ABSTRACT
In this supplement, we show a comprehensive anatomic atlas of the human cerebrum demonstrating all 180 distinct regions comprising the cerebral cortex. The location, functional connectivity, and structural connectivity of these regions are outlined, and where possible a discussion is included of the functional significance of these areas. In this chapter, we specifically address the regions integrating to form the frontal aslant tract.
Keywords: Anatomy, Cerebrum, Connectivity, DTI, Functional connectivity, Human, Parcellations
ABBREVIATIONS
- DSI
diffusion spectrum imaging
- DTI
diffusion tensor imaging
- FAT
frontal aslant tract
- IFG
inferior frontal gyrus
- MR
magnetic resonance
- ROI
region of interest
- SFG
superior frontal gyrus
- SMA
supplementary motor area
The frontal aslant tract (FAT) was first described in 2008 by two independent groups constructing and studying white matter atlases of the human cerebrum.1,2 They noted at the time short white matter tracts within the frontal lobe running between the superior and inferior frontal gyri that appeared to be connecting parts of the supplementary motor area (SMA) to Broca's area.1,2 Subsequent studies have demonstrated the FAT’s importance as part of the language network, including functional roles in motor planning and speech production.3-5
While diffusion tensor imaging (DTI) and gross anatomic dissection studies have clarified the structural anatomy of the FAT in some detail,3,6 little is known about its various cortical terminations. Recently, the Human Connectome Project published parcellation data redefining the human cortex.7 This provides a unique opportunity to elucidate the macro-connectome of the human cerebrum, in that high-resolution DTI tractography has been shown to accurately illustrate the anatomy of different white matter tracts in the brain.8-10
In this study, we delineate the boundaries of the FAT utilizing the parcellation scheme developed under the Human Connectome Project.7 Through diffusion spectrum imaging (DSI), we show the relationship between these parcellations and the FAT. We also provide a simplified tract map summarizing those regions with white matter connections integrating within this white matter tract. The purpose of this study is to present the structural connectivity of the FAT in an indexed, illustrated, and tractographically aided series of figures and tables for anatomic and clinical reference.
METHODS
Identification of Relevant Cortical Regions
The parcellation data entries within the first nine chapters of this supplement were reviewed to determine the specific cortical regions with structural connectivity in the distribution of the FAT. These data were tabulated, and connections between individual parcellations within the FAT were recorded. These results served as the basis for constructing simplified tractography maps of the SLF and performing deterministic tractography.
Deterministic Tractography
Publicly available imaging data from the Human Connectome Project was obtained for this study from the HCP database (http://humanconnectome.org, release Q3). Diffusion imaging with corresponding T1-weighted images from 10 healthy, unrelated controls were analyzed (Subjects IDs: 100307, 103414, 105115, 110411, 111312, 113619, 115320, 117112, 118730, 118932). A multishell diffusion scheme was used, and the b-values were 990, 1985, and 1980 s/mm2. Each b-value was sampled in 90 directions. The in-plane resolution was 1.25 mm. The diffusion data was reconstructed using generalized q-sampling imaging with a diffusion sampling length ratio of 1.25.11
We performed brain registration to MNI space, wherein imaging is warped to fit a standardized brain model comparison between subjects. Tractography was performed in DSI studio using a region of interest approach to initiate fiber tracking from a user-defined seed region. A two region of interest (ROI) approach was used to isolate tracts. Voxels within each ROI were automatically traced with a maximum angular threshold of 45 degrees. When a voxel was approached with no tract direction or a direction change of greater than 45 degrees, the tract was halted. Tractography was stopped after reaching a maximum length of 800 mm. In some instances, exclusion ROIs were placed to exclude obvious spurious tracts that were not involved in the white matter pathway of interest. Tractographic results are shown only for regions of interest within the left cerebral hemisphere.
