ABSTRACT
In this supplement, we show a comprehensive anatomic atlas of the human cerebrum demonstrating all 180 distinct regions comprising the cerebral cortex. The location, functional connectivity, and structural connectivity of these regions are outlined, and where possible a discussion is included of the functional significance of these areas. In this chapter, we specifically address the regions integrating to form the uncinate fasciculus.
Keywords: Anatomy, Cerebrum, Connectivity, DTI, Functional connectivity, Human, Parcellations
ABBREVIATIONS
- DSI
diffusion spectrum imaging
- DTI
diffusion tensor imaging
- HCP
Human Connectome Project
- ROI
region of interest
- UF
uncinate fasciculus
The uncinate fasciculus (UF) is a “J”-shaped white matter tract that hooks around the Sylvian fissure as it courses from the anterior temporal pole and amygdala to the lateral aspect of the orbitofrontal gyri and inferior frontal lobe.1-3 This tract is thought to be involved in a variety of critical sociopsychological, limbic, and cognitive functions including decision making,4,5 emotional regulation and understanding,1,6 semantic memory retrieval,3,7,8 and language processing.9-12 Damage and structural abnormalities to the UF have also been linked to a number of neuropsychiatric disorders, including Alzheimer's disease,13,14 social anxiety disorder,15 post-traumatic stress disorder following traumatic brain injury,16 obsessive compulsive disorder,17 bipolar disorder,18 depression,19,20 schizophrenia,21-24 and antisocial behavior.25,26 Given its role in multiple clinical disease states and language processing, a better understanding of the cortical connections integrated within the UF is important.
While diffusion tensor imaging (DTI) and gross anatomic dissection studies have clarified the structural anatomy of the UF in some detail,27 little is known about its various cortical terminations. Recently, the Human Connectome Project (HCP) published parcellation data redefining the human cortex.28 This provides a unique opportunity to elucidate the macro-connectome of the human cerebrum, in that high-resolution DTI tractography has been shown to accurately illustrate the anatomy of different white matter tracts in the brain.29-31
In this study, we delineate the boundaries of the UF utilizing the parcellation scheme developed under the HCP.28 Through diffusion spectrum imaging (DSI), we show the relationship between these parcellations and the UF. We also provide a simplified tract map summarizing those regions with white matter connections specific to the UF. The purpose of this study is to present the structural connectivity of the UF in an indexed, illustrated, and tractographically aided series of figures and tables for anatomic and clinical reference.
METHODS
Identification of Relevant Cortical Regions
The parcellation data entries within the first 9 chapters of this supplement were reviewed to determine the specific cortical regions with structural connectivity in the distribution of the UF. These data were tabulated, and connections between individual parcellations within the UF were recorded. These results served as the basis for constructing a simplified tractography map of the UF and performing deterministic tractography.
Deterministic Tractography
Publicly available imaging data from the HCP was obtained for this study from the HCP database (http://humanconnectome.org, release Q3). Diffusion imaging with corresponding T1-weighted images from 10 healthy, unrelated controls were analyzed (Subjects IDs: 100307, 103414, 105115, 110411, 111312, 113619, 115320, 117112, 118730, 118932). A multishell diffusion scheme was used, and the b-values were 990, 1985, and 1980 s/mm2. Each b-value was sampled in 90 directions. The in-plane resolution was 1.25 mm. The diffusion data was reconstructed using generalized q-sampling imaging with a diffusion sampling length ratio of 1.25.32
We performed brain registration to MNI space, wherein imaging is warped to fit a standardized brain model comparison between subjects. Tractography was performed in DSI studio using a region of interest approach to initiate fiber tracking from a user-defined seed region. A 2-ROI-approach was used to isolate tracts. Voxels within each region of interest (ROI) were automatically traced with a maximum angular threshold of 45º. When a voxel was approached with no tract direction or a direction change of greater than 45º, the tract was halted. Tractography was stopped after reaching a maximum length of 800 mm. In some instances, exclusion ROIs were placed to exclude obvious spurious tracts that were not involved in the white matter pathway of interest. Tractographic results are shown only for regions of interest within the left cerebral hemisphere.
