ABSTRACT
The inferior fronto-occipital fasciculus (IFOF) is a large white matter tract of the human cerebrum with functional connectivity associated with semantic language processing and goal-oriented behavior. However, little is known regarding the overall connectivity of this tract. Recently, the Human Connectome Project parcellated the human cortex into 180 distinct regions. In our other work, we have shown these various regions in relation to clinically applicable anatomy and function. Utilizing Diffusion Spectrum Magnetic Resonance Imaging tractography coupled with the human cortex parcellation data presented earlier in this supplement, we aim to describe the macro-connectome of the IFOF in relation to the linked parcellations present within the human cortex. The purpose of this study is to present this information in an indexed, illustrated, and tractographically aided series of figures and tables for anatomic and clinical reference.
Keywords: Anatomy, Cerebrum, Connectivity, diffusion tensor imaging, Functional connectivity, Human, Parcellations
ABBREVIATIONS
- DSI
diffusion spectrum imaging
- IFOF
inferior fronto-occipital fasciculus
- MR
magnetic resonance
The inferior fronto-occipital fasciculus (IFOF) is a large white matter tract which originates in the occipital and parietal lobes and terminates in the inferior frontal lobe.1-3 This white matter tract courses, along with the uncinate fasciculus, adjacent to the infero-lateral insula via the extreme and external capsules.4 While its role is primarily associated with semantic language processing and transmission,3,5-8 other studies have demonstrated that the IFOF connects the salience network to the executive control network, thus potentially playing an important role in goal-oriented behavior.9
Although multiple studies have described the structural anatomy of the IFOF,7,10 none have described the specific cortical connectivity associated with this white matter pathway. Rece-ntly, the Human Connectome Project published parcellation data redefining the human cortex.11 This provides a unique opportunity to elucidate the macro-connectome of the human cerebrum, in that high-resolution diffusion tensor imaging tractography has been shown to accurately illustrate the anatomy and structure of various white matter tracts in the human brain.12-14
In this anatomic study, we utilized high resolution diffusion spectrum imaging (DSI) tractography in conjunction with the Glasser parcellation scheme to illustrate the macro-connectivity between various associated funct-ionally and anatomically connected areas of the cerebrum within the confines of the IFOF. The purpose of this study is to present the structural connectivity of the IFOF in an indexed, illustrated, and tractographically aided series of figures and tables for anatomic and clinical reference.
METHODS
Identification of Relevant Cortical Regions
The parcellation data entries within the first 9 chapters of this supplement were reviewed to determine the specific cortical regions with structural connectivity in the distribution of the middle longitudinal fasciculus. These data were tabulated, and connections between individual parcellations within the MdLF were recorded. These results served as the basis for constructing a simplified tractography map of the MdLF and performing deterministic tractography.
Deterministic Tractography
Publicly available imaging data from the Human Connectome Project was obtained for this study from the HCP database (http://humanconnectome.org, release Q3). Diffusion imaging with corresponding T1-weighted images from 10 healthy, unrelated controls were analyzed (Subjects IDs: 100307, 103414, 105115, 110411, 111312, 113619, 115320, 117112, 118730, 118932). A multi-shell diffusion scheme was used, and the b-values were 990, 1985, and 1980 s/mm2. Each b-value was sampled in 90 directions. The in-plane resolution was 1.25 mm. The diffusion data was reconstructed using generalized q-sampling imaging with a diffusion sampling length ratio of 1.25.15
We performed brain registration to MNI space, wherein imaging is warped to fit a standardized brain model comparison between subjects. Tractography was performed in DSI studio using a region of interest approach to initiate fiber tracking from a user-defined seed region. A 2-ROI-approach was used to isolate tracts. Voxels within each ROI were automatically traced with a maximum angular threshold of 45º.
When a voxel was approached with no tract direction or a direction change of greater than 45º, the tract was halted. Tractography was stopped after reaching a maximum length of 800 mm. In some instances, exclusion ROIs were placed to exclude obvious spurious tracts that were not involved in the white matter pathway of interest. Tractographic results are shown only for regions of interest within the left cerebral hemisphere.