CONNECTIVITY OVERVIEW
Four parcellations of the superior frontal gyrus (SFG) show structural connectivity in the distribution of the FAT: 6ma, 8BL, S6-8, and SFL. Table summarizes the relevant cortical regions that integrate to form the tract. These regions show variable connections to regions 44, 6r, FOP1, FOP3, and FOP4 of the inferior frontal gyrus (IFG) and frontal operculum, as well as region MI of the anterior insula. Figure 1 illustrates a simplified tract map of the relevant structural connectivity of these cerebral parcellation data within the confines of the FAT. In addition, Figures 2 and 3 illustrate key DSI-based fiber tracking examples chosen for the strength and breadth of linked parcellation data. It should be noted that the figures and tables presented in this study do not imply directionality. Instead, supposed information transit is utilized as a simplified means for connectivity description.
TABLE.
Regions Integrating Within the FAT
| Original parcellation | Terminations |
|---|---|
| 6ma | 44 |
| FOP4 | |
| FOP3 | |
| MI | |
| 8BL | 44 |
| MI | |
| s6-8 | 44 |
| FOP4 | |
| 6r | |
| SFL | FOP4 |
| FOP3 | |
| FOP1 | |
| MI |
FIGURE 1.
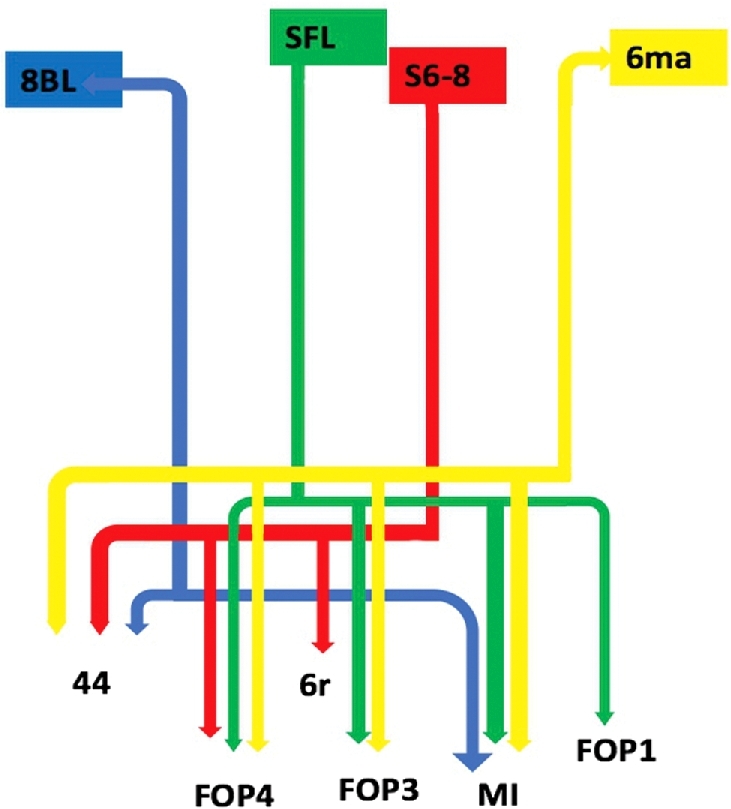
Simplified tract map showing the structural connections that integrate within the FAT. Connections between cortical areas are color-coded based on the parcellation of origin (eg, blue arrows indicate structural connections from origin 8BL to areas 44 and MI). Note that arrows are not meant to imply the direction of information transmit.