CONNECTIVITY OVERVIEW
Two temporal pole parcellations have connections that contribute to the UF: STGa and TGd. Table summarizes the relevant cortical regions that integrate to form the UF. Both parcellations show connections to frontal polar areas 47L and 47S. Area TGd also has connections to the inferior frontal gyrus (areas 44 and 45), the frontal operculum (areas FOP4 and FOP5), and the orbitofrontal cortex (areas OFC and pOFC).
TABLE.
Regions Integrating within the UF
| Original Parcellation | Terminations |
|---|---|
| TGd | 44 |
| 45 | |
| 47l | |
| 47s | |
| FOP4 | |
| FOP5 | |
| OFC | |
| pOFC | |
| STGa | 47l |
Figure 1 illustrates a simplified tract map of the relevant structural connectivity of the cerebral parcellation data contributing to the UF. In addition, Figures 2-5 illustrate key DSI-based fiber tracking examples chosen for the strength and breadth of their linked parcellation data. In short, the UF arises in the anterior temporal lobe, gradually curves 180º as it courses medial to the insula in the extreme and external capsules to terminate in the orbitofrontal gyri, frontal pole, and inferior frontal gyrus. It should be noted that the figures and tables presented in this study do not imply directionality. Instead, supposed information transit is utilized as a simplified means for connectivity description.
FIGURE 1.
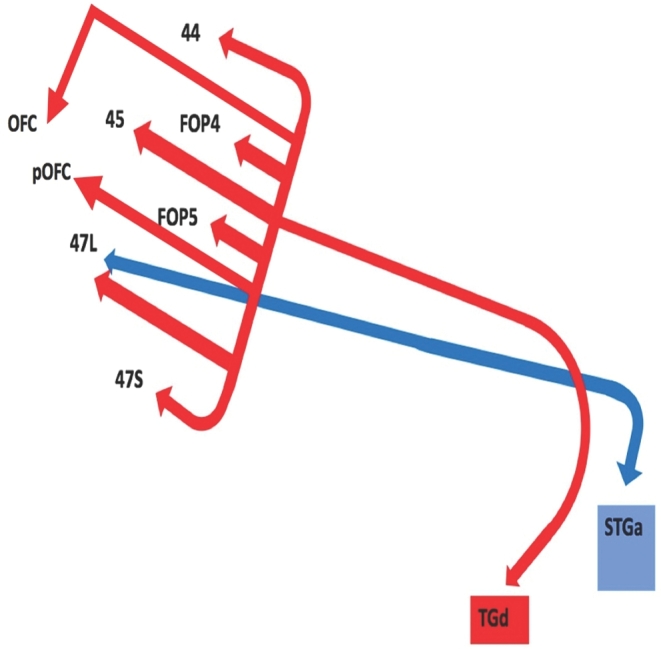
Simplified tract map showing the structural connections that integrate within the UF. Connections between cortical areas are color-coded based on the parcellation of origin (eg, red arrows indicate structural connections from origin TGd to areas 44, FOP4, 45, FOP5, OFC, pOFC, 47L, and 47S). Note that arrows are not meant to imply the direction of information transmit.
FIGURE 2.
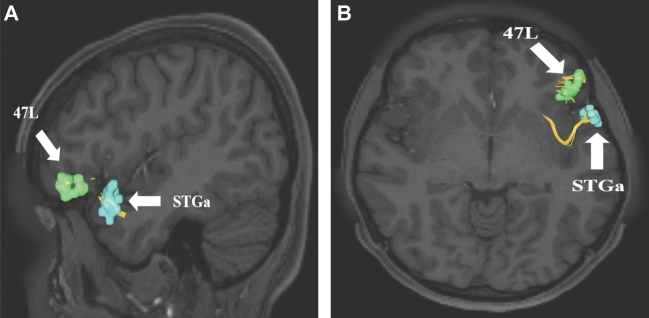
Uncinate connections from temporal polar region STGa. Area STGa only has 1 structural connection integrated within the uncinate to area 47L. This connection is shown in the left cerebral hemisphere on T1-weighted MR images in the A, sagittal and B, axial planes. All parcellations are identified with white arrows and corresponding labels.