CONNECTIVITY OVERVIEW
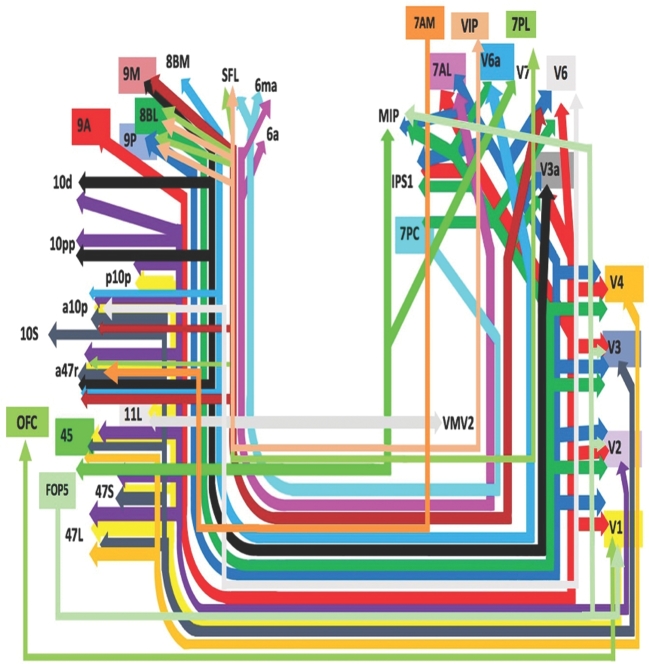
Presented in Figure 1, we demonstrate the functionally relevant and anatomically connected cerebral parcellation data that integrates within the confines of the IFOF. Pertinent examples of tractographically connected parcellations are shown in Figures 2-5. It should be noted that the figures and tables presented in this study do not imply directionality. Instead, supposed information transit is utilized as a simplified means for connectivity description and reference. Table summarizes the macro-connected parcellated areas of the human cerebrum that integrate to form the IFOF. No attempt has been made to subdivide the IFOF into smaller white matter bundles as our methods preclude accurate subdivision of the various white matter tracts of the brain. In general, the IFOF connects early visual processing parcellations in the cuneus and lingual gyrus as well as parts of the parietal lobe to frontal lobe regions.
FIGURE 1.
Simplified tract map showing the structural connections that integrate within the IFOF. Connections between cortical areas are color-coded based on the parcellation of origin (eg, black arrows indicate structural connections from origin V3a to areas 9m, 10d, 10pp, and a47r). Note that arrows are not meant to imply the direction of information transmit.
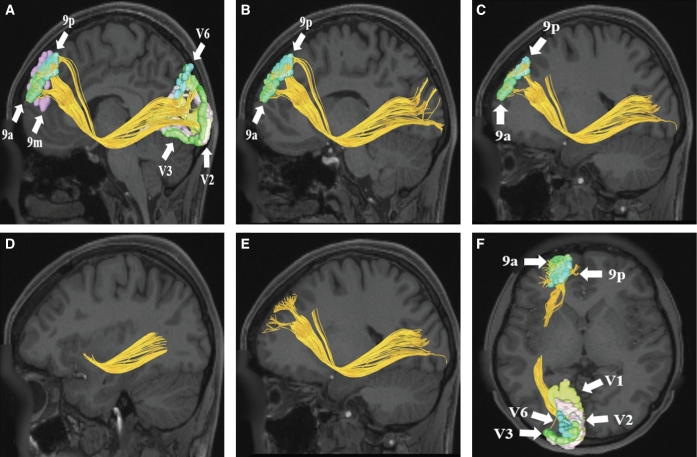
FIGURE 2.
IFOF connections from areas 9p, 9a, and 9m in the left cerebral hemisphere. Area 9 regions have connections to early visual processing areas including V1, V2, and V3 in this subject brain. Area 9p demonstrates structural connections to area V6 as well. Connections are shown on T1-weighted magnetic resonance (MR) images in the A-E, sagittal and F, axial planes. All parcellations are indicated with white arrows and corresponding labels. The IFOF courses inferiorly to pass adjacent to the insula in the extreme capsule before reaching its terminations in the occipital lobe.
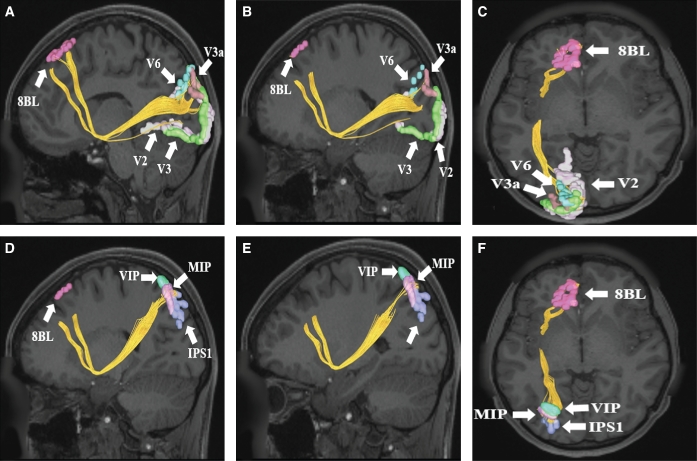
FIGURE 5.