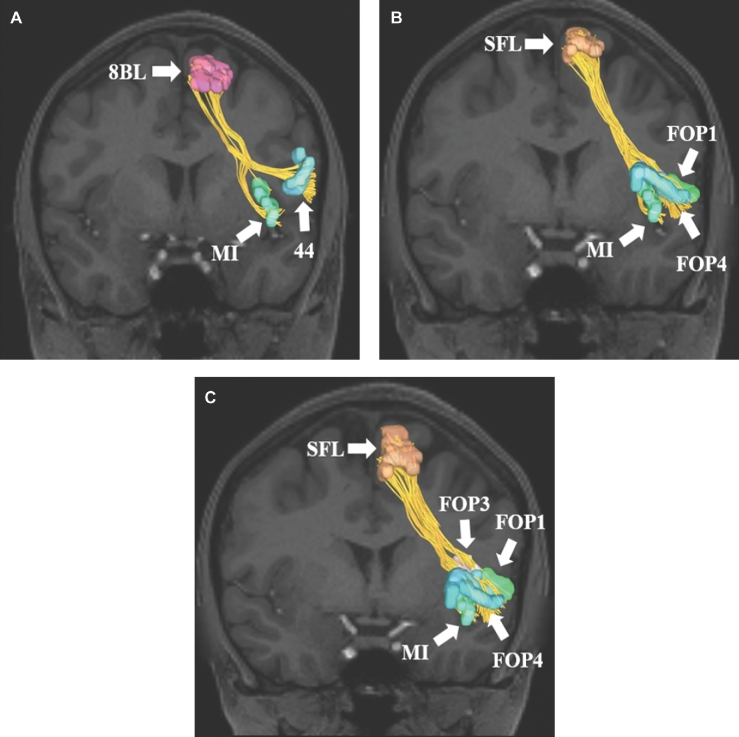
FIGURE 2.
Structural connections from cortical regions integrating within the FAT. A, Connections from area 8BL to regions 44 and MI of the IFG and anterior insula, respectively. B and C, Connections from SFL to areas FOP1, FOP3, FOP4, and MI of the insular-opercular cortex. C is an oblique view of SFL connections to better identify region FOP3 which lies behind areas MI and FOP4. All tractography images are shown on T1-weighted magnetic resonance (MR) images in the coronal plan.
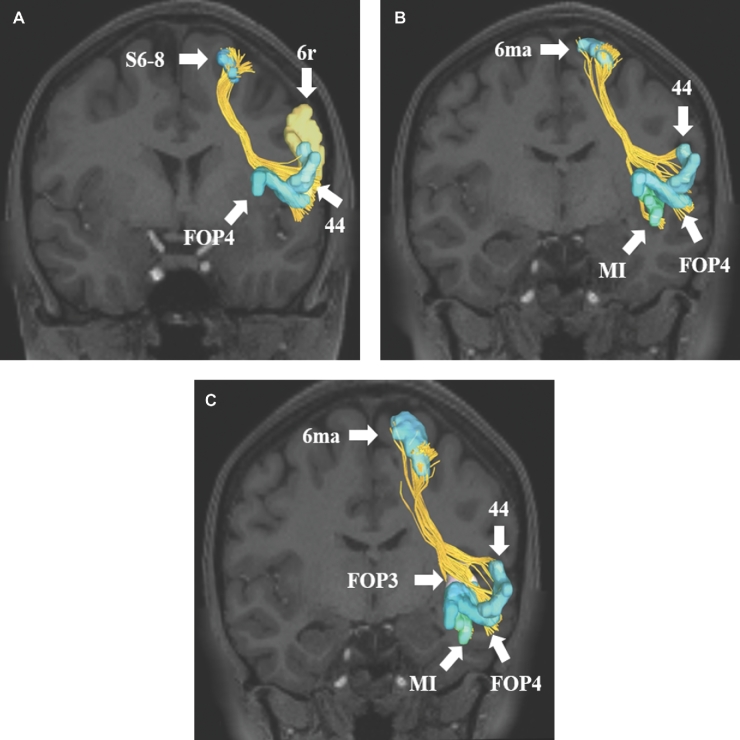
FIGURE 3.
Structural connections from cortical regions integrating within the FAT. A, Connections from area S6-8 to regions 44, 6r, and FOP4 of the IFG and frontal operculum. B and C, Connections from 6ma to areas 44, FOP3, FOP4, and MI of the IFG, frontal operculum and anterior insula. C is an oblique view of 6ma connections to better identify region FOP3 which lies behind areas MI and FOP4. All tractography images are shown on T1-weighted MR images in the coronal plan.