FIGURE 5.
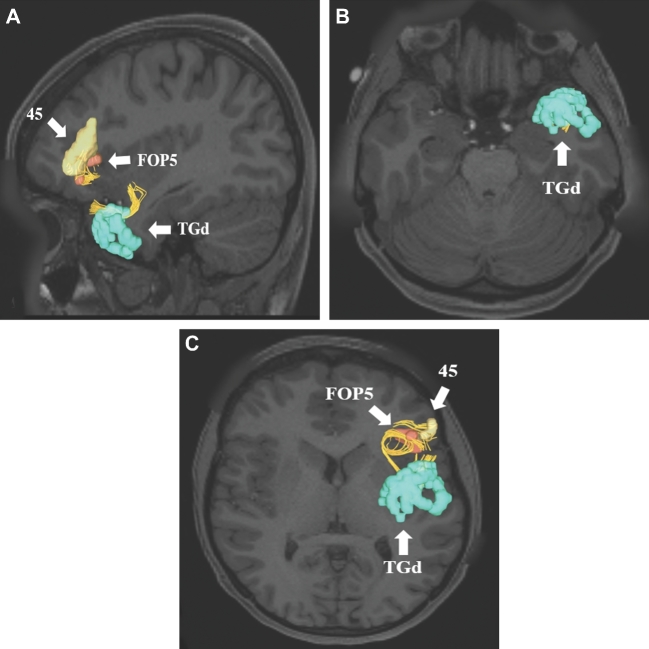
Uncinate connections from temporal polar region TGd. Area TGd has multiple structural connections integrated within the uncinate to areas 44, FOP4, 45, FOP5, OFC, pOFC, 47L, and 47S. A subset of these connections to regions 45 and FOP5 are shown in the left cerebral hemisphere on T1-weighted MR images in the A, sagittal and B and C, axial planes. All parcellations are identified with white arrows and corresponding labels.
FIGURE 3.
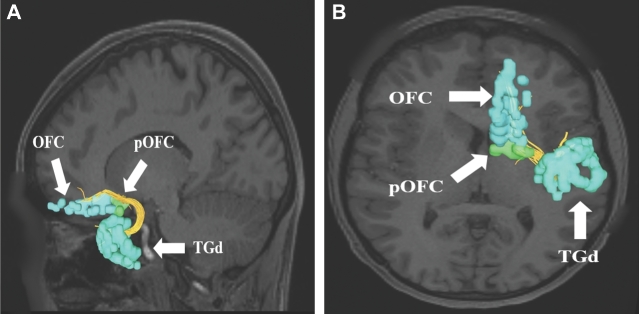
Uncinate connections from temporal polar region TGd. Area TGd has multiple structural connections integrated within the uncinate to areas 44, FOP4, 45, FOP5, OFC, pOFC, 47L, and 47S. A subset of these connections to regions OFC and pOFC are shown in the left cerebral hemisphere on T1-weighted MR images in the A, sagittal and B, axial planes. All parcellations are identified with white arrows and corresponding labels.
FIGURE 4.
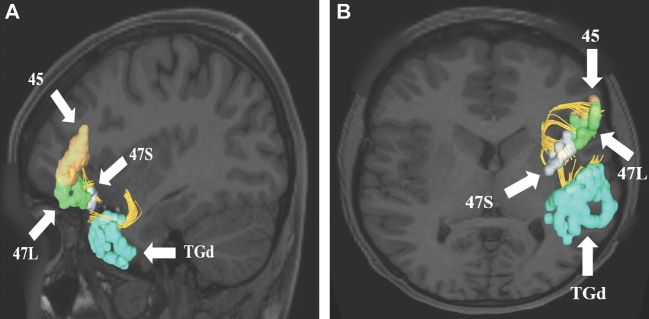
Uncinate connections from temporal polar region TGd. Area TGd has multiple structural connections integrated within the uncinate to areas 44, FOP4, 45, FOP5, OFC, pOFC, 47L, and 47S. A subset of these connections to regions 45, 47S, and 47L are shown in the left cerebral hemisphere on T1-weighted MR images in the A, sagittal and B, axial planes. All parcellations are identified with white arrows and corresponding labels.
