SUMMARY
SETTING:
Fifty-six public clinics in Limpopo Province, South Africa.
OBJECTIVE:
To evaluate the association between tuberculosis (TB) patient costs and poverty as measured by a multidimensional poverty index.
DESIGN:
We performed cross-sectional interviews of consecutive patients with TB. TB episode costs were estimated from self-reported income, travel costs, and care-seeking time. Poverty was assessed using the South African Multidimensional Poverty Index (SAMPI) deprivation score, a 12-item household-level index, with higher scores indicating greater poverty. We used multivariable linear regression to adjust for age, sex, HIV status and travel time.
RESULTS:
Among 323 participants, 108 (33%) were ‘deprived’ (deprivation score>0.33). For each 0.1-unit increase in deprivation score, absolute TB episode costs were 1.11 times greater (95% confidence interval, CI 0.97–1.26). TB episode costs were 1.19 times greater with each quintile of higher deprivation score (95%CI 1.00–1.40) but 0.54 times lower with each quintile of lower self-reported income (higher poverty, 95%CI 0.46–0.62).
CONCLUSION:
Individuals experiencing multidimensional poverty faced equal or higher costs of TB than non-impoverished patients. Individuals with lower self-reported income experienced higher costs as a proportion of household income but lower absolute costs. Targeted interventions are needed to reduce the economic burden of TB on patients with multidimensional poverty.
Keywords: multidimensional poverty index, patient costs, income
INTRODUCTION
Tuberculosis (TB) is the leading infectious cause of mortality worldwide.1 In addition to morbidity and mortality, an economic cost is shouldered by people affected by TB, particularly the poor. A primary aim of the END TB strategy of the World Health Organization (WHO) is zero TB-affected families facing catastrophic costs due to TB (defined as ≥20% of the annual household income) by 2020.1 Catastrophic costs include out-of-pocket payments for clinic costs, reductions in income and lost wages as a result of time spent care-seeking. In pursuing this ambitious goal, it is important to understand associations between these costs and poverty.
Although many studies have measured patient costs of TB illness, few have analyzed the relationship between poverty and TB costs.2–5 While some studies suggest that absolute costs are higher for those with lowest income,6 most highlight that costs for lower-income families represent a larger proportion of their household income.4,7,8 Nearly all investigators agree on the need for interventions (e.g. social protection) to mitigate the effects of high TB costs on those at highest financial risk.2–4,6,7 Specific policy approaches may depend on absolute costs of illness faced by the most impoverished rather than costs as a proportion of income. Self-reported income – the most widely used measure of poverty in assessing TB illness costs – may not capture all elements of poverty such as access to education and public utilities, and costs of living. Self-reported income can be difficult to assess in settings with informal economies.9–11 Multidimensional poverty indices offer an alternative measure of poverty that could identify individuals who might benefit most from social protection.
Here we used the South African Multidimensional Poverty Index (SAMPI)12 to evaluate the relationship between poverty and cost of TB illness in rural South Africa.
METHODS
Study setting and participants
This study was nested in Kharitode TB, an ongoing cluster-randomized trial of active TB case finding strategies in 56 public clinics in the Vhembe and Waterberg districts of Limpopo Province, South Africa.13
Limpopo is a largely rural province of northern South Africa with the second highest proportion of households involved in agriculture.14 It has the lowest average annual household income (56,844 South African Rand [ZAR] = 4,213 US dollars per year)15 and the lowest provincial annual TB incidence in South Africa (301 per 100,000 population in 2016).16
Potential participants were identified consecutively from presumptive TB case registries in the study clinics. Inclusion criteria were: enrollment within two months of diagnosis of active TB; initiation of TB treatment; age 18 years or older; proficiency in English, Sepedi, Xitsonga, or Tshivenda; and presentation to study clinics between October 2017 and January 2018. Patients transferred from other healthcare facilities to study clinics for treatment of active TB were also included. As a reference group, a random sample of individuals who presented to the same clinics for TB evaluation but were negative on sputum Xpert MTB/RIF testing and not started on TB treatment were interviewed in the same time period.