A-C, IFOF connections from area 8BL to early visual cortex parcellations in the left cerebral hemisphere. Connections are shown on T1-weighted MR images in the A and B, sagittal and C, axial planes. Area 8BL has connections to regions V2, V3, V3a, and V6 in this subject brain. D-F, IFOF connections from area 8BL to superior parietal lobe parcellations in the left cerebral hemisphere. Connections are shown on T1-weighted MR images in D and E, sagittal and F, axial planes. Area 8BL has connections to regions VIP, MIP, and IPS1in this subject brain. All parcellations in each panel are indicated with white arrows and corresponding labels.
TABLE.
Regions Integrating within the Inferior Fronto-Occipital Fasciculus
| Original Parcellation | Terminations |
|---|---|
| 7AL | 6a |
| 6ma | |
| 7AM | a47r |
| 7PC | 6ma |
| SFL | |
| 7PL | 8BL |
| 9p | |
| a47r | |
| SFL | |
| 8BL | 7PC |
| IPS1 | |
| MIP | |
| V2 | |
| V3 | |
| V3a | |
| V4 | |
| V6 | |
| V6a | |
| 9a | 7AL |
| IPS1 | |
| V1 | |
| V2 | |
| V3 | |
| V3a | |
| V4 | |
| V6 | |
| 9m | V6 |
| 9p | 7AL |
| IPS1 | |
| MIP | |
| V1 | |
| V2 | |
| V3 | |
| V3a | |
| V4 | |
| V6 | |
| 11l | VMV2 |
| 45 | MIP |
| V7 | |
| FOP5 | MIP |
| V1 | |
| V2 | |
| V3 | |
| V1 | 11l |
| 45 | |
| 47l | |
| 47s | |
| a47r | |
| a10p | |
| p10p | |
| OFC | |
| V2 | 10d |
| 10pp | |
| 45 | |
| 47l | |
| 47s | |
| a47r | |
| a10p | |
| p10p | |
| V3 | 10s |
| 45 | |
| 47l | |
| 47s | |
| a47r | |
| a10p | |
| V3a | 9m |
| 10d | |
| 10pp | |
| a47r | |
| V4 | 45 |
| 47l | |
| V6 | a10p |
| a47r | |
| V6a | 8BM |
| a10p | |
| a47r |
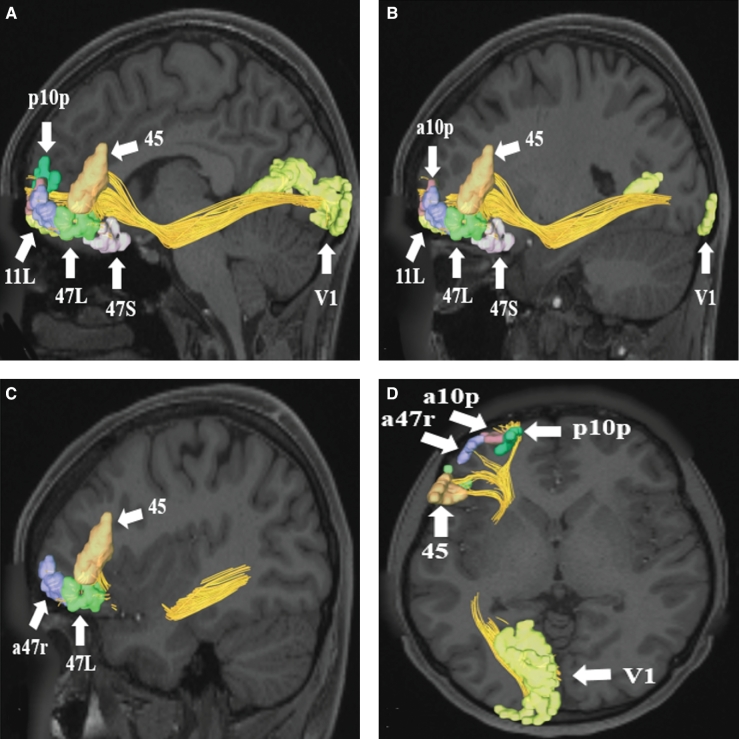
FIGURE 3.
