ABSTRACT
In this supplement, we show a comprehensive anatomic atlas of the human cerebrum demonstrating all 180 distinct regions comprising the cerebral cortex. The location, functional connectivity, and structural connectivity of these regions are outlined, and where possible a discussion is included of the functional significance of these areas. In this chapter, we specifically address regions integrating to form the cingulum.
Keywords: Anatomy, Cerebrum, Connectivity, DTI, Functional connectivity, Human, Parcellations
We end our discussion of the major white matter tracts of the brain with the cingulum, an important pathway that interconnects medial parts of the frontal, parietal, and temporal lobes.1 While often described as a continuous fiber bundle spanning from the subcallosal region to the entorhinal cortex, some have suggested that the cingulum actually consists of several white matter tracts, including parahippocampal, retrosplenial, and subgenual components.2 Its role in connecting the anterior thalamic nucleus to the entorhinal cortex was first described by Papez3 in his proposed circuit of the limbic system in 1937,3 implicating the cingulum in emotional processing. Since then, our understanding of the this white matter tract has changed, and within the last two decades, the cingulum has been identified as the major white matter tract connecting the anterior cingulate and posterior cingulate cortices of the brain's default mode network.4
While diffusion tensor imaging (DTI) and gross anatomic dissection studies have clarified the structural anatomy of the cingulum in some detail,1,5-7 little is known about its various cortical terminations. Recently, the Human Connectome Project (HCP) published parcellation data redefining the human cortex.8 This provides a unique opportunity to elucidate the macro-connectome of the human cerebrum, in that high-resolution DTI tractography has been shown to accurately illustrate the anatomy of different white matter tracts in the brain.9-11
In this study, we delineate the boundaries of the cingulum utilizing the parcellation scheme developed under the HCP.8 Through diffusion spectrum Imaging (DSI), we show the relationship between these parcellations and the cingulum. We also provide a simplified tract map summarizing those regions with white matter connections specific to the cingulum. The purpose of this study is to present the structural connectivity of the cingulum in an indexed, illustrated, and tractographically aided series of figures and tables for anatomic and clinical reference.
METHODS
Identification of Relevant Cortical Regions
The parcellation data entries within the first 9 chapters of this supplement were reviewed to determine the specific cortical regions with structural connectivity in the distribution of the cingulum. These data were tabulated, and connections between individual parcellations within the cingulum were recorded. These results served as the basis for constructing a simplified tractography map of the cingulum and performing deterministic tractography.
Deterministic Tractography
Publicly available imaging data from the HCP was obtained for this study from the HCP database (http://humanconnectome.org, release Q3). Diffusion imaging with corresponding T1-weighted images from 10 healthy, unrelated controls were analyzed (Subjects IDs: 100307, 103414, 105115, 110411, 111312, 113619, 115320, 117112, 118730, and 118932). A multishell diffusion scheme was used, and the b-values were 990, 1985, and 1980 s/mm2. Each b-value was sampled in 90 directions. The in-plane resolution was 1.25 mm. The diffusion data were reconstructed using generalized q-sampling imaging with a diffusion sampling length ratio of 1.25.12
We performed brain registration to MNI space, wherein imaging is warped to fit a standardized brain model comparison between subjects. Tractography was performed in DSI studio using a region of interest approach to initiate fiber tracking from a user-defined seed region. A two-ROI-approach was used to isolate tracts. Voxels within each ROI were automatically traced with a maximum angular threshold of 45°. When a voxel was approached with no tract direction or a direction change of greater than 45°, the tract was halted. Tractography was stopped after reaching a maximum length of 800 mm. In some instances, exclusion ROIs were placed to exclude obvious spurious tracts that were not involved in the white matter pathway of interest. Tractographic results are shown only for regions of interest within the left cerebral hemisphere.
CONNECTIVITY OVERVIEW
Presented in Figure 1, we demonstrate the functionally relevant and anatomically connected cerebral parcellation data that integrates within the confines of the cingulum.
FIGURE 1.