Cost estimates
Participants were asked to self-report income, travel costs to the clinic, and time spent at the clinic. We used a shortened version of the 2015 World Health Organization (WHO) tool for assessing TB-related catastrophic costs, streamlined to reflect participants’ time constraints.17 Further details of costing methods are provided elsewhere.18
The total cost of each TB episode was estimated based on three components: 1) out-of-pocket (OOP) costs, 2) lost wages resulting from time spent in care-seeking and treatment and 3) decreased monthly household income as a result of illness.
OOP costs were based on self-reported expenses related to clinic visits including transportation and other costs. Lost wages due to time spent in care-seeking and treatment were estimated from patient-reported door-to-door time between leaving and returning home, with time valued as described below. The OOP costs and lost wages were summed to provide an estimated diagnostic visit cost. Based on a prior costing study in South Africa, the cost of subsequent intensive-phase treatment visits was estimated at 24% of the diagnostic visit cost, and continuation-phase visits were estimated at 8% of the diagnostic visit cost, assuming four intensive phase and eight continuation phase visits to complete therapy.6 The reference group (patients without TB) were assumed not to incur any costs for TB treatment. Pre-diagnostic costs were estimated from the number of patient-reported care-seeking visits prior to the diagnostic visit (for the same episode of illness), multiplied by the cost of the diagnostic visit as estimated by the patient.
In the primary analysis, lost wages were based on time spent seeking care and was valued by dividing the participant population into quintiles of self-reported hourly wages and assigning each participant a wage corresponding to the median hourly wage of her/his corresponding income quintile. Several other estimates of time value were used for sensitivity analyses, including: (a) the median hourly wage for all study participants, (b) zero lost wages, and (c) self-reported estimates of lost wages and income-earning opportunities during health care-seeking.
Decreased monthly income as a result of illness was estimated as the difference between participants’ reported current monthly household incomes at the time of survey (during the intensive phase of treatment) and their reported monthly incomes prior to the onset of TB symptoms. This monthly income differential was multiplied by half of the total self-reported duration of symptoms and estimated duration of treatment, based on the assumption that participants’ symptoms would be sufficiently severe to affect income halfway into their disease course and would, on average, recover sufficiently to allow resumption of work halfway through TB treatment.
The total estimated cost for TB patients was therefore the sum of these three components:
The conversion rate between US Dollars (USD) and South African Rand (ZAR) was estimated using International Monetary Fund archives from October 2017 (1 USD = 13.6 ZAR).19
SAMPI deprivation score calculation
Participants answered questions based on the South African Multidimensional Poverty Index (SAMPI) – a 12-item index including health, education, employment, and standard of living.12 As recommended by Statistics South Africa,12 participants were asked questions at the household level, and a household deprivation score was calculated ranging from 0 to 1. A higher score indicates greater poverty, and a score ≥ 0.33 defines a household as “deprived”.12 At this threshold, 10.1% of Limpopo’s population was deprived in 2011, compared to 8.0% of the entire country.12
Our questions reflected the official SAMPI questions with one exception: we asked if one or more household members ≥15 years old had not completed 5 years of schooling, whereas the official SAMPI asks if no household member ≥ 15 years old had completed 5 years of schooling. This led to higher scores than the validated SAMPI. To resolve this issue, we used other survey questions about highest grade completed by the participant and head of household, if different from the participant. Only those households where neither the participant nor head of household had achieved at least a 5th grade education were counted as deprived in the dimension of adult education access. We performed sensitivity analyses using the original questionnaire item, which did not affect the value of any primary result by more than 5%.
