy rate for different overlap periods are provided in the online supplement.
Figure 1. Polypharmacy prevalence.
Summary statistics for the explanatory variables are presented in Table 2. About 35% of patients had at least one of the Charlson index morbidities and 13% were diagnosed with both schizophrenia and bipolar during the observation period.
Table 2. Descriptive statistics for the explanatory variables.
| Variable | N (patients) | (%) |
|---|---|---|
| Age at entry date | ||
| 19-35 | 4,484 | 26 |
| 36-45 | 3,718 | 22 |
| 46-55 | 3,017 | 17 |
| 56-65 | 2,341 | 14 |
| >65 | 3,695 | 21 |
| Index of multiple deprivation | ||
| Quintile 1 | 2,618 | 15 |
| Quintile 2 | 3,093 | 18 |
| Quintile 3 | 3,238 | 19 |
| Quintile 4 | 4,064 | 24 |
| Quintile 5 | 4,242 | 25 |
| Male | 8,171 | 47 |
| White | 12,521 | 73 |
| GP practice in rural area | 1,921 | 11 |
| Number of primary care contacts in year preceding FSDT | ||
| 0-4 | 3,798 | 22 |
| 5-9 | 4,496 | 26 |
| 10-14 | 3,271 | 19 |
| 15-19 | 2,158 | 13 |
| >=20 | 3,532 | 20 |
| Distance from GP to nearest acute provider | ||
| 0-3km | 7,489 | 43 |
| 3-6km | 4,925 | 29 |
| 6-9km | 2,160 | 13 |
| >9km | 2,681 | 16 |
| Distance from GP to nearest MH provider | ||
| 0-3km | 3,441 | 20 |
| 3-6km | 4,330 | 25 |
| 6-9km | 3,178 | 18 |
| >9km | 6,306 | 37 |
| Number of Charlson Index comorbidities at FSDT | ||
| 0 | 11,273 | 65 |
| 1 | 4,441 | 26 |
| 2 | 1,079 | 6 |
| >=3 | 462 | 3 |
| History of depression at FSDT | 9,746 | 56 |
| Current or ex-smoker | 12,556 | 73 |
| Current or ex-alcohol consumption | 11,062 | 64 |
| Schizophrenia | 9,653 | 56 |
| Bipolar | 5,342 | 31 |
| Both schizophrenia and bipolar | 2,260 | 13 |
Table 3 presents the survival analysis estimates for the two time-varying variables for our main specification assuming an overlap period of 30 days. Being on polypharmacy (relative to monotherapy) was not statistically significantly associated with the risk of unplanned admission, death or ED presentation. Not being prescribed any antipsychotic substance increases the hazard (relative to monotherapy) of an unplanned admission to hospital by 8.2% (95%CI=3% –3.6%) and the hazard of an ED presentation by 18.6% (95%CI=13.5%–23.9%) but has no effect on mortality risk. For estimates of the other explanatory variables see online supplement. Having both diagnosis of schizophrenia and bipolar increases the hazard of an unplanned admission by 20% (HR=1.20; 95%CI=1.12–1.29).
Table 3. Hazard Ratios for polypharmacy from the base case analysis.
| Unplanned admissions | Death | ED presentations | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Polypharmacy | 1.14 | (.98–1.32) | 1.02 | (.76–1.37) | .95 | (.80–1.14) |
| No antipsychotic substance | 1.08** | (1.03–1.14) | 1.02 | (.94–1.10) | 1.19*** | (1.14–1.24) |
p<0.01
p<0.001; 95% CI in parentheses
Table 4 shows the results of sensitivity analyses that explore the impact of changing the length of overlap in the definition of polypharmacy. The estimated relationships are generally insensitive to the length of overlap. The only exception is unplanned admissions: when the lower boundary of the overlap duration is reduced to 14 days, polypharmacy is associated with an increased hazard of unplanned admission of about 21%.
Table 4. Hazard Ratios for polypharmacy from the Sensitivity analyses.
| Unplanned admissions | Death | ED presentations | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| 14 days | 1.21** | (1.08–1.37) | 1.11 | (.87–1.40) | .94 | (.80–1.11) |
| 30 days | 1.14 | (.98–1.32) | 1.02 | (.76–1.37) | .95 | (.80–1.14) |
| 60 days | 1.08 | (.90–1.30) | .90 | (.63–1.28) | .98 | (.79–1.21) |
| 90 days | 1.02 | (.80–1.29) | .83 | (.54–1.28) | .80 | (.62–1.03) |
p<0.01; 95% CI in parentheses
We also estimated our survival models for psychiatric hospitalisations, which are a subset of all unplanned admissions. The results are reported in the online supplement and show no association between psychiatric hospitalisations and polypharmacy.