DISCUSSION
In this study, we describe a detailed map of the macro-connectivity of the UF and its relevant cerebral parcellations. It can be surmised from this data that actionable future studies and surgical planning may be better outlined. Models of the anatomy of the UF generally describe a fiber bundle coursing from the anterior temporal lobes to the lateral frontal lobe and inferior orbitofrontal cortex via the extreme and external capsules medial to the insula.1-3 Our findings are consistent with these descriptions of the anatomy, as parcellations TGd and STGa are located in the anterior temporal pole and connect to various parts of the inferior frontal lobe and orbitofrontal gyri via a short, hook-shaped fiber bundle running medial to the insula.
As with most of the long-range white matter tracts in the brain, the uncinate has been studied in the context of language processing.3,33 Some have proposed that the UF is the major white matter pathway connecting the dorsal and ventral language streams in the inferior frontal lobe,12 although this has not been fully validated in the literature. Despite this, there is some evidence linking the UF to language functionality. For example, in one study of patients with left hemispheric lesions and aphasia, the researchers found an association between the fractional anisotropy score of the left UF and performance on 2 different semantic-related language tasks.34 Still others have reported a significant reduction in fractional anisotropy scores of the left UF in patients with semantic dementia.9,10 In addition to these studies, consideration of the anatomy of the left UF suggests it plays an important role in semantic memory and its retrieval, as both the temporal lobes and the frontal lobes have been described as participating in these processes.3,7,8 Despite the evidence for the role of the UF in human language, others have reported contradictory results. For example, it is well known that transecting the UF during left temporal lobectomy does not lead to severe semantic memory problems.35,36
Beyond its role in language processing on the left, the UF on the right is associated with emotional understanding and empathy.6 In their study, Oishi et al6 were able to demonstrate that acute lesions to the right uncinate were independently associated with impaired empathy, which was measured via a series of yes-no and multiple choice questions requiring patients to make emotional inferences. The right uncinate also appears to play a role in autonoetic self-awareness,37 meaning the process of being able to consciously place oneself in his or her past experiences,37 thereby allowing for self-reflection and re-examination of past events. The UF has also been proposed as part of the limbic system, specifically playing a role in emotional regulation,1 as well as subsuming a role in reversal learning processes related to impulsive decision making.4,5
CONCLUSION
While the structural parcellation scheme of the uncinate presented in this study may appear quite simple, the composite of functions attributed to the UF is quite complex. With functional roles ranging from semantic language processing to impulsive decision making, the uncinate likely participates in several critical cognitive behavioral networks in the brain. In addition to this functional complexity, structural damage and abnormalities in the UF have been identified in a significant number of neuropsychiatric disease states. Further, subtract guided functional and anatomic studies are needed to enhance our understanding of the functional connectivity of the UF. However, our tractographic map of this white matter pathway can serve as a reference point for these future studies as we move forward to describe these complex networks.
Disclosures
Synaptive Medical assisted in the funding of all 18 chapters of this supplement. No other funding sources were utilized in the production or submission of this work.
Acknowledgments
Data were provided [in part] by the Human Connectome Project, WU- Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blue print for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University. We would also like to thank Brad Fernald, Haley Harris, and Alicia McNeely of Synaptive Medical for their assistance in constructing the network figures for Chapter 18 and for coordinating the completion and submission of this supplement.