Statistical analysis
From the eligible participants, we included in our analysis any individual with a TB diagnosis who was not missing cost or poverty data. For univariate comparisons, we used Mann-Whitney U tests for continuous variables and Fisher’s exact tests for categorical variables. Our a priori primary analysis was the association between poverty, measured continuously using the SAMPI deprivation score, and cost of TB illness, measured as described above. Our primary measure of association was the relative cost per 0.1 increase in SAMPI score. The estimates of total TB cost were log transformed to better meet the assumption of normality based on the positively skewed distribution and to generate coefficients interpretable as relative costs per unit increase in poverty. Age, sex, HIV status, and travel time (categorized based on distributions in exploratory data analysis) were chosen as covariates based on a priori selection as potential confounders of the poverty-cost relationship. Analyses were performed using linear regression.
We performed a post hoc secondary analysis using self-reported (pre-illness) income rather than SAMPI score as the measure of poverty. Since self-reported income contained notable outlier values, we modeled self-reported income by creating quintiles of equal size based on self-reported pre-illness income. We performed sensitivity analyses to ensure that associations persisted when modeling self-reported income as a continuous variable. To ensure a fair comparison of this analysis with that of SAMPI scores, we performed a regression in which SAMPI score was modeled in quintiles. All analyses were performed in Stata v15 (StataCorp, College Station, TX, USA).
Ethical approval
The study protocol was approved by the University of the Witwatersrand’s Human Research Ethics Committee and the Limpopo Health Research Committee. Informed consent was provided by all study participants.
RESULTS
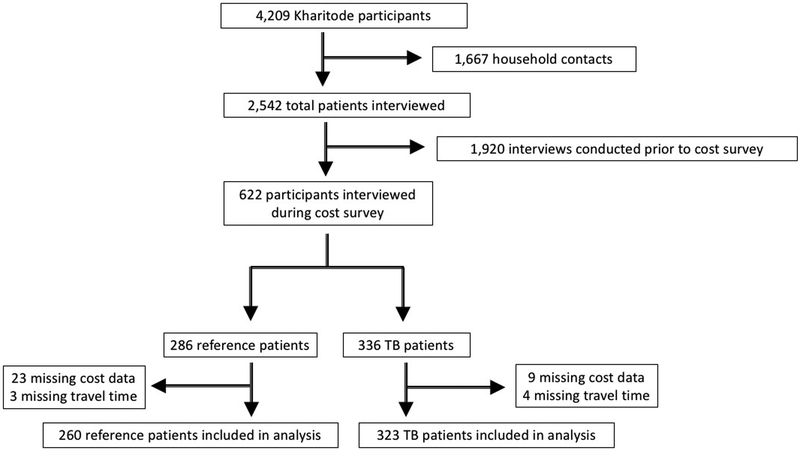
From October 1, 2017 to January 31, 2018, we interviewed 336 TB patients; 323 (96%) provided complete cost data (Table 1, Figure 1) and were included in the analysis. Of these patients, 108 (33%) were deprived as defined by a SAMPI deprivation score >0.33. The deprived group was younger (median age 38 vs 41 years) and more likely to have HIV (67% vs 50%) than the non-deprived group. The monthly income did not differ significantly between these two groups (median ZAR 1,920 [141 USD] versus ZAR 2,360 [174 USD]).
Table 1.