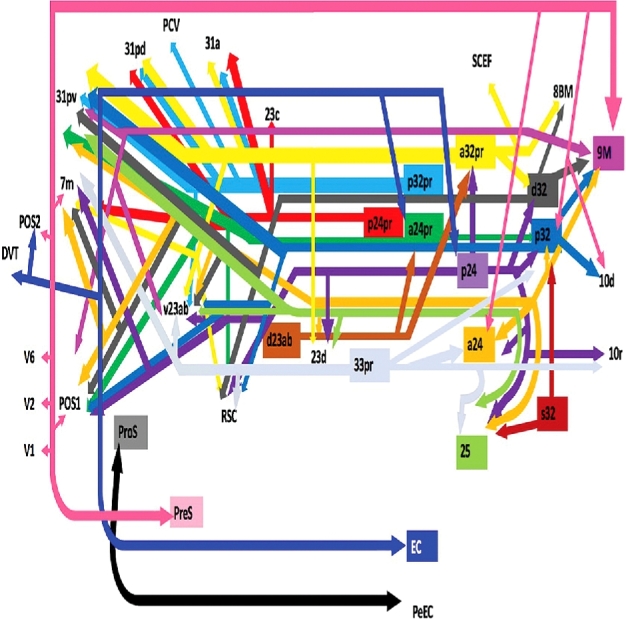
Simplified tract map showing the structural connections that integrate within the cingulum. Connections between cortical areas are color-coded based on the parcellation of origin (eg, turquoise arrows indicate structural connections from origin p32 to areas 9M, 10d, 31pv, POS1, RSC, and v23ab). Note that arrows are not meant to imply the direction of information transmit.
Pertinent examples of tractographically connected parcellations are shown in Figures 2–5. It should be noted that the figures and tables presented in this study do not imply directionality. Instead, supposed information transit is utilized as a simplified means for connectivity description and reference. Table summarizes the macro-connected parcellated areas of the human cerebrum that integrate to form the cingulum. No attempt has been made to subdivide the cingulum into smaller white matter bundles as has been described by others,2 as our methods preclude accurate subdivision of the various white matter tracts of the brain. In general, the cingulum can be seen to connect parcellations of the medial temporal lobe to those of the precuneus, cingulate gyrus, and medial frontal lobe.
FIGURE 2.
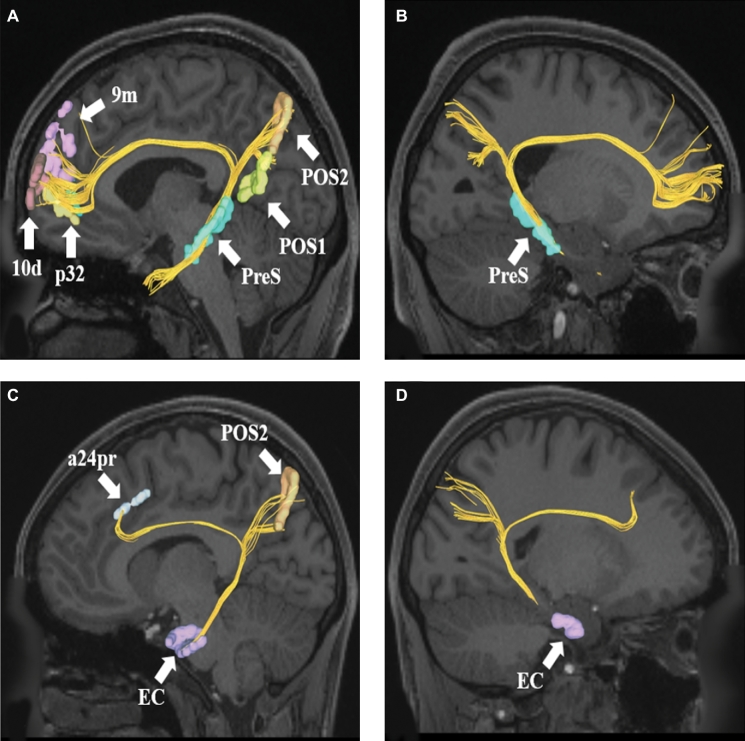
A and B, Cingulate connections from area PreS in the left cerebral hemisphere. Connections are shown on T1-weighted MR images in the sagittal plane. Area PreS has connections to POS1, POS2, 9m, p32, and 10d in this subject brain. PanelBis the same image rotated 180° to demonstrate the precise location of PreS in the medial temporal lobe.CandD, Cingulate connections from area EC in the left cerebral hemisphere. Connections are shown on T1-weighted MR images in the sagittal plane. Area EC has connections to POS2 and a24pr in this subject brain. PanelDis the same image rotated 180° to demonstrate the precise location of EC in the medial temporal lobe.