DISCUSSION
The SFG Parcellations
Of the four parcellations of the SFG that contribute to the FAT, two, areas 6ma and SFL, are part of the SMA. While these two parcellations are newly described by the HCP authors,7 the SMA is known to contribute to the initiation, planning, and production of ordered movements.12 The two other regions, areas 8BL and S6-8, are part of the dorsolateral prefrontal cortex and appear to play a role in maintaining and processing spatial information.13
The Principle Connections of the FAT
SFG Connections to the IFG
All four SFG parcellations have connections to region 44 of the IFG. Area 44 is a principle component of the motor network that helps to guide the production of verbal and manual motion.14 Immediately posterior to region 44 is 6r, a parcellation that is not well studied, but appears to be functionally related to Broca's complex, a region of the cortex that includes Brodmann areas 44, 45, 46, 47, and the mesial supplementary motor region of area 6.15 Broca's complex has long been thought essential to the proper processing and function of language in humans.15
SFG Connections to the Frontal Operculum
The SFG parcellations also have connections to the frontal operculum. Three regions (6ma, S6-8, and SFL) have connections to at least one of the FOP parcellations, all of which are newly described by the HCP authors.7 Areas 6ma, S6-8, and SFL connect to FOP3 and FOP4. SFL also connects to FOP1. The frontal operculum is involved in the initiation of language as well as in language learning through the processes of lexical retrieval.16,17 Area FOP1 also appears to play a role in the imagination of abstract movement from a third-person perspective.18 It may also serve a nociceptive function, specifically the perception and transmission of pain information to limbic areas.19
SFG Connections to the Insula
Finally, three SFG parcellations, 6ma, 8BL, and SFL, have connections to area MI of the insula. The precise function of MI is not well understood, but the insula itself has been implicated in a diverse array of neural functions including autonomic nervous system control, human awareness and self-recognition, time perception, and decision making.20,21 What, if any influence, the SFG has on the anterior insula in modulating, inhibiting, or activating such functions remains unclear.
The Anatomic and Functional Significance of the FAT
The FAT has previously been described as a lateralized fiber bundle that connects the ipsilateral superior and inferior frontal gyri, connecting parts of the SMA to Broca's area.22,23 The connections between parcellations 6ma and SFL to area 44 are consistent with these results. The FAT is also known to be left lateralized and highly integrated into the pars opercularis, helping to explain the significance of the FAT in left-sided language processes such as the initiation of speech and verbal fluency.3 Several other studies have also implicated the FAT in orofacial movement,4 hand movement,5 and movement inhibition.5
Disruption of the FAT is also well known to cause language motor deficits. For example, awake brain surgery involving electrical stimulation of the left FAT has led to stuttering and speech arrest in multiple studies.24,25 Another study found that patients who underwent resection of frontal tumors abutting the FAT experienced transient speech initiation problems in the postoperative period.26 Damage to the FAT also appears to play a role in the verbal fluency deficits in primary progressive aphasia as well as poststroke aphasia.27,28 White matter tracts, including the FAT, are also appear altered in autism spectrum disorder.29 Reductions in tract integrity are specifically associated with impaired social interaction capability and communication suggesting the FAT plays a role in language development.29
Beyond the language domain of human cognition, the FAT remains largely understudied. While we have identified connections to the frontal operculum and anterior insula, little is known about the activity of the FAT in relation to the functions of these regions and their corresponding parcellations. It is possible that the output from the SFG is modulating the activity of these regions or vice versa, but we do not yet know in what ways this modulation is occurring. In addition, there does not appear be any literature regarding the role of the FAT on decision making, human awareness, or self-recognition. Additional studies into the connectomics of the FAT and its modulatory effects on these regions will help explain these effects and refine our knowledge of the FAT.