Demographics of study participants, stratified by deprivation based on a SAMPI score of 0.33 (n = 323)
| Characteristic | Deprived† (n = 108, 33%) n(%) | Not deprived (n = 215, 67%) n(%) | P-value‡ |
|---|---|---|---|
| Age, years, median [IQR] | 38 [31–45] | 41 [30–50] | 0.124 |
| Female sex | 50 (46) | 81 (38) | 0.150 |
| Black African ethnicity | 108 (100) | 214 (100) | 1.0 |
| HIV positive | 72 (67) | 107 (50) | 0.004 |
| Travel time to clinic | 0.027 | ||
| <20 minutes | 33 (31) | 98 (46) | |
| 20–60 minutes | 50 (46) | 73 (34) | |
| >60 minutes | 25 (23) | 44 (20) | |
| TB symptoms§ | |||
| Number of symptoms, n, median [IQR] | 5 [3–6] | 4 [2–5] | 0.001 |
| Duration of symptoms and treatment, months, median [IQR] | 7.0 [6.5–9.0] | 7.0 [6.5–9.0] | 0.971 |
| Monthly income, South African Rand#, median [IQR] | 1920 [790–3500] | 2360 [1160–4260] | 0.128 |
| Smoking status | 0.911 | ||
| Current | 23 (21) | 43 (20) | |
| Ever | 27 (25) | 51 (24) | |
| Never | 58 (54) | 121 (56) | |
| Education, highest grade, median [IQR] | 10 [7–11] | 10 [7–12] | 0.371 |
Deprived as defined by SAMPI deprivation score ≥ 0.33
p-values were calculated using the Mann-Whitney U test for continuous variables and Fisher’s exact test for categorical variables
Of 10 possible symptoms: cough, fever, weight loss, night sweats, chest pain, body pain, skin, GI, urogenital, other
1 USD = 13.6 ZAR (October 2017)19
SAMPI = South African Multidimensional Poverty Index; IQR = interquartile range; HIV = human immunodeficiency virus; TB = tuberculosis; ZAR = South African Rands
Figure 1.
Study flowchart. Of the nine TB patients excluded for missing cost data, five could not provide sufficient income information, three were unable to provide transportation costs, and one was missing both types of data. Of the 23 reference patients (i.e., those without TB) excluded for missing cost data, 15 were unable to provide sufficient income information, seven were unable to provide transportation costs and 1 was missing both types of data. TB = tuberculosis.
In univariate analysis, the absolute TB episode cost was 1.14 times greater (95%CI 1.01–1.29) for each 0.1 unit increase in SAMPI deprivation score. Other variables associated with increased costs included clinic travel times over 60 minutes (relative cost 2.05, 95%CI 1.11–3.78) and HIV co-infection (relative cost 1.76, 95%CI 1.11–2.79) (Table 2). After multivariable adjustment, the relative cost per 0.1 unit increase in SAMPI deprivation score was 1.11 (95%CI 0.97–1.26).