IFOF connections from area V1 in the left cerebral hemisphere to parcellations in the frontal pole and inferior frontal gyrus. Connections are shown on T1-weighted MR images in A-C, sagittal and D, axial planes. Area V1 has connections to regions 45, 47S, 47L, a47r, 11L, a10p, and p10p in this subject brain. All parcellations are indicated with white arrows and corresponding labels. The IFOF courses anteriorly to pass adjacent to the insula in the extreme capsule before reaching its terminations in the frontal lobe.
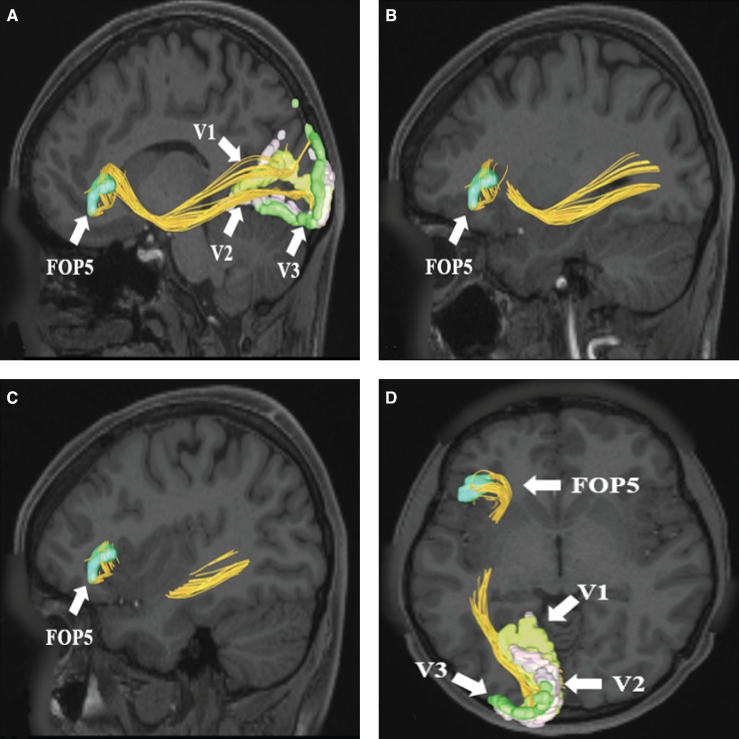
FIGURE 4.
IFOF connections from FOP5 in the left cerebral hemisphere to early visual cortex areas. Connections are shown on T1-weighted MR images in the A-C, sagittal and D, axial planes. Area FOP5 has connections to regions V1, V2, and V3 in this subject brain. All parcellations are indicated with white arrows and corresponding labels.
DISCUSSION
In this study, we provide a detailed map of the macro-connectivity of the IFOF and its relevant cerebral parcellations. It can easily be concluded from this data that actionable future studies and surgical planning methodologies may be better outlined. As supported by multiple other studies, we demonstrate that the IFOF more or less begins in the parietal and occipital lobes, traverses anteriorly lateral to the insula via the extreme and external capsule, and terminates in the inferior frontal lobe along the opercular gyri.1-3 Given this anatomic course, it can be hypothesized that the IFOF might play an important role in language processing and transmission. Many have surmised that the IFOF participates in the direct ventral pathway of language function, and that this white matter tract is focally involved in semantic speech processing.5-7,16-19
While the IFOF is thought to be a key contributor to the language network, others have described the tract's role in the visual recognition system.16,20,21 This idea likely stems from the tract's anatomic terminations in the occipital lobe, along with the theory of the dual stream model of speech and language processing.5,8,22 To summarize, the dual stream model of language function encompasses the idea that the human language network is split into a dorsal and ventral stream, with the dorsal stream being served by the superior longitudinal fasciculus/arcuate fasciculus complex (SLF/AC) and the more ventral stream being served by the IFOF.23 Within this paradigm, the ventral stream manages the semantic or “what” pathway, while the dorsal stream manages the phonemic or “where and how (motor articulation)” pathway.5,8,22 Within the ventral stream model, the IFOF’s role in semantic processing is supported by its anatomic constraints within the occipital lobe and visual system, ie, naming, object/word recognition, and the relay of this information to the more frontally located speech production centers, ie, Broca's area.5,8,22 However, given the size and inherent multi-site connectivity demonstrated by the IFOF, other functional networks are likely subserved by this white matter tract.