FIGURE 5.
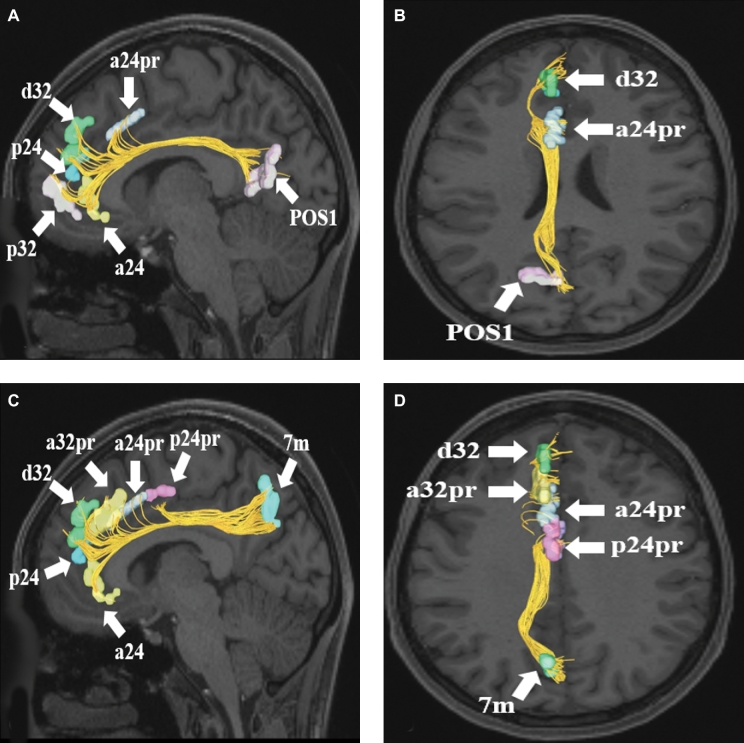
A and B, Cingulate connections from area POS1 in the left cerebral hemisphere. Connections are shown on T1-weighted MR images in theA, sagittal andB, axial planes. Area POS1 has connections to a24, p32, p24, d32, and a24pr in this subject brain.CandD, Cingulate connections from area 7m in the left cerebral hemisphere. Connections are shown on T1-weighted MR images in theC, sagittal andD, axial planes. Area 7m has connections to a24, p24, d32, a32pr, a24pr, and p24pr in this subject brain. All parcellations are identified with white arrows and corresponding labels.
TABLE.
Regions Integrating Within the Cingulum.
| Original parcellation | Terminations |
|---|---|
| 9m | 31pv |
| POS1 | |
| v23ab | |
| 25 | 23d |
| 31pv | |
| v23ab | |
| a24 | 7M |
| 9m | |
| 23d | |
| 25 | |
| 31pv | |
| p32 | |
| POS1 | |
| v23ab | |
| a24pr | 31pv |
| p32 | |
| POS1 | |
| RSC | |
| a32pr | 7m |
| 8BM | |
| 23d | |
| 31a | |
| 31pd | |
| 31pv | |
| d32 | |
| RSC | |
| SCEF | |
| v23ab | |
| d32 | 7m |
| 8BM | |
| 9m | |
| 31pv | |
| POS1 | |
| RSC | |
| v23ab | |
| p24 | 7m |
| 10r | |
| 23d | |
| 25 | |
| a24 | |
| a32pr | |
| d32 | |
| p32 | |
| POS1 | |
| RSC | |
| v23ab | |
| p24pr | 7m |
| 23c | |
| 31a | |
| 31pd | |
| p32 | 9m |
| 10d | |
| 31pv | |
| POS1 | |
| RSC | |
| v23ab | |
| p32pr | 31a |
| 31pd | |
| 31pv | |
| PCV | |
| v23ab | |
| 33pr | 7m |
| 10r | |
| 25 | |
| a24 | |
| p32 | |
| RSC | |
| v23ab | |
| d23ab | a24pr |
| a32pr | |
| EC | a24pr |
| DVT | |
| p24 | |
| POS2 | |
| PreS | 7m |
| 9m | |
| 10d | |
| a24 | |
| p32 | |
| POS1 | |
| POS2 | |
| V1 | |
| V2 | |
| V6 | |
| ProS | PEEC |
| s32 | 25 |
| p32 |
FIGURE 3.
