ABSTRACT
In this supplement, we show a comprehensive anatomic atlas of the human cerebrum demonstrating all 180 distinct regions comprising the cerebral cortex. The location, functional connectivity, and structural connectivity of these regions are outlined, and where possible a discussion is included of the functional significance of these areas. In this chapter, we specifically address regions integrating to form the vertical occipital fasciculus.
Keywords: Anatomy, Cerebrum, Connectivity, DTI, Functional connectivity, Human, Parcellations
ABBREVIATIONS
- DSI
diffusion spectrum imaging
- DTI
diffusion tensor imaging
- HCP
Human Connectome Project
- IPL
inferior parietal lobe
- VOF
vertical occipital fasciculus
The vertical occipital fasciculus (VOF) is a short white matter tract that courses obliquely from parts of the inferior parietal lobule and superior occipital lobe to the inferolateral occipital cortex.1-4 The VOF runs vertically behind the arcuate division of the superior longitudinal fasciculus and lateral to the inferior longitudinal fasciculus which courses orthogonally to the VOF as it traverses the cerebrum to the cuneus and lingual gyrus.1-3,5,6 Given this anatomy, the VOF appears to be connecting the dual streams of the visual processing system,3,5,7,8 suggesting important functional roles for this tract in vision and perception.3,5
While diffusion tensor imaging (DTI) and gross anatomic dissection studies have clarified the structural anatomy of the VOF in some detail,9 little is known about its various cortical terminations. Recently, the Human Connectome Project (HCP) published parcellation data redefining the human cortex.10 This provides a unique opportunity to elucidate the macro-connectome of the human cerebrum, in that high-resolution DTI tractography has been shown to accurately illustrate the anatomy of different white matter tracts in the brain.11-13
In this study, we delineate the boundaries of the VOF utilizing the parcellation scheme developed under the HCP.10 Through diffusion spectrum imaging (DSI), we show the relationship between these parcellations and the VOF. We also provide a simplified tract map summarizing those regions with white matter connections specific to the VOF. The purpose of this study is to present the structural connectivity of the VOF in an indexed, illustrated, and tractographically aided series of figures and tables for anatomic and clinical reference.
METHODS
Identification of Relevant Cortical Regions
The parcellation data entries within the first 9 chapters of this supplement were reviewed to determine the specific cortical regions with structural connectivity in the distribution of the VOF. These data were tabulated, and connections between individual parcellations within the VOF were recorded. These results served as the basis for constructing a simplified tractography map of the VOF and performing deterministic tractography.
Deterministic Tractography
Publicly available imaging data from the HCP was obtained for this study from the HCP database (http://humanconnectome.org, release Q3). Diffusion imaging with corresponding T1-weighted images from 10 healthy, unrelated controls were analyzed (Subjects IDs: 100307, 103414, 105115, 110411, 111312, 113619, 115320, 117112, 118730, 118932). A multi-shell diffusion scheme was used, and the b-values were 990, 1985, and 1980 s/mm2. Each b-value was sampled in 90 directions. The in-plane resolution was 1.25 mm. The diffusion data were reconstructed using generalized q-sampling imaging with a diffusion sampling length ratio of 1.25.14
We performed brain registration to MNI space, wherein imaging is warped to fit a standardized brain model comparison between subjects. Tractography was performed in DSI studio using a region of interest approach to initiate fiber tracking from a user-defined seed region. A two-ROI-approach was used to isolate tracts. Voxels within each ROI were automatically traced with a maximum angular threshold of 45°. When a voxel was approached with no tract direction or a direction change of greater than 45°, the tract was halted. Tractography was stopped after reaching a maximum length of 800 mm. In some instances, exclusion ROIs were placed to exclude obvious spurious tracts that were not involved in the white matter pathway of interest. Tractographic results are shown only for regions of interest within the left cerebral hemisphere.
CONNECTIVITY OVERVIEW
Table summarizes the relevant cortical regions that integrate to form the VOF. Four parcellations in the superolateral occipital cortex show structural connectivity in the distribution of this tract, including V7, V3a, V3b, and V3cd. These parcellations have variable connections to areas V8, VVC, VMV3, VMV2, and VMV1, all of which are located on the inferior/basal surface of the occipital lobe. Area PIT also demonstrates structural connections in the distribution of the VOF to the superior aspects of early visual areas V2 and V3.
TABLE .