REFERENCES
- 1. Von Der Heide RJ, Skipper LM, Klobusicky E, Olson IR. Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain. 2013;136(6):1692-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kier EL, Staib LH, Davis LM, Bronen RA. MR imaging of the temporal stem: Anatomic dissection tractography of the uncinate fasciculus, inferior occipitofrontal fasciculus, and Meyer's loop of the optic radiation. AJNR Am J Neuroradiol. 2004;25(5):677-691. [PMC free article] [PubMed] [Google Scholar]
- 3. Olson IR, Von Der Heide RJ, Alm KH, Vyas G. Development of the uncinate fasciculus: Implications for theory and developmental disorders. Dev Cogn Neurosci. 2015;14:50-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rudebeck PH, Saunders RC, Prescott AT, Chau LS, Murray EA. Prefrontal mechanisms of behavioral flexibility, emotion regulation and value updating. Nat Neurosci. 2013;16(8):1140-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alm KH, Rolheiser T, Mohamed FB, Olson IR. Fronto-temporal white matter connectivity predicts reversal learning errors. Front Hum Neurosci. 2015;9:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oishi K, Faria AV, Hsu J, Tippett D, Mori S, Hillis AE. Critical role of the right uncinate fasciculus in emotional empathy. Ann Neurol. 2015;77(1):68-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Catani M, Mesulam M. The arcuate fasciculus and the disconnection theme in language and aphasia: History and current state. Cortex. 2008;44(8):953-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grossman M, McMillan C, Moore P et al. . What's in a name: voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer's disease, frontotemporal dementia and corticobasal degeneration. Brain. 2004;127(Pt 3):628-649. [DOI] [PubMed] [Google Scholar]
- 9. Agosta F, Galantucci S, Canu E et al. . Disruption of structural connectivity along the dorsal and ventral language pathways in patients with nonfluent and semantic variant primary progressive aphasia: A DT MRI study and a literature review. Brain Lang. 2013;127(2):157-166. [DOI] [PubMed] [Google Scholar]
- 10. Agosta F, Henry RG, Migliaccio R et al. . Language networks in semantic dementia. Brain. 2010;133(1):286-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chang EF, Raygor KP, Berger MS. Contemporary model of language organization: an overview for neurosurgeons. J Neurosurg. 2015;122(2):250-261. [DOI] [PubMed] [Google Scholar]
- 12. Dick AS, Bernal B, Tremblay P. The language connectome: new pathways, new concepts. Neuroscientist. 2014;20(5):453-467. [DOI] [PubMed] [Google Scholar]
- 13. Bozzali M, Serra L, Cercignani M. Quantitative MRI to understand Alzheimer's disease pathophysiology. Curr Opin Neurol. 2016;29(4):437-444. [DOI] [PubMed] [Google Scholar]
- 14. Yasmin H, Nakata Y, Aoki S et al. . Diffusion abnormalities of the uncinate fasciculus in Alzheimer's disease: diffusion tensor tract-specific analysis using a new method to measure the core of the tract. Neuroradiology. 2008;50(4):293-299. [DOI] [PubMed] [Google Scholar]
- 15. Bas-Hoogendam JM, Blackford JU, Bruhl AB, Blair KS, van der Wee NJ, Westenberg PM. Neurobiological candidate endophenotypes of social anxiety disorder. Neurosci Biobehav Rev. 2016;71:362-378. [DOI] [PubMed] [Google Scholar]
- 16. Williamson JB, Heilman KM, Porges EC, Lamb DG, Porges SW. A possible mechanism for PTSD symptoms in patients with traumatic brain injury: central autonomic network disruption. Front. Neuroeng. 2013;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Peng Z, Lui SS, Cheung EF et al. . Brain structural abnormalities in obsessive-compulsive disorder: converging evidence from white matter and grey matter. Asian J Psychiatry. 2012;5(4):290-296. [DOI] [PubMed] [Google Scholar]
- 18. McIntosh AM, Munoz Maniega S, Lymer GK et al. . White matter tractography in bipolar disorder and schizophrenia. Biol Psychiatry. 2008;64(12):1088-1092. [DOI] [PubMed] [Google Scholar]