Table 2.
Association between poverty and mean cost of a TB episode
| Relative cost of TB episode | |||||
|---|---|---|---|---|---|
| Unadjusted | Adjusted*, by poverty measure | ||||
| SAMPI continuous† | SAMPI quintile‡ | Income quintile§ | |||
| Predictors | |||||
| SAMPI deprivation score (per 0.1 unit) | 1.14 (1.01–1.29) | 1.11 (0.97–1.26) | |||
| SAMPI deprivation score (per quintileˆ) | 1.24 (1.06–1.46) | 1.19 (1.00–1.40) | |||
| Self-reported income (per quintileˆ) | 0.54 (0.47–0.63) | 0.54 (0.46–0.62) | |||
| Age (per year) | 1.01 (0.99–1.02) | 1.01 (0.99–1.03) | 1.01 (0.99–1.03) | 1.01 (0.99–1.02) | |
| Female sex (vs male sex) | 1.13 (0.71–1.81) | 1.01 (0.63 – 1.62) | 1.02 (0.64–1.63) | 1.06 (0.69–1.63) | |
| HIV positive (vs negative or unknown) | 1.76 (1.11–2.79) | 1.68 (1.05–2.70) | 1.64 (1.02–2.62) | 1.89 (1.24–2.88) | |
| Trip duration | |||||
| <20 minutes | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | |
| 20–59 minutes | 1.39 (0.83–2.34) | 1.21 (0.72–2.04) | 1.19 (0.70–2.00) | 1.40 (0.88–2.24) | |
| ≥60 minutes | 2.05 (1.11–3.78) | 1.92 (1.04–3.55) | 1.85 (1.00–3.42) | 2.34 (1.35–4.06) | |
Adjusted for age (continuous), female sex (binary), HIV positive (binary), and trip duration (binary per category)
SAMPI deprivation score modeled as a continuous variable
SAMPI deprivation score modeled as a quintile
Income modeled as a quintile
Per quintile of increasing poverty (increasing SAMPI deprivation score or lower self-reported income)
TB = tuberculosis; SAMPI = South African multidimensional poverty index; HIV = human immunodeficiency virus
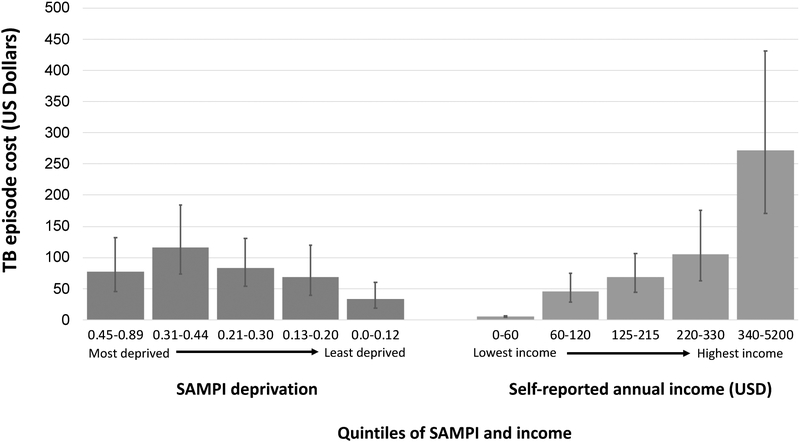
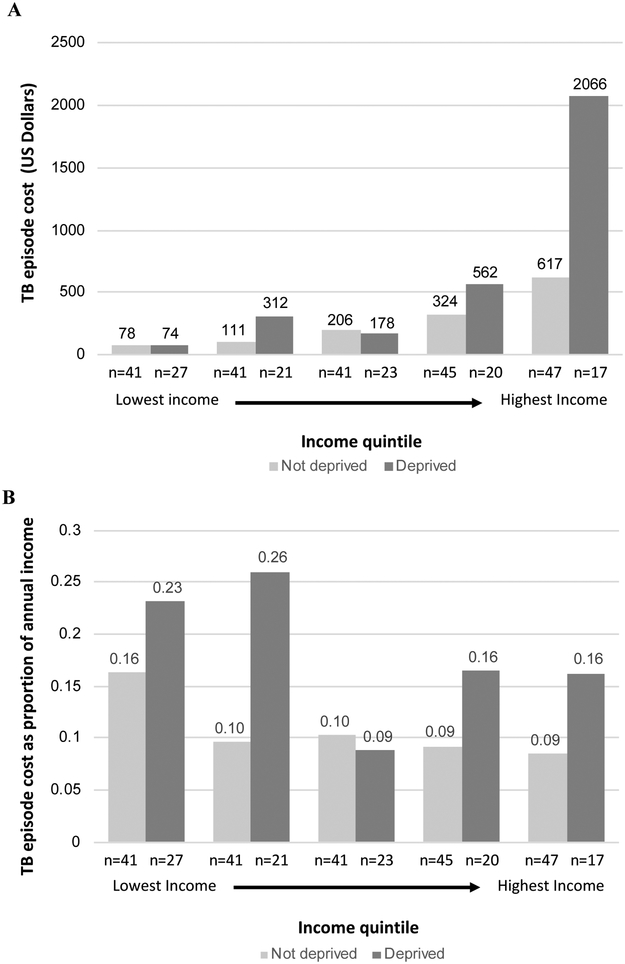
Similar results were found when measuring SAMPI scores in quintiles (relative cost per quintile of increasing deprivation 1.19, 95%CI 1.00–1.40). However, when modeling poverty according to quintiles of self-reported income, each quintile of lower income (higher poverty) was associated with significantly lower absolute TB-related costs (0.54, 95%CI 0.46–0.62, Figure 2). In each quintile of self-reported income, those with a SAMPI score >0.33 (i.e., “deprived”) had equal or higher costs of illness, both absolute and proportional (Figure 3). The most deprived quintile of patients suffered the greatest absolute costs of TB disease, just as the lowest-income quintile suffered the greatest costs as a proportion of household income (Figures 2 and 3).