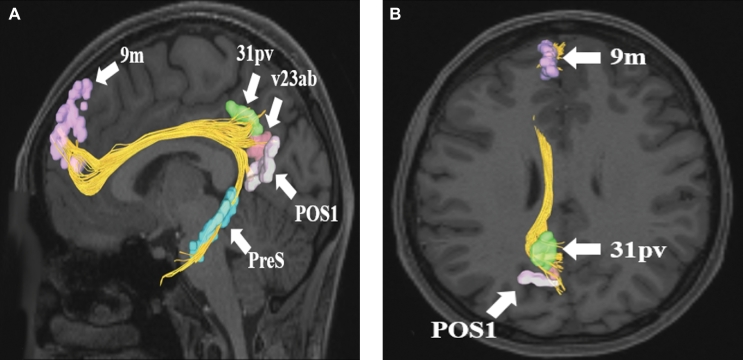
Cingulate connections from area 9m in the left cerebral hemisphere. Connections are shown on T1-weighted MR images in the A, sagittal andB, axial planes. Area 9m has connections to 31pv, v23ab, POS1, and PreS in this subject brain. All parcellations are identified with white arrows and corresponding labels.
FIGURE 4.
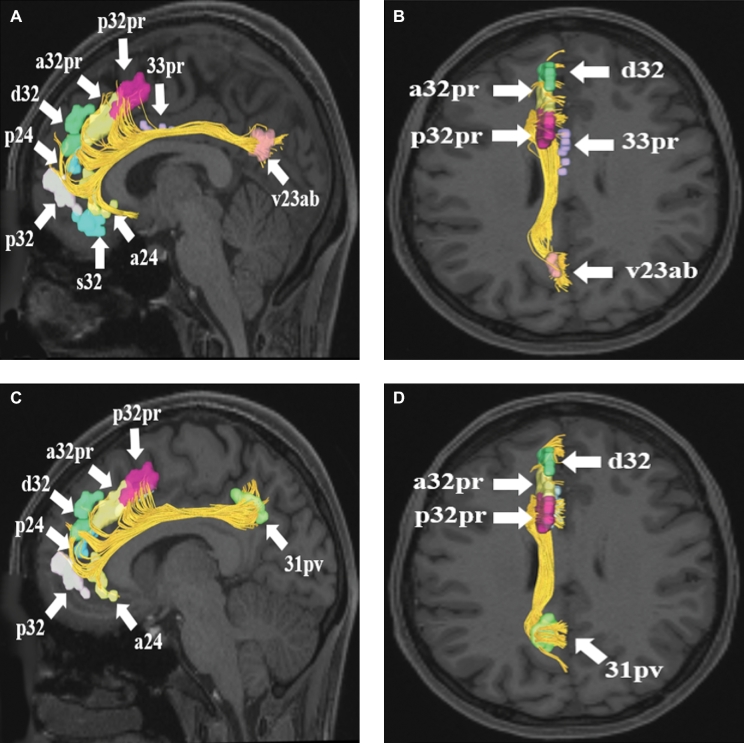
A and B, Cingulate connections from area v23ab in the left cerebral hemisphere. Connections are shown on T1-weighted MR images in theA, sagittal andB, axial planes. Area v23ab has connections to a24, s32, p32, p24, d32, a32pr, p32pr, and 33pr in this subject brain.CandD, Cingulate connections from area 31pv in the left cerebral hemisphere. Connections are shown on T1-weighted MR images in theC, sagittal andD, axial planes. Area 31pv has connections to a24, p32, p24, d32, a32pr, and p32pr in this subject brain. All parcellations are identified with white arrows and corresponding labels.
DISCUSSION
In this study, we describe a detailed map of the macro-connectivity of the cingulum and its relevant cerebral parcellations. Anatomic models of the cingulum generally begin in the subcallosal cortex inferior to the rostrum of the corpus callosum from which the cingulum bends superocaudally around the genu to continue its course superior to the body and splenium of the corpus callosum within the deep white matter of the cingulate gyrus.1 At the level of the splenium, the cingulum bends again, passing inferocranially into the medial parahippocampal gyrus and uncus where it terminates within the temporal lobe.1
The cingulum has long been thought of as a part of the limbic system, mediating important aspects of behavior, including emotional processing and memory.3,13 As described by Papez,3 the multimodal sensory processing that occurs in the hippocampus is eventually relayed to the mammillary bodies via the fornix before entering the anterior thalamic nucleus via the mammillothalamic tract.13 The cingulum bundle serves as the major point of information outflow at this point, transferring emotionally relevant information from the thalamus to the cingulate cortex and eventually to the enterorhinal cortex from which it is retransmitted to the hippocampus. While modifications to the circuit have been proposed over time,14 the cingulum remains an important part of the limbic system.