CONCLUSION
The FAT is a relatively new white matter tract within the cerebrum connecting key parts of the SMA in the SFG to parcellations in the IFG, frontal operculum, and anterior insula. While the FAT is critical to the language network for appropriate speech production, its significance in other functional networks has yet to be fully elucidated. Further, sub-tract guided functional and anatomic studies are needed to enhance our understanding of the functional connectivity of the FAT. However, our tractographic map of this white matter pathway can serve as a reference point moving forward.
Disclosures
Synaptive Medical assisted in the funding of all 18 chapters of this supplement. No other funding sources were utilized in the production or submission of this work.
Acknowledgments
Data were provided [in part] by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University. We would also like to thank Brad Fernald, Haley Harris, and Alicia McNeely of Synaptive Medical for their assistance in constructing the network figures for Chapter 18 and for coordinating the completion and submission of this supplement.
REFERENCES
- 1. Lawes INC, Barrick TR, Murugam V et al. . Atlas-based segmentation of white matter tracts of the human brain using diffusion tensor tractography and comparison with classical dissection. Neuroimage. 2008;39(1):62-79. [DOI] [PubMed] [Google Scholar]
- 2. Oishi K, Zilles K, Amunts K et al. . Human brain white matter atlas: identification and assignment of common anatomical structures in superficial white matter. Neuroimage. 2008;43(3):447-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Szmuda T, Rogowska M, Sloniewski P et al. . Frontal aslant tract projections to the inferior frontal gyrus. Folia Morphol. 2017:(4). [DOI] [PubMed] [Google Scholar]
- 4. Martino J, de Lucas EM, Ibanez-Plagaro FJ, Valle-Folgueral JM, Vazquez-Barquero A. Foix-Chavany-Marie syndrome caused by a disconnection between the right pars opercularis of the inferior frontal gyrus and the supplementary motor area. J Neurosurg. 2012;117(5):844-850. [DOI] [PubMed] [Google Scholar]
- 5. Budisavljevic S, Dell’Acqua F, Djordjilovic V, Miotto D, Motta R, Castiello U. The role of the frontal aslant tract and premotor connections in visually guided hand movements. Neuroimage. 2017;146:419-428. [DOI] [PubMed] [Google Scholar]
- 6. Baker CM, Burks JD, Briggs RG et al. . The crossed frontal aslant tract: A possible pathway involved in the recovery of supplementary motor area syndrome. Brain Behav. 2018;8(3):e00926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Glasser MF, Coalson TS, Robinson EC et al. . A multi-modal parcellation of human cerebral cortex. Nature. 2016;536(7615):171-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kamali A, Flanders AE, Brody J, Hunter JV, Hasan KM. Tracing superior longitudinal fasciculus connectivity in the human brain using high resolution diffusion tensor tractography. Brain Struct Funct. 2014;219(1):269-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Menjot de Champfleur N, Lima Maldonado I, Moritz-Gasser S et al. . Middle longitudinal fasciculus delineation within language pathways: a diffusion tensor imaging study in human. Eur J Radiol. 2013;82(1):151-157. [DOI] [PubMed] [Google Scholar]
- 10. Lemaire JJ, Cosnard G, Sakka L et al. . White matter anatomy of the human deep brain revisited with high resolution DTI fibre tracking. Neurochirurgie. 2011;57(2):52-67. [DOI] [PubMed] [Google Scholar]
- 11. Yeh FC, Wedeen VJ, Tseng WY. Generalized q-sampling imaging. IEEE Trans Med Imaging. 2010;29(9):1626-1635. [DOI] [PubMed] [Google Scholar]
- 12. Ziegler W, Kilian B, Deger K. The role of the left mesial frontal cortex in fluent speech: evidence from a case of left supplementary motor area hemorrhage. Neuropsychologia. 1997;35(9):1197-1208. [DOI] [PubMed] [Google Scholar]