- 19. Bracht T, Linden D, Keedwell P. A review of white matter microstructure alterations of pathways of the reward circuit in depression. J Affect Disord. 2015;187:45-53. [DOI] [PubMed] [Google Scholar]
- 20. Taylor WD, MacFall JR, Gerig G, Krishnan RR. Structural integrity of the uncinate fasciculus in geriatric depression: relationship with age of onset. Neuropsychiatr Dis Treat. 2007;3(5):669-674. [PMC free article] [PubMed] [Google Scholar]
- 21. Nakamura M, McCarley RW, Kubicki M et al. . Fronto-temporal disconnectivity in schizotypal personality disorder: a diffusion tensor imaging study. Biol Psychiatry. 2005;58(6):468-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Park HJ, Westin CF, Kubicki M et al. . White matter hemisphere asymmetries in healthy subjects and in schizophrenia: a diffusion tensor MRI study. Neuroimage. 2004;23(1):213-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gurrera RJ, Nakamura M, Kubicki M et al. . The uncinate fasciculus and extraversion in schizotypal personality disorder: a diffusion tensor imaging study. Schizophr Res. 2007;90(1-3):360-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chiapponi C, Piras F, Fagioli S, Piras F, Caltagirone C, Spalletta G. Age-related brain trajectories in schizophrenia: a systematic review of structural MRI studies. Psychiatry Res. 2013;214(2):83-93. [DOI] [PubMed] [Google Scholar]
- 25. Waller R, Dotterer HL, Murray L, Maxwell AM, Hyde LW. White-matter tract abnormalities and antisocial behavior: a systematic review of diffusion tensor imaging studies across development. NeuroImage. 2017;14:201-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Craig MC, Catani M, Deeley Q et al. . Altered connections on the road to psychopathy. Mol Psychiatry. 2009;14(10):946-953. [DOI] [PubMed] [Google Scholar]
- 27. Jellison BJ, Field AS, Medow J, Lazar M, Salamat MS, Alexander AL. Diffusion tensor imaging of cerebral white matter: a pictorial review of physics, fiber tract anatomy, and tumor imaging patterns. AJNR Am J Neuroradiol. 2004;25(3):356-369. [PMC free article] [PubMed] [Google Scholar]
- 28. Glasser MF, Coalson TS, Robinson EC et al. . A multi-modal parcellation of human cerebral cortex. Nature. 2016;536(7615):171-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kamali A, Flanders AE, Brody J, Hunter JV, Hasan KM. Tracing superior longitudinal fasciculus connectivity in the human brain using high resolution diffusion tensor tractography. Brain Struct Funct. 2014;219(1):269-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Menjot de Champfleur N, Lima Maldonado I, Moritz-Gasser S et al. . Middle longitudinal fasciculus delineation within language pathways: a diffusion tensor imaging study in human. Eur J Radiol. 2013;82(1):151-157. [DOI] [PubMed] [Google Scholar]
- 31. Lemaire JJ, Cosnard G, Sakka L et al. . White matter anatomy of the human deep brain revisited with high resolution DTI fibre tracking. Neurochirurgie. 2011;57(2):52-67. [DOI] [PubMed] [Google Scholar]
- 32. Yeh FC, Wedeen VJ, Tseng WY. Generalized q-sampling imaging. IEEE Trans Med Imaging. 2010;29(9):1626-1635. [DOI] [PubMed] [Google Scholar]
- 33. Chang EF, Raygor KP, Berger MS. Contemporary model of language organization: an overview for neurosurgeons. J Neurosurg. 2015;122(2):250-261. [DOI] [PubMed] [Google Scholar]
- 34. Harvey DY, Wei T, Ellmore TM, Hamilton AC, Schnur TT. Neuropsychological evidence for the functional role of the uncinate fasciculus in semantic control. Neuropsychologia. 2013;51(5):789-801. [DOI] [PubMed] [Google Scholar]
- 35. Papagno C, Miracapillo C, Casarotti A et al. . What is the role of the uncinate fasciculus? Surgical removal and proper name retrieval. Brain. 2011;134(2):405-414. [DOI] [PubMed] [Google Scholar]
- 36. Drane DL, Ojemann GA, Aylward E et al. . Category-specific naming and recognition deficits in temporal lobe epilepsy surgical patients. Neuropsychologia. 2008;46(5):1242-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Levine B, Black SE, Cabeza R et al. . Episodic memory and the self in a case of isolated retrograde amnesia. Brain. 1998;121 (10):1951-1973. [DOI] [PubMed] [Google Scholar]