Discussion
The present study is a step forward towards understanding the links between polypharmacy and healthcare utilization and mortality. As with all observational studies, validity relies on rigorous design and adjustment of confounding factors to minimise selection bias. We address this fundamental issue employing a three-step strategy.
First, we construct the antipsychotic prescribing profile of patients from primary care records.
In the UK, family practices provide the majority of care for patients with serious mental illness (21) including the management of long-term prescribing. Therefore, unlike previous studies that used solely hospital data to investigate polypharmacy (22) we define polypharmacy and monotherapy from primary care data. Second, we link primary care data with hospital and mortality data at patient level to determine the sequence of polypharmacy episodes and hospital utilization and mortality. Third, we employ a Cox survival analysis model that analyses time to each outcome adjusting for both time invariant confounders and time dependent polypharmacy and monotherapy (16). By specifying polypharmacy as a time-dependent variable, we address the statistical challenge arising in cases where the exposure is not present throughout the entire time of observation.
The use of a large linked dataset coupled with a suitable survival analysis model provides more robust estimates of the effects of polypharmacy on outcomes than would be possible with aggregate data or a cross sectional design.
We found that the annual polypharmacy prevalence fluctuates over time between 5% and 6%. It is not straightforward to compare this figure with other studies due to diversity in the definition of polypharmacy, and differences in the sample characteristics and methodology. A large international study estimated a global median of 20% (23) but there is considerable variation between and within geographic locations (23, 24). Higher rates of polypharmacy have been estimated for the UK but the patients included in those studies were prescribed at least one antipsychotic at the date of data collection and polypharmacy was defined as the concurrent use of more than one antipsychotic on that single date (25, 26), a definition that is likely to overestimate polypharmacy. A more comparable approach by Kadra et al. (22) found polypharmacy to be 11.5% using a six weeks overlap. The lower estimate of polypharmacy prevalence in our study may be because patients can be at risk of polypharmacy for a fraction of a calendar year while in Kadra et al. patients were followed for an entire 6-month period.
Current UK guidance (1) recommends antipsychotic monotherapy as a treatment option and our results provide further supportive evidence establishing a negative association between antipsychotic monotherapy and hospitalizations. This may be due to the fact that drug therapy helps to stabilise the patients’ condition and allows better management of their physical health. Being prescribed an antipsychotic may be associated with closer or more regular clinical monitoring in the primary care setting, as set out in the guidelines which recommend that prescription of an antipsychotic should be considered as “an explicit individual therapeutic trial” [8], accompanied by detailed requirements for monitoring. The latter may facilitate timely diagnosis and treatment of health problems, avoiding the need for hospital care.
It is widely believed that polypharmacy increases mortality and hospitalisations but there is a lack of methodologically sound studies to support this assumption. To our knowledge, the only previous study that used nationwide data of medication prescriptions and appropriate methods to adjust for confounding factors was conducted by Tihonen et al (12). They investigated the impact of polypharmacy on mortality using a cohort of 2,588 patients from Finnish hospital data and concluded that polypharmacy is not associated with increased mortality. This conclusion is reinforced by the present study using a significantly larger cohort of 17,255 patients with a record of serious mental illness diagnosis in primary care. Our study further concludes no association between polypharmacy and inpatient hospitalizations or ED attendances contrasting the positive correlations found in previous studies’(13–15).
That polypharmacy is not significantly associated with any of the three outcomes, suggests that the effectiveness of polypharmacy and monotherapy are comparable. For a shorter overlap period (14 days or longer) which captures more cross-tapering in the definition of polypharmacy, we observe an increase in the risk of unplanned admission. One explanation is that patients who change drugs might have more unstable disease profiles and/or that changing drugs further destabilises their condition. This suggests a need for close monitoring in the first few weeks of cross-tapering when the risk of unplanned hospitalization is higher.
UK guidelines (1) recommend against combining antipsychotic drugs except as a last resort. These recommendations are based on limited supportive evidence for superior efficacy of polypharmacy over monotherapy as well as concerns that combined antipsychotics are associated with an increased risk of side effects. Our study cannot draw conclusions on the polypharmacy effect in terms of efficacy and tolerability and bearing in mind the limitations of an observational study (despite its advanced design) cannot substitute for RCTs. Its contribution lies in providing real-world evidence on the effectiveness of polypharmacy.