Interestingly, the IFOF also appears to be involved in reading and writing tasks within the human cerebrum. This is thought to correlate with the tract's association with the inferior parietal and occipital lobes.3 In addition, the tract has been demonstrated to connect the cingulo-opercular salience network to the frontoparietal executive network, serving to coordinate salient stimuli and coordinate goal-oriented behaviors.9 This role is further supported clinically via multiple studies demonstrating correlation between IFOF degeneration and Alzheimer's disease along with degeneration of the cingulate bundle.19,24 IFOF degeneration has also been demonstrated in patients with neuropsychological behavioral disorders, including antisocial personality disorder and obsessive compulsive disorder.9,22,25 Finally, progressive supranuclear palsy has also been shown to correlate with IFOF degeneration.1
Given the IFOF’s multiple, connectivity-related links with various networks, some have proposed subdividing the IFOF into distinct subunits: superficial, middle, and deep.26 This schema was primarily devised to account for reported functional connectivity differences related to the IFOF and emotional and behavioral elements.26 However, we would argue that this adds credence to the idea that the IFOF serves diverse roles within multiple functional networks within the human brain. While precise subdivision of the IFOF is beyond the scope of the tractographic description of the tract provided in this study, future endeavors will likely redefine the IFOF in the context of the data presented here.
CONCLUSION
The IFOF is a large and complex white matter tract connecting the parietal and occipital lobes to the frontal lobe via its complex route lateral to the insula. It plays a critical role in semantic language processing, goal-oriented behavior, and visual switching tasks. Given the breadth of connectivity inherent to the tract, it can be easily assumed that the IFOF is involved in additional functional networks within the human cerebrum. Further microconnectivity studies, parcellation-related studies, and network analyses are necessary to fully comprehend the IFOF’s role in human function and cognition.
Disclosures
Synaptive Medical assisted in the funding of all 18 chapters of this supplement. No other funding sources were utilized in the production or submission of this work.
Acknowledgments
Data were provided [in part] by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University.We would also like to thank Brad Fernald, Haley Harris, and Alicia McNeely of Synaptive Medical for their assistance in constructing the network figures for Chapter 18 and for coordinating the completion and submission of this supplement.
REFERENCES
- 1. Kvickström P, Eriksson B, van Westen D, Lätt J, Elfgren C, Nilsson C. Selective frontal neurodegeneration of the inferior fronto-occipital fasciculus in progressive supranuclear palsy (PSP) demonstrated by diffusion tensor tractography. BMC Neurol. 2011;11(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Schotten MT, Dell’Acqua F, Valabregue R, Catani M. Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex. 2012;48(1):82-96. [DOI] [PubMed] [Google Scholar]
- 3. Motomura K, Fujii M, Maesawa S, Kuramitsu S, Natsume A, Wakabayashi T. Association of dorsal inferior frontooccipital fasciculus fibers in the deep parietal lobe with both reading and writing processes: a brain mapping study. J Neurosurg. 2014;121(1):142-148. [DOI] [PubMed] [Google Scholar]
- 4. Kier EL, Staib LH, Davis LM, Bronen RA. MR imaging of the temporal stem: anatomic dissection tractography of the uncinate fasciculus, inferior occipitofrontal fasciculus, and Meyer's loop of the optic radiation. Am J Neuroradiol. 2004;25(5):677-691. [PMC free article] [PubMed] [Google Scholar]
- 5. Almairac F, Herbet G, Moritz-Gasser S, de Champfleur NM, Duffau H. The left inferior fronto-occipital fasciculus subserves language semantics: a multilevel lesion study. Brain Struct Funct. 2015;220(4):1983-1995. [DOI] [PubMed] [Google Scholar]
- 6. Caverzasi E, Papinutto N, Amirbekian B, Berger MS, Henry RG. Q-ball of inferior fronto-occipital fasciculus and beyond. PLoS One. 2014;9(6):e100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martino J, Brogna C, Robles SG, Vergani F, Duffau H. Anatomic dissection of the inferior fronto-occipital fasciculus revisited in the lights of brain stimulation data. Cortex. 2010;46(5):691-699. [DOI] [PubMed] [Google Scholar]