- 13. Petrides M, Pandya DN. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur J Neurosci. 2002;16(2):291-310. [DOI] [PubMed] [Google Scholar]
- 14. van Schie HT, Toni I, Bekkering H. Comparable mechanisms for action and language: Neural systems behind intentions, goals, and means. Cortex. 2006;42(4):495-498. [DOI] [PubMed] [Google Scholar]
- 15. Ardila A, Bernal B, Rosselli M. How localized are language brain areas? A review of brodmann areas involvement in oral language. Arch Clin Neuropsychol. 2016;31(1):112-122. [DOI] [PubMed] [Google Scholar]
- 16. Zhang Y, Song H, Liu X, Tang D, Chen YE, Zhang X. Language learning enhanced by massive multiple online role-playing games (MMORPGs) and the underlying behavioral and neural mechanisms. Front Hum Neurosci. 2017;11:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Steinmetz H, Seitz RJ. Functional anatomy of language processing: neuroimaging and the problem of individual variability. Neuropsychologia. 1991;29(12):1149-1161. [DOI] [PubMed] [Google Scholar]
- 18. Binkofski F, Buccino G. Motor functions of the Broca's region. Brain Lang. 2004;89(2):362-369. [DOI] [PubMed] [Google Scholar]
- 19. Sawamoto N, Honda M, Okada T et al. . Expectation of pain enhances responses to nonpainful somatosensory stimulation in the anterior cingulate cortex and parietal operculum/posterior insula: an event-related functional magnetic resonance imaging study. J Neurosci. 2000;20(19):7438-7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nelson SM, Dosenbach NU, Cohen AL, Wheeler ME, Schlaggar BL, Petersen SE. Role of the anterior insula in task-level control and focal attention. Brain Struct Funct. 2010;214(5-6):669-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Craig AD. How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10(1):59-70. [DOI] [PubMed] [Google Scholar]
- 22. Martino J, De Lucas EM. Subcortical anatomy of the lateral association fascicles of the brain: a review. Clin Anat. 2014;27(4):563-569. [DOI] [PubMed] [Google Scholar]
- 23. Dick AS, Bernal B, Tremblay P. The language connectome: New pathways, new concepts. Neuroscientist. 2014;20(5):453-467. [DOI] [PubMed] [Google Scholar]
- 24. Kemerdere R, de Champfleur NM, Deverdun J et al. . Role of the left frontal aslant tract in stuttering: A brain stimulation and tractographic study. J Neurol. 2016;263(1):157-167. [DOI] [PubMed] [Google Scholar]
- 25. Fujii M, Maesawa S, Motomura K et al. . Intraoperative subcortical mapping of a language-associated deep frontal tract connecting the superior frontal gyrus to Broca's area in the dominant hemisphere of patients with glioma. J Neurosurg. 2015;122(6):1390-1396. [DOI] [PubMed] [Google Scholar]
- 26. Kinoshita M, de Champfleur NM, Deverdun J, Moritz-Gasser S, Herbet G, Duffau H. Role of fronto-striatal tract and frontal aslant tract in movement and speech: An axonal mapping study. Brain Struct Funct. 2015;220(6):3399-3412. [DOI] [PubMed] [Google Scholar]
- 27. Catani M, Mesulam MM, Jakobsen E et al. . A novel frontal pathway underlies verbal fluency in primary progressive aphasia. Brain. 2013;136(8):2619-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Basilakos A, Fillmore PT, Rorden C, Guo D, Bonilha L, Fridriksson J. Regional white matter damage predicts speech fluency in chronic post-stroke aphasia. Front Hum Neurosci. 2014;8:845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lo YC, Chen YJ, Hsu YC, Tseng WI, Gau SS. Reduced tract integrity of the model for social communication is a neural substrate of social communication deficits in autism spectrum disorder. J Child Psychol Psychiatr. 2017;58(5):576-585. [DOI] [PubMed] [Google Scholar]