There are three main limitations to the study. First, the measures of health status and healthcare utilization prior to diagnosis of serious mental illness may not fully depict the complexities of health status, including severity of the condition. Second, imputing the treatment duration for a number of prescriptions may introduce measurement error in the calculation of polypharmacy. Lastly, we have explored the effect of polypharmacy on broadly defined outcomes. Future research could investigate whether effects vary by reason for admission or for particular combinations of antipsychotic medication.
Conclusions
Our study examined the overall effectiveness of polypharmacy relative to monotherapy by investigating associations between polypharmacy and three patient outcomes. We found no evidence of a positive or negative effect of polypharmacy on mortality, inpatient hospitalizations, and ED presentations. At a policy level these findings do not rule out polypharmacy options but highlight the need for further research on the appropriateness of polypharmacy.
Supplementary Material
Key points.
Where clinicians have deemed polypharmacy necessary - despite guidance discouraging its use - healthcare utilization and mortality are not affected.
Relative to those on monotherapy patients who are not on antipsychotic medication have higher hazard of an unplanned hospital admission and ED presentations.
The prevalence of antipsychotic polypharmacy varies significantly across studies due to diversity in the definition of polypharmacy, differences in sample characteristics and methodology.
Acknowledgements
We are grateful to the researchers who extracted and provided the CPRD data.
We would like to thank all members of our Scientific Steering Committee (SSC) for their invaluable support and feedback on this study.
The multidisciplinary team responsible for this study included two patient representatives with serious mental illness, who contributed to the design of the research questions, the methodological approach, the interpretation of findings, and drafting of the manuscript.
Due to the sensitive and confidential nature of the data used for this analysis, and the permissions required to access it, the dataset is not publicly available.
Funding: This project was funded by the National Institute for Health Research HS&DR programme (project number 13/54/40). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnotes
Declarations of interest: None
References
- 1.National Clinical Guideline Number 178. National Institute for Health and Care Excellence, National Collaborating Centre for Mental Health; 2014. Psychosis and schizophrenia in adults. Treatment and management. [Google Scholar]
- 2.Hasan A, Falkai P, Wobrock T, et al. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, Part 1: Update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. The World Journal of Biological Psychiatry. 2012;13:318–78. doi: 10.3109/15622975.2012.696143. [DOI] [PubMed] [Google Scholar]
- 3.Faries D, Ascher-Svanum H, Zhu B, et al. Antipsychotic monotherapy and polypharmacy in the naturalistic treatment of schizophrenia with atypical antipsychotics. BMC Psychiatry. 2005;5:26. doi: 10.1186/1471-244X-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paton C, Barnes T, Cavanagh M, et al. High-dose and combination antipsychotic prescribing in acute adult wards in the UK: the challenges posed by p.r.n. prescribing. The British Journal of Psychiatry. 2008;192:435–39. doi: 10.1192/bjp.bp.107.042895. [DOI] [PubMed] [Google Scholar]
- 5.Correll C, Rummel-Kluge C, Corve C, et al. Antipsychotic combinations vs monotherapy in schizophrenia: A meta-analysis of randomized controlled trials. Schizophrenia Bulletin. 2009;35:443–57. doi: 10.1093/schbul/sbn018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hou C, Ma X, Zang Y, et al. Antipsychotic polypharmacy and quality of life in patients with schizophrenia treated in primary care in China. International Journal of Clinical Pharmacology and Therapeutics. 2016;54(1):36–42. doi: 10.5414/CP202413. [DOI] [PubMed] [Google Scholar]
- 7.Misawa F, Shimizu K, Fujii Y, et al. Is antipsychotic polypharmacy associated with metabolic syndrome even after adjustment for lifestyle effects?: a cross-sectional study. BMC Psychiatry. 2011;11:118. doi: 10.1186/1471-244X-11-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lochmann van Bennekom M, Gijsman H, Zitman F. Antipsychotic polypharmacy in psychotic disorders: a critical review of neurobiology, efficacy, tolerability and cost effectiveness. Journal of Psychopharmacology. 2013;27(4):327–36. doi: 10.1177/0269881113477709. [DOI] [PubMed] [Google Scholar]