- 8. Duffau H, Gatignol P, Mandonnet E, Capelle L, Taillandier L. Intraoperative subcortical stimulation mapping of language pathways in a consecutive series of 115 patients with Grade II glioma in the left dominant hemisphere. J Neurosurg. 2008. 109(3):461-471. [DOI] [PubMed] [Google Scholar]
- 9. Waller R, Dotterer HL, Murray L, Maxwell AM, Hyde LW. White-matter tract abnormalities and antisocial behavior: A systematic review of diffusion tensor imaging studies across development. NeuroImage. 2017;14:201-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martino J, Vergani F, Robles SG, Duffau H. New insights into the anatomic dissection of the temporal stem with special emphasis on the inferior fronto-occipital fasciculus: Implications in surgical approach to left mesiotemporal and temporoinsular structures. Neurosurgery. 2010;66(3 Suppl Operative):4-12. [DOI] [PubMed] [Google Scholar]
- 11. Glasser MF, Coalson TS, Robinson EC et al. . A multi-modal parcellation of human cerebral cortex. Nature. 2016;536(7615):171-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kamali A, Flanders AE, Brody J, Hunter JV, Hasan KM. Tracing superior longitudinal fasciculus connectivity in the human brain using high resolution diffusion tensor tractography. Brain Struct Funct. 2014;219(1):269-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Menjot de Champfleur N, Lima Maldonado I, Moritz-Gasser S et al. . Middle longitudinal fasciculus delineation within language pathways: a diffusion tensor imaging study in human. Eur J Radiol. 2013;82(1):151-157. [DOI] [PubMed] [Google Scholar]
- 14. Lemaire JJ, Cosnard G, Sakka L et al. . White matter anatomy of the human deep brain revisited with high resolution DTI fibre tracking. Neurochirurgie. 2011;57(2):52-67. [DOI] [PubMed] [Google Scholar]
- 15. Yeh FC, Wedeen VJ, Tseng WY. Generalized q-sampling imaging. IEEE Trans Med Imaging. 2010;29(9):1626-1635. [DOI] [PubMed] [Google Scholar]
- 16. Vandermosten M, Boets B, Wouters J, Ghesquiere P. A qualitative and quantitative review of diffusion tensor imaging studies in reading and dyslexia. Neurosci Biobehav Rev. 2012;36(6):1532-1552. [DOI] [PubMed] [Google Scholar]
- 17. Khan OH, Herbet G, Moritz-Gasser S, Duffau H. The role of left inferior fronto-occipital fascicle in verbal perseveration: a brain electrostimulation mapping study. Brain Topogr. 2014;27(3):403-411. [DOI] [PubMed] [Google Scholar]
- 18. Friederici AD, Gierhan SM. The language network. Curr Opin Neurobiol. 2013;23(2):250-254. [DOI] [PubMed] [Google Scholar]
- 19. Agosta F, Pievani M, Sala S et al. . White matter damage in Alzheimer disease and its relationship to gray matter atrophy. Radiology. 2011;258(3):853-863. [DOI] [PubMed] [Google Scholar]
- 20. Dick AS, Tremblay P. Beyond the arcuate fasciculus: consensus and controversy in the connectional anatomy of language. Brain. 2012;135(12):3529-3550. [DOI] [PubMed] [Google Scholar]
- 21. de Schotten MT, Bizzi A, Dell’Acqua F et al. . Atlasing location, asymmetry and inter-subject variability of white matter tracts in the human brain with MR diffusion tractography. Neuroimage. 2011;54(1):49-59. [DOI] [PubMed] [Google Scholar]
- 22. Dick AS, Bernal B, Tremblay P. The language connectome: new pathways, new concepts. Neuroscientist. 2014;20(5):453-467. [DOI] [PubMed] [Google Scholar]
- 23. Agosta F, Galantucci S, Canu E et al. . Disruption of structural connectivity along the dorsal and ventral language pathways in patients with nonfluent and semantic variant primary progressive aphasia: a DT MRI study and a literature review. Brain Lang. 2013;127(2):157-166. [DOI] [PubMed] [Google Scholar]
- 24. Gold BT, Johnson NF, Powell DK, Smith CD. White matter integrity and vulnerability to Alzheimer's disease: preliminary findings and future directions. Biochim Biophys Acta. 2012;1822(3):416-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Peng Z, Lui SS, Cheung EF et al. . Brain structural abnormalities in obsessive-compulsive disorder: converging evidence from white matter and grey matter. Asian J Psychiatry. 2012;5(4):290-296. [DOI] [PubMed] [Google Scholar]
- 26. Sarubbo S, De Benedictis A, Maldonado IL, Basso G, Duffau H. Frontal terminations for the inferior fronto-occipital fascicle: anatomical dissection, DTI study and functional considerations on a multi-component bundle. Brain Struct Funct. 2013;218(1):21-37. [DOI] [PubMed] [Google Scholar]