Figure 2.
Mean costs of TB episode in US Dollars (USD) by SAMPI deprivation score and income quintile in order of decreasing poverty (defined as either decreasing SAMPI deprivation score or increasing self-reported pre-illness income). Error bars represent 95% confidence intervals. Income given as annual US Dollars. For October 2017, 1 US Dollar = 13.6 South African Rand19. SAMPI = South African multidimensional poverty index
Figure 3.
Absolute (panel A) and proportional (panel B) TB costs by income quintile and deprivation status. Absolute costs are given in US Dollars (1 US Dollar = 13.6 South African Rand).19 Sample size n is listed for each subgroup. Income was assessed at the household level prior to the onset of illness by self-report. Deprivation was defined as a SAMPI deprivation score ≥ 0.33. TB = tuberculosis; USD = US dollar; SAMPI = South African Multidimensional Poverty Index; ZAR = South African rand
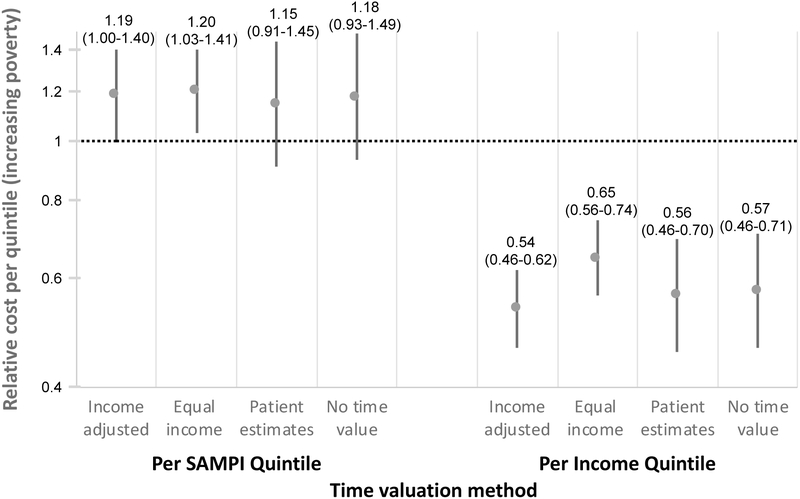
Relative cost estimates were similar regardless of time valuation method for lost wages (Figure 4). Among individuals presenting to the same clinic but negative for TB and able to provide complete cost data (n = 260), there was no association between diagnostic episode cost and quintiles of increasing SAMPI deprivation score (0.96, 95%CI 0.80–1.15), but there was a positive association with quintiles of decreasing self-reported income (0.50, 95%CI 0.34–0.66).
Figure 4.
Relative cost estimates based on different methods of time valuation for lost wages. Relative cost represents the relative change in absolute disease cost for each quintile of increasing poverty in either SAMPI deprivation score or income. The y-axis is on a log scale. Error bars represent 95% confidence intervals. “Income adjusted” denotes valuation of time as median hourly wage of the patient’s income quintile (primary analysis). “Equal income” denotes equal valuation of time for all patients using the study median as the hourly wage. “Patient estimates” denotes valuation of time based directly on patient estimates rather than an hourly wage. “No time value” refers to ignoring lost wages due to time loss and considering only out-of-pocket costs and changes to income as a result of illness. SAMPI = South African Multidimensional Poverty Index
DISCUSSION
This cross-sectional analysis of 323 TB patients in rural South Africa found that, in addition to proportional costs being higher for those with the lowest income, absolute TB costs were equal or greater for patients with greater poverty as measured by SAMPI deprivation score. These findings suggest that a multidimensional poverty index may identify a population of TB patients who are at risk of catastrophic economic consequences resulting from TB illness. Long travel times to the clinic and infection with HIV were also significant predictors of mean TB costs. This understanding may be important in refining appropriate definitions of TB-related catastrophic costs and setting social protection policies for people affected by TB and their households.
One important finding from our study is that the estimated association between poverty and cost of TB illness differed qualitatively according to how poverty was measured. When measured by self-reported income, we found that although TB illness costs were higher as a proportion of income among the lowest income quintiles – consistent with previous findings in urban Malawi – the absolute costs of TB illness were lower among these patients.20 However, when measuring poverty according to a multidimensional poverty index, we found that those who were most poor faced equal if not higher absolute costs. This is consistent with previous findings using self-reported income in a South African setting.6 Given the paucity of studies that have stratified TB costs by poverty status,4,7 additional efforts should elucidate the relationship between poverty and costs of TB illness in a variety of settings.
In designing further studies of TB costs and poverty, one must understand the differences between self-reported income and multidimensional poverty indices. The Oxford Poverty and Human Development Initiative (which led to the development of the multidimensional poverty index) cites numerous examples in which these two measures of poverty disagree.21 For example, 13% of income-poor households in Uruguay did not face unmet economic needs, 53% of malnourished Indian children did not live in income-poor households, and 69% of multi-dimensionally poor households in China were not income-poor.21,22 In 2015, the National Statistics Service of South Africa estimated poverty in Limpopo province to be 15.4% according to income measures but 10.1% according to SAMPI measures.23 We found little correlation between self-reported income and SAMPI deprivation (Figure 3). This discrepancy highlights the difference in these two measures, and is likely related to local variations in assets and costs of living. In considering the goal of eliminating catastrophic costs due to TB worldwide,1 future high-level policy discussions should consider whether catastrophic costs are better benchmarked as a proportion of annual household income or considered in light of multidimensional poverty. Longitudinal changes in multidimensional poverty might also be a useful measure for tracking future impoverishment resulting from catastrophic costs of TB disease.
Our study had three main limitations. First, our data were cross-sectional, self-reported, and collected using tools (e.g. SAMPI) that are intentionally simple. As a result, our results are susceptible to recall bias, unmeasured confounding, and potential misclassification of poverty or cost of illness. Future studies could evaluate these measures in more detailed, longitudinal fashion. Nevertheless, our data may better reflect those that could be collected in the context of interventions (e.g., social protection) designed to alleviate catastrophic TB costs. Second, our findings are specific to rural South Africa and may not generalize to other settings. Finally, the ultimate goal of measuring TB costs in the context of poverty is to design interventions to ameliorate the economic burden imposed by TB on the most economically vulnerable,24,25 and our analysis cannot be interpreted as supporting the effect of any specific intervention.
In summary, our study findings suggest that the absolute cost of TB illness is similar (if not higher) for rural South African adults who are most deprived on a multidimensional poverty index. By contrast, using self-reported income to measure poverty resulted in a strong negative correlation between poverty and absolute TB costs. Our results highlight the importance of considering appropriate metrics of poverty as we seek to alleviate the economic burden of TB for the most vulnerable patients.
ACKNOWLEDGMENTS
We are grateful to the patients who made this work possible. This project was funded by the United States National Institutes of Health (R01AI116787).
Footnotes
Conflicts of interest: none declared
REFERENCES
- 1.World Health Organization. Global tuberculosis report 2017. WHO. 2017. [Google Scholar]
- 2.Barter DM, Agboola SO, Murray MB, Barnighausen T. Tuberculosis and poverty: The contribution of patient costs in sub-Saharan Africa--a systematic review. BMC Public Health. 2012;12:980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Needham DM, Godfrey-Faussett P, Foster SD. Barriers to tuberculosis control in urban Zambia: The economic impact and burden on patients prior to diagnosis. Int J Tuberc Lung Dis. 1998;2(10):811–817. [PubMed] [Google Scholar]
- 4.Ukwaja KN, Modebe O, Igwenyi C, Alobu I. The economic burden of tuberculosis care for patients and households in Africa: A systematic review. Int J Tuberc Lung Dis. 2012;16(6):733–739. [DOI] [PubMed] [Google Scholar]
- 5.Ramma L, Cox H, Wilkinson L, et al. Patients’ costs associated with seeking and accessing treatment for drug-resistant tuberculosis in south africa. Int J Tuberc Lung Dis. 2015;19(12):1513–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foster N, Vassall A, Cleary S, Cunnama L, Churchyard G, Sinanovic E. The economic burden of TB diagnosis and treatment in South Africa. Soc Sci Med. 2015;130:42–50. [DOI] [PubMed] [Google Scholar]
- 7.Russell S. The economic burden of illness for households in developing countries: A review of studies focusing on malaria, tuberculosis, and human immunodeficiency virus/acquired immunodeficiency syndrome. Am J Trop Med Hyg. 2004;71(2):147–155. [PubMed] [Google Scholar]
- 8.Tanimura T, Jaramillo E, Weil D, Raviglione M, Lonnroth K. Financial burden for tuberculosis patients in low- and middle-income countries: A systematic review. Eur Respir J. 2014;43(6):1763–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sweeney S, Mukora R, Candfield S, Guinness L, Grant AD, Vassall A. Measuring income for catastrophic cost estimates: Limitations and policy implications of current approaches. Social Science & Medicine. 2018;215:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sen A. A decade of human development. Journal of Human Development. 2000;1(1):17–23. [Google Scholar]
- 11.Victor Bart, Blevins Meridith, Green Ann F, et al. Multidimensional poverty in rural Mozambique: A new metric for evaluating public health interventions. PLoS One. 2014;9(9):e108654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Statistics South Africa. The South African MPI: Creating a multidimensional poverty index using census data. StatsSA. 2014. [Google Scholar]
- 13.Hanrahan C, Nonyane B, Mmolawa L. Kharitode TB: A pragmatic cluster randomized trial of contact tracing versus enhanced facility screening for active TB case finding in rural South Africa. Under review. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Statistics South Africa. Community survey 2016. StatsSA. 2016. [Google Scholar]
- 15.Statistics South Africa. Census 2011. StatsSA. 2012. [Google Scholar]
- 16.Annual progress report 2015/16: Provincial strategic plan 2012–2016. Limpopo Provincial AIDS Council. 2017. [Google Scholar]
- 17.World Health Organization, Global TB Programme November 2015. Protocol for survey to determine direct and indirect costs due to TB and to estimate proportion of TB-affected households experiencing catastrophic total costs due to TB field testing version. . [Google Scholar]
- 18.Stracker N, Hanrahan C, Mmolawa L, et al. Risk factors for catastrophic costs associated with tuberculosis in rural South Africa Int J Tuberc Lung Dis. 2018. (In press; ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.International Monetary Fund. Representative exchange rates for selected currencies for October 2017. https://www.imf.org/external/np/fin/data/rms_mth.aspx?SelectDate=2017-10-31&reportType=REP. Accessed Aug 19, 2018.
- 20.Kemp JR, Mann G, Simwaka BN, Salaniponi FM, Squire SB. Can malawi’s poor afford free tuberculosis services? patient and household costs associated with a tuberculosis diagnosis in Lilongwe. Bulletin of the World Health Organization. 2007;85(8):580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foster J, Santos ME, Roche JM, Alkire S, Ballon P, Seth S. Multidimensional poverty measurement and analysis. First edition ed. Oxford: Oxford University Press; 2015. [Google Scholar]
- 22.Wang X, Feng H, Xia Q, Alkire S. On the relationship between income poverty and multidimensional poverty in China. OPHI working paper 101 2016. [Google Scholar]
- 23.Statistics South Africa. Census 2011: Income dynamics and poverty status of household in South Africa. 2015. [Google Scholar]
- 24.Lonnroth K, Glaziou P, Weil D, Floyd K, Uplekar M, Raviglione M. Beyond UHC: Monitoring health and social protection coverage in the context of tuberculosis care and prevention. PLoS Med. 2014;11(9):e1001693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benatar SR, Upshur R. Tuberculosis and poverty: What could (and should) be done? Int J Tuberc Lung Dis. 2010;14(10):1215–1221. [PubMed] [Google Scholar]