- 9.Fleischhacker W, Uchida H. Critical review of antipsychotic polypharmacy in the treatment of schizophrenia. International Journal of Neuropsychopharmacology. 2014;17(7):1083–93. doi: 10.1017/S1461145712000399. [DOI] [PubMed] [Google Scholar]
- 10.Stahl S. Antipsychotic polypharmacy, Part 1: Therapeutic option or dirty little secret? Journal of Clinical Psychiatry. 1999;60:425–26. doi: 10.4088/jcp.v60n0701. [DOI] [PubMed] [Google Scholar]
- 11.Baandrup L, Gasse C, Jensen V, et al. Antipsychotic polypharmacy and risk of death from natural causes in patients with schizophrenia: a population-based nested case-control study. J Clin Psychiatry. 2010;71(2):103–08. doi: 10.4088/JCP.08m04818yel. [DOI] [PubMed] [Google Scholar]
- 12.Tihonen J, Suokas JT, Suvisaari JM, et al. Polypharmacy with antipsychotics, antidepressants, or benzodiazepines and mortality in schizophrenia. Arch Gen Psychiatry. 2012;69(5):476–83. doi: 10.1001/archgenpsychiatry.2011.1532. [DOI] [PubMed] [Google Scholar]
- 13.Todd P, Gilmer TP, Dolder CR, Folsom DP, et al. Antipsychotic polypharmacy trends among Medi-Cal beneficiaries with schizophrenia in San Diego County, 1999–2004. Psychiatric Services. 2007;58(7):1007–10. doi: 10.1176/ps.2007.58.7.1007. [DOI] [PubMed] [Google Scholar]
- 14.Kreyenbuhl JA, Valenstein M, McCarthy JF, et al. Long-term antipsychotic polypharmacy in the VA health system: patient characteristics and treatment patterns. Psychiatric Services. 2007;58(4):489–95. doi: 10.1176/appi.ps.58.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Velligan D, Carroll C, Lage M, et al. Outcomes of medicaid beneficiaries with schizophrenia receiving clozapine only or antipsychotic combinations. Psychiatric Services. 2015;66(2):127–33. doi: 10.1176/appi.ps.201300085. [DOI] [PubMed] [Google Scholar]
- 16.Crowley J, Hu M. Covariance analysis of heart transplant survival data. Journal of the American Statistical Association. 1977;72:27–36. [Google Scholar]
- 17.Springate D, Kontopantelis E, Ashcroft D, et al. ClinicalCodes: An online clinical codes repository to improve the validity and reproducibility of research using electronic medical recors. PloS ONE. 2014;9(6) doi: 10.1371/journal.pone.0099825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Windfuhr K, While D, Kapur N, et al. Suicide risk linked with clinical consultation frequency, psychiatric diagnoses and psychotropic medication prescribing in a national study of primary-care patients. Psychological Medicine. 2016;46:3407–17. doi: 10.1017/S0033291716001823. [DOI] [PubMed] [Google Scholar]
- 19.Khan NF, Perera R, Harper S, et al. Adaptation and validation of the Charlson Index for Read/OXMIS coded databases. BMC Family Practice. 2010;11(1):1. doi: 10.1186/1471-2296-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cox D. Regression models and life tables. Journal of the Royal Statistical Society. 1972;B34:187–220. [Google Scholar]
- 21.Reilly S, Planner C, Hann M, et al. The role of primary care in service provision for people with severe mental illness in the United Kingdom. PLoS one. 2012;7(5):e36468. doi: 10.1371/journal.pone.0036468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kadra G, Stewart R, Shetty H, et al. Extracting antipsychotic polypharmacy data from electronic health records: developing and evaluating a novel process. BMC Psychiatry. 2015;15(166) doi: 10.1186/s12888-015-0557-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallego J, Bonetti J, Zhang J, et al. Prevalence and correlates of antipsychotic polypharmacy: A systematic review and meta-regression of global and regional trends from the 1970s to 2009. Schizophrenia Research. 2012;138:18–28. doi: 10.1016/j.schres.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malandain L, Thibaut F, Grimaldi-Bensouda L, et al. Correlates and predictors of antipsychotic drug polypharmacy in real-life settings: Results from a nationwide cohort study. Schizophrenia Research. 2017 doi: 10.1016/j.schres.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 25.Lelliott P, Paton C, Harrington M, et al. The influence of patient variables on polypharmacy and combined high dose of antipsychotic drugs prescribed for in-patients. Psychiatric Bulletin. 2002;26:411–14. [Google Scholar]
- 26.Connolly A, Taylor D. Factors associated with non evidence-based prescribing of antipsychotics. Therapeutic Advances in Psychopharmacology. 2014;4(6):247–56. doi: 10.1177/2045125314540298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.