Structural abnormalities within the cingulum have also been identified in several clinical disease states. For example, white matter damage to the cingulum (as well as the uncinate and fornix) has been demonstrated in patients with Alzheimer's disease,15 including reductions in fractional anisotropy in mild cognitive impairment states that are further reduced in Alzheimer's.16 These abnormalities are often correlated with adjacent gray matter atrophy and worsening cognitive ability.15 Reductions in cingulum and corpus callosum fractional anisotropy have also been reported in patients with bipolar disorder,17 while another study reported changes in the microstructure of the cingulum and uncinate in patients with negative emotionality traits, traits that are more commonly associated with mood and anxiety disorders.18 Both of these studies suggest that the cingulum subserves an important functional role in emotional regulation. Altered structural integrity to the cingulum has also been identified in patients with schizophrenia, although numerous long-range white matter bundles can be affected in this disease process.19 Finally, decreased fractional anisotropy and increased mean diffusivity of the cingulum and corpus callosum have been reported in patients with autism spectrum disorder.20
Discovery of a default resting state in 2001 demonstrated for the first time a base-line functional brain network in individuals who were neither sleeping nor performing tasks during functional magnetic resonance imaging testing.21,22 The nodes of this network, called the default mode or DMN, have been localized to the anterior cingulate and medial frontal cortex, the posterior cingulate cortex and precuneus, and the lateral parietal lobe.23 Study of the structural connections between these functionally correlated nodes identified the cingulum as the primary white matter pathway connecting the anterior and posterior cingulate cortices.4 Increased average fractional anisotropy of the cingulum has also been shown to correlate positively with the level of functional connectivity between regions of the DMN.4 This suggests that the structural integrity of the cingulum is important for proper DMN functional connectivity, and that the functional significance of the cingulum extends beyond simple emotional regulation. For example, there is some evidence to suggest that the DMN is involved during mental exercises in which individuals consider memories and future planning to construct and manipulate hypothetical scenarios.24 Additional studies are necessary to further characterize the role of the cingulum in DMN-related activity.
CONCLUSION
The cingulum is an important white matter tract coursing around the corpus callosum as it connects medial aspects of the frontal, parietal, and temporal lobes. It has long been described as part of the limbic system, a series of structures important in memory, emotion, and behavioral regulation. The cingulum has also been identified as part of the default mode network, suggesting its role in cerebral connectomics is more complex than mere emotional processing. Further, subtract guided functional and anatomic studies are needed to enhance our understanding of the functional connectivity of the cingulum. However, our tractographic map of this white matter pathway can serve as a reference point for these future studies.
Disclosures
Synaptive Medical assisted in the funding of all 18 chapters of this supplement. No other funding sources were utilized in the production or submission of this work.
Acknowledgments
Data were provided [in part] by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research and by the McDonnell Center for Systems Neuroscience at Washington University. We would also like to thank Brad Fernald, Haley Harris, and Alicia McNeely of Synaptive Medical for their assistance in constructing the network figures for Chapter 18 and for coordinating the completion and submission of this supplement.