Regions Integrating Within the Vertical Occipital Fasciculus
| Original parcellation | Terminations |
|---|---|
| PIT | V2 |
| V3 | |
| V3a | PIT |
| V8 | |
| VMV1 | |
| VMV2 | |
| VMV3 | |
| V3b | V8 |
| VMV3 | |
| VVC | |
| V3cd | V8 |
| V7 | V8 |
Figure 1 illustrates a simplified tract map of the relevant structural connectivity of the cerebral parcellation data within the confines of the VOF. In addition, Figures 2–4 illustrate key DSI-based fiber tracking examples chosen for the strength and breadth of linked parcellation data. In short, the VOF can be seen to arise from the basal surface of the occipital lobe as it courses obliquely and medially to terminate in the aforementioned parcellations. It should be noted that the figures and tables presented in this study do not imply directionality. Instead, supposed information transit is utilized as a simplified means for connectivity description.
FIGURE 1.
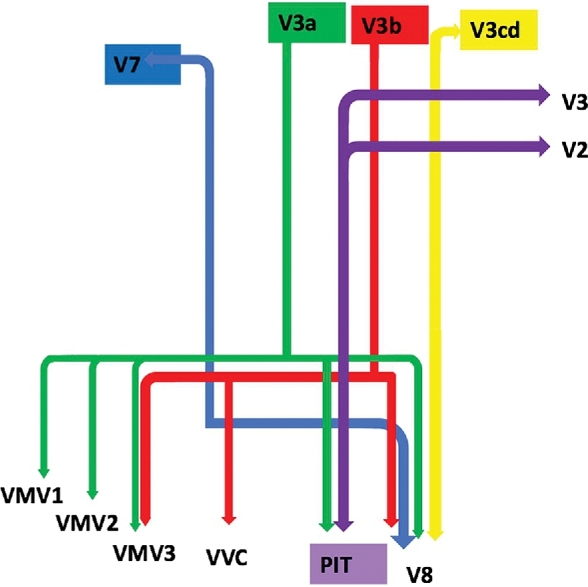
Simplified tract map showing the structural connections that integrate within the VOF. Connections between cortical areas are color-coded based on the parcellation of origin (eg, red arrows indicate structural connections from origin V3b to areas VMV3, VVC, and V8). Note that arrows are not meant to imply the direction of information transmit.
FIGURE 2.
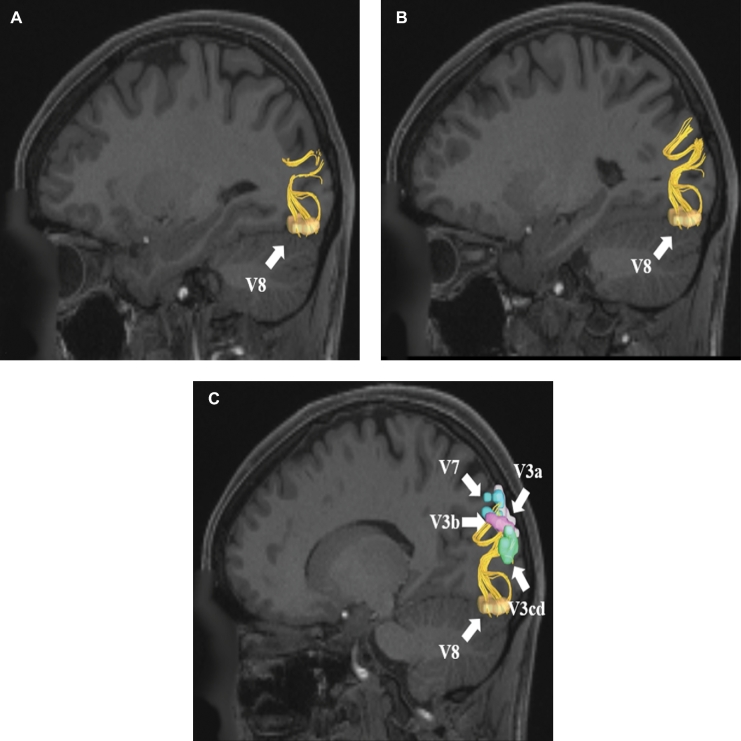
VOF connections from region V8. Area V8 is located in the lateral occipital cortex and has structural connections to areas V3a, V3b, V3cd, and V7. These connections are shown in the left cerebral hemisphere on T1-weighted MR images in the sagittal plane: A, medial view, B, lateral view without regions of interest, C, lateral view with regions of interest. All parcellations are identified with white arrows and corresponding labels.
FIGURE 4.
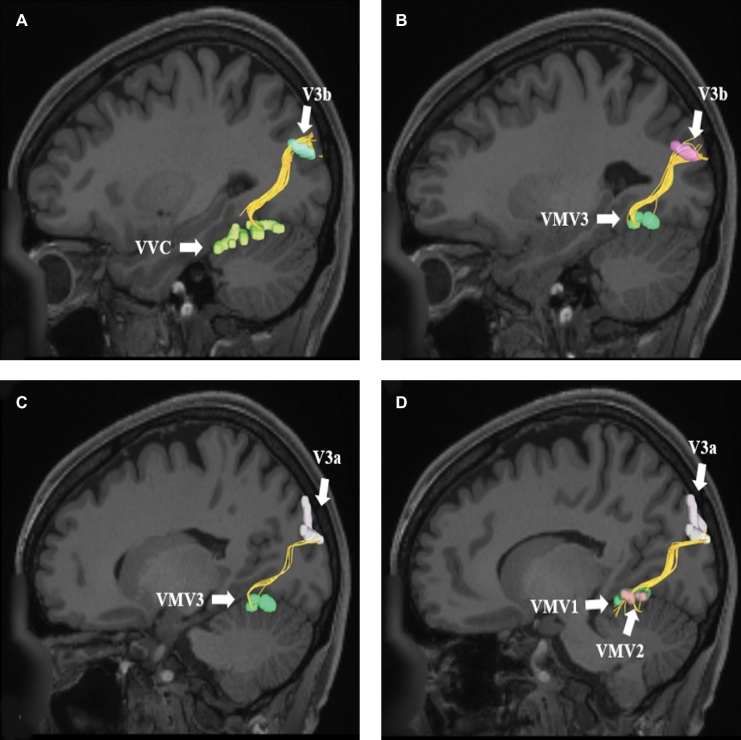
VOF connections from regions A and B, V3b and C and D, V3a. Area V3b exhibits structural connections to areas A, VVC and B, VMV3. These connections are shown in the left cerebral hemisphere on T1-weighted MR images in the sagittal plane. Area V3a exhibits structural connections to areas C, VMV3 and D, VMV1 and VMV2. These connections are shown in the left cerebral hemisphere on T1-weighted MR images in the sagittal plane. All parcellations are identified with white arrows and corresponding labels.
FIGURE 3.
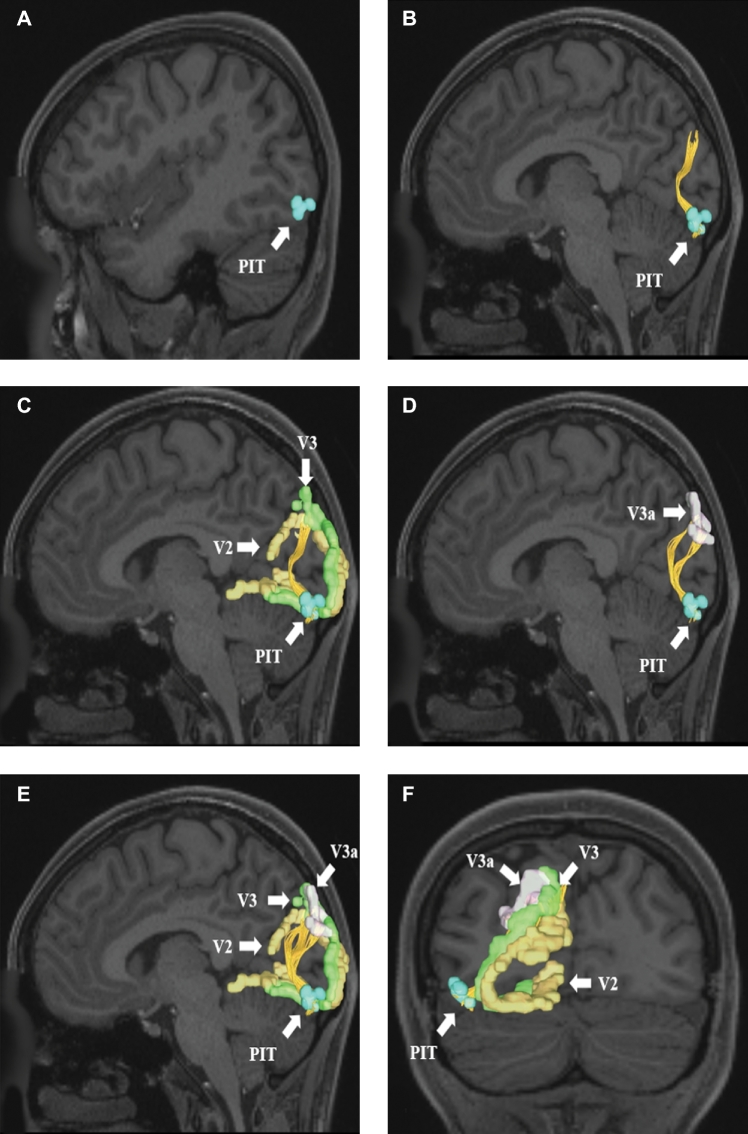
VOF connections from region PIT. Area PIT is located in the lateral occipital cortex and has structural connections to areas V2, V3, and V3a. These connections are shown in the left cerebral hemisphere on T1-weighted MR images in the A–E, sagittal and F, coronal planes: A, medial view of PIT, B, lateral view of PIT with the VOF readily identified, C, VOF connections to V2 and V3, D, VOF connections to V3a, E, entire set of VOF connections from area PIT, and F, posterior coronal view of the early visual processing regions connected to PIT. All parcellations are identified with white arrows and corresponding labels.
DISCUSSION
In this study, we provide a detailed map of the macro-connectivity of the VOF and its relevant cerebral parcellations. As has been demonstrated by others, we show that the VOF begins in parts of the inferolateral occipital lobe and courses superiorly to terminate in the superior occipital cortex.1-3 While some report terminations in the inferior parietal lobe (IPL), specifically the angular gyrus,1,2,4 others report that few of such fibers from the VOF actually terminate in the IPL.3 This latter finding is consistent with the tractographic description of the VOF presented here.
Given the VOF’s structural connections between superior and inferolateral parts of the occipital lobe, it is likely that this white matter pathway participates in information transfer between the dual visual processing streams.3,5,7,8 Briefly, the dual stream model of visual processing was first proposed by Mishkin and Ungerleider15 in 1982 in their seminal paper discussing their electrical stimulation work in non-human primates. These studies revealed distinct visual processing streams for objects and object positions.15 They termed these processing streams the “what” and the “where” pathways, respectively, corresponding to the ventral and dorsal visual streams.15 While it is likely that the VOF is modulating or transmitting “what” and “where” information between these distinct cortical streams based on its structural connectivity, such functionality has not yet been elucidated.
Despite our limited understanding of the functional role of the VOF in the dual stream model, it is clear that the distinct cortical areas integrated within this fiber bundle belong either to the dorsal or ventral visual streams. For example, it is known that area V3a demonstrates sensitivity to motion and contrast in the central visual field while integrating spatial information from visual inputs.16,17 Area V3b has been implicated as a relay point for motion-sensitive information, and is involved in the processing of motion and discernment of kinetic boundaries.18 Meanwhile, area V7 is involved in the integration of spatial information within the central visual field around the fovea.19 In contrast to these dorsal stream parcellations, other areas such as V8 are responsible for the perception and processing of color in the visual field.20 Area PIT is involved in the recognition of basic color characteristics of objects, including hue, saturation, and brightness.21 Finally, Area VVC is implicated in color perception, and shows increased responsiveness to the detection of color in monochromatic fields.18 Area VVC is also essential for the integration of color, contrast, and textural information for the recognition of places.22
One critical function of the VOF is its relevance to reading.1,4,23 Damage to this tract has been linked to pure word blindness, also known as pure alexia,1,23 a condition in which reading capacity is impaired while other language functionalities are preserved, including writing. Beyond this, little else is known regarding the function of the VOF and its clinical significance.
CONCLUSION
The VOF is a short white matter tract contained within the occipital lobe that connects parcellations within the dorsal and ventral visual processing streams. While the VOF is critical to reading capacity, its role in exchanging, modifying, or transmitting information between the dual streams of visual processing remains unclear. Further, sub-tract guided functional and anatomic studies are needed to enhance our understanding of the functional connectivity of the VOF. However, our tractographic map of this white matter pathway can serve as a reference point for these future studies.
Disclosures
Synaptive Medical assisted in the funding of all 18 chapters of this supplement. No other funding sources were utilized in the production or submission of this work.
Acknowledgments
Data were provided [in part] by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University. We would also like to thank Brad Fernald, Haley Harris, and Alicia McNeely of Synaptive Medical for their assistance in constructing the network figures for Chapter 18 and for coordinating the completion and submission of this supplement.
REFERENCES
- 1. Gungor A, Baydin S, Middlebrooks EH, Tanriover N, Isler C, Rhoton AL Jr. The white matter tracts of the cerebrum in ventricular surgery and hydrocephalus. J Neurosurg. 2017;126(3):945-971. [DOI] [PubMed] [Google Scholar]
- 2. Wu Y, Sun D, Wang Y, Wang Y, Wang Y. Tracing short connections of the temporo-parieto-occipital region in the human brain using diffusion spectrum imaging and fiber dissection. Brain Res. 2016;1646:152-159. [DOI] [PubMed] [Google Scholar]
- 3. Yeatman JD, Weiner KS, Pestilli F, Rokem A, Mezer A, Wandell BA. The vertical occipital fasciculus: A century of controversy resolved by in vivo measurements. Proc Natl Acad Sci USA. 2014;111(48):E5214-E5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Price CJ. Current themes in neuroimaging studies of reading. Brain Lang. 2013;125(2):131-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weiner KS, Yeatman JD, Wandell BA. The posterior arcuate fasciculus and the vertical occipital fasciculus. Cortex. 2017;97:274-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Takemura H, Pestilli F, Weiner KS et al. . Occipital white matter tracts in human and macaque. Cereb Cortex. 2017;27(6):3346-3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Freud E, Plaut DC, Behrmann M. ‘What’ is happening in the dorsal visual pathway. Trends Cogn Sci. 2016;20(10):773-784. [DOI] [PubMed] [Google Scholar]
- 8. Takemura H, Rokem A, Winawer J, Yeatman JD, Wandell BA, Pestilli F. A major human white matter pathway between dorsal and ventral visual cortex. Cereb Cortex. 2016;26(5):2205-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Keser Z, Ucisik-Keser FE, Hasan KM. Quantitative mapping of human brain vertical-occipital fasciculus. J Neuroimaging. 2016;26(2):188-193. [DOI] [PubMed] [Google Scholar]
- 10. Glasser MF, Coalson TS, Robinson EC et al. . A multi-modal parcellation of human cerebral cortex. Nature. 2016;536(7615):171-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kamali A, Flanders AE, Brody J, Hunter JV, Hasan KM. Tracing superior longitudinal fasciculus connectivity in the human brain using high resolution diffusion tensor tractography. Brain Struct Funct. 2014;219(1):269-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Menjot de Champfleur N, Lima Maldonado I, Moritz-Gasser S et al. . Middle longitudinal fasciculus delineation within language pathways: A diffusion tensor imaging study in human. Eur J Radiol. 2013;82(1):151-157. [DOI] [PubMed] [Google Scholar]
- 13. Lemaire JJ, Cosnard G, Sakka L et al. . White matter anatomy of the human deep brain revisited with high resolution DTI fibre tracking. Neurochirurgie. 2011;57(2):52-67. [DOI] [PubMed] [Google Scholar]
- 14. Yeh FC, Wedeen VJ, Tseng WY. Generalized q-sampling imaging. IEEE Trans Med Imaging. 2010;29(9):1626-1635. [DOI] [PubMed] [Google Scholar]
- 15. Mishkin M, Ungerleider LG. Contribution of striate inputs to the visuospatial functions of parieto-preoccipital cortex in monkeys. Behav Brain Res. 1982;6(1):57-77. [DOI] [PubMed] [Google Scholar]
- 16. Tootell RB, Mendola JD, Hadjikhani NK et al. . Functional analysis of V3A and related areas in human visual cortex. J Neurosci. 1997;17(18):7060-7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tootell RB, Mendola JD, Hadjikhani NK, Liu AK, Dale AM. The representation of the ipsilateral visual field in human cerebral cortex. Proc Natl Acad Sci USA. 1998;95(3):818-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wandell BA, Brewer AA, Dougherty RF. Visual field map clusters in human cortex. Philos Trans R Soc Lond B Biol Sci. 2005;360(1456):693-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Press WA, Brewer AA, Dougherty RF, Wade AR, Wandell BA. Visual areas and spatial summation in human visual cortex. Vision Res. 2001;41(10-11):1321-1332. [DOI] [PubMed] [Google Scholar]
- 20. Hadjikhani N, Liu AK, Dale AM, Cavanagh P, Tootell RB. Retinotopy and color sensitivity in human visual cortical area V8. Nat Neurosci. 1998;1(3):235-241. [DOI] [PubMed] [Google Scholar]
- 21. Conway BR. Color signals through dorsal and ventral visual pathways. Vis Neurosci. 2014;31(02):197-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grill-Spector K, Malach R. The human visual cortex. Annu Rev Neurosci. 2004;27(1):649-677. [DOI] [PubMed] [Google Scholar]
- 23. Yeatman JD, Rauschecker AM, Wandell BA. Anatomy of the visual word form area: Adjacent cortical circuits and long-range white matter connections. Brain Lang. 2013;125(2):146-155. [DOI] [PMC free article] [PubMed] [Google Scholar]