REFERENCES
- 1. Jellison BJ, Field AS, Medow J, Lazar M, Salamat MS, Alexander AL. Diffusion tensor imaging of cerebral white matter: A pictorial review of physics, fiber tract anatomy, and tumor imaging patterns. AJNR Am J Neuroradiol. 2004;25(3):356-369. [PMC free article] [PubMed] [Google Scholar]
- 2. Jones DK, Christiansen KF, Chapman RJ, Aggleton JP. Distinct subdivisions of the cingulum bundle revealed by diffusion MRI fibre tracking: Implications for neuropsychological investigations. Neuropsychologia. 2013;51(1):67-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Papez JW. A proposed mechanism of emotion. 1937. J Neuropsychiatry Clin Neurosci. 1995;7(1):103-112. [DOI] [PubMed] [Google Scholar]
- 4. van den Heuvel M, Mandl R, Luigjes J, Hulshoff Pol H. Microstructural organization of the cingulum tract and the level of default mode functional connectivity. J Neurosci. 2008;28(43):10844-10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burks JD, Bonney PA, Conner AK et al. . A method for safely resecting anterior butterfly gliomas: The surgical anatomy of the default mode network and the relevance of its preservation. J Neurosurg. 2017;126(6):1795-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Makris N, Pandya DN, Normandin JJ et al. . Quantitative DT-MRI investigations of the human cingulum bundle. CNS Spectr. 2002;7(07):522-528. [Google Scholar]
- 7. Shah A, Jhawar SS, Goel A. Analysis of the anatomy of the Papez circuit and adjoining limbic system by fiber dissection techniques. J Clin Neurosci. 2012;19(2):289-298. [DOI] [PubMed] [Google Scholar]
- 8. Glasser MF, Coalson TS, Robinson EC et al. . A multi-modal parcellation of human cerebral cortex. Nature. 2016;536(7615):171-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kamali A, Flanders AE, Brody J, Hunter JV, Hasan KM. Tracing superior longitudinal fasciculus connectivity in the human brain using high resolution diffusion tensor tractography. Brain Struct Funct. 2014;219(1):269-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Menjot de Champfleur N, Lima Maldonado I, Moritz-Gasser S et al. . Middle longitudinal fasciculus delineation within language pathways: A diffusion tensor imaging study in human. Eur J Radiol. 2013;82(1):151-157. [DOI] [PubMed] [Google Scholar]
- 11. Lemaire JJ, Cosnard G, Sakka L et al. . White matter anatomy of the human deep brain revisited with high resolution DTI fibre tracking. Neurochirurgie. 2011;57(2):52-67. [DOI] [PubMed] [Google Scholar]
- 12. Yeh FC, Wedeen VJ, Tseng WY. Generalized q-sampling imaging. IEEE Trans Med Imaging. 2010;29(9):1626-1635. [DOI] [PubMed] [Google Scholar]
- 13. Mega MS, Cummings JL, Salloway S, Malloy P. The limbic system: An anatomic, phylogenetic, and clinical perspective. J Neuropsychiatry Clin Neurosci. 1997;9(3):315-330. [DOI] [PubMed] [Google Scholar]
- 14. Catani M, Dell’acqua F, Thiebaut de Schotten M. A revised limbic system model for memory, emotion and behaviour. Neurosci Biobehav Rev. 2013;37(8):1724-1737. [DOI] [PubMed] [Google Scholar]
- 15. Bozzali M, Serra L, Cercignani M. Quantitative MRI to understand Alzheimer's disease pathophysiology. Curr Opin Neurol. 2016;29(4):437-444. [DOI] [PubMed] [Google Scholar]
- 16. Zhang Y, Schuff N, Jahng G-H et al. . Diffusion tensor imaging of cingulum fibers in mild cognitive impairment and Alzheimer disease. Neurology. 2007;68(1):13-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duarte JA, de Araujo ESJQ, Goldani AA, Massuda R, Gama CS. Neurobiological underpinnings of bipolar disorder focusing on findings of diffusion tensor imaging: A systematic review. Rev Bras Psiquiatr. 2016;38(2):167-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mincic AM. Neuroanatomical correlates of negative emotionality-related traits: A systematic review and meta-analysis. Neuropsychologia. 2015;77:97-118. [DOI] [PubMed] [Google Scholar]
- 19. Wheeler AL, Voineskos AN. A review of structural neuroimaging in schizophrenia: From connectivity to connectomics. Front Hum Neurosci. 2014;8:653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Travers BG, Adluru N, Ennis C et al. . Diffusion tensor imaging in autism spectrum disorder: A review. Autism Res. 2012;5(5):289-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98(2):676-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Raichle ME. The brain's default mode network. Annu Rev Neurosci. 2015;38(1):433-447. [DOI] [PubMed] [Google Scholar]
- 23. Harrison BJ, Pujol J, López-Solà M et al. . Consistency and functional specialization in the default mode brain network. Proc Natl Acad Sci USA. 2008;105(28):9781-9786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Poerio GL, Sormaz M, Wang HT, Margulies D, Jefferies E, Smallwood J. The role of the default mode network in component processes underlying the wandering mind. Soc Cogn Affect Neurosci. 2017;12(7